Abstract
Two experiments examined the impact of encoding conditions and information content in memory for positive, neutral, and negative pictures. We examined the hypotheses that the positivity effect in memory (i.e., a bias in favor of positive or against negative information in later life) would be reduced when (a) pictures were viewed under structured as opposed to unstructured conditions, and (b) contained social as opposed to nonsocial content. Both experiments found that the positivity effect observed with nonsocial stimuli was absent with social stimuli. In addition, little evidence was obtained that encoding conditions affected the strength of the positivity effect. We argue that some types of social stimuli may engage different types of processing than nonsocial stimuli, perhaps encouraging self-referential processing that engages attention and supports memory. This processing may then conflict with the goal-driven, top-down processing that is hypothesized to drive the positivity effect. Thus, our results identify further boundary conditions associated with the positivity effect in memory, arguing that stimulus factors as well as situational goals may affect its occurrence. Further research awaits to determine if this effect is specific to all social stimuli or specific subsets.
Emotional content has privileged status in memory across adulthood. In contrast to relatively neutral stimuli, individuals of all ages remember emotional stimuli better and report more vivid memories for such stimuli (for review, see Kensinger, 2009). Studies of aging suggest that emotional content may have an even greater advantage in later life (e.g., Carstensen & Turk-Charles, 1994), and that the impact of affective content may be qualitatively different from that seen in young adulthood when valence is considered. Specifically, in laboratory studies of memory for pictures and words, younger adults exhibit enhanced memory for both positive and negative stimuli, with either no difference in retention rates by valence or better memory for negative than for positive items (i.e., a negativity bias). In contrast, older adults are often found to exhibit enhanced memory for positive information (i.e., a positivity bias) or reduced memory for negative information (e.g., Charles, Mather, & Carstensen, 2003; Mather & Carstensen, 2003; Mather & Knight, 2005). This age-based trend towards an increased ratio of positive to negative information in memory has traditionally been referred to as the positivity effect. According to socioemotional selectivity theory (SST), this effect is thought to reflect emotion regulatory functions that contribute to enhanced well-being in later adulthood (Mather & Carstensen, 2005).
Whereas the positivity effect has been found in several studies, it is not always observed (e.g., Comblain, D’Argembeau, Van der Linden, & Aldenhoff, 2004; Denburg, Buchanan, Tranel, & Adolphs, 2003; Grühn, Smith, & Baltes, 2005; Kensinger, Brierley, Medford, Growdon, & Corkin, 2002; Leigland, Schulz, & Janowsky, 2004). As described above, when the effect does occur, it is sometimes due to a reduced negativity bias with age, whereas it is sometimes due to an increased positivity bias with age. This variability in both the size and nature of the positivity effect suggests that unstudied moderating factors may have influenced past results. In the present set of studies, we explored how two such factors—stimulus content and encoding instructions—might influence both memory for positive relative to negative information and the moderation of this valence effect by age.
Concerning content, our primary interest was in examining the impact of social versus non-social stimuli on memory for emotional material. Many previous studies of the positivity effect used pictures from the International Affective Picture System (IAPS; Lang, Bradley, & Cuthbert, 2001) as emotional stimuli. The IAPS pictures contain a variety of images that differ in content, including some that contain people and others that do not. Although most studies examining positivity effects in memory using visual stimuli have used pictures both with and without people, we have not been able to identify reports where the impact of content has been systematically examined. Several types of evidence, however, suggest that social stimuli (e.g., pictures involving people) are processed differently than nonsocial stimuli, which in turn could result in modifications of valence-based effects on memory. For example, research with young adults has demonstrated a bias in attention toward the social elements of visual scenes (e.g., Humphrey & Underwood, 2010), and social stimuli (e.g., faces) have been found to be more distracting than nonsocial stimuli (e.g., Langton, Law, Burton, & Schweinberger, 2008), suggesting that social stimuli receive additional attentional resources . The processing of social stimuli versus nonsocial stimuli is also associated with differential activation of structures in the brain (e.g., medial prefrontal cortex [mPFC]), and this activation is relatively independent of differential activation of the amygdala in response to emotional content (Harvey, Fossati, & Lepage, 2007). Finally, there is evidence that social information processing activates the same cortical structures as self-referential processing (e.g., Fossati et al., 2003; Gutchess, Kensinger, & Schacter, 2007). Harvey et al. (2007) speculate that this may reflect the fact that social stimuli engage self-referential processes as individuals attempt to understand the mental states of others. Importantly, self-referential processing—which involves encoding information in relationship to oneself—tends to improve memory (Bower & Gilligan, 1979; Rogers, Kuiper, & Kirker, 1977). Thus, to the extent that social and nonsocial stimuli evoke different encoding processes associated with different levels of retention, it is possible that the impact of valence might also vary across picture content.
How might the presence of social content moderate age differences in the impact of valence on memory? Based on the previously cited research, one possibility is that social stimuli may be processed in a relatively obligatory manner that is different from the more motivated processing hypothesized to underlie the positivity effect. Alternatively, engagement of empathic processes, which have been shown to remain stable or even increase with age in adulthood (Bailey, Henry, & von Hippel, 2008; Grühn, Rebucal, Diehl, Lumley, & Labouvie-Vief, 2008; Richter & Kunzmann, 2011), may bias attention to social stimuli. It might even be expected that such empathic responses would be stronger to negative social images involving emotions such as sadness and suffering. It is possible that the nature of this stimulus-initiated processing may conflict with motivated processing associated with chronic emotional goals, resulting in reduction or elimination of the positivity effect. Within the context of SST—a theory originally developed to explain age differences in social behavior (e.g., Carstensen, 1991)—an argument might also be made for motivated attention to negative social information in that it may be diagnostic of the potential affective outcomes associated with interactions with others (e.g., Hess & Kotter-Grühn, 2011). Thus, a motivated bias by older adults away from negative information might be more likely with nonsocial information than with social information.
Although no previous studies have directly examined the impact of stimulus content, two studies using only social stimuli obtained little evidence for the positivity effect. Denberg et al. (2003) found that young, middle-aged, and older adults exhibited a clear negativity bias in an unexpected free recall test. Note that participants in this study viewed the pictures under structured conditions, which have been hypothesized to be associated with reductions in positivity. However, the nature of the encoding task—to experience the emotion depicted in the pictures—appeared to encourage processing of stimuli by older adults in a manner consistent with chronic affective goals. In addition, two experiments by Emery and Hess (2008) in which memory was tested under a variety of instructional and test conditions found no or weak evidence for a positivity effect. This was true even under “natural” viewing conditions mimicking those used by Charles et al. (2003). A third study by Tomaszczyk, Fernandes, and MacLeod, (2008) germane to our line of reasoning observed a positivity effect in memory for stimuli rated low in personal relevance, but not for stimuli high in personal relevance. Although these researchers did not examine the social content of the pictures, these findings imply that the regulatory processes underlying the positivity effect may be less influential when stimulus content other than valence engages attention. Taken together, these results suggest that the nature of processing for social and nonsocial stimuli might differ, with the positivity effect perhaps reduced or eliminated for social stimuli. We explicitly tested this hypothesis by comparing young and older adults’ memory for positive, negative, and neutral IAPS pictures possessing social versus nonsocial content.
