Abstract
Background
Traditional forms of cancer therapy, which includes chemotherapy, have largely been overhauled due to the significant degree of toxicity they pose to normal, otherwise healthy tissue. It is hoped that use of biological agents, most of which are endogenously present in the body, will lead to safer treatment outcomes, without sacrificing efficacy.
Objective
The finding that PEDF, a naturally-occurring protein, was a potent angiogenesis inhibitor became the basis for studying the role of PEDF in tumours that are highly resistant to chemotherapy. The determination of the direct role of PEDF against cancer paved the way for understanding and developing PEDF as a novel drug. This review focuses on the patent applications behind testing the anticancer therapeutic effect of PEDF via its receptors as an antiangiogenic agent and as a direct anticancer agent.
Conclusions
The majority of the PEDF patents describe its and/or its fragments’ antiangiogenic ability and the usage of recombinant vectors as the mode of treatment delivery. PEDF’s therapeutic potential against different diseases and the discovery of its receptors opens possibilities for improving PEDF-based peptide design and drug delivery modes.
Keywords: angiogenesis, cancer, drug, PEDF, PEDF receptor, therapy, patent
1. Introduction
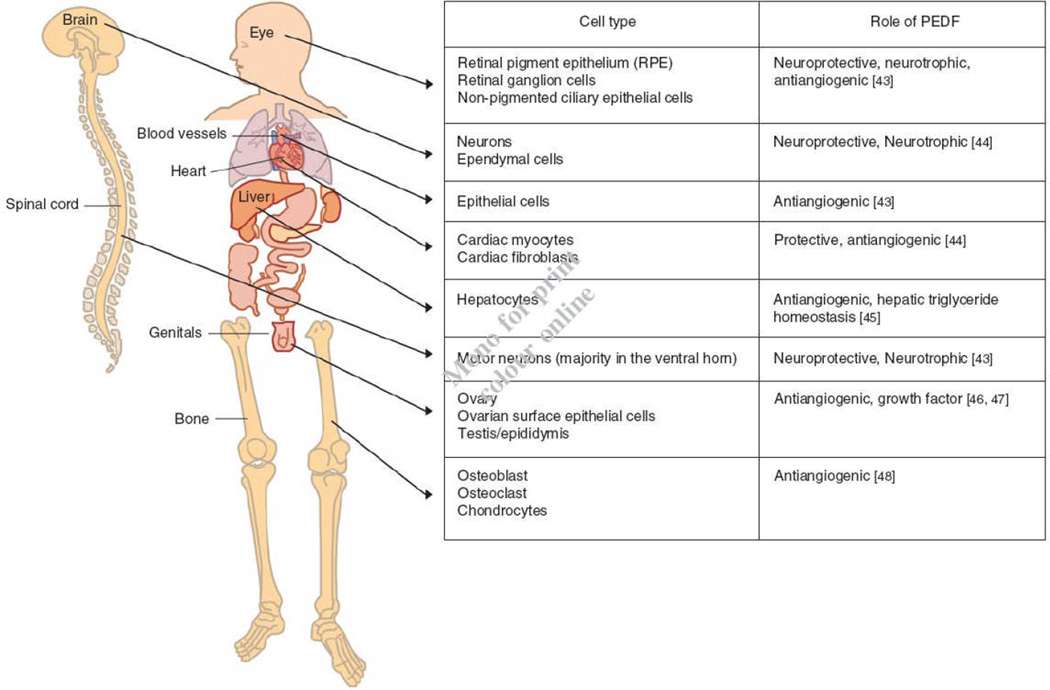
Pigment epithelium-derived factor (PEDF), a 50kDa glycoprotein, belongs to the serpin superfamily of proteins, which have common structural homology. Most of the serpin members are serine protease inhibitors like antitrypsin, plasmin inhibitor and antichymotrypsin. However, there is subgroup of serpins that lack protease inhibitory activity, and PEDF is classified as a non-inhibitory serpin [1]. It is thought that during evolution several non inhibitory serpins, including PEDF, had lost their protease inhibitory ability while at the same time gained other properties. In particular, PEDF does not undergo the serpin conformational change that inhibitory serpins do upon interacting with target proteases [1]. However, because PEDF is expressed by different cell lines (Figure 1), PEDF interacts with a variety of cellular components and its structure-function relationships have been revealed. PEDF is able to interact with multiple signalling cascades and molecular systems enabling PEDF as a multi-functional protein with therapeutic effects in several diseases, especially in different cancer types (Table 1).
Figure 1.
Main body distribution of PEDF.
Table 1.
