Abstract
Caffeine is a naturally occurring methylxanthine that acts as a non-selective adenosine receptor antagonist. Epidemiological studies demonstrated habitual coffee drinking to be significantly associated with liver cancer survival. We aimed to investigate the effects of caffeine and its analog CGS 15943 on hepatocellular carcinoma (HCC) and pancreatic cancer adenocarcinoma (PDAC). We demonstrate that caffeine and CGS 15943 block proliferation in HCC and PDAC cell lines by inhibiting the PI3K/Akt pathway. Importantly a kinase profiling assay reveals that CGS 15943 targets specifically the catalytic subunit of the class IB PI3K isoform (p110γ). These data give mechanistic insight into the action of caffeine and its analogs and they identify these compounds as promising lead compounds to develop drugs that can specifically target this PI3K isoform whose key role in cancer progression is emerging.
Keywords: caffeine, CGS 15943, hepatocellular carcinoma, pancreatic ductal adenocarcinoma, phosphoinositide 3-kinase
Introduction
Hepatocellular carcinoma (HCC) is a primary liver cancer and the third most common cause of cancer mortality worldwide.1 It occurs more often in men than women, and it is usually seen in people aged over 50.2-4 Indeed in 2011 HCC was estimated as the fifth most common cancer and the second leading cause of cancer-related death in men and the seventh most common and sixth leading cause of death in women.5 Most cases occur in the sub-Saharan Africa and eastern Asia but the incidence is rising in developed regions, including western countries with previous low or intermediate incidence,6 mainly because of alcoholic liver disease and hepatitis C infection.7-9 Liver cirrhosis, which can be caused by hepatitis B and C, alcohol abuse, and obesity, is considered a premalignant condition for developing HCC, as about 80% of HCC is associated with liver cirrhosis.4,6,10 Surgery, either hepatic resection or liver transplantation, remains the most effective treatment for HCC11 but only a small percentage of patients are eligible for this treatment since the majority of patients present with advanced or unresectable disease.9,11 For many years systemic chemotherapy also proved to be only minimally effective12,13 and currently only the inhibitor sorafenib is approved for advanced HCC patients.11 Several additional agents are currently being tested in clinical trials11 but there is a clear and urgent need to identify novel potential chemotherapeutic agents. Interestingly, several studies have identified an association between coffee/caffeine intake and reduced risk of HCC.14-18
Caffeine is a naturally occurring methylxanthine non-selective adenosine receptor antagonist that can be found in many beverages like coffee, tea, and Coke and in some medications like pain remedies. Global consumption of caffeine has been estimated at 120 000 tons per year, making it the most widely consumed pharmacologically active substance in the world.19 Many epidemiological studies (case-control and cohort studies) demonstrated habitual coffee drinking to be significantly inversely associated with HCC mortality.14-18,20,21 According to National Health and Nutrition Examination Survey (NHANES III), two cups of coffee per day were sufficient to reduce the risk of fibrosis progression markedly.22 This effect was presumably mediated by caffeine. In addition to its preventive properties, studies have also proposed caffeine to be an anti-cancer agent due to its inhibitory effect on cell growth and induction of apoptosis in some cancer cell lines.23,24 Indeed the potential chemotherapeutic benefit of caffeine was identified as early as 1974 when topical administration was shown to inhibit skin carcinogenesis in mice.25 Subsequently, inhibition of carcinogenesis by caffeine was reported in lung, stomach, and breast.26-28 Taken together all these studies suggest that caffeine can be considered as both chemopreventive and chemotherapeutic agent. Importantly caffeine has also been show to enhance the toxicity of radiation29-33 and of chemotherapeutic agents in cancer cells including hepatoma cell lines.34-36 Caffeine has been reported to affect cell cycle and induce apoptosis23,24,37-39 but the molecular mechanisms of its anti-carcinogenic effect are still not completely elucidated. Interestingly, caffeine has been shown to inhibit various isoforms of phosphoinositide 3-kinases (PI3Ks) in vitro.40
Phosphoinositide 3 kinases (PI3K) are a family of lipid kinases divided into three classes.41,42 Class I exist as heterodimers consisting of a catalytic subunit and a regulatory subunit; class IA comprises three catalytic subunits (p110α, p110β, and p110δ) and three regulatory subunits (p85α, p85β, and p55γ) while p110γ is the only catalytic subunit of class IB and it can associate with two regulatory subunits (p84 and p101). Class II comprises three monomeric isoforms (PI3K-C2α, PI3K-C2β, and PI3K-C2γ)42 whereas hVps34 is the only member of class III.41,42 PI3Ks catalyze the phosphorylation of the 3-hydroxyl group within the inositol ring of phosphatidylinositol and some of its derivatives phosphoinositides. Specifically, class I PI3Ks catalyze the phosphorylation of phosphatidylinositol 4,5-bisphosphate (PtdIns[4,5]P2) to generate phosphatidylinositol 3,4,5-trisphosphate (PtdIns[3,4,5]P3) downstream of receptor tyrosine kinase or G-protein-coupled receptors activation. PtdIns(3,4,5)P3 then interacts and activates specific kinases, including the most studied protein kinase B/Akt that in turn control several cellular functions including cell survival, proliferation, and migration. The PI3K pathway is mainly regulated by the enzyme phosphatase and tensin homolog (PTEN), which is a tumor suppressor and a lipid phosphatase that dephosphorylates PtdIns(3,4,5)P3 to PtdIns(4,5)P2 therefore antagonizing the action of PI3Ks.43
Hyperactivation of PI3K-dependent pathways occurs in many types of cancer, including HCC, either as a result of mutation/deletion of PTEN gene43-45 or due to gain of function/mutation of PI3Ks or amplification of its downstream effector Akt.46-48 Indeed the PI3K pathway has increasingly become an attractive target in cancer therapy development49-51 and PI3Ks inhibitors are being tested in clinical trials. It is important to notice that for many years most of the studies investigating the role of PI3Ks in cancer have been almost exclusively focused on the class IA isoform p110α, since gain of function of this specific isoform through mutation was detected in several human cancers.52 Only recently has an increased interest emerged to determine the potential contribution of other PI3K isoforms to cancer development and progression and to identify alternative and possibly more specific therapeutic strategies. In this respect we have recently demonstrated that the class IB isoform p110γ plays an important role in pancreatic53 and liver cancer.54 Similarly other groups have shown that p110γ may play a role in medulloblastoma55 and breast cancer.56 These data have revealed a novel important role for p110γ in tumorigenesis and have highlighted the importance of developing potential novel strategies specifically targeting this isoform.57
Here we show that caffeine and the analog CGS 15943 inhibits proliferation of HCC and pancreatic ductal adenocarcinoma (PDAC) cells. Our data show that caffeine and CGS 15943 have an anti-carcinogenic effect on HCC and PDAC cells by acting on the PI3K/Akt signaling pathway. Importantly we show that CGS 15943 selectively targets p110γ indicating that it may represent an important lead compound to develop drugs that can specifically target this PI3K isoform whose key role in cancer progression is emerging.
