Abstract
T cell recognition of antigen presenting cells depends on their expression of a spectrum of peptides bound to Major Histocompatibility Complex class I (MHC-I) and class II (MHC-II) molecules. Conversion of antigens from pathogens or transformed cells into MHC-I and MHC-II-bound peptides is critical for mounting protective T cell responses, and similar processing of self proteins is necessary to establish and maintain tolerance. Cells use a variety of mechanisms to acquire protein antigens, from translation in the cytosol to variations on the theme of endocytosis, and to degrade them once acquired. In this review we highlight the aspects of MHC-I and MHC-II biosynthesis and assembly that have evolved to intersect these pathways and sample the peptides that are produced.
Keywords: Cross-presentation, MHC class I, MHC class II, proteolysis, peptide
INTRODUCTION
The T cell arm of the adaptive immune response has evolved to recognize the products of partial intracellular proteolysis. CD8+ T cells recognize protein-derived peptides in association with Major Histocompatibility Complex (MHC) class I molecules (MHC-I) while CD4+ T cells recognize peptides bound to MHC class II molecules (MHC-II). There are also T cells that recognize lipid antigens associated with CD1 molecules (1), but CD1 functions and the processing mechanisms that regulate their interaction with lipids will not be considered here.
All vertebrates possess an MHC, a large multigenic region with many conserved genes in addition to MHC-I and MHC-II molecules. Some of these encode products essential to MHC-I and MHC–II function. In many species the MHC encodes multiple MHC-I and MHC-II molecules, presumed to have arisen by gene duplication. For example, in mice, depending on the strain, there are two to three genes encoding ‘classical’ MHC-I molecules, called H2-D, -K and –L, within the H2 complex, and most strains have two MHC-II molecules, called I-A and I-E. Humans have three ‘classical’ MHC-I genes within the HLA complex, called HLA-A, -B, and –C, and there are three MHC-II molecules, called HLA-DR, -DQ, and –DP. In both mice and man there are other class I genes present in the MHC. These are known as class Ib genes and are discussed elsewhere in this volume (2).
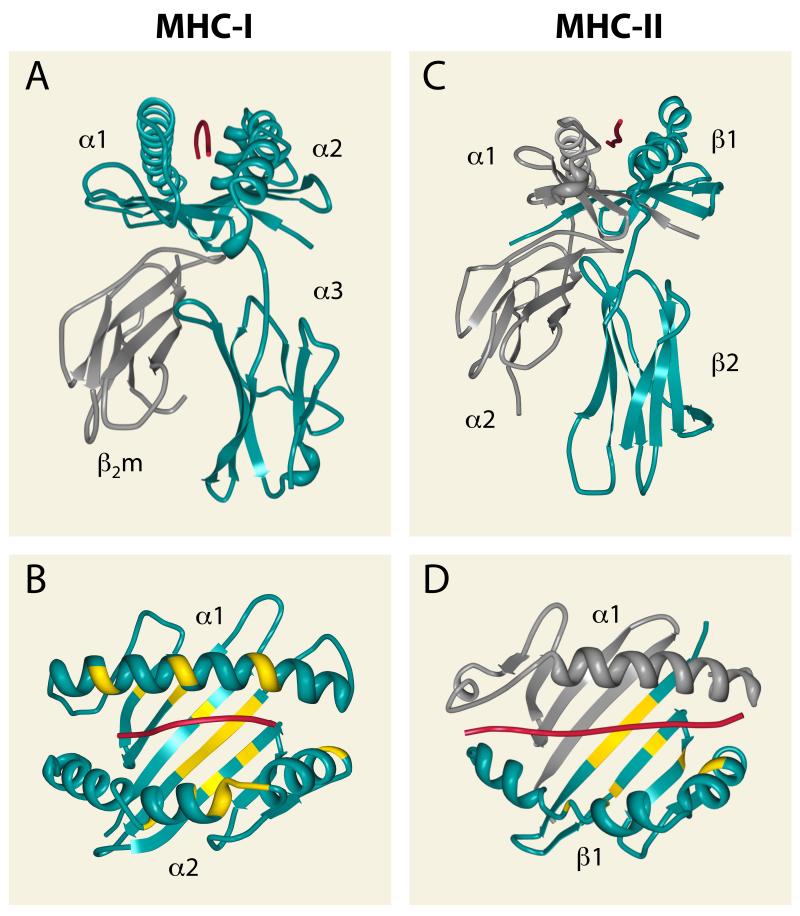
Multiple structures of MHC-I and MHC-II molecules have been determined, and a schematic structure of each is presented in Figure 1. MHC-I and MHC-II genes exhibit enormous allelic polymorphism, and amino acid sequence variation is heavily concentrated in the part of each structure that interacts with peptides, allowing different alleles to bind a different range of peptides. The peptide-binding structure consists of a membrane-distal groove formed by two anti-parallel α-helices overlaying an eight-strand β-sheet. In the case of MHC-I the groove corresponds to a contiguous amino acid sequence formed by the N-terminal region of the single MHC-encoded subunit, or heavy chain, while for MHC-II it is formed by the juxtaposition of the N-terminal regions of two MHC-encoded α- and β-chains. For both molecules the membrane-proximal region consists of two conserved domains that are homologous to Ig constant region domains. For MHC-I, one is provided by the heavy chain and the other is a separate protein, β2-microglobulin (β2m), a soluble product of a non-MHC-linked gene. For MHC-II one conserved domain is part of the α-subunit and the other part of the β-subunit. The MHC-I heavy chain and the MHC -II α- and β-subunits are transmembrane glycoproteins with short cytoplasmic domains. The theme that emerges is that MHC-I and -II molecules each have a structurally homologous platform capable of binding peptides with very high affinity that can engage the T cell receptor. A significant difference is that for MHC-I the peptide is confined by binding groove interactions at both the N and C-termini, while for MHC-II each end of the peptide can overhang the binding groove.
Figure 1.
Three dimensional structures of MHC-I and MHC-II molecules with peptide ligands. A and B) Structure of the MHC-I molecule (HLA-A2 complexed with residues 58-66 of the influenza matrix protein, (229)). MHC-I heavy chain, blue; β2m, grey; peptide, red. C and D) Structure of the MHC-II molecule (HLA-DR1 complexed with residues 306-318 of influenza haemagglutinin, (230)). MHC-II α chain, grey; MHC-II β chain, blue; peptide, red. Ribbon diagrams were generated with the Protein Workshop software available from the RSCB Protein Data Bank (http://www.rcsb.org). Highly polymorphic residues of HLA-A (B) and HLA-DR (D) proximal to the peptide binding groove (http://hla.alleles.org) are highlighted in yellow. Note that polymorphism of the MHC-II alpha chains is limited, and they are essentially non-polymorphic for HLA-DR alpha chains.
Peptides are the products of proteolysis, and there are two major proteolytic systems operating within the cell that contribute to MHC-dependent T cell recognition (Figure 2). In the cytosol the vast majority of proteolysis is mediated by the proteasome. The proteasome (reviewed in (3)) will not be discussed extensively, but in brief its core is a barrel-shaped 20S structure consisting of four stacked rings of seven subunits each. The outer rings are composed of α-subunits and the middle two of β-subunits, three of which, β1, β2 and β5, constitute the active proteolytic components. Variants of the active β-subunits are induced by interferon-γ (IFN-γ) and replace the constitutive versions. These were historically called LMP1, LMP2 and MECL1, and the genes encoding LMP1 and LMP2 are MHC-linked. Commonly the IFN-γ–inducible subunits are now called β1i, β2i and β5i, and proteasomes that contain them are called immunoproteasomes. The cleavage specificities of standard protaseomes and immunoproteosomes differ. The 20S core is capped at each end by an additional 19S multi-subunit complex that recognizes ubiquitin-conjugated proteins targeted for degradation. The 19S component has deubiquitinase activity and an unfoldase activity that allows the targeted proteins to enter the channel in the center of the barrel where the β-subunit active sites reside. The unfolding function, in particular, necessitates that proteolysis by the capped (26S) proteasome is ATP-dependent. There is an alternative capping structure (11S) comprised of a different set of IFN-γ-inducible proteins that allow a level of ATP-independent proteolysis of peptides but not of folded proteins. The end products of proteolysis by the 26S proteasome (20S plus 19S) form the dominant source of peptides for MHC-I binding.
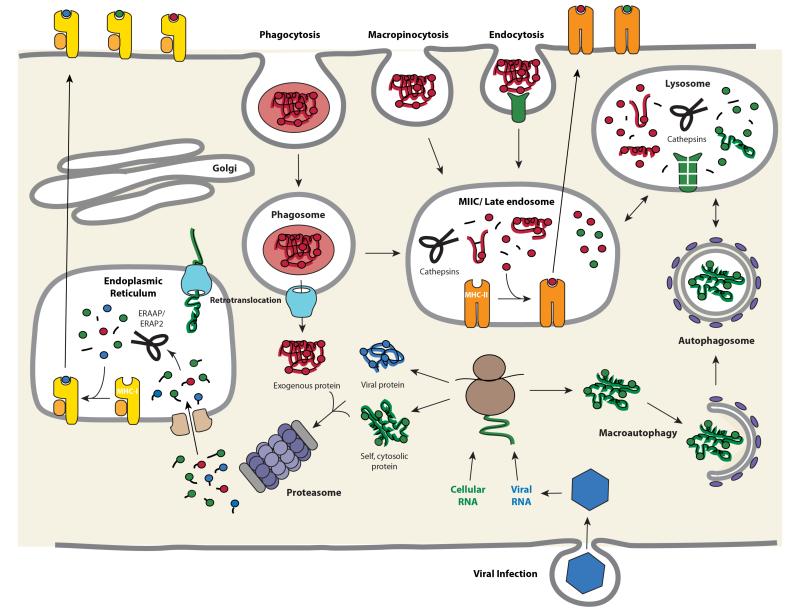
Figure 2.
Trafficking of antigens for processing and presentation with MHC molecules: basic pathways and exceptions to the “rules”. Cytosolic proteins are processed primarily by the action of the proteasome. The short peptides are then transported into the ER by TAP for subsequent assembly with MHC-I molecules. In certain antigen presenting cells, particularly dendritic cells, exogenous proteins can also be fed into this pathway by retrotranslocation from phagosomes, a phenomenon known as cross-presentation. The retrotranslocation channels may be recruited from the ER, where they are used for ER-associated degradation, or ERAD, of misfolded transmembrane or secretory proteins. Exogenous proteins, however, are primarily presented by MHC-II molecules. Antigens are internalized by several pathways, including phagocytosis, macropinocytosis, and endocytosis, and eventually traffic to a mature or late endosomal compartment where they are processed and loaded onto MHC-II molecules. Cytoplasmic/nuclear antigens can also be trafficked into the endosomal network via autophagy for subsequent processing and presentation with MHC-II molecules.
Proteins that are internalized by a cell from exogenous sources are degraded by lysosomal proteolysis (Figure 2). In brief, endocytosed proteins enter a vesicular pathway consisting of progressively more acidic and proteolytically active compartments classically described as early endosomes, late endosomes and lysosomes (4). Particles internalized by phagocytosis follow a similar path, terminating in phagolysosomes that are formed by the fusion of phagosomes and lysosomes. Lysosomes and phagolysosomes have a pH of 4 to 4.5, and contain a number of acid pH-optimum proteases generically called cathepsins (5). In highly degradative cells such as macrophages, successive cleavages by these enzymes result in very short peptides and free amino acids that are translocated into the cytosol to replenish tRNAs for new protein synthesis, but in less proteolytically active antigen presenting cells (APCs), larger intermediates form the dominant source of peptides for MHC-II binding.
The trafficking of exogenous and endogenous proteins for antigen processing and presentation are summarized in Figure 2. In general, MHC-I molecules bind peptides generated by proteasomal proteolysis and they bind them in the endoplasmic reticulum (ER) after the peptides are translocated from the cytosol. Peptide binding by MHC-I is integrated into the assembly pathway of the heavy chain-β2m dimer. MHC-II molecules generally bind peptides generated by lysosomal proteolysis in the endocytic and phagocytic pathways. However, both can access peptides from endogenous and exogenous antigens. For example, MHC-II binds peptides derived from endogenous membrane proteins that are degraded in the lysosome. In addition, MHC-I can bind peptides derived from exogenous proteins internalized by endocytosis or phagocytosis, a phenomenon called cross-presentation. Specific subsets of dendritic cells (DCs) are particularly adept at mediating this process, which is critically important for the initiation of a primary response by naïve CD8+ T cells when it is termed cross-priming.
PEPTIDE BINDING TO MHC-I MOLECULES
Peptides generated in the cytosol are translocated into the ER by the Transporter associated with Antigen Processing (TAP), which is a member of the ATP-Binding Cassette (ABC) family of transporters (6). TAP is a heterodimeric protein, and the TAP1 and TAP2 subunits are encoded by closely linked genes in the MHC. These are widely distributed in both prokaryotes and eukaryotes and transfer a variety of molecules across membranes. Biochemical evidence combined with molecular modeling suggests that each TAP subunit consist of a central core domain of 6 transmembrane α-helices, which constitute the channel, that is immediately N-terminal to the Nucleotide Binding Domain (NBD) (7). The NBD structure is known for TAP1 and it is similar to that of other ABC family members, with the classical Walker A and B motifs present in many ATPases (8). Cytosolic loops in the core domains that are proximal to the NBDs constitute the peptide recognition site, and ATP hydrolysis mediates the translocation event (7). Both subunits have additional N-terminal domains (N-domains), comprising 4 transmembrane segments for TAP1 and 3 for TAP2, which have no counterparts in other members of the ABC family of transporters (7).
The TAP heterodimer associates with a number of other proteins to form the Peptide Loading Complex, or PLC (Figure 3). The transmembrane glycoprotein tapasin, which is encoded by an MHC-linked gene (9), interacts within the membrane with the N- domains (10-13). Tapasin has a bridging function, recruiting MHC-I-β2m dimers and the chaperone calreticulin (CRT) to the PLC (14). Recent experiments have confirmed that there are two tapasin molecules in the PLC, one associated with each TAP subunit (13, 15). Tapasin in turn is stably linked via a disulfide bond to a second molecule, the protein disulfide isomerase homologue ERp57, and the structure of the lumenal region of human tapasin conjugated to ERp57 has been solved (16). The N-terminal domain of tapasin consists of a β barrel fused to an Ig-like domain, and, as for the MHC-I and -II proteins, the membrane proximal domain is Ig-like. ERp57 has a slightly twisted U-shaped structure and tapasin is inserted into the U in a way that results in extensive protein-protein interactions with ERp57, particularly with the a and a’ domains, each of which contains a double cysteine ‘CXXC’ motif that constitute its two redox active sites. As predicted by earlier biochemical experiments (17) a disulfide bond connects cysteine 95 of tapasin with cysteine 57 of ERp57, which is the N-terminal cysteine residue of the a domain CXXC motif. Normally, disulfide bonds involving cysteine 57 are transiently formed during the reduction of a disulfide-containing ERp57 substrate protein, and reduction of this enzyme-substrate bond by the second cysteine in the motif releases the substrate. The interactions of tapasin with the a domain and a’ domains appear to trap the disulfide linked species, explaining the stability of the tapasin-ERp57 disulfide bond.
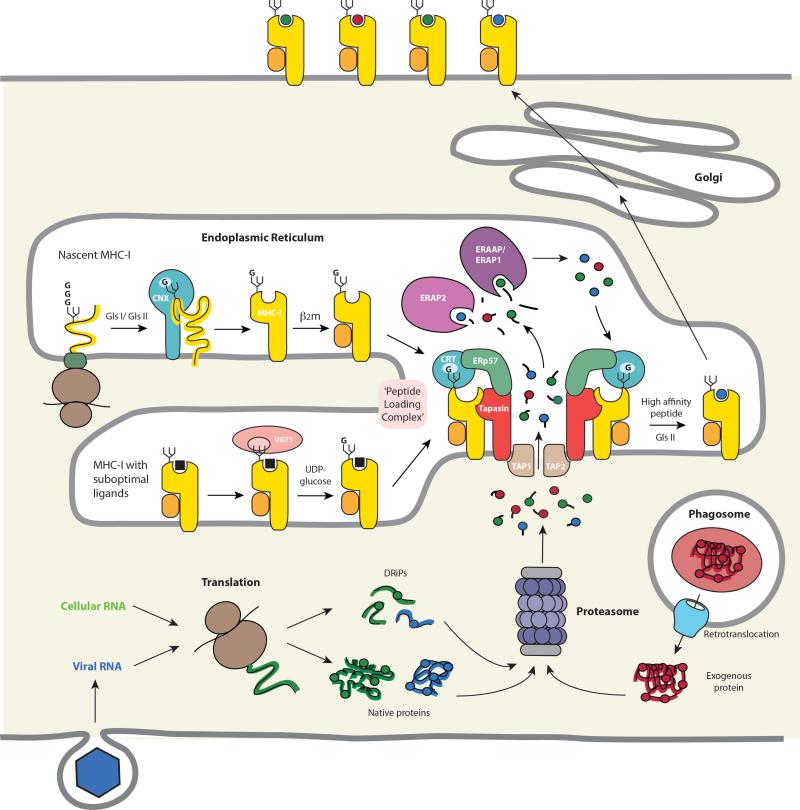
Figure 3.
