Abstract
Objective
The objective of this study was to assess the effects of HAART initiation on CD4+ T-cell repopulation and T-cell immune activation in rectal and duodenal mucosa.
Design
The effects of HAART on the gastrointestinal (GI) tract remain controversial, and studies have reached different conclusions regarding its effectiveness at restoring mucosal CD4+ T-cells depending upon time of initiation, duration of treatment, and GI tract region studied.
Methods
We obtained blood, rectal biopsies, and duodenal biopsies from fourteen chronically infected individuals at baseline and at four to nine months post HAART initiation. We examined CD4+ T-cell frequencies in blood, rectum and duodenum at both time points, and performed a detailed assessment of CD4+ T-cell phenotype, immune activation marker expression, and HIV-specific CD8+ T-cell responses in blood and rectal mucosa.
Results
CD4+ T-cell percentages increased significantly in blood, rectal, and duodenal mucosa after four to nine months of HAART (p = 0.02, 0.0005, 0.0002), but remained lower than in uninfected controls. HIV-specific CD8+ T-cell responses in blood and rectal mucosa declined following HAART initiation (p=0.0015, 0.021). CD8+ T-cell coexpression of CD38 and HLA-DR in blood and mucosa, as well as plasma sCD14, declined significantly. CD28 expression on blood and mucosal CD8+ T-cells increased, while PD-1 expression on blood HIV-specific CD4+ and CD8+ T-cells decreased.
Conclusions
Within the first months of HAART, limited CD4+ T-cell reconstitution occurs in small and large intestinal mucosa. Nevertheless, decreased immune activation and increased CD28 expression suggest rapid immunological benefits of HAART despite incomplete CD4+ T-cell reconstitution.
Keywords: HIV, HAART, Gut, mucosa, T-cell, immune activation
Introduction
Primary HIV-1 infection is associated with rapid and massive depletion of mucosal CD4+ T-cells in the gastrointestinal (GI) tract, home to the majority of the body’s lymphocytes [1]. CD4+ T-cell loss occurs more rapidly in the gut than in peripheral blood [2–5]. With recent advances in highly active antiretroviral therapy (HAART), many HIV-infected individuals are able to maintain plasma viremia at levels undetectable by most assays. This viral suppression is also associated with significant reconstitution of blood CD4+ T-cells. However, CD4 recovery is often incomplete, failing to reach levels observed in uninfected individuals. The extent of recovery may vary widely depending on such factors as age and extent of immunodeficiency at the time of HAART initiation [6, 7]. In lymph nodes, immune activation, contributing to collagen deposition and lymphoid tissue fibrosis, may severely limit CD4+ T-cell reconstitution [8]. Similar mechanisms likely impact CD4+ T-cell reconstitution in the gut lamina propria [9]. However, the kinetics and extent of HAART-induced CD4+ T-cell reconstitution in the gut are less well understood. To date, most studies have focused on a single portion of the GI tract, such as jejunum (12, 16, 23), ileum (3), or colon [10–16]. Sheth et al. observed near complete recovery of CD4+ T-cells in sigmoid colon following long-term HAART [10, 15], while Chun et al. saw incomplete recovery in terminal ileum [15]. These discrepancies may be related to differences between patient groups and treatment protocols, and/or to differences between small and large intestine. To our knowledge, no studies have longitudinally tracked changes in mucosal memory/effector T-cell subsets, immune activation and antigen-specific T-cell responses during the weeks and months immediately following HAART initiation.
Based upon earlier studies, we predicted that initiation of suppressive HAART in chronically HIV-infected individuals would lead to measurable reductions in lymphocyte activation and begin to elicit mucosal CD4+ T-cell reconstitution in both small and large intestine. To address these issues, we sampled rectal and duodenal mucosa from fourteen chronically infected individuals prior to initiation of HAART, then at a second time point 4 to 9 months post-initiation. We also performed a detailed analysis of HIV-specific CD8+ T-cell responses and expression of T-cell activation and phenotypic markers in blood and rectal mucosa at both time points. Our data revealed incomplete CD4+ T-cell reconstitution; nevertheless, significant increases in CD4+ T-cell percentages were detected in blood, rectal and duodenal mucosa, along with decreased expression of immune activation markers and a decline in HIV-specific T-cell response magnitudes in blood and mucosa.
Methods
Subjects and HAART
Fourteen HIV-seropositive subjects, all infected for a minimum of 1 year and naïve to antiretroviral therapy (ART), or who had been briefly exposed to ART (less than 30 days) in the past were enrolled. The only exclusion criteria were safety parameters related to upper endoscopy with biopsies (subjects with Grade II anemia or abnormal coagulation parameters were excluded). After baseline clinical parameters were established, the subjects were prescribed a HAART regimen. The 14 HIV positive subjects in this research study were also participating in a separate clinical trial comparing the effects of a raltegravir (RGV)-based HAART regimen versus a non-nucleoside reverse transcriptase inhibitor (NNRTI)-based HAART regimen on gut immune reconstitution in HAART-naïve subjects (Clinical Trial Registry Number NCT00661960). The primary findings from the clinical trial have been reported elsewhere [17]. Written informed consent for phlebotomy, rectal and duodenal biopsy was obtained through study protocols approved by the Institutional Review Board, School of Medicine, University of California, Davis and the UC Davis CTSC Clinical Research Center (CCRC).
Sample preparation
Samples were obtained from gastrointestinal mucosa and peripheral blood at baseline or four to nine months after initiation of HAART. Blood was collected by sterile venipuncture using ethylenediamine tetraacetic acid (EDTA) as an anticoagulant. Rectal biopsies were obtained via flexible sigmoidoscopy at 10–15 cm from the anal verge as previously described [18]. Duodenal biopsies were obtained via upper endoscopy. Rectal and duodenal biopsies and blood were immediately transported to the laboratory and processed on the day of collection.
Peripheral blood mononuclear cells (PBMC) were isolated from blood using Ficoll-Paque™ (Pfizer-Pharmacia, New York, NY). Isolation of mononuclear cells from rectal and duodenal biopsies was performed as previously described [19]. Following isolation, PBMC, duodenal (DMC) and rectal mononuclear cells (RMC) were either stained the same day for cell surface phenotypic analysis, or incubated overnight in complete medium [RPMI-1640 supplemented with fetal calf serum (15%), penicillin (100 U/ml), streptomycin (100 µg/ml), and glutamine (2 mM)]. DMC and RMC were treated with Piperacillin-Tazobactam (0.5 mg/ml) (Zosyn®, Wyeth Pharmaceuticals, Philadelphia, PA) to inhibit overgrowth of intestinal bacteria.