Different molecular pathways targeted by PEDF in different types of diseases
| Molecular pathway | Function | Diseases involved | Role of PEDF |
|---|---|---|---|
| uPA/uPAR (urokinase plasminogen activator and its receptor) | The binding of uPA to uPAR activates proteolytic cascades resulting to blood vessel invasion of tumour cells leading in metastasis. Serpins plasminogen activator inhibitor-1 and -2 (PAI-1 and PAI-2) are known inhibitors of the interaction of uPA to uPAR. | Osteosarcoma | PEDF in combination with uPAR downregulation showed a decrease in osteosarcoma invasion, growth and metastasis, The presence of PEDF internalized the distribution of uPA/uPAR from the surface of the cell hence, reducing the ability of the osterosarcoma cells to migrate [10] |
| Prostate cancer | PAI-2 is upregulated by PEDF inhibiting the activity of uPA and uPAR [11] | ||
| Lung cancer | Reduced lung cancer cell adhesion and motility [11] | ||
| VEGF/VEGFR (vascular endothelial growth factor) | Overexpression generates leaky blood vessels allowing recruitment of endothelial cells forming new blood vessels, uneven distribution of oxygen and nutrients. For cancer, the effects of VEGF overexpression can influence tumour growth and survival, and can leak out delivered chemotherapeutic drugs. | Cardiovascular disease | PEDF inhibited VEGF-induced uPA/uPAR activation affecting vascular permeability [40] |
| Diabetic retinopathy | PEDF downregulated VEGF expression at the transcription level. PEDF was also shown to compete against VEGF in binding to VEGF receptor 2 [41] | ||
| Lung cancer, prostate cancer, | PEDF resulted in a reduced tumour microvessel density, and reduced tumour growth and weight [11] | ||
| Melanoma | Tumour growth, tumour cell survival and microvessel density is decreased [11] | ||
| Pancreatic cancer | Microvessel density is decreased, tumour growth is inhibited and the proliferation and migration of endothelial cells decreased [11] | ||
| Fas/FasL | Fas ligand when bound to Fas creates an activator complex activating caspase 8, The activation of caspase 8 and its release in the cytosol allows the activation of other caspases, which leads to cell apoptosis. | Melanoma | PEDF lead to increased levels of apoptosis which was prevented after the addition of neutralizing FasL antibodies [11] |
| p53/PPARγ | p53 is a tumour suppressor protein which, upon signals of DNA damage, is involved in arresting cell cycling activity or initiates apoptosis. PPARγ, a transcription factor, which upon activation by its ligand, 15d-PGJ2, activates caspase-mediated endothelial cell apoptosis. | Choroidal neovascularisation | PEDF increased the expression of PPARγ in human umbilical vein endothelial cells (HUVECs) initiating endothelial cell apoptosis and upon the inhibition of PPARγ activity using PPARγ antagonists, abolished the apoptotic effect of PEDF. PEDF also induced overexpression of p53 for apoptosis. Inhibition of either PPARγ or p53 attenuates apoptosis [23] |
| c-jun N-terminal kinase (JNK) | JNK responses to inflammatory cytokines, growth factors and environmental stress. JNK plays a role in cell differentiation, apoptosis, and inflammation, to name a few. It modifies proteins acting in the nucleus or in the mitochondria to regulate cell function and protein synthesis. | Neovascularization | PEDF regulated the expression of JNK leading to the apoptosis of endothelial cell inhibiting neovascularisation and tumour growth [42] |
| Obesity | PEDF activated JNK in the muscle and liver resulting to insulin expression inhibition [43] |
Tombran-Tink and Johnson [2] first discovered that media conditioned by retinal pigment epithelial cell induced Y79 retinoblastoma tumour cell differentiation into a non-proliferating type with an increase in neurite outgrowth in the neuronal morphology of the tumour cells. Then Tombran-Tink, Chader and Johnson [3] identified the protein responsible for such activity in the retinal pigment epithelial conditioned media and termed it PEDF. This marked the role of PEDF as a neurotrophic factor, where apart from playing a role in changing the morphology of the cells; PEDF was also shown to protect spinal cord motor neurons and immature cerebellar granule cells from degeneration and apoptosis as well as photoreceptors from light damage [4–7]. Soon after, PEDF was defined as the most potent inhibitor for angiogenesis among other well-characterised antiangiogenic factors like angiostatin and endostatin [8]. It was demonstrated that the action of PEDF as to whether it will act as a neurotrophic agent or an antiangiogenic agent reflects on the phosphorylation state of PEDF [9]. Two different kinases, casein kinase (CK2) and protein kinase A (PKA), phosphorylates PEDF. CK2-phosphorylated PEDF at sites Ser24 and Ser114 follows a molecular conformational change in PEDF preventing phosphorylation of PKA to Ser227 which results in the antiangiogenic activity of PEDF and eliminates PEDF’s neurotrophic function [9]. On the other hand, PKA-phosphorylated PEDF at site Ser227 induces the neurotrophic functions of PEDF reducing PEDF’s antiangiogenic function [9]. However, the PKA-phosphorylation site at PEDF can be phosphorylated by CK2 which results in PEDF as a potent antiangiogenic factor which does not reduce PEDF’s neurotrophic activity [9]. As an antiangiogenic factor, PEDF has shown great potential in inhibiting endothelial cell proliferation and migration through various mechanisms such as initiating endothelial cell apoptosis via the Fas/FasL intrinsic death pathway and disrupting the balance between pro- and anti-angiogenic factors present in the bloodstream via the inhibitory effect of PEDF on vascular endothelial growth factor (VEGF) by inhibiting its receptor 1 (VEGFR-1) (Table 1) [10]. But what seems to be one of the most important therapeutic actions of PEDF is its potential as a novel drug candidate against cancer (Table 1).
PEDF as an anticancer agent branched out from the discovery of its antiangiogenic properties. From here, PEDF has been explored in different types of cancers and was found to have an indirect effect as an antiangiogenic agent as well as a direct anti-cancer effect in different tumours (Table 1) [11]. Patent applications have been published where PEDF can be used in the prevention and treatment for melanoma [12] and osteosarcoma [13]. Further tests on PEDF have generated patents in using plasmid vectors to deliver the PEDF gene [14–16] and the detection of the different forms of PEDF phosphorylation states have led to the search of more than one PEDF receptor (PEDF-R) which could explain the neurotrophic and antiangiogenic functions of PEDF [9, 17]. The purpose of this article aims to summarise the current patent applications in addressing the therapeutic potential and the different treatment method deliveries used for PEDF. Furthermore, this article also aims to acknowledge the benefits associated with further understanding of the role of the receptors for PEDF.
2.1 PEDF: An antiangiogenic and anticancer agent
Research on antiangiogenic factors against cancer has increased as it has been acknowledged that angiogenesis plays a factor in the growth, survival and metastasis of a tumour [18, 19]. The tumour can activate the overexpression of angiogenic factors, especially VEGF, which allows for the production of blood vessels supplying nutrients and oxygen to the tumour [18, 19]. The inhibitory role of PEDF against VEGF as well as fibroblast growth factor (FGF); its action in targeting only new blood vessels and not affecting pre-existing blood vessels [20]; and its wide distribution (Figure 1) and actions in different types of cells and molecular systems (Table 1) make PEDF a suitable antiangiogenic drug against cancer. This was the rationale behind the PEDF patents published in regards to the use of full length PEDF [16] or derived fragments as anticancer agents [21, 22].
