Abstract
Target of Rapamycin (TOR) is involved in cellular and organismal aging. Rapamycin extends lifespan and delays cancer in mice. It is important to determine the minimum effective dose and frequency of its administration that still extends lifespan and prevents cancer. Previously we tested 1.5 mg/kg of rapamycin given subcutaneously 6 times per two weeks followed by a two-week break (1.5 × 6/bi-weekly schedule: total of 6 injections during a 4-week period). This intermittent treatment prolonged lifespan and delayed cancer in cancer-prone female FVB/N HER-2/neu mice. Here, the dose was decreased from 1.5 mg/kg to 0.45 mg/kg per injection. This treatment was started at the age of 2 months (group Rap-2), 4 months (Rap-4), and 5 months (Rap-5). Three control groups received the solvent from the same ages. Rapamycin significantly delayed cancer and decreased tumor burden in Rap-2 and Rap-5 groups, increased mean lifespan in Rap-4 and Rap-5 groups, and increased maximal lifespan in Rap-2 and Rap-5 groups. In Rap-4 group, mean lifespan extension was achieved without significant cancer prevention. The complex relationship between life-extension and cancer-prevention depends on both the direct effect of rapamycin on cancer cells and its anti-aging effect on the organism, which in turn prevents cancer indirectly. We conclude that total doses of rapamycin that are an order of magnitude lower than standard total doses can detectably extend life span in cancer-prone mice.
Keywords: longevity, anti-aging agents, cancer, rapalogs
Introduction
Rapamycin is an anti-cancer and anti-aging drug.1 Rapamycin extends life span in mice.2-20 Furthermore, rapamycin delays the onset of cancer in mice.3-7,9-13,15,18 Rapamycin and everolimus (a rapamycin analog) decrease the risk of cancer in humans, who receive these rapalogs to prevent transplant rejection.1,21-24 Therefore, rapalogs such as rapamycin are considered for both prevention of cancer and extension of healthy life span in humans.25-31 There is a concern that potential side effects may limit rapamycin use as anti-aging drug. This concern is exaggerated. First, metabolic side effects of high chronic doses seem to be benevolent.32 In fact, rapamycin extends (not shortens) lifespan in all animal studies. Second, instead of daily treatment, rapamycin can be used intermittently. In organ transplant patients, rapamycin and evirolimus are administrated in high doses daily to achieve full and steady inhibition of mTOR complex 1 (mTORC1). For prevention of aging and its diseases, there may be no need in complete effect. Furthermore, in theory, pulse treatment with rapamycin can improve stem cell function and wound healing.33 In fact, short-term treatment with rapamycin preserved stem cell function.2,34-36 Rapamycin can improve and stimulate the immune response37
In most studies, mice were treated with 1.5–2 mg/kg rapamycin. Importantly, the clearance of rapamycin is much faster in mice than in humans. For example, in mice levels of rapamycin drop 20-folds the next day after injection,14 whereas in humans its terminal half-life is about 2.5 d.38,39 It was estimated that a 1.5 mg/kg injection in mice corresponds to the therapeutic oral dose in humans.40
Every other day (e.o.d.) administration of 1.5 mg/kg rapamycin dramatically prevented cancer induced by tobacco-carcinogen.40 We introduced intervals between treatments: e.o.d. (for practical convenience: 3 times per week) rapamycin was administrated bi-weekly (every other two weeks). Thus, mice were treated with 1.5 mg/kg × 3 times a week for 2 wk followed by a 2-wk break. We showed that this schedule delayed cancer and extended mean and maximal lifespan in mice.4,6 This treatment was started from a very young age (2 mo). In the current study, treatment was started from the age of 2, 4, and 5 mo. Importantly, the dose of rapamycin was reduced from 1.5 mg/kg to 0.45 mg/kg (Fig. 1). We evaluated the effect of low-dose treatment on lifespan and cancer onset.
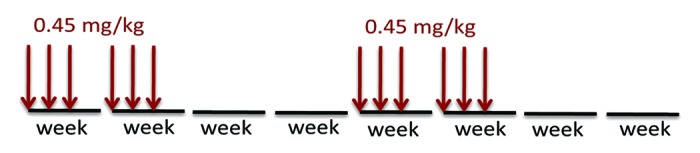
Figure 1. The intermittent low doses treatment. A dose of 0.45 mg/kg rapamycin was injected subcutaneously 3 times per week for 2 wk, followed by a two-week interval.
