Abstract
Objective
To determine whether NICU admission hypothermia is associated with an intrauterine inflammatory response.
Methods
We analyzed a cohort of 309 very low birthweight infants to determine relationships between admission hypothermia, chorioamnionitis, and serum and cerebrospinal fluid interleukin(IL)-1β, IL-6, and tumor necrosis factor-α.
Results
Admission hypothermia <36°C occurred in 72% of patients <26 weeks and 44% of patients ≥26 weeks gestational age. NICU admission hypothermia was not associated with histologic chorioamnionitis or with elevated serum cytokine concentrations. Cerebrospinal fluid IL-6 concentrations ≥ 6.3 pg/ml were associated with admission hypothermia in infants <26 weeks' gestation. Clinical chorioamnionitis was associated with a lower risk of admission hypothermia, while cesarean section delivery was associated with increased risk.
Conclusions
NICU admission hypothermia is common among preterm infants and is not associated with the fetal inflammatory response syndrome. Hypothermia is less common in the setting of clinical chorioamnionitis and more common in cesarean section deliveries, identifying two groups in whom extra attention to appropriate thermoregulation is warranted.
Keywords: hypothermia, fetal inflammatory response, chorioamnionitis, cytokines, intraventricular hemorrhage
Introduction
Hypothermia on admission to the neonatal intensive care unit (NICU) is common among preterm infants. Depending on the selected temperature cutoff (usually ranging from 35.5°C to 36.5°C), the incidence of NICU admission hypothermia among very low birthweight (<1500g) infants ranges from 30-80% with significant inter-institution variability [4, 14, 18]. Multiple factors including immature skin and thermoregulatory defenses and need for resuscitative measures contribute to the drop in core temperature in preterm infants between birth and NICU admission. Indeed, the incidence and degree of NICU admission hypothermia are directly related to gestational age (GA) [6, 14] and illness severity [18]. Some previous epidemiologic surveys have linked short-duration hypothermia following birth to mortality and morbidities[2, 6, 14]. Whether a causal link exists between hypothermia and adverse outcomes or simply multiple etiologies and risk factors are shared remains to be determined.
Intrauterine infection is a common cause of preterm birth, but the contributions of clinical chorioamnionitis (the definition of which typically includes maternal fever) and the fetal inflammatory response (characterized by umbilical cord inflammation and elevated concentrations of circulating cytokines) to initial infant thermoregulation are unknown. Cytokines modulate core temperature, and, conversely, changes in temperature can alter production of inflammatory cytokines [5, 9, 11]. In adults, interleukin (IL)-1β and IL-6 are generally considered pyrogenic (fever-inducing) while tumor necrosis factor-α (TNFα) has been associated with both fever and hypothermia [1, 11]. Neonates have differences in production of and responses to cytokines compared with adults [8, 9], and are more likely to manifest hypothermia than fever in response to infection. It is thus plausible that NICU admission hypothermia could reflect a systemic inflammatory response and that this inflammatory response could contribute to adverse outcomes.
We previously reported the relationship of inflammation in intrauterine, blood, and CSF compartments and adverse neurologic and pulmonary outcomes in a cohort of preterm infants less than 33 weeks gestation [19]. Utilizing this dataset, we undertook the current study to test the hypothesis that NICU admission hypothermia is associated with an intrauterine inflammatory response including chorioamnionitis and elevation of temperature-regulating cytokines in serum and cerebrospinal fluid.
Methods
Sample
The current study includes 309 of 327 very low birth weight infants enrolled in a prospective study designed to assess association between fetal and neonatal inflammation and risk for cranial ultrasound abnormalities or bronchopulmonary dysplasia (BPD) [19]. The parent study included inborn babies with gestational age (GA) <33 wk and birth weight (BW) <1501 g admitted to the neonatal intensive care units at the University of Maryland Medical System and Mercy Medical Center, Baltimore, MD between June 1999 and July 2003. Babies with congenital brain/ neural tube defects or confirmed congenital TORCH infections were excluded. Parental consent was obtained and the Institutional Review Boards of the participating centers approved the study protocol.