The overproduction of VEGF allows the recruitment and proliferation of endothelial cells and form blood vessel branches surrounding the tumour enabling the tumour to grow and evolve from benign to malignant [18]. Apart from the tumour’s ability to switch on the expression of proangiogenic factors for its growth and survival, the tumour also has the ability to switch off transcription of antiangiogenic factors [18]. Thus, measuring PEDF’s concentration within the tissue or fluid of a cancer patient compared to healthy individuals may determine whether the tumour is on its early or advanced stage of tumorigenesis [16], though this remains a hypothesis at present.
Introducing exogenous full-length or fragments of PEDF enables it/them to target endothelial cells by inhibiting their proliferation and migration into the bloodstream through apoptosis of endothelial cells recruited by the tumour [16, 23, 24]. This method does not only prevent the expansion of blood vessels to the tumour but also inhibits tumour growth by restricting the tumour’s supply of nutrients and oxygen [16]. Not only does PEDF play a role in inhibiting the growth of a primary tumour, PEDF also prevents tumour cell invasion, adhesion and migration which all contributes to the suppression of cancer metastasis in order for the development of a secondary tumour (Figure 2). For instance, the treatment of exogenous full length PEDF inhibits VEGF expression in cells and in mice, administration or overexpression of PEDF leads to the inhibition of osteosarcoma tumour growth, which also disables metastasis to the lungs [25]. The same result was also evident when PEDF fragments, StVOrth-2 and -3, were given to mice with orthotopic osteosarcoma. Furthermore, StVOrth-3 and -4 were found to repress VEGF expression [26]. Apart from osteosarcoma, several other studies also showed the efficacy of PEDF against the growth of primary tumours and inhibition of tumour cell metastasis (Table 1).
Figure 2.
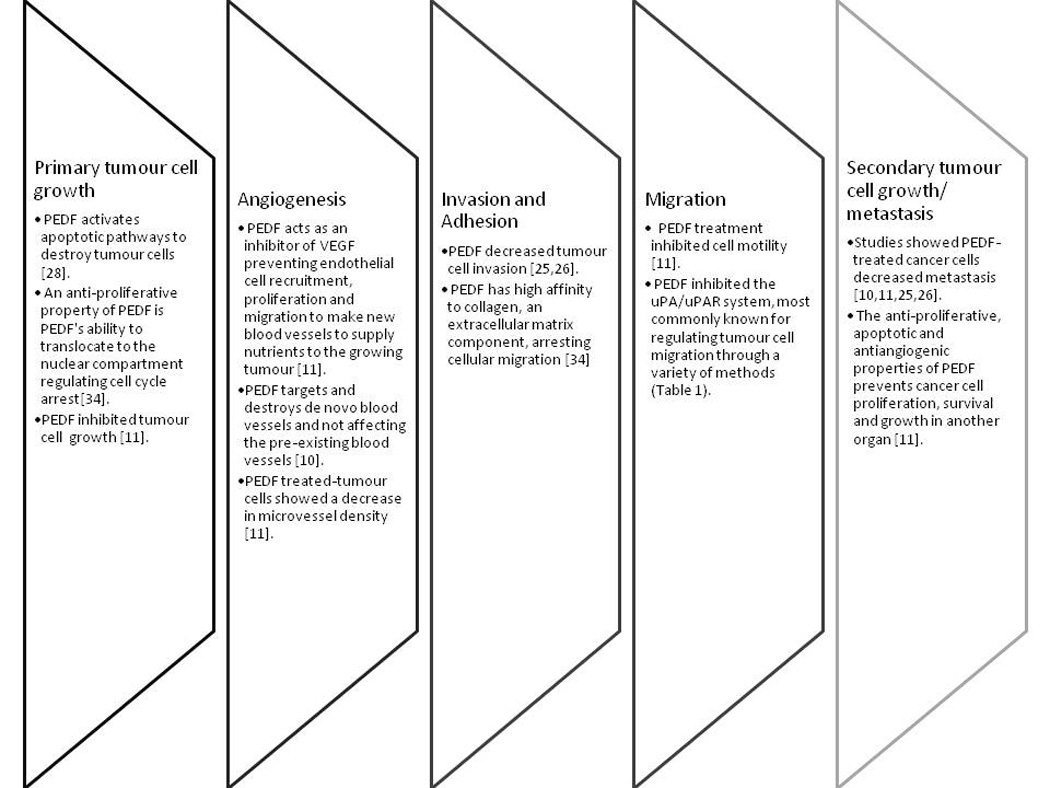
Overview of the key roles of PEDF during the metastatic process showing the efficacy of PEDF in not only reducing primary tumour growth but also in preventing metastasis.
It has been recommended that if PEDF is combined with other antiangiogenic factors, tumorigenesis can be effectively eradicated [16]. Given that PEDF is already a potent antiangiogenic factor in itself, testing it in combination with a chemotherapeutic agent, doxorubicin, against a chemo-resistant tumour –osteosarcoma made the tumour more susceptible to treatment [27]. Using PEDF as an antiangiogenic factor has been such a success that researchers focused at the direct anticancer properties of PEDF. Two patent applications demonstrated that treating melanoma and osteosarcoma with PEDF results in decreasing the proliferative property of these cancer cells with increasing doses of PEDF concentration [12, 13]. The antiproliferative property of PEDF was attributed to its ability to induce Fas ligand-dependent apoptosis [12, 28] and regulate cell cycling [29]. Treatment of osteosarcoma cell lines results in a decrease in cell proliferation and invasion and an increase in tumour cell apoptosis and adhesion. The ability of PEDF to directly target cancer cells enables a potential research on using PEDF as a direct drug against cancers and eliminates the use of conventional and toxic chemotherapy.