MHC-I biosynthesis and antigenic peptide binding in the ER. Trimming of the N-linked glycan by glucosidases I and II (GlsI/ GlsII) to a single terminal glucose residue (“G”) permits the interaction of the MHC-I heavy chain with lectin-like chaperones at several stages during folding and assembly. The initial folding events involve the chaperone calnexin (CNX) and allow subsequent assembly with β2m. The empty heterodimer, which is inherently unstable, is then recruited by calreticulin (CRT) via the monoglucosylated N-linked glycan to the PLC. The association of MHC-I/β2m heterodimers with the PLC both stabilizes the empty MHC-I molecule and maintains the binding groove in a conformation that favors high affinity peptide loading. These functions are mediated by direct interactions between the MHC-I heavy chain and tapasin and are supported by coordinating interactions with CRT and ERp57 in the PLC. MHC-I molecules with suboptimal peptides are substrates for UGT1 which reglucosylates the heavy chain glycan, allowing re-entry of the MHC-I into the PLC and exchange for high affinity peptides. Peptides translocated into the ER by TAP originate primarily from the proteasomal degradation of endogenous proteins or DRiPs. These proteins may arise from the translation of either self or foreign (i.e. viral) RNA or, in the case of cross-presentation, by translocation into the cytosol from endosomes or phagosomes. Many of the peptides that are delivered into the ER are longer than the 8-10 residues preferred by MHC-I molecules and undergo trimming by ER aminopeptidases known as ERAAP/ERAP1 and ERAP2. Finally, high affinity peptides bind preferentially to MHC-I molecules in the PLC by a tapasin-mediated editing process, MHC-I-peptide complexes are released and then transit to the cell surface for T cell recognition by CD8+ T cells.
ERp57 assists the folding of newly synthesized glycoproteins in the ER by mediating disulfide bond isomerization. Its specificity for glycoproteins results from its ability to associate via its b’ domain with CRT and a second lectin-like ER chaperone, the transmembrane CRT homologue calnexin (CNX). Both CNX and CRT are important in MHC-I assembly (Figure 3). CNX and CRT normally function in a quality control cycle that depends on their interactions with the N-linked glycans of the glycoproteins (18). They then recruit ERp57, which mediates proper disulfide bond formation in the folding glycoprotein. Glycan binding to CNX or CRT is dependent on the precise structure of the N-linked glycan, which must bear a single terminal glucose residue and is a biosynthetic intermediate maintained in this form by the competing actions of two enzymes. One, glucosidase II, removes the glucose and the other, UDP-glucose glycoprotein transferase-1 (UGT1), replaces the glucose only if the glycoprotein bearing the glycan is partially unfolded (19-21). This cycle does play a role in MHC-I peptide loading (Figure 3), but the one step that does not appear to be involved is the reduction-oxidation cycle mediated by ERp57 (see below).
Cells that lack TAP1 or TAP2 do not form MHC-I-peptide complexes because no peptides are imported into the ER. There are a few published exceptions to this rule, some of which lead to CD8+ T cell recognition (22, 23), but the only major one, in terms of quantitative effects on MHC-I assembly, is the unusual and specific ability of HLA-A2 molecules to bind peptides derived from signal sequences of certain ER-targeted molecules (24). Because of the inherent instability of ‘empty’ MHC-I molecules, and because they do not fold into a transport-competent structure in the ER, TAP-negative cells express very little surface MHC-I. Cells that lack tapasin also exhibit reduced surface MHC-I, but the defect is much less drastic than in TAP-negative cells and the magnitude of the effect depends on the individual MHC-I allele expressed (25, 26). Data from tapasin knockout mice showed an essential function for tapasin in generating CD8+ T cell responses, and data based on T cell recognition demonstrated that tapasin plays a ‘peptide editing’ role, mediating the binding of high affinity peptides at the expense of peptides with lower but still significant affinity, and that because of this surface MHC-I molecules on tapasin-negative cells are less stable than those on tapasin-positive cells (27-29). Subsequently, in vitro data produced using recombinant tapasin-ERp57 conjugates confirmed that tapasin facilitates high affinity peptide binding and further showed that its association with ERp57 is essential (30). The addition of tapasin-ERp57 conjugates to extracts of human tapasin-negative cells expressing HLA-B8 was found to facilitate the binding of added high affinity peptides to HLA-B8-β2m dimers. Lower affinity peptides were much less successful competitors for binding in the presence of the conjugate than in its absence, indicative of a peptide editing effect. The tapasin-ERp57 conjugate was also found to mediate peptide binding to purified, soluble, recombinant HLA-B8-β2m dimers provided the HLA-B8 molecules expressed a monoglucosylated N-linked glycan (31). While this reaction depended on the addition of recombinant CRT, presumably to provide a bridge between MHC-I and the tapasin-associated ERp57, no other components were required. In a more simplified in vitro system neither CRT nor tapasin-associated ERp57 were needed for peptide binding when the MHC-I heavy chain and tapasin were artificially coupled by the addition of leucine zippers to their C-termini (32).
ERp57-negative cells, as well as CRT-negative cells, also have reduced numbers of MHC-I molecules on the cell surface (33, 34). The initial identification of ERp57 in the PLC led to considerable speculation suggesting that its redox activity was important for generating stable MHC-I-peptide complexes. However, the structural data indicated that tapasin obstructs both of the ERp57 active sites, rendering this unlikely. In fact, when the second active site cysteine in the a domain and both active site cysteine residues in the a’ domain were mutated to serine residues, the combined substitutions had no effect on the ability of tapasin to reconstitute MHC-I cell surface expression when it was introduced into an ERp57-deficient cell line (35). This triply mutated ERp57 was still disulfide-linked to tapasin. However, further analysis in both cell-free systems and intact cells using ERp57 mutated in the b’ domain showed that the ability of ERp57 to bind CRT is essential for MHC-I recruitment to the PLC and normal MHC-I peptide loading (31). In addition to the CRT-dependent interactions with the MHC-I glycan and ERp57 that mediate MHC-I binding to the PLC, there is also a direct interaction between MHC-I and tapasin. Mutagenesis of specific tapasin residues and expression of the mutants as recombinant tapasin-ERp57 conjugates revealed a ‘patch’ on the surface of tapasin that binds to the MHC-I molecule, and there was a positive correlation between the relative abilities of different mutants to bind MHC-I and their efficiency in mediating peptide binding to MHC-I in vitro (16). In addition, a tapasin mutant that was non-functional in cell-free assays also failed to function when expressed as a full-length protein in a tapasin-negative cell.
The PLC consists of the TAP heterodimer and two tapasin-ERp57 conjugates, and up to two CRT molecules and MHCI-β2m dimers can be recruited (Figure 3). The MHC class I glycan must be in the monoglucosylated form, consistent with the CRT requirement (31). Cellular expression of UGT1 is essential for optimal MHC-I peptide loading and, in vitro, the enzyme can discriminate between MHC-I molecules bound to high affinity peptides and those associated with lower affinity peptides (36). This suggests a mechanism that resembles the normal CRT/CNX quality control cycle. A plausible model is that there are two discriminatory events that regulate peptide editing (Figure 3). First, after peptide-free MHC-I-β2m dimers bearing a monoglucosylated N-linked glycan are recruited to the PLC by CRT, there is a direct interaction of the MHC-I molecule with tapasin. This interaction is sensitive to the peptide occupancy of the MHC-I molecule such that when a peptide is bound the affinity of the MHC-I interaction with tapasin is reduced, perhaps by a conformational change in the MHC-I heavy chain similar to that proposed to explain the ability of DM molecules to regulate peptide binding to MHC-II (Figure 4, see below). Thus peptide binding induces dissociation of the MHC-I molecule from tapasin, and because the affinity of the CRT interaction with the monoglucosylated MHC-I glycan is low, the glucose residue becomes accessible to the enzyme glucosidase II, which removes it. If the peptide affinity is sufficiently high, the MHC-I molecule can be transported from the ER through the Golgi apparatus and ultimately to the cell surface. If the affinity of the peptide is low there are two possible scenarios for the second stage. Either the peptide dissociates and the transiently ‘empty’ MHC-I molecule now becomes a substrate for UGT1 and glucose is added back to the N-linked glycan, or the UGT1 can recognize that the conformation of the MHC-I-peptide complex is in some way imperfect and re-glucosylates the glycan. In either case the consequence of the addition of the glucose residue is that the MHC-I molecule re-associates with CRT, re-integrates completely into the PLC, and is subjected to further rounds of tapasin-mediated peptide binding and selection. Ultimately the MHC-I molecule will escape with a high affinity peptide, or, in common with other glycoproteins that are subject to the CRT/CNX/ERp57 quality control cycle, enzymatic removal of mannose residues from the N-linked glycan will render it unsusceptible to re-glucosylation by UGT1. This acts as a ‘timer’, leading to irreversible dissociation of the MHC-I from the PLC and its degradation by the ER-associated degradation (ERAD) pathway (37).
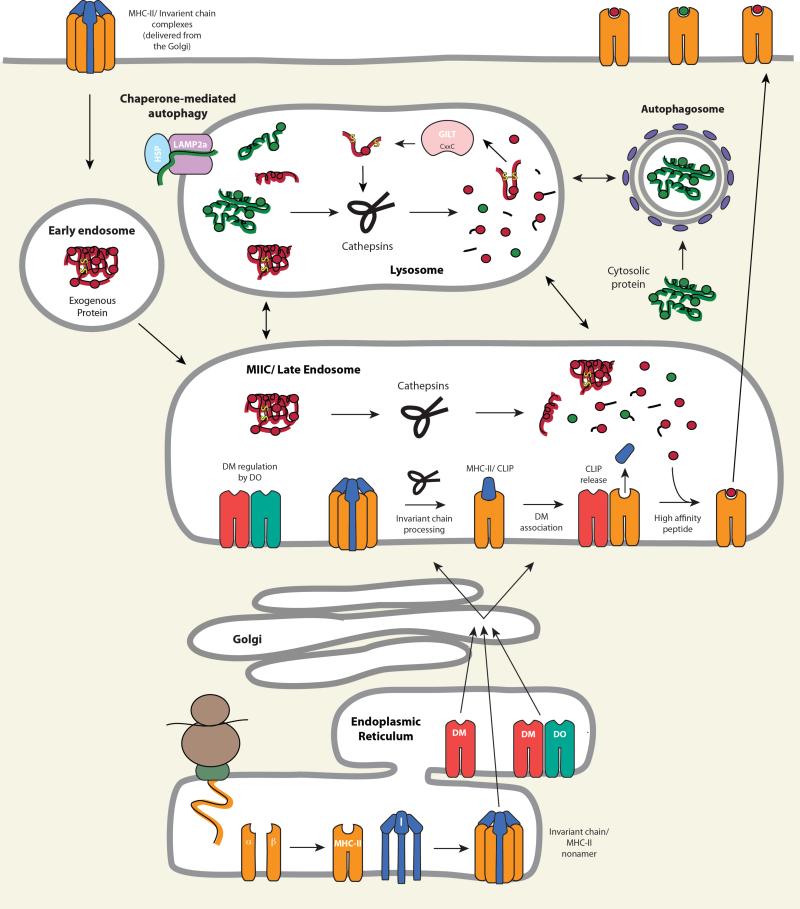
Figure 4.
MHC-II biosynthesis and antigenic peptide binding in the endocytic pathway. MHC-II α and β associate with I chain trimers to form nonamers. These complexes transit to mature endosomes either via the TGN or by recycling from the cell surface. Within endosomes, I chain is sequentially proteolyzed to yield the residual I chain fragment, CLIP. Displacement of CLIP from the ligand groove of MHC-II αβ is mediated by DM and blunted by DO. Expression of DO and regulation of DM function involves the assembly of DM-DO complexes in the ER and co-transport to endocytic compartments. Antigens delivered to late endosomes by phagocytosis, pinocytosis, endocytosis, and autophagy, are processed by cathepsins and the thiol oxidoreductase GILT, and acquisition of high affinity peptides by MHC-II is facilitated by DM. The MHC-II-peptide complexes are subsequently transported to the cell surface for T cell recognition by CD4+ T cells.
One other ER luminal component that is critically important for the proper generation of MHC-I-peptide complexes is an aminopeptidase, in the mouse called ER Aminopeptidase associated with Antigen Processing (ERAAP) and in humans called ER Aminopeptidase-1 (ERAP1) (Figure 3) (38, 39). A second aminopeptidase, ERAP2, is present in humans but not in mice and can also play a role (40). Peptides associated with MHC-I are generally 8-10 amino acids in length, but TAP can translocate peptides into the ER that are significantly longer (41). These peptides can be amino-terminally trimmed in the ER by ERAAP/ERAP1 to yield peptides of the appropriate length for MHC-I binding. A structural change required for cleavage that can only be induced by a longer peptide prevents ERAP1 from ‘overtrimming’ TAP-translocated peptides to a length that would eliminate their ability to bind MHC-I (42). Many of the peptides associated with MHC-I molecules expressed on cells derived from ERAAP knockout mice are elongated, and the MHC-I molecules are relatively unstable (43-45) The absence of ERAAP results in such a severe alteration in the range of bound peptides that wildtype and knockout mice on the same background are actually histo-incompatible, with wildtype mice able to generate CD8+ T cell responses, and even antibody responses, against knockout cells (44). The antibodies generated recognize the MHC-I molecules complexed with elongated peptides and can block recognition of ERAAP-negative cells by the ERAAP-positive CD8+ T cells.
PEPTIDE BINDING TO MHC-II MOLECULES
MHC-II molecules assemble within the ER, followed by functional maturation in endosomal compartments rich in antigenic peptides. Upon ER translocation MHC-II α and β subunits associate in a process facilitated by a specific chaperone, the invariant chain (I chain) or CD74 (Figure 4). Studies using I chain-deficient cells and animals have shown that I chain promotes MHC-II αβ folding, protects the MHC-II ligand binding groove, and directs MHC-II molecules to endosomal compartments for ligand capture. I chain is a non-polymorphic, type II transmembrane glycoprotein not encoded in the MHC. Several forms of I chain exist due to alternative splicing and the use of alternate start codons (46). Nomenclature for the variants is based on their molecular mass, with the shortest form, p33, being most abundantly expressed. A larger splice variant p41 contains a glycosylated domain, homologous to domains present in thyrogloblulin, which can inhibit the activity of the protease cathepsin L (47). All forms of I chain contain a conserved di-leucine motif in the N-terminal cytoplasmic domain required for targeting I chain and associated MHC-II to late endosomal compartments (48, 49). In humans an alternate upstream translational start site gives rise to two additional forms of I chain, p35 and p43, each with an N-terminal 16 amino acid extension. This extended cytoplasmic domain encodes an ER retention motif, which may facilitate ER accumulation and the folding of nascent MHC-II αβ. A limited number of I chain molecules are also modified via linkage of a chondroitin sulfate chain; these molecules reach the cell surface and facilitate cell-cell adhesion (50, 51). Several other molecules involved in antigen presentation or transport have been reported to associate with I chain, including CD1, MHC-I, and the neonatal Fc gamma receptor (52-54). While I chain expression is not required for the function of CD1 or MHC-I, it may enhance antigen presentation by these molecules (55, 56). I chain expression negatively regulates DC motility in vitro, but it is unknown whether this facilitates antigen presentation or if it is related to the role of I chain as a receptor for the macrophage and stem cell chemoattractant migration inhibitory factor (MIF) (54, 55).
Newly synthesized I chain variants form homo- or mixed trimers, involving p33, p35, p41 and p43 in humans, which accumulate in the ER (57). These multimers act as a nucleus for MHC-II α and β assembly giving rise to nonamers with 3 α, 3 β and 3 I chains (Figure 4) (58). Distinct MHC-II alleles have different affinities and requirements for I chain binding that can influence their expression and function. In the absence of I chain, some MHC-II αβ complexes are unstable, resulting in their aggregation, retention in the ER, and failure to reach cell surface (59-61). Association of I chain with MHC-II αβ dimers prevents antigenic peptide binding, consistent with minimal peptide acquisition early in MHC-II biosynthesis (62, 63). After assembly the MHC-II-I chain complexes leave the ER and are routed to the endocytic pathway by the I chain di-leucine motifs (46). This may occur by direct targeting from the Trans Golgi Network (TGN) or by endocytosis from the plasma membrane (Figure 4) (64).
