Abstract
Objective
Earlier diagnosis and treatment of necrotizing enterocolitis (NEC) in preterm infants, prior to clinical deterioration, might improve outcomes. A monitor that measures abnormal heart rate characteristics (HRC) of decreased variability and repetitive decelerations was developed as an early warning system for sepsis. Since NEC shares pathophysiologic features with sepsis, we tested the hypothesis that abnormal HRC occur prior to clinical diagnosis of NEC.
Study Design
Retrospective review of Bells stage II to III NEC cases among infants <34 weeks gestation enrolled in a prospective randomized clinical trial of HRC monitoring at 3 neonatal intensive care units.
Results
Of 97 infants with NEC and HRC data, 33 underwent surgical intervention within one week of diagnosis. The baseline HRC index from 1 to 3 days before diagnosis was higher in patients who developed surgical versus medical NEC (2.06 ± 1.98 vs. 1.22 ± 1.10, p=0.009). The HRC index increased significantly 16 hours prior to the clinical diagnosis of surgical NEC and 6 hours prior to medical NEC. At the time of clinical diagnosis, the HRC index was higher in patients with surgical versus medical NEC (3.3 ± 2.2 vs 1.9 ± 1.7, p<0.001).
Conclusions
Abnormal HRC occur before clinical diagnosis of NEC, suggesting that continuous HRC monitoring may facilitate earlier detection and treatment.
Keywords: neonatal intensive care unit, preterm infant, heart rate variability, predictive monitoring
Introduction
Necrotizing enterocolitis (NEC) is the most common acquired intestinal disease of preterm infants, and despite advances in neonatal intensive care unit (NICU) care, mortality may be as high as 30%. 1, 2, 3 Survivors of NEC have longer hospital stay and are at risk for long-term nutritional and neurologic problems. 4, 5, 6 A major challenge toward improving outcomes is identifying and treating infants in the early stages of NEC, when clinical signs may be subtle and nonspecific. Once more definitive signs appear, adverse outcomes such as extensive bowel necrosis leading to death or short bowel syndrome are more likely.7 Discovering markers of NEC that appear prior to overt clinical signs would facilitate earlier goal-directed therapies which could improve outcomes.
Abnormal heart rate characteristics (HRC) have been established as a biomarker for late-onset neonatal sepsis and mortality in NICU patients.8 Our group has shown that reduced heart rate variability and repetitive transient decelerations often occur in the preclinical phase of sepsis.9 This finding inspired the development of a monitor that calculates an HRC index, a mathematical representation of decreased beat-to-beat variability and decelerations which is the fold-increase in probability of sepsis being diagnosed in the next 24 hours. 10, 11 In a recent randomized clinical trial of 3003 infants, HRC monitoring reduced mortality in VLBW infants from 10.2% to 8.1%, a statistically significant and clinically meaningful result. 12
Necrotizing enterocolitis and sepsis share pathophysiologic features, including a systemic inflammatory response,13 hemodynamic compromise, and organ hypoperfusion.14 We and others have shown that elevated circulating levels of proinflammatory cytokines contribute to decreased heart rate variability 15, 16, 17, 18 In NEC, serum cytokines are elevated, particularly when there is significant intestinal necrosis or associated bacteremia.19 Intestinal hypomotility, ileus, and bowel distension have also been associated with increased vagal tone and altered heart rate characteristics in adults.20, 21 Our studies in preclinical models indicate that peritoneal administration of bacteria or fungi activates vagus nerve pathways leading to transient heart rate decelerations similar to those seen in neonates with sepsis.22 Heart rate characteristics changes may thus reflect activation of the cholinergic anti-inflammatory pathway which plays a critical role in host defense.23
Abnormal heart rate characteristics have not previously been studied in infants with NEC, and we therefore undertook a retrospective review of cases of NEC in VLBW infants during a prospective randomized clinical trial of HRC monitoring. Our goal was to determine whether the HRC index could identify patients in the early, preclinical phase of disease when aggressive treatment may improve outcomes.
