Abstract
Background
Active management of the third stage of labour involves giving a prophylactic uterotonic, early cord clamping and controlled cord traction to deliver the placenta. With expectant management, signs of placental separation are awaited and the placenta is delivered spontaneously. Active management was introduced to try to reduce haemorrhage, a major contributor to maternal mortality in low-income countries.
Objectives
To compare the effectiveness of active versus expectant management of the third stage of labour.
Search methods
We searched the Cochrane Pregnancy and Childbirth Group Trials Register (15 February 2011).
Selection criteria
Randomised and quasi-randomised controlled trials comparing active versus expectant management of the third stage of labour.
Data collection and analysis
Two review authors independently assessed the studies for inclusion, assessed risk of bias and carried out data extraction.
Main results
We included seven studies (involving 8247 women), all undertaken in hospitals, six in high-income countries and one in a low-income country. Four studies compared active versus expectant management, and three compared active versus a mixture of managements. We used random-effects in the analyses because of clinical heterogeneity. There was an absence of high quality evidence for our primary outcomes. The evidence suggested that for women at mixed levels of risk of bleeding, active management showed a reduction in the average risk of maternal primary haemorrhage at time of birth (more than 1000 mL) (average risk ratio (RR) 0.34, 95% confidence interval (CI) 0.14 to 0.87, three studies, 4636 women) and of maternal haemoglobin (Hb) less than 9 g/dL following birth (average RR 0.50, 95% CI 0.30 to 0.83, two studies, 1572 women). We also found no difference in the incidence in admission of infants to neonatal units (average RR 0.81, 95% CI 0.60 to 1.11, two studies, 3207 women) nor in the incidence of infant jaundice requiring treatment (0.96, 95% CI 0.55 to 1.68, two studies, 3142 women). There were no data on our other primary outcomes of very severe postpartum haemorrhage (PPH) at the time of birth (more than 2500 mL), maternal mortality, or neonatal polycythaemia needing treatment.
Active management also showed a significant decrease in primary blood loss greater than 500 mL, and mean maternal blood loss at birth, maternal blood transfusion and therapeutic uterotonics during the third stage or within the first 24 hours, or both and significant increases in maternal diastolic blood pressure, vomiting after birth, after-pains, use of analgesia from birth up to discharge from the labour ward and more women returning to hospital with bleeding (outcome not pre-specified). There was also a decrease in the baby’s birthweight with active management, reflecting the lower blood volume from interference with placental transfusion.
In the subgroup of women at low risk of excessive bleeding, there were similar findings, except there was no significant difference identified between groups for severe haemorrhage or maternal Hb less than 9 g/dL (at 24 to 72 hours).
Hypertension and interference with placental transfusion might be avoided by using modifications to the active management package, e.g. omitting ergot and deferring cord clamping, but we have no direct evidence of this here.
Authors’ conclusions
Although there is a lack of high quality evidence, active management of the third stage reduced the risk of haemorrhage greater than 1000 mL at the time of birth in a population of women at mixed risk of excessive bleeding, but adverse effects were identified. Women should be given information on the benefits and harms of both methods to support informed choice. Given the concerns about early cord clamping and the potential adverse effects of some uterotonics, it is critical now to look at the individual components of third-stage management. Data are also required from low-income countries.
BACKGROUND
Description of the condition
The third stage of labour is the time from the birth of the baby to the expulsion of the placenta and membranes. Once the baby is born, the uterus continues to contract and reduce in size. There is a lack of full understanding of the physiology of the third stage of labour, but recent work using ultrasonography has demonstrated that the process of placental separation has three distinct phases (Herman 2002). The first, or latent phase, consists of strong uterine contractions, which lead to thickening of the uterine muscle, thus causing a shearing force to occur between the elastic uterine wall and the more rigid placenta (Herman 2002). Continued contractions lead to gradual separation of the placenta, commencing at one of the poles (most commonly the lower) and spreading slowly during the contraction or detachment phase until full separation occurs. This is followed by delivery of the placenta in the expulsion phase (Herman 2002). Muscle fibres surrounding the maternal vessels contract to prevent excessive bleeding (Inch 1985) and the mother’s coagulation system is activated temporarily (Bonnar 1970).
There is always some blood loss during the third stage of labour as the placenta separates and is delivered, but what might be considered a normal amount of loss is the subject of debate (Gyte 1992). Nevertheless, some women can suffer from considerable blood loss during or after the third stage of labour. This can be a primary haemorrhage (within the first 24 hours) (Mousa 2007) or a secondary haemorrhage (between 24 hours and six weeks) (McDonald 2003). Postpartum haemorrhage (PPH) is commonly defined as a blood loss in excess of 500 mL (WHO 2003), with severe haemorrhage being a loss of 1000 mL or more and very severe haemorrhage being a loss of 2500 mL or more (Bloomfield 1990; Greer 1998; Penney 2005). However, the impact of blood loss at birth on an individual woman can vary considerably and will depend not only on the volume of blood lost, but also on her general state of health, the speed of the loss, her haemoglobin (Hb) levels at the time and her coagulation system. It is well documented that blood loss is consistently under- or over-estimated by clinicians (Razvi 2008), although many centres do try to measure and record blood loss accurately. In well-nourished women, some consider that, in general, there is little impact from a blood loss of 500 mL (Bloomfield 1990), this being equivalent to a routine blood donation (Burnley 2006), but in women in low-income countries who may be poorly nourished and anaemic, this loss can cause considerable morbidity or mortality. It has been estimated that at least 25% of maternal deaths in a number of countries are due to haemorrhage - most due to PPH (Abouzaher 1998; Khan 2006). The vast majority of these happen in the developing world, and PPH is the leading cause of maternal mortality in sub-Saharan Africa (Lazarus 2005). However, a study in Mexico (Romero-Gutierrez 2007) reported that while the leading cause of maternal death was haemorrhage, two-thirds of bleeding-related deaths resulted from placental abruption, placenta accreta, placenta praevia, and peripartum hysterectomy, rather than uterine atony. Significant morbidity does occur, though, from major bleeding due to uterine atony (poor contraction of the muscles in the uterus), which is far more common than the other causes of bleeding listed above. The seriousness with which PPH is viewed by professionals is evidenced in joint policy statements between the International Confederation of Midwives (ICM) and the International Federation of Gynaecology and Obstetrics (FIGO) (ICM-FIGO 2003; ICM-FIGO 2006), and the World Health Organization (WHO 2003), all of which recommend active management of the third stage of labour. Debate continues among women and practitioners on the optimum method of management of the third stage of labour to balance the benefits and harms.
There are two distinct approaches to the clinical management of the third stage of labour: expectant and active management. However, a third approach is sometimes used that consists of a combination of components of both expectant and active management: this has been referred to as ‘mixed management’ or the ‘piecemeal approach’ (Prendiville 1989). Expectant, active and mixed management approaches, and comparisons of different types of active management, have been the subject of a number of critical reviews (Elbourne 1995; Gyte 1994; Maughan 2006; McDonald 2007a; Prendiville 1989; Prendiville 1996; Soltani 2008).
Description of the intervention
(a) Expectant management of the third stage of labour
Expectant management is also known as conservative or physiological management and is popular in some northern European countries (Nordstrom 1997). It is also practised on occasion in midwife-led units and in home births in the United Kingdom and Ireland (Blackburn 2008; Fry 2007; Kanikosmay 2007), and is the usual practice in domiciliary care in some parts of the developing world. The main principle of expectant management is a ‘hands off’ approach, where signs of placental separation are awaited and the placenta is delivered spontaneously or with the aid of gravity, maternal pushing or, sometimes, nipple stimulation (Inch 1985; Prendiville 1989), hence:
a prophylactic uterotonic agent is not administered;
ideally the umbilical cord is neither clamped nor cut until the placenta has been delivered but, as a minimum, caregivers have waited until cord pulsation has ceased; and
the placenta is delivered spontaneously with the aid of gravity and sometimes by maternal effort (Rogers 1998).
There can be variations within expectant management. For example, some caregivers will wait for the placenta to be delivered before clamping and cutting the cord whilst others, for convenience, just wait until pulsation has finished. Breastfeeding or other means of stimulating the physiological release of oxytocin, such as nipple stimulation, is sometimes also used (Bullough 1989) but is not an essential component of expectant management.
(b) Active management of the third stage of labour
In active management of the third stage of labour, the clinician intervenes by using the following package of interventions (Prendiville 1989):
the routine administration of a prophylactic uterotonic drug just before, with, or immediately after, the birth of the baby;
early cord clamping and cutting* (i.e. prior to, alongside, or immediately after administration of an oxytocic, which is before cord pulsation ceases); and
-
controlled cord traction to deliver the placenta.
* current WHO recommendations (WHO 2006) are to delay cord clamping, although the National Institute for Health and Clinical Excellence (NICE) still supports early cord clamping (NICE 2007).
These interventions are implemented routinely and prophylactically in an attempt to reduce the blood loss associated with the third stage of labour and to reduce the risk of PPH. There are many possible variations with this package of interventions.
There are different uterotonic drugs that can be used, e.g. oxytocin (intravenous (IV) or intramuscular (IM)); syntometrine (IM); ergometrine (IV or IM); misoprostol (IM) (Cotter 2001; Gülmezoglu 2007; Liabsuetrakul 2007; McDonald 2007b; Su 2007). There is also debate over the route of administration and dosage of the drugs used. However, recent guidelines from WHO, FIGO, ICM and NICE all recommend the use of 10 IU oxytocin IM ((ICM-FIGO 2003; NICE 2007; WHO 2006). Misoprostol is potentially the most important uterotonic for use in some low-income countries because it is stable at ambient temperatures and is inexpensive (Parsons 2007). However, it does have adverse side effects (Mousa 2007) such as shivering, nausea and headaches, and it has been shown to be less effective than other agents (Gülmezoglu 2007).
There are differing timings for giving the prophylactic uterotonic drug, e.g. with the crowning of the baby’s head; with the birth of the anterior shoulder; immediately after the birth of the baby; after the birth of the baby but before the placenta is delivered (Harris 2004) and after the placenta is delivered (Winter 2007). The timing of administration of uterotonic drugs is the subject of another Cochrane review (Soltani 2006).
There can be variation in the time when the cord is clamped and cut; this can be immediately the baby is born; within a set time after the birth, e.g. within 30 seconds, or a minute; or anytime before umbilical cord pulsation ceases (McDonald 2008; Rabe 2004; Van Rheenan 2007).
There are also different timings for the initiation of controlled cord traction, such as waiting for signs of placental separation or not (McDonald 2003).
There can also be a delay in using the whole package of active management until after cord pulsation ceases, which has been described as ‘delayed active management’ (Gyte 2006).
Some guidelines (e.g. ICM-FIGO 2003) add uterine massage to the active management package although there is little evidence to support this (Abdel-Aleem 2010).
Placental cord drainage is sometimes used with active management of the third stage. This involves releasing the clamp on the maternal end of the umbilical cord to allow the blood from the placental side to drain, thus reducing the size of the placenta and thereby hoping to help separation and reduce the chance of a retained placenta (Prendiville 1989; Soltani 2005).
Some of these variations in the components of active management of the third stage of labour may no longer be considered good practice (e.g.,early cord clamping), but may, nonetheless, be used in included studies identified for this review.
(c) Mixed management of the third stage of labour
Mixed management of the third stage of labour (or ‘combined’ or ‘piecemeal’ management) consists of a mixture of some of the components of both active and expectant management of the third stage, but without exclusively containing all the components of either. Although active management of the third stage is usually recommended (ICM-FIGO 2006; NICE 2007; WHO 2003), there are many variations, and in practice some women may actually receive mixed management (Harris 2006; Mercer 2000). Mixed management of the third stage might include, for example: (1) early uterotonic administration, cord clamping after pulsation ceases and controlled cord traction; or (2) delayed uterotonic administration until cord pulsation ceases, then cord clamping and controlled cord traction. These forms of mixed management of the third stage are of interest because of the evidence of benefits from delayed cord clamping for the baby (McDonald 2008; Mercer 2008; Rabe 2004).
How the intervention might work
Expectant management
Expectant management of the third stage relies on the natural contractions of the uterus, stimulated by a surge of physiological oxytocin at birth, and anything that interferes with this oxytocin release may reduce the effectiveness of the physiological process in the third stage (Inch 1985). Release of oxytocin can, for example, be inhibited by anxiety through the excess release of adrenaline (Buckley 2004).
Hence, expectant management of the third stage of labour is commonly only considered appropriate following a labour where there has been no interference with the natural release of oxytocin, e.g. where oxytocin augmentation, induction, epidural or narcotic analgesia, or both, have not been used (Buckley 2004; Fry 2007); but some will consider that these aspects still need to be assessed in well-designed studies. This type of labour is more likely when the woman has positive psychological support from her midwife, or other trained supporter, who encourages her to listen to her body’s messages about movement, positioning, hydration and nutrition (Buckley 2004; Hatem 2008; Hodnett 2003).
Active management
In active management of the third stage of labour, it is suggested that the prophylactic administration of a uterotonic will reduce bleeding and the risk of severe haemorrhage (Greer 1998; Prendiville 1989). The role of early cord clamping and controlled cord traction in the reduction of bleeding is less clear, but it is thought that once the uterotonic drug has been administered, it is important to deliver the placenta quickly to prevent it being retained. Applying a clamp to the cord thus gives the caregiver something to grasp in order to deliver the placenta quickly by applying controlled cord traction. Active management of the third stage has been standard practice in many parts of the world for many years (Prendiville 1989). Recently, however, arguments have been put forward for a delay in cord clamping, pointing out that it is not an evidence-based part of the package of active management (Weeks 2007). A Cochrane review found that neither early nor late cord clamping showed any significant difference in PPH rates (McDonald 2008).
A number of Cochrane reviews have been conducted examining different aspects of active management of the third stage of labour. These include reviews on prophylactic oxytocin in the third stage of labour (Cotter 2001); prophylactic ergometrineoxytocin versus oxytocin for the third stage of labour (McDonald 2007b); prophylactic use of ergot alkaloids in the third stage of labour (Liabsuetrakul 2007); prostaglandins for preventing PPH (Gülmezoglu 2007); oxytocin agonists for preventing PPH (Su 2007); timing of cord clamping in term infants (McDonald 2008) and timing of cord clamping in preterm infants (Rabe 2004).
Potential adverse effects
Interventions used in active management of third stage have some adverse effects, due mainly to the uterotonic drugs used and to the common practice of early clamping of the cord.
Uterotonic drugs can increase the risk of hypertension, nausea and vomiting for women (Maughan 2006), and which appear to be related to the use of ergometrine-based drugs. Active management in many countries has moved away from ergometrine-based uterotonics, for this reason, and possibly also due to clinicians’ fear of retained placenta, although a review of ergometrine-based drugs compared with other uterotonics showed no difference in rates of manual removal of placentae (McDonald 2007b).
The potential consequences for the newborn infant of active management of the third stage of labour relate mainly to the timing of cord clamping. The effects on the neonate of early versus deferred cord clamping have been explored in Cochrane and other systematic reviews (Hutton 2007; McDonald 2008; Rabe 2004). Early cord clamping reduces the volume of placental blood transfusion and thus reduces the baby’s blood volume at birth by about 20% for term infants (RCOG 2009; Werner 2005). This results in lower blood haematocrit (HCT) levels and Hb concentrations after birth in term infants but the long-term importance of this effect is unknown (Hutton 2007; McDonald 2008; Prendiville 1989; Van Rheenan 2007). Potentially, placental transfusion may be more important for infants born in low- and middle-income settings where iron-deficiency anaemia exacerbated by nutritional and infectious insults may have substantial and long-term adverse effects on growth and development (Van Rheenan 2007). For preterm infants, another specific concern is the effect of postnatal placental transfusion on neonatal haemodynamic transition processes. The Cochrane review of early versus delayed cord clamping for preterm infants found some evidence that infants who had early cord clamping had a higher risk of hypotension treated with volume-transfusion and of intraventricular haemorrhage (Rabe 2004).
In contrast, early cord clamping also results in lower postnatal levels of plasma bilirubin and a lower incidence of neonatal jaundice that requires phototherapy (McDonald 2008; Rabe 2004). Treatment of neonatal jaundice may result in mother-infant separation that delays the initiation and establishment of breastfeeding and disrupts early neonatal metabolic adaptation (Mercer 2001). For infants born in low- or middle-income settings, or in rural or remote settings distant from healthcare facilities, the need for phototherapy (or its lack of availability) may be of greater clinical importance.
If uterotonic drugs are administered before delivery of the infant, for example, inadvertently prior to the birth of an undiagnosed twin, then disruption of the placental-uterine wall interface and interruption of placental-umbilical blood flow may cause acute perinatal asphyxia compromising neonatal cardio-respiratory transition. Newborn infants compromised at birth are more likely to need transition support (cardio-respiratory resuscitation). If an asphyxial insult has been severe or prolonged (for example, if exacerbated by obstructed labour such as shoulder dystocia) then other potential consequences may include neonatal encephalopathy with its associated risk of mortality and long-term neurodevelopmental morbidity.
Why it is important to do this review
This review was undertaken because of the need to determine if active, expectant management, or a mixed management package, was most likely to be of overall benefit. It is important to assess the impact of all these forms of care on both the mother and baby. We believe that this review is highly relevant to families and clinicians, as women frequently enquire about the differences in third-stage management during the antenatal period.
OBJECTIVES
To compare the effects of active versus expectant management of the third stage of labour on severe primary PPH and other maternal and infant outcomes.
To compare variations in the packages of active and expectant management of the third stage of labour on severe primary PPH and other maternal and infant outcomes.
METHODS
Criteria for considering studies for this review
Types of studies
We included all randomised, and quasi-randomised, controlled trials of active versus expectant management of the third stage of labour.
Types of participants
All women who expected a vaginal birth at 24 weeks’ gestation or later. We looked at women in high-income countries separately from women in low-/middle-income countries.
Types of interventions
- Active management of the third stage of labour, which is here defined as the package of interventions comprising:
- the administration of a prophylactic uterotonic just before, with, or immediately after the birth of the baby;
- early cord clamping and cutting (from immediately after the birth of the baby’s head in the case of a nuchal cord, or immediately after the birth of the baby to, usually, within a minute of birth);
- controlled cord traction to aid the delivery of the placenta.
- Expectant management of the third stage of labour, which is here defined as:
- no prophylactic administration of a uterotonic;
- the umbilical cord is neither clamped nor cut until the placenta has been delivered or until cord pulsation has ceased; and
- the placenta is delivered spontaneously with the aid of gravity and sometimes by maternal effort;
- none of the components of active management, described above, are employed routinely.
Mixed management of the third stage of labour which consists of a mixture of some of the components of both active and expectant management of the third stage, but without exclusively containing all the components of either (Table 1).
Table 1. Terms and definitions used in this review.
| Terms | Definitions used in this review |
|---|---|
| Expectant management of third stage of labour |
|
| Active management of third stage of labour |
|
| Mixed man-agement of third stage of labour | A mixture of some of the components of both active and expectant management of third stage, but without exclusively containing all the components of either. There can be a number of different mixed third stage managements, for example:
|
| Early prophylactic uterotonic | Prophylactic uterotonic drug administered just before, with, or immediately after, the birth of the infant |
| Delayed prophylactic uterotonic | Prophylactic uterotonic drug administered after the cord pulsation has ceased |
| Early cord clamping | The application of a clamp to the umbilical cord within 60 seconds of the birth of the infant (McDonald 2008). |
| Delayed cord clamping | The application of a clamp to the umbilical cord greater than 1 minute after birth or when cord pulsation has ceased (McDonald 2008). |
| Sarnat staging for hypoxic ischaemic encephalopathy (Sarnat 1976) | Stage 1 (mild): hyper-alertness, hyper-reflexia, dilated pupils, tachycardia, absence of seizures Stage 2 (moderate): lethargy, hyper-reflexia, miosis, bradycardia, seizures, hypotonia with weak suck and Moro reflexes Stage 3 (severe): stupor, flaccidity, small to mid-position pupils which react poorly to light, decreased stretch reflexes, hypothermia and absent Moro |
Comparisons
Active versus expectant management of the third stage of labour.
Active versus mixed management with early prophylactic uterotonic administration, delayed cord clamping and controlled cord traction.
Active versus mixed management, with delayed prophylactic uterotonic administration, delayed cord clamping and controlled cord traction.
Active management with prophylactic uterotonic given before or with the birth of baby versus active management with prophylactic uterotonic drug given immediately after birth of the baby.
Expectant versus mixed management with early prophylactic uterotonic administration, delayed cord clamping and controlled cord traction.
Expectant versus mixed management with delayed prophylactic uterotonic administration, delayed cord clamping and controlled cord traction.
Comparisons two to six were included because of the review team’s awareness of these different forms of clinical management of the third stage of labour and following the results of two reviews that indicated the benefits of delaying cord clamping for the baby (McDonald 2008; Rabe 2004). There are other variations of mixed management that could also be considered, e.g. variations in controlled cord traction, but we considered the above to be the most commonly used and thus important to review.
Types of outcome measures
We selected outcome measures in order of importance with due recognition of the core data set of outcome measures identified by Devane et al (Devane 2007).
Primary outcomes
Maternal
*Severe primary PPH at time of birth (clinically estimated or measured blood loss greater than or equal to 1000 mL).
*Very severe primary PPH at time of birth (clinically estimated or measured blood loss greater than or equal to 2500 mL).
Maternal mortality.
Maternal Hb concentration less than 9 g/dL 24 to 72 hours postpartum.
Infant
Admission to neonatal special care or intensive care unit.
Neonatal jaundice requiring phototherapy or exchange transfusion.
Neonatal polycythaemia treated with dilutional exchange transfusion.
Secondary outcomes
Maternal
*Severe primary PPH after delivery of placenta and up to 24 hours (clinically estimated or measured blood loss greater than or equal to 1000 mL).
*Severe primary PPH at time of birth and up to 24 hours (clinically estimated or measured blood loss greater than or equal to 1000 mL).
*Primary blood loss equal to or greater than 500 mL at time of birth (clinically estimated or measured).
*Primary blood loss equal to or greater than 500 mL after delivery of placenta and up to 24 hours (clinically estimated or measured).
*Primary blood loss equal to or greater than 500 mL at time of birth and up to 24 hours (clinically estimated or measured).
*Mean blood loss (mL) at time of birth (clinically estimated or measured).
*Mean blood loss (mL) after delivery of placenta and up to 24 hours (clinically estimated or measured).
*Mean blood loss (mL) at time of birth and up to 24 hours (clinically estimated or measured)
Maternal blood transfusion.
Clinical signs of severe blood loss at the time of birth, e.g. woman feeling breathless, weak, faint, pale, exhausted.
Therapeutic uterotonics during the third stage or within the first 24 hours, or both.
Mean length of the third stage (minutes).
Manual removal of the placenta as defined by authors.
Diastolic blood pressure greater than 90 mmHg between birth of baby and discharge from the labour ward.
Vomiting between birth of baby and discharge from the labour ward.
Any analgesia between birth of the baby and discharge from the labour ward.
Women’s assessment of pain during the third stage as reported by authors.
Secondary blood loss/any vaginal bleeding needing treatment (after 24 hours and before six weeks).
Amount of lochia either estimated or measured after 24 hours and up to discharge from hospital.
Surgical evacuation of retained products of conception.
Afterpains - abdominal pain associated with the contracting uterus in the postpartum period.
Infant
Apgar score less than seven at five minutes.
Birthweight.
Neonatal encephalopathy assessed using Sarnat staging (Sarnat 1976; Table 1).
Neonatal mortality.
Intraventricular haemorrhage - papille grade III/IV (for infants born before 34 weeks gestation only).
Number of infants who received a red blood cell transfusion.
Infant Hb level at 24 to 72 hours.
Infant Hb level at three to six months.
Infant iron indices (ferritin) at three to six month.
Exclusive breastfeeding at discharge.
-
Neurodevelopmental, cognitive or developmental outcomes assessed after age 18 months.
* All PPH amounts and mean blood losses are now expressed at three time periods “at the time of the birth”, “after delivery of the placenta and up to 24 hours”, and “at the time of birth and up to 24 hours.”
Search methods for identification of studies
Electronic searches
We contacted the Trials Search Co-ordinator to search the Cochrane Pregnancy and Childbirth Group’s Trials Register (15 February 2011)
The Cochrane Pregnancy and Childbirth Group’s Trials Register is maintained by the Trials Search Co-ordinator and contains trials identified from:
quarterly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
weekly searches of MEDLINE;
weekly searches of EMBASE;
handsearches of 30 journals and the proceedings of major conferences;
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Details of the search strategies for CENTRAL, MEDLINE and EMBASE, the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service can be found in the ‘Specialized Register’ section within the editorial information about the Cochrane Pregnancy and Childbirth Group. Trials identified through the searching activities described above are each assigned to a review topic (or topics). The Trials Search Co-ordinator searches the register for each review using the topic list rather than keywords.
In the protocol, we proposed to do a supplementary search in addition to that described above. On further discussion, we decided that the Trials Search Co-ordinator search would be adequate. We did retrieve additional relevant references cited in papers identified through the above search strategy and assessed their suitability for inclusion in the review. We did not apply any language restrictions.
Data collection and analysis
We based the methodology for data collection and analysis on the Cochrane Handbook of Systematic Reviews of Interventions (Higgins 2011).
Selection of studies
We obtained all potentially eligible trials identified by the search strategy as full-text papers and two review authors assessed each study for potential inclusion. We resolved any disagreements through discussion with at least one additional review author. One paper and one abstract required translation. We sought missing information from nine authors, and received five replies.
Data extraction and management
We designed a form to extract data. Two review authors (Cecily Begley (CB), GG (Gill Gyte) extracted the data independently from each study using the agreed form. We resolved discrepancies through discussion with at least one additional review author. Two review authors (GG, Declan Devane (DD)) and a member of the Cochrane Pregnancy and Childbirth Group’s staff independently reviewed Begley’s paper (Begley 1990) and the lead author of this review was not involved in any discussions of the paper’s inclusion, or assessment of its risk of bias status. We used the Review Manager software (RevMan 2011) to enter all data, which we checked independently.
Assessment of risk of bias in included studies
Two review authors (GG, DD or GG, CB) independently assessed risk of bias as a measure of methodological quality of included studies using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). When information regarding any of the criteria was unclear, we contacted the authors of the original reports to provide further details. Where these data were unobtainable, and the missing data were thought to introduce serious bias, we explored the impact of including such studies in the overall assessment of results by sensitivity analysis. We used the following criteria in the assessment of bias.
(1) Sequence generation (randomisation)
We assessed the quality of method of randomisation using the following headings:
low risk of bias - random-number table, computer random-number generator;
high risk of bias - systematic non-random approach, e.g. use of case record numbers, dates of birth; or days of the week;
unclear risk of bias.
(2) Allocation concealment (selection bias)
We assessed the quality of allocation concealment under the following headings:
low risk of bias - telephone or central randomisation, consecutively numbered, sealed opaque envelopes;
high risk of bias-open list of random-number tables, alternation or rotation;
unclear risk of bias.
(3) Blinding (checking for possible performance bias)
It is not possible to blind participants or personnel in these trials, as the fact that a uterotonic has been given (rather than a placebo, or nothing) is usually apparent to both women (who feel a strong contraction or pain) and clinicians (who can see or feel a strongly contracted uterus) following injection of a uterotonic. In addition, it is clear, in many cases, to both women and clinicians if early versus late cord clamping is practised or if cord traction versus maternal effort is used. We, therefore, did not assess methods for blinding of participants or personnel.
(4) Incomplete data collection (checking for possible attrition bias through withdrawals, dropouts, protocol violations)
We describe for each included study and for each outcome the completeness of data, including attrition and exclusions from the analysis. We state whether attrition and exclusions were reported, the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported or was sought and supplied by the trial authors, we re-included missing data in the analyses. No studies required re-analysis with the original allocated treatment groups being restored to their correct groups. Following these steps, studies were assessed as:
low risk of bias - less than 10% attrition at any stage, or 10% to 15% attrition in small sections of data, equal in both groups and due to natural fall-out of long-term follow-up;
high risk of bias - more than 20% attrition, or more than 15% exclusion at any stage when the reason for missing data was likely to be related to true outcomes;
unclear risk of bias.
Acknowledging that with long-term follow-up, complete data are difficult to attain, we discussed whether missing data greater than 20% might (a) be reasonably expected, and (b) impact on outcomes; if the latter, we excluded such studies. We subjected studies where attrition levels were unclear, or missing data greater than 15% occurred, to sensitivity analysis.
(5) Selective reporting bias
We describe for each included study how we examined the possibility of selective outcome reporting bias and we assessed reporting methods as:
low risk of bias (where it is clear that all of the study’s pre-specified outcomes and all expected outcomes of interest to the review have been reported);
high risk of bias (where not all the study’s pre-specified outcomes have been reported; one or more reported primary outcomes were not pre-specified; outcomes of interest were reported incompletely and so could not be used; study failed to include results of a key outcome that would be expected to have been reported);
unclear risk of bias.
(6) Other sources of bias
We also assessed and describe for each included study any important concerns we had about other possible sources of bias (e.g. specific study design, trial stopped early; extreme baseline imbalances). We thus assessed studies as being:
low risk of bias;
high risk of bias (problems detailed);
unclear risk of bias.
(7) Overall risk of bias
We made explicit judgements about whether or not studies were at high risk of bias, according to the criteria given in the Cochrane Handbook for Systematic Reviews of Interventions with reference to (1) to (6) above (Higgins 2011). As necessary, we explored the impact of the level of bias through undertaking sensitivity analyses.
Measures of treatment effect
We conducted statistical analysis using the Review Manager software (RevMan 2011).
Dichotomous data
For dichotomous data, we presented results as summary risk ratio with (RR) 95% confidence intervals (CIs).
Continuous data
For continuous data, we used the mean difference (MD) if outcomes were measured in the same way between trials. We used the standardised mean difference (SMD) to combine trials that measured the same outcome, but used different scales.
Unit of analysis issues
Cluster-randomised trials
We identified no cluster-randomised trials in this review.
Dealing with missing data
We analysed data on all participants with available data in the group to which they were allocated, regardless of whether or not they received the allocated intervention. If, in the original reports, participants were not analysed in the group to which they were randomised, and there was sufficient information in the trial report or in information obtained from the trial authors, we planned to restore them to the correct group and analyse accordingly (i.e. intention-to-treat (ITT) analysis). No studies required re-analysis with the original allocated treatment groups being restored to their correct groups. We used the number of women randomised minus the number of participants known to have missing data as the denominators. Where loss to follow up was greater than 20%, or where trial authors had excluded participants at a level greater than 15% and for reasons that were deemed to impact on outcomes, we excluded that study.
Assessment of heterogeneity
We assessed statistical heterogeneity in each meta-analysis using the T2 (tau-squared), I2 and Chi2 statistics. We regarded heterogeneity as substantial if T2 was greater than zero and either I2 was greater than 30% or there was a low P value (< 0.10) in the Chi2 test for heterogeneity.
Assessment of reporting biases
If there had been 10 or more studies in the meta-analysis, we would have investigated reporting biases (such as publication bias) using funnel plots. We would have assessed funnel plot asymmetry visually, and used formal tests for funnel plot asymmetry. For continuous outcomes we would have used the test proposed by Egger 1997, and for dichotomous outcomes we would have used the test proposed by Harbord 2006. If asymmetry had been detected in any of these tests or was suggested by a visual assessment, we would have performed exploratory analyses to investigate it.
Where we suspected reporting bias (see ‘Selective reporting bias’ above), we contacted study authors asking them to provide missing outcome data. If this had not been possible, and the missing data were thought to introduce serious bias, we would have explored the impact of including such studies in the overall assessment of results by conducting a sensitivity analysis.
Data synthesis
We carried out statistical analysis using the Review Manager software (RevMan 2011). We used random-effect meta-analyses for combining data because we considered that there was clinical heterogeneity sufficient to expect that the underlying treatment effects differed between trials. The random-effects summary has been treated as the average range of possible treatment effects and we have discussed the clinical implications of treatment effects differing between trials. If we had considered that the average treatment effect was not clinically meaningful, we would not have combined trials. We have presented the results as the average treatment effect with its 95% CI, and the estimates of T2, Chi2 P value and I2 (Higgins 2009). We found significant clinical and/or methodological heterogeneity between studies sufficient to suggest that treatment effects might differ between trials, which supported our choice of random-effects meta-analysis.
Subgroup analysis and investigation of heterogeneity
We did not undertake any interaction tests (Deeks 2001), as we were unable to conduct all subgroup analyses as planned, due to lack of usable data. We had planned the following subgroup analyses:
low risk of PPH versus high risk of PPH;
spontaneous versus operative vaginal birth;
nulliparous versus multiparous women;
low-income versus high-income setting;
full-term versus preterm birth (including outcomes specific to preterm babies).
In the end, we were able to look at the data on women at low risk of bleeding separate from the data on women irrespective of risk of bleeding. In addition, all the included studies in the main analysis were undertaken in high-income countries, although an on-going study on controlled cord traction is being undertaken in eight low-income countries (Gülmezoglu 2009).
Sensitivity analysis
We performed sensitivity analysis based on trial quality, separating high-quality trials from trials of lower quality. ‘High quality’ was, for the purposes of this sensitivity analysis, defined as a trial having adequate sequence generation, allocation concealment and an attrition rate of less than 20%, given the stated importance of attrition as a quality measure (Tierney 2005).
RESULTS
Description of studies
See: Characteristics of included studies; Characteristics of excluded studies; Characteristics of ongoing studies.
Results of the search
Our search of the literature identified 19 citations relating to 13 studies of potential relevance.
Included studies
We included seven studies involving 8247 women (Begley 1990; Jangsten 2011; Jerbi 2007; Khan 1997; Prendiville 1988; Rogers 1998; Thilaganathan 1993) (see Characteristics of included studies). Included studies were conducted in the UK (Prendiville 1988; Rogers 1998; Thilaganathan 1993), Ireland (Begley 1990), Sweden (Jangsten 2011), Tunisia (Jerbi 2007) and Abu Dhabi (Khan 1997). All studies took place in hospital settings.
Four studies (4829 women) compared active versus expectant management (Begley 1990; Prendiville 1988; Rogers 1998;Thilaganathan 1993), and three studies (3418 women) compared active versus mixed management (Jangsten 2011; Jerbi 2007; Khan 1997). In all trials, participants were healthy pregnant women expected to give birth vaginally. Three studies included only women classified as being at low risk to bleeding or its effects (Begley 1990;Rogers 1998; Thilaganathan 1993), and four (Jangsten 2011; Jerbi 2007; Khan 1997; Prendiville 1988) included women irrespective of their risk of bleeding.
There was one ongoing trial identified of mixed management for the third stage, looking at active management with or without controlled cord traction. This is being undertaken in eight centres in low-income countries throughout the world (Gülmezoglu 2009).
Considerable differences were seen in the protocols for both active and expectant management in the various trials (Table 2).
