Abstract
BACKGROUND
Associations of either insulin receptor substrate 1 (IRS1) variants or circulating 25-hydroxyvitamin D [25(OH)D] with type 2 diabetes (T2D) and insulin resistance (IR) are inconsistent. This study sought to determine whether circulating 25(OH)D modulates the association of a potentially functional variant at IRS1 (rs2943641) with insulin resistance.
METHOD
Interaction between IRS1 rs2943641 and circulating 25(OH)D on homeostasis model assessment for IR (HOMA-IR) was examined in the Boston Puerto Rican Health Study (BPRHS) (n = 1144). Replication was performed in the African-American (n = 1126), non-Hispanic white (n = 1967), and Hispanic (n = 1241) populations of the Multi-Ethnic Study of Atherosclerosis (MESA) with genotypes of 3 IRS1 variants, rs2972144, rs1515104, and rs2673142, which are tag single nucleotide polymorphisms (SNPs) and in strong linkage disequilibrium with rs2943641.
RESULTS
Higher circulating 25(OH)D was associated with lower risk of T2D and IR in BPRHS women homozygous for minor allele rs2943641T. Consistently, in each of 3 MESA populations, HOMA-IR and insulin decreased more evidently with higher circulating 25(OH)D in women of the rs2943641TT genotype than in carriers of the major allele (rs2943641C). Metaanalysis indicated significant and consistent interactions between circulating 25(OH)D and IRS1 variants on HOMA-IR (log transformed) [pooled β = −0.008, 95% CI: −0.016 to −0.001, P interaction = 0.004] and insulin (log transformed) (pooled β = −0.006, 95% CI: −0.011 to −0.002, P interaction = 0.023) in 3065 women of the 4 populations.
CONCLUSIONS
Participants with different genotypes of IRS1 rs2943641 exhibit differential benefit from high circulating 25(OH)D for the reduction of insulin resistance and T2D risk. This gene–nutrient interaction, which appears to be limited to women, warrants further examination in randomized controlled trials of vitamin D supplementation.
Recent genome-wide association studies (GWAS)4 have identified >30 novel genetic loci associated with type 2 diabetes (T2D) (1). Most of these loci preferentially affect T2D risk through β-cell function (2). One of the few T2D loci associated with insulin resistance (IR) encodes insulin receptor substrate 1 (IRS1), a key protein central to the insulin signaling pathway (3). A common genetic variant (rs2943641) in the neighborhood of IRS1 is associated with T2D, IR, and hyperinsulinemia in GWAS of Euro-pean populations, and this variant may disrupt the insulin signaling pathway (4). However, other large-scale GWAS or candidate gene studies have not been consistent in detecting an association between rs2943641 and T2D (5, 6). One recent trial (7) suggests that rs2943641 interacts with high-carbohydrate and low-fat diets to affect IR. Whether the association of rs2943641 with T2D or IR could be modulated by other nutrients or nutrient status is still unclear.
Vitamin D is not only paramount in maintaining calcium and phosphorus homeostasis and bone health, but is also important for the preservation of insulin secretion and insulin sensitivity (8). Accumulating evidence suggests that circulating 25-hydroxyvitamin D [25(OH)D], the best indicator of vitamin D status, is inversely associated with T2D risk (9). However, results from randomized controlled trials investigating the effects of vitamin D supplementation on insulin sensitivity and β-cell function are inconsistent (10–13). On the basis of a metaanalysis, there is a significant inverse association between 25(OH)D concentrations and T2D prevalence in a dose-responsive manner, but the association is rather weak (14). Such inconsistencies and the weak correlation might be attributable to individual genetic differences in genes and gene–environment interactions responsible for the vitamin D absorption and the separate metabolic processes involving vitamin D, energy homeostasis, and insulin.
In the kidneys, conversion of 25(OH)D to 1,25 dihydroxyvitamin D [1,25(OH)2D] activates the vitamin D receptor (VDR), which then forms a het-erodimer with retinoid-X receptor (RXR), and the 1,25(OH)2D-VDR-RXR complex regulates expression of hundreds of genes (15). Specifically, the presence of VDRs in human pancreatic β-cells (16) and the presence of a vitamin D response element in the human insulin receptor gene promoter (17) increase the biological plausibility that vitamin D participates in regulating glucose metabolism. In the present study, we aimed to test whether circulating 25(OH)D could modify associations of IRS1 variant rs2943641 with T2D and IR in Boston-based Puerto Rican adults. Replication of the results was conducted in African-American, non-Hispanic white, and Hispanic adults participating in the Multi-Ethnic Study of Atherosclerosis (MESA).
Materials and Methods
PARTICIPANTS
Participants were drawn from the Boston Puerto Rican Health Study (BPRHS), a longitudinal cohort study on stress, nutrition, health, and aging (18). The ancestry composition for the BPRHS is 57.2% European, 27.4% African, and 15.4% Native American (19). The current cross-sectional study consisted of 1144 self-identified Puerto Rican adults (336 men and 808 women), for whom baseline demographics and biochemical and rs2943641 genotype information were available. Dietary intake was measured with a semiquantitative food frequency questionnaire (FFQ), adapted and validated in this population (20). Data collection for demographics, health status, medications, and lifestyle factors have been described (18). The study protocol was approved by the Institutional Review Boards at Tufts University and Northeastern University. All participants gave informed consent.
Replication was conducted among the MESA participants, whose data were obtained from dbGaP (database of Genotypes and Phenotypes, http://www.ncbi.nlm.nih.gov/gap). MESA is a population-based prospective cohort, which recruited 6814 participants, ages 45–84 years, from 6 US centers between 2000 and 2002. MESA is designed to evaluate the presence, extent, and progression of subclinical cardiovascular disease and consists of participants who are non-Hispanic white (38%), African-American (28%), Hispanic (23%), or Asian (11%). The detailed objectives and design of MESA have been described (21). Our present cross-sectional analysis included 4334 participants, who were either non-Hispanic white (946 men and 1021 women), African-American (525 men and 601 women), or Hispanic (606 men and 635 women), but not Asian, as the minor allele frequency of rs2943641 is too low in this group. All dietary or biochemical measures used in this study were collected from the baseline MESA examination. Demographics, health status, medications, and lifestyle factors were collected by standard questionnaires. The MESA protocol was approved by the institutional review boards of all collaborating institutions and the National Heart, Lung, and Blood Institute. All participants gave informed consent.
