Abstract
The molecular mechanisms underlying colorectal cancer (CRC) tumorigenesis remain incompletely understood, partially contributing to the mortality of CRC. Advances in identification of novel mechanisms are therefore in an urgent need to fill the gap of our knowledge in CRC development. Here, we performed both in vitro and in vivo experiments along with in silico analysis to identify a new regulatory circuit that stimulated CRC tumorigenesis. In this report, we, for the first time, analyzed the correlation of FIH-1 level with clinicopathological features of CRC. The finding that FIH-1 was not only significantly decreased in tumor tissue as compared with the adjacent normal tissue but also was significantly correlated with tumor T stage status, indicated the role of FIH-1 as a tumor suppressor in CRC development. Moreover, we found the expression of miR-31, a short non-coding RNA which played a critical role in CRC development, was negatively correlated with FIH-1 expression in CRC samples and cell lines. Together with the result from luciferase report assay, it was demonstrated that miR-31 could directly regulate FIH-1 expression in CRC. This miR-31/FIH-1 nexus was further shown to control cell proliferation, migration and invasion in vitro and to control tumor growth in vivo. Additionally, correlation of the miR-31 expression with clinicopathologic features in CRC samples was examined in support of the driving role of newly identified miR-31/FIH-1 nexus in CRC tumorigenesis. These findings highlight the critical role of miR-31/FIH-1 nexus in CRC and reveal the contribution of miR-31 to CRC development by targeting FIH-1.
Keywords: FIH-1, miRNA-31, regulatory circuit, colorectal cancer, tumorigenesis
Introduction
Colorectal cancer (CRC) is one of the most common cancers and the third leading cause of cancer-related deaths worldwide.1 However, the molecular mechanisms underlying CRC tumorigenesis remain elusive. Therefore, importance lies in the identification of the specific molecular mechanisms underlying CRC development. Insights gained from these mechanistic studies are important for the development of new strategies and therapeutic targets for effective treatment of CRC.
Hypoxia-inducible transcription factor 1α (HIF-1α) is a master regulator of oxygen homeostasis, which under hypoxic conditions, controls angiogenesis, erythropoiesis, and glycolysis via transcriptional activation of target genes. Additionally, HIF-1α also plays a pivotal role in maintaining cellular activities even during normoxia.2 According to the Warburg effect, a hallmark of malignant tumors that characterized by increased activity of aerobic glycolysis, HIF-1α is constitutively activated in malignant tumor cells and regulates cellular glycolytic activity.3 Also, overexpression and activation of HIF-1α is associated with tumor angiogenesis, tumor cell proliferation, and tumor cell invasion in CRC.4,5 Previous studies illustrated that HIF-1α could activate a transcription program that promoted aggressive tumor phenotypes by triggering the expression of critical genes, including vascular endothelial growth factor (VEGF), lactate dehydrogenase A (LDH-A), glucose transporter 1 (GLUT1), and others.6 As a key regulator of HIF-1α, factor inhibiting HIF-1α (FIH-1) hydroxylates HIF-1α protein at asparagine 803, inactivates the C-terminal transactivation domain (C-TAD) region, suppresses its interaction with transcription coactivators, and reduces the transcriptional activity of the protein.7-9 Recently, several studies identified that FIH-1 was also associated with neoplastic progression by repressing the HIF-1α pathway.10-12 Hence, the FIH–HIF cascade plays a critical role in cancer development and the factors that influence this axis should be elucidated to generate more effective therapeutic targets for CRC patients.
The molecular mechanisms that underlie FIH–HIF aberration remain unclear and the regulatory factors that control FIH-1 expression are not fully understood. MicroRNA (miRNA) are a class of 19–24 nt short non-coding RNAs, which regulate translation and degradation of target mRNAs by direct interaction with 3′-untranslated region (3′UTR) of the target mRNA. Up until now, miRNA are known as a vital factor in carcinogenesis and tumor progression, and many miRNA were found aberrantly expressed in various tumor types. In CRC, miR-31 functions as an oncogenic miRNA, and has been shown to be associated with tumor prognosis.13,14 In the present study, we identified that FIH-1 expression was negatively correlated with miR-31 and was directly regulated by miR-31 in CRC. Aberration of the miR-31/FIH-1 nexus was further shown to control cell proliferation, migration and invasion in CRC cell lines and was found to control tumor growth in HCT116 xenografts. Correlation of the miR-31 expression with clinicopathologic features in CRC samples was further examined in support of the driving role of newly identified miR-31/FIH-1 nexus in CRC tumorigenesis. Together, our data provide strong evidence that the miR-31/FIH-1 nexus plays a critical role in human CRC tumorigenesis and indicate that miR-31 contributes to CRC development by targeting FIH-1.
