Abstract
The ER Ca2+ sensor STIM1 recruits and activates the plasma membrane (PM) Ca2+ channel Orai1 at ER-PM junctions for store-operated Ca2+ entry (SOCE). Reporting in Nature, Sharma et al. (2013) showed that septins are necessary for Orai1 recruitment and SOCE, implicating these scaffolding proteins in signaling at ER-PM junctions.
Store-operated Ca2+ entry (SOCE) is a ubiquitous signaling pathway that controls a range of cellular functions in different cell types (Lewis, 2011). SOCE is triggered by stimulation of diverse cell surface receptors that induce release of Ca2+ from endoplasmic reticulum (ER) Ca2+ stores. The depletion of ER Ca2+ subsequently leads to opening of Ca2+ channels in the plasma membrane (PM), resulting in Ca2+ influx from the extracellular space into the cytosol. SOCE is important to refill ER Ca2+ store after depletion to prevent ER stress responses and to enable repeated release of ER Ca2+. In addition, SOCE can persistently elevate cytosolic Ca2+ signals to activate Ca2+ effectors including the transcription factor NFAT. Patients with defective SOCE suffer severe combined immunodeficiency, muscular dystrophy and other diseases (Feske, 2010).
Two key regulators of SOCE were identified to be a luminal ER Ca2+ sensor STIM1 and a PM Ca2+ channel Orai1 (Carrasco and Meyer, 2011). Over-expression of STIM1 and Orai1 in the same cells results in a large amplification of SOCE, indicating that STIM1 and Orai1 are the two limiting components of SOCE. There were initially two competing mechanisms for the activation of STIM1, one based on TIRF imaging of YFP-STIM1 which showed that STIM1 senses ER luminal Ca2+ and translocates within the ER to specific sites where the ER membrane is in close proximity to the PM but without inserting into the PM (Liou et al, 2005) and one based on the insertion of the ER localized STIM1 into the PM after ER store depletion (Zhang et al., 2005). Most recent data supported the first model, arguing that a reduction in ER Ca2+ levels causes Ca2+ dissociation from the EF hand STIM1 Ca2+ binding domain in the ER lumen, leading to STIM1 oligomerization, STIM1 activation, and its translocation to ER-PM junctions in a process that involves binding of its C-terminal polybasic domain to PM phosphoinositide lipids (Liou et al., 2007). In addition, STIM1 oligomerization exposes the Orai1-interacting SOAR/CAD/ccb9 domain that is responsible for recruiting Orai1 to ER-PM junctions (Lewis, 2011). The same interaction between STIM1 and Orai1 at ER-PM junctions activates Orai1 and induces a local Ca2+ influx into the cytosol.
SOCE is dependent on the existence of ER-PM junctions since STIM1 is an ER membrane protein that can only reach the PM channel Orai1 at these junctions. Thus, it is expected that molecules that affect the number, size, gap distance, and distribution of ER-PM junctions may regulate SOCE (Lewis, 2011). ER-PM junctions are sites where the ER is in close proximity with the PM allowing direct interactions of molecules in the heterologous membranes. We are only at the beginning of understanding how these evolutionarily conserved subcellular structures are formed and regulated. Two recent reports showed that extended synaptotagmins (E-Syts) and their yeast orthologs tricalbins contribute to the formation and maintenance of ER-PM junctions (Giordano et al., 2013; Manford et al., 2012). Interestingly, SOCE is not affected in E-Syt-depleted HeLa cells even when the amount of ER-PM junctions was significantly reduced (Giordano et al., 2013). Thus, at least in HeLa cells, there is an excess of ER-PM junctions enabling STIM1 and Orai1 to interact even if the number of contacts is significantly reduced. Nevertheless, regulation of other structural aspects of ER-PM junctions may still modulate SOCE.
The SOCE mediator STIM1 was independently identified in focused RNA interference (RNAi) screens for putative human signaling proteins and putative Drosophila ion channel proteins while Orai1 was identified in three independent genome-wide Drosophila RNAi screens (Carrasco and Meyer, 2011). The success of these different RNAi screening strategies was a motivation for the recent work described in Nature from Sharma et al. (2013), where the authors performed a human genome-wide SOCE and NFAT signaling screen using a NFAT1-GFP reporter that senses persistent Ca2+ increases from SOCE. They found that, siSEPT, a pool of small interfering RNA that knocked down the expression of septin 4, septin 5, and partially septin 2, was effective at blocking nuclear translocation of NFAT1-GFP. Re-expression of either septin 4 or septin 5 rescued the defect. Sharma et al. (2013) further demonstrated that siSEPT treatment resulted in a selective reduction of SOCE, indicating that septins are necessary regulators of SOCE. Although STIM1 translocation to ER-PM junctions was only partially affected by the loss of septins, the subsequent recruitment of Orai1 to ER-PM junctions was significantly compromised in siSEPT-treated cells (Figure 1). The defective SOCE in siSEPT-treated cells could be rescued by expressing a soluble STIM1 fragment containing the Orai1-interacting domain, suggesting that the SOCE defect is likely a result of inefficient recruitment of Orai1 to STIM1 at ER-PM junctions. Finally, the authors observed a relocalization of septin 4 at the PM after ER Ca2+ store depletion.