BIOCHEMISTRY AND ANTHROPOMETRIC MEASUREMENTS
Blood samples were drawn after an overnight fast. For BPRHS, fasting serum glucose was measured with the Olympus Au400e with Olympus glucose reagents (Olympus America), and fasting serum insulin was measured with an IMMULITE 1000 (Siemens Medical Solutions Diagnostics). Plasma 25(OH)D was measured with a competitive protein binding reaction with an LKB Wallac Rackbeta 1215 Counter (Perkin Elmer) and Packard COBRA Software (22, 23). For MESA, fasting serum glucose was measured by the Vitros 950 analyzer (Johnson & Johnson Ortho-Clinical Diagnostics), and serum insulin was measured by the Linco Human Insulin Specific RIA kit (Linco Research). Serum 25(OH)D was measured with an RIA (25-Hydroxyvitamin D 125I RIA Kit; Diasorin) with reference to NIST standards, as described (24).
For both BPRHS and MESA, homeostasis model assessment of IR (HOMA-IR) was calculated as (fasting insulin × fasting glucose)/22.5. T2D was defined as fasting glucose ≥ 126 mg/dL (7 mmol/L) or use of diabetes medication. Both traits were natural log transformed to a normal distribution before analysis. Anthropometric data, including weight, height, and waist circumference, were measured by standard techniques, and body mass index was calculated as weight (kg)/ height (m)2.
GENOTYPING
For BPRHS, genomic DNA was extracted from blood samples with QIAamp DNA Blood mini kit (Qiagen), and single nucleotide polymorphism (SNP) rs2943641 was genotyped with the Applied Biosystems TaqMan SNP genotyping system. Details of genomic DNA extraction and genotyping have been described (25).
For MESA, DNA was extracted with a commercially available DNA isolation kit (Puregene, Gentra System). Affymetrix Genome-Wide Human SNP Array 6.0 was used for genome-wide genotyping. All genotype data and related information were requested from dbGaP after registration. As SNP rs2943641 was not available from the genome-wide genotyping data, 3 SNPs in high linkage disequilibrium (LD) with rs2943641, rs2972144 (for African-Americans), rs1515104 (for non-Hispanic whites), and rs2673142 (for Hispanics), were selected for replication in MESA. SNPs rs2972144, rs1515104, and rs2673142 are 7.7, 1.3, and 62.9 kb from rs2943641, respectively. In a Euro-pean population (CEU), rs2943641 is in complete LD (r2 = 1) with rs2972144 and rs1515104. In the His-panic population, rs2943641 is in complete LD (r2 =1) with rs2673142. In an African population (YRI), the LD (r2) of rs2943641 with rs2972144 is 0.91.
Population structure, known as genetic subgroups within a population, may exist because of admixed ancestries of participants and lead to a false association (19). Thus, we measured population structure in all 4 populations using principal component analysis with a selected set of informative ancestry markers and a program called SVS (GoldenHelix). The key eigenvalues of principal component analyses were included in models for adjustment for population structure in all analyses (19).
STATISTICAL ANALYSES
We used SAS 9.2 (SAS Institute Inc) to conduct statistical analyses. Fasting glucose, insulin, and HOMA-IR were natural log-transformed to achieve normal distribution before statistical analysis. Hardy–Weinberg equilibria of the corresponding SNPs were assessed by χ2 tests. We estimated Spearman correlation coefficients between circulating 25(OH)D and T2D traits after adjustment for potential confounders. Logistic regression models were used to test the interaction between rs2943641 and circulating 25(OH)D on T2D risk. General linear models were used to examine the interaction between rs2943641 and circulating 25(OH)D on T2D traits. In BPRHS, the potential confounders in these statistical models were age, sex, waist circumference, physical activity, alcohol drinking, smoking status, hormone replacement therapy, diabetes medication, total energy intake, total fat intake (% energy), renal function (serum creatinine), season of blood draw, and population structure. In MESA, potential confounders included age, sex, waist circumference, physical activity, alcohol drinking, smoking status, hormone replacement therapy, diabetes medication, total energy intake, total fat intake (% energy), renal function (serum creatinine), season of blood draw, study center, race, and population structure.
We carried out metaanalysis of the interaction effects by combining study-specific regression estimates (β), weighted by the inverse of their variance, with STATA software (version 12, StataCorp LP). The DerSimonian and Laird random-effects model, which takes both within- and between-study variation into consideration, was used for the metaanalysis.
As a sex-specific interaction between the IRS1 variant rs2943641 and dietary factors on risk of type 2 diabetes was observed in a prior report (26), statistical analysis was performed in men and women separately in both BPRHS and MESA. As significant interactions for T2D or T2D-related traits were observed only among women, only the results from women participants are reported in our present study. In addition, because of the low prevalence of T2D in all 3 MESA populations, replication was conducted only for T2D traits, including fasting insulin, glucose, and HOMA-IR.
Results
STUDY CHARACTERISTICS
In both BPRHS and MESA, no significant difference for anthropometric traits was observed among different IRS1 genotypes (see Supplemental Tables 1 and 2, which accompany the online version of this article at http://www.clinchem.org/content/vol60/issue1). In BPRHS, no significant association between rs2943641 and T2D or related traits was observed (6). The frequency of the minor T allele of SNP rs2943641 was 0.318. In MESA, no significant association was observed between rs2972144, rs1515104, rs2673142, and T2D traits. The minor allele frequencies of rs2972144 (T), rs1515104 (A), and rs2673142 (C) are 0.275, 0.35, and 0.293 for MESA African-American, non-Hispanic white, and Hispanic participants, respectively. None of the genotype frequencies for IRS1 variants in BPRHS or MESA deviate from Hardy–Weinberg equilibrium (P > 0.05).