Results
Caffeine and CGS 15943 inhibit proliferation of human HCC
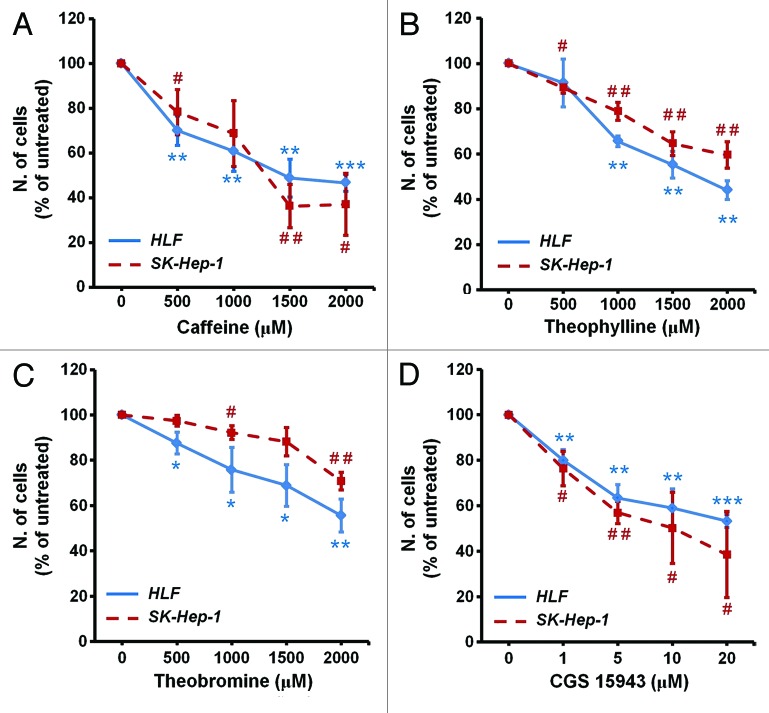
To determine whether caffeine affected proliferation, distinct HCC cell lines were incubated with increasing concentrations of caffeine and cell number was determined by counting after 72 h. Results show that caffeine strongly inhibited cell growth in HLF and SK-Hep-1 cell lines (Fig. 1A). Similar results were obtained in HepG2 and PLC-PRF-5 cells (Fig. S1A). A reduction in cell viability was also detected by MTT assay in HLF and HepG2 upon treatment with increasing concentrations of caffeine (Fig. S1B). We then determined the effect of distinct methyl xanthines on cell proliferation of HCC cell lines. Treatment of HLF and SK-Hep-1 with increasing concentrations of theophylline (Fig. 1B) and, to a lesser extent, theobromine (Fig. 1C) also reduced cell number as assessed by cell counting (Fig. 1B and C). HLF cell viability assessed by MTT was also inhibited upon treatment with increasing concentrations of theophylline (Fig. S1C) and, to a lesser extent, theobromine (Fig. S1D). Once assessed the inhibitory effect of methyl xanthines we then investigated the effect of caffeine analogs on HCC cell growth. Among these analogs we observed that the compound CGS 15943 inhibited growth of HLF and SK-Hep-1 (Fig. 1D) as well as HepG2 and PLC-PRF-5 cells (Fig. S1E) assessed by cell counting. Similarly, viability assessed by MTT was reduced in PLC-PRF-5 and HLF upon treatment with CGS 15943 (Fig. S1F). Taken together these data indicated that methyl-xanthines, and more potently the caffeine analog CGS 15943 inhibit growth of four distinct HCC cell lines.
Figure 1. In vitro activity of xanthines and CGS 15943 on HCC cell lines. HLF and SK-Hep-1 cell lines were treated for 72 h with increasing concentrations of the indicated compounds in the presence of serum and cell proliferation was assessed by cell counting. In all panels data are expressed as percentage of untreated cells and are means ± s.e.m. (A) Results are from n = 4–7 (HLF) and n = 3–5 (Sk-Hep-1) independent experiments. (B) Results are from n = 3 (HLF) and n = 4 (Sk-Hep-1) independent experiments. (C) For both cell lines results are from n = 4 independent experiments. (D) Results are from n = 4–7 (HLF) and n = 3–4 (Sk-Hep-1) independent experiments. HLF: *P < 0.05, **P < 0.01, ***P < 0.001 vs corresponding untreated cells as assessed by the Student t test (paired, one-tailed distribution). SK-Hep-1: #P < 0.05, ##P < 0.01 vs corresponding untreated cells as assessed by the Student t test (paired, one-tailed distribution).
CGS 15943 only slightly induces apoptosis in HCC
We next investigated whether the reduction in cell number detected in HCC cell lines upon treatment with CGS 15943 or caffeine was due to increased apoptosis or to inhibition of cell proliferation. HLF and SK-Hep-1 cells were treated with CGS 15943 for 72 h in the presence of serum and the percentage of apoptotic cells was measured by flow cytometry using AnnexinV-conjugated FITC and PI stain. Treatment with CGS 15943 only slightly induced apoptosis when used at concentrations of 20 μM in SK-Hep-1 (Fig. 2). On the other hand, caffeine did not appear to induce apoptosis when used at a concentration able to block proliferation in SK-Hep-1 cells (Fig. 2). The relatively modest effect detected in these experiments suggests that induction of apoptosis only partially contributes to the reduction of cell number detected upon treatment with CGS 15943 or caffeine. Taken together these data suggest that both compounds mainly affect cell proliferation, possibly through cell cycle impairment, whereas they only slightly induce apoptosis.
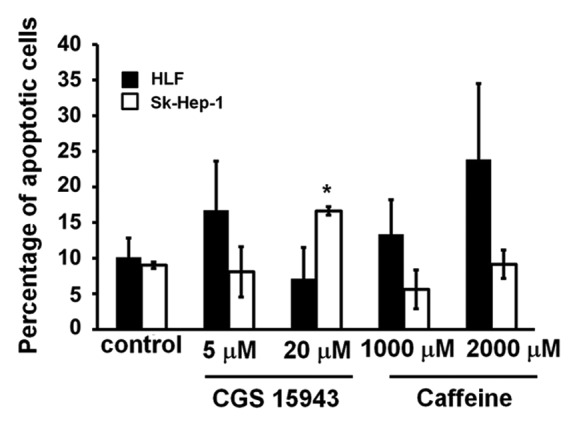
Figure 2. Anti-apoptotic activity of CGS 154943 and caffeine in HCC cells. HCC cell lines were treated for 72 h with the indicated concentrations of CGS 15943 and caffeine. The number of surviving cells was assessed by flow cytometry using Annexin V-conjugated FITC and PI stain. Data are means ± s.e.m. of n = 3 (SK-Hep-1) and n = 2 (HLF) independent experiments. *P < 0.01.
Caffeine and CGS 15943 inhibit the PI3K/Akt pathway
Since it has been previously shown that both caffeine and CGS could affect the PI3K lipid kinase in vitro activity40 we decided to investigate whether the detected effects of CGS 15943 in HCC were ascribable to inhibition of the PI3K pathway in vivo. To test this hypothesis, HLF and Sk-Hep-1 were treated with increasing concentrations of CGS 15943 for 24 h in the presence of serum and phosphorylation of the PI3K downstream effector Akt was assessed by western blotting analysis. CGS 15943 reduced the phosphorylation of Akt at its residues Ser473 (Fig. 3A and B) and Thr308 (Fig. 3C and D) in HLF and Sk-Hep-1. Similarly, both compounds inhibited Akt Ser473 phosphorylation in HepG2 cells (Fig. S2A) and in PLC-PRF-5 (Fig. S2B). No effect was detected on phosphorylation of ERK1/2 in HLF (Fig. 3E), Sk-Hep-1 (Fig. 3F), and HepG2 (Fig. S2A). Inhibition of Akt phosphorylation at both residues Ser473 and Thr308 was also observed in Sk-Hep-1 treated with increasing concentrations of caffeine (Fig. S3A). Treatment of SK-Hep-1 with theophylline inhibited Ser473 Akt phosphorylation (Fig. S3B and D) and theobromine also slightly affected Ser473 Akt phosphorylation in SK-Hep-1 (Fig. S3C and E). Both theophylline and theobromine also reduced Ser473 Akt phosphorylation in HLF (Fig. S3D and E). None of these treatments affected ERK1/2 phosphorylation (Fig. S3B–E). These data demonstrate that caffeine and CGS 15943 inhibit the PI3K/Akt pathway in HCC cells.
