Abstract
The mineralocorticoid receptor (MR) is a key regulator of blood pressure. MR-antagonist drugs are used to treat hypertension and heart failure, resulting in decreased mortality by mechanisms that are not completely understood. In addition to the kidney, MR is also expressed in the smooth muscle cells (SMCs) of the vasculature, where it is activated by the hormone aldosterone and affects the expression of genes involved in vascular function at the cellular and systemic levels. Following vascular injury due to mechanical or physiological stresses, vessels undergo remodeling resulting in SMC hypertrophy, migration, and proliferation, as well as vessel fibrosis. Exuberant vascular remodeling is associated with poor outcomes in cardiovascular patients. This review compiles recent findings on the specific role of SMC-MR in the vascular remodeling process. The development and characterization of a SMC-specific MR-knockout mouse has demonstrated a direct role for SMC-MR in vascular remodeling. Additionally, several novel mechanisms contributing to SMC-MR-mediated vascular remodeling have been identified and are reviewed here, including Rho-kinase signaling, placental growth factor signaling through vascular endothelial growth factor type 1 receptor, and galectin signaling.
Keywords: Mineralocorticoid receptor, Aldosterone, Vascular injury, Vascular remodeling, Smooth muscle cells
Introduction
In addition to the critical role of the vasculature as a conduit to deliver oxygen and nutrients, blood vessels also regulate regional blood flow, modulate vascular tone and systemic blood pressure, and function as a barrier to prevent thrombosis and infection. The vessel includes two communicating layers: the inner lining of endothelial cells that are in contact with circulating blood, and the outer smooth muscle cells (SMCs) that control vascular contraction. In larger vessels, this is further surrounded by adventitia that is composed of fibroblasts and extracellular matrix (ECM) and contributes to vascular structural characteristics.
When the endothelium is damaged, the vessel undergoes a healing process, termed vascular remodeling. When remodeling is exuberant, it can result in vascular stiffening (as seen in hypertension and aging) and can contribute to atherosclerosis, vein graft failure, restenosis after percutaneous vascular procedures, and cardiac transplant vasculopathy (reviewed in [1]). Endothelial damage can be caused by mechanical injury or by cardiovascular risk factors, including dyslipidemia, hypertension, diabetes, or toxins from smoking. In the area of endothelial damage, the normally quiescent SMCs proliferate and produce ECM that contributes to vascular fibrosis, stenosis, and stiffening. These adversely remodeled vessels have decreased compliance and decreased ability to vasodilate to enhance local blood flow, and may have decreased luminal area, thereby contributing to hypertension, regional ischemia, and decreased functional capacity. Although we have some knowledge about the mechanisms of vascular remodeling, our current cardiovascular therapies are still limited by the effects of adverse remodeling. Thus there is still a need to identify novel contributors to vascular remodeling that may serve as effective therapeutic targets to prevent cardiovascular disease.
The mineralocorticoid receptor (MR) is an intracellular transcription factor that binds the steroid hormone aldosterone, which acts in the kidney to enhance sodium retention and increase blood pressure [2]. Therefore, MR antagonists (such as spironolactone and eplerenone) are often used as antihypertensive drugs and to treat heart failure, a state of volume excess. However, in clinical trials, patients treated with MR antagonists have greater improvements in their cardiovascular health than would be attributable to modest declines in volume and blood pressure [3–6], thus prompting further research into the roles of aldosterone and MR in vascular pathologies. Clinical data reveal that elevated aldosterone levels are associated with an increased incidence of atherosclerotic ischemic events (myocardial infarction and stroke) and cardiovascular mortality [7, 8]. Additionally, animal models of vascular injury show enhanced vascular remodeling with aldosterone infusion and decreased remodeling with MR antagonist treatment [9–12].
Due to the critical role of aldosterone and MR in regulating blood pressure, their detrimental effects on vascular remodeling have been previously attributed to hypertension with secondary vascular consequences. However, in addition to its presence in the kidneys, MR is also expressed in endothelial cells and SMCs of the vasculature in humans and rodents, supporting the possibility that MR in the vessels could contribute directly to the remodeling process. Activation of MR in SMCs in vitro has been shown to regulate the expression of genes involved in vascular inflammation, fibrosis, and calcification [13–15] and to promote SMC proliferation [16,17]. Recent advances in our understanding of the specific and direct role of SMC-MR in the process of vascular remodeling in vivo and novel molecular mechanisms by which SMC-MR contribute to vascular remodeling will be summarized in this review.