Table 2. Varying managements used in studies compared with study protocols.
| Active management protocol | Expectant management protocol | Active management used | Expectant management used | |
|---|---|---|---|---|
| Prendiville 1988 (Bristol trial) |
|
|
|
|
| Begley 1990 (Dublin trial) |
|
|
|
|
| Rogers 1998 (Hinchingbrooke trial) |
|
|
|
|
| Thilaganathan 1993 (Brighton trial) |
|
|
|
|
| Khan 1997 (Abu Dhabi trial) |
|
|
|
|
BP: blood pressure
CCT: controlled cord traction
IM: intramuscular
IV: intravenous
Interventions in the ‘active’ management groups
In the studies there were various uterotonic regimens used. These were intravenous (IV) ergometrine 0.5 mg (Begley 1990), IM syntometrine (5 units oxytocin + 0.5 mg ergometrine) (Thilaganathan 1993), IM syntometrine (5 units oxytocin + 0.5 mg ergometrine) or IM 10 units oxytocin if the woman had raised blood pressure (Prendiville 1988; Rogers 1998), IM 10 units oxytocin for all women (Khan 1997), IV oxytocin 5 units (Jerbi 2007) and IV oxytocin 10 units (Jangsten 2011). The descriptions of timing of administration of uteronic agent also varied and included “at the delivery of the anterior shoulder”, “as soon as possible after birth of anterior shoulder”, “immediately after the birth of the anterior shoulder” (which in practice probably equate to the same time), “immediately following birth”, “as soon as baby is born” (which is, in practice, very similar in timing to the preceding descriptions, perhaps 10 to 20 seconds later) and “within 2 minutes of birth”.
All trials stated that the cord was clamped and cut either within 30 seconds or “immediately”, which in practice is likely to be approximately similar timing. Controlled cord traction was attempted once the uterus was contracted in all trials. Two studies included maternal effort as an option (Jangsten 2011; Rogers 1998), and one included fundal pressure (Jerbi 2007).
Protocols in the ‘expectant’ management groups
In all trials, no uterotonic was to be given routinely prior to delivery of placenta. However, in one trial, an IV infusion of oxytocin 10 units in 500 mL normal saline was given slowly to all women following delivery of the placenta (Khan 1997). In one trial, 2 mL of placebo (saline solution) was administered intravenously within two minutes (Jangsten 2011). Practice varied widely as to how many women did, in fact, receive a uterotonic, either prophylactically: 0% (Begley 1990), 2.5% (Rogers 1998),and 20% (Jangsten 2011; Prendiville 1988), and/or as a treatment 14% (Begley 1990), 21% (Rogers 1998), 30% (Prendiville 1988), and 38% (Jangsten 2011), with no information given in the other three trials.
In four trials, clinicians were asked to try not to cut or clamp the cord until after pulsation ceased (Begley 1990; Prendiville 1988;Rogers 1998; Thilaganathan 1993), although this was achieved in only 42% to 70% of participants; in three trials, the cord was to be clamped and cut after birth of the baby (Jangsten 2011; Jerbi 2007; Khan 1997). Maternal effort was to be used in five trials (Begley 1990; Jangsten 2011; Prendiville 1988; Rogers 1998; Thilaganathan 1993), with the option in some of gentle controlled cord traction once the placenta had separated (Begley 1990) or assisting the placenta out once it was felt in the vagina (Thilaganathan 1993). Maternal effort was used by 32% to 88% of participants. In one trial, controlled cord traction was used, with gentle fundal pressure (Jerbi 2007) and in another uterine massage was used after placental delivery (Jangsten 2011).
Given the differences in uterotonics used in the active groups and the wide variation in the proportion of women in expectant management groups who actually received a uterotonic, it was decided to use a random-effects model due to the degree of clinical heterogeneity.
Excluded studies
We excluded six studies (Hoffman 2006; Kashanian 2010;Magann 2006; Muller 1996; Ramirez 2001; Vasegh 2005 (see Characteristics of excluded studies)). One study was only available as a conference abstract with no information on the number of women randomised to each group, and the authors of the previous review had been unable to obtain further information from the study authors (Muller 1996). Although we were able to contact one of the authors, we obtained no further useful information. One study assessed the timing for manual removal of the placenta, so did not fit the criteria for inclusion (Magann 2006). We excluded the third study because of the high number of women excluded after randomisation (48%) (Kashanian 2010). The fourth study (Hoffman 2006) was excluded due to concerns regarding the number of women withdrawn, after randomisation, due to caesarean section. Only a conference abstract was available, but further information on methodology was obtained from authors. We excluded the fifth study due to insufficient information on the numbers included in each of the three arms, and the method of management for the expectant arm (Ramirez 2001). Vasegh 2005 was excluded due to insufficient information in the published study and inability to elicit a response from the authors.
Risk of bias in included studies
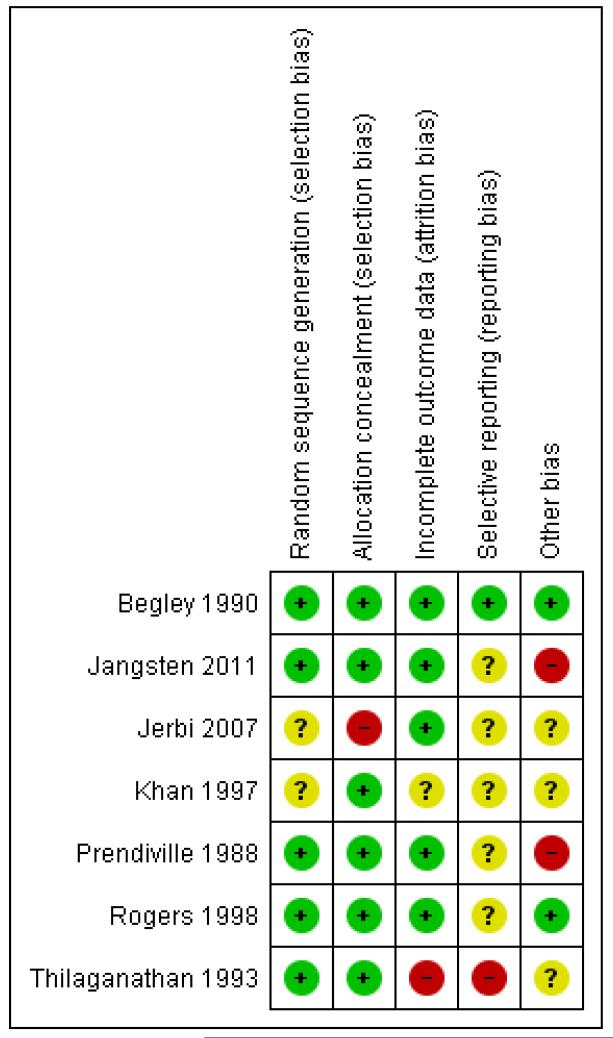
Of the seven studies included, we assessed one as having low risk of bias across all six aspects of the assessment (Begley 1990). We considered four studies as having low risk of bias in the main aspects of sequence generation, allocation concealment and completeness of data collection (Begley 1990; Jangsten 2011; Prendiville 1988;Rogers 1998), this being our criteria for overall quality for sensitivity analyses. We considered one study at high risk of bias for completeness of data collection, selective reporting and other biases (Thilaganathan 1993) and assessed one study as unclear on five of the assessment criteria, although allocation concealment seemed acceptable (Khan 1997). One study was assessed as unclear for sequence generation, selective reporting bias and other biases, at high risk of bias for allocation concealment and acceptable for completeness of data (Jerbi 2007). See Figure 1.
Figure 1. Methodological quality summary: review authors’ judgements about each methodological quality item for each included study.
Allocation
Five studies used adequate sequence generation using random-number tables or computer random-number generators (Begley 1990; Jangsten 2011; Prendiville 1988; Rogers 1998;Thilaganathan 1993) and in two the method was unclear (Jerbi 2007; Khan 1997). Adequate allocation concealment by telephone or central randomisation or consecutively numbered, sealed opaque envelopes was used in six studies (Begley 1990; Jangsten 2011; Khan 1997; Prendiville 1988; Rogers 1998; Thilaganathan 1993) and in one study allocation was not concealed (Jerbi 2007).
Blinding
Blinding was not possible when assessing the management of third stage of labour, for either women or clinicians, and so we did not assess this in the individual studies in the ‘Risk of bias’ tables (Characteristics of included studies). The assessment of many outcomes, particularly blood loss, could, therefore, have been unconsciously affected by people’s beliefs. Having chosen maternal Hb less than 9 g/dL as a hard outcome relating to blood loss at the protocol stage, we have now also included the mean postnatal Hb values to help in understanding the blood loss estimations. Haemoglobin assessment would usually be performed by a technician who would be blinded to the trial allocation.
In the Dublin trial (Begley 1990), and Swedish study (Jangsten 2011), blood loss was measured, but in all other studies it was estimated and therefore open to subjective inaccuracies, which should, however, have been the same across both groups; in addition, both blood loss estimation and measurement were open to bias. For certain outcomes such as Hb concentration, which could be measured by a blinded outcome assessor, we attempted to assess how such blinding had occurred. In practice, we found that almost all studies did not mention how such assessors were blinded; this category was therefore excluded from the ‘Risk of bias’ tables.
Incomplete outcome data
Five trials presented complete outcome data (Begley 1990;Jangsten 2011; Jerbi 2007; Prendiville 1988; Rogers 1998), with acceptable levels of attrition except for some follow-up measures such as postnatal Hb levels. One trial was considered at high risk of bias for complete data in that it was not clear how many were randomised and an unknown number of women were withdrawn following randomisation, due to caesarean section, operative delivery and cervical tears (Thilaganathan 1993). In the remaining study, it was unclear how many data were missing (Khan 1997). In both these trials, the denominator used was the number given by trial authors as taking part in the study after withdrawals had been made.
Selective reporting
We assessed one trial as free of selective reporting bias (Begley 1990) and categorised five others as ‘unclear’ as the trial protocols were not viewed (Jangsten 2011; Jerbi 2007; Khan 1997;Prendiville 1988; Rogers 1998). We deemed one study to have used selective reporting as PPH rates were not presented (Thilaganathan 1993).
Other potential sources of bias
We judged two studies as free of other apparent sources of bias (Begley 1990; Rogers 1998) and in three studies it was unclear whether or not other sources of bias existed (Jerbi 2007; Khan 1997; Thilaganathan 1993). We judged two studies to have other sources of bias (Jangsten 2011; Prendiville 1988). In one of these (Prendiville 1988) the trial included women at increased risk of PPH (high parity, all age groups, previous PPH, epidural, long labour, operative delivery), a problem with the previous study also (Khan 1997). Women at increased risk of PPH will have a higher blood loss, by definition, using expectant management; clinicians experiencing this may respond by anxiety in subsequent births, even of low-risk women, which may result in higher intervention (mixed management) rates. In Prendiville 1988, 50% of the expectant management group received an oxytocic, a proportion of intervention incompatible with the philosophy of expectant management. In one study, although 11,000 women were available, of whom at least half would usually be considered potentially eligible, only 1802 were entered into the study (Jangsten 2011). The majority were excluded due to “excessive workload”. This has the potential to have biased the study, as midwives would have had the choice of not asking the women to participate and may unconsciously have not offered participation to some women who they felt were not suitable for physiological management.
Midwives in all studies were more used to using active than expectant management, which is likely to have had an influence on results in the expectant arm. This influence may have been that they a) reverted to a type of active management, potentially reducing blood loss and narrowing the difference between trial arms in terms of blood loss outcomes or b) used mixed management, which, from the data, was more likely to increase blood loss or c) would have conducted a type of expectant management that was not ideal and resulted in increased blood loss in the expectant arm. In one study (Rogers 1998), a questionnaire administered to 92 of the 153 mid-wives prior to the trial commencement showed that 84% felt “very confident” of active management, whilst only 41% were “very confident” of expectant management. Similarly, Prendiville 1988 states that, before the trial commenced, the midwives were trained in the use of expectant management. Only six (13%), however, said that they were very confident in using expectant management before the trial started and 22 (46%) afterwards. In addition, of 49 midwives responding to a questionnaire regarding this study, 30 (61%) had never managed a third stage physiologically. Among the remaining 19, only one had practised physiological management as defined in the report (Harding 1989). In Begley 1990, the PPH rate in the expectant arm fell during the trial from 21% in the pilot study and 12% over the first four months, to 7% in the last six months, as midwives developed their skill (Begley 1990). No information on skill levels was provided in the other studies.
Finally, in one trial (Prendiville 1988) the protocol was changed after 425 births, but all births were included in the results, which may have affected the findings.
Effects of interventions
See: Summary of findings for the main comparison Active versus expectant management of the third stage of labour (all women)
The review includes seven studies involving 8247 women. We used a random-effects model for pooling data because of clinical heterogeneity seen in the included studies. In the forest plots, for six of the outcomes the “Favours expectant” label is on the left rather than the right. This is dictated by whether we are reporting negative (e.g. PPH) or positive (e.g. breastfeeding) outcomes.
01 Active versus expectant management of 3rd stage of labour - all women (four studies, 4829 women)
This comparison included four studies (Begley 1990; Prendiville 1988; Rogers 1998; Thilaganathan 1993). Three studies included only women at low risk of bleeding (Begley 1990; Rogers 1998;Thilaganathan 1993) and one study included women irrespective of risk of bleeding (Prendiville 1988). We assessed two studies as being of high methodological quality (Begley 1990; Rogers 1998); one raised concerns regarding high risk of bias in terms of midwives’ comfort with expectant management, and other possible biases (Prendiville 1988); and we considered one study to have high risk of bias in terms of incomplete outcome data and selective reporting bias (Thilaganathan 1993). We used random-effects meta-analyses due to the clinical heterogeneity involved. The random-effects summary gives an average for ‘active’ methods versus ‘expectant’ methods, and it is important to note that the treatment effect found by comparing any two specific techniques may differ from this. For a number of outcomes, there was very little statistical heterogeneity found (T2 = 0 and I2 = 0%), so there appears to be a single common treatment effect for these outcomes.
Primary outcomes
Compared with expectant management, active management showed a statistically significant average reduction in:
severe primary PPH (≥ 1000 mL at time of birth) (average RR 0.34, 95% CI 0.14 to 0.87, three studies, 4636 women, random-effects (T2 = 0.38, Chi2 P = 0.08, I2 = 60%) Analysis 1.1)
maternal Hb less than 9 g/dL at 24 to 72 hours (average RR 0.50, 95% CI 0.30 to 0.83, two studies, 1572 women, random effects T2 = 0.02, Chi2 P = 0.31, I2 = 3% Analysis 1.4).
None of the studies reported on the other primary maternal outcomes of very severe primary PPH (≥ 2500 mL) (except Begley 1990, who found no instances of such extreme blood loss in either group) or maternal mortality.
There was no statistically significant difference identified in:
admission to neonatal special care or intensive care unit (average RR 0.81, 95% CI 0.60 to 1.11, two studies, 3207 infants, random-effects T2 = 0.00, Chi2 P = 0.40, I2 = 0%, Analysis 1.5);
neonatal jaundice requiring phototherapy or exchange transfusion (average RR 0.96, 95% CI 0.55 to 1.68, two studies, 3142 infants, random-effects (T2 = 0.11, Chi2 P = 0.09, I2 = 66%), Analysis 1.6);
None of the studies reported on the other primary neonatal outcome of neonatal polycythaemia treated with dilutional exchange transfusion.
It should be noted that the evidence presented on the primary outcomes selected is based on results of a small number of studies with relatively small numbers of participants. The lack of consistent quality of evidence for these outcomes should be borne in mind when considering the overall results (see Summary of findings for the main comparison).
Secondary outcomes
Compared with expectant management, active management showed a statistically significant average reduction in:
primary blood loss ≥ 500 mL (clinically estimated or measured at birth) (average RR 0.34, 95% CI 0.27 to 0.44, three studies, 4636 women, random-effects (T2 = 0.02, Chi2 P = 0.23, I2 = 32%), Analysis 1.10);
mean maternal blood loss (average mean difference (MD) in mL −78.80, 95% CI −95.96 to −61.64, two studies, 2941 women, random-effects (T2 = 34.92, Chi2 P = 0.26, I2 = 22%), Analysis 1.13);
maternal blood transfusion (average RR 0.35, 95% CI 0.22 to 0.55, four studies, 4829 women, random-effects (T2 = 0.00, Chi2 P = 0.46, I2 = 0%), Analysis 1.16);
therapeutic uterotonics during the third stage and/or within the first 24 hours (average RR 0.19, 95% CI 0.15 to 0.23, four studies, 4829 women, random-effects (T2 = 0.00, Chi2 P = 0.47, I2 = 0%), Analysis 1.18);
mean birthweight (average MD in g −76.90, 95% CI −108.51 to −45.30, two studies, 3207 infants, random-effects (T2 = 0.00, Chi2 P = 0.58, I2 = 0%, Analysis 1.30).
Compared with expectant management, active management showed a statistically significant average increase in:
postnatal diastolic blood pressure > 90 mmHg up to discharge from labour ward (average RR 4.10, 95% CI 1.63 to 10.30, three studies, 4636 women, random-effects (T2 = 0.32, Chi2 P = 0.14, I2 = 49%), Analysis 1.21);
vomiting from birth of baby to discharge from labour ward (RR 2.47, 95% CI 1.36 to 4.48, three studies, 4636 women, random effects T2 = 0.14, Chi2 P = 0.09, I2 = 59%) Analysis 1.22)
administration of any analgesia from birth up to discharge from labour ward (RR 2.53, 95% CI 1.34 to 4.78, one study, 1429 women, Analysis 1.23);
afterpains (RR 2.53, 95% CI 1.34 to 4.78, one study, 1429 women, Analysis 1.28).
return to hospital as an in- or outpatient because of bleeding (outcome not pre-specified) (average RR 2.21, 95% CI 1.29 to 3.79, two studies, 2941 women, random-effects (T2 = 0.00, Chi2 P = 0.82, I2 = 0%), Analysis 1.40);
postnatal maternal Hb (outcome not pre-specified) (average MD 0.52, 95% CI 0.44 to 0.60, three studies, 4062 women, random-effects (T2 = 0.00, Chi2 P = 0.66, I2 = 0%), Analysis 1.41);
There was no statistically significant difference identified in:
mean length of the third stage in minutes (MD −0.30, 95% CI −1.87 to 1.27, one study, 1429 women, Analysis 1.19);
manual removal of placenta (average RR 1.78, 95% CI 0.57 to 5.56, four studies, 4829 women, random-effects (T2 = 0.82, Chi2 P = 0.01, I2 = 73%), Analysis 1.20);
secondary blood loss/any vaginal bleeding needing treatment (after 24 hours and before six weeks). (average RR 1.10, 95% CI 0.40 to 2.99, three studies, 4636 women, random-effects (T2 = 0.67, Chi2 P = 0.0005, I2 = 87%), Analysis 1.25);
surgical evacuation of retained products of conception (average RR 0.74, 95% CI 0.32 to 1.71, three studies, 4636 women, random-effects (T2 = 0.26, Chi2 P = 0.15, I2 = 47%), Analysis 1.27);
Apgar scores less than seven at five minutes (RR 1.00, 95% CI 0.38 to 2.66, one study, 1695 infants, Analysis 1.29).
exclusive breastfeeding at discharge (RR 1.01, 95% CI 0.96 to 1.07, one study, 1695 women, Analysis 1.38).
Authors of the included studies did not assess any of the review’s other secondary outcomes.
02 Active versus expectant management of 3rd stage of labour - women at low risk of bleeding (three studies, 3134 women)
This comparison included three studies (Begley 1990; Rogers 1998; Thilaganathan 1993). We considered two studies to be of high methodological quality (Begley 1990; Rogers 1998) and one study to have high risk of bias in terms of incomplete outcome data and selective reporting bias (Thilaganathan 1993). All meta-analyses used random-effects meta-analyses due to the clinical heterogeneity involved. For a number of outcomes, there was very little heterogeneity found (T2 = 0 and I2 = 0%), so there appears to be a single common treatment effect for these outcomes.
Primary outcomes
There was no statistically significant average difference identified in:
severe primary PPH (≥ 1000 mL at time of birth) (average RR 0.31, 95% CI 0.05 to 2.17, two studies, 2941 women, random-effects (T2 = 1.46, Chi2 P = 0.06, I2 = 71%), Analysis 2.1);
maternal Hb < 9 g/dL (at 24 to 72 hours) (RR 0.17, 95% CI 0.02 to 1.47, one study, 193 women, Analysis 2.4).
None of the studies reported on the other primary outcomes of: very severe primary PPH (≥ 2500 mL) (except Begley 1990, who found no instances of such extreme blood loss in either group) or maternal mortality.
There was no statistically significant average difference identified in:
admission to neonatal special care or intensive care unit (RR 1.02, 95% CI 0.55 to 1.88, one study, 1512 women, Analysis 2.5);
neonatal jaundice requiring phototherapy or exchange transfusion (RR 1.31, 95% CI 0.78 to 2.18, one study, 1447 women, Analysis 2.6);
None of the studies reported on the other primary outcome of neonatal polycythaemia.
Secondary outcomes
Compared with expectant management, active management in women at low risk showed a statistically significant average reduction in:
primary blood loss ≥ 500 mL (clinically estimated or measured at time of birth) (average RR 0.33, 95% CI 0.20 to 0.56, two studies, 2941 women, random-effects (T2 = 0.10, Chi 2 P = 0.10, I2 = 63%), Analysis 2.10);
mean maternal blood loss (mLl) (average MD −78.80, 95% CI −95.96 to −61.64, two studies, 2941 women, random-effects (T2 = 34.93, Chi2 P = 0.26, I2 = 22%), Analysis 2.13);
maternal blood transfusions (average RR 0.30, 95% CI 0.10 to 0.88, three studies, 3134 women, random-effects (T2 = 0.14, Chi2 P = 0.32, I2 = 11%), Analysis 2.16);
therapeutic uterotonics during the third stage and/or within the first 24 hours (average RR 0.15, 95% CI 0.11 to 0.21, three studies, 3134 women, random-effects (T2 = 0.00, Chi2 P = 0.98, I2 = 0%), Analysis 2.18);
mean birthweight in g (MD −67.00, 95% CI −114.13 to −19.87, one study, 1512 women, Analysis 2.30).
Compared with expectant management, active management for women at low risk showed a statistically significant average increase in:
postnatal diastolic blood pressure > 90 mm Hg (average RR 7.00, 95% CI 2.99 to 16.43, two studies, 2941 women, random-effects (T2 = 0.00, Chi2 P = 0.89, I2 = 0%), Analysis 2.21);
administration of any analgesia between birth of the baby and discharge from labour ward (RR 2.53, 95% CI 1.34 to 4.78, one study, 1429 women, Analysis 2.23);
afterpains (RR 2.53, 95% CI 1.34 to 4.78, one study, 1429 women, Analysis 2.28).
return to hospital as an in- or outpatient because of bleeding (outcome not pre-specified) (average RR 2.21, 95% CI 1.29 to 3.79, two studies, 2941 women, random-effects (T2 = 0.00, Chi2 P = 0.82, I2 = 0%), Analysis 2.40);
postnatal maternal mean Hb (outcome not pre-specified) (average MD in g/dL 0.50, 95% CI 0.41 to 0.59, two studies, 2683 women, random-effects (T2 = 0.00, Chi2 P = 1.00, I2 = 0%), Analysis 2.41).
There was no statistically significant average difference identified in:
length of the third stage in minutes (MD −0.30, 95% CI −1.87 to 1.27, one study, 1429 women, Analysis 2.19);
manual removal of placenta (average RR 3.58, 95% CI 0.42 to 30.61, three studies, 3134 women, random-effects (T2 = 2.58, Chi2 P = 0.02, I2 = 75%], Analysis 2.20);
postnatal vomiting (average RR 5.63, 95% CI 0.69 to 46.08, two studies, 2941 women, random-effects (T2 = 1.60, Chi2 P = 0.12, I2 = 60%), Analysis 2.22);
Secondary blood loss/any vaginal bleeding needing treatment (after 24 hours and before six weeks) (average RR 1.78, 95% CI 0.69 to 4.60, two studies, 2941 women, random-effects (T2 = 0.39, Chi2 P = 0.01, I2 = 84%), Analysis 2.25);
surgical evacuation of retained products of conception (average RR 0.69, 95% CI 0.12 to 3.98, two studies, 2941 women, random-effects (T2 = 1.17, Chi2 P = 0.06, I2 = 72%), Analysis 2.27);
Authors of the included studies did not assess any of the review’s other secondary outcomes.
03 Active versus mixed management of 3rd stage - all women (early uterotonic, delayed cord clamping, controlled cord traction) (no studies)
There were no studies that assessed this comparison.
04 Active versus mixed management of 3rd stage - all women (delayed uterotonic, delayed cord clamping, controlled cord traction) (no studies)
There were no studies that assessed this comparison.
05 Active versus mixed management of 3rd stage - all women (delayed uterotonic, delayed cord clamping, no controlled cord traction) (no studies)
There were no studies that assessed this comparison.
06 Active management with prophylactic uterotonic given before birth versus active management with uterotonic given after birth - all women (no studies)
There were no studies that assessed this comparison.
07 Mixed management - all women (early uterotonic, delayed cord clamping, controlled cord traction) versus expectant management (no studies)
There were no studies that assessed this comparison.
08 Mixed management - all women (delayed uterotonic, delayed cord clamping, controlled cord traction) versus expectant management (no studies)
There were no studies that assessed this comparison.
09 Mixed management - all women (delayed uterotonic, delayed cord clamping, no controlled cord traction) versus expectant management (no studies)
There were no studies that assessed this comparison.
10 Active versus mixed management of 3rd stage of labour - all women (immediate cord clamping, no controlled cord traction, uterotonic after placental delivery) (one study, 1657 women, comparison not pre-specified)
This comparison included one study (Khan 1997).
Primary outcomes
Compared with mixed management, active management showed a statistically significant reduction in:
severe primary PPH (blood loss ≥ 1000 mL at time of birth) (RR 0.23, 95% CI 0.09 to 0.55, one study, 1648 women, Analysis 10.1).
The study did not report on the other primary outcomes of: very severe primary PPH (≥ 2500 mL); maternal Hb < 9 g/dL at 24 to 72 hours; maternal mortality, neonatal jaundice requiring phototherapy or exchange transfusion; or neonatal polycythaemia treated with dilutional exchange transfusion.
Secondary outcomes
Compared with mixed management, active management showed a statistically significant reduction in:
primary blood loss ≥ 500 mL (clinically estimated or measured at time of birth) (RR 0.53, 95% CI 0.38 to 0.74, one study, 1648 women, Analysis 10.10);
therapeutic uterotonics during the third stage and/or within the first 24 hours (RR 0.45, 95% CI 0.26 to 0.77, one study, 1648 women, Analysis 10.18);
length of the third stage in minutes (MD −10.00, 95% CI −10.24 to −9.76, one study, 1648 women, Analysis 10.19).
There was no statistically significant difference identified in:
maternal blood transfusions (RR 0.25, 95% CI 0.03 to 2.22, one study, 1648 women, Analysis 10.16);
clinical signs of severe blood loss (RR 0.25, 95% CI 0.05 to 1.17, one study, 1648 women, Analysis 10.17).
Authors of the included studies did not assess any of the review’s other secondary outcomes.
11 Active versus mixed management of 3rd stage of labour - all women (no routine uterotonic, immediate cord clamping, no controlled cord traction) (one study, 1631 women, comparison not pre-specified)
This comparison included one study (Jangsten 2011). The study was judged to be at low risk of bias for sequence generation, allocation concealment and incomplete outcome data. It was unclear for selective reporting bias but considered at high risk of bias for other biases (see Characteristics of included studies).
Primary outcomes
Compared with mixed management, no statistically significant difference was identified in active management for:
severe primary PPH at time of birth (≥ 1000 mL) (risk ratio (RR) 0.78, 95% CI 0.48 to 1.24 Analysis 11.1)
maternal Hb less than 9 g/dL at 24 to 72 hours (RR 1.23, 95% CI 0.75 to 2.01 Analysis 11.4).
The study did not report on the other primary outcomes of: very severe primary PPH (≥ 2500 mL); maternal mortality; neonatal jaundice requiring phototherapy or exchange transfusion; neonatal polycythaemia treated with dilutional exchange transfusion.
Secondary outcomes
Compared with mixed management, active management showed a statistically significant reduction in:
primary blood loss ≥ 500 mL at time of birth (clinically estimated or measured at birth) (RR 0.51, 95% CI 0.40 to 0.66 Analysis 11.10);
mean maternal blood loss at time of birth (mean difference (MD) in mL −94.00, 95% CI −126.57 to −61.43, one study, 1621 women, Analysis 11.13);
therapeutic uterotonics during the third stage and/or within the first 24 hours (RR 0.39, 95% CI 0.33 to 0.48 Analysis 11.18);
mean length of third stage in minutes (MD −1.60, 95% CI −3.08 to −0.12 Analysis 11.19);
severe primary PPH after delivery of placenta and up to two hours (clinically estimated or measured blood loss ≥ 1000 mL) (outcome not pre-specified) (RR 0.54, 95% CI 0.39 to 0.74, Analysis 11.42).
severe primary PPH at time of birth and up to two hours (clinically estimated or measured blood loss ≥ 1000 mL) (outcome not pre-specified) (RR 0.60, 95% CI 0.47 to 0.78,.Analysis 11.43).
mean maternal blood loss after delivery of the placenta and up to two hours (outcome not pre-specified) (mean difference (MD) in mL −49, 95% CI −75.52 to −22.48, one study, 1621 women, Analysis 11.44).
Compared with mixed management, active management showed a statistically significant increase in:
postnatal maternal mean Hb (outcome not pre-specified) (MD in g/dL 0.28, 95% CI 0.14 to 0.42, one study, 1631 women, Analysis 11.41).
There was no statistically significant difference identified in:
maternal blood transfusion (RR 0.79, 95% CI 0.43 to 1.46 Analysis 11.16);
manual removal of placenta (RR 1.25, 95% CI 0.71 to 2.21 Analysis 11.20);
mean birthweight (MD in g 15.00, 95% CI −28.88 to 58.88 Analysis 11.30).
The study did not assess any of the review’s other secondary outcomes.
12 Active versus mixed management of 3rd stage of labour - all women (no routine uterotonic, immediate cord clamping, controlled cord traction) (one study, 130 women, comparison not pre-specified)
This comparison included one study (Jerbi 2007).
Primary outcomes
Compared with mixed management, no statistically significant difference was identified in active management for:
maternal Hb less than 9 g/dL at 24 to 72 hours (RR 0.80, 95% CI 0.34 to 1.90 Analysis 12.4).
The study did not report on the other primary outcomes of: severe primary PPH (≥ 1000 mL time of birth); very severe primary PPH (≥ 2500 mL); maternal mortality; neonatal jaundice requiring phototherapy or exchange transfusion; neonatal polycythaemia treated with dilutional exchange transfusion.
Secondary outcomes
Compared with mixed management, active management showed a statistically significant reduction in:
mean length of the third stage in minutes (MD −8.12 95% CI −9.72 to −6.52 Analysis 12.19);
There was no statistically significant difference identified in:
manual removal of placenta (RR 1.00, 95% CI 0.06 to 15.65 Analysis 12.20);
The study did not assess any of the review’s other secondary outcomes.
Sensitivity analysis
We undertook sensitivity analyses including only the four studies with adequate sequence generation, allocation concealment and complete outcome reporting (Begley 1990; Jangsten 2011;Prendiville 1988; Rogers 1998). There were no differences in the overall direction of the findings.
DISCUSSION
This review includes seven trials conducted in hospital settings in five countries involving 8247 women. There were no maternal deaths nor any very severe PPH (greater than 2500 mL) reported in any of the studies, and also no neonatal mortality reported. It should be noted that the random-effects summaries presented are the average effects found for ‘active’ versus ‘expectant’ management. Thus, it may not necessarily be true that all methods of active management will have the reported size of advantage in terms of PPH, or other outcomes, over all methods of expectant management.
Summary of main results
Active versus expectant management of the third stage in women irrespective of their risk of bleeding
Active management in hospitals in high-income countries led to a reduction in severe primary PPH equal to or greater than 1000 mL and maternal Hb less than 9 g/dL at 24 to 72 hours. Indices of maternal blood loss were also significantly improved; for example, mean Hb was higher by 0.5 g/dL in the active group. However, the average difference may not be clinically important, as routine blood donation reduces Hb levels by approximately 0.6 g/dL (Burnley 2006) without ill effects and postnatal women undergo a diuresis postnatally that reverses the haemodilution of pregnancy, thus increasing their Hb levels within a few days after birth (Hytten 2001; Taylor 1981). The more clinically important effects are the reduction in severe PPH rate, need for transfusion and uterotonic therapy, which confirms its effectiveness in lessening the severe bleeding that can prove life-threatening if left untreated.
However, active management resulted in a lower average birth-weight for the baby (possibly due to decreased placental transfusion at birth arising from the early cord clamping component, Farrar 2009a), and an increase in the incidence of maternal post-partum diastolic blood pressure greater than 90 mmHg (possibly due to the use of ergometrine-based uterotonics), afterpains, need for postpartum analgesia in the labour ward, and an increase in women having to return to hospital because of bleeding. Using data from only the good quality studies showed similar results.
The main findings may also be presented using the ‘number needed to treat’. There would be one fewer severe PPH for every 66 women who had active management (95% CI 44 to 127) and one woman fewer with a postnatal Hb under 9 g/dL for every 28 women who had active management (95% CI 17 to 73). Conversely, there would be one more woman with a diastolic blood pressure greater than 90mmHg for every 52 women who had active management (95% CI 38 to 83) and one more woman who had to return to hospital because of bleeding for every 65 women who had active management (95% CI 39 to 192).
Although this analysis considers women irrespective of risk of bleeding, the studies mainly included women at low risk of bleeding with just one out of the four studies specifically including women of any risk. There were no specific data on women considered to be at high risk of bleeding.
Overall, active management reduced the risk of severe bleeding, but it would be important to investigate if this benefit arose from the uterotonic component of the active management alone. The negative effects of active management appear, in the main, to be due either to 1) the administration of a specific uterotonic (e.g. hypertension due to ergometrine-containing preparations and hypotension due to IV oxytocin boluses (Lewis 2007)) or 2) possibly to controlled cord traction leading to retained shreds of membrane or placenta, thus causing the increased incidence of return to hospital due to bleeding or 3) early cord clamping leading to a 20% reduction in the baby’s blood volume. Different uterotonics will have differing effects, and clinicians will need to assess the optimum one for use in their circumstances. Recent international guidelines have turned to IM oxytocin as a uterotonic that provides effective prophylaxis but without the associated side effects (ICM-FIGO 2003; NICE 2007; WHO 2006). The increased incidence of women in the active management group having to return to hospital due to bleeding is of concern, as such bleeding takes place away from immediate access to medical assistance. This would, again, be of greater significance for women in low-income countries.
The reduction in the average birthweight of babies following active management is possibly due to a reduction in placental transfusion following early cord clamping (McDonald 2008; Rabe 2004;RCOG 2009). The evidence from the trials we identified is that the average volume of transfused blood was 77 mL (45 to 108 mL) based on differences in the birthweight between the groups. This estimate is consistent with historical data (Yao 1974), and also with more recent data (Farrar 2009a). Farrar’s small study (n = 26) weighing babies at birth on accurate scales to calculate placental transfusion showed that 79 mL (interquartile range 50 to 163 mL) of extra blood was transfused to babies following vaginal births and 84 mL (interquartile range 59 to 165 mL) following caesarean births. Placental transfusion for most of these babies was completed by about three minutes after birth, but transfusion continued for up to five minutes for some babies (Farrar 2009a). As all babies in this study breathed normally at birth, further studies are necessary to ascertain whether or not the cord remains pulsating for longer if the baby has not commenced respiration.