Results
Tumor development and lifespan in three control groups
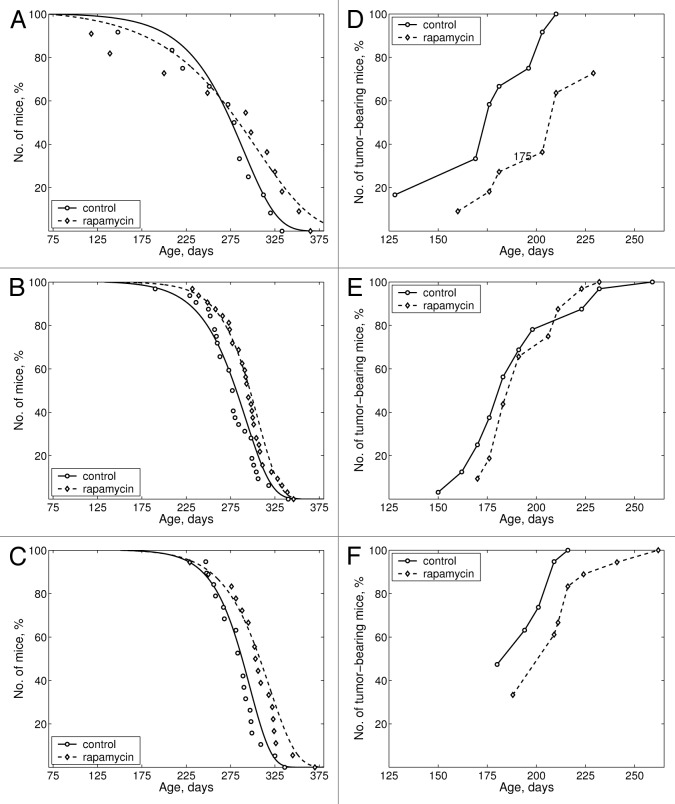
The distribution of survivors was similar in three control groups (Table 1). The mean life span, life span of last 10% survivors and maximum life span were similar in all control groups (Fig. 2 A–C; Table 2). Tumor incidence and multiplicity (a number of tumors per mouse), the mean latent period of the first tumor and the incidence of metastases were also similar in all control groups of mice (Table 3). There was the tendency to earlier cancer in mice treated with solvent from the earlier age (group C-2) than in C-4 and especially in C-5 groups (Table 2) consistent with the observation that injections per se may accelerate carcinogenesis. In C-2 control group, the first tumor was detected by the age of 128 d. By the age of 6 mo mammary carcinomas were detected in 3 of 8 mice in C-2 group (37.5%). In C-4 control group, first tumor was detected at the age of 150 d. At the age of 6 mo tumors were developed in 19 of 32 mice (59.4%). In C-5 control group, by 6 mo of age 9 of 19 mice (47.4%) had tumors. Thus, the rapid development of detectable, macroscopic tumors began by the age of 5–6 mo. Treatment with rapamcyin was started at the age of 2, 4 and 5 mo.
Table 1. Survival distribution in mice treated with rapamycin and solvent (control) from different age.
| Group | Age at start, mo | Age of mice, months | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | ||
| Control-2 | 2 | 12 | 12 | 12 | 11 | 11 | 10 | 9 | 8 | 3 | 1 | 0 | 0 |
| Rapamycin-2 | 2 | 11 | 11 | 10 | 9 | 9 | 8 | 8 | 7 | 5 | 3 | 1 | 0 |
| Control-4 | 4 | - | - | 32 | 32 | 32 | 31 | 29 | 22 | 6 | 2 | 0 | 0 |
| Rapamycin-4 | 4 | - | - | 32 | 32 | 32 | 32 | 30 | 27a | 13a | 3 | 0 | 0 |
| Control-5 | 5 | - | - | - | 19 | 19 | 19 | 19 | 13 | 3 | 1 | 0 | 0 |
| Rapamycin-5 | 5 | - | - | - | 19 | 18 | 18 | 17 | 16a | 12a | 2 | 1 | 0 |
a Significant difference with the corresponding control group: P < 0.01 (Fischer exact test)
Figure 2. Effects of rapamycin on life span and tumor incidence. (A–C) Effects of rapamycin on mice survival. (D and E) Effects of rapamycin on tumor yield curves. (A and D) Upper panel (groups C-2 and Rap-2): treatment was started at the age of 2 mo. (B and E) Middle panel (groups C-4 and Rap-4): treatment was started at the age of 4 mo. (C and F) Middle panel (groups C-5 and Rap-5): treatment was started at the age of 5 mo.
Table 2. Parameters of life span in mice treated with rapamycin and solvent (control) from different age.
| Parameters | Control-2 | Rap-2 | Control-4 | Rap-4 | Control-5 | Rap-5 |
|---|---|---|---|---|---|---|
| No. of mice | 12 | 11 | 32 | 32 | 19 | 18 |
| Mean life span, d | 268 ± 15.2 | 272 ± 25.5 | 278 ± 5.4 | 293 ± 4.7 +5.4%; t = 2.09 |
285 ± 5.5 | 304 ± 7.7 +6.7%; t = 2.01 |
| Median life span, d | 282 | 298 | 278 | 295 | 289 | 305 |
| Mean life span of last 10%, d | 333 ± 0 | 365 ± 0 +9.6% | 333 ± 7.3 | 339 ± 3.8 | 331 ± 5.5 | 358 ± 12.5 (+8.2%); t = 1.98 |
| Maximum life span, d | 333 | 365 +9.6% | 340 | 346 | 336 | 370 (+10.1%) |
The difference with relevant controls is significant, P < 0.05.