Direct role for SMC-MR in vascular remodeling
The recent creation of mouse models in which the MR can be specifically deleted from the SMCs (SMC-MR-KO) has allowed directed study of the effects of SMC-MR in vascular remodeling in the setting of normal MR function in the kidney, other cells of the vasculature, and elsewhere. Our lab developed a model that allows for inducible deletion of SMC-MR in adulthood, thereby eliminating the potential that developmental effects of MR could have led to the observed changes in vascular function later in life. SMC-MR-KO mice have normal blood pressure when they are young and exhibit decreased blood pressure with aging relative to their wild-type counterparts, despite unchanged renal MR function [18]. Additionally, aged SMC-MR-KO mice have decreased vascular tone, and the aged vessels exhibit decreased contractile responses to thromboxane, angiotensin II, and calcium channel agonists [18]. Finally, SMC-MR-KO mice have an attenuated increase in blood pressure and superoxide production in response to angiotensin II infusion [18]. These findings suggest a role for extra-renal—and specifically SMC—MR in the vascular contractile changes that are associated with aging and oxidative stress. The characterization of this animal model has served as a foundation for further research on the role of SMC-MR in vascular injury and the remodeling process.
Vascular remodeling in response to injury can be modeled in animals by manually denuding a blood vessel of its endothelial cell layer. In multiple models of mechanical endothelial injury, aldosterone has been found to enhance the remodeling process, while MR antagonists attenuate it [9–12]. The specific role of SMC-MR has recently been explored using a wire-induced carotid endothelial denudation model in mice. In this model, aldosterone was infused at a low dose to achieve levels seen in patients with cardiovascular disease. Although this dose of aldosterone did not increase blood pressure, it still caused a significant increase in SMC proliferation, ECM deposition, and medial vessel thickening after injury, supporting the concept that the enhanced remodeling might be due to direct effects of aldosterone on the vasculature [10]. Indeed, in SMC-MR-KO mice, aldosterone infusion did not have any significant effect on vascular remodeling compared to vehicle infusion, supporting that SMC-MR is necessary for aldosterone-enhanced remodeling [19]. Additionally, even in the absence of excess aldosterone infusion, SMC-MR-KO mice have an attenuated fibrotic response to carotid wire injury relative to MR-intact littermates [19]. These data indicate that SMC-MR directly contributes to the vascular fibrotic response to mechanical injury in the presence of physiological levels of aldosterone and mediates the enhanced vascular remodeling response to aldosterone excess.
Another animal model of vascular remodeling is the response to hypertension induced by uni-nephrectomy/aldosterone/salt challenge. In this model, wild-type mice exposed to hypertension for 4 weeks develop increased stiffness and decreased distensibility of the carotid artery. Using a model with constitutive SMC-MR deletion, it was recently demonstrated that these changes in vascular properties induced by the hypertensive stimulus in MR intact littermates are not observed in SMC-MR-KO mice [20]. This difference was not due to changes in the collagen-to-elastin ratio of the vessels, but may be attributable to the observation that this hypertension model induced an increase in expression of the integrin alpha-5-subunit in the carotid arteries that is not induced in the SMC-MR-KO mice [20]. Thus the presence of the MR in the SMCs is necessary for the vascular response to hypertension that results in altered vascular distensibility and increased stiffness, perhaps through its regulation of integrin protein expression. Since vascular stiffness is an independent risk factor for cardiovascular ischemic events and mortality in humans [21, 22], further exploration of the mechanisms by which SMC-MR directly contributes to vascular remodeling has important clinical implications.
Novel mechanisms by which SMC-MR contributes to vascular remodeling
The data presented above support a specific and necessary role for SMC-MR in the vascular injury response, including vascular remodeling and stiffening. A variety of mechanisms by which MR contributes to vascular remodeling have been previously identified and reviewed [23]. Here we focus on very recent data that has specifically implicated SMC-MR in new cellular signaling pathways associated with vascular remodeling. In addition to the integrin mechanism alluded to by Galmiche et al. [20], we review three additional novel SMC-MR regulated signaling pathways that contribute to vascular remodeling. These include Rho-kinase signaling, placental growth factor (PGF) signaling through vascular endothelial growth factor type 1 receptor (VEGFR1), and galectin signaling.