Results
FIH-1 expression is correlated with CRC pathologic and miR-31
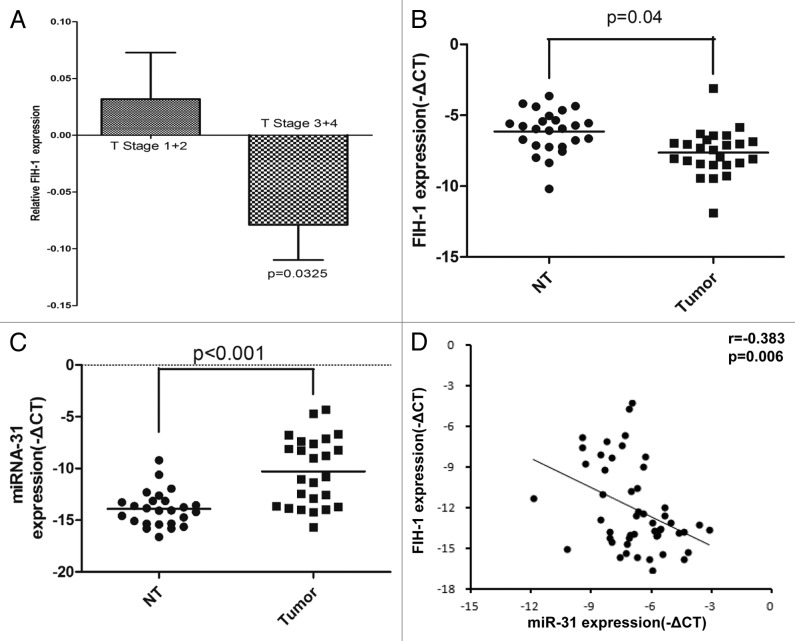
We first investigated the clinical correlation of FIH-1 mRNA expression in The Cancer Genome Atlas (TCGA) sample cohort15 and found that FIH-1 mRNA expression was significantly correlated with tumor T stage status (2 samples having no T stage information, Fig. 1A, T1/2 vs. T3/4, P = 0.03, unpaired t test with Welch correction). By using real-time PCR, we further screened FIH-1 mRNA expression in 25 pairs of CRC and their corresponding adjacent normal tissues. The mRNA level of FIH-1 was significantly downregulated in the CRC samples, compared with the adjacent normal samples. This result independently confirmed the role of FIH-1 mRNA in CRC tumorigenesis (Fig. 1B). Meanwhile, miR-31 expression was significantly higher in CRC tumors (Fig. 1C). We then found that the FIH-1 mRNA expression was significantly and negatively correlated with miR-31 expression by analyzing the above 50 paired CRC and adjacent normal tissues (Fig. 1D, r = −0.383, P = 0.006), suggesting that FIH-1 might be the downstream target of miR-31.
Figure 1. FIH-1 and miR-31 expressions in human CRC tissues and their correlation. (A) Clinical correlation of FIH-1 mRNA expression and T stage of CRC in the TCGA sample cohort. (B) mRNA expression of FIH-1 was decreased in 25 CRC tissues compared with their corresponding normal tissues. (C) miR-31 level was increased in those above 25 CRC tissues compared with their corresponding normal tissues. (D) Expression patterns of FIH-1 with miR-31 in 50 samples including CRC tissues and their corresponding normal tissues.
FIH-1 is a target of miR-31
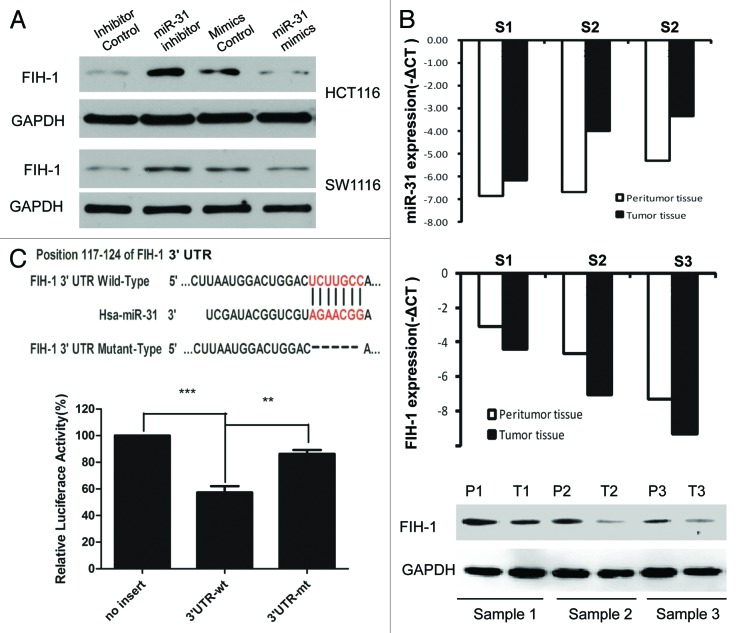
Both Target Scan and Pictar systems predicted FIH-1, an important hypoxia-associated gene, to be a putative target of miR-31. To verify whether miR-31 directly targeted FIH-1 mRNA in CRC tumors and cell lines, in vitro and in silico analysis were conducted. First, siRNA against miR-31 or control was transfected into HCT116 and SW1116 cells, and we found that inhibited expression of miR-31 increased FIH-1 protein levels in both lines (Fig. 2A). Then, miR-31 mimic or control was transfected into the above 2 cell lines. We found that forced expression of miR-31 decreased FIH-1 protein levels in both cell lines consistently (Fig. 2A). To further confirm the regulatory relationship between miR-31 and FIH-1, we examined the expression of FIH-1 in CRC samples and their corresponding normal tissues. Using qRT-PCR, we found FIH-1 mRNA was negatively correlated with miR-31 (Fig. 2B). This trend was also observed when we employed western blotting and immunohistochemistry (IHC) to detect FIH-1 protein levels in these tissues (Fig. 2B; Fig. S1). To evaluate the ability of miR-31 binding to the 3′UTR of FIH-1, we performed a luciferase reporter assay and observed a significant decrease (Fig. 2C) in luciferase activity in the presence of miR-31-FIH-1 in HCT116 cell, compared with the controls. To validate whether FIH-1 was a direct target of miR-31, we mutated the miR-31 binding sites in the 3′UTR of FIH-1 and observed the loss of repression (Fig. 2C). Taken together, the data suggested that FIH-1 was a direct target of miR-31.
Figure 2. FIH-1 is one of the miR-31 targets and is negatively regulated by miR-31. (A) FIH-1 expression in HCT116 or SW1116 cells after transfection with anti-miR-31 siRNA or miR-31 mimics detected by western blotting. (B) FIH-1 expression was negatively correlated with miR-31 in 3 pairs of CRC tumor and peritumor tissues. (C) Predicted consequential pairing of target 3′UTR region of FIH-1 (wild-type or mutated) and miR-31 mature sequence. Luciferase activity on the presence of both wild-type FIH-1 3′UTR or mutant and miR-31 was compared with the control.
MiR-31/FIH-1 nexus promotes CRC cell proliferation, migration, and invasion in vitro by regulating FIH-1
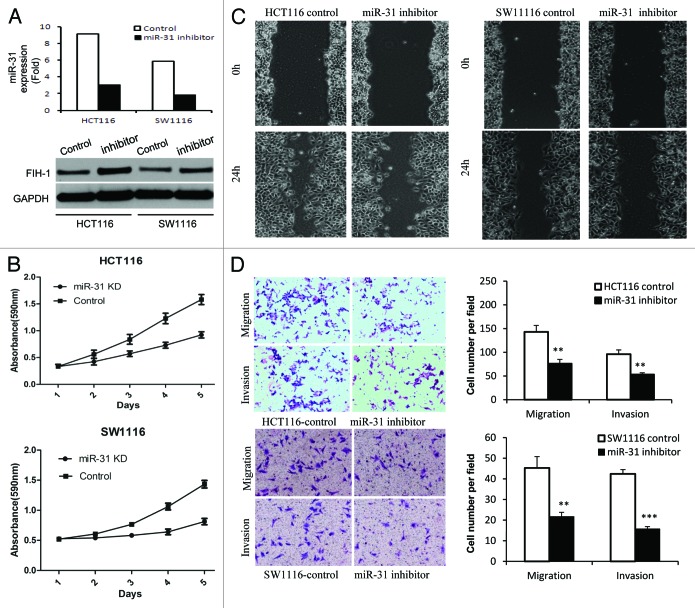
Thus far we have shown that miR-31 can directly regulate FIH-1 expression in both CRC tissue and cell lines. We then examined the biological role of the miR-31/FIH-1 nexus in vitro by performing functional assays. First, siRNA against miR-31 was transfected in HCT116 and SW1116 cell lines. We observed a decrease of miR-31 levels and a subsequent increase of FIH-1 protein levels (Fig. 3A), which was consistent with observation stated above. As a consequence, a significant decrease in cell proliferation was observed in both cell lines after transfection of siRNA against miR-31 (Fig. 3B). A subsequent wound-healing assay showed that the rate of wound-healing of miR-31 knocked down cells was significantly slower than that of untreated cells (Fig. 3C). At 24 h, a wound in HCT116 cells was almost closed, while cells treated with siRNA against miR-31 still showed a noticeable wound. A similar trend was observed in SW1116 cells, though not so prominent as HCT116 cells, probably because of the difference in FIH-1 protein levels in these two cell lines (Fig. 3A). Furthermore, downregulation of miR-31 along with upregulation of FIH-1 resulted in significant inhibition of HCT116 and SW1116 cell migration and invasion evaluated via cell migration and invasion assays (Fig. 3D). Taken together, the data suggested that the miR-31/FIH-1 nexus that newly identified in CRC from this study played an oncogenetic role in CRC development.