With expectant management, term infants receive about 80 mL more blood from placental transfusion than with active management, with its early cord clamping - thus adding about 20% more to the infant’s blood volume (Werner 2005). This may be associated with a lower incidence of anaemia in infancy (Cernadas 2006; Chaparro 2006; McDonald 2008; Van Rheenan 2007). It is possible that anaemia in early infancy may have adverse effects for the infant’s longer term growth and development, particularly for infants born in low-income countries. Although it is also plausible that placental transfusion with expectant management may increase the risk of neonatal jaundice requiring phototherapy (McDonald 2008), but there was no evidence for this in the two studies that assessed this outcome. Finally, it needs to be considered that in the context of the administration of a powerful uterotonic, early cord clamping may protect the baby by preventing a sudden increase of blood volume into the transitional circulation, that might disrupt physiological processes such as duct closure, lung fluid reabsorption and cerebral haemodynamic autoregulation. This may be particularly important to infants who are slow to breathe (Mercer 2008). This problem can be avoided by giving the uterotonic drug immediately following the deferred cord clamping. The included studies did not report on these specific neonatal adverse outcomes, but this review did not find evidence that admission rates to special care baby units (SCBU)/neonatal intensive care units (NICU) were affected by the types of third-stage management included (average RR 0.81, 95% CI 0.60 to 1.11, two studies, 3207 babies).
In the three studies that documented both severe PPH (equal to or greater than 1,000 mL) and number of blood transfusions (Begley 1990; Prendiville 1988; Rogers 1998), it is noted that a total of 78 out of the 4636 women had a severe PPH, whereas 94 received a blood transfusion, perhaps indicating an over-use of this treatment in a healthy population, under-estimation of blood loss, or undetected low antenatal haemoglobins in some women. Given that a woman’s body is well prepared for normal blood loss at birth by the haemodilution of pregnancy (Mims 2005), and that 600 mL to 750 mL of diluted blood is equivalent to a routine blood donation, it is possible that the impact of blood losses less than 750 mL are not severe in normal, healthy women, but this needs investigation. In addition, the decrease in plasma volume in the early days after birth increases postnatal Hb concentration in a reversal of haemodilution (Hytten 2001; Mims 2005; Taylor 1981).
Active versus expectant management of the third stage in women at low risk of bleeding
In hospitals in high-income countries, we found no statistically significant difference in maternal primary PPH greater than 1000 mL, although the number of women was insufficient to assess this outcome with confidence (two studies, 2941 women) and further studies would be needed to study this association.
One small study (n = 193) did compare the number of women with Hb levels less than 9 g/dL postnatally (Thilaganathan 1993) but the high risk of bias identified means the findings cannot be relied upon. None of the studies assessed Apgar scores less than seven at five minutes. Other indices of maternal blood loss were still statistically significantly decreased with the use of active management in this population. Active management again resulted in a lower birthweight (possibly due to reduced placental transfusion), an increase in the incidence of postpartum diastolic blood pressure greater than 90 mmHg, afterpains, need for postpartum analgesia in the labour ward, and having to return to hospital as an in- or outpatient because of bleeding.
In this comparison, active management of the third stage of labour reduced blood loss at the time of birth (and concomitant treatments required) but increased hypertension, pain and discomfort and increased return to hospital due to postnatal bleeding following discharge to the community. In addition, it decreased the baby’s birthweight, possibly due to clamping the cord before pulsation ceased, with a possible loss of approximately 80 mL of blood. There was no statistically significant reduction or increase in severe PPH for women at low risk to bleeding.
Mixed managements around the timing of cord clamping in combination with the timing of uterotonic drug
There were no studies identified that looked at these aspects of third-stage management.
Active versus mixed management of the third stage (immediate cord clamping, no controlled cord traction and uterotonic after placental delivery) in women irrespective of their risk of bleeding
The one study assessing this comparison was of uncertain quality (sequence generation was unclear) (Khan 1997). The results indicated that active management led to a reduction in severe primary PPH. The rate of blood loss greater than 500 mL, use of therapeutic uterotonics postpartum and length of the third stage were all decreased with the use of active management, with no statistically significant difference identified in number of blood transfusions. However, these findings are uncertain due to the ambiguity around sequence generation and other aspects of quality used in the study (Figure 1).
Active versus mixed management of 3rd stage of labour - all women (no routine uterotonic, immediate cord clamping, no controlled cord traction) (one study, 1631 women, comparison not pre-specified)
The one study included was of good quality (Jangsten 2011). The results indicated that no statistically significant difference was identified between active and mixed management in severe primary PPH or in maternal Hb less than 9 g/dL at 24 to 72 hours. The rate of blood loss greater than 500 mL and the use of therapeutic uterotonics during the third stage were decreased with the use of active management, with no statistically significant difference identified in the number of maternal blood transfusions, mean length of the third stage, mean birthweight or rate of manual removal of placenta.
Active versus mixed management of 3rd stage of labour - all women (no routine uterotonic, immediate cord clamping, controlled cord traction) (one study, 130 women, comparison not pre-specified)
The one study included was of moderate quality (Jerbi 2007). The results indicated that no statistically significant difference was identified between active and mixed management in maternal Hb less than 9 g/dL at 24 to 72 hours. The mean length of the third stage was decreased with the use of active management, with no statistically significant difference identified in manual removal of placenta.
Overall completeness and applicability of evidence
Four trials compared active versus expectant management of the third stage of labour and three compared active versus mixed management. All were conducted in hospitals, six in high-income countries, and one in a low-income country (n = 130). Three trials involved women at low risk of bleeding and four included women irrespective of their risk of bleeding. Given these factors, the results of the meta-analysis can only be applied to care given in high-income countries. As only three trials (one of uncertain quality) compared active versus mixed management, and all mixed managements differed, firm conclusions cannot be drawn on any apparent differences between these two managements. Also, for many of the outcomes there is heterogeneity in the treatment effects, so there is no information as to the specific factors that might affect the difference between active and expectant management.
Quality of the evidence
We assessed the methodological quality of the included trials as ‘high quality’ in terms of sequence generation, allocation concealment and complete outcome data for five trials (Begley 1990;Jangsten 2011, Prendiville 1988; Rogers 1998; Thilaganathan 1993), ‘high quality’ for sequence generation only for one trial (Jerbi 2007) and ‘unclear’ for one trial (Khan 1997). We under-took sensitivity analysis by trial quality to assess for any substantial difference in the main results. This made little difference to the overall findings except to indicate that there was no good quality evidence for the results of comparisons between active management and mixed management of the third stage.
In all studies, there is the problem of assessment of blood loss by clinicians where no blinding is possible. However, studies used other indices of blood loss such as postnatal Hb, which may have been assessed by technicians blinded to treatment allocation. For this reason, we included an outcome that had not been pre-specified, mean postnatal Hb, in addition to comparing the number of women with Hb levels less than 9 g/dL at 24 to 48 hours, which not all studies had measured.
Adherence to trial protocols and, in particular, to the management proposed for the two study arms was mixed (Table 2). In the four high-quality studies (Begley 1990; Jangsten 2011; Prendiville 1988; Rogers 1998), the majority of women in the active management arm (93% to 100%) received a uterotonic as directed. In the expectant management arm, however, practice varied widely, with 86% receiving ‘no uterotonic’ in one study (Begley 1990), 76.5% in another (Rogers 1998), 62% in a third (Jangsten 2011) and only 50% in the last study (Prendiville 1988), with no information given in the other three trials. The protocol for the expectant arm of the Prendiville study (Prendiville 1988) did allow for administration of a uterotonic if necessary, as it stated “Try not to give a uterotonic”; however, the actual proportion that received the drug appears incompatible with a philosophy of expectant management. In addition, the fact that the administration of the same uterotonic that constitutes the main treatment in active management to half of the ‘expectant’ management group as well, does raise questions as to the usefulness of the findings. The resulting ‘mixed’ management may also increase levels of blood loss and other complications, as changing the trial protocol after the first 425 births to exclude participants when mixed management had to be used due to cutting the cord early because of nuchal cord, concerns about meconium or baby needing resuscitation, resulted in a decrease in PPH rate.
In addition, this study (Prendiville 1988) included women at increased risk of bleeding. The total numbers involved are not clear, but 84 (5%) had previous third-stage problems, 212 (13%) had an epidural in labour, and 230 (14%) had assisted births. As these women are likely to have a higher blood loss when expectant management is used, clinicians experiencing this may respond by anxiety in subsequent births using expectant management, even for women at low risk of bleeding, and be more likely to give a uterotonic early; this may have increased the intervention or ‘mixed management’ rates in women allocated to expectant management in this study (30% received uterotonic for treatment, and 20% prophylactically), and would also have decreased the opportunity for those midwives to keep up their skill level in expectant management.
A second study, comparing active with mixed management, also included women at increased risk of bleeding (Jangsten 2011), with similar possible results as above. Seven per cent (131) had a caesarean section, 714 (40%) had an epidural in labour, and 148 (8%) had a ventouse birth.
The skill of the midwives in both forms of care in all studies is of interest. The midwives in Roger’s study (Rogers 1998) were said to be “similarly confident” in active and expectant management. However, the questionnaire administered to 92 of the 153 midwives prior to the trial commencement showed that, whereas 84% felt “very confident” of active management, only 41% were “very confident” of expectant management. Similarly, Prendiville 1988 states that, before the trial commenced, the researchers sought the advice of midwives in the UK who were “known to practise physiological management” and then used this advice to train the midwives. Only six (13%) of the midwives in this trial, however, said that they were very confident of physiological management before the trial started and 22 (46%) afterwards. Harding et al found that, of 49 midwives responding to a questionnaire regarding this study, 30 (61%) had never managed a third stage physiologically. Among the remaining 19, only one had practised physiological management as defined in the trial (Harding 1989). Those in Begley’s study (Begley 1990) were also more used to active management and the PPH rate in the expectant arm fell during the trial from 21% in the pilot study to 7% in the last six months, as they developed their skills (Begley 1990).
Midwives’ skill and the accurate measurement of blood loss obviously has an impact on documented blood loss in the third stage of labour. For example, the mean (estimated) blood loss in the active arm of one study (269 mL, standard deviation (SD) 246) (Rogers 1998) was actually greater than the mean (measured) blood loss in the expectant arm of another (235 mL, SD 224) (Begley 1990). This indicates that results of all these trials need to be examined in conjunction with local practice, to ascertain whether or not similar results will be found in different settings.
Finally, in one trial (Prendiville 1988), the protocol was changed after 425 births, but all births were included in the results, which may have affected the findings.
As previously noted, the evidence presented on the primary outcomes selected is based on results of a small number of studies with relatively small numbers of participants. The lack of consistent quality of evidence for these outcomes should be borne in mind when considering the overall results (see Summary of findings for the main comparison).
Potential biases in the review process
All review authors have an interest in third-stage management, and each brings different views on the methods that might be used. C Begley conducted one of the trials included in the review, and is a member of a team who conducted a systematic review for the ICM on physiological management of the third stage of labour. G Gyte has written on the third stage and is a involved in a study on timing of cord clamping in preterm birth, and other members have written on third-stage management. W McGuire is also involved in further studies on timing of cord clamping. A Weeks is involved in running clinical trials of the timing of cord clamping and misoprostol for third-stage prophylaxis in low resource settings. However, the review authors’ views differed and, during the review process, discussion and consensus was necessary to reach a final conclusion acceptable to all. Although all humans bring potential biases to any endeavour, we did try to ensure that our eventual conclusion arose solely from the data. Feedback from an international audience will serve to improve the next review update.
Agreements and disagreements with other studies or reviews
Previous version of this review
Our assessment of the evidence differs from the previous authors of this review (Prendiville 2000) who concluded that active management was “superior” to expectant management. For the main comparison, we have no additional studies but we analysed one small trial (Khan 1997) in the category of “active compared with mixed management,” and therefore did not include this in the main analysis. We have, however, used the recent methodology introduced for Cochrane reviews in 2008, which assesses risk of bias in the individual studies more carefully than in the past (Higgins 2011). We also chose to use a random-effects model for analysis due to the clear variations in the specific forms of both active and expectant management used in the included studies (clinical heterogeneity). Our findings, therefore, differ for some outcomes because of this decision (particularly for the group of women at low risk of bleeding) but we have reported on Tau2 and I2, regardless of level of heterogeneity. We believe the evidence shows both benefits and harms for active management, and believe it is now critical to look at the advantages and disadvantages of the individual components of third-stage management to see if the benefits can be achieved with fewer harms.
The previous authors of this review recommended active management of the third stage due to the benefit identified in terms of reduced incidence of severe bleeding. We agree that bleeding is a very important component when balancing the benefits and harms of active compared to expectant management of the third stage of labour. However, we consider that the number of harms caused by active management also deserve consideration. In particular, the increased rate of hypertension, increased numbers of mothers returning to hospital due to bleeding and the possible decrease in average blood volume of newborns reflected in the lower birthweight for babies where the mother has received active management of the third stage, are of concern. In the population of women at low risk to bleeding such harms are of more concern as there was no statistical evidence that severe bleeding was reduced by active management. Further studies would be needed to confirm if there is a difference or not. Our analysis of women at low risk of bleeding differs from the previous version of the review in that we excluded the Prendiville data (Prendiville 1988) as we considered there to be a high risk of bias because the randomisation was not stratified by high and low risk of bleeding and there were no specific data in the published study to enable us to use the data had we chosen to do so.
Other systematic reviews on active versus expectant management of the third stage
Active management in the included studies involved, in general, clamping and cutting the cord soon after the baby’s birth. A number of women in the expectant management groups also had their baby’s cord clamped and cut before pulsation had ceased. A recent Cochrane review has recommended that, in future, the umbilical cord should not be clamped and cut until after pulsation has ceased, regardless of the type of management used (McDonald 2008); if this change in practice is implemented it may negate the harms of active management due to early cord clamping. However, the optimum timing of the administration of the uterotonic still needs to be determined clearly.
Despite the evidence from the Cochrane review regarding timing of cord clamping (McDonald 2008), a recent survey of 1176 members of the Royal College of Obstetricians and Gynaecologists (RCOG) and 1445 members of the Royal College of Midwives (RCM) in the UK found that the majority (94% and 71%, respectively) ‘always’ or ‘usually’ use active management, and 73% and 40% clamp the cord within 20 seconds (Farrar 2009b). It would thus appear that there is considerable variation in practice and not all practitioners use the complete package of ‘active’ or ‘expectant’ management as described in these trials.
Guidelines issued by various policy-making bodies promote different aspects of third-stage management. The RCM provides information on the benefits and harms of both methods, drawing on the published literature, including the previous version of the Cochrane review. They recommend providing information for women and state that physiological management of the third stage can be seen as the logical ending to a normal labour (RCM 2008). The National Institute for Health and Clinical Excellence (NICE) contains recommendations on the management of the third stage of labour in their guideline on intrapartum care (NICE 2007). Using the previous Cochrane review and other published research evidence, NICE conclude that active management of the third stage is to be recommended, including the use of oxytocin (10 international units by IM injection), early clamping and cutting of the cord and controlled cord traction. They recommend that women should be told that active management reduces the risk of maternal bleeding and shortens the third stage, but no mention is made of the need to inform them of the documented harms of active management. It is, however, recommended that women who request expectant management and are at low risk of PPH “should be supported in their choice” (NICE 2007 p 183). The World Health Organization (WHO) also bases its recommendations on the previous Cochrane review, promoting the offering of active management to all women, provided they are cared for by skilled attendants (WHO 2006). The International Confederation of Midwives states that “Every midwife is required to attend the birth of the placenta without the aid of uterotonics” and that knowledge of physiological management of the third stage is “a basic midwifery competency”. It further recommends that “when a woman makes an active choice to experience a non-interventionist placental birth, the midwife will ensure the woman and her family have all the information necessary on which to make the decision” (ICM 2008).
Two issues bear further discussion in relation to published work: the experience of midwives in using expectant management and the use of expectant management of the third stage when the first and second stages of labour have not been normal. The RCM defines a normal birth as one where a woman commences, continues and completes labour physiologically at term (RCM 2004 Part 4e). Anecdotally, midwives experienced in expectant management say that only women who have had a normal, physiological labour and birth should have expectant management of the third stage. It has also been suggested in the literature that women who require induction or augmentation of labour with oxytocin (Sheiner 2005), or misoprostol (Phillip 2004), are prone to higher blood loss postpartum. In three of the four studies included in this review, high percentages of women had received a uterotonic for induction or acceleration of labour, and in all of them expectant management was not the norm for the midwives involved. It was recommended at the time of the first Cochrane review on this topic that trials be conducted in areas where midwives were skilled at using expectant or physiological management. The Netherlands and New Zealand are two such places, and observational studies emanating from these countries are worth examining as their results indicate no increase in blood loss in conjunction with the use of expectant management.
In Holland, a descriptive study was conducted to determine the incidence and risk factors for PPH in 3464 nulliparous women giving birth vaginally. The women were stratified for high- and low-risk factors for PPH, with 1416 stratified as low risk (41%) (Bais 2004). Approximately 50% of these women received prophylactic oxytocin and 50% did not, with no significant difference found in blood loss (Bais 2004). The New Zealand College of Midwives conducted a population-based, retrospective cohort study, which reported on the management provided by midwives during the third stage of labour (NZCM 2009). The study included 33,752 women who experienced a physiologically normal labour and birth. Almost half of the women (48.1%) had expectant management in the third stage while 51.9% had an actively managed third stage. Women who had expectant management were more likely to have a blood loss of less than 500 mL (96.3%) than those who had active management (93.1%). Mean blood loss was 213.6 mL (95% CI 211.6 mL to 215.5 mL) for the expectant management group and 241.6 mL (95% CI 239.4 mL to 243.8 mL) for active management. Similar findings were seen in an Irish study comparing midwife-led with consultant-led care (Begley 2009). Thirty per cent (n = 136) of the 446 women who received mid-wife-led care throughout their pregnancy and birth, and received no epidurals or oxytocin in labour, had expectant management. None of these 136 women had a PPH, whereas the PPH rate for the women having active management was 1% (Begley 2009). Although these are observational data, there is an interesting link here between normal blood loss and expectant management of the third stage, when such management follows a physiologically normal first and second stage, and care is given by midwives skilled in the technique. This is an area that would benefit from further research.
AUTHORS’ CONCLUSIONS
Implications for practice
Active management of the third stage of labour in hospitals in high-income countries brings benefits to women of mixed levels of risk of bleeding in terms of reducing severe blood loss (greater than 1000 mL) and the incidence of blood transfusions, but can cause a number of sequelae such as postnatal hypertension, pain and return to hospital due to bleeding. In addition, active management is associated with a reduction in birthweight possibly reflecting a reduction in neonatal blood volume due to early cord clamping. In women at low risk of bleeding, we identified no reduction or increase in severe blood loss (greater than 1000 mL) with active management of the third stage although there was still a reduction in the use of blood transfusions. Nor did we identify any change in postnatal anaemia, but active management still caused decreased birthweight, an increase in postnatal hypertension and pain, and increased the likelihood of women having to return to hospital as an in- or outpatient because of bleeding. It must be emphasised that this review includes only a small number of studies with relatively small numbers of participants, and the quality of evidence for primary outcomes is low or very low.
In the context of these studies, in high-income countries with high levels of clinician expertise and adequate access to emergency care, healthy women do not appear to suffer unduly from the results of above average blood loss (about 500 mL) that does not reach the level of severe primary postpartum haemorrhage (PPH) (greater than 1000 mL) (Bloomfield 1990). For example, this review found only a small number of low-risk women (29 out of 3134, 0.9%) had a sufficiently large blood loss to require a blood transfusion. There are also both benefits and harms from active management of the third stage and it is also unclear whether all three components of the active management package are required to gain the benefit of reduced PPH.
Drawing on evidence from the Cochrane review on ‘Prophylactic ergometrine-oxytocin versus oxytocin for the third stage of labour’ (McDonald 2007b), and other research on ergot compounds (Maughan 2006), we would expect that omitting the ergot component of the prophylactic uterotonic drug used as part of active management of the third stage should reduce the adverse effects of hypertension identified for active management. Similarly, the Cochrane review on ‘Effect of timing of umbilical cord clamping of term infants on maternal and neonatal outcomes’ (McDonald 2008), shows that deferring cord clamping should reduce the adverse effects of the reduced placental transfusion that come with early cord clamping as part of active management of the third stage. However, the optimum timing of the administration of the uterotonic drug still needs clarification.
Healthcare providers should, therefore, present information to all women in the antenatal period on the advantages and disadvantages of both methods of third-stage management to facilitate their discussion and informed choice of care. This information should include not only the benefits of active management (reduces the risk of severe blood loss, blood transfusions and postnatal anaemia in women at mixed risk of bleeding) but also the harms to the mother (increases the risk of hypertension if using ergot compounds, increases afterpains, need for analgesia, and bleeding following discharge). In addition, information regarding the effects on the baby of early versus deferred cord clamping should be provided whilst acknowledging the uncertainty deferred cord clamping brings due to the lack of evidence around optimal timing of the prophylactic uterotonic.
Although the studies in the review did not assess women at increased risk of bleeding specifically, it can be deduced from Prendiville’s study (Prendiville 1988) that for these women the benefit of reduced blood loss is likely to outweigh the harms. This may lead clinicians to suggest active management of the third stage with a prophylactic uterotonic that contains no ergot, and also deferred cord clamping, though women’s choice should always be respected.
For women at low risk of bleeding, the benefits seen were the reduction in number of blood transfusions and a reduced primary blood loss greater than 500 mL, but less than 1000 mL. The negative sequelae of decreased baby’s birthweight, increased incidence of hypertension, postpartum pain and return to hospital due to postnatal bleeding following discharge still occurred in this group. The outcomes “blood loss” and “number of blood transfusions” are susceptible to bias, due to the lack of blinding of clinicians. The decrease in baby’s birthweight and increased incidence of hypertension are considered avoidable if the cord is left unclamped and ergometrine-containing uterotonics are not used. Women at low risk of PPH should, therefore, be informed of the potential benefits and adverse effects of both physiological and active management of the third stage of labour, and how adverse effects can be minimised. When expectant management is used, it is important that the option of using a uterotonic (non-ergot based initially) as treatment at any time is available if excess bleeding occurs. These results cannot, and should not, be extrapolated to other contexts such as low-income countries where access to care is often severely restricted, or those countries with insufficient trained clinicians or inadequate emergency care.
Implications for research
All future studies should consider the results of the Cochrane review on timing of cord clamping (McDonald 2008) and consider inclusion of the element of leaving the cord unclamped until it has stopped pulsating in their protocols for both active and expectant management. It is important to establish, when deferred cord clamping is used as a variation of active management, whether the prophylactic uterotonic should be administered as the baby is born or deferred and administered immediately after the cord is clamped. Individual components of both types of management should be examined in further trials. Many of the outcomes that the review authors considered important, and listed in the protocol for this review, had not been assessed in the published trials. All future trials should aim to include maternal, fetal and infant outcomes, as listed in this review, as far as is practicable. Studies are needed in developing or resource-constrained countries, similar to the ongoing trial (Gülmezoglu 2009). In our view, it is critical to establish whether, in order to reduce bleeding for the mother, a uterotonic drug is what is needed rather than all three components of active management of the third stage. The ongoing study in eight low-income countries is assessing whether or not controlled cord traction needs to be part of the active management package (Gülmezoglu 2009), which will assist in answering this question.
It is also critical to establish if a uterotonic on its own is the effective part of active management, whether or not a routine uterotonic is really necessary for healthy women at low risk of bleeding, and what is the optimum time for administration. In addition, it is important to consider the adverse effects on the mother’s blood pressure, pain, and returning to hospital after the birth because of bleeding. The specific uterotonic drugs used, and resolving the above questions, may hold the answer in trying to reduce these adverse effects.
Future studies may compare different types of expectant management; for example, comparing expectant management with expectant management followed by uterotonic, or expectant management using maternal effort only with expectant management using maternal effort and gentle cord traction. Given the possibility that women who require induction or augmentation of labour with a uterotonic are prone to higher postpartum blood loss, researchers should consider if such women should be excluded from future trials including an expectant management arm, as the natural release of oxytocin may be inhibited by the administration of exogenous oxytocics or other uterotonics.
Studies involving active management should compare different types of active management, or different components of active management e.g., delaying administration of uterotonic until after cord clamping compared with giving uterotonic immediately and then delaying cord clamping.
PLAIN LANGUAGE SUMMARY.
Delivering the placenta with active, expectant or mixed management in the third stage of labour
Once a baby is born, the womb (uterus) continues to contract, causing the placenta to separate from the uterine wall. The mother then delivers the placenta, or ‘after-birth’. This is called expectant management of the third stage of labour. Active management of the third stage involves giving a drug (uterotonic) to contract the uterus and clamping the cord early (usually prior to, alongside, or immediately after giving the uterotonic, before cord pulsation ceases). In addition, traction is applied to the cord with counter-pressure on the uterus to deliver the placenta (controlled cord traction). Specific ways the three components are applied often vary. Mixed management uses some, but not all, of the three components. Active management was introduced to try to reduce haemorrhage, which is a major cause of women dying in low-income countries where women are more likely to be poorly nourished, anaemic and have infectious diseases. In high-income countries, bleeding occurs much less often, yet active management has become standard practice in many countries.
This review looked at different ways of managing the third stage of labour, for all women and specifically for women at low risk of bleeding. Seven studies were identified (8247 women), all in hospitals, six in high-income countries and one in a low-income country. Four studies compared active with expectant management and three compared active with mixed management.
Overall more data are needed to be confident in the findings. However, for all women, irrespective of their risk of bleeding, active management reduced severe bleeding and anaemia. However, it also reduced the baby’s birthweight and increased the mother’s blood pressure, afterpains, vomiting and the number of women returning to hospital with bleeding.
For women at low risk of bleeding, findings were similar though there was no difference in risk of severe bleeding.
Women should be given information antenatally to help them make informed choices. Some of the adverse effects experienced by mothers may possibly be avoided by using specific uterotonic drugs. Delaying cord clamping may benefit the baby by preventing the reduction in blood volume from early cord clamping, but more research is needed to be sure. Also it may be that just giving a uterotonic might reduce severe bleeding without reducing the baby’s blood volume. More research is also needed on the third stage in low-income countries.
ACKNOWLEDGEMENTS
We acknowledge the work of the previous review team, upon which the protocol was based (Prendiville 2000), and also the contribution of Deirdre Murphy and Sue McDonald in the previous version of this review. We thank the trial authors who provided additional information on request: Beck (on behalf of Muller), Begley, Hoffman, Rogers/Elbourne, Thilaganathan.
Particular thanks to Therese Dowswell, who contributed greatly to data extraction and tables.
As part of the pre-publication editorial process, this review has been commented on by three peers (an editor and two referees who are external to the editorial team), a member of the Pregnancy and Childbirth Group’s international panel of consumers and the Group’s Statistical Adviser.
Thanks to Nazie AmirAnsari and Alireza Karbalaei who translated Vasegh 2005a and Emily Lemon who translated Muller 1996.
SOURCES OF SUPPORT
Internal sources
(GG) The University of Liverpool, UK.
External sources
(GG) National Institute for Health Research, UK.
NIHR NHS Cochrane Collaboration Programme Grant Scheme award for NHS-prioritised centrally-managed, pregnancy and childbirth systematic reviews: CPGS02
Appendix
CHARACTERISTICS OF STUDIES
Characteristics of included studies [ordered by study ID]
| Methods | RCT with randomisation of individual women. | |
|---|---|---|
| Participants |
Irish hospital setting. High-income country. Inclusion criteria: all women at low risk of haemorrhage (< 35 years; parity < 5; 1st stage of labour <15 hours; no previous history of PPH; Hb >11 g/dL (or 10.6 g/dL for capillary sample)) with singleton, cephalic presentation, 35 to 36 weeks at recruitment; no medical complications which would contraindicate ergometrine or would increase the risk of bleeding (cardiac disease, use of heparin, hypertension), and expected to give birth vaginally 1429 women randomised out of 2901 eligible. Exclusion criteria: women with hypertension in pregnancy or 1st or 2nd stage; epidural anaesthesia (included in separate study); APH; 1st stage >15 hours; OVB; women attending private care Clinician responsible for third stage: midwives. |
|
| Interventions |
Intervention: active management of third stage (N = 705):
For retained placenta: 1 hour after birth:
Comparison: expectant management of third stage (N = 724):
Special circumstances: if baby’s cord is clamped and cut before pulsation ceases (due to cord round neck, asphyxia etc) do not give ergometrine. Milk any placental blood into bowl and discard it. Watch for signs of placental separation and deliver placenta by controlled cord traction Retained placenta > 1 hour after birth:
Data entered into Comparisons 1 and 2 |
|
| Outcomes | Pre-specified outcomes: manual removal of placenta; PPH (> 500 mL); mean blood loss; length 3rd stage; Hb <10 g/dL at 48-72 hours; and difference between 32 weeks and 4872 hours PP: PP blood transfusions; side effects 1-2 hours post birth; PP complications; breastfeeding; serum prolactin; women’s views. No neonatal outcomes (Information from Oxford Database of Perinatal Trials registration sheet) and from [Begley 1990]): morbidity; blood loss during 3rd stage; method of placental delivery; complications occurring in first 1-2 hours post birth (haemorrhage, nausea, vomiting, raised BP, pain); Hb on 3rd postnatal day; prolactin levels on 3rd postnatal day, duration of breastfeeding. | |
| Notes | Between 1 Oct ‘87 and 31 Oct ‘88, 2901 women were deemed eligible for initial inclusion, 2650 agreed to take part. 1221 of these were excluded prior to randomisation because of epidural (399); OVB (354); CS (132); rapid birth (95); hypertension (77); missed (53); low Hb (40); woman’s request (28); miscellaneous (23); breech (20) Actual management used in the active arm: all given IV ergometrine 0.5 mg before delivery of placenta; 89% cord clamped and cut; 93% CCT and 5% maternal effort; 7% upright and 93% recumbent Actual management used in the expectant arm: 14% got ergometrine for treatment, not prophylactically, 6 (0.83%) before placenta delivered; cord left unclamped till pulsation ceased 42%; placenta delivered by maternal effort 32% and CCT 66%; 11% upright |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Unpublished information from author: random number tables were used (Fleiss 1981). The first number was selected from the table by a disinterested observer and the numbers were allocated in blocks of 100 following in sequence |
| Allocation concealment (selection bias) | Low risk | “…a numbered, sealed envelope containing the randomly allocated group was stapled to the woman’s chart in readiness for admis-sion…The envelope remained sealed until the women was in second stage of labour and the midwife was certain a normal delivery would ensue. The envelope was then opened…” |
| “When a woman was excluded from the study, her envelope was returned, unopened, to the researcher. All returned envelopes were re-allocated in numerical order prior to starting the next batch of 100 envelopes.” | ||
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Some missing data for some postnatal haemoglobin measurements (618 out of 705 in the active group (12% attrition) and 645 out of 724 in the expectant (11% attrition) ITT not mentioned but no loss to follow up for outcomes measured during labour. Some outcome data are taken from the unpublished thesis |
| Selective reporting (reporting bias) | Low risk | None apparent, outcomes on trial registration sheet all reported |
| Other bias | Low risk | No significant difference in baseline characteristics, but more women in the physiological arm had pethidine in labour (46% cf 52%, P = 0.05). This may impact on outcomes in the physiological arm where the sight and sound of the baby may be the stimulus for the hormonal release needed for natural 3rd stage and pethidine may impact here. 1st and 2nd stage management similar and no obvious differences overall |
| Methods | RCT with randomisation of individual women | ||
|---|---|---|---|
| Participants |
Setting: University hospital, Sweden. High-income country. Inclusion criteria: healthy women with normal pregnancies, a gestational age of 34 to 43 weeks, singleton, cephalic presentation and expected vaginal birth (included ventouse deliveries). Exclusion criteria: non-Swedish speaking, previous PPH, elective CS, pre-eclampsia, grand multiparity (> 5) or intrauterine fetal death Subgroups: High-income, not low-risk Clinician responsible for third stage: midwives |
||
| Interventions |
Experimental intervention: Active management of third stage (N = 903, but analysed 810)
Control/Comparison intervention: Mixed management of third stage (N = 899, but analysed 821) Description: Mixed: (no routine uterotonic; early cord clamping, no CCT):
Data used in Comparison 11. |
||
| Outcomes |
Pre-specified outcomes: primary outcome was the incidence of blood loss > 1000 mL during the third stage of labour. Other outcomes: Hb at 24 hours and women’s views Reported outcomes: blood loss > 1000 mL and > 500 mL during the third stage of labour, blood loss > 1000 mL and > 500 mL in first two hours postpartum, Hb at 24 hours, change in Hb from antenatal to 24 hours postnatal, retained placenta/retained part of placenta or membranes, additional uterotonics, duration of 3rd stage . Blood transfusion, units transfused, experience of mothers, after-pains Outcomes obtained by email response 28th April 2011 manual removal of placenta alone, cross-over in additional uterotonics, Hb <9 g/dL at 24-48 hours, length of third stage > 60 minutes Outcomes obtained by email response 26th May 2011 clarification of cross-over in additional uterotonics, blood loss > 500 mL and > 1000 mL during third stage oflabour separated from blood loss > 500 mL and > 1000 mL in first two hours postpartum |
||
| Notes | We wrote to the author for additional information which was provided as follows: “In the active group, 41 women had extra Synt, therapeutically, before the placenta (some went on to have a second dose, and/or Methergin) = 41 767 had no extra Synt before the placenta. Of these, 26 had a second dose after the placenta was delivered, 6 had a second dose of Synt and a dose of Methergin and 48 had Methergin but no Synt = 80 IN TOTAL, IN THE ACTIVE GROUP, 121 WOMEN HAD THERAPEUTIC UTEROTONICS. In the expectant group, 160 women had a dose of Synt, therapeutically, before the placenta (some went on to have a second dose, and/or Methergin) = 160 655 had no Synt before the placenta. Of these, 102 had a dose of Synt after the placenta was delivered, 32 had a dose of Synt and a dose of Methergin and 17 had Methergin but no Synt. = 151 IN TOTAL, IN THE EXPECTANT GROUP, 311 WOMEN HAD THERAPEUTIC UTEROTONICS Blood loss before and during placenta delivery: IN ACTIVE GROUP: 0 to 500 mL = 86.57% ( n = 696) > 500 mL = 13.43% (n = 108) and 0 to 1000 mL = 96.39% (n = 775) > 1000 mL = 3.61% (n = 29) Blood loss before and during placenta delivery: IN EXPECTANT GROUP: 0 to 500 mL = 76.25% (n = 623) > 500 mL = 23.75% (n = 194) and 0 to 1000 mL = 95.3% (n = 779) > 1000 mL = 4.65% (n = 38) 26 women in the active group and 21 in the expectant had manual removal of placenta 34 women in the active and 28 in the expectant group had maternal haemoglobin <9 g/ dL at 24-48 hrs 31 women in the active and 23 in the expectant group had third stage > 60 minutes “mean blood loss before placenta” was clarified as meaning “mean blood loss ‘before and during expulsion’ of the placenta.” |
||
| Risk of bias | |||
| Bias | Authors’ judgement | Support for judgement | |
| Random sequence generation (selection bias) | Low risk | Computer-generated randomisation. | |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes containing the computer-generated randomisation group were prepared in consecutive order and kept in another unit. At randomisation, midwives phoned the staff at the other unit who opened the sealed envelopes and disclosed the assigned intervention and trial number | |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Of the 903 women randomised to the active group and 899 women randomised to the mixed management group:
|
|
| Selective reporting (reporting bias) | Unclear risk | ||
| Other bias | High risk | There were more inductions in the active group (10% versus 7%). Describe any differential diagnosis: none. Although 11,000 women were potentially eligible, of whom at least half would usually be considered eligible, only 1802 were entered into the study. Numbers excluded due to ineligibility are not recorded, and other reasons given are ‘excessive workload’ or ‘admission in advanced labour’. Email response from Jangsten 28th April 2001: “the hard workload was one reason that the women were not included and that all eligible women were not asked to participate. Few women refused in participating but I don’t have the number.” This has the potential to have biased the study, as midwives would have had the choice of not asking the women to participate and may unconsciously have not offered participation to some women who they felt were not suitable for physiological management |
|
| Methods | Randomised controlled trial of individual women in low-income setting | |
|---|---|---|
| Participants |
Setting: Sousse, Tunisia. Low-income country. Women with singleton pregnancies expecting to give birth vaginally Exclusion criteria: placenta praevia, APH, non-cephalic presentation, intrauterine death, parity > 5, uterine fibroids, anticoagulation therapy, history of PPH, history of CS |
|
| Interventions |
Intervention: active management of 3rd stage (N = 65):
Data included in Comparison 12 |
|
| Outcomes | Pre-specified: reduction in HCT and Hb. | |
| Notes | We contacted the authors again on 14th March 2011, for further information on the management in the comparison arm, the methodology they used and data obtained. Reply received 19th March 2011. No publication has emanated, no further data were provided, but methodology was clarified | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | As per www.randomization.com (not stated in publication but information provided by author on 19 March 2011) |
| Allocation concealment (selection bias) | High risk | Not concealed in any way (not stated in publication, but in information provided by author on 19 March 2011) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss described in publication and confirmed by information from author on 19 March 2011. Authors also provided information that the analysis was by ‘intention to treat’ |
| Selective reporting (reporting bias) | Unclear risk | We did not assess the trial protocol. |
| Other bias | Unclear risk | Women reported to have been allocated to groups after placental delivery yet active management group supposedly had oxy-tocin with the anterior shoulder |
| Methods | RCT with randomisation of individual women. | |
|---|---|---|
| Participants |
Abu Dhabi hospital setting. High-income country. Inclusion criteria: all women expected to give birth vaginally. 1657 women randomised out of a possible 4239 Exclusion criteria: refusal or caesarian section in second stage (9 excluded, final sample 1648) |
|
| Interventions |
Intervention: active management of 3rd stage (N = 827):
Data included in Comparison 10. |
|
| Outcomes |
Primary: PPH. Secondary: duration of third stage, retained placenta, shock, blood transfusion, methy-lergonovine or 15-methyl-a-prostaglandin to control haemorrhage |
|
| Notes | Not readily comparable to other studies as IV oxytocin infusion given to all women in expectant management group after delivery of placenta. This is the practice in the US but not elsewhere | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No detail. |
| Allocation concealment (selection bias) | Low risk | Numbered sealed opaque envelopes. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 2582 excluded prior to randomisation due to refusal. 9 excluded after randomisation due to emergency CS. |
| Selective reporting (reporting bias) | Unclear risk | No trial protocol reviewed. Most reported outcomes were pre-specified. Mean change in haematocrit was reported, but not pre-specified |
| Other bias | Unclear risk | 2582 out of 4239 refused to participate. Those that did agree may have been biased It is unknown whether or not the mid-wives had sufficient training in physiological third stage before the trial started This trial has been criticised for including all women (including high parity, all age groups, previous PPH, epidural, long labour, operative delivery) and not confining inclusion criteria to women who were low risk. Women at high risk of PPH will have a higher blood loss using expectant management; clinicians experiencing this may respond by anxiety in subsequent births, even oflow-risk women, which may result in higher intervention (mixed management) rates Also, the minimal intervention (control) group had the cord clamped and cut immediately after delivery, which is suspected to lead to an increase in blood loss |
| Methods | RCT with randomisation of individual wonen. | |
|---|---|---|
| Participants |
UK hospital setting. High-income country. Inclusion criteria: all women expected to give birth vaginally. 1695 women randomised out of a possible 4709 Exclusion criteria: refusal, cardiac disease, APH, non-cephalic presentation, multiple pregnancy, IUFD, if clinician had good reason not to include women After the first 5 months, exclusions included women with ritodrine given 2 hours before birth; anticoagulant treatment; any condition needing a particular management of 3rd stage (e.g. meconium-stained liquor, dural tap) |
|
| Interventions |
Intervention: active management of 3rd stage (N = 846):
Comparison: expectant management of 3rd stage (N = 849).
