Abstract
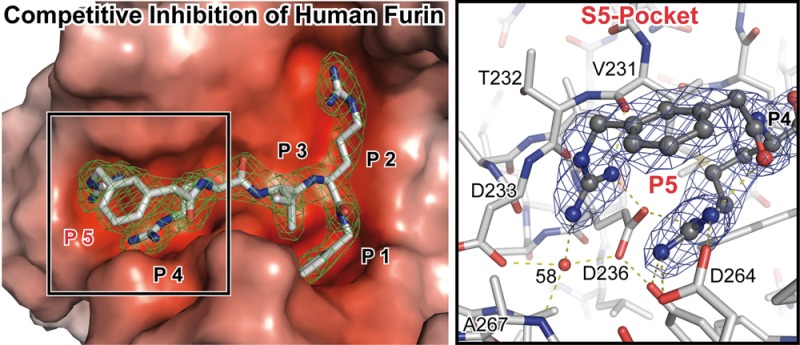
Furin inhibitors are promising therapeutics for the treatment of cancer and numerous infections caused by bacteria and viruses, including the highly lethal Bacillus anthracis or the pandemic influenza virus. Development and improvement of inhibitors for pharmacological use require a detailed knowledge of the protease’s substrate and inhibitor binding properties. Here we present a novel preparation of human furin and the first crystal structures of this enzyme in complex with noncovalent inhibitors. We show the inhibitor exchange by soaking, allowing the investigation of additional inhibitors and substrate analogues. Thus, our work provides a basis for the rational design of furin inhibitors.
Furin is a member of the pro-hormone/pro-protein convertase family (PCs) of subtilisin-like endoproteinases.1 PCs are required for activation and maturation of many secreted proteins. Target proteins include peptide hormones, growth factors, matrix metalloproteases, blood clotting factors, regulators of the cholesterol metabolism, bacterial toxins, and viral capsid proteins.2,3 Therefore furin and other PCs are intensively investigated as pharmacological targets for the treatment of many diseases, e.g., atherosclerosis, hypercholesterolaemia, and cancer, as well as viral and bacterial infections.4 Proteolysis by furin is highly specific and occurs C-terminal to a multibasic recognition motive. The extended substrate binding site gives rise to diverging specificities, strongly favoring arginine at P1 and basic amino acid side chains at P2, P4, and/or P6, whereby R-[X]-(R/K)-R↓ is the most common recognition sequence.
Up to now several compound classes have been identified as promising starting points for drug development. In addition to small molecules and peptide based inhibitors,5 also camelid VHH-antibodies were found to selectively inhibit furin.6 It was shown that furin inhibitors are indeed suitable to prevent the growth and invasiveness of tumors (e.g., refs (7 and 8)), the replication of viruses (e.g., refs (9 and 10)), or the toxicity of bacterial toxins (e.g., refs (11 and 12)). For their broad pharmacological application, next generation compounds require, however, improvements of their stability, selectivity, bioavailability, and/or pharmacokinetics.5
Structure-guided drug design provides the possibility for rational modification and directed development of enhanced inhibitors. This approach requires an in-depth structural understanding of furin–inhibitor complexes. So far, structures of mouse furin13 and of its yeast homologue kexin14 are available only in complex with covalently attached peptides. The mouse furin structure showed the interaction with a prototypical R-V-K-R↓ recognition motive. Investigation of other furin substrate analogues or inhibitors by exchange of the initially co-crystallized compound, however, was not possible.
Peptidomimetic compounds based on a phenylacetyl-Arg-Val-Arg-4-(amidomethyl)benzamidine (Phac-RVR-4-Amba) core structure (15) belong to the strongest noncovalent inhibitors available so far. Upon variation of the P5 position, dramatic changes of the Ki values were observed that cannot be explained by the known recognition motive. The Ki improved by approximately 2 orders of magnitude after addition of basic substituents, e.g., by modification of the Phac-moiety at P5 by a m- or p-guanidinomethyl group.15
Here we describe a novel preparation of human furin and two crystal structures of this enzyme in complex with competitive, noncovalent inhibitors. The tight binding observed for the inhibitor complexes is accompanied by a very strong increase of the structural stability in thermal denaturation experiments. The structures explain the different affinities of the inhibitors and the related specificity of the protease for substrates with Arg/Lys residues at the P5 position.
Methods
The coding sequence of human furin was inserted into the plasmid pHLsec28 and expressed by transient transfection of human embryonic kidney cells. The protein was purified in a three-step chromatography scheme, employing metal affinity chromatography, inhibitor based affinity chromatography,17 and size exclusion chromatography. Finally a ∼300-fold enrichment of human furin was observed, corresponding to a specific activity of 57 ± 1 u. One unit corresponds to 1 μmol AMC (h × mg)−1 released from the peptide pGlu-Arg-Thr-Lys-Arg-AMC (200 μM) at 37 °C in 100 mM Hepes, pH 7.0, 5 mM CaCl2, 0.5% (v/v) TritonX-100. Details of the expression, preparation, kinetic analyses, and thermal denaturation assays are described in Supporting Information.
