Abstract
Background
Observational studies have suggested that antioxidant nutrients may reduce cancer and overall mortality risks. However, most randomized trials have failed to demonstrate survival benefits. Examining non-linear associations between antioxidant levels and health outcomes may explain these discrepant findings.
Methods
We evaluated all-cause, cancer and cardiovascular mortality risks associated with quintiles (Q1–Q5) of serum antioxidant (vitamins C and E, beta-carotene, and selenium) and vitamin A levels, in 16,008 adult NHANES III (The Third National Health and Nutrition Examination survey, 1988–1994) participants.
Results
Over a median follow-up period of 14.2 years, there were 4,225 deaths, including 891 from cancer, and 1,891 from cardiovascular disease. We observed a dose-response decrease in cancer and overall mortality risks with higher vitamin C levels. In contrast, for vitamin A, risk of cancer death decreased from Q1–Q2, with no further decline in risk at higher levels. For vitamin E, having levels in Q4 were associated with the lowest cancer mortality risk. Both vitamin A and E had U-shaped associations with all-cause mortality. Cancer mortality risks decreased from Q1–Q2 for beta-carotene and from Q1–Q4 for selenium. However, for beta-carotene and selenium, overall mortality risks decreased from Q1–Q2 but then did not change significantly with higher levels.
Conclusions
Antioxidant supplement use should be studied in the context of overall mortality and other competing mortality risks.
Impact
These data suggest the possible use of novel intervention studies where doses of these agents are individualized based on serum levels, and possibly, markers of oxidative stress and systemic inflammatory response.
Keywords: Antioxidants, vitamin A, mortality, NHANES, dietary supplements
Introduction
Recent data show that between 28–30% US adults use supplements containing vitamins A, C and E, while 18–19% report using selenium (1, 2). Higher serum antioxidant levels have been associated with lower overall mortality risk (3–15), and fruit and vegetable intake (a rich source of antioxidants) has also been shown to predict better health outcomes in observational studies (16–20). In contrast, most randomized controlled trials have either failed to demonstrate significant health benefits from taking these supplements or have reported possible harm. A recent systematic review of 78 primary and secondary prevention trials by the Cochrane Collaboration concluded that use of beta-carotene, vitamin E, or high doses of vitamin A supplements was associated with increased all-cause mortality, whereas the role of vitamin C or selenium supplementation was not clear (21). Although the Physicians’ Health Study II (22) recently showed a small reduction in total cancer incidence with multivitamin supplement use, most large clinical trials assessing cancer incidence or mortality have also failed to show any beneficial effects of taking these supplements (23–29).
Many authors have suggested that the reason for these discrepant findings is that antioxidants have different effects on various disease processes, and that beyond a certain “threshold” level, they can be potentially toxic (30–40). Surprising little literature exists however examining non-linear effects between serum antioxidant nutrient levels and all-cause and cause-specific mortality outcomes. There are also few studies examining competing risks of mortality in the context of antioxidant nutrient use.
Using data from NHANES III (The Third National Health and Nutrition Examination survey, 1988 – 1994), a large nationally representative cohort of US adults, we examined whether serum levels of micronutrients with antioxidant properties (vitamins C and E, beta-carotene, and selenium) and vitamin A, predicted the risks of all-cause, cancer, and cardiovascular disease mortality outcomes. Further, by assessing nonlinear associations, we explored the reasons behind the inconsistency in findings between observational studies and randomized trials regarding the role of these agents on health outcomes.
Materials and Methods
Study Population
NHANES III was conducted from October 1988 through October 1994 by the Centers for Disease Control and Prevention (CDC) to provide national health estimates of the United States’ civilian population (41). The overall sample size was 39,695; interview and examination response rates were 86% (33,974 participants) and 78% (30,818 participants) respectively (42). Follow-up was from the date of survey participation (1988–1994) to December 31, 2006. Of the 16,573 NHANES III participants aged 20 years and above who underwent medical examination, our study included 16,008 (97%) participants for whom data on serum antioxidant nutrient levels and vital status were available. Missing data on covariates ranged from less than 1% to 8% of the study participants. In the analysis, we included only those individuals with complete information available for these covariates. NHANES III was approved by the Institutional Review Board at the CDC. Written informed consent was obtained from all study participants (43).
Measures
Our primary exposures of interest were serum levels of vitamin A, and micronutrients with antioxidant properties. Levels of vitamin A (retinol), vitamin C, vitamin E (alpha-tocopherol), and beta-carotene were measured by isocratic high performance liquid chromatography (44). Serum selenium levels were measured with atomic absorption spectrometry (44). NHANES III laboratory procedures including quality control systems have been described elsewhere (45).
Our primary outcomes of interest were cause-specific and all-cause mortality. Mortality data were primarily obtained by probabilistic matching to the National Death Index records using the 2010 public release version of the NHANES III Linked Mortality File (46). NHANES III also used various other sources of information to determine the final mortality status and causes of death for survey participants including death certificates, Social Security Administration data, and records from the Centers for Medicare and Medicaid Services. The 9th and 10th revisions of the International Statistical Classification of Diseases (ICD-9 and ICD-10) were used to classify deaths due to cancer (C00–C97) and cardiovascular disease (I00–I78) (46–48). Final mortality status was determined for more than 99% of the study participants (46).
Statistical Analysis
To accommodate the complex survey design of NHANES III, we applied appropriate statistical weights in our analyses (42). Cox Proportional Hazards Models were used to estimate the hazard ratios, and the proportional-hazards assumption was tested using the Kolmogorov-type supremum test (49). To examine whether the associations had a dose-response relationship, we modeled serum levels of antioxidants as quintiles, using the first quintile as the reference group.
Covariates used in the analysis were selected a priori based on their suspected roles as confounders. We first fit a basic model adjusted for age and gender (Model 1). For multivariable analysis, Model 2 additionally included race-ethnicity, education, income, body mass index, smoking status, serum cotinine levels, alcohol consumption, fruit and vegetable intake, physical activity, serum total cholesterol levels, hypertension status (systolic or diastolic blood pressure ≥140 or ≥90 mmHg respectively, use of anti-hypertensive drugs or hypertension medical history), diabetes mellitus status (glycosylated hemoglobin ≥6.5%, use of anti-diabetic drugs or diabetes medical history), history of heart attack, congestive heart failure, stroke or cancer, hormone use among women (use of any estrogen or progesterone including oral contraceptive pills in the past one month), and use of vitamin or mineral supplements (in the past one month). Categories of variables used in the analysis were consistent with the NHANES III survey design (42).
Additional analyses examined these associations in the first 5 or 10 years of follow-up, after excluding deaths within the first three years of the survey, and after excluding current smokers. We also assessed the independent effects of these agents by adjusting for other micronutrients in the multivariable models. Since systemic inflammatory response has been shown to affect plasma micronutrient measurements, in an additional analysis, we further adjusted for C – reactive protein levels (50). Because participants with self-reported history of comorbidities may have changed their dietary habits and supplement usage, we conducted separate analyses after excluding those with known history of heart attack, congestive heart failure, stroke or cancer. Finally, to investigate dose-response associations using vitamin A and antioxidant nutrient levels as continuous variables, we created restricted cubic spline functions for all-cause, cancer, and cardiovascular disease mortality outcomes (51). All analyses were done using SAS (Version 9.3; SAS Institute Inc., Cary, NC).
