One hypothesis about how infants process face stimuli is that early mechanisms, which have evolved from our ancestral history, predispose us to initially attend to faces at birth and subsequently to learn about faces via visual experience (Pascalis & Kelly, 2009; Slater & Quinn, 2001). Newborns’ attraction toward typical faces compared to scrambled faces might be an illustration of those early mechanisms. Johnson (2005) has proposed that this early orientation may be driven at birth by a face detector system: CONSPEC, that is a Low Spatial Frequency (LSF) subcortical system responding to very basic information regarding the visual structural characteristics of a human face, such as positive stimulus contrast, a bounded oval, two eyes, a nose, and a mouth. A cortical system, which retains fine details regarding the visual characteristics of individual conspecifics via experience, CONLERN, will emerge around 2 months of age.
If a crude representation of the human face (2 eyes, a nose, and a mouth) attracts infant’s attention, any faces that share the same general arrangement, should have the same power. Heron-Delaney, Wirth, and Pascalis (2011) found, however, a neonatal preference toward human faces compared to macaque faces using colored pictures, concluding that a few days of exposure to human faces was sufficient to allow them to differentiate human from non-human primate faces. Di Giorgio, Leo, Pascalis, and Simion (2012) using similar stimuli equated for low-level perceptual properties failed to replicate such a preference for human faces in newborns. However, Di Giorgio, Meary, Pascalis, and Simion (2013) reported it as early as 3 months of age. In the latter study, most of the infants’ fixations were toward the eye region of the faces, but infants were even more focused on the eye area of the human faces.
The scanning path observed in Di Giorgio et al. (2013) suggests that human eyes engaged the visual attention of 3-month-olds more so than monkey eyes. An attraction toward human eyes early in development would be consistent with the fact that the human eye is distinctive relative to that in non-human primates and other animals: It has a widely exposed white sclera that is paler than the facial skin or iris (Kobayashi & Kohshima, 1997). The unique anatomical evolution of the human eye may be linked to the emergence of an elaborate system of social cognition (Emery, 2000). This hypothesis is in line with Baron-Cohen’s theory (1995) that human eyes play a predominant role in the early face processing system via the existence of an “Eye Direction Detector”. The system first detects the presence of eyes, and then codes their direction. The first of these functions was argued by Baron-Cohen to be innate, whereas the second should emerge later.
An issue raised by the prior work on face preference and attention to the eyes is whether the early preference for human faces is driven mainly by a preference for human eyes. The importance of eyes for categorization and social cognition has been well documented in adults. Eyes attract attention, convey an extensive amount of information, and are well known to play a central role in face processing and social communication in general (Emery, 2000). Adults ‘electrophysiological studies suggest that the early perceptual stage of face processing, elicited approximately 170 milliseconds after face presentation, is driven by the eye region, which would be processed first, between 100 and 150 milliseconds (e.g., Bentin, Allison, Puce, Perez, & McCarthy, 1996; Itier, Alain, Sedore, & McIntosh, 2007; Schyns, Petro, & Smith, 2007). Interestingly, it has been shown that the early perceptual stage of face processing is human specific and been suggested that human eyes contribute to a large extent to this species-specificity (Itier, Van Roon, & Alain, 2011; Shibata et al., 2002).
The importance of the eye region in face processing is also supported by empirical studies in infants. Newborns prefer to look at faces with open eyes or gaze directed straight at them when paired with the same faces with closed eyes or averted gaze respectively (Batki, Baron-Cohen, Wheelwright, Connellan, & Ahluwalia, 2000; Farroni, Csibra, Simion, & Johnson, 2002). Farroni and collaborators (2005) have shown that the preference for upright schematic faces in newborns (Simion, Turati, Valenza, & Leo, 2006) requires the contrast polarity characteristic of the eyes. Finally, electrophysiological data indicate that the brain response to isolated eyes is mature well before the brain response to a full face, suggesting that the importance of the eyes may be even more apparent early in development than in adults (Taylor, Edmonds, McCarthy, & Allison, 2001). Taken together, these studies reveal the crucial role of the eye region from an early age.