Methods
Study population
We retrospectively identified all cases of NEC Bell's stage II to III24 in very low birth weight (VLBW <1500 grams) infants admitted from January 2005 to April 2010 to 3 centers participating in a prospective randomized clinical trial (RCT) of HRC monitoring. In the HRC (HeRO) RCT, 3003 VLBW infants in 9 NICUs had HRC continuously monitored and were randomized to having their HRC index displayed to clinicians, or not displayed, then followed for 120 days or until death or NICU discharge. Results of this study were published in 2011.12 Institutional Review Boards of each institution approved the RCT, which required parental consent, and approved retrospective review of deidentified patient data for the current analysis. The three centers participating in the current analysis were the University of Virginia, Wake Forest University (Brenner Children's Hospital and Forsyth Medical Center), and Vanderbilt University. At the University of Virginia, the principle site for the RCT, VLBW infants not enrolled in the HRC trial had HRC monitored but not displayed to clinicians. At the other units, infants not enrolled in the RCT did not have HRC monitored.
NEC diagnosis and clinical features
Through database review, we identified all cases of NEC in the three centers in the date range indicated above, and then identified cases meeting the following criteria: less than 34 weeks gestational age, clinical and radiographic evidence of Bell's stage II to III NEC, at least 7 days of age at diagnosis, and at least 7 days of treatment with bowel rest and antibiotics. Clinical signs included abdominal distention, ileus, and hematochezia. Radiographic signs included fixed dilated loops, pneumatosis intestinalis, portal venous gas, and pneumoperitoneum. We excluded patients in whom a diagnosis of spontaneous intestinal perforation was made at the time of laparotomy, as well as repeat episodes of NEC in the same patient.
Medical records were reviewed for demographic and clinical variables. The time of NEC diagnosis was defined as the time of the initial abdominal radiograph that led to the diagnosis of NEC. Cases were classified as medical or surgical, the latter defined as peritoneal drain placement or laparotomy within 7 days of NEC diagnosis. Mortality was considered within 14 days of NEC diagnosis.
Heart rate characteristics index monitoring
The heart rate characteristics (HeRO) monitor (Medical Predictive Science Corporation, Charlottesville, VA) uses the electrocardiogram from standard NICU bedside monitors to continuously calculate an HRC index. The mathematical algorithm from which the HRC index is derived incorporates decreased variability and transient decelerations and, using logistic regression, is the fold increase in probability of sepsis being diagnosed in the next 24 hours. The monitor continuously displays the HRC index which is updated hourly and represents HRC over the previous 12 hours. In the randomized trial, clinicians were educated about the HRC index but no course of action was mandated for patients with a rising score.
Statistical analysis
Comparisons of hourly HRC index between the two groups (medical and surgical NEC) were made by t-test for continuous variables and Fisher's exact test for categorical variables. Paired signed rank test was used to analyze the rise in the HRC index before NEC diagnosis. Average HRC index ± standard deviation is given, unless otherwise noted. Statistical analyses were performed in MatLab (MathWorks, Natick, MA) with a two-tailed level of significance of 0.05.
Results
Patient demographics and clinical course
We identified 97 episodes of NEC Bell's stage II to III in VLBW infants <34 weeks gestation with heart rate characteristics data available at the time of diagnosis. 33 of these patients underwent either laparotomy or peritoneal drain placement within 7 days of diagnosis and were classified as surgical NEC for this analysis. Clinical characteristics and outcomes are shown in Table 1. Patients that had surgical intervention (laparotomy or peritoneal drain placement) within one week of NEC diagnosis were of lower gestational age, were more likely to have a positive blood culture, and were more likely to die within 14 days of NEC diagnosis.
Table 1.
Clinical characteristics and outcomes of patients with medical and surgical NEC
| Medical NEC (n = 64) | Surgical NEC (n = 33) | p = | |
|---|---|---|---|
| Gestational age (weeks) | 27.0 ± 2.7 | 25.8 ± 2.3 | 0.03 |
| Birth weight (grams) | 914 ± 236 | 827 ± 231 | 0.09 |
| Female gender (n,%) | 28 (44%) | 13 (39%) | 0.83 |
| Age at NEC (days) | 30 ± 17 | 31 ± 22 | 0.95 |
| HRC at diagnosis | 1.9 ± 1.7 | 3.3 ± 2.2 | 0.002 |
| Positive blood culture | 11 (17%) | 11 (33%) | 0.08 |
| Mortality (n,%) | 4 (6%) | 15 (46%) | <0.001 |
Mean ± SD or n (%), where indicated
Figure 1 shows screen shots from the HRC monitor, with representative examples of normal HRC and abnormal HRC occurring prior to clinical presentation of NEC.
