Abstract
Accumulating evidence indicates that, analogous to omega-6 polyunsaturated fatty acids (PUFA), omega-3 PUFA are enzymatically converted into diverse families of bioactive mediators that play numerous roles in physiology. These mediators, which include the resolvins, protectins and maresins, are particularly important in resolving acute inflammation and also appear to play a role in enhancing host defense. Given the protective actions of omega-3 PUFA in humans and in animal models of disease, active generation of bioactive mediators may in part underlie these protective effects. Several studies have demonstrated that bioactive autacoids generated from omega-3 PUFA have direct anti-inflammatory and pro-resolution actions, and the structures of many of these endogenous mediators have been elucidated. The diverse roles of these lipid mediators in health and disease, regulation of their biosynthesis, as well as identification of specific receptors and cellular targets, are emerging. This brief review will highlight the biosynthesis of resolvins, protectins and maresins, and discuss their receptor-mediated biological actions in promoting the resolution of inflammation. Their potential use as a new class of pro-resolution therapeutics, as well as gaps in knowledge and challenges for future research, will also be discussed. Overall, the identification of these novel families of lipid mediators has yielded insight into the protective actions of omega-3 PUFA and may lead to the development of an entirely new class of therapeutics aimed at regulating inflammation and host defense.
Keywords: Resolvins, Resolution of inflammation, DHA, EPA
Introduction
Polyunsaturated fatty acids (PUFA) of both the omega-6 and omega-3 classes are essential to health and must be obtained in the diet. The concept that certain types of fat are essential in the diet was put forth initially in studies of rodents consuming a fat-depleted diet, which was associated with a marked appearance of dry skin, brittle hair and skin rashes; effects that were reversed by fat replenishment(1). A similar phenotype is apparent in humans with essential fatty acid deficiency (EFAD), with symptoms appearing rapidly (< 1 week) in infants when their essential fatty acid levels are below 5% of total caloric intake(2). More than 80 years of research has now defined that essential fatty acids, which are components of major lipid classes including phospholipids, triglycerides and cholesterol esters, serve numerous roles in physiology(3, 4). These diverse functions include the regulation of cell membrane dynamics and cell signaling, providing a source of energy (through beta-oxidation), and serving as the precursors to bioactive lipid mediators(4–6).
While mammals have the ability to elongate and desaturate both omega-3 and omega-6 PUFA, the biosynthetic starting materials for these pathways, namely alpha-linolenic acid (ALA; 18:3n-3) and linoleic acid (18:2n-6), respectively, must be obtained in the diet(7–9). Linoleic acid can be further converted into arachidonic acid (AA; 20:4n-6) through a series of elongation and desaturation reactions, while ALA is similarly converted into eicosapentaeonoic acid (EPA; 20:5n-3) and docosahexaenoic acid (DHA; 22:6n-3)(9). As such, EPA and DHA are not considered essential in the diet, but it should be noted that the conversion of ALA into EPA and DHA is very low in humans and thus nutritional strategies designed to address deficiencies in omega-3 PUFA generally utilize marine-based sources that are naturally high in EPA and DHA(2, 8, 9). Importantly, studies of the biological role of omega-3 PUFA in health and disease have been aided by the generation of transgenic mice that express an omega-3 desaturase gene (denoted fat-1) not normally found in mammals that enables endogenous generation of omega-3 PUFA from omega-6 PUFA(10). These mice show substantial increases in tissue levels of both EPA and DHA and are protected from inflammation and tissue injury in a myriad of animal models of disease, including colitis, cancer and pathologic angiogenesis(10–12).
While both omega-6 and omega-3 PUFA are essential for health, it is well-documented that the typical Western-type diet is substantially enriched in omega-6 PUFA, shifting the balance from the optimal n-6:n-3 ratio of 1–2:1 to nearly 20:1(13). This altered ratio of PUFA is associated with the development of numerous chronic diseases, including cardiovascular disease (CVD) and rheumatoid arthritis, potentially because of an imbalance between bioactive lipid mediators that are involved in regulating inflammation(8) (see below). Indeed, several clinical studies have determined that enriching the diet in omega-3 PUFA improves outcomes in diseases such as rheumatoid arthritis(3, 8). Omega-3 PUFA are important during fetal and infant development and may be effective in preventing EFAD in infants requiring parenteral nutrition(2). The protective effects of omega-3 PUFA are particularly convincing for CVD, such as atherosclerosis and its associated cardiac manifestations including acute myocardial infarction and heart failure(14, 15). Building upon observational studies demonstrating a low incidence of CVD in Greenland Eskimos, who have a diet rich in omega-3 PUFA from marine sources, interventional studies have shown that dietary supplementation with purified fish oil extracts reduces the incidence of CVD(15). In particular, the Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto miocardico (GISSI) Prevenzione trial, which enrolled patients with a history of myocardial infarction, demonstrated reduced risk of cardiovascular death with fish oil supplementation(16). A similar improvement in survival was also seen in heart failure patients(17). As a testament to these and related clinical studies, the American Heart Association currently recommends eating fish rich in omega-3 PUFA for the prevention of CVD (www.americanheart.org).
While several epidemiological and interventional studies have established a beneficial role for omega-3 PUFA in health and disease, the specific mechanisms whereby they exert protective actions are still emerging. Certainly, anti-arrhyrthmogenic effects via direct membrane incorporation of omega-3 PUFA have been demonstrated and this may be related to the protective effects of omega-3 PUFA in the context of sudden cardiac death(7). Other mechanisms include the generation of less-potent inflammatory mediators (i.e. 3-series prostanoids and 5-series leukotrienes) that compete for AA metabolism and pro-inflammatory effects of eicosanoids(4, 8). However, over the last decade, it has also emerged that omega-3 PUFA, including both EPA and DHA, are converted into novel families of bioactive lipid mediators that are potent immunomodulatory agonists. This short review will highlight the biosynthesis and biological actions of these mediators, which include the resolvins, protectins and maresins, and discuss the potential for targeted therapeutics based on these specific mediators.
Bioactive lipid mediators generated from omega-3 polyunsaturated fatty acids: Biosynthetic pathways
It is well established that bioactive lipid autacoids are generated from polyunsaturated fatty acids in an enzymatic manner. Prominent examples include the generation of prostanoids and leukotrienes derived from cyclooxygenase (COX)-and lipoxygenase (LOX)-mediated conversion of arachidonic acid (20:4, n-6). Similarly, arachidonic acid is also a substrate for monoxygenases (i.e., cytochrome P450) that give rise to epoxyeicosatrienoic acids (EETs). Collectively, these mediators play numerous physiologic roles, including regulation of hemodynamics, inflammatory cell trafficking and blood coagulation(5, 18–20). More recently, it has been demonstrated that, like n-6 PUFA, n-3 PUFA, including EPA and DHA, can also serve as substrates for both COX and LOX enzymes and give rise to several new families of bioactive mediators(6, 21).