Our secondary focus in the present research concerned the impact of encoding instructions on the size of the positivity effect. Several researchers have suggested that this effect represents older adults’ use of a specific regulatory strategy aimed at maintaining positive affective states, and thus is reflective of a differential deployment of controlled processing across younger versus older adults (e.g., Kensinger, 2012; Mather & Knight, 2005). We therefore also tested the hypothesis that the positivity effect will be most evident when there are few external constraints on performance and participants are free to deploy controlled attention based on their own goals (e.g., Mather, 2006). In contrast, when task demands encourage use of other types of strategies, the positivity effect should be reduced as situational goals result in individuals being less likely to engage in behaviors promoting chronic goals. We note here, however, that Emery and Hess (2008)—who used only social stimuli—did not find support for the hypothesis that open-ended encoding instructions would enhance the positivity effect. One possible interpretation of this finding is that social images result in processing that conflicts with the more top-down deployment of attention assumed to underlie the positivity effect.
EXPERIMENT 1
Our first study examined these ideas by having young, middle-aged, and older adults view positive, neutral, or negative images that were either social (i.e., contained people) or nonsocial. Participants viewed the pictures under unconstrained or constrained (i.e., instructions to remember) conditions, followed by a memory test. We expected any observed age-based positivity effect in memory—as reflected in an Age X Valence interaction—to be moderated by instructions, with positivity greatest under unconstrained conditions. Picture content was also expected to moderate this positivity effect, with positivity being attenuated with social compared to nonsocial stimuli.
Method
Participants
Participants were recruited from the Raleigh, NC community through newspaper and internet advertisements. Individuals received an honorarium of $35 for participation. The final sample consisted of 64 younger (32 women; age range = 20 – 44), 66 middle-aged (44 women; age range = 45 – 64), and 63 older (33 women; age range = 65 – 91) adults.
Materials
Picture stimuli
A series of 36 images (12 positive, 12 neutral, 12 negative) from the International Affective Picture System (IAPS; Lang et al, 2008) were used as target stimuli. Within each level of valence, half of the images were social (i.e., featured people), the other half nonsocial (i.e., did not contain people). For continuity, we used the same images as Charles et al. (2003, Experiment 1), with one exception: we presented equal numbers of pictures from each level of valence, adding four positive and four negative images to Charles et al.’s original stimulus set and removing four neutral pictures from this set. Mean valence and arousal ratings are presented in Table 1.
Table 1.
Mean IAPS Valence and Arousal Ratings for Stimulus Pictures
| Valence | Arousal | |
|---|---|---|
| Positive Pictures | ||
| Social | 7.58 | 4.55 |
| Nonsocial | 7.61 | 4.77 |
| Neutral Pictures | ||
| Social | 4.85 | 3.50 |
| Nonsocial | 4.94 | 2.86 |
| Negative Pictures | ||
| Social | 2.26 | 4.77 |
| Nonsocial | 2.85 | 5.71 |
Note. Ratings are on a 9-pt scale. Higher numbers represent more positive valence or greater arousal.
Affective state
Mood over the prior 30 days was assessed with the Positive and Negative Affect Schedule (PANAS; Watson, Clark, & Tellegen, 1988). We also created a mood scale for use during testing, which contained the following items rated on an 8-point Likert scale (1 = not at all, 8 = very much): interested, happy, focused, unhappy, calm, involved, tense, and bored.
Ability measures
Several cognitive ability measures were used. Working memory was assessed with the operation span task, in which participants simultaneously solved a series of arithmetic equations while attempting to remember words. Inhibitory control was measured via a Stroop task, whereby mean response time for congruent trials was subtracted from that of incongruent trials. Task-shifting ability was assessed with the Plus/Minus task specifically as the time required to complete a set of alternating trials entailing adding and subtracting 3 to a series of two-digit numbers minus the mean time required to solve two other sets of problems using only addition or subtraction. The Digit-Symbol Substitution and Vocabulary Subtests from the Wechsler Adult Intelligence Scale-III (WAIS-III; Wechsler, 1997) were used to measure processing speed and verbal ability, respectively.
Health
Self-rated physical and mental health were measured with the SF-36 Health Survey (Ware, 1993). The Geriatric Depression Scale (GDS; Sheikh & Yesavage, 1986) was included as a measure of negative affect, with higher scores representing greater levels of depressive symptoms.
Procedure
Prior to coming to the lab, participants were mailed and completed a basic background questionnaire, the SF-36, the 30-day PANAS, and GDS scales, plus several questionnaires unrelated to the present study. At the beginning of the testing session, participants were given the mood scale and asked to rate how they were feeling right now in the present moment, making sure “not to think too much about how [they were] feeling since we [were] interested in relatively spontaneous reactions.” During the subsequent 15 min, participants completed the NEO personality inventory (Costa & McCrae, 2003) as a filler task.
Participants were then told that they would view a series of pictures, with approximately half of the participants assigned to each of two instructional conditions. Participants in the intentional condition were given explicit instructions to remember the pictures because they would be asked to recall them later, whereas individuals in the incidental condition were told to “simply watch the pictures as you would a television”, with no mention of the subsequent memory test. These latter instructions were similar to those used by Charles et al. (2003). Participants were asked to not think about their responses to the pictures and to react as they would under normal circumstances. Just prior to viewing the pictures, participants completed the mood scale a second time. They then completed a short filler task. Each picture was displayed on the computer screen for 5 s each, with a 1 s interval between pictures. Images were presented in quasi-random order such that no more than two pictures of the same valence were presented in a row.
After all pictures had been viewed, participants rated their mood a third time. This was followed by a 15- to 20-min delay in which the Plus/Minus, Digit-Symbol Substitution, and Stroop tasks were administered. Participants were then given a minimum of 3 min to write down a brief description of each picture that they could recall, such that a person unfamiliar with the pictures would be able to unambiguously identify which target picture was being described. Following this, participants also completed a recognition memory test. Unexpectedly, performance on this task was close to ceiling, and thus we do not report these results. They then rated their mood one final time.
Finally, as manipulation checks, participants were presented with all the target pictures used in the memory task and rated the valence of each using a 5-point Likert scale (1 = negative, 5 = positive). Pictures were presented on a computer screen at a rate of 5-sec each, after which participants pressed a button on the response box corresponding to the way the picture made them feel. They were also asked whether they expected to be prompted to recall the pictures.
Results
Participant Characteristics
Participant characteristics are displayed in Table 2. The pattern of age differences is generally consistent with known normative trends. Tests for differences between conditions revealed no significant effects, suggesting that variations within groups were randomly distributed across the two experimental conditions.
Table 2.
Experiment 1: Participant characteristics
| Measure | Young | Middle-Aged | Older | |||
|---|---|---|---|---|---|---|
|
| ||||||
| M | SD | M | SD | M | SD | |
| Age | 34.0 | 6.9 | 55.3 | 5.9 | 74.9 | 6.7 |
| Education | 15.5 | 2.3 | 15.7 | 2.7 | 15.9 | 2.6 |
| SF36 Physical health* | 50.7 | 5.9 | 48.9 | 8.0 | 43.2 | 9.8 |
| SF36 Mental health* | 51.3 | 7.4 | 50.7 | 11.1 | 54.7 | 7.3 |
| GDS* | 2.2 | 2.5 | 3.4 | 4.0 | 1.8 | 2.0 |
| Operation span | 26.2 | 9.0 | 23.8 | 9.6 | 23.0 | 10.4 |
| Digit Symbol | 80.6 | 16.6 | 68.3 | 13.1 | 58.3 | 15.7 |
| Vocabulary* | 45.3 | 11.0 | 55.2 | 9.4 | 49.0 | 11.3 |
| Plus-Minus | 29.3 | 22.6 | 28.6 | 22.8 | 34.2 | 21.8 |
| PANAS Positive affect | 3.6 | .8 | 3.3 | .8 | 3.5 | .6 |
| PANAS Negative affect* | 1.7 | .6 | 1.7 | .6 | 1.5 | .5 |
Age group difference significant at p < .05.