Rho-kinase signaling
Rho proteins are small G-proteins that activate Rho-associated kinases (ROCKs), which have been observed as important regulators of a range of SMC functions, including contraction, migration, proliferation, and apoptosis [24]. Additionally, ROCK activity has been linked to a range of clinical conditions involving vascular remodeling, including atherosclerosis, restenosis, and pulmonary hypertension [24]. Since 2008, substantial new data has revealed a role for Rho kinase in the pathogenesis of aldosterone-mediated vascular remodeling [25]. In cultured rat SMCs, aldosterone increases Rho-kinase activity, stress fiber formation, and SMC migration in an SMC-MR-dependent manner (evaluated through the attenuation achieved with the MR-antagonist eplerenone). These data implicate SMC-MR activation of Rho-kinase in the process of SMC migration. A mechanism for the activation of the Rho-kinase pathway by aldosterone has been proposed that involves c-Src phosphorylation [26]. Src is kinase that is synergistically phosphorylated in response to aldosterone and angiotensin II in cultured rat SMCs [26]. This effect is attenuated by eplerenone, irbesartan (an angiotensin II receptor antagonist), or PP2 (a selective Src inhibitor). Angiotensin II is also observed to act synergistically with aldosterone on SMCs to increase superoxide anion generation via NADPH oxidase and to increase translocation of RhoA to the membrane. Finally, co-stimulation with aldosterone and angiotensin II increase SMC migration in a c-Src and Rho-kinase-dependent manner. These data support aldosterone-activated SMC-MR and angiotensin II acting synergistically in vascular SMCs to promote proliferation and migration via activation of a c-Src-dependent, redox-sensitive, Rho-kinase pathway (see Fig. 1, Panel A).
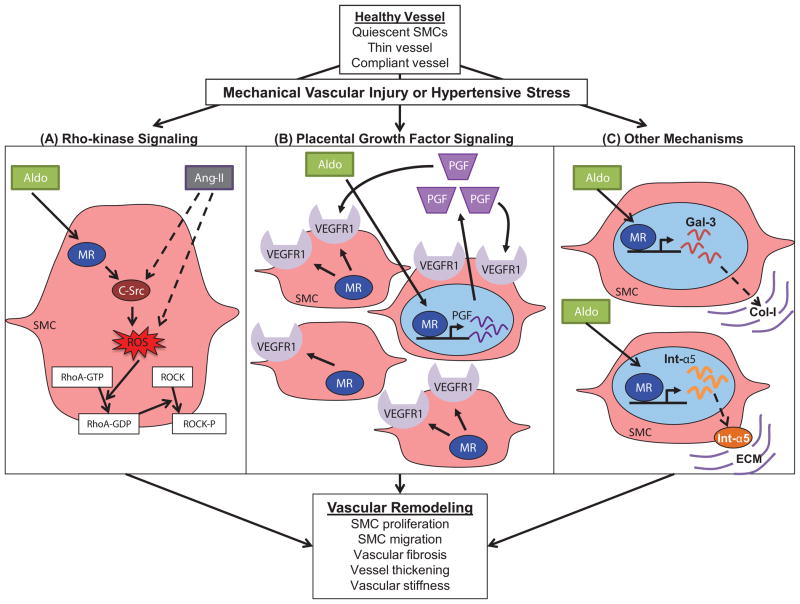
Figure 1. New mechanisms by which smooth muscle cell (SMC) mineralocorticoid receptor (MR) directly contributes to vascular remodeling.
(A) Rho-kinase signaling is synergistically activated by aldosterone (Aldo) via SMC-MR and by angiotensin-II (Ang-II), leading to Rho-associated kinase (ROCK) activation and SMC migration. ROS = reactive oxygen species, ROCK-P = phosphorylated ROCK (activated form). (B) Placental growth factor (PGF) signaling contributes to vascular remodeling specifically in areas of vascular injury, where the vascular endothelial growth factor type 1 receptor (VEGFR1) is up-regulated in the SMC by MR. (C) Other mechanisms include galectin-3 (Gal-3) regulation by SMC-MR resulting in enhanced type I collagen (Col-I) expression in the extracellular matrix (ECM) and increased expression of the alpha-5 subunit of integrin (Int-α5) that interacts with the ECM to enhance vascular stiffness.