Resolvins
Resolvins are a family of lipid mediators generated in an enzymatic manner from EPA (E-series) or DHA (D-series)(21–24). They are so named because they were originally identified during the resolution phase of inflammation and were found to potently regulate this critical process(23) (see below). While EPA can serve as a substrate for COX-2 to give rise to 3-series prostanoids, acetylation of COX-2 by aspirin permits the generation of 18-hydoxyeicosapentaenoic acid (18-HEPE)(25, 26). Of note, 18-HEPE can also be formed through a P450-dependent route(26). This product can be further converted by 5-LOX to 5S,12R,18R-trihydroxy-6Z,8E,10E,14Z,16E-eicosapentaenoic acid, which was later coined resolvin E1 (RvE1; Figure 1A)(23, 25). This latter biosynthetic route is similar to that of leukotriene B4, in that the 5,6 epoxide formed by 5-LOX can be enzymatically hydrolyzed by leukotriene A4 hydrolase to yield RvE1(27). More recent studies using chiral chromatography demonstrated that both 18S-HEPE and 18R-HEPE are generated by acetylated COX-2 and are associated with the generation of 18S-RvE1 and 18R-RvE1, respectively(27). Importantly, both mediators are bioactive and share the same specific receptor (see below). While the formation of an epoxide group and enzymatic hydrolysis mediates RvE1 biosynthesis, the 5-hydoperoxy group can also be directly reduced to give rise to 5S,18R-dihydroxy-6E,8Z,11Z,14Z,16E-eicosapentaenoic acid, which is denoted RvE2(28, 29). This structurally unique mediator is also bioactive. More recently, a third member of the E-series resolvin family was discovered and its structure was determined. Like RvE2, RvE3 is a dihydroxy-containing product generated from EPA by a 12/15-LOX pathway as opposed to the 5-LOX pathway involved in RvE1 and RvE2 biosynthesis(30). This new pathway, which is operative in eosinophils, generates two distinct stereoisomers, namely 17R, 18S-diHEPE and 17R, 18R-diHEPE, both of which are bioactive in murine models of acute inflammation(30).
Figure 1. Biosynthesis of pro-resolving lipid mediators from EPA and DHA.
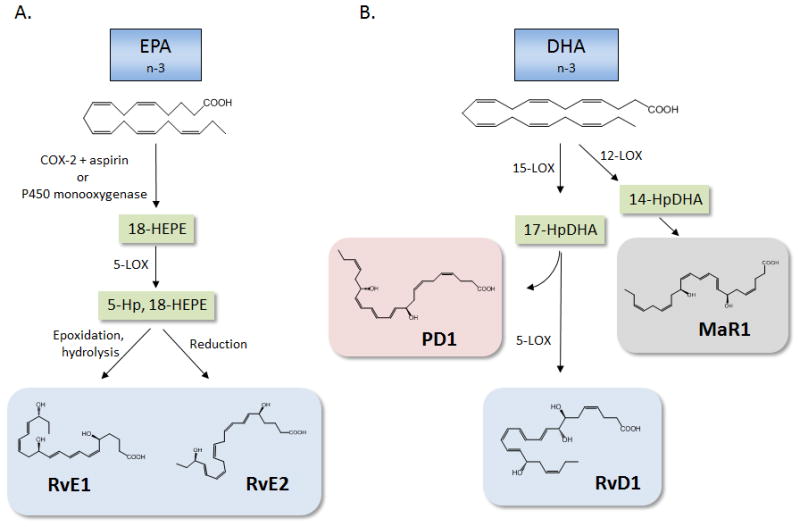
(A) EPA serves as the substrate precursor for the E-series resolvins. In the presence of aspirin, acetylated cyclooxygenase (COX)-2 utilizes EPA as a substrate and produces 18-hydroxy eicosapentaenoic acid (18-HEPE). This intermediate, which can also be generated by a P450 route, can serve as a substrate for 5-lipoxygenase (LOX) to give rise to 5-hydroperoxy (Hp)-18-HEPE. Epoxidation and enzymatic hydrolysis generates RvE1, while 5-Hp, 18-HEPE can also be directly reduced to generate RvE2. (B). DHA is converted to 17-HpDHA by 15-LOX, which, through the formation of an epoxide intermediate, can form protectin D1 (PD1). Conversely, 17-HpDHA can be further converted by 5-LOX to generate resolvin D1 (RvD1). In addition to 15-LOX, DHA can also serve as a substrate for 12-LOX, giving rise to 14-HpDHA, which through enzymatic epoxidation and hydrolysis, gives rise to maresin 1 (MaR1).
Like EPA, DHA can also be converted into a family of bioactive lipid mediators generated during the resolution phase of inflammation, which are denoted D-series resolvins(6). The common biosynthetic intermediate in D-series resolvin biosynthesis is 17-hydroperoxy (Hp) DHA, which can be generated by either 15-LOX or acetylated COX-2 (Figure 1B). However, while 15-LOX generates predominately 17S-HpDHA, acetylated COX-2 generates 17R-HpDHA and thus gives rise to “aspirin-triggered” resolvins(31). In either case, transcellular biosynthesis of D-series resolvins proceeds through 5-LOX, which generates a series of distinct compounds denoted RvD1-RvD6. Resolvins D1-D4 are trihydroxy-containing regioisomers generated via enzymatic hydrolysis of either a 7, 8 epoxide (RvD1 & D2) or a 4, 5 epoxide (RvD3 & 4)(21, 32). In contrast, RvD5 and RvD6 are dihydroxy-containing mediators that arise through a peroxidase mechanism in which the carbon-4 or carbon-7 hydroperoxide formed prior to epoxidation is converted into a hydroxyl group at position 4 in the case of RvD6 or position 7 in the case of RvD5(21). For a detailed description of the precise stereochemistry, total organic synthesis and biosynthetic pathways giving rise to D-series resolvins, the reader is referred to a recent review by Serhan and Petasis(21). In all cases, these diverse biosynthetic routes giving rise to D-series resolvins generate mediators with distinct stereochemistry and double bond geometry that in turn dictate their biological actions.
Protectins
The protectins, or neuroprotectins when generated in neural tissues, are so named because they were initially documented to have tissue protective actions in the context of neural injury associated with ischemic stroke(33–35). The main member of this family is protectin D1 (PD1), which like D-series resolvins, is generated from DHA (Figure 1B). The biosynthesis of PD1 proceeds via 15-LOX-mediated conversion of DHA to 17-HpDHA that, in the absence of 5-LOX (which enables biosynthesis of D-series resolvins), forms a 16,17 epoxide that is hydrolyzed to 10R, 17S-diHDHA through the conjugated triene system(21). Analogous to the leukotriene biosynthetic pathway, an isomer of PD1 can be generated from 17-HpDHA via double dioxygenation(36, 37). This isomer has an E,Z,E double bond geometry, in contrast to the E,E,Z geometry of the conjugated triene system in PD1 and the stereochemistry of the hydroxyl group at position 10 is in the S configuration. Although less potent than PD1, this double dioxygenation product reduced PMN infiltration in an acute model of sterile inflammation and also appears to regulate platelet activation(36, 37).