For the current study, NICU admission temperatures were retrospectively collected from medical records. Delivery room temperature was not recorded, but both vaginal and cesarean deliveries occurred in an operating room. NICU admission temperature was recorded as “<96°F” (<35.5°C) for 54 patients in whom temperature was measured using a thermometer that did not give lower readings; all others had actual temperature recorded. Rectal temperatures were taken immediately after the infant was placed on a radiant warmer on admission to the NICU. As defined by the World Health Organization, a core temperature <36°C (96.8°F) was categorized as moderate neonatal hypothermia [13].
Serum and CSF cytokines analysis
Cord blood (2 ml) or venous blood obtained within 12 h of delivery was drawn aseptically and the serum separated, aliquoted, and stored at −80°C for cytokine measurements. Cerebrospinal fluid (1.0 ml) was obtained from study infants who had a lumbar puncture performed as part of a sepsis evaluation within 72 h of delivery. TNFα, IL-1β, and IL-6 levels were measured in duplicate in serum and CSF samples using standard two-antibody ELISA with commercial antibody pairs and recombinant standards (Endogen, Boston, MA) as previously described. A curve was fit to the standards using a computer program (Softpro: Molecular Devices), and cytokine concentrations from each sample were calculated from the standard curve. Assay sensitivities were 3, 0.78, and 1.5 pg/ml for TNFα, IL-1β, and IL-6, respectively.
Chorioamnionitis diagnosis
Clinical chorioamnionitis was defined as maternal temperature ≥ 38°C and two of the following: uterine tenderness, malodorous vaginal discharge, fetal tachycardia >160 bpm, or maternal white blood cell count >15,000/mm3. Placental pathology studies were performed in 257 of the 309 study subjects (83%). Formalin-fixed, paraffin-embedded, and hematoxylin- and eosin-stained sections of umbilical cord, membrane roll, placental disc near the cord insertion site, and midway between cord insertion and periphery of the placental disc were prepared. The sections were reviewed in a blinded fashion according to a standard protocol. Histologic chorioamnionitis was separated into maternal and fetal involvement and assigned a stage according to the scheme proposed by Redline et al [17]. Fetal vasculitis was defined as polymorphonuclear infiltration of the chorionic vessels or umbilical cord.
Cranial ultrasound scans
Serial CUS examinations were performed using a high frequency variable bandwidth transducer (5.5-8.5 MHz) (Acuson Sequoia 512, Mountain View, CA) for detection of white matter damage (WMD) and intraventricular hemorrhage (IVH) at the following time points 1) between 3 to 7 postnatal days, 2) at 28-30 postnatal days, 3) at 34-36 postmenstrual age (PMA) or pre-transfer or pre-discharge and as clinically indicated. The CUS diagnoses were classified as a) germinal matrix/ intraventricular hemorrhage (GM/IVH); b) transient periventricular echodensities (resolution without progressing to echolucencies); c) persistent periventricular echodensities (echodensities present on 2 or more scans without resolution or progression to echolucencies); d) periventricular echolucencies, and e) ventricular enlargement. White matter damage included periventricular echodensities and echolucencies and ventricular enlargement.
Neonatal Outcomes
Demographic, obstetric, and neonatal variables were recorded as previously described [19]. Hypotension was defined as mean blood pressure <10th percentile for weight and postnatal age sustained for ≥2 consecutive hours during the first 96 h of age [7]. BPD was defined as supplemental oxygen at 36 wk post menstrual age.
Statistical Analysis
The Student's t test or one-way ANOVA was used to analyze continuous variables and the Chi-square test for categorical variables. Because of the wide range of cytokine concentrations, values in pg/ml were dichotomized around the median value for IL-6 and IL-1β and around the lower limit of detection for TNFα. Univariate odds ratios and 95% confidence intervals were calculated for all variables for hypothermic versus non-hypothermic patients stratified by GA <26 wk and ≥26 wk or presence and absence of clinical chorioamnionitis, with hypothermia defined as NICU admission temperature <36°C (<96.8°F). The p values were not corrected for multiple comparisons. Statistical analysis was performed with STATA 7.0 (College Station, TX) with p value < 0.05 considered significant.
Results
Cohort characteristics and outcomes
From the initial prospective parent study of 327 patients, NICU admission temperature was retrospectively retrieved from the medical record for 309 patients (94%). The 18 patients for whom no admission temperature was recorded had similar gestational age and survival compared with the 309 patients included in this study.