For crystallization furin was concentrated to 140–150 μM (∼7.5 mg mL–1), and I1 was added to a final concentration of 290 μM. Crystals were grown at 30 °C in 50 mM Tris, pH 8.5, 2.8 M sodium formate and 0.015 mM Cymal-7. For the structural investigation of the complex of furin with I2, crystals were soaked in crystallization solution supplemented with 3 mM of I2. Diffraction data were collected at 100 K at the BESSY-II beamline 14.1 of the Helmholtz-Zentrum Berlin (HZB)29 and processed with XDS (v.03/201330). Model building was carried out in COOT (v.0.6.231). CNS (v.1.332) was used for refinement of the structures of furin in complex with I1 and I2 up to 2.3 and 2.7 Å resolution, respectively.
Acknowledgments
We acknowledge the Helmholtz Zentrum Berlin BESSY II for provision of synchrotron radiation at the beamline BL 14.1 and thank the scientific staff for assistance. This work was supported by EMBO (to S.O.D., ASTF 513 - 2011).
Supporting Information Available
Supplementary figures, supplementary tables, and supplementary methods, containing a detailed description of the expression procedure, crystallographic work, enzymatic tests, and thermal denaturation assays. This material is available free of charge via the Internet at http://pubs.acs.org.
Accession Codes
Structure factors and coordinates for the complex structures of human furin in complex with inhibitor I1 and I2 have been deposited to the protein databank (PDB) with the accession codes 4OMC and 4OMD, respectively.
The authors declare no competing financial interest.
Supplementary Material
References
- Siezen R. J.; Leunissen J. A. (1997) Subtilases: the superfamily of subtilisin-like serine proteases. Protein Sci. 6, 501–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas G. (2002) Furin at the cutting edge: from protein traffic to embryogenesis and disease. Nat. Rev. Mol. Cell. Biol. 3, 753–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artenstein A. W.; Opal S. M. (2011) Proprotein convertases in health and disease. N. Engl. J. Med. 365, 2507–2518. [DOI] [PubMed] [Google Scholar]
- Seidah N. G.; Prat A. (2012) The biology and therapeutic targeting of the proprotein convertases. Nat. Rev. 11, 367–383. [DOI] [PubMed] [Google Scholar]
- Couture F.; D’Anjou F.; Day R. (2011) On the cutting edge of proprotein convertase pharmacology: from molecular concepts to clinical applications. Biomol. Concepts 2, 421–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J.; Declercq J.; Roucourt B.; Ghassabeh G. H.; Meulemans S.; Kinne J.; David G.; Vermorken A. J.; Van de Ven W. J.; Lindberg I.; Muyldermans S.; Creemers J. W. (2012) Generation and characterization of non-competitive furin-inhibiting nanobodies. Biochem. J. 448, 73–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez de Cicco R.; Bassi D. E.; Zucker S.; Seidah N. G.; Klein-Szanto A. J. (2005) Human carcinoma cell growth and invasiveness is impaired by the propeptide of the ubiquitous proprotein convertase furin. Cancer Res. 65, 4162–4171. [DOI] [PubMed] [Google Scholar]
- Coppola J. M.; Bhojani M. S.; Ross B. D.; Rehemtulla A. (2008) A small-molecule furin inhibitor inhibits cancer cell motility and invasiveness. Neoplasia (N. Y., NY, U. S.) 10, 363–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallenberger S.; Bosch V.; Angliker H.; Shaw E.; Klenk H. D.; Garten W. (1992) Inhibition of furin-mediated cleavage activation of HIV-1 glycoprotein gp160. Nature 360, 358–361. [DOI] [PubMed] [Google Scholar]
- Becker G. L.; Sielaff F.; Than M. E.; Lindberg I.; Routhier S.; Day R.; Lu Y.; Garten W.; Steinmetzer T. (2010) Potent inhibitors of furin and furin-like proprotein convertases containing decarboxylated P1 arginine mimetics. J. Med. Chem. 53, 1067–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarac M. S.; Peinado J. R.; Leppla S. H.; Lindberg I. (2004) Protection against anthrax toxemia by hexa-D-arginine in vitro and in vivo. Infection Immunity 72, 602–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiryaev S. A.; Remacle A. G.; Ratnikov B. I.; Nelson N. A.; Savinov A. Y.; Wei G.; Bottini M.; Rega M. F.; Parent A.; Desjardins R.; Fugere M.; Day R.; Sabet M.; Pellecchia M.; Liddington R. C.; Smith J. W.; Mustelin T.; Guiney D. G.; Lebl M.; Strongin A. Y. (2007) Targeting host cell furin proprotein convertases as a therapeutic strategy against bacterial toxins and viral pathogens. J. Biol. Chem. 282, 20847–20853. [DOI] [PubMed] [Google Scholar]
- Henrich S.; Cameron A.; Bourenkov G. P.; Kiefersauer R.; Huber R.; Lindberg I.; Bode W.; Than M. E. (2003) The crystal structure of the proprotein processing proteinase furin explains its stringent specificity. Nat. Struct. Biol. 10, 520–526. [DOI] [PubMed] [Google Scholar]