Figure 3. Effect of miR-31/FIH-1 nexus on proliferation, migration, invasion of HCT116 and SW1116 cells. (A) The transfection efficiency of anti-miR-31 siRNA and the promotion of FIH-1 expression in HCT116 and SW1116 cells. (B) Inhibition of miR-31 (promotion of FIH-1 expression) had significant effect on decreasing proliferation rate of both cell lines. (C) Wound closure was delayed in cells transfected with anti-miR-31 siRNA as compared with negative control in 24 h time points in both types of CRC cells. (D) Representative fields of invasion (down) or migration (up) cells on the membrane was on the left (magnification of 200×). Average invasion or migration cell number per field was on the right. The invasion or migration cell number of HCT116 and SW1116 transfected with anti-miR-31 siRNA was drastically decreased than that transfected with pairing negative control. **P < 0.01, ***P < 0.001.
MiR-31/FIH-1 nexus controls the tumor growth of HCT116 xenografts in vivo
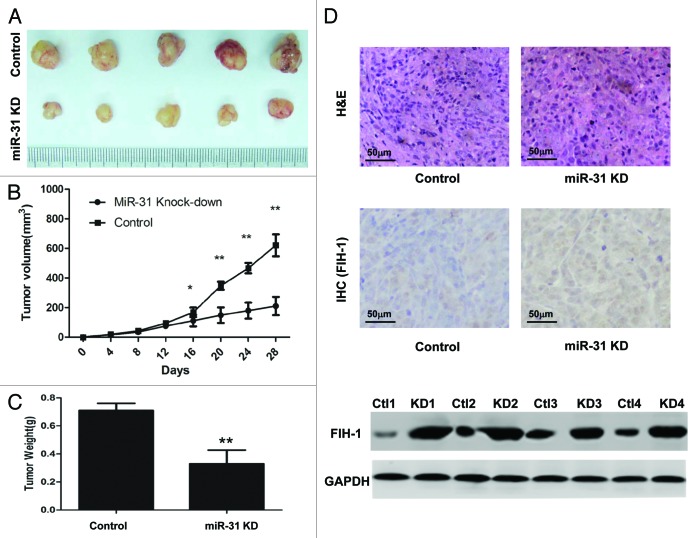
To further investigate whether the miR-31/FIH-1 nexus affects tumor formation in vivo, HCT116 cells were stably transfected with anti-miR-31-LV or anti-NC-LV. The cells were then implanted subcutaneously into nude mice and the tumors were monitored every 4 d. At the end of the fourth week, all mice were sacrificed, and the tumor volumes and weights were measured. There was a significant difference in average tumor volume and weight between the two groups (Fig. 4A). The tumors were larger and heavier in the control group than those in the miR-31 knockdown group (Fig. 4B and C). To further determine whether the growth inhibition was through FIH-1, we performed IHC and western blotting assay on tumor tissues, and found that the expression level of FIH-1 protein was increased in the tumors of miR-31 knock-down mice (Fig. 4D). These data agreed with in vitro results implicating the role of miR-31 and FIH-1 in CRC tumorigenesis.
Figure 4. MiR-31/FIH-1 nexus controls the tumor growth of HCT116 xenografts in vivo. (A) Downexpression of miR-31 strikingly decreased the growth of HCT116 cells xenografted in nude mice. (B) The tumors were much bigger in control group than that in miR-31 knockdown group. (C) The tumors were much heavier in control group than that in miR-31 knockdown (miR-31KD) group. (D) Representative H&E-stained sections of the subcutaneous tumor tissues collected from control and miR-31KD groups were shown (up). FIH-1 expressions in the subcutaneous tumor tissues collected from control and miR-31KD groups were detected by IHC (middle, magnification of 200×) and western blotting (down). *P < 0.05, **P < 0.01.