However, an attraction for human eyes per se, which could drive the preference observed for human faces compared to animal faces, has not been directly shown in newborns or older infants. Regardless of the reason for such attraction (e.g., low-level visual cues or a more sophisticated human eye detector), our main objective was to determine whether human eyes can trigger a heightened level of attention early in development. The current study aims to investigate this question and determine whether and when the importance and attraction for human eyes might appear during the first year after birth. We created stimuli that differed only by the presence or absence of human eyes. To avoid interference from the overall structure of the human face, we inserted human eyes into a monkey or ape face, which has the advantage of having a structure similar to human faces, but with eyes that do not present the human species’ specificity (e.g., sclera). If human eyes per se trigger a heightened level of attention, infants should orient more toward a non-human primate face with human eyes (HumanEyes-Face) when paired with the original non-human primate face with non-human eyes (NonHumanEyes-Face). Furthermore, given the high contrast of the human eye, such behavior would be expected from the first week after birth. We thus tested newborns along with 3-, 6-, 9-, and 12-month-olds.
Method
Participants
One hundred and forty five healthy, full-term newborns and 3-, 6-, 9-, and 12-month-old infants were included in the final analysis. There were 29 newborns (Mean age = 3 days, SD = 1; 16 females), 30 3-month-olds (Mean age = 113 days, SD = 5, 16 females), 30 6- month-olds (Mean age = 194 days, SD = 5; 15 females), 30 9-month-olds (Mean age = 283 days, SD = 6; 13 females), and 26 12-month-olds (Mean age = 376 days, SD = 7; 11 females). A further 85 infants were eliminated from the analysis due to technical problems or mother’s interference during recording (6 newborns, 6 3-month-olds, 5 6-month-olds, 1 9-month-olds, and 2 12-month-olds), changing state during the test (8 newborns, 2 3-month-olds, 7 6-month-olds, 6 9-month-olds, and 1 12-month-old), strong position bias (i.e., the child looked in one direction for more than 95% of the time; 11 newborns, 5 3-month-olds, 9 6-month-olds, 2 9-month-olds, and 2 12-month-olds), or insufficient looking time toward the stimuli (3 newborns, 4 3-month-olds, 2 6-month-olds, and 3 9-month-olds). An additional 18 infants were randomly eliminated from the analysis in order to equalize sample sizes.
Stimuli
Stimuli were fully colored face stimuli of three Barbary macaques and three chimpanzees (21 cm × 17 cm i.e., 41° × 33° for newborns and 20° × 16° for older infants). Non-human primate faces were duplicated by replacing the original eyes with human eyes. Two different exemplars of human eyes were used to create two pairs of stimuli for each individual, pairing the face with original eyes with the face with human eyes. This yielded a total of 12 different pairs of stimuli (2 species × 3 individuals × 2 pairs of human eyes). Analysis of the low-level visual properties of the pictures showed that inserting human eyes resulted in a significant increase of luminance and contrast in the eye region (Figure 1).
Figure 1.
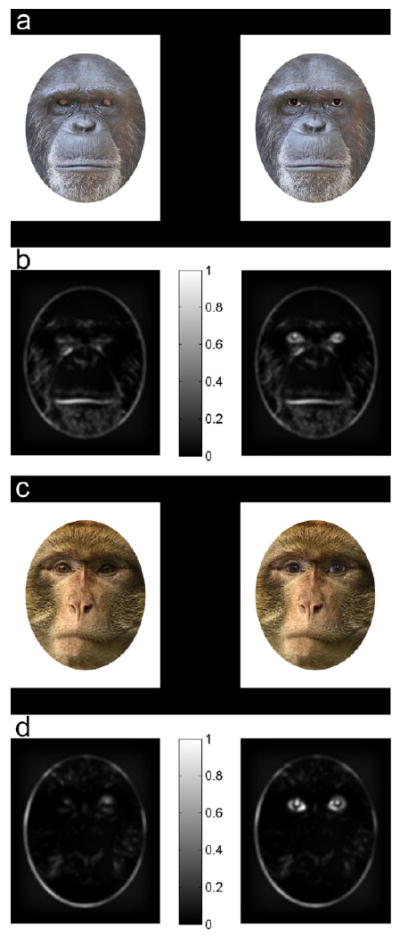