We also examined subjective reports of mood during the task—reflected by the mean of ratings for happiness and unhappiness (reverse scored)—using a 3 × 2 × 4 (Age group × Instructions × Time of assessment) ANOVA. Ratings were generally positive, with the only significant effect obtained due to time, F(3,558) = 5.14, p = .002, η2 = .03. Mean ratings for the four times of assessment were 6.88, 7.01, 6.86, and 7.03. Importantly, there were no age differences in mood, ruling out a potential confound in interpreting valence-based effects in memory.
Memory
Each description produced by each participant was coded for recall by two trained judges, who determined whether it could be unambiguously assigned to a specific target picture. Cases in which descriptions were either too vague or ambiguous to match to a particular picture, or did not correspond to any of the displayed pictures were not coded, with the incidence of such cases relatively constant across ages: old—M = 0.67, SD = 1.06; middle-aged—M = 0.64, SD = 1.31; young—M = 0.42, SD = 0.66. Coding disagreements were settled by a third trained rater. Inter-rater reliability was high (Fleiss’ kappa = .92).
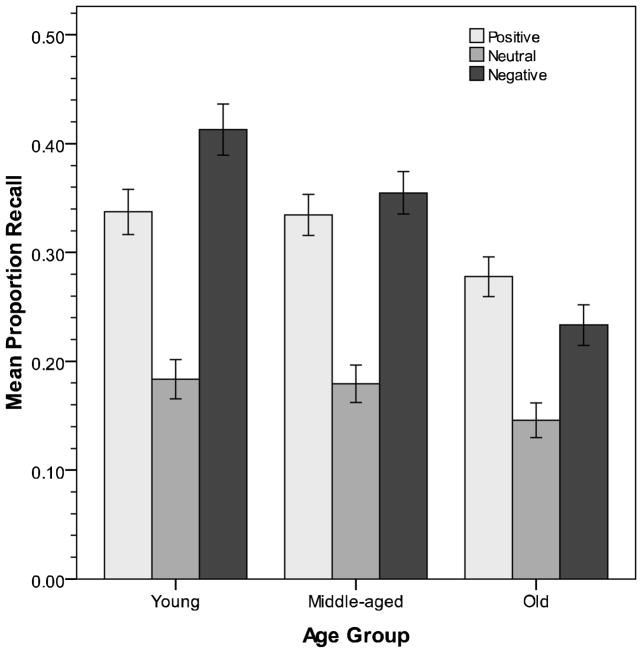
The proportion of each type of picture recalled was then entered into a 3 × 2 × 2 × 3 (Age Group X Instructions X Picture Content X Picture Valence) ANOVA.1 Significant effects were observed for age, F(2,184) = 10.77, p < .001, η2 = .11, with older adults (M = .22) recalling less than either younger (M = .31) or middle-aged (M = .29) adults. A significant main effect of valence was also obtained, F(2,368) = 119.04, p < .001, η2 = .39, with positive and negative pictures (Ms = .32 & .33) recalled better than neutral pictures (M = .17). The Age X Valence interaction was also significant, F(4,368) = 7.06, p < .001, η2 = .07 (Figure 1). In all age groups, positive and negative pictures were recalled significantly better than neutral ones. However, younger adults recalled significantly more negative than positive pictures (p = .01), middle-aged adults’ recall rates did not differ for these two types of pictures (p = .37), and older adults recalled significantly more positive than negative pictures (p = .04). Thus, we replicated the positivity effect in older adults’ memory. Significant effects were also obtained due to instructions, F(1,184) = 12.61, p < .001, η2 = .06, and its interaction with age, F(2,184) = 3.32, p = .04, η2 = .04. These effects reflected the facts that the intentional memory instructions facilitated recall when compared to incidental instructions in the young (Ms = .37 vs. .25) and middle-aged (Ms = .32 vs. .27) groups, but not in the older group (Ms = .23 vs. .21). However, instructions did not moderate the form of valence effects across age groups, F(4,368) = 1.10, p = .35, η2 = .01. Although the power associated with this test was somewhat low (.39), the effect size was also quite small. Thus, inconsistent with expectations, intentional learning instructions did not moderate the impact of emotional content on memory, with evidence for positivity being just as strong under structured as opposed to unstructured viewing conditions.
Figure 1.
Mean recall in Experiment 1 (error bars ± 1 SE).
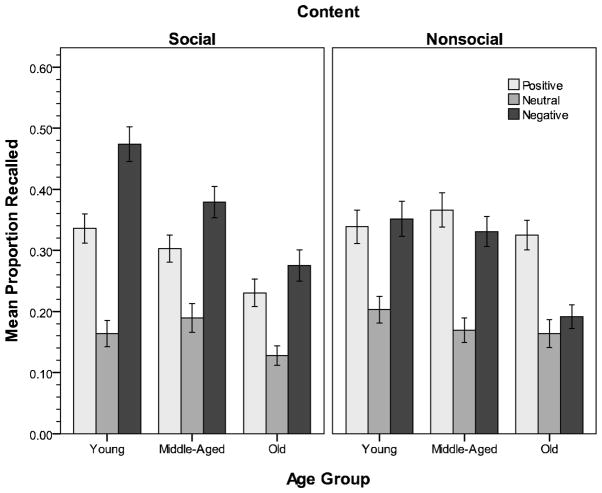
Consistent with expectations, picture content moderated the impact of valence on recall, F(2,368) = 16.37, p < .001, η2 = .08 (Figure 2). Specifically, positive nonsocial pictures were recalled significantly better than negative nonsocial pictures, whereas the opposite was true for social pictures (ps < .01). From a slightly different perspective, positive nonsocial pictures were recalled significantly better than positive social pictures, whereas negative social pictures were recalled significantly better than negative nonsocial pictures (ps < .005). Although the expected Age X Content X Valence interaction was not significant (p = .12), the quadratic component associated with this effect was significant, F(2,184) = 3.69, p = .03, η2 = .04, suggesting that content did moderate the nature of the critical Age X Valence interaction. Given this effect plus our interest in the impact of content, we conducted follow-up contrasts comparing levels of recall for positive and negative pictures within each type of picture content.2 For nonsocial pictures, positive pictures were remembered better than negative pictures, F(1,184) = 7.32, p = .007, η2 = .04, but age moderated this effect, F(2,184) = 4.92, p = .008, η2 = .05. This was due to the valence effect being significant in the old (p < .001), but not in the young and middle-aged groups (ps > .30). In contrast, negative social pictures were remembered better than positive social pictures, F(1,184) = 20.85, p < .001, η2 = .10, but the effect was not moderated by age (p = .12). In other words, the recall advantage for negative relative to positive stimuli for older adults was not significantly different than that observed for the two younger groups. These results thus support our hypothesis that the positivity effect in memory is dependent upon content.
Figure 2.
Experiment 1 recall for social (left) and nonsocial (right) pictures. Error bars = ± 1 SE.