Data included in Comparison 1. |
|
| Outcomes | Pre-specified outcomes: PPH (and “more objective measures of blood loss”, presumably Hb); length 3rd stage; need for therapeutic oxytocics; manual removal placenta; ERPC; side effects of oxytocics (nausea, vomiting, headaches, hypertension); Apgar scores; PCV; SCBU; jaundice; breastfeeding. Views of a subsample of women | |
| Notes |
Actual management used in the active arm: 99% given prophylactic uterotonic before delivery of placenta; 99% cord clamped and cut before delivery of placenta; 99% CCT; 26% upright Actual management used in the expectant arm: 30% received uterotonic for treatment, and 20% prophylactically; cord left unclamped till pulsation ceased 48%; placenta delivered by maternal effort 60% and CCT 40%; 49% upright |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | No description of the randomisation given. However, we believe that unclear sequence generation was a consequence of reporting omission rather than methodological inadequacies and therefore does not give rise to bias |
| Allocation concealment (selection bias) | Low risk | “On admission to the labour ward… Correspondingly numbered, sealed opaque envelopes were placed in the woman’s notes.. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing data for some outcomes, e.g. 19% of Hb results missing in active arm and 18% in the physiological; 25% of antenatal and/or postnatal Hb results (used to calculate drop in mean Hb) missing in the active arm and 26% in the physiological Apparently no women were excluded after randomisation but 182 are described as having not entered in the trial due to the cord being cut early for fetal safety reasons. The allocation details, however, state that when the clinician was ready to prepare for delivery, the envelope was opened and “all women for whom an envelope was opened were deemed to have entered the trial and were followed up”. The envelope would have been opened before any neonatal need for attention became apparent |
| Selective reporting (reporting bias) | Unclear risk | Trial protocol not assessed. Reported outcomes not pre-specified: blood transfusions; number of units transfused; days’ stay; serious problems with 3rd stage;neonatal respiratory problems; birth-weight; number with “serious problems in 3rd stage” (not pre-specified), described as PPH, transfusion, manual removal of placenta, ERPC is reported. This is misleading as a number of those problems would co-exist |
| Other bias | High risk | Protocol was modified after 5 months (425 births), due to high blood loss in expectant management group, to allow women in the control arm who needed some active management to be switched to fully active management. However, data for the first 5 months were still included in analysis Trial was stopped early because of potential harm. Sample size was meant to be 3900 but stopped after 1695 30 women in the control group gave a late maternal refusal, whereas only 1 in the experimental group did so. The outcomes of these women are included in analysis It is questioned whether the midwives had sufficient training in physiological third stage before the trial started. Harding et al found that, of 49 midwives responding to a questionnaire, only one had practised physiological management as defined in the trial. Only 6 (13%) of the mid-wives said that they were very confident of physiological management before the trial and 22 (46%) afterwards (Harding 1989; Prendiville 1988 paper). This trial has been criticised for including all women (including high parity, all age groups, previous PPH, epidural, long labour, operative delivery) and not confining inclusion criteria to women who were low risk. Women at high risk of PPH will have a higher blood loss using expectant management; clinicians experiencing this may respond by anxiety in subsequent births, even oflow-risk women, which may result in higher intervention (mixed management) rates Only 47% (403/849) of women in physiological arm received the full physiological package (a problem with other studies also). But, in particular, 168/849 = 20% had prophylactic oxytocic, which is a large number for a “prophylactic” treatment as opposed to one in response to clinical need; In addition, 252 (30%) had a uterotonic as a treatment, so in total, 50% of the expectant management group received an oxyto-cic However, 99% (838/846) of women in active management group received allocated management |
| Methods | RCT with randomisation of individual women in balanced blocks, with allocation to 1 of 2 delivery postures within each arm | |
|---|---|---|
| Participants |
UK hospital setting. High-income country. Inclusion criteria: 1512 women at low risk of PPH giving birth at study hospital (including water births). Exclusion criteria: placenta praevia, previous PPH, APH after 20 weeks’ gestation, Hb <10 g/dL or MCV < 75 fL, non-cephalic presentation, multiple pregnancy, intrauterine death, epidural anaesthesia, parity > 5, uterine fibroid, oxytocin augmentation infusion, anticoagulation therapy, intended instrumental or OVB, duration of gestation < 32 weeks, (plus any other contraindication, in clinician’s view) |
|
| Interventions |
Intervention: active management of 3rd stage (N = 748). Two arms: active management - upright position (N = 374); Active management - supine position (N = 374):
Control: expectant management of 3rd stage (N = 764). Two arms: expectant management - upright position (N = 381); expectant management - supine position (N = 383):
Data included in Comparisons 1 & 2. |
|
| Outcomes | Pre-specified outcomes: PPH (> 500 mL) as assessed/estimated by the attending MW (used for power calculation); severe PPH 1000 mL), blood transfusion, iron tablets post-natally, Hb at 24-48 hours P/N, self-completed questionnaire on maternal fatigue and depression at 6 weeks P/N, nausea, vomiting, headache, hypertension, manual removal of placenta, ERPC, neonatal outcomes, views of mothers and staff | |
| Notes |
High-income setting Actual management used in the active arm: 699 (93.4%) had full active management; 95% given prophylactic uterotonic before delivery of placenta; 93% cord clamped before pulsation ceased; 46% CCT; 44% upright Actual management used in the expectant arm: 488 (63.9%) had full expectant management; 21% received oxytocic for treatment, and 2.5% prophylactically; cord left un-clamped till pulsation ceased 70%; placenta delivered by CCT 12%; 43% upright The setting is described as one where the midwives were “similarly confident” in active and expectant management. However, the questionnaire administered to 92 of the 153 midwives prior to the trial commencement showed that, whereas 84% felt “very confident” of active management, only 41% were “very confident” of expectant management Maternal mean Hb levels were reported with SEs and so SDs were calculated |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Variable sized balanced blocks “…randomisation envelopes were prepared in advance.." in an external academic unit -National Perinatal Epidemiology Unit, Oxford |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered, opaque, sealed envelopes stored on the ward. Entry to the trial occurred when an envelope was opened |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data available on 1507 out of 1512 at discharge (less than 0.5% attrition, approximately equal losses in both groups) At 6 weeks follow-up less than 5% attrition. |
| Selective reporting (reporting bias) | Unclear risk | Both significant and non-significant results presented but we have not been able to check the trial protocol. Most as above reported plus others. Only a few neonatal outcomes reported |
| Other bias | Low risk | Initial power calculation suggested a sample size of 2000. Interim analysis showed a higher PPH rate than expected, so sample size was revised to 1500 and the trial stopped earlier than expected once the recalculated sample size was reached - no suggestion of bias |
| Methods | RCT with randomisation of individual women. | |
|---|---|---|
| Participants |
UK hospital setting. High-income country. Inclusion criteria: women at “low risk of PPH” (defined only by the exclusion criteria for study) and at term (37-42 weeks). 193 women randomised, from an unknown population Exclusion criteria: grand multiparity; malpresentation, multiple pregnancy; previous CS or PPH; APH; pregnancy-induced hypertension and intrauterine death. Then after randomisation: women who had had augmentation, instrumental or OVB, 3rd degree tear and cervical laceration |
|
| Interventions |
Intervention: active management of 3rd stage (N = 103):
(Authors’ information by letter states:
Data included in Comparisons 1. and 2. |
|
| Outcomes |
Pre-specified outcomes: estimated blood loss; Hb in labour and 3rd postnatal day; length of 3rd stage; complications. Reported outcomes: as above plus therapeutic uterotonics, blood transfusion. |
|
| Notes | Drop in Hb is not calculated correctly. Maternal mean postnatal Hb reported as median and range. Active: 11.7 g/dL (10.7 to 12.6 g/dL) and expectant 11.7 g/dL (10.9 to 12.6 g/dL) Worrying problems with methodology and analysis. |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "…randomly allocated using standard randomisation tables…" |
| Allocation concealment (selection bias) | Low risk | Not described. Not clear when randomisation occurred “…the midwife responsible for the management of her patient was not aware of the proposed allocation until her patient was entered into the study” (authors’ information states: “randomised in the late 1st stage of labour when it was apparent that they were likely to delivery normally”) |
| Incomplete outcome data (attrition bias) All outcomes | High risk | It is not clear how many were randomised. P. 20 states “A total of 193 women completed the study AND had all results available for complete analysis”. This could mean that a larger number were included but that some of their results were missing, and they were therefore excluded. This could lead to significant bias. It is very unlikely that all participants received the allocated management, yet this is not presented. The study groups were also very different sizes (103 and 93), which sounds unlikely Women withdrawn after randomisation for operative delivery, 3rd degree tears and cervical lacerations. Numbers were not given; there is a significant risk of bias here It is not stated in the published paper whether or not “intention to treat” analysis was used, but the response to Diana El-bourne’s letter of April 1991 states that they did not analyse on ‘intention to treat’ as it would not answer the aims of this preliminary study |
| Selective reporting (reporting bias) | High risk | PPH rates not given. Mean blood loss equal in both groups, and very round numbers, which seems unlikely. |
| Other bias | Unclear risk | Variables age, birthweight and parity said to be equal between the groups but no details given. No power calculation done Not a null hypothesis. The study groups were very different sizes (103 and 90). |
APH: antepartum haemorrhage
BP: blood pressure
CCT: controlled cord traction
CS: caesarean section
ERPC: evacuation of retained products of conception
GA: general anaesthesia
Hb: haemoglobin
HCT: haematocrit (= packed cell volume)
IM: intramuscular
IUFD: intrauterine fetal death
IV: intravenous
MCV: mean corpuscular volume
MW: midwife
OVB: operative vaginal birth
PCV: packed cell volume (= haematocrit)
P/N: postnatal
PPH: postpartum haemorrhage
PP: postpartum
RCT: randomised controlled trial
SCBU: special care baby unit
SD: standard deviation
SE: standard error
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abdel-Aleem 2010 | This study looked at uterine massage compared with active management and active management with uterine massage, so it was not a comparison of active and expectant management within the definitions used in the review |
| Hoffman 2006 | Conference abstract available only, but further information on methodology obtained from authors. Concerns re number of women withdrawn, after randomisation, due to CS |
| Kashanian 2010 | 48% of participants excluded in both arms following randomisation |
| Magann 2006 | This study looked at different times of undertaking manual removal of placenta to try to reduce PPH, so it was not a comparison of active and expectant management within the definitions used in the review |
| Muller 1996 | French conference abstract only, no full publication identified. The translation provided no information on the number of women randomised to each group and so it was not possible to use these data. Previous review authors wrote for further information but had no response. We wrote and received a response from the coauthor, but no further details to add to the published information |
| Ramirez 2001 | Insufficient information on the numbers included in each of the three arms, and the method of management for the expectant arm |
| Vasegh 2005 | Insufficient information. |
CS: caesarean section
PPH: postpartum haemorrhage
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods |
| Participants |
| Interventions |
| Outcomes |
| Notes |
APH: antepartum haemorrhage
CCT: controlled cord traction
Hb: haemoglobin
IM: iIntramuscular
IU: international unit
IV: intravenous
PPH: postpartum haemorrhage
RCT: randomised controlled trial
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | Active management of the third stage of labour without controlled cord traction: a randomised non-inferiority controlled trial |
| Methods | Multi-centre RCT. |
| Participants | Women giving birth vaginally. |
| Interventions |
Intervention: active management of third stage of labour without CCT: 1)10 units oxytocin IM immediately after birth of baby; 2) delayed cord clamping and 3) placenta delivered by gravity or maternal effort. Comparison: active management of third stage of labour: 1) 10 units oxytocin IM immediately after birth of baby; 2) delayed cord clamping and 3) placenta delivered by controlled cord traction |
| Outcomes |
Primary: blood loss > 1000 mL. Secondary: blood transfusions, additional uterotonic; blood loss > 500 mL; postpartum maternal Hb; maternal death; manual removal of placenta; additional surgical procedures (hysterectomy, ligation of vessels); composite outcome of maternal death and serious morbidity; initiation of breastfeeding; side effects of nausea and abdominal pain |
| Starting date | November-December 2008. |
| Contact information | A Metin Gulmezoglu. Email: gulmezoglum@who.int |
| Notes | Eight centres located in Argentina, Egypt, India, Kenya, Philippines, South Africa, Thailand and Uganda |
CCT: controlled cord traction
Hb; haemoglobin
IM: intramuscular
IV: intravenous
RCT: randomised controlled trial
DATA AND ANALYSES
Comparison 1. Active versus expectant management of 3rd stage of labour (all women).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Severe primary postpartum haemorrhage (PPH) at time of birth (clinically estimated or measured blood loss ≥ 1000 mL) | 3 | 4636 | Risk Ratio (M-H, Random, 95% CI) | 0.34 [0.14, 0.87] |
| 1.1 High-income setting | 3 | 4636 | Risk Ratio (M-H, Random, 95% CI) | 0.34 [0.14, 0.87] |
| 1.2 Low-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Very severe primary PPH at time of birth (clinically estimated or measured blood loss ≥ 2500 mL) | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.1 High-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 Low-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Maternal mortality | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.1 High-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.2 Low-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Maternal Hb < 9 g/dL 24-72 hours postpartum | 2 | 1572 | Risk Ratio (M-H, Random, 95% CI) | 0.50 [0.30, 0.83] |
| 4.1 High-income setting | 2 | 1572 | Risk Ratio (M-H, Random, 95% CI) | 0.50 [0.30, 0.83] |
| 4.2 Low-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Admission to neonatal special/intensive care | 2 | 3207 | Risk Ratio (M-H, Random, 95% CI) | 0.81 [0.60, 1.11] |
| 5.1 High-income setting | 2 | 3207 | Risk Ratio (M-H, Random, 95% CI) | 0.81 [0.60, 1.11] |
| 5.2 Low-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Neonatal jaundice requiring phototherapy or exchange transfusion | 2 | 3142 | Risk Ratio (M-H, Random, 95% CI) | 0.96 [0.55, 1.68] |
| 6.1 High-income setting | 2 | 3142 | Risk Ratio (M-H, Random, 95% CI) | 0.96 [0.55, 1.68] |
| 6.2 Low-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Neonatal polycythaemia treated with dilutional exchange transfusion | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7.1 High-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7.2 Low-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Severe primary PPH after placental delivery and up to 24 hours (clinically estimated or measured blood loss ≥ 1000 mL) | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8.1 High-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8.2 Low-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Severe primary PPH at time of birth and up to 24 hours (clinically estimated or measured blood loss ≥ 1000 mL) | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9.1 High-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9.2 Low-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Primary blood loss ≥ 500 mL at time of birth (clinically estimated or measured) | 3 | 4636 | Risk Ratio (M-H, Random, 95% CI) | 0.34 [0.27, 0.44] |
| 10.1 High-income setting | 3 | 4636 | Risk Ratio (M-H, Random, 95% CI) | 0.34 [0.27, 0.44] |
| 10.2 Low-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Primary blood loss ≥ 500 mL after delivery of placenta and up to 24 hours (clinically estimated or measured) | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11.1 High-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11.2 Low-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 12 Primary blood loss ≥ 500 mL at time of birth and up to 24 hours (clinically estimated or measured) | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 12.1 High-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 12.2 Low-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 13 Mean maternal blood loss (mL) at time of birth (clinically estimated or measured) | 2 | 2941 | Mean Difference (IV, Random, 95% CI) | -78.80 [-95.96, -61. 64] |
| 13.1 High-income setting | 2 | 2941 | Mean Difference (IV, Random, 95% CI) | -78.80 [-95.96, -61. 64] |
| 13.2 Low-income setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14 Mean maternal blood loss (mL) after delivery of placenta and up to 24 hours (clinically estimated or measured) | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14.1 High-income setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14.2 Low-income setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15 Mean maternal blood loss (mL at time of birth and up to 24 hours (clinically estimated or measured) | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15.1 High-income setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15.2 Low-income setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16 Maternal blood transfusion | 4 | 4829 | Risk Ratio (M-H, Random, 95% CI) | 0.35 [0.22, 0.55] |
| 16.1 High-income setting | 4 | 4829 | Risk Ratio (M-H, Random, 95% CI) | 0.35 [0.22, 0.55] |
| 16.2 Low-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17 Clinical signs of severe blood loss | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17.1 High-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17.2 Low-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 18 Therapeutic uterotonics during third stage and/or within 24 hours | 4 | 4829 | Risk Ratio (M-H, Random, 95% CI) | 0.19 [0.15, 0.23] |
| 18.1 High-income setting | 4 | 4829 | Risk Ratio (M-H, Random, 95% CI) | 0.19 [0.15, 0.23] |
| 18.2 Low-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 19 Mean length of third stage | 1 | 1429 | Mean Difference (IV, Random, 95% CI) | -0.30 [-1.87, 1.27] |
| 19.1 High-income setting | 1 | 1429 | Mean Difference (IV, Random, 95% CI) | -0.30 [-1.87, 1.27] |
| 19.2 Low-income setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 20 Manual removal of placenta as defined by authors | 4 | 4829 | Risk Ratio (M-H, Random, 95% CI) | 1.78 [0.57, 5.56] |
| 20.1 High-income setting | 4 | 4829 | Risk Ratio (M-H, Random, 95% CI) | 1.78 [0.57, 5.56] |
| 20.2 Low-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 21 Postnatal diastolic blood pressure > 90 mmHg between birth of baby and discharge from the labour ward. | 3 | 4636 | Risk Ratio (M-H, Random, 95% CI) | 4.10 [1.63, 10.30] |
| 21.1 High-income setting | 3 | 4636 | Risk Ratio (M-H, Random, 95% CI) | 4.10 [1.63, 10.30] |
| 21.2 Low-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 22 Postnatal vomiting between birth of baby and discharge from the labour ward. | 3 | 4636 | Risk Ratio (M-H, Random, 95% CI) | 2.47 [1.36, 4.48] |
| 22.1 High-income setting | 3 | 4636 | Risk Ratio (M-H, Random, 95% CI) | 2.47 [1.36, 4.48] |
| 22.2 Low-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 23 Any analgesia between birth of the baby and discharge from labour ward | 1 | 1429 | Risk Ratio (M-H, Random, 95% CI) | 2.53 [1.34, 4.78] |
| 23.1 High-income setting | 1 | 1429 | Risk Ratio (M-H, Random, 95% CI) | 2.53 [1.34, 4.78] |
| 23.2 Low-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 24 Women’s assessment of pain during third stage as reported by authors | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 24.1 High-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 24.2 Low-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 25 Secondary blood loss/any vaginal bleeding needing treatment (after 24 hhours and up to 6 weeks) | 3 | 4636 | Risk Ratio (M-H, Random, 95% CI) | 1.10 [0.40, 2.99] |
| 25.1 High-income setting | 3 | 4636 | Risk Ratio (M-H, Random, 95% CI) | 1.10 [0.40, 2.99] |
| 25.2 Low-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 26 Amount of lochia either estimated or measured after 24 hours and up to discharge from hospital | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 26.1 High-income setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 26.2 Low-income setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 27 Surgical evacuation of retained products of conception | 3 | 4636 | Risk Ratio (M-H, Random, 95% CI) | 0.74 [0.32, 1.71] |
| 27.1 High-income setting | 3 | 4636 | Risk Ratio (M-H, Random, 95% CI) | 0.74 [0.32, 1.71] |
| 27.2 Low-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 28 Afterpains - abdominal pain associated with the contracting uterus in the postpartum period. | 1 | 1429 | Risk Ratio (M-H, Random, 95% CI) | 2.53 [1.34, 4.78] |
| 28.1 High-income setting | 1 | 1429 | Risk Ratio (M-H, Random, 95% CI) | 2.53 [1.34, 4.78] |
| 28.2 Low-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 29 Apgar score < 7 at 5 minutes | 1 | 1695 | Risk Ratio (M-H, Random, 95% CI) | 1.00 [0.38, 2.66] |
| 29.1 High-income setting | 1 | 1695 | Risk Ratio (M-H, Random, 95% CI) | 1.00 [0.38, 2.66] |
| 29.2 Low-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 30 Birthweight | 2 | 3207 | Mean Difference (IV, Random, 95% CI) | -76.90 [-108.51, -45.30] |
| 30.1 High-income setting | 2 | 3207 | Mean Difference (IV, Random, 95% CI) | -76.90 [-108.51, -45.30] |
| 30.2 Low-income setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 31 Neonatal encephalopathy assessed using Sarnat staging (Sarnat 1976; Table 1). | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 31.1 High-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 31.2 Low-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 32 Neonatal mortality | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 32.1 High-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 32.2 Low-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 33 Intraventricular haemorrhage - Papille grade III/IV - (for infants born before 34 weeks gestation only) | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 33.1 High-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 33.2 Low-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 34 Number of infants who received a red blood cell transfusion | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 34.1 High-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 34.2 Low-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 35 Infant Hb level at 24 to 72 hours | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 35.1 High-income setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 35.2 Low-income setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 36 Infant Hb level at 3-6 months | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 36.1 High-income setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 36.2 Low-income setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 37 Infant iron indices (ferritin) at 3 to 6 months. | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 37.1 High-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 37.2 Low-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 38 Exclusive breastfeeding at discharge from hospital | 1 | 1695 | Risk Ratio (M-H, Random, 95% CI) | 1.01 [0.96, 1.07] |
| 38.1 High-income setting | 1 | 1695 | Risk Ratio (M-H, Random, 95% CI) | 1.01 [0.96, 1.07] |
| 38.2 Low-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 39 Neurodevelopmental, cognitive or developmental outcomes assessed after age 18 months | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 39.1 High-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 39.2 Low-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 40 Return to hospital as in- or outpatient because of bleeding (not pre-specified) | 2 | 2941 | Risk Ratio (M-H, Random, 95% CI) | 2.21 [1.29, 3.79] |
| 40.1 High-income setting | 2 | 2941 | Risk Ratio (M-H, Random, 95% CI) | 2.21 [1.29, 3.79] |
| 40.2 Low-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 41 Postnatal maternal mean Hb (outcome not pre-specified) | 3 | 4062 | Mean Difference (IV, Random, 95% CI) | 0.52 [0.44, 0.60] |
| 41.1 High-income setting | 3 | 4062 | Mean Difference (IV, Random, 95% CI) | 0.52 [0.44, 0.60] |
| 41.2 Low-income setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 2. Active versus expectant management of 3rd stage of labour (women at low risk).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Severe primary PPH at time of birth (clinically estimated or measured blood loss ≥ 1000 mL) | 2 | 2941 | Risk Ratio (M-H, Random, 95% CI) | 0.31 [0.05, 2.17] |
| 1.1 High-income setting | 2 | 2941 | Risk Ratio (M-H, Random, 95% CI) | 0.31 [0.05, 2.17] |
| 1.2 Low-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Very severe primary PPH at time of birth (clinically estimated or measured blood loss ≥ 2500 mL) | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.1 High-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 Low-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Maternal mortality | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.1 High-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.2 Low-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Maternal Hb < 9 g/dL at 24-72 hr | 1 | 193 | Risk Ratio (M-H, Random, 95% CI) | 0.17 [0.02, 1.47] |
| 4.1 High-income setting | 1 | 193 | Risk Ratio (M-H, Random, 95% CI) | 0.17 [0.02, 1.47] |
| 4.2 Low-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Admission to neonatal special/intensive care | 1 | 1512 | Risk Ratio (M-H, Random, 95% CI) | 1.02 [0.55, 1.88] |
| 5.1 High-income setting | 1 | 1512 | Risk Ratio (M-H, Random, 95% CI) | 1.02 [0.55, 1.88] |
| 5.2 Low-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Neonatal jaundice requiring phototherapy or exchange transfusion | 1 | 1447 | Risk Ratio (M-H, Random, 95% CI) | 1.31 [0.78, 2.18] |
| 6.1 High-income setting | 1 | 1447 | Risk Ratio (M-H, Random, 95% CI) | 1.31 [0.78, 2.18] |
| 6.2 Low-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Neonatal polycythaemia treated with dilutional exchange transfusion | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7.1 High-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7.2 Low-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Severe primary PPH after placental delivery and up to 24 hours (clinically estimated or measured blood loss ≥ 1000 mL) | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8.1 High-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8.2 Low-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Severe primary PPH at time of birth and up to 24 hours (clinically estimated or measured blood loss ≥1000 mL) | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9.1 High-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9.2 Low-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Primary blood loss ≥ 500 mL at time of birth (clinically estimated or measured) | 2 | 2941 | Risk Ratio (M-H, Random, 95% CI) | 0.33 [0.20, 0.56] |
| 10.1 High-income setting | 2 | 2941 | Risk Ratio (M-H, Random, 95% CI) | 0.33 [0.20, 0.56] |
| 10.2 Low-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Primary blood loss ≥ 500 mL after delivery of placenta and up to 24 hours (clinically estimated or measured) | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11.1 High-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11.2 Low-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 12 Primary blood loss ≥ 500 mL at time of birth and up to 24 hours (clinically estimated or measured) | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 12.1 High-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 12.2 Low-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 13 Mean maternal blood loss (mL at the time of birth, clinically estimated or measured | 2 | 2941 | Mean Difference (IV, Random, 95% CI) | −78.80 [−95.96, −61 64] |
| 13.1 High-income setting | 2 | 2941 | Mean Difference (IV, Random, 95% CI) | −78.80 [−95.96, −61 64] |
| 13.2 Low-income setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14 Mean maternal blood loss (mL) after delivery of placenta and up to 24 hours (clinically estimated or measured) | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14.1 High-income setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14.2 Low-income setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15 Mean maternal blood loss (mL) at time of birth and up to 24 hours (clinically estimated or measured) | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15.1 High-income setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15.2 Low-income setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16 Maternal blood transfusion | 3 | 3134 | Risk Ratio (M-H, Random, 95% CI) | 0.30 [0.10, 0.88] |
| 16.1 High-income setting | 3 | 3134 | Risk Ratio (M-H, Random, 95% CI) | 0.30 [0.10, 0.88] |
| 16.2 Low-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17 Clinical signs of severe blood loss | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17.1 High-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17.2 Low-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 18 Therapeutic uterotonics during third stage and/or within 24 hours | 3 | 3134 | Risk Ratio (M-H, Random, 95% CI) | 0.15 [0.11, 0.21] |
| 18.1 High-income setting | 3 | 3134 | Risk Ratio (M-H, Random, 95% CI) | 0.15 [0.11, 0.21] |
| 18.2 Low-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 19 Mean length of third stage | 1 | 1429 | Mean Difference (IV, Random, 95% CI) | −0.30 [−1.87, 1.27] |
| 19.1 High-income setting | 1 | 1429 | Mean Difference (IV, Random, 95% CI) | −0.30 [−1.87, 1.27] |
| 19.2 Low-income setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 20 Manual removal of placenta as defined by authors | 3 | 3134 | Risk Ratio (M-H, Random, 95% CI) | 3.58 [0.42, 30.61] |
| 20.1 High-income setting | 3 | 3134 | Risk Ratio (M-H, Random, 95% CI) | 3.58 [0.42, 30.61] |
| 20.2 Low-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 21 Postnatal diastolic blood pressure > 90 mmHg between birth of baby and discharge from the labour ward. | 2 | 2941 | Risk Ratio (M-H, Random, 95% CI) | 7.00 [2.99, 16.43] |
| 21.1 High-income setting | 2 | 2941 | Risk Ratio (M-H, Random, 95% CI) | 7.00 [2.99, 16.43] |
| 21.2 Low-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 22 Postnatal vomiting between birth of baby and discharge from the labour ward. | 2 | 2941 | Risk Ratio (M-H, Random, 95% CI) | 5.63 [0.69, 46.08] |
| 22.1 High-income setting | 2 | 2941 | Risk Ratio (M-H, Random, 95% CI) | 5.63 [0.69, 46.08] |
| 22.2 Low-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 23 Any analgesia between birth of the baby and up to discharge from labour ward | 1 | 1429 | Risk Ratio (M-H, Random, 95% CI) | 2.53 [1.34, 4.78] |
| 23.1 High-income setting | 1 | 1429 | Risk Ratio (M-H, Random, 95% CI) | 2.53 [1.34, 4.78] |
| 23.2 Low-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 24 Women’s assessment of pain during third stage as reported by authors | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 24.1 High-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 24.2 Low-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 25 Secondary blood loss/any vaginal bleeding needing treatment (after 24 hhours and up to 6 weeks) | 2 | 2941 | Risk Ratio (M-H, Random, 95% CI) | 1.78 [0.69, 4.60] |
| 25.1 High-income setting | 2 | 2941 | Risk Ratio (M-H, Random, 95% CI) | 1.78 [0.69, 4.60] |
| 25.2 Low-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 26 Amount of lochia either estimated or measured after 24 hours and up to discharge from hospital | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 26.1 High-income setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 26.2 Low-income setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 27 Surgical evacuation of retained products of conception | 2 | 2941 | Risk Ratio (M-H, Random, 95% CI) | 0.69 [0.12, 3.98] |
| 27.1 High-income setting | 2 | 2941 | Risk Ratio (M-H, Random, 95% CI) | 0.69 [0.12, 3.98] |
| 27.2 Low-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 28 Afterpains - abdominal pain associated with the contracting uterus in the postpartum period. | 1 | 1429 | Risk Ratio (M-H, Random, 95% CI) | 2.53 [1.34, 4.78] |
| 28.1 High-income setting | 1 | 1429 | Risk Ratio (M-H, Random, 95% CI) | 2.53 [1.34, 4.78] |
| 28.2 Low-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 29 Apgar score < 7 at 5 minutes | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 29.1 High-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 29.2 Low-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 30 Birthweight | 1 | 1512 | Mean Difference (IV, Random, 95% CI) | −67.0 [−114.13, −19. 87] |
| 30.1 High-income setting | 1 | 1512 | Mean Difference (IV, Random, 95% CI) | −67.0 [−114.13, −19. 87] |
| 30.2 Low-income setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 31 Neonatal encephalopathy assessed using Sarnat staging (Sarnat 1976; Table 1) | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 31.1 High-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 31.2 Low-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 32 Neonatal mortality | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 32.1 High-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 32.2 Low-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 33 Intraventricular haemorrhage - Papille grade III/IV - (for infants born before 34 weeks gestation only) | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 33.1 High-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 33.2 Low-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 34 Number of infants exposed to one or more red blood cell transfusions | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 34.1 High-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 34.2 Low-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 35 Infant Hb level at 24 to 72 hours | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 35.1 High-income setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 35.2 Low-income setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 36 Infant Hb level at 3-6 months | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 36.1 High-income setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 36.2 Low-income setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 37 Infant iron indices (ferritin) at 3 to 6 months. | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 37.1 High-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 37.2 Low-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 38 Exclusive breastfeeding at discharge from hospital | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 38.1 High-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 38.2 Low-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 39 Neurodevelopmental, cognitive or developmental outcomes assessed after age 18 months | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 39.1 High-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 39.2 Low-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 40 Return to hospital as in- or outpatient because of bleeding (not pre-specified) | 2 | 2941 | Risk Ratio (M-H, Random, 95% CI) | 2.21 [1.29, 3.79] |
| 40.1 High-income setting | 2 | 2941 | Risk Ratio (M-H, Random, 95% CI) | 2.21 [1.29, 3.79] |
| 40.2 Low-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 41 Postnatal maternal mean Hb (outcome not pre-specified) | 2 | 2683 | Mean Difference (IV, Random, 95% CI) | 0.5 [0.41, 0.59] |
| 41.1 High-income setting | 2 | 2683 | Mean Difference (IV, Random, 95% CI) | 0.5 [0.41, 0.59] |