2.2 Treatment delivery approaches for PEDF
Many patent applications have focused on using gene delivery of PEDF either full-length or derived fragments via the use of recombinant vectors [12, 13, 15, 16]. To effectively ensure PEDF’s activity especially in deep and hard to reach tumour locations, the normal introduction of plasmid vectors through virus-mediated delivery becomes inefficient and ineffective. Zhou and colleagues [30] made use of PEDF gene transfer through ultrasound-mediated microbubble destruction where choroidal neovascularisation’s development was inhibited. Chitosan microparticles containing a PEDF-expressing plasmid (pPEDF) demonstrated a reduction in primary tumour growth, bone lysis and pulmonary metastasis [31]. Using the same technique with the addition of administering a chemotherapeutic agent, doxorubicin, in osteosarcoma has shown effective in decreasing primary and secondary tumour development as well as decreasing the toxic side-effects of doxorubicin [27]. Though these methods are novel, research on these techniques with PEDF is starting to become apparent and soon enough, patent applications for these methods will emerge.
2.3 PEDF receptors
Extracellular PEDF is known to be involved in inducing or mediating intracellular signalling transduction mechanisms and different molecular pathways. PEDF actions are dependent on interactions with cell surfaces [35]. Little is known about the identification of receptors for PEDF (PEDF-Rs) until recent studies initiated a search for cell-surface proteins with high affinity for PEDF [35]. As mentioned previously, due to PEDF’s variable phosphorylation states affecting how PEDF exerts either its neurotrophic or antiangiogenic function have lead the authors to believe that there might be two different receptors for PEDF which caters for the two different mechanistic actions of PEDF [9]. It was suggested that heparin and heparan sulfate regulate the ligand: receptor interactions of PEDF, likely because these glycosaminoglycans can promote a conformational change enabling an epitope of PEDF to be exposed and bind to its receptor [32, 33]. Two distinct receptors are proposed for PEDF, a 80kDa PEDF putative receptor (PEDF-RN) localised on motor neurons with high affinity to the 44-mer PEDF peptide involved in neurotrophic activity and a 60kDa PEDF putative receptor (PEDF-RA) localised on endothelial cells with high affinity to the 34-mer PEDF peptide involved in the antiangiogenesis [34–36].
The first identified receptor, PEDF-RN, was found in the retina and is a novel phospholipase and triglyceride lipase involved in triglyceride metabolism [34]. It is also termed PNPLA2, adipose triglyceraide lipase, desnutrin, iPLAζ and human Transport Secretion Protein-2.2 (TTS-2.2)/independent phospholipase Aζ [37]. The effect of the interaction of PEDF to PNPLA2 activating a molecular signalling pathway is still under investigation, although a number of propositions have already been put forward: (1) the localisation of PNPLA2 around the neural retina and the central nervous system depicts the neurotrophic role of this receptor upon activation of PEDF; (2) the triglyceride lipase domain is known to facilitate energy mobilisation and adipocyte storage; (3) upon PEDF binding, this novel receptor was found to induce phospholipase A2 liberating fatty acids and lysophosphatidic acid from phospholipids [34, 37, 38] which could act as second messegers or precursor in mediating signal transduction for neuronal cell development and survival or in eliciting antitumorigenic (e.g. DHA’s apoptotic role in tumour cells) and antiangiogenic functions (e.g. PLA2 mediating PPAR-gamma ligands to activate the PPAR-gamma receptor in vascular endothelial cells) [23, 37].
A second receptor for PEDF was identified more recently [36]. This receptor, PEDF-RA,also a receptor for laminin, binds to PEDF at a site which contains residues 46–70, the PEDF-interacting domain on this receptor is located between amino acids 120 and 210, which includes the laminin-1 binding domain of the receptor, binding of a 25-mer (P46) short peptide within the 34-mer region of PEDF, which also binds specifically to EC membranes, inhibits bFGF-induced angiogenesis. Upon binding of the 25-mer PEDF region to the laminin receptor, EC apoptosis is initiated while angiogenesis, migration, tumour cell adhesion and proliferation are inhibited. Through the binding of PEDF to the laminin receptor, the authors claimed that their results reveal a new signalling pathway for PEDF’s anti-angiogenic activities. Furthermore, the authors were able to postulate that PEDF binding to the laminin receptor also activates multiple apoptotic pathways independent of the Fas/FasL death pathway such as MAPK, JNK, p38 and caspase-3. True enough, a recent paper demonstrated PEDF initiating apoptosis via the JNK pathway and inhibiting migration via the p38 pathway [42]Even more recently, it has been identified that PEDF, like angiostatin, interacts and inhibits endothelial and tumor cell surface F1-ATP synthase [39]. The interaction of PEDF and angiostatin occurs at the same location on the F1-ATP synthase and prevents the formation of ATP from ADP and inorganic phosphate by the enzyme. ATP and ADP have receptors on cell surfaces and would constitute mediators of PEDF. A change in ATP levels could affect cell viability and proliferation negatively. Given that the enzyme is also a proton pump, it is proposed that these antiangiogenic factors could prevent the exit of protons from cells resulting in an decrease of intracellular pH leading to cell death. Agents that inhibit exclusively the cell-surface F1-ATP synthase, like PEDF, could be used as agents to prevent endothelial and tumour viability. This ectopic enzyme is being considered another receptor for PEDF.
This discovery of PEDF-Rs enables researchers to further probe PEDF’s therapeutic activities and provide researchers with a finer tool for identifying and isolating PEDF sequences, creating antibodies that will allow specific localisation of PEDF [17], determining the pathways involved after PEDF-R activation/inhibition [34], and most important of all, design novel drugs using PEDF fragments that can bind to PEDF-R that could lead to a possible breakthrough in cancer treatment research. Furthermore, modulation of PEDF activity via its receptor could provide an attractive option for treating angiogenesis-dependent diseases, of which metastatic cancer is a prime example.
3. Conclusion
The therapeutic role of PEDF has shown great potential against different diseases because of its potent activity in inhibiting angiogenesis. This antiangiogenic property of PEDF makes PEDF an attractive antiangiogenic drug to be used in cancer research creating patent applications for its use in research. Direct anticancer effects of PEDF have only started to emerge after the testing of PEDF as a direct drug across a range of malignancies which also lead to patent applications for using PEDF in preventing and treating melanoma and osteosarcoma. In addition, treatment methods in inducing exogenous PEDF is also starting to evolve in terms of creating effective ways of delivering PEDF and ensuring its uptake in hard to reach tissues. Furthermore, the discovery of PEDF-R and the continuous research in dissecting its role upon PEDF binding yields a positive road towards designing novel drugs against cancer.