A potential concern in interpreting these results is the possibility that stimulus factors other than valence and social content that vary across stimuli could account for the observed effects in memory. Our own examination of the stimuli along with data collected elsewhere, however, provide little support for this alternative explanation of the results. Positive and negative social pictures used in our study did not differ in terms of the number, sex, and age of the people depicted. In addition, examination of ratings on a number of dimensions associated with memory (distinctiveness, familiarity, importance, meaningfulness, memorability, surprise) available for 35 of the 36 target pictures (Libkuman, Otani, Kern, Viger, & Novak, 2007) revealed that social stimuli were rated as significantly more meaningful than nonsocial stimuli (p = .006) and negative stimuli were rated as significantly more surprising and distinctive but less familiar than positive pictures (ps < .01). Importantly, however, the interaction between picture content and valence was not significant on any of these dimensions (ps > .25), suggesting that the reversal of the valence effect across social versus nonsocial stimuli could not easily be attributed to important differences in stimulus characteristics that covaried with memory performance.
Manipulation Checks
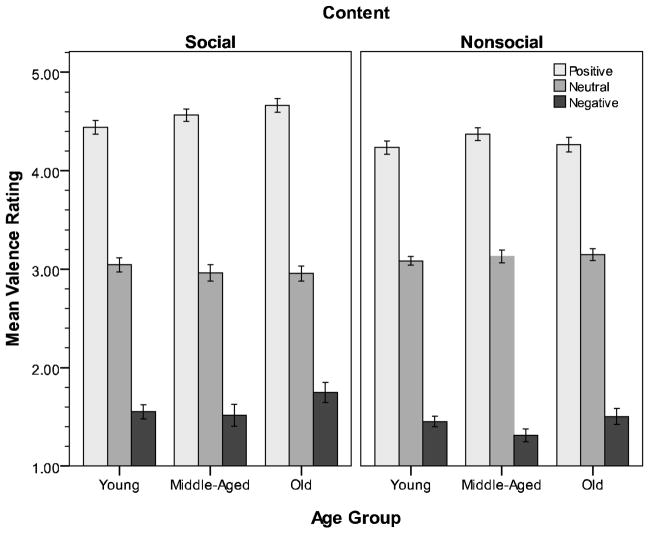
An Age Group X Instructions X Picture Content X Valence ANOVA was performed on valence ratings of pictures collected at the end of the study.3 As seen in Figure 3, a strong valence effect was obtained, F(2,258) = 1540.15, p < .001, η2 = .92, with participants’ ratings of positive, neutral, and negative target pictures (Ms = 4.43, 3.06, & 1.51, respectively) conforming to expectation. Although there was some variation in ratings as a function of instructions, F(2,258) = 3.08, p = .05, η2 = .02, and content, F(2,258) = 23.32, p < .001, η2 = .15, the effects did not dramatically alter the general pattern of ratings across conditions. Importantly, age did not have a significant main or moderating effect on ratings (ps > .08).
Figure 3.
Experiment 1 valence ratings (1 = negative, 5 = positive) for social (left) and nonsocial (right) pictures. Error bars = ± 1 SE.
The absence of age differences in valence ratings is inconsistent with results reported by Grühn and Sheibe (2008) and Keil and Freund (2009) using a larger sample of IAPS pictures, but replicates findings in other studies of memory (e.g., Emery & Hess, 2008; Tomaszczyk et al., 2008). The differences across studies may relate to the stimulus subsets examined or the nature of the sample (e.g., German vs. North American). The reasons for the discrepancy are beyond the scope of the present research. Of importance, however, is the finding that different-aged participants in our study viewed the affective nature of the stimuli in a similar fashion.
We also examined whether participants expected their memory to be tested, and found that 74.5% of those in the intentional condition and 87.4% of those in the incidental condition had expectations consistent with the instructions they received. Importantly, the results of our analyses were not significantly altered if we either (a) only used data from those participants with instructions-consistent expectations or (b) examined performance as a function of expectations about a memory test (as opposed to instructional condition).
Discussion
One main finding emerged from this study. Specifically, we found that, in addition to age, stimulus content influences the nature of emotional biases in memory. When picture content was not considered, we found that the oft-observed recall advantage for positive over negative information was significantly reduced in older adults. Importantly, however, this positivity effect was primarily evident for pictures that did not depict people. For pictures containing people, all age groups recalled more negative than positive pictures, and the strength of this negativity bias did not vary with age. In other words, the SST-derived expectation that older adults will bias attention and memory away from negative and toward positive information in service of emotional goals was only confirmed with nonsocial stimuli. We also failed to find support for the hypothesis that the positivity effect would be stronger under unconstrained viewing conditions.
Experiment 2
The primary novel result of the first study was the impact of picture content on emotional biases in memory. We conducted a second study in an attempt to both establish the reliability of the effect and explore a possible mechanism for its basis. Although social content did eliminate the age-based positivity effect in Experiment 1, its influence was through induction of a negativity bias in recall rather than a general elevation of memory. Thus, an important question concerns what might be unique about the negative social stimuli that would result in this somewhat dramatic reversal of valence-based biases in older adults’ recall.
We had hypothesized that social content might be more likely to engage self-referential processing, which in turn might facilitate retention. (Interestingly, Kensinger [2012] has suggested that the positivity effect can be viewed as a reflection of older adults’ self-referential processing of positive information in the service of maintaining a positive mood.) One possibility is that negative social stimuli might be particularly likely to engage processes related to self-referential processing. For example, Williams et al. (2006) found that mPFC activation in older adults was higher for negative than for positive social stimuli. Given that mPFC is also associated with self-referential processes (Fossati et al., 2003), the heightened responding associated with negative stimuli might suggest greater engagement of such processing for negative relative to positive social stimuli. This, in turn, might account for the negativity bias in memory.
To test these ideas, we had younger and older adults view the same pictures as in the first study, but under two new instructional conditions. The first was a self-referential condition, in which participants were asked to imagine themselves or someone close to them in the situation depicted in the pictures. The second was a condition in which people simply judged the quality of the pictures. If self-referential processing boosts memory for social information and is a factor in the negativity bias for social stimuli observed in Experiment 1, then a positive impact of such processing on memory should be observed. We anticipated that this effect would be greater for positive than for negative pictures if such processing was the basis for the observed negativity bias for social stimuli. Our expectations regarding the impact of instructions on memory for pictures without people were less clear, but it might be assumed that the impact would be smaller since it would be more difficult to process such pictures in a self-referential fashion, thereby resulting in a three-way interaction between instructions, picture content, and valence.
Method
Participants
A new sample of older adults (33 women, 27 men; age range = 63 – 85) was recruited and compensated as in Experiment 1. The younger group (36 women, 30 men; age range = 18–31) consisted of undergraduates recruited from Introductory Psychology classes who fulfilled a course option through participation.
Materials and Procedure
The materials and procedure were similar to that of Experiment 1, with the following exceptions. Participants viewed the target pictures under one of two new randomly assigned instructional conditions. In the quality condition, participants were asked to adopt a neutral, detached attitude as they examined each image, evaluating them for vividness of color, clarity, and degree of detail as a photography instructor might, while ignoring the subject matter of the images. In the referential condition, participants were asked to imagine themselves, a close family member, or a friend in the situation depicted in the images, thereby attempting to experience the associated emotions evoked by the images. Participants in the former condition rated the quality of the picture whereas those in the self-referential condition rated the extent to which they could imagine themselves or a close family member in the situation depicted in the picture. All participants also rated the ease with which they were able to make their task-specific judgments. All ratings used 5-point scales. Given the nature of these instructions, the subsequent recall test was unexpected for all participants. Mood ratings were not collected during testing, nor were facial expressions recorded, given our failure to find performance associations involving these stimuli. However, the PANAS was administered just prior to initial viewing of the pictures to assess current affective status. In addition, the WAIS III Letter-Number Sequencing task was administered instead of the operation span task.