Maresins
The maresins are the newest family of pro-resolving lipid mediators generated from DHA and are so named because they are generated by macrophages (macrophage mediators in resolving inflammation)(38). Maresin-1 (MaR1) is the first member of this family and is generated from DHA by a lipoxygenase-dependent mechanism (Figure 1B)(38). While 15-LOX forms predominately 17-HpDHA when DHA is the substrate, the murine isoform of 15-LOX, namely 12/15-LOX, also generates a 14-hydroperoxide. Similarly, this product is also generated by 12-LOX, which is likely involved in the biosynthesis of 14-HpDHA in humans. This 14-HpDHA product can undergo epoxidation to form a 13,14 epoxide, which similar to the biosynthesis of PD1, can be hydrolyzed to generate 7R, 14S dihydroxyDHA (MaR1) through the conjugated double bond system (Figure 1B)(38–40). Of note, 14-HpDHA can also serve as a substrate for 5-LOX to generate double dioxygenation product, 7S, 14S-diHDHA, which is markedly less potent than MaR1 in resolving acute inflammation in vivo(38).
Resolving inflammation and omega-3 PUFA derived mediators
Complete resolution is the normal outcome of acute inflammation and is required to re-establish tissue homeostasis. Key checkpoints in the resolution of inflammation include the termination of polymorphonuclear neutrophil (PMN) infiltration into the injured tissue, the timely apoptosis of PMN, active clearance of apoptotic PMN, microbes and other dead tissue by macrophages, and the efflux of phagocytes from the injured area(41, 42). If these events are not precisely controlled, inadvertent tissue damage can occur and the later events of the wound healing response, such as angiogenesis and re-epithelialization are impaired. It is these cell-mediated events in active resolving inflammation that are regulated by specialized pro-resolving lipid mediators (SPM), which include the resolvins, protectins and maresins.
As detailed above, these lipid mediators are generated in an enzymatic manner by enzymes expressed primarily in leukocytes, although other cell types, such as epithelial and endothelial cells could also be involved in transcellular SPM biosynthesis. While our current understanding of the precise mechanisms governing a switch from pro-inflammatory lipid mediator to SPM biosynthesis is incomplete, it should be noted that LOX and COX enzymes are subject to dynamic transcriptional regulation. For example, it is well documented that engagement of pattern recognition receptors initiates inflammatory signaling pathways giving rise to increased transcriptional regulators of 5-LOX and COX-2 enzymes, such as the nuclear factor-κB (NFκB) and activator protein-1 (AP-1) pathways(43, 44). Moreover, cytokines generated during a type 2 inflammatory response, such as IL-4 regulate 15-LOX expression in mononuclear leukocytes(45). Indeed, Th2-skewed peripheral blood mononuclear cells generate SPM and alternatively activated macrophages (M2) generate more SPM in totality than classically activated (M1) macrophages(46, 47). It should be noted that engulfment of apoptotic cells by macrophages is also a stimulus for SPM generation. Thus, SPM biosynthesis is regulated in a temporal manner and is dependent upon the particular leukocyte subsets and enzymatic pathways operative at specific points in time. Interestingly, different types of inflammation are associated with specific SPM signatures. For example, bacterial infection biases production of RvD5, while viral infection regulates PD1 biosynthesis(48, 49).
Once generated, SPM act locally to promote resolution of inflammation and they have multiple cellular targets. Most SPM identified to date block PMN infiltration into sites of inflammation. Mechanisms involved include the downregulation of adhesion receptors (i.e. CD11b), cytoskeletal structural rearrangements, and endothelial production of anti-adhesive mediators, such as nitric oxide (NO)(21, 31, 50). Modulation of this phase of the acute inflammatory response is essential to prevent excessive PMN accumulation in tissues. Next, some SPM, including RvE1, override PMN survival signaling to allow timely apoptosis to occur(51). Like aberrant PMN infiltration, prolonged PMN survival can delay resolution of inflammation(42). One of the most prominent defining features of actively resolving inflammation is the macrophage-dependent clearance of apoptotic cells(42). As alluded to, this process is required to prevent post-apoptotic secondary necrosis of lingering apoptotic cells. Like the regulation of PMN chemotaxis, most SPM identified to date actively promote macrophage efferocytosis. Controlling both the magnitude of PMN infiltration, while also stimulating their removal by macrophages is a characteristic “dual action” of SPM(31). In addition to the clearance of apoptotic cells, SPM also promote macrophage phagocytosis of bacteria and promote the efflux of phagocytes from sites of inflammation(48, 50, 52). This was shown in animal models of peritonitis and sepsis, in which treatment with SPM, such as PD1 and RvD2, was shown to enhance the appearance of phagocytes in the spleen and lymph nodes(50, 52).
In addition to these well-defined endpoints in leukocyte trafficking, SPM also regulate other processes that allow for resolution of inflammation. For example, SPM, including PD1, stimulate the upregulation of chemokine receptors on apoptotic cells, which promotes scavenging of soluble chemokines in inflammatory exudates(53). Moreover, RvE1 was shown to stimulate the upregulation of CD55 on epithelial cells to promote PMN clearance(54). The stimulatory actions of SPM distinguish them from other omega-3 PUFA-derived mediators, such as the 3-series prostanoids or the 5-series leukotrienes, which are primarily less potent pro-inflammatory mediators or potentially endogenous receptor antagonists(8). While discussion of all documented biological roles of SPM are beyond the scope of this review, the key biological roles of SPM in the resolution of inflammation have identified them as a new class of mediators and have helped to study the process of resolution itself.
Pro-resolving lipid mediators: Identification of specific receptors
The biological actions of most lipid mediators, including the leukotrienes and prostaglandins, are mediated primarily by G-protein coupled receptors (GPCRs). Initial studies interrogating the mechanisms whereby certain SPM elicit their biological actions demonstrated that pertussis toxin (PTX) abolished the effects of SPM in many cases. Indeed, stimulation of macrophage phagocytosis by RvD1 is completely blocked by PTX, as is the stimulation of NO and prostacyclin production in endothelial cells stimulated by RvD2(50, 55). This suggested that SPM may bind to specific GPCRs that couple to Gαi. The first SPM demonstrated to bind a specific GPCR was RvE1. Based on the fact that RvE1 reduced PMN recruitment during acute inflammation in vivo and that this process is both promoted and counter-regulated by other lipid mediators such as LTB4 and lipoxin A4 (LXA4), a search for an RvE1 receptor began by screening receptors closely related to GPCRs for these other lipid mediators. Using this unbiased approach, it was determined that RvE1 inhibited TNF-α-stimulated activation of NFκB in cells transfected with a GPCR denoted ChemR23(25). Radioligand binding studies demonstrated that RvE1 specifically binds ChemR23 with high affinity (Kd~11nM). In peripheral blood monocytes and ChemR23-transfected HEK293 cells, RvE1 stimulated phosphorylation of ERK(56). Similarly, stimulation of macrophage phagocytosis by RvE1 is blocked by an ERK inhibitor, providing further evidence for receptor-mediated pro-resolving actions of RvE1. In vivo, RvE1 inhibited PMN infiltration in a murine model of peritonitis and the doses of RvE1 required to elicit such actions was log-orders of magnitude lower in mice with transgenic overexpression of ChemR23(57).