I chain release is initiated by progressive proteolysis in acidic endosomes (65). This culminates in a variably extended peptide of roughly 20 residues that is associated with the MHC-II binding groove (Figure 4). This is called CLIP, for class II-associated invariant chain peptide (66, 67). The structure of CLIP bound to HLA-DR3 is virtually identical to the structure of MHC-II bound to antigenic peptides indicated in Figure 1. (68). There are some MHC-II alleles with a low affinity for CLIP and they are genetically associated with the development of autoimmunity (69). This may reflect a role for MHC-II-CLIP complexes in regulating thymic selection, or skewing of T helper cell subset differentiation (70, 71). Alternatively, premature release of CLIP from these disease-associated MHC-II alleles may favor the selection of epitopes from autoantigens or the capture of self peptides within distinct endosomal compartments (72, 73).
CLIP release from MHC-II is facilitated by another MHC-encoded heterodimeric glycoprotein, DM, which is highly homologous to conventional MHC-II (Figure 4) (74, 75). In humans DM is known as HLA-DM and in mice as H2-DM. The DM α and β subunits display limited genetic polymorphism and the assembled dimer lacks an open or accessible ligand binding groove (76, 77). The cytoplasmic domain of the DM β chain contains a tyrosine motif which is responsible for sorting assembled DM molecules to late endosomes; DM may also bind I chain which may facilitate but is not required for DM assembly and stability (78-80). DM interaction with MHC-II-CLIP complexes occurs in late endosomes where DM acts to promote a conformational change that induces CLIP dissociation (Figure 4), analogous to the role of tapasin in MHC-1 peptide editing discussed above (Figure 3). This reaction can be replicated using purified MHC-II-CLIP and DM, and displays Michaelis-Menten kinetics and an acidic pH optimum (75, 81, 82). CLIP removal facilitates MHC-II loading with antigenic peptides, which influences the repertoire of CD4+ T cells selected in the thymus (83, 84). DM can remove any low affinity peptides from MHC-II, and, analogous to the role of tapasin in MHC-I peptide editing discussed above, repetitive interactions with DM lead to the accumulation MHC-II complexes with high affinity peptides (85). While MHC-II binding to peptides derived from endocytosed antigens is inefficient in the absence of DM, there is a slow release of CLIP from MHC-II even in DM-negative APCs. As a consequence synthetic peptides bind efficiently to surface MHC-II in these cells and presentation of endogenous antigens can be detected, while in B cells BcR-mediated targeting of antigens can overcome the loss of DM, presumably by increasing the amount internalized over a critical threshold (86-88).
The function of DM is modulated by another MHC-encoded MHC-II-like αβ heterodimer, DO, and it is generally accepted that that DO inhibits DM function (89, 90). DO is expressed in B cells and thymic epithelium and at low levels in select DC subsets, where there is evidence that it is regulated by Toll-like receptor (TLR) agonists (91-94). DO αβ dimers associate tightly with DM molecules, and are retained in the ER in the absence of DM, suggesting that in DO-positive cells DM and DO move in concert to endosomes (Figure 4) (95). Studies using Förster (Fluorescence) Resonance Energy Transfer (FRET) and mutational analysis that defined the DM/DR interface suggested that that DO and DR bind to the same region of DM (96). Recently the crystal structure of the DO/DM complex confirmed this, and demonstrated an apparent displacement of a segment of the DO α-chain α-helix compared with that of this α-helix in MHC-II-peptide complexes, which may reflect the conformational alteration that DM imparts to induce the dissociation of low affinity peptides (97).
A precise biological function for DO has been hard to define. Studies in mice deficient in DO have revealed subtle defects in MHC-II antigen presentation, although the effects observed were influenced by the genetic background of the mice and MHC-II allele examined (90, 98). In vivo, over-expression of DO in dendritic cells can impair MHC-II presentation of antigenic epitopes and, presumably because of this, reduce type I diabetes development in NOD mice (99, 100).
ANTIGEN INTRODUCTION AND PROTEOLYSIS IN THE ENDOCYTIC PATHWAY
Exploiting conserved pathways established for nutrient and growth factor uptake, APCs sample soluble and particulate matter from extracellular fluids. Many pathogens, including viruses, bacteria and fungi use these same pathways as conduits into cells, favoring immune recognition and antigen presentation. Pathogen driven disruption of these pathways allows immune evasion (101-103). Among these transport pathways three routes, clathrin-mediated endocytosis, phagocytosis, and macropinocytosis, efficiently promote antigen internalization and sorting to vesicular organelles for processing and presentation by MHC molecules (Figure 2). During clathrin-mediated endocytosis, cell surface receptor-ligand complexes, membrane proteins and soluble macromolecules are internalized. Regulated capture of particulate antigens and pathogens is mediated by phagocytosis, a process which synchronizes engulfment with delivery into a microenvironment containing reactive oxygen species (ROS), proteases, and anti-microbial agents to promote pathogen destruction. The non-selective process of macropinocytosis captures larger quantities of extracellular material, including proteins, bacteria, and viruses, via plasma membrane ruffling and folding. All of these pathways exist in DCs, macrophages and B lymphocytes, although there are variations in efficiency and regulation. For example, B cells are less efficient at fluid phase endocytosis than DCs or macrophages (104). However, soluble antigen uptake and MHC-II presentation by B cells can be detected in vivo using antibodies recognizing specific MHC-II-peptide complexes (49). Surface Ig as a component of the BcR promotes rapid and efficient internalization of antigens, enhancing the potency of antigen-specific B cells 103-104 fold as stimulators of CD4+ T cells (105).
APCs in general display multiple cell surface receptors that can capture antigens or intact pathogens to promote internalization and processing. Enhanced antigen presentation by MHC-II has been observed following antigen uptake via several receptors that cluster in clathrin-coated domains, including the BcR, Fc receptors and the C-type lectin family receptor DEC205, as well as mannose and transferrin receptors (106-110). MHC-I cross-presentation was also increased following the internalization of ovalbumin (OVA) via the mannose receptor on DCs and macrophages (111). DEC205 can promote efficient antigen internalization and presentation by both MHC-I and MHC-II, and conjugation of antigens to antibodies recognizing DEC205 has been used to induce tolerance (108). APCs also express receptors for self and microbial heat shock proteins (HSP) such as Hsp70, Hsp90, and gp96, which promote endocytic uptake of these chaperones and associated ligands (including peptides and antigens) for MHC-I and MHC-II presentation (112-115).
Receptors on the surface of APCs promote the phagocytosis of bacteria, fungi, select viruses, and apoptotic or necrotic cells (116-118). Macrophages and DCs are well-established phagocytes but this process can also be observed in B cells, which can present phagocytosed antigens to CD4+ T cells (119-121). MHC-I cross-presentation as well as MHC-II presentation of opsonized antigens is enhanced by receptor engagement upon phagocytosis, which may reflect intracellular receptor signaling rather than simply enhanced uptake of these particles. Thus, IgG-coated bacteria were effectively presented to CD8+ T cells while complement C3 opsonization of bacteria facilitated phagocytosis but not antigen presentation (122). Signaling by receptors such as the C-type lectin family receptor DNGR-1 promotes MHC-I and MHC-II presentation of antigens from phagocytosed necrotic cells (123, 124). Internalization and presentation of self-antigens associated with necrotic cells may contribute to autoimmunity or allograft rejection. Indeed, while all the above pathways promote uptake of extracellular antigens by APCs, internalization and recycling of the plasma membrane also delivers endogenous proteins for processing; peptides derived from membrane proteins, such transferrin receptor and MHC-I heavy chain, are abundantly associated with MHC-II molecules (125).
Endocytic Compartments in Antigen Processing and Presentation
Internalized antigens enter organelles with microenvironments favoring protein denaturation and proteolysis. While these pathways permit MHC-II access to exogenous antigens, MHC-I molecules also use these routes to acquire antigens for cross-presentation (Figure 2). Electron microscopy initially revealed an abundance of MHC-II molecules distributed in the endocytic pathway, concentrated in late endosomal vesicles, originally defined as MHC-II compartments, or MIICs (Figure 2), in contrast to only limited amounts of MHC-I (126-128). The role of this MHC-I in cross-presentation has been debated. Disrupting expression of HS-1, a modulator of endocytic invaginations, demonstrated that endocytosis delivers extracellular antigens for presentation by MHC-I as well as MHC-II in DCs (129). However, in DCs antigens can transit from within endosomes to the cytoplasm or the ER, raising questions as to the role of endocytosed MHC-I in antigen cross-presentation (130). A tyrosine motif in the cytoplasmic tail of MHC-I heavy chain facilitates recycling of low levels of these molecules from the cell surface into endosomes, but direct delivery of immature MHC-I from the ER may also occur in DCs, possibly facilitated by associated I chain (56, 131-134).
Early endosomes mature into late endosomes and lysosomes driven in part by processes such as increased luminal acidification and fusion with TGN-derived vesicles delivering enzymes that promote antigen denaturation and proteolysis. Low temperature (18°C) can block the maturation step and disrupts the presentation of several exogenous antigens by MHC-II (135). However, MHC-II presentation of select antigenic epitopes processed within early endosomes can be detected (136, 137). MHC-I-restricted cross-presentation via the mannose receptor was favored by its delivery of antigen into early endosomes (111). Whether this is due to limited antigen processing in these vesicles, favoring epitope recovery by endocytic MHC-I, or enhanced translocation of antigens into the cytoplasm for re-direction via TAP to MHC-I, is not clear. Co-localization of MHC-I in endosomes with the insulin-regulated aminopeptidase (IRAP), potentially a substitute for ERAP1, also promoted antigen cross-presentation (138). MHC-I presentation was also facilitated by liposome-mediated antigen delivery into early but not late endosomes, and neutralization of the acidic pH in the latter enhanced antigen presentation by MHC-I. By contrast, antigens delivered via liposomes into early or late endosomes were processed for MHC-II presentation (139).
Mature or late endosomal vesicles are heterogeneous in morphology and content, and include translucent and electron dense vesicles, multi-vesicular bodies (MVB) containing intra-lumenal vesicles (ILV), multi-lamellar vesicles, and pre-lysosomes. Antigen processing in these vesicles is influenced by their pH, which regulates the activity of resident proteases and other relevant enzymes, such as gamma interferon inducible lysosomal thiol reductase (GILT, Figure 4) (140-142). Differences in the ability of distinct APCs to regulate endocytic processing have also been documented. For example, the limited protease content and higher pH of DC endocytic compartments may enhance their capacity for presenting antigens via MHC-I and MHC-II compared with macrophages (143). The precise steps in I chain processing vary between APC types, consistent with their differential expression of cathepsins. Studies using protease inhibitors and protease-deficient mice revealed that several enzymes including cathepsins (S, L, F) and asparaginyl endopeptidase (AEP) mediate I chain cleavage (5). While cathepsin S plays a key role in the late stages of I processing in DCs and B cells, in macrophages cathepsin F is required. Cathepsin L or V is necessary for terminal I chain proteolysis in cortical thymic epithelial cells. Disruptions in I chain processing can impede MHC-II binding to peptides as well as the transit of the complexes to the cell surface (144).
While it is well established that I chain guides MHC-II to endosomes, the regulation of MHC-II transport within and out of endosomal compartments is not well understood and may differ between APC types. Myosin II, an actin-based motor, may modulate this process in B cells, while in DCs MHC-II internalization is mediated by ubiquitination of the cytoplasmic tail of the β chain; DC maturation promotes the expression of MHC-II-peptide complexes on the cell surface (145, 146). Recently down-regulation of the MIR family ubiquitin ligase MARCH-1 has been implicated in the reduction of MHC-II ubiquitination and retention of surface expression (147, 148). Sub-compartments within mature endosomes may also regulate MHC-II acquisition of peptides. In MVB, the interaction of DM and DO favors their co-localization with HLA-DR in the outer or limiting membrane of these endosomes, whereas DM without DO migrates into internal vesicles which can be shed from cells as exosomes (149, 150). At the cell surface MHC-II peptide presentation is greatly enhanced by the clustering in lipid raft microdomains (151).
Phagocytosis and Macropinocytosis and Antigen Presentation
MHC-I as well as MHC-II is detectable within phagosomes (131, 152, 153). Phagosomal antigen processing and MHC-II presentation are well established and newly formed MHC II-peptide complexes can be detected in these organelles (154). In contrast with endocytosed antigens, MHC-II presentation of phagocytosed antigens was impaired in DCs lacking the cytoplasmic adaptor, AP-3, due to defective transit of MHC-II-peptide complexes to the cell surface (155). Recent studies have revealed the importance of phagocytosis in cross-presentation (116), which typically leads to antigen translocation into the cytoplasm for processing and subsequent delivery for presentation by MHC-I (Figure 2). Processing of phagocytosed antigen by cathepsins has been observed to promote MHC-I cross-presentation, in some cases by a vacuolar peptide exchange pathway (116, 156). In DCs, antigen cross-presentation by MHC-I is enhanced within newly formed phagosomes, which maintain a neutral pH by regulated delivery of NADPH oxidase to the phagosomal membrane (156). In contrast, phagosome maturation and acidification can facilitate MHC-II presentation of pathogen-associated antigens (157).
Exposure of APCs to TLR ligands and pro-inflammatory cytokines can influence the microenvironment within phagosomes by reducing protease content, controlling luminal pH, and modulating the binding of cytoplasmic regulatory proteins such as LC3 and GTPases which mediate phagosome maturation (158-161). In macrophages, phagosome maturation was found to be independent of TLR2 or TLR4 signaling (162), while in DCs, TLR4 activation within a specific phagosome drives maturation and MHC-II-restricted antigen presentation within the organelle (163). The pH is higher and the protease content lower within DC endosomes and phagosomes than in macrophages, which preserves epitopes and favors antigen presentation (143). Macrophages, on the other hand, are more proficient in killing engulfed pathogens, at least partly because of their higher phagosomal protease content and more acidic phagosomal pH (164).
Macropinocytosis does not rely on receptors (Figure 2), but nevertheless captures large antigens and extracellular material into vesicles termed pinosomes (165). These vesicles share features with early and late endosomes but are distinct, although pinosomes eventually fuse with lysosomes (166). TLR ligands can promote a rapid burst of macropinocytosis in DCs which then abruptly halts, stimulating preferential MHC-I and MHC-II presentation of the bolus of internalized antigen (167). A lack of specific inhibitors has limited analysis of macropinocytosis in APC, although studies suggest a role for this pathway in MHC-II presentation of the autoantigen type II collagen and liposome-coupled antigen presentation via MHC-I (168, 169).
Autophagy and Antigen Presentation
Between 10-30% of the peptides bound to MHC-II are derived from cytoplasmic and nuclear proteins (170). Within APC, three routes of autophagy promote the delivery of proteins and peptides from the cytoplasm and nucleus into the endosomal network (170, 171). In macroautophagy, nuclear and cytoplasmic material, including mitochondria, peroxisomes, and some intracellular bacteria, are engulfed by isolation membranes to form autophagosomes. These fuse with endosomes and lysosomes, facilitating antigen presentation by MHC-II (Figure 4) as well as the delivery of nucleic acids to TLRs. MHC-II presentation of Epstein Barr virus nuclear antigen I as well as ectopically expressed recombinant viral and bacterial antigens, was perturbed in APCs deficient in macroautophagy (172). Macroautophagy is readily detected in thymic epithelial cells and disruption of Atg5, a regulator of this process, perturbed the selection of thymic CD4+ but not CD8+ T cells, implying an effect on MHC-II but not on MHC-I processing (173). The induction of macroautophagy in macrophages and DCs also enhanced MHC-II presentation of mycobacteria, likely due to more efficient phagosome maturation (174). In B cells, chaperone-mediated autophagy also promoted MHC-II presentation of autoantigens to CD4+ T cells (170). In this pathway, cytoplasmic chaperones such as Hsc70 and Hsp90, together with the lysosomal transmembrane protein LAMP-2A, selectively deliver epitopes to MHC-II (Figure 4). Proteins may also be captured by microautophagy for delivery into endosomes via Hsc70 and the ESCRT system, although whether this contributes to antigen presentation is unclear (171).
APCs readily acquire and present antigens from target or dying cells for MHC-I and MHC-II, promoting graft rejection and autoimmunity as well as immune responses to pathogens. In APCs, MHC-II presentation of cytoplasmic antigens derived from target cells with diminished TAP, ERAAP, and proteasome activity was enhanced, suggesting a role for these molecules in subverting cross-presentation of cytoplasmic antigens (175). In addition, induction of macroautophagy in tumor or target cells can enhance their phagocytosis and MHC-I cross-presentation to CD8+ T cells (176). By contrast in DCs, MHC-II direct presentation of membrane antigens from influenza virus required TAP and proteasome activity (177). A requirement for proteasomal processing of some cytoplasmic antigens and a role for ERAAP in MHC-II presentation has been documented, but the mechanisms by which these components influence the MHC-II pathway remain unclear (175, 178-180).