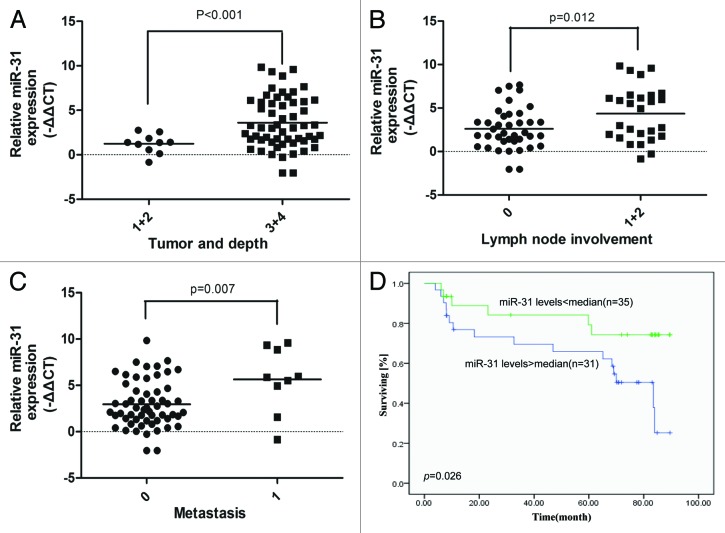
Increased miR-31 expression in CRC tissue is associated with disease progression and poor prognosis
Both the in vitro and in vivo results from this study strongly suggested that the miR31/FIH-1 nexus played an oncogenic role in CRC tumorigenesis. Given the fact that miR-31 acts as the driving element of this nexus, we speculated that miR-31 expression might be a prognostic factor in CRC patient samples. To prove above speculation, we collected 66 CRC patient samples including the 25 samples shown in Figure 1 with well-annotated clinical information (Table S1), and measured the relative levels of miR-31 expression by using real-time PCR. Statistical analysis showed that high miR-31 expression was significantly correlated with invading depth (P < 0.001, Fig. 5A), lymph node involvement (P = 0.012, Fig. 5B), and metastasis (P = 0.007, Fig. 5C). Kaplan–Meier analysis showed that the patients with high miR-31 expression had worse prognosis than those with low miR-31 expression (P = 0.026, Fig. 5D). These data suggest that miR-31 is a potential therapeutic target for CRC patient treatment.
Figure 5. Increased miR-31 expression in CRC tissue is associated with disease progression and poor prognosis. (A) Increased miR-31 expression was correlated with CRC invading depth. (B) Increased miR-31 expression was correlated with CRC lymph node involvement. (C) Increased miR-31 expression was correlated with CRC metastasis. (D) Kaplan–Meier survival curves for CRC patients. The patients with high miR-31 expression had worse prognosis than those with low miR-31 expression.
Discussion
HIF-1α controls the expression of several genes during adaptation to hypoxic conditions and also plays a pivotal role in maintaining cellular activities during normoxia. HIF-1α is constitutively activated in malignant tumor cells. Previous studies demonstrated that HIF-1α expression was associated with human cancer progression and was overexpressed in colon, breast, gastric, lung, skin, ovarian, pancreatic, prostate, and renal carcinomas.16,17 The activity or stability of HIF-1α is maintained by FIH-1. Hydroxylation of HIF-1α by FIH-1 blocks the association of HIF-1α with the transcriptional co-activators, thus inhibiting transcriptional activation. However, the clinical correlation and biological role of FIH-1 expression in CRC have not been reported yet. In this study, we, for the first time, analyzed the correlation of FIH-1 level with clinicopathological features of CRC. We found that FIH-1 expression not only significantly decreased in tumor tissues as compared with the adjacent normal tissues, but was also significantly correlated with tumor T stage status. This result suggests the role of FIH-1 as tumor suppressor in CRC development.
Recently, accumulated evidence suggests that miRNA regulate gene expression through posttranscriptional mechanisms, and are involved in both normoxia and hypoxia.18,19 In this study, we found that miR-31 was highly expressed in CRC tissues and was significantly associated with tumor development and prognosis, consistent with previous reports.13,14 In addition, previous studies demonstrated that miR-31 was upregulated, even in premalignant lesions such as colonic adenomas, which were the target lesions of CRC screening.20,21 By analyzing clinical data, miR-31 expression was obviously upregulated in CRC tissues, as expected. Results from both in silico analysis and functional assays strongly suggested that miR-31 could directly target FIH-1 and regulate FIH-1 expression in vitro and in vivo. Aberration in this miR-31/FIH-1 nexus was shown to alter cell proliferation, migration, and invasion in CRC cell lines and to alter tumor growth in HCT116 xenografts. This regulatory nexus agrees with previous studies that had shown FIH-1 was the target gene of miR-31.12,22,23 In light of these observations, we consider that upregulation of miR-31 suppresses FIH-1 expression and causes HIF-1α activation in CRC. We believe that this newly identified pathway plays a critical role in CRC progression.
In support of the driving role of the miR-31/FIH-1 nexus in CRC tumorigenesis, we further examined miR-31 expression levels in CRC patient samples and found miR-31 level was significantly correlated with CRC TNM stages. Additionally, the Kaplan–Meier survival curve showed that the patients with high miR-31 expression exhibited poorer prognosis, suggesting that miR-31 expression was also associated with the clinical outcome of CRC patients.