Further studies on the receptor-mediated biological actions of RvE1 demonstrated that direct receptor crosstalk is an important mechanism whereby RvE1 elicits its effects. In human platelets, RvE1 blocks activation, aggregation and ADP-stimulated P-selectin mobilization(58, 59). While direct binding of ADP receptor, P2Y12 was ruled out, it was demonstrated that ChemR23-dependent activation by RvE1 inhibits P2Y12 signaling. Indeed, co-transfection of P2Y12 expressing cells with ChemR23 blocked ADP-stimulated calcium mobilization in the presence of RvE1, whereas RvE1 failed to block P2Y12 signaling in mock-transfected cells(59). Of interest, ChemR23 is not highly expressed on PMN and later studies demonstrated that in addition to serving as an agonist of this receptor, RvE1 also blocks LTB4-induced activation of PMN in a BLT-1 (B leukotriene receptor-1)-dependent manner(60). While a specific GPCR for RvE2 has not yet been identified, it is noteworthy that RvE2 shows specific binding with human PMN(28).
In addition to RvE1, other SPM have also been recently shown to bind specific GPCRs. In humans, two GPCRs have been shown to mediate the pro-resolving actions of D-series resolvin, RvD1(55). Building upon observations that regulation of actin polymerization and adhesion receptor expression by RvD1 in human PMN was sensitive to PTX, a screening approach revealed that RvD1 binds both GPR32 (a previous orphan receptor) as well as the LXA4 receptor, FPR2 (also denoted ALX). Radioligand binding studies demonstrated that RvD1 binds human leukocytes with high affinity (Kd= 0.17nM) and binding was observed on both PMN and monocytes. In macrophages, stimulation of phagocytosis by RvD1 was blocked by shRNA-mediated knock-down of FPR2 and GPR32, establishing the involvement of receptor-mediated signaling(55). The effects of RvD1 in regulating PMN infiltration during acute inflammation in vivo were enhanced in mice with transgenic overexpression of human FPR2, whereas the regulation of PMN trafficking by RvD1 is abolished in mice deficient in the closely related murine homolog of FPR2(61, 62). Collectively, these results unequivocally demonstrate that the pro-resolving actions of RvD1 are mediated by specific binding to GPCRs. Lastly, while no specific GPCR for DHA product, PD1, has been identified, specific binding studies using radiolabeled PD1 have documented that PD1 specifically binds both retinal pigment epithelial cells and human PMN(63). Importantly, PD1 binding was displaced in homoligand displacement assays, whereas its isomer, 10S, 17S-diHDHA was less effective in competing for PD1 binding, and a Δ15-trans isomer of PD1 was essentially inactive(63). Future studies are likely to reveal important new insights into the specific receptors that mediate the biological actions of SPM and open up exciting new avenues for targeted pro-resolution therapeutics.
Pro-resolving lipid mediators and animal models of disease
Consistent with the potent actions of SPM on human leukocytes, these novel mediators have beneficial actions that have been demonstrated in several distinct animal models of disease. As resolvins were originally identified during the resolution phase of acute inflammation in mice, initial studies into the biological role of these mediators aimed to determine why they are generated during this specific phase and whether they are active mediators of resolution. Moving from HPLC isolates to geometrically and stereochemically pure compounds prepared by total organic synthesis, SPM including E-series resolvins, D-series resolvins, protectins and maresins, have been shown to promote resolution of acute inflammation in vivo(6, 21, 22, 31). Specifically, these SPM shorten the resolution interval, the time during which PMN decline from their maximum value by 50%(6). This parameter, which is indicative of reduced PMN infiltration and enhanced removal of apoptotic PMN by macrophages, is a critical defining feature of pro-resolving mediators. Moving beyond acute models of sterile inflammation, multiple studies have now reported that SPM also combat bacterial infection by controlling leukocyte trafficking and actively promoting both PMN and macrophage-mediated phagocytosis and bacterial killing. This translates into improved survival in multiple distinct models of infection, including polymicrobial sepsis induced by cecal ligation and puncture, as well as direct instillation of live gram-negative bacteria(48, 50). In the latter case, SPM were shown to lower the threshold of antibiotic therapy, suggesting that they may be effective adjunctive therapeutics in the context of infection(48). By selectively enhancing effector functions of leukocytes and at the same time preventing overzealous inflammatory cytokine production and leukocyte recruitment, SPM have been shown to be tissue protective in several other distinct models of inflammation as well, including colitis, cancer, periodontal disease, diabetes, stroke and pathological angiogenesis. The reader is referred to more in-depth reviews regarding the role of SPM in animal models of inflammation and disease(6, 21, 22, 31).
More recent studies on isolated SPM have shown that in addition to promoting bacterial containment and preventing excessive leukocyte accumulation in tissues, these resolution agonists may be particularly important for later phases of wound healing (i.e. tissue repair). For example, studies in diabetic rodents, which are used as a model of the delayed wound healing that occurs in human diabetics, have demonstrated that treatment with RvD1 increases the rate of wound closure and promotes granulation tissue formation(64). In the context of diabetes, the time to wound closure is critical to prevent secondary infection. Moreover, RvD2 prevents tissue necrosis in rodent models of burn injury by preventing thrombosis and PMN sequestration and thereby enhancing microvascular access to the healing dermis(65, 66). Other SPM, such as RvD1, have been shown to stimulate keratinocyte migration in vitro, indicating that SPM likely have other diverse cellular targets within wounds and that they may promote other phases of wound repair beyond regulating leukocyte trafficking(67). Lastly, the newest member of the SPM genus, namely MaR1, directly stimulates tissue regeneration in brown planaria (D. tigrina) subject to surgical injury(40). Given that MaR1 was also biosynthesized during the tissue regeneration process and rescued altered regeneration induced by LOX inhibition, MaR1 may also be a critical endogenous mediator of the regeneration process.
Animal models of disease have also been important to determine the endogenous role of SPM in the protective actions of omega-3 PUFA. In particular, feeding a diet rich in omega-3 PUFA (particularly EPA and DHA), increases endogenous production of SPM in acute sterile peritonitis, obesity, non-alcoholic fatty liver disease (NAFLD) and pathologic retinal angiogenesis(6, 11, 21, 68–71). Moreover, genetic manipulation of mice to increase endogenous production of EPA and DHA via fat-1 transgenesis (discussed above), also increases production of SPM in the context of inflammation associated with colitis, melanoma growth and pathologic angiogenesis(10–12). In these studies, isolated SPM largely recapitulate the actions of increasing omega-3 PUFA, suggesting that, not only are they generated endogenously from omega-3 PUFA, but that they may in part underlie the protective actions of omega-3 PUFA in these scenarios. Future studies designed to establish this cause-effect relationship using specific SPM-receptor knock-out mice for example, are likely to lend important insights into the role of omega-3 PUFA in different disease states.
Human clinical studies: Are resolvins a new class of pro-resolution therapeutics?
