Abstract
Preterm birth (PTB) is a leading cause of infant mortality and morbidity in the U.S. and across the globe. Infection and associated inflammation are important initiators for PTB pathways; an estimated 40% of PTBs are attributed to amniochorionic-decidual or systemic inflammation. Historically, intrauterine infections have been implicated in PTB; recent evidence suggests that infections remote from the fetal site may also be causative. There is strong epidemiological evidence that bacterial vaginosis and periodontitis -- two syndromes characterized by perturbations in the normal vaginal and oral bacterial microflora respectively-- are linked to infection-associated PTB. Oral and vaginal environments are similar in their bacterial microbiology; identical bacterial species have been independently isolated in periodontitis and bacterial vaginosis. Periodontitis and bacterial vaginosis also share many behavioral and sociodemographic risk factors suggesting a possible common pathophysiology. Genetic polymorphisms in host inflammatory responses to infection are shared between bacterial vaginosis, periodontitis and PTB, suggesting common mechanisms through which host genotype modify the effect of abnormal bacterial colonization on preterm birth. We review the state of knowledge regarding the risk of PTB attributable to perturbations in bacterial flora in oral and vaginal sites and the role of host genetics in modifying the risk of infection-related PTB. We posit that bacterial species that are common in perturbed vaginal and oral sites are associated with PTB through their interaction with the host immune system.
Keywords: Prematurity, infection, genotype
Introduction
Preterm birth (PTB) is a leading cause of infant mortality and morbidity in the U.S. and across the globe. Infection and associated inflammation are important initiators for PTB pathways; an estimated 40% of PTB are attributed to amniochorionic-decidual or systemic inflammation (1). PTB can be spontaneous or indicated; spontaneous PTB include births that occur at < 37 weeks gestation following preterm labor with or without premature rupture of membranes (PROM). Genital tract infections such as pyelonephritis and sexually transmitted infections such as trichomoniasis have long been associated with increased risk of PTB (2–7). More recently, a growing body of evidence indicates that even low levels of chronic infection and associated perturbations in the bacterial flora in the mouth or vaginal cavity are sufficient to stimulate a maternal inflammatory response, ultimately leading to PTB (8–11). It is not known when these sub acute infections are acquired and how they lead to PTB; infections early in gestation or even before pregnancy are likely important. The inflammatory response is oftentimes subclinical; the lack of specific histology and apparent clinical symptoms make diagnosis difficult. The association of sub-acute infection and sub-clinical inflammation has been used to explain the strong and consistent two-fold association of PTB with bacterial vaginosis (BV)(12) and periodontitis(13), two clinical syndromes associated with microbial shifts away from the normal bacterial flora in the vaginal and oral sites. Treatment trials of both BV and periodontitis during pregnancy have given mixed results, showing both positive and negative effects on preterm delivery (14–19).
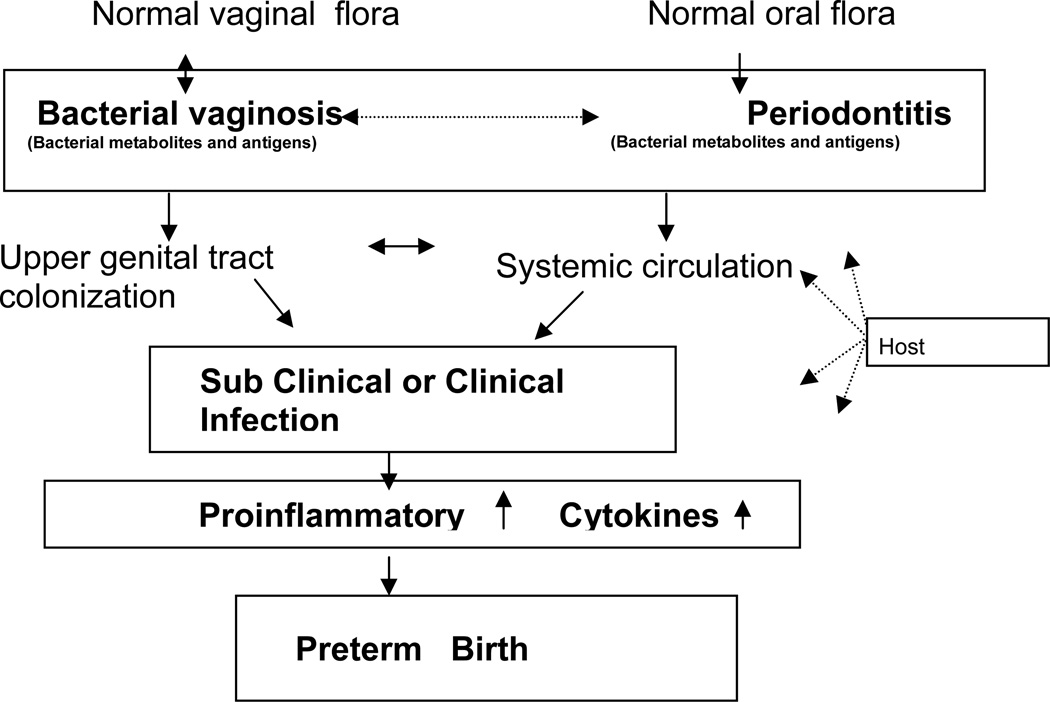
The generally accepted hypothesis for the pathophysiology of infection-associated PTB is that bacteria or bacterial products enter the uterine cavity by ascending from the proximal lower genital tract or systemically from remote sites such as the oral cavity. The pregnant body responds to infection by mounting an immune response to clear pathogen(s) and repair the associated injury; elevated local levels of cytokines and chemokines are important mediators of this process that results in PTB. High cytokine levels are used to indicate subclinical infection even when clinical symptoms of inflammation such as fever are absent (20). Clinical and subclinical bacterial infection trigger a cascade of events that can lead to PTB either when the bacteria directly ascend into the maternal uterine cavity and invade the fetal unit invoking a massive fetal inflammatory response (as measured by elevated cytokines, metalloproteinases and C reactive protein in fetal plasma (21–23)) and/or by triggering a proinflammatory maternal host response in the uterine tissues (1, 23–31). These processes up regulate prostaglandin synthesis and culminate in the onset of myometrial contractility (Figure 1). Invasion of the amniotic cavity through an ascending infection from the vagina is most proximal to the fetal unit; however, the consistent association of PTB with periodontal disease even in the absence of recovery of oral microbiota from the amniotic cavity suggests that periodontal disease may invoke a host response that triggers pathways to PTB (32). Alternatively, PTB may be triggered through initial colonization by bacteria shared between oral and vaginal sites (Figure 1). If the latter is true than personal hygiene and sexual behavioral factors that may aid sharing of bacterial flora between oral and vaginal sites will be strongly associated with risk of PTB. Regardless of the mechanism via which infection triggers PTB, the degree of inflammatory response to acute or sub acute infection is likely modified by innate host immunity; the interaction between host immune status (genotype) and bacterial exposure (environment) is critical for illuminating the etiology of infection associated PTB. In this review we will focus on the current state of knowledge regarding the risk of PTB attributable to perturbations in bacterial flora shared between oral and vaginal sites and the role of host genetics in modifying the risk of infection-related PTB.
Figure 1.
Hypothesized parallel potential pathways for vaginal and oral flora leading to preterm birth
Bacteria in the amniotic fluid and PTB
80% of women who deliver preterm have infections of the amnion or choramnion compared to 30% of women who deliver at term (33); detection of microorganisms in the amniotic fluid is a marker for upper genital tract infection and associated with a higher risk of PTB (34). Maternal upper genital tract infections can occur early on and remain asymptomatic for most of gestation; bacteria recovered from amniotic fluid in the absence of clinical symptoms have been associated with subsequent clinical chorioamnionitis and premature rupture of membranes (PROM) (35). Alternatively, bacterial organisms that colonize the lower genital tract and the vagina ascend to the uterus and cause infection; colonization by low virulence non-commensal bacteria in the vaginal sites are thought to be markers of upper genital tract infection (36–40). Additionally, colonization by these microorganisms cause increased vaginal concentrations of pro inflammatory cytokines (41). Microorganisms recovered from the uterine cavity (including from amniotic fluid) following PTB include Ureaplasma urealyticum (42), Chlamydia trachomatis (43), Trichomonas vaginalis44), Streptococcus agalactiae (43), Escherichia coli (2, 45, 46) and various anaerobes (Table 1). Some of them including species of Fusobacterium and Streptococcus are also found in the oral cavity although it is not clear whether a) these organisms share identity at the strain level and b) these organisms are representative of normal or diseased oral conditions (47) . Detection of bacteria in amniotic fluid correlates to histological inflammation; higher grades of histological lesions are associated with total colony count of bacteria in amniotic fluid (p < 0.05) and with “high-virulence” bacteria in amniotic fluid (p < 0.05)(48). However, in approximately 30% of PROM cases, the recovery of bacterial organisms does not correlate with inflammatory changes found during histological chorioamnionitis (23, 49, 50). The detection of bacterial colonization in the absence of host inflammation might result from contamination of the chorion by vaginal organisms at the time of delivery, bacterial infection close to delivery time, or colonization by relatively ‘avirulent’ organisms or strains. Conversely, inflammation occurring in the absence of detected bacterial colonization might result from colonization by non-cultivable organisms, a generalized fetal inflammatory response to infection or from a non-infectious process.
Table 1.
Organisms isolated from the amniotic cavity of pregnant women with preterm birth.
| Organism | Species | Reference |
|---|---|---|
| Acenitobacter | ||
| Bacteroides | B.urealyticus | (46, 73, 156, 157) |
| Candida | (29, 158) | |
| Capnocytophaga | C.sputigena | (155, 159) |
| Eikenella | E.corrodens | (160, 161) |
| Escherichia | E.coli | (2, 45, 46) |
| Fusobacterium | F.nucleatum | (47, 155, 162) |
| Gardnerella | G.vaginalis | (73, 157) |
| Lactobacillus | (73) | |
| Leptotrichia | L.amnionii | (46, 163) |
| Mobiluncus | (73) | |
| Mycoplasma | M.hominis | (46, 52, 73, 157) |
| Peptostreptococcus | (73, 164) | |
| Staphylococcus | S.aureus | (164) |
| Streptococcus |
S.agalactiae S.millerii S.acidomimus S.intermedius S.constellatus S.sanguis S.mutans S.uberis |
(45, 73, 164–166) |
| Ureaplasma | U.urealyticum | (42) |
Bacterial organisms recovered from amniotic fluid of women who went on to deliver preterm have been recovered from the vagina as early as in the first trimester. Single or mixed vaginal colonization with U.urealyticum, C. trachomatis or T. vaginalis are commonly associated with PTB, although there is variation between studies (51–55). The inconsistent results across studies may reflect racial and ethnic differences in study populations, since the prevalence of bacterial colonization vary by racial/ethnic group (56, 57); in a study of pregnant women, vaginal colonization with Mycoplasma hominis was more prevalent in African American (18.9%) and Hispanic (20.9%) women than in Caucasian women (4.2%, p = 0.01) (57). The timing of detection of non commensal bacterial organisms is important in attributing risk of PTB. One study found levels G.vaginalis, M.hominis and U.urealyticum are acquired at a low rate but are highly persistent and significantly associated with PTB while species of Peptostreptococcus and Bacteroides are frequently acquired only in late pregnancy and found not to be associated with PTB (58). These results imply that abnormal colonization by certain bacteria early in pregnancy or perhaps even before gestation might be important in determining PTB outcome. PTB risk was elevated in women with BV who were also colonized by U. urealyticum (OR 3.1, 1.8-5.4) compared to the rate in the presence of U. urealyticum only, indicating the potential importance of mixed infections (59).
Antibiotic treatment of patients with PROM without labor decreases risk of chorioamnionitis from 69% to 46%, p < 0.05 (60), suggesting that regardless of whether the colonizing bacteria caused rupture of membranes, they contribute to subsequent PTB. There is some indication that it is the relative loads of bacteria rather than their presence or absence per seis associated with increased risk of PTB; florescence in situ hybridization experiments using a DNA probe specific to the conserved 16S rDNA of bacteria detected higher numbers of bacteria detected in PROM; bacteria were also found to colonize 13–16% of term deliveries, albeit in lesser numbers (61).
Paradoxically, treatment regimens targeting PTB associated bacteria have not changed PTB outcome for the most part (Table 2); this has been variously attributed to differences in type and mechanism of action of antibiotic and formulation of antibiotics prescribed, duration and timing of administration (37, 62–67). These results imply that once abnormal bacteria colonize and trigger inflammatory maternal and/or fetal response, eradication of the bacteria in itself is insufficient to fix the cascade of damaging processes that culminate in PTB.
Table 2.
Effect of antibiotic treatment on specific bacterial organisms in preterm birth outcomes in the years 1990–2007.
| Organism | Preterm birth Indicators |
Association | Treatment Effect | Antibiotic(s) Prescribed |
References |
|---|---|---|---|---|---|
| S. agalactiae | PTB<37 weeks | 1.1 (0.9–1.4) | 1.1 (0.8–1.2) | Assorted i | (63) |
| LBW | 1.3 (1.05–1.7) | 1.0 (0.7–1.5) | |||
| 0.9 (0.6 – 1.3) | Erythromycin j | (62) | |||
| Chorioamnionitis | 7.2 (2.4–21.2) | 1.2 (0.34– 4.4) | Not available | (64) | |
| U. urealyticum | LBW b | Not reported | 0.70 (0.46–1.07) | Erythromycin | (65) |
| Clindamycin a | |||||
| C.trachomatis | PTB | 9.0, p= 0.05 | Erythromycin c | (37) | |
| PPROM | 1.3 (1.1–1.5) | 0.37, p<0.01 | |||
| PTB | 0.6(0.2–1.7) | Erythromycin | (84) | ||
| 0.39 (0.14–1.1) | Erythromycin | (67) | |||
| T.vaginalis | PTB | 1.8 (1.2,2.7)d,f | Metronidazole | (14, 84) | |
| 1.45(0.8–2.7) | Metronidazole | (67) | |||
| T. vaginalis + BV e | 3.3 (1.01–10.7) | 0.6 (0.19–1.9) | Clindamycin | (67) | |
| 1.4 (0.4–5.5) | |||||
| (167) | |||||
| T.vaginalis + C. trachomatis +BV | PTB<37 weeks | 3.6 (1.8–7.5) | 0.13 (0.02–1.0) | Clindamycin | (167) g |
| Asymptomatic bacteruria E.coli a | LBW | Not reported | 0.66 (0.49–0.89) | Assorted k | (168) |
Results of meta analysis
PTB outcome not measured
Erythromycin treatment at 26 to 30 weeks. U.urealyticum colonization not associated with PTB.
Metronidazole treatment was not associated with increased risk of PTB at <35 or <32 weeks
Individual effects not evaluated due to low numbers
Metronidazole treatment associated with increase in PTB
Study conducted in african american women
Heavy colonization, defined as growth of S.agalactiae in non-selective media
Antibiotics effective against S. agalactiae - penicillins, cephalosporins, erythromycin, clindamycin, trimethoprim-sulfa, sulfisoxazole, and triple sulfa vaginal cream were used in different studies
Treatment during the third trimester and before 30 weeks and continuing for 10 weeks or until 35 weeks 6 days of pregnancy
Treatments from different studies with one of the following: nitrofurantoin,sulphadimidine, penicillin and Sulphonamides
Bacterial Vaginosis and PTB
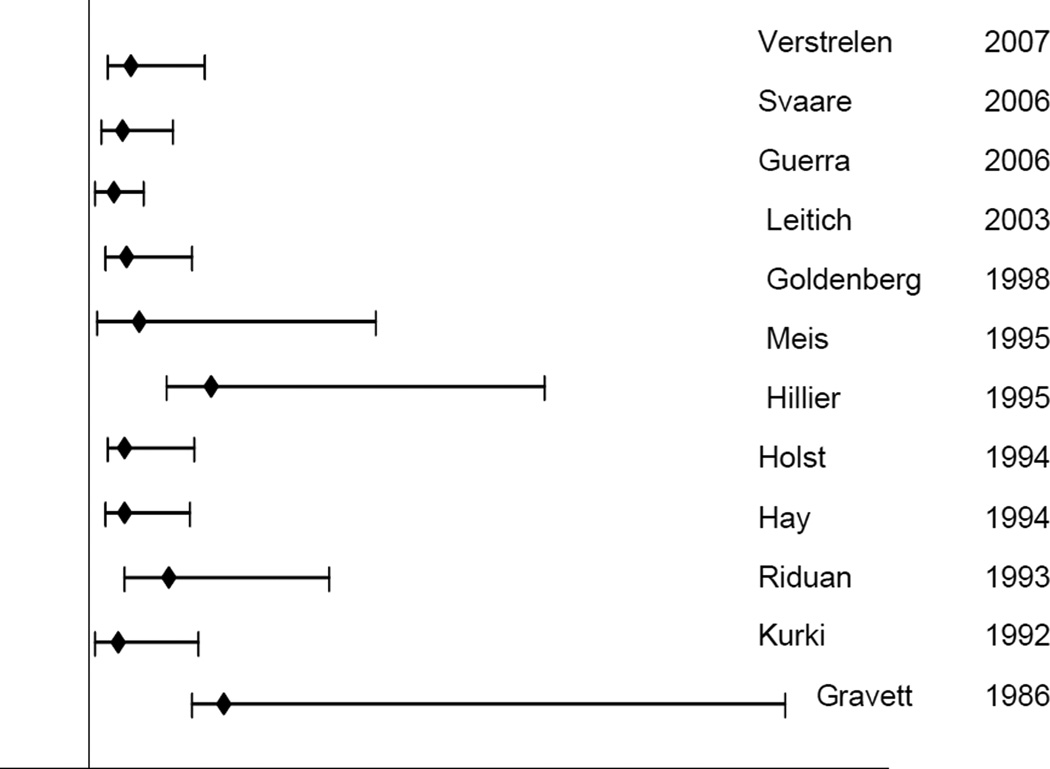
Several studies have shown a two to five fold increased risk of PTB in women with bacterial vaginosis (BV) during gestation; this increased risk is independent of previous PTB, smoking and black race (Figure 2) (29, 44, 47, 68–72). BV diagnosed during gestation is also associated with chorioamnionitis and preterm PROM (73). BV is a complex polymicrobial disorder characterized by depletion of Lactobacilli dominated flora and overgrowth of a mixed and variable anaerobic and facultative flora, including Gardnerella vaginalisPrevotella spp, Bacteroides spp, Mobiluncus sppgram-positive cocci, and genital Mycoplasma. The number and types of species found in BV are diverse: a metagenomics study found 35 unique species (some not related to any known species) in samples from 27 women with BV (74).