Flow Cytometry
For immunophenotypic analysis, freshly isolated PBMC and RMC were labeled with fluorochrome-conjugated antibodies. Cells were stained with Aqua Amine Reactive Dye (Invitrogen Molecular Probes, Eugene, OR) to discriminate dead cells and the following fluorescently-conjugated monoclonal antibodies: CD3 clone UCHT1 (Pacific Blue), CD8 clone SK1 (QDot 605), CD38 clone HIT2 (PE-Cy5), CCR7 clone 3D12 (PE-Cy7), HLA-DR clone Tu-39 (FITC), CXCR4 clone 12G5 (APC), and CCR5 clone 2D7 (PE) (BD Biosciences, San Jose, CA); CD45RA clone 2H4 (ECD) (Beckman Coulter, Fullerton, CA); CD4 clone S3.5 (APC-Cy5.5), (Invitrogen Caltag, Burlingame, CA). In some experiments, CD28 clone CD28.2 (PE-Cy7) (eBioscience) was included. In each experiment, fluorescence minus one controls (FMO) were included to facilitate gating [20]. Stained cells were washed, fixed in 1% formaldehyde and stored at 4°C until data acquisition (within 24 hours).
Intracellular cytokine staining (ICS) was performed on PBMC and RMC rested overnight at 37°C and 5% CO2 as described previously [21]. Briefly, cells were stimulated with HIV-1 Consensus B Gag overlapping peptides (JPT Peptide Technologies, Berlin, Germany) at 3.5 µg/ml for each peptide and with the costimulatory antibodies CD28 (2.5 µg/ml) and CD49d (5 µg/ml), in the presence of 5 µg/ml brefeldin A (Sigma-Aldrich, St. Louis, MO) and 1 µM monensin (GolgiStop™, BD Biosciences). PE-Cy5-labelled CD107a antibody clone H4A3 (PE-Cy5) (BD Biosciences) was also added, and the cells were incubated for 5 hours at 37° and 5% CO2. A negative control well (cells stimulated with costimulatory antibodies and DMSO alone) and a positive control well (CEF peptides: immunodominant peptides from cytomegalovirus, Epstein-Barr virus, and influenza virus, JPT Peptide Technologies) were run in parallel for each sample.
Following stimulation, cells were stained for surface antigens CD4, CD8, and PD-1 clone J105 (PE) (eBiosciences, San Diego, CA) and for viability (Aqua Amine Reactive Dye, AARD, Invitrogen). Cells were fixed and then permeabilized using FACS Perm 2 (BD Biosciences). For intracellular staining, cells were incubated with antibodies against CD3, IFNγ clone B27 (PE-Cy7), IL-2 clone 5344.111 (APC), and TNF-α clone Mab11 (Alexa 700) (all from BD Biosciences). Cells were then washed and stored at 4°C in 1% formaldehyde until acquisition. Flow cytometry data were acquired on an LSRII (BD Immunocytometry Systems, San Jose, CA) and analyzed using FlowJo Software, V.8 (TreeStar, Ashland, OR). For experiments measuring four functional responses, individual responses were evaluated alone and also processed through Boolean combinations to partition responding cells into one of 16 possible specific response categories. Cytometry data were biexponentially transformed in order to include all events. SPICE software (Mario Roederer, Vaccine Research Center, NIAID/NIH, Bethesda, MD) was used to graph response data [22].
Plasma viral loads
Plasma was separated from EDTA-anti-coagulated blood. The quantification of HIV RNA copy number was performed by reverse transcriptase PCR using the Amplicor HIV-1 Monitor Standard and UltraSensitive kits (Roche Diagnostic, Branchburg, New Hampshire).
Soluble CD14 assay
Soluble CD14 levels in plasma samples were quantified by ELISA with the Quantikine Human sCD14 Immunoassay (R&D Systems, Minneapolis, MN, USA) according to the manufacturer’s protocol. Samples were assayed in duplicate.
Statistical methods
Mean values of CD4+ T-cell count, gut CD4+ T-cell percentages, HIV viral load, T-cell differentiation status, costimulatory molecule expression, and immune activation levels were compared before and after HAART initiation. Statistical analyses were performed using paired Student's t test or Mann-Whitney tests, when appropriate, and Wilcoxon's signed rank test. P values were two-tailed and were considered significant when less than 0.05. GraphPad Prism (GraphPad Software, San Diego, CA) and XLStat software (Addinsoft SARL, Paris, France) were used for statistical analyses.
Results
Baseline patient characteristics
The study participants included 3 females and 11 males with a median age of 38 years (Table 1). HAART-naïve patients had median absolute CD4+ T-cell counts of 328 cells per µL and a median viral load of 29,000 RNA copies per mL plasma. Peripheral blood and rectal mucosa CD4+ T-cell data from 10 seronegative subjects enrolled in previous studies were used as historical controls along with data from two HIV-negative volunteers enrolled in the present study to provide reference values. Seronegatives included 6 females and 4 males with an average age of 41 years; whenever possible, these individuals were recruited from the same risk groups as HIV positive subjects.
Table 1.
Baseline patient characteristics.
| Patient ID | Gendera | Raceb | Age | CD4 countc | Plasma VLd | HAARTe |
|---|---|---|---|---|---|---|
| 104 | M | C | 42 | 293 | 29,000 | NVP |
| 105 | F | AA | 38 | 495 | 11,300 | RGV |
| 107 | M | C | 29 | 415 | 66,701 | RGV |
| 110 | M | C | 28 | 454 | 974 | EFV to ETV |
| 111 | M | C | 33 | 485 | 16,800 | NVP |
| 115 | M | CH | 32 | 36 | 552,000 | EFV |
| 116 | F | AA | 47 | 258 | 19,149 | RGV |
| 117 | M | C | 46 | 304 | 121,916 | RGV |
| 94 | M | C | 56 | 593 | 30,500 | EFV |
| 118 | F | CH | 26 | 328 | 26,168 | EFV to ETV |
| 119 | M | C | 47 | 361 | 48,400 | EFV |
| 124 | M | C | 46 | 165 | 117,600 | RGV |
| 125 | M | C | 37 | 407 | 159,000 | RGV |
| 131 | M | CH | 25 | 190 | 23,800 | RGV |
M, male; F, female
C, Caucasian; AA, African-American; CH, Caucasian with Hispanic ethnicity
CD4+ T-cells/uL;
RNA copies/mL;
Compound on which HAART regimen was based: NVP, nevirapine; RGV, raltegravir; EFV, efavirenz; ETV, etavirine.