A myriad of studies in rodents and isolated human leukocytes have unequivocally established that SPM are generated from omega-3 PUFA and that they have potent immunomodulatory actions that are consistent with the protective effects of omega-3 PUFA. That generation of SPM could underlie the beneficial effects of omega-3 PUFA supplementation have been strengthened by recent studies in humans. In healthy human volunteers taking a fish oil supplement for just 3 weeks (4g/day; 35% EPA, 25% DHA), SPM including RvD1, RvD2, PD1 and SPM biosynthetic precursors, 17-HDHA and 18-HEPE, were identified(72). Moreover, the levels of these mediators identified using a specific LC-MS/MS based approach, were found to be within the range at which they are biologically active (e.g. ~20–40pg/ml for RvD1 and RvD2). In addition, other studies have established that 18-HEPE and RvE1 are generated in healthy human subjects within 3–4h of EPA and aspirin administration(25, 27).
In contrast to the generation of SPM in healthy volunteers given omega-3 PUFA supplements, other human studies suggest that SPM generation may be deficient in the context of certain diseases associated with chronic, unresolved inflammation. For instance, PD1 and 17-HDHA were identified in exhaled breath condensates from healthy individuals, whereas only trace amounts were detected in humans with clinical exacerbation of asthma(73). Similarly, SPM, including RvD1, RvD2 and PD1 were identified in human subcutaneous adipose tissue samples obtained from healthy individuals, whereas the levels of PD1 and 17-HDHA were significantly reduced in subcutaneous adipose tissue obtained from patients with peripheral vascular disease(70). This result is similar to deficiencies observed in other pro-resolving lipid mediators, such as LXA4, observed in asthma and other chronic inflammatory diseases including peripheral artery disease(74, 75). Lastly, our recent studies in a rodent model of diabetic wound healing also demonstrated a reduced SPM biosynthetic capacity despite similar levels of DHA in the wounds of diabetic and non-diabetic mice(64). Given that omega-3 PUFA improve disease outcomes in some diseases but not others (e.g., asthma, diabetes and inflammatory bowel disease), it is possible that impaired downstream metabolism of omega-3 PUFA could in part underlie this lack of efficacy and the development of a comprehensive understanding of the mediators of the protective actions of omega-3 PUFA may help to identify responders vs. non-responders(8). Further human clinical studies will be required to elucidate these relationships fully.
Building upon evidence that the generation of SPM may in part be responsible for the protective actions of omega-3 PUFA in humans, clinical studies have begun to determine whether SPM might be effective targeted therapeutics. Resolvyx (www.resolvyx.com) has been on the forefront of this effort, initiating the first clinical studies with omega-3 PUFA-derived SPM, including RvE1. The company’s product pipeline includes a synthetic RvE1 analog, denoted RX-10045, which has been successfully used in a phase 2 clinical study for the treatment of dry eye. In 2009, Resolvyx reported positive results of the 28-day, randomized, placebo-controlled trial, which enrolled 232 patients (www.clinicaltrials.gov). The primary endpoint of the trial was the Worst Symptom Score, which is a composite indicator of dryness, ocular discomfort, stinging, burning and grittiness. Patients treated with RX-10045 showed significant improvements in this primary endpoint compared with placebo and RX-10045 was safe and well-tolerated. This particular analog and clinical program has now been licensed to Celtic Therapeutics for further clinical development. In addition to RX-10045, Resolvyx is also developing a program around synthetic RvE1 (RX-10001) and PD1 (RX-20001) for other indications, such as asthma, inflammatory bowel disease and rheumatoid arthritis, and Phase 1 studies have been initiated to assess safety, tolerability, pharmacodynamics and pharmacokinetics (see www.clinicaltrials.gov).
In addition to treating chronic inflammatory pathologies, there is also considerable potential for SPM as a new genus of pain management therapeutics(76). Recent studies have demonstrated that certain SPM, such as RvE1, RvD1 and RvD2, are potent analgesics in animal models of inflammatory pain. In particular, intrathecal or intraplantar administration of SPM have been shown to decrease pain induced by a variety of stimuli, including formalin, complete Freund’s adjuvant, TNF-α and capsaicin without affecting basal pain perception(76–78). These SPM, such as RvE1, were more potent than morphine or non-steroidal anti-inflammatory drugs (NSAIDs) in vivo. Mechanistically, SPM have been shown to regulate activation of transient receptor potential villinoid subtype-1 (TRPV-1) and TRP ankyryn 1 (TRPA1) in primary sensory neurons with IC50 values in the low nanomolar range, suggesting that these SPM may be endogenous regulators of pain(76–78). Although clinical studies have not yet evaluated the efficacy of SPM in regulating pain, this particular therapeutic area holds great promise as chronic pain management continues to be a challenging area of clinical medicine.
Concluding remarks
Studies over the last 10–15 years have defined that omega-3 PUFA are enzymatically converted into a diverse array of bioactive autacoids that have potent immunomodulatory and tissue protective actions. These mediators bind specific receptors, have multiple cellular targets and their biosynthesis and actions have been demonstrated in both rodents and humans. The elucidation of these new pathways has provided a mechanistic understanding of the protective roles of omega-3 PUFA in health and disease and may lead to the development of effective targeted therapeutics aimed at treating chronic inflammatory diseases.
Acknowledgments
Financial Support
The support of National Institutes of Health grants HL106173 and GM103492 is gratefully acknowledged. The National Institutes of Health had no role in the design, analysis or writing of this article
Abbreviations
- SPM
Specialized pro-resolving mediator
- PMN
Polymorphonuclear neutrophil
- LC-UV-MS/MS
liquid chromatography-ultraviolet spectrometry-tandem mass spectrometry
- LTB4
leukotriene B4 (5S, 12R-dihydroxy-eicosa-6Z, 8E, 10E, 14Z-tetraenoic acid)
- LXA4
lipoxin A4 (5S, 6R, 15S-trihydroxy-eicosa-7E, 9E, 11Z, 13E-tetraenoic acid)
- PD1/NPD1
protectin D1/neuroprotectin D1 (10R, 17S-dihydroxy-docosa-4Z, 7Z, 11E, 13E, 15Z, 19Z-hexaenoic acid)
- PGE2
9-oxo-11a, 15S-dihydroxy-prosta-5Z, 13E-dien-1-oic acid
- RvE1
Resolvin E1 (5S, 12R, 18R-trihydroxy-eicosa-6Z, 8E, 10E, 14Z, 16E pentaenoic acid)
- RvE2
Resolvin E2 (5S, 18R-dihydroxy-eicosa-6E, 8Z, 11Z, 14Z, 16E, pentaenoic acid)
- RvE3
Resolvin E3 (17,18-dihydroxy-eicosa-5Z, 8Z, 11Z, 13E, 15E, pentaenoic acid)
- RvD1
Resolvin D1 (7S, 8R, 17S-trihydroxy-docosa-4Z, 9E, 11E, 13Z, 15E, 19Z-hexaenoic acid)
- RvD2
Resolvin D2 (7S, 16R, 17S-trihydroxy-docosa-4Z, 8E, 10Z, 12E, 14E, 19Z-hexaenoic acid)
- RvD3
Resolvin D3 (4S, 11R, 17S- trihydroxy-docosa-5Z, 7E, 9E, 13Z, 15E, 19Z-hexaenoic acid)
- RvD5
Resolvin D5 (7S, 17S-dihydroxy-docosa-4Z, 8E, 10Z, 13Z, 15E, 19Z-hexaenoic acid)
Footnotes
Conflicts of Interest
The author has no conflicts of interest to declare.