Figure 2. Relative risk of preterm birth with bacterial vaginosis.
11 observational studies conducted between 1986 and 2007 on populations varying in sociodemographic variables and sample size were included. Y-axis intersects x-axis at relative risk = 1.
Notably, BV elicits only a low or minimal inflammatory response. The clinical syndrome is characterized by low levels of Lactobacillus, discharge, and vaginal odor, but the characteristic microbiology is often detected in the absence of the clinical syndrome. When the diagnosis of abnormal bacterial flora is extended beyond the clinical diagnosis of BV and includes atypical gram-positive rods and lactobacilli-dominated smears showing heavy leukorrhea of unknown cause, the risk of PTB increases from 25% to 60%. “Normal” flora by this modified criterion is associated with a four-fold decrease in PTB (95%CI 0.1-0.6, P < .001) and an abnormal Gram stain with an overall adjusted odds ratio for PTB of 5.2 (95%CI 1.8-14.5, P < .001) (75).
The prevalence of BV during pregnancy ranges from 4.9% to 49%, (70, 72, 76–78) and varies by clinical setting, sociodemographic factors, diagnostic criteria and gestational age. In the Vaginal Infections and Prematurity (VIP) study of 13,914 pregnant women (23–26 weeks gestation) enrolled at seven US academic medical centers from 1984 to 1989, 16% (1645/10,397) of women had BV with center-specific prevalence ranging from 9% to 28%. (70) Prevalence of BV was higher among African American (23%) than Caucasian (9%) participants.(79) As BV is very common, and is strongly associated with PTB, treating BV seems a sound strategy to prevent PTB. However, results from clinical treatment trials are conflicting (72, 80–84), and in some cases suggest harm, so treatment of BV during pregnancy currently is not recommended to prevent PTB. Given the importance and substantial attributable risk of preterm birth for BV, there has been intense speculation about the reasons for the failure (16).
BV is reported more frequently among women who are poor (70), less educated (70), young (36, 70), or unmarried (70). Until recently, the only behavioral factors reported were early age at first intercourse(70) and smoking (85). Psychosocial stress was found to be strongly and independently associated with BV prevalence in a cross sectional multi-racial sample of pregnant women. (76) An increased risk of BV was associated with stress measured using the Perceived Stress Scale in a one-year prospective study of multi-racial nonpregnant women aged 15 to 44. Behavioral factors such as douching may also influence PTB outcomes. In the 1988 National Survey of Family Growth, women who reported currently douching two to three times per week were more likely to have a history of delivering a low birth weight infant, but that women who douched less often experienced no increase in risk. (86) However other case control studies show contradicting results (87). In a recent study of preterm birth among a cohort of low-income African-American women, women who reported douching less than three times per month in the six months before pregnancy were significantly less likely to deliver preterm than women who reported never douching (prevalence ratio (PR), 95% CI: 0.63, 0.42-0.95) after adjusting for potential confounding. Compared to women reporting never douching, women reporting douching during the pregnancy were at a higher risk of delivering preterm, although not statistically significant (PR, 95% CI: 1.64, 0.97-2.76) (88). It is not known whether women with vaginal infections are more likely to douche because of associated symptoms or whether douching leads to an increase in vaginal infections; consequently the association of douching with PTB is not yet explained.
Most pregnant women with BV do not deliver prematurely and a high percentage of PTB women whose placenta/ membranes show inflammation do not have BV. This has led investigators to hypothesize that BV is a marker for upper genital tract infection. Elastase, mucinase, sialidase, prolidase and other proteolytic enzymes produced by anaerobic bacteria involved in the pathogenesis of BV likely alter the immune signals and promote the degradation of host mucosal epithelial barrier, permitting bacteria access to the uterus as well as impairing fetal membrane strength and elasticity; elevated levels of these enzymes are implicated in increased risk of PTB (89–91). The deleterious effects of these bacterial byproducts have been shown to induce preterm birth in rat models (92, 93). BV is associated with higher levels of sialidase activity (84% of 50 women with BV compared to none in 19 normal women) and > 70 % of sialidase activity can be accounted for by the presence of Prevotella, Bacteroides and Gardnerella species (94). High rates of phospholipase A2 (a precursor of prostaglandin synthesis) are produced by Bacteroides spp., anaerobic Streptococci, Fusobacterium spp., and G. vaginalis and high concentrations of these anaerobes as seen in BV may induce prostaglandin synthesis and resultant PTB (reviewed in 73). A study on Danish pregnant women showed that high sialidase and/or prolidase activity combined with elevated vaginal pH 5 or greater, are strong risk factors for early preterm birth (< 32 weeks of gestation), low birthweight (LBW), and very LBW (< 1500 g) (95). Although BV organisms are known to produce high levels of these enzymes, clinically diagnosed BV was not found to be associated with risk of PTB in this population; understanding the functional determinants of BV organisms may allow for a better quantitative measure of the risk of BV associated PTB. In the same population, high levels of specific immunoglobulin A (IgA) against the toxin produced by G. vaginalis (anti- Gvh IgA) were protective for adverse pregnancy outcomes (90). In contrast, Lactobacilli are found not to increase synthesis of prostaglandins in fetal membranes and may have a protective effect against PTB. This has led investigators to postulate that preventing perturbations in Lactobacilli dominated vaginal flora is critical to achieving a decreased risk of PTB. Clinical trials using L. acidophilus and L. reuterii strains along with antibiotics for treating BV, revealed that use of the Lactobacilli strains lengthened the time to BV recurrence for women who were initially cured using antibiotics, however the effect of probiotics on PTB outcomes has not been reported (96). Recent studies using 16S rDNA phylogenetic analysis indicates that vaginal bacterial flora of healthy African American may differ from Caucasian women. Lactobacilli were less likely to be the dominant organisms in healthy African American women (68% vs. 91% in healthy Caucasian women) and African American women were more likely to be colonized primarily by anaerobes (32% in African American women vs. 8% in Caucasians) (97). This implies that a non-Lactobacilli dominated vaginal flora in itself is not indicative of poor vaginal health; establishing the bacterial signatures of normal (and altered flora) is an essential first step towards determining the subset of bacteria that contribute to infection associated PTB.
Oral flora and PTB
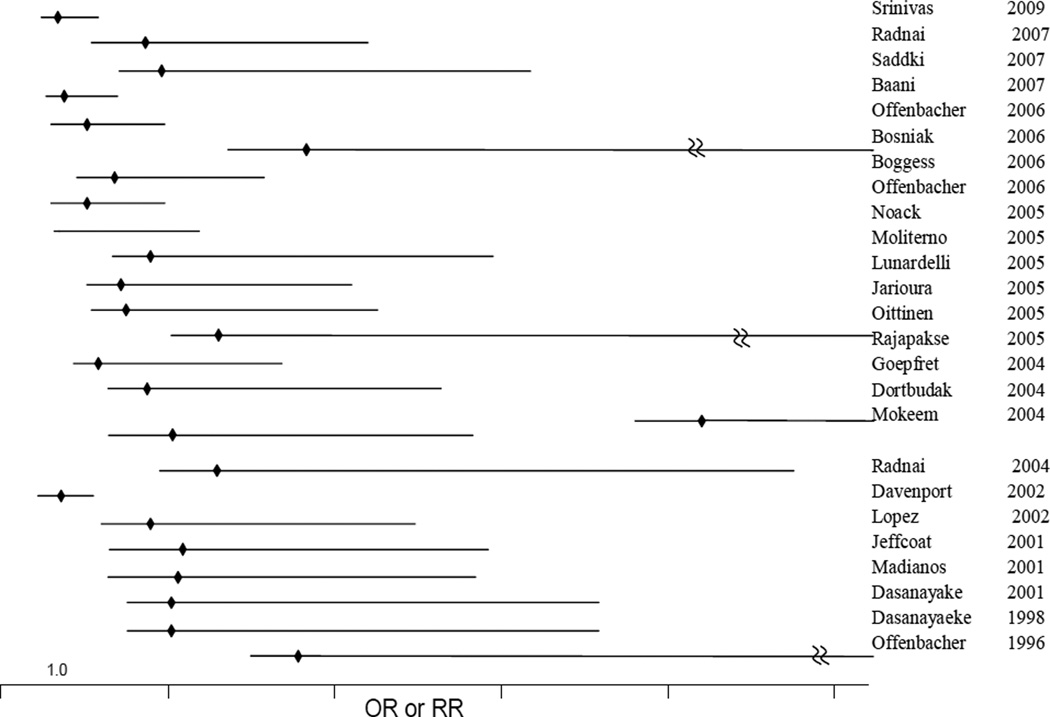
A strong association has been observed between poor oral health and PTB although the results are not uniform (13, 98–101). In 1996, Offenbacher first reported a case-control study where women with PTB and low birth weight babies were found to be more likely to have significant periodontal disease compared to control women with normal birth weight babies. In a meta-analysis of 17 observational studies on periodontal disease and preterm birth conducted in 2007, a positive association was found between periodontitis and preterm birth (pooled odds ratio, 95% confidence interval, p-value: 2.27 (1.06-4.85), p < 0.001) but the authors recommended that large multi-center trials be completed prior to instituting changes in clinical practice (102). The meta-analysis identified considerable statistical heterogeneity, at least some of which was accounted for by differences in study quality, and study population. The observational studies that were included did not examine specific microbes or their relative abundance; periodontal disease was measured using a variety of measures including bleeding on probing, pocket depth, clinical attachment loss, plaque index, and gingival index (102). Results from a pilot clinical treatment trial showed a 3.8-fold reduction in the rate of preterm delivery, a decrease in periodontal pathogen load, and a decrease in local and serum inflammatory factors (32). Bacterial organisms which are significantly associated with periodontal disease in the “red cluster”(Porphyromonas gingivalis, Tanerella forsythensis, and Tanerella denticola) and “orange cluster” (Prevotella intermedia, Prevotella nigrescens, Campylobacter rectus were also associated with PTB, albeit at borderline significance (p = 0.012-0.069) (103). Postpartum levels of all selected bacteria were at least two times higher in the preterm group than in the term group, with significant differences in P. gingivalis, T. forsythensis, T. denticola, P. intermedia, P. nigrescens, and C. rectus (P < 0.05). Subsequently many studies have confirmed this association of periodontitis with PTB, although a few results from large studies have contradicting results (Figure 3).
Figure 3. Relative risk of preterm birth associated with periodontitis.
25 observational studies conducted between 1996 and 2009 on populations varying in sociodemographic characteristics and sample sizes were included. Y-axis intersects X-axis at relative risk = 1.
A wide range of gram positive and gram negative bacterial species, yeasts, mycoplasmas and protozoa are found in the healthy oral cavity (104). The hard enamel surface of teeth provides surfaces for microbial colonization in a three dimensional architecture resulting in plaque. From initial colonization by aerobic and facultative anaerobes in infancy, mainly Streptococcus oralis, Streptococcus mitis and Streptococcus salivarius, the diversity of oral microbes increases to contain gram positive bacteria from Actinomyces, Lactobacillus, Rothia genus and gram negative organisms including Neisseria, Canocytpphaga and Prevotella genus (105). Diet and social behaviors such as smoking affect the composition of the dental flora; diets rich in sugar provide for acidification of teeth surfaces that encourage colonization by acid tolerant cariogenic species such as Streptococcus mutans. Smoking increases the risk of periodontitis and levels of periodontal pathogens such as P. gingivalis and A. actinomcytemcomitans. Using 16S rRNA analysis, several species of Fusobacteria, Peptostreptococcus, Prevotella, Capnocytophaga, Eikenella and Porphyromonas are found to be distributed differently between healthy and diseased sites on teeth (105–109). There is some evidence for racial differences in periodontal pathogens; P.gingivalis and P. anaerobius were more significantly associated with black subjects in the adult periodontitis group, while F. nucleatum was associated with white subjects in both the adult periodontitis and early onset periodontitis groups (110).
Oral prophylaxis and periodontal treatment in three clinical trials demonstrated a 57% reduction in preterm low birth weight (pooled RR 0.43; 95% CI 0.24-0.78) and a 50% reduction in preterm births (RR 0.5; 95% CI 0.20-1.30) (111) but results of other studies show little or no effect (112). A recent meta analysis of periodontal treatment on PTB reported a significant effect of root planning and scaling on PTB in the absence of history of PTB (OR, 0.48; 95% CI, 0.29-0.77; P = .003). The authors also reported that treatment effect on PTB was more in patients with less severe disease as defined by probing depth and bleeding on probing (OR, 0.49; 95% CI, 0.28-0.87 and OR, 0.37; 95% CI, 0.14- 0.95 respectively) (113). Specific microbes are both positively or negatively associated with increased risk of PTB: in a case control study of 53 Turkish women with preterm low birth weight babies and 128 women with term babies, P. micros and C. rectus significantly increased and P. nigrescens and A. actinomycetemcomitans significantly decreased risk of preterm delivery of low birth weight babies (114). A study among 161 Hungarian women found higher levels of periodontal pathogens, P. gingivalis, P. nigrescens, T.forsythensis, A. actinomycetemcomitans, F. nucleatum, T.denticola, M. micros, C. rectus, E. corrodens, E. nodatum, and S. intermedius in the PTB cases than controls (115). However, a recent study in the USA did not show significant differences between periodontal pathogens in preterm and term births (116).
Host immune response to oral pathogens appears to modify the risk of PTB; both maternal and fetal immunity appears to play an important role in determining PTB risk. In a study of 812 births, maternal immunoglobulin G (IgG) antibody to oral organisms was associated with a decreased rate of preterm delivery and an increase in birth weight, and therefore, provided protection to mothers and fetuses from exposure to bacterial pathogens (117) . In contrast, in a predominantly African American group of 448 women, women with elevated second trimester serum IgG levels against P. gingivalis were more likely to give birth to a preterm low birth weight infant compared to those with lower serum values (118). In a predominantly Hispanic population (N= 203) there was no association of PTB with IgG levels against specific oral pathogens (119). While the host immune response appears to depend on race and ethnicity; the extent of this association with risk of PTB is not known.
Host genetics and PTB
A familial component to preterm birth has been established (120, 121) and includes an increased risk to PTB if the mother was premature herself (122), if the sister of the mother had a premature child (123) and if there is a sibling born from a previous spontaneous preterm delivery (124). A history of one previous preterm birth is associated with a recurrence risk, i.e., an odds ratio (OR) of 4.58 with a 95% confidence interval (CI) of 4.40-4.78 for 16 to 36 week gestation infants. Recurrence risk alone, however, may be explained by persistence of environmental risk factors and is not necessarily explained by genetic factors. Other studies provide more specific support for the role of genes in the causation of preterm birth. Ward showed a 50-fold increase in the coefficient of kinship for grandparents of preterm infants (121). Using a national cohort of female twin pairs in Sweden (2089 pairs) a significant genetic component was identified for PTB with a heritability of 0.36 (125). Treloar showed heritability of 0.27 in a large twin study of preterm labor in Australia (126). Overall, there is strong support for the role of genes present in the mother and/or fetus in PTB (127).