Figure 1. Heart rate characteristics monitor screen shots showing normal HRC and abnormal HRC in a patient with NEC.
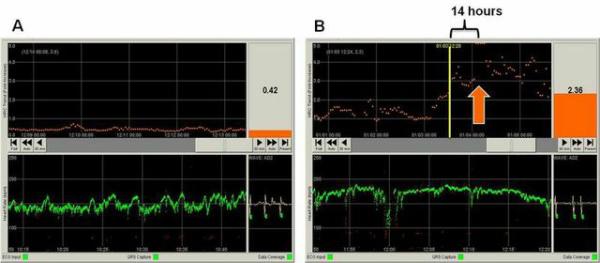
A) HRC monitor screen for an individual patient with normal heart rate characteristics. The bottom panel shows the last 30 minutes of heart rate (green) with a baseline HR of 150 beats per minute and normal frequent small accelerations and decelerations. The top panel shows the 5-day trend in the HRC index, which is derived from measures of heart rate variability and repetitive decelerations and is the probability the patient will be diagnosed with sepsis in the next 24 hours. In this patient, the HRC index has been low (<1) for 5 days indicating normal HRC and low probability of sepsis. The current HRC index is displayed in the upper right corner (0.42). B) HRC monitor screen for a patient with necrotizing enterocolitis and E. coli bacteremia. The time of clinical diagnosis of NEC is indicated by the orange arrow, at which time the HRC index was >5. Fourteen hours before NEC diagnosis (yellow vertical line) the HRC index had risen to 2.36 reflecting decreased accelerations and transient decelerations as seen in the corresponding HR tracing (green, bottom).
HRC index in infants with medical and surgical NEC
The baseline HRC index from 1 to 3 days before NEC diagnosis was higher in patients who developed surgical versus medical NEC (2.06 ± 1.98 vs. 1.22 ± 1.10, p=0.009), and this difference persisted after adjustment for gestational age and birth weight. In the hours leading up to the clinical diagnosis of NEC, there was a significant rise in the HRC index over patients’ prior 24 hour baseline (Figure 2). This occurred 16 hours before clinical diagnosis of surgical NEC and 6 hours prior to diagnosis of medical NEC.
Figure 2. Heart rate characteristics index in very low birth weight infants with medical or surgical necrotizing enterocolitis.
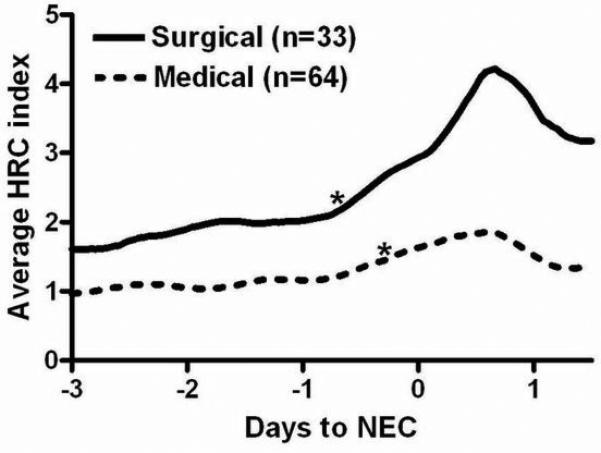
97 VLBW infants with Bell's stage II or III NEC at 3 centers had HRC index monitoring data available around the time of NEC diagnosis (day 0). *indicates significant increase in average HRC index compared to the previous 24 hour average (p<0.01).
At the time of diagnosis, patients with surgical NEC had a higher average HRC index compared to those with medical NEC (3.3 ± 2.2 vs 1.9 ± 1.7, p<0.001), and patients who died had a higher HRC index compared with survivors (4.0 ± 1.9 vs. 2.5 ± 1.7, p=0.004) (Table 1). Blood culture within 24 hours of NEC diagnosis was positive in 22 cases, negative in 74, and not obtained in 1. The HRC index in patients with a positive blood culture was 3.0 ± 1.5 and in those with a negative blood culture was 2.6 ± 1.9, (p=0.44). Considering only patients with a negative blood culture at the time of NEC diagnosis, there was a significantly higher HRC index in those with surgical compared to those with medical NEC (3.8 ± 2.0 vs. 2.2 ± 1.7, p=0.007).