Authorship
M.S. wrote and edited the manuscript.
References
- 1.Burr GO, Burr MM. Nutrition classics from The Journal of Biological Chemistry 82:345–67, 1929. A new deficiency disease produced by the rigid exclusion of fat from the diet. Nutr Rev. 1973;31:248–249. doi: 10.1111/j.1753-4887.1973.tb06008.x. [DOI] [PubMed] [Google Scholar]
- 2.Le HD, Meisel JA, de Meijer VE, et al. The essentiality of arachidonic acid and docosahexaenoic acid. Prostaglandins Leukot Essent Fatty Acids. 2009;81:165–170. doi: 10.1016/j.plefa.2009.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fritsche K. Fatty acids as modulators of the immune response. Annu Rev Nutr. 2006;26:45–73. doi: 10.1146/annurev.nutr.25.050304.092610. [DOI] [PubMed] [Google Scholar]
- 4.Harbige LS. Fatty acids, the immune response, and autoimmunity: a question of n-6 essentiality and the balance between n-6 and n-3. Lipids. 2003;38:323–341. doi: 10.1007/s11745-003-1067-z. [DOI] [PubMed] [Google Scholar]
- 5.Samuelsson B, Dahlen SE, Lindgren JA, et al. Leukotrienes and lipoxins: structures, biosynthesis, and biological effects. Science. 1987;237:1171–1176. doi: 10.1126/science.2820055. [DOI] [PubMed] [Google Scholar]
- 6.Serhan CN, Chiang N, Van Dyke TE. Resolving inflammation: dual anti-inflammatory and pro-resolution lipid mediators. Nat Rev Immunol. 2008;8:349–361. doi: 10.1038/nri2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.London B, Albert C, Anderson ME, et al. Omega-3 fatty acids and cardiac arrhythmias: prior studies and recommendations for future research: a report from the National Heart, Lung, and Blood Institute and Office Of Dietary Supplements Omega-3 Fatty Acids and their Role in Cardiac Arrhythmogenesis Workshop. Circulation. 2007;116:e320–335. doi: 10.1161/CIRCULATIONAHA.107.712984. [DOI] [PubMed] [Google Scholar]
- 8.Calder PC. Omega-3 polyunsaturated fatty acids and inflammatory processes: nutrition or pharmacology? Br J Clin Pharmacol. 2013;75:645–662. doi: 10.1111/j.1365-2125.2012.04374.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sprecher H. Metabolism of highly unsaturated n-3 and n-6 fatty acids. Biochim Biophys Acta. 2000;1486:219–231. doi: 10.1016/s1388-1981(00)00077-9. [DOI] [PubMed] [Google Scholar]
- 10.Hudert CA, Weylandt KH, Lu Y, et al. Transgenic mice rich in endogenous omega-3 fatty acids are protected from colitis. Proc Natl Acad Sci U S A. 2006;103:11276–11281. doi: 10.1073/pnas.0601280103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Connor KM, SanGiovanni JP, Lofqvist C, et al. Increased dietary intake of omega-3-polyunsaturated fatty acids reduces pathological retinal angiogenesis. Nat Med. 2007;13:868–873. doi: 10.1038/nm1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xia S, Lu Y, Wang J, et al. Melanoma growth is reduced in fat-1 transgenic mice: impact of omega-6/omega-3 essential fatty acids. Proc Natl Acad Sci U S A. 2006;103:12499–12504. doi: 10.1073/pnas.0605394103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.DeFilippis AP, Sperling LS. Understanding omega-3’s. Am Heart J. 2006;151:564–570. doi: 10.1016/j.ahj.2005.03.051. [DOI] [PubMed] [Google Scholar]
- 14.Thies F, Garry JM, Yaqoob P, et al. Association of n-3 polyunsaturated fatty acids with stability of atherosclerotic plaques: a randomised controlled trial. Lancet. 2003;361:477–485. doi: 10.1016/S0140-6736(03)12468-3. [DOI] [PubMed] [Google Scholar]
- 15.De Caterina R. n-3 fatty acids in cardiovascular disease. N Engl J Med. 2011;364:2439–2450. doi: 10.1056/NEJMra1008153. [DOI] [PubMed] [Google Scholar]
- 16.Dietary supplementation with n-3 polyunsaturated fatty acids and vitamin E after myocardial infarction: results of the GISSI-Prevenzione trial. Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto miocardico. Lancet. 1999;354:447–455. [PubMed] [Google Scholar]
- 17.Tavazzi L, Maggioni AP, Marchioli R, et al. Effect of n-3 polyunsaturated fatty acids in patients with chronic heart failure (the GISSI-HF trial): a randomised, double-blind, placebo-controlled trial. Lancet. 2008;372:1223–1230. doi: 10.1016/S0140-6736(08)61239-8. [DOI] [PubMed] [Google Scholar]
- 18.Samuelsson B. Role of basic science in the development of new medicines: examples from the eicosanoid field. J Biol Chem. 2012;287:10070–10080. doi: 10.1074/jbc.X112.351437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Capdevila JH, Harris RC, Falck JR. Microsomal cytochrome P450 and eicosanoid metabolism. Cell Mol Life Sci. 2002;59:780–789. doi: 10.1007/s00018-002-8466-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hata AN, Breyer RM. Pharmacology and signaling of prostaglandin receptors: multiple roles in inflammation and immune modulation. Pharmacol Ther. 2004;103:147–166. doi: 10.1016/j.pharmthera.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 21.Serhan CN, Petasis NA. Resolvins and protectins in inflammation resolution. Chem Rev. 2011;111:5922–5943. doi: 10.1021/cr100396c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang MJ, Spite M. Resolvins: anti-inflammatory and proresolving mediators derived from omega-3 polyunsaturated fatty acids. Annu Rev Nutr. 2012;32:203–227. doi: 10.1146/annurev-nutr-071811-150726. [DOI] [PubMed] [Google Scholar]
- 23.Serhan CN, Clish CB, Brannon J, et al. Novel functional sets of lipid-derived mediators with antiinflammatory actions generated from omega-3 fatty acids via cyclooxygenase 2-nonsteroidal antiinflammatory drugs and transcellular processing. J Exp Med. 2000;192:1197–1204. doi: 10.1084/jem.192.8.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Serhan CN, Hong S, Gronert K, et al. Resolvins: a family of bioactive products of omega-3 fatty acid transformation circuits initiated by aspirin treatment that counter proinflammation signals. J Exp Med. 2002;196:1025–1037. doi: 10.1084/jem.20020760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arita M, Bianchini F, Aliberti J, et al. Stereochemical assignment, antiinflammatory properties, and receptor for the omega-3 lipid mediator resolvin E1. J Exp Med. 2005;201:713–722. doi: 10.1084/jem.20042031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arita M, Clish CB, Serhan CN. The contributions of aspirin and microbial oxygenase to the biosynthesis of anti-inflammatory resolvins: novel oxygenase products from omega-3 polyunsaturated fatty acids. Biochem Biophys Res Commun. 2005;338:149–157. doi: 10.1016/j.bbrc.2005.07.181. [DOI] [PubMed] [Google Scholar]