Although both periodontitis and bacterial vaginosis have a strong bacterial etiology, it is clear that in addition to environmental factors, genetic predisposition plays an important role in these diseases. A number of human gene polymorphisms studied to date for periodontal disease and bacterial vaginosis are involved in maternal and fetal inflammatory pathways and have been reviewed elsewhere (128, reviewed in 129, 130–134). These genetic polymorphisms likely result in functional changes (for eg increased expression of pro inflammatory factors), although this has not been demonstrated for all the gene polymorphisms studied to date (135, 136). Most of the research to date has focused on gene variations in pro- and anti-inflammatory cytokine genes and their respective receptors because these cytokines increase expression of matrix degrading metalloproteinases. A recent study determine increased levels of IL-2, IL-4, IL-5, IL-8, IL-10, monocyte chemoattractant protein 1, macrophage inflammatory protein -alpha, MIP-1β, soluble IL-6 receptor α , TNFα, soluble TNFR I, and TREM-1 (triggering receptor expressed on myeloid cells) were associated with PTB, while levels of IL-1β, IL-18, matrix metalloproteinase 9, and neurotrophin 3 decreased in PTB (137). Increased expression of matrix metalloproteinases results in degradation of the extracellular matrix and contributes to cervical ripening and labor (138). Both periodontitis and bacterial vaginosis share a common association with a number of alleles of inflammation-associated genes; some of these have also been implicated in PTB (Table 5). Genetic polymorphisms in these host immune factors that result in increased production of inflammatory cytokines TNFα and IL-6 and decreased production of IL-10 are associated with increased risk of PTB (27). Recent studies imply that some of these differences are race-specific; levels of IL-6 (measured shortly before birth) were found to be higher in PTB (defined as < 35 weeks gestation)in Caucasian women (p < 0.0003) compared to African American women (p < 0.6), suggesting that elevated IL-6 concentrations are associated with preterm birth in Caucasians but not African Americans (139). In the same population levels of IL- 8 were higher in PTB in Caucasians and IL-1beta levels were higher in African American PTB cases (140). It is not known to what extent gene-gene or gene-environment interactions play a role in racial differences seen in cytokine levels between Caucasians and African Americans. A recent study of PTB risk in African Americans suggests a strong role for maternal IL-12 and fetal IL-12RB, indicating that fetal and maternal factors may together contribute to genetic risks for PTB (141).
Table 5.
Polymorphisms in host genetic factors that play a role in the inflammatory immune response to bacterial infection
| Function | Polymorphism | Bacterial vaginosis association |
Periodontitis association |
PTB association |
|---|---|---|---|---|
| Pro and anti-inflammatory cytokines | TNF(−308) | x (229) | ns (230, 231) | x (232) c |
| TNF-B1(codon 25) | nd | x (233) | nd | |
| IL-6 (−174) | nd b | x (233, 234) | x (235) | |
| IL-4(−590) | nd | x (236) c | nd | |
| IL1Beta (−511) | x (132) b | ns | x | |
| IL1Beta (+3954) | x (132) b | x (237) | nd | |
| IL-2 (−330) | nd | x (238) | x (239) | |
| IL-10 (1082) | nd | x (240) | ||
| IL-10 (819) | nd | x (240) | ns (241) | |
| IL-10(−590) | nd | x (240) | ||
| IL-1RN (VNTR) | x (242) a | x (237) | x (242) | |
| IFN-γR1 | nd | x (243) | nd | |
| Innate defence against bacteria | Toll like receptors | |||
| TLR4 896 | x (244) | x(245) | x (246) | |
| TLR2 | nd b | nd | x | |
| Metalloproteinase | x b | |||
| MMP1(−1607) | x(247) (248) d | x (142) | ||
| MMP9(−1562) | x (249, 250) | x (251) | ||
| MMP9(CA repeat) | x (251) | |||
TNFα is involved in remodeling the cervix and fetal membranes by promoting production of collagen-degrading matrix metalloproteinases (MMPs), including MMP1 and MMP9 and levels of MMPs are higher in PTB and PPROM (142) (138, 143). TNF polymorphisms are not significantly associated with periodontitis; however plasma concentration and gingival cervicular fluid levels of TNFα are higher in patients with aggressive periodontitis, which causes more severe inflammation in periodontitis lesions (144, 145).
Bacterial endotoxin and LPS produced by periodontitis and BV organisms activate pattern recognition molecules TLR2 and TLR4 on host cell surfaces and stimulate increased levels of TNF-α and matrix metalloproteinase’s (146–148). In a murine model for pregnancy, oral infection with periodontal pathogen C. rectus resulted in increased levels of placental TLR4 levels (149). Gene environment interactions are likely to be important; carriers of TNF-2 allele were at a significantly increased risk of spontaneous preterm birth in a case-control study of 375 pregnant women (OR 2.7, 95% CI 1.7-4.5] and this risk was increased significantly when the women also had BV (OR 6.1, 95% CI 1.9-21.0) (150). Interactions between candidate genes in PTB outcome are also significant; a study demonstrating multilocus interaction between SNPs -3448 of TNFα, −7227 of IL-6, and 33314 of IL-6R was successful in predicting low risk of PTB genotypes in European-American women (RR 3.50, 95% CI 2.52-4.87) (151).
The increased risk of PTB due to exposure to bacterial factors is modified by the host’s genetic background indicating that successful prevention of PTB may require antibiotic therapy to eradicate the bacterial organisms as well as immunomodulators to decrease the levels of pro inflammatory immune factors that trigger processes leading to PTB. Studies on non human, primate models of PTB support the notion of preventing PTB by treating both the infection and the associated inflammation; administration of ampicillin together with dexamethasone/indomethacin delayed preterm birth induced by intraamniotic infection by group B Streptococcus (P = .004) (152).
Hypothesis : Oral-Vaginal Health Interaction
In the past decade there has been an explosion in non-culture based molecular methods to defining the microflora of the oral and vaginal sites; some of the newly discovered bacteria are not amenable to culture such as those belonging to the Clostridiales order, others are very fastidious such as Atopobium. The discovery of these non cultivable organisms has enabled molecular biologists to study archived tissues from studies and attempt to classify the breadth of bacterial diversity present in the oral and vaginal sites. As we begin to apply these methods to understand the complex microflora involved in bacterial vaginosis and periodontitis there is preliminary evidence pointing to subsets of bacterial organisms that can colonize both the vaginal and oral cavity and that increase risk of PTB (Table 3). Although there are several reports on the associations of PTB independently with perturbed oral flora (periodontitis) and perturbed vaginal flora (BV), little is known about the combined effects of altered vaginal and oral health on PTB. We found only one report that studied both BV and gingivitis which reported that women with BV were more likely to have gingivitis and the bacterial loads of women with BV and gingivitis were higher than in women with BV alone (153). In women with BV and gingivitis, the vaginal samples had higher counts of bacteria commonly associated with periodontal disease including: A. actinomycetemcomitans , Fusobacterium sp., P. micro, P. intermeda, P. gingivalis, and T. forsythia in comparison with those with BV but not gingivitis, suggesting that oral disease may exacerbate the level of bacterial perturbations associated with BV. The association of BV and gingivitis with PTB was not reported.
Table 3.
Genus and species of organisms found in the vaginal and oral cavity and association with preterm birth.
| Genus | Species | Found in Vaginal flora b |
Found in Oral flora b |
Associated with PTB? b |
References |
|---|---|---|---|---|---|
| Bacteroides | B. forsythus (T.forsythensis) | x | x | x | (47, 169–173) |
| B. bivius | |||||
| B. assacharolyticus | |||||
| B. capillosus | |||||
| Fusobacterium | F. nucleatum sub nucleatum | x | x | x | (47, 155, 162, 173–175) |
| F. nucleatum sub vincentii | |||||
| F. nucleatum var polymorphum | |||||
| Gardnerella | G. vaginalis | x | x | ||
| Lactobacillus | L. fermentum | x | x | x | (174, 176–182) |
| L. crispatus | |||||
| L. jensenii | |||||
| L. gasserii | |||||
| Peptostreptococcus | P. micros | x | x | x | (47, 173–175) |
| P. anaerobius | |||||
| P. lacrimalis | |||||
| P. ivorii | |||||
| P. assacharolyticus | |||||
| P. magnus | |||||
| P. prevotti | |||||
| Porphyromonas | P.gingivalis | x | x | x | (47, 172–175) |
| Prevotella | P.intermedia | x | x | x | (172–174, 183–185) |
| P.nigrescens | |||||
| P.bivia | |||||
| P.bucalis | |||||
| Actinomyces | A. Actinomycetemcomita | x | x | (178, 186) | |
| ns A. naeslundii | |||||
| Campylobacter | C. gracilis | x | x | (173, 175) | |
| C. sputorum | |||||
| C. rectus | |||||
| C. showae | |||||
| Capnocytophaga | C. sputigena | x | x | (186) | |
| Mobiluncus | M. curtisii | x | x | (174, 182, 187) | |
| M. mulieris | |||||
| Leptotrichia | L. amnionii | x | x | (188, 189) | |
| Atopobium | A. rimae | x | x | ? | (175, 181, 182, 190–192) |
| A. vaginae | |||||
| Megasphaera | x | ? | (74, 193) | ||
| Veillonella | V. parvula | x | (194) | ? | (195) |
| Clostridiales order | BVAB1, BVAB2, BVAB3 a | x | ? | (74) | |
BVAB1-3 are uncultivable phylotypes of unknown genera in the Clostridiales order that are found highly associated with BV.
Genus but not necessarily all species listed
The multifactorial etiology of periodontitis and BV are strikingly similar. Both BV and periodontitis are characterized by dynamic colonization by a number of opportunistic bacterial pathogens on squamous epithelial cells in the oral or vaginal cavities. Health behaviors and socioeconomic risk factors are similar between the two conditions (Table 4). Oral pathogens can spread to the vaginal cavity within the female host via the gastrointestinal tract; alternatively there may be transmission between individuals via oro-genital contact. Oral sex has been associated with gum disease (154). Dixon et al reported the isolation of C. sputigena and F. nucleatum from the amniotic cavity in a single case of PTB with clinical chorioamnionitis; a temporal relation was noted between orogenital contact with the male partner with periodontitis and the onset of clinical infection (155), suggesting exchange of PTB related microflora between oral and genital sites. Thus vaginal and oral cavities possibly share a subset of microbes, making the origin of bacteria isolated from PTB potentially hard to establish. The methodological differences in studies included use bacterial identification at different resolutions; while some studies report genus level identification of bacteria, others classify bacteria at the species level. A systematic analysis of bacterial diversity at oral and vaginal sites at the same taxonomic resolution will allow us to determine the extent of shared bacteria between oral and vaginal sites and its association with PTB. Establishing the interaction of host genotype in modulating the combined risk of BV and periodontitis to PTB will help identify the subset of women who are at most risk of PTB and who are most likely to benefit from clinical interventions to prevent PTB.
Table 4.
Commonality of sociodemographic and behavioral risk factors for bacterial vaginosis and periodontitis
| Bacterial Vaginosis | Periodontitis |
|---|---|
| Stress (196–198) | Stress (199, 200) |
| Smoking (201–203) | Smoking (204–209) |
| Poor nutrition (210, 211) | Poor nutrition (212) |
| Ethnicity : African Americans at higher risk of BV(213, 214) | Ethnicity; African Americans have a higher risk of periodontitis (215–217) |
| Microbiology of infection not fully characterized; decreased levels of H2O2 producing Lactobacilli (218–220) | Microbiology of infection not fully characterized; decreased levels of H2O2 producing Lactobacilli (176, 221, 222) |
| Invokes local proinflammatory immune response (223–225) | Invokes local and perhaps systemic immune response (226–228) |
Conclusion
Perturbations in bacterial flora resulting in both BV and periodontitis are potentially related to infection associated PTB. To date, infection associated PTB has been studied in a reductionist framework: oral and vaginal health are treated as being independent of each other. The combined effect of exposure to bacteria that are shared between the oral and vaginal sites and the modifying effect of host genetics on their association with PTB has yet to be studied. Normal bacterial flora represents a dynamic equilibrium of commensal bacteria and opportunistic pathogens. Host genetics and host behaviors modify the bacterial ecology of the vaginal and oral environments and host genetics. Further, there are striking similarities between host genetic factors that predispose to BV, periodontitis and PTB. Newer studies on infection associated PTB will benefit from recognizing the interrelationships between bacterial populations at vaginal and oral sites.
Acknowledgements
The authors wish to acknowledge Carl Marrs for reviewing the manuscript and Dawn Reed for help with formatting. Funding was provided by National Institutes for Health (R01 DE014899, M.L.M, B.F, U.S).