ASSOCIATION OF CIRCULATING 25(OH)D WITH T2D AND RELATED TRAITS
In BPRHS women, 25(OH)D was not correlated with fasting glucose (r = −0.036, P = 0.32), insulin (r = −0.044, P = 0.22), or HOMA-IR (r = −0.05, P = 0.16), after controlling for potential confounders. In the multivariate adjusted model, 25(OH)D (higher vs lower than the population median) was not significantly associated with the risk of T2D [odds ratio (OR) = 0.83, 95% CI: 0.55–1.26]. In MESA women (combining the 3 MESA populations), 25(OH)D was inversely associated with fasting glucose (r = −0.09, P < 0.001) and HOMA-IR (r = −0.064, P = 0.004). However, 25(OH)D itself (higher vs lower than the population median) was not significantly associated with the risk of T2D (OR = 0.83, 95% CI: 0.59–1.17).
INTERACTION BETWEEN CIRCULATING 25(OH)D AND RS2943641 ON T2D RISK AND RELATED TRAITS IN BPRHS WOMEN
No significant gene–nutrient interaction of 25(OH)D for T2D or related traits was observed in BPRHS men. To assess the interaction between circulating 25(OH)D and rs2943641 on T2D risk, we tested different genetic models in BPRHS women (Table 1), and 25(OH)D was dichotomized according to the population median [17 ng/mL (42.4 nmol/L)] to maximize statistical power. Significant interactions were observed under both an additive genetic model (P = 0.006) and a recessive genetic model (P = 0.007) for rs2943641 minor T allele, but not for a dominant model. The rs2943641 minor allele T homozygotes had lower risk of T2D compared with C allele carriers only when 25(OH)D was higher than the median [>17 ng/mL (42.4 nmol/L)] (OR = 0.20, 95% CI: 0.07–0.55, P = 0.002). On the other hand, compared to subjects with circulating 25(OH)D ≤ 17 ng/mL (42.4 nmol/L), individuals with 25(OH)D >17 ng/mL (42.4 nmol/L) showed lower T2D risk (OR = 0.14, 95% CI: 0.02–0.76, P = 0.024) only in rs2943641 T homozygotes. Using the recommendation from the Institute of Medicine (22) of a circulating 25(OH)D concentration of 20 ng/mL (49.9 nmol/L), we dichotomized on this value. There was a significant interaction between circulating 25(OH)D and rs2943641 on T2D only under the T allele recessive model (P interaction = 0.023) (Table 1).
Table 1.
Interaction between circulating 25(OH)D and rs2943641 on risk of T2D in BPRHS women.
| 25(OH)D ≤17 ng/mL
|
25(OH)D >17 ng/mLa
|
25(OH)D ≤20 ng/mLb
|
25(OH)D >20 ng/mLb
|
|||||
|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | Pc | OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | |
| Association between rs2943641 and T2D by circulating 25(OH)D category | ||||||||
|
| ||||||||
| TT vs CC | 0.99 (0.35–2.83) | 0.989 | 0.19 (0.06–0.54) | 0.002 | 0.82 (0.34–1.95) | 0.648 | 0.13 (0.03–0.51) | 0.003 |
|
| ||||||||
| n | 41 vs 184 | 37 vs 185 | 54 vs 245 | 25 vs 127 | ||||
|
| ||||||||
| TT vs CT | 2.93 (0.98–8.75) | 0.054 | 0.21 (0.07–0.60) | 0.004 | 1.39 (0.57–3.37) | 0.465 | 0.21 (0.05–0.82) | 0.025 |
|
| ||||||||
| n | 41 vs 174 | 37 vs 181 | 54 vs 229 | 25 vs 128 | ||||
|
| ||||||||
| TT vs CC + CT | 1.58 (0.59–4.23) | 0.365 | 0.20 (0.07–0.55) | 0.002 | 1.05 (0.46–2.40) | 0.909 | 0.16 (0.04–0.60) | 0.007 |
|
| ||||||||
| n | 41 vs 358 | 37 vs 366 | 54 vs 474 | 25 vs 255 | ||||
|
| ||||||||
| Association between circulating 25(OH)D and T2D [OR of T2D for high vs low 25(OH)D) by rs2943641 genotype] | ||||||||
|
| ||||||||
| TT | 1 (ref)d | NA | 0.14 (0.02–0.76) | 0.024 | 1 (ref) | NA | 0.15 (0.03–0.86) | 0.034 |
|
| ||||||||
| n | 41 | 37 | 54 | 25 | ||||
|
| ||||||||
| CC | 1 (ref) | NA | 0.62 (0.32–1.24) | 0.176 | 1 (ref) | NA | 0.70 (0.35–1.42) | 0.325 |
|
| ||||||||
| n | 184 | 185 | 245 | 127 | ||||
|
| ||||||||
| CC + CT | 1 (ref) | NA | 1.00 (0.64–1.57) | 0.984 | 1 (ref) | NA | 0.77 (0.49–1.22) | 0.263 |
|
| ||||||||
| n | 358 | 366 | 474 | 255 | ||||
Circulating 25(OH)D was dichotomized based on the median concentration (17 ng/mL) in the BPRHS female population; P values for the interaction between circulating 25(OH)D and rs2943641 on the risk of T2D were 0.006, 0.007, and 0.429 for additive, recessive, and dominant genetic model of rs2943641 T allele, respectively.
Circulating 25(OH)D was dichotomized based on the Institute of Medicine recommended cutoff point (20 ng/mL); P values for the interaction between circulating 25(OH)D and rs2943641 on the risk of type 2 diabetes were 0.076, 0.023, and 0.55 for additive, recessive, and dominant genetic model of rs2943641 T allele, respectively.
P values were adjusted for age, waist circumference, physical activity, alcohol drinking, smoking status, hormone replacement therapy, diabetes medication, total energy intake, total fat intake (% energy), renal function (serum creatinine), season of blood draw. and population structure.
ref, Reference; NA, not applicable.
To assess modification of circulating 25(OH)D (continuous variable) on the association between rs2943641 with T2D traits, we tested different genetic models. For the whole BPRHS population, significant interactions between 25(OH)D and rs2943641 on insulin (P interaction = 0.019) and HOMA-IR (P interaction = 0.03) were observed. A 3-way interaction between sex, rs2943641, and 25(OH)D was observed for insulin (P interaction = 0.023) and HOMA-IR (P interaction = 0.084). Among women, but not men, a marginally significant interaction was observed between rs2943641 and circulating 25(OH)D on fasting insulin (P interaction = 0.072) and HOMA-IR (P interaction = 0.086) only under the recessive genetic model for the T allele. With higher 25(OH)D, rs2943641 T homozygotes, but not C allele carriers, had lower fasting insulin (P = 0.066) and HOMA-IR (P = 0.053) (Figs. 1 and 2).