Results
Of the 16,008 study participants, 4225 died over a median follow-up period of 14.2 years. 891 deaths were due to cancer and 1891 were due to cardiovascular disease (CVD). Table 1 shows the mean baseline serum levels subdivided by socio-demographic, lifestyle and health related variables. There were significant differences in serum micronutrient levels for different participant characteristics, especially for BMI categories and smoking status, with current smokers and those with BMI ≥30 kg/m2 having significantly lower vitamin C and beta-carotene levels. NHANES III Analytic and Reporting Guidelines provide further details about the characteristics of the study population (42).
Table 1.
Serum Antioxidant Nutrient Levels among NHANES III Participants
| Mean Serum Levels (95% CI) | ||||||
|---|---|---|---|---|---|---|
| Characteristic | n | Vitamin A (μmol/L) | Vitamin C (mmol/L) | Vitamin E (μmol/L) | Beta-carotene (μmol/L) | Selenium (nmol/L) |
| Age | ||||||
| 20–39 years | 6425 | 1.93 (1.90–1.96) | 39.77 (37.95–41.60) | 22.84 (22.45–23.22) | 0.29 (0.28–0.31) | 1.58 (1.55–1.60) |
| 40–59 years | 4252 | 2.08 (2.05–2.11) | 41.94 (39.90–43.97) | 28.80 (27.89–29.72) | 0.39 (0.37–0.41) | 1.60 (1.57–1.62) |
| ≥60 years | 5331 | 2.24 (2.20–2.28) | 49.52 (47.35–51.69) | 32.85 (32.21–33.49) | 0.49 (0.47–0.52) | 1.59 (1.56–1.61) |
| Gender | ||||||
| Male | 7510 | 2.18 (2.15–2.21) | 38.24 (36.58–39.90) | 26.38 (25.82–26.93) | 0.31 (0.30–0.33) | 1.61 (1.58–1.63) |
| Female | 8498 | 1.93 (1.90–1.95) | 46.59 (44.60–48.58) | 27.52 (26.95–28.09) | 0.42 (0.41–0.43) | 1.57 (1.54–1.59) |
| Race-ethnicity | ||||||
| Non-Hispanic white | 6783 | 2.09 (2.07–2.12) | 43.80 (41.70–45.89) | 27.77 (27.19–28.35) | 0.37 (0.36–0.39) | 1.60 (1.57–1.63) |
| Non-Hispanic black | 4270 | 1.90 (1.88–1.93) | 34.27 (33.27–35.26) | 22.91 (22.51–23.30) | 0.36 (0.33–0.38) | 1.50 (1.49–1.52) |
| Mexican-American | 4325 | 1.86 (1.83–1.89) | 39.34 (37.61–41.08) | 25.06 (24.64–25.49) | 0.32 (0.29–0.34) | 1.57 (1.55–1.59) |
| Other | 630 | 1.92 (1.84–2.00) | 43.68 (39.63–47.73) | 25.77 (24.48–27.06) | 0.39 (0.36–0.43) | 1.57 (1.54–1.60) |
| Level of education | ||||||
| Less than high school | 6556 | 2.06 (2.02–2.09) | 37.85 (34.73–40.97) | 26.77 (26.36–27.18) | 0.35 (0.33–0.37) | 1.56 (1.54–1.58) |
| High school or more | 9344 | 2.05 (2.02–2.07) | 44.08 (42.57–45.59) | 27.03 (26.47–27.60) | 0.37 (0.36–0.39) | 1.60 (1.57–1.62) |
| Annual family income | ||||||
| <$20,000 | 7730 | 2.02 (1.98–2.05) | 38.54 (36.67–40.40) | 25.71 (25.31–26.11) | 0.34 (0.33–0.36) | 1.56 (1.54–1.58) |
| ≥$20,000 | 8018 | 2.06 (2.04–2.09) | 44.53 (42.75–46.30) | 27.59 (27.02–28.17) | 0.38 (0.37–0.40) | 1.60 (1.57–1.62) |
| Body mass index | ||||||
| <25 kg/m2 | 6314 | 1.98 (1.95–2.01) | 45.07 (43.27–46.87) | 25.37 (24.78–25.96) | 0.41 (0.40–0.43) | 1.60 (1.57–1.62) |
| 25–29.9 kg/m2 | 5609 | 2.13 (2.10–2.15) | 42.44 (40.51–44.37) | 28.27 (27.70–28.85) | 0.36 (0.35–0.37) | 1.58 (1.56–1.61) |
| ≥30 kg/m2 | 4042 | 2.06 (0.03–2.10) | 37.79 (35.60–39.97) | 28.27 (27.50–29.03) | 0.29 (0.27–0.31) | 1.57 (1.54–1.60) |
| Smoking status | ||||||
| Current smoker | 4086 | 1.99 (1.96–2.03) | 31.67 (29.28–34.05) | 24.27 (23.82–24.72) | 0.25 (0.23–0.26) | 1.56 (1.53–1.58) |
| Former smoker | 4018 | 2.18 (2.15–2.21) | 45.99 (44.31–47.66) | 29.68 (28.91–30.44) | 0.41 (0.38–0.43) | 1.61 (1.59–1.64) |
| Never smoker | 7903 | 2.01 (1.98–2.03) | 47.45 (45.79–49.10) | 27.12 (26.54–27.70) | 0.42 (0.41–0.44) | 1.59 (1.57–1.61) |
| Alcohol consumption | ||||||
| Yes | 7598 | 2.09 (2.06–2.12) | 41.50 (39.94–43.05) | 26.03 (25.54–26.53) | 0.34 (0.32–0.35) | 1.60 (1.57–1.62) |
| No | 8410 | 1.99 (1.97–2.02) | 43.95 (41.62–46.29) | 28.15 (27.45–28.86) | 0.41 (0.39–0.43) | 1.57 (1.55–1.60) |
| Physical activitya | ||||||
| More active | 4958 | 2.11 (2.08–2.14) | 46.11 (44.26–47.96) | 28.52 (27.71–29.34) | 0.44 (0.42–0.46) | 1.60 (1.57–1.62) |
| Less active | 3518 | 2.00 (1.96–2.03) | 39.04 (37.59–40.49) | 25.66 (25.12–26.20) | 0.30 (0.29–0.32) | 1.57 (1.54–1.60) |
| About the same | 7217 | 2.03 (2.00–2.05) | 41.44 (39.27–43.61) | 26.48 (25.96–26.99) | 0.35 (0.33–0.36) | 1.59 (1.56–1.61) |
| Hypertension status | ||||||
| Yes | 5484 | 2.23 (2.20–2.26) | 42.90 (40.71–45.09) | 30.88 (30.29–31.46) | 0.39 (0.37–0.42) | 1.59 (1.57–1.62) |
| No | 10522 | 1.98 (1.96–2.00) | 42.45 (40.78–44.12) | 25.47 (25.01–25.94) | 0.36 (0.35–0.37) | 1.58 (1.56–1.61) |
| Diabetes Mellitus status | ||||||
| Yes | 1770 | 2.13 (2.07–2.19) | 38.37 (36.00–40.74) | 32.24 (30.79–33.68) | 0.37 (0.35–0.40) | 1.60 (1.57–1.62) |
| No | 14238 | 2.04 (2.02–2.07) | 42.89 (41.12–44.66) | 26.57 (26.10–27.04) | 0.37 (0.36–0.38) | 1.59 (1.56–1.61) |
| Hypercholesterolemia | ||||||
| Yes | 4875 | 2.25 (2.23–2.28) | 44.53 (42.28–46.78) | 33.91 (33.12–34.69) | 0.44 (0.41–0.46) | 1.61 (1.59–1.64) |
| No | 11124 | 1.95 (1.93–1.98) | 41.69 (39.99–43.38) | 23.81 (23.40–24.22) | 0.34 (0.32–0.35) | 1.57 (1.55–1.60) |
| Hormone use in women | ||||||
| Yes | 818 | 2.30 (2.25–2.36) | 51.16 (47.51–54.80) | 30.93 (29.14–32.72) | 0.38 (0.34–0.41) | 1.65 (1.62–1.68) |
| No | 7494 | 2.03 (2.01–2.05) | 41.89 (40.17–43.60) | 26.66 (26.23–27.10) | 0.37 (0.36–0.38) | 1.58 (1.56–1.60) |
| Supplement use | ||||||
| Yes | 6050 | 2.13 (2.10–2.16) | 52.43 (50.31–54.55) | 31.22 (30.46–31.98) | 0.46 (0.44–0.48) | 1.59 (1.56–1.61) |
| No | 9948 | 1.99 (1.96–2.01) | 35.33 (33.56–37.11) | 23.85 (23.50–24.21) | 0.30 (0.29–0.31) | 1.59 (1.56–1.61) |
Abbreviations: NHANES III, the Third National Health and Nutrition Examination Survey; CI, Confidence Interval.