Abbreviations
- PTB
Preterm Birth
- PPROM
Preterm premature rupture of membranes
- BV
Bacterial vaginosis
- RR
Risk ratio
- IgG
Immunoglobulin
- MMP
matrix metalloproteinases
- TNF
Tumor necrosis factor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lockwood CJ, Kuczynski E. Risk stratification and pathological mechanisms in preterm delivery. Paediatr Perinat Epidemiol. 2001 Jul;15(Suppl 2):78–89. doi: 10.1046/j.1365-3016.2001.00010.x. 2001. [DOI] [PubMed] [Google Scholar]
- 2.Naeye RL. Causes of the excessive rates of perinatal mortality and prematurity in pregnancies complicated by maternal urinary-tract infections. N Engl J Med. 1979 Apr 12;300(15):819–823. doi: 10.1056/NEJM197904123001503. [DOI] [PubMed] [Google Scholar]
- 3.Ovalle A, Levancini M. Urinary tract infections in pregnancy. Curr Opin Urol. 2001 Jan;11(1):55–59. doi: 10.1097/00042307-200101000-00008. [DOI] [PubMed] [Google Scholar]
- 4.Herraiz MA, Hernandez A, Asenjo E, Herraiz I. Urinary tract infection in pregnancy. Enferm Infecc Microbiol Clin. 2005 Dec;23 Suppl 4:40–46. doi: 10.1157/13091447. [DOI] [PubMed] [Google Scholar]
- 5.Maclean AB. Urinary tract infection in pregnancy. Int J Antimicrob Agents. 2001;17(4):273–277. doi: 10.1016/s0924-8579(00)00354-x. [DOI] [PubMed] [Google Scholar]
- 6.Millar LK, DeBuque L, Wing DA. Uterine contraction frequency during treatment of pyelonephritis in pregnancy and subsequent risk of preterm birth. J Perinat Med. 2003;31(1):41–46. doi: 10.1515/JPM.2003.006. [DOI] [PubMed] [Google Scholar]
- 7.Heine PM, McGregor JAM. Trichomonas Vaginalis: A Reemerging Pathogen. Clin Obstet Gynecol. 1993;36:137–144. doi: 10.1097/00003081-199303000-00019. [DOI] [PubMed] [Google Scholar]
- 8.Romero R, Espinoza J, Goncalves LF, Kusanovic JP, Friel LA, Nien JK. Inflammation in preterm and term labour and delivery. Semin Fet Neonat Med. 2006 Oct;11(5):317–326. doi: 10.1016/j.siny.2006.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Romero R, Chaiworapongsa T, Kuivaniemi H, Tromp G. Bacterial vaginosis, the inflammatory response and the risk of preterm birth: a role for genetic epidemiology in the prevention of preterm birth. Am J Obstet Gynecol. 2004 Jun;190(6):1509–1519. doi: 10.1016/j.ajog.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 10.Gotsch F, Romero R, Kusanovic JP, Mazaki-Tovi S, Pineles BL, Erez O, et al. The fetal inflammatory response syndrome. Clin Obstet Gynecol. 2007 Sep;50(3):652–683. doi: 10.1097/GRF.0b013e31811ebef6. [DOI] [PubMed] [Google Scholar]
- 11.Offenbacher S, Jared HL, O'Reilly PG, Wells SR, Salvi GE, Lawrence HP, et al. Potential pathogenic mechanisms of periodontitis associated pregnancy complications. Ann Periodontol. 1998 Jul;3(1):233–250. doi: 10.1902/annals.1998.3.1.233. [DOI] [PubMed] [Google Scholar]
- 12.Hillier SL, Nugent RP, Eschenbach DA, Krohn MA, Gibbs RS, Martin DH, et al. Association between bacterial vaginosis and preterm delivery of a low-birth-weight infant. The Vaginal Infections and Prematurity Study Group. N. Engl. J. Med. 1995 Dec 28;333(26):1737–1742. doi: 10.1056/NEJM199512283332604. [DOI] [PubMed] [Google Scholar]
- 13.Jeffcoat MK, Geurs NC, Reddy MS, Goldenberg RL, Hauth JC. Current evidence regarding periodontal disease as a risk factor in preterm birth. Ann Periodontol. 2001 Dec;6(1):183–188. doi: 10.1902/annals.2001.6.1.183. [DOI] [PubMed] [Google Scholar]
- 14.Okun N, Gronau KA, Hannah ME. Antibiotics for bacterial vaginosis or Trichomonas vaginalis in pregnancy: a systematic review. Obstet Gynecol. 2005 Apr;105(4):857–868. doi: 10.1097/01.AOG.0000157108.32059.8f. [DOI] [PubMed] [Google Scholar]
- 15.Andrews WW, Klebanoff MA, Thom EA, Hauth JC, Carey JC, Meis PJ, et al. Midpregnancy genitourinary tract infection with Chlamydia trachomatis: association with subsequent preterm delivery in women with bacterial vaginosis and Trichomonas vaginalis. Am J Obstet Gynecol. 2006 Feb;194(2):493–500. doi: 10.1016/j.ajog.2005.08.054. [DOI] [PubMed] [Google Scholar]
- 16.Morency AM, Bujold E. The effect of second-trimester antibiotic therapy on the rate of preterm birth. J Obstet Gynaecol Can. 2007 Jan;29(1):35–44. doi: 10.1016/s1701-2163(16)32350-7. [DOI] [PubMed] [Google Scholar]
- 17.Xiong X, Buekens P, Fraser WD, Beck J, Offenbacher S. Periodontal disease and adverse pregnancy outcomes: a systematic review. BJOG: An International Journal of Obstetrics & Gynaecology. 2006 Feb;113(2):135–143. doi: 10.1111/j.1471-0528.2005.00827.x. [DOI] [PubMed] [Google Scholar]
- 18.Offenbacher S, Lieff S, Boggess KA, Murtha AP, Madianos PN, Champagne CM, et al. Maternal periodontitis and prematurity. Part I: Obstetric outcome of prematurity and growth restriction. Ann Periodontol. 2001 Dec;6(1):164–174. doi: 10.1902/annals.2001.6.1.164. [DOI] [PubMed] [Google Scholar]
- 19.Offenbacher S, Beck J. Has Periodontal Treatment Failed to Reduce Adverse Pregnancy Outcomes? The Answer May Be Premature. J Periodontol. 2007;78(2):195–197. doi: 10.1902/jop.2007.060506. [DOI] [PubMed] [Google Scholar]
- 20.Sweet RL, Eschenbach DA, Hillier SL, Romero R, Gibbs RS. A review of premature birth and subclinical infection. Am J Obstet Gynecol. 1992;166(5):1515–1528. doi: 10.1016/0002-9378(92)91628-n. [DOI] [PubMed] [Google Scholar]
- 21.Romero R, Chaiworapongsa TEJ, Gomez R. Fetal plasma MMP-9 concentrations are elevated in preterm premature rupture of the membranes. Am J Obstet Gynecol. 2002;187:1125–1130. doi: 10.1067/mob.2002.127312. [DOI] [PubMed] [Google Scholar]
- 22.Ismail MMA, Zinaman MMJ, Lowensohn RRI, Moawad AAH. The significance of C-reactive protein levels in women with premature rupture of membranes. Am J Obstet Gynecol. 1985;151(4):541–544. doi: 10.1016/0002-9378(85)90285-6. [DOI] [PubMed] [Google Scholar]
- 23.Hillier SL, Witkin SS, Krohn MA, Watts DH, Kiviat NB, Eschenbach DA. The relationship of amniotic fluid cytokines and preterm delivery, amniotic fluid infection, histologic chorioamnionitis, and chorioamnion infection. Obstet Gynecol. 1993 Jun;81(6):941–948. [PubMed] [Google Scholar]
- 24.St John E, Mares D, Spear GT. Bacterial vaginosis and host immunity. Curr HIV/AIDS Rep. 2007 Feb;4(1):22–28. doi: 10.1007/s11904-007-0004-y. [DOI] [PubMed] [Google Scholar]
- 25.Romero R, Gomez R, Chaiworapongsa T, Conoscenti G, Kim JC, Kim YM. The role of infection in preterm labour and delivery. Paediatr Perinat Epidemiol. 2001 Jul;15 Suppl 2:41–56. doi: 10.1046/j.1365-3016.2001.00007.x. [DOI] [PubMed] [Google Scholar]
- 26.Romero R, Espinoza J, Kusanovic JP, Gotsch F, Hassan S, Erez O, et al. The preterm parturition syndrome. Br J Obstet Gynecol. 2006;113:17–42. doi: 10.1111/j.1471-0528.2006.01120.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Romero R, Espinoza J, Goncalves LF, Kusanovic JP, Friel LA, Nien JK. Inflammation in preterm and term labour and delivery. Semin Fetal Neonatal Med. 2006 Oct;11(5):317–326. doi: 10.1016/j.siny.2006.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Klein LL, Gibbs RS. Infection and preterm birth. Obstet Gynecol Clin North Am. 2005 Sep;32(3):397–410. doi: 10.1016/j.ogc.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 29.Gravett MG, Hummel D, Eschenbach DA, Holmes KK. Preterm labor associated with subclinical amniotic fluid infection and with bacterial vaginosis. Obstet Gynecol. 1986;67:229–237. doi: 10.1097/00006250-198602000-00013. [DOI] [PubMed] [Google Scholar]
- 30.Goldenberg R, Hauth J, Andrews W. Intrauterine infection and preterm delivery. N Engl J Med. 2000;342:1500–1507. doi: 10.1056/NEJM200005183422007. [DOI] [PubMed] [Google Scholar]
- 31.Gibbs RS, Romero R, Hillier SL, Eschenbach DA, Sweet RL. A review of premature birth and subclinical infection. Am J Obstet Gynecol. 1992 May;166(5):1515–1528. doi: 10.1016/0002-9378(92)91628-n. [DOI] [PubMed] [Google Scholar]
- 32.Offenbacher S, Katz V, Fertik G, Collins J, Boyd D, Maynor G, et al. Periodontal infection as a possible risk factor for preterm low birth weight. J Periodontol. 1996 Oct;67(10 Suppl):1103–1113. doi: 10.1902/jop.1996.67.10s.1103. [DOI] [PubMed] [Google Scholar]
- 33.Gibbs RS, Blanco JD, St Clair PJ, Castaneda YS. Quantitative bacteriology of amniotic fluid from women with clinical intraamniotic infection at term. J Infect Dis. 1982 Jan;145(1):1–8. doi: 10.1093/infdis/145.1.1. [DOI] [PubMed] [Google Scholar]
- 34.Watts DHKMA, Hillier SL, Eschenbach DA. The association of occult amniotic fluid infection with gestational age and neonatal outcome among women in preterm labor. Obstet Gynecol. 1992;79(3):351–357. doi: 10.1097/00006250-199203000-00005. [DOI] [PubMed] [Google Scholar]
- 35.Goldenberg RL, Andrews WW. Intrauterine infection and why preterm prevention programs have failed. Am J Public Health. 1996 Jun 1;86(6):781–783. doi: 10.2105/ajph.86.6.781. 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hay PE, Lamont RF, Taylor-Robinson D, Morgan DJ, Ison C, Pearson J. Abnormal bacterial colonisation of the genital tract and subsequent preterm delivery and late miscarriage. Br Med J. 1994;308:295–298. doi: 10.1136/bmj.308.6924.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McGregor JA, French JI, Richter R, Vuchetich M, Bachus V, Seo K, et al. Cervicovaginal microflora and pregnancy outcome: results of a double-blind, placebo-controlled trial of erythromycin treatment. Am J Obstet Gynecol. 1990 Nov;163(5 Pt 1):1580–1591. doi: 10.1016/0002-9378(90)90632-h. [DOI] [PubMed] [Google Scholar]
- 38.Krohn MA, Thwin SS, Rabe LK, Brown Z, Hillier SL. Vaginal colonization by Escherichia coli as a risk factor for very low birth weight delivery and other perinatal complications. J Infect Dis. 1997 Mar;175(3):606–610. doi: 10.1093/infdis/175.3.606. [DOI] [PubMed] [Google Scholar]
- 39.Witkin SS, Linhares IM, Giraldo P. Bacterial flora of the female genital tract: function and immune regulation. Best Pract Res Clin Obstet Gynaecol. 2007;21(3):347–354. doi: 10.1016/j.bpobgyn.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 40.McGregor JA, French JI, Richter R, Franco-Buff A, Johnson A, Hillier S, et al. Antenatal microbiologic and maternal risk factors associated with prematurity. Am J Obstet Gynecol. 1990 Nov;163(5 Pt 1):1465–1473. doi: 10.1016/0002-9378(90)90607-9. [DOI] [PubMed] [Google Scholar]
- 41.Genc MR, Witkin SS, Delaney ML, Paraskevas L-R, Tuomala RE, Norwitz ER, et al. A disproportionate increase in IL-1beta over IL-1ra in the cervicovaginal secretions of pregnant women with altered vaginal microflora correlates with preterm birth. Am J Obstet Gynecol. 2004 May;190(5):1191–1197. doi: 10.1016/j.ajog.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 42.Yoon BH, Romero R, Park JS, Chang JW, Kim YA, Kim JC, et al. Microbial invasion of the amniotic cavity with Ureaplasma urealyticum is associated with a robust host response in fetal, amniotic, and maternal compartments. Am J Obstet Gynecol. 1998 Nov;179(5):1254–1260. doi: 10.1016/s0002-9378(98)70142-5. [DOI] [PubMed] [Google Scholar]
- 43.Gibbs RS, Romero R, Eschenbach DA, Sweet RL. A review of premature birth and subclinical infection. Am J Obstet Gynecol. 1992;166:1515–1528. doi: 10.1016/0002-9378(92)91628-n. [DOI] [PubMed] [Google Scholar]
- 44.French J, McGregor J, Draper D, Parker R, McFee J. Gestational bleeding, bacterial vaginosis, and common reproductive tract infections: Risk for preterm birth and benefit of treatment. Obstet Gynecol. 1999;93:715–724. doi: 10.1016/s0029-7844(98)00557-2. [DOI] [PubMed] [Google Scholar]
- 45.Straka M, Dela Cruz W, Blackmon C, Johnson O, Stassen S, Streitman D, et al. Rapid detection of group B Streptococcus and Escherichia coli in amniotic fluid using real-time fluorescent PCR. Infect Dis Obstet Gynecol. 2004 Sep-Dec;12(3–4):109–114. doi: 10.1080/10647440400020679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McDonald HM, Chambers HM. Intrauterine infection and spontaneous midgestation abortion: is the spectrum of microorganisms similar to that in preterm labor? Infect Dis Obstet Gynecol. 2000;8(5–6):220–227. doi: 10.1155/S1064744900000314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hill GB. Preterm birth: associations with genital and possibly oral microflora. Ann Periodontol. 1998 Jul;3(1):222–232. doi: 10.1902/annals.1998.3.1.222. [DOI] [PubMed] [Google Scholar]
- 48.Dong Y, St Clair PJ, Ramzy I, Kagan-Hallet KS, Gibbs RS. A microbiologic and clinical study of placental inflammation at term. Obstet Gynecol. 1987 Aug;70(2):175–182. [PubMed] [Google Scholar]
- 49.Hillier SL, Krohn MA, Kiviat NB, Watts DH, Eschenbach DA. Microbiologic causes and neonatal outcomes associated with chorioamnion infection. Am J Obstet Gynecol. 1991 Oct;165(4 Pt 1):955–961. doi: 10.1016/0002-9378(91)90447-y. [DOI] [PubMed] [Google Scholar]
- 50.Hillier SL, Martius J, Krohn M, Kiviat N, Holmes KK, Eschenbach DA. A case-control study of chorioamnionic infection and histologic chorioamnionitis in prematurity. N Engl J Med. 1988 Oct 13;319(15):972–978. doi: 10.1056/NEJM198810133191503. [DOI] [PubMed] [Google Scholar]
- 51.Holst E, Goffeng AR, Andersch B. Bacterial vaginosis and vaginal microorganisms in idiopathic premature labor and association with pregnancy outcome. J Clin Microbiol. 1994;32:176–186. doi: 10.1128/jcm.32.1.176-186.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Perni SC, Vardhana S, Korneeva I, Tuttle SL, Paraskevas LR, Chasen ST, et al. Mycoplasma hominis and Ureaplasma urealyticum in midtrimester amniotic fluid: association with amniotic fluid cytokine levels and pregnancy outcome. Am J Obstet Gynecol. 2004 Oct;191(4):1382–1386. doi: 10.1016/j.ajog.2004.05.070. [DOI] [PubMed] [Google Scholar]
- 53.Kataoka S, Yamada T, Chou K, Nishida R, Morikawa M, Minami M, et al. Association between preterm birth and vaginal colonization by mycoplasmas in early pregnancy. J Clin Microbiol. 2006 Jan;44(1):51–55. doi: 10.1128/JCM.44.1.51-55.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Aaltonen R, Heikkinen J, Vahlberg T, Jensen JS, Alanen A. Local inflammatory response in choriodecidua induced by Ureaplasma urealyticum. Bjog. 2007 Nov;114(11):1432–1435. doi: 10.1111/j.1471-0528.2007.01410.x. [DOI] [PubMed] [Google Scholar]