Experimental stimuli (a, c) and their averaged saliency maps (b, d). Chimpanzee (a) and Barbary macaque (c) faces with their original eyes and human eyes were prepared using Adobe Photoshop 12.0. Inserting human eyes resulted in a slight but significant increase of luminance in the eye region compared to non-human primate faces with original eyes (23.36 candela/m2 for NonHumanEyes-face vs. 25.17 candela/m2 for HumanEyes-face, t(5) = 3.02, p < .05). Luminance values were estimated for each face on the basis of spectrophotometric measurements of screen emittance (SpectraScan PR650, PhotoResearch). With respect to contrast, the effect of inserting human eyes into a non-human primate face is illustrated here by the Chimpanzee (b) and Barbary macaque (d) saliency maps, expressed in arbitrary units normalized for the maximum saliency found over the set of stimuli (Ho Phuoc, Guyader, & Guérin-dugué, 2010). As shown, inserting human eyes into non-human primate faces resulted in a significant increase of contrast in the eye region where saliency for each pair was, on average, multiplied by 2.8 compared to stimuli with original eyes (t[5] = 6.81, p < .005).1
Procedure
Overall, the same procedure was used for all age groups. The infants were tested in a quiet room where they were seated on their parent’s lap approximately 60 cm (30 cm for newborns) away from a screen onto which the pairs of face stimuli were projected using E-prime2 software. All parents were instructed to fixate centrally above the screen and to remain quiet during testing. A video camera (specialized for low light conditions) was used to film the infant’s eye movements during stimulus presentation. The film was then digitized to be analyzed offline, frame by frame, by two blind independent observers. Inter-observer agreement was calculated on 33% of the participants from the final sample and showed high reliability (Pearson r = .96).
Half of the participants saw chimpanzees’ faces, and the other half saw macaques’ faces. There were two test trials during which two pairs of different individuals were presented. Images were displayed side-by-side, separated with a 13 cm gap. The different pairs of stimuli were counterbalanced across participants. The left-right position of the original and human eyes was counterbalanced across infants on the first trial and reversed on the following trial.
Before each trial, an attention getter attracted infant’ gaze toward the screen middle. The trial started when the infant looked at one of the two stimuli and ended after 10 seconds had elapsed for the 3-, 6-, 9-, and 12-month-olds. For newborns, it ended after a cumulative 10 seconds of looking time duration. This procedural difference in presentation time is not unusual (e.g., Quinn et al., 2008) and allowed us to obtain maximum as well as similar looking durations for newborns and older infants (see Figure 2).
Figure 2.
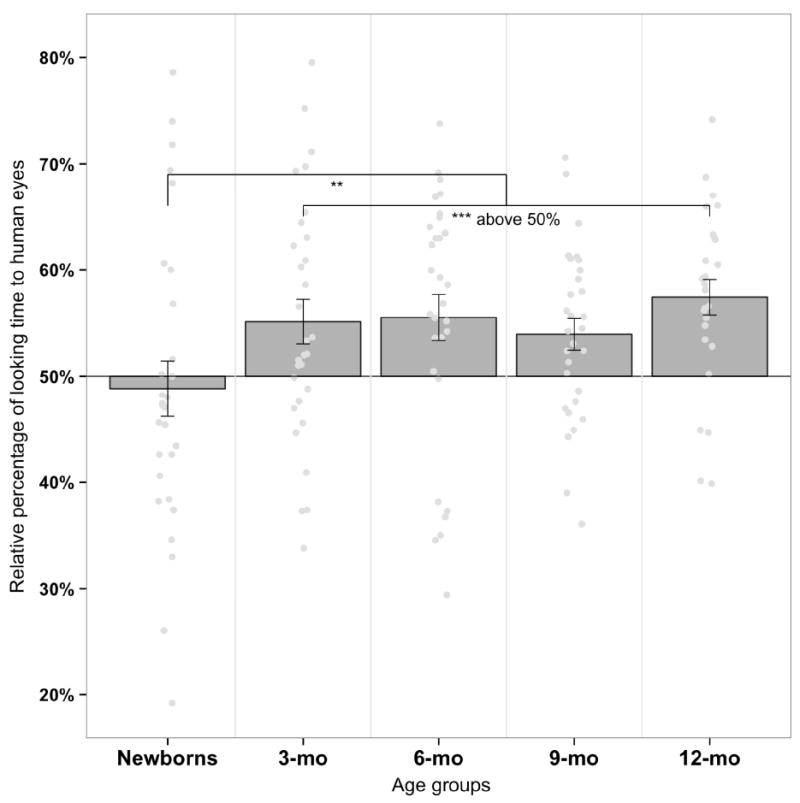
Percentage of time spent looking at the HumanEyes-Face for each age group. Dark grey bars indicate mean scores (± SE) while light grey dots illustrate individual scores. Percentage scores greater than 50% mean that infants looked longer toward non-human primate faces with human eyes than non-human primate faces with their original eyes. Those percentages were relative to the total time spent looking at the stimuli (i.e., both faces), which was high and similar in each age group: Newborns spent 10 seconds looking at the stimuli (as required in the procedure) while the mean total looking time in older infants reached 8.8 seconds (SD = 1) for 3-month-olds, 8.6 seconds (SD = 0.7) for 6-month-olds, 8.6 seconds (SD = 0.8) for 9-month-olds, and 8.8 seconds (SD = 1) for 12-month-olds. As shown, older infants looked significantly longer at the HumanEyes-Face with a mean percentage of 55.1% for 3-month-olds, 55.5% for 6-month-olds, 54% for 9-month-olds, and 57.4% for 12- month-olds; *p < .05, **p < .01, ***p < .001. Regarding the individual scores, twenty of the 30 3-month-olds (P[A] = 0.67, p = .07), 23 of the 30 6-month-olds (P[A] = 0.77, p = .004), 21 of the 30 9-month-olds (P[A] = 0.70, p = .029), and 22 of the 26 12-month-olds (P[A] = 0.85, p = .0004) displayed individual preferences for the HumanEyes-Face.