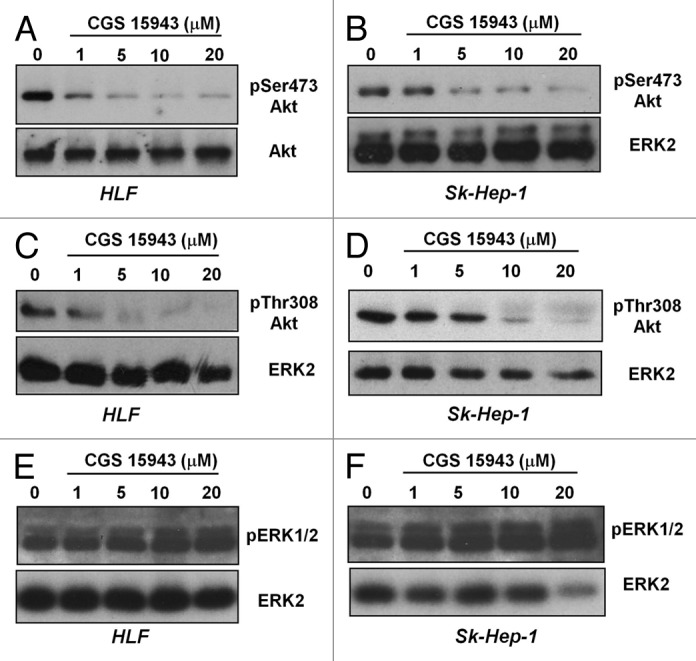
Figure 3. In vitro activity of CGS and caffeine on Akt phosphorylation. HLF and SK-Hep-1 cells were treated for 24 h with the indicated concentrations of CGS 15943 in the presence of serum. Akt activation was assessed by monitoring phosphorylation at its residues Ser473 (A and B) and Thr308 (C and D). Equal loading was assessed using an anti-ERK2 antibody. Alternatively membranes were stripped and re-incubated with an anti-Akt antibody. Phosphorylation of ERK1/2 was also assessed by using a specific antibody (E and F). Membranes were then stripped and re-incubated with an anti-ERK2 antibody.
Selective adenosine receptor antagonists did not inhibit proliferation
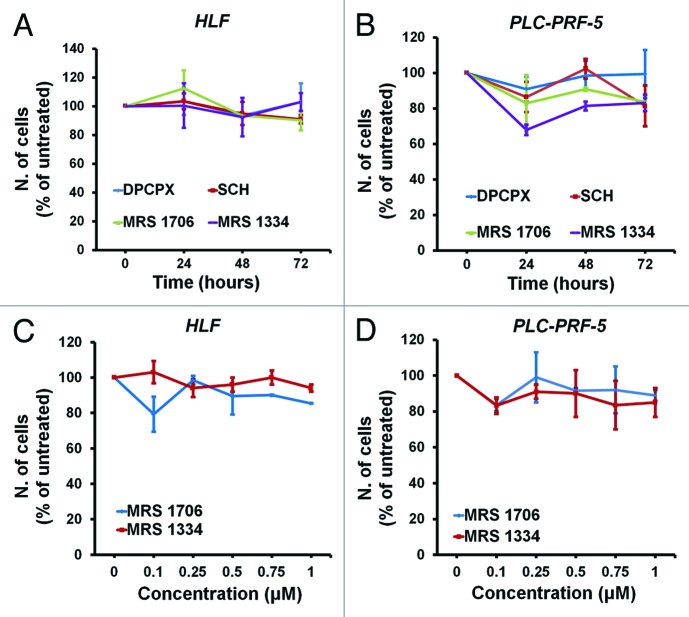
Since caffeine and CGS 15943 are known non-selective adenosine receptor antagonists we next investigated whether the detected effects on proliferation and on the PI3K pathway could result from inhibition of adenosine receptors. To test this hypothesis, we determined the effect of specific antagonists of the four adenosine receptors on proliferation of HCC cell lines. We first performed a time course of the effects of DPCPX (A1 receptor antagonist), SCH (A2A receptor antagonist), MRS 1706 (AAB receptor antagonist), and MRS 1334 (A3 receptor antagonist) on proliferation of HLF (Fig. 4A) and PLC-PRF-5 (Fig. 4B) cells. MRS 1706 and MRS 1334 were then tested at concentrations ranging from 0.01 µM to 1 µM in the same cells (Fig. 4C and D). Interestingly, we did not observe significant inhibition of cell proliferation in either PLC-PRF-5 or HLF cells upon treatment with the adenosine receptor inhibitors and at the concentrations tested. These data rule out a role for these receptors in caffeine- and CGS 15943-induced inhibition of proliferation of HCC cells.
Figure 4. In vitro activity of adenosine receptors-specific antagonists on HCC cell lines. HLF and PLC-PRF-5 cells were treated for the indicated time points with 0.1 μM of the indicated adenosine receptors antagonists (A and B) or for 72 h with increasing concentrations of receptors-specific antagonists (C and D) in the presence of serum. Cell growth was assessed by cell counting. In all panels data are expressed as percentage of untreated cells and are means ± s.e.m. of n = 2 (A and C) (except for treatment with MRS 1796 and MRS 1334 at 72 h for which n = 4) and n = 2 (B and D) (except for treatment at 0.1 μM for which n = 4) independent experiments.
Kinase profiling assay
To gain further insight into the mechanism by which CGS 15943 inhibits the PI3K/Akt pathway we performed an in vitro kinase profiling assay (SelectScreenTM, Invitrogen). The effect of CGS 15943 was tested on 23 kinases including members of the PI3K family (Table 1), PI3K downstream effectors (Table 2) as Akt and 3-phosphoinositide-dependent protein kinase (PDK1) and several receptor tyrosine kinases (Table 3). The assay revealed that CGS 15943 inhibited the kinase activity of the class IB PI3K isoform p110γ with an IC50 of 1.1 μM (Fig. S4). A slight inhibition was also detected on the class IA PI3K isoform p110δ with an IC50 of 8.47 μM. These data indicate that CGS 15943 can directly inhibit the lipid kinase activity of p110γ.
Table 1. Results from SelectScreenTM (Invitrogen) kinase profiling of CGS 15943 − PI3K isoforms.
| [ATP] tested μM | Kinase tested | % of inhibition (point 1) | % of inhibition (point 2) | % of inhibition (mean) | Difference between data points (point 1 − point 2) | Z’ |
|---|---|---|---|---|---|---|
| Km app | PIK3C2A (PI3K-C2α) | −1 | 8 | 3 | 9 | 0.73 |
| 100 | PIK3C2B (PI3K-C2β) | 3 | 26 | 15 | 23 | 0.55 |
| Km app | PIK3C3 (hVPS34) | 2 | 4 | 3 | 2 | 0.78 |
| Km app | PIK3CA/PIK3R1 (p110 α/p85 α) | 15 | 6 | 10 | 9 | 0.76 |
| Km app | PIK3CD/PIK3R1 (p110 δ/p85 α) | 9 | 24 | 16 | 15 | 0.41 |
| Km app | PIK3CG (p110 γ) | 41 | 34 | 38 | 7 | 0.62 |
Table 2. Results from SelectScreenTM (Invitrogen) kinase profiling of CGS 15943 − PI3K downstream effectors.