Virus suppression and CD4+ T-cell reconstitution
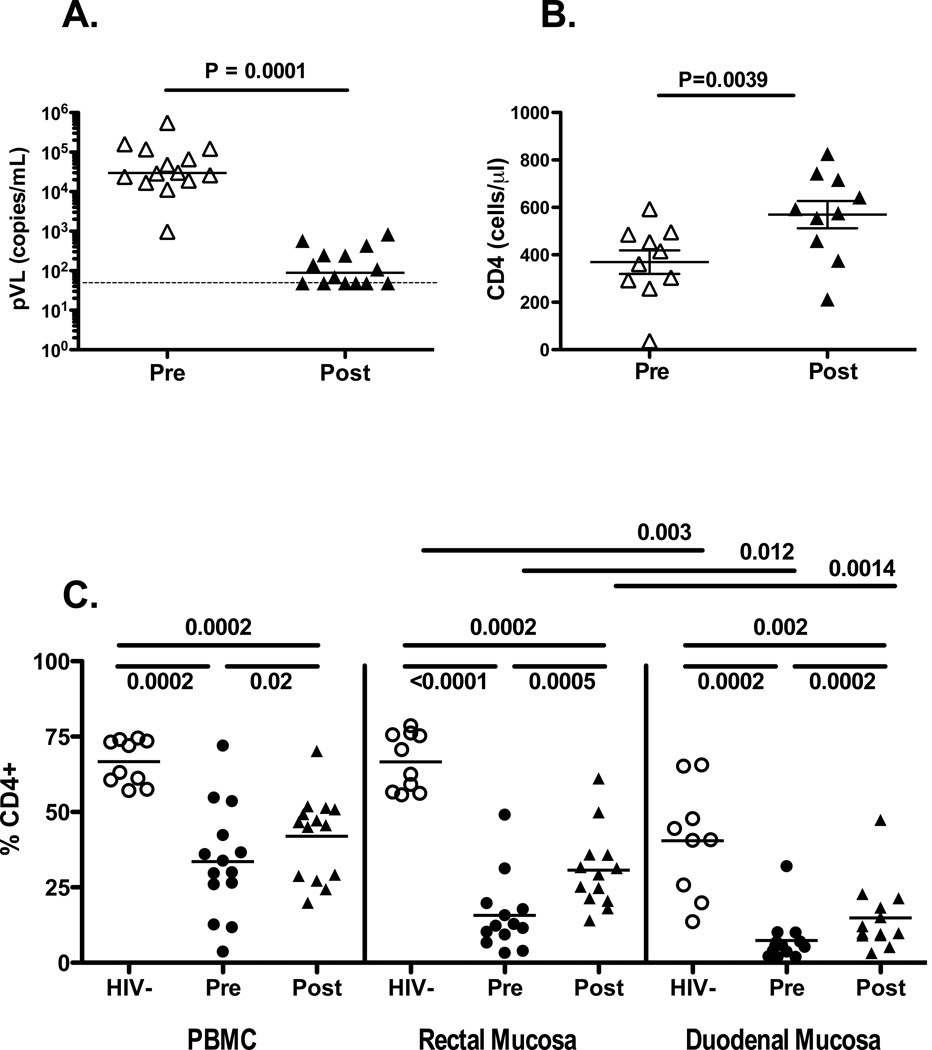
The initial median plasma virus load was 29,000 RNA copies/mL with a range of 974 to 552,000 copies/mL (Table 1). HAART significantly reduced median plasma virus load to 108 copies/mL (Figure 1A) with no detectable virus in six individuals. Median pre-HAART mucosal CD4+ T-cell percentages, presented here as proportion of CD3+ cells expressing CD4 but not CD8, were 12.3% in rectal mucosa and 5.6% in duodenal mucosa. In blood, rectal and duodenal mucosa, significant increases were observed in total CD4+ T-cell percentages after HAART, although in all three cases, post-therapy levels were still significantly lower than CD4+ T-cell percentages in uninfected controls (Figure 1B). It is important to note that the percentage of CD4+ T-cells in duodenal mucosa was significantly lower than in rectal mucosa; this was true for healthy control individuals as well as for HIV-positive subjects pre and post-HAART.
Figure 1.
(A) Viral load suppression in patients on HAART. Values on the y-axis indicate plasma viral load (VL), as HIV vRNA copies/mL. Each triangle corresponds to a single patient. Open figures represent pre-HAART viral load; filled figures show post-HAART viral load. Horizontal lines indicate median values. The dashed line represents the limit of detection of the assay. P value was determined by Wilcoxon signed rank test. (B) Changes in blood CD4+ T-cell counts. Clinical laboratory values for blood CD4 counts (cells/µL) are summarized pre- and post-HAART. P value was determined by Wilcoxon signed rank test. (C) Changes in CD4+ T-cell frequency following HAART. Flow cytometry data are summarized as percentages, gating on CD3+CD4+ T-cells (y-axis). Data from seronegative controls are presented as open circles; pre-HAART data are presented as filled circles; post-HAART data are shown as filled triangles. Horizontal lines indicate mean values. P values were determined by Mann-Whitney U test or Wilcoxon signed rank test, as appropriate.
Using linear regression analysis, we tested for significant correlations between baseline CD4 count, baseline VL, and immune reconstitution in blood and gut. There were no significant relationships between either baseline CD4 count or VL and CD4 reconstitution in blood, rectal or duodenal mucosa.
Given that the time of evaluation post-HAART varied from 4 to 9 months, we used regression analysis to check for any significant relationships or trends between time of evaluation post HAART and CD4+ T-cell reconstitution in blood and rectal mucosa. No significant relationships were detected between time of evaluation and any of the following: change in blood CD4+ T-cell count, blood CD4+ T-cells as a percentage of CD3+ T-cells, or rectal mucosa CD4+ T-cells as a percentage of CD3+ T-cells.
Changes in T-cell memory/effector phenotype after HAART initiation
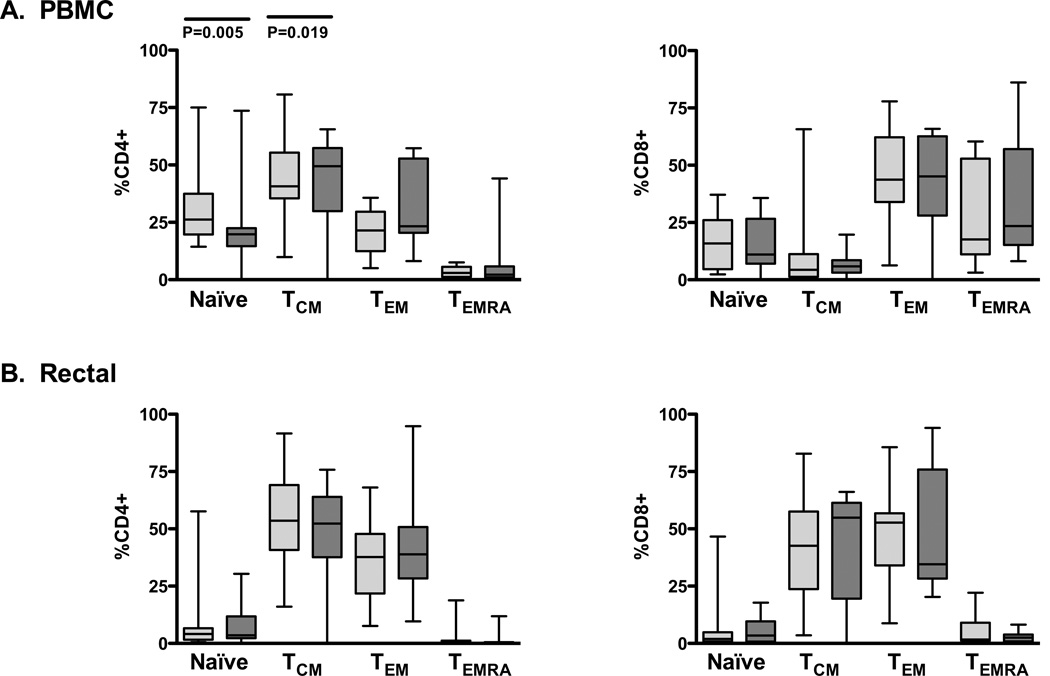
To examine the effect of HAART initiation on T-cell differentiation profiles, blood and rectal CD4+ and CD8+ T-cells were analyzed by flow cytometry for expression of maturation markers CCR7 and CD45RA (Figure 2) [23]. Cells expressing both CCR7 and CD45RA were considered naïve, cells positive for CCR7 but negative for CD45RA were considered central memory (TCM), cells expressing neither antigen were designated as effector memory (TEM), and cells expressing CD45RA but not CCR7 were considered terminally differentiated effectors (TEMRA) [23, 24]. Changes in these subsets were monitored longitudinally in each patient before and after HAART. Few significant differences in memory/effector phenotype were observed between pre- and post-HAART T cell subsets. After HAART initiation, a significant increase was observed in the blood CD4+ TCM population along with a concomitant decrease in the peripheral CD4+ naïve T-cell subset. However, significant early HAART effects were not apparent in other CD4+ or CD8+ T-cell memory subsets in either blood or rectal mucosa.