In humans, MR antagonism with eplerenone has also been shown to be beneficial in hypertensive patients with high levels of ROCK activation. In comparing different therapies for patients with hypertension, eplerenone and nifedipine (a calcium channel blocker) resulted in decreased ROCK activity and blood pressure, while losartan (an angiotensin II receptor blocker) exhibited only hypotensive effects [27]. Thus MR-antagonist treatment exerts modest effects on blood pressure (similar to other anti-hypertensive therapies), but provide additional vascular protection, perhaps in part through a reduction of SMC Rho/ROCK activity.
Placental growth factor signaling via VEGFR1
Placental growth factor (PGF) is a member of the vascular endothelial growth factor (VEGF) family that is involved in post-embryonic angiogenesis and wound healing. PGF has been previously identified as a vascular aldosterone-regulated gene [15]. PGF expression and vascular protein secretion are up-regulated in response to aldosterone in ex vivo treated mouse vessels and diseased human vessels [10]. Attenuation of this effect by spironolactone treatment and identification of an aldosterone-responsive MR binding site sequence upstream of the Pgf gene suggest that MR is the direct mediator between aldosterone and PGF expression. Aldosterone induction of PGF expression (and expression of its receptor VEGFR1) was further enhanced in vessels that had undergone endothelial denudation injury [10]. Furthermore, aldosterone-induction of PGF expression was prevented in vessels from SMC-MR-KO mice [19]. PGF is thus identified as a novel target of SMC-MR with enhanced expression in the setting of endothelial injury.
Further studies have revealed additional details about the mechanism by which the SMC-MR/PGF/VEGFR1 pathway contributes to the mechanism of aldosterone-enhanced vascular remodeling in vivo. The enhanced SMC proliferation, ECM deposition, and medial vessel thickening induced by aldosterone after wire injury in wild-type mice is prevented in PGF-KO mice [10]. Additionally, aldosterone-induced remodeling following wire injury is inhibited by treatment with VEGFR1- (but not VEGFR2-) blocking antibody [19]. Immunohistochemistry revealed that VEGFR1 is expressed exclusively on endothelial cells in healthy vessels and is expressed on SMCs only in response to vascular injury. Induction of VEGFR1 after injury is substantially attenuated in the SMC-MR-KO mice [19]. Collectively, these findings provide an explanation for why aldosterone enhances vascular remodeling only after endothelial injury and not when the endothelium is healthy and intact. MR activation of PGF is enhanced in the setting of vascular injury and disease, and the effects of PGF on SMC proliferation are only realized when its receptor, VEGFR1, is expressed on SMC in the area of injury in a SMC-MR-dependent manner (see Fig. 1, Panel B).
Galectin-3-mediated fibrosis
Galectin-3 is a small lectin protein that is expressed in many tissues. Previous studies have implicated galectin-3 in the development of cardiac fibrosis in heart failure models and have suggested that the detrimental effects of aldosterone on cardiac fibrosis may also be mediated by galectin-3 (reviewed in [28]). Recently, a role for galectin-3 was identified in mediating the vascular remodeling effects in the aldosterone/salt hypertension model [29].
In cultured SMCs, aldosterone increased the expression of galectin-3 in a concentration-dependent and MR-dependent manner, suggesting that galectin-3 may be another SMC-MR-regulated gene [29]. In a rat model of hypertension induced by 3 weeks of aldosterone-salt challenge, inhibition of either MR or galectin-3 showed similar effects in attenuating the hypertension, inflammation, and vascular fibrosis. Finally, galectin-3 knock-out mice do not exhibit the aldosterone-induced increases in collagen I synthesis, vascular inflammation, or vessel fibrosis that are observed in their wild-type counterparts [29]. Thus MR regulates galectin in vascular SMCs, and both MR and galectin-3 are directly involved in the mechanism of aldosterone-mediated vascular fibrosis in this rat hypertension model (see Fig. 1, Panel C).