This study identifies a new regulatory network of the miR-31/FIH-1 nexus that is not reported in CRC. Additionally, the miR-31/FIH-1 nexus importantly functions as an oncogenic role in CRC tumor development. Further considerations are needed to determine how miR-31 regulates the metabolism of CRC in normoxic and hypoxic conditions. Nevertheless, our data illustrates the important finding that miR-31 contributes to CRC development and progression through FIH-1 mediated HIF-1α activation in tumor regions. Further understanding of the pathogenic significance of miR-31/FIH-1 nexus may contribute to a better diagnosis and treatment of CRC.
Materials and Methods
Patient samples
Written informed consent was obtained from each patient and the investigation was approved by the institutional review board of Zhongshan Hospital, Shanghai Fudan University. Human CRC samples and their paired noncancerous matched tissues were collected at the time of surgical resection, immediately snapfrozen in liquid nitrogen and stored at −80 °C. The patients’ clinicopathological data were also collected (Table S1). In addition, a total of 244 CRC samples with both gene expression data and clinical data were obtained from TCGA.15
Cell culture
HCT116 and SW1116 human CRC cells were obtained from the Institute of Cell Biology at the Chinese Academy of Sciences. HCT116 cells were cultured in McCoy’s 5A medium supplemented with 10% fetal bovine serum (FBS), whereas SW1116 cells were cultured in L15 medium supplemented with 10% FBS. All cells were cultured in a humidified incubator at 37 °C with 5% CO2.
RNA extraction and qRT-PCR assay
Total RNA was isolated using TRIzol reagent (Invitrogen) according to the manufacturer’s instructions. TaqMan real-time PCR assays for miR-31, U6, FIH, and GAPDH were from Applied Biosciences. All reactions, including the no template controls, were run in triplicate. After the reactions were completed, the CT values were determined using fixed threshold settings. Data were analyzed using the 2−ΔΔCT method.
Proliferation assay
Cells were plated in 96-well plates with 1 × 103 cells/well in regular growth medium. Proliferation of cancer cells was measured 5 d after treatment by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay.
Wound healing assay
Cells (1 × 105 per well) were seeded in 6-well plates and grown to confluence. Then, the cells were wounded with a pipette tip. After being washed with PBS for three times, the cells were cultured in full medium and allowed to close the wound for 24 h. Photos of the wound were taken immediately after scratching 24 later in the same marked location of the dish.
Cell migration and invasion assays
A 24-well transwell plate (8-mm pore size, Corning) was used to measure each cell line’s migratory and invasive ability. For the migration assay, 5 × 104 cells were plated in the top chamber lined with a non-coated membrane. For the invasion assay, chamber inserts were coated with 200 mg/ml of matrigel and dried overnight under sterile conditions. Then, 1 × 105 cells were plated in the top chamber. Both assays were performed according to standard procedures.
Knockdown of miR-31
The siRNA against miR-31 and negative control were purchased from Pharma Gene. Cells were seeded at 2 × 105 per well in 6-well plates and allowed to attach for at least 16 h. The siRNA or negative control was transfected using lipofectamine 2000 (Invitrogen). The stable cell lines were established by lentiviral infection.24 Anti-miR-31-LV and anti-NC-LV were obtained from GeneChem.
Luciferase reporter assay
To test the targeting activity of miR-31, the 3′ untranslated region (3′UTR) sequence of FIH-1 containing predictive miR-31 target sites was cloned into the p-MIR-reporter plasmid (3′UTR-WT). The mutant FIH-1 3′UTR reporter vector (3′UTR-MT) that lacked the binding sites for miR-31 was created through site-directed mutagenesis using a QuikChange kit (Stratagene). Luciferase reporter assay was performed as described previously.25 Each transfection experiment was performed in triplicate.
Protein extraction and western blotting
Cellular proteins were extracted and separated in SDS-PAGE gels, and western blot analyses were performed according to standard procedures as previously described.24 Western blotting of GAPDH on the same membrane was used as a loading control. The antibodies used were anti-FIH-1 (1:500) and anti-GAPDH (1:1000), both from Santa Cruz Biotechnology.
Immunodeficient mouse xenograft tumor model
All animal experiments were performed according to the regulations of PR China and Fudan University, and approved by the animal care and use committee of Fudan University. HCT116 cells, which were infected with anti-miR-31-LV or anti-NC-LV, were harvested and injected subcutaneously into the right flank of male nude (nu/nu) mice (2 × 106 viable tumor cells/mouse). The animals were equally divided into control and treated groups (5 mice per group). Tumor volume (V) was measured every 4 d and it was calculated as V = (length × width2)/2. The mice were sacrificed at 28 d post-implantation; then xenograft tumors were removed for further investigation.