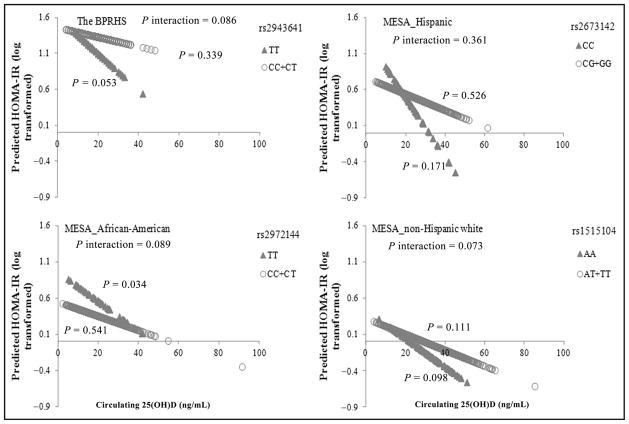
Fig. 1. Circulating 25(OH)D interacts with IRS1 variant on HOMA-IR in women in 4 populations of different ancestries. Predicted values for HOMA-IR (both natural log-transformed) were calculated with regression models, adjusting for potential confounders in the female participants of the BPRHS and MESA Hispanic, African-American, and non-Hispanic white populations.
With higher circulating 25(OH)D, predicted HOMA-IR decreased more evidently in the IRS1 variant minor allele homozygotes than the major allele carriers in each population. The P values of the interaction for each population were calculated in a general linear model including an interaction term [genotype by 25(OH)D] in the model, and the P values are shown in the respective panel. Number of participants in each population: BPRHS, n = 79 for rs2943641 TT carriers and n = 729 for C allele carriers; MESA Hispanic, n = 62 for rs2673142 CC carriers and n = 573 for G allele carriers; MESA African-American, n = 44 for rs2972144 TT carriers and n = 557 for C allele carriers; and MESA non-Hispanic white, n = 118 for rs1515104 AA carriers and n = 903 for T allele carriers.
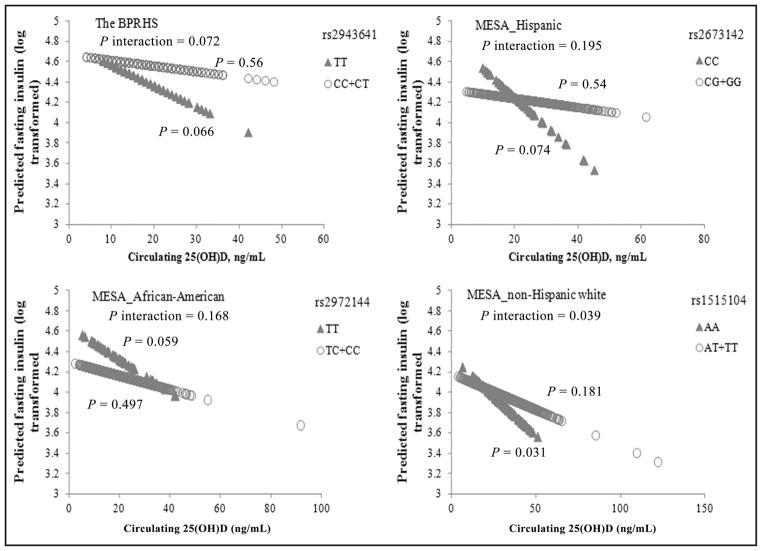
Fig. 2. Circulating 25(OH)D interacts with IRS1 variant on fasting insulin in women in 4 populations of different ancestries. Predicted values for HOMA-IR (both natural log-transformed) were calculated with regression models, adjusting for potential confounders in the female participants of the BPRHS and MESA Hispanic, African-American, and non-Hispanic white populations.
With higher circulating 25(OH)D, predicted insulin decreased more clearly in the IRS1 variant minor allele homozygotes than the major allele carriers in each population. For each population, the P values of the interaction were calculated in a general linear model by including an interaction term [genotype by 25(OH)D] in the model, and the P values are presented in the respective panel. Number of participants in each population: BPRHS, n = 79 for rs2943641 TT carriers and n = 729 for C allele carriers; MESA Hispanic, n = 62 for rs2673142 CC carriers and n = 573 for G allele carriers; MESA African-American, n = 44 for rs2972144 TT carriers and n = 557 for C allele carriers; and MESA non-Hispanic white, n = 118 for rs1515104 AA carriers and n = 903 for T allele carriers.
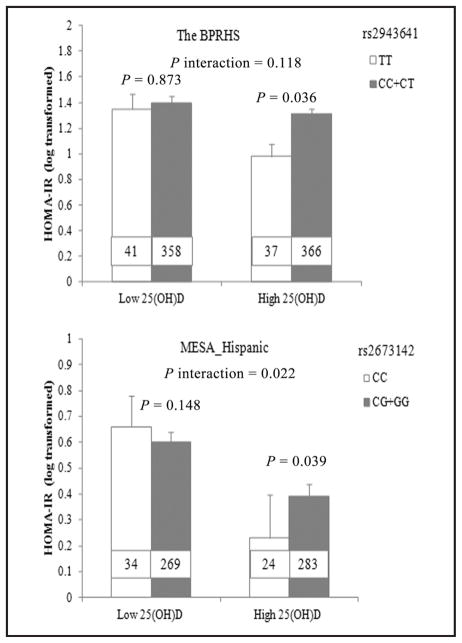
Circulating 25(OH)D was also dichotomized on the basis of the population median to assess its interaction with rs2943641. HOMA-IR was significantly higher in rs2943641 minor T allele homozygotes than major C allele carriers (P =0.036), only when circulating 25(OH)D was higher than the population median [17 ng/mL (42.4 nmol/L)] (Fig. 3).
Fig. 3. Interaction of circulating 25(OH)D, as a categorical variable, with IRS1 variant on HOMA-IR in BPRHS and MESA Hispanic women. In BPRHS, minor T homozygotes of rs2943641 had higher HOMA-IR than C carriers only when circulating 25(OH)D was higher than the population median (P = 0.036).