Figure 2. HAART-induced maturation changes in peripheral and mucosal T-cells.
Box plots show interquartile ranges with horizontal lines at the median. Pale gray boxes represent pre-HAART data points while dark gray boxes represent post-HAART data. P values were determined by Wilcoxon signed rank test. Four subsets are shown: Naïve T-cells (CCR7+CD45RA+), central memory T-cells (TCM, CCR7+CD45RA-), effector memory T-cells (TEM, CCR7-CD45RA-), and terminally differentiated effectors (TEMRA, CCR7-CD45RA+).
Expression of T-cell costimulatory markers
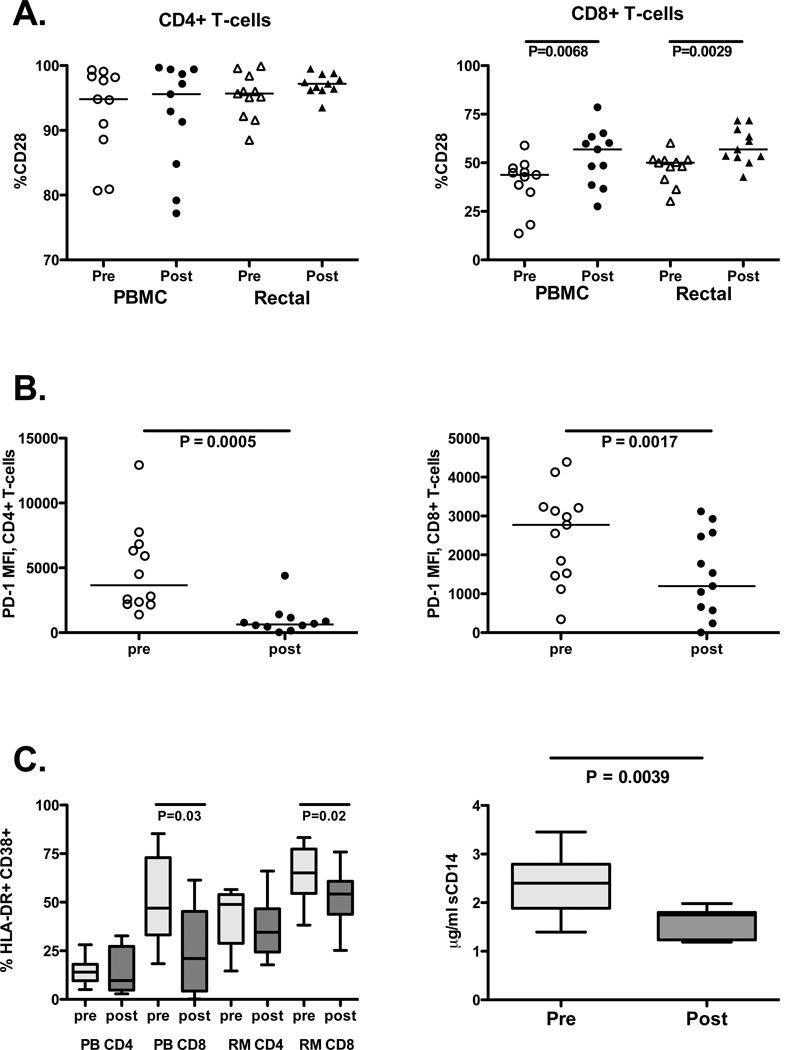
Costimulatory molecules provide a vital “second signal” for activation of T-cells [25] when they encounter antigen presenting cells. CD28 ligation provides an important second signal to naïve T cells [26], and memory CD8+ T-cells require CD28 costimulation for maximal responsiveness in vivo [27]. Furthermore, T-cells from HIV-infected individuals with impaired CD4+ T-cell recovery during HAART may express decreased levels of CD28 [28]. Changes in expression of CD28 on peripheral and mucosal T-cells were monitored before and after HAART initiation (Figure 3A). No significant changes were observed on CD4+ T-cells in either compartment. However, a significant increase in the proportion of CD8+ T-cells expressing CD28 was measured after HAART in both blood and rectal mucosa.
Figure 3. Effects of HAART on costimulatory molecules and immune activation markers.
(A) CD28 expression pre and post-HAART in CD4+ (left) and CD8+ T-cells (right) in PBMC and rectal mucosa. Open shapes indicate pre-HAART values; closed shapes indicate post-HAART values. Horizontal lines indicate median values. P values were determined by and Wilcoxon signed rank test. (B) Median fluorescence intensity (MFI) of PD-1 on IFNγ-producing, Gag-specific CD4+ (left) and CD8+ T-cells (right) in PBMC. Lines indicate median values. P values were determined by Wilcoxon signed rank test. (C) Immune activation markers. Left: CD4+ and CD8+ T-cells in PBMC (PB) and rectal mucosa (RM) co-expressing HLA-DR and CD38. Box plots indicate interquartile ranges, with lines at the median. Pale gray boxes indicate pre-HAART; dark boxes indicate post-HAART values. Right: Plasma sCD14 concentrations pre- and post-HAART (interquartile ranges, with lines at the median). P values were determined by Wilcoxon test.
Programmed death receptor-1 (PD-1), another member of the CD28 family of receptors, has been implicated in T-cell exhaustion in the context of chronic viral infection [29] and impaired HIV-specific T-cell responses [30–32], due in part to increased spontaneous apoptosis of HIV-specific T-cells [31]. We assessed the expression of PD-1 by HIV-specific CD8+ T-cells by determining the median fluorescence intensity (MFI) of PD-1 staining on the population of cells that produced IFNγ in response to HIV Gag peptide stimulation (Figure 3B). In peripheral blood, PD-1 MFI was found to decrease following HAART initiation in both HIV-specific CD4+ and CD8+ T-cells (Figure 3B). Due to the small number of HIV peptide-responsive cells in rectal mucosa, particularly after HAART initiation, this comparison was not feasible on rectal HIV-specific CD8+ T-cells.
Decreased immune activation after HAART initiation
Immune activation is an important prognostic indicator of disease progression in HIV infection [33, 34]. To address the effects of HAART initiation on immune activation, the expression of HLA-DR and CD38 on was measured on CD4+ and CD8+ T-cells in blood and rectal mucosa. Additionally, soluble CD14 (sCD14), a marker of activation that is released by monocytes upon activation by LPS [35], was measured in plasma. Immune activation was significantly diminished in both blood and rectal CD8+ T-cells following HAART (Figure 3C). A similar, but not significant decrease was observed in blood and rectal CD4+ T-cells. Plasma sCD14 also decreased after initiation of therapy (Figure 3C).