After NEC diagnosis, the HRC index increased in both groups, peaking 15 hours after clinical diagnosis (Figure 2). Of note, general anesthesia and surgery lead to abnormal heart rate characteristics, and the figure therefore displays the HRC index only prior to surgical intervention.
HRC index display versus non-display
Of the 97 patients with NEC, 88 had been enrolled, with parental consent, in a randomized clinical trial in which the HRC index was either displayed to clinicians or not displayed (47 randomized to HRC display and 41 to non-display). The remaining 9 patients were at the University of Virginia and were not enrolled in the RCT but had HRC monitored and not displayed to clinicians, per unit protocol. Thus, there were 47 patients with HRC displayed and 50 non-displayed. Figure 3 shows the HRC index around the time of NEC diagnosis in patients in the non-display group compared to the HRC display group..
Figure 3. HRC index in infants with NEC with HRC index displayed or not displayed to clinicians.
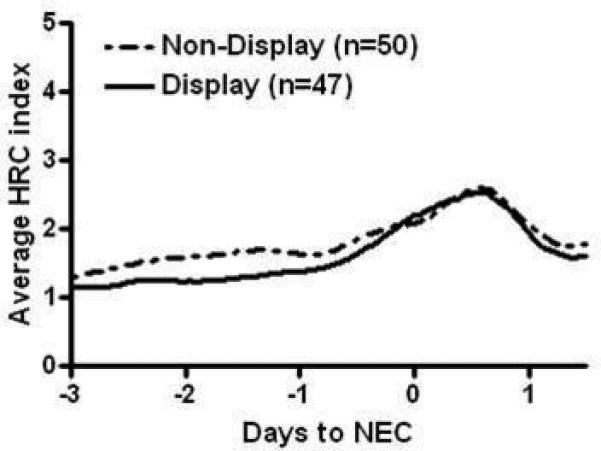
Of 97 infants with NEC, 88 were enrolled in a clinical trial of HRC monitoring in which infants were randomized to having their HRC index displayed to clinicians, and 9 had HRC monitored but not displayed outside the clinical trial. Average HRC index is shown before and after NEC diagnosis at day 0 for infants with HRC displayed, (solid line, n=47) or not displayed (dashed line, n=50).
Discussion
Identification of biomarkers for prediction or early detection of NEC has been the subject of intense investigation, which is appropriate given the high incidence and potentially devastating consequences of the disease. In the current analysis of VLBW infants admitted to 3 centers during a randomized clinical trial of heart rate characteristics monitoring, we found that abnormal HRC similar to those seen in sepsis also occur in preterm infants prior to clinical presentation of NEC. If clinicians are alerted to this physiologic change they may be prompted to closely evaluate the patient and consider further testing and treatment, potentially leading to improved outcomes through early intervention.
Prior studies of NEC biomarkers have not yet yielded clinically useful tests. Daily calculation of the Score for Neonatal Acute Physiology-II (SNAP-II), which includes measures of hypotension, hypothermia, acidosis, and oxygenation, was shown not to predict or detect NEC. 25 Genetic susceptibility testing, breath hydrogen sampling, and testing for inflammatory markers in blood or stool have all been shown to have limited diagnostic value.26 Measurements of altered mesenteric blood flow27, 28 and changes in intestinal microbiota 29 show promise for predicting or detecting NEC but are in early stages of investigation. Our study indicates that a commercially available monitor that is FDA-cleared for reporting on abnormal heart rate characteristics in neonates can detect biological changes associated with NEC up to a day before clinicians recognize that a patient is ill.