Results
Preference for HumanEyes-Face compared to NonHumanEyes-Face was assessed for each participant by calculating the relative percentage of time spent looking at the HumanEyes-Face. This score corresponded to the summed looking time to HumanEyes-Face divided by the summed looking time to both HumanEyes- and NonHumanEyes-Faces, converted then to a percentage score. On average, newborns spent 49% of the time looking at HumanEyes-Face while older infants looked longer at the HumanEyes-Face with mean scores going above 50% (see Figure 2). An ANOVA with Age (Newborns, 3-, 6-, 9-, and 12-month-olds) and Species (Chimpanzee vs. Macaque) as between-subject factors was conducted using STATISTICA v10 in order to test whether and when a preference for human eyes appeared during the first year. The analysis revealed a main effect of age (F[4, 135] = 2.52, p < .05, ηp2 = .07). The main effect of species (F[1, 135] = 1.37, p > .05) and the age × species interaction (F[4, 135] < 1) were not significant.
The age effect was further explored using a contrast analysis (decomposition of the omnibus effect in its one degree of freedom components). Bonferroni post-hoc corrections for all possible pairwise comparisons (c = 10) were applied on the alpha threshold because we did not have a clear-cut a-priori hypothesis about when the preference for human eyes might appear. The analysis showed that newborns differed from older infants (i.e., 3-, 6-, 9-, 12-month-olds, F[1, 135] = 8.92, p = .003, ηp2 = .06, αBonferroni-adjusted = .005). It explained 88.5 % of the total variance of the age effect, and the test of the residual treatment (the unexplained variance) was not significant (F[3, 135] < 1), showing that older infants did not differ from each other. The contrast analysis thus showed that the time spent looking at the HumanEyes-Face increased significantly at 3 months after birth and remained stable until the end of the first year.
To confirm that the age effect was associated with the emergence of a reliable preference for HumanEyes-Face in older infants, we tested the mean percentage scores of looking time against chance (i.e., 50%) by performing one sample Student t-tests. Because our design comprised an unusually high number of age groups, performing multiple t-tests, one in each group, would have substantially increased type I or II errors. As the ANOVA revealed a theoretically coherent two-step function, which contrasted newborns and older infants without any species effect, we overcame this problem by performing two independent t-tests, respectively, on newborns and on the combination of older infants’ data, collapsed across species. We applied Bonferroni post-hoc corrections because the combinations of age groups were a posteriori defined. Consistent with the contrast analysis, the newborns’ scores did not differ from 50% (t < 1), showing no preference for any faces whereas older infants’ scores were significantly greater than chance (t[115] = 5.80, p = 1E-07, αBonferoni-adjusted =.01), demonstrating that older infants as a whole systematically preferred looking at HumanEyes-Face. Also, separate analyses in each of these older infant groups confirmed a significant preference for human eyes in all groups (ps < .05). In addition, the proportion of infants displaying individual preference for the human eyes was significantly greater than 50% in each older infant group except for the 3-month-olds in which the proportion was marginally significant (Figure 2).