- 55.Carey JC, Blackwelder WC, Nugent RP, Matteson MA, Rao AV, Eschenbach DA, et al. Antepartum cultures for Ureaplasma urealyticum are not useful in predicting pregnancy outcome. The Vaginal Infections and Prematurity Study Group. Am J Obstet Gynecol. 1991 Mar;164(3):728–733. doi: 10.1016/0002-9378(91)90505-l. [DOI] [PubMed] [Google Scholar]
- 56.Cotch MF, Pastorek JG, 2nd, Nugent RP, Yerg DE, Martin DH, Eschenbach DA. Demographic and behavioral predictors of Trichomonas vaginalis infection among pregnant women. The Vaginal Infections and Prematurity Study Group. Obstet Gynecol. 1991 Dec;78(6):1087–1092. [PubMed] [Google Scholar]
- 57.Doh K, Barton PT, Korneeva I, Perni SC, Bongiovanni AM, Tuttle SL, et al. Differential vaginal expression of interleukin-1 system cytokines in the presence of Mycoplasma hominis and Ureaplasma urealyticum in pregnant women. Infect Dis Obstet Gynecol. 2004 Jun;12(2):79–85. doi: 10.1080/10647440400003667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.McDonald HM, O'Loughlin JA, Jolley PT, Vigneswaran R, McDonald PJ. Changes in vaginal flora during pregnancy and association with preterm birth. Journal of Infectious Diseases. 1994 Sep;170(3):724–728. doi: 10.1093/infdis/170.3.724. [DOI] [PubMed] [Google Scholar]
- 59.Vogel I, Thorsen P, Hogan VK, Schieve LA, Jacobsson B, Ferre CD. The joint effect of vaginal Ureaplasma urealyticum and bacterial vaginosis on adverse pregnancy outcomes. Acta Obstet Gynecol Scand. 2006;85(7):778–785. doi: 10.1080/00016340500442423. [DOI] [PubMed] [Google Scholar]
- 60.Ovalle A, Martinez MA, Kakarieka E, Gomez R, Rubio R, Valderrama O, et al. Antibiotic administration in patients with preterm premature rupture of membranes reduces the rate of histological chorioamnionitis: a prospective, randomized, controlled study. J Matern Fetal Neonatal Med. 2002 Jul;12(1):35–41. doi: 10.1080/jmf.12.1.35.41. [DOI] [PubMed] [Google Scholar]
- 61.Steel JH, Malatos S, Kennea N, Edwards AD, Miles L, Duggan P, et al. Bacteria and inflammatory cells in fetal membranes do not always cause preterm labor. Pediatr Res. 2005 Mar;57(3):404–411. doi: 10.1203/01.PDR.0000153869.96337.90. [DOI] [PubMed] [Google Scholar]
- 62.Klebanoff MA, Regan JA, Rao AV, Nugent RP, Blackwelder WC, Eschenbach DA, et al. Outcome of the Vaginal Infections and Prematurity Study: results of a clinical trial of erythromycin among pregnant women colonized with group B streptococci. Am J Obstet Gynecol. 1995 May;172(5):1540–1545. doi: 10.1016/0002-9378(95)90493-x. [DOI] [PubMed] [Google Scholar]
- 63.Regan JA, Klebanoff MA, Nugent RP, Eschenbach DA, Blackwelder WC, Lou Y, et al. Colonization with group B streptococci in pregnancy and adverse outcome. VIP Study Group. Am J Obstet Gynecol. 1996 Apr;174(4):1354–1360. doi: 10.1016/s0002-9378(96)70684-1. [DOI] [PubMed] [Google Scholar]
- 64.Anderson BL, Simhan HN, Simons KM, Wiesenfeld HC. Untreated asymptomatic group B streptococcal bacteriuria early in pregnancy and chorioamnionitis at delivery. American Journal of Obstetrics and Gynecology. 2007;196(6):524.e1–524.e5. doi: 10.1016/j.ajog.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 65.Raynes-Greenow CH, Roberts CL, Bell JC, Peat B, Gilbert GL. Antibiotics for Ureaplasma in the vagina in pregnancy. Cochrane Database Syst Rev. 2004;(1):CD003767. doi: 10.1002/14651858.CD003767.pub2. [DOI] [PubMed] [Google Scholar]
- 66.Klebanoff MA, Carey JC, Hauth JC, Hillier SL, Nugent RP, Thom EA, et al. Failure of Metronidazole to Prevent Preterm Delivery among Pregnant Women with Asymptomatic Trichomonas vaginalis Infection. N Engl J Med. 2001 Aug 16;345(7):487–493. doi: 10.1056/NEJMoa003329. 2001. [DOI] [PubMed] [Google Scholar]
- 67.McGregor JA, French JI, Parker R, Draper D, Patterson E, Jones W, et al. Prevention of premature birth by screening and treatment for common genital tract infections: Results of a prospective controlled evaluation. Am J Obstet Gynecol. 1995;173(1):157–167. doi: 10.1016/0002-9378(95)90184-1. [DOI] [PubMed] [Google Scholar]
- 68.Gravett MG, Nelson HP, DeRouen T, Critchlow CW, Eschenbach DA, Holmes KK. Independent associations of bacterial vaginosis and Chlamydia trachomatis infection with adverse pregnancy outcome. JAMA. 1986;256:1899–1903. [PubMed] [Google Scholar]
- 69.McDonald HM, O'Loughlin JA, Jolley PT, Vigneswaran R, McDonald PJ. Changes in vaginal flora during pregnancy and association with preterm birth. J Infect Dis. 1994 Sep;170(3):724–728. doi: 10.1093/infdis/170.3.724. [DOI] [PubMed] [Google Scholar]
- 70.Hillier SL, Nugent RP, Eschenbach DA, Krohn MA, Gibbs RS, Martin DH, et al. Association between bacterial vaginosis and preterm delivery of a low-birth-weight infant. N Engl J Med. 1995;333:1737–1742. doi: 10.1056/NEJM199512283332604. [DOI] [PubMed] [Google Scholar]
- 71.Leitich H, Bodner-Adler B, Brunbauer M, Kaider A, Egarter C, Husslein P. Bacterial vaginosis as a risk factor for preterm delivery: a meta-analysis. Am J Obstet Gynecol. 2003 Jul;189(1):139–147. doi: 10.1067/mob.2003.339. [DOI] [PubMed] [Google Scholar]
- 72.Klebanoff MA, Hillier SL, Nugent RP, MacPherson CA, Hauth JC, Carey JC, et al. Is bacterial vaginosis a stronger risk factor for preterm birth when it is diagnosed earlier in gestation? Am J Obstet Gynecol. 2005 Feb;192(2):470–477. doi: 10.1016/j.ajog.2004.07.017. [DOI] [PubMed] [Google Scholar]
- 73.Martius J, Eschenbach DA. The role of bacterial vaginosis as a cause of amniotic fluid infection, chorioamnionitis and prematurity--a review. Arch Gynecol Obstet. 1990;247(1):1–13. doi: 10.1007/BF02390649. [DOI] [PubMed] [Google Scholar]
- 74.Fredricks DN, Fiedler TL, Marrazzo JM. Molecular Identification of Bacteria Associated with Bacterial Vaginosis. N Engl J Med. 2005 Nov 3;353(18):1899–1911. doi: 10.1056/NEJMoa043802. 2005. [DOI] [PubMed] [Google Scholar]
- 75.Verstraelen H, Verhelst R, Roelens K, Claeys G, Weyers S, De Backer E, et al. Modified classification of Gram-stained vaginal smears to predict spontaneous preterm birth: a prospective cohort study. Am J Obstet Gynecol. 2007 Jun;196(6):528 e1–528 e6. doi: 10.1016/j.ajog.2006.12.026. [DOI] [PubMed] [Google Scholar]
- 76.Culhane JF, Rauh V, McCollum KF, Hogan VK, Agnew K, Wadhwa PD. Maternal stress is associated with bacterial vaginosis in human pregnancy. Matern Child Health J. 2001 Jun;5(2):127–134. doi: 10.1023/a:1011305300690. [DOI] [PubMed] [Google Scholar]
- 77.Cristiano L, Rampello S, Noris C, Valota V. Bacterial vaginosis: prevalence in an Italian population of asymptomatic pregnant women and diagnostic aspects. Eur J Epidemiol. 1996 Aug;12(4):383–390. doi: 10.1007/BF00145302. [DOI] [PubMed] [Google Scholar]
- 78.Kurki T, Sivonen A, Renkonen O-V, Savia E, Ylikorkala O. Bacterial vaginosis in early pregnancy and pregnancy outcome. Obstet Gynecol. 1992;80:173–177. [PubMed] [Google Scholar]
- 79.Goldenberg RL, Klebanoff MA, Nugent R, Krohn MA, Hillier S, Andrews WW. Bacterial colonization of the vagina during pregnancy in four ethnic groups. American Journal of Obstetrics and Gynecology. 1996;174:1618–1621. doi: 10.1016/s0002-9378(96)70617-8. [DOI] [PubMed] [Google Scholar]
- 80.Kekki M, Kurki T, Pelkonen J, Kurkinen-Raty M, Cacciatore B, Paavonen J. Vaginal clindamycin in preventing preterm birth and peripartal infections in asymptomatic women with bacterial vaginosis: a randomized, controlled trial. Obstet Gynecol. 2001 May;97(5 Pt 1):643–648. doi: 10.1016/s0029-7844(01)01321-7. [DOI] [PubMed] [Google Scholar]
- 81.Carey J, Klebanoff M, Hauth J, et al. Metronidazole to prevent preterm delivery in pregnant women with asymptomatic bacterial vaginosis. National Institute of Child Health and Human Development Network of Maternal and Fetal Medicine Units. N Engl J Med. 2000;342:534–540. doi: 10.1056/NEJM200002243420802. [DOI] [PubMed] [Google Scholar]
- 82.Leitich H, Brunbauer M, Bodner-Adler B, Kaider A, Egarter C, Husslein P. Antibiotic treatment of bacterial vaginosis in pregnancy: a meta-analysis. Am J Obstet Gynecol. 2003 Mar;188(3):752–758. doi: 10.1067/mob.2003.167. [DOI] [PubMed] [Google Scholar]
- 83.Ugwumadu A, Manyonda I, Reid F, Hay P. Effect of early oral clindamycin on late miscarriage and preterm delivery in asymptomatic women with abnormal vaginal flora and bacterial vaginosis: a randomised controlled trial. Lancet. 2003 Mar 22;361(9362):983–988. doi: 10.1016/S0140-6736(03)12823-1. [DOI] [PubMed] [Google Scholar]
- 84.Klebanoff M, Carey J, Hauth J, Hillier S, Nugent R, Thom E, et al. Failure of metronidazole to prevent preterm delivery among pregnant women with asymptomatic trichomonas vaginalis infection. N Engl J Med. 2001;345:487–493. doi: 10.1056/NEJMoa003329. [DOI] [PubMed] [Google Scholar]
- 85.Hillier SL, Nugent RP, Eschenbach DA, Krohn MA, Gibbs RS, Martin DH, et al. Association between Bacterial Vaginosis and Preterm Delivery of a Low-Birth-Weight Infant. N Engl J Med. 1995 Dec 28;333(26):1737–1742. doi: 10.1056/NEJM199512283332604. 1995. [DOI] [PubMed] [Google Scholar]
- 86.Fiscella K, Franks P, Kendrick J, Bruce F. The risk of low birth weight associated with vaginal douching. Obstetrics and Gynecology. 1998;92:913–917. doi: 10.1016/s0029-7844(98)00325-1. [DOI] [PubMed] [Google Scholar]
- 87.Fiscella K, Franks P, Kendrick J, Meldrum M, Kieke B. Risk of preterm birth that is associated with vaginal douching. Am J Obstet Gynecol. 2002;186:1345–1350. doi: 10.1067/mob.2002.122406. [DOI] [PubMed] [Google Scholar]
- 88.Misra DP, Trabert B. Vaginal douching and risk of preterm birth among African American women. Am J Obstet Gynecol. 2007 Feb;196(2):140 e1–140 e8. doi: 10.1016/j.ajog.2006.10.880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cauci S, Culhane JF. Modulation of vaginal immune response among pregnant women with bacterial vaginosis by Trichomonas vaginalis, Chlamydia trachomatis, Neisseria gonorrhoeae, and yeast. Am J Obstet Gynecol. 2007 Feb;196(2):133 e1–133 e7. doi: 10.1016/j.ajog.2006.08.033. [DOI] [PubMed] [Google Scholar]
- 90.Cauci S, Hitti J, Noonan C, Agnew K, Quadrifoglio F, Hillier SL, et al. Vaginal hydrolytic enzymes, immunoglobulin A against Gardnerella vaginalis toxin, and risk of early preterm birth among women in preterm labor with bacterial vaginosis or intermediate flora. Am J Obstet Gynecol. 2002 Oct;187(4):877–881. doi: 10.1067/mob.2002.127454. [DOI] [PubMed] [Google Scholar]
- 91.Howe L, Wiggins R, Soothill PW, Millar MR, Horner PJ, Corfield AP. Mucinase and sialidase activity of the vaginal microflora: implications for the pathogenesis of preterm labour. Int J STD AIDS. 1999 Jul;10(7):442–447. doi: 10.1258/0956462991914438. [DOI] [PubMed] [Google Scholar]
- 92.Bennett WA, Terrone DA, Rinehart BK, Kassab S, Martin JN, Jr, Granger JP. Intrauterine endotoxin infusion in rat pregnancy induces preterm delivery and increases placental prostaglandin F2alpha metabolite levels. Am J Obstet Gynecol. 2000 Jun;182(6):1496–1501. doi: 10.1067/mob.2000.106848. [DOI] [PubMed] [Google Scholar]
- 93.Celik H, Ayar A. Effects of erythromycin on pregnancy duration and birth weight in lipopolysaccharide-induced preterm labor in pregnant rats. Eur J Obstet Gynecol Reprod Biol. 2002 Jun 10;103(1):22–25. doi: 10.1016/s0301-2115(02)00018-0. [DOI] [PubMed] [Google Scholar]
- 94.Briselden AM, Moncla BJ, Stevens CE, Hillier SL. Sialidases (neuraminidases) in bacterial vaginosis and bacterial vaginosis-associated microflora. J Clin Microbiol. 1992 Mar;30(3):663–666. doi: 10.1128/jcm.30.3.663-666.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Cauci S, McGregor J, Thorsen P, Grove J, Guaschino S. Combination of vaginal pH with vaginal sialidase and prolidase activities for prediction of low birth weight and preterm birth. Am J Obstet Gynecol. 2005 Feb;192(2):489–496. doi: 10.1016/j.ajog.2004.07.023. [DOI] [PubMed] [Google Scholar]
- 96.Larsson P-G, Stray-Pedersen B, Ryttig K, Larsen S. Human lactobacilli as supplementation of clindamycin to patients with bacterial vaginosis reduce the recurrence rate; a 6-month, double-blind, randomized, placebo-controlled study. BMC Women's Health. 2008;8(1):3. doi: 10.1186/1472-6874-8-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhou X, Brown CJ, Abdo Z, Davis CC, Hansmann MA, Joyce P, et al. Differences in the composition of vaginal microbial communities found in healthy Caucasian and black women. The ISME Journal. 2007;1:121–133. doi: 10.1038/ismej.2007.12. [DOI] [PubMed] [Google Scholar]
- 98.Boggess KA, Beck JD, Murtha AP, Moss K, Offenbacher S. Maternal periodontal disease in early pregnancy and risk for a small-for-gestational-age infant. Am J Obstet Gynecol . 2006 May;194(5):1316–1322. doi: 10.1016/j.ajog.2005.11.059. [DOI] [PubMed] [Google Scholar]
- 99.Boggess KA. Pathophysiology of preterm birth: emerging concepts of maternal infection. Clin Perinatol. 2005 Sep;32(3):561–569. doi: 10.1016/j.clp.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 100.Dasanayake AP, Russell S, Boyd D, Madianos PN, Forster T, Hill E. Preterm low birth weight and periodontal disease among African Americans. Dent Clin N Am. 2003 Jan;47(1):115–125. doi: 10.1016/s0011-8532(02)00056-3. [DOI] [PubMed] [Google Scholar]
- 101.Metlay J, Elovitz M, Macones G, Parry S, Jeffcoat M, Clothier B, et al. Periodontal disease and adverse pregnancy outcomes: is there an association? Am j obstet gynecol. 2009;200(5):497–498. doi: 10.1016/j.ajog.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 102.Vergnes JN, Sixou M. Preterm low birth weight and maternal periodontal status: a meta-analysis. Am J Obstet Gynecol. 2007 Feb;196(2):135 e1–135 e7. doi: 10.1016/j.ajog.2006.09.028. [DOI] [PubMed] [Google Scholar]
- 103.Lin D, Moss K, Beck JD, Hefti A, Offenbacher S. Persistently high levels of periodontal pathogens associated with preterm pregnancy outcome. J Periodontol. 2007 May;78(5):833–841. doi: 10.1902/jop.2007.060201. [DOI] [PubMed] [Google Scholar]
- 104.Marsh PD, Percival RS. The oral microflora – friend or foe? Can we decide? Internat dent j. 2006;56:233–239. doi: 10.1111/j.1875-595x.2006.tb00107.x. [DOI] [PubMed] [Google Scholar]
- 105.Paster BJ, Olsen I, Aas JA, Dewhirst FE. The breadth of bacterial diversity in the human periodontal pocket and other oral sites. Periodontol 2000. 2006;42:80–87. doi: 10.1111/j.1600-0757.2006.00174.x. [DOI] [PubMed] [Google Scholar]