In the MESA Hispanic, minor C homozygotes of rs2673142 had higher HOMA-IR than G carriers only when circulating 25(OH)D was higher than the population median (P = 0.039). The P values of the interaction were calculated in a general linear model by including an interaction term [genotype by 25(OH)D] in the model. Number inside the bar indicates the number of subjects in that group. Values are means ± SE.
INTERACTION BETWEEN CIRCULATING 25(OH)D AND RS2943641 ON T2D TRAITS IN MESA WOMEN
The MESA population consists of participants of 4 ancestries. We examined 1 tag SNP of IRS1 rs2943641 in each of 3 populations separately (Figs. 1 and 2). No significant interaction between IRS1 variant and 25(OH)D on T2D traits was observed for men in any of the 3 MESA populations. A potential 3-way interaction between sex, IRS1 variant, and 25(OH)D for HOMA-IR (P interaction = 0.065) was observed for MESA Hispanic participants. Consistent with the BPRHS, interactions between the IRS1 variant and 25(OH)D on fasting insulin (P interaction = 0.168, 0.04, and 0.195 for African-American, non-Hispanic white, and Hispanic populations, respectively) and HOMA-IR (P interaction = 0.089, 0.073, and 0.361 for African-American, non-Hispanic white, and Hispanic populations, respectively) approached significance in women, and the most significant interaction was observed under the recessive genetic model for the minor allele of corresponding IRS1 variant. With the increase of 25(OH)D, predicted HOMA-IR (Fig. 1) decreased more evidently in rs2673142 minor C allele homozygotes (compared with major G allele carriers, P trend = 0.171 vs 0.526) in MESA Hispanic women, in rs2972144 minor T allele homozygotes (compared with major C allele carriers, P trend = 0.089 vs 0.541) in MESA African-American women, and in rs1515104 minor A allele homozygotes (compared with major T allele carriers, P trend = 0.089 vs 0.111) in MESA non-Hispanic white women (Fig. 1). A consistent trend was observed for fasting insulin (Fig. 2).
When circulating 25(OH)D was dichotomized on the basis of the population median in each MESA sub-population, significant interactions for fasting insulin (P interaction = 0.003) and HOMA-IR (P interaction = 0.022) were observed only in MESA Hispanic women, not in other MESA subpopulations. In MESA Hispanic women, HOMA-IR (P = 0.039) (Fig. 3) was significantly lower in rs2673142 minor C allele homozygotes than G allele carriers only when 25(OH)D was higher than the population median [23.6 ng/mL (58.9 nmol/L)].
METAANALYSIS FOR THE INTERACTION BETWEEN CIRCULATING 25(OH)D AND RS2943641 ON HOMA-IR AND FASTING INSULIN
Metaanalysis indicated that the circulating 25(OH)D (continuous) showed a significant interaction with IRS1 variant rs2943641 on fasting insulin (pooled β = −0.006, 95% CI: −0.011 to −0.002, P interaction = 0.004) and HOMA-IR (pooled β = −0.008, 95% CI: − 0.016 to −0.001, P interaction = 0.023) in women. For each of the 4 populations, with the increase of circulating 25(OH)D, the IRS1 variant minor allele (T) homozygotes showed a greater decrease in fasting insulin and HOMA-IR than the major allele (C) carriers (Figs. 4 and 5). However, among men, meta-analysis did not find significant interaction (P interaction = 0.226 for insulin; P interaction = 0.211 for HOMA-IR).
Fig. 4. Metaanalysis of the interaction between IRS1 variant and circulating 25(OH)D on HOMA-IR in 4 populations of different ancestries. Random-effects model was used to obtain the overall effect estimate.
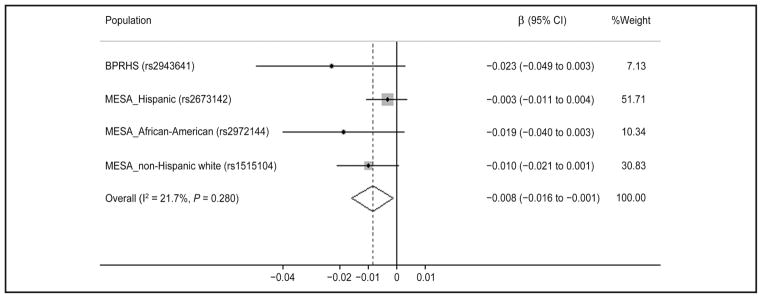
Gray square stands for study-specific β, with the square size reflecting the corresponding weight and horizontal bars reflecting 95% CIs.
Fig. 5. Metaanalysis of the interaction between IRS1 variant and circulating 25(OH)D on fasting insulin in 4 populations of different ancestries. Random-effects model was used to get the overall effect estimate.
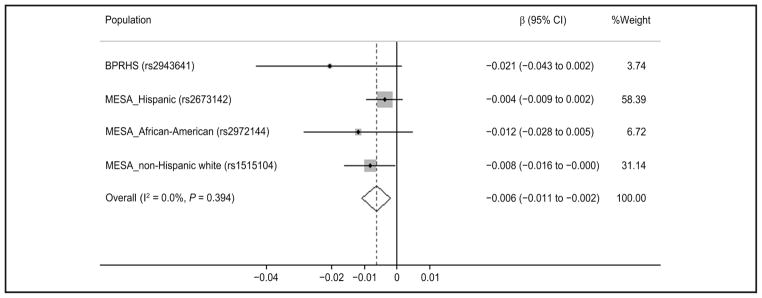
Gray square stands for study-specific β, with the square size reflecting the corresponding weight and horizontal bars reflecting 95% CIs.
Discussion
To the best of our knowledge, this is the first study identifying an IRS1 variant that interacts with circulating 25(OH)D on risk of T2D and IR. In BPRHS women, higher circulating 25(OH)D was associated with lower risk of T2D and lower IR in the rs2943641 minor allele T homozygotes, but not in the other genotype groups. This trend of interaction was further confirmed in the female participants of 3 MESA populations. No significant interaction was observed in men in BPRHS or MESA populations. Present results suggest that participants with different genotypes of rs2943641 exhibit differential benefits from high circulating 25(OH)D for the reduction of T2D and IR risk.