The mean gestational age of the cohort was 27.5 ± 2.4 (SD) weeks and mean birth weight was 985 ± 277 grams. Fifty-three percent were male. Overall mortality was 7%. Of the survivors, 21% were diagnosed with BPD [12]. Cranial ultrasounds showed IVH Grade ≥3 in 15% and white matter damage in 14%. Culture-confirmed early-onset neonatal sepsis was present in 2% of cases.
Hypothermia incidence and clinical associations
Admission temperatures ranged from 33.2 – 38.2°C (91.8-100.8°F). Admission hypothermia <36°C, was present in 51% of all patients while hyperthermia >37.5°C was present in only 6 patients (1.4%). Figure 1 shows the mean temperature and number of patients at each gestational age with NICU admission hypothermia <36°C. This degree of hypothermia occurred in 72% of infants <26 weeks gestation and 44% of infants ≥26 weeks.
Figure 1. NICU admission hypothermia as a function of gestational age.
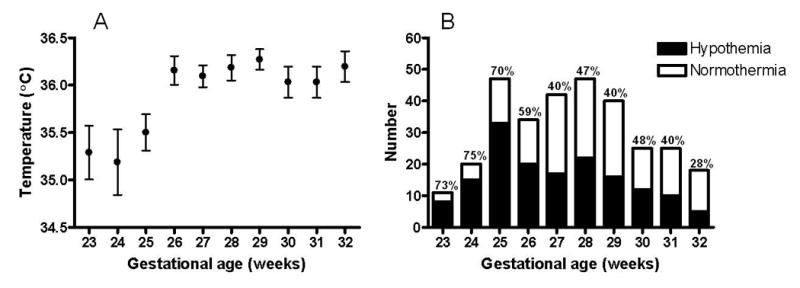
NICU admission temperatures were recorded in 309 very low birth weight infants. Mean temperature (°C) ± SD at each gestational age in weeks is shown on the left. Number of patients at each gestational age with “hypothermia” (<36°C) versus “normothermia” (≥36°C) on NICU admission is shown on the right. NICU= neonatal intensive care unit.
Table 1 shows clinical variables significantly associated with NICU admission hypothermia in unadjusted analyses. The strongest association was gestational age, with birth at <26 weeks gestation associated with a 3.2-fold increased chance of NICU admission temperature <36°C. Clinical chorioamnionitis and preterm premature rupture of membranes were protective against hypothermia. Cesarean section was the only obstetric factor found to be positively associated with NICU admission hypothermia before gestational age stratification. Delivery room resuscitation and requirement for surfactant administration in the delivery room increased the risk of hypothermia. Neonatal outcomes associated with NICU admission temperature <36°C in the non-stratified analyses were hypotension in the first four days after birth, mechanical ventilation beyond one week of age, and severe cranial ultrasound findings of Grade 3 or 4 intraventricular hemorrhage and/or white matter damage.
Table 1. Relationship of clinical variables and admission hypothermia *.
| <36°C n=158 |
≥ 36° C n=151 |
OR* | 95% CI | p value | |
|---|---|---|---|---|---|
| PPROM | 58 (37) | 74 (49) | 0.60 | 0.38-0.95 | 0.03 |
| Clinical chorioamnionitis | 33 (21) | 47 (31) | 0.58 | 0.35-0.98 | 0.04 |
| GA < 26 wk | 56 (35) | 22 (15) | 3.23 | 1.85-5.56 | <0.001 |
| Cesarean section | 96 (61) | 69 (46) | 1.84 | 1.17-2.89 | 0.01 |
| Resuscitation | 138 (85) | 109 (72) | 2.16 | 1.22-3.77 | 0.01 |
| Surfactant administration | 114 (72) | 92 (61) | 1.66 | 1.03-2.68 | 0.04 |
| Hypotension <96 h age | 59 (38) | 40 (27) | 1.67 | 1.03-2.71 | 0.04 |
| IMV > 7d | 88 (56) | 60 (40) | 1.91 | 1.21-2.99 | 0.01 |
| Severe CUS | 45 (28) | 27 (18) | 1.83 | 1.06-3.14 | 0.03 |
unadjusted OR
Data are presented as n (%)
GA=gestational age; Resuscitation=any delivery room interventions beyond suction and stimulation; Surfactant administration =surfactant administration in the delivery room; Hypotension= mean arterial pressure <10th percentile for age for ≥2 consecutive hours; IMV= intermittent mandatory ventilation; severe cranial ultrasound (CUS) abnormality=Grade 3 and 4 intraventricular hemorrhage and/or white matter damage
Hypothermia associations stratified by gestational age
Since gestational age appeared to be the predominant factor contributing to admission hypothermia (Figure 1), further statistical analyses were stratified by <26 and ≥26 weeks gestational age. Table 2 shows demographic, obstetric, delivery room, and neonatal factors stratified by gestational age and admission temperature <36°C. Birth weight was significantly associated with hypothermia only in neonates ≥26 weeks gestation, although there was a trend toward an association between lower birthweight and lower NICU admission temperature in infants <26 weeks gestation (p=0.09). The frequencies of preterm labor, preterm prelabor rupture of membranes, maternal antibiotics, and clinical chorioamnionitis were higher in the normothermic ≥26 wk GA infants, but not in the less mature group. As shown in Table 2, after stratification for gestational age, NICU admission hypothermia was not significantly associated with any adverse neonatal outcomes studied.