For the 10 patients biopsied at baseline and 9 months post-HAART, there was no significant correlation between either baseline VL or CD4 and baseline CD8 T-cell immune activation or change in blood or rectal CD8 T-cell activation following HAART. There was also no relationship between baseline CD4 or plasma VL and either baseline rectal CD4 T-cell activation or change in rectal CD4 T-cell activation following HAART. However, there was a trend towards an inverse correlation between baseline CD4 count and blood CD4 T-cell activation (P=0.054, R2=0.388), and a similar trend between baseline CD4 and the change in blood CD4 T-cell activation following HAART (P=0.099, R2=0.304). There was also a positive correlation between baseline plasma VL and blood CD4 T-cell activation (P=0.017, R2=0.530), as well as a trend towards a positive correlation between baseline plasma VL and reduction in blood CD4 T-cell activation (P=0.115, R2=0.281).
HIV-specific CD8+ T-cell responses
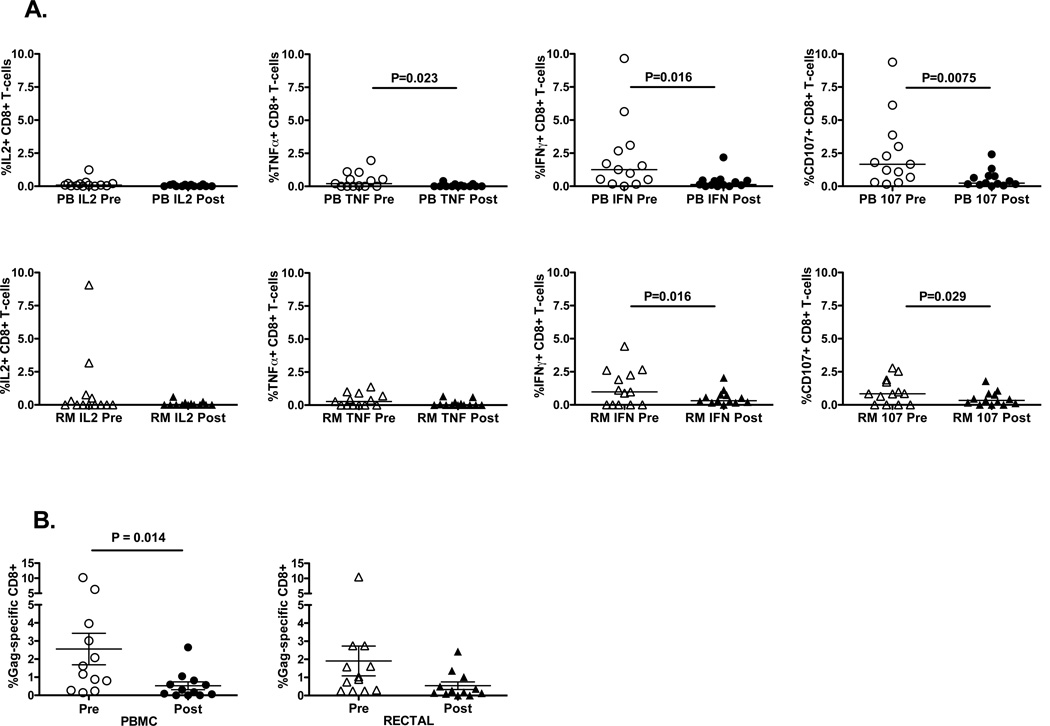
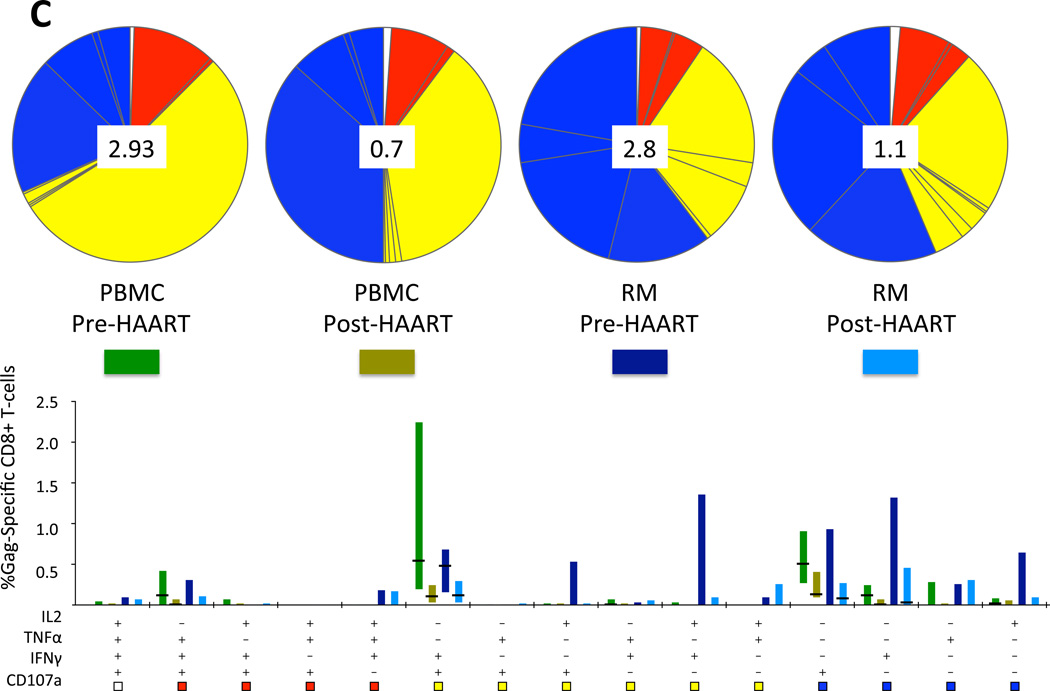
Lower viral antigen levels as a result of HAART lead to decreased frequencies of HIV-specific CD8+ T-cell responses in blood [36, 37] and rectal mucosa [21]. To examine the effect of HAART initiation on HIV-specific CD8+ T-cell response magnitude and polyfunctionality, blood and rectal mucosal CD8+ T-cells were stimulated with HIV Gag peptide and stained with fluorescent monoclonal antibodies to measure the production of three cytokines and a marker of degranulation. In both compartments, HIV Gag-specific CD8+ T-cell response magnitudes decreased significantly after beginning HAART: the percentage of Gag-responding CD8+ T-cells producing either IFNγ or CD107 was markedly reduced (Figure 4A). In PBMC, the percentage of Gag-specific cells producing TNFα was also reduced. Using Boolean gating and SPICE software, we then determined the magnitude of the total Gag-specific response, including cells positive for any one, two, three or all four factors, counting each cell only once (Figure 4B). This analysis revealed that the total Gag-specific CD8+ T-cell response declined in both compartments after HAART, reaching significance in PBMC. We also evaluated response complexity by graphing populations positive for each of the 15 possible combinations of factors (Figure 4C). This analysis demonstrated that HAART initiation did not lead to an increase in response polyfunctionality within the time frame studied. Instead, minor shifts in functionality were observed in both PBMC and rectal mucosa, with monofunctional responses dominating in rectal mucosa at both time points, and in PBMC following HAART.