| 41.2 Low-income setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 3. Active versus mixed management of 3rd stage (early uterotonic, delayed cord clamping, CCT).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Severe primary PPH at time of birth (clinically estimated or measured blood loss ≥ 1000 mL) | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.1 High-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 Low-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Very severe primary PPH at time of birth (clinically estimated or measured blood loss ≥ 2500 mL) | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.1 High-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 Low-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Maternal mortality | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.1 High-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.2 Low-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Maternal Hb < 9 g/dL at 24-72 hr | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.1 High-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 Low-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Admission to neonatal special/ intensive care | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.1 High-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.2 Low-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Neonatal jaundice requiring phototherapy or exchange transfusion | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6.1 High-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6.2 Low-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Neonatal polycythaemia treated with dilutional exchange transfusion | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7.1 High-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7.2 Low-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 4. Active versus mixed management of 3rd stage (delayed uterotonic, delayed cord clamping, CCT).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Severe primary PPH at time of birth (clinically estimated or measured blood loss ≥ 1000 mL) | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.1 High-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 Low-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Very severe primary PPH at time of birth (clinically estimated or measured blood loss ≥ 2500 mL) | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.1 High-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 Low-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Maternal mortality | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.1 High-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.2 Low-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Maternal Hb < 9 g/dL at 24-72 hr | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.1 High-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 Low-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Admission to neonatal special/ intensive care | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.1 High-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.2 Low-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Neonatal jaundice requiring phototherapy or exchange transfusion | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6.1 High-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6.2 Low-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Neonatal polycythaemia treated with dilutional exchange transfusion | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7.1 High-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7.2 Low-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 5. Active versus mixed management of 3rd stage (delayed uterotonic, delayed cord clamping, no CCT).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Severe primary PPH at time of birth (clinically estimated or measured blood loss ≥ 1000 mL) | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.1 High-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 Low-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Very severe primary PPH at time of birth (clinically estimated or measured blood loss ≥ 2500 mL) | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.1 High-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 Low-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Maternal mortality | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.1 High-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.2 Low-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Maternal Hb < 9 g/dL at 24-72 hr | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.1 High-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 Low-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Admission to neonatal special/ intensive care | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.1 High-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.2 Low-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Neonatal jaundice requiring phototherapy or exchange transfusion | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6.1 High-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6.2 Low-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Neonatal polycythaemia treated with dilutional exchange transfusion | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7.1 High-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7.2 Low-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 6. Active management with prophylactic uterotonic given before birth versus active management with uterotonic given after birth.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Severe primary PPH at time of birth (clinically estimated or measured blood loss ≥ 1000 mL) | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.1 High-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 Low-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Very severe primary PPH at time of birth (clinically estimated or measured blood loss ≥ 2500 mL) | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.1 High-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 Low-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Maternal mortality | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.1 High-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.2 Low-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Maternal Hb < 9 g/dL at 24-72 hr | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.1 High-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 Low-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Admission to neonatal special/ intensive care | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.1 High-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.2 Low-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Neonatal jaundice requiring phototherapy or exchange transfusion | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6.1 High-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6.2 Low-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Neonatal polycythaemia treated with dilutional exchange transfusion | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7.1 High-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7.2 Low-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 7. Mixed management (early uterotonic, delayed cord clamping, CCT) versus expectant management.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Severe primary PPH at time of birth (clinically estimated or measured blood loss ≥ 1000 mL) | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.1 High-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 Low-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Very severe primary PPH at time of birth (clinically estimated or measured blood loss ≥ 2500 mL) | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.1 High-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 Low-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Maternal mortality | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.1 High-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.2 Low-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Maternal Hb < 9 g/dL at 24-72 hr | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.1 High-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 Low-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Admission to neonatal special/ intensive care | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.1 High-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.2 Low-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Neonatal jaundice requiring phototherapy or exchange transfusion | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6.1 High-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6.2 Low-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Neonatal polycythaemia treated with dilutional exchange transfusion | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7.1 High-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7.2 Low-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 8. Mixed management (delayed uterotonic, delayed cord clamping, CCT) versus expectant management.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Severe primary PPH at time of birth (clinically estimated or measured blood loss ≥ 1000 mL) | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.1 High-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 Low-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Very severe primary PPH at time of birth (clinically estimated or measured blood loss ≥ 2500 mL) | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.1 High-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 Low-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Maternal mortality | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.1 High-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.2 Low-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Maternal Hb < 9 g/dL at 24-72 hr | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.1 High-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 Low-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Admission to neonatal special/ intensive care | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.1 High-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.2 Low-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Neonatal jaundice requiring phototherapy or exchange transfusion | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6.1 High-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6.2 Low-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Neonatal polycythaemia treated with dilutional exchange transfusion | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7.1 High-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7.2 Low-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 9. Mixed management (delayed uterotonic, delayed cord clamping, no CCT) versus expectant management.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Severe primary PPH at time of birth (clinically estimated or measured blood loss ≥ 1000 mL) | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.1 High-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 Low-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Very severe primary PPH at time of birth (clinically estimated or measured blood loss ≥ 2500 mL) | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.1 High-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 Low-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Maternal mortality | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.1 High-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.2 Low-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Maternal Hb < 9 g/dL at 24-72 hr | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.1 High-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 Low-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Admission to neonatal special/ intensive care | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.1 High-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.2 Low-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Neonatal jaundice requiring phototherapy or exchange transfusion | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6.1 High-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6.2 Low-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Neonatal polycythaemia treated with dilutional exchange transfusion | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7.1 High-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7.2 Low-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 10. Active versus mixed management (uterotonic after placental delivery, immediate cord clamping and no CCT).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Severe primary PPH at time of birth (clinically estimated or measured blood loss ≥ 1000 mL) | 1 | 1648 | Risk Ratio (M-H, Random, 95% CI) | 0.23 [0.09, 0.55] |
| 1.1 High-income setting | 1 | 1648 | Risk Ratio (M-H, Random, 95% CI) | 0.23 [0.09, 0.55] |
| 1.2 Low-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Very severe primary PPH at time of birth (clinically estimated or measured blood loss ≥ 2500 mL) | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.1 High-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 Low-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Maternal mortality | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.1 High-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.2 Low-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Maternal Hb < 9 g/dL at 24-72 hr | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.1 High-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 Low-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Admission to neonatal special/ intensive care | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.1 High-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.2 Low-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Neonatal jaundice requiring phototherapy or exchange transfusion | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6.1 High-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6.2 Low-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Neonatal polycythaemia treated with dilutional exchange transfusion | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7.1 High-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7.2 Low-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Severe primary PPH after placental delivery and up to 24 hours (clinically estimated or measured blood loss ≥ 1000 mL) | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8.1 High-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8.2 Low-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Severe primary PPH at time of birth and up to 24 hours (clinically estimated or measured blood loss ≥1000 mL) | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9.1 High-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9.2 Low-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Primary blood loss ≥ 500 mL at time of birth (clinically estimated or measured) | 1 | 1648 | Risk Ratio (M-H, Random, 95% CI) | 0.53 [0.38, 0.74] |
| 10.1 High-income setting | 1 | 1648 | Risk Ratio (M-H, Random, 95% CI) | 0.53 [0.38, 0.74] |
| 10.2 Low-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Primary blood loss ≥ 500 mL after delivery of placenta and up to 24 hours (clinically estimated or measured) | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11.1 High-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11.2 Low-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 12 Primary blood loss ≥ 500 mL at time of birth and up to 24 hours (clinically estimated or measured) | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 12.1 High-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 12.2 Low-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 13 Mean maternal blood loss (mL) at time of birth (clinically estimated or measured) | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 13.1 High-income setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 13.2 Low-income setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14 Mean maternal blood loss (mL) after delivery of placenta and up to 24 hours (clinically estimated or measured) | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14.1 High-income setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14.2 Low-income setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15 Mean maternal blood loss (mL) at time of birth and up to 24 hours (clinically estimated or measured) | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15.1 High-income setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15.2 Low-income setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16 Maternal blood transfusion | 1 | 1648 | Risk Ratio (M-H, Random, 95% CI) | 0.25 [0.03, 2.22] |
| 16.1 High-income setting | 1 | 1648 | Risk Ratio (M-H, Random, 95% CI) | 0.25 [0.03, 2.22] |
| 16.2 Low-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17 Clinical signs of severe blood loss | 1 | 1648 | Risk Ratio (M-H, Random, 95% CI) | 0.25 [0.05, 1.17] |
| 17.1 High-income setting | 1 | 1648 | Risk Ratio (M-H, Random, 95% CI) | 0.25 [0.05, 1.17] |
| 17.2 Low-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 18 Therapeutic uterotonics during third stage and/or within 24 hours | 1 | 1648 | Risk Ratio (M-H, Random, 95% CI) | 0.45 [0.26, 0.77] |
| 18.1 High-income setting | 1 | 1648 | Risk Ratio (M-H, Random, 95% CI) | 0.45 [0.26, 0.77] |
| 18.2 Low-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 19 Mean length of third stage | 1 | 1648 | Mean Difference (IV, Random, 95% CI) | -10.0 [-10.24, -9.76] |
| 19.1 High-income setting | 1 | 1648 | Mean Difference (IV, Random, 95% CI) | -10.0 [-10.24, -9.76] |
| 19.2 Low-income setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 20 Manual removal of placenta as defined by authors | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 20.1 High-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 20.2 Low-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 21 Postnatal diastolic blood pressure > 90 mmHg between birth of baby and discharge from the labour ward | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 21.1 High-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 21.2 Low-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 22 Postnatal vomiting between birth of baby and discharge from the labour ward | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 22.1 High-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 22.2 Low-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 23 Any analgesia between birth of the baby and discharge from labour ward | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 23.1 High-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 23.2 Low-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 24 Women’s assessment of pain during third stage as reported by authors | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 24.1 High-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 24.2 Low-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 25 Secondary blood loss/any vaginal bleeding needing treatment (after 24 hours and up to 6 weeks) | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 25.1 High-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 25.2 Low-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 26 Amount of lochia either estimated or measured after 24 hours and up to discharge from hospital | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 26.1 High-income setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 26.2 Low-income setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 27 Surgical evacuation of retained products of conception | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 27.1 High-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 27.2 Low-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 28 Afterpains - abdominal pain associated with the contracting uterus in the postpartum period | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 28.1 High-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 28.2 Low-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 29 Apgar score < 7 at 5 minutes | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 29.1 High-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 29.2 Low-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 30 Birthweight | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 30.1 High-income setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 30.2 Low-income setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 31 Neonatal encephalopathy assessed using Sarnat staging (Sarnat 1976; Table 1) | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 31.1 High-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 31.2 Low-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 32 Neonatal mortality | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 32.1 High-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 32.2 Low-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 33 Intraventricular haemorrhage - Papille grade III/IV - (for infants born before 34 weeks gestation only) | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 33.1 High-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 33.2 Low-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 34 Number of infants who received a red blood cell transfusion | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 34.1 High-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 34.2 Low-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 35 Infant Hb level at 24 to 72 hours | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 35.1 High-income setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 35.2 Low-income setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 36 Infant Hb level at 3-6 months | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 36.1 High-income setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 36.2 Low-income setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 37 Infant iron indices (ferritin) at 3 to 6 months. | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 37.1 High-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 37.2 Low-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 38 Exclusive breastfeeding at discharge from hospital | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 38.1 High-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 38.2 Low-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 39 Neurodevelopmental, cognitive or developmental outcomes assessed after age 18 months | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 39.1 High-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 39.2 Low-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 40 Return to hospital as in- or outpatient because of bleeding (not pre-specified) | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 40.1 High-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 40.2 Low-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 41 Postnatal maternal mean Hb (outcome not pre-specified) | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 41.1 High-income setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 41.2 Low-income setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 11. Active versus mixed management (no routine uterotonic, early cord clamping, no CCT).
| Outcome or subgroup title | No. of No. of studies participants | Statistical method | Effect size | |
|---|---|---|---|---|
| 1 Severe primary PPH at time of birth (clinically estimated or measured blood loss ≥ 1000 mL) | 1 | 1621 | Risk Ratio (M-H, Random, 95% CI) | 0.78 [0.48, 1.24] |
| 1.1 High-income setting | 1 | 1621 | Risk Ratio (M-H, Random, 95% CI) | 0.78 [0.48, 1.24] |
| 1.2 Low-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Very severe primary PPH at time of birth (clinically estimated or measured blood loss ≥ 2500 mL) | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.1 High-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 Low-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Maternal mortality | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.1 High-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.2 Low-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Maternal Hb < 9 g/dL at 24-72 hr | 1 | 1631 | Risk Ratio (M-H, Random, 95% CI) | 1.23 [0.75, 2.01] |
| 4.1 High-income setting | 1 | 1631 | Risk Ratio (M-H, Random, 95% CI) | 1.23 [0.75, 2.01] |
| 4.2 Low-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Admission to neonatal special/ intensive care | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.1 High-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.2 Low-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Neonatal jaundice requiring phototherapy or exchange transfusion | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6.1 High-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6.2 Low-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Neonatal polycythaemia treated with dilutional exchange transfusion | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7.1 High-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7.2 Low-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Severe primary PPH after placental delivery and up to 24 hours (clinically estimated or measured blood loss ≥ 1000 mL) | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8.1 High-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8.2 Low-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Severe primary PPH at time of birth and up to 24 hours (clinically estimated or measured blood loss ≥1000 mL) | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9.1 High-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9.2 Low-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Primary blood loss ≥ 500 mL at time of birth (clinically estimated or measured) | 1 | 1621 | Risk Ratio (M-H, Random, 95% CI) | 0.51 [0.40, 0.66] |
| 10.1 High-income setting | 1 | 1621 | Risk Ratio (M-H, Random, 95% CI) | 0.51 [0.40, 0.66] |
| 10.2 Low-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Primary blood loss ≥ 500 mL after delivery of placenta and up to 24 hours (clinically estimated or measured) | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11.1 High-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11.2 Low-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 12 Primary blood loss ≥ 500 mL at time of birth and up to 24 hours (clinically estimated or measured) | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 12.1 High-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 12.2 Low-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 13 Mean maternal blood loss (mL) at time of birth (clinically estimated or measured) | 1 | 1621 | Mean Difference (IV, Random, 95% CI) | −94.0 [−126.57, −61 43] |
| 13.1 High-income setting | 1 | 1621 | Mean Difference (IV, Random, 95% CI) | −94.0 [−126.57, −61 43] |
| 13.2 Low-income setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14 Mean maternal blood loss (mL) after delivery of placenta and up to 24 hours (clinically estimated or measured) | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14.1 High-income setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14.2 Low-income setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15 Mean maternal blood loss (mL) at time of birth and up to 24 hours (clinically estimated or measured) | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15.1 High-income setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15.2 Low-income setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16 Maternal blood transfusion | 1 | 1631 | Risk Ratio (M-H, Random, 95% CI) | 0.79 [0.43, 1.46] |
| 16.1 High-income setting | 1 | 1631 | Risk Ratio (M-H, Random, 95% CI) | 0.79 [0.43, 1.46] |
| 16.2 Low-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17 Clinical signs of severe blood loss | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17.1 High-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17.2 Low-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 18 Therapeutic uterotonics during third stage and/or within 24 hours | 1 | 1631 | Risk Ratio (M-H, Random, 95% CI) | 0.39 [0.33, 0.48] |
| 18.1 High-income setting | 1 | 1631 | Risk Ratio (M-H, Random, 95% CI) | 0.39 [0.33, 0.48] |
| 18.2 Low-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 19 Mean length of third stage | 1 | 1631 | Mean Difference (IV, Random, 95% CI) | −1.60 [−3.08, −0.12] |
| 19.1 High-income setting | 1 | 1631 | Mean Difference (IV, Random, 95% CI) | −1.60 [−3.08, −0.12] |
| 19.2 Low-income setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 20 Manual removal of placenta as defined by authors | 1 | 1631 | Risk Ratio (M-H, Random, 95% CI) | 1.25 [0.71, 2.21] |
| 20.1 High-income setting | 1 | 1631 | Risk Ratio (M-H, Random, 95% CI) | 1.25 [0.71, 2.21] |
| 20.2 Low-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 21 Postnatal diastolic blood pressure > 90 mmHg between birth of baby and discharge from the labour ward | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 21.1 High-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 21.2 Low-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 22 Postnatal vomiting between birth of baby and discharge from the labour ward | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 22.1 High-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 22.2 Low-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 23 Any analgesia between birth of the baby and discharge from labour ward | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 23.1 High-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 23.2 Low-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 24 Women’s assessment of pain during third stage as reported by authors | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 24.1 High-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 24.2 Low-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 25 Secondary blood loss/any vaginal bleeding needing treatment (after 24 hhours and up to 6 weeks) | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 25.1 High-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 25.2 Low-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 26 Amount of lochia either estimated or measured after 24 hours and up to discharge from hospital | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 26.1 High-income setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 26.2 Low-income setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 27 Surgical evacuation of retained products of conception | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 27.1 High-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 27.2 Low-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 28 Afterpains - abdominal pain associated with the contracting uterus in the postpartum period | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 28.1 High-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 28.2 Low-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 29 Apgar score < 7 at 5 minutes | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 29.1 High-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 29.2 Low-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 30 Birthweight | 1 | 1631 | Mean Difference (IV, Random, 95% CI) | 15.0 [-28.88, 58.88] |
| 30.1 High-income setting | 1 | 1631 | Mean Difference (IV, Random, 95% CI) | 15.0 [−28.88, 58.88] |
| 30.2 Low-income setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 31 Neonatal encephalopathy assessed using Sarnat staging (Sarnat 1976; Table 1) | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 31.1 High-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 31.2 Low-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 32 Neonatal mortality | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 32.1 High-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 32.2 Low-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 33 Intraventricular haemorrhage - Papille grade III/IV - (for infants born before 34 weeks gestation only) | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 33.1 High-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 33.2 Low-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 34 Number of infants who received a red blood cell transfusion | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 34.1 High-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 34.2 Low-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 35 Infant Hb level at 24 to 72 hours | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 35.1 High-income setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 35.2 Low-income setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 36 Infant Hb level at 3-6 months | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 36.1 High-income setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 36.2 Low-income setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 37 Infant iron indices (ferritin) at 3 to 6 months. | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 37.1 High-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 37.2 Low-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 38 Exclusive breastfeeding at discharge from hospital | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 38.1 High-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 38.2 Low-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 39 Neurodevelopmental, cognitive or developmental outcomes assessed after age 18 months | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 39.1 High-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 39.2 Low-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 40 Return to hospital as in- or outpatient because of bleeding (not pre-specified) | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 41 Postnatal maternal mean Hb (outcome not pre-specified) | 1 | 1631 | Mean Difference (IV, Random, 95% CI) | 0.28 [0.14, 0.42] |
| 41.1 High-income setting | 1 | 1631 | Mean Difference (IV, Random, 95% CI) | 0.28 [0.14, 0.42] |
| 41.2 Low-income setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 42 Severe primary PPH after placental delivery and up to 2 hours (clinically estimated or measured blood loss ≥ 1000 mL) - not pre-specified | 1 | 1621 | Risk Ratio (M-H, Random, 95% CI) | 0.54 [0.39, 0.74] |
| 42.1 High-income setting | 1 | 1621 | Risk Ratio (M-H, Random, 95% CI) | 0.54 [0.39, 0.74] |
| 42.2 Low-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 43 Severe primary PPH at time of birth and up to 2 hours (clinically estimated or measured blood loss ≥1000 mL) - not pre-specified | 1 | 1621 | Risk Ratio (M-H, Random, 95% CI) | 0.60 [0.47, 0.78] |
| 43.1 High-income setting | 1 | 1621 | Risk Ratio (M-H, Random, 95% CI) | 0.60 [0.47, 0.78] |
| 43.2 Low-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 44 Mean blood loss (mL) (clinically estimated or measured at birth and up to 2 hours (not pre-specified) | 1 | 1621 | Mean Difference (IV, Random, 95% CI) | −49.0 [−75.52, −22. 48] |
| 44.1 High-income setting | 1 | 1621 | Mean Difference (IV, Random, 95% CI) | −49.0 [−75.52, −22. 48] |
| 44.2 Low-income setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 12. Active versus mixed management (no routine uterotonic, early cord clamping, CCT).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Severe primary PPH at time of birth (clinically estimated or measured blood loss ≥ 1000 mL) | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.1 High-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 Low-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Very severe primary PPH at time of birth (clinically estimated or measured blood loss ≥ 2500 mL) | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.1 High-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 Low-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Maternal mortality | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.1 High-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.2 Low-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Maternal Hb < 9 g/dL at 24-72 hr | 1 | 130 | Risk Ratio (M-H, Random, 95% CI) | 0.8 [0.34, 1.90] |
| 4.1 High-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 Low-income setting | 1 | 130 | Risk Ratio (M-H, Random, 95% CI) | 0.8 [0.34, 1.90] |
| 5 Admission to neonatal special/ intensive care | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.1 High-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.2 Low-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Neonatal jaundice requiring phototherapy or exchange transfusion | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6.1 High-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6.2 Low-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Neonatal polycythaemia treated with dilutional exchange transfusion | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7.1 High-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7.2 Low-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Severe primary PPH after placental delivery and up to 24 hours (clinically estimated or measured blood loss ≥ 1000 mL) | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8.1 High-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8.2 Low-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Severe primary PPH at time of birth and up to 24 hours (clinically estimated or measured blood loss ≥1000 mL) | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9.1 High-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9.2 Low-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Primary blood loss ≥ 500 mL at time of birth (clinically estimated or measured) | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.1 High-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.2 Low-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Primary blood loss ≥ 500 mL after delivery of placenta and up to 24 hours (clinically estimated or measured) | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11.1 High-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11.2 Low-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 12 Primary blood loss ≥ 500 mL at time of birth and up to 24 hours (clinically estimated or measured) | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 12.1 High-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 12.2 Low-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 13 Mean maternal blood loss (mL) at time of birth (clinically estimated or measured) | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 13.1 High-income setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 13.2 Low-income setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14 Mean maternal blood loss (mL) after delivery of placenta and up to 24 hours (clinically estimated or measured) | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14.1 High-income setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14.2 Low-income setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15 Mean maternal blood loss (mL) at time of birth and up to 24 hours (clinically estimated or measured) | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15.1 High-income setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15.2 Low-income setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16 Maternal blood transfusion | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16.1 High-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16.2 Low-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17 Clinical signs of severe blood loss | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17.1 High-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17.2 Low-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 18 Therapeutic uterotonics during third stage and/or within 24 hours | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 18.1 High-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 18.2 Low-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 19 Mean length of third stage | 1 | 130 | Mean Difference (IV, Random, 95% CI) | -8.12 [-9.72, -6.52] |
| 19.1 High-income setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 19.2 Low-income setting | 1 | 130 | Mean Difference (IV, Random, 95% CI) | -8.12 [-9.72, -6.52] |
| 20 Manual removal of placenta as defined by authors | 1 | 130 | Risk Ratio (M-H, Random, 95% CI) | 1.0 [0.06, 15.65] |
| 20.1 High-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 20.2 Low-income setting | 1 | 130 | Risk Ratio (M-H, Random, 95% CI) | 1.0 [0.06, 15.65] |
| 21 Postnatal diastolic blood pressure > 90 mmHg between birth of baby and discharge from the labour ward | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 21.1 High-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 21.2 Low-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 22 Postnatal vomiting between birth of baby and discharge from the labour ward | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 22.1 High-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 22.2 Low-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 23 Any analgesia between birth of the baby and discharge from labour ward | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 23.1 High-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 23.2 Low-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 24 Women’s assessment of pain during third stage as reported by authors | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 24.1 High-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 24.2 Low-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 25 Secondary blood loss/any vaginal bleeding needing treatment (after 24 hours and up to 6 weeks) | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 25.1 High-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 25.2 Low-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 26 Amount of lochia either estimated or measured after 24 hours and up to discharge from hospital | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 26.1 High-income setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 26.2 Low-income setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 27 Surgical evacuation of retained products of conception | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 27.1 High-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 27.2 Low-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 28 Afterpains - abdominal pain associated with the contracting uterus in the postpartum period | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 28.1 High-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 28.2 Low-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 29 Apgar score < 7 at 5 minutes | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 29.1 High-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 29.2 Low-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 30 Birthweight | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 30.1 High-income setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 30.2 Low-income setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 31 Neonatal encephalopathy assessed using Sarnat staging (Sarnat 1976; Table 1) | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 31.1 High-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 31.2 Low-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 32 Neonatal mortality | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 32.1 High-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 32.2 Low-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 33 Intraventricular haemorrhage - Papille grade III/IV - (for infants born before 34 weeks gestation only) | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 33.1 High-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 33.2 Low-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 34 Number of infants who received a red blood cell transfusion | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 34.1 High-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 34.2 Low-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 35 Infant Hb level at 24 to 72 hours | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 35.1 High-income setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 35.2 Low-income setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 36 Infant Hb level at 3-6 months | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 36.1 High-income setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 36.2 Low-income setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 37 Infant iron indices (ferritin) at 3 to 6 months. | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 37.1 High-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 37.2 Low-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 38 Exclusive breastfeeding at discharge from hospital | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 38.1 High-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 38.2 Low-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 39 Neurodevelopmental, cognitive or developmental outcomes assessed after age 18 months | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 39.1 High-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 39.2 Low-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 40 Return to hospital as in- or outpatient because of bleeding (not pre-specified) | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 40.1 High-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 40.2 Low-income setting | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 41 Postnatal maternal mean Hb (outcome not pre-specified) | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 41.1 High-income setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 41.2 Low-income setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
Analysis 1.1. Comparison 1 Active versus expectant management of 3rd stage of labour (all women), Outcome 1 Severe primary postpartum haemorrhage (PPH) at time of birth (clinically estimated or measured blood loss ≥ 1000 mL).
Review: Active versus expectant management for women in the third stage of labour
Comparison: 1 Active versus expectant management of 3rd stage of labour (all women)
Outcome: 1 Severe primary postpartum haemorrhage (PPH) at time of birth (clinically estimated or measured blood loss ≥ 1000 mL)
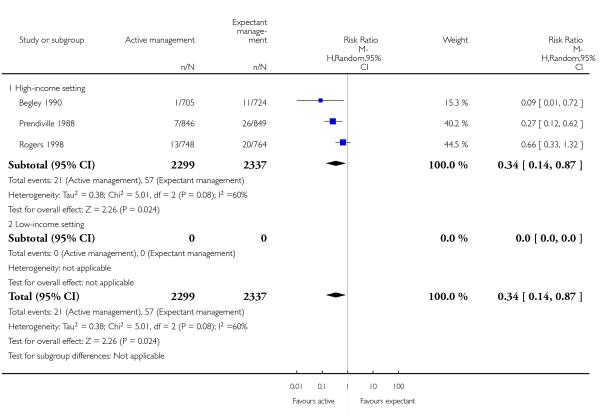 |
Analysis 1.4. Comparison 1 Active versus expectant management of 3rd stage of labour (all women), Outcome 4 Maternal Hb < 9 g/dL 24-72 hours postpartum.
Review: Active versus expectant management for women in the third stage of labour
Comparison: 1 Active versus expectant management of 3rd stage of labour (all women)
Outcome: 4 Maternal Hb < 9 g/dL 24-72 hours postpartum
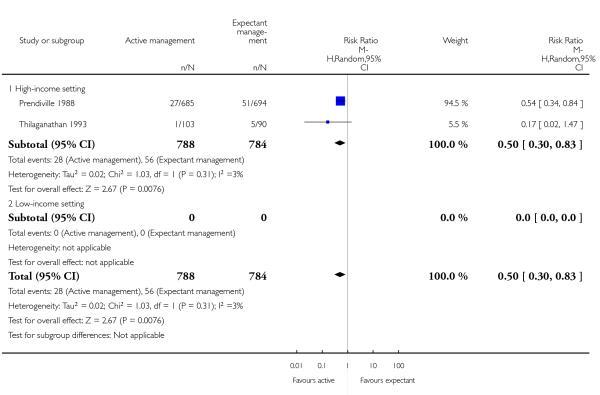 |
Analysis 1.5. Comparison 1 Active versus expectant management of 3rd stage of labour (all women), Outcome 5 Admission to neonatal special/intensive care.
Review: Active versus expectant management for women in the third stage of labour
Comparison: 1 Active versus expectant management of 3rd stage of labour (all women)
Outcome: 5 Admission to neonatal special/intensive care
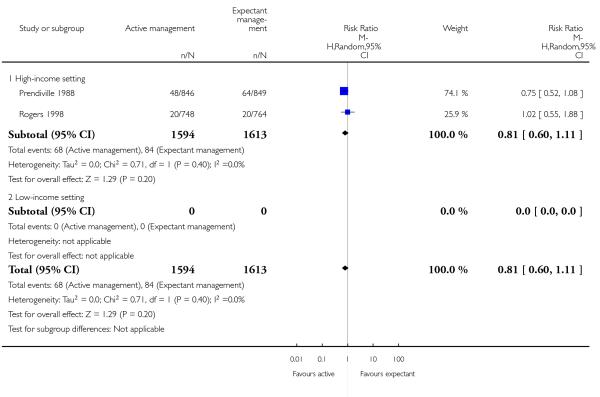 |
Analysis 1.6. Comparison 1 Active versus expectant management of 3rd stage of labour (all women), Outcome 6 Neonatal jaundice requiring phototherapy or exchange transfusion.
Review: Active versus expectant management for women in the third stage of labour
Comparison: 1 Active versus expectant management of 3rd stage of labour (all women)
Outcome: 6 Neonatal jaundice requiring phototherapy or exchange transfusion
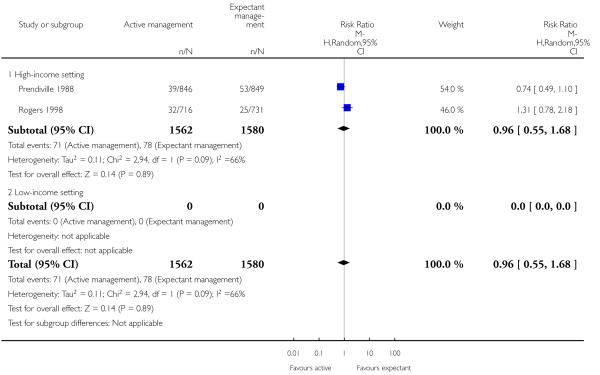 |
Analysis 1.10. Comparison 1 Active versus expectant management of 3rd stage of labour (all women), Outcome 10 Primary blood loss ≥ 500 mL at time of birth (clinically estimated or measured).
Review: Active versus expectant management for women in the third stage of labour
Comparison: 1 Active versus expectant management of 3rd stage of labour (all women)
Outcome: 10 Primary blood loss ≥ 500 mL at time of birth (clinically estimated or measured)
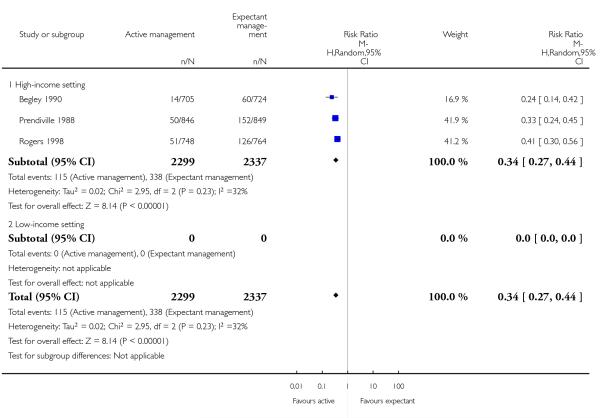 |
Analysis 1.13. Comparison 1 Active versus expectant management of 3rd stage of labour (all women), Outcome 13 Mean maternal blood loss (mL) at time of birth (clinically estimated or measured).
Review: Active versus expectant management for women in the third stage of labour
Comparison: 1 Active versus expectant management of 3rd stage of labour (all women)
Outcome: 13 Mean maternal blood loss (mL) at time of birth (clinically estimated or measured)
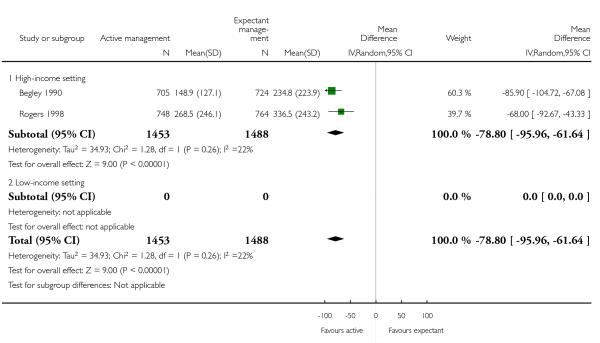 |
Analysis 1.16. Comparison 1 Active versus expectant management of 3rd stage of labour (all women), Outcome 16 Maternal blood transfusion.
Review: Active versus expectant management for women in the third stage of labour
Comparison: 1 Active versus expectant management of 3rd stage of labour (all women)
Outcome: 16 Maternal blood transfusion
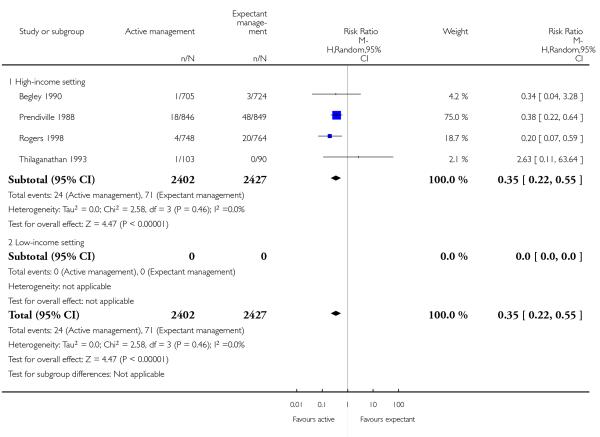 |
Analysis 1.18. Comparison 1 Active versus expectant management of 3rd stage of labour (all women), Outcome 18 Therapeutic uterotonics during third stage and/or within 24 hours.
Review: Active versus expectant management for women in the third stage of labour
Comparison: 1 Active versus expectant management of 3rd stage of labour (all women)
Outcome: 18 Therapeutic uterotonics during third stage and/or within 24 hours
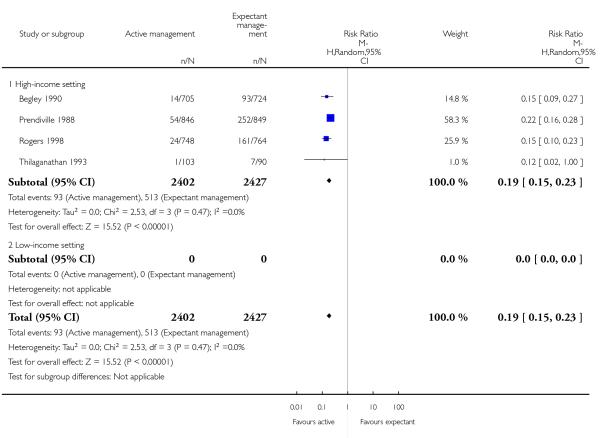 |
Analysis 1.19. Comparison 1 Active versus expectant management of 3rd stage of labour (all women), Outcome 19 Mean length of third stage.