Results
Participant Characteristics
Information about the sample is presented in Table 3. Once again, age differences on the included measures are as expected. Additional Age Group X Instructions (quality vs. referential processing) ANOVAs on each measure revealed no effects due to instructions.
Table 3.
Experiment 2: Participant characteristics
| Measure | Young Adults | Older Adults | ||
|---|---|---|---|---|
|
| ||||
| M | SD | M | SD | |
| Age | 19.2 | 1.8 | 72.5 | 5.6 |
| Education* | 13.0 | 1.1 | 16.4 | 2.7 |
| SF36 Physical health* | 51.2 | 5.6 | 44.0 | 8.8 |
| SF36 Mental health* | 48.7 | 9.0 | 56.4 | 6.7 |
| GDS* | 2.1 | 2.3 | 1.3 | 1.8 |
| Letter-number sequencing* | 12.4 | 2.5 | 9.9 | 3.0 |
| Digit Symbol* | 85.0 | 15.1 | 60.2 | 12.2 |
| Vocabulary | 46.7 | 7.1 | 49.0 | 10.4 |
| Plus-Minus* | 23.5 | 20.6 | 36.7 | 26.1 |
| PANAS Positive affect | 3.5 | .7 | 3.7 | .6 |
| PANAS Negative affect* | 1.9 | .6 | 1.4 | .4 |
Age group difference significant at p < .05.
Memory
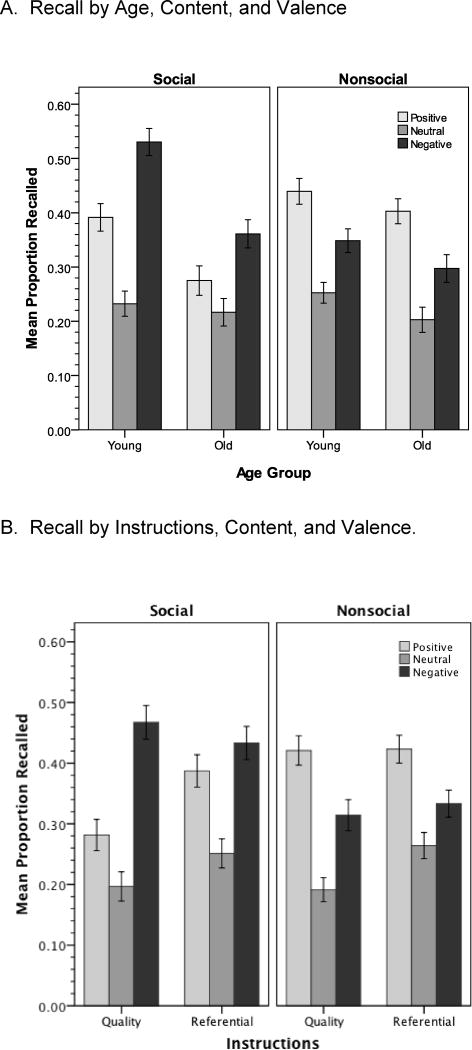
Recalled picture descriptions were coded as in Experiment 1, with high interrater reliability (Fleiss’ kappa = .93). Older adults (M = 0.46, SD = 0.90) were more likely to produce uncodable descriptions than were younger adults (M = 0.15, SD = 0.44), t(92.27) = −2.49, p = .01. The resulting proportion recall scores were submitted to a 2 × 2 × 2 × 3 (Age Group X Instructions X Picture Content X Picture Valence) ANOVA. The 4-way interaction was not significant (p =.57); as such, we concentrate our analysis below on the two questions outlined above: (a) does social content influence the relative recall of positive and negative pictures, replicating Experiment 1, and (b) do self-referential encoding instructions increase recall for positive, but not negative, social pictures?
As before, young adults recalled more pictures than older adults (Ms = .37 vs. .29), F(1,122) = 15.03, p < .001, η2 = .11, and positive and negative pictures were recalled better than neutral pictures (Ms = .38 & .38 vs. .23), F(2,244) = 74.80, p < .001, η2 = .38. Significant interactions were also observed between age and valence, F(2,244) = 3.30, p = .04, η2 = .03, age and content, F(2,244) = 4.77, p = .03 η2 = .04, and age, content, and valence, F(2,244) = 3.02, p = .05, η2 = .02. The effects of content on recall are illustrated in the top panel of Figure 4. Overall, older adults exhibited a slight positivity bias (Mpositive = .34, Mnegative = .33) compared to a slight negativity bias for younger adults (Mpositive = .42, Mnegative = .44). In addition, the emotional memory bias reversal with content was once again observed, with negative social pictures recalled better than positive social pictures (Ms = .45 vs .33), whereas the opposite was true for nonsocial pictures (Ms = .32 vs .42). The three-way interaction appears due to the fact that the negativity bias (i.e., negative recall minus positive recall) for social pictures was somewhat stronger for the young than for the older adults (.14 vs. .08), whereas the positivity bias (i.e., positive recall minus negative recall) for nonsocial pictures was slighty stronger for older adults (.10 vs. .09). Follow-up analyses revealed, however, that age did not reliably moderate the just-noted significant impact of valence on recall for either type of content (ps > .29). In other words, the strength of the valence-based effects within content conditions did not vary across age groups.
Figure 4.
Experiment 2 recall by (A) age, cotent, and valence, and (B) instructions, content, and valence. (bottom). Error bars = ± 1 SE.
Of prime interest was the impact of instructions (Figure 4, bottom). Consistent with expectations, referential instructions boosted recall relative to quality instructions (Ms = .35 vs .31), F(1,122) = 4.19, p = .04, η2 = .03, and significant Instructions X Valence, F(2,244) = 3.26, p = .04, η2 = .03, and Instructions X Content X Valence, F(2,244) = 3.41, p = .04, η2 = .03, interactions were obtained. For social pictures, there was a significant interaction between instructions and valence, F(2,244) = 5.01, p = .01, η2 = .04. As expected, relative to the quality condition, referential instructions significantly (p = .001) increased memory for positive pictures, and marginally (p = .06) increased memory for neutral pictures. In contrast, there was no impact of instructions on negative pictures (p = .71). For nonsocial pictures, referential instructions only enhanced memory for neutral pictures (p = .01). The positivity bias in recall of these pictures was similar for both types of instructions.
Finally, as in the first study, we specifically compared memory for positive versus negative pictures within each type of picture content. For nonsocial pictures, positive pictures were remembered better than negative pictures, F(1,122) = 14.73, p < .001, η2 = .11, and this effect was moderated by neither age nor instructions (ps > .66). For social pictures, a significant valence effect in the opposite direction was obtained, F(1,122) = 22.04, p < .001, η2 = .15, and this effect was moderated by instructions, F(1,122) = 10.89, p = .001, η2 = .08. This was due to the enhancement of recall of positive social pictures in the referential condition, resulting in a nonsignificant difference in recall of positive and negative pictures in this condition (Figure 4, bottom).