- 106.Kumar PS, Griffen AL, Moeschberger ML, Leys EJ. Identification of candidate periodontal pathogens and beneficial species by quantitative 16S clonal analysis. J Clin Microbiol. 2005 Aug;43(8):3944–3955. doi: 10.1128/JCM.43.8.3944-3955.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.de Lillo A, Ashley FP, Palmer RM, Munson MA, Kyriacou L, Weightman AJ, et al. Novel subgingival bacterial phylotypes detected using multiple universal polymerase chain reaction primer sets. Oral Microbiol Immunol. 2006 Feb;21(1):61–68. doi: 10.1111/j.1399-302X.2005.00255.x. [DOI] [PubMed] [Google Scholar]
- 108.Siqueira JF, Jr, Rocas IN, Paiva SS, Magalhaes KM, Guimaraes-Pinto T. Cultivable bacteria in infected root canals as identified by 16S rRNA gene sequencing. Oral Microbiol Immunol. 2007 Aug;22(4):266–271. doi: 10.1111/j.1399-302X.2007.00355.x. [DOI] [PubMed] [Google Scholar]
- 109.Zuger J, Luthi-Schaller H, Gmur R. Uncultivated Tannerella BU045 and BU063 are slim segmented filamentous rods of high prevalence but low abundance in inflammatory disease-associated dental plaques. Microbiology. 2007 Nov;153(Pt 11):3809–3816. doi: 10.1099/mic.0.2007/010926-0. [DOI] [PubMed] [Google Scholar]
- 110.Schenkein HA, Burmeister JA, Koertge TE, Brooks CN, Best AM, Moore LV, et al. The influence of race and gender on periodontal microflora. J Periodontol. 1993 Apr;64(4):292–296. doi: 10.1902/jop.1993.64.4.292. [DOI] [PubMed] [Google Scholar]
- 111.Xiong X, Buekens P, Fraser WD, Beck J, Offenbacher S. Periodontal disease and adverse pregnancy outcomes: a systematic review. Br J Obstet Gynecol. 2006 Feb;113(2):135–143. doi: 10.1111/j.1471-0528.2005.00827.x. [DOI] [PubMed] [Google Scholar]
- 112.Michalowicz BSH, JS, DiAngelis AJ, Lupo VR, Novak MJ, Ferguson JE, Buchanan W, Bofill J, Papapanou PN, Mitchell DA, Matseoane S, Tschida PA. Treatment of Periodontal Disease and The Risk of Preterm Birth. N Engl J Med. 2006;355(18):1885–1894. doi: 10.1056/NEJMoa062249. 2006. [DOI] [PubMed] [Google Scholar]
- 113.Casazza G, Cortinovis I, Tsappi M, Tzioras S, Mauri D, Polyzos I, et al. Effect of periodontal disease treatment during pregnancy on preterm birth incidence: a metaanalysis of randomized trials. A j obstet gynecol. 2009;200(3):225–232. doi: 10.1016/j.ajog.2008.09.020. [DOI] [PubMed] [Google Scholar]
- 114.Buduneli N, Baylas H, Buduneli E, Turkoglu O, Kose T, Dahlen G. Periodontal infections and pre-term low birth weight: a case-control study. J Clin Periodontol. 2005 Feb;32(2):174–181. doi: 10.1111/j.1600-051X.2005.00670.x. [DOI] [PubMed] [Google Scholar]
- 115.Urban E, Radnai M, Novak T, Gorzo I, Pal A, Nagy E. Distribution of anaerobic bacteria among pregnant periodontitis patients who experience preterm delivery. Anaerobe. 2006;12(1):52–57. doi: 10.1016/j.anaerobe.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 116.Novak MJ, Novak KF, Hodges JS, Kirakodu S, Govindaswami M, DiAngelis A, et al. Periodontal Bacterial Profiles in Pregnant Women: Response to Treatment and Associations With Birth Outcomes in the Obstetrics and Periodontal Therapy (OPT) Study. J Periodontol. 2008;79(10):1870–1879. doi: 10.1902/jop.2008.070554. [DOI] [PubMed] [Google Scholar]
- 117.Madianos PN, Lieff S, Murtha AP, Boggess KA, Auten RL, Jr, Beck JD, et al. Maternal periodontitis and prematurity. Part II: Maternal infection and fetal exposure. Ann Periodontol. 2001 Dec;6(1):175–182. doi: 10.1902/annals.2001.6.1.175. [DOI] [PubMed] [Google Scholar]
- 118.Dasanayake AP, Russell S, Boyd D, Madianos PN, Forster T, Hill E. Preterm low birth weight and periodontal disease among African Americans. Dent Clin N Am 2003. 2003 Jan;47(1):115–125. doi: 10.1016/s0011-8532(02)00056-3. [DOI] [PubMed] [Google Scholar]
- 119.Jarjoura K, Devine PC, Perez-Delboy A, Herrera-Abreu M, D'Alton M, Papapanou PN. Markers of periodontal infection and preterm birth. Am J Obstet Gynecol. 2005 Feb;192(2):513–519. doi: 10.1016/j.ajog.2004.07.018. [DOI] [PubMed] [Google Scholar]
- 120.Varner MW, Esplin MS. Current understanding of genetic factors in preterm birth. BJOG: An International Journal of Obstetrics & Gynaecology. 2005 Mar;112 Suppl 1:28–31. doi: 10.1111/j.1471-0528.2005.00581.x. [DOI] [PubMed] [Google Scholar]
- 121.Ward K, Argyle V, Meade M, Nelson L. The heritability of preterm delivery. Obstet Gynecol. 2005 Dec;106(6):1235–1239. doi: 10.1097/01.AOG.0000189091.35982.85. [DOI] [PubMed] [Google Scholar]
- 122.Porter TF, Fraser AM, Hunter CY, Ward RH, Varner MW. The risk of preterm birth across generations. Obstet Gynecol. 1997 Jul 1;90(1):63–67. doi: 10.1016/S0029-7844(97)00215-9. 1997. [DOI] [PubMed] [Google Scholar]
- 123.Winkvist A, Mogren I, Hogberg U. Familial patterns in birth characteristics: impact on individual and population risks. Int J Epidemiol. 1998 Apr 1;27(2):248–254. doi: 10.1093/ije/27.2.248. 1998. [DOI] [PubMed] [Google Scholar]
- 124.Mercer BM, Goldenberg RL, Moawad AH, Meis PJ, Iams JD, Das AF, et al. The preterm prediction study: effect of gestational age and cause of preterm birth on subsequent obstetric outcome. National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Am J Obstet Gynecol. 1999 Nov;181(5 Pt 1):1216–1221. doi: 10.1016/s0002-9378(99)70111-0. [DOI] [PubMed] [Google Scholar]
- 125.Clausson B, Lichtenstein P, Cnattingius S. Genetic influence on birthweight and gestational length determined by studies in offspring of twins. Br J Obstet Gynecol. 2000;107(3):375–381. doi: 10.1111/j.1471-0528.2000.tb13234.x. [DOI] [PubMed] [Google Scholar]
- 126.Treloar SA, Macones GA, Mitchell LE, Martin NG. Genetic influences on premature parturition in an Australian twin sample. Twin Res. 2000 Jun;3(2):80–82. doi: 10.1375/136905200320565526. [DOI] [PubMed] [Google Scholar]
- 127.Cotten CM, Ginsburg GS, Goldberg RN, Speer MC. Genomic analyses: a neonatology perspective. Journal of Pediatrics. 2006 Jun;148(6):720–726. doi: 10.1016/j.jpeds.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 128.Hettne KM, Weeber M, Laine ML, Cate Ht, Boyer S, Kors JA, et al. Automatic mining of the literature to generate new hypotheses for the possible link between periodontitis and atherosclerosis: lipopolysaccharide as a case study. J Clin Periodontol. 2007;34(12):1016–1024. doi: 10.1111/j.1600-051X.2007.01152.x. [DOI] [PubMed] [Google Scholar]
- 129.Yoshie H, Kobayashi T, Tai H, Galicia JC. The role of genetic polymorphisms in periodontitis. Periodontol 2000. 2007;43(1):102–132. doi: 10.1111/j.1600-0757.2006.00164.x. [DOI] [PubMed] [Google Scholar]
- 130.Kilian M, Frandsen EVG, Haubek D, Poulsen K. The etiology of periodontal disease revisited by population genetic analysis. Periodontol 2000. 2006;42(1):158–179. doi: 10.1111/j.1600-0757.2006.00159.x. [DOI] [PubMed] [Google Scholar]
- 131.Genc MR, Schantz-Dunn J. The role of gene-environment interaction in predicting adverse pregnancy outcome. Best Pract Res Clin Obstet Gynaecol. 2007;21(3):491–504. doi: 10.1016/j.bpobgyn.2007.01.009. [DOI] [PubMed] [Google Scholar]
- 132.Cauci S, Di Santolo M, Casabellata G, Ryckman K, Williams SM, Guaschino S. Association of interleukin-1{beta} and interleukin-1 receptor antagonist polymorphisms with bacterial vaginosis in non-pregnant Italian women. Mol Hum Reprod. 2007 Apr 1;13(4):243–250. doi: 10.1093/molehr/gam002. 2007. [DOI] [PubMed] [Google Scholar]
- 133.Holst D, Garnier Y. Preterm birth and inflammation--The role of genetic polymorphisms. Eur J Obstet Gynecol Reprod Biol. 2008;141(1):3–9. doi: 10.1016/j.ejogrb.2008.07.020. [DOI] [PubMed] [Google Scholar]
- 134.Plunkett J, Muglia LJ. Genetic contributions to preterm birth: Implications from epidemiological and genetic association studies. Ann Med. 2008;40(3):167–179. doi: 10.1080/07853890701806181. [DOI] [PubMed] [Google Scholar]
- 135.González S, Rodrigo L, Martínez-Borra J, López-Vázquez A, Fuentes D, Niño P, et al. TNF-[alpha]-308A promoter polymorphism is associated with enhanced TNF-[alpha] production and inflammatory activity in Crohn's patients with fistulizing disease. Am J Gastroenterol. 2003;98(5):1101–1106. doi: 10.1111/j.1572-0241.2003.07416.x. [DOI] [PubMed] [Google Scholar]
- 136.Menon R, Merialdi M, Betrán AP, Dolan S, Jiang L, Fortunato SJ, et al. Analysis of association between maternal tumor necrosis factor-[alpha] promoter polymorphism (-308), tumor necrosis factor concentration, and preterm birth. Am J Obstet Gynecol. 2006;195(5):1240–1248. doi: 10.1016/j.ajog.2006.05.037. [DOI] [PubMed] [Google Scholar]
- 137.Matoba N, Yu Y, Mestan K, Pearson C, Ortiz K, Porta N, et al. Differential Patterns of 27 Cord Blood Immune Biomarkers Across Gestational Age. Pediatrics. 2009 May 1;123(5):1320–1328. doi: 10.1542/peds.2008-1222. 2009. [DOI] [PubMed] [Google Scholar]
- 138.Weiss A, Goldman S, Shalev E. The matrix metalloproteinases (MMPS) in the decidua and fetal membranes. Front Biosci. 2007;12:649–659. doi: 10.2741/2089. [DOI] [PubMed] [Google Scholar]
- 139.Menon R, Camargo MC, Thorsen P, Lombardi SJ, Fortunato SJ. Amniotic fluid interleukin-6 increase is an indicator of spontaneous preterm birth in white but not black Americans. Am J Obstet Gynecol. 2008;198(1):77.e1–77.e7. doi: 10.1016/j.ajog.2007.06.071. [DOI] [PubMed] [Google Scholar]
- 140.Menon R, Williams SM, Fortunato SJ. Amniotic Fluid Interleukin-1{beta} and Interleukin-8 Concentrations: Racial Disparity in Preterm Birth. Reprod Sci. 2007 Apr 1;14(3):253–259. doi: 10.1177/1933719107301336. 2007. [DOI] [PubMed] [Google Scholar]
- 141.Menon R, Williams S, Lombardi S, Thorsen P, Fortunato S, Velez D. Spontaneous preterm birth in African Americans is associated with infection and inflammatory response gene variants. Am j obstet gynecol. 2009;200(2):209–227. doi: 10.1016/j.ajog.2008.08.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Fujimoto T, Parry S, Urbanek M, Sammel M, Macones G, Kuivaniemi H, et al. A single nucleotide polymorphism in the matrix metalloproteinase-1 (MMP-1) promoter influences amnion cell MMP-1 expression and risk for preterm premature rupture of the fetal membranes. J Biol Chem. 2002 Feb 22;277(8):6296–6302. doi: 10.1074/jbc.M107865200. [DOI] [PubMed] [Google Scholar]
- 143.Ferrand PE, Parry S, Sammel M, Macones GA, Kuivaniemi H, Romero R, et al. A polymorphism in the matrix metalloproteinase-9 promoter is associated with increased risk of preterm premature rupture of membranes in African Americans. Molr Hum Reprod. 2002 May;8(5):494–501. doi: 10.1093/molehr/8.5.494. [DOI] [PubMed] [Google Scholar]
- 144.Havemose-Poulsen A, Sorensen LK, Stolte K, Bendtzen K, Holmstrup P. Cytokine profiles in peripheral blood and whole blood cell cultures associated with aggressive periodontitis, juvenile idiopathic arthritis, and rheumatoid arthritis. J Periodontol. 2005;76:2276–2285. doi: 10.1902/jop.2005.76.12.2276. [DOI] [PubMed] [Google Scholar]
- 145.Kurtis B, Tuter G, Serdar M, Akdemir P, Uygur C, Firatli E, Bal B. Gingival crevicular fluid levels of monocyte chemoattractant protein-1 and tumor necrosis factor-alpha in patients with chronic and aggressive periodontitis. J Periodontol. 2005;76:1849–1855. doi: 10.1902/jop.2005.76.11.1849. [DOI] [PubMed] [Google Scholar]
- 146.Liu H, Redline RW, Han YW. Fusobacterium nucleatum induces fetal death in mice via stimulation of TLR4-mediated placental inflammatory response. J Immunol. 2007 Aug 15;179(4):2501–2508. doi: 10.4049/jimmunol.179.4.2501. [DOI] [PubMed] [Google Scholar]
- 147.Bodet C, Andrian E, Tanabe S, Grenier D. Actinobacillus actinomycetemcomitans lipopolysaccharide regulates matrix metalloproteinase, tissue inhibitors of matrix metalloproteinase, and plasminogen activator production by human gingival fibroblasts: a potential role in connective tissue destruction. J Cell Physiol. 2007 Jul;212(1):189–194. doi: 10.1002/jcp.21018. [DOI] [PubMed] [Google Scholar]
- 148.Amory JH, Hitti J, Lawler R, Eschenbach DA. Increased tumor necrosis factor-alpha production after lipopolysaccharide stimulation of whole blood in patients with previous preterm delivery complicated by intra-amniotic infection or inflammation. Am J Obstet Gynecol. 2001 Nov;185(5):1064–1067. doi: 10.1067/mob.2001.117637. [DOI] [PubMed] [Google Scholar]
- 149.Offenbacher S, Moss K, Peters B, Wacker B, Barros SP, Arce RM. Increased TLR4 expression in murine placentas after oral infection with periodontal pathogens. Placenta. 2009;30(2):156–162. doi: 10.1016/j.placenta.2008.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Macones GA, Parry S, Elkousy M, Clothier B, Ural SH, Strauss JF., 3rd A polymorphism in the promoter region of TNF and bacterial vaginosis: preliminary evidence of gene-environment interaction in the etiology of spontaneous preterm birth. Am J Obstet Gynecol. 2004 Jun;190(6):1504–1508. doi: 10.1016/j.ajog.2004.01.001. discussion 3A. [DOI] [PubMed] [Google Scholar]
- 151.Menon R, Velez DR, Simhan H, Ryckman K, Jiang L, Thorsen P, et al. Multilocus interactions at maternal tumor necrosis factor-[alpha], tumor necrosis factor receptors, interleukin-6 and interleukin-6 receptor genes predict spontaneous preterm labor in European-American women. Am J Obstet Gynecol. 2006;194(6):1616–1624. doi: 10.1016/j.ajog.2006.03.059. [DOI] [PubMed] [Google Scholar]
- 152.Gravett MG, Adams KM, Sadowsky DW, Grosvenor AR, Witkin SS, Axthelm MK, et al. Immunomodulators plus antibiotics delay preterm delivery after experimental intraamniotic infection in a nonhuman primate model. Am J Obstet Gynecol. 2007;197(5):518.e1–518.e8. doi: 10.1016/j.ajog.2007.03.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Persson R, Hitti J, Verhelst R, Vaneechoutte M, Persson R, Hirschi R, et al. The vaginal microflora in relation to gingivitis. BMC Infect Dis. 2009;9(1):6. doi: 10.1186/1471-2334-9-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Harville EWZJ, Hatch MC. Oral sex and gum disease. Sex Transm Infect. 2004;80(5):418–419. doi: 10.1136/sti.2003.009027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Dixon NG, Ebright D, Defrancesco MA, Hawkins RE. Orogenital contact: a cause of chorioamnionitis? Obstet Gynecol. 1994 Oct;84(4 Pt 2):654–655. [PubMed] [Google Scholar]