Epitope Selection and Guided Antigen Processing
Proteins can contain multiple sequences capable of binding MHC molecules, but only a handful of peptides are selected for presentation to T cells. T cell responses are influenced by the diversity of the T cell repertoire but the steps in antigen processing and presentation play a major role. The concept that a hierarchy of antigenic epitopes is recognized by the immune system is well established; the strongest are called immunodominant, and there are subdominant and cryptic epitopes. Immunodominant epitopes are important for immunity to tumors and pathogens, while a shift in the hierarchy of T cell responses to subdominant epitopes is associated with autoimmune disorders (181, 182). Multiple factors contribute to the process of epitope selection by MHC-I and MHC-II molecules. In the case of MHC-I, the specificity of the proteasome, ERAAP/ERAP1, tapasin, and TAP can influence epitope generation and transport to receptive MHC-I molecules (181). For MHC-II, antigen unfolding and proteolysis influence processing and epitope presentation (183, 184). Multiple endocytic proteases have been implicated in processing antigens for MHC-II, including cathepsins B, D, L, S and AEP, and several of these enzymes also function in I chain processing (5). Antigen reduction facilitates protease access for processing, influencing the generation of antigenic epitopes, and GILT is the key enzyme implicated in this process (141). In melanoma cells, the hierarchy of epitopes presented by MHC-II is GILT dependent (142). GILT expression also influences autoantigen processing and the development of experimental autoimmune encephalomyelitis and tolerance development to melanocyte antigens (185, 186). MHC-I and MHC-II epitopes can also be destroyed by proteases, which may result in differential epitope presentation by different APC types as well as tissue specific differences in presentation (5, 187).
The open groove of MHC-II allows large fragments of antigen to bind (Figure 1) (188). This led to the concept of guided antigen processing, in which MHC-II binding to epitopes within antigens shapes proteolytic cleavage (189, 190). In B cells the specific interaction of antigens with the Ig component of the BcR also influences processing and presentation by MHC-II (191). An in vitro system reconstituting antigen binding to the BcR followed by digestion with the enzyme AEP favored epitope capture by proximal MHC-II (190). Similarly MHC-II binding to immunodominant epitopes from an intact protein was reconstituted in vitro using soluble purified components, including cathepsins to yield peptides and DM to promote editing of the resulting MHC-II-peptide complexes (192). Epitopes may bind MHC-II in an unstable conformation, and editing of these complexes by DM alters the hierarchy of peptides displayed to CD4+ T cells (193). Notably, DM-independent epitope conformations can persist, particularly when the antigen is available to APCs as a peptide rather than an intact protein, and may induce unusual CD4+ T cells (so-called Type B T cells) that can lead to autoimmunity (73, 194). Far less is known about the endosomal factors that influence epitope selection for MHC-I cross-presentation, although GILT expression is required for cross-presentation of a disulfide-containing glycoprotein antigen from herpes simplex virus 1 (195). Notably, during cross-presentation innate signaling via TLRs appears to influence antigen presentation, as suggested by a shift in the dominant CD8+ T cell epitopes during LCMV infection (196).
ANTIGEN INTRODUCTION AND PROTEOLYSIS IN THE CYTOSOL
Protein antigens are conventionally introduced into the cytosol by the cellular protein synthetic machinery. When a virus infects a cell the viral genes are transcribed into mRNAs and these are translated on host ribosomes to generate viral proteins. While autophagic mechanisms can give them access to the MHC-II pathway, cytosolic antigens are the prime source of MHC-I-associated peptides. Their proteolysis generates peptides that are translocated into the ER by TAP, and ultimately bind to MHC-I molecules (Figure 3). If they are too long they are trimmed in the ER by ERAAP/ERAP1 as described above. This process is not specific for viral proteins; host proteins are similarly degraded and generate peptides that bind to MHC-I. In fact, in the case of autoimmunity or tumor immunity MHC-I-associated, host protein-derived peptides can be recognized by CD8+ T cells. For example, CD8+ T cell-mediated killing of melanoma cells, which is exploited for immunotherapy, often involves the recognition of MHC -I-associated peptides derived from melanocyte-specific glycoproteins (197). These proteins are found in melanosomes, the pigment-containing organelles of melanocytes from which melanomas originate. In an infected cell viral proteins must compete with host proteins for representation in the peptide profile presented to CD8+ T cells.
Protein sources of MHC-I-associated peptides
Epitopes from viral glycoproteins, as well as melanosomal glycoproteins, can be recognized by CD8+ T cells. These peptides are generally derived from parts of the antigen that are luminal, not cytosolic (198, 199). Nevertheless, the generation of these MHC-I-peptide complexes is virtually always TAP- and proteasome-dependent. This implies that, in spite of the presence of a signal sequence and the potential for translocation into the ER, the processing mechanisms at work are no different from those involved in the generation of peptides from exclusively cytosolic antigens. These observations have contributed to the hypothesis that intact, folded, cytosolic proteins are not the major source of peptides that bind to MHC-I. Instead the sources are proteins that are either incomplete, perhaps because of premature termination, or misfolded because cytosolic chaperones are not 100% effective in mediating the folding of newly synthesized proteins. In mammalian cells approximately 30% of total proteins are degraded extremely rapidly following synthesis (Figure 3) (200). Yewdell has been a strong advocate of the hypothesis that this rapidly degraded pool is the primary source of MHC-I-associated peptides, and coined the acronym DRiP, for Defective Ribosomal Product, to describe them, and has recently reviewed the evidence supporting the hypothesis (201). Briefly, very early experiments showed that expression in cells of truncated proteins, which are unstable, generated MHC-I-peptide complexes as effectively as full-length proteins. In fact, the experiments that mapped and defined the first MHC-I-restricted epitope, an influenza nucleoprotein-derived peptide that binds to H2-Db, relied on the expression of truncated proteins (202). Work by Neefjes and co-workers suggested that newly synthesized proteins are the primary source of TAP-translocated peptides (203). They showed by FRAP (Fluorescence Recovery After Photobleaching) analysis that the lateral mobility of TAP in the ER membrane decreases when active peptide translocation is occurring, and that inhibiting protein synthesis by cycloheximide addition rapidly enhanced TAP mobility. Kinetic analysis of the synthetic rates of cytosolic antigens versus the rates at which complexes of MHC-I and peptide derived from them are generated confirmed a general principle that the accumulation of the protein lags considerably behind the acquisition of the complexes (204). Using the SILAC (Stable isotope labeling with aminoacids in cell culture) technique, in which cellular proteins, and the peptides derived from them, are labeled with specific isotopic variants of amino acids upon synthesis and identified by mass spectrometry, it has been observed that there is no clear relationship between the abundance of MHC-I-bound peptides and the abundance of the proteins from which they derive (205). In fact, some MHC-I-associated peptides are derived from proteins that are undetectable in the cell.
Exactly what the mechanisms are that drive DRiP formation are still not entirely clear, although there are increasing suggestions that one component may involve modifications to normal translational processes. Work by Fahraeus and co-workers adapted the phenomenon of nonsense mediated decay, in which mRNA with a premature stop codon is degraded after only a single round of translation, to show that an epitope encoded by such an mRNA is produced with high efficiency for T cell recognition (206, 207). More recently, Granados et al. (208) used the SILAC method to analyze MHC-I-associated peptides in human EBV-transformed B cell lines and made the intriguing observations that, first, many of the peptides were derived from proteins associated with B cell differentiation rather than more abundant house-keeping proteins, and second, that the peptides were preferentially derived from proteins encoded by transcripts that were the targets of microRNAs (miRNAs), which are known to regulate transcript stability. Analysis of literature data covering multiple epitopes and their sources determined that this is a general phenomenon, not specific for transformed B cell lines. The precise mechanistic connection between mRNA instability and the generation of MHC-I-associated peptides remains unknown.
Chaperones and cytosolic peptide generation
While DRiPs are a significant, perhaps major, source, MHC-I-associated peptides can be derived from intact proteins. Proteins introduced directly into the cytosol of a cell, for example proteins such as listeriolysin and other proteins secreted by Listeria monocytogenes after its internalization by macrophages (209), can be processed and recognized by CD8+ T cells. What then are the intracellular processing steps that proteins, or DRiPs, follow before they degenerate into the peptides that are translocated into the ER by TAP? Shastri and co-workers developed exceptionally clever techniques to identify the cytosolic precursors of MHC-I binding peptides and have shown that they are associated with cytosolic chaperones. The approach draws on the ability of exogenous MHC-I-binding peptides to sensitize cells for recognition by CD8+ T cells. In the most refined version of the method, the epitope, derived from OVA, is flanked with lysine residues and embedded in a protein that is then expressed in cells. The precise epitope (SIINFEHL, a modification of the classical H2-Kb-associated SIINFEKL epitope with histidine substituted for the normal internal lysine residue) is released from any cytosolic precursor of the peptide by digestion with trypsin, which produces the correct N-terminal amino acid, and carboxypeptidase B, which removes the C-terminal lysine. The exceptional sensitivity of a T cell hybridoma recognizing this epitope allowed the identification of precursors which co-immunoprecipitated with anti-chaperone antibodies, assaying the proteolytically released epitope by sensitization of an H2-Kb-positive target cell. Large intermediate degradation fragments of the protein were found in association with the chaperone Hsp90α (210). shRNA-mediated knockdown of Hsp90α inhibited accumulation of the fragments and processing of the antigen, as well as its recognition by CD8+ T cells, as did knockdown of a co-chaperone, CHIP, which ubiquitinates Hsp70 or Hsp90α-associated proteins and delivers them to proteasomes for degradation. This suggests that these fragments are pre-proteasomal. Consistent with this, the addition of a proteasome inhibitor to the cell increased the amounts of the fragments, and they were extended at the C-terminus beyond the actual epitope; the C-terminal residue of peptides translocated by TAP and associated with MHC-I is generally generated by proteasomal cleavage (211). Other fragments were associated with another chaperone, the Tailless Complex Polypeptide-1 (TCP-1) Ring Complex or TRiC (212). These fragments were N-terminally extended but not C-terminally extended, i.e. all of them ended with the precise epitope sequence that was originally embedded in the protein. This indicates that they are post-proteasomal. Thus the pathway that has emerged is that a cytosolic protein, usually a recently synthesized or somehow defective one (a DRiP), associates with Hsp90α, is ubiquitinated by CHIP, and is degraded by the proteasome to yield truncated fragments which then associate with TRiC. Cytosolic aminoterminal trimming, for example by leucine aminopeptidase (213), can then reduce them to an appropriate size for TAP mediated transport into the ER. For individual epitopes, cytosolic peptidases, including leucine aminopeptidase and/or tripeptidyl peptidase II, may facilitate or inhibit their generation (214).
Nonconventional sources of MHC-I-associated peptides
The extraordinary sensitivity of T cell recognition is well established. Very low numbers of MHC-I peptide complexes are required; even a single complex may be sufficient to trigger a T cell (215). Possibly because of this some MHC-I-associated peptides have origins that do not depend on conventional translation. There are examples of antigenic peptides that are out of frame with regard to their proteins of origin and others derived from sequences embedded in introns (216). There are peptides that derive from translation initiated at codons other that the conventional methionine codon, ATG. Shastri and co-workers have identified a novel translational mechanism that involves leucine-tRNA-mediated initiation of translation at a CUG codon and suggest that other codons may be functional (217). These experiments constitute recent examples of a historically common phenomenon; immunological studies often enhance our understanding of molecular biological processes.
There are also examples of peptide epitopes derived from non-contiguous sequences in proteins. Many of these have derived from studies of human epitopes recognized by patient-derived tumor specific CD8+ T cells. Vigneron et al. (218) described an HLA-A32-associated epitope derived from the melanosomal glycoprotein gp100 (or pmel17) that was a nonamer but was derived from a 13 amino acid precursor by removal of four internal residues. They showed that this excision/splicing event was mediated by the proteasome and involved a mechanism in which the hydrolysis of a bond between the peptide and a threonine residue in the active site of the proteasome β-subunit, normally the final step of proteolysis, is replaced by reaction with the N-terminal amino group of a second peptide instead of water. A number of other examples of this have been described, including one peptide in which the N-terminal sequence of the peptide is actually C-terminal to the N-terminal peptide sequence in the intact protein (219).
Another example of an epitope that does not represent the primary sequence of a protein also involves a melanosomal glycoprotein. In this case, an asparagine residue present in the melanosomal enzyme tyrosinase was replaced by an aspartic acid residue in a tyrosinase-derived HLA-A2.1-associated nonameric peptide (220). This occurs because the peptide is generated from the protein after its signal sequence-mediated entry into the ER and subsequent degradation following retrotranslocation into the cytosol. This is the conventional mechanism for disposal of misfolded proteins and glycoproteins and is known as ER-associated degradation, or ERAD (221). The proteasome is the normal destination for such retrotranslocated proteins. A component of the pathway for glycoproteins involves their cytosolic deglysosylation by an N-glycanase that converts the glycan-bearing asparagine residue to an aspartic acid (222); the epitope encompassed a glycosylated sequence in tyrosinase that was deglycosylated in the cytosol.
Implications of ERAD for cross-presentation
A pathway in which proteins that enter the ER are retrotranslocated into the cytosol and generate peptides that are potentially available for MHC-I-restricted T cell recognition has a clear parallel to the dominant mechanism involved in cross-presentation. Here the compartment is an endosome or phagosome rather than the ER, but the underlying principle is the same (Figure 3). A luminal protein internalized by a DC must enter the cytosol and be degraded by the proteasome to generate the relevant peptide, in principle the same peptide that would be generated by a normal cell expressing the protein as an endogenously translated protein. Thus a CD8+ T cell induced by cross-presentation of a viral protein would recognize the epitope generated in the infected cell, allowing its destruction. The seductive logic of this argument has led to a considerable body of work suggesting, although not without controversy, that the mechanisms responsible for cross-presentation are an adaptation of ERAD (Figure 2). This was first suggested by the work of Desjardin and coworkers, who identified ER-derived proteins in phagosomes purified from a macrophage cell line (223), with the implication that the ERAD retrotranslocation machinery could be recruited to phagosomes from the ER. Experimental evidence supporting this rapidly followed. DCs and DC-like cell lines were found to be capable of transferring proteins into the cytosol from endosomes or phagosomes, including enzymes such as luciferases, as well as cytochrome C (224-226). The addition of cytochrome C to DCs and its entry into the cytosol caused apoptosis, mimicking the effect of cytochrome C released from mitochondria in conventionally induced apoptosis (225). Processing and presentation of soluble, exogenous OVA by H2-Kb expressed in a human DC-like cell line, KG-1, could be blocked by a cytosolically expressed dominant-negative, ATPase-defective, mutant version of the AAA-ATPase p97, which normally mediates the extraction of proteins from the ER during ERAD. In addition, phagosomes derived from KG-1 were capable of extruding luciferases that were internalized along with the phagocytic substrate, a latex bead, into the external milieu, topologically equivalent to the cytosol (224). This was ATP-dependent, could be enhanced by recombinant p97, and inhibited by recombinant dominant-negative p97, all of which is consistent with an ERAD-like mechanism. TAP and other PLC components were also identified in purified phagosomes, and they were capable of internalizing peptides via TAP and assembling them with MHC-I molecules present in the phagosome (131, 153). This led to the concept that phagosomes in DCs are compartments specialized for MHC-I-restricted antigen processing, later extended to endosomes (227). This is an interesting but not essential element of a coherent hypothesis involving ER recruitment to phagosomes. The critical step is the role of ERAD in mediating cytosolic access; after that proteolytic degradation and TAP-mediated transport of peptides into the ER would be sufficient. However, as mentioned earlier the ERAP1-like aminopeptidase IRAP is present in DC phagosomes and can facilitate cross-presentation, which is consistent with the idea that they may be dedicated cross-presenting organelles (138).
Vesicular fusion events in cells are regulated by the interactions of SNARE proteins present on the vesicles involved. Recently it has been shown that recruitment of ER resident proteins to the phagosome, and cross-presentation, in DCs is dependent on Sec22b, a SNARE localized to the ER-Golgi intermediate compartment (ERGIC), that interacts with a partner SNARE, syntaxin 4, normally present on the plasma membrane but present on phagosomes in DCs (228). This appears to have resolved some of the controversies surrounding the connection of ERAD and cross-presentation, although the nature of the channel that mediates the translocation of internalized antigens into the cytosol of the cross-presenting cell remains unknown.