Abnormal heart rate characteristics of decreased variability and decelerations were discovered by our group to be strongly associated with late-onset sepsis in preterm infants.9, 30, 31, 32 Since NEC shares pathophysiologic features with sepsis it is not surprising that HRC are abnormal in infants at the time of diagnosis of NEC. The systemic inflammatory response, intestinal distension, and tissue necrosis may all contribute indirectly (via autonomic nervous system activation and dysfunction) or directly (via effects on sinoatrial node pacemaker cells) to abnormal heart rate characteristics. In the current analysis we found that the HRC index continued to rise after diagnosis and the start of treatment of NEC, in contrast to sepsis where we previously have shown that peak HRC occurs near the time of diagnosis and declines after antibiotics and other therapies are started. The continued worsening of HRC in patients with NEC parallels the clinical course in these infants in whom a dramatic systemic inflammatory response and, in some cases, significant bowel necrosis, often lead to progressive clinical deterioration even after initiation of therapy. The clinical relevance of this finding is that, in NICU patients started on antibiotics for suspected infection, a continued rise in the HRC index could be a sign of an ongoing inflammatory process and should lead to careful clinical assessment and consideration of additional studies or therapies.
Most patients in the current analysis were participating in a randomized clinical trial of 3003 VLBW infants in which the HRC index was monitored in all infants but displayed to clinicians in only half. In this large clinical trial, mortality was significantly reduced in patients who had their HRC index continuously displayed. In the current analysis, we did not find a statistically significant difference in the average HRC index prior to NEC diagnosis in infants in the HRC display and non-display groups. A limitation of the current analysis is that the number of infants was small, and in the randomized clinical trial there was no mandated intervention for infants with an acute rise in their HRC index. A larger number of infants with NEC would be required to determine whether display of the HRC index leads to earlier treatment or improved outcomes
Conclusion
Earlier diagnosis of NEC and institution of therapies to decrease the metabolic demand (bowel rest), reduce intestinal distension (gastric decompression), treat infection (broad-spectrum antibiotics), and optimize tissue oxygen delivery (fluid resuscitation and blood pressure support) may improve outcomes. Newer therapies for NEC such as anti-inflammatory agents 33 may also be more effective if patients can be identified in the early phases of illness. Continuous monitoring of heart rate characteristics provides clinicians with information about subtle physiologic changes associated with the systemic inflammatory response that proceed clinical deterioration associated with NEC, raising the possibility of improving patient outcomes through earlier intervention.
Acknowledgments
Funding: National Institutes of Health (grant R01-HD48562 to J.R.M.)
References
- 1.Guthrie SO, Gordon PV, Thomas V, Thorp JA, Peabody J, Clark RH. Necrotizing enterocolitis among neonates in the United States. Journal of perinatology: official journal of the California Perinatal Association. 2003;23(4):278–285. doi: 10.1038/sj.jp.7210892. [DOI] [PubMed] [Google Scholar]
- 2.Thyoka M, de Coppi P, Eaton S, Khoo K, Hall NJ, Curry J, et al. Advanced necrotizing enterocolitis part 1: mortality. European journal of pediatric surgery: official journal of Austrian Association of Pediatric Surgery [et al] = Zeitschriftfur Kinderchirurgie. 2012;22(1):8–12. doi: 10.1055/s-0032-1306263. [DOI] [PubMed] [Google Scholar]