Figure 4. HIV-specific T-cell responses decline during HAART.
(A) HIV Gag-specific CD8+ T-cell responses in PBMC (PB) and rectal mucosa (RM) pre- and post-HAART, showing individual factors (IL-2, TNF-α, IFN-γ, CD107) produced in response to Gag peptide stimulation. Pale gray boxes indicate pre-HAART; dark boxes indicate post-HAART values. (B) Total Gag-specific CD8+ T-cell response in PBMC (PB) and rectal mucosa (RM), pre- and post-HAART, when all non-overlapping responses are combined. P values were determined by Wilcoxon test. (C) Polyfunctionality of CD8+ T-cell responses to HIV Gag peptides. The percentage value shown in the center of each pie diagram denotes the total percentage of CD8+ T-cells responding in any way to Gag peptide stimulation. Individual color-coded pie slices represent the fraction of cells within the total responding population displaying 4 (white), 3 (red), 2 (yellow), or 1 (blue) function(s), beginning at the top of each pie and moving clock-wise, as compiled from the bar graph data. The bar graph provides fine detail on frequencies within each of the individual 15 categories. Individual bars are color-coded in bright green (PBMC, pre-HAART), avocado (PBMC, post-HAART), dark blue (Rectal Mucosa, pre-HAART), or aqua (Rectal Mucosa, post-HAART). Bar heights show interquartile ranges (25%–75%), with horizontal black lines indicating median values.
Discussion
Effects of SIV/HIV infection on gut mucosal CD4+ T-cells have been extensively documented [3, 4, 38–43]. However, the effects of HAART on CD4+ T-cell recovery in the gut have remained controversial, with studies reaching varied conclusions depending on the site of the GI tract studied, time of sampling, duration of HAART, specific HAART regimens, and the stage of HIV infection in which HAART was initiated [10, 13–16, 38, 44, 45]. These variables make comparisons difficult, particularly as most studies have relied on sampling at a single mucosal site, and several have used cross-sectional rather than longitudinal sampling. It is generally accepted that HAART does not lead to full restoration of mucosal CD4+ T-cells, particularly when initiated during chronic infection [9, 11, 13, 15, 38, 45]; however, one study found that long-term HAART was associated with rectal mucosal CD4+ T-cell populations that approximated levels found in healthy controls [10]. It should also be noted that when flow cytometry is used to gauge changes in T-cell subset percentages, as was done for this study, it is not possible to differentiate recovery of CD4+ T-cells from a decline in CD8+ T-cells. Nevertheless, recruitment and/or expansion of CD8+ T-cells in the gastrointestinal lamina propria occurs during HIV/SIV infection, beginning during the acute phase [41], contributing to the observed decreased percentages of CD4+ T-cells in mucosal lamina propria. In the parallel clinical trial in which these subjects were also enrolled, analysis of duodenal tissue samples by immunohistochemistry revealed a significant decline in CD8+ T-cell numbers following HAART initiation. These findings, discussed in detail elsewhere, show that much of the apparent CD4+ T-cell reconstitution observed in mucosal tissue during HAART is in fact due to a normalization (i.e., reduction) of CD8+ T-cell density [17].
In the present study, effects of HAART on CD4+ T-cell recovery in multiple anatomic sites were measured in individuals initiating therapy. T-cell differentiation, expression of costimulatory molecules, markers of immune activation, and HIV-specific CD8+ T-cell responses were analyzed before and during HAART in blood and rectal mucosa of 14 individuals. Early effects of HAART were apparent, as significant increases in CD4+ T-cell populations were evident in blood, small intestine (duodenum), and large intestine (rectum). In blood CD4+ T-cells, a significant increase in TCM, typically cells with high proliferative capacity, was observed. Significant decreases in immune activation were measured in CD8+ T-cells from blood and rectal mucosa; plasma levels of sCD14, a marker of macrophage activation, also declined with HAART initiation. Despite these changes, CD4+ T-cell populations in all three compartments failed to recover to levels seen in uninfected, healthy controls.
Mucosal HIV Gag-specific CD8+ T-cell responses decreased in magnitude after four to nine months of therapy. This apparent contraction of the HIV-specific memory T-cell pool was expected based on previous reports showing a rapid decline of HIV-specific T-cell responses in blood following initiation of therapy [36, 37], and is also consistent with prior cross-sectional studies from our group [18, 21]. However, HAART also led to significant increases in CD28 expression on peripheral and mucosal CD8+ T-cells and significant decreases in PD-1 on peripheral HIV-specific T-cells, suggesting enhancement in the capacity of the remaining HIV-specific CD8+ T-cells to survive and respond to antigenic stimulation [30–32, 46].
The present study examined immune reconstitution in blood and both small and large intestine in chronically HIV-infected individuals during the first four to nine months following HAART initiation. By examining the same individuals longitudinally, HAART-specific effects could be determined for CD4+ vs CD8+ T-cell subset normalization, T-cell costimulatory molecule expression, differentiation status, immune activation, and CD8+ T-cell function. Early effects of HAART included significant CD4 recovery in blood, duodenal and rectal mucosa, decreases in immune activation of T-cells and monocytes, changes in T-cell differentiation profiles and costimulatory molecule expression, and decreased HIV-specific CD8+ T-cell responses. These results suggest that initiation of HAART during chronic infection provides modest yet measurable immunologic benefit to mucosal tissues, despite incomplete CD4+ T-cell recovery.
Acknowledgments
The authors thank the study volunteers for their participation, the CARES clinic, Sacramento, CA, and the Gastroenterology Laboratory, UC Davis Medical Center.
Funding: This research was supported by NIH/NIAID (R01-AI057020 to BLS) and the California Universitywide AIDS Research Program (UARP Center Grant CH05-D-606 to RBP). The study was also supported in part by a research grant from the Investigator-Initiated Studies Program of Merck & Co., Inc. to DMA. The opinions expressed in this paper are those of the authors and do not necessarily represent those of Merck & Co., Inc. The UC Davis CCRC is supported by Grant Number UL1 RR024146 from the National Center for Research Resources (NCRR), NIH. This investigation was conducted in a facility constructed with support from the Research Facilities Improvement Program (grant C06 RR-12088-01) from the National Center for Research Resources, National Institutes of Health. The LSR-II violet laser was upgraded with funding from the James B. Pendleton Charitable Trust.
Footnotes
Preliminary data from this study were presented at the Keystone Conference on HIV Vaccines, Banff, Alberta, March 2010 (Poster X5-211), and at AIDS Vaccine 2010, Atlanta, GA (Poster P10.08).
Author contributions: DMA and BLS designed the study with input from RBP. DMA, RBP, TY, DHM and JCG enrolled patients and procured samples. TLH performed laboratory work with assistance from THK and DHM. TLH analyzed data with assistance from JWC and BEM. TLH and BLS wrote the paper. All authors reviewed the manuscript.