Referential Processing
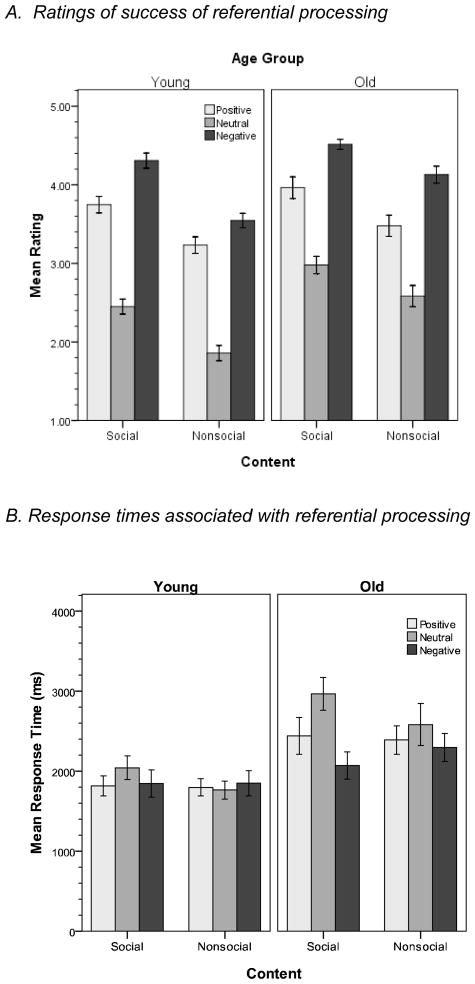
We next examined the judgments that participants made while initially viewing the pictures. We focused on ratings in the self-referential group given that these were most directly related to our questions of interest, with specific interest in ratings of the degree to which individuals were able to imagine themselves or a close other in the depicted situation. An Age X Content X Valence ANOVA on these ratings revealed that older adults had higher success rates than younger adults (Ms = 3.6 vs. 3.2), F(1,63) = 14.90, p < .001, η2 = .19, and success was higher for social than for nonsocial stimuli (Ms = 3.7 vs. 3.1), F(1,63) = 143.62, p < .001, η2 = .69. A significant valence effect was also obtained, F(2,126) = 270.35, p < .001, η2 = .81, with ratings for negative images (M = 4.1) being significantly higher than those for positive images (M = .36), both of which were significantly greater than ratings for neutral images (M = 2.5; ps < 001). Age moderated the impact of both valence, F(2,126) = 3.72, p = .03, η2 = .06, and content, F(1,63) = 5.34, p = .02, η2 = .08. These interactions reflected the facts that the previously described age difference was greater for neutral stimuli than for valenced-stimuli, and that the overall higher success ratings of older adults relative to younger adults was greater for nonsocial than for social pictures (Figure 5, top).
Figure 5. Ratings (A) and response times (B) in referential instructions group relating to success of making self-referential judgments.
Error bars = ± 1 SE.
A similar analysis was conducted on response times associated with these judgments (Figure 5, bottom). A significant valence effect was obtained, F(2,126) = 6.62, p = .002, η2 = .10, with significantly faster response times (ps ≤ .005) for negative and positive images than for neutral images. A significant Age X Valence interaction was also obtained, F(2,126) = 6.62, p = .002, η2 = .10, due to the just-described valence effect being significant for old adults (p = .002), but not for young adults (p = .60). In correspondence with the ratings, where encoding success was greater with negative than with positive pictures, there was a nonsignificant tendency for response times to be faster for negative than for positive pictures. Finally, a significant Content X Valence interaction was also obtained, F(2,126) = 4.73, p = .01, η2 = .07, due to the valence effect being specific to social pictures.
In sum, all participants reported more successful referential processing for social than for nonsocial stimuli, and success rates were greatest for negative images. Interestingly older adults reported generating stronger referential responses than younger adults, particularly for stimuli that might lack social relevance (e.g., pictures without people, neutral pictures). Further, referential processing was faster for valenced stimuli, with this effect being specific to older adults and social stimuli.
Discussion
We once again observed that valence-based biases in memory were dependent not only on age, but also on content. A slight positivity effect (i.e., Age X Valence interaction) was observed when overall recall was examined. However, subsequent examination taking into account instructions and content revealed a more complex picture. Young and old adults alike demonstrated a positivity bias in memory for nonsocial information, regardless of viewing instructions. In addition, the strength of this bias was similar across age groups. In contrast, when recall of social stimuli was examined, both younger and older adults exhibited clear negativity biases in recall. In support of our hypothesis that this effect reflected the greater probability of self-referential processing for social stimuli, the negativity bias was significantly reduced through enhancement of recall of positive images when participants were required to process the pictures in a self-referential manner. The fact that recall of nonsocial pictures appeared not to be influenced by instructions may reflect the difficulty of engaging in self-referential processing with such stimuli. Notably, the effects of valence and instructions were similar across age groups.
One notable difference between these results and those of the first experiment is the fact that younger adults also exhibited a strong positivity bias in recall of nonsocial stimuli. The reasons for this are unclear, perhaps reflecting the greater influence of the instructional sets in the present experiment. In contrast to those in the first study, the instructions used here may have placed greater constraints on processing, thereby reducing age differences in memory biases. Of primary significance, however, is the fact that we once again found that the nature of these biases varied with the degree of social content in the pictures.
Examination of ratings and response times in the referential condition also provided some indirect support for the hypothesis that self-referential processing accounted for the observed negativity bias for social images. Such processing was easier—both in terms of success ratings and response times—for social than for nonsocial stimuli. There was also some indication that self-referential processing was particularly efficient for negative images, although somewhat inconsistent with our perspective was the fact that this was true for both social and nonsocial images. Regardless, the ease of self-referential processing associated with social stimuli may suggest a higher probability of engaging in such processing for images containing people than for those that do not.
GENERAL DISCUSSION
Studies of the impact of stimulus valence on cognitive processes have revealed interesting insights about adaptive functions. From a general perspective, research has suggested that biologically relevant information (e.g., threatening stimuli with survival implications) is processed in a proprietary fashion (e.g., Öhman, Flykt, & Esteves, 2001), perhaps reflective of evolutionary roots. More germane to the present research, valence influences may also reflect individual and developmental differences in goals. In the field of aging, relative to younger adults, older adults have shown a bias toward positive and away from negative information. This pattern of performance is consistent with expectations derived from SST and the hypothesized enhancement of affective goals in later life. Researchers have also found, however, that this positivity effect is not observed in all situations.
A primary goal of the present research was to further delineate the conditions associated with this effect. We investigated two such factors: content of the information to be remembered and encoding conditions. Of greatest significance is the finding that stimulus content is associated with both the nature of valence-based memory biases and the strength of the positivity effect in memory. We found that the positivity effect was most evident when the to-be-remembered stimuli did not contain images of people. In contrast, when memory for social stimuli was examined, both young and older adults exhibited clear memory superiority for negative over positive images, with this negativity bias being similar across age groups. We hypothesize that this may reflect the greater likelihood social stimuli engaging attention—particularly those reflecting negative affect—which may evoke empathy and engage self-referential processing of the stimuli. Support for this hypothesis was bolstered in Experiment 2, where we found that referential processing of the stimuli selectively enhanced memory for positive social images. A reasonable inference for this selective effect is that participants were already likely to process negative social stimuli in a self-referential fashion (e.g., Williams, et al., 2006), resulting in less benefit when specific instructions were given to do so. Referential processing did not have a dramatic effect on memory for nonsocial stimuli, perhaps reflecting the greater difficulty in processing such stimuli in this manner. Indeed, participants were generally slower and reported more difficulty in processing nonsocial as opposed to social stimuli in a nonreferential fashion. This may, in part, explain the absence of a negativity bias in their recall of such material. The difficulty in engaging in self-referential processing may also help to explain their apparent ability to disengage from negative nonsocial stimuli and focus on positive stimuli.