Although the strong protective association of rs2943641 T allele with T2D and IR has been established in several European populations, and potential functionality of this SNP has been proposed (4), other studies have shown inconsistent results (5, 6) between this SNP and T2D. Our previous study (6) did not find significant associations of rs2943641 with T2D and IR in this BPRHS population. In addition, evidence from observational and intervention studies showed inverse or no associations of circulating 25(OH)D with T2D or related traits (9, 13). These discrepancies could be explained by the interaction between rs2943641 and 25(OH)D, as indicated in the current study. Significant inverse associations of 25(OH)D with IR and fasting insulin were observed only in the rs2943641 T homozygotes in the BPRHS. Consistent with those observations, the results were replicated in the women of MESA African-American, non-Hispanic white, and Hispanic populations. The genetic effect of IRS1 variant rs2943641 on IR and T2D was susceptible to various dietary factors, as has been demonstrated in 3 other studies (7, 26, 27). One intervention study (7) found that rs2943641 interacted with dietary carbohydrate and fat on IR, and that rs2943641 CC carriers saw more benefit in terms of improved insulin sensitivity when choosing a high-carbohydrate and low-fat diet. In contrast, Zheng and colleagues (27) found that rs2943641 T allele carriers had lower risk of IR and metabolic syndrome when their dietary saturated fat-to-carbohydrate ratio was low. Another recent observational study (26) suggested a 3-way interaction between sex, rs2943641, and carbohydrate intake on incident T2D, and men and women showed opposite directions of the interaction between rs2943641 and carbohydrate intake. Our present study reports an interaction of rs2943641 with status of a micronutri-ent (vitamin D) on insulin resistance. It is noteworthy that this interaction did not change when we further adjusted for dietary fat or saturated fat-to-carbohydrate ratio in either the BPRHS or the MESA populations.
The gene–nutrient interaction observed in the BPRHS women was replicated only in MESA women, not in men, which is quite conceivable. The sex-specific interaction between rs2943641 with diet on T2D has been reported in a prospective study (26). Another study (28) also reported an interaction between 1 IRS1 variant (rs1522813) and physical activity on T2D only in women. These studies together with our present study suggest that biological differences between men and women may influence the T2D effects of variants at IRS1. Indeed, endogenous sex hormones have important roles in the pathogenesis of T2D for men and women (29). Furthermore, men and women usually have quite different lifestyles, including energy intake, alcohol drinking, and smoking. Although these situations, or others described below, may facilitate the IRS1–vitamin D interaction on T2D traits and risk, the precise mechanism by which these variants interact with circulating 25(OH)D to influence T2D traits is still unclear.
Although the same interaction pattern was observed in the BPRHS and the 3 MESA populations, slightly different strengths of the associations were found among the 4 populations. This may be because of the proxy markers (rs1515104, rs2972144, and rs2673142) of rs2943641 used in MESA and different genetic backgrounds and demographic and cultural characteristics of the BPRHS and MESA populations. For example, HOMA-IR and fasting insulin were substantially lower in MESA compared with the BPRHS population, and prevalence of T2D was higher in BPRHS than in the MESA populations. Although we adjusted for these differences in the interaction tests, those adjustments may not have been complete.
The interaction of rs2943641 with circulating 25(OH)D is biologically plausible. The IRS1 variant rs2943641 C allele has been shown to disrupt the insulin signaling pathway by reducing IRS1 protein expression and its downstream phosphatidylinositol-3-OH kinase activity in a functional test (4). In contrast, 1,25(OH)D may stimulate the activity of phosphatidylinositol-3-OH kinase (30). Therefore, the beneficial effect of higher circulating 25(OH)D on the insulin signaling pathway and insulin sensitivity may be blocked by this diabetogenic risk allele, while individuals carrying the nonrisk TT genotype could still see benefit from high 25(OH)D status. More research is warranted to reveal a precise mechanism for the observed interaction.
Strengths of the present study include its successful replication in populations of 3 ancestries. In addition, MESA includes participants from Hispanic, African-American, and non-Hispanic white populations. Usually, a gene–nutrient interaction is difficult to replicate, especially among populations of different ancestries. Therefore, our observed interactions of circulating 25(OH)D with rs2943641 on IR and T2D provides support of the finding that it is likely to be of importance to health of different populations. Nevertheless, there are several limitations with this study. First, a causal relation between 25(OH)D and T2D or related traits cannot be concluded because of the observational study design. Second, a different tag SNP was used in each of 3 populations of different ethnicities for replication, which may lead to underestimation of the genetic effect. Third, circulating vitamin D concentrations may be influenced by other genetic factors, and these may interact with IRS1 variants, which was not considered in this study. These unknown genetic variants may affect the estimated effect of gene-by-nutrient interaction on IR. Therefore, future work is needed to establish a causal relation between 25(OH)D and T2D or related traits, including genotype-selected randomized controlled trials of vitamin D supplementation, as well as gene-by-gene and gene-by-environment interaction analyses at the genome-wide level.
The present study has important public health implications. Vitamin D deficiency, defined as circulating 25(OH)D ≤20 ng/mL (49.9 nmol/L), is common in US adults, and the prevalence is especially high in minorities, such as African-Americans (82.1%) and Hispanics (69.2%) (31). This agrees with values from Boston Puerto Rican adults, a minority population participating in our study, where 66.5% of this population was vitamin D deficient [on the basis of the cutoff point of 20 ng/mL (49.9 nmol/L)]. Given the high prevalence of vitamin D deficiency and emerging evidence of its multiple health-related effects, high vitamin D intake (≥1000 IU) has been recommended to achieve a serum 25(OH)D of 40 ng/mL (99.8 nmol/L) (32). However, our study indicates that 1 recommendation may not be optimal for all adults. Genetic variation at IRS1 interacts with circulating 25(OH)D on T2D risk and IR. In terms of personalized nutrition, developing vitamin D dietary recommendations based on personal genotype information could, one day, improve dietary strategies for the prevention of T2D.
In conclusion, circulating 25(OH)D modulated the associations of IRS1 variant rs2943641 with T2D and IR in Puerto Rican female adults. Higher circulating 25(OH)D was associated with lower risk of T2D and IR in rs2943641 minor allele T homozygotes, but the associations were greatly attenuated in the other genotype groups. Replication was successfully achieved in the female participants of MESA African-American, non-Hispanic white, and Hispanic populations. This study suggests critical roles for both the IRS1 gene and vitamin D in regulating insulin resistance and T2D risk. The observed interaction between IRS1 genotype and vitamin D status, likely restricted to women, may have significant implications for vitamin D supplementation in the prevention of T2D.