REFERENCES
- 1.Mowat AM, Viney JL. The anatomical basis of intestinal immunity. Immunol Rev. 1997;156:145–166. doi: 10.1111/j.1600-065x.1997.tb00966.x. [DOI] [PubMed] [Google Scholar]
- 2.Schneider T, Jahn HU, Schmidt W, Riecken EO, Zeitz M, Ullrich R. Loss of CD4 T lymphocytes in patients infected with human immunodeficiency virus type 1 is more pronounced in the duodenal mucosa than in the peripheral blood. Berlin Diarrhea/Wasting Syndrome Study Group. Gut. 1995;37:524–529. doi: 10.1136/gut.37.4.524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brenchley JM, Schacker TW, Ruff LE, Price DA, Taylor JH, Beilman GJ, et al. CD4+ T cell depletion during all stages of HIV disease occurs predominantly in the gastrointestinal tract. J Exp Med. 2004;200:749–759. doi: 10.1084/jem.20040874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clayton F, Snow G, Reka S, Kotler DP. Selective depletion of rectal lamina propria rather than lymphoid aggregate CD4 lymphocytes in HIV infection. Clin Exp Immunol. 1997;107:288–292. doi: 10.1111/j.1365-2249.1997.236-ce1111.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lim SG, Condez A, Lee CA, Johnson MA, Elia C, Poulter LW. Loss of mucosal CD4 lymphocytes is an early feature of HIV infection. Clin Exp Immunol. 1993;92:448–454. doi: 10.1111/j.1365-2249.1993.tb03419.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smith K, Aga E, Bosch RJ, Valdez H, Connick E, Landay A, et al. Long-term changes in circulating CD4 T lymphocytes in virologically suppressed patients after 6 years of highly active antiretroviral therapy. AIDS. 2004;18:1953–1956. doi: 10.1097/00002030-200409240-00012. [DOI] [PubMed] [Google Scholar]
- 7.Kaufmann GR, Perrin L, Pantaleo G, Opravil M, Furrer H, Telenti A, et al. CD4 T-lymphocyte recovery in individuals with advanced HIV-1 infection receiving potent antiretroviral therapy for 4 years: the Swiss HIV Cohort Study. Arch Intern Med. 2003;163:2187–2195. doi: 10.1001/archinte.163.18.2187. [DOI] [PubMed] [Google Scholar]
- 8.Zeng M, Smith AJ, Wietgrefe SW, Southern PJ, Schacker TW, Reilly CS, et al. Cumulative mechanisms of lymphoid tissue fibrosis and T cell depletion in HIV-1 and SIV infections. J Clin Invest. 2011;121:998–1008. doi: 10.1172/JCI45157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Estes J, Baker JV, Brenchley JM, Khoruts A, Barthold JL, Bantle A, et al. Collagen deposition limits immune reconstitution in the gut. J Infect Dis. 2008;198:456–464. doi: 10.1086/590112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sheth PM, Chege D, Shin LY, Huibner S, Yue FY, Loutfy M, et al. Immune reconstitution in the sigmoid colon after long-term HIV therapy. Mucosal Immunol. 2008;1:382–388. doi: 10.1038/mi.2008.23. [DOI] [PubMed] [Google Scholar]
- 11.Guadalupe M, Sankaran S, George MD, Reay E, Verhoeven D, Shacklett BL, et al. Viral suppression and immune restoration in the gastrointestinal mucosa of human immunodeficiency virus type 1-infected patients initiating therapy during primary or chronic infection. J Virol. 2006;80:8236–8247. doi: 10.1128/JVI.00120-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.George MD, Reay E, Sankaran S, Dandekar S. Early antiretroviral therapy for simian immunodeficiency virus infection leads to mucosal CD4+ T-cell restoration and enhanced gene expression regulating mucosal repair and regeneration. J Virol. 2005;79:2709–2719. doi: 10.1128/JVI.79.5.2709-2719.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mehandru S, Poles MA, Tenner-Racz K, Jean-Pierre P, Manuelli V, Lopez P, et al. Lack of mucosal immune reconstitution during prolonged treatment of acute and early HIV-1 infection. PLoS Med. 2006;3:e484. doi: 10.1371/journal.pmed.0030484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Macal M, Sankaran S, Chun TW, Reay E, Flamm J, Prindiville TJ, et al. Effective CD4+ T-cell restoration in gut-associated lymphoid tissue of HIV-infected patients is associated with enhanced Th17 cells and polyfunctional HIV-specific T-cell responses. Mucosal Immunol. 2008;1:475–488. doi: 10.1038/mi.2008.35. [DOI] [PubMed] [Google Scholar]
- 15.Chun TW, Nickle DC, Justement JS, Meyers JH, Roby G, Hallahan CW, et al. Persistence of HIV in gut-associated lymphoid tissue despite long-term antiretroviral therapy. J Infect Dis. 2008;197:714–720. doi: 10.1086/527324. [DOI] [PubMed] [Google Scholar]
- 16.Ciccone EJ, Read SW, Mannon PJ, Yao MD, Hodge JN, Dewar R, et al. Cycling of gut mucosal CD4+ T cells decreases after prolonged anti-retroviral therapy and is associated with plasma LPS levels. Mucosal Immunol. 2010;3:172–181. doi: 10.1038/mi.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Asmuth DM, Ma ZM, Mann S, Knight TH, Yotter T, Albanese A, et al. Gastrointestinal-associated lymphoid tissue immune reconstitution in a randomized clinical trial of raltegravir versus non-nucleoside reverse transcriptase inhibitor-based regimens. AIDS. 2012;26:1625–1634. doi: 10.1097/QAD.0b013e3283546595. [DOI] [PubMed] [Google Scholar]
- 18.Critchfield JW, Lemongello D, Walker DH, Garcia JC, Asmuth DM, Pollard RB, et al. Multifunctional human immunodeficiency virus (HIV) gag-specific CD8+ T-cell responses in rectal mucosa and peripheral blood mononuclear cells during chronic HIV type 1 infection. J Virol. 2007;81:5460–5471. doi: 10.1128/JVI.02535-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shacklett BL, Yang O, Hausner MA, Elliott J, Hultin L, Price C, et al. Optimization of methods to assess human mucosal T-cell responses to HIV infection. J Immunol Methods. 2003;279:17–31. doi: 10.1016/s0022-1759(03)00255-2. [DOI] [PubMed] [Google Scholar]
- 20.Roederer M. Spectral compensation for flow cytometry: visualization artifacts, limitations, and caveats. Cytometry. 2001;45:194–205. doi: 10.1002/1097-0320(20011101)45:3<194::aid-cyto1163>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 21.Critchfield JW, Young DH, Hayes TL, Braun JV, Garcia JC, Pollard RB, et al. Magnitude and complexity of rectal mucosa HIV-1-specific CD8+ T-cell responses during chronic infection reflect clinical status. PLoS One. 2008;3:e3577. doi: 10.1371/journal.pone.0003577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roederer M, Nozzi JL, Nason MC. SPICE: exploration and analysis of post-cytometric complex multivariate datasets. Cytometry A. 2011;79:167–174. doi: 10.1002/cyto.a.21015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 24.Michie CA, McLean A, Alcock C, Beverley PC. Lifespan of human lymphocyte subsets defined by CD45 isoforms. Nature. 1992;360:264–265. doi: 10.1038/360264a0. [DOI] [PubMed] [Google Scholar]