Table 3. Effects of rapamycin on tumor development.
| Parameters | Control-2 | Rap-2 | Control-4 | Rap-4 | Control-5 | Rap-5 |
|---|---|---|---|---|---|---|
| No. of mice | 12 | 11 | 32 | 32 | 19 | 18 |
| 1st tumor detection | 128 | 160 | 150 | 170 | 180 | 188 |
| No. effective mice | 12 | 9 | 32 | 32 | 19 | 18 |
| No. of tumors | 12 (100%) | 8 (88.9%) | 32 (100%) | 32 (100%) | 19 (100%) | 18 (100%) |
| Mean latency of the 1st tumor, d | 176 ± 7.7 | 197 ± 8.1 +11.9% | 191 ± 4.5 | 194 ± 3.1 | 192 ± 3.1 | 209 ± 4.7a +8.9% |
| Total number of tumors | 76 | 59 | 212 | 216 | 144 | 123 |
| No. of tumors per mouse | 6.3 | 7.4 | 6.6 | 6.8 | 7.6 | 6.8 |
| No. of mice with metastases into lung | 8 (66.7%) | 7 (63.7%) | 16 (50.0%) | 24 (75.0%) | 13 (68.4%) | 12 (66.7%) |
a The difference with relevant controls is significant, P < 0.05.
The effect of rapamycin on lifespan
Rapamycin treatment started at the age of 4 and 5 mo significantly increased the mean life span (Fig. 2B and C; Table 2). The lack of the effect of rapamycin on the mean life span in Rap-2 group can be explained by the death of some mice early in life (Table 1; Fig. 2A) before cancer developed (cancer-unrelated death), which may be accidental or due to effects of rapamycin at very young age (2 mo). Still in Rap-2 group, rapamycin extended maximal lifespan by 9.6%. Similarly, in Rap-5 group, rapamycin increased the mean life span of the last 10% survivors (Fig. 2C; Table 2).
The effect of rapamycin on carcinogenesis
In groups Rap-2 and Rap-5, the mean latent period of the first tumor development was significantly increased. The kinetic of tumor incidence in rapamycin-treated mice (R2 and R5) was slower than the kinetic in control animals (Fig. 2D–F). Thus, 50% of all control mice developed tumors by the age of 175–180 d (Fig. 2D and F), whereas in Rap-2 and Rap-5 groups this period was extended to 200–205 d. These data demonstrate that rapamycin not only increased lifespan, but also reduced mammary carcinogenesis in cancer-prone mice. In Rap-4 group, rapamycin increased the number of mice with multiple metastases (40.6% and 18.8%, correspondingly, P < 0.02, exact Fischer test), probably due to an increase in life span of tumor-bearing mice. A number of tumors per animal was similar in rapamycin-treated and control groups (Table 3). Yet, 6.3% of C-4 mice were bearing 1–4 tumors per mouse, and 40.6% had 5–6 tumors per mouse, whereas in rapamycin-treated groups these numbers were 15.6% and 24.4%, respectively.
Discussion
Rapamycin slows down aging, prevents cancer and extends lifespan in mice. Noteworthy, metformin, which affects the AMPK/mTOR pathway, also extends life span in mice and prevents cancer in mice41-49 Unlike metformin, which is effective mostly when started early in life,45 rapamycin extends life span when the treatment started both in young and in old animals (Table 4). In order to develop low-dose anti-aging therapy for humans, one needs to determine the minimum effective dose of rapamycin. Currently in the clinic, rapamycin is mostly administered daily in high dose. In most animal studies, rapamycin was also used as daily treatment either with food or by injection. Such treatments extended life span (Table 4). In some studies, rapamycin was administrated every other day (e.o.d.) and even weekly or biweekly (Table 4). Previously, we have tested double intermittent schedule 1.5 mg/kg × 6/4 wk in 2-mo-old inbred6 and cancer-prone4 female mice. In both studies, rapamycin delayed cancer, decreased multiplicity of tumors per mice and extended life span. Here the dose was decreased from 1.5 to 0.45 mg/kg. The current study additionally included groups Rap-4 and Rap-5, with treatment started at the age of 4 and 5 mo, respectively. Noteworthy, rapamycin treatment started at the age of 2 mo (but not at 4 or 5 mo) was associated with cancer-unrelated death at a young age, blunting the effect of rapamycin on median (but not maximal) lifespan (Fig. 1A). Remarkably, Johnson et al. demonstrated extraordinary life extension by high-dose rapamcyin treatment started at the age 20 d in very short-lived Leigh syndrome mice.14 So there may be no negative effect in very young age (albeit rapamycin-treated mice growth was slowed down14).