Review: Active versus expectant management for women in the third stage of labour
Comparison: 1 Active versus expectant management of 3rd stage of labour (all women)
Outcome: 19 Mean length of third stage
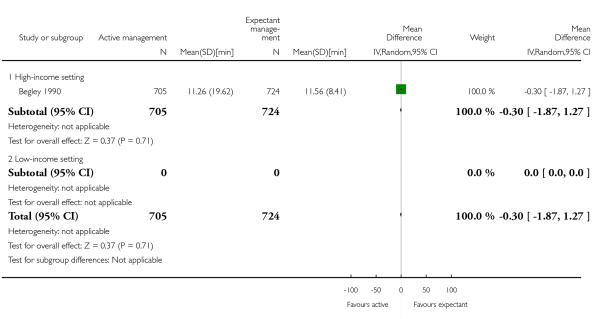 |
Analysis 1.20. Comparison 1 Active versus expectant management of 3rd stage of labour (all women), Outcome 20 Manual removal of placenta as defined by authors.
Review: Active versus expectant management for women in the third stage of labour
Comparison: 1 Active versus expectant management of 3rd stage of labour (all women)
Outcome: 20 Manual removal of placenta as defined by authors
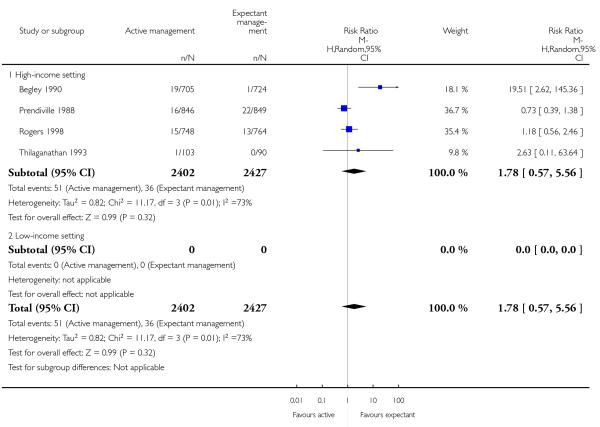 |
Analysis 1.21. Comparison 1 Active versus expectant management of 3rd stage of labour (all women), Outcome 21 Postnatal diastolic blood pressure > 90 mmHg between birth of baby and discharge from the labour ward.
Review: Active versus expectant management for women in the third stage of labour
Comparison: 1 Active versus expectant management of 3rd stage of labour (all women)
Outcome: 21 Postnatal diastolic blood pressure > 90 mmHg between birth of baby and discharge from the labour ward.
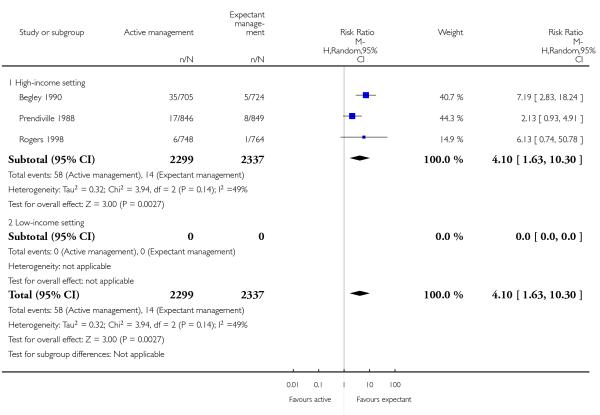 |
Analysis 1.22. Comparison 1 Active versus expectant management of 3rd stage of labour (all women), Outcome 22 Postnatal vomiting between birth of baby and discharge from the labour ward.
Review: Active versus expectant management for women in the third stage of labour
Comparison: 1 Active versus expectant management of 3rd stage of labour (all women)
Outcome: 22 Postnatal vomiting between birth of baby and discharge from the labour ward.
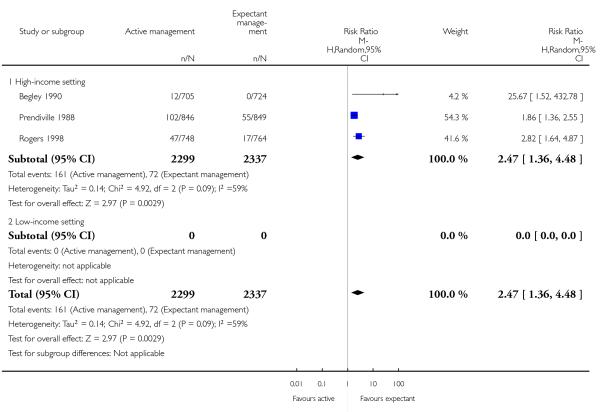 |
Analysis 1.23. Comparison 1 Active versus expectant management of 3rd stage of labour (all women), Outcome 23 Any analgesia between birth of the baby and discharge from labour ward.
Review: Active versus expectant management for women in the third stage of labour
Comparison: 1 Active versus expectant management of 3rd stage of labour (all women)
Outcome: 23 Any analgesia between birth of the baby and discharge from labour ward
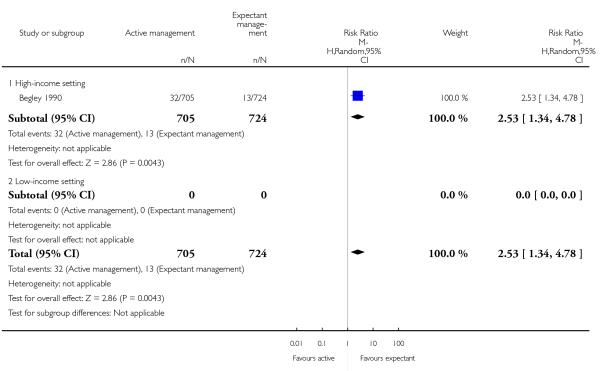 |
Analysis 1.25. Comparison 1 Active versus expectant management of 3rd stage of labour (all women), Outcome 25 Secondary blood loss/any vaginal bleeding needing treatment (after 24 hhours and up to 6 weeks).
Review: Active versus expectant management for women in the third stage of labour
Comparison: 1 Active versus expectant management of 3rd stage of labour (all women)
Outcome: 25 Secondary blood loss/any vaginal bleeding needing treatment (after 24 hhours and up to 6 weeks)
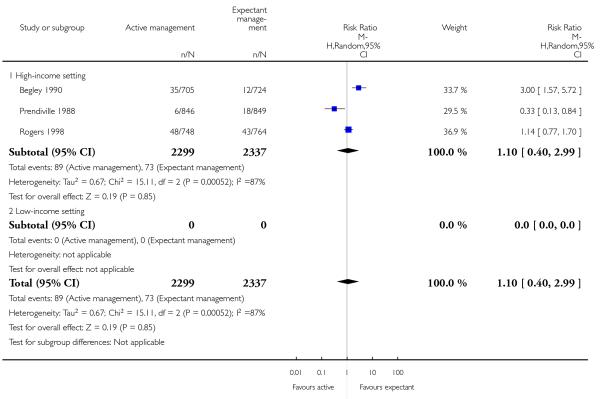 |
Analysis 1.27. Comparison 1 Active versus expectant management of 3rd stage of labour (all women), Outcome 27 Surgical evacuation of retained products of conception.
Review: Active versus expectant management for women in the third stage of labour
Comparison: 1 Active versus expectant management of 3rd stage of labour (all women)
Outcome: 27 Surgical evacuation of retained products of conception
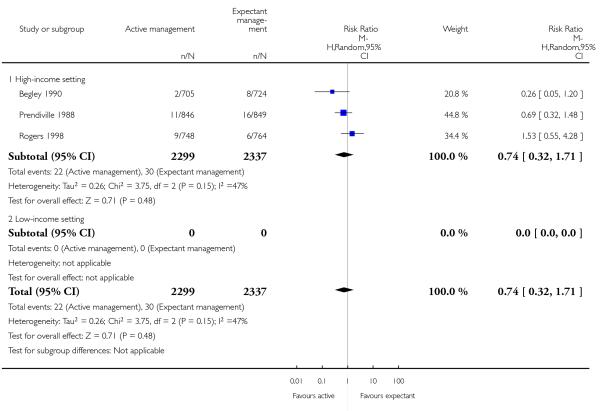 |
Analysis 1.28. Comparison 1 Active versus expectant management of 3rd stage of labour (all women), Outcome 28 Afterpains - abdominal pain associated with the contracting uterus in the postpartum period.
Review: Active versus expectant management for women in the third stage of labour
Comparison: 1 Active versus expectant management of 3rd stage of labour (all women)
Outcome: 28 Afterpains - abdominal pain associated with the contracting uterus in the postpartum period.
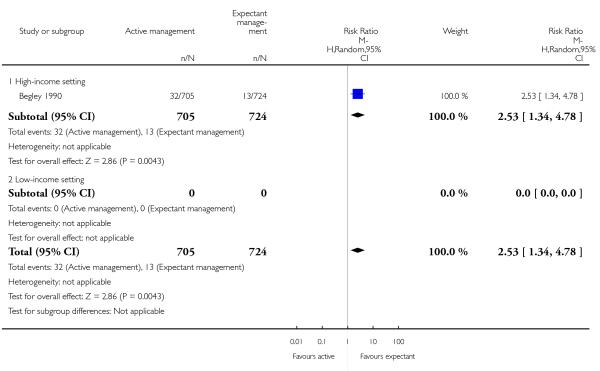 |
Analysis 1.29. Comparison 1 Active versus expectant management of 3rd stage of labour (all women), Outcome 29 Apgar score < 7 at 5 minutes.
Review: Active versus expectant management for women in the third stage of labour
Comparison: 1 Active versus expectant management of 3rd stage of labour (all women)
Outcome: 29 Apgar score < 7 at 5 minutes
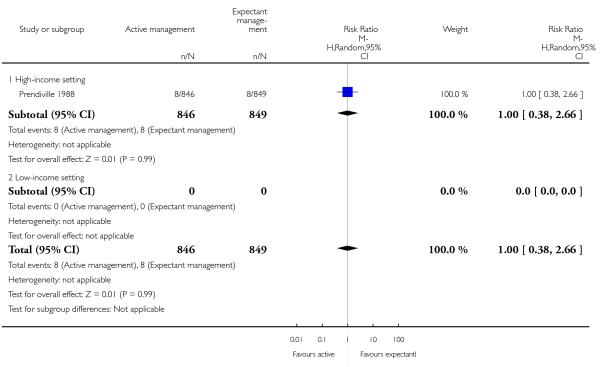 |
Analysis 1.30. Comparison 1 Active versus expectant management of 3rd stage of labour (all women), Outcome 30 Birthweight.
Review: Active versus expectant management for women in the third stage of labour
Comparison: 1 Active versus expectant management of 3rd stage of labour (all women)
Outcome: 30 Birthweight
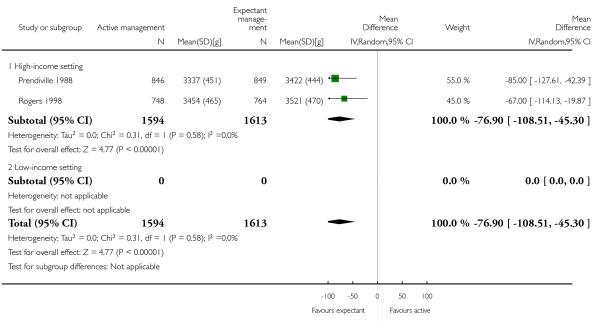 |
Analysis 1.38. Comparison 1 Active versus expectant management of 3rd stage of labour (all women), Outcome 38 Exclusive breastfeeding at discharge from hospital.
Review: Active versus expectant management for women in the third stage of labour
Comparison: 1 Active versus expectant management of 3rd stage of labour (all women)
Outcome: 38 Exclusive breastfeeding at discharge from hospital
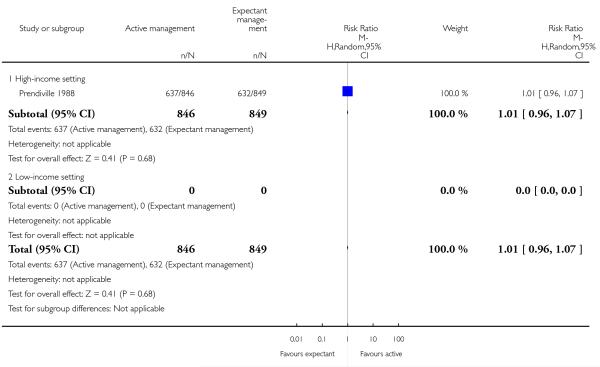 |
Analysis 1.40. Comparison 1 Active versus expectant management of 3rd stage of labour (all women), Outcome 40 Return to hospital as in- or outpatient because of bleeding (not pre-specified).
Review: Active versus expectant management for women in the third stage of labour
Comparison: 1 Active versus expectant management of 3rd stage of labour (all women)
Outcome: 40 Return to hospital as in- or outpatient because of bleeding (not pre-specified)
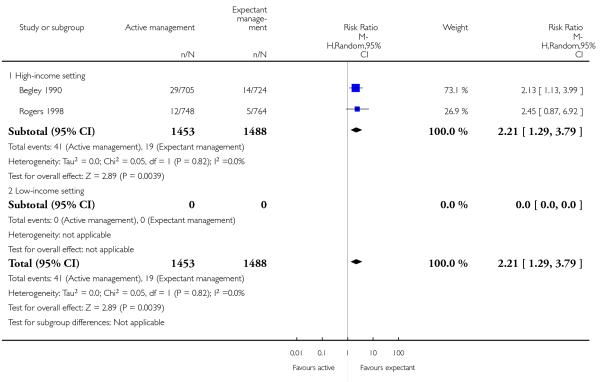 |
Analysis 1.41. Comparison 1 Active versus expectant management of 3rd stage of labour (all women), Outcome 41 Postnatal maternal mean Hb (outcome not pre-specified).
Review: Active versus expectant management for women in the third stage of labour
Comparison: 1 Active versus expectant management of 3rd stage of labour (all women)
Outcome: 41 Postnatal maternal mean Hb (outcome not pre-specified)
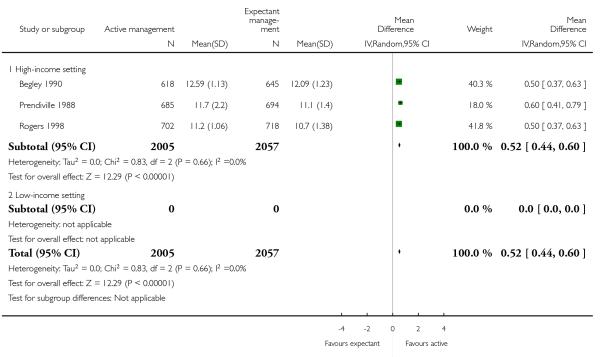 |
Analysis 2.1. Comparison 2 Active versus expectant management of 3rd stage of labour (women at low risk), Outcome 1 Severe primary PPH at time of birth (clinically estimated or measured blood loss ≥ 1000 mL).
Review: Active versus expectant management for women in the third stage of labour
Comparison: 2 Active versus expectant management of 3rd stage of labour (women at low risk)
Outcome: 1 Severe primary PPH at time of birth (clinically estimated or measured blood loss ≥ 1000 mL)
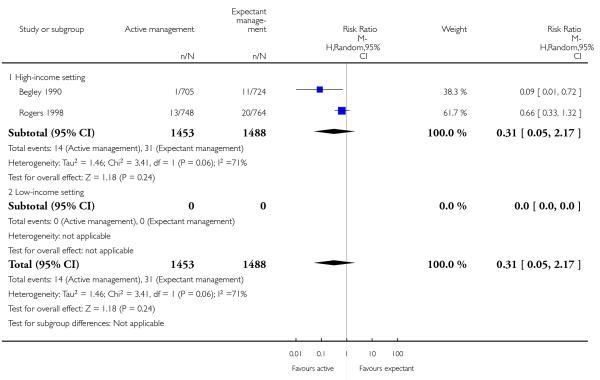 |
Analysis 2.4. Comparison 2 Active versus expectant management of 3rd stage of labour (women at low risk), Outcome 4 Maternal Hb < 9 g/dL at 24-72 hr.
Review: Active versus expectant management for women in the third stage of labour
Comparison: 2 Active versus expectant management of 3rd stage of labour (women at low risk)
Outcome: 4 Maternal Hb < 9 g/dL at 24-72 hr
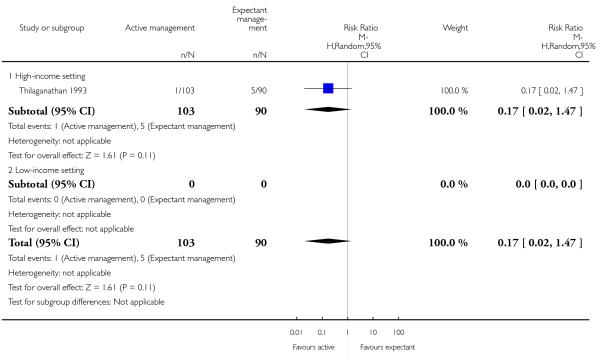 |
Analysis 2.5. Comparison 2 Active versus expectant management of 3rd stage of labour (women at low risk), Outcome 5 Admission to neonatal special/intensive care.
Review: Active versus expectant management for women in the third stage of labour
Comparison: 2 Active versus expectant management of 3rd stage of labour (women at low risk)
Outcome: 5 Admission to neonatal special/intensive care
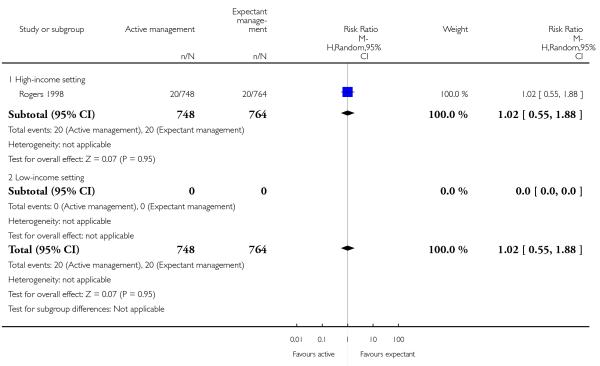 |
Analysis 2.6. Comparison 2 Active versus expectant management of 3rd stage of labour (women at low risk), Outcome 6 Neonatal jaundice requiring phototherapy or exchange transfusion.
Review: Active versus expectant management for women in the third stage of labour
Comparison: 2 Active versus expectant management of 3rd stage of labour (women at low risk)
Outcome: 6 Neonatal jaundice requiring phototherapy or exchange transfusion
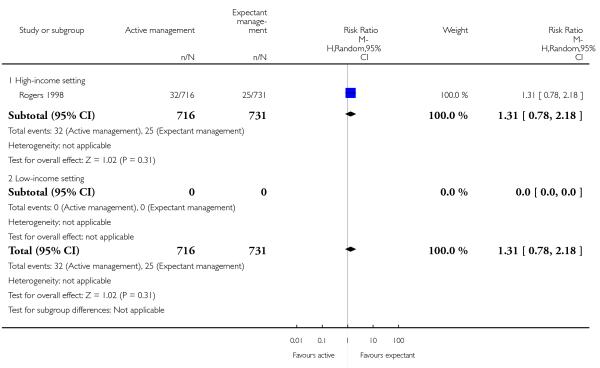 |
Analysis 2.10. Comparison 2 Active versus expectant management of 3rd stage of labour (women at low risk), Outcome 10 Primary blood loss ≥ 500 mL at time of birth (clinically estimated or measured).
Review: Active versus expectant management for women in the third stage of labour
Comparison: 2 Active versus expectant management of 3rd stage of labour (women at low risk)
Outcome: 10 Primary blood loss ≥ 500 mL at time of birth (clinically estimated or measured)
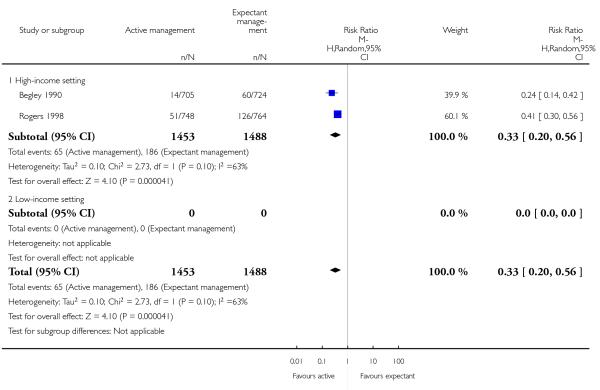 |
Analysis 2.13. Comparison 2 Active versus expectant management of 3rd stage of labour (women at low risk), Outcome 13 Mean maternal blood loss (mL at the time of birth, clinically estimated or measured.
Review: Active versus expectant management for women in the third stage of labour
Comparison: 2 Active versus expectant management of 3rd stage of labour (women at low risk)
Outcome: 13 Mean maternal blood loss (mL at the time of birth, clinically estimated or measured
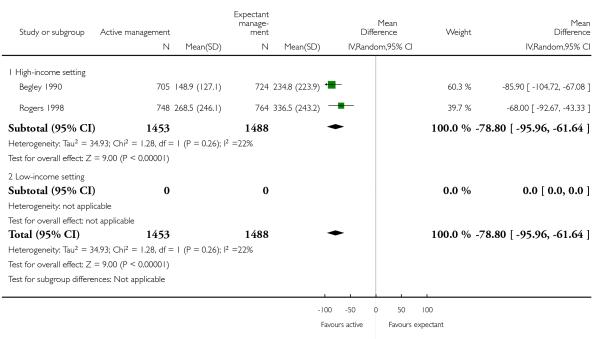 |
Analysis 2.16. Comparison 2 Active versus expectant management of 3rd stage of labour (women at low risk), Outcome 16 Maternal blood transfusion.
Review: Active versus expectant management for women in the third stage of labour
Comparison: 2 Active versus expectant management of 3rd stage of labour (women at low risk)
Outcome: 16 Maternal blood transfusion
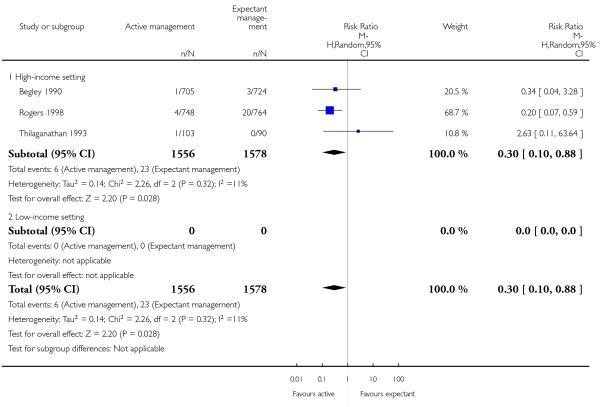 |
Analysis 2.18. Comparison 2 Active versus expectant management of 3rd stage of labour (women at low risk), Outcome 18 Therapeutic uterotonics during third stage and/or within 24 hours.
Review: Active versus expectant management for women in the third stage of labour
Comparison: 2 Active versus expectant management of 3rd stage of labour (women at low risk)
Outcome: 18 Therapeutic uterotonics during third stage and/or within 24 hours
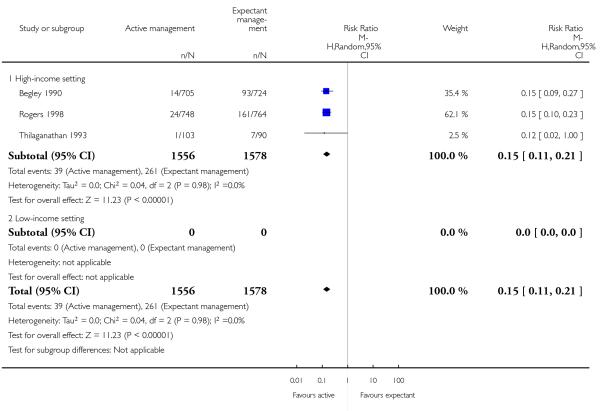 |
Analysis 2.19. Comparison 2 Active versus expectant management of 3rd stage of labour (women at low risk), Outcome 19 Mean length of third stage.
Review: Active versus expectant management for women in the third stage of labour
Comparison: 2 Active versus expectant management of 3rd stage of labour (women at low risk)
Outcome: 19 Mean length of third stage
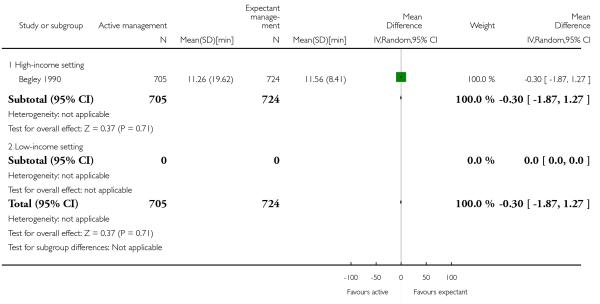 |
Analysis 2.20. Comparison 2 Active versus expectant management of 3rd stage of labour (women at low risk), Outcome 20 Manual removal of placenta as defined by authors.
Review: Active versus expectant management for women in the third stage of labour
Comparison: 2 Active versus expectant management of 3rd stage of labour (women at low risk)
Outcome: 20 Manual removal of placenta as defined by authors
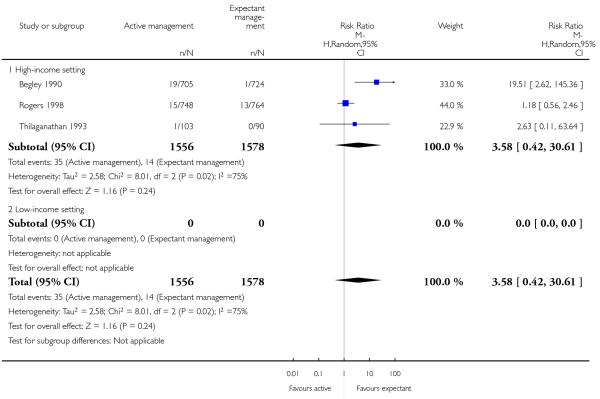 |
Analysis 2.21. Comparison 2 Active versus expectant management of 3rd stage of labour (women at low risk), Outcome 21 Postnatal diastolic blood pressure > 90 mmHg between birth of baby and discharge from the labour ward.
Review: Active versus expectant management for women in the third stage of labour
Comparison: 2 Active versus expectant management of 3rd stage of labour (women at low risk)
Outcome: 21 Postnatal diastolic blood pressure > 90 mmHg between birth of baby and discharge from the labour ward.
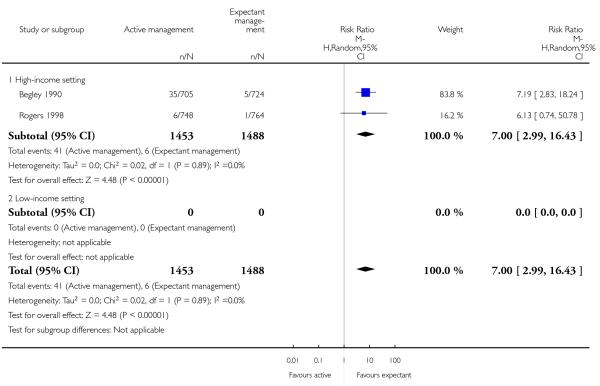 |
Analysis 2.22. Comparison 2 Active versus expectant management of 3rd stage of labour (women at low risk), Outcome 22 Postnatal vomiting between birth of baby and discharge from the labour ward.
Review: Active versus expectant management for women in the third stage of labour
Comparison: 2 Active versus expectant management of 3rd stage of labour (women at low risk)
Outcome: 22 Postnatal vomiting between birth of baby and discharge from the labour ward.
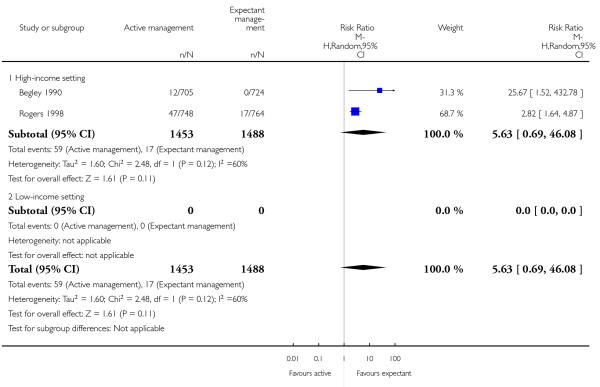 |
Analysis 2.23. Comparison 2 Active versus expectant management of 3rd stage of labour (women at low risk), Outcome 23 Any analgesia between birth of the baby and up to discharge from labour ward.
Review: Active versus expectant management for women in the third stage of labour
Comparison: 2 Active versus expectant management of 3rd stage of labour (women at low risk)
Outcome: 23 Any analgesia between birth of the baby and up to discharge from labour ward
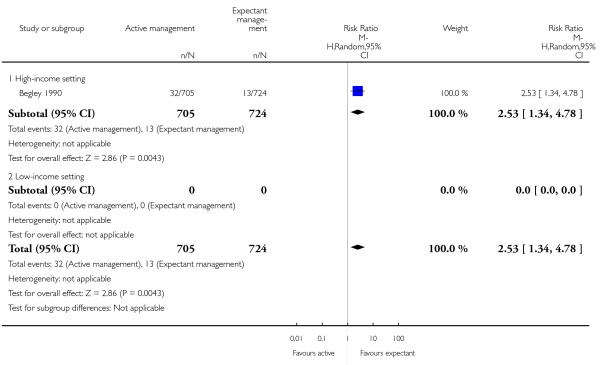 |
Analysis 2.25. Comparison 2 Active versus expectant management of 3rd stage of labour (women at low risk), Outcome 25 Secondary blood loss/any vaginal bleeding needing treatment (after 24 hhours and up to 6 weeks).
Review: Active versus expectant management for women in the third stage of labour
Comparison: 2 Active versus expectant management of 3rd stage of labour (women at low risk)
Outcome: 25 Secondary blood loss/any vaginal bleeding needing treatment (after 24 hhours and up to 6 weeks)
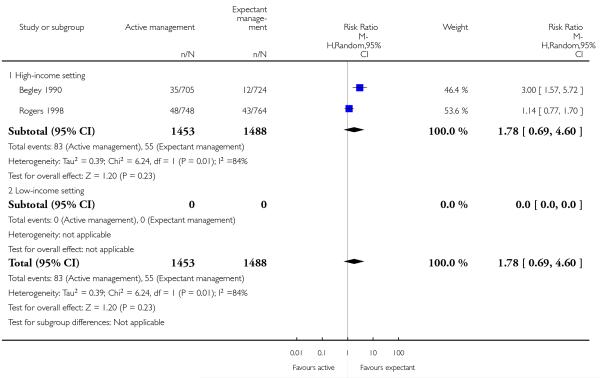 |
Analysis 2.27. Comparison 2 Active versus expectant management of 3rd stage of labour (women at low risk), Outcome 27 Surgical evacuation of retained products of conception.
Review: Active versus expectant management for women in the third stage of labour
Comparison: 2 Active versus expectant management of 3rd stage of labour (women at low risk)
Outcome: 27 Surgical evacuation of retained products of conception
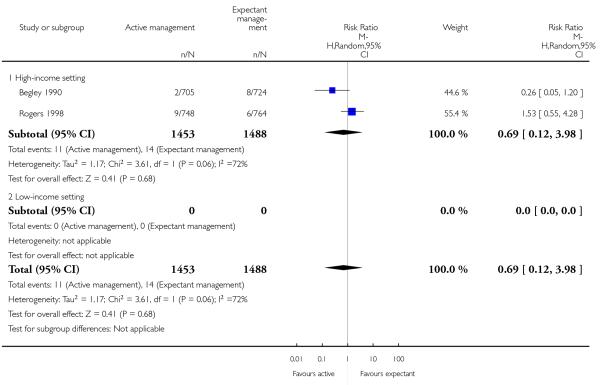 |
Analysis 2.28. Comparison 2 Active versus expectant management of 3rd stage of labour (women at low risk), Outcome 28 Afterpains - abdominal pain associated with the contracting uterus in the postpartum period.
Review: Active versus expectant management for women in the third stage of labour
Comparison: 2 Active versus expectant management of 3rd stage of labour (women at low risk)
Outcome: 28 Afterpains - abdominal pain associated with the contracting uterus in the postpartum period
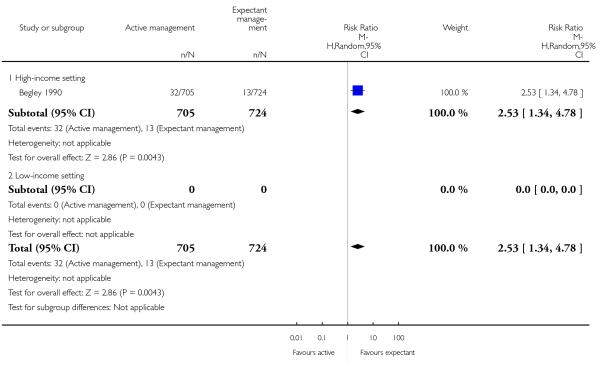 |
Analysis 2.30. Comparison 2 Active versus expectant management of 3rd stage of labour (women at low risk), Outcome 30 Birthweight.
Review: Active versus expectant management for women in the third stage of labour
Comparison: 2 Active versus expectant management of 3rd stage of labour (women at low risk)
Outcome: 30 Birthweight
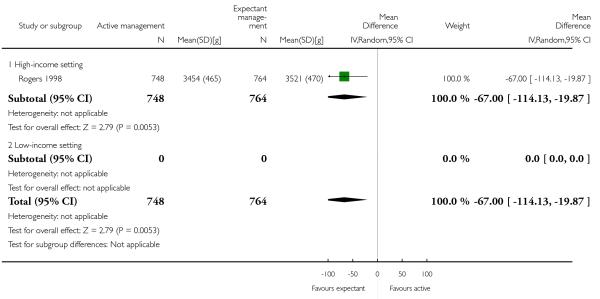 |
Analysis 2.40. Comparison 2 Active versus expectant management of 3rd stage of labour (women at low risk), Outcome 40 Return to hospital as in- or outpatient because of bleeding (not pre-specified).
Review: Active versus expectant management for women in the third stage of labour
Comparison: 2 Active versus expectant management of 3rd stage of labour (women at low risk)
Outcome: 40 Return to hospital as in- or outpatient because of bleeding (not pre-specified)
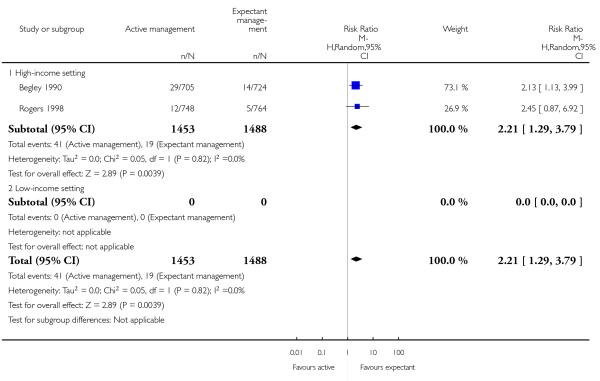 |
Analysis 2.41. Comparison 2 Active versus expectant management of 3rd stage of labour (women at low risk), Outcome 41 Postnatal maternal mean Hb (outcome not pre-specified).