CONCLUDING REMARKS
The study of antigen processing is now over three decades old, yet novel findings continue to surprise and delight those of us working in the field. For MHC-I the mechanisms of cross-presentation and the precise mechanisms that regulate DRiP formation and the cytosolic generation of peptides are areas in need of clarification. For MHC-II the mechanisms that regulate the formation of the peptide complexes recognized by Type A and Type B CD4+ T cells, in particular the role of DM in the process, and the precise function of DO remain to be uncovered. In addition, recent demonstrations of phagocytosis in B cells underlines the need for additional work to determine how this modulates MHC-I and -II functions in these cells, given the clear differences in the process between B cells, DCs and macrophages. Applications of mechanistic insights to vaccine development are likely to be important. For example, how do we best incorporate immunogenic epitopes into recombinant vaccines and how can they be moved into the cytosol for effective sensitization of CD8+ T cells? Over the next few years many of these problems will be solved, and questions not yet asked will undoubtedly be answered.
ACKNOWLEDGMENTS
This review is an attempt to synthesize the antigen processing field into a semi-coherent whole. As a result we have been forced to focus on some aspects and give short shrift to others. We apologize to our many colleagues who may disagree with our points of emphasis and to those who will no doubt believe their work is inadequately cited. We thank Nancy Dometios for assistance with manuscript preparation. Work by the authors has been supported by grants from the National Institutes of Health (P01 AI056097, R01AI079065, P01AI084853, U01DK085505, RO1AI069085, R01AI059167, R01AI097206) and by the Howard Hughes Medical Institute.
Acronyms and Definitions
- TAP
transporter associated with antigen processing composed of two subunits TAP1 and TAP2
- Peptide Loading Complex
protein complex which facilitates MHC-I peptide loading consisting of the MHC-I heavy chain and β2microglobulin, TAP, tapasin, and ERp-57
- ERp57
an endoplasmic reticulum resident homologue of protein disulfide isomerase
- ERAD
endoplasmic reticulum-associated degradation pathway which promotes the translocation of mis-folded proteins into the cytoplasm for proteolysis
- ERAP
murine endoplasmic reticulum aminopeptidase associated with antigen processing also known as ER aminopeptidase-1/2 in humans
- CLIP
class II-associated invariant chain peptide
- BcR
B cell receptor for antigen
- GILT
gamma interferon inducible lysosomal thiol reductase
- DRiP
defective ribosomal product include peptides and truncated proteins which arise as a result of pre-mature termination of translation in host cells
Footnotes
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
REFERENCES
- 1.Cohen NR, Garg S, Brenner MB. Antigen Presentation by CD1 Lipids, T Cells, and NKT Cells in Microbial Immunity. Adv Immunol. 2009;102:1–94. doi: 10.1016/S0065-2776(09)01201-2. [DOI] [PubMed] [Google Scholar]
- 2.Adams E. Biology and Structure of Nonclassical MHC Class I Molecules. Ann Rev. Immunol. 2013 doi: 10.1146/annurev-immunol-032712-095912. in press. [DOI] [PubMed] [Google Scholar]
- 3.Maupin-Furlow J. Proteasomes and protein conjugation across domains of life. Nat Rev Microbiol. 2012;10:100–11. doi: 10.1038/nrmicro2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huotari J, Helenius A. Endosome maturation. EMBO J. 2011;30:3481–500. doi: 10.1038/emboj.2011.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Watts C. The exogenous pathway for antigen presentation on major histocompatibility complex class II and CD1 molecules. Nat Immunol. 2004;5:685–92. doi: 10.1038/ni1088. [DOI] [PubMed] [Google Scholar]
- 6.Oancea G, O’Mara ML, Bennett WF, Tieleman DP, Abele R, Tampe R. Structural arrangement of the transmission interface in the antigen ABC transport complex TAP. Proc Natl Acad Sci U S A. 2009;106:5551–6. doi: 10.1073/pnas.0811260106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hinz A, Tampe R. ABC Transporters and Immunity: Mechanism of Self-Defense. Biochemistry. 2012;51:4981–9. doi: 10.1021/bi300128f. [DOI] [PubMed] [Google Scholar]
- 8.Gaudet R, Wiley DC. Structure of the ABC ATPase domain of human TAP1, the transporter associated with antigen processing. EMBO J. 2001;20:4964–72. doi: 10.1093/emboj/20.17.4964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ortmann B, Copeman J, Lehner PJ, Sadasivan B, Herberg JA, Grandea AG, Riddell SR, Tampe R, Spies T, Trowsdale J, Cresswell P. A critical role for tapasin in the assembly and function of multimeric MHC class I-TAP complexes. Science. 1997;277:1306–9. doi: 10.1126/science.277.5330.1306. [DOI] [PubMed] [Google Scholar]
- 10.Tan P, Kropshofer H, Mandelboim O, Bulbuc N, Hammerling GJ, Momburg F. Recruitment of MHC class I molecules by tapasin into the transporter associated with antigen processing-associated complex is essential for optimal peptide loading. J Immunol. 2002;168:1950–60. doi: 10.4049/jimmunol.168.4.1950. [DOI] [PubMed] [Google Scholar]
- 11.Procko E, Raghuraman G, Wiley DC, Raghavan M, Gaudet R. Identification of domain boundaries within the N-termini of TAP1 and TAP2 and their importance in tapasin binding and tapasin-mediated increase in peptide loading of MHC class I. Immunol Cell Biol. 2005;83:475–82. doi: 10.1111/j.1440-1711.2005.01354.x. [DOI] [PubMed] [Google Scholar]
- 12.Koch J, Guntrum R, Heintke S, Kyritsis C, Tampe R. Functional dissection of the transmembrane domains of the transporter associated with antigen processing (TAP) J Biol Chem. 2004;279:10142–7. doi: 10.1074/jbc.M312816200. [DOI] [PubMed] [Google Scholar]
- 13.Leonhardt RM, Keusekotten K, Bekpen C, Knittler MR. Critical role for the tapasin-docking site of TAP2 in the functional integrity of the MHC class I-peptide-loading complex. J Immunol. 2005;175:5104–14. doi: 10.4049/jimmunol.175.8.5104. [DOI] [PubMed] [Google Scholar]
- 14.Sadasivan B, Lehner PJ, Ortmann B, Spies T, Cresswell P. Roles for calreticulin and a novel glycoprotein, tapasin, in the interaction of MHC class I molecules with TAP. Immunity. 1996;5:103–14. doi: 10.1016/s1074-7613(00)80487-2. [DOI] [PubMed] [Google Scholar]
- 15.Panter MS, Jain A, Leonhardt RM, Ha T, Cresswell P. Dynamics of Major Histocompatibility Complex Class I Association with the Human Peptide-Loading Complex. J. Biol. Chem. 2012 doi: 10.1074/jbc.M112.387704. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dong G, Wearsch PA, Peaper DR, Cresswell P, Reinisch KM. Insights into MHC class I peptide loading from the structure of the tapasin-ERp57 thiol oxidoreductase heterodimer. Immunity. 2009;30:21–32. doi: 10.1016/j.immuni.2008.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dick TP, Bangia N, Peaper DR, Cresswell P. Disulfide bond isomerization and the assembly of MHC class I-peptide complexes. Immunity. 2002;16:87–98. doi: 10.1016/s1074-7613(02)00263-7. [DOI] [PubMed] [Google Scholar]
- 18.Hebert DN, Garman SC, Molinari M. The glycan code of the endoplasmic reticulum: asparagine-linked carbohydrates as protein maturation and quality-control tags. Trends in Cell Biology. 2005;15:364–70. doi: 10.1016/j.tcb.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 19.Caramelo JJ, Castro OA, de Prat-Gay G, Parodi AJ. The endoplasmic reticulum glucosyltransferase recognizes nearly native glycoprotein folding intermediates. J Biol Chem. 2004;279:46280–5. doi: 10.1074/jbc.M408404200. [DOI] [PubMed] [Google Scholar]
- 20.Ritter C, Quirin K, Kowarik M, Helenius A. Minor folding defects trigger local modification of glycoproteins by the ER folding sensor GT. EMBO J. 2005;24:1730–8. doi: 10.1038/sj.emboj.7600645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Solda T, Galli C, Kaufman RJ, Molinari M. Substrate-specific requirements for UGT1-dependent release from calnexin. Mol Cell. 2007;27:238–49. doi: 10.1016/j.molcel.2007.05.032. [DOI] [PubMed] [Google Scholar]
- 22.Gabathuler R, Reid G, Kolaitis G, Driscoll J, Jefferies WA. Comparison of cell lines deficient in antigen presentation reveals a functional role for TAP-1 alone in antigen processing. J Exp Med. 1994;180:1415–25. doi: 10.1084/jem.180.4.1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lampen MH, Verweij MC, Querido B, van der Burg SH, Wiertz EJ, van Hall T. CD8+ T cell responses against TAP-inhibited cells are readily detected in the human population. J Immunol. 2010;185:6508–17. doi: 10.4049/jimmunol.1001774. [DOI] [PubMed] [Google Scholar]
- 24.Wei ML, Cresswell P. HLA-A2 molecules in an antigen-processing mutant cell contain signal sequence-derived peptides. Nature. 1992;356:443–6. doi: 10.1038/356443a0. [DOI] [PubMed] [Google Scholar]
- 25.Peh CA, Laham N, Burrows SR, Zhu Y, McCluskey J. Distinct functions of tapasin revealed by polymorphism in MHC class I peptide loading. J Immunol. 2000;164:292–9. doi: 10.4049/jimmunol.164.1.292. [DOI] [PubMed] [Google Scholar]
- 26.Purcell AW, Gorman JJ, Garcia-Peydro M, Paradela A, Burrows SR, et al. Quantitative and qualitative influences of tapasin on the class I peptide repertoire. J Immunol. 2001;166:1016–27. doi: 10.4049/jimmunol.166.2.1016. [DOI] [PubMed] [Google Scholar]
- 27.Grandea AG, 3rd, Golovina TN, Hamilton SE, Sriram V, Spies T, et al. Impaired assembly yet normal trafficking of MHC class I molecules in Tapasin mutant mice. Immunity. 2000;13:213–22. doi: 10.1016/s1074-7613(00)00021-2. [DOI] [PubMed] [Google Scholar]
- 28.Williams AP, Peh CA, Purcell AW, McCluskey J, Elliott T. Optimization of the MHC class I peptide cargo is dependent on tapasin. Immunity. 2002;16:509–20. doi: 10.1016/s1074-7613(02)00304-7. [DOI] [PubMed] [Google Scholar]
- 29.Howarth M, Williams A, Tolstrup AB, Elliott T. Tapasin enhances MHC class I peptide presentation according to peptide half-life. Proc Natl Acad Sci U S A. 2004;101:11737–42. doi: 10.1073/pnas.0306294101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wearsch PA, Cresswell P. Selective loading of high-affinity peptides onto major histocompatibility complex class I molecules by the tapasin-ERp57 heterodimer. Nat Immunol. 2007;8:873–81. doi: 10.1038/ni1485. [DOI] [PubMed] [Google Scholar]
- 31.Wearsch PA, Peaper DR, Cresswell P. Essential glycan-dependent interactions optimize MHC class I peptide loading. Proc Natl Acad Sci U S A. 2011;108:4950–5. doi: 10.1073/pnas.1102524108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen M, Bouvier M. Analysis of interactions in a tapasin/class I complex provides a mechanism for peptide selection. EMBO J. 2007;26:1681–90. doi: 10.1038/sj.emboj.7601624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gao B, Adhikari R, Howarth M, Nakamura K, Gold MC, et al. Assembly and antigen-presenting function of MHC class I molecules in cells lacking the ER chaperone calreticulin. Immunity. 2002;16:99–109. doi: 10.1016/s1074-7613(01)00260-6. [DOI] [PubMed] [Google Scholar]
- 34.Garbi N, Tanaka S, Momburg F, Hammerling GJ. Impaired assembly of the major histocompatibility complex class I peptide-loading complex in mice deficient in the oxidoreductase ERp57. Nat Immunol. 2006;7:93–102. doi: 10.1038/ni1288. [DOI] [PubMed] [Google Scholar]
- 35.Peaper DR, Cresswell P. The redox activity of ERp57 is not essential for its functions in MHC class I peptide loading. Proc Natl Acad Sci U S A. 2008;105:10477–82. doi: 10.1073/pnas.0805044105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang W, Wearsch PA, Zhu Y, Leonhardt RM, Cresswell P. A role for UDP-glucose glycoprotein glucosyltransferase in expression and quality control of MHC class I molecules. Proc Natl Acad Sci U S A. 2011;108:4956–61. doi: 10.1073/pnas.1102527108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ruddock LW, Molinari M. N-glycan processing in ER quality control. J Cell Sci. 2006;119:4373–80. doi: 10.1242/jcs.03225. [DOI] [PubMed] [Google Scholar]
- 38.Serwold T, Gonzalez F, Kim J, Jacob R, Shastri N. ERAAP customizes peptides for MHC class I molecules in the endoplasmic reticulum. Nature. 2002;419:480–3. doi: 10.1038/nature01074. [DOI] [PubMed] [Google Scholar]
- 39.Saric T, Chang SC, Hattori A, York IA, Markant S, et al. An IFN-gamma-induced aminopeptidase in the ER, ERAP1, trims precursors to MHC class I-presented peptides. Nat Immunol. 2002;3:1169–76. doi: 10.1038/ni859. [DOI] [PubMed] [Google Scholar]
- 40.Saveanu L, Carroll O, Lindo V, Del Val M, Lopez D, et al. Concerted peptide trimming by human ERAP1 and ERAP2 aminopeptidase complexes in the endoplasmic reticulum. Nat Immunol. 2005;6:689–97. doi: 10.1038/ni1208. [DOI] [PubMed] [Google Scholar]
- 41.Koopmann JO, Post M, Neefjes JJ, Hammerling GJ, Momburg F. Translocation of long peptides by transporters associated with antigen processing (TAP) Eur J Immunol. 1996;26:1720–8. doi: 10.1002/eji.1830260809. [DOI] [PubMed] [Google Scholar]
- 42.Nguyen TT, Chang SC, Evnouchidou I, York IA, Zikos C, et al. Structural basis for antigenic peptide precursor processing by the endoplasmic reticulum aminopeptidase ERAP1. Nat Struct Mol Biol. 2011;18:604–13. doi: 10.1038/nsmb.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hammer GE, Gonzalez F, Champsaur M, Cado D, Shastri N. The aminopeptidase ERAAP shapes the peptide repertoire displayed by major histocompatibility complex class I molecules. Nat Immunol. 2006;7:103–12. doi: 10.1038/ni1286. [DOI] [PubMed] [Google Scholar]
- 44.Hammer GE, Gonzalez F, James E, Nolla H, Shastri N. In the absence of aminopeptidase ERAAP, MHC class I molecules present many unstable and highly immunogenic peptides. Nat Immunol. 2007;8:101–8. doi: 10.1038/ni1409. [DOI] [PubMed] [Google Scholar]
- 45.Blanchard N, Kanaseki T, Escobar H, Delebecque F, Nagarajan NA, et al. Endoplasmic reticulum aminopeptidase associated with antigen processing defines the composition and structure of MHC class I peptide repertoire in normal and virus-infected cells. J Immunol. 2010;184:3033–42. doi: 10.4049/jimmunol.0903712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Landsverk OJ, Bakke O, Gregers TF. MHC II and the endocytic pathway: regulation by invariant chain. Scand J Immunol. 2009;70:184–93. doi: 10.1111/j.1365-3083.2009.02301.x. [DOI] [PubMed] [Google Scholar]
- 47.Fineschi B, Sakaguchi K, Appella E, Miller J. The proteolytic environment involved in MHC class II-restricted antigen presentation can be modulated by the p41 form of invariant chain. J Immunol. 1996;157:3211–5. [PubMed] [Google Scholar]
- 48.Pond L, Kuhn LA, Teyton L, Schutze MP, Tainer JA, et al. A role for acidic residues in di-leucine motif-based targeting to the endocytic pathway. J Biol Chem. 1995;270:19989–97. doi: 10.1074/jbc.270.34.19989. [DOI] [PubMed] [Google Scholar]
- 49.Zhong G, Romagnoli P, Germain RN. Related leucine-based cytoplasmic targeting signals in invariant chain and major histocompatibility complex class II molecules control endocytic presentation of distinct determinants in a single protein. J Exp Med. 1997;185:429–38. doi: 10.1084/jem.185.3.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ceman S, Sant AJ. The function of invariant chain in class II-restricted antigen presentation. Semin Immunol. 1995;7:373–87. doi: 10.1006/smim.1995.0042. [DOI] [PubMed] [Google Scholar]