Table 4. Effects of rapamycin on life span and spontaneous carcinogenesis in mice.
| Strain | Sex | No. of mice, C/Ta | Age at start of treatment, mo | Drug, dose and route of treatmentb | Effect on mean life span, % | Effect on carcinogenesisc | References |
|---|---|---|---|---|---|---|---|
| mTOR (Δ/Δ) | M | 10/17 | 0 | No drugs, mTOR hypomorphic model | +22% | ↓ | Wu et al., 201352 |
| F | 24/26 | +10% | ↓ | ||||
| UM-HET3 | M | 357/134 | 20 | In foodd | +9% | No data | Harrison et al., 20093 |
| UM-HET3 | F | 289/144 | 20 | +14% | No data | ||
| UM-HET3 | M | 50/50 | 9 | +10% | No data | Miller et al., 20115 | |
| UM-HET3 | F | 50/50 | 9 | +18% | No data | ||
| HER-2/neu | F | 28/30 | 2 | 1.5 mg/kg, s.c. 6 times per 4 wk | +4% | ↓ | Anisimov et al., 20104 |
| HER-2/neu | F | 12/11 | 2 | 0.45 mg/kg, s.c. 6 times per 4 wk | 0 | ↓ | Present paper |
| 32/32 | 4 | +5% | ↓ | ||||
| 19/18 | 5 | +7% | ↓ | ||||
| 129/Sv | F | 31/35 | 2 | 1.5 mg/kg, s.c. | +4% | ↓ | Anisimov et al., 20116 |
| С57BL/6 | M | 10/10 | 22–24 | 4 mg/kg b.w., i.p. bidaily for 6 wk | Increase | No data | Chen et al., 20092 |
| С57BL/6J | M | 20/20 | 4 | In food | Increase | ↓ | Neff et al., 201313 |
| M | 21/21 | 13 | |||||
| M | 27/27 | 20–22 | |||||
| C57BL/6Nia | M | 44/45 | 19 | In food | No effect | ↓ | Zhang et al., 201312 |
| F | 43/45 | Increase | ↓ | ||||
| Ndufs4−/− | M | No data | 20 d | 8 mg/kg i.p. e.o.d. | +25% | No data | Johnson et al., 201314 |
| F | +38% | ||||||
| M+F | 8 mg/kg i.p. daily | +100% | No data | ||||
| С57BL/6J p53+/− | M | 38/37 | <5 | 1.5 mg/kg, d.w. | +28% | ↓ | Komarova et al., 201210 |
| C57BL/6J p53+/− | M | 38/37 | >5 | 1.5 mg/kg, d.w. | +10% | ↓ | Komarova et al., 201210 and Comas et al., 20129 |
| C57BL/6J p53−/− | M | 17/21 | 2 | 0.5 mg/kg, p.o. × 5 d; break 9 d | +30% | ↓ | |
| Rb1+/− | M | 97/98 | 2–3 | 14 mg/kg, p.o. or or in food | +14% | ↓ | Livi et al., 201311 |
| F | 2–3 | +9% | ↓ | ||||
| С57BL/6J Bmal1−/− | M+F | 73/31 | 3.3 | 0.5 mg/kg d.w. | +50% | No data | Khapre et al., 201453 |
| 129Sv-C57BL/6J Lmna−/− | M+F | 23/23 | 1 | In food | +23% | No data | Ramos et al.8 |
| 11/11 | 1 | 8 mg/kg i.p. e.o.d. | +57% | ||||
| 9/11 | 1 | 8 mg/kg i.p. weekly | +53% | ||||
| C57BL/6J Lmna−/− | M/F | 11/11 | 1 | 8 mg/kg i.p. e.o.d. | +56% |
a C/T, Control/Treatment; bd.w., with drinking water; s.c., subcutaneously; i.p., intraperitoneally; p.o. (per os), gavage; e.o.d., every other day; c↑, increases; ↓, decreases; dIn food, around 14 mg/kg encapsulated rapamycin with food.
In the current study, 0.45 mg/kg rapamycin exerted similar effects as 1.5 mg/kg rapamycin used by us previously, yet, as may be expected, the effects of lower doses were less pronounced.4 The extension of maximal life span was observed in groups Rap-2 and Rap-5. In groups Rap-4 and Rap-5, rapamycin increased medium lifespan. In group Rap-5, both medium and maximal lifespan were increased. Significant tumor prevention was observed in groups Rap-2 and Rap-5. The extension of the medium lifespan was associated with the extension of lifespan of cancer-bearing mice, explaining an increase of a number of mice with metastasis. In brief, 0.45 mg/kg × 6/biweekly treatment delayed cancer in two groups and extended either maximal or medium lifespan, or both. The difference between groups may depend on potential negative effects of rapamycin injections at very early age (2 mo), direct anti-cancer effect, selection for resistance of premalignant cells, and indirect anti-cancer effect via anti-aging effects of rapamycin. Although many explanations are possible, we cannot provide the evidence. The simplest explanation is that doses used in this study exerted mild effects, which statistical significance varied from group to group. Therefore, in some groups cancer-prevention was not statistically significant, while rapamycin still significantly extended median life span. In general, we can conclude that low-dose treatment exerted modest effects, probably reaching its threshold of statistically significance in small groups of animals.