Table 2. Clinical factors stratified by gestational age and admission hypothermia <36°C.
| Variable | <26 wk GA (n=78) |
≥26 wk GA (n=231) |
||||
|---|---|---|---|---|---|---|
| <36°C (n=56) |
≥ 36° C (n=22) |
P value |
<36°C (n=102) |
≥ 36° C (n=129) |
p value |
|
| Demographics | ||||||
| Birth weight (g) | 671 ± 97* | 714 ± 108 | 0.09 | 1042 ± 235 | 1123 ± 239 | 0.01 |
| GA (wk) | 24.45 ± 0.74 | 24.5 ± 0.74 | 0.77 | 28.3 ± 1.78 | 28.7 ± 1.83 | 0.09 |
| Race (African-American) | 45 (80) | 17 (77) | 0.76 | 77 (75.5) | 86 (66.7) | 0.14 |
| Sex (male) | 22 (39) | 13 (59) | 0.14 | 55 (54) | 74 (57.4) | 0.60 |
| Obstetric factors | ||||||
| Pregnancy-induced hypertension | 2 (4) | 1 (5) | 0.84 | 27 (26) | 15 (12) | 0.004 |
| Preterm labor | 51 (91) | 21 (95) | 0.51 | 70 (69) | 107 (82) | 0.01 |
| PPROM | 22 (39) | 11 (50) | 0.39 | 36 (35) | 63 (49) | 0.04 |
| Maternal antibiotics | 50 (89) | 18 (82) | 0.38 | 65 (64) | 99 (77) | 0.03 |
| Clinical chorioamnionitis | 15 (27) | 9 (41) | 0.22 | 18 (18) | 38 (30) | 0.04 |
| Delivery room factors | ||||||
| Delivery route (C/S) | 29 (52) | 9 (41) | 0.39 | 67 (66) | 60 (46) | 0.004 |
| Resuscitation | 56 (100) | 22 (100) | 78 (76) | 87 (69) | 0.02 | |
| Surfactant administration | 55 (98) | 22 (100) | 0.53 | 59 (58) | 70 (54) | 0.59 |
| Neonatal outcomes | ||||||
| Hypotension <96 h of age | 34 (62) | 14 (64) | 0.88 | 25 (25) | 26 (20) | 0.43 |
| Early onset sepsis | 3 (5) | 1 (5) | 0.88 | 3 (3) | 8 (6) | 0.24 |
| NEC | 2 (4) | 1 (5) | 0.86 | 9 (10) | 6 (5) | 0.17 |
| IMV (d) | 36 ± 23 | 31 ± 23 | 0.35 | 8.9 ± 13.9 | 7.9 ± 1.2 | 0.60 |
| Supplemental O2 (d) | 70 ± 39 | 59 ± 33 | 0.24 | 27 ± 26 | 28 ± 28 | 0.80 |
| Survival | 49 (88) | 17 (77) | 0.26 | 96 (94) | 125 (97) | 0.30 |
| CLD | 24 (46) | 7 (41) | 0.72 | 13 (13) | 19 (15) | 0.68 |
| Severe CUS abnormalities | 21 (37) | 8 (36) | 0.93 | 24 (24) | 19 (15) | 0.09 |
Data are presented as mean ± SD or n (%). GA=gestational age, PPROM=preterm prelabor rupture of membranes, NEC=necrotizing enterocolitis; IMV=intermittent mandatory ventilation, CLD=chronic lung disease
Hypothermia, placental histology, and cytokines
Placental pathology was performed in 257 cases (83% of the entire cohort), and histologic chorioamnionitis was present in 161/257 (63%). Histologic chorioamnionitis and fetal vasculitis were significantly more common among infants <26 weeks gestation (CA 90% vs 53% and FV 69% vs 43% in patients <26 wks vs. ≥26 wks, respectively, p<0.001). Table 3 shows that there was no association between NICU admission temperature and histologic chorioamnionitis or fetal vasculitis in either gestational age stratum.