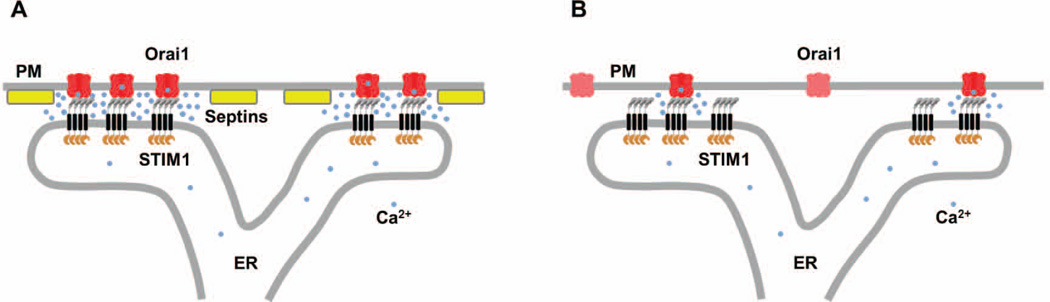
Figure 1. Septins regulate Orai1 recruitment to STIM1 at ER-PM junctions during SOCE.
(A) Sharma et al. (2013) showed that septins regulate the recruitment of the PM Ca2+ channel Orai1 to the ER membrane protein STIM1 at ER-PM junctions, where the ER is closely opposed to the PM, following ER Ca2+ store depletion. STIM1 directly interacts with Orai1 via a C terminal domain in the cytosolic region. The interaction between STIM1 and Orai1 results in Orai1 activation and Ca2+ entry into the cytosol.
(B) In cells treated with siRNA to deplete septins, ER Ca2+ store depletion triggers STIM1 translocation to ER-PM junctions. However, the colocalization of Orai1 with STIM1 is significantly reduced, resulting in decreased SOCE. Similar defects in Orai1 recruitment and SOCE are observed in cells treated with forchlorfenuron that hyperpolymerizes and stabilizes septin filaments.
How do septins regulate Orai1 distribution in the PM? Septins are a family of GTP-binding proteins that form hetero-oligomeric complexes that can further assemble into higher-order structures such as filaments and rings (Mostowy and Cossart, 2012). Septins can create lateral diffusion barriers for membrane compartmentalization as has been shown at the base of cilia and at the midbody during cytokinesis. Although a complete mechanistic explanation is still missing,Sharma et al. (2013) now propose a new functional role of septins. They observed that septin 4 relocalizes to the PM and thereby promotes interaction of Orai1 with ER-PM junction localized STIM1 following ER Ca2+ store depletion. They proposed that the effects of septin 4 may be mediated by its binding to phosphatidylinositol (4,5)-bisphosphate (PIP2) at the PM. As potential mechanisms, it is plausible that septins directly or indirectly put Orai1 in a state in which it can freely diffuse within the PM to bind STIM1 at ER-PM junctions following ER Ca2+ store depletion. Additionally, rearrangement of septin filaments around ER-PM junctions during SOCE may stabilize the formation of STIM1-Orai1 complexes. Nevertheless, alternative more indirect effects may also need to be considered. It has for example been shown that SOCE and Ca2+ increases enhance cell adhesion which can bring the PM closer to the adhesion surface, which may in turn increase signals from fluorescently tagged septins or PIP2 sensors in selected regions of the cell, potentially explaining some of the apparent recruitment data. While the presented data are intriguing, more mechanistic data will be needed to understand how septins contribute to signaling at ER-PM junctions.
In conclusion, Sharma et al. (2013) provide a genome-wide dataset of putative regulators of SOCE and NFAT nuclear translocation and identify septins as new regulators of SOCE that promote efficient interactions of Orai1 with STIM1. These findings add an interesting structural and regulatory protein to the known players controlling signaling at ER-PM junctions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Carrasco S, Meyer T. Annu Rev Biochem. 2011;80:973–1000. doi: 10.1146/annurev-biochem-061609-165311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feske S. Pflugers Arch. 2010;460:417–435. doi: 10.1007/s00424-009-0777-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giordano F, Saheki Y, Idevall-Hagren O, Colombo SF, Pirruccello M, Milosevic I, Gracheva EO, Bagriantsev SN, Borgese N, De Camilli P. Cell. 2013;153:1494–1509. doi: 10.1016/j.cell.2013.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis RS. Cold Spring Harb Perspect Biol. 2011;3 doi: 10.1101/cshperspect.a003970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liou J, Kim ML, Heo WD, Jones JT, Myers JW, Ferrell JE, Jr, Meyer T. Curr Biol. 2005;15:1235–1241. doi: 10.1016/j.cub.2005.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liou J, Fivaz M, Inoue T, Meyer T. Proc Natl Acad Sci U S A. 2007;104:9301–9306. doi: 10.1073/pnas.0702866104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manford AG, Stefan CJ, Yuan HL, Macgurn JA, Emr SD. Dev Cell. 2012;23:1129–1140. doi: 10.1016/j.devcel.2012.11.004. [DOI] [PubMed] [Google Scholar]
- Mostowy S, Cossart P. Nat Rev Mol Cell Biol. 2012;13:183–194. doi: 10.1038/nrm3284. [DOI] [PubMed] [Google Scholar]
- Sharma S, Quintana A, Findlay GM, Mettlen M, Baust B, Jain M, Nilsson R, Rao A, Hogan PG. Nature. 2013;499(7457):238–42. doi: 10.1038/nature12229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang SL, Yu Y, Roos J, Kozak JA, Deerinck TJ, Ellisman MH, Stauderman KA, Cahalan MD. Nature. 2005;437:902–5. doi: 10.1038/nature04147. [DOI] [PMC free article] [PubMed] [Google Scholar]