In this study, we tested the lowest doses/frequencies of rapamycin in mice compared with all previous studies. In heterogeneous mice, rapamycin-containing food extended female medium life span by 13–18%, when treatment started at 600 d3 and 270 d,5 respectively. Expressed as life expectancy at 600 d (the age of first exposure to rapamycin), the effect size was 38% for females.3 In studies that included males and females rapamycin extended life span in females more significantly. One explanation is that the mTOR pathway is less sensitive to rapamycin in males than in females.50 Given that we treated breast cancer-prone mice, all mice in our study were females.
In study by Johnson,14 the effect of high-dose daily rapamycin was so dramatic that maximal life span was extended 300% and mean life span 100%.14 In contrast, e.o.d. rapamycin extended life span just by 38%14 Yet, it was a special model of mitochondrial disease with extremely short life span in control.14 To compensate for profound mitochondrial dysfunction, a steady full inhibition of mTOR was necessary. In our studies, cancer was modestly prevented by intermittent low-dose administration of rapamycin, although no comparison with daily doses is available. We assume that higher doses and daily administration would be more potent. Yet, low-dose intermittent rapamycin seems to be a practical approach to prevent cancer and extend life span in healthy human population.
Material and Methods
Animals and experimental design
Homozygous FVB/N HER-2/neu transgenic mice originally obtained from Charles River by the Italian National Research Center for Aging (INRCA) were housed and breed in the Department of Carcinogenesis and Oncogerontology, N.N. Petrov Research Institute of Oncology. Mice received standard laboratory chow and tap water ad libitum.51 All studies were conducted in accordance with the ethical standards and according to national and international guidelines and have been approved by the authors’ institutional review board.
Longevity study
One hundred and twenty-four (124) female FVB/N HER-2/neu mice were under observation. Sixty-one mice received 0.45 mg/kg rapamycin (LC Laboratories) subcutaneously (s.c.) 3 times a week for a period of 2 wk followed by 2-wk intervals starting at the age of 2 mo (11 mice), 4 mo (32 mice) or 5 mo (18 mice). Rapamycin was dissolved in 95% ethanol and then diluted with apyrogenic sterile water to a final concentration of 11.4 μg in 0.1 ml of 2% ethanol. Sixty-three mice in the second group received s.c. 0.1 of solvent without rapamycin starting at the same age and served as a control (C-2, C-4, C-5). Once a week all mice were palpated for detection of mammary tumors appearance. The localization and the size of tumors were registered. The neoplastic masses were measured with a caliper and progressively growing masses of >3 mm in mean diameter were regarded as tumors. The mean number of palpable mammary carcinomas/mouse was calculated as the cumulative number of incident tumors/number of tumor-bearing mice. The number of mice with metastases into lungs was also registered. Animals were weighed once a month and were observed throughout their lifespan.4,51
Pathomorphological examination
All animals were autopsied. All tumors, as well as the tissues and organs with suspected tumor development were excised, fixed in 10% buffered formalin and embedded into paraffin. Five micrometer histological sections were stained with hematoxylin and eosine and were microscopically examined. Tumors were classified according to the International Agency for Research on Cancer recommendations.
Statistics
Experimental results were statistically processed by the methods of variation statistics with the use of STATGRAPH statistic program kit as previously described.4,43 The significance of the discrepancies was defined according to the Student t criterion, Fischer exact method, χ2, non-parametric Wilcoxon–Mann–Whitney, and Friedman RM Anova on Ranks. The Student–Newman–Keuls method was used for all pairwise multiple comparisons. Coefficient of correlation was estimated by the Spearman method. Differences in tumor incidence were evaluated by the Mantel–Haenszel log-rank test.