Review: Active versus expectant management for women in the third stage of labour
Comparison: 2 Active versus expectant management of 3rd stage of labour (women at low risk)
Outcome: 41 Postnatal maternal mean Hb (outcome not pre-specified)
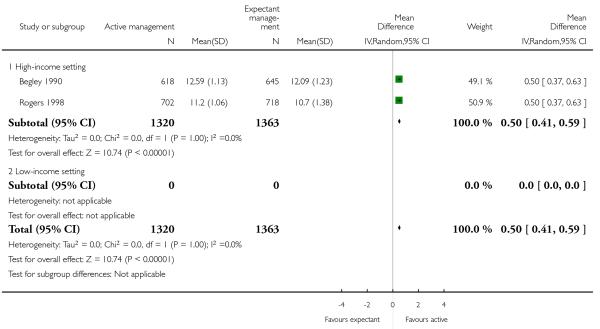 |
Analysis 10.1. Comparison 10 Active versus mixed management (uterotonic after placental delivery, immediate cord clamping and no CCT) , Outcome 1 Severe primary PPH at time of birth (clinically estimated or measured blood loss ≥ 1000 mL).
Review: Active versus expectant management for women in the third stage of labour
Comparison: 10 Active versus mixed management (uterotonic after placental delivery, immediate cord clamping and no CCT)
Outcome: 1 Severe primary PPH at time of birth (clinically estimated or measured blood loss ≥ 1000 mL)
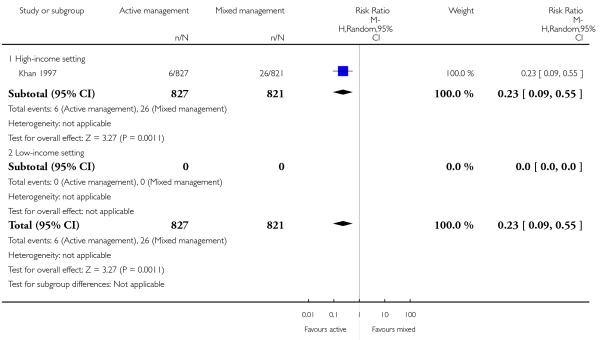 |
Analysis 10.10. Comparison 10 Active versus mixed management (uterotonic after placental delivery, immediate cord clamping and no CCT) , Outcome 10 Primary blood loss ≥ 500 mL at time of birth (clinically estimated or measured).
Review: Active versus expectant management for women in the third stage of labour
Comparison: 10 Active versus mixed management (uterotonic after placental delivery, immediate cord clamping and no CCT)
Outcome: 10 Primary blood loss ≥ 500 mL at time of birth (clinically estimated or measured)
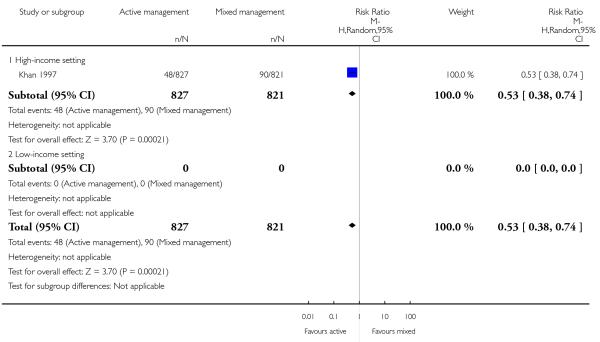 |
Analysis 10.16. Comparison 10 Active versus mixed management (uterotonic after placental delivery, immediate cord clamping and no CCT) , Outcome 16 Maternal blood transfusion.
Review: Active versus expectant management for women in the third stage of labour
Comparison: 10 Active versus mixed management (uterotonic after placental delivery, immediate cord clamping and no CCT)
Outcome: 16 Maternal blood transfusion
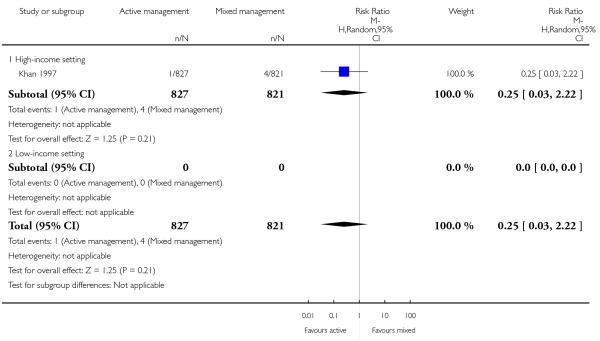 |
Analysis 10.17. Comparison 10 Active versus mixed management (uterotonic after placental delivery, immediate cord clamping and no CCT) , Outcome 17 Clinical signs of severe blood loss.
Review: Active versus expectant management for women in the third stage of labour
Comparison: 10 Active versus mixed management (uterotonic after placental delivery, immediate cord clamping and no CCT)
Outcome: 17 Clinical signs of severe blood loss
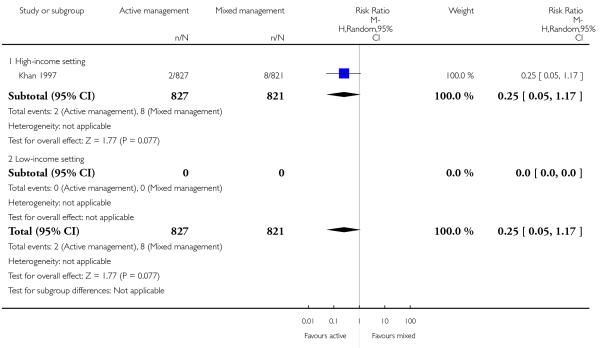 |
Analysis 10.18. Comparison 10 Active versus mixed management (uterotonic after placental delivery, immediate cord clamping and no CCT) , Outcome 18 Therapeutic uterotonics during third stage and/or within 24 hours.
Review: Active versus expectant management for women in the third stage of labour
Comparison: 10 Active versus mixed management (uterotonic after placental delivery, immediate cord clamping and no CCT)
Outcome: 18 Therapeutic uterotonics during third stage and/or within 24 hours
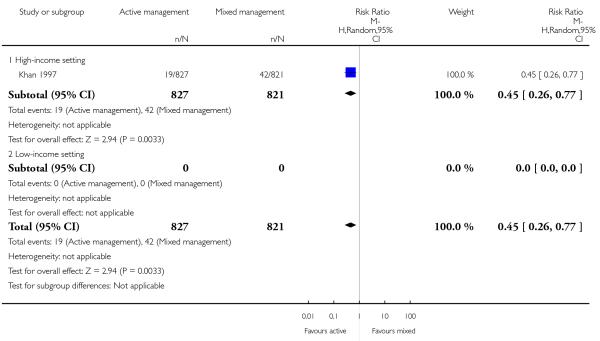 |
Analysis 10.19. Comparison 10 Active versus mixed management (uterotonic after placental delivery, immediate cord clamping and no CCT) , Outcome 19 Mean length of third stage.
Review: Active versus expectant management for women in the third stage of labour
Comparison: 10 Active versus mixed management (uterotonic after placental delivery, immediate cord clamping and no CCT)
Outcome: 19 Mean length of third stage
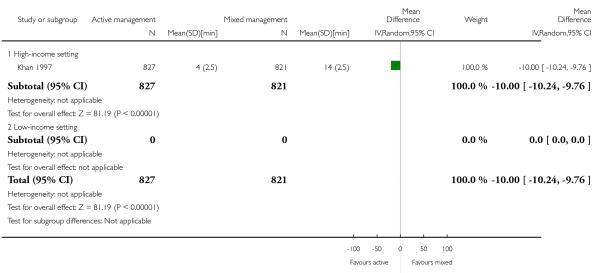 |
Analysis 11.1. Comparison 11 Active versus mixed management (no routine uterotonic, early cord clamping, no CCT), Outcome 1 Severe primary PPH at time of birth (clinically estimated or measured blood loss ≥ 1000 mL).
Review: Active versus expectant management for women in the third stage of labour
Comparison: 11 Active versus mixed management (no routine uterotonic, early cord clamping, no CCT)
Outcome: 1 Severe primary PPH at time of birth (clinically estimated or measured blood loss ≥ 1000 mL)
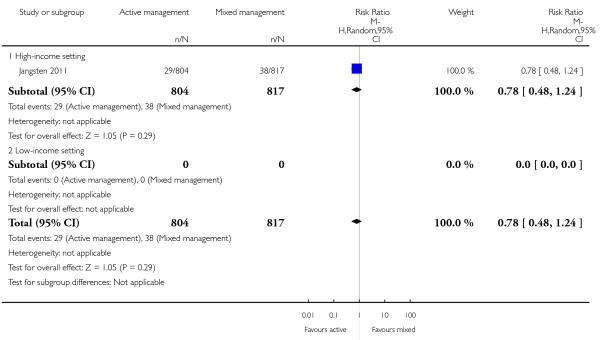 |
Analysis 11.4. Comparison 11 Active versus mixed management (no routine uterotonic, early cord clamping, no CCT), Outcome 4 Maternal Hb < 9 g/dL at 24-72 hr.
Review: Active versus expectant management for women in the third stage of labour
Comparison: 11 Active versus mixed management (no routine uterotonic, early cord clamping, no CCT)
Outcome: 4 Maternal Hb < 9 g/dL at 24-72 hr
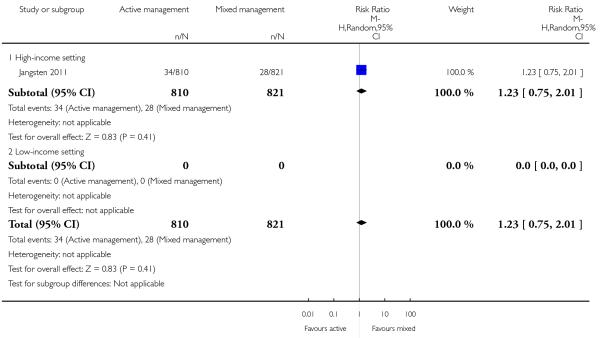 |
Analysis 11.10. Comparison 11 Active versus mixed management (no routine uterotonic, early cord clamping, no CCT), Outcome 10 Primary blood loss ≥ 500 mL at time of birth (clinically estimated or measured).
Review: Active versus expectant management for women in the third stage of labour
Comparison: 11 Active versus mixed management (no routine uterotonic, early cord clamping, no CCT)
Outcome: 10 Primary blood loss ≥ 500 mL at time of birth (clinically estimated or measured)
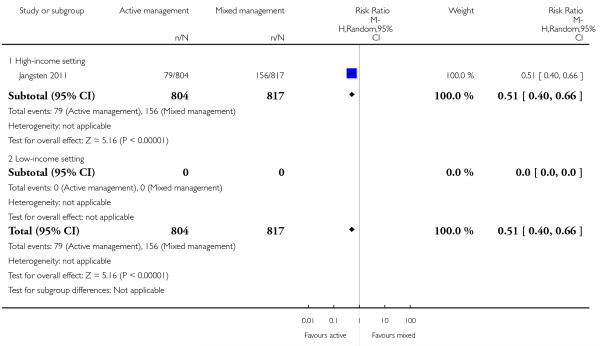 |
Analysis 11.13. Comparison 11 Active versus mixed management (no routine uterotonic, early cord clamping, no CCT), Outcome 13 Mean maternal blood loss (mL) at time of birth (clinically estimated or measured).
Review: Active versus expectant management for women in the third stage of labour
Comparison: 11 Active versus mixed management (no routine uterotonic, early cord clamping, no CCT)
Outcome: 13 Mean maternal blood loss (mL) at time of birth (clinically estimated or measured)
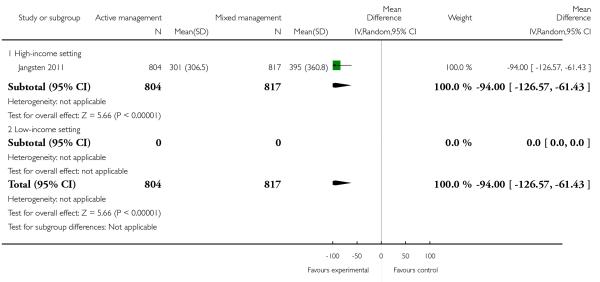 |
Analysis 11.16. Comparison 11 Active versus mixed management (no routine uterotonic, early cord clamping, no CCT), Outcome 16 Maternal blood transfusion.
Review: Active versus expectant management for women in the third stage of labour
Comparison: 11 Active versus mixed management (no routine uterotonic, early cord clamping, no CCT)
Outcome: 16 Maternal blood transfusion
 |
Analysis 11.18. Comparison 11 Active versus mixed management (no routine uterotonic, early cord clamping, no CCT), Outcome 18 Therapeutic uterotonics during third stage and/or within 24 hours.
Review: Active versus expectant management for women in the third stage of labour
Comparison: 11 Active versus mixed management (no routine uterotonic, early cord clamping, no CCT)
Outcome: 18 Therapeutic uterotonics during third stage and/or within 24 hours
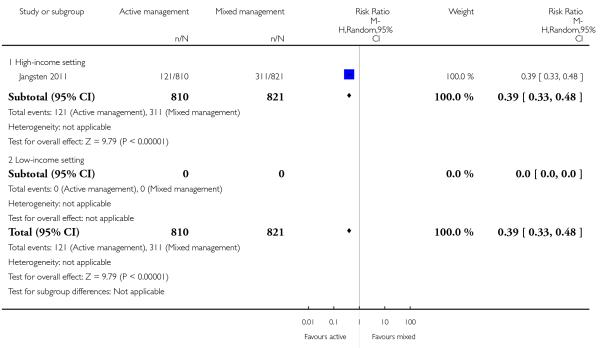 |
Analysis 11.19. Comparison 11 Active versus mixed management (no routine uterotonic, early cord clamping, no CCT), Outcome 19 Mean length of third stage.
Review: Active versus expectant management for women in the third stage of labour
Comparison: 11 Active versus mixed management (no routine uterotonic, early cord clamping, no CCT)
Outcome: 19 Mean length of third stage
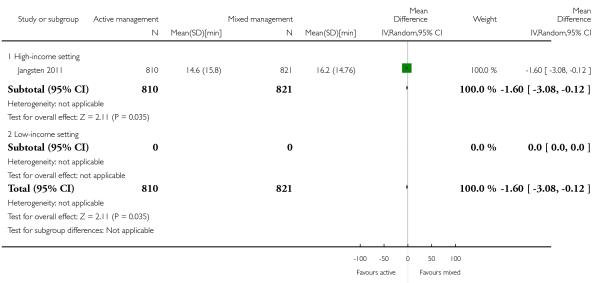 |
Analysis 11.20. Comparison 11 Active versus mixed management (no routine uterotonic, early cord clamping, no CCT), Outcome 20 Manual removal of placenta as defined by authors.
Review: Active versus expectant management for women in the third stage of labour
Comparison: 11 Active versus mixed management (no routine uterotonic, early cord clamping, no CCT)
Outcome: 20 Manual removal of placenta as defined by authors
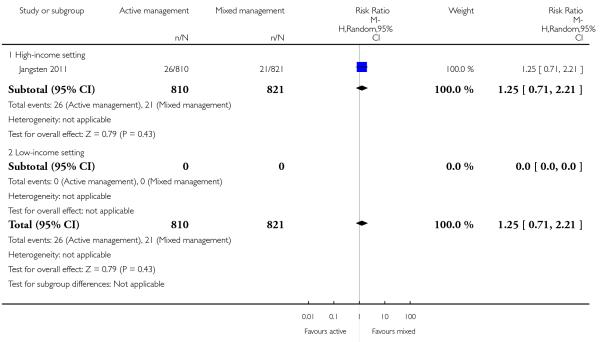 |
Analysis 11.30. Comparison 11 Active versus mixed management (no routine uterotonic, early cord clamping, no CCT), Outcome 30 Birthweight.
Review: Active versus expectant management for women in the third stage of labour
Comparison: 11 Active versus mixed management (no routine uterotonic, early cord clamping, no CCT)
Outcome: 30 Birthweight
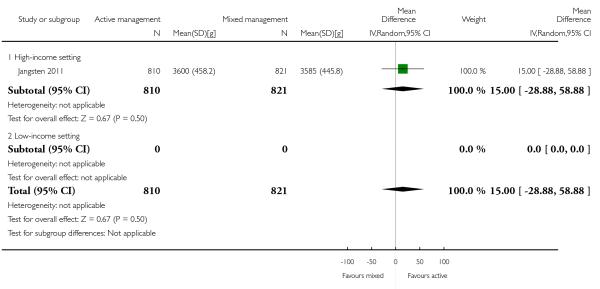 |
Analysis 11.41. Comparison 11 Active versus mixed management (no routine uterotonic, early cord clamping, no CCT), Outcome 41 Postnatal maternal mean Hb (outcome not pre-specified).
Review: Active versus expectant management for women in the third stage of labour
Comparison: 11 Active versus mixed management (no routine uterotonic, early cord clamping, no CCT)
Outcome: 41 Postnatal maternal mean Hb (outcome not pre-specified)
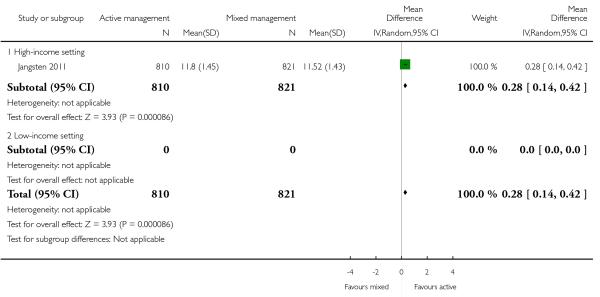 |
Analysis 11.42. Comparison 11 Active versus mixed management (no routine uterotonic, early cord clamping, no CCT), Outcome 42 Severe primary PPH after placental delivery and up to 2 hours (clinically estimated or measured blood loss ≥ 1000 mL) - not pre-specified.
Review: Active versus expectant management for women in the third stage of labour
Comparison: 11 Active versus mixed management (no routine uterotonic, early cord clamping, no CCT)
Outcome: 42 Severe primary PPH after placental delivery and up to 2 hours (clinically estimated or measured blood loss ≥ 1000 mL) - not pre-specified
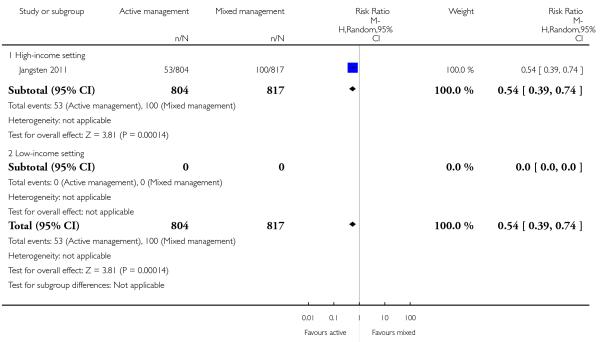 |
Analysis 11.43. Comparison 11 Active versus mixed management (no routine uterotonic, early cord clamping, no CCT), Outcome 43 Severe primary PPH at time of birth and up to 2 hours (clinically estimated or measured blood loss ≥1000 mL) - not pre-specified.
Review: Active versus expectant management for women in the third stage of labour
Comparison: 11 Active versus mixed management (no routine uterotonic, early cord clamping, no CCT)
Outcome: 43 Severe primary PPH at time of birth and up to 2 hours (clinically estimated or measured blood loss ≥1000 mL) - not pre-specified
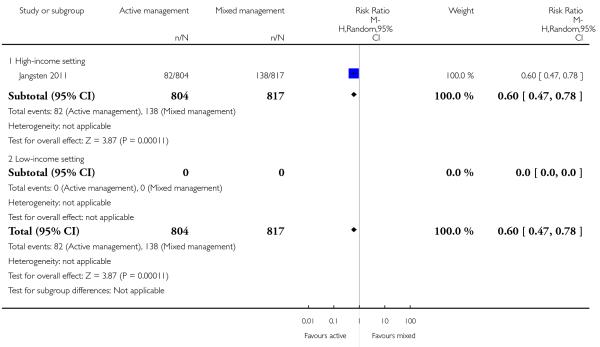 |
Analysis 11.44. Comparison 11 Active versus mixed management (no routine uterotonic, early cord clamping, no CCT), Outcome 44 Mean blood loss (mL) (clinically estimated or measured at birth and up to 2 hours (not pre-specified).
Review: Active versus expectant management for women in the third stage of labour
Comparison: 11 Active versus mixed management (no routine uterotonic, early cord clamping, no CCT)
Outcome: 44 Mean blood loss (mL) (clinically estimated or measured at birth and up to 2 hours (not pre-specified)
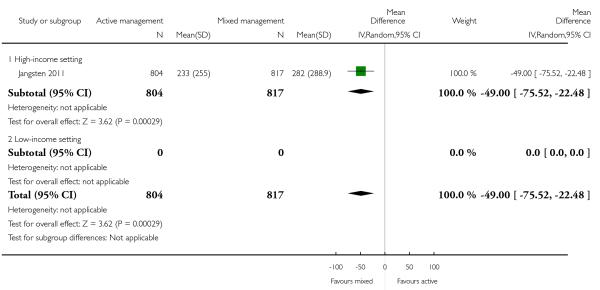 |
Analysis 12.4. Comparison 12 Active versus mixed management (no routine uterotonic, early cord clamping, CCT), Outcome 4 Maternal Hb < 9 g/dL at 24-72 hr.
Review: Active versus expectant management for women in the third stage of labour
Comparison: 12 Active versus mixed management (no routine uterotonic, early cord clamping, CCT)
Outcome: 4 Maternal Hb > 9 g/dL at 24-72 hr
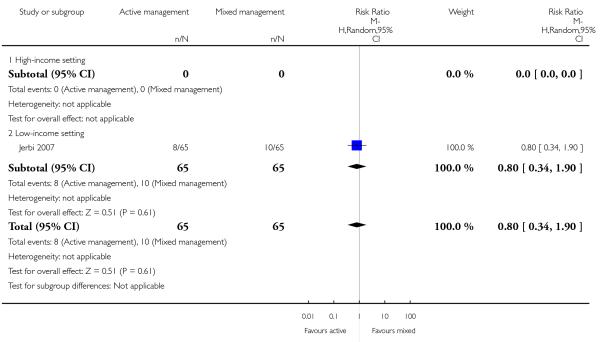 |
Analysis 12.19. Comparison 12 Active versus mixed management (no routine uterotonic, early cord clamping, CCT), Outcome 19 Mean length of third stage.
Review: Active versus expectant management for women in the third stage of labour
Comparison: 12 Active versus mixed management (no routine uterotonic, early cord clamping, CCT)
Outcome: 19 Mean length of third stage
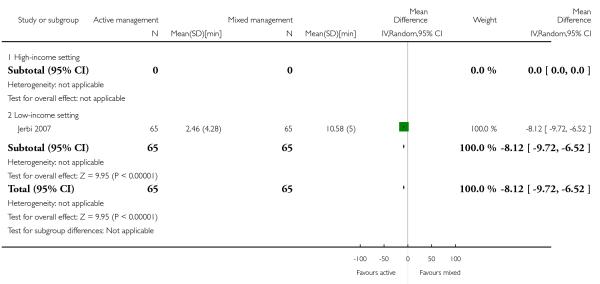 |
Analysis 12.20. Comparison 12 Active versus mixed management (no routine uterotonic, early cord clamping, CCT), Outcome 20 Manual removal of placenta as defined by authors.
Review: Active versus expectant management for women in the third stage of labour
Comparison: 12 Active versus mixed management (no routine uterotonic, early cord clamping, CCT)
Outcome: 20 Manual removal of placenta as defined by authors
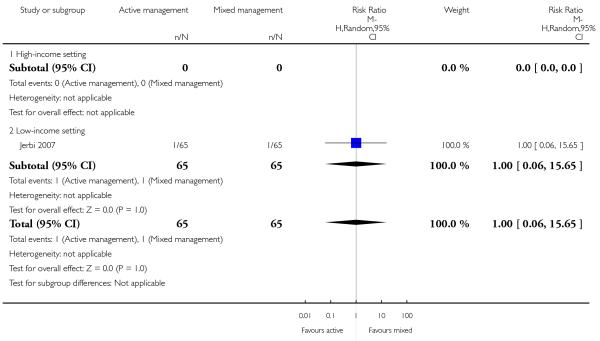 |
FEEDBACK
Mc’Alpine, 31 August 2002
Summary
I have some questions. In the four included studies, how many women were in each study and when were the studies done? Was a comparison made between maternity hospitals, birth centres, and home delivery? For postpartum haemorrhage of more than 500 mls, what does “relative risk 0.38, 95% confidence interval 0.32 to 0.46” mean in terms of numbers?
Why do you conclude that active management should be the ‘routine’ management of choice in a maternity hospital? What are the implications for other settings?
[Summary of comments from Elizabeth McAlpine, August 2002]
Reply
Reply from Cecily Begley in December 2009: The number of women in each study can be found in the analyses graphs in this updated review, and the overall numbers of studies and women included for each outcome are also reported in the text. Dates for the studies are included in the references. All the studies were in hospital settings and we found no studies of midwifery-led birth centres or home births. We have included this information on study settings in the ‘Characteristics of Included studies’ and at appropriate places in the text of the review. Our estimate of the relative risk of blood loss greater than 500 mL is slightly different from the previous version of the review because we have used a random-effects analysis. We also report the ‘Numbers needed to treat’ under ‘Summary of main results’ for the outcomes of severe primary PPH and postnatal Hb < 9 g/dL; we hope this addresses the meaning of the relative risk and confidence intervals for these outcomes. Our conclusions differ from the previous review as we have included additional outcomes which show a balance of benefits and harms and we have used new systematic review methodology. Our findings refer to hospital settings because this is where the included studies were undertaken. We cannot provide evidence-based information for other settings.
Contributors
Cecily Begley
Matthews, December 2004
Summary
My anecdotal observation, having changed my practice to include physiological management of the third stage, is that women who choose this option have a decrease in the amount of lochia postpartum and a shorter duration of vaginal discharge. I have not seen any studies that could confirm or refute this. [Comment received from Mary Jo Matthews, December 2004]
Reply
We have included in the update of the review the outcome ‘Amount of lochia > 24 hours and up to discharge from hospital [mL]’; however, none of the included studies assessed this outcome, so we have, therefore, no evidence to confirm or refute this observation.
Contributors
Cecily Begley
Van Wyk, 4 March 2009
Summary
This review was last updated in 2000, and comments sent in 2002 and 2004 have not been addressed. The authors stated in 2007 that an update is in progress. We are concerned about the validity of the review findings. [Summary of feedback from Susan van Wyk on behalf of the Masters Clinical Epidemiology class, Stellenbosch University, February 2009]
Reply
A new team took over this review in December 2008. We have now updated this review and addressed these comments from 2002 and 2004. Our conclusions differ from the previous review as we have included additional outcomes which show a balance of benefits and harms and we have used new systematic review methodology. The delay has arisen from our needing to produce a new protocol for the review prior to undertaking the update.
Contributors
Reply from Cecily Begley, December 2009
WHAT’S NEW
Last assessed as up-to-date: 26 September 2011.
| Date | Event | Description |
|---|---|---|
| 17 August 2011 | New citation required but conclusions have not changed | Deirdre Murphy and Susan MacDonald stepped down as authors. Andrew Weeks joined the team |
| 17 August 2011 | New search has been performed | Search updated in February 2011: two new studies included (Jangsten 2011; Jerbi 2007); four excluded (Abdel-Aleem 2010; Hoffman 2006; Ramirez 2001; Vasegh 2005); and one added to Studies awaiting classification. |
| Protocol section updated - see Differences between protocol and review. | ||
HISTORY
Protocol first published: Issue 4, 2008
Review first published: Issue 7, 2010
| Date | Event | Description |
|---|---|---|
| 10 May 2010 | Feedback has been incorporated | Feedback on the previous version of this review added, along with replies from the new review team |
DIFFERENCES BETWEEN PROTOCOL AND REVIEW
We did not carry out additional searching as proposed. We decided after looking at the variation in the interventions and controls used in the studies that we had clinical heterogeneity between the studies (Table 1) and so we have used a random-effects meta analysis throughout the review.
We have changed the labels on the secondary outcomes of ‘Primary postpartum haemorrhage (> 500 mL)’ and ‘Secondary postpartum haemorrhage (> 500 mL)’ to ‘Primary maternal blood loss > 500 mL’ and ‘Secondary maternal blood loss > 500 mL’ respectively. This is because we believe that in research the term ‘haemorrhage’ should be reserved for excessive blood loss. This is also in keeping with the terminology used in the ongoing trial in low-income countries (Gülmezoglu 2009). We have also included reference to the mean Hb values in order to provide an outcome that was calculated by blinded personnel.
We have modified the wording in the methods sections for ‘Assessment of heterogeneity’, ‘Assessment of reporting biases’ and ‘Data synthesis’ to update them with the new methods being used by the group, developed in conjunction with the group’s statisticians, Simon Gates and Richard Riley. We have used these new methods in the review.
We decided to reduce the number of outcomes, in line with the Cochrane Handbook of Systematic Reviews of Interventions recommendations. We made the following changes to outcomes.
Nine primary outcomes reduced to seven
Clinical signs of severe blood loss at the time of birth, e.g. woman feeling breathless, weak, faint, pale, exhausted: moved to secondary outcomes list.
Evidence of acidaemia indicated by a pH less than seven or base deficit greater than 12 mmol/L in umbilical arterial cord blood, or (c) neonatal blood sample in first hour of life, or both: removed.
Apgar score less than seven at five minutes, and neonatal (’hypoxic ischaemic’) encephalopathy assessed using Sarnat staging: moved to secondary outcomes list.
Changes to primary outcomes
All PPH amounts and mean blood losses are now expressed at three time periods “at the time of the birth”, “after delivery of placenta and up to 24 hours”, and “at the time of birth and up to 24 hours.” This was done because it was noted that the two primary outcomes “Severe primary PPH (clinically estimated or measured blood loss greater than or equal to 1000 mL at time of birth and up to 24 hours)” and “Very severe primary PPH (clinically estimated or measured blood loss greater than or equal to 2500 mL at time of birth and up to 24 hours),” which were based on the international definition of PPH could, in fact, provide misleading results if study authors measured or estimated blood loss at birth, and over a period of some hours in the first 24 hours, and added all amounts together to provide an overall PPH rate. While this estimate could also be useful, it raises the PPH rate artificially in comparison with studies that do not do this. Accordingly, we have changed the first two primary outcomes to “Severe primary postpartum haemorrhage (PPH) (clinically estimated or measured blood loss greater than or equal to 1000 mL at time of birth)” and “Very severe primary PPH (clinically estimated or measured blood loss greater than or equal to 2500 mL at time of birth)” and have included the original definitions as secondary outcomes.
“Maternal Hb concentration less than 9 g/dL 24 to 48 hours postpartum” changed to “Maternal Hb concentration less than 9 g/dL 24 to 72 hours postpartum” - as Hb levels may be taken within the first three days postnatal, rather than the first two.
Secondary outcomes deleted (to reduce number of outcomes)
Iron therapy during the puerperium.
Length of the third stage greater than or equal to 60 minutes.
Nausea between birth of baby and discharge from the labour ward.
Headache between birth of baby and discharge from the labour ward.
Maternal views of third-stage management (assessed using a validated questionnaire).
Maternal Hb concentration less than 9 g/dL postdischarge and up to six weeks.
Sequelae of PPH (length of stay; infection; re-admission).
Infant Hb level and iron indices beyond three months.
Changes to secondary outcomes
“Administration of oral or rectal analgesia (e.g. paracetamol, codeine, non-steroidals) between birth of the baby and discharge from the labour ward” and “Administration of opiate analgesia between birth of the baby and discharge from the labour ward” combined as “Administration of any analgesia between birth of the baby and discharge from the labour ward”.
“Secondary blood loss equal to or greater than 500 mL (clinically estimated or measured after 24 hours and before six weeks)” and “Any vaginal bleeding needing treatment (after 24 hours and before six weeks)” and “Uterotonic treatment after 24 hours and before six weeks” combined as “Secondary blood loss/any vaginal bleeding needing treatment (after 24 hours and before six weeks)”.
“Infant Hb level (Hb) at 24 to 48 hours” changed to “at 24 to 72 hours,” as Hb levels may be taken within the first three days postnatal, rather than the first two.
“Intraventricular haemorrhage (preterm infants): (i) grade III/IV; (ii) all grades (Sarnat 1976)” changed to “Papille grade III/IV intraventricular haemorrhage (for infants born before 34 weeks gestation only).”
“Transfusion requirements (preterm infants): (i) number of infants exposed to one or more red blood cell transfusions; (ii) number of transfusions per infant; (iii) number of donors to whom the infant was exposed” changed to “Number of infants who received a red blood cell transfusion.”
“Breastfeeding at discharge from hospital and at interval assessments until six months” changed to “Exclusive breastfeeding at discharge from hospital”
New secondary outcomes included
“Neonatal mortality” included.
SUMMARY OF FINDINGS FOR THE MAIN COMPARISON
Active versus expectant management of the third stage of labour (all women).
Population: All women who expected a vaginal birth at 24 weeks’ gestation or later
>Intervention: Active management of the third stage of labour
Comparison: Expectant management of the third stage of labour
| Outcomes | Illustrative comparative risks* (95% CI) |
Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
|---|---|---|---|---|---|---|
| Assumed risk | Corresponding risk | |||||
| Expectant management Active management of of the third stage of the third stage of labour labour | ||||||
| Severe primary postpartum haemorrhage (PPH) (clinically estimated or measured blood loss ≥ to 1000 mL at time of birth) | 24 per 1000 | 8 per 1000 (3 to 21) | RR 0.34 (0.14 to 0.87) | 4636 (3 studies) | ⊕○○○ very low1 | |
| Very severe primary PPH (clinically estimated or measured blood loss ≥ to 2500 mL at time of birth) | See comment | See comment | Not estimable | 0 (0) | See comment | No data |
| Maternal mortality | See comment | See comment | Not estimable | 0 (0) | See comment | No data |
| Maternal Hb <9 g/dL 24 to 72 hours postpartum | 71 per 1000 | 36 per 1000 (21 to 59) | RR 0.5 (0.3 to 0.83) | 1572 (2 studies) | ⊕⊕○○ low2 | |
| Admission to neonatal special/intensive care | 52 per 1000 | 42 per 1000 (31 to 58) | RR 0.81 (0.6 to 1.11) | 3207 (2 studies) | ⊕⊕○○ low3 | |
| Neonatal jaundice requiring phototherapy or exchange transfusion | 49 per 1000 | 47 per 1000 (27 to 83) | RR 0.96 (0.55 to 1.68) | 3142 (2 studies) | ⊕○○○ very low4 | |
| Neonatal polycythaemia treated with dilutional exchange transfusion | See comment | See comment | Not estimable | 0 (0) | See comment | No data |
The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI).
CI: Confidence interval; RR: Risk ratio;
GRADE Working Group grades of evidence
High quality: Further research is very unlikely to change our confidence in the estimate of effect.
Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate.
Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate.
Very low quality: We are very uncertain about the estimate.
Risk of bias: Of the 3 trials providing data for this outcome, all 3 are at low risk of bias for sequence generation (selection bias), allocation concealment (selection bias) and for incomplete outcome data (attrition bias). Two are at low-risk of selective reporting (reporting bias) and one is unclear. One study is at high risk of other bias (see Risk of bias tables).