In humans, elevated serum levels of galectin-3 have been associated with adverse outcomes in cardiovascular patients [30]. However, the role of galectin-3 in vascular remodeling in humans remains unclear. In a study within the HF-ACTION clinical trial (which examined the effects of exercise training versus “usual” treatments for patients with chronic heart failure), there was no observed correlation between galectin-3 plasma levels and MR-antagonist use after adjusting for other prognostic predictors [31]. Further research is needed to clarify how the plasma levels of galectin-3 (and other molecules or proteins implicated in the vascular fibrosis process) are related to vascular outcome and/or response to different therapies. It is possible that galectin-3, PGF, VEGFR1, and other SMC-MR-regulated genes may be novel biomarkers of aldosterone-mediated vascular disease that could be used to predict and/or follow the effects of different doses of MR antagonists and other therapies.
Summary and clinical implications
In summary, recent advances in the study of mineralocorticoid receptors in vascular SMCs have enhanced our understanding of their role in the vascular injury response. The development and characterization of the SMC-MR-KO mouse has led to the discovery that SMC-MR is necessary for the adverse vascular effects of aldosterone on the responses to mechanical injury and to hypertension. In the absence of SMC-MR, aldosterone does not mediate its detrimental effects on SMC proliferation, vessel wall thickening, vascular fibrosis, and vascular stiffening. New mechanisms by which SMC-MR contributes to the vascular injury response have also recently been described, including pathways involving integrin alpha 5, Rho-kinase, PGF-VEGFR1, and galectin-3 (see Fig. 1). While further research is needed to elucidate the complete mechanism of the aldosterone-mediated vascular remodeling, the data presented above defend an important and direct role for the vascular SMC-MR and implicate several new players that may contribute to this phenomenon at a molecular and cellular level.
MR antagonists have traditionally been used to treat hypertension and heart failure; however, the data presented above demonstrating a direct role for SMC-MR in vascular remodeling may support the use of MR antagonists to prevent or treat vascular fibrosis and stiffening that result from vascular injury in other disorders (reviewed in [33]). Indeed, in animal models, MR antagonists prevent coronary stent restenosis [12], adverse vein graft remodeling [9], and pulmonary vessel remodeling in pulmonary hypertension [32]. However, the use of systemic MR antagonists can cause unwanted effects, including hyperkalemia and gynecomastia, through MR inhibition in extra-vascular tissues. Since we are currently unable to directly target and specifically inhibit SMC-MR in a clinical setting, these extravascular effects limit the use of MR antagonists for vascular protection, and thus further work in cell type-specific drug delivery mechanisms is required. The potential to use MR antagonists to coat stents could be considered for localized mechanical vascular injury induced by angioplasty and stenting, particularly since aldosterone appears to specifically contribute to SMC proliferation but not to endothelial regrowth after vascular injury [19]. As cell type-targeted therapy is far from a reality, additional research on the cellular mechanisms by which SMC-MR contributes to vascular remodeling has the potential to identify the downstream molecules that could be the targets of novel anti-fibrosis drugs, including VEGFR1, Rho-kinase, galectin, and likely others.
In addition to drug targets, the vascular remodeling mediators downstream of SMC-MR may be valuable as biomarkers. Current MR-antagonist treatment is titrated to its effects on blood pressure lowering. However, MR antagonists may be able to have effects on vascular protection at different doses than those needed to decrease blood pressure. Treatment with MR antagonists for vascular indications could be initiated or monitored based on plasma levels of these SMC-MR-regulated biomarkers. Further studies are needed to determine whether secreted SMC-MR targets such as PGF or galectin-3 might be biomarkers to identify patients who could benefit from MR antagonists or as markers for treatment effect. If such biomarkers become available, patients may be able to gain the substantial vascular benefits of MR antagonism while avoiding potentially unnecessary effects on the kidneys or other tissues in the body.
Acknowledgments
This work was supported by the US National Institutes of Health (NIH HL095590 [I.Z.J.] and T32 GM008448 [J.B.K.]) and the American Heart Association (GRNT7240000 [I.Z.J.]).
Footnotes
Conflict of Interest
Jenny B. Koenig and Iris Z. Jaffe declare that they have no conflict of interest.