- Holyoak T.; Wilson M. A.; Fenn T. D.; Kettner C. A.; Petsko G. A.; Fuller R. S.; Ringe D. (2003) 2.4 A resolution crystal structure of the prototypical hormone-processing protease Kex2 in complex with an Ala-Lys-Arg boronic acid inhibitor. Biochemistry 42, 6709–6718. [DOI] [PubMed] [Google Scholar]
- Becker G. L.; Lu Y.; Hardes K.; Strehlow B.; Levesque C.; Lindberg I.; Sandvig K.; Bakowsky U.; Day R.; Garten W.; Steinmetzer T. (2012) Highly potent inhibitors of proprotein convertase furin as potential drugs for treatment of infectious diseases. J. Biol. Chem. 287, 21992–22003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves P. J.; Callewaert N.; Contreras R.; Khorana H. G. (2002) Structure and function in rhodopsin: high-level expression of rhodopsin with restricted and homogeneous N-glycosylation by a tetracycline-inducible N-acetylglucosaminyltransferase I-negative HEK293S stable mammalian cell line. Proc. Natl. Acad. Sci. U.S.A. 99, 13419–13424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuester M.; Becker G. L.; Hardes K.; Lindberg I.; Steinmetzer T.; Than M. E. (2011) Purification of the proprotein convertase furin by affinity chromatography based on PC-specific inhibitors. Biol. Chem. 392, 973–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krissinel E.; Henrick K. (2004) Secondary-structure matching (SSM), a new tool for fast protein structure alignment in three dimensions. Acta Crystallogr. 60, 2256–2268. [DOI] [PubMed] [Google Scholar]
- Henrich S.; Lindberg I.; Bode W.; Than M. E. (2005) Proprotein convertase models based on the crystal structures of furin and kexin: explanation of their specificity. J. Mol. Biol. 345, 211–227. [DOI] [PubMed] [Google Scholar]
- Creemers J. W.; Choquet H.; Stijnen P.; Vatin V.; Pigeyre M.; Beckers S.; Meulemans S.; Than M. E.; Yengo L.; Tauber M.; Balkau B.; Elliott P.; Jarvelin M. R.; Van Hul W.; Van Gaal L.; Horber F.; Pattou F.; Froguel P.; Meyre D. (2012) Heterozygous mutations causing partial prohormone convertase 1 deficiency contribute to human obesity. Diabetes 61, 383–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Than M. E.; Henrich S.; Bourenkov G. P.; Bartunik H. D.; Huber R.; Bode W. (2005) The endoproteinase furin contains two essential Ca2+ ions stabilizing its N-terminus and the unique S1 specificity pocket. Acta Crystallogr. 61, 505–512. [DOI] [PubMed] [Google Scholar]
- Zheng H.; Chordia M. D.; Cooper D. R.; Chruszcz M.; Muller P.; Sheldrick G. M.; Minor W. (2014) Validation of metal-binding sites in macromolecular structures with the CheckMyMetal web server. Nat. Protoc. 9, 156–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheatley J. L.; Holyoak T. (2007) Differential P1 arginine and lysine recognition in the prototypical proprotein convertase Kex2. Proc. Natl. Acad. Sci. U.S.A. 104, 6626–6631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells C. M.; Di Cera E. (1992) Thrombin is a Na(+)-activated enzyme. Biochemistry 31, 11721–11730. [DOI] [PubMed] [Google Scholar]
- Di Cera E. (2004) Thrombin: a paradigm for enzymes allosterically activated by monovalent cations. C. R. Biol. 327, 1065–1076. [DOI] [PubMed] [Google Scholar]
- Gros P.; Kalk K. H.; Hol W. G. (1991) Calcium binding to thermitase. Crystallographic studies of thermitase at 0, 5, and 100 mM calcium. J. Biol. Chem. 266, 2953–2961. [DOI] [PubMed] [Google Scholar]
- Tian S.; Huang Q.; Fang Y.; Wu J. (2011) FurinDB: A database of 20-residue furin cleavage site motifs, substrates and their associated drugs. Int. J. Mol. Sci. 12, 1060–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aricescu A. R.; Lu W.; Jones E. Y. (2006) A time- and cost-efficient system for high-level protein production in mammalian cells. Acta Crystallogr. 62, 1243–1250. [DOI] [PubMed] [Google Scholar]
- Mueller U.; Darowski N.; Fuchs M. R.; Forster R.; Hellmig M.; Paithankar K. S.; Puhringer S.; Steffien M.; Zocher G.; Weiss M. S. (2012) Facilities for macromolecular crystallography at the Helmholtz-Zentrum Berlin. J. Synchrotron Radiat. 19, 442–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabsch W. (2010) Xds. Acta Crystallogr. 66, 125–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emsley P.; Lohkamp B.; Scott W. G.; Cowtan K. (2010) Features and development of Coot. Acta Crystallogr. 66, 486–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunger A. T. (2007) Version 1.2 of the Crystallography and NMR system. Nat. Protoc. 2, 2728–2733. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