Histological analyses
After mice were sacrificed according to experimental protocol, tumor tissues were isolated, fixed, and embedded in paraffin for histopathological analysis. Hematoxylin and eosin (H&E) staining and immunohistochemistery were performed according to standard procedures. Imaging from tumor tissue was detected with microscope.
Statistical analysis
All experiments were performed in triplicate. Differences between groups were calculated using the Student t test or the Fisher exact test. Additionally, P < 0.05 was selected to indicate a significant difference. These data were analyzed using SPSS version 17.0 (SPSS).
Supplementary Material
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
Project supported by the National Natural Science Foundation of China (81101566), Shanghai’s Health Bureau (XYQ2011017), and Shanghai Science and Technology Commission (11411950501 and 12QA1400600). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Glossary
Abbreviations:
- CRC
colorectal cancer
- miRNA
microRNA
- FIH-1
factor inhibiting HIF-1α
- HIF-1α
hypoxia-inducible transcription factor 1α
- VEGF
vascular endothelial growth factor
- LDH-A
lactate dehydrogenase A
- GLUT1
glucose transporter 1
- C-TAD
C terminal transactivation domain
- 3′UTR
3′-untranslated region
- TCGA
The Cancer Genome Atlas
- MTT
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide
- NC
negative control
- LV
lentivirus
Footnotes
Previously published online: www.landesbioscience.com/journals/cbt/article/28017
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Sakamoto T, Niiya D, Seiki M. Targeting the Warburg effect that arises in tumor cells expressing membrane type-1 matrix metalloproteinase. J Biol Chem. 2011;286:14691–704. doi: 10.1074/jbc.M110.188714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Semenza GL. HIF-1: upstream and downstream of cancer metabolism. Curr Opin Genet Dev. 2010;20:51–6. doi: 10.1016/j.gde.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kuwai T, Kitadai Y, Tanaka S, Onogawa S, Matsutani N, Kaio E, Ito M, Chayama K. Expression of hypoxia-inducible factor-1alpha is associated with tumor vascularization in human colorectal carcinoma. Int J Cancer. 2003;105:176–81. doi: 10.1002/ijc.11068. [DOI] [PubMed] [Google Scholar]
- 5.Furlan D, Sahnane N, Carnevali I, Cerutti R, Uccella S, Bertolini V, Chiaravalli AM, Capella C. Up-regulation and stabilization of HIF-1alpha in colorectal carcinomas. Surg Oncol. 2007;16(Suppl 1):S25–7. doi: 10.1016/j.suronc.2007.10.014. [DOI] [PubMed] [Google Scholar]
- 6.Harris AL. Hypoxia--a key regulatory factor in tumour growth. Nat Rev Cancer. 2002;2:38–47. doi: 10.1038/nrc704. [DOI] [PubMed] [Google Scholar]
- 7.Mahon PC, Hirota K, Semenza GL. FIH-1: a novel protein that interacts with HIF-1alpha and VHL to mediate repression of HIF-1 transcriptional activity. Genes Dev. 2001;15:2675–86. doi: 10.1101/gad.924501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cockman ME, Lancaster DE, Stolze IP, Hewitson KS, McDonough MA, Coleman ML, Coles CH, Yu X, Hay RT, Ley SC, et al. Posttranslational hydroxylation of ankyrin repeats in IkappaB proteins by the hypoxia-inducible factor (HIF) asparaginyl hydroxylase, factor inhibiting HIF (FIH) Proc Natl Acad Sci U S A. 2006;103:14767–72. doi: 10.1073/pnas.0606877103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coleman ML, McDonough MA, Hewitson KS, Coles C, Mecinovic J, Edelmann M, Cook KM, Cockman ME, Lancaster DE, Kessler BM, et al. Asparaginyl hydroxylation of the Notch ankyrin repeat domain by factor inhibiting hypoxia-inducible factor. J Biol Chem. 2007;282:24027–38. doi: 10.1074/jbc.M704102200. [DOI] [PubMed] [Google Scholar]
- 10.Couvelard A, Deschamps L, Rebours V, Sauvanet A, Gatter K, Pezzella F, Ruszniewski P, Bedossa P. Overexpression of the oxygen sensors PHD-1, PHD-2, PHD-3, and FIH Is associated with tumor aggressiveness in pancreatic endocrine tumors. Clin Cancer Res. 2008;14:6634–9. doi: 10.1158/1078-0432.CCR-07-5258. [DOI] [PubMed] [Google Scholar]