- 3.Clark RH, Gordon P, Walker WM, Laughon M, Smith PB, Spitzer AR. Characteristics of patients who die of necrotizing enterocolitis. J Perinatol. 2012;32(3):199–204. doi: 10.1038/jp.2011.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Martin CR, Dammann O, Allred EN, Patel S, O'Shea TM, Kuban KC, et al. Neurodevelopment of extremely preterm infants who had necrotizing enterocolitis with or without late bacteremia. The Journal of pediatrics. 2010;157(5):751–756. e751. doi: 10.1016/j.jpeds.2010.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Catlin A. Extremely long hospitalizations of newborns in the United States: data, descriptions, dilemmas. Journal of perinatology : official journal of the California Perinatal Association. 2006;26(12):742–748. doi: 10.1038/sj.jp.7211617. [DOI] [PubMed] [Google Scholar]
- 6.Roze E, Ta BD, MH VDR, Tanis JC, VANB KN, Hulscher JB, et al. Functional impairments at school age of children with necrotizing enterocolitis or spontaneous intestinal perforation. Pediatr Res. 2011;70(6):619–625. doi: 10.1203/PDR.0b013e31823279b1. [DOI] [PubMed] [Google Scholar]
- 7.Abdullah F, Zhang Y, Camp M, Mukherjee D, Gabre-Kidan A, Colombani PM, et al. Necrotizing enterocolitis in 20,822 infants: analysis of medical and surgical treatments. Clin Pediatr (Phila) 2010;49(2):166–171. doi: 10.1177/0009922809349161. [DOI] [PubMed] [Google Scholar]
- 8.Griffin MP, Lake DE, Bissonette EA, Harrell FE, Jr., O'Shea TM, Moorman JR. Heart rate characteristics: novel physiomarkers to predict neonatal infection and death. Pediatrics. 2005;116(5):1070–1074. doi: 10.1542/peds.2004-2461. [DOI] [PubMed] [Google Scholar]
- 9.Griffin MP, Moorman JR. Toward the early diagnosis of neonatal sepsis and sepsis-like illness using novel heart rate analysis. Pediatrics. 2001;107(1):97–104. doi: 10.1542/peds.107.1.97. [DOI] [PubMed] [Google Scholar]
- 10.Kovatchev BP, Farhy LS, Cao H, Griffin MP, Lake DE, Moorman JR. Sample asymmetry analysis of heart rate characteristics with application to neonatal sepsis and systemic inflammatory response syndrome. Pediatric research. 2003;54(6):892–898. doi: 10.1203/01.PDR.0000088074.97781.4F. [DOI] [PubMed] [Google Scholar]
- 11.Moorman JR, Delos JB, Flower AA, Cao H, Kovatchev BP, Richman JS, et al. Cardiovascular oscillations at the bedside: early diagnosis of neonatal sepsis using heart rate characteristics monitoring. Physiological measurement. 2011;1821-1832;32(11) doi: 10.1088/0967-3334/32/11/S08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moorman JR, Carlo WA, Kattwinkel J, Schelonka RL, Porcelli PJ, Navarrete CT, et al. Mortality reduction by heart rate characteristic monitoring in very low birth weight neonates: a randomized trial. The Journal of pediatrics. 2011;159(6):900–906. e901. doi: 10.1016/j.jpeds.2011.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nanthakumar N, Meng D, Goldstein AM, Zhu W, Lu L, Uauy R, et al. The mechanism of excessive intestinal inflammation in necrotizing enterocolitis: an immature innate immune response. PLoS One. 2011;6(3):e17776. doi: 10.1371/journal.pone.0017776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Caplan MS, Jilling T. New concepts in necrotizing enterocolitis. Curr Opin Pediatr. 2001;13(2):111–115. doi: 10.1097/00008480-200104000-00004. [DOI] [PubMed] [Google Scholar]
- 15.Aronson D, Mittleman MA, Burger AJ. Interleukin-6 levels are inversely correlated with heart rate variability in patients with decompensated heart failure. J Cardiovasc Electrophysiol. 2001;12(3):294–300. doi: 10.1046/j.1540-8167.2001.00294.x. [DOI] [PubMed] [Google Scholar]
- 16.Fairchild KD, Saucerman JJ, Raynor LL, Sivak JA, Xiao Y, Lake DE, et al. Endotoxin depresses heart rate variability in mice: cytokine and steroid effects. Am J Physiol Regul Integr Comp Physiol. 2009;297(4):R1019–1027. doi: 10.1152/ajpregu.00132.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jan BU, Coyle SM, Macor MA, Reddell M, Calvano SE, Lowry SF. Relationship of basal heart rate variability to in vivo cytokine responses after endotoxin exposure. Shock. 2010;33(4):363–368. doi: 10.1097/SHK.0b013e3181b66bf4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Raynor LL, Saucerman JJ, Akinola MO, Lake DE, Moorman JR, Fairchild KD. Cytokine screening identifies NICU patients with Gram-negative bacteremia. Pediatr Res. 2012;71(3):261–266. doi: 10.1038/pr.2011.