- 51.Naujokas MF, Morin M, Anderson MS, Peterson M, Miller J. The chondroitin sulfate form of invariant chain can enhance stimulation of T cell responses through interaction with CD44. Cell. 1993;74:257–68. doi: 10.1016/0092-8674(93)90417-o. [DOI] [PubMed] [Google Scholar]
- 52.Kang SJ, Cresswell P. Regulation of intracellular trafficking of human CD1d by association with MHC class II molecules. EMBO J. 2002;21:1650–60. doi: 10.1093/emboj/21.7.1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sugita M, Brenner MB. Association of the invariant chain with major histocompatibility complex class I molecules directs trafficking to endocytic compartments. J Biol Chem. 1995;270:1443–8. doi: 10.1074/jbc.270.3.1443. [DOI] [PubMed] [Google Scholar]
- 54.Ye L, Liu X, Rout SN, Li Z, Yan Y, et al. The MHC class II-associated invariant chain interacts with the neonatal Fc gamma receptor and modulates its trafficking to endosomal/lysosomal compartments. J Immunol. 2008;181:2572–85. doi: 10.4049/jimmunol.181.4.2572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jayawardena-Wolf J, Benlagha K, Chiu YH, Mehr R, Bendelac A. CD1d endosomal trafficking is independently regulated by an intrinsic CD1d-encoded tyrosine motif and by the invariant chain. Immunity. 2001;15:897–908. doi: 10.1016/s1074-7613(01)00240-0. [DOI] [PubMed] [Google Scholar]
- 56.Basha G, Omilusik K, Chavez-Steenbock A, Reinicke AT, Lack N, et al. A CD74-dependent MHC class I endolysosomal cross-presentation pathway. Nat Immunol. 2012;13:237–45. doi: 10.1038/ni.2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Marks MS, Blum JS, Cresswell P. Invariant chain trimers are sequestered in the rough endoplasmic reticulum in the absence of association with HLA class II antigens. J Cell Biol. 1990;111:839–55. doi: 10.1083/jcb.111.3.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Roche PA, Marks MS, Cresswell P. Formation of a nine-subunit complex by HLA class II glycoproteins and the invariant chain. Nature. 1991;354:392–4. doi: 10.1038/354392a0. [DOI] [PubMed] [Google Scholar]
- 59.Anderson MS, Miller J. Invariant chain can function as a chaperone protein for class II major histocompatibility complex molecules. Proc Natl Acad Sci U S A. 1992;89:2282–6. doi: 10.1073/pnas.89.6.2282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bikoff EK, Huang LY, Episkopou V, van Meerwijk J, Germain RN, Robertson EJ. Defective major histocompatibility complex class II assembly, transport, peptide acquisition, and CD4+ T cell selection in mice lacking invariant chain expression. J Exp Med. 1993;177:1699–712. doi: 10.1084/jem.177.6.1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.van Lith M, McEwen-Smith RM, Benham AM. HLA-DP, HLA-DQ, and HLA-DR have different requirements for invariant chain and HLA-DM. J Biol Chem. 2010;285:40800–8. doi: 10.1074/jbc.M110.148155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Roche PA, Cresswell P. Invariant chain association with HLA-DR molecules inhibits immunogenic peptide binding. Nature. 1990;345:615–8. doi: 10.1038/345615a0. [DOI] [PubMed] [Google Scholar]
- 63.Teyton L, O’Sullivan D, Dickson PW, Lotteau V, Sette A, et al. Invariant chain distinguishes between the exogenous and endogenous antigen presentation pathways. Nature. 1990;348:39–44. doi: 10.1038/348039a0. [DOI] [PubMed] [Google Scholar]
- 64.Roche PA, Teletski CL, Stang E, Bakke O, Long EO. Cell surface HLA-DR-invariant chain complexes are targeted to endosomes by rapid internalization. Proc Natl Acad Sci U S A. 1993;90:8581–5. doi: 10.1073/pnas.90.18.8581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Watts C. The endosome-lysosome pathway and information generation in the immune system. Biochim Biophys Acta. 2012;1824:14–21. doi: 10.1016/j.bbapap.2011.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Riberdy JM, Newcomb JR, Surman MJ, Barbosa JA, Cresswell P. HLA-DR molecules from an antigen-processing mutant cell line are associated with invariant chain peptides. Nature. 1992;360:474–7. doi: 10.1038/360474a0. [DOI] [PubMed] [Google Scholar]
- 67.Sette A, Ceman S, Kubo RT, Sakaguchi K, Appella E, Hunt DF, et al. Invariant chain peptides in most HLA-DR molecules of an antigen-processing mutant. Science. 1992;258:1801–4. doi: 10.1126/science.1465617. [DOI] [PubMed] [Google Scholar]
- 68.Ghosh P, Amaya M, Mellins E, Wiley DC. The structure of an intermediate in class II MHC maturation: CLIP bound to HLA-DR3. Nature. 1995;378:457–62. doi: 10.1038/378457a0. [DOI] [PubMed] [Google Scholar]
- 69.Busch R, Rinderknecht CH, Roh S, Lee AW, Harding JJ, et al. Achieving stability through editing and chaperoning: regulation of MHC class II peptide binding and expression. Immunol Rev. 2005;207:242–60. doi: 10.1111/j.0105-2896.2005.00306.x. [DOI] [PubMed] [Google Scholar]
- 70.Miyazaki T, Wolf P, Tourne S, Waltzinger C, Dierich A, et al. Mice lacking H2-M complexes, enigmatic elements of the MHC class II peptide-loading pathway. Cell. 1996;84:531–41. doi: 10.1016/s0092-8674(00)81029-6. [DOI] [PubMed] [Google Scholar]
- 71.Rohn TA, Boes M, Wolters D, Spindeldreher S, Muller B, et al. Upregulation of the CLIP self peptide on mature dendritic cells antagonizes T helper type 1 polarization. Nat Immunol. 2004;5:909–18. doi: 10.1038/ni1108. [DOI] [PubMed] [Google Scholar]
- 72.Mohan JF, Petzold SJ, Unanue ER. Register shifting of an insulin peptide-MHC complex allows diabetogenic T cells to escape thymic deletion. J Exp Med. 2011;208:2375–83. doi: 10.1084/jem.20111502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pu Z, Lovitch SB, Bikoff EK, Unanue ER. T cells distinguish MHC-peptide complexes formed in separate vesicles and edited by H2-DM. Immunity. 2004;20:467–76. doi: 10.1016/s1074-7613(04)00073-1. [DOI] [PubMed] [Google Scholar]
- 74.Morris P, Shaman J, Attaya M, Amaya M, Goodman S, et al. An essential role for HLA-DM in antigen presentation by class II major histocompatibility molecules. Nature. 1994;368:551–4. doi: 10.1038/368551a0. [DOI] [PubMed] [Google Scholar]
- 75.Denzin LK, Cresswell P. HLA-DM induces CLIP dissociation from MHC class II alpha beta dimers and facilitates peptide loading. Cell. 1995;82:155–65. doi: 10.1016/0092-8674(95)90061-6. [DOI] [PubMed] [Google Scholar]
- 76.Fremont DH, Crawford F, Marrack P, Hendrickson WA, Kappler J. Crystal structure of mouse H2-M. Immunity. 1998;9:385–93. doi: 10.1016/s1074-7613(00)80621-4. [DOI] [PubMed] [Google Scholar]
- 77.Mosyak L, Zaller DM, Wiley DC. The structure of HLA-DM, the peptide exchange catalyst that loads antigen onto class II MHC molecules during antigen presentation. Immunity. 1998;9:377–83. doi: 10.1016/s1074-7613(00)80620-2. [DOI] [PubMed] [Google Scholar]
- 78.Marks MS, Roche PA, van Donselaar E, Woodruff L, Peters PJ, Bonifacino JS. A lysosomal targeting signal in the cytoplasmic tail of the beta chain directs HLA-DM to MHC class II compartments. J Cell Biol. 1995;131:351–69. doi: 10.1083/jcb.131.2.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lindstedt R, Liljedahl M, Peleraux A, Peterson PA, Karlsson L. The MHC class II molecule H2-M is targeted to an endosomal compartment by a tyrosine-based targeting motif. Immunity. 1995;3:561–72. doi: 10.1016/1074-7613(95)90127-2. [DOI] [PubMed] [Google Scholar]
- 80.Pierre P, Shachar I, Matza D, Gatti E, Flavell RA, Mellman I. Invariant chain controls H2-M proteolysis in mouse splenocytes and dendritic cells. J Exp Med. 2000;191:1057–62. doi: 10.1084/jem.191.6.1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sloan VS, Cameron P, Porter G, Gammon M, Amaya M, et al. Mediation by HLA-DM of dissociation of peptides from HLA-DR. Nature. 1995;375:802–6. doi: 10.1038/375802a0. [DOI] [PubMed] [Google Scholar]
- 82.Sherman MA, Weber DA, Jensen PE. DM enhances peptide binding to class II MHC by release of invariant chain-derived peptide. Immunity. 1995;3:197–205. doi: 10.1016/1074-7613(95)90089-6. [DOI] [PubMed] [Google Scholar]
- 83.Fung-Leung WP, Surh CD, Liljedahl M, Pang J, Leturcq D, et al. Antigen presentation and T cell development in H2-M-deficient mice. Science. 1996;271:1278–81. doi: 10.1126/science.271.5253.1278. [DOI] [PubMed] [Google Scholar]
- 84.Martin WD, Hicks GG, Mendiratta SK, Leva HI, Ruley HE, Van Kaer L. H2-M mutant mice are defective in the peptide loading of class II molecules, antigen presentation, and T cell repertoire selection. Cell. 1996;84:543–50. doi: 10.1016/s0092-8674(00)81030-2. [DOI] [PubMed] [Google Scholar]
- 85.Kropshofer H, Vogt AB, Moldenhauer G, Hammer J, Blum JS, Hammerling GJ. Editing of the HLA-DR-peptide repertoire by HLA-DM. EMBO J. 1996;15:6144–54. [PMC free article] [PubMed] [Google Scholar]
- 86.Kovats S, Whiteley PE, Concannon P, Rudensky AY, Blum JS. Presentation of abundant endogenous class II DR-restricted antigens by DM-negative B cell lines. Eur J Immunol. 1997;27:1014–21. doi: 10.1002/eji.1830270431. [DOI] [PubMed] [Google Scholar]
- 87.Kovats S, Grubin CE, Eastman S, deRoos P, Dongre A, et al. Invariant chain-independent function of H-2M in the formation of endogenous peptide-major histocompatibility complex class II complexes in vivo. J Exp Med. 1998;187:245–51. doi: 10.1084/jem.187.2.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ma C, Blum JS. Receptor-mediated endocytosis of antigens overcomes the requirement for HLA-DM in class II-restricted antigen presentation. J Immunol. 1997;158:1–4. [PubMed] [Google Scholar]
- 89.Denzin LK, Sant’Angelo DB, Hammond C, Surman MJ, Cresswell P. Negative regulation by HLA-DO of MHC class II-restricted antigen processing. Science. 1997;278:106–9. doi: 10.1126/science.278.5335.106. [DOI] [PubMed] [Google Scholar]
- 90.Liljedahl M, Winqvist O, Surh CD, Wong P, Ngo K, et al. Altered antigen presentation in mice lacking H2-O. Immunity. 1998;8:233–43. doi: 10.1016/s1074-7613(00)80475-6. [DOI] [PubMed] [Google Scholar]
- 91.Chen X, Jensen PE. The expression of HLA-DO (H2-O) in B lymphocytes. Immunol Res. 2004;29:19–28. doi: 10.1385/IR:29:1-3:019. [DOI] [PubMed] [Google Scholar]
- 92.Hornell TM, Burster T, Jahnsen FL, Pashine A, Ochoa MT, et al. Human dendritic cell expression of HLA-DO is subset specific and regulated by maturation. J Immunol. 2006;176:3536–47. doi: 10.4049/jimmunol.176.6.3536. [DOI] [PubMed] [Google Scholar]
- 93.Fallas JL, Yi W, Draghi NA, O’Rourke HM, Denzin LK. Expression patterns of H2-O in mouse B cells and dendritic cells correlate with cell function. J Immunol. 2007;178:1488–97. doi: 10.4049/jimmunol.178.3.1488. [DOI] [PubMed] [Google Scholar]
- 94.Porter GW, Yi W, Denzin LK. TLR agonists downregulate H2-O in CD8alpha-dendritic cells. J Immunol. 2011;187:4151–60. doi: 10.4049/jimmunol.1003137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Liljedahl M, Kuwana T, Fung-Leung WP, Jackson MR, Peterson PA, Karlsson L. HLA-DO is a lysosomal resident which requires association with HLA-DM for efficient intracellular transport. EMBO J. 1996;15:4817–24. [PMC free article] [PubMed] [Google Scholar]
- 96.Yoon T, Macmillan H, Mortimer SE, Jiang W, Rinderknecht CH, et al. Mapping the HLA-DO/HLA-DM complex by FRET and mutagenesis. Proc Natl Acad Sci U S A. 2012;109:11276–81. doi: 10.1073/pnas.1113966109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Guce AI, Mortimer SE, Yoon T, Painter CA, Mellins ED, Stern LJ. Crystal structure of the HLA-DO/HLA-DM complex shows that HLA-DO acts as a substrate mimic to inhibit antigen presentation by a competitive mechanism. 2012 Submitted. [Google Scholar]
- 98.Perraudeau M, Taylor PR, Stauss HJ, Lindstedt R, Bygrave AE, et al. Altered major histocompatibility complex class II peptide loading in H2-O-deficient mice. Eur J Immunol. 2000;30:2871–80. doi: 10.1002/1521-4141(200010)30:10<2871::AID-IMMU2871>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 99.Fallas JL, Tobin HM, Lou O, Guo D, Sant’Angelo DB, Denzin LK. Ectopic expression of HLA-DO in mouse dendritic cells diminishes MHC class II antigen presentation. J Immunol. 2004;173:1549–60. doi: 10.4049/jimmunol.173.3.1549. [DOI] [PubMed] [Google Scholar]
- 100.Yi W, Seth NP, Martillotti T, Wucherpfennig KW, Sant’Angelo DB, Denzin LK. Targeted regulation of self-peptide presentation prevents type I diabetes in mice without disrupting general immunocompetence. J Clin Invest. 2010;120:1324–36. doi: 10.1172/JCI40220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Alix E, Mukherjee S, Roy CR. Subversion of membrane transport pathways by vacuolar pathogens. J Cell Biol. 2011;195:943–52. doi: 10.1083/jcb.201105019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ham H, Sreelatha A, Orth K. Manipulation of host membranes by bacterial effectors. Nat Rev Microbiol. 2011;9:635–46. doi: 10.1038/nrmicro2602. [DOI] [PubMed] [Google Scholar]
- 103.Kuballa P, Nolte WM, Castoreno AB, Xavier RJ. Autophagy and the immune system. Annu Rev Immunol. 2012;30:611–46. doi: 10.1146/annurev-immunol-020711-074948. [DOI] [PubMed] [Google Scholar]
- 104.Watts C. Capture and processing of exogenous antigens for presentation on MHC molecules. Annu Rev Immunol. 1997;15:821–50. doi: 10.1146/annurev.immunol.15.1.821. [DOI] [PubMed] [Google Scholar]
- 105.Lanzavecchia A. Antigen-specific interaction between T and B cells. Nature. 1985;314:537–9. doi: 10.1038/314537a0. [DOI] [PubMed] [Google Scholar]
- 106.Davidson HW, West MA, Watts C. Endocytosis, intracellular trafficking, and processing of membrane IgG and monovalent antigen/membrane IgG complexes in B lymphocytes. J Immunol. 1990;144:4101–9. [PubMed] [Google Scholar]
- 107.Gil-Torregrosa BC, Lennon-Dumenil AM, Kessler B, Guermonprez P, Ploegh HL, et al. Control of cross-presentation during dendritic cell maturation. Eur J Immunol. 2004;34:398–407. doi: 10.1002/eji.200324508. [DOI] [PubMed] [Google Scholar]
- 108.Bonifaz LC, Bonnyay DP, Charalambous A, Darguste DI, Fujii S, et al. In vivo targeting of antigens to maturing dendritic cells via the DEC-205 receptor improves T cell vaccination. J Exp Med. 2004;199:815–24. doi: 10.1084/jem.20032220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lorenz RG, Blum JS, Allen PM. Constitutive competition by self proteins for antigen presentation can be overcome by receptor-enhanced uptake. J Immunol. 1990;144:1600–6. [PubMed] [Google Scholar]
- 110.McCoy KL, Page MS, Merkel BJ, Inman JK, Stutzman R. Differences among various lineages of antigen-presenting cells in processing exogenous antigen internalized through transferrin receptors. J Immunol. 1993;151:6757–68. [PubMed] [Google Scholar]
- 111.Burgdorf S, Kautz A, Bohnert V, Knolle PA, Kurts C. Distinct pathways of antigen uptake and intracellular routing in CD4 and CD8 T cell activation. Science. 2007;316:612–6. doi: 10.1126/science.1137971. [DOI] [PubMed] [Google Scholar]