Supplementary Material
Acknowledgments
Role of Sponsor: The funding organizations played no role in the design of study, choice of enrolled patients, review and interpretation of data, or preparation or approval of manuscript.
We thank the investigators, staff, and participants of MESA for their contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org. The MESA data sets used for the analyses described in this article were obtained from dbGaP (http://www.ncbi.nlm.nih.gov/sites/entrez?Dbgap) through dbGaP accession numbers for MESA Cohort (phs000209.v11.p3) and for Health/ Medical/Biomedical (phs000209.v11.p3.c1).
Footnotes
Nonstandard abbreviations: GWAS, genome-wide association studies; T2D, type 2 diabetes; IR, insulin resistance; IRS1, insulin receptor substrate 1; 25(OH)D, 25-hydroxyvitamin D; 1,25(OH)2D, 1,25 dihydroxyvitamin D; VDR, vitamin D receptor; RXR, retinoid-X receptor; MESA, Multi-Ethnic Study of Atherosclerosis; BPRHS, Boston Puerto Rican Health Study; FFQ, food frequency questionnaire; dbGaP, database of Genotypes and Phenotypes; HOMA, homeostasis model assessment; SNP, single nucleotide polymorphism; LD, linkage disequilibrium; OR, odds ratio.
Disclaimers: Mention of trade names or commercial products in this publication is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the U.S. Department of Agriculture. The USDA is an equal opportunity provider and employer.
Author Contributions: All authors confirmed they have contributed to the intellectual content of this paper and have met the following 3 requirements: (a) significant contributions to the conception and design, acquisition of data, or analysis and interpretation of data; (b) drafting or revising the article for intellectual content; and (c) final approval of the published article.
- Employment or Leadership: None declared.
- Consultant or Advisory Role: None declared.
- Stock Ownership: None declared.
- Honoraria: None declared.
- Research Funding: This study was supported by the National Basic Research Program of China (973 Program: 2011CB504002); National Heart, Lung, and Blood Institute (NHLBI) grant numbers HL54776 and HL078885; and contracts 53-K06-5-10 and 58-1950-9-001 from the US Department of Agriculture, Agriculture Research Service.
- Expert Testimony: None declared.
- Patents: None declared.
References
- 1.Hindorff LA, Sethupathy P, Junkins HA, Ramos EM, Mehta JP, Collins FS, Manolio TA. Potential etiologic and functional implications of genome-wide association loci for human diseases and traits. Proc Natl Acad Sci U S A. 2009;106:9362–7. doi: 10.1073/pnas.0903103106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Florez JC. Newly identified loci highlight beta cell dysfunction as a key cause of type 2 diabetes: where are the insulin resistance genes? Diabeto-logia. 2008;51:1100–10. doi: 10.1007/s00125-008-1025-9. [DOI] [PubMed] [Google Scholar]
- 3.Tamemoto H, Kadowaki T, Tobe K, Yagi T, Sakura H, Hayakawa T, et al. Insulin resistance and growth retardation in mice lacking insulin receptor substrate-1. Nature. 1994;372:182–6. doi: 10.1038/372182a0. [DOI] [PubMed] [Google Scholar]
- 4.Rung J, Cauchi S, Albrechtsen A, Shen L, Roche-leau G, Cavalcanti-Proenca C, et al. Genetic variant near IRS1 is associated with type 2 diabetes, insulin resistance and hyperinsulinemia. Nat Genet. 2009;41:1110–5. doi: 10.1038/ng.443. [DOI] [PubMed] [Google Scholar]
- 5.Yiannakouris N, Cooper JA, Shah S, Drenos F, Ireland HA, Stephens JW, et al. IRS1 gene variants, dysglycaemic metabolic changes and type-2 diabetes risk. Nutr Metab Cardiovasc Dis. 2012;22:1024–30. doi: 10.1016/j.numecd.2011.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Feng X, Tucker KL, Parnell LD, Shen J, Lee YC, Ordovas JM, et al. Insulin receptor substrate 1 (IRS1) variants confer risk of diabetes in the Boston Puerto Rican Health Study. Asia Pac J Clin Nutr. 2013;22:150–9. doi: 10.6133/apjcn.2013.22.1.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Qi Q, Bray GA, Smith SR, Hu FB, Sacks FM, Qi L. Insulin receptor substrate 1 gene variation modifies insulin resistance response to weight-loss diets in a 2-year randomized trial: the Preventing Overweight Using Novel Dietary Strategies (POUNDS LOST) trial. Circulation. 2011;124:563–71. doi: 10.1161/CIRCULATIONAHA.111.025767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alvarez JA, Ashraf A. Role of vitamin D in insulin secretion and insulin sensitivity for glucose ho-meostasis. Int J Endocrinol. 2010;2010:351385. doi: 10.1155/2010/351385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Forouhi NG, Ye Z, Rickard AP, Khaw KT, Luben R, Langenberg C, Wareham NJ. Circulating 25-hydroxyvitamin D concentration and the risk of type 2 diabetes: results from the European Prospective Investigation into Cancer (EPIC)-Norfolk cohort and updated meta-analysis of prospective studies. Diabetologia. 2012;55:2173–82. doi: 10.1007/s00125-012-2544-y. [DOI] [PubMed] [Google Scholar]
- 10.Pittas AG, Harris SS, Stark PC, Dawson-Hughes B. The effects of calcium and vitamin D supplementation on blood glucose and markers of inflammation in nondiabetic adults. Diabetes Care. 2007;30:980–6. doi: 10.2337/dc06-1994. [DOI] [PubMed] [Google Scholar]
- 11.de Boer IH, Tinker LF, Connelly S, Curb JD, How-ard BV, Kestenbaum B, et al. Calcium plus vitamin D supplementation and the risk of incident diabetes in the Women’s Health Initiative. Diabetes Care. 2008;31:701–7. doi: 10.2337/dc07-1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nagpal J, Pande JN, Bhartia A. A double-blind, randomized, placebo-controlled trial of the short-term effect of vitamin D3 supplementation on insulin sensitivity in apparently healthy, middle-aged, centrally obese men. Diabet Med. 2009;26:19–27. doi: 10.1111/j.1464-5491.2008.02636.x. [DOI] [PubMed] [Google Scholar]