Compared to others of same age.
Table 2 shows the hazard ratios (HRs) and 95% confidence intervals (CIs) for all-cause mortality by quintiles (Q1–Q5) of micronutrient levels. Table 2 also provides the cut-points used for dividing serum levels into quintiles. Hazard ratios for cancer and CVD mortality are shown in Table 3.
Table 2.
Hazard Ratios for All-Cause Mortality by Quintiles of Serum Levels
| Quintiles of Serum Levels | ||||||
|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | Q5 | P for Trend | |
| Vitamin A levels, μmol/L | ≤1.50 | 1.54–1.78 | 1.82–2.06 | 2.09–2.41 | ≥2.44 | |
| All-cause mortality, no. of events | 641 | 629 | 804 | 916 | 1218 | |
| Model 1a | 1 [Reference] | 0.80 (0.67–0.96) | 0.73 (0.61–0.86) | 0.70 (0.59–0.82) | 0.83 (0.70–0.98) | 0.16 |
| Model 2b | 1 [Reference] | 0.89 (0.73–1.09) | 0.82 (0.68–0.98) | 0.82 (0.69–0.97) | 0.95 (0.78–1.15) | 0.93 |
| Vitamin C levels, mmol/L | ≤15.33 | 15.90–31.80 | 32.36–45.42 | 45.99–59.62 | ≥60.19 | |
| All-cause mortality, no. of events | 949 | 691 | 651 | 662 | 838 | |
| Model 1 | 1 [Reference] | 0.78 (0.65–0.93) | 0.63 (0.51–0.78) | 0.53 (0.45–0.62) | 0.55 (0.47–0.65) | <0.01 |
| Model 2 | 1 [Reference] | 0.90 (0.77–1.06) | 0.80 (0.63–1.01) | 0.75 (0.61–0.91) | 0.75 (0.62–0.91) | <0.01 |
| Vitamin E levels, μmol/L | ≤18.65 | 18.67–22.08 | 22.11–26.05 | 26.08–32.16 | ≥32.18 | |
| All-cause mortality, no. of events | 555 | 620 | 803 | 979 | 1251 | |
| Model 1 | 1 [Reference] | 0.80 (0.66–0.97) | 0.69 (0.57–0.84) | 0.69 (0.56–0.84) | 0.71 (0.60–0.84) | <0.01 |
| Model 2 | 1 [Reference] | 0.84 (0.67–1.04) | 0.81 (0.68–0.97) | 0.84 (0.66–1.07) | 0.89 (0.70–1.13) | 0.77 |
| Beta-carotene levels, μmol/L | ≤0.13 | 0.15–0.20 | 0.22–0.32 | 0.34–0.50 | ≥0.52 | |
| All-cause mortality, no. of events | 693 | 662 | 814 | 912 | 1127 | |
| Model 1 | 1 [Reference] | 0.72 (0.61–0.85) | 0.65 (0.56–0.76) | 0.60 (0.51–0.72) | 0.58 (0.49–0.69) | <0.01 |
| Model 2 | 1 [Reference] | 0.75 (0.61–0.93) | 0.76 (0.65–0.89) | 0.75 (0.63–0.89) | 0.75 (0.63–0.90) | 0.01 |
| Selenium levels, nmol/L | ≤1.38 | 1.40–1.50 | 1.51–1.60 | 1.61–1.71 | ≥1.73 | |
| All-cause mortality, no. of events | 930 | 858 | 763 | 679 | 856 | |
| Model 1 | 1 [Reference] | 0.70 (0.60–0.82) | 0.67 (0.57–0.79) | 0.58 (0.48–0.70) | 0.66 (0.57–0.76) | <0.01 |
| Model 2 | 1 [Reference] | 0.74 (0.63–0.87) | 0.77 (0.66–0.91) | 0.69 (0.56–0.85) | 0.79 (0.68–0.92) | 0.03 |
Abbreviations: Q1 to Q5, Quintiles 1 to 5.
Data are given as: Hazard Ratio (95% Confidence Interval).
Model 1: Adjusted for age and gender.
Model 2: Adjusted for variables in Model 1 and race-ethnicity, level of education, annual family income, body mass index, smoking status, serum cotinine level, alcohol consumption, fruit and vegetable intake, physical activity, serum total cholesterol levels, hypertension status, diabetes status, history of heart attack, congestive heart failure, stroke or cancer, hormone use in women, and supplement use.
Table 3.