- 25.Lafferty KJ, Misko IS, Cooley MA. Allogeneic stimulation modulates the in vitro response of T cells to transplantation antigen. Nature. 1974;249:275–276. doi: 10.1038/249275a0. [DOI] [PubMed] [Google Scholar]
- 26.Sharpe AH. Mechanisms of costimulation. Immunol Rev. 2009;229:5–11. doi: 10.1111/j.1600-065X.2009.00784.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Borowski AB, Boesteanu AC, Mueller YM, Carafides C, Topham DJ, Altman JD, et al. Memory CD8+ T cells require CD28 costimulation. J Immunol. 2007;179:6494–6503. doi: 10.4049/jimmunol.179.10.6494. [DOI] [PubMed] [Google Scholar]
- 28.Erikstrup C, Kronborg G, Lohse N, Ostrowski SR, Gerstoft J, Ullum H. T-cell dysfunction in HIV-1-infected patients with impaired recovery of CD4 cells despite suppression of viral replication. J Acquir Immune Defic Syndr. 2010;53:303–310. doi: 10.1097/QAI.0b013e3181ca3f7c. [DOI] [PubMed] [Google Scholar]
- 29.Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, Sharpe AH, et al. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439:682–687. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- 30.Day CL, Kaufmann DE, Kiepiela P, Brown JA, Moodley ES, Reddy S, et al. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature. 2006;443:350–354. doi: 10.1038/nature05115. [DOI] [PubMed] [Google Scholar]
- 31.Petrovas C, Casazza JP, Brenchley JM, Price DA, Gostick E, Adams WC, et al. PD-1 is a regulator of virus-specific CD8+ T cell survival in HIV infection. J Exp Med. 2006;203:2281–2292. doi: 10.1084/jem.20061496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Trautmann L, Janbazian L, Chomont N, Said EA, Gimmig S, Bessette B, et al. Upregulation of PD-1 expression on HIV-specific CD8+ T cells leads to reversible immune dysfunction. Nat Med. 2006;12:1198–1202. doi: 10.1038/nm1482. [DOI] [PubMed] [Google Scholar]
- 33.Giorgi JV, Hultin LE, McKeating JA, Johnson TD, Owens B, Jacobson LP, et al. Shorter survival in advanced human immunodeficiency virus type 1 infection is more closely associated with T lymphocyte activation than with plasma virus burden or virus chemokine coreceptor usage. J Infect Dis. 1999;179:859–870. doi: 10.1086/314660. [DOI] [PubMed] [Google Scholar]
- 34.Deeks SG, Kitchen CM, Liu L, Guo H, Gascon R, Narvaez AB, et al. Immune activation set point during early HIV infection predicts subsequent CD4+ T-cell changes independent of viral load. Blood. 2004;104:942–947. doi: 10.1182/blood-2003-09-3333. [DOI] [PubMed] [Google Scholar]
- 35.Kitchens RL, Thompson PA. Modulatory effects of sCD14 and LBP on LPS-host cell interactions. J Endotoxin Res. 2005;11:225–229. doi: 10.1179/096805105X46565. [DOI] [PubMed] [Google Scholar]
- 36.Ogg GS, Jin X, Bonhoeffer S, Moss P, Nowak MA, Monard S, et al. Decay kinetics of human immunodeficiency virus-specific effector cytotoxic T lymphocytes after combination antiretroviral therapy. J Virol. 1999;73:797–800. doi: 10.1128/jvi.73.1.797-800.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kalams SA, Goulder PJ, Shea AK, Jones NG, Trocha AK, Ogg GS, et al. Levels of human immunodeficiency virus type 1-specific cytotoxic T-lymphocyte effector and memory responses decline after suppression of viremia with highly active antiretroviral therapy. J Virol. 1999;73:6721–6728. doi: 10.1128/jvi.73.8.6721-6728.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Guadalupe M, Reay E, Sankaran S, Prindiville T, Flamm J, McNeil A, et al. Severe CD4+ T-cell depletion in gut lymphoid tissue during primary human immunodeficiency virus type 1 infection and substantial delay in restoration following highly active antiretroviral therapy. J Virol. 2003;77:11708–11717. doi: 10.1128/JVI.77.21.11708-11717.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mehandru S, Poles MA, Tenner-Racz K, Horowitz A, Hurley A, Hogan C, et al. Primary HIV-1 infection is associated with preferential depletion of CD4+ T lymphocytes from effector sites in the gastrointestinal tract. J Exp Med. 2004;200:761–770. doi: 10.1084/jem.20041196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Smit-McBride Z, Mattapallil JJ, McChesney M, Ferrick D, Dandekar S. Gastrointestinal T lymphocytes retain high potential for cytokine responses but have severe CD4(+) T-cell depletion at all stages of simian immunodeficiency virus infection compared to peripheral lymphocytes. J Virol. 1998;72:6646–6656. doi: 10.1128/jvi.72.8.6646-6656.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Veazey RS, DeMaria M, Chalifoux LV, Shvetz DE, Pauley DR, Knight HL, et al. Gastrointestinal tract as a major site of CD4+ T cell depletion and viral replication in SIV infection. Science. 1998;280:427–431. doi: 10.1126/science.280.5362.427. [DOI] [PubMed] [Google Scholar]
- 42.Li Q, Duan L, Estes JD, Ma ZM, Rourke T, Wang Y, et al. Peak SIV replication in resting memory CD4+ T cells depletes gut lamina propria CD4+ T cells. Nature. 2005;434:1148–1152. doi: 10.1038/nature03513. [DOI] [PubMed] [Google Scholar]
- 43.Mattapallil JJ, Douek DC, Hill B, Nishimura Y, Martin M, Roederer M. Massive infection and loss of memory CD4+ T cells in multiple tissues during acute SIV infection. Nature. 2005;434:1093–1097. doi: 10.1038/nature03501. [DOI] [PubMed] [Google Scholar]
- 44.Miao YM, Hayes PJ, Gotch FM, Barrett MC, Francis ND, Gazzard BG. Elevated mucosal addressin cell adhesion molecule-1 expression in acquired immunodeficiency syndrome is maintained during antiretroviral therapy by intestinal pathogens and coincides with increased duodenal CD4 T cell densities. J Infect Dis. 2002;185:1043–1050. doi: 10.1086/340235. [DOI] [PubMed] [Google Scholar]
- 45.Gordon SN, Cervasi B, Odorizzi P, Silverman R, Aberra F, Ginsberg G, et al. Disruption of Intestinal CD4+ T Cell Homeostasis Is a Key Marker of Systemic CD4+ T Cell Activation in HIV-Infected Individuals. J Immunol. 2010 doi: 10.4049/jimmunol.1001801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Parish ST, Wu JE, Effros RB. Sustained CD28 Expression Delays Multiple Features of Replicative Senescence in Human CD8 T Lymphocytes. J Clin Immunol. 2010 doi: 10.1007/s10875-010-9449-7. [DOI] [PMC free article] [PubMed] [Google Scholar]