Table 3. Inflammatory factors in infants with and without admission hypothermia stratified by gestational age.
| <26 wk GA (N=78) |
≥26 wk GA (N=231) |
|||||
|---|---|---|---|---|---|---|
| < 36°C (n=56) |
≥ 36°C (n=22) |
P value |
< 36°C (n=102) |
≥ 36°C (n=129) |
p value |
|
| Histologic chorioamnionitis | 43 (89)* | 19 (90) | 0.91 | 38 (46) | 61 (57) | 0.13 |
| Fetal vasculitis | 31 (66) | 16 (76) | 0.40 | 33 (40) | 50 (47) | 0.34 |
| Serum IL-6≥ 18.2 pg/ml | 30 (73) | 9 (64) | 0.53 | 33 (42) | 40 (43) | 0.88 |
| Serum IL-1β ≥ 0.322 pg/ml | 25 (69) | 8 (73) | 0.84 | 34 (48) | 31 (38) | 0.21 |
| Serum TNFα ≥ 3 pg/ml | 17 (44) | 5 (45) | 0.97 | 28 (41) | 28 (38) | 0.74 |
| CSF IL-6 ≥ 6.3 pg/ml | 20 (77) | 4 (36) | 0.02 | 28 (52) | 36 (43) | 0.33 |
| CSF IL-1β ≥ 0.78 pg/ml | 10 (44) | 3 (27) | 0.36 | 21 (44) | 24 (31) | 0.15 |
| CSF TNFα ≥3 pg/ml | 7 (28) | 4 (36) | 0.62 | 10 (20) | 16 (20) | 0.93 |
Data are expressed as n (%). GA=gestational age, CSF=cerebrospinal fluid
Blood and CSF samples were available for cytokine analysis in 256 (83%) and 183 (59%) patients, respectively. Cord blood was collected in 159 patients and venous blood was obtained within 12h of delivery in another 97 patients. There were no significant differences in cytokine levels in cord blood compared with venous blood.Table 3 shows that NICU admission hypothermia <36°C was not significantly associated with blood concentrations of IL-6, IL-1β, or TNFα in either gestational age group. Interleukin-1β and TNFα concentrations were low in CSF, but CSF IL-6 concentrations ≥ 6.3 pg/ml were associated with admission hypothermia in infants <26 weeks' gestation.(Table 3).
Correlations of obstetric variables
We analyzed the correlations of the obstetric factors associated with admission hypothermia. PPROM (r2=0.301, p<0.001) and maternal antibiotics (r2=0.224, p=0.0005) were positively correlated with clinical chorioamnionitis, while cesarean section was inversely correlated (r2= -0.26, p=<0.001). Pregnancy-induced hypertension (PIH) was significantly correlated with cesarean section delivery (r2=0.228, p<0.001).
Discussion
In contrast to our initial hypothesis, we did not find that NICU admission hypothermia was reflective of the fetal inflammatory response syndrome (FIRS). We did, however, find that clinical chorioamnionitis was associated with lower risk for NICU admission hypothermia, while cesarean section delivery was associated with increased risk. The combination of fetal vasculitis and elevated serum cytokines at birth, referred to as the fetal inflammatory response syndrome (FIRS), has been shown by us and others to be associated with adverse pulmonary and neurologic outcomes in preterm infants [10, 19]. Inflammation-modulating cytokines may affect core temperature and, conversely, temperature may affect cytokine production. We did not find a correlation between NICU admission temperature and histologic chorioamnionitis, fetal vasculitis, or serum levels of TNFα, IL-1β, and IL-6. Among infants <26 weeks' gestation, CSF IL-6 levels, were higher in those who were hypothermic on NICU admission. This finding is based on a relatively small number of patients and deserves further consideration in larger studies since IL-6 has neuromodulatory properties [3, 15, 16].