Hazard Ratios for Cancer and Cardiovascular Disease Mortality by Quintiles of Serum Levels
| Quintiles of Serum Levels | ||||||
|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | Q5 | P for Trend | |
| Vitamin A levels, μmol/L | ≤1.50 | 1.54–1.78 | 1.82–2.06 | 2.09–2.41 | ≥2.44 | |
| Cancer, no. of deaths | 150 | 137 | 171 | 215 | 216 | |
| Model 1a | 1 [Reference] | 0.83 (0.58–1.18) | 0.79 (0.61–1.02) | 0.80 (0.61–1.06) | 0.72 (0.53–0.98) | 0.08 |
| Model 2b | 1 [Reference] | 0.89 (0.59–1.34) | 0.87 (0.65–1.15) | 0.96 (0.72–1.28) | 0.91 (0.64–1.28) | 0.89 |
| Cardiovascular disease, no. of deaths | 250 | 265 | 365 | 404 | 596 | |
| Model 1 | 1 [Reference] | 0.87 (0.71–1.07) | 0.90 (0.71–1.13) | 0.78 (0.64–0.96) | 1.08 (0.86–1.36) | 0.24 |
| Model 2 | 1 [Reference] | 0.97 (0.78–1.21) | 0.93 (0.72–1.20) | 0.84 (0.66–1.06) | 1.09 (0.83–1.43) | 0.45 |
| Vitamin C levels, mmol/L | ≤15.33 | 15.90–31.80 | 32.36–45.42 | 45.99–59.62 | ≥60.19 | |
| Cancer, no. of deaths | 234 | 161 | 147 | 141 | 140 | |
| Model 1 | 1 [Reference] | 0.79 (0.61–1.04) | 0.56 (0.37–0.85) | 0.49 (0.37–0.64) | 0.40 (0.29–0.57) | <0.01 |
| Model 2 | 1 [Reference] | 0.86 (0.64–1.15) | 0.70 (0.45–1.09) | 0.69 (0.51–0.91) | 0.55 (0.39–0.79) | <0.01 |
| Cardiovascular disease, no. of deaths | 409 | 288 | 267 | 281 | 416 | |
| Model 1 | 1 [Reference] | 0.73 (0.56–0.94) | 0.62 (0.48–0.81) | 0.49 (0.39–0.61) | 0.60 (0.49–0.73) | <0.01 |
| Model 2 | 1 [Reference] | 0.83 (0.64–1.08) | 0.76 (0.58–1.01) | 0.66 (0.49–0.89) | 0.77 (0.60–1.00) | 0.04 |
| Vitamin E levels, μmol/L | ≤18.65 | 18.67–22.08 | 22.11–26.05 | 26.08–32.16 | ≥32.18 | |
| Cancer, no. of deaths | 117 | 155 | 192 | 195 | 230 | |
| Model 1 | 1 [Reference] | 0.98 (0.64–1.51) | 0.85 (0.59–1.22) | 0.68 (0.42–1.09) | 0.68 (0.49–0.94) | <0.01 |
| Model 2 | 1 [Reference] | 1.04 (0.64–1.68) | 1.01 (0.67–1.55) | 0.88 (0.50–1.53) | 0.93 (0.57–1.50) | 0.52 |
| Cardiovascular disease, no. of deaths | 210 | 249 | 356 | 469 | 596 | |
| Model 1 | 1 [Reference] | 1.06 (0.78–1.44) | 0.91 (0.64–1.30) | 1.00 (0.75–1.32) | 1.03 (0.78–1.35) | 0.73 |
| Model 2 | 1 [Reference] | 1.05 (0.76–1.45) | 1.01 (0.69–1.48) | 1.10 (0.83–1.47) | 1.16 (0.84–1.59) | 0.29 |
| Beta-carotene levels, μmol/L | ≤0.13 | 0.15–0.20 | 0.22–0.32 | 0.34–0.50 | ≥0.52 | |
| Cancer, no. of deaths | 173 | 150 | 180 | 181 | 205 | |
| Model 1 | 1 [Reference] | 0.67 (0.49–0.93) | 0.63 (0.47–0.86) | 0.60 (0.46–0.77) | 0.51 (0.39–0.67) | <0.01 |
| Model 2 | 1 [Reference] | 0.74 (0.50–1.09) | 0.76 (0.53–1.08) | 0.83 (0.59–1.16) | 0.74 (0.54–1.01) | 0.25 |
| Cardiovascular disease, no. of deaths | 263 | 265 | 354 | 437 | 561 | |
| Model 1 | 1 [Reference] | 0.73 (0.57–0.93) | 0.71 (0.55–0.92) | 0.70 (0.54–0.89) | 0.69 (0.53–0.90) | 0.03 |
| Model 2 | 1 [Reference] | 0.75 (0.56–1.01) | 0.84 (0.64–1.10) | 0.86 (0.67–1.12) | 0.91 (0.68–1.21) | 0.82 |
| Selenium levels, nmol/L | ≤1.38 | 1.40–1.50 | 1.51–1.60 | 1.61–1.71 | ≥1.73 | |
| Cancer, no. of deaths | 194 | 191 | 169 | 128 | 182 | |
| Model 1 | 1 [Reference] | 0.79 (0.56–1.12) | 0.77 (0.58–1.02) | 0.49 (0.34–0.69) | 0.73 (0.54–0.97) | 0.01 |
| Model 2 | 1 [Reference] | 0.76 (0.52–1.12) | 0.81 (0.62–1.08) | 0.53 (0.36–0.79) | 0.86 (0.62–1.20) | 0.20 |
| Cardiovascular disease, no. of deaths | 400 | 387 | 335 | 320 | 383 | |
| Model 1 | 1 [Reference] | 0.71 (0.57–0.90) | 0.67 (0.53–0.85) | 0.73 (0.58–0.93) | 0.71 (0.58–0.88) | 0.04 |
| Model 2 | 1 [Reference] | 0.76 (0.61–0.95) | 0.77 (0.63–0.96) | 0.89 (0.71–1.12) | 0.83 (0.67–1.04) | 0.58 |
Abbreviations: Q1 to Q5, Quintiles 1 to 5.
Data are given as: Hazard Ratio (95% Confidence Interval).
Model 1: Adjusted for age and gender.
Model 2: Adjusted for variables in Model 1 and race-ethnicity, level of education, annual family income, body mass index, smoking status, serum cotinine level, alcohol consumption, fruit and vegetable intake, physical activity, serum total cholesterol levels, hypertension status, diabetes status, history of heart attack, congestive heart failure, stroke or cancer, hormone use in women, and supplement use.
We observed U-shaped associations between serum levels of vitamins A and E, and all-cause mortality (Figure 1) with those with levels in Q1 or Q5 having higher mortality risks compared to those with levels in Q2–Q4. For vitamin A, risk of cancer death decreased from Q1 to Q2, with no further decline in risk at higher levels, whereas for vitamin E, having levels ≥26.08 μmol/L (Q4–Q5) were associated with the lowest cancer mortality risk. The increased all-cause mortality risk for those with levels in Q5 for vitamin A was mainly driven by higher CVD mortality (Figure 2), and for vitamin E, by higher stroke mortality.
Figure 1.
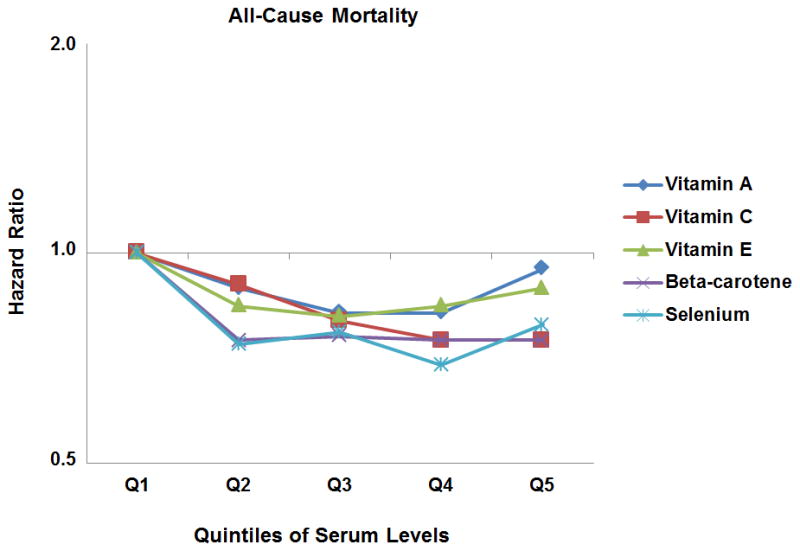
Hazard Ratios for All-Cause Mortality
Abbreviations: Q1 to Q5, Quintiles 1 to 5. Hazard Ratios are adjusted for variables in Model 2: age, gender, race-ethnicity, level of education, annual family income, body mass index, smoking status, serum cotinine level, alcohol consumption, fruit and vegetable intake, physical activity, serum total cholesterol levels, hypertension status, diabetes status, history of heart attack, congestive heart failure, stroke or cancer, hormone use in women, and supplement use. The y-axes are shown on a log scale.
Figure 2.