| [ATP] tested μM | Kinase tested | % of inhibition (point 1) | % of inhibition (point 2) | % of inhibition (mean) | Difference between data points (point 1 − point 2) | Z’ |
|---|---|---|---|---|---|---|
| Km app | AKT1 (PKB α) | 3 | −2 | 0 | 6 | 0.56 |
| Km app | AKT2 (PKB β) | 8 | 4 | 6 | 4 | 0.79 |
| Km app | AKT3 (PKB γ) | 13 | 13 | 13 | 0 | 0.63 |
| 100 | PDK1 | 12 | 13 | 13 | 1 | 0.71 |
Table 3. Results from SelectScreenTM (Invitrogen) kinase profiling of CGS 15943 − other kinases.
| [ATP] tested μM | Kinase tested | % of inhibition (point 1) | % of inhibition (point 2) | % of inhibition (mean) | Difference between data points (point 1 − point 2) | Z’ |
|---|---|---|---|---|---|---|
| Km app | SPHK1 | −6 | −1 | −4 | 5 | 0.65 |
| Km app | EGFR (ErbB1) | −3 | 4 | 1 | 7 | 0.87 |
| Km app | FGFR1 | 0 | 0 | 0 | 1 | 0.82 |
| Km app | FRAP1 (mTOR) | −4 | 14 | 5 | 17 | 0.92 |
| Km app | IGF1R | −6 | −4 | −5 | 2 | 0.77 |
| Km app | INSR | −2 | −4 | −3 | 2 | 0.81 |
| Km app | KIT | −13 | −25 | −19 | 12 | 0.62 |
| Km app | MET (cMet) | −9 | −20 | −15 | 10 | 0.79 |
| Km app | PDGFRA (PDGFR α) | 19 | 13 | 16 | 7 | 0.78 |
| Km app | PTK2 (FAK) | 4 | 5 | 5 | 0 | 0.89 |
| 100 | RAF1 (cRAF) Y340D Y341D | 14 | 11 | 12 | 3 | 0.57 |
| Km app | RET | 4 | 3 | 4 | 1 | 0.91 |
| Km app | SRC | −1 | −6 | −3 | 4 | 0.90 |
Caffeine and CGS 15943 inhibit proliferation of human PDAC cell lines
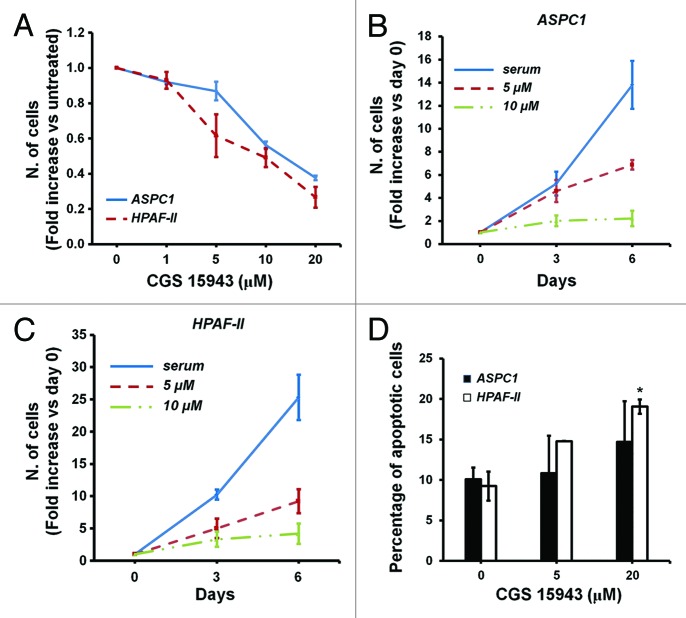
The inhibitory effect of CGS 15943 on proliferation of HCC cells together with the specific effect of this compound on p110γ kinase activity in vitro are consistent with our previous data demonstrating a key role for this specific PI3K isoform on proliferation of HCC cells.54 Since we also reported that p110γ is critical for proliferation of pancreatic cancer cells,53 we next investigated the effect of CGS 15943 on pancreatic cancer cell lines ASPC1 and HPAF-II. Consistent with the ability of the compound to inhibit p110γ activity, treatment with increasing concentrations of CGS 15943 for 72 h reduced the number of ASPC1 and HPAF-II assessed by cell counting (Fig. 5A). Time course experiments confirmed the inhibitory effect of CGS 15943 on growth of both cell lines (Fig. 5B and C). A slight increase in apoptosis was also detected in HPAF-II upon treatment with CGS (Fig. 5D). Reduced cell number (Fig. S5A) and viability (Fig. S5B) were also observed in ASPC1 cells treated with increasing concentrations of theophylline. Both CGS 15943 (Fig. S5C) and caffeine (Fig. S5D) inhibited Akt phosphorylation in ASPC1. Taken together, these data support the hypothesis that CGS 15943 inhibits proliferation of HCC and PDAC cell lines by specifically targeting the class IB isoform p110γ and its downstream target Akt. More important, these data suggest that CGS 15943 may represent a valid pharmacophore for the development of specific and potent inhibitors of this PI3K isoform as novel anti-cancer drugs for HCC and PDAC treatment.
Figure 5. In vitro activity of caffeine and CGS 15943 on PDAC cell lines. (A) ASPC1 and HPAF-II cells were treated for 72 h with the indicated concentrations of caffeine and CGS 15943 in the presence of serum and cell number was assessed by counting. Data are expressed as fold change vs. untreated cells and are means ± s.e.m. of n = 2–4 (ASPC1) and 2–5 (HPAF-II) independent experiments. (B and C) ASPC1 (B) and HPAF-II (C) cells were incubated with 5 μM or 20 μM CGS 15943 in the presence of serum. The number of cells was determined by cell counting after 3 and 6 d of incubation. Data are expressed as fold increase over number of cells at day 0 (start treatment) and are means ± s.e.m. of n = 4 (B) and n = 3–4 (C) independent experiments. (D) ASPC1 and HPAF-II cells were treated for 72 h with the indicated concentrations of CGS 15943 in serum-containing medium. The number of surviving cells was assessed by flow cytometry using Annexin V-conjugated FITC and PI stain. Data are means ± s.e.m. of n = 3 independent experiments. *P < 0.05.
Discussion
Several epidemiological studies have reported an association between coffee/caffeine intake and reduced risk of different types of cancer. For instance some of the most recent prospective studies have reported that caffeinated coffee is inversely associated with oral/pharyngeal cancer mortality58 and that coffee consumption, in particular caffeinated coffee, is associated with lower risk of type I endometrial cancer in obese postmenopausal women.59 Inverse association between coffee consumption and the risk of basal cell carcinoma has also been reported60 in particular in participants with prior skin cancers61 with no association for squamous cell carcinomas60,61 and melanoma.61 Specifically to our study, coffee/caffeine consumption has been inversely associated with HCC.14-18,20-22 One of the most recent metaanalyses has reported a 40% reduction in the risk of HCC for any coffee consumption compared with no consumption.62 A very similar percentage of reduction in risk of HCC (44%) for individuals who consumed three or more cups of coffee compared with non-drinkers has also been recently reported in the Singapore Chinese Health Study.63 These studies suggest that caffeine may represent a chemopreventive agent in HCC. Consistent with this hypothesis caffeine was reported to inhibit diethhylnitrosamine-induced hepatocarcinogenesis in rats64 and coffee, especially containing caffeine, was shown to prevent the formation of pre-neoplastic liver lesions induced by aflatoxin B1.65
In the present study we showed that caffeine was able to inhibit proliferation of four distinct HCC cell lines in vitro consistent with previous studies demonstrating anti-proliferative properties of caffeine in hepatic cancer cells.23,36-38 Other methylxanthines tested, namely theophylline and theobromine, were also able to reduce cell proliferation although to a lesser extent compared to caffeine. Interestingly, although it was previously reported that caffeine slightly induces apoptosis in pancreatic cancer cells,66 in a neuroblastoma cell line67 and in a mouse epithelial cell line JB624 we observed that caffeine was not able to induce apoptosis in hepatic cancer cells at the concentrations used in our study. This was consistent with previous studies reporting an effect on cell proliferation with no effect on apoptosis in hepatic cancer cells23,34-36 Our data support the conclusion that caffeine could have anti-carcinogenic effects in HCC in addition to chemopreventive properties. However the high concentrations of caffeine (1–2 mM) necessary to exert its inhibitory effect clearly indicate that caffeine itself is not likely to represent a suitable chemotherapeutic agent. Indeed over 100 cups of coffee per day would be required to achieve a concentration of 1–2 mM caffeine physiologically.68 This high concentration of caffeine in blood would be followed by adverse side effects, since caffeine can only reach 50–100 µM before being highly toxic.68
We therefore decided to investigate whether analogs of caffeine might have similar anti-proliferative properties on HCC. Our data revealed that, among these analogs, CGS 15943 was able to inhibit in vitro growth of all HCC cell lines examined at concentrations (1 µM–20 µM) that were much lower than those necessary for caffeine to inhibit. Consistent with data obtained with caffeine the effect of CGS 15943 in HCC cell lines was mainly due to inhibition of cell proliferation than induction of cell apoptosis. These data suggested that CGS 15943 may represent a potential lead compound for development of novel therapeutic strategies.