Discussion
When presented with two non-human primate faces that differed only by the nature of the eyes, human infants, from 3-months of age, looked longer toward the stimuli containing human eyes. No looking preference was, however, observed in newborns. As human infants would not have seen many live monkeys or apes, they would not likely have formed a representation of “normal” non-human primate faces (i.e., with non-human eyes). The “strangeness” of HumanEyes-Face stimuli is thus an improbable explanation of the attraction observed. Our results therefore demonstrate the importance and the attraction of the human eyes for infants, even embedded in a non-human face.
As hypothesized in adult studies, low-level visual cues like the high contrast of the human eyes may largely contribute in making human eyes salient (e.g., Itier et al., 2011). This observation is not contradictory with the emergence of an early elaborate system for detecting eyes (Baron-Cohen, 1995; Emery, 2000): Low-level cues could favor the emergence of such a system and its specialization in humans, but could also help to quickly detect human eyes. To test this hypothesis, one could have equalized the contrast between the human eyes and the non-human primate eyes, but that would have reduced the contrast between the sclera and iris in human eyes, thereby removing the factor that gives human eyes their distinctiveness (Kobayashi & Kohshima, 1997).
Our data have implications for face perception research in general. The fact that human eyes alone, embedded in a non-human primate face, are sufficient to attract an infant’s attention suggests that the infant’s attraction toward human eyes is not entirely driven by human face processing. It suggests that human eyes may explain or at least contribute to the early preference for human faces observed from 3 months of age (Di Giorgio, Meary, Pascalis, & Simion, 2013). In addition, given that the preference for human eyes remains stable after 3 months of age, eyes may remain critical for face processing, at least until the end of the first year. This hypothesis is in agreement with the experimental evidence and theory supporting the critical role and precedence of the eyes in face processing (Bentin et al., 1996; Itier et al., 2007; Schyns et al., 2007; Shibata et al., 2002).
From a developmental perspective, how can we explain the absence of preference in newborns? To discount any explanation based on procedural difference between newborns and older infants (cumulated vs. fixed 10-second trials), we performed additional analyses on the first ten seconds of newborns’ looking time, similar to what was done for older infants. This new analysis still showed no preference for either of the non-human primate faces (main age effect: F[4, 120] = 4.02, p < .05, ηp2 = .12, t-test against chance: t[13] = -1.49, p > .05), thereby rendering as unlikely such an explanation. In agreement with previous findings (Di Giorgio et al., 2013), our study instead supports the idea that attraction for human eyes is not inborn and that it develops during the first months after birth.
A possible explanation for this developmental change could be a lack of sensitivity for the human eyes at birth related to their immature visual system. In effect, newborns might be unable to detect a difference between human eyes and other species’ eyes. A low-level-based capacity to detect human eyes would emerge later with the improvements in acuity, contrast sensitivity, and color vision (Slater, 2001). However, this proposal appears inconsistent with our luminance and contrast analysis showing that infants and newborns should be able to discriminate the NonHumanEyes-Face with the HumanEyes-Face (see Figure 1 and Footnote 1). Alternatively, the lack of sensitivity to human eyes at birth could be related to face context. Human eyes might be of importance, but having them embedded as internal features in a face limits their processing, with neonates paying more attention to the external parts of faces (Pascalis, de Schonen, Morton, Deruelle, & Fabre-Grenet, 1995), and maybe especially when embedded in a non-human primate face. In other words, newborns would be able to detect eyes and their direction in faces (Farroni et al., 2005), but their processing may not be sufficient for the visual system to notice more subtle information like the specificity of human eyes.
A second explanation, also based on face context, is that neonates may not even have attended to the eye region, thereby rendering the side-by-side images identical looking. A general lack of attention to the eye region is unlikely since previous work has shown that eyes are important at birth when embedded in a human-like face context (Farroni et al., 2002; Farroni et al., 2005). Nevertheless, it is possible that attention to the eye region is dependent upon the face context, newborns being inattentive to the eye region when embedded in a non-human primate face with salient external contours emphasized by fur. A possible avenue of further investigation would be to determine with an eye tracking system if newborns even notice the human eye in the non-human primate faces stimuli or if they are just not scanning the non-human primate face with human eyes in the same way as they do human faces.