For experimental group the Cox regression model was used to estimate relative risk of death and tumor development under the treatment compared with the control group: h(t,z) = h0(t) exp(zβ), where h(t,z) and h0(t) denote the conditional hazard and baseline hazard rates, respectively, β is the unknown parameter for treatment group, and z takes values 0 and 1, being an indicator variable for two samples—the control and treatment group.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/cbt/article/28164
References
- 1.Blagosklonny MV. Rapalogs in cancer prevention: anti-aging or anticancer? Cancer Biol Ther. 2012;13:1349–54. doi: 10.4161/cbt.22859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen C, Liu Y, Liu Y, Zheng P. mTOR regulation and therapeutic rejuvenation of aging hematopoietic stem cells. Sci Signal. 2009;2:ra75. doi: 10.1126/scisignal.2000559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harrison DE, Strong R, Sharp ZD, Nelson JF, Astle CM, Flurkey K, Nadon NL, Wilkinson JE, Frenkel K, Carter CS, et al. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature. 2009;460:392–5. doi: 10.1038/nature08221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anisimov VN, Zabezhinski MA, Popovich IG, Piskunova TS, Semenchenko AV, Tyndyk ML, Yurova MN, Antoch MP, Blagosklonny MV. Rapamycin extends maximal lifespan in cancer-prone mice. Am J Pathol. 2010;176:2092–7. doi: 10.2353/ajpath.2010.091050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miller RA, Harrison DE, Astle CM, Baur JA, Boyd AR, de Cabo R, Fernandez E, Flurkey K, Javors MA, Nelson JF, et al. Rapamycin, but not resveratrol or simvastatin, extends life span of genetically heterogeneous mice. J Gerontol A Biol Sci Med Sci. 2011;66:191–201. doi: 10.1093/gerona/glq178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anisimov VN, Zabezhinski MA, Popovich IG, Piskunova TS, Semenchenko AV, Tyndyk ML, Yurova MN, Rosenfeld SV, Blagosklonny MV. Rapamycin increases lifespan and inhibits spontaneous tumorigenesis in inbred female mice. Cell Cycle. 2011;10:4230–6. doi: 10.4161/cc.10.24.18486. [DOI] [PubMed] [Google Scholar]
- 7.Wilkinson JE, Burmeister L, Brooks SV, Chan CC, Friedline S, Harrison DE, Hejtmancik JF, Nadon N, Strong R, Wood LK, et al. Rapamycin slows aging in mice. Aging Cell. 2012;11:675–82. doi: 10.1111/j.1474-9726.2012.00832.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ramos FJ, Chen SC, Garelick MG, Dai DF, Liao CY, Schreiber KH, MacKay VL, An EH, Strong R, Ladiges WC, et al. Rapamycin reverses elevated mTORC1 signaling in lamin A/C-deficient mice, rescues cardiac and skeletal muscle function, and extends survival. Sci Transl Med. 2012;4:144ra103. doi: 10.1126/scitranslmed.3003802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Comas M, Toshkov I, Kuropatwinski KK, Chernova OB, Polinsky A, Blagosklonny MV, Gudkov AV, Antoch MP. New nanoformulation of rapamycin Rapatar extends lifespan in homozygous p53−/− mice by delaying carcinogenesis. Aging (Albany NY) 2012;4:715–22. doi: 10.18632/aging.100496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Komarova EA, Antoch MP, Novototskaya LR, Chernova OB, Paszkiewicz G, Leontieva OV, Blagosklonny MV, Gudkov AV. Rapamycin extends lifespan and delays tumorigenesis in heterozygous p53+/− mice. Aging (Albany NY) 2012;4:709–14. doi: 10.18632/aging.100498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Livi CB, Hardman RL, Christy BA, Dodds SG, Jones D, Williams C, Strong R, Bokov A, Javors MA, Ikeno Y, et al. Rapamycin extends life span of Rb1+/− mice by inhibiting neuroendocrine tumors. Aging (Albany NY) 2013;5:100–10. doi: 10.18632/aging.100533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang Y, Bokov A, Gelfond J, Soto V, Ikeno Y, Hubbard G, Diaz V, Sloane L, Maslin K, Treaster S, et al. Rapamycin extends life and health in C57BL/6 mice. J Gerontol A Biol Sci Med Sci. 2014;69:119–30. doi: 10.1093/gerona/glt056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Neff F, Flores-Dominguez D, Ryan DP, Horsch M, Schröder S, Adler T, Afonso LC, Aguilar-Pimentel JA, Becker L, Garrett L, et al. Rapamycin extends murine lifespan but has limited effects on aging. J Clin Invest. 2013;123:3272–91. doi: 10.1172/JCI67674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johnson SC, Yanos ME, Kayser EB, Quintana A, Sangesland M, Castanza A, Uhde L, Hui J, Wall VZ, Gagnidze A, et al. mTOR inhibition alleviates mitochondrial disease in a mouse model of Leigh syndrome. Science. 2013;342:1524–8. doi: 10.1126/science.1244360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miller RA, Harrison DE, Astle CM, Fernandez E, Flurkey K, Han M, Javors MA, Li X, Nadon NL, Nelson JF, et al. Rapamycin-mediated lifespan increase in mice is dose and sex dependent and metabolically distinct from dietary restriction. Aging Cell. 2013 doi: 10.1111/acel.12194. Forthcoming. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ye L, Widlund AL, Sims CA, Lamming DW, Guan Y, Davis JG, Sabatini DM, Harrison DE, Vang O, Baur JA. Rapamycin doses sufficient to extend lifespan do not compromise muscle mitochondrial content or endurance. Aging (Albany NY) 2013;5:539–50. doi: 10.18632/aging.100576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fok WC, Chen Y, Bokov A, Zhang Y, Salmon AB, Diaz V, Javors M, Wood WH, 3rd, Zhang Y, Becker KG, et al. Mice fed rapamycin have an increase in lifespan associated with major changes in the liver transcriptome. PLoS One. 2014;9:e83988. doi: 10.1371/journal.pone.0083988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hasty P, Livi CB, Dodds SG, Jones D, Strong R, Javors M, Fischer KE, Sloane L, Murthy K, Hubbard G, et al. eRapa Restores a Normal Life Span in a FAP Mouse Model. Cancer Prev Res (Phila) 2014;7:169–78. doi: 10.1158/1940-6207.CAPR-13-0299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fang Y, Bartke A. Prolonged rapamycin treatment led to beneficial metabolic switch. Aging (Albany NY) 2013;5:328–9. doi: 10.18632/aging.100554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Luo LL, Xu JJ, Fu YC. Rapamycin prolongs female reproductive lifespan. Cell Cycle. 2013;12:3353–4. doi: 10.4161/cc.26578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mathew T, Kreis H, Friend P. Two-year incidence of malignancy in sirolimus-treated renal transplant recipients: results from five multicenter studies. Clin Transplant. 2004;18:446–9. doi: 10.1111/j.1399-0012.2004.00188.x. [DOI] [PubMed] [Google Scholar]
- 22.Stallone G, Schena A, Infante B, Di Paolo S, Loverre A, Maggio G, Ranieri E, Gesualdo L, Schena FP, Grandaliano G. Sirolimus for Kaposi’s sarcoma in renal-transplant recipients. N Engl J Med. 2005;352:1317–23. doi: 10.1056/NEJMoa042831. [DOI] [PubMed] [Google Scholar]
- 23.Campistol JM, Eris J, Oberbauer R, Friend P, Hutchison B, Morales JM, Claesson K, Stallone G, Russ G, Rostaing L, et al. Sirolimus therapy after early cyclosporine withdrawal reduces the risk for cancer in adult renal transplantation. J Am Soc Nephrol. 2006;17:581–9. doi: 10.1681/ASN.2005090993. [DOI] [PubMed] [Google Scholar]
- 24.Euvrard S, Morelon E, Rostaing L, Goffin E, Brocard A, Tromme I, Broeders N, del Marmol V, Chatelet V, Dompmartin A, et al. TUMORAPA Study Group Sirolimus and secondary skin-cancer prevention in kidney transplantation. N Engl J Med. 2012;367:329–39. doi: 10.1056/NEJMoa1204166. [DOI] [PubMed] [Google Scholar]
- 25.Zoncu R, Efeyan A, Sabatini DM. mTOR: from growth signal integration to cancer, diabetes and ageing. Nat Rev Mol Cell Biol. 2011;12:21–35. doi: 10.1038/nrm3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kaeberlein M. Longevity and aging. F1000Prime Rep. 2013;5:5. doi: 10.12703/P5-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kaeberlein M. mTOR Inhibition: From Aging to Autism and Beyond. Scientifica (Cairo). 2013;2013:849186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kennedy BK, Pennypacker JK. Drugs that modulate aging: the promising yet difficult path ahead. Transl Res. 2013 doi: 10.1016/j.trsl.2013.11.007. Forthcoming. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Blagosklonny MV. Prevention of cancer by inhibiting aging. Cancer Biol Ther. 2008;7:1520–4. doi: 10.4161/cbt.7.10.6663. [DOI] [PubMed] [Google Scholar]
- 30.Blagosklonny MV. Rapamycin and quasi-programmed aging: four years later. Cell Cycle. 2010;9:1859–62. doi: 10.4161/cc.9.10.11872. [DOI] [PubMed] [Google Scholar]
- 31.Blagosklonny MV. Increasing healthy lifespan by suppressing aging in our lifetime: preliminary proposal. Cell Cycle. 2010;9:4788–94. doi: 10.4161/cc.9.24.14360. [DOI] [PubMed] [Google Scholar]
- 32.Blagosklonny MV. Once again on rapamycin-induced insulin resistance and longevity: despite of or owing to. Aging (Albany NY) 2012;4:350–8. doi: 10.18632/aging.100461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Blagosklonny MV. Aging, stem cells, and mammalian target of rapamycin: a prospect of pharmacologic rejuvenation of aging stem cells. Rejuvenation Res. 2008;11:801–8. doi: 10.1089/rej.2008.0722. [DOI] [PubMed] [Google Scholar]