In order to determine the specific mechanisms of action of this compound we first investigated the possibility that the anti-proliferative effects of CGS 15943 were due to its function as a non-selective adenosine receptor antagonists. Indeed it has been shown that CGS 15943 is approximately 3 to 10 times more potent than caffeine as a behavioral stimulant after intramuscular or intravenous administration in squirrel monkeys.69 However we observed that treatment of HCC cells with specific inhibitors of adenosine receptor receptors does not recapitulate the inhibitory effects of caffeine or CGS 15943 ruling out the possibility that the detected reduction of proliferation of HCC cell lines is due to inhibition of the adenosine receptors. On the other hand we found that caffeine and CGS 15943 were able to inhibit Akt phosphorylation indicating that these compounds exert their effect by targeting the PI3K/Akt pathway. Inhibition of Akt phosphorylation was previously reported in HepG2 and PLC/PRF/5 cells treated with caffeine at concentrations as high as 2.5 mM.34 Here we further extended this observation by showing that CGS 15943 was also able to affect Akt phosphorylation in HCC cell lines by acting on the PI3K/Akt pathway.
It is now well established that deregulation of PI3K/Akt pathway plays a pivotal role in development and progression of several types of cancer.43-52 Drugs targeting several components of the PI3K/Akt pathway are currently either in preclinical studies or are being tested in clinical trials.49-51 Few of them, for instance drugs targeting the Akt downstream effector mammalian target of rapamycin, have also been approved for specific cancer types. Although the importance of targeting PI3Ks in cancer is widely recognized and well accepted, a huge interest has currently emerged toward determining the specific role of each PI3K isoform in cancer. Indeed selective inhibitors targeting only one PI3K isoform would guarantee a lower toxicity and higher specificity than pan-PI3K inhibitors.
In this respect we decided to further investigate the mechanisms of action of CGS 15943 by performing an in vitro screening of the effect of CGS 15943 on 23 different kinases including members of the PI3K family and PI3K downstream effectors ad Akt and PDK1. Interestingly this screening revealed a selective inhibitory activity of the compound toward the class IB PI3K isoform p110γ. A lower inhibitory activity toward the class IA isoform p110δ was also detected consistent with a previous report.40 It must be noted that in this previous study the activity toward p110γ was not assessed. No inhibition of the other PI3Ks tested was detected in our screening, consistent with previous results40 indicating specificity of CGS 15943 for p110γ.
Importantly we have recently demonstrated that p110γ is a critical enzyme for regulation of proliferation of HCC54 strongly suggesting that CGS 15943 affects proliferation of HCC cells by specifically inhibiting p110γ and its downstream effector Akt. Consistent with this hypothesis, we observed that CGS 15943 was also able to inhibit proliferation of pancreatic cancer cells, a process that we previously demonstrated to be highly dependent on p110γ.53 Even if the in vitro IC50 of CGS 15943 toward the kinase activity of p110γ does not indicate a strong potency of this compound, our data suggest that CGS 15943 is a good lead compound for the development of novel derivatives with higher potency and better pharmacokinetic properties.
In conclusion, the present data not only support our previous conclusions that targeting p110γ can represent an important, novel strategy to inhibit growth of HCC and pancreatic cancer cells57 but they also indicate that the caffeine analog CGS 15943 represents a promising molecule for further development of novel chemotherapeutic agents specifically targeting this enzyme whose key role in cancer progression is emerging. Since there are very few systemic chemotherapy available for HCC and PDAC, these results provide important information to develop novel potential anti-cancer strategies.
Materials and Methods
Cell lines and culture
Hepatic cancer cell lines Sk-Hep-1, HLF, HepG2, and PLC-PRF-5 and pancreatic cancer cell lines ASPC1 and HPAF-II were from American Type Culture Collection (ATCC). Cells were cultured in RPMI medium supplemented with 10% FBS and 1% penicillin, streptomycin, glutamine (all from GIBCO®, Life TechnologiesTM) at 37 °C in a 5% CO2 incubator.
Materials
Caffeine, CGS 15943 (Sigma-Aldrich Co Ltd.) were dissolved in PBS and DMSO respectively. Theophylline and Theobromine (Sigma-Aldrich Co Ltd.) were dissolved in 0.1 M NaOH. Adenosine receptor antagonists DPCPX, SCH, MRS 1706 and MRS 1334 (Tocris Bioscience) were dissolved in DMSO and were used at concentrations ranging from 0.01 µM to 1 µM. For western blotting analysis, the following primary antibodies were used: anti-phosphoSer473 Akt (Cell Signaling Technology), anti-phosphoThr308 Akt (Santa Cruz Biotechnology Inc.), anti-phospho-p44/42 MAPK (Erk1/2) (Thr202/Tyr204) (Cell Signaling Technology), anti-ERK2 (Santa Cruz Biotechnology Inc.), and anti-Akt (Santa Cruz Biotechnology Inc.). HRP-conjugated secondary anti-rabbit and anti-mouse IgG antibodies were from Sigma-Aldrich Co Ltd. whereas anti-goat IgG was from Dako UK Ltd.
Western blot analysis
Cells were washed with ice-cold PBS and lysed in lysis buffer (100 mM NaCl, 50 mM TRIS-HCl [pH 7.5], 1 mM EDTA, 1 mM phenylmethylsulphonyl fluoride, 1 mM sodium orthovanadate, 1% Triton X-100 and protease and phosphatase inhibitor cocktails [Sigma-Aldrich Co Ltd]) for 30 min on ice. Protein levels were quantified by Bradford protein assay. Proteins were separated by SDS/PAGE and transferred onto a nitrocellulose membrane (Whatman®). To block the unspecific binding of proteins, the membranes were incubated with blocking buffer containing 5% milk (Fluka Analytical GmbH) in PBS-Tween 20 for 30–60 min at room temperature. After blocking, membranes were washed once with PBS and incubated with primary antibodies overnight at 4 °C. Membranes were then washed 3× with PBS-Tween 20 and then incubated with secondary antibodies in PBS-Tween 20 for 1 h at room temperature. ECL (GE Healthcare Life Sciences) was used to visualize HRP-conjugated secondary antibodies.