We found that the diagnosis of clinical chorioamnionitis was associated with higher NICU admission temperature in preterm infants. Maternal fever ≥38°C was a requirement for the diagnosis of clinical chorioamnionitis, and thus we speculate that these neonates had a higher core temperature at birth and were at lower risk for falling to the hypothermic range in the course of delivery room stabilization and transport to the NICU. Maternal fever has been associated with adverse neurologic outcomes, thus the finding that clinical chorioamnionitis is protective against early hypothermia highlights the complexity of determining associations between NICU admission temperature and long-term outcomes of preterm infants.
Two obstetric factors, cesarean section delivery and PIH were correlated with each other and were associated with a higher risk for admission hypothermia. A previously reported link [3] between cesarean section and hypothermia in preterm neonates may be been explained, in part, by anesthetic agents' effects on maternal and fetal temperature or by lower ambient temperature in an operating room than in a birthing room. An alternative explanation may be that cesarean section is more common among women delivering preterm without chorioamnionitis (such as those with PIH) as observed in the current study, therefore there is lower risk of fetal hyperthermia and consequently a higher risk for iatrogenic neonatal hypothermia.
Our study has several notable strengths and limitations. We performed a comprehensive analysis of the fetal inflammatory response in a large cohort of VLBW infants, with cytokine analysis and placental pathology in over 80% of cases. We also excluded outborn patients, eliminating the contribution of neonatal transport to both temperature disturbances and adverse outcomes. A limitation is that admission temperatures were collected retrospectively and in many cases we were not able to determine the precise time elapsed between birth and the first recorded temperature. Duration of hypothermia and speed of rewarming could not be assessed and it is conceivable that these factors could portend or contribute to adverse outcomes. While we did not find an association between NICU admission hypothermia and death or intraventricular hemorrhage, after adjusting for gestational age, other studies have reported these associations [2, 6, 14].
While it is not clear whether there is a causal association between NICU admission hypothermia and adverse outcomes, it is nonetheless reasonable to assume that even short-duration hypothermia should be avoided in preterm infants. Several small studies have shown that use of warm gel packs or heat loss barriers such as plastic wraps increase NICU admission temperatures of preterm infants by ∼0.5-1°C [15], and a large randomized clinical trial is underway to study the impact of heat loss prevention on outcomes of preterm infants (NCT00607464). An important caveat is that overzealous warming measures resulting in hyperthermia could have adverse consequences and should also be avoided [16]. Results of our study suggest that this risk may be greatest in cases of maternal fever and close monitoring of temperature is warranted to achieve normothermia in these patients.
Acknowledgments
This work was supported by NIH grants HL071113 and HL087166 (RMV) and HD051609 (KDF)17
References
- 1.Arons MM, Wheeler AP, Bernard GR, Christman BW, Russell JA, Schein R, et al. Effects of ibuprofen on the physiology and survival of hypothermic sepsis. Ibuprofen in Sepsis Study Group Crit Care Med. 1999;27(4):699–707. doi: 10.1097/00003246-199904000-00020. [DOI] [PubMed] [Google Scholar]
- 2.Bartels DB, Kreienbrock L, Dammann O, Wenzlaff P, Poets CF. Population based study on the outcome of small for gestational age newborns. Arch Dis Child Fetal Neonatal Ed. 2005;90(1):F53–59. doi: 10.1136/adc.2004.053892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bauer J, Hentschel R, Zahradnik H, Karck U, Linderkamp O. Vaginal delivery and neonatal outcome in extremely-low-birth-weight infants below 26 weeks of gestational age. Am J Perinatol. 2003;20(4):181–188. doi: 10.1055/s-2003-40608. [DOI] [PubMed] [Google Scholar]