- 11.Giatromanolaki A, Koukourakis MI, Pezzella F, Turley H, Sivridis E, Bouros D, Bougioukas G, Harris AL, Gatter KC. Expression of prolyl-hydroxylases PHD-1, 2 and 3 and of the asparagine hydroxylase FIH in non-small cell lung cancer relates to an activated HIF pathway. Cancer Lett. 2008;262:87–93. doi: 10.1016/j.canlet.2007.11.041. [DOI] [PubMed] [Google Scholar]
- 12.Liu CJ, Tsai MM, Hung PS, Kao SY, Liu TY, Wu KJ, Chiou SH, Lin SC, Chang KW. miR-31 ablates expression of the HIF regulatory factor FIH to activate the HIF pathway in head and neck carcinoma. Cancer Res. 2010;70:1635–44. doi: 10.1158/0008-5472.CAN-09-2291. [DOI] [PubMed] [Google Scholar]
- 13.Slaby O, Svoboda M, Fabian P, Smerdova T, Knoflickova D, Bednarikova M, Nenutil R, Vyzula R. Altered expression of miR-21, miR-31, miR-143 and miR-145 is related to clinicopathologic features of colorectal cancer. Oncology. 2007;72:397–402. doi: 10.1159/000113489. [DOI] [PubMed] [Google Scholar]
- 14.Wang CJ, Zhou ZG, Wang L, Yang L, Zhou B, Gu J, Chen HY, Sun XF. Clinicopathological significance of microRNA-31, -143 and -145 expression in colorectal cancer. Dis Markers. 2009;26:27–34. doi: 10.1155/2009/921907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cancer Genome Atlas Network Comprehensive molecular characterization of human colon and rectal cancer. Nature. 2012;487:330–7. doi: 10.1038/nature11252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Talks KL, Turley H, Gatter KC, Maxwell PH, Pugh CW, Ratcliffe PJ, Harris AL. The expression and distribution of the hypoxia-inducible factors HIF-1alpha and HIF-2alpha in normal human tissues, cancers, and tumor-associated macrophages. Am J Pathol. 2000;157:411–21. doi: 10.1016/S0002-9440(10)64554-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhong H, De Marzo AM, Laughner E, Lim M, Hilton DA, Zagzag D, Buechler P, Isaacs WB, Semenza GL, Simons JW. Overexpression of hypoxia-inducible factor 1alpha in common human cancers and their metastases. Cancer Res. 1999;59:5830–5. [PubMed] [Google Scholar]
- 18.Crosby ME, Kulshreshtha R, Ivan M, Glazer PM. MicroRNA regulation of DNA repair gene expression in hypoxic stress. Cancer Res. 2009;69:1221–9. doi: 10.1158/0008-5472.CAN-08-2516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pocock R. Invited review: decoding the microRNA response to hypoxia. Pflugers Arch. 2011;461:307–15. doi: 10.1007/s00424-010-0910-5. [DOI] [PubMed] [Google Scholar]
- 20.Oberg AL, French AJ, Sarver AL, Subramanian S, Morlan BW, Riska SM, Borralho PM, Cunningham JM, Boardman LA, Wang L, et al. miRNA expression in colon polyps provides evidence for a multihit model of colon cancer. PLoS One. 2011;6:e20465. doi: 10.1371/journal.pone.0020465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cekaite L, Rantala JK, Bruun J, Guriby M, Agesen TH, Danielsen SA, Lind GE, Nesbakken A, Kallioniemi O, Lothe RA, et al. MiR-9, -31, and -182 deregulation promote proliferation and tumor cell survival in colon cancer. Neoplasia. 2012;14:868–79. doi: 10.1593/neo.121094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cekaite L, Rantala JK, Bruun J, Guriby M, Agesen TH, Danielsen SA, Lind GE, Nesbakken A, Kallioniemi O, Lothe RA, et al. MiR-9, -31, and -182 deregulation promote proliferation and tumor cell survival in colon cancer. Neoplasia. 2012;14:868–79. doi: 10.1593/neo.121094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peng H, Hamanaka RB, Katsnelson J, Hao LL, Yang W, Chandel NS, Lavker RM. MicroRNA-31 targets FIH-1 to positively regulate corneal epithelial glycogen metabolism. FASEB J. 2012;26:3140–7. doi: 10.1096/fj.11-198515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sun D, Yu F, Ma Y, Zhao R, Chen X, Zhu J, Zhang CY, Chen J, Zhang J. MicroRNA-31 activates the RAS pathway and functions as an oncogenic MicroRNA in human colorectal cancer by repressing RAS p21 GTPase activating protein 1 (RASA1) J Biol Chem. 2013;288:9508–18. doi: 10.1074/jbc.M112.367763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yu J, Ryan DG, Getsios S, Oliveira-Fernandes M, Fatima A, Lavker RM. MicroRNA-184 antagonizes microRNA-205 to maintain SHIP2 levels in epithelia. Proc Natl Acad Sci U S A. 2008;105:19300–5. doi: 10.1073/pnas.0803992105. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.