Cell proliferation/survival
To determine the effect of each specific inhibitor, cells were cultured in 12-well plates at 37 °C in a 5% CO2 incubator and treated with the indicated xanthine or CGS 15943 for 72 h or with adenosine receptor antagonists (DPCPX, SCH, MRS 1706, and MRS 1334) for 24, 48, and 72 h. The number of surviving cells was determined by manual counting using a hemocytometer. Differences between groups were analyzed by the Student t test (paired, one-tailed distribution).
Alternatively, cells were cultured in 96-well plates at 37 °C in a 5% CO2 incubator and treated with the compounds for 72 h before incubation with MTT (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide) for 4 h. Cells were then dissolved in DMSO and the absorbance was measured using a spectrophotometer (Synergy HT, BioTek). Differences between groups were analyzed by the Student t test (paired, one-tailed distribution).
Apoptosis assay
Cells were treated with caffeine and CGS 15943 for 72 h in the presence of serum. Cells were then trypsinized and pellets were washed with cold PBS before being resuspended in binding buffer and incubated with Annexin V-conjugated FITC and propidium iodide (PI) at room temperature for 15 min in the dark. Stained cells were analyzed by flow cytometry on BD FACS Canto II analyzer.
SelectScreenTM kinase profiling
The effect of CGS 15943 on the activity of various kinases was assessed by SelectScreenTM Kinase Profiling Service (Life Technologies™). Assays were performed using 1 μM of the tested compound and ATP concentration as indicated in Tables 1–3.
Supplementary Material
Disclosure of Potential Conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
This work was supported by Pancreatic Cancer Research Fund.
Glossary
Abbreviations:
- HCC
hepatocellular carcinoma
- PDAC
pancreatic ductal adenocarcinoma
- PI
propidium iodide
- PI3K
phosphoinositide 3-kinase
- PtdIns(4,5)P2
phosphatidylinositol 4,5-bisphosphate
- PtdIns(3,4,5)P3
phosphatidylinositol 3,4,5-trisphosphate
- PTEN
phosphatase and tensin homolog
Footnotes
Previously published online: www.landesbioscience.com/journals/cbt/article/28018
References
- 1.Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet. 2012;379:1245–55. doi: 10.1016/S0140-6736(11)61347-0. [DOI] [PubMed] [Google Scholar]
- 2.Di Bisceglie AM, Carithers RL, Jr., Gores GJ. Hepatocellular carcinoma. Hepatology. 1998;28:1161–5. doi: 10.1002/hep.510280436. [DOI] [PubMed] [Google Scholar]
- 3.Bosch FX, Ribes J, Cléries R, Díaz M. Epidemiology of hepatocellular carcinoma. Clin Liver Dis. 2005;9:191–211, v. doi: 10.1016/j.cld.2004.12.009. [DOI] [PubMed] [Google Scholar]
- 4.Farazi PA, DePinho RA. Hepatocellular carcinoma pathogenesis: from genes to environment. Nat Rev Cancer. 2006;6:674–87. doi: 10.1038/nrc1934. [DOI] [PubMed] [Google Scholar]
- 5.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 6.El-Serag HB. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology. 2012;142:1264–73, e1. doi: 10.1053/j.gastro.2011.12.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Taylor-Robinson SD, Foster GR, Arora S, Hargreaves S, Thomas HC. Increase in primary liver cancer in the UK, 1979-94. Lancet. 1997;350:1142–3. doi: 10.1016/S0140-6736(05)63789-0. [DOI] [PubMed] [Google Scholar]
- 8.Deuffic S, Poynard T, Buffat L, Valleron AJ. Trends in primary liver cancer. Lancet. 1998;351:214–5. doi: 10.1016/S0140-6736(05)78179-4. [DOI] [PubMed] [Google Scholar]
- 9.Singh S, Singh PP, Roberts LR, Sanchez W. Chemopreventive strategies in hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol. 2014;11:45–54. doi: 10.1038/nrgastro.2013.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kuper H, Tzonou A, Kaklamani E, Hsieh CC, Lagiou P, Adami HO, Trichopoulos D, Stuver SO. Tobacco smoking, alcohol consumption and their interaction in the causation of hepatocellular carcinoma. Int J Cancer. 2000;85:498–502. doi: 10.1002/(SICI)1097-0215(20000215)85:4<498::AID-IJC9>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 11.Thomas MB, Jaffe D, Choti MM, Belghiti J, Curley S, Fong Y, Gores G, Kerlan R, Merle P, O’Neil B, et al. Hepatocellular carcinoma: consensus recommendations of the National Cancer Institute Clinical Trials Planning Meeting. J Clin Oncol. 2010;28:3994–4005. doi: 10.1200/JCO.2010.28.7805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ryder SD, British Society of Gastroenterology Guidelines for the diagnosis and treatment of hepatocellular carcinoma (HCC) in adults. Gut. 2003;52(Suppl 3):iii1–8. doi: 10.1136/gut.52.suppl_3.iii1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thomas MB, O’Beirne JP, Furuse J, Chan AT, Abou-Alfa G, Johnson P. Systemic therapy for hepatocellular carcinoma: cytotoxic chemotherapy, targeted therapy and immunotherapy. Ann Surg Oncol. 2008;15:1008–14. doi: 10.1245/s10434-007-9705-0. [DOI] [PubMed] [Google Scholar]
- 14.Kurozawa Y, Ogimoto I, Shibata A, Nose T, Yoshimura T, Suzuki H, Sakata R, Fujita Y, Ichikawa S, Iwai N, et al. JACC Study Group Coffee and risk of death from hepatocellular carcinoma in a large cohort study in Japan. Br J Cancer. 2005;93:607–10. doi: 10.1038/sj.bjc.6602737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gallus S, Bertuzzi M, Tavani A, Bosetti C, Negri E, La Vecchia C, Lagiou P, Trichopoulos D. Does coffee protect against hepatocellular carcinoma? Br J Cancer. 2002;87:956–9. doi: 10.1038/sj.bjc.6600582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bravi F, Bosetti C, Tavani A, Bagnardi V, Gallus S, Negri E, Franceschi S, La Vecchia C. Coffee drinking and hepatocellular carcinoma risk: a meta-analysis. Hepatology. 2007;46:430–5. doi: 10.1002/hep.21708. [DOI] [PubMed] [Google Scholar]
- 17.Gelatti U, Covolo L, Franceschini M, Pirali F, Tagger A, Ribero ML, Trevisi P, Martelli C, Nardi G, Donato F, Brescia HCC Study Group Coffee consumption reduces the risk of hepatocellular carcinoma independently of its aetiology: a case-control study. J Hepatol. 2005;42:528–34. doi: 10.1016/j.jhep.2004.11.039. [DOI] [PubMed] [Google Scholar]
- 18.Inoue M, Yoshimi I, Sobue T, Tsugane S, JPHC Study Group Influence of coffee drinking on subsequent risk of hepatocellular carcinoma: a prospective study in Japan. J Natl Cancer Inst. 2005;97:293–300. doi: 10.1093/jnci/dji040. [DOI] [PubMed] [Google Scholar]