Inconsistency: There is some overlap of confidence intervals of the 3 trials. However, Tau² = 0.38, the P value for the Chi² test of heterogeneity is 0.08 and I² = 60%. These suggest the presence of heterogeneity, which cannot be explained by any of the subgroups or sensitivity analyses performed.
Indirectness: Directly answers the question.
Imprecision: Total (cumulative) sample size 4,636 is less than the optimal information size (OIS) of 18,590 (assuming α = 0.05, 1-β = 0.80, relative risk reduction (RRR) of 25% from control event rate).
Risk of bias: Of the 2 trials providing data for this outcome, both are at low risk of bias for sequence generation (selection bias) and allocation concealment (selection bias), One is at low risk of bias for incomplete outcome data (attrition bias) while the other is high-risk. One is at high-\-risk of selective reporting (reporting bias) and one is unclear. Both studies are at high risk of other bias (see Risk of bias tables).
Inconsistency: The confidence intervals of the 2 trials overlap. Tau² = 0.02, the p value for the Chi² test of heterogeneity is 0.31 and I² = 3%. Although Tau² is non-zero, tests suggest an absence of unexplained heterogeneity. Indirectness: Directly answers the question.
Imprecision: Total (cumulative) sample size 1,572 is less than the optimal information size (OIS) of 5,804 (assuming α = 0.05,1-β = 0.80, RRR of 25% from control event rate).
Publication bias: Assessment of funnel plot asymmetry not performed due to <10 studies included for this outcome.
Risk of bias: Of the 2 trials providing data for this outcome, both are at low risk of bias for sequence generation (selection bias), allocation concealment (selection bias) and for incomplete outcome data (attrition bias). One is at low-risk of selective reporting (reporting bias) and one is unclear. One study is at high risk of other bias and the other is at low-risk of bias (see Risk of bias tables).
Inconsistency: The confidence intervals of the 2 trials overlap. Heterogeneity: Tau² = 0.00; Chi² P value = 0.40, I² = 0%. This suggests an absence of unexplained heterogeneity.
Indirectness: Directly answers the question.
Imprecision: Total (cumulative) sample size 3,207 is less than the optimal information size (OIS) of 8,066 (assuming α = 0.05, 1-β =0.80, RRR of 25% from control event rate).
Publication bias: Assessment of funnel plot asymmetry not performed due to <10 studies included for this outcome.
Risk of bias: Of the 2 trials providing data for this outcome, both are at low risk of bias for sequence generation (selection bias), allocation concealment (selection bias) and for incomplete outcome data (attrition bias). One is at low-risk of selective reporting (reporting bias) and one is unclear. One study is at high risk of other bias and the other is at low-risk of bias (see Risk of bias tables).
Inconsistency: There is some overlap of confidence intervals of the 2 trials. However, Heterogeneity: Tau² = 0.11, P value for heterogeneity = 0.09) and I² = 66%. These suggest the presence of heterogeneity, which cannot be explained by any of the subgroups or sensitivity analyses performed.
Indirectness: Directly answers the question.
Imprecision: Total (cumulative) sample size 3,142 is less than the optimal information size (OIS) of 8,584 (assuming α = 0.05, 1-β = 0.80, RRR of 25% from control event rate).
Publication bias: Assessment of funnel plot asymmetry not performed due to <10 studies included for this outcome.
Footnotes
DECLARATIONS OF INTEREST
Cecily Begley was the lead researcher on the ‘Dublin trial’ (Begley 1990).
Gill Gyte has written extensively on third-stage management and is currently involved in the development of trial protocols to assess the impact on mother and infant of deferred cord clamping in preterm infants.
William McGuire is also involved in the development of the same trial protocols to assess the impact on mother and infant of deferred cord clamping in term and preterm infants.
Andrew Weeks is also on the same trial team related to the timing of cord clamping as well as investigating the use of misoprostol for PPH prophylaxis in rural Uganda. He is also one of nine inventors of a small resuscitation trolley (the BASICS trolley) that allows neonatal resuscitation with an intact cord.
INDEX TERMS
Medical Subject Headings (MeSH)
*Oxytocics [administration & dosage; adverse effects]; *Watchful Waiting; Birth Weight; Constriction; Delivery, Obstetric [adverse effects; *methods]; Labor Stage, Third [*physiology]; Placenta; Postpartum Hemorrhage [*prevention & control]; Randomized Controlled Trials as Topic
MeSH check words
Female; Humans; Pregnancy
REFERENCES
* Indicates the major publication for the study
References to studies included in this review
- Begley 1990.Begley CM. Comparative studies in the third stage of labour [thesis] Trinity College, University of Dublin, Ireland; Dublin: 1989. [Google Scholar]; *; Begley CM. A comparison of ‘active’ and ‘physiological’ management of the third stage of labour. Midwifery. 1990;6:3–17. doi: 10.1016/s0266-6138(05)80091-9. [DOI] [PubMed] [Google Scholar]; Begley CM. Personal communication. 1987. A comparison of physiological and pharmacological methods of managing the third stage of labour. [Google Scholar]; Begley CM. The effect of ergometrine on breast feeding. Midwifery. 1990;6:60–72. doi: 10.1016/s0266-6138(05)80150-0. [published and unpublished data] [DOI] [PubMed] [Google Scholar]
- Jangsten 2011.Jangsten E, Mattsson LA, Lyckestam I, Hellstrom AL, Berg M. A comparison of active management and expectant management of the third stage of labour: a Swedish randomised controlled trial. BJOG: an international journal of obstetrics and gynaecology 2011. 2011;118(3):362–369. doi: 10.1111/j.1471-0528.2010.02800.x. [published and unpublished data] [DOI] [PubMed] [Google Scholar]
- Jerbi 2007.Jerbi M, Hidar S, Elmoueddeb, Chaieb A, Khairi H. Oxytocin in the third stage of labor. International Journal of Gynecology & Obstetrics. 2007;96(3):198–9. doi: 10.1016/j.ijgo.2006.11.019. [published data only] [DOI] [PubMed] [Google Scholar]
- Khan 1997.Khan GQ, John IS, Wani S, Doherty T, Sibai BM. Controlled cord traction versus minimal intervention techniques in delivery of the placenta: a randomised controlled trial. American Journal of Obstetrics and Gynecology. 1997;177(4):770–4. doi: 10.1016/s0002-9378(97)70266-7. [published data only] [DOI] [PubMed] [Google Scholar]
- Prendiville 1988.Elbourne DR, Harding J. The Bristol Third Stage Trial; Proceedings of Research and the Midwives Conference; Manchester, UK. 1989.1989. pp. 19–31. [Google Scholar]; Harding JE, Elbourne DR, Prendiville WJ. Views of mothers and midwives participating in the Bristol randomized, controlled trial of active management of the third stage of labor. Birth. 1989;16:1–6. doi: 10.1111/j.1523-536x.1989.tb00846.x. [DOI] [PubMed] [Google Scholar]; *; Prendiville WJ, Harding JE, Elbourne DR, Stirrat GM. The Bristol third stage trial: active versus physiological management of third stage of labour. BMJ. 1988;297:1295–300. doi: 10.1136/bmj.297.6659.1295. [published data only] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers 1998.*; Rogers J, Wood J, McCandlish R, Ayers S, Truesdale A, Elbourne D. Active versus expectant management of third stage of labour: the Hinchingbrooke randomised controlled trial. Lancet. 1998;351(9104):693–9. doi: 10.1016/S0140-6736(97)09409-9. see comments. [DOI] [PubMed] [Google Scholar]; Wood J, Rogers J, Elbourne D, McCandlish R, Truesdale A. The Hinchingbrooke third stage trial; International Confederation of Midwives 24th Triennial Congress; Oslo, Norway. 1996 May 26-31; 1996. p. 140. [published data only] [Google Scholar]
- Thilaganathan 1993.Thilaganathan B, Cutner A, Latimer J, Beard R. Management of the third stage of labour in women at low risk of postpartum haemorrhage. European Journal of Obstetrics & Gynecology and Reproductive Biology. 1993;48:19–22. doi: 10.1016/0028-2243(93)90048-h. [published data only] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
- Abdel-Aleem 2010.Abdel-Aleem H, Singata M, Abdel-Aleem M, Mshweshwe N, Williams X, Hofmeyr GJ. Uterine massage to reduce postpartum hemorrhage after vaginal delivery. International Journal of Gynecology & Obstetrics. 2010;111(1):32–6. doi: 10.1016/j.ijgo.2010.04.036. [published data only] [DOI] [PubMed] [Google Scholar]
- Hoffman 2006.*; Hoffman M, Castagnola D, Naqvi F. A randomized trial of active versus expectant management of the third stage of labor [abstract] American Journal of Obstetrics and Gynecology. 2006;195(6 Suppl 1):S107. [Google Scholar]; Hoffman M, Naqvi F, Sciscione A. A randomized trial of active versus expectant management of the third stage of labor [abstract] American Journal of Obstetrics and Gynecology. 2004;191(6 Suppl 1):S82. [published data only] [Google Scholar]
- Kashanian 2010.Kashanian M, Fekrat M, Masoomi Z, Ansari NS. Comparison of active and expectant management on the duration of the third stage of labour and the amount of blood loss during the third and fourth stage of labour: a randomised controlled trial. Midwifery. 2010;26(2):241–5. doi: 10.1016/j.midw.2008.03.004. [published data only] [DOI] [PubMed] [Google Scholar]
- Magann 2006.Magann EF, Doherty DA, Briery CM, Niederhauser A, Chauhan SP, Morrison JC. Obstetric characteristics for a prolonged third stage of labor and risk for postpartum hemorrhage. Gynecologic and Obstetric Investigation. 2008;65(3):201–5. doi: 10.1159/000112227. [published data only] [DOI] [PubMed] [Google Scholar]; *; Magann EF, Doherty DA, Briery CM, Niederhauser A, Morrison JC. Timing of placental delivery to prevent post-partum haemorrhage: lessons learned from an abandoned randomised clinical trial. Australian and New Zealand Journal of Obstetrics and Gynaecology. 2006;46(6):549–51. doi: 10.1111/j.1479-828X.2006.00658.x. [published data only] [DOI] [PubMed] [Google Scholar]
- Muller 1996.Muller R, Beck G. Active management of the third stage of labour; 19th Swiss Congress of the Swiss Society of Gynecology and Obstetrics; Interlaken, Switzerland. 1996 June; 1996. [published data only] [Google Scholar]
- Ramirez 2001.Ramirez O, Benito V, Jimenez R, Valido C, Hernandez C, Garcia JA. Third stage of labour: active or expectant management? preliminary results [abstract] Journal of Perinatal Medicine. 2001;(Suppl 1(Pt 2)):364. [published data only] [Google Scholar]
- Vasegh 2005.Vasegh FR, Bahiraie A, Mahmoudi M, Salehi L. Comparison of active and physiologic management of third stage of labor. HAYAT: The Journal of Tehran Faculty of Nursing & Midwifery. 2005;10(23):102. [published data only] [Google Scholar]
References to studies awaiting assessment
- Rosario 1973.Rosario YP Do, Jain CK. Active management of third stage of labour. Journal of Obstetrics and Gynaecology of India. 1973;23(1):66–9. [published data only] [Google Scholar]
References to ongoing studies
- Gülmezoglu 2009.Gülmezoglu AM, Widmer M, Merialdi M, Qureshi Z, Piaggio G, Elbourne D, et al. Active management of the third stage of labour without controlled cord traction: a randomized non-inferiority controlled trial. Reproductive Health. 2009;6:2. doi: 10.1186/1742-4755-6-2. DOI: 10.1186/1742-4755-6-2. [published data only] [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
- Abouzaher 1998.Abouzaher C. Antepartum and postpartum haemorrhage. In: Murray CJL, Lopez AD, editors. Health Dimensions of Sex and Reproduction. Harvard University Press; Boston: 1998. pp. 172–4. [Google Scholar]
- Bais 2004.Bais J, Eskes M, Pel M, Bonsel G, Bleker O. Postpartum haemorrhage in nulliparous women: incidence and risk factors in low and high risk women. European Journal of Obstetrics & Gynecology and Reproductive Biology. 2004;115(2):166–72. doi: 10.1016/j.ejogrb.2003.12.008. [DOI] [PubMed] [Google Scholar]
- Begley 2009.Begley C, Devane D, Clarke M. An evaluation of midwifery led care in the Health Service Executive North Eastern area: the report of the MidU study. Health Service Executive; Dublin: Dec, 2009. [Google Scholar]
- Blackburn 2008.Blackburn S. Physiological third stage of labour and birth at home. In: Edwins J, editor. Community Midwifery Practice. Blackwell Publishing; Oxford: 2008. [Google Scholar]
- Bloomfield 1990.Bloomfield TH, Gordon H. Reaction to blood loss at delivery. Journal of Obstetrics and Gynaecology. 1990;10(Suppl 2):S13–S16. [Google Scholar]
- Bonnar 1970.Bonnar J, McNicol GP, Douglas AS. Coagulation and fibrinolysis mechanisms during and after normal childbirth. British Medical Journal. 1970;2(103):200–3. doi: 10.1136/bmj.2.5703.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley 2004.Buckley SJ. Undisturbed birth - nature’s hormone blueprint for safety, ease and ecstasy. MIDIRS Midwifery Digest. 2004;14(2):203–9. [Google Scholar]
- Bullough 1989.Bullough CH, Msuku RS, Karonde L. Early suckling and postpartum haemorrhage: controlled trial in deliveries by traditional birth attendants. Lancet. 1989;2(8662):522–5. doi: 10.1016/s0140-6736(89)90652-1. [DOI] [PubMed] [Google Scholar]
- Burnley 2006.Burnley M, Roberts CL, Thatcher R, Doust JH, Jones AM. Influence of blood donation on O2uptake on-kinetics, peak O2 uptake and time to exhaustion during severe-intensity cycle exercise in humans. Experimental Physiology. 2006;91:499–509. doi: 10.1113/expphysiol.2005.032805. [DOI] [PubMed] [Google Scholar]
- Cernadas 2006.Cernadas JM, Carroli G, Pellegrini L, Otano L, Ferreira M, Ricci C, et al. The effect of timing of cord clamping on neonatal venous hematocrit values and clinical outcome at term: a randomized, controlled trial. Pediatrics. 2006;117(4):e779–e786. doi: 10.1542/peds.2005-1156. [DOI] [PubMed] [Google Scholar]
- Chaparro 2006.Chaparro CM, Neufeld LM, Tena Alavez G, Eguia-Líz Cedillo R, Dewey KG. Effect of timing of umbilical cord clamping on iron status in Mexican infants: a randomised controlled trial. Lancet. 2006;367(9527):1997–2004. doi: 10.1016/S0140-6736(06)68889-2. [DOI] [PubMed] [Google Scholar]
- Cotter 2001.Cotter A, Ness A, Tolosa J. Prophylactic oxytocin for the third stage of labour. Cochrane Database of Systematic Reviews. 2001;(4) doi: 10.1002/14651858.CD001808.pub3. DOI: 10.1002/14651858.CD001808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deeks 2001.Deeks JJ, Altman DG, Bradburn MJ. Statistical methods for examining heterogeneity and combining results from several studies in meta-analysis. In: Egger M, Davey Smith G, Altman DG, editors. Systematic Reviews in Health Care: Meta-analysis in Context. BMJ Books; London: 2001. [Google Scholar]
- Devane 2007.Devane D, Begley C, Clarke M, Horey D, OBoyle C. Evaluating maternity care: a core set of outcome measures. Birth. 2007;34(2):164–72. doi: 10.1111/j.1523-536X.2006.00145.x. [DOI] [PubMed] [Google Scholar]
- Egger 1997.Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–34. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbourne 1995.Elbourne D. Care in the third stage of labour. In: Robinson S, Thomson AM, editors. Midwives, Research and Childbirth. Vol. 4. Chapman & Hall; London: 1995. pp. 192–207. [Google Scholar]
- Farrar 2009a.Farrar D, Airey R, Tuffnell D, Law G, Cattle B, Duley L. Measuring placental transfusion for term births: weighing babies with the cord intact. Archives of Disease in Childhood. Fetal and Neonatal Edition. 2009;94(Suppl 1):Fa7. doi: 10.1111/j.1471-0528.2010.02781.x. [DOI] [PubMed] [Google Scholar]
- Farrar 2009b.Farrar D, Airey R, Tuffnell D, Duley L. Care during the third stage of labour: a postal survey of obstetricians and midwives. Archives of Disease in Childhood. Fetal and Neonatal Edition. 2009;94(Suppl 1):Fa40. [Google Scholar]
- Fleiss 1981.Fleiss JL. Statistical Methods for Rates and Proportions. 2nd Edition John Wiley & Sons; New York: 1981. [Google Scholar]
- Fry 2007.Fry J. Physiological third stage of labour: support it or lose it. British Journal of Midwifery. 2007;15(11):693–5. [Google Scholar]
- Greer 1998.Greer I, Lang G, Patel N. [accessed 2008];The management of postpartum haemorrhage: a clinical practice guideline for professionals involved in maternity care in Scotland. 2002 1998 http://www.abdn.ac.uk/spcerh/pubs.shtml# SPCERH publication 6.
- Gyte 1992.Gyte G. The significance of blood loss at delivery. MIDIRS Midwifery Digest. 1992;2(1):88–92. [Google Scholar]
- Gyte 1994.Gyte GM. Evaluation of the meta-analyses on the effects, on both mother and baby, of the various components of ‘active’ management of the third stage of labour. Midwifery. 1994;10(4):183–99. doi: 10.1016/0266-6138(94)90054-x. [DOI] [PubMed] [Google Scholar]
- Gyte 2006.Gyte G. The third stage of labour. Part 2: active management of third stage. National Childbirth Trust New Digest. 2006;36:22–8. [Google Scholar]
- Gülmezoglu 2007.Gülmezoglu AM, Forna F, Villar J, Hofmeyr GJ. Prostaglandins for preventing postpartum haemorrhage. Cochrane Database of Systematic Reviews. 2007;(3) doi: 10.1002/14651858.CD000494.pub3. DOI: 10.1002/14651858.CD000494.pub3. [DOI] [PubMed] [Google Scholar]
- Harbord 2006.Harbord RM, Egger M, Sterne JA. A modified test for small-study effects in meta-analyses of controlled trials with binary endpoints. Statistics in Medicine. 2006;25:3443–57. doi: 10.1002/sim.2380. [DOI] [PubMed] [Google Scholar]
- Harding 1989.Harding JE, Elbourne DR, Prendiville WJ. Views of mothers and midwives participating in the Bristol randomized, controlled trial of active management of the third stage of labor. Birth. 1989;16:1–6. doi: 10.1111/j.1523-536x.1989.tb00846.x. [DOI] [PubMed] [Google Scholar]
- Harris 2004.Harris T. Care in the third stage of labour. In: Henderson C, MacDonald S, editors. Mayes Midwifery. Bailliere Tindall; Edinburgh: 2004. pp. 507–23. [Google Scholar]
- Harris 2006.Harris T. An explanation for third stage practice variation: The theory of contingent decision making; Normal labour and birth: 3rd Research Conference; Grange-over-Sands, England, UK. 2006 June 7-9.2006. [Google Scholar]
- Hatem 2008.Hatem M, Sandall J, Devane D, Soltani H, Gates S. Midwife-led versus other models of care for childbearing women. Cochrane Database of Systematic Reviews. 2008;(4) doi: 10.1002/14651858.CD004667.pub2. DOI: 10.1002/14651858.CD004667.pub2. [DOI] [PubMed] [Google Scholar]
- Herman 2002.Herman A, Zimerman A, Arieli S, Tovbin Y, Bezer M, Bukovsky I, et al. Down-up sequential separation of the placenta. Ultrasound in Obstetrics & Gynecology. 2002;19:278–81. doi: 10.1046/j.1469-0705.2002.00557.x. [DOI] [PubMed] [Google Scholar]
- Higgins 2009.Higgins JPT, Thompson SG, Spiegelhalter DJ. A reevaluation of random-effects meta-analysis. Journal of the Royal Statistical Society. 2009;172:137–59. doi: 10.1111/j.1467-985X.2008.00552.x. Series A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins 2011.The Cochrane Collaboration Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011] 2011 Available from www.cochrane-handbook.org.
- Hodnett 2003.Hodnett ED, Gates S, Hofmeyr GJ, Sakala C. Continuous support for women during childbirth. Cochrane Database of Systematic Reviews. 2003;(3) doi: 10.1002/14651858.CD003766. DOI: 10.1002/14651858.CD003766. [DOI] [PubMed] [Google Scholar]
- Hutton 2007.Hutton EK, Hassan ES. Late vs early clamping of the umbilical cord in full-term neonates: systematic review and meta-analysis of controlled trials. JAMA. 2007;297:1241–52. doi: 10.1001/jama.297.11.1241. [DOI] [PubMed] [Google Scholar]
- Hytten 2001.Hytten F. The physiology of the puerperium. In: Chamberlain G, Steer P, editors. Turnbull’s Obstetrics. 3rd Edition Churchill Livingstone; Edinburgh: 2001. pp. 635–46. [Google Scholar]
- ICM 2008.International Confederation of Midwives . Role of the Midwife in Physiological Third Stage Labour: Position Statement. International Confederation of Midwives; The Hague: 2008. [Google Scholar]
- ICM-FIGO 2003.International Confederation of Midwives (ICM) International Federation of Gynaecology and Obstetrics (FIGO) [accessed 9 May 2008];Management of the third stage of labour to prevent post-partum haemorrhage. Joint statement. 2008 http://www.figo.org/docs/PPH%20Joint%20Statement.pdf.
- ICM-FIGO 2006.International Confederation of Midwives (ICM) International Federation of Gynaecology and Obstetrics (FIGO) [accessed 9 May 2008];Prevention and treatment of post-partum haemorrhage: new advances for low resource settings. Joint statement. 2006 http://www.figo.org/docs/PPH%20Joint%20Statement%202%20English.pdf.
- Inch 1985.Inch S. Management of third stage of labour - another cascade of intervention? Midwifery. 1985;1(2):114–22. [Google Scholar]
- Kanikosmay 2007.Kanikosmay F. Third stage: the why of physiological practice. Midwives, the official journal of the Royal College of Midwives. 2007;10(9):422–5. [PubMed] [Google Scholar]
- Khan 2006.Khan KS, Wojdyla D, Say L, Gülmezoglu AM, Van Look PF. WHO analysis of causes of maternal death: a systematic review. Lancet. 2006;367(9516):1066–74. doi: 10.1016/S0140-6736(06)68397-9. [DOI] [PubMed] [Google Scholar]
- Lazarus 2005.Lazarus JV, Lalonde A. Reducing postpartum haemorrhage in Africa. International Journal of Gynecology & Obstetrics. 2005;88(1):89–90. doi: 10.1016/j.ijgo.2004.09.016. [DOI] [PubMed] [Google Scholar]
- Lewis 2007.Lewis G. The Confidential Enquiry into Maternal and Child Health (CEMACH). Saving Mothers’ Lives: reviewing maternal deaths to make motherhood safer-2003-2005. The Seventh Report on Confidential Enquiries into Maternal Deaths in the United Kingdom. CEMACH; London: 2007. [Google Scholar]
- Liabsuetrakul 2007.Liabsuetrakul T, Choobun T, Peeyananjarassri K, Islam QM. Prophylactic use of ergot alkaloids in the third stage of labour. Cochrane Database of Systematic Reviews. 2007;(2) doi: 10.1002/14651858.CD005456.pub2. DOI: 10.1002/14651858.CD005456.pub2. [DOI] [PubMed] [Google Scholar]
- Maughan 2006.Maughan KL, Heim SW, Galazka SS. Preventing postpartum hemorrhage: managing the third stage of labor. American Family Physician. 2006;73(6):1025–8. [PubMed] [Google Scholar]
- McDonald 2003.McDonald S. Physiology and management of the third stage of labour. In: Fraser DM, Cooper MA, editors. Myles Textbook for Midwives. Churchill Livingstone; Edinburgh: 2003. pp. 507–30. [Google Scholar]
- McDonald 2007a.McDonald S. Management of the third stage of labour. Journal of Midwifery and Women’s Health. 2007;52(3):254–61. doi: 10.1016/j.jmwh.2007.02.012. [DOI] [PubMed] [Google Scholar]
- McDonald 2007b.McDonald SJ, Abbott JM, Higgins SP. Prophylactic ergometrine-oxytocin versus oxytocin for the third stage of labour. Cochrane Database of Systematic Reviews. 2004;(1) doi: 10.1002/14651858.CD000201.pub2. DOI: 10.1002/14651858.CD000201.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald 2008.McDonald SJ, Middleton P. Effect of timing of umbilical cord clamping of term infants on maternal and neonatal outcomes. Cochrane Database of Systematic Reviews. 2008;(3) doi: 10.1002/14651858.CD004074.pub2. DOI: 10.1002/14651858.CD004074.pub2. [DOI] [PubMed] [Google Scholar]
- Mercer 2000.Mercer JS, Nelson CC, Skovgaard RL. Umbilical cord clamping: beliefs and practices of American nurse-midwives. Journal of Midwifery & Women’s Health. 2000;45(1):58–66. doi: 10.1016/s1526-9523(99)00004-5. [DOI] [PubMed] [Google Scholar]
- Mercer 2001.Mercer JS. Current best evidence: a review of the literature on umbilical cord clamping. Journal of Midwifery & Women’s Health. 2001;46(6):402–14. doi: 10.1016/s1526-9523(01)00196-9. [DOI] [PubMed] [Google Scholar]
- Mercer 2008.Mercer J, Skovgaard R, Erickson-Owens D. Normal Childbirth: Evidence and Debate. 2nd Edition Churchill Livingstone; Edinburgh: 2008. Fetal to neonatal transition: first do no harm. In: Downe S editor (s) pp. 149–74. [Google Scholar]
- Mims 2005.Mims MP, Prchal JT. Hematology during pregnancy. In: Lichtman MA, Williams WJ, Beutler E, Kaushansky K, Kipps TJ, Seligsohn U, et al., editors. Williams Haematology. 7th Edition McGraw Hill Medical; New York: 2005. pp. 101–10. [Google Scholar]
- Mousa 2007.Mousa HA, Alfirevic Z. Treatment for primary postpartum haemorrhage. Cochrane Database of Systematic Reviews. 2007;(1) doi: 10.1002/14651858.CD003249.pub2. DOI: 10.1002/14651858.CD003249.pub2. [DOI] [PubMed] [Google Scholar]
- NICE 2007.National Institute for Health and Clinical Excellence . Intrapartum care: care of healthy women and their babies during childbirth, Guideline 55. National Institute for Health and Clinical Excellence; London: 2007. Normal labour: third stage. [PubMed] [Google Scholar]
- Nordstrom 1997.Nordstrom L, Fogelstam K, Fridman G, Larsson A, Rydhstroem H. Routine oxytocin in the third stage of labour: a placebo controlled randomised trial. British Journal of Obstetrics and Gynaecology. 1997;104(7):781–6. doi: 10.1111/j.1471-0528.1997.tb12020.x. [DOI] [PubMed] [Google Scholar]
- NZCM 2009.New Zealand College of Midwives . Third stage management practices of midwife lead maternity carers: an analysis of the New Zealand College of Midwives Midwifery Database Information 2004 -2008. New Zealand College of Midwives; Christchurch: 2009. [Google Scholar]
- Parsons 2007.Parsons SM, Walley RL, Crane JM, Matthews K, Hutchens D. Rectal misoprostol versus oxytocin in the management of the third stage of labour. Journal of Obstetrics and Gynaecology. 2007;29(9):711–8. doi: 10.1016/s1701-2163(16)32594-4. [DOI] [PubMed] [Google Scholar]
- Penney 2005.Penney G, Adamson L, Kernaghan D. [accessed 2005];Scottish confidential audit of severe maternal morbidity. Second Annual Report 2004. Aberdeen. Scottish Programme for Clinical Effectiveness in Reproductive Health. 2005 http://www.abdn.ac.uk/spcerh/pubs.htm#2005
- Phillip 2004.Phillip H, Fletcher H, Reid M. The impact of induced labour on postpartum blood loss. Journal of Obstetrics and Gynaecology. 2004;24(1):12–5. doi: 10.1080/01443610310001620215. [DOI] [PubMed] [Google Scholar]
- Prendiville 1989.Prendiville WJ, Elbourne DR. Care during the third stage of labour. In: Chalmers I, Enkin M, Keirse MJNC, editors. Effective Care in Pregnancy and Childbirth. Oxford University Press; Oxford: 1989. pp. 1145–69. [Google Scholar]
- Prendiville 1996.Prendiville WJ. The prevention of post partum haemorrhage: optimising routine management of the third stage of labour. European Journal of Obstetrics & Gynecology and Reproductive Biology. 1996;69:19–24. doi: 10.1016/0301-2115(95)02529-4. [DOI] [PubMed] [Google Scholar]
- Rabe 2004.Rabe H, Reynolds G, Diaz-Rossello J. Early versus delayed umbilical cord clamping in preterm infants. Cochrane Database of Systematic Reviews. 2004;(4) doi: 10.1002/14651858.CD003248.pub2. DOI: 10.1002/14651858.CD003248.pub2. [DOI] [PubMed] [Google Scholar]
- Razvi 2008.Razvi K, Chua S, Arulkumaran S, Ratnam SS. A comparison between visual estimation and laboratory determination of blood loss during the third stage of labour. Australian and New Zealand Journal of Obstetrics and Gynaecology. 2008;36(2):152–4. doi: 10.1111/j.1479-828x.1996.tb03273.x. [DOI] [PubMed] [Google Scholar]
- RCM 2004.Royal College of Midwives . Normal Childbirth: Position Statement. Royal College of Midwives; London: 2004. [Google Scholar]
- RCM 2008.Royal College of Midwives . Third Stage of Labour: Midwifery Practice Guideline. RCM; London: 2008. [Google Scholar]
- RCOG 2009.Duley LMM, Weeks AD, Hey EN, Drife JO. Clamping of the umbilical cord and placental transfusion. RCOG Scientific Advisory Committee; May 14, 2009. Opinion Paper. issue http://www.rcog.org.uk/clamping-umbilicalcord-and-placental-transfusion. [Google Scholar]
- RevMan 2011.The Nordic Cochrane Centre. The Cochrane Collaboration . Review Manager (RevMan). 5.1. The Nordic Cochrane Centre, The Cochrane Collaboration; Copenhagen: 2011. [Google Scholar]
- Romero-Gutierrez 2007.Romero-Gutierrez G, Espitia-Vera A, Ponce-Ponce de Leon AL, Huerta-Vargas LF. Risk factors of maternal death in Mexico. Birth. 2007;34(1):21–5. doi: 10.1111/j.1523-536X.2006.00142.x. [DOI] [PubMed] [Google Scholar]
- Sarnat 1976.Sarnat HB, Sarnat MS. Neonatal encephalopathy following fetal distress: a clinical and electrographic study. Archives of Neurology. 1976;33:696. doi: 10.1001/archneur.1976.00500100030012. [DOI] [PubMed] [Google Scholar]
- Sheiner 2005.Sheiner E, Sarid L, Levy A, Seidman DS, Hallak M. Obstetric risk factors and outcome of pregnancies complicated with early postpartum hemorrhage: a population-based study. Journal of Maternal-Fetal & Neonatal Medicine. 2005;18(3):149–54. doi: 10.1080/14767050500170088. [DOI] [PubMed] [Google Scholar]
- Soltani 2005.Soltani H, Dickinson F, Symonds I. Placental cord drainage after spontaneous vaginal delivery as part of the management of third stage of labour. Cochrane Database of Systematic Reviews. 2005;(4) doi: 10.1002/14651858.CD004665.pub2. DOI: 10.1002/14651858.CD004665.pub2. [DOI] [PubMed] [Google Scholar]
- Soltani 2006.Soltani H, Hutchon DR, Poulose TA. Timing of prophylactic oxytocics for the third stage of labour after vaginal birth. Cochrane Database of Systematic Reviews. 2006;(4) doi: 10.1002/14651858.CD006173.pub2. DOI: 10.1002/14651858.CD006173. [DOI] [PubMed] [Google Scholar]
- Soltani 2008.Soltani H. Global implications of evidence based practice: management of the third stage of labour. Midwifery. 2008;24:138–42. doi: 10.1016/j.midw.2008.03.002. [DOI] [PubMed] [Google Scholar]
- Su 2007.Su LL, Chong YS, Samuel M. Oxytocin agonists for preventing postpartum haemorrhage. Cochrane Database of Systematic Reviews. 2007;(3) doi: 10.1002/14651858.CD005457.pub2. DOI: 10.1002/14651858.CD005457.pub2. [DOI] [PubMed] [Google Scholar]
- Taylor 1981.Taylor DJ, Phillips P, Lind T. Puerperal haematological indices. British Journal of Obstetrics and Gynaecology. 1981;88(6):601–6. doi: 10.1111/j.1471-0528.1981.tb01215.x. [DOI] [PubMed] [Google Scholar]
- Tierney 2005.Tierney JF, Stewart LA. Investigating patient exclusion bias in meta-analysis. International Journal of Epidemiology. 2005;34:79–87. doi: 10.1093/ije/dyh300. [DOI] [PubMed] [Google Scholar]
- Van Rheenan 2007.Van Rheenen P, De Moor L, Eschbach S, De Grooth H, Brabin B. Delayed cord clamping and haemoglobin levels in infancy: a randomised controlled trial in term babies. Tropical Medicine & International Health. 2007;12(5):603–16. doi: 10.1111/j.1365-3156.2007.01835.x. [DOI] [PubMed] [Google Scholar]
- Weeks 2007.Weeks A. Umbilical cord clamping after birth. BMJ. 2007;335:312–3. doi: 10.1136/bmj.39282.440787.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner 2005.Werner EJ. Disorders of the fetomaternal unit. In: de Alarcon PA, Werner EJ, editors. Neonatal Hematology. Cambridge University Press; Cambridge: 2005. pp. 10–39. [Google Scholar]
- WHO 2003.World Health Organization . Managing Complications in Pregnancy and Childbirth: A Guide for Midwives and Doctors. World Health Organization; Geneva: 2003. [Google Scholar]
- WHO 2006.World Health Organization . WHO Recommendations for the Prevention of Postpartum Haemorrhage. World Health Organization; Geneva: 2006. [Google Scholar]
- Winter 2007.Winter C, Macfarlane A, Deneux-Tharaux C, Zhang W-H, Alexander S, Brocklehurst P, et al. Variations in policies for management of the third stage of labour and the immediate management of postpartum haemorrhage in Europe. BJOG: an international journal of obstetrics and gynaecology. 2007;114:845–54. doi: 10.1111/j.1471-0528.2007.01377.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao 1974.Yao AC, Lind J. Placental transfusion. American Journal of Diseases of Children. 1974;127:128–41. doi: 10.1001/archpedi.1974.02110200130021. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
- Begley 2010.Begley CM, Gyte GML, Murphy DJ, Devane D, McDonald SJ, McGuire W. Active versus expectant management for women in the third stage of labour. Cochrane Database of Systematic Reviews. 2010;(7) doi: 10.1002/14651858.CD007412.pub2. DOI: 10.1002/14651858.CD007412.pub2. [DOI] [PubMed] [Google Scholar]
- Prendiville 2000.Prendiville WJ, Elbourne D, McDonald S. Active versus expectant management in the third stage of labour. Cochrane Database of Systematic Reviews. 2000;(3) doi: 10.1002/14651858.CD000007. DOI: 10.1002/14651858.CD000007. [DOI] [PubMed] [Google Scholar]