- 112.Castellino F, Boucher PE, Eichelberg K, Mayhew M, Rothman JE, et al. Receptor-mediated uptake of antigen/heat shock protein complexes results in major histocompatibility complex class I antigen presentation via two distinct processing pathways. J Exp Med. 2000;191:1957–64. doi: 10.1084/jem.191.11.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Tobian AA, Harding CV, Canaday DH. Mycobacterium tuberculosis heat shock fusion protein enhances class I MHC cross-processing and -presentation by B lymphocytes. J Immunol. 2005;174:5209–14. doi: 10.4049/jimmunol.174.9.5209. [DOI] [PubMed] [Google Scholar]
- 114.Houlihan JL, Metzler JJ, Blum JS. HSP90alpha and HSP90beta isoforms selectively modulate MHC class II antigen presentation in B cells. J Immunol. 2009;182:7451–8. doi: 10.4049/jimmunol.0804296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Matsutake T, Sawamura T, Srivastava PK. High efficiency CD91- and LOX-1-mediated re-presentation of gp96-chaperoned peptides by MHC II molecules. Cancer Immun. 2010;10:7. [PMC free article] [PubMed] [Google Scholar]
- 116.Ramachandra L, Simmons D, Harding CV. MHC molecules and microbial antigen processing in phagosomes. Curr Opin Immunol. 2009;21:98–104. doi: 10.1016/j.coi.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Schelhaas M. Come in and take your coat off - how host cells provide endocytosis for virus entry. Cell Microbiol. 2010;12:1378–88. doi: 10.1111/j.1462-5822.2010.01510.x. [DOI] [PubMed] [Google Scholar]
- 118.Schulz O, Reis e Sousa C. Cross-presentation of cell-associated antigens by CD8alpha+ dendritic cells is attributable to their ability to internalize dead cells. Immunology. 2002;107:183–9. doi: 10.1046/j.1365-2567.2002.01513.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Vidard L, Kovacsovics-Bankowski M, Kraeft SK, Chen LB, Benacerraf B, Rock KL. Analysis of MHC class II presentation of particulate antigens of B lymphocytes. J Immunol. 1996;156:2809–18. [PubMed] [Google Scholar]
- 120.Gao J, Ma X, Gu W, Fu M, An J, et al. Novel functions of murine B1 cells: active phagocytic and microbicidal abilities. Eur J Immunol. 2012;42:982–92. doi: 10.1002/eji.201141519. [DOI] [PubMed] [Google Scholar]
- 121.Parra D, Rieger AM, Li J, Zhang YA, Randall LM, et al. Pivotal advance: peritoneal cavity B-1 B cells have phagocytic and microbicidal capacities and present phagocytosed antigen to CD4+ T cells. J Leukoc Biol. 2012;91:525–36. doi: 10.1189/jlb.0711372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kim SH, Visser A, Cruijsen C, van der Velden AW, Boes M. Recruitment of Rab27a to phagosomes controls microbial antigen cross-presentation by dendritic cells. Infect Immun. 2008;76:5373–80. doi: 10.1128/IAI.01044-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Sancho D, Joffre OP, Keller AM, Rogers NC, Martinez D, et al. Identification of a dendritic cell receptor that couples sensing of necrosis to immunity. Nature. 2009;458:899–903. doi: 10.1038/nature07750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Joffre OP, Sancho D, Zelenay S, Keller AM, Reis e Sousa C. Efficient and versatile manipulation of the peripheral CD4+ T-cell compartment by antigen targeting to DNGR-1/CLEC9A. Eur J Immunol. 2010;40:1255–65. doi: 10.1002/eji.201040419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Chicz RM, Urban RG, Gorga JC, Vignali DA, Lane WS, Strominger JL. Specificity and promiscuity among naturally processed peptides bound to HLA-DR alleles. J Exp Med. 1993;178:27–47. doi: 10.1084/jem.178.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Guagliardi LE, Koppelman B, Blum JS, Marks MS, Cresswell P, Brodsky FM. Co-localization of molecules involved in antigen processing and presentation in an early endocytic compartment. Nature. 1990;343:133–9. doi: 10.1038/343133a0. [DOI] [PubMed] [Google Scholar]
- 127.Harding CV, Unanue ER, Slot JW, Schwartz AL, Geuze HJ. Functional and ultrastructural evidence for intracellular formation of major histocompatibility complex class II-peptide complexes during antigen processing. Proc Natl Acad Sci U S A. 1990;87:5553–7. doi: 10.1073/pnas.87.14.5553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Peters PJ, Neefjes JJ, Oorschot V, Ploegh HL, Geuze HJ. Segregation of MHC class II molecules from MHC class I molecules in the Golgi complex for transport to lysosomal compartments. Nature. 1991;349:669–76. doi: 10.1038/349669a0. [DOI] [PubMed] [Google Scholar]
- 129.Huang Y, Biswas C, Klos Dehring DA, Sriram U, Williamson EK, et al. The actin regulatory protein HS1 is required for antigen uptake and presentation by dendritic cells. J Immunol. 2011;187:5952–63. doi: 10.4049/jimmunol.1100870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ackerman AL, Kyritsis C, Tampe R, Cresswell P. Access of soluble antigens to the endoplasmic reticulum can explain cross-presentation by dendritic cells. Nat Immunol. 2005;6:107–13. doi: 10.1038/ni1147. [DOI] [PubMed] [Google Scholar]
- 131.Ackerman AL, Kyritsis C, Tampe R, Cresswell P. Early phagosomes in dendritic cells form a cellular compartment sufficient for cross presentation of exogenous antigens. Proc Natl Acad Sci U S A. 2003;100:12889–94. doi: 10.1073/pnas.1735556100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Chiu I, Davis DM, Strominger JL. Trafficking of spontaneously endocytosed MHC proteins. Proc Natl Acad Sci U S A. 1999;96:13944–9. doi: 10.1073/pnas.96.24.13944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Reid PA, Watts C. Cycling of cell-surface MHC glycoproteins through primaquine-sensitive intracellular compartments. Nature. 1990;346:655–7. doi: 10.1038/346655a0. [DOI] [PubMed] [Google Scholar]
- 134.Lizee G, Basha G, Tiong J, Julien JP, Tian M, et al. Control of dendritic cell cross-presentation by the major histocompatibility complex class I cytoplasmic domain. Nat Immunol. 2003;4:1065–73. doi: 10.1038/ni989. [DOI] [PubMed] [Google Scholar]
- 135.Harding CV, Unanue ER. Low-temperature inhibition of antigen processing and iron uptake from transferrin: deficits in endosome functions at 18 degrees C. Eur J Immunol. 1990;20:323–9. doi: 10.1002/eji.1830200214. [DOI] [PubMed] [Google Scholar]
- 136.Griffin JP, Chu R, Harding CV. Early endosomes and a late endocytic compartment generate different peptide-class II MHC complexes via distinct processing mechanisms. J Immunol. 1997;158:1523–32. [PubMed] [Google Scholar]
- 137.Pathak SS, Blum JS. Endocytic recycling is required for the presentation of an exogenous peptide via MHC class II molecules. Traffic. 2000;1:561–9. doi: 10.1034/j.1600-0854.2000.010706.x. [DOI] [PubMed] [Google Scholar]
- 138.Saveanu L, Carroll O, Weimershaus M, Guermonprez P, Firat E, et al. IRAP identifies an endosomal compartment required for MHC class I cross-presentation. Science. 2009;325:213–7. doi: 10.1126/science.1172845. [DOI] [PubMed] [Google Scholar]
- 139.Harding CV, Unanue ER. Modulation of antigen presentation and peptide-MHC-specific, LFA-1-dependent T cell-macrophage adhesion. J Immunol. 1991;147:767–73. [PubMed] [Google Scholar]
- 140.Muller S, Dennemarker J, Reinheckel T. Specific functions of lysosomal proteases in endocytic and autophagic pathways. Biochim Biophys Acta. 2012;1824:34–43. doi: 10.1016/j.bbapap.2011.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Maric M, Arunachalam B, Phan UT, Dong C, Garrett WS, et al. Defective antigen processing in GILT-free mice. Science. 2001;294:1361–5. doi: 10.1126/science.1065500. [DOI] [PubMed] [Google Scholar]
- 142.Haque MA, Li P, Jackson SK, Zarour HM, Hawes JW, et al. Absence of gamma-interferon-inducible lysosomal thiol reductase in melanomas disrupts T cell recognition of select immunodominant epitopes. J Exp Med. 2002;195:1267–77. doi: 10.1084/jem.20011853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Delamarre L, Pack M, Chang H, Mellman I, Trombetta ES. Differential lysosomal proteolysis in antigen-presenting cells determines antigen fate. Science. 2005;307:1630–4. doi: 10.1126/science.1108003. [DOI] [PubMed] [Google Scholar]
- 144.Neefjes JJ, Stollorz V, Peters PJ, Geuze HJ, Ploegh HL. The biosynthetic pathway of MHC class II but not class I molecules intersects the endocytic route. Cell. 1990;61:171–83. doi: 10.1016/0092-8674(90)90224-3. [DOI] [PubMed] [Google Scholar]
- 145.Vascotto F, Lankar D, Faure-Andre G, Vargas P, Diaz J, et al. The actin-based motor protein myosin II regulates MHC class II trafficking and BCR-driven antigen presentation. J Cell Biol. 2007;176:1007–19. doi: 10.1083/jcb.200611147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.van Niel G, Wubbolts R, Ten Broeke T, Buschow SI, Ossendorp FA, et al. Dendritic cells regulate exposure of MHC class II at their plasma membrane by oligoubiquitination. Immunity. 2006;25:885–94. doi: 10.1016/j.immuni.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 147.De Gassart A, Camosseto V, Thibodeau J, Ceppi M, Catalan N, et al. MHC class II stabilization at the surface of human dendritic cells is the result of maturation-dependent MARCH I down-regulation. Proc Natl Acad Sci U S A. 2008;105:3491–6. doi: 10.1073/pnas.0708874105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Ishido S, Goto E, Matsuki Y, Ohmura-Hoshino M. E3 ubiquitin ligases for MHC molecules. Curr Opin Immunol. 2009;21:78–83. doi: 10.1016/j.coi.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 149.van Lith M, van Ham M, Griekspoor A, Tjin E, Verwoerd D, et al. Regulation of MHC class II antigen presentation by sorting of recycling HLA-DM/DO and class II within the multivesicular body. J Immunol. 2001;167:884–92. doi: 10.4049/jimmunol.167.2.884. [DOI] [PubMed] [Google Scholar]
- 150.Xiu F, Cote MH, Bourgeois-Daigneault MC, Brunet A, Gauvreau ME, et al. Cutting edge: HLA-DO impairs the incorporation of HLA-DM into exosomes. J Immunol. 2011;187:1547–51. doi: 10.4049/jimmunol.1100199. [DOI] [PubMed] [Google Scholar]
- 151.Anderson HA, Hiltbold EM, Roche PA. Concentration of MHC class II molecules in lipid rafts facilitates antigen presentation. Nat Immunol. 2000;1:156–62. doi: 10.1038/77842. [DOI] [PubMed] [Google Scholar]
- 152.Harding CV, Geuze HJ. Class II MHC molecules are present in macrophage lysosomes and phagolysosomes that function in the phagocytic processing of Listeria monocytogenes for presentation to T cells. J Cell Biol. 1992;119:531–42. doi: 10.1083/jcb.119.3.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Houde M, Bertholet S, Gagnon E, Brunet S, Goyette G, et al. Phagosomes are competent organelles for antigen cross-presentation. Nature. 2003;425:402–6. doi: 10.1038/nature01912. [DOI] [PubMed] [Google Scholar]
- 154.Muraille E, Gounon P, Cazareth J, Hoebeke J, Lippuner C, et al. Direct visualization of peptide/MHC complexes at the surface and in the intracellular compartments of cells infected in vivo by Leishmania major. PLoS Pathog. 2010;6:e1001154. doi: 10.1371/journal.ppat.1001154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Mantegazza AR, Guttentag SH, El-Benna J, Sasai M, Iwasaki A, et al. Adaptor protein-3 in dendritic cells facilitates phagosomal toll-like receptor signaling and antigen presentation to CD4(+) T cells. Immunity. 2012;36:782–94. doi: 10.1016/j.immuni.2012.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Amigorena S, Savina A. Intracellular mechanisms of antigen cross presentation in dendritic cells. Curr Opin Immunol. 2010;22:109–17. doi: 10.1016/j.coi.2010.01.022. [DOI] [PubMed] [Google Scholar]
- 157.Singh CR, Moulton RA, Armitige LY, Bidani A, Snuggs M, et al. Processing and presentation of a mycobacterial antigen 85B epitope by murine macrophages is dependent on the phagosomal acquisition of vacuolar proton ATPase and in situ activation of cathepsin D. J Immunol. 2006;177:3250–9. doi: 10.4049/jimmunol.177.5.3250. [DOI] [PubMed] [Google Scholar]
- 158.Yates RM, Hermetter A, Taylor GA, Russell DG. Macrophage activation downregulates the degradative capacity of the phagosome. Traffic. 2007;8:241–50. doi: 10.1111/j.1600-0854.2006.00528.x. [DOI] [PubMed] [Google Scholar]
- 159.Jutras I, Houde M, Currier N, Boulais J, Duclos S, et al. Modulation of the phagosome proteome by interferon-gamma. Mol Cell Proteomics. 2008;7:697–715. doi: 10.1074/mcp.M700267-MCP200. [DOI] [PubMed] [Google Scholar]
- 160.Sanjuan MA, Dillon CP, Tait SW, Moshiach S, Dorsey F, et al. Toll-like receptor signalling in macrophages links the autophagy pathway to phagocytosis. Nature. 2007;450:1253–7. doi: 10.1038/nature06421. [DOI] [PubMed] [Google Scholar]
- 161.Kim BH, Shenoy AR, Kumar P, Das R, Tiwari S, MacMicking JD. A family of IFN-gamma-inducible 65-kD GTPases protects against bacterial infection. Science. 2011;332:717–21. doi: 10.1126/science.1201711. [DOI] [PubMed] [Google Scholar]
- 162.Yates RM, Russell DG. Phagosome maturation proceeds independently of stimulation of toll-like receptors 2 and 4. Immunity. 2005;23:409–17. doi: 10.1016/j.immuni.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 163.Blander JM. Phagocytosis and antigen presentation: a partnership initiated by Toll-like receptors. Ann Rheum Dis. 2008;67(Suppl 3):iii44–9. doi: 10.1136/ard.2008.097964. [DOI] [PubMed] [Google Scholar]
- 164.Savina A, Jancic C, Hugues S, Guermonprez P, Vargas P, et al. NOX2 controls phagosomal pH to regulate antigen processing during crosspresentation by dendritic cells. Cell. 2006;126:205–18. doi: 10.1016/j.cell.2006.05.035. [DOI] [PubMed] [Google Scholar]
- 165.Lim JP, Gleeson PA. Macropinocytosis: an endocytic pathway for internalising large gulps. Immunol Cell Biol. 2011;89:836–43. doi: 10.1038/icb.2011.20. [DOI] [PubMed] [Google Scholar]
- 166.Hewlett LJ, Prescott AR, Watts C. The coated pit and macropinocytic pathways serve distinct endosome populations. J Cell Biol. 1994;124:689–703. doi: 10.1083/jcb.124.5.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.West MA, Wallin RP, Matthews SP, Svensson HG, Zaru R, et al. Enhanced dendritic cell antigen capture via toll-like receptor-induced actin remodeling. Science. 2004;305:1153–7. doi: 10.1126/science.1099153. [DOI] [PubMed] [Google Scholar]
- 168.von Delwig A, Hilkens CM, Altmann DM, Holmdahl R, Isaacs JD, et al. Inhibition of macropinocytosis blocks antigen presentation of type II collagen in vitro and in vivo in HLA-DR1 transgenic mice. Arthritis Res Ther. 2006;8:R93. doi: 10.1186/ar1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Tanaka Y, Taneichi M, Kasai M, Kakiuchi T, Uchida T. Liposome-coupled antigens are internalized by antigen-presenting cells via pinocytosis and cross-presented to CD8 T cells. PLoS One. 2010;5:e15225. doi: 10.1371/journal.pone.0015225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Crotzer VL, Blum JS. Autophagy and adaptive immunity. Immunology. 2010;131:9–17. doi: 10.1111/j.1365-2567.2010.03321.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Sahu R, Kaushik S, Clement CC, Cannizzo ES, Scharf B, et al. Microautophagy of cytosolic proteins by late endosomes. Dev Cell. 2011;20:131–9. doi: 10.1016/j.devcel.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Munz C. Antigen Processing for MHC Class II Presentation via Autophagy. Front Immunol. 2012;3:9. doi: 10.3389/fimmu.2012.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Nedjic J, Aichinger M, Emmerich J, Mizushima N, Klein L. Autophagy in thymic epithelium shapes the T-cell repertoire and is essential for tolerance. Nature. 2008;455:396–400. doi: 10.1038/nature07208. [DOI] [PubMed] [Google Scholar]