A third explanation would be that sensitivity for human eyes emerges several months after birth based on accrual of experience with conspecifics. Given the lack of age effect after 3 months of age, it appears that 3 months of exposure to human faces and eyes is sufficient to drive infants’ attraction toward the familiar human eyes even when they are embedded in unfamiliar non-human primate faces. Sensitivity to eyes in general at birth, which may become specialized to humans from 3 months of age, is supported by the well-known idea that the system underlying face perception at birth is broad and develops according to the type of input received, thereby tuning face-space dimensions toward the category to which infants are predominantly exposed (Nelson, 2001). In particular, it is consistent with the data demonstrating the emergence, at 3-months of age, of a preference for faces that match the gender of their primary caregiver (Quinn, Yahr, Kuhn, Slater, & Pascalis, 2002) and for faces of their own-ethnic group (e.g., Kelly et al., 2005). Our results are the first to suggest a preference toward own-species internal features from 3 months of age.
Regardless of which of these or other accounts comes to be confirmed on the basis of additional research, the present investigation indicates that there is a preference for human eyes per se in infancy, which is not likely innate and may instead develop over the course of the first several months after birth.
We investigated infant attraction for human eyes during the first year.
Human eyes were inserted into monkey faces.
Preference for the original picture versus that with human eyes was tested.
No preference was observed in newborns.
Preference for non-human primate faces with human eyes emerged at 3 months.
Acknowledgments
This research was supported by NIH Grant HD-46526. We thank the infants and parents who participated in the study, Valérie Belin, Sylvie Caraby, and all the staff from the maternity Couple-Enfant from of the University Hospital Centre (CHU). We also thank Scott P. Johnson and an anonymous reviewer for their helpful comments on earlier draft of the manuscript.
Footnotes
Because saliency models are derived from an adult model of low-level visual processing, the saliency maps used here can only approximate local energy according to an infant model. The overall lower contrast sensitivity of infants up to 8 months of age and their lack of sensitivity for spatial frequencies over 3 CPD (Banks, 1982) would result in lower saliency values for the high spatial frequency components of the image. Still, the maps capture the gist of the infant saliency maps because the human eyes inserted into the monkey faces subtended about 3 degrees of visual angle for the newborns and about 1.5 degrees for the infants. We can estimate the main spatial frequency of the high contrast region formed by the sclera and iris at respectively 0.5 and 1 CPD for the newborns and infants. In sum, the images are large enough for the detection of the high contrast region formed by the human eyes even for our younger participants.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Banks MS. The development of spatial and temporal contrast sensitivity. Current Eye Research. 1982;2:191–198. doi: 10.3109/02713688208997694. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S. Mindblindness: An essay on autism and theory of mind. Cambridge, MA: MIT Press; 1995. [Google Scholar]
- Batki A, Baron-Cohen S, Wheelwright S, Connellan J, Ahluwalia J. Is there an innate gaze module? Evidence from human neonates. Infant Behavior & Development. 2000;23:223–229. doi: 10.1016/S0163-63830100037-6. [DOI] [Google Scholar]
- Bentin S, Allison T, Puce A, Perez E, McCarthy G. Electrophysiological studies of face perception in humans. Journal of Cognitive Neuroscience. 1996;8:551–565. doi: 10.1162/jocn.1996.8.6.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Giorgio E, Leo I, Pascalis O, Simion F. Is the face-perception system human-specific at birth? Developmental psychology. 2012;48:1083–1090. doi: 10.1037/a0026521. [DOI] [PubMed] [Google Scholar]
- Di Giorgio E, Meary D, Pascalis G, Simion F. The face perception system becomes species-specific at three months: An eye-tracking study. International Journal of Behavioral Development. 2013;13:95–99. [Google Scholar]
- Emery NJ. The eyes have it: the neuroethology, function and evolution of social gaze. Neuroscience and Biobehavioral Reviews. 2000;24:581–604. doi: 10.1016/S0149-76340000025-7. [DOI] [PubMed] [Google Scholar]