Compliance with Ethics Guidelines
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
References
** denotes key references that have been published recently.
- 1.Goel SA, Guo LW, Liu B, Kent KC. Mechanisms of post-intervention arterial remodelling. Cardiovasc Res. 2012;96:363–371. doi: 10.1093/cvr/cvs276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rogerson FM, Fuller PJ. Mineralocorticoid action. Steroids. 2000;65:61–73. doi: 10.1016/s0039-128x(99)00087-2. [DOI] [PubMed] [Google Scholar]
- 3.Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A, Palensky J, Wittes J. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med. 1999;341:709–717. doi: 10.1056/NEJM199909023411001. [DOI] [PubMed] [Google Scholar]
- 4.Pitt B, Remme W, Zannad F, Neaton J, Martinez F, Roniker B, Bittman R, Hurley S, Kleiman J, Gatlin M. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med. 2003;348:1309–1321. doi: 10.1056/NEJMoa030207. [DOI] [PubMed] [Google Scholar]
- 5.Pitt B, Reichek N, Willenbrock R, Zannad F, Phillips RA, Roniker B, Kleiman J, Krause S, Burns D, Williams GH. Effects of eplerenone, enalapril, and eplerenone/enalapril in patients with essential hypertension and left ventricular hypertrophy: the 4E-left ventricular hypertrophy study. Circulation. 2003;108:1831–1838. doi: 10.1161/01.CIR.0000091405.00772.6E. [DOI] [PubMed] [Google Scholar]
- 6.Zannad F, Mcmurray JJ, Krum H, van Veldhuisen DJ, Swedberg K, Shi H, Vincent J, Pocock SJ, Pitt B EMPHASIS-HF Study Group. Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med. 2011;364(1):11–21. doi: 10.1056/NEJMoa1009492. [DOI] [PubMed] [Google Scholar]
- 7.Ivanes F, Susen S, Mouquet F, Pigny P, Cuilleret F, Sautiere K, Collet JP, Beygui F, Hennache B, Ennezat PV, Juthier F, Richard F, Dallongeville J, Hillaert MA, Doevendans PA, Jude B, Bertrand M, Montalescot G, Van BE. Aldosterone, mortality, and acute ischaemic events in coronary artery disease patients outside the setting of acute myocardial infarction or heart failure. Eur Heart J. 2012;33:191–202. doi: 10.1093/eurheartj/ehr176. [DOI] [PubMed] [Google Scholar]
- 8.Milliez P, Girerd X, Plouin PF, Blacher J, Safar ME, Mourad JJ. Evidence for an increased rate of cardiovascular events in patients with primary aldosteronism. J Am Coll Cardiol. 2005;45:1243–1248. doi: 10.1016/j.jacc.2005.01.015. [DOI] [PubMed] [Google Scholar]
- 9.Ehsan A, McGraw AP, Aronovitz M, Galayda C, Conte MS, Karas RH, Jaffe IZ. Mineralocorticoid receptor antagonism inhibits vein graft remodeling in mice. J Thorac Cardiovasc Surg. 2012;145:1642–1649. doi: 10.1016/j.jtcvs.2012.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jaffe IZ, Newfell BG, Aronovitz M, Mohammad NN, McGraw AP, Perreault RE, Ehsan A, Mendelsohn ME. Placental growth factor mediates aldosterone-dependent vascular injury in mice. J Clin Invest. 2010;120:3891–3900. doi: 10.1172/JCI40205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Van Belle E, Bauters C, Wernert N, Hamon M, McFadden EP, Racadot A, Dupuis B, Lablanche JM, Bertrand ME. Neointimal thickening after balloon denudation is enhanced by aldosterone and inhibited by spironolactone, and aldosterone antagonist. Cardiovasc Res. 1995;29(1):27–32. [PubMed] [Google Scholar]
- 12.Ward MR, Kanellakis P, Ramsey D, Funder J, Bobik A. Eplerenone suppresses constrictive remodeling and collagen accumulation after angioplasty in porcine coronary arteries. Circulation. 2001;104(4):467–72. doi: 10.1161/hc3001.091458. [DOI] [PubMed] [Google Scholar]
- 13.Jaffe IZ, Mendelsohn ME. Angiotensin II and aldosterone regulate gene transcription via functional mineralocortocoid receptors in human coronary artery smooth muscle cells. Circ Res. 2005;96(6):643–50. doi: 10.1161/01.RES.0000159937.05502.d1. [DOI] [PubMed] [Google Scholar]