4. Expert Opinion
For cancer, faced with toxicity of current drugs and due to lack of efficacy of others, various biological agents, most of which are endogenously present in the body, are being tested in the hope of finding forms of therapy that are safer to administer, but have efficacy. One such promising molecule, PEDF, a naturally-occurring protein, a known potent angiogenesis inhibitor, is a leading protein candidate for certain tumours, including osteosarcoma, a type of bone tumour. Due to several studies showing PEDF’s therapeutic role directly and indirectly against a wide range of tumours, it is not surprising that several patents have been published indicating the different experimental applications and treatment methods for PEDF. Elucidating the specific molecular pathways for PEDF action against cancer has become the focus of several groups of researchers around the globe. These are surely helping to develop PEDF as a novel anticancer drug candidate. However, in this case, understanding not only the ligand (PEDF) is crucial, but its two receptors, given the ability of PEDF to have versatile roles in cells. The latest research showing the discovery of PEDF receptors shows how the field in PEDF research is progressing. Although there are still missing pieces of the puzzle that are yet to be explored, especially the molecular pathways involving PEDF and PEDF binding to its receptors, there is no denying that patenting of PEDF for various biomedical applications attests to the latent potential of this protein.
5. Article highlights (Figure 3)
Figure 3.
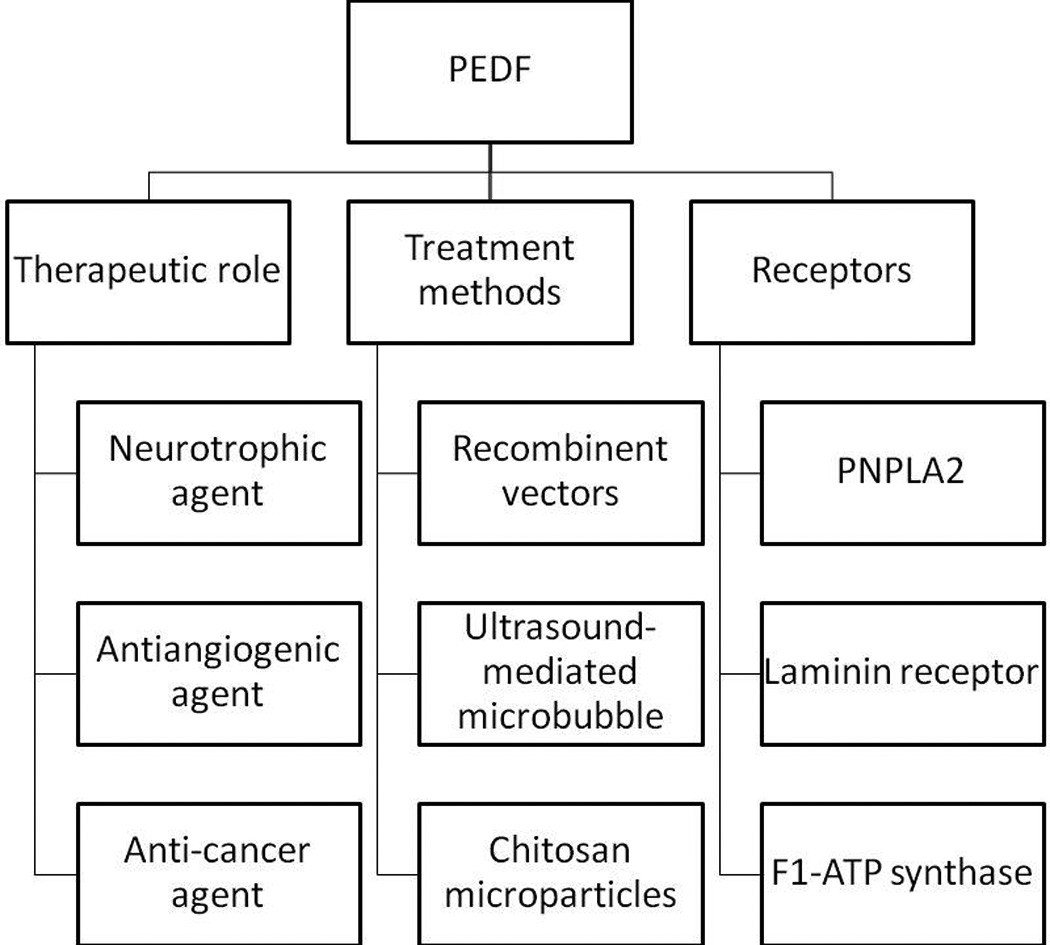
Summary of all the important information in the paper.
-
The role of pigment epithelium-derived factor (PEDF) in tumorigenesis AQ5 (Figure 3).
The concentration of endogenous PEDF can be used to determine the stage of tumorigenesis.
PEDF treatment results in apoptosis of recruited endothelial cells preventing tumorigenesis, restricting tumour growth and inhibiting metastasis.
PEDF can be combined with other antiangiogenic or chemotherapeutic agents for better tumour efficacy.
-
PEDF as an anticancer agent.
PEDF decreases tumour cell proliferation by decreasing cell cycling and inducing Fas ligand-dependent apoptosis.
PEDF plays multiple roles in different stages of metastasis making it a powerful agent against secondary tumour growth.
PEDF gene delivery through viral-mediated plasmid vectors has been the most conventional way of inducing exogenous PEDF.
New methods have been introduced for PEDF to reach deep tissues such as ultrasound-mediated microbubble destruction and chitosan microparticles containing PEDF-expressing plasmid.
PEDF receptors (PEDF-Rs) with a high affinity binding towards PEDF are proposed.
PEDF-RN (PNPLA2) plays a role in initiating PEDF’s neurotrophic activity.