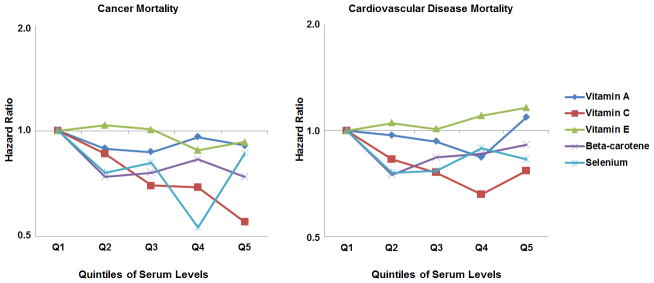
Hazard Ratios for Cancer and Cardiovascular Disease Mortality
Abbreviations: Q1 to Q5, Quintiles 1 to 5. Hazard Ratios are adjusted for variables in Model 2: age, gender, race-ethnicity, level of education, annual family income, body mass index, smoking status, serum cotinine level, alcohol consumption, fruit and vegetable intake, physical activity, serum total cholesterol levels, hypertension status, diabetes status, history of heart attack, congestive heart failure, stroke or cancer, hormone use in women, and supplement use. The y-axes are shown on a log scale.
For vitamin C (Model 2), all-cause mortality risk decreased with increases in serum levels from Q1–Q4 (P<0.001 for trend) with no further decline in risk with higher levels (≥60.19 mmol/L). For cancer mortality, we observed a dose-response relationship between higher levels and reduced risk (P<0.001 for trend). CVD mortality decreased with higher vitamin C levels, except for those with levels in Q5.
For beta-carotene and selenium, there was a significant decrease in the overall mortality risk from Q1–Q2 (HR for Q2 vs. Q1 for beta-carotene: 0.75; 95% CI: 0.61–0.93; for selenium: 0.74; 95% CI: 0.63–0.87); however, higher levels (≥0.15 μmol/L for beta carotene; ≥1.40 μmol/L for selenium) did not significantly change the risk. Cancer mortality risks decreased from Q1 to Q2 for beta-carotene and from Q1 to Q4 for selenium. Beyond Q1, higher levels appeared to increase the CVD mortality risk for both beta-carotene and selenium.
In our analysis, in multivariable models, hazard ratios did not change significantly after further adjusting for Serum C-reactive protein levels (Supplementary Table S1) or after adjusting for other micronutrients in the study (Supplementary Table S2). Excluding NHANES III participants who are current smokers (Supplementary Table S3), or those with history of heart attack, congestive heart failure, stroke or cancer (Supplementary Table S4) also did not materially change the findings. We observed similar patterns for mortality risks after excluding deaths within the first three years of the survey (Supplementary Table S5) or after limiting follow-up to 5 or 10 years (Supplementary Table S6). We also examined hazard ratios for all-cause mortality stratified by age, gender, race-ethnicity, body mass index, and supplement use (Supplementary Table S7). More detailed results for cancer and CVD mortality outcomes are provided in Supplementary Tables S8 and S9, respectively. Restricted Cubic Splines for all-cause mortality, cancer mortality, and cardiovascular mortality, are presented in Supplementary Figures S1, S2 and S3, respectively.
Discussion
Oxidative stress has been implicated in the pathogenesis of several chronic diseases, including various cancers (52–55). Vitamin A and antioxidant nutrients such as vitamins C and E, beta-carotene, and selenium have been hypothesized to prevent or delay this damage (56–61). Consequently, use of these agents is rising, and almost 40% of US adults take them for their perceived health benefits (1, 2).
In this study, using data from a large, nationally representative cohort of 16,008 US adults, we observed threshold effects and non-linear associations between serum levels of these micronutrients, and all-cause and cause-specific mortality outcomes. These results help to explain some of the discrepant findings between observational studies and randomized trials regarding the role of these agents on health outcomes.
We found that higher levels were generally associated with a modest decrease in all-cancer mortality risk. However, the most significant decline in risk was generally from the first to the second quintile. A number of high profile trials assessing antioxidants as cancer prevention agents have failed to show significant mortality benefits with taking these supplements (23–27) and some have reported possible harm (28, 29). A recent meta-analysis of 22 primary and secondary prevention trials concluded that there was no evidence to support preventive effects of any of the antioxidant supplements on any cancer type (62). The Physicians’ Health Study II also recently showed only a small reduction in total cancer incidence (HR=0.92; 95% CI: 0.86–0.998) and a non-significant reduction in mortality (HR=0.88; 95% CI: 0.77–1.01) with more than a decade of daily multivitamin use and follow-up (22). Further, most of these large prevention trials did not consider baseline nutrition level in their inclusion criteria (63, 64).
The importance of assessing baseline nutritional status is underscored by the Linxian Study in China, which specifically targeted a geographic area where subjects had chronically low blood levels of multiple micronutrients. It is the only prevention trial to our knowledge so far that has reported statistically significant reductions in all-cancer (RR=0.87, 95% CI: 0.75–1.00) as well as overall mortality (RR=0.91; 95%CI 0.84–0.99) risks with antioxidant supplementation (65).
Interestingly, in the Physicians’ Health Study I, there was no decrease in total cancer incidence with beta-carotene supplementation. However, in subgroup analyses, lower baseline beta-carotene levels were associated with alcohol consumption and higher BMI, and overall cancer incidence was modestly reduced with supplementation in these subgroups (among daily alcohol drinkers, RR= 0.9, 95% CI: 0.8–1.0; among those in the highest BMI quartile, RR = 0.9, 95% CI: 0.7–1.0) (23). Similarly, in the SU.VI.MAX trial, lower total cancer incidence (RR=0.69, 95% CI: 0.53–0.91) and all-cause mortality (RR=0.63, 95% CI: 0.42–0.93) with multivitamin use in men was attributable to their lower baseline antioxidant status (27). Although some of these results could be due to testing multiple hypotheses, the findings presented in our study as well as the results from others examining a nutritionally deficient population further support the hypothesis that supplementation might only be useful for those who have low serum antioxidant levels, and that beyond a threshold, higher levels do not lead to additional benefit, and may potentially be toxic.
Our results also have broader implications for assessing overall mortality benefits from the use of dietary supplements. For example, using antioxidant levels as continuous variables, a study in the British National Diet and Nutrition Survey found that higher levels of vitamin C, selenium and beta-carotene were associated with lower overall mortality risk (HR per standard deviation for vitamin C = 0.81, P < 0.001; for selenium = 0.76, P < 0.001; and for beta-carotene = 0.92, P = 0.08) whereas the results for vitamins A and E were not significant (HR for vitamin A = 0.96, P = 0.4; alpha-tocopherol = 0.96, P = 0.4) (6). If we had used antioxidant levels as continuous variables, we would have obtained similar results. However, by assessing non-linear associations, we were able to show that for selenium and beta-carotene, beyond the first quintile, there was no apparent decrease in the mortality risk with higher levels (Figure 1). Moreover, we demonstrated a U-shaped association between levels of vitamins A and E, and all-cause mortality.
These results for overall mortality are also consistent with the findings of the recent systematic review by the Cochrane Collaboration, which concluded that supplementation with beta-carotene, vitamin E or high doses of vitamin A was associated with increased mortality risk, whereas the role of vitamin C or selenium supplement use was not clear (21). Figure 1 in our study shows that except for vitamin C, having antioxidant levels beyond the first quintile did not lead to any further decrease in all-cause mortality risk. Moreover, for vitamins A and E, higher levels increased the overall risk of death.
Our study has a number of strengths compared to previous studies. First, this is the largest study to date assessing serum vitamin A and antioxidant nutrient levels, and cause-specific and all-cause mortality outcomes. Therefore, instead of examining serum levels as continuous variables, we were able to divide them into quintiles, and assess threshold and non-linear relationships with mortality. Because of small sample sizes and/or short follow-up durations, most observational studies have been unable to demonstrate such associations (3–15). Further, NHANES III used a nationally representative sample of the US population, and final mortality status was available for more than 99% of the participants, minimizing the possibility of selection bias. Assessing serum levels of these agents instead of using dietary history, along with standardized and validated laboratory methods, reduced the potential for information bias. Finally, use of appropriate sampling weights in the analysis helped to obtain statistical estimates similar to those if the entire sampling frame (the United States) had been surveyed.