- 14.Jaffe IZ, Tintut Y, Newfell BG, Demer LL, Mendelsohn ME. Mineralocorticoid receptor activation promotes vascular cell calcification. Arterioscler Thromb Vasc Biol. 2007;27(4):799–805. doi: 10.1161/01.ATV.0000258414.59393.89. [DOI] [PubMed] [Google Scholar]
- 15.Newfell BG, Iyer LK, Mohammad NN, McGraw AP, Ehsan A, Rosano G, Huang PL, Mendelsohn ME, Jaffe IZ. Aldosterone Regulates Vascular Gene Transcription via Oxidative Stress-Dependent And -Independent Pathways. Arterioscler Thromb Vasc Biol. 2011;31:1871–1880. doi: 10.1161/ATVBAHA.111.229070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Min LJ, Mogi M, Li JM, Iwanami J, Iwai M, Horiuchi M. Aldosterone and angiotensin II synergistically induce mitogenic response in vascular smooth muscle cells. Circ Res. 2005;97(5):434–42. doi: 10.1161/01.RES.0000180753.63183.95. [DOI] [PubMed] [Google Scholar]
- 17.Xiao F, Puddefoot JR, Vinson GP. Aldosterone mediates angiotensin II-stimulated rat vascular smooth muscle cell proliferation. J Endocrinol. 2000;165:533–536. doi: 10.1677/joe.0.1650533. [DOI] [PubMed] [Google Scholar]
- 18**.McCurley A, Pires PW, Bender SB, Aronovitz M, Zhao MJ, Metzger D, Chambon P, Hill MA, Dorrance AM, Mendelsohn ME, Jaffe IZ. Direct Regulation of Blood Pressure by Smooth Muscle Cell Mineralocorticoid Receptors. Nat Med. 2012;18:1429–1433. doi: 10.1038/nm.2891. This paper describes the phenotype of the inducible SMC-MR-KO mouse which has decreased blood pressure, vascular tone, and angiotensin II-mediated oxidative stress with aging. This is a novel and important model for studying the specific role of SMC-MR in aldosterone-mediated vascular remodeling. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19**.Pruthi D, McCurley A, Aronovitz M, Galayda C, Karumanchi SA, Jaffe IZ. Aldosterone Promotes Vascular Remodeling by Direct Effects on Smooth Muscle Cell Mineralocorticoid Receptors. Arterioscler Thromb Vasc Biol. 2013 doi: 10.1161/ATVBAHA.113.302854. [Epub ahead of print]. This paper demonstrates that SMC-MR is necessary for aldosterone-induced vascular remodeling and for injury-induced vascular fibrosis, even with physiological levels of aldosterone. This study also implicates SMC-MR regulation of PGF and VEGFR1 in the mechanism of aldosterone-induced vascular remodeling in vivo. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20**.Galmiche G, Pizard A, Gueret A, Moghrabi SE, Ouvrard-Pascaud A, Berger S, Challande P, Jaffe IZ, Labat C, Lacolley P, Jaisser F. Smooth Muscle Cell Mineralocorticoid Receptors Are Mandatory for Aldosterone-Salt to Induce Vascular Stiffness. Hypertension. 2013 doi: 10.1161/HYPERTENSIONAHA.113.01967. [Epub ahead of print]. This paper shows that SMC-MR is necessary for the development of vascular stiffness in response to hypertension in the uninephrectomy/aldosterone/salt hypertension model and suggests that the mechanism involves increased expression of the integrin alpha-5 subunit. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Duprez DA. Arterial stiffness/elasticity in the contribution to progression of heart failure. Heart Fail Clin. 2012;8:135–141. doi: 10.1016/j.hfc.2011.08.001. [DOI] [PubMed] [Google Scholar]
- 22.Marti CN, Gheorghiade M, Kalogeropoulos AP, Georgiopoulou VV, Quyyumi AA, Butler J. Endothelial dysfunction, arterial stiffness, and heart failure. J Am Coll Cardiol. 2012;60:1455–1469. doi: 10.1016/j.jacc.2011.11.082. [DOI] [PubMed] [Google Scholar]