- 4.Bhatt DR, White R, Martin G, Van Marter LJ, Finer N, Goldsmith JP, et al. Transitional hypothermia in preterm newborns. J Perinatol. 2007;27 Suppl 2:S45–47. doi: 10.1038/sj.jp.7211842. [DOI] [PubMed] [Google Scholar]
- 5.Cooper ZA, Ghosh A, Gupta A, Maity T, Benjamin IJ, Vogel SN, et al. Febrile-range temperature modifies cytokine gene expression in LPS-stimulated macrophages by differentially modifying NF-{kappa}B recruitment to cytokine gene promoters. Am J Physiol Cell Physiol. 2010;298(1):C171–181. doi: 10.1152/ajpcell.00346.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Costeloe K, Hennessy E, Gibson AT, Marlow N, Wilkinson AR. The EPICure study: outcomes to discharge from hospital for infants born at the threshold of viability. Pediatrics. 2000;106(4):659–671. doi: 10.1542/peds.106.4.659. [DOI] [PubMed] [Google Scholar]
- 7.Dammann O, Leviton A. Maternal intrauterine infection, cytokines, and brain damage in the preterm newborn. Pediatr Res. 1997;42(1):1–8. doi: 10.1203/00006450-199707000-00001. [DOI] [PubMed] [Google Scholar]
- 8.Dembinski J, Behrendt D, Martini R, Heep A, Bartmann P. Modulation of pro- and anti-inflammatory cytokine production in very preterm infants. Cytokine. 2003;21(4):200–206. doi: 10.1016/s1043-4666(02)00498-2. [DOI] [PubMed] [Google Scholar]
- 9.Fairchild KD, Viscardi RM, Hester L, Singh IS, Hasday JD. Effects of hypothermia and hyperthermia on cytokine production by cultured human mononuclear phagocytes from adults and newborns. J Interferon Cytokine Res. 2000;20(12):1049–1055. doi: 10.1089/107999000750053708. [DOI] [PubMed] [Google Scholar]
- 10.Gotsch F, Romero R, Kusanovic JP, Mazaki-Tovi S, Pineles BL, Erez O, et al. The fetal inflammatory response syndrome. Clin Obstet Gynecol. 2007;50(3):652–683. doi: 10.1097/GRF.0b013e31811ebef6. [DOI] [PubMed] [Google Scholar]
- 11.Hasday JD, Fairchild KD, Shanholtz C. The role of fever in the infected host. Microbes Infect. 2000;2(15):1891–1904. doi: 10.1016/s1286-4579(00)01337-x. [DOI] [PubMed] [Google Scholar]
- 12.Jobe AH, Bancalari E. Bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2001;163(7):1723–1729. doi: 10.1164/ajrccm.163.7.2011060. [DOI] [PubMed] [Google Scholar]
- 13.Kumar V, Shearer JC, Kumar A, Darmstadt GL. Neonatal hypothermia in low resource settings: a review. J Perinatol. 2009;29(6):401–412. doi: 10.1038/jp.2008.233. [DOI] [PubMed] [Google Scholar]
- 14.Laptook AR, Salhab W, Bhaskar B. Admission temperature of low birth weight infants: predictors and associated morbidities. Pediatrics. 2007;119(3):e643–649. doi: 10.1542/peds.2006-0943. [DOI] [PubMed] [Google Scholar]
- 15.McCall EM, Alderdice FA, Halliday HL, Jenkins JG, Vohra S. Interventions to prevent hypothermia at birth in preterm and/or low birthweight infants. Cochrane Database Syst Rev. 2008;(1):CD004210. doi: 10.1002/14651858.CD004210.pub3. [DOI] [PubMed] [Google Scholar]
- 16.Newton T, Watkinson M. Preventing hypothermia at birth in preterm babies: at a cost of overheating some? Arch Dis Child Fetal Neonatal Ed. 2003;88(3):F256. doi: 10.1136/fn.88.3.F256-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Redline RW, Wilson-Costello D, Borawski E, Fanaroff AA, Hack M. Placental lesions associated with neurologic impairment and cerebral palsy in very low-birth-weight infants. Arch Pathol Lab Med. 1998;122(12):1091–1098. [PubMed] [Google Scholar]
- 18.Richardson DK, Shah BL, Frantz ID, 3rd, Bednarek F, Rubin LP, McCormick MC. Perinatal risk and severity of illness in newborns at 6 neonatal intensive care units. Am J Public Health. 1999;89(4):511–516. doi: 10.2105/ajph.89.4.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Viscardi RM, Muhumuza CK, Rodriguez A, Fairchild KD, Sun CC, Gross GW, et al. Inflammatory markers in intrauterine and fetal blood and cerebrospinal fluid compartments are associated with adverse pulmonary and neurologic outcomes in preterm infants. Pediatr Res. 2004;55(6):1009–1017. doi: 10.1203/01.pdr.0000127015.60185.8a. [DOI] [PubMed] [Google Scholar]