The second goal of the present research was to further explore the impact of encoding conditions on memory. Given that the positivity effect is hypothesized to reflect goal-based controlled processing mechanisms, it should be most evident in situations involving few constraints on the manner in which stimuli are encoded (Mather, 2006; Kensinger, 2012). We obtained no support for this hypothesis in Experiment 1, where valence-based effects on memory performance under unconstrained viewing conditions were similar to those obtained when participants received explicit instructions to memorize the pictures being viewed. These results replicate the null effects obtained by both Emery and Hess (2008) and Tomaszczyk et al. (2008). In Experiment 2, we obtained little evidence of a positivity effect when instructions and picture content were taken into account. Given that we used two different structured viewing conditions in that study, this could be taken as support for such conditions eliminating the positivity effect. However, older adults continued to exhibit a positive bias under certain conditions (i.e., nonsocial pictures) even when given specific instructions for viewing. The interesting result is that younger adults exhibited a bias of similar strength in the same condition, suggesting that stimulus content might moderate the strength of the bias and age differences therein.
The impact of information content on age differences in valence-based effects on memory performance, and the failure of instructions to moderate the strength of the positivity effect appear inconsistent with SST-based predictions regarding aging and the processing of affective stimuli. They do not necessarily negate the possibility that older adults will differentially focus on positive versus negative stimuli as an emotion-regulation strategy. Our results do, however, suggest that stimulus characteristics may be important moderators of the conditions under which such differential processing can occur. If we accept that chronic emotional goals motivate older adults to focus on positive as opposed to negative stimuli, an interesting question concerns the mechanisms associated with social stimuli that appear to counteract this focus. One possibility is that negative social stimuli are more likely to engage stimulus-driven bottom-up processing that interferes with goal-driven top-down processing. As noted before, there is evidence that social stimuli and negative stimuli have some priority in attention, and the confluence of these two factors may account for such bottom-up processing. Alternatively, it is also possible that such processing may be more top-down in nature, reflecting either attempts to regulate emotional responses to such negative social stimuli (e.g., Williams et al, 2006) or reflect an adjustment in motivated processing based in empathic responding to suffering, which appears to be relatively stable through adulthood (e.g., Grühn et al., 2008). Unfortunately, we do not have data that would enable us to discriminate between these two possibilities, both of which may be involved.
Although the present study provides evidence that the positivity effect is eliminated or reduced with social stimuli, a valid question remains as to whether this effect is general to all social stimuli. Unfortunately, we cannot test this in the current study given that our negative social stimuli were relatively homogeneous in terms of emotional content. Based on classifications of IAPS pictures by Mikels et al. (2005), the dominant emotion displayed in our negative social stimuli was sadness, as was also the case in Emery and Hess (2008). Such stimuli might be especially likely to induce empathic responses and associated self-referential processing, thereby modulating the differential focus on positive information. In addition, there is some evidence that older adults have stronger emotional responses to sad stimuli than do younger adults (Streubel & Kunzmann, 2011). Other research, however, suggests that older adults are more likely to divert attention from displays of anger than from those of other negative emotions (e.g., Isaacowitz, Wadlinger, Goren, & Wilson, 2006) and are better able than younger adults to suppress attention to such displays (Hahn, Carlson, Singer, & Gronlund, 2006). This suggests that the observed negativity bias may be less evident with more threatening social stimuli than other types of negative social stimuli. This may not necessarily reflect differences in the degree to which these various types of stimuli initially attract attention. Older adults may, however, be more likely to disengage from threatening stimuli than from nonthreatening social stimuli, resulting in less processing and poorer memory. These ideas await further test. Even if the negativity bias in memory for social stimuli is found to be specific to the emotional content of the pictures, however, it does not negate the fact that stimulus content moderates the positivity effect in memory.
In conclusion, the present findings provide additional insight into the factors that moderate the positivity effect in memory. In particular, our results underscore the importance of considering the content of the information to-be-remembered, which may impact attentional processes and create potential processing conflicts with emotion regulation processes assumed to underlie this effect. While not negating the validity of the regulation processes associated with SST that are assumed to undergird the positivity effect, our results suggest a further narrowing of the conditions under which they are likely to occur.
Acknowledgments
Support for this study was provided by NIA grant R01 AG020153.
We would like to thank Logan Collins, Carla Strickland-Hughes, Katie Bigelow, Devin Bueker, Charlotte Dominguez, Dana Mueller, Molly Rogowski, Ashley Van Alstyne, and Erin Winsch for their assistance with data collection and other aspects of the projects.
Footnotes
The data from three older adults who did not recall anything were excluded from this analysis.
We excluded neutral pictures in these focused analyses due to the possibility of Age X Valence interactions emerging due to the overall lower levels of recall in older adults, which resulted in smaller differences between valenced and neutral stimuli in older relative to young and middle-aged adults. Comparing just positive and negative stimuli still allows for an examination of the theoretically meaningful reversal of valence effects across picture content while controlling for this potential confound.
Due to a revision in procedures, the first 57 participants tested did not provide these ratings.
Contributor Information
Thomas M. Hess, Email: thomas_hess@ncsu.edu, Department of Psychology, North Carolina State University, Raleigh, NC 27695-7650, (919) 515-1729
Lauren E. Popham, North Carolina State University
Paul A. Dennis, North Carolina State University
Lisa Emery, Appalachian State University.