PEDF-RA (laminin receptor) is involved in blocking angiogenesis.
Proteins are identified as receptors for PEDF.
A lipase-linked cell membrane PEDF-R is involved in PEDF’s neurotrophic activity, fatty acid liberation and phospholipase A(2) enzymatic activity.
PEDF interacts with cell-surface F1-ATP synthase.
Acknowledgement
The authors would like to thank Danica Ronina Revote (Bachelor of Communication, Media (Hons); RMIT University) for making the Figure 1 illustration used in this article.
Abbreviations
- PEDF
pigment epithelium-derived factor
- RCL
reactive centre loop
- FasL
Fas ligand
- VEGF
vascular endothelial growth factor
- VEGFR-1
vascular endothelial growth factor receptor 1
- PEDF-R
PEDF receptor
- pPEDF
PEDF-expressing plasmid
- PEDF-RN
PEDF-R involved in neuroprotection
- PEDF-RA
PEDF-R involved in blocking angiogenesis
- TTS-2.2
human Transport Secretion Protein-2.2
- FGF
fibroblast growth factor
- PNPLA2
patatin-like phospholipase domain containing protein 2
- iPLAζ
calcium-independent phospholipase Aζ
- CK2
casein kinase
- PKA
protein kinase A
- uPa
urokinase plasminogen activator
- uPAR
uPA receptor
- PAI-1
plasminogen activator inhibitor type I
- PAI-2
PAI type II
- JNK
c-jun N-terminal kinase
- p53
protein 53 or tumour protein 53
- PPARγ
perxisome proliferator-activated receptor γ
- 15d-PGJ2
15-deoxy-delta-12,14-prostaglandin J2
- RPE
retinal pigment epithelium
Footnotes
Declaration of interest
The authors declare no conflict of interest and have received no payment in preparation for this manuscript.
References
Papers of special note have been highlighted as:
* of importance
** of considerable importance
- 1. Becerra SP, Sagasti A, Spinella P, Notario V. Pigment epithelium derived factor behaves like a noninhibitory serpin. Neurotrophic activity does not require the serpin reactive loop. J Biol Chem. 1995;270:25992–25999. doi: 10.1074/jbc.270.43.25992. ** One of the first papers showing the structure and function of PEDF.
- 2. Tombran-Tink J, Johnson LV. Neuronal differentiation of retinoblastoma cells induced by medium conditioned by human RPE cells. Invest Opthalmol Vis Sci. 1989;30:1700–1707. ** A paper demonstrating the discovery of the neurotrophic role of PEDF in differentiating retinoblastoma cells.
- 3. Tombran-Tink J, Chader GG, Johnson LV. PEDF: A pigment epitherlium-derived factor with potent neuronal differentiative activity. Exp Eye Res. 1991;53(3):411–414. doi: 10.1016/0014-4835(91)90248-d. ** A paper demonstrating the neurotrophic role of PEDF.
- 4. Cao W, Tombran-Tink J, Elias R, et al. In vivo protection of photoreceptors from light damage by pigment epithelium-derived factor. Invest Opthalmol Vis Sci. 2001;42:1646–1652. * A research paper demonstrating the neuroprotective role of PEDF in the eye.
- 5. Bilak MM, Corse AM, Bilak SR, et al. Pigment epithelium-derived factor (PEDF) protects motor neurons from chronic glutamate-mediated neurodegeneration. Journal of Neuropathol Exp Neurol. 1999;58(7):719–728. doi: 10.1097/00005072-199907000-00006. * A research paper demonstrating the neuroprotective role of PEDF in neurons.
- 6. Araki T, Taniwaki T, Becerra SP, et al. Pigment epithelium-derived factor (PEDF) differentially protects immature but not mature cerebellar granule cells against apoptotic cell death. J Neurosci Res. 1998;53(1):7–15. doi: 10.1002/(SICI)1097-4547(19980701)53:1<7::AID-JNR2>3.0.CO;2-F. * A research paper demonstrating the neuroprotective role of PEDF towards cerebellar granule cells.
- 7. Cayouette M, Smith SB, Becerra SP, Gravel C. Pigment epithelium-derived factor delays the death of photoreceptors in mouse models of inherited retinal degenerations. Neurobiol Dis. 1999;6(6):523–532. doi: 10.1006/nbdi.1999.0263. * A research paper demonstrating the neuroprotective role of PEDF in the eye.
- 8. Dawson DW, Volpert OV, Gillis P, et al. Pigment epithelium-derived factor: A potent inhibitor of angiogenesis. Science. 1999;285:245–248. doi: 10.1126/science.285.5425.245. ** A paper showing the antiangiogenic role of PEDF.
- 9. Maik-Rachline G, Seger R. Variable phosphorylation states of pigment-epithelim-derived factor differentially regulate its function. Blood. 2006;107:2745–2752. doi: 10.1182/blood-2005-06-2547. ** A research paper demonstrating the different phosphorylation states of PEDF affecting PEDF’s role as a neurotrophic agent or an antiangiogenic agent.
- 10. Dass CR, Ek ET, Choong PF. PEDF as an emerging therapeutic candidate for osteosarcoma. Curr Cancer Drug Targets. 2008;8(8):683–90. doi: 10.2174/156800908786733487. ** A literature review summarising the antiangiogenic and antitumorigenic property of PEDF as a drug candidate against osteosarcoma.
- 11. Broadhead ML, Dass CR, Choong PF. In vitro and in vivo biological activity of PEDF against a range of tumors. Expert Opin Ther Targets. 2009;13(12):1429–1438. doi: 10.1517/14728220903307475. ** A literature review summarising all the research papers which reported the therapeutic role of PEDF in different cancer cell lines.
- 12. Yamagishi S, Imaizumi T, Shimizu H, Abe R. Method for preventing or treating malignant melanoma. US20050222031. 2005 ** A patent paper showing the therapeutic role of PEDF against melanoma.