- 27.Oh SF, Pillai PS, Recchiuti A, et al. Pro-resolving actions and stereoselective biosynthesis of 18S E-series resolvins in human leukocytes and murine inflammation. J Clin Invest. 2011;121:569–581. doi: 10.1172/JCI42545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oh SF, Dona M, Fredman G, et al. Resolvin E2 formation and impact in inflammation resolution. J Immunol. 2012;188:4527–4534. doi: 10.4049/jimmunol.1103652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tjonahen E, Oh SF, Siegelman J, et al. Resolvin E2: identification and anti-inflammatory actions: pivotal role of human 5-lipoxygenase in resolvin E series biosynthesis. Chem Biol. 2006;13:1193–1202. doi: 10.1016/j.chembiol.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 30.Isobe Y, Arita M, Matsueda S, et al. Identification and structure determination of novel anti-inflammatory mediator resolvin E3, 17,18-dihydroxyeicosapentaenoic acid. J Biol Chem. 2012;287:10525–10534. doi: 10.1074/jbc.M112.340612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Spite M, Serhan CN. Novel lipid mediators promote resolution of acute inflammation: impact of aspirin and statins. Circ Res. 2010;107:1170–1184. doi: 10.1161/CIRCRESAHA.110.223883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dalli J, Winkler JW, Colas RA, et al. Resolvin D3 and aspirin-triggered resolvin D3 are potent immunoresolvents. Chem Biol. 2013;20:188–201. doi: 10.1016/j.chembiol.2012.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hong S, Gronert K, Devchand PR, et al. Novel docosatrienes and 17S-resolvins generated from docosahexaenoic acid in murine brain, human blood, and glial cells. Autacoids in anti-inflammation. J Biol Chem. 2003;278:14677–14687. doi: 10.1074/jbc.M300218200. [DOI] [PubMed] [Google Scholar]
- 34.Marcheselli VL, Hong S, Lukiw WJ, et al. Novel docosanoids inhibit brain ischemia-reperfusion-mediated leukocyte infiltration and pro-inflammatory gene expression. J Biol Chem. 2003;278:43807–43817. doi: 10.1074/jbc.M305841200. [DOI] [PubMed] [Google Scholar]
- 35.Mukherjee PK, Marcheselli VL, Serhan CN, et al. Neuroprotectin D1: a docosahexaenoic acid-derived docosatriene protects human retinal pigment epithelial cells from oxidative stress. Proc Natl Acad Sci U S A. 2004;101:8491–8496. doi: 10.1073/pnas.0402531101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Serhan CN, Gotlinger K, Hong S, et al. Anti-inflammatory actions of neuroprotectin D1/protectin D1 and its natural stereoisomers: assignments of dihydroxy-containing docosatrienes. J Immunol. 2006;176:1848–1859. doi: 10.4049/jimmunol.176.3.1848. [DOI] [PubMed] [Google Scholar]
- 37.Chen P, Fenet B, Michaud S, et al. Full characterization of PDX, a neuroprotectin/protectin D1 isomer, which inhibits blood platelet aggregation. FEBS Lett. 2009;583:3478–3484. doi: 10.1016/j.febslet.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 38.Serhan CN, Yang R, Martinod K, et al. Maresins: novel macrophage mediators with potent antiinflammatory and proresolving actions. J Exp Med. 2009;206:15–23. doi: 10.1084/jem.20081880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dalli J, Zhu M, Vlasenko NA, et al. The novel 13S, 14S-epoxy-maresin is converted by human macrophages to maresin1 (MaR1), inhibits leukotriene A4 hydrolase (LTA4H), and shifts macrophage phenotype . FASEB J. 2013 doi: 10.1096/fj.13-227728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Serhan CN, Dalli J, Karamnov S, et al. Macrophage proresolving mediator maresin 1 stimulates tissue regeneration and controls pain. FASEB J. 2012;26:1755–1765. doi: 10.1096/fj.11-201442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Buckley CD, Gilroy DW, Serhan CN, et al. The resolution of inflammation. Nat Rev Immunol. 2013;13:59–66. doi: 10.1038/nri3362. [DOI] [PubMed] [Google Scholar]
- 42.Serhan CN, Brain SD, Buckley CD, et al. Resolution of inflammation: state of the art, definitions and terms. FASEB J. 2007;21:325–332. doi: 10.1096/fj.06-7227rev. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kang YJ, Mbonye UR, DeLong CJ, et al. Regulation of intracellular cyclooxygenase levels by gene transcription and protein degradation. Prog Lipid Res. 2007;46:108–125. doi: 10.1016/j.plipres.2007.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Silverman ES, Drazen JM. The biology of 5-lipoxygenase: function, structure, and regulatory mechanisms. Proc Assoc Am Physicians. 1999;111:525–536. doi: 10.1046/j.1525-1381.1999.t01-1-99231.x. [DOI] [PubMed] [Google Scholar]
- 45.Conrad DJ, Kuhn H, Mulkins M, et al. Specific inflammatory cytokines regulate the expression of human monocyte 15-lipoxygenase. Proc Natl Acad Sci U S A. 1992;89:217–221. doi: 10.1073/pnas.89.1.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ariel A, Li PL, Wang W, et al. The docosatriene protectin D1 is produced by TH2 skewing and promotes human T cell apoptosis via lipid raft clustering. J Biol Chem. 2005;280:43079–43086. doi: 10.1074/jbc.M509796200. [DOI] [PubMed] [Google Scholar]
- 47.Dalli J, Serhan CN. Specific lipid mediator signatures of human phagocytes: microparticles stimulate macrophage efferocytosis and pro-resolving mediators. Blood. 2012;120:e60–72. doi: 10.1182/blood-2012-04-423525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chiang N, Fredman G, Backhed F, et al. Infection regulates pro-resolving mediators that lower antibiotic requirements. Nature. 2012;484:524–528. doi: 10.1038/nature11042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Morita M, Kuba K, Ichikawa A, et al. The lipid mediator protectin d1 inhibits influenza virus replication and improves severe influenza. Cell. 2013;153:112–125. doi: 10.1016/j.cell.2013.02.027. [DOI] [PubMed] [Google Scholar]
- 50.Spite M, Norling LV, Summers L, et al. Resolvin D2 is a potent regulator of leukocytes and controls microbial sepsis. Nature. 2009;461:1287–1291. doi: 10.1038/nature08541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.El Kebir D, Gjorstrup P, Filep JG. Resolvin E1 promotes phagocytosis-induced neutrophil apoptosis and accelerates resolution of pulmonary inflammation. Proc Natl Acad Sci U S A. 2012;109:14983–14988. doi: 10.1073/pnas.1206641109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schwab JM, Chiang N, Arita M, et al. Resolvin E1 and protectin D1 activate inflammation-resolution programmes. Nature. 2007;447:869–874. doi: 10.1038/nature05877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ariel A, Fredman G, Sun YP, et al. Apoptotic neutrophils and T cells sequester chemokines during immune response resolution through modulation of CCR5 expression. Nat Immunol. 2006;7:1209–1216. doi: 10.1038/ni1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Campbell EL, Louis NA, Tomassetti SE, et al. Resolvin E1 promotes mucosal surface clearance of neutrophils: a new paradigm for inflammatory resolution. FASEB J. 2007;21:3162–3170. doi: 10.1096/fj.07-8473com. [DOI] [PubMed] [Google Scholar]