Our study has several limitations. We tested multiple hypotheses. Therefore, results must be interpreted with caution. We limited our analysis to vitamin A and antioxidant nutrients that are commonly used as dietary supplements. Other potential agents with antioxidant properties such as zinc, lycopene, and other carotenoids need further evaluation. As with any observational study, residual confounding by socioeconomic status, lifestyle variables and other factors cannot be excluded. However, NHANES III assessed a large number of health related variables, which enabled us to control for many potential confounders. Further, we obtained different results for different micronutrients as well as for different health outcomes. These findings would be difficult to explain entirely on the basis of residual confounding. In addition to assessing cardiovascular mortality, there are many other possible competing risks for mortality that we could have assessed. We assessed cardiovascular mortality as an example of potential different effects of antioxidants for different disease processes.
Finally, in this study, we used a single measurement of serum levels to assess long term status. In our analysis (Supplementary Table S3), we observed similar hazard ratios for 5-year, 10-year, and for the entire duration of follow-up. This suggests that at the population level, a single measurement of serum levels may provide a reliable estimate of long term antioxidant status. A major limitation of randomized trials assessing use of supplements for primary prevention is that the participants typically have to be kept on intervention for long periods in order to significantly affect health outcomes. Therefore, observational studies such as this may facilitate the assessment of the relationship between long term nutritional status and health outcomes.
In summary, using data from a nationally representative cohort of over 16,000 US adults, we were able to identify specific plasma levels of vitamin A and various antioxidant nutrients which were associated with maximum cancer-related and overall survival. We found that beyond a certain threshold, there was generally no additional benefit with higher serum levels with respect to overall mortality. Specifically, for vitamins A and E, higher levels increased the overall mortality risk. These data support the findings of recent randomized trials that have generally failed to demonstrate health benefits with taking these supplements (21, 62, 66). We also showed that having low serum antioxidant nutrient levels were generally also associated with higher mortality risk, suggesting that supplementation might still be useful for those who are nutritionally deficient.
Our findings underscore the need to assess safety of these agents like other drugs, rather than classifying them as ‘dietary supplements’, which affects their regulatory oversight (67). Although the current Institute of Medicine guidelines provide recommended dietary allowances and tolerable upper intake levels for these agents, we also highlight the potential significance of measuring serum levels to guide their use as supplements (68). Novel intervention studies might then be planned where doses of these agents are individualized based on serum levels, lifestyle behaviors such as smoking which affect levels, and possibly, markers of oxidative stress and systemic inflammatory response (50, 69, 70). Such a strategy would help ensure that those who are deficient get the required micronutrients, while also preventing toxic levels of antioxidants that could potentially lead to worse health outcomes.
Supplementary Material
Acknowledgments
Financial support: Steven J. Levinson Medical Research Foundation, and NIH K23 CA149084-01A1 (to A Siegel).
We thank Dr. Zhezhen Jin from the Department of Biostatistics, Mailman School of Public Health, Columbia University, for his help with restricted cubic spline functions.
Footnotes
Conflicts of interest: None
References
- 1.Gahche J, Bailey R, Burt V, Hughes J, Yetley E, Dwyer J, et al. Dietary supplement use among U.S. adults has increased since NHANES III (1988–1994) NCHS Data Brief. 2011 Apr;(61):1–8. Epub 2011/05/20.eng. [PubMed] [Google Scholar]
- 2.Bailey RL, Gahche JJ, Lentino CV, Dwyer JT, Engel JS, Thomas PR, et al. Dietary supplement use in the United States, 2003–2006. J Nutr. 2011 Feb;141(2):261–6. doi: 10.3945/jn.110.133025. Epub 2010/12/24.eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buijsse B, Feskens EJ, Schlettwein-Gsell D, Ferry M, Kok FJ, Kromhout D, et al. Plasma carotene and alpha-tocopherol in relation to 10-y all-cause and cause-specific mortality in European elderly: the Survey in Europe on Nutrition and the Elderly, a Concerted Action (SENECA) The American journal of clinical nutrition. 2005 Oct;82(4):879–86. doi: 10.1093/ajcn/82.4.879. Epub 2005/10/08.eng. [DOI] [PubMed] [Google Scholar]
- 4.Buijsse B, Feskens EJ, Kwape L, Kok FJ, Kromhout D. Both alpha- and beta-carotene, but not tocopherols and vitamin C, are inversely related to 15-year cardiovascular mortality in Dutch elderly men. J Nutr. 2008 Feb;138(2):344–50. doi: 10.1093/jn/138.2.344. Epub 2008/01/22.eng. [DOI] [PubMed] [Google Scholar]
- 5.Bleys J, Navas-Acien A, Guallar E. Serum selenium levels and all-cause, cancer, and cardiovascular mortality among US adults. Archives of internal medicine. 2008 Feb 25;168(4):404–10. doi: 10.1001/archinternmed.2007.74. Epub 2008/02/27.eng. [DOI] [PubMed] [Google Scholar]
- 6.Bates CJ, Hamer M, Mishra GD. Redox-modulatory vitamins and minerals that prospectively predict mortality in older British people: the National Diet and Nutrition Survey of people aged 65 years and over. Br J Nutr. 2011 Jan;105(1):123–32. doi: 10.1017/S0007114510003053. Epub 2010/09/03.eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Akbaraly NT, Arnaud J, Hininger-Favier I, Gourlet V, Roussel AM, Berr C. Selenium and mortality in the elderly: results from the EVA study. Clin Chem. 2005 Nov;51(11):2117–23. doi: 10.1373/clinchem.2005.055301. Epub 2005/08/27.eng. [DOI] [PubMed] [Google Scholar]
- 8.Akbaraly TN, Favier A, Berr C. Total plasma carotenoids and mortality in the elderly: results of the Epidemiology of Vascular Ageing (EVA) study. Br J Nutr. 2009 Jan;101(1):86–92. doi: 10.1017/S0007114508998445. Epub 2008/05/30.eng. [DOI] [PubMed] [Google Scholar]