- 19.Weinberg BA, Bealer BK, eds. The world of caffeine: the science and culture of the world's most popular drug. New York:Routledge, 2001. [Google Scholar]
- 20.Kurozawa Y, Ogimoto I, Shibata A, Nose T, Yoshimura T, Suzuki H, Sakata R, Fujita Y, Ichikawa S, Iwai N, et al. Dietary habits and risk of death due to hepatocellular carcinoma in a large scale cohort study in Japan. Univariate analysis of JACC study data. Kurume Med J. 2004;51:141–9. doi: 10.2739/kurumemedj.51.141. [DOI] [PubMed] [Google Scholar]
- 21.Shimazu T, Tsubono Y, Kuriyama S, Ohmori K, Koizumi Y, Nishino Y, Shibuya D, Tsuji I. Coffee consumption and the risk of primary liver cancer: pooled analysis of two prospective studies in Japan. Int J Cancer. 2005;116:150–4. doi: 10.1002/ijc.20989. [DOI] [PubMed] [Google Scholar]
- 22.Ruhl CE, Everhart JE. Coffee and tea consumption are associated with a lower incidence of chronic liver disease in the United States. Gastroenterology. 2005;129:1928–36. doi: 10.1053/j.gastro.2005.08.056. [DOI] [PubMed] [Google Scholar]
- 23.Okano J, Nagahara T, Matsumoto K, Murawaki Y. Caffeine inhibits the proliferation of liver cancer cells and activates the MEK/ERK/EGFR signalling pathway. Basic Clin Pharmacol Toxicol. 2008;102:543–51. doi: 10.1111/j.1742-7843.2008.00231.x. [DOI] [PubMed] [Google Scholar]
- 24.He Z, Ma WY, Hashimoto T, Bode AM, Yang CS, Dong Z. Induction of apoptosis by caffeine is mediated by the p53, Bax, and caspase 3 pathways. Cancer Res. 2003;63:4396–401. [PubMed] [Google Scholar]
- 25.Rothwell K. Dose-related inhibition of chemical carcinogenesis in mouse skin by caffeine. Nature. 1974;252:69–70. doi: 10.1038/252069a0. [DOI] [PubMed] [Google Scholar]
- 26.Nishikawa A, Furukawa F, Imazawa T, Ikezaki S, Hasegawa T, Takahashi M. Effects of caffeine on glandular stomach carcinogenesis induced in rats by N-methyl-N’-nitro-N-nitrosoguanidine and sodium chloride. Food Chem Toxicol. 1995;33:21–6. doi: 10.1016/0278-6915(95)80243-6. [DOI] [PubMed] [Google Scholar]
- 27.Theiss JC, Shimkin MB. Inhibiting effect of caffeine on spontaneous and urethan-induced lung tumors in strain A mice. Cancer Res. 1978;38:1757–61. [PubMed] [Google Scholar]
- 28.VanderPloeg LC, Welsch CW. Inhibition by caffeine of ovarian hormone-induced mammary gland tumorigenesis in female GR mice. Cancer Lett. 1991;56:245–50. doi: 10.1016/0304-3835(91)90009-7. [DOI] [PubMed] [Google Scholar]
- 29.Tolmach LJ, Jones RW, Busse PM. The action of caffeine on X-irradiated HeLa cells. I. Delayed inhibition of DNA synthesis. Radiat Res. 1977;71:653–65. doi: 10.2307/3574633. [DOI] [PubMed] [Google Scholar]
- 30.Dillehay LE, Chang G, Williams JR. Effects of methylxanthines on cell-cycle redistribution and sensitization to killing by low-dose-rate radiation. NCI Monogr. 1988;6:173–6. [PubMed] [Google Scholar]
- 31.Boonkitticharoen V, Laohathai K, Puribhat S. Differential radiosensitization of radioresistant human cancer cells by caffeine. J Med Assoc Thai. 1993;76:271–7. [PubMed] [Google Scholar]
- 32.Busse PM, Bose SK, Jones RW, Tolmach LJ. The action of caffeine on X-irradiated HeLa cells. II. Synergistic lethality. Radiat Res. 1977;71:666–77. doi: 10.2307/3574634. [DOI] [PubMed] [Google Scholar]
- 33.Busse PM, Bose SK, Jones RW, Tolmach LJ. The action of caffeine on X-irradiated HeLa cells. III. Enhancement of X-ray-induced killing during G2 arrest. Radiat Res. 1978;76:292–307. doi: 10.2307/3574780. [DOI] [PubMed] [Google Scholar]
- 34.Fujimaki S, Matsuda Y, Wakai T, Sanpei A, Kubota M, Takamura M, Yamagiwa S, Yano M, Ohkoshi S, Aoyagi Y. Blockade of ataxia telangiectasia mutated sensitizes hepatoma cell lines to sorafenib by interfering with Akt signaling. Cancer Lett. 2012;319:98–108. doi: 10.1016/j.canlet.2011.12.043. [DOI] [PubMed] [Google Scholar]
- 35.Kawano Y, Nagata M, Kohno T, Ichimiya A, Iwakiri T, Okumura M, Arimori K. Caffeine increases the antitumor effect of Cisplatin in human hepatocellular carcinoma cells. Biol Pharm Bull. 2012;35:400–7. doi: 10.1248/bpb.35.400. [DOI] [PubMed] [Google Scholar]
- 36.Chae S, Kim YB, Lee JS, Cho H. Resistance to paclitaxel in hepatoma cells is related to static JNK activation and prohibition into entry of mitosis. Am J Physiol Gastrointest Liver Physiol. 2012;302:G1016–24. doi: 10.1152/ajpgi.00449.2011. [DOI] [PubMed] [Google Scholar]
- 37.Miura Y, Ono K, Okauchi R, Yagasaki K. Inhibitory effect of coffee on hepatoma proliferation and invasion in culture and on tumor growth, metastasis and abnormal lipoprotein profiles in hepatoma-bearing rats. J Nutr Sci Vitaminol (Tokyo) 2004;50:38–44. doi: 10.3177/jnsv.50.38. [DOI] [PubMed] [Google Scholar]
- 38.Ito K, Nakazato T, Miyakawa Y, Yamato K, Ikeda Y, Kizaki M. Caffeine induces G2/M arrest and apoptosis via a novel p53-dependent pathway in NB4 promyelocytic leukemia cells. J Cell Physiol. 2003;196:276–83. doi: 10.1002/jcp.10289. [DOI] [PubMed] [Google Scholar]
- 39.Hashimoto T, He Z, Ma WY, Schmid PC, Bode AM, Yang CS, Dong Z. Caffeine inhibits cell proliferation by G0/G1 phase arrest in JB6 cells. Cancer Res. 2004;64:3344–9. doi: 10.1158/0008-5472.CAN-03-3453. [DOI] [PubMed] [Google Scholar]
- 40.Foukas LC, Daniele N, Ktori C, Anderson KE, Jensen J, Shepherd PR. Direct effects of caffeine and theophylline on p110 delta and other phosphoinositide 3-kinases. Differential effects on lipid kinase and protein kinase activities. J Biol Chem. 2002;277:37124–30. doi: 10.1074/jbc.M202101200. [DOI] [PubMed] [Google Scholar]
- 41.Vanhaesebroeck B, Guillermet-Guibert J, Graupera M, Bilanges B. The emerging mechanisms of isoform-specific PI3K signalling. Nat Rev Mol Cell Biol. 2010;11:329–41. doi: 10.1038/nrm2882. [DOI] [PubMed] [Google Scholar]
- 42.Falasca M, Maffucci T. Regulation and cellular functions of class II phosphoinositide 3-kinases. Biochem J. 2012;443:587–601. doi: 10.1042/BJ20120008. [DOI] [PubMed] [Google Scholar]
- 43.Salmena L, Carracedo A, Pandolfi PP. Tenets of PTEN tumor suppression. Cell. 2008;133:403–14. doi: 10.1016/j.cell.2008.04.013. [DOI] [PubMed] [Google Scholar]
- 44.Keniry M, Parsons R. The role of PTEN signaling perturbations in cancer and in targeted therapy. Oncogene. 2008;27:5477–85. doi: 10.1038/onc.2008.248. [DOI] [PubMed] [Google Scholar]