- 13.George PS, Pearson ER, Witham MD. Effect of vitamin D supplementation on glycaemic control and insulin resistance: a systematic review and meta-analysis. Diabet Med. 2012;29:e142–50. doi: 10.1111/j.1464-5491.2012.03672.x. [DOI] [PubMed] [Google Scholar]
- 14.Song Y, Wang L, Pittas AG, Del Gobbo LC, Zhang C, Manson JE, Hu FB. Blood 25-hydroxy vitamin D levels and incident type 2 diabetes: a meta-analysis of prospective studies. Diabetes Care. 2013;36:1422–8. doi: 10.2337/dc12-0962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ramagopalan SV, Heger A, Berlanga AJ, Maugeri NJ, Lincoln MR, Burrell A, et al. A ChIP-seq defined genome-wide map of vitamin D receptor binding: associations with disease and evolution. Genome Res. 2010;20:1352–60. doi: 10.1101/gr.107920.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johnson JA, Grande JP, Roche PC, Kumar R. Immu-nohistochemical localization of the 1,25(OH)2D3 receptor and calbindin D28k in human and rat pancreas. Am J Physiol. 1994;267:E356–60. doi: 10.1152/ajpendo.1994.267.3.E356. [DOI] [PubMed] [Google Scholar]
- 17.Maestro B, Davila N, Carranza MC, Calle C. Identification of a vitamin D response element in the human insulin receptor gene promoter. J Steroid Biochem Mol Biol. 2003;84:223–30. doi: 10.1016/s0960-0760(03)00032-3. [DOI] [PubMed] [Google Scholar]
- 18.Tucker KL, Mattei J, Noel SE, Collado BM, Men-dez J, Nelson J, et al. The Boston Puerto Rican Health Study, a longitudinal cohort study on health disparities in Puerto Rican adults: challenges and opportunities. BMC Public Health. 2010;10:107. doi: 10.1186/1471-2458-10-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lai CQ, Tucker KL, Choudhry S, Parnell LD, Mattei J, Garcia-Bailo B, et al. Population admixture associated with disease prevalence in the Boston Puerto Rican Health Study. Hum Genet. 2009;125:199–209. doi: 10.1007/s00439-008-0612-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tucker KL, Bianchi LA, Maras J, Bermudez OI. Adaptation of a food frequency questionnaire to assess diets of Puerto Rican and non-Hispanic adults. Am J Epidemiol. 1998;148:507–18. doi: 10.1093/oxfordjournals.aje.a009676. [DOI] [PubMed] [Google Scholar]
- 21.Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, et al. Multi-ethnic study of atherosclerosis: objectives and design. Am J Epi-demiol. 2002;156:871–81. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 22.Mayer E, Schmidt-Gayk H. Interlaboratory comparison of 25-hydroxyvitamin D determination. Clin Chem. 1984;30:1199–204. [PubMed] [Google Scholar]
- 23.Chen TC, Turner AK, Holick MF. Methods for the determination of the circulating concentration of 25-hydroxyvitamin D. J Nutr Biochem. 1990;1:315–9. doi: 10.1016/0955-2863(90)90067-u. [DOI] [PubMed] [Google Scholar]
- 24.Adeney KL, Siscovick DS, Ix JH, Seliger SL, Shlipak MG, Jenny NS, Kestenbaum BR. Association of serum phosphate with vascular and valvular calcification in moderate CKD. J Am Soc Nephrol. 2009;20:381–7. doi: 10.1681/ASN.2008040349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lai CQ, Tucker KL, Parnell LD, Adiconis X, Garcia-Bailo B, Griffith J, et al. PPARGC1A variation associated with DNA damage, diabetes, and cardiovascular diseases: the Boston Puerto Rican Health Study. Diabetes. 2008;57:809–16. doi: 10.2337/db07-1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ericson U, Rukh G, Stojkovic I, Sonestedt E, Gull-berg B, Wirfalt E, et al. Sex-specific interactions between the IRS1 polymorphism and intakes of carbohydrates and fat on incident type 2 diabetes. Am J Clin Nutr. 2013;97:208–16. doi: 10.3945/ajcn.112.046474. [DOI] [PubMed] [Google Scholar]
- 27.Zheng JS, Arnett DK, Parnell LD, Smith CE, Li D, Borecki IB, et al. Modulation by dietary fat and carbohydrate of IRS1 association with type 2 diabetes traits in two populations of different ancestries. Diabetes Care. 2013;36:2621–7. doi: 10.2337/dc12-2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.He MA, Workalemahu T, Cornelis MC, Hu FB, Qi L. Genetic variants near the IRS1 gene, physical activity and type 2 diabetes in US men and women. Diabetologia. 2011;54:1579–82. doi: 10.1007/s00125-011-2123-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ding EL, Song Y, Malik VS, Liu S. Sex differences of endogenous sex hormones and risk of type 2 diabetes: a systematic review and meta-analysis. JAMA. 2006;295:1288–99. doi: 10.1001/jama.295.11.1288. [DOI] [PubMed] [Google Scholar]
- 30.Hughes PJ, Lee JS, Reiner NE, Brown G. The vitamin D receptor-mediated activation of phos-phatidylinositol 3-kinase (PI3Kalpha) plays a role in the 1alpha,25-dihydroxyvitamin D3-stimulated increase in steroid sulphatase activity in myeloid leukaemic cell lines. J Cell Biochem. 2008;103:1551–72. doi: 10.1002/jcb.21545. [DOI] [PubMed] [Google Scholar]
- 31.Forrest KY, Stuhldreher WL. Prevalence and correlates of vitamin D deficiency in US adults. Nutr Res. 2011;31:48–54. doi: 10.1016/j.nutres.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 32.Bischoff-Ferrari HA, Giovannucci E, Willett WC, Dietrich T, Dawson-Hughes B. Estimation of optimal serum concentrations of 25-hydroxyvitamin D for multiple health outcomes. Am J Clin Nutr. 2006;84:18–28. doi: 10.1093/ajcn/84.1.18. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.