- Farroni T, Csibra G, Simion F, Johnson MH. Eye contact detection in humans from birth. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:9602–9605. doi: 10.1073/pnas.152159999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farroni T, Johnson MH, Menon E, Zulian L, Faraguna D, Csibra G. Newborns’ preference for face-relevant stimuli: effects of contrast polarity. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:17245–17250. doi: 10.1073/pnas.0502205102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heron-Delaney M, Wirth S, Pascalis O. Infants’ knowledge of their own species. Philosophical Transactions of the Royal Society of London Series B, Biological sciences. 2011;366:1753–1763. doi: 10.1098/rstb.2010.0371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho Phuoc T, Guyader N, Guérin-dugué A. A functional and statistical bottom-up saliency model to reveal the relative contributions of low-level visual guiding factors. Cognitive Computation. 2010;2:344–359. [Google Scholar]
- Itier RJ, Alain C, Sedore K, McIntosh AR. Early face processing specificity: It’s in the eyes! Journal of Cognitive Neuroscience. 2007;19:1815–1826. doi: 10.1162/jocn.2007.19.11.1815. [DOI] [PubMed] [Google Scholar]
- Itier RJ, Van Roon P, Alain C. Species sensitivity of early face and eye processing. Neuroimage. 2011;54:705–713. doi: 10.1016/j.neuroimage.2010.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MH. Subcortical face processing. Nature Reviews Neuroscience. 2005;6:766–774. doi: 10.1038/Nrn1766. [DOI] [PubMed] [Google Scholar]
- Kelly DJ, Quinn PC, Slater AM, Lee K, Gibson A, Smith M, Pascalis O. Three-month-olds, but not newborns, prefer own-race faces. Developmental Science. 2005;8:F31–F36. doi: 10.1111/J.1467-7687.2005.0434a.X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi H, Kohshima S. Unique morphology of the human eye. Nature. 1997;387:767–768. doi: 10.1038/42842. [DOI] [PubMed] [Google Scholar]
- Nelson CA. The development and neural bases of face recognition. Infant and Child Development. 2001;10:3–18. doi: 10.1002/Icd.239. [DOI] [Google Scholar]
- Pascalis O, de Schonen S, Morton J, Deruelle C, Fabre-Grenet M. Mother’s face recognition by neonates: A replication and an extension. Infant Behavior and Development. 1995;18:79–85. [Google Scholar]
- Pascalis O, Kelly DJ. The origins of face processing in humans: Phylogeny and ontogeny. Perspectives on Psychological Science. 2009;4:200–209. doi: 10.1111/J.1745-6924.2009.01119.X. [DOI] [PubMed] [Google Scholar]
- Quinn PC, Uttley L, Lee K, Gibson A, Smith M, Slater AM, Pascalis O. Infant preference for female faces occurs for same- but not other-race faces. Journal of Neuropsychology (Special Issue on Face Processing) 2008;2:15–26. doi: 10.1348/174866407x231029. [DOI] [PubMed] [Google Scholar]
- Quinn PC, Yahr J, Kuhn A, Slater AM, Pascalis O. Representation of the gender of human faces by infants: A preference for female. Perception. 2002;31:1109–1121. doi: 10.1068/P3331. [DOI] [PubMed] [Google Scholar]
- Schyns PG, Petro LS, Smith ML. Dynamics of visual information integration in the brain for categorizing facial expressions. Current Biology. 2007;17:1580–1585. doi: 10.1016/j.cub.2007.08.048. [DOI] [PubMed] [Google Scholar]
- Shibata T, Nishijo H, Tamura R, Miyamoto K, Eifuku S, Endo S, Ono T. Generators of visual evoked potentials for faces and eyes in the human brain as determined by dipole localization. Brain Topography. 2002;15:51–63. doi: 10.1023/a:1019944607316. [DOI] [PubMed] [Google Scholar]
- Simion F, Turati C, Valenza E, Leo I. The emergence of cognitive specialization in infancy: The case of face preference. In: Munakata Y, Johnson MH, editors. Processes of change in brain and cognitive development: Attention and performance XXI. Oxford: Oxford Univ. Press; 2006. pp. 189–208. [Google Scholar]
- Slater A. Visual perception. In: Bremner G, Fogel A, editors. Backwell handbook of infant development. Oxford, UK: Blackwell Publishers; 2001. pp. 5–34. [Google Scholar]
- Slater A, Quinn PC. Face recognition in the newborn infant. Infant and Child Development. 2001;10:21–24. doi: 10.1002/Icd.241. [DOI] [Google Scholar]
- Taylor MJ, Edmonds GE, McCarthy G, Allison T. Eyes first! Eye processing develops before face processing in children. Neuroreport. 2001;12:1671–1676. doi: 10.1097/00001756-200106130-00031. [DOI] [PubMed] [Google Scholar]