References
- Bailey PE, Henry JD, von Hippel W. Empathy and social functioning in late adulthood. Aging and Mental Health. 2008;12:499–503. doi: 10.1080/13607860802224243. [DOI] [PubMed] [Google Scholar]
- Bower GH, Gilligan SG. Remembering information related to one’s self. Journal of Research in Personality. 1979;13:420–432. doi: 10.1016/0092-6566(79)90005-9. [DOI] [Google Scholar]
- Carstensen LL. Selectivity theory: Social activity in life-span context. In: Schaie KW, editor. Annual review of gerontology and geriatrics. Vol. 11. New York: Springer; 1991. pp. 195–217. [Google Scholar]
- Carstensen LL, Turk-Charles S. The salience of emotion across the adult life span. Psychology and Aging. 1994;9:259–264. doi: 10.1037/0882-7974.9.2.259. [DOI] [PubMed] [Google Scholar]
- Charles ST, Mather M, Carstensen LL. Aging and emotional memory: The forgettable nature of negative images for older adults. Journal of Experimental Psychology: General. 2003;132:310–324. doi: 10.1037/0096-3445.132.2.310. [DOI] [PubMed] [Google Scholar]
- Comblain C, D’Argembeau A, Van der Linden M, Aldenhoff L. The effect of ageing on the recollection of emotional and neutral pictures. Memory. 2004;12:673–684. doi: 10.1080/09658210344000477. [DOI] [PubMed] [Google Scholar]
- Denburg NL, Buchanan TW, Tranel D, Adolphs R. Evidence for preserved emotional memory in normal older persons. Emotion. 2003;3:239–253. doi: 10.1037/1528-3542.3.3.239. [DOI] [PubMed] [Google Scholar]
- Emery L, Hess TM. Viewing instructions impact emotional memory differently in older and young adults. Psychology and Aging. 2008;23:2–12. doi: 10.1037/0882-974.23.1.2. [DOI] [PubMed] [Google Scholar]
- Fossati P, Hevenor SJ, Graham SJ, Grady C, Keightley ML, Craik F, Mayberg H. In search of the emotional self: an fMRI study using positive and negative emotional words. The American Journal of Psychiatry. 2003;160:1938–1945. doi: 10.1176/appi.ajp.160.11.1938. [DOI] [PubMed] [Google Scholar]
- Grühn D, Rebucal K, Diehl M, Lumley MA, Labouvie-Vief G. Empathy across the adult lifespan: Longitudinal and experience-sampling findings. Emotion. 2008;8:753–765. doi: 10.1037/a0014123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grühn D, Smith J, Baltes PB. No aging bias favoring memory for positive material: Evidence form a heterogeneity-homogeneity list paradigm using emotionally toned words. Psychology and Aging. 2005;20:579–588. doi: 10.1037/0882-7974.20.4.579. [DOI] [PubMed] [Google Scholar]
- Gutchess AH, Kensinger EA, Schacter DL. Ageing, self-referencing, and medial pre-frontal cortex. Social Neuroscience. 2007;2:117–133. doi: 10.1080/17470910701399029. [DOI] [PubMed] [Google Scholar]
- Hahn S, Carlson C, Singer S, Gronlund SD. Aging and visual search: Automatic and controlled attentional bias to threat faces. Acta Psychologica. 2006;123:312–336. doi: 10.1016/j.actpsy.2006.01.008. [DOI] [PubMed] [Google Scholar]
- Harvey PO, Fossati P, Lepage M. Modulation of memory formation by stimulus content: specific role of the medial prefrontal cortex in the successful encoding of social pictures. Journal of Cognitive Neuroscience. 2007;19:351–362. doi: 10.1162/jocn.2007.19.2.351. [DOI] [PubMed] [Google Scholar]
- Hess TM, Kotter-Grühn D. Social knowledge and goal-based influences on social information processing in adulthood. Psychology and Aging. 2011;26:792–802. doi: 10.1037/a0023775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphrey K, Underwood G. The potency of people in pictures: Evidence from sequences of eye fixations. Journal of Vision. 2010;10 doi: 10.1167/10.10.19. article 10. [DOI] [PubMed] [Google Scholar]
- Isaacowitz DM, Wadlinger HA, Goren D, Wilson HR. Selective preference in visual fixation away from negative images in old age? An eye tracking study. Psychology and Aging. 2006;21:40–48. doi: 10.1037/0882-7974.21.1.40. 1037/0882-7974.21.1.40. [DOI] [PubMed] [Google Scholar]
- Kensinger EA. Emotional memory across the adult lifespan. Psychology Press; New York, NY: 2009. [Google Scholar]
- Kensinger EA. Emotion-memory interactions in older adulthood. In: Naveh-Benjamin M, Ohtao N, editors. Memory and aging: Current issues and future directions. New York, NY: Psychology Press; 2012. pp. 215–243. [Google Scholar]
- Kensinger EA, Brierley B, Medford N, Growdon JH, Corkin S. Effects of normal aging and Alzheimer’s Disease on emotional memory. Emotion. 2002;2:118–134. doi: 10.1037/1528-3542.2.2.118. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. International affective picture system (IAPS): Affective ratings of pictures and instruction manual. Gainesville: University of Florida; 2001. [Google Scholar]
- Langton SR, Law AS, Burton AM, Schweinberger SR. Attention capture by faces. Cognition. 2008;107:330–342. doi: 10.1016/j.cognition.2007.07.012. [DOI] [PubMed] [Google Scholar]
- Leigland LA, Schulz LE, Janowsky JS. Age related changes in emotional memory. Neurobiology of Aging. 2004;25:1117–1124. doi: 10.1016/j.neurobiolaging.2003.10.015. [DOI] [PubMed] [Google Scholar]
- Mather M. Why memories may become more positive as people age. In: Uttl B, Ohta N, Siegenthaler AL, editors. Memory and emotion: Interdisciplinary perspectives. Malden: Blackwell Publishing; 2006. pp. 135–158. 2006. [Google Scholar]
- Mather M, Carstensen LL. Aging and attentional biases for emotional faces. Psychological Science. 2003;14:409–415. doi: 10.1111/1467-9280.01455. [DOI] [PubMed] [Google Scholar]
- Mather M, Carstensen LL. Aging and motivated cognition: The positivity effect in attention and memory. Trends in Cognitive Sciences. 2005;9:496–502. doi: 10.1016/j.tics.2005.08.005. [DOI] [PubMed] [Google Scholar]
- Mather M, Knight MR. Goal-directed memory: The role of cognitive control in older adults’ emotional memory. Psychology and Aging. 2005;20:554–570. doi: 10.1037/0882-7974.20.4.554. [DOI] [PubMed] [Google Scholar]
- Mikels JA, Fredrickson BL, Larkin GR, Lindberg CM, Maglio SJ, Reuter-Lorenz PA. Emotional category data on images from the International Affective Picture System. Behavior Research Methods. 2005;37:626–630. doi: 10.3758/bf03192732. 2006-03539-007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Öhman A, Flykt A, Esteves F. Emotion drives attention: Detecting the snake in the grass. Journal of Experimental Psychology: General. 2001;130:466–478. doi: 10.1037/0096-3445.130.3.466. [DOI] [PubMed] [Google Scholar]
- Richter D, Kunzmann U. Age differences in three facets of empathy: Performance-based evidence. Psychology and Aging. 2011;26:60–70. doi: 10.1037/a0021138. [DOI] [PubMed] [Google Scholar]
- Rogers TB, Kuiper NA, Kirker WS. Self-reference and the encoding of personal information. Journal of Personality and Social Psychology. 1977;35:677–688. doi: 10.1016/0092-6566(79)90006-0. [DOI] [PubMed] [Google Scholar]
- Sheikh JI, Yesavage JA. Geriatric depression scale (GDS): Recent evidence and development of a shorter version. Clinical Gerontologist: The Journal of Aging and Mental Health. 1986;5:165–173. doi: 10.1300/J018v05n01_09. [DOI] [Google Scholar]
- Streubel B, Kunzmann U. Age differences in emotional reactions: Arousal and age-relevance count. Psychology and Aging. 2011;26:966–978. doi: 10.1037/a0023424. [DOI] [PubMed] [Google Scholar]
- Tomaszczyk JC, Fernandes MA, MacLeod CM. Personal relevance modulates the positivity bias in recall of emotional pictures in older adults. Psychonomic Bulletin and Review. 2008;15:191–196. doi: 10.3758/PBR.15.1.191. [DOI] [PubMed] [Google Scholar]
- Ware JE., Jr . SF-36 Health Survey. Boston: New England Medical Center Health Institute; 1993. [Google Scholar]
- Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: The PANAS scales. Journal of Personality & Social Psychology. 1988;54:1063–1070. doi: 10.1037/0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale. New York: Psychological Corporation; 1997. [Google Scholar]
- Williams LM, Brown KJ, Palmer D, Liddell BJ, Kemp AH, Olivieri G, Gordon E. The mellow years?: Neural basis of improving emotional stability over age. The Journal of Neuroscience. 2006;26:6422–6430. doi: 10.1523/JNEUROSCI.0022-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]