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chan KY, Leung FW, Lam HS, Tam YH, To KF, Cheung HM, et al. Immunoregulatory protein profiles of necrotizing enterocolitis versus spontaneous intestinal perforation in preterm infants. PloS one. 2012;7(5):e36977. doi: 10.1371/journal.pone.0036977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pancoto JA, Correa PB, Oliveira-Pelegrin GR, Rocha MJ. Autonomic dysfunction in experimental sepsis induced by cecal ligation and puncture. Autonomic neuroscience: basic & clinical. 2008;138(1-2):57–63. doi: 10.1016/j.autneu.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 21.Budzynski J, Klopocka M, Bujak R, Swiatkowski M, Pulkowski G, Sinkiewicz W. Autonomic nervous function in Helicobacter pylori-infected patients with atypical chest pain studied by analysis of heart rate variability. European journal of gastroenterology & hepatology. 2004;16(5):451–457. doi: 10.1097/00042737-200405000-00003. [DOI] [PubMed] [Google Scholar]
- 22.Fairchild KD, Srinivasan V, Moorman JR, Gaykema RP, Goehler LE. Pathogen-induced heart rate changes associated with cholinergic nervous system activation. Am J Physiol Regul Integr Comp Physiol. 2011;300(2):R330–339. doi: 10.1152/ajpregu.00487.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huston JM, Tracey KJ. The pulse of inflammation: heart rate variability, the cholinergic anti-inflammatory pathway and implications for therapy. J Intern Med. 2011;269(1):45–53. doi: 10.1111/j.1365-2796.2010.02321.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bell MJ, Ternberg JL, Feigin RD, Keating JP, Marshall R, Barton L, et al. Neonatal necrotizing enterocolitis. Therapeutic decisions based upon clinical staging. Ann Surg. 1978;187(1):1–7. doi: 10.1097/00000658-197801000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lim L, Rozycki HJ. Postnatal SNAP-II scores in neonatal intensive care unit patients: relationship to sepsis, necrotizing enterocolitis, and death. J Matern Fetal Neonatal Med. 2008;21(6):415–419. doi: 10.1080/14767050802046481. [DOI] [PubMed] [Google Scholar]
- 26.Young C, Sharma R, Handfield M, Mai V, Neu J. Biomarkers for infants at risk for necrotizing enterocolitis: clues to prevention? Pediatric research. 2009;65(5 Pt 2):91R–97R. doi: 10.1203/PDR.0b013e31819dba7d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cortez J, Gupta M, Amaram A, Pizzino J, Sawhney M, Sood BG. Noninvasive evaluation of splanchnic tissue oxygenation using near-infrared spectroscopy in preterm neonates. The journal of maternal-fetal & neonatal medicine: the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstet. 2011;24(4):574–582. doi: 10.3109/14767058.2010.511335. [DOI] [PubMed] [Google Scholar]
- 28.Oh S, Young C, Gravenstein N, Islam S, Neu J. Monitoring technologies in the neonatal intensive care unit: implications for the detection of necrotizing enterocolitis. JPerinatol. 2010;30(11):701–708. doi: 10.1038/jp.2010.9. [DOI] [PubMed] [Google Scholar]
- 29.Mai V, Young CM, Ukhanova M, Wang X, Sun Y, Casella G, et al. Fecal microbiota in premature infants prior to necrotizing enterocolitis. PloS one. 2011;6(6):e20647. doi: 10.1371/journal.pone.0020647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Griffin MP, Lake DE, O'Shea TM, Moorman JR. Heart rate characteristics and clinical signs in neonatal sepsis. Pediatr Res. 2007;61(2):222–227. doi: 10.1203/01.pdr.0000252438.65759.af. [DOI] [PubMed] [Google Scholar]
- 31.Griffin MP, Lake DE, Moorman JR. Heart rate characteristics and laboratory tests in neonatal sepsis. Pediatrics. 2005;115(4):937–941. doi: 10.1542/peds.2004-1393. [DOI] [PubMed] [Google Scholar]
- 32.Griffin MP, O'Shea TM, Bissonette EA, Harrell FE, Jr., Lake DE, Moorman JR. Abnormal heart rate characteristics preceding neonatal sepsis and sepsislike illness. Pediatr Res. 2003;53(6):920–926. doi: 10.1203/01.PDR.0000064904.05313.D2. [DOI] [PubMed] [Google Scholar]
- 33.Grave GD, Nelson SA, Walker WA, Moss RL, Dvorak B, Hamilton FA, et al. New therapies and preventive approaches for necrotizing enterocolitis: report of a research planning workshop. Pediatr Res. 2007;62(4):510–514. doi: 10.1203/PDR.0b013e318142580a. [DOI] [PubMed] [Google Scholar]