- 9.Gale CR, Martyn CN, Winter PD, Cooper C. Vitamin C and risk of death from stroke and coronary heart disease in cohort of elderly people. Bmj. 1995 Jun 17;310(6994):1563–6. doi: 10.1136/bmj.310.6994.1563. Epub 1995/06/17.eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hu P, Reuben DB, Crimmins EM, Harris TB, Huang MH, Seeman TE. The effects of serum beta-carotene concentration and burden of inflammation on all-cause mortality risk in high-functioning older persons: MacArthur studies of successful aging. J Gerontol A Biol Sci Med Sci. 2004 Aug;59(8):849–54. doi: 10.1093/gerona/59.8.m849. Epub 2004/09/04.eng. [DOI] [PubMed] [Google Scholar]
- 11.Ito Y, Suzuki K, Ishii J, Hishida H, Tamakoshi A, Hamajima N, et al. A population-based follow-up study on mortality from cancer or cardiovascular disease and serum carotenoids, retinol and tocopherols in Japanese inhabitants. Asian Pac J Cancer Prev. 2006 Oct-Dec;7(4):533–46. Epub 2007/01/26.eng. [PubMed] [Google Scholar]
- 12.Khaw KT, Bingham S, Welch A, Luben R, Wareham N, Oakes S, et al. Relation between plasma ascorbic acid and mortality in men and women in EPIC-Norfolk prospective study: a prospective population study. European Prospective Investigation into Cancer and Nutrition. Lancet. 2001 Mar 3;357(9257):657–63. doi: 10.1016/s0140-6736(00)04128-3. Epub 2001/03/15.eng. [DOI] [PubMed] [Google Scholar]
- 13.Loria CM, Klag MJ, Caulfield LE, Whelton PK. Vitamin C status and mortality in US adults. The American journal of clinical nutrition. 2000 Jul;72(1):139–45. doi: 10.1093/ajcn/72.1.139. Epub 2000/06/29.eng. [DOI] [PubMed] [Google Scholar]
- 14.Ray AL, Semba RD, Walston J, Ferrucci L, Cappola AR, Ricks MO, et al. Low serum selenium and total carotenoids predict mortality among older women living in the community: the women’s health and aging studies. J Nutr. 2006 Jan;136(1):172–6. doi: 10.1093/jn/136.1.172. Epub 2005/12/21.eng. [DOI] [PubMed] [Google Scholar]
- 15.Sahyoun NR, Jacques PF, Russell RM. Carotenoids, vitamins C and E, and mortality in an elderly population. American journal of epidemiology. 1996 Sep 1;144(5):501–11. doi: 10.1093/oxfordjournals.aje.a008957. Epub 1996/09/01.eng. [DOI] [PubMed] [Google Scholar]
- 16.Agudo A, Cabrera L, Amiano P, Ardanaz E, Barricarte A, Berenguer T, et al. Fruit and vegetable intakes, dietary antioxidant nutrients, and total mortality in Spanish adults: findings from the Spanish cohort of the European Prospective Investigation into Cancer and Nutrition (EPIC-Spain) The American journal of clinical nutrition. 2007 Jun;85(6):1634–42. doi: 10.1093/ajcn/85.6.1634. Epub 2007/06/09.eng. [DOI] [PubMed] [Google Scholar]
- 17.Genkinger JM, Platz EA, Hoffman SC, Comstock GW, Helzlsouer KJ. Fruit, vegetable, and antioxidant intake and all-cause, cancer, and cardiovascular disease mortality in a community-dwelling population in Washington County, Maryland. American journal of epidemiology. 2004 Dec 15;160(12):1223–33. doi: 10.1093/aje/kwh339. Epub 2004/12/08.eng. [DOI] [PubMed] [Google Scholar]
- 18.Nothlings U, Schulze MB, Weikert C, Boeing H, van der Schouw YT, Bamia C, et al. Intake of vegetables, legumes, and fruit, and risk for all-cause, cardiovascular, and cancer mortality in a European diabetic population. J Nutr. 2008 Apr;138(4):775–81. doi: 10.1093/jn/138.4.775. Epub 2008/03/22.eng. [DOI] [PubMed] [Google Scholar]
- 19.Dauchet L, Amouyel P, Hercberg S, Dallongeville J. Fruit and vegetable consumption and risk of coronary heart disease: a meta-analysis of cohort studies. J Nutr. 2006 Oct;136(10):2588–93. doi: 10.1093/jn/136.10.2588. Epub 2006/09/22.eng. [DOI] [PubMed] [Google Scholar]
- 20.Hercberg S, Galan P, Preziosi P, Alfarez MJ, Vazquez C. The potential role of antioxidant vitamins in preventing cardiovascular diseases and cancers. Nutrition. 1998 Jun;14(6):513–20. doi: 10.1016/s0899-9007(98)00040-9. Epub 1998/07/01.eng. [DOI] [PubMed] [Google Scholar]
- 21.Bjelakovic G, Nikolova D, Gluud LL, Simonetti RG, Gluud C. Antioxidant supplements for prevention of mortality in healthy participants and patients with various diseases. Cochrane Database Syst Rev. 2012;3:CD007176. doi: 10.1002/14651858.CD007176.pub2. Epub 2012/03/16.eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gaziano JM, Sesso HD, Christen WG, Bubes V, Smith JP, MacFadyen J, et al. Multivitamins in the prevention of cancer in men: the Physicians’ Health Study II randomized controlled trial. JAMA: the journal of the American Medical Association. 2012 Nov 14;308(18):1871–80. doi: 10.1001/jama.2012.14641. Epub 2012/11/20.eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cook NR, Le IM, Manson JE, Buring JE, Hennekens CH. Effects of beta-carotene supplementation on cancer incidence by baseline characteristics in the Physicians’ Health Study (United States) Cancer causes & control: CCC. 2000 Aug;11(7):617–26. doi: 10.1023/a:1008995430664. Epub 2000/09/08.eng. [DOI] [PubMed] [Google Scholar]
- 24.Lippman SM, Klein EA, Goodman PJ, Lucia MS, Thompson IM, Ford LG, et al. Effect of selenium and vitamin E on risk of prostate cancer and other cancers: the Selenium and Vitamin E Cancer Prevention Trial (SELECT) JAMA: the journal of the American Medical Association. 2009 Jan 7;301(1):39–51. doi: 10.1001/jama.2008.864. Epub 2008/12/11.eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lonn E, Bosch J, Yusuf S, Sheridan P, Pogue J, Arnold JM, et al. Effects of long-term vitamin E supplementation on cardiovascular events and cancer: a randomized controlled trial. JAMA: the journal of the American Medical Association. 2005 Mar 16;293(11):1338–47. doi: 10.1001/jama.293.11.1338. Epub 2005/03/17.eng. [DOI] [PubMed] [Google Scholar]
- 26.Lee IM, Cook NR, Manson JE, Buring JE, Hennekens CH. Beta-carotene supplementation and incidence of cancer and cardiovascular disease: the Women’s Health Study. Journal of the National Cancer Institute. 1999 Dec 15;91(24):2102–6. doi: 10.1093/jnci/91.24.2102. Epub 1999/12/22.eng. [DOI] [PubMed] [Google Scholar]
- 27.Hercberg S, Galan P, Preziosi P, Bertrais S, Mennen L, Malvy D, et al. The SU.VI.MAX Study: a randomized, placebo-controlled trial of the health effects of antioxidant vitamins and minerals. Archives of internal medicine. 2004 Nov 22;164(21):2335–42. doi: 10.1001/archinte.164.21.2335. Epub 2004/11/24.eng. [DOI] [PubMed] [Google Scholar]
- 28.The effect of vitamin Ebeta carotene on the incidence of lung cancerother cancers in male smokers. The Alpha-Tocopherol, Beta Carotene Cancer Prevention Study Group. The New England journal of medicine. 1994 Apr 14;330(15):1029–35. doi: 10.1056/NEJM199404143301501. Epub 1994/04/14.eng. [DOI] [PubMed] [Google Scholar]