- 45.Vinciguerra M, Foti M. PTEN at the crossroad of metabolic diseases and cancer in the liver. Ann Hepatol. 2008;7:192–9. [PubMed] [Google Scholar]
- 46.Miller TW, Rexer BN, Garrett JT, Arteaga CL. Mutations in the phosphatidylinositol 3-kinase pathway: role in tumor progression and therapeutic implications in breast cancer. Breast Cancer Res. 2011;13:224. doi: 10.1186/bcr3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Whittaker S, Marais R, Zhu AX. The role of signaling pathways in the development and treatment of hepatocellular carcinoma. Oncogene. 2010;29:4989–5005. doi: 10.1038/onc.2010.236. [DOI] [PubMed] [Google Scholar]
- 48.Thompson JE, Thompson CB. Putting the rap on Akt. J Clin Oncol. 2004;22:4217–26. doi: 10.1200/JCO.2004.01.103. [DOI] [PubMed] [Google Scholar]
- 49.Hennessy BT, Smith DL, Ram PT, Lu Y, Mills GB. Exploiting the PI3K/AKT pathway for cancer drug discovery. Nat Rev Drug Discov. 2005;4:988–1004. doi: 10.1038/nrd1902. [DOI] [PubMed] [Google Scholar]
- 50.Falasca M. PI3K/Akt signalling pathway specific inhibitors: a novel strategy to sensitize cancer cells to anti-cancer drugs. Curr Pharm Des. 2010;16:1410–6. doi: 10.2174/138161210791033950. [DOI] [PubMed] [Google Scholar]
- 51.Falasca M. Phosphoinositide 3-kinase pathway inhibitors: pharmacology, metabolism & drug development. Curr Med Chem. 2011;18:2673. doi: 10.2174/092986711796011210. [DOI] [PubMed] [Google Scholar]
- 52.Zhao L, Vogt PK. Class I PI3K in oncogenic cellular transformation. Oncogene. 2008;27:5486–96. doi: 10.1038/onc.2008.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Edling CE, Selvaggi F, Buus R, Maffucci T, Di Sebastiano P, Friess H, Innocenti P, Kocher HM, Falasca M. Key role of phosphoinositide 3-kinase class IB in pancreatic cancer. Clin Cancer Res. 2010;16:4928–37. doi: 10.1158/1078-0432.CCR-10-1210. [DOI] [PubMed] [Google Scholar]
- 54.Dituri F, Mazzocca A, Lupo L, Edling CE, Azzariti A, Antonaci S, Falasca M, Giannelli G. PI3K class IB controls the cell cycle checkpoint promoting cell proliferation in hepatocellular carcinoma. Int J Cancer. 2012;130:2505–13. doi: 10.1002/ijc.26319. [DOI] [PubMed] [Google Scholar]
- 55.Guerreiro AS, Fattet S, Kulesza DW, Atamer A, Elsing AN, Shalaby T, Jackson SP, Schoenwaelder SM, Grotzer MA, Delattre O, et al. A sensitized RNA interference screen identifies a novel role for the PI3K p110γ isoform in medulloblastoma cell proliferation and chemoresistance. Mol Cancer Res. 2011;9:925–35. doi: 10.1158/1541-7786.MCR-10-0200. [DOI] [PubMed] [Google Scholar]
- 56.Brazzatti JA, Klingler-Hoffmann M, Haylock-Jacobs S, Harata-Lee Y, Niu M, Higgins MD, Kochetkova M, Hoffmann P, McColl SR. Differential roles for the p101 and p84 regulatory subunits of PI3Kγ in tumor growth and metastasis. Oncogene. 2012;31:2350–61. doi: 10.1038/onc.2011.414. [DOI] [PubMed] [Google Scholar]
- 57.Falasca M, Selvaggi F, Buus R, Sulpizio S, Edling CE. Targeting phosphoinositide 3-kinase pathways in pancreatic cancer--from molecular signalling to clinical trials. Anticancer Agents Med Chem. 2011;11:455–63. doi: 10.2174/187152011795677382. [DOI] [PubMed] [Google Scholar]
- 58.Hildebrand JS, Patel AV, McCullough ML, Gaudet MM, Chen AY, Hayes RB, Gapstur SM. Coffee, tea, and fatal oral/pharyngeal cancer in a large prospective US cohort. Am J Epidemiol. 2013;177:50–8. doi: 10.1093/aje/kws222. [DOI] [PubMed] [Google Scholar]
- 59.Uccella S, Mariani A, Wang AH, Vierkant RA, Cliby WA, Robien K, Anderson KE, Cerhan JR. Intake of coffee, caffeine and other methylxanthines and risk of Type I vs Type II endometrial cancer. Br J Cancer. 2013;109:1908–13. doi: 10.1038/bjc.2013.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Song F, Qureshi AA, Han J. Increased caffeine intake is associated with reduced risk of basal cell carcinoma of the skin. Cancer Res. 2012;72:3282–9. doi: 10.1158/0008-5472.CAN-11-3511. [DOI] [PubMed] [Google Scholar]
- 61.Miura K, Hughes MC, Green AC, van der Pols JC. Caffeine intake and risk of basal cell and squamous cell carcinomas of the skin in an 11-year prospective study. Eur J Nutr. 2013 doi: 10.1007/s00394-013-0556-0. Forthcoming. [DOI] [PubMed] [Google Scholar]
- 62.Bravi F, Bosetti C, Tavani A, Gallus S, La Vecchia C. Coffee reduces risk for hepatocellular carcinoma: an updated meta-analysis. Clin Gastroenterol Hepatol. 2013;11:1413–1421. doi: 10.1016/j.cgh.2013.04.039. [DOI] [PubMed] [Google Scholar]
- 63.Johnson S, Koh WP, Wang R, Govindarajan S, Yu MC, Yuan JM. Coffee consumption and reduced risk of hepatocellular carcinoma: findings from the Singapore Chinese Health Study. Cancer Causes Control. 2011;22:503–10. doi: 10.1007/s10552-010-9725-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fujise Y, Okano J, Nagahara T, Abe R, Imamoto R, Murawaki Y. Preventive effect of caffeine and curcumin on hepato-carcinogenesis in diethylnitrosamine-induced rats. Int J Oncol. 2012;40:1779–88. doi: 10.3892/ijo.2012.1343. [DOI] [PubMed] [Google Scholar]
- 65.Ferk F, Huber WW, Grasl-Kraupp B, Speer K, Buchmann S, Bohacek R, Mišík M, Edelbauer L, Knasmüller S. Protective effects of coffee against induction of DNA damage and pre-neoplastic foci by aflatoxin B1. Mol Nutr Food Res. 2013(Forthcoming) doi: 10.1002/mnfr.201300154. [DOI] [PubMed] [Google Scholar]
- 66.Gururajanna B, Al-Katib AA, Li YW, Aranha O, Vaitkevicius VK, Sarkar FH. Molecular effects of taxol and caffeine on pancreatic cancer cells. Int J Mol Med. 1999;4:501–7. doi: 10.3892/ijmm.4.5.501. [DOI] [PubMed] [Google Scholar]
- 67.Jang MH, Shin MC, Kang IS, Baik HH, Cho YH, Chu JP, Kim EH, Kim CJ. Caffeine induces apoptosis in human neuroblastoma cell line SK-N-MC. J Korean Med Sci. 2002;17:674–8. doi: 10.3346/jkms.2002.17.5.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lelo A, Miners JO, Robson R, Birkett DJ. Assessment of caffeine exposure: caffeine content of beverages, caffeine intake, and plasma concentrations of methylxanthines. Clin Pharmacol Ther. 1986;39:54–9. doi: 10.1038/clpt.1986.10. [DOI] [PubMed] [Google Scholar]
- 69.Howell LL, Byrd LD. Effects of CGS 15943, a nonxanthine adenosine antagonist, on behavior in the squirrel monkey. J Pharmacol Exp Ther. 1993;267:432–9. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.