- 55.Krishnamoorthy S, Recchiuti A, Chiang N, et al. Resolvin D1 binds human phagocytes with evidence for proresolving receptors. Proc Natl Acad Sci U S A. 2010;107:1660–1665. doi: 10.1073/pnas.0907342107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ohira T, Arita M, Omori K, et al. Resolvin E1 receptor activation signals phosphorylation and phagocytosis. J Biol Chem. 2010;285:3451–3461. doi: 10.1074/jbc.M109.044131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gao L, Faibish D, Fredman G, et al. Resolvin E1 and chemokine-like receptor 1 mediate bone preservation. J Immunol. 2013;190:689–694. doi: 10.4049/jimmunol.1103688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dona M, Fredman G, Schwab JM, et al. Resolvin E1, an EPA-derived mediator in whole blood, selectively counterregulates leukocytes and platelets. Blood. 2008;112:848–855. doi: 10.1182/blood-2007-11-122598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fredman G, Van Dyke TE, Serhan CN. Resolvin E1 regulates adenosine diphosphate activation of human platelets. Arterioscler Thromb Vasc Biol. 2010;30:2005–2013. doi: 10.1161/ATVBAHA.110.209908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Arita M, Ohira T, Sun YP, et al. Resolvin E1 selectively interacts with leukotriene B4 receptor BLT1 and ChemR23 to regulate inflammation. J Immunol. 2007;178:3912–3917. doi: 10.4049/jimmunol.178.6.3912. [DOI] [PubMed] [Google Scholar]
- 61.Krishnamoorthy S, Recchiuti A, Chiang N, et al. Resolvin D1 receptor stereoselectivity and regulation of inflammation and proresolving microRNAs. Am J Pathol. 2012;180:2018–2027. doi: 10.1016/j.ajpath.2012.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Norling LV, Dalli J, Flower RJ, et al. Resolvin D1 limits polymorphonuclear leukocyte recruitment to inflammatory loci: receptor- dependent actions. Arterioscler Thromb Vasc Biol. 2012;32:1970–1978. doi: 10.1161/ATVBAHA.112.249508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Marcheselli VL, Mukherjee PK, Arita M, et al. Neuroprotectin D1/protectin D1 stereoselective and specific binding with human retinal pigment epithelial cells and neutrophils. Prostaglandins Leukot Essent Fatty Acids. 2010;82:27–34. doi: 10.1016/j.plefa.2009.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tang Y, Zhang MJ, Hellmann J, et al. Proresolution therapy for the treatment of delayed healing of diabetic wounds. Diabetes. 2013;62:618–627. doi: 10.2337/db12-0684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bohr S, Patel SJ, Sarin D, et al. Resolvin D2 prevents secondary thrombosis and necrosis in a mouse burn wound model. Wound Repair Regen. 2013;21:35–43. doi: 10.1111/j.1524-475X.2012.00853.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kurihara T, Jones CN, Yu YM, et al. Resolvin D2 restores neutrophil directionality and improves survival after burns. FASEB J. 2013 doi: 10.1096/fj.12-219519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Norling LV, Spite M, Yang R, et al. Cutting edge: Humanized nano- proresolving medicines mimic inflammation-resolution and enhance wound healing. J Immunol. 2011;186:5543–5547. doi: 10.4049/jimmunol.1003865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Claria J, Dalli J, Yacoubian S, et al. Resolvin D1 and resolvin D2 govern local inflammatory tone in obese fat. J Immunol. 2012;189:2597–2605. doi: 10.4049/jimmunol.1201272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Claria J, Gonzalez-Periz A, Lopez-Vicario C, et al. New insights into the role of macrophages in adipose tissue inflammation and Fatty liver disease: modulation by endogenous omega-3 Fatty Acid-derived lipid mediators. Front Immunol. 2011;2:49. doi: 10.3389/fimmu.2011.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Claria J, Nguyen BT, Madenci A, et al. Diversity of lipid mediators in human adipose tissue depots. Am J Physiol Cell Physiol. 2013 doi: 10.1152/ajpcell.00351.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gonzalez-Periz A, Horrillo R, Ferre N, et al. Obesity-induced insulin resistance and hepatic steatosis are alleviated by omega-3 fatty acids: a role for resolvins and protectins. FASEB J. 2009;23:1946–1957. doi: 10.1096/fj.08-125674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mas E, Croft KD, Zahra P, et al. Resolvins D1, D2, and other mediators of self-limited resolution of inflammation in human blood following n-3 fatty acid supplementation. Clin Chem. 2012;58:1476–1484. doi: 10.1373/clinchem.2012.190199. [DOI] [PubMed] [Google Scholar]
- 73.Levy BD, Kohli P, Gotlinger K, et al. Protectin D1 is generated in asthma and dampens airway inflammation and hyperresponsiveness. J Immunol. 2007;178:496–502. doi: 10.4049/jimmunol.178.1.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ho KJ, Spite M, Owens CD, et al. Aspirin-triggered lipoxin and resolvin E1 modulate vascular smooth muscle phenotype and correlate with peripheral atherosclerosis. Am J Pathol. 2010;177:2116–2123. doi: 10.2353/ajpath.2010.091082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sanak M, Levy BD, Clish CB, et al. Aspirin-tolerant asthmatics generate more lipoxins than aspirin-intolerant asthmatics. Eur Respir J. 2000;16:44–49. doi: 10.1034/j.1399-3003.2000.16a08.x. [DOI] [PubMed] [Google Scholar]
- 76.Ji RR, Xu ZZ, Strichartz G, et al. Emerging roles of resolvins in the resolution of inflammation and pain. Trends Neurosci. 2011;34:599–609. doi: 10.1016/j.tins.2011.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Park CK, Xu ZZ, Liu T, et al. Resolvin D2 is a potent endogenous inhibitor for transient receptor potential subtype V1/A1, inflammatory pain, and spinal cord synaptic plasticity in mice: distinct roles of resolvin D1, D2, and E1. J Neurosci. 2011;31:18433–18438. doi: 10.1523/JNEUROSCI.4192-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Xu ZZ, Zhang L, Liu T, et al. Resolvins RvE1 and RvD1 attenuate inflammatory pain via central and peripheral actions. Nat Med. 2010;16:592–597. doi: 10.1038/nm.2123. 591p following 597. [DOI] [PMC free article] [PubMed] [Google Scholar]