- 13. Yamagishi S, Imaizumi T. Method for preventing or treating osteosarcoma. US20060122112. 2006 ** A patent paper showing the therapeutic role of PEDF against osteosarcoma.
- 14. Kovesdi I, Brough DE, McVey DL, Wei L. Viral vector encoding pigment epithelium-derived factor. US20050101018. 2005 ** A patent paper showing the experimental methods behind recombinant gene delivery vectors of PEDF.
- 15. Manning WC, Dwarki VJ, Rendahl K, et al. Use of recombinant gene delivery vectors for treating or preventing diseases of the eye. US6943153. 2005 ** A patent paper showing the experimental methods behind recombinant gene delivery vectors of PEDF in the eye.
- 16. Bouck NP, Dawson DW, Gillis PR, et al. Methods and compositions for inhibiting angiogenesis. US 7105496. 2006 ** A patent paper showing the experimental methods behind the investigation of the antiangiogenic factor of PEDF.
- 17. Becerra SP, Notari L, Laborda J, Escribano-Martinez J. PEDF-R receptor and uses. US20090162363. 2009 ** A patent paper showing the use and function of PEDF receptors.
- 18. Gordon MS, Mendelson DS, Kato G. Tumor angiogenesis and novel antiangiogenic strategies. Int J Cancer. 2010;126(8):1777–1787. doi: 10.1002/ijc.25026. ** A literature review paper summarising the mechanism behind tumorigenesis and a list of the antiangiogenic factors currently under investigation in clinical trials.
- 19. Folkman J. Fighting cancer by attacking its blood supply. Sci Am. 1996;275(3):150–154. doi: 10.1038/scientificamerican0996-150. ** The first paper published proposing the role of angiogenesis as an important factor in cancer cell growth and survival.
- 20. Bouck N. PEDF: anti-angiogenic guardian of ocular function. Trends Mol Med. 2002;8:330–334. doi: 10.1016/s1471-4914(02)02362-6. ** A literature review paper summarising PEDF’s antiangiogenic role in the eye.
- 21. Volz K, Filleur S, Volpert O, Zaichuk T. Anti-angiogenic fragments of pigment epitheliumderived factor (PEDF) US20090118191. 2009 ** A patent paper showing the use and function of PEDF peptides.
- 22. Seger R, Maik-rachline G. Variants of pigment epithelium derived factor and uses thereof. US20090269320. 2009 ** A patent paper showing the use and function of PEDF peptides.
- 23. Ho TC, Chen SL, Yang YC, et al. PEDF induces p53-mediated apoptosis through PPAR gamma signaling in human umbilican vein endothelial cells. Cardiovasc Res. 2007;76:213–223. doi: 10.1016/j.cardiores.2007.06.032. ** A paper showing the apoptotic pathway activated by PEDF.
- 24. Gao D, Nolan D, McDonnell K, et al. Bone marrow-derived endothelial progenitor cells contribute to the angiogenic switch in tumor growth and metastatic progression. Biochim Biophys Acta. 2009;1796:33–40. doi: 10.1016/j.bbcan.2009.05.001. * A paper showing the involvement of bone marrow-derived endothelial cells in tumorigenesis.
- 25. Ek ET, Dass CR, Contreras KG, Choong PF. Inhibition of orthotopic osteosarcoma growth and metastasis by multitargeted antitumor activities of pigment epithelium-derived factor. Clin Exp Metastasis. 2007;24:93–106. doi: 10.1007/s10585-007-9062-1. * A research paper showing the therapeutic role of PEDF against osteosarcoma.
- 26. Ek ET, Dass CR, Contreras KG, Choong PF. PEDF-derived synthetic peptides exhibit antitumor activity in an orthotopic model of human osteosarcoma. J Orthop Res. 2007;25:1671–1680. doi: 10.1002/jor.20434. * A paper showing the therapeutic effects of PEDF peptides against osteosarcoma.
- 27. Ta HT, Dass CR, Larson I, et al. A chitosan-dipotassium orthophosphate hydrogel for the delivery of Doxorubicin in the treatment of osteosarcoma. Biomaterials. 2009;30(21):3605–3613. doi: 10.1016/j.biomaterials.2009.03.022. ** A paper showing the use of chitosan microparticles in combination with a hydrogel in PEDF treatment on bone.
- 28. Broadhead ML, Dass CR, Choong PF. Cancer cell apoptotic pathways mediated by PEDF: Prospects for therapy. Trends Mol Med. 2009;15(10):461–467. doi: 10.1016/j.molmed.2009.08.003. ** A literature review summarising all the research papers which reported the apoptotic role of PEDF.
- 29. Pignolo RJ, Francis MK, Rotenberg MO, Cristofalo VJ. Putative role for EPC-1/PEDF in the G0 growth arrest of human diploid fibroblasts. J Cell Physiol. 2003;195:12–20. doi: 10.1002/jcp.10212. ** A paper showing one of the mechanisms behind PEDF’s role in inhibiting proliferation and cell cycling.
- 30. Zhou XY, Liao Q, Pu YM, et al. Ultrasound-mediated microbubble delivery of pigment epithelium-derived factor gene into retina inhibits choroidal neovascularization. Chin Med J (Engl) 2009;122(22):2711–2717. ** A paper showing the use of ultrasound-mediated microbubble technique in PEDF treatment.
- 31. Dass CR, Contreras K, Dunstan DE, Choong PF. Chitosan microparticles encapsulating PEDF plasmid demonstrate efficacy in an orthotopic metastatic model of osteosarcoma. Biomaterials. 2007;28:3026–3033. doi: 10.1016/j.biomaterials.2007.03.016. ** A paper showing the use of chitosan microparticles in PEDF treatment on bone.
- 32. Valnickova Z, Petersen SV, Nielsen SB, et al. Heparin binding induces a conformational change in pigment epithelium-derived factor. J Biol Chem. 2007;282:6661–6667. doi: 10.1074/jbc.M610471200. * A paper showing the mechanism of PEDF to induce a conformational change in its structure.