- 23.McCurley A, Jaffe IZ. Mineralocorticoid Receptors in Vascular Function and Disease. Mol Cell Endocrinol. 2011;350:256–265. doi: 10.1016/j.mce.2011.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Loirand G, Guerin P, Pacaud P. Rho kinases in cardiovascular physiology and pathophysiology. Circ Res. 2006;98:322–334. doi: 10.1161/01.RES.0000201960.04223.3c. [DOI] [PubMed] [Google Scholar]
- 25.Miyata K, Hitomi H, Guo P, Zhang GX, Kimura S, Kiyomoto H, Hosomi N, Kagami S, Kohno M, Nishiyama A. Possible involvement of Rho-kinase in aldosterone-induced vascular smooth muscle cell remodeling. Hypertens Res. 2008;31:1407–1413. doi: 10.1291/hypres.31.1407. [DOI] [PubMed] [Google Scholar]
- 26**.Montezano AC, Callera GE, Yogi A, He Y, Tostes RC, He G, Schiffrin EL, Touyz RM. Aldosterone and angiotensin II synergistically stimulate migration in vascular smooth muscle cells through c-Src-regulated redox-sensitive RhoA pathways. Arterioscler Thromb Vasc Biol. 2008;28(8):1511–8. doi: 10.1161/ATVBAHA.108.168021. This paper proposes a mechanism for vascular remodeling involving the synergistic activation of a c-Src-dependent, redox-sensitive, Rho-kinase pathway by aldosterone/SMC-MR and angiotensin-II in SMCs. [DOI] [PubMed] [Google Scholar]
- 27.Fujimura N, Noma K, Hata T, Soga J, Hidaka T, Idei N, Fujii Y, Mikami S, Maruhashi T, Iwamoto Y, Kihara Y, Chayama K, Kato H, Liao JK, Higashi Y ROCK Study Group. Mineralocorticoid receptor blocker eplerenone improves endothelial function and inhibits Rho-associated kinase activity in patients with hypertension. Clin Pharmacol Ther. 2012;91:289–297. doi: 10.1038/clpt.2011.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.de Boer RA, Edelmann F, Cohen-Solal A, Mamas MA, Maisel A, Pieske B. Galectin-3 in heart failure with preserved ejection fraction. Eur J Heart Fail. 2014;15:1095–1101. doi: 10.1093/eurjhf/hft077. [DOI] [PubMed] [Google Scholar]
- 29**.Calvier L, Miana M, Reboul P, Cachofeiro V, Martinez-Martinez E, de Boer RA, Poirier F, Lacolley P, Zannad F, Rossignol P, Lopez-Andres N. Galectin-3 mediates aldosterone-induced vascular fibrosis. Arterioscler Thromb Vasc Biol. 2013;33:67–75. doi: 10.1161/ATVBAHA.112.300569. This paper identifies galectin-3 as a SMC-MR-regulated gene and demonstrates that hypertension-induced vascular remodeling occurs via -MR-mediated upregulation of galectin-3, resulting in increased vascular collagen-I production and fibrosis. [DOI] [PubMed] [Google Scholar]
- 30.Felker GM, Fiuzat M, Shaw LK, Clare R, Whellan DJ, Bettari L, Shirolkar SC, Donahue M, Kitzman DW, Zannad F, Pina IL, O’Connor CM. Galectin-3 in ambulatory patients with heart failure: results from the HF-ACTION study. Circ Heart Fail. 2012;5:72–78. doi: 10.1161/CIRCHEARTFAILURE.111.963637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fiuzat M, Schulte PJ, Felker GM, Ahmad T, Neely M, Adams KF, Donahue MP, Kraus WE, Pina IL, Whellan DL, O’Connor CM. Relationship between Galectin-3 Levels and Mineralocorticoid Receptor Antagonist Use in Heart Failure: Analysis from the HF-ACTION Study. J Card Fail. 2013 doi: 10.1016/j.cardfail.2013.11.011. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Preston IR, Sagliani KD, Warburton RR, Hill NS, Fanburg BL, Jaffe IZ. Mineralocorticoid receptor antagonism attenuates experimental pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol. 2013;304:L678–L688. doi: 10.1152/ajplung.00300.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McGraw AP, McCurley A, Preston IR, Jaffe IZ. Mineralocorticoid receptors in vascular disease: connecting molecular pathways to clinical implications. Curr Atheroscler Rep. 2013;15:340. doi: 10.1007/s11883-013-0340-x. [DOI] [PMC free article] [PubMed] [Google Scholar]