- 156.Hitti J, Hillier SL, Agnew KJ, Krohn MA, Reisner DP, Eschenbach DA. Vaginal indicators of amniotic fluid infection in preterm labor. Obstet Gynecol. 2001 Feb;97(2):211–219. doi: 10.1016/s0029-7844(00)01146-7. [DOI] [PubMed] [Google Scholar]
- 157.Krohn MA, Hillier SL, Nugent RP, Cotch MF, Carey JC, Gibbs RS, et al. The genital flora of women with intraamniotic infection. Vaginal Infection and Prematurity Study Group. J Infect Dis. 1995 Jun;171(6):1475–1480. doi: 10.1093/infdis/171.6.1475. [DOI] [PubMed] [Google Scholar]
- 158.Chaim W, Mazor M, Wiznitzer A. The prevalence and clinical significance of intraamniotic infection with Candida species in women with preterm labor. Arch Gynecol Obstet. 1992;251(1):9–15. doi: 10.1007/BF02718273. [DOI] [PubMed] [Google Scholar]
- 159.Alanen A, Laurikainen E. Second-trimester abortion caused by Capnocytophaga sputigena: case report. Am J Perinatol. 1999;16(4):181–183. doi: 10.1055/s-2007-993854. [DOI] [PubMed] [Google Scholar]
- 160.Andres MT, Martin MC, Fierro JF, Mendez FJ. Chorioamnionitis and neonatal septicaemia caused by Eikenella corrodens. J Infect. 2002 Feb;44(2):133–134. doi: 10.1053/jinf.2001.0871. [DOI] [PubMed] [Google Scholar]
- 161.Kostadinov S, Pinar H. Amniotic fluid infection syndrome and neonatal mortality caused by Eikenella corrodens. Pediatr Dev Pathol. 2005 Jul-Aug;8(4):489–492. doi: 10.1007/s10024-005-0010-2. [DOI] [PubMed] [Google Scholar]
- 162.Bearfield C, Davenport ES, Sivapathasundaram V, Allaker RP. Possible association between amniotic fluid micro-organism infection and microflora in the mouth. Br J Obstet Gynecol. 2002 May;109(5):527–533. doi: 10.1111/j.1471-0528.2002.01349.x. [DOI] [PubMed] [Google Scholar]
- 163.Shukla SK, Meier PR, Mitchell PD, Frank DN, Reed KD. Leptotrichia amnionii sp. nov., a novel bacterium isolated from the amniotic fluid of a woman after intrauterine fetal demise. J Clin Microbiol. 2002 Sep;40(9):3346–3349. doi: 10.1128/JCM.40.9.3346-3349.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Miralles R, Hodge R, McParland PC, Field DJ, Bell SC, Taylor DJ, et al. Relationship between antenatal inflammation and antenatal infection identified by detection of microbial genes by polymerase chain reaction. Pediatr Res. 2005 Apr;57(4):570–577. doi: 10.1203/01.PDR.0000155944.48195.97. [DOI] [PubMed] [Google Scholar]
- 165.Rabe LK, Winterscheid KK, Hillier SL. Association of viridans group streptococci from pregnant women with bacterial vaginosis and upper genital tract infection. J Clin Microbiol. 1988 Jun;26(6):1156–1160. doi: 10.1128/jcm.26.6.1156-1160.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Bearfield C, Davenport ES, Sivapathasundaram V, Allaker RP. Possible association between amniotic fluid micro-organism infection and microflora in the mouth. Br J Obstet Gynecol. 2002 May;109(5):527–533. doi: 10.1111/j.1471-0528.2002.01349.x. [DOI] [PubMed] [Google Scholar]
- 167.French JI, McGregor JA, Parker R. Readily treatable reproductive tract infections and preterm birth among black women. Am J Obstet Gynecol. 2006;194(6):1717–1726. doi: 10.1016/j.ajog.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 168.Smaill F, Vazquez JC. Antibiotics for asymptomatic bacteriuria in pregnancy. Cochrane Database Syst Rev. 2007;(2):CD000490. doi: 10.1002/14651858.CD000490.pub2. [DOI] [PubMed] [Google Scholar]
- 169.Gibbons RJ, Socransky SS, de Araujo WC, van Houte J. Studies of the predominant cultivable microbiota of dental plaque. Arch Oral Biol. 1964;9(3):365–370. doi: 10.1016/0003-9969(64)90069-x. [DOI] [PubMed] [Google Scholar]
- 170.Boggess KA, Trevett TN, Madianos PN, Rabe L, Hillier SL, Beck J, et al. Use of DNA hybridization to detect vaginal pathogens associated with bacterial vaginosis among asymptomatic pregnant women. Am J Obstet Gynecol. 2005 Sep;193(3 Pt 1):752–756. doi: 10.1016/j.ajog.2005.01.068. [DOI] [PubMed] [Google Scholar]
- 171.Minkoff H, Grunebaum AN, Schwarz RH, Feldman J, Cummings M, Crombleholme W, et al. Risk factors for prematurity and premature rupture of membranes: a prospective study of the vaginal flora in pregnancy. Am J Obstet Gynecol. 1984 Dec 15;150(8):965–972. doi: 10.1016/0002-9378(84)90392-2. [DOI] [PubMed] [Google Scholar]
- 172.Germain M, Krohn MA, Hillier SL, Eschenbach DA. Genital flora in pregnancy and its association with intrauterine growth retardation. J Clin Microbiol. 1994 Sep;32(9):2162–2168. doi: 10.1128/jcm.32.9.2162-2168.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Socransky SS, Haffajee AD, Cugini MA, Smith C, Kent RL., Jr Microbial complexes in subgingival plaque. J Clin Periodontol. 1998 Feb;25(2):134–144. doi: 10.1111/j.1600-051x.1998.tb02419.x. [DOI] [PubMed] [Google Scholar]
- 174.Holst E, Goffeng AR, Andersch B. Bacterial vaginosis and vaginal microorganisms in idiopathic premature labor and association with pregnancy outcome. J Clin Microbiol. 1994 Jan;32(1):176–186. doi: 10.1128/jcm.32.1.176-186.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Kumar PS, Griffen AL, Moeschberger ML, Leys EJ. Identification of candidate periodontal pathogens and beneficial species by quantitative 16S clonal analysis. J Clin Microbiol. 2005 Aug;43(8):3944–3955. doi: 10.1128/JCM.43.8.3944-3955.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Koll-Klais P, Mandar R, Leibur E, Marcotte H, Hammarstrom L, Mikelsaar M. Oral lactobacilli in chronic periodontitis and periodontal health: species composition and antimicrobial activity. Oral Microbiol Immunol. 2005;20(6):354–361. doi: 10.1111/j.1399-302X.2005.00239.x. [DOI] [PubMed] [Google Scholar]
- 177.Antonio MA, Hawes SE, Hillier SL. The identification of vaginal Lactobacillus species and the demographic and microbiologic characteristics of women colonized by these species. J Infect Dis. 1999 Dec;180(6):1950–1956. doi: 10.1086/315109. [DOI] [PubMed] [Google Scholar]
- 178.Dasanayake AP, Li Y, Wiener H, Ruby JD, Lee M-J. Salivary Actinomyces naeslundii genospecies 2 and Lactobacillus casei levels predict pregnancy outcomes. Journal of Periodontology. 2005 Feb;76(2):171–177. doi: 10.1902/jop.2005.76.2.171. [DOI] [PubMed] [Google Scholar]
- 179.Kim YH, Kim CH, Cho MK, Na JH, Song TB, Oh JS. Hydrogen peroxide-producing Lactobacilli in the vaginal flora of pregnant women with preterm labor with intact membranes. Int J Gynaecol Obstet. 2006 Apr;93(1):22–27. doi: 10.1016/j.ijgo.2006.01.013. [DOI] [PubMed] [Google Scholar]
- 180.Vasquez A, Jakobsson T, Ahrne S, Forsum U, Molin G. Vaginal Lactobacillus flora of healthy Swedish women. J Clin Microbiol. 2002 Aug;40(8):2746–2749. doi: 10.1128/JCM.40.8.2746-2749.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Zhou X, Bent SJ, Schneider MG, Davis CC, Islam MR, Forney LJ. Characterization of vaginal microbial communities in adult healthy women using cultivation-independent methods. Microbiology. 2004 Aug;150(Pt 8):2565–2573. doi: 10.1099/mic.0.26905-0. [DOI] [PubMed] [Google Scholar]
- 182.Thies FL, Konig W, Konig B. Rapid characterization of the normal and disturbed vaginal microbiota by application of 16S rRNA gene terminal RFLP fingerprinting. J Med Microbiol. 2007 Jun 1;56(6):755–761. doi: 10.1099/jmm.0.46562-0. 2007. [DOI] [PubMed] [Google Scholar]
- 183.Finegold SM, Vaisanen M-L, Rautio M, Eerola E, Summanen P, Molitoris D, et al. Porphyromonas uenonis sp. nov., a pathogen for humans distinct from P. asaccharolytica and P. endodontalis. J Clin Microbiol. 2004 Nov;42(11):5298–5301. doi: 10.1128/JCM.42.11.5298-5301.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Gomes BPFA, Jacinto RC, Pinheiro ET, Sousa ELR, Zaia AA, Ferraz CCR, et al. Porphyromonas gingivalis, Porphyromonas endodontalis, Prevotella intermedia and Prevotella nigrescens in endodontic lesions detected by culture and by PCR. Oral Microbiol Immunol. 2005 Aug;20(4):211–215. doi: 10.1111/j.1399-302X.2005.00214.x. [DOI] [PubMed] [Google Scholar]
- 185.Vaisanen ML, Kiviranta M, Summanen P, Finegold SM, Jousimies-Somer HR. Porphyromonas endodontalis-like organisms from extraoral sources. Clin Infect Dis . 1997 Sep;25(Suppl 2):S191–S193. doi: 10.1086/516223. [DOI] [PubMed] [Google Scholar]
- 186.Urban E, Radnai M, Novak T, Gorzo I, Pal A, Nagy E. Distribution of anaerobic bacteria among pregnant periodontitis patients who experience preterm delivery. Anaerobe. 2006 Feb;12(1):52–57. doi: 10.1016/j.anaerobe.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 187.Schwebke JR, Lawing LF. Prevalence of Mobiluncus spp among women with and without bacterial vaginosis as detected by polymerase chain reaction. Sex Transm Dis. 2001 Apr;28(4):195–199. doi: 10.1097/00007435-200104000-00002. [DOI] [PubMed] [Google Scholar]
- 188.Thilesen CM, Nicolaidis M, Lokebo JE, Falsen E, Jorde AT, Muller F. Leptotrichia amnionii, an emerging pathogen of the female urogenital tract. J Clin Microbiol. 2007 Jul;45(7):2344–2347. doi: 10.1128/JCM.00167-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Fredricks DN, Fiedler TL, Marrazzo JM. Molecular identification of bacteria associated with bacterial vaginosis.[see comment] N Engl J Med. 2005 Nov 3;353(18):1899–1911. doi: 10.1056/NEJMoa043802. [DOI] [PubMed] [Google Scholar]
- 190.Verhelst R, Verstraelen H, Claeys G, Verschraegen G, Delanghe J, Van Simaey L, et al. Cloning of 16S rRNA genes amplified from normal and disturbed vaginal microflora suggests a strong association between Atopobium vaginae, Gardnerella vaginalisand bacterial vaginosis. BMC Microbiol. 2004 Apr 21;4:16. doi: 10.1186/1471-2180-4-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Verstraelen H, Verhelst R, Claeys G, Temmerman M, Vaneechoutte M. Culture-independent analysis of vaginal microflora: the unrecognized association of Atopobium vaginae with bacterial vaginosis. Am J Obstet Gynecol. 2004 Oct;191(4):1130–1132. doi: 10.1016/j.ajog.2004.04.013. [DOI] [PubMed] [Google Scholar]
- 192.Bradshaw CS, Tabrizi SN, Fairley CK, Morton AN, Rudland E, Garland SM. The Association of Atopobium vaginae and Gardnerella vaginalis with Bacterial Vaginosis and Recurrence after Oral Metronidazole Therapy. J Infect Dis. 2006 Sep 15;194(6):828–836. doi: 10.1086/506621. 2006. [DOI] [PubMed] [Google Scholar]
- 193.Fredricks DN, Fiedler TL, Thomas KK, Oakley BB, Marrazzo JM. Targeted PCR for detection of vaginal bacteria associated with bacterial vaginosis. J Clin Microbiol. 2007 Oct;45(10):3270–3276. doi: 10.1128/JCM.01272-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Faveri M, Feres M, Shibli JA, Hayacibara RF, Hayacibara MM, de Figueiredo LC. Microbiota of the dorsum of the tongue after plaque accumulation: an experimental study in humans. J Periodontol. 2006 Sep;77(9):1539–1546. doi: 10.1902/jop.2006.050366. [DOI] [PubMed] [Google Scholar]
- 195.Piot P, Van Dyck E, Godts P, Vanderheyden J. The vaginal microbial flora in non-specific vaginitis. Eur J ClinMicrobiol. 1982 Oct;1(5):301–306. doi: 10.1007/BF02019976. [DOI] [PubMed] [Google Scholar]
- 196.Harville EW, Hatch MC, Zhang J. Perceived life stress and bacterial vaginosis. J Womens Health (Larchmt) 2005 Sep;14(7):627–633. doi: 10.1089/jwh.2005.14.627. [DOI] [PubMed] [Google Scholar]
- 197.Ruiz RJ, Fullerton J, Brown CE, Schoolfield J. Relationships of cortisol, perceived stress, genitourinary infections, and fetal fibronectin to gestational age at birth. Biol Res Nurs. 2001 Jul;3(1):39–48. doi: 10.1177/109980040100300106. [DOI] [PubMed] [Google Scholar]
- 198.Wadhwa PD, Culhane JF, Rauh V, Barve SS, Hogan V, Sandman CA, et al. Stress, infection and preterm birth: a biobehavioural perspective. Paediatr Perinat Epidemiol. 2001 Jul;15 Suppl 2:17–29. doi: 10.1046/j.1365-3016.2001.00005.x. [DOI] [PubMed] [Google Scholar]
- 199.Pistorius A, Krahwinkel T, Willershausen B, Boekstegen C. Relationship between stress factors and periodontal disease. Eur J Med Res. 2002 Sep 30;7(9):393–398. [PubMed] [Google Scholar]
- 200.Monteiro da Silva AM, Newman HN, Oakley DA, O'Leary R. Psychosocial factors, dental plaque levels and smoking in periodontitis patients. J Clin Periodontol. 1998 Jun;25(6):517–523. doi: 10.1111/j.1600-051x.1998.tb02481.x. [DOI] [PubMed] [Google Scholar]
- 201.Larsson PG, Fahraeus L, Carlsson B, Jakobsson T, Forsum U. Predisposing factors for bacterial vaginosis, treatment efficacy and pregnancy outcome among term deliveries; results from a preterm delivery study. BMC Womens Health. 2007;7:20. doi: 10.1186/1472-6874-7-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Koumans EH, Sternberg M, Bruce C, McQuillan G, Kendrick J, Sutton M, et al. The prevalence of bacterial vaginosis in the United States, 2001–2004; associations with symptoms, sexual behaviors, and reproductive health. Sex Transm Dis. 2007 Nov;34(11):864–869. doi: 10.1097/OLQ.0b013e318074e565. [DOI] [PubMed] [Google Scholar]
- 203.Hellberg D, Nilsson S, Mardh PA. Bacterial vaginosis and smoking. Int J STD AIDS. 2000 Sep;11(9):603–606. doi: 10.1258/0956462001916461. [DOI] [PubMed] [Google Scholar]
- 204.Fisher S, Kells L, Picard JP, Gelskey SC, Singer DL, Lix L, et al. Progression of Periodontal Disease in a Maintenance Population of Smokers and Non-Smokers: A 3-Year Longitudinal Study. J Periodontol. 2008 Mar;79(3):461–468. doi: 10.1902/jop.2008.070296. [DOI] [PubMed] [Google Scholar]
- 205.Pitiphat W, Joshipura KJ, Gillman MW, Williams PL, Douglass CW, Rich-Edwards JW. Maternal periodontitis and adverse pregnancy outcomes. Community Dent Oral Epidemiol. 2008 Feb;36(1):3–11. doi: 10.1111/j.1600-0528.2006.00363.x. [DOI] [PubMed] [Google Scholar]
- 206.Kibayashi M, Tanaka M, Nishida N, Kuboniwa M, Kataoka K, Nagata H, et al. Longitudinal study of the association between smoking as a periodontitis risk and salivary biomarkers related to periodontitis. J Periodontol. 2007 May;78(5):859–867. doi: 10.1902/jop.2007.060292. [DOI] [PubMed] [Google Scholar]
- 207.Duncan HF, Pitt Ford TR. The potential association between smoking and endodontic disease. Int Endod J. 2006 Nov;39(11):843–854. doi: 10.1111/j.1365-2591.2006.01141.x. [DOI] [PubMed] [Google Scholar]
- 208.Tomar SL, Asma S. Smoking-attributable periodontitis in the United States: findings from NHANES III. National Health and Nutrition Examination Survey. J Periodontol. 2000 May;71(5):743–751. doi: 10.1902/jop.2000.71.5.743. [DOI] [PubMed] [Google Scholar]