- 33. Alberdi EMWJ, Becerra SP. Glycosaminoglycans in human retinoblastoma cells: Hepara sulfate, a modulator of the pigment epithelium-derived factor-receptor interactions. BMC Biochem. 2003;19(4):1. doi: 10.1186/1471-2091-4-1. * A paper showing the role of glycosaminoglycans in the conformational change exerted by PEDF.
- 34. Filleur S, Nelius T, de Riese W, Kennedy RC. Characterization of PEDF: A multi-functional serpin family protein. J Cellular Biochem. 2009;106:769–775. doi: 10.1002/jcb.22072. ** A review summarising PEDF’s biological role and the discovery of two PEDF receptors.
- 35. Alberdi E, Aymerich MS, Becerra SP. Binding of pigment epithelium-derived factor (PEDF) to retinoblastoma cells and cerebellar granule neurons. Evidence for a PEDF receptor. J Biol Chem. 1999;274(44):31605–31612. doi: 10.1074/jbc.274.44.31605. ** One of the first paper to report the discovery of a PEDF receptor.
- 36. Bernard A, Gao-Li J, Franco CA, et al. Laminin receptor involvement in the anti-angiogenic activity of pigment epithelium-derived factor. J Biol Chem. 2009;284(16):10480–10490. doi: 10.1074/jbc.M809259200. ** A research paper showing the antiangiogenic effects of PEDF upon its binding to the laminin receptor.
- 37. Notari L, Baladron V, Aroca-Aguilar JD, et al. Identification of a lipase-linked cell membrane receptor for pigment epithelium-derived factor. J Biol Chem. 2006;49:38022–38037. doi: 10.1074/jbc.M600353200. ** One of the first papers published demonstrating the identification, structure and localisation of the PNPLA2 PEDF receptor and its affinity on PEDF.
- 38. Subramanian P, Notario PM, Becerra SP. Pigment epithelium-derived factor receptor (PEDF-R): A plasma membrane-linked phospholipase with PEDF binding affinity. Adv Exp Med Biol. 2010;664:29–37. doi: 10.1007/978-1-4419-1399-9_4. ** A paper showing the biological role of the PNPLA2 PEDF receptor upon PEDF binding.
- 39. Notari L, Arakaki N, Mueller D, et al. Pigment epithelium-derived factor binds to cell-surface F(1)-ATP synthase. FEBS J. 2010;277(9):2192–2205. doi: 10.1111/j.1742-4658.2010.07641.x. ** A recent research paper showing a possible new PEDF receptor.
- 40. Yang J, Duh EJ, Caldwell RB, et al. Antipermeability function of PEDF involves blockade of the MAP kinase/GSK/beta-catenin signalling pathway and uPAR expression. Invest Opthalmol Vis Sci. 2010;51(6):3273–3280. doi: 10.1167/iovs.08-2878. ** A recent research paper showing the molecular pathways activated by PEDF.
- 41. Zhang SX, Wang JJ, Gao G, et al. Pigment epithelium-derived factor downregulates vascular endothelial growth factor (VEGF) expression and inhibits VEGF- VEGF receptor 2 binding in diabetic retinopathy. J Mol Endocrinol. 2006;37(1):1–12. doi: 10.1677/jme.1.02008. ** A research papers which showed PEDF’s inhibitory role against VEGFR and its receptor.
- 42. Konson A, Pradeep S, D’Acunto CW, at el. Pigment epithelium-derived factor and its phosphomimetic mutant induce JNK-dependent apoptosis and p38-mediated migration arrest. J Biol Chem. 2010 doi: 10.1074/jbc.M110.151548. (in press). ** A recent paper and one of the first research papers which showed the molecular pathways associated with PEDF binding to the laminin receptor.
- 43. Crowe S, Wu LE, Economou C, et al. Pigment epithelium-derived factor contributes to insulin resistance in obesity. Cell Metabolism. 2009;10(1):40–47. doi: 10.1016/j.cmet.2009.06.001. * A research paper showing the biological role of PEDF in obesity.
- 44. Tombran-Tink J. The neuroprotective and angiogenesis inhibitory serpin, PEDF: New insights into phylogeny, function, and signalling. Front Biosci. 2005;10:2131–2149. doi: 10.2741/1686. ** An excellent review summarising the structure, function and localisation of PEDF as a neurotrophic and antiangiogenic factor.
- 45. Rychli K, Kaun C, Hohensinner PJ, et al. The anti-angiogenic factor PEDF if present in the human heart and is regulated by anoxia in cardiac myocytes and fibroblasts. J Cell Mol Med. 2010;14(1-2):198–205. doi: 10.1111/j.1582-4934.2009.00731.x. * A research paper showing the biological role of PEDF and its localisation in the heart.
- 46. Chung C, Doll JA, Gattu AK, et al. Anti-angiogenic pigment epithelium-derived factor regulates hepatocyte triglyceride content through adipose triglyceride lipase (ATGL) J Hepatol. 2008;48(3):471–478. doi: 10.1016/j.jhep.2007.10.012. * A research paper showing the biological role of PEDF and its localisation in the liver.
- 47. Cheugh L, Au S, Cheung A, et al. Pigment epithelium-derived factor is estrogen sensitive and inhibits the growth of human ovarian cancer and ovarian surface epithelial cells. Endocrinology. 2006;147(9):4179–4191. doi: 10.1210/en.2006-0168. * A research paper showing the biological role of PEDF and its localisation in the female reproductive system.
- 48. Grayhack JT, Smith ND, Ilio K, et al. Pigment epithelium-derived factor, a human testis epididymis secretory product, promotes human prostate stromal cell growth in culture. J Urol. 2004;171(1):434–438. doi: 10.1097/01.ju.0000088774.80045.c4. * A research paper showing the biological role of PEDF and its localisation in the male reproductive system.
- 49. Quan G, Ojaimi J, Li Y, et al. Localization of pigment epithelim-derived factor in growing mouse bone. Calcif Tissue Int. 2005;76:146–153. doi: 10.1007/s00223-004-0068-2. * A research paper showing the biological role of PEDF and its localisation in bone.