- 34.Iglesias-Bartolome R, Patel V, Cotrim A, Leelahavanichkul K, Molinolo AA, Mitchell JB, Gutkind JS. mTOR inhibition prevents epithelial stem cell senescence and protects from radiation-induced mucositis. Cell Stem Cell. 2012;11:401–14. doi: 10.1016/j.stem.2012.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Iglesias-Bartolome R, Gutkind SJ. Exploiting the mTOR paradox for disease prevention. Oncotarget. 2012;3:1061–3. doi: 10.18632/oncotarget.712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Luo Y, Li L, Zou P, Wang J, Shao L, Zhou D, Liu L. Rapamycin enhances long-term hematopoietic reconstitution of ex vivo expanded mouse hematopoietic stem cells by inhibiting senescence. Transplantation. 2014;97:20–9. doi: 10.1097/TP.0b013e3182a7fcf8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bravo-San Pedro JM, Senovilla L. Immunostimulatory activity of lifespan-extending agents. Aging (Albany NY) 2013;5:793–801. doi: 10.18632/aging.100619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zimmerman JJ, Kahan BD. Pharmacokinetics of sirolimus in stable renal transplant patients after multiple oral dose administration. J Clin Pharmacol. 1997;37:405–15. doi: 10.1002/j.1552-4604.1997.tb04318.x. [DOI] [PubMed] [Google Scholar]
- 39.MacDonald A, Scarola J, Burke JT, Zimmerman JJ. Clinical pharmacokinetics and therapeutic drug monitoring of sirolimus. Clin Ther. 2000;22(Suppl B):B101–21. doi: 10.1016/S0149-2918(00)89027-X. [DOI] [PubMed] [Google Scholar]
- 40.Granville CA, Warfel N, Tsurutani J, Hollander MC, Robertson M, Fox SD, Veenstra TD, Issaq HJ, Linnoila RI, Dennis PA. Identification of a highly effective rapamycin schedule that markedly reduces the size, multiplicity, and phenotypic progression of tobacco carcinogen-induced murine lung tumors. Clin Cancer Res. 2007;13:2281–9. doi: 10.1158/1078-0432.CCR-06-2570. [DOI] [PubMed] [Google Scholar]
- 41.Anisimov VN, Berstein LM, Egormin PA, Piskunova TS, Popovich IG, Zabezhinski MA, Kovalenko IG, Poroshina TE, Semenchenko AV, Provinciali M, et al. Effect of metformin on life span and on the development of spontaneous mammary tumors in HER-2/neu transgenic mice. Exp Gerontol. 2005;40:685–93. doi: 10.1016/j.exger.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 42.Anisimov VN, Egormin PA, Bershtein LM, Zabezhinskii MA, Piskunova TS, Popovich IG, Semenchenko AV. Metformin decelerates aging and development of mammary tumors in HER-2/neu transgenic mice. Bull Exp Biol Med. 2005;139:721–3. doi: 10.1007/s10517-005-0389-9. [DOI] [PubMed] [Google Scholar]
- 43.Anisimov VN, Berstein LM, Egormin PA, Piskunova TS, Popovich IG, Zabezhinski MA, Tyndyk ML, Yurova MV, Kovalenko IG, Poroshina TE, et al. Metformin slows down aging and extends life span of female SHR mice. Cell Cycle. 2008;7:2769–73. doi: 10.4161/cc.7.17.6625. [DOI] [PubMed] [Google Scholar]
- 44.Anisimov VN. Metformin for aging and cancer prevention. Aging (Albany NY) 2010;2:760–74. doi: 10.18632/aging.100230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Anisimov VN, Berstein LM, Popovich IG, Zabezhinski MA, Egormin PA, Piskunova TS, Semenchenko AV, Tyndyk ML, Yurova MN, Kovalenko IG, et al. If started early in life, metformin treatment increases life span and postpones tumors in female SHR mice. Aging (Albany NY) 2011;3:148–57. doi: 10.18632/aging.100273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Anisimov VN. Metformin: do we finally have an anti-aging drug? Cell Cycle. 2013;12:3483–9. doi: 10.4161/cc.26928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Moiseeva O, Deschênes-Simard X, Pollak M, Ferbeyre G. Metformin, aging and cancer. Aging (Albany NY) 2013;5:330–1. doi: 10.18632/aging.100556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Martin-Montalvo A, Mercken EM, Mitchell SJ, Palacios HH, Mote PL, Scheibye-Knudsen M, Gomes AP, Ward TM, Minor RK, Blouin MJ, et al. Metformin improves healthspan and lifespan in mice. Nat Commun. 2013;4:2192. doi: 10.1038/ncomms3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Anisimov VN. Metformin and rapamycin are master-keys for understanding the relationship between cell senescent, aging and cancer. Aging (Albany NY) 2013;5:337–8. doi: 10.18632/aging.100561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Leontieva OV, Paszkiewicz GM, Blagosklonny MV. Mechanistic or mammalian target of rapamycin (mTOR) may determine robustness in young male mice at the cost of accelerated aging. Aging (Albany NY) 2012;4:899–916. doi: 10.18632/aging.100528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Anisimov VN, Popovich IG, Zabezhinski MA. Methods of testing pharmacological drugs effects on aging and life span in mice. Methods Mol Biol. 2013;1048:145–60. doi: 10.1007/978-1-62703-556-9_12. [DOI] [PubMed] [Google Scholar]
- 52.Wu JJ, Liu J, Chen EB, Wang JJ, Cao L, Narayan N, Fergusson MM, Rovira II, Allen M, Springer DA, et al. Increased mammalian lifespan and a segmental and tissue-specific slowing of aging after genetic reduction of mTOR expression. Cell Rep. 2013;4:913–20. doi: 10.1016/j.celrep.2013.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Khapre RV, Kondratova AA, Patel S, Dubrovsky Y, Wrobel M, Antoch MP, Kondratov RV. MAL1-dependent regulation of the mTOR signaling pathway delays aging. Aging (Albany, NY Online) 2014;6 doi: 10.18632/aging.100633. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]