- 209.Palmer RM, Wilson RF, Hasan AS, Scott DA. Mechanisms of action of environmental factors--tobacco smoking. J Clin Periodontol. 2005;32 Suppl 6:180–195. doi: 10.1111/j.1600-051X.2005.00786.x. [DOI] [PubMed] [Google Scholar]
- 210.Ahluwalia N, Grandjean H. Nutrition, an under-recognized factor in bacterial vaginosis. J Nutr. 2007 Sep;137(9):1997–1998. doi: 10.1093/jn/137.9.1997. [DOI] [PubMed] [Google Scholar]
- 211.Neggers YH, Nansel TR, Andrews WW, Schwebke JR, Yu KF, Goldenberg RL, et al. Dietary intake of selected nutrients affects bacterial vaginosis in women. J Nutr. 2007 Sep;137(9):2128–2133. doi: 10.1093/jn/137.9.2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Enwonwu CO, Sanders C. Nutrition: impact on oral and systemic health. Compend Contin Educ Dent. 2001 Jul;22(3 Spec No):12–18. [PubMed] [Google Scholar]
- 213.Ness RB, Hillier S, Richter HE, Soper DE, Stamm C, Bass DC, et al. Can known risk factors explain racial differences in the occurrence of bacterial vaginosis? J Natl Med Assoc. 2003 Mar;95(3):201–212. [PMC free article] [PubMed] [Google Scholar]
- 214.Simhan HN, Bodnar LM, Krohn MA. Paternal race and bacterial vaginosis during the first trimester of pregnancy. Am J Obstet Gynecol. 2008 Feb;198(2):196 e1–196 e4. doi: 10.1016/j.ajog.2007.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Craig RG, Boylan R, Yip J, Bamgboye P, Koutsoukos J, Mijares D, et al. Prevalence and risk indicators for destructive periodontal diseases in 3 urban American minority populations. J Clin Periodontol. 2001 Jun;28(6):524–535. doi: 10.1034/j.1600-051x.2001.028006524.x. [DOI] [PubMed] [Google Scholar]
- 216.Borrell LN, Burt BA, Warren RC, Neighbors HW. The role of individual and neighborhood social factors on periodontitis: the third National Health and Nutrition Examination Survey. J Periodontol. 2006 Mar;77(3):444–453. doi: 10.1902/jop.2006.050158. [DOI] [PubMed] [Google Scholar]
- 217.Borrell LN, Lynch J, Neighbors H, Burt BA, Gillespie BW. Is there homogeneity in periodontal health between African Americans and Mexican Americans? Ethn Dis. 2002 Winter;12(1):97–110. [PubMed] [Google Scholar]
- 218.Strus M, Malinowska M, Heczko PB. In vitro antagonistic effect of Lactobacillus on organisms associated with bacterial vaginosis. J Reprod Med. 2002 Jan;47(1):41–46. [PubMed] [Google Scholar]
- 219.Hillier SL, Krohn MA, Rabe LK, Klebanoff SJ, Eschenbach DA. The normal vaginal flora, H2O2-producing lactobacilli, and bacterial vaginosis in pregnant women. Clin Infect Dis. 1993 Jun;16(Suppl 4):S273–S281. doi: 10.1093/clinids/16.supplement_4.s273. [DOI] [PubMed] [Google Scholar]
- 220.Puapermpoonsiri S, Kato N, Watanabe K, Ueno K, Chongsomchai C, Lumbiganon P. Vaginal microflora associated with bacterial vaginosis in Japanese and Thai pregnant women. Clin Infect Dis. 1996 Oct;23(4):748–752. doi: 10.1093/clinids/23.4.748. [DOI] [PubMed] [Google Scholar]
- 221.Riccia DN, Bizzini F, Perilli MG, Polimeni A, Trinchieri V, Amicosante G, et al. Anti-inflammatory effects of Lactobacillus brevis (CD2) on periodontal disease. Oral Dis. 2007 Jul;13(4):376–385. doi: 10.1111/j.1601-0825.2006.01291.x. [DOI] [PubMed] [Google Scholar]
- 222.Hojo K, Mizoguchi C, Taketomo N, Ohshima T, Gomi K, Arai T, et al. Distribution of salivary Lactobacillus and Bifidobacterium species in periodontal health and disease. Biosci Biotechnol Biochem. 2007 Jan;71(1):152–157. doi: 10.1271/bbb.60420. [DOI] [PubMed] [Google Scholar]
- 223.Cauci S. Vaginal Immunity in Bacterial Vaginosis. Curr Infect Dis Rep. 2004 Dec;6(6):450–456. doi: 10.1007/s11908-004-0064-8. [DOI] [PubMed] [Google Scholar]
- 224.Cherpes TL, Marrazzo JM, Cosentino LA, Meyn LA, Murray PJ, Hillier SL. Hormonal contraceptive use modulates the local inflammatory response to bacterial vaginosis. Sex Transm Infect. 2008 Feb;84(1):57–61. doi: 10.1136/sti.2007.026625. [DOI] [PubMed] [Google Scholar]
- 225.Cauci S, Guaschino S, De Aloysio D, Driussi S, De Santo D, Penacchioni P, et al. Interrelationships of interleukin-8 with interleukin-1beta and neutrophils in vaginal fluid of healthy and bacterial vaginosis positive women. Mol Hum Reprod. 2003 Jan;9(1):53–58. doi: 10.1093/molehr/gag003. [DOI] [PubMed] [Google Scholar]
- 226.Kinane DF, Lappin DF. Immune processes in periodontal disease: a review. Ann Periodontol. 2002 Dec;7(1):62–71. doi: 10.1902/annals.2002.7.1.62. [DOI] [PubMed] [Google Scholar]
- 227.Preshaw PM, Seymour RA, Heasman PA. Current concepts in periodontal pathogenesis. Dent Update. 2004 Dec;31(10):570–572. 4–8. doi: 10.12968/denu.2004.31.10.570. [DOI] [PubMed] [Google Scholar]
- 228.Tatakis DN, Kumar PS. Etiology and pathogenesis of periodontal diseases. Dent Clin North Am. 2005 Jul;49(3):491–516. doi: 10.1016/j.cden.2005.03.001. , v. [DOI] [PubMed] [Google Scholar]
- 229.Genc MR, Vardhana S, Delaney ML, Witkin SS, Onderdonk AB. TNFA-308G>A polymorphism influences the TNF-alpha response to altered vaginal flora. Eur J Obstet Gynecol Reprod Biol. 2007 Oct;134(2):188–191. doi: 10.1016/j.ejogrb.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 230.de Freitas N, Imbronito A, AC N, Nunes F, Pustiglioni FE, RFM L. Analysis of IL-1A(-889) and TNFA(-308) gene polymorphism in Brazilian patients with generalized aggressive periodontitis. Eur Cyt Net. 2007;18:142–147. doi: 10.1684/ecn.2007.0100. [DOI] [PubMed] [Google Scholar]
- 231.Schulz S, Machulla HKG, Altermann W, Klapproth J, Zimmermann U, Glaser C, et al. Genetic markers of tumour necrosis factor α in aggressive and chronic periodontitis. J Clin Periodontol. 2008;35(6):493–500. doi: 10.1111/j.1600-051X.2008.01226.x. [DOI] [PubMed] [Google Scholar]
- 232.Roberts AK, Monzon-Bordonaba F, Van Deerlin PG, Holder J, Macones GA, Morgan MA, et al. Association of polymorphism within the promoter of the tumor necrosis factor [alpha] gene with increased risk of preterm premature rupture of the fetal membranes. Am J Obstet Gynecol. 1999;180(5):1297–1302. doi: 10.1016/s0002-9378(99)70632-0. [DOI] [PubMed] [Google Scholar]
- 233.Babel N, Cherepnev G, Babel D, Tropmann A, Hammer M, Volk HD, et al. Analysis of tumor necrosis factor-alpha, transforming growth factor-beta, interleukin-10, IL-6, and interferon-gamma gene polymorphisms in patients with chronic periodontitis. J Periodontol. 2006 Dec;77(12):1978–1983. doi: 10.1902/jop.2006.050315. [DOI] [PubMed] [Google Scholar]
- 234.Tervonen T, Raunio T, Knuuttila M, Karttunen R. Polymorphisms in the CD14 and IL-6 genes associated with periodontal disease. Journal of Clinical Periodontology. 2007;34(5):377–383. doi: 10.1111/j.1600-051X.2007.01067.x. [DOI] [PubMed] [Google Scholar]
- 235.Simhan HN, Krohn MA, Roberts JM, Zeevi A, Caritis SN. Interleukin-6 promoter-174 polymorphism and spontaneous preterm birth. Am J Obstet Gynecol. 2003 Oct;189(4):915–918. doi: 10.1067/s0002-9378(03)00843-3. [DOI] [PubMed] [Google Scholar]
- 236.Gonzales JR, Mann M, Stelzig J, Bodeker RH, Meyle J. Single-nucleotide polymorphisms in the IL-4 and IL-13 promoter region in aggressive periodontitis. J Clini Periodontol. 2007;34(6):473–479. doi: 10.1111/j.1600-051X.2007.01086.x. [DOI] [PubMed] [Google Scholar]
- 237.Havemose-Poulsen A, Sorensen LK, Bendtzen K, Holmstrup P. Polymorphisms within the IL-1 gene cluster: effects on cytokine profiles in peripheral blood and whole blood cell cultures of patients with aggressive periodontitis, juvenile idiopathic arthritis, and rheumatoid arthritis. J Periodontol. 2007 Mar;78(3):475–492. doi: 10.1902/jop.2007.060135. [DOI] [PubMed] [Google Scholar]
- 238.Scarel-Caminaga RM, Trevilatto PC, Souza AP, Brito RB, Line SRP. Investigation of an IL-2 polymorphism in patients with different levels of chronic periodontitis. J Clin Periodontol. 2002;29(7):587–591. doi: 10.1034/j.1600-051x.2002.290701.x. [DOI] [PubMed] [Google Scholar]
- 239.Wilke C, Renz H, Tekesin I, Hellmeyer L, Herz U, Schmidt S. Suppression of IL-2 and IFN-gamma production in women with spontaneous preterm labor. J Perinat Med. 2006;34(1):20–27. doi: 10.1515/JPM.2006.003. [DOI] [PubMed] [Google Scholar]
- 240.Reichert S, Machulla HKG, Klapproth J, Zimmermann U, Reichert Y, Glaser CH, et al. The interleukin-10 promoter haplotype ATA is a putative risk factor for aggressive periodontitis. J Periodont Res. 2008;43(1):40–47. doi: 10.1111/j.1600-0765.2007.00992.x. [DOI] [PubMed] [Google Scholar]
- 241.Stonek F, Metzenbauer M, Hafner E, Philipp K, Tempfer C. Interleukin-10-1082 G/A promoter polymorphism and pregnancy complications: results of a prospective cohort study in 1,616 pregnant women. Acta Obstetricia et Gynecologica Scandinavica. 2008;87(4):430–433. doi: 10.1080/00016340801995657. [DOI] [PubMed] [Google Scholar]
- 242.Genc MR, Onderdonk AB, Vardhana S, Delaney ML, Norwitz ER, Tuomala RE, et al. Polymorphism in intron 2 of the interleukin-1 receptor antagonist gene, local midtrimester cytokine response to vaginal flora, and subsequent preterm birth. Am J Obstet Gynecol. 2004 Oct;191(4):1324–1330. doi: 10.1016/j.ajog.2004.05.074. [DOI] [PubMed] [Google Scholar]
- 243.Hooshmand B, Hajilooi M, Rafiei A, Mani-Kashani KH, Ghasemi R. Interleukin-4 (C-590T) and interferon-γ (G5644A) gene polymorphisms in patients with periodontitis. J Periodont Res. 2008;43(1):111–115. doi: 10.1111/j.1600-0765.2007.01006.x. [DOI] [PubMed] [Google Scholar]
- 244.Genc MR, Vardhana S, Delaney ML, Onderdonk A, Tuomala R, Norwitz E, et al. Relationship between a toll-like receptor-4 gene polymorphism, bacterial vaginosis-related flora and vaginal cytokine responses in pregnant women. Eur J Obstet Gynecol Reprod Biol. 2004 Oct 15;116(2):152–156. doi: 10.1016/j.ejogrb.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 245.James JA, Poulton KV, Haworth SE, Payne D, McKay IJ, Clarke FM, et al. Polymorphisms of TLR4 but not CD14 are associated with a decreased risk of aggressive periodontitis. Journal of Clinical Periodontology. 2007;34(2):111–117. doi: 10.1111/j.1600-051X.2006.01030.x. [DOI] [PubMed] [Google Scholar]
- 246.Lorenz E, Hallman M, Marttila R, Haataja, Schwartz D. Association between the Asp299Gly Polymorphisms in the Toll-like Receptor 4 and Premature Births in the Finnish. Population. 2002;52(3):373–376. doi: 10.1203/00006450-200209000-00011. [DOI] [PubMed] [Google Scholar]
- 247.Cao Z, Li C, Jin L, Corbet EF. Association of matrix metalloproteinase-1 promoter polymorphism with generalized aggressive periodontitis in a Chinese population. J Periodont Res. 2005;40(6):427–431. doi: 10.1111/j.1600-0765.2005.00806.x. [DOI] [PubMed] [Google Scholar]
- 248.de Souza AP, Trevilatto PC, Scarel-Caminaga RM, Brito RB, Line SRP. MMP-1 promoter polymorphism: association with chronic periodontitis severity in a Brazilian population. J Clin Periodontol. 2003;30(2):154–158. doi: 10.1034/j.1600-051x.2003.300202.x. [DOI] [PubMed] [Google Scholar]
- 249.Gurkan A, Emingil G, Saygan BH, Atilla G, C1narc1k S, Kose T, et al. Matrix Metalloproteinase-2, -9, and -12 Gene Polymorphisms in Generalized Aggressive Periodontitis. J Periodontol. 2007;78(12):2338–2347. doi: 10.1902/jop.2007.070148. [DOI] [PubMed] [Google Scholar]
- 250.Keles GC, Gunes S, Sumer AP, Sumer M, Kara N, Bagci H, et al. Association of Matrix Metalloproteinase-9 Promoter Gene Polymorphism With Chronic Periodontitis. J Periodontol. 2006;77(9):1510–1514. doi: 10.1902/jop.2006.050378. [DOI] [PubMed] [Google Scholar]
- 251.Ferrand P, Parry SSM, Macones GA, et al. A polymorphism in the matrix metalloproteinase-9 promoter is associated with increased risk of preterm premature rupture of membranes in African Americans. Mol Hum Reprod. 2002;8:494–501. doi: 10.1093/molehr/8.5.494. [DOI] [PubMed] [Google Scholar]
- 252.Diaz-Cueto L, Cuica-Flores A, Ziga-Cordero F, Ayala-Mendez JA, Tena-Alavez G, Dominguez-Lopez P, et al. Vaginal matrix metalloproteinase levels in pregnant women with bacterial vaginosis. J Soc Gynecol Investig. 2006 Sep;13(6):430–434. doi: 10.1016/j.jsgi.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 253.Beigi R, Yudin M, Cosentino L, Meyn L, Hillier S. Cytokines, Pregnancy, and Bacterial Vaginosis: Comparison of Levels of Cervical Cytokines in Pregnant and Nonpregnant Women with Bacterial Vaginosis. J Infect Dis. 2007;196(9):1355–1360. doi: 10.1086/521628. [DOI] [PubMed] [Google Scholar]
- 254.Mares D, Simoes JA, Novak RM, Spear GT. TLR2-mediated cell stimulation in bacterial vaginosis. J Reproduct Immunol. 2008;77(1):91–99. doi: 10.1016/j.jri.2007.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Michel J, Gonzales JR, Wunderlich D, Diete A, Herrmann JM, Meyle J. Interleukin-4 polymorphisms in early onset periodontitis. J Clin Periodontol. 2001 May;28(5):483–488. doi: 10.1034/j.1600-051x.2001.028005483.x. [DOI] [PubMed] [Google Scholar]
- 256.Gonzales JR, Kobayashi T, Michel J, Mann M, Yoshie H, Meyle J. Interleukin-4 gene polymorphisms in Japanese and Caucasian patients with aggressive periodontitis. J Clin Periodontol. 2004 May;31(5):384–389. doi: 10.1111/j.1600-051X.2004.00492.x. [DOI] [PubMed] [Google Scholar]
- 257.Hooshmand B, Hajilooi M, Rafiei A, Mani-Kashani KH, Ghasemi R. Interleukin-4 (C-590T) and interferon-gamma (G5644A) gene polymorphisms in patients with periodontitis. J Periodontal Res. 2008 Feb;43(1):111–115. doi: 10.1111/j.1600-0765.2007.01006.x. [DOI] [PubMed] [Google Scholar]
- 258.Kara N, Keles GC, Sumer P, Gunes SO, Bagci H, Koprulu H, et al. Association of the polymorphisms in promoter and intron regions of the interleukin-4 gene with chronic periodontitis in a Turkish population. Acta Odontol Scand. 2007 Oct;65(5):292–297. doi: 10.1080/00016350701644040. [DOI] [PubMed] [Google Scholar]
- 259.Menon R, Merialdi M, Betran AP, Dolan S, Jiang L, Fortunato SJ, et al. Analysis of association between maternal tumor necrosis factor-alpha promoter polymorphism (-308), tumor necrosis factor concentration, and preterm birth. Am J Obstet Gynecol. 2006 Nov;195(5):1240–1248. doi: 10.1016/j.ajog.2006.05.037. [DOI] [PubMed] [Google Scholar]
- 260.Astolfi CM, Shinohara AL, da Silva RA, Santos MCLG, Line SRP, de Souza AP. Genetic polymorphisms in the MMP-1 and MMP-3 gene may contribute to chronic periodontitis in a Brazilian population. Journal of Clinical Periodontology. 2006;33(10):699–703. doi: 10.1111/j.1600-051X.2006.00979.x. [DOI] [PubMed] [Google Scholar]