- 174.Jagannath C, Lindsey DR, Dhandayuthapani S, Xu Y, Hunter RL, Jr., Eissa NT. Autophagy enhances the efficacy of BCG vaccine by increasing peptide presentation in mouse dendritic cells. Nat Med. 2009;15:267–76. doi: 10.1038/nm.1928. [DOI] [PubMed] [Google Scholar]
- 175.Dragovic SM, Hill T, Christianson GJ, Kim S, Elliott T, et al. Proteasomes, TAP, and endoplasmic reticulum-associated aminopeptidase associated with antigen processing control CD4+ Th cell responses by regulating indirect presentation of MHC class II-restricted cytoplasmic antigens. J Immunol. 2011;186:6683–92. doi: 10.4049/jimmunol.1100525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Li Y, Wang LX, Pang P, Cui Z, Aung S, et al. Tumor-derived autophagosome vaccine: mechanism of cross-presentation and therapeutic efficacy. Clin Cancer Res. 2011;17:7047–57. doi: 10.1158/1078-0432.CCR-11-0951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Tewari MK, Sinnathamby G, Rajagopal D, Eisenlohr LC. A cytosolic pathway for MHC class II-restricted antigen processing that is proteasome and TAP dependent. Nat Immunol. 2005;6:287–94. doi: 10.1038/ni1171. [DOI] [PubMed] [Google Scholar]
- 178.Lich JD, Elliott JF, Blum JS. Cytoplasmic processing is a prerequisite for presentation of an endogenous antigen by major histocompatibility complex class II proteins. J Exp Med. 2000;191:1513–24. doi: 10.1084/jem.191.9.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Mukherjee P, Dani A, Bhatia S, Singh N, Rudensky AY, et al. Efficient presentation of both cytosolic and endogenous transmembrane protein antigens on MHC class II is dependent on cytoplasmic proteolysis. J Immunol. 2001;167:2632–41. doi: 10.4049/jimmunol.167.5.2632. [DOI] [PubMed] [Google Scholar]
- 180.Dorfel D, Appel S, Grunebach F, Weck MM, Muller MR, et al. Processing and presentation of HLA class I and II epitopes by dendritic cells after transfection with in vitro-transcribed MUC1 RNA. Blood. 2005;105:3199–205. doi: 10.1182/blood-2004-09-3556. [DOI] [PubMed] [Google Scholar]
- 181.Akram A, Inman RD. Immunodominance: a pivotal principle in host response to viral infections. Clin Immunol. 2012;143:99–115. doi: 10.1016/j.clim.2012.01.015. [DOI] [PubMed] [Google Scholar]
- 182.Moudgil KD, Sercarz EE. Crypticity of self antigenic determinants is the cornerstone of a theory of autoimmunity. Discov Med. 2005;5:378–82. [PubMed] [Google Scholar]
- 183.Dai YD, Jensen KP, Marrero I, Li N, Quinn A, Sercarz EE. N-terminal flanking residues of a diabetes-associated GAD65 determinant are necessary for activation of antigen-specific T cells in diabetes-resistant mice. Eur J Immunol. 2008;38:968–76. doi: 10.1002/eji.200737703. [DOI] [PubMed] [Google Scholar]
- 184.Mimura Y, Mimura-Kimura Y, Doores K, Golgher D, Davis BG, et al. Folding of an MHC class II-restricted tumor antigen controls its antigenicity via MHC-guided processing. Proc Natl Acad Sci U S A. 2007;104:5983–8. doi: 10.1073/pnas.0701307104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Bergman CM, Marta CB, Maric M, Pfeiffer SE, Cresswell P, Ruddle NH. A Switch in Pathogenic Mechanism in Myelin Oligodendrocyte Glycoprotein-Induced Experimental Autoimmune Encephalomyelitis in IFN-gamma-Inducible Lysosomal Thiol Reductase-Free Mice. J Immunol. 2012;188:6001–9. doi: 10.4049/jimmunol.1101898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Rausch MP, Irvine KR, Antony PA, Restifo NP, Cresswell P, Hastings KT. GILT accelerates autoimmunity to the melanoma antigen tyrosinase-related protein 1. J Immunol. 2010;185:2828–35. doi: 10.4049/jimmunol.1000945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Delamarre L, Couture R, Mellman I, Trombetta ES. Enhancing immunogenicity by limiting susceptibility to lysosomal proteolysis. J Exp Med. 2006;203:2049–55. doi: 10.1084/jem.20052442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Davidson HW, Reid PA, Lanzavecchia A, Watts C. Processed antigen binds to newly synthesized MHC class II molecules in antigen-specific B lymphocytes. Cell. 1991;67:105–16. doi: 10.1016/0092-8674(91)90575-j. [DOI] [PubMed] [Google Scholar]
- 189.Sercarz EE, Maverakis E. Mhc-guided processing: binding of large antigen fragments. Nat Rev Immunol. 2003;3:621–9. doi: 10.1038/nri1149. [DOI] [PubMed] [Google Scholar]
- 190.Moss CX, Tree TI, Watts C. Reconstruction of a pathway of antigen processing and class II MHC peptide capture. EMBO J. 2007;26:2137–47. doi: 10.1038/sj.emboj.7601660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Brooks K, Knight AM. Lowering the affinity between antigen and the B cell receptor can enhance antigen presentation. Eur J Immunol. 2004;34:837–43. doi: 10.1002/eji.200324357. [DOI] [PubMed] [Google Scholar]
- 192.Hartman IZ, Kim A, Cotter RJ, Walter K, Dalai SK, et al. A reductionist cell-free major histocompatibility complex class II antigen processing system identifies immunodominant epitopes. Nat Med. 2010;16:1333–40. doi: 10.1038/nm.2248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Nanda NK, Sant AJ. DM determines the cryptic and immunodominant fate of T cell epitopes. J Exp Med. 2000;192:781–8. doi: 10.1084/jem.192.6.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Mohan JF, Levisetti MG, Calderon B, Herzog JW, Petzold SJ, Unanue ER. Unique autoreactive T cells recognize insulin peptides generated within the islets of Langerhans in autoimmune diabetes. Nat Immunol. 2010;11:350–4. doi: 10.1038/ni.1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Singh R, Cresswell P. Defective cross-presentation of viral antigens in GILT-free mice. Science. 2010;328:1394–8. doi: 10.1126/science.1189176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Siddiqui S, Basta S. CD8+ T cell immunodominance in lymphocytic choriomeningitis virus infection is modified in the presence of toll-like receptor agonists. J Virol. 2011;85:13224–33. doi: 10.1128/JVI.05996-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Boon T, Coulie PG, Van den Eynde BJ, van der Bruggen P. Human T cell responses against melanoma. Annu Rev Immunol. 2006;24:175–208. doi: 10.1146/annurev.immunol.24.021605.090733. [DOI] [PubMed] [Google Scholar]
- 198.Hahn YS, Braciale VL, Braciale TJ. Presentation of viral antigen to class I major histocompatibility complex-restricted cytotoxic T lymphocyte. Recognition of an immunodominant influenza hemagglutinin site by cytotoxic T lymphocyte is independent of the position of the site in the hemagglutinin translation product. J Exp Med. 1991;174:733–6. doi: 10.1084/jem.174.3.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Hanke T, Graham FL, Rosenthal KL, Johnson DC. Identification of an immunodominant cytotoxic T-lymphocyte recognition site in glycoprotein B of herpes simplex virus by using recombinant adenovirus vectors and synthetic peptides. J Virol. 1991;65:1177–86. doi: 10.1128/jvi.65.3.1177-1186.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Schubert U, Anton LC, Gibbs J, Norbury CC, Yewdell JW, Bennink JR. Rapid degradation of a large fraction of newly synthesized proteins by proteasomes. Nature. 2000;404:770–4. doi: 10.1038/35008096. [DOI] [PubMed] [Google Scholar]
- 201.Yewdell JW. DRiPs solidify: progress in understanding endogenous MHC class I antigen processing. Trends Immunol. 2011;32:548–58. doi: 10.1016/j.it.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Townsend AR, Gotch FM, Davey J. Cytotoxic T cells recognize fragments of the influenza nucleoprotein. Cell. 1985;42:457–67. doi: 10.1016/0092-8674(85)90103-5. [DOI] [PubMed] [Google Scholar]
- 203.Reits EA, Vos JC, Gromme M, Neefjes J. The major substrates for TAP in vivo are derived from newly synthesized proteins. Nature. 2000;404:774–8. doi: 10.1038/35008103. [DOI] [PubMed] [Google Scholar]
- 204.Princiotta MF, Finzi D, Qian SB, Gibbs J, Schuchmann S, et al. Quantitating protein synthesis, degradation, and endogenous antigen processing. Immunity. 2003;18:343–54. doi: 10.1016/s1074-7613(03)00051-7. [DOI] [PubMed] [Google Scholar]
- 205.Mackay LK, Long HM, Brooks JM, Taylor GS, Leung CS, et al. T cell detection of a B-cell tropic virus infection: newly-synthesised versus mature viral proteins as antigen sources for CD4 and CD8 epitope display. PLoS Pathog. 2009;5:e1000699. doi: 10.1371/journal.ppat.1000699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Apcher S, Daskalogianni C, Lejeune F, Manoury B, Imhoos G, et al. Major source of antigenic peptides for the MHC class I pathway is produced during the pioneer round of mRNA translation. Proc Natl Acad Sci U S A. 2011;108:11572–7. doi: 10.1073/pnas.1104104108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Apcher S, Manoury B, Fahraeus R. The role of mRNA translation in direct MHC class I antigen presentation. Curr Opin Immunol. 2012;24:71–6. doi: 10.1016/j.coi.2012.01.007. [DOI] [PubMed] [Google Scholar]
- 208.Granados DP, Yahyaoui W, Laumont CM, Daouda T, Muratore-Schroeder TL, et al. MHC I-associated peptides preferentially derive from transcripts bearing miRNA response elements. Blood. 2012;119:e181–91. doi: 10.1182/blood-2012-02-412593. [DOI] [PubMed] [Google Scholar]
- 209.Villanueva MS, Sijts AJ, Pamer EG. Listeriolysin is processed efficiently into an MHC class I-associated epitope in Listeria monocytogenes-infected cells. J Immunol. 1995;155:5227–33. [PubMed] [Google Scholar]
- 210.Kunisawa J, Shastri N. Hsp90alpha chaperones large C-terminally extended proteolytic intermediates in the MHC class I antigen processing pathway. Immunity. 2006;24:523–34. doi: 10.1016/j.immuni.2006.03.015. [DOI] [PubMed] [Google Scholar]
- 211.Cascio P, Hilton C, Kisselev AF, Rock KL, Goldberg AL. 26S proteasomes and immunoproteasomes produce mainly N-extended versions of an antigenic peptide. EMBO J. 2001;20:2357–66. doi: 10.1093/emboj/20.10.2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Kunisawa J, Shastri N. The group II chaperonin TRiC protects proteolytic intermediates from degradation in the MHC class I antigen processing pathway. Mol Cell. 2003;12:565–76. doi: 10.1016/j.molcel.2003.08.009. [DOI] [PubMed] [Google Scholar]
- 213.Beninga J, Rock KL, Goldberg AL. Interferon-gamma can stimulate post-proteasomal trimming of the N terminus of an antigenic peptide by inducing leucine aminopeptidase. J Biol Chem. 1998;273:18734–42. doi: 10.1074/jbc.273.30.18734. [DOI] [PubMed] [Google Scholar]
- 214.Urban S, Textoris-Taube K, Reimann B, Janek K, Dannenberg T, et al. The Efficiency of Human Cytomegalovirus pp65495-503 CD8+ T Cell Epitope Generation Is Determined by the Balanced Activities of Cytosolic and Endoplasmic Reticulum-Resident Peptidases. J Immunol. 2012;189:529–38. doi: 10.4049/jimmunol.1101886. [DOI] [PubMed] [Google Scholar]
- 215.Sykulev Y, Joo M, Vturina I, Tsomides TJ, Eisen HN. Evidence that a single peptide-MHC complex on a target cell can elicit a cytolytic T cell response. Immunity. 1996;4:565–71. doi: 10.1016/s1074-7613(00)80483-5. [DOI] [PubMed] [Google Scholar]
- 216.Vigneron N, Van den Eynde BJ. Insights into the processing of MHC class I ligands gained from the study of human tumor epitopes. Cell Mol Life Sci. 2011;68:1503–20. doi: 10.1007/s00018-011-0658-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Starck SR, Jiang V, Pavon-Eternod M, Prasad S, McCarthy B, et al. Leucine-tRNA initiates at CUG start codons for protein synthesis and presentation by MHC class I. Science. 2012;336:1719–23. doi: 10.1126/science.1220270. [DOI] [PubMed] [Google Scholar]
- 218.Vigneron N, Stroobant V, Chapiro J, Ooms A, Degiovanni G, et al. An antigenic peptide produced by peptide splicing in the proteasome. Science. 2004;304:587–90. doi: 10.1126/science.1095522. [DOI] [PubMed] [Google Scholar]
- 219.Warren EH, Vigneron NJ, Gavin MA, Coulie PG, Stroobant V, et al. An antigen produced by splicing of noncontiguous peptides in the reverse order. Science. 2006;313:1444–7. doi: 10.1126/science.1130660. [DOI] [PubMed] [Google Scholar]
- 220.Skipper JC, Hendrickson RC, Gulden PH, Brichard V, Van Pel A, et al. An HLA-A2-restricted tyrosinase antigen on melanoma cells results from posttranslational modification and suggests a novel pathway for processing of membrane proteins. J Exp Med. 1996;183:527–34. doi: 10.1084/jem.183.2.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Smith MH, Ploegh HL, Weissman JS. Road to ruin: targeting proteins for degradation in the endoplasmic reticulum. Science. 2011;334:1086–90. doi: 10.1126/science.1209235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Suzuki T, Seko A, Kitajima K, Inoue Y, Inoue S. Purification and enzymatic properties of peptide:N-glycanase from C3H mouse-derived L-929 fibroblast cells. Possible widespread occurrence of post-translational remodification of proteins by N-deglycosylation. J Biol Chem. 1994;269:17611–8. [PubMed] [Google Scholar]
- 223.Gagnon E, Duclos S, Rondeau C, Chevet E, Cameron PH, et al. Endoplasmic reticulum-mediated phagocytosis is a mechanism of entry into macrophages. Cell. 2002;110:119–31. doi: 10.1016/s0092-8674(02)00797-3. [DOI] [PubMed] [Google Scholar]
- 224.Ackerman AL, Giodini A, Cresswell P. A role for the endoplasmic reticulum protein retrotranslocation machinery during crosspresentation by dendritic cells. Immunity. 2006;25:607–17. doi: 10.1016/j.immuni.2006.08.017. [DOI] [PubMed] [Google Scholar]
- 225.Lin ML, Zhan Y, Proietto AI, Prato S, Wu L, et al. Selective suicide of cross-presenting CD8+ dendritic cells by cytochrome c injection shows functional heterogeneity within this subset. Proc Natl Acad Sci U S A. 2008;105:3029–34. doi: 10.1073/pnas.0712394105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Giodini A, Cresswell P. Hsp90-mediated cytosolic refolding of exogenous proteins internalized by dendritic cells. EMBO J. 2008;27:201–11. doi: 10.1038/sj.emboj.7601941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Burgdorf S, Scholz C, Kautz A, Tampe R, Kurts C. Spatial and mechanistic separation of cross-presentation and endogenous antigen presentation. Nat Immunol. 2008;9:558–66. doi: 10.1038/ni.1601. [DOI] [PubMed] [Google Scholar]
- 228.Cebrian I, Visentin G, Blanchard N, Jouve M, Bobard A, et al. Sec22b regulates phagosomal maturation and antigen crosspresentation by dendritic cells. Cell. 2011;147:1355–68. doi: 10.1016/j.cell.2011.11.021. [DOI] [PubMed] [Google Scholar]
- 229.Madden DR, Garboczi DN, Wiley DC. The antigenic identity of peptide-MHC complexes: a comparison of the conformations of five viral peptides presented by HLA-A2. Cell. 1993;75:693–708. doi: 10.1016/0092-8674(93)90490-h. [DOI] [PubMed] [Google Scholar]
- 230.Stern LJ, Brown JH, Jardetzky TS, Gorga JC, Urban RG, et al. Crystal structure of the human class II MHC protein HLA-DR1 complexed with an influenza virus peptide. Nature. 1994;368:215–21. doi: 10.1038/368215a0. [DOI] [PubMed] [Google Scholar]