- 29.Omenn GS, Goodman GE, Thornquist MD, Balmes J, Cullen MR, Glass A, et al. Risk factors for lung cancer and for intervention effects in CARET, the Beta-Carotene and Retinol Efficacy Trial. Journal of the National Cancer Institute. 1996 Nov 6;88(21):1550–9. doi: 10.1093/jnci/88.21.1550. Epub 1996/11/06.eng. [DOI] [PubMed] [Google Scholar]
- 30.Rock CL, Jacob RA, Bowen PE. Update on the biological characteristics of the antioxidant micronutrients: vitamin C, vitamin E, and the carotenoids. J Am Diet Assoc. 1996 Jul;96(7):693–702. doi: 10.1016/S0002-8223(96)00190-3. quiz 3–4. Epub 1996/07/01.eng. [DOI] [PubMed] [Google Scholar]
- 31.Padayatty SJ, Katz A, Wang Y, Eck P, Kwon O, Lee JH, et al. Vitamin C as an antioxidant: evaluation of its role in disease prevention. J Am Coll Nutr. 2003 Feb;22(1):18–35. doi: 10.1080/07315724.2003.10719272. Epub 2003/02/06.eng. [DOI] [PubMed] [Google Scholar]
- 32.Burton GW, Traber MG. Vitamin E: antioxidant activity, biokinetics, and bioavailability. Annual review of nutrition. 1990;10:357–82. doi: 10.1146/annurev.nu.10.070190.002041. Epub 1990/01/01.eng. [DOI] [PubMed] [Google Scholar]
- 33.Tapiero H, Townsend DM, Tew KD. The antioxidant role of selenium and seleno-compounds. Biomed Pharmacother. 2003 May-Jun;57(3–4):134–44. doi: 10.1016/s0753-3322(03)00035-0. Epub 2003/06/24.eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Burton GW, Ingold KU. beta-Carotene: an unusual type of lipid antioxidant. Science. 1984 May 11;224(4649):569–73. doi: 10.1126/science.6710156. Epub 1984/05/11.eng. [DOI] [PubMed] [Google Scholar]
- 35.Hathcock JN, Hattan DG, Jenkins MY, McDonald JT, Sundaresan PR, Wilkening VL. Evaluation of vitamin A toxicity. The American journal of clinical nutrition. 1990 Aug;52(2):183–202. doi: 10.1093/ajcn/52.2.183. Epub 1990/08/01.eng. [DOI] [PubMed] [Google Scholar]
- 36.Galli F, Azzi A. Present trends in vitamin E research. Biofactors. 2010 Jan-Feb;36(1):33–42. doi: 10.1002/biof.75. Epub 2010/01/29.eng. [DOI] [PubMed] [Google Scholar]
- 37.Omaye ST, Krinsky NI, Kagan VE, Mayne ST, Liebler DC, Bidlack WR. beta-carotene: friend or foe? Fundam Appl Toxicol. 1997 Dec;40(2):163–74. doi: 10.1006/faat.1997.2387. Epub 1998/01/27.eng. [DOI] [PubMed] [Google Scholar]
- 38.Zwolak I, Zaporowska H. Selenium interactions and toxicity: a review. Selenium interactions and toxicity. Cell Biol Toxicol. 2012 Feb;28(1):31–46. doi: 10.1007/s10565-011-9203-9. Epub 2011/09/14.eng. [DOI] [PubMed] [Google Scholar]
- 39.Rutkowski M, Grzegorczyk K. Adverse effects of antioxidative vitamins. Int J Occup Med Environ Health. 2012 Jun;25(2):105–21. doi: 10.2478/S13382-012-0022-x. Epub 2012/04/25.eng. [DOI] [PubMed] [Google Scholar]
- 40.Rogovik AL, Vohra S, Goldman RD. Safety considerations and potential interactions of vitamins: should vitamins be considered drugs? Ann Pharmacother. 2010 Feb;44(2):311–24. doi: 10.1345/aph.1M238. Epub 2009/12/31.eng. [DOI] [PubMed] [Google Scholar]
- 41.National Center for Health Statistics. The National Health and Nutrition Examination Survey (NHANES) http://www.cdc.gov/nchs/nhanes.htm.
- 42.National Center for Health Statistics. The Third National Health and Nutrition Examination Survey (NHANES III), 1988–94. Analytic and Reporting Guidelines. http://www.cdc.gov/nchs/data/nhanes/nhanes3/nh3gui.pdf.
- 43.National Center for Health Statistics. Research Ethics Review Board (ERB) Approval. http://www.cdc.gov/nchs/nhanes/irba98.htm.
- 44.Gunter EW, Lewis BG, Koncikowski SM. Laboratory Procedures Used for the Third National Health and Nutrition Examination Survey (NHANES III), 1988–1994. 1996. [Google Scholar]
- 45.Gunter EW, McQuillan G. Quality control in planning and operating the laboratory component for the Third National Health and Nutrition Examination Survey. J Nutr. 1990 Nov;120(Suppl 11):1451–4. doi: 10.1093/jn/120.suppl_11.1451. Epub 1990/11/01.eng. [DOI] [PubMed] [Google Scholar]
- 46.National Center for Health Statistics. The Third National Health and Nutrition Examination Survey (NHANES III) Linked Mortality File, Mortality follow-up through 2006: Matching Methodology May 2009. http://www.cdc.gov/nchs/data/datalinkage/matching_methodology_nhanes3_final.pdf.
- 47.National Center for Health Statistics. The Third National Health and Nutrition Examination Survey (NHANES III) Linked Mortality File. http://www.cdc.gov/nchs/data/datalinkage/nh3_file_layout_public_2010.pdf.
- 48.World Health Organization. International statistical classification of diseases and related health problems, 10th revision. Geneva, Switzerland: World Health Organization; 1992. [Google Scholar]
- 49.Lin DY, Wei LJ, Ying Z. Checking the Cox model with cumulative sums of martingale-based residuals. Biometrika. 1993 Sep 1;80(3):557–72. [Google Scholar]
- 50.Duncan A, Talwar D, McMillan DC, Stefanowicz F, O’Reilly DS. Quantitative data on the magnitude of the systemic inflammatory response and its effect on micronutrient status based on plasma measurements. The American journal of clinical nutrition. 2012 Jan;95(1):64–71. doi: 10.3945/ajcn.111.023812. Epub 2011/12/14.eng. [DOI] [PubMed] [Google Scholar]
- 51.Desquilbet L, Mariotti F. Dose-response analyses using restricted cubic spline functions in public health research. Statistics in medicine. 2010 Apr 30;29(9):1037–57. doi: 10.1002/sim.3841. English. [DOI] [PubMed] [Google Scholar]
- 52.Finkel T, Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature. 2000 Nov 9;408(6809):239–47. doi: 10.1038/35041687. Epub 2000/11/23.eng. [DOI] [PubMed] [Google Scholar]
- 53.Griendling KK, Alexander RW. Oxidative stress and cardiovascular disease. Circulation. 1997 Nov 18;96(10):3264–5. Epub 1997/12/13.eng. [PubMed] [Google Scholar]
- 54.Klaunig JE, Kamendulis LM. The role of oxidative stress in carcinogenesis. Annu Rev Pharmacol Toxicol. 2004;44:239–67. doi: 10.1146/annurev.pharmtox.44.101802.121851. Epub 2004/01/28.eng. [DOI] [PubMed] [Google Scholar]
- 55.Halliwell B. Free radicals, antioxidants, and human disease: curiosity, cause, or consequence? Lancet. 1994 Sep 10;344(8924):721–4. doi: 10.1016/s0140-6736(94)92211-x. Epub 1994/09/10.eng. [DOI] [PubMed] [Google Scholar]
- 56.Willcox JK, Ash SL, Catignani GL. Antioxidants and prevention of chronic disease. Crit Rev Food Sci Nutr. 2004;44(4):275–95. doi: 10.1080/10408690490468489. Epub 2004/10/07.eng. [DOI] [PubMed] [Google Scholar]
- 57.Halliwell B. Antioxidants in human health and disease. Annual review of nutrition. 1996;16:33–50. doi: 10.1146/annurev.nu.16.070196.000341. Epub 1996/01/01.eng. [DOI] [PubMed] [Google Scholar]
- 58.Halliwell B. Antioxidant defence mechanisms: from the beginning to the end (of the beginning) Free Radic Res. 1999 Oct;31(4):261–72. doi: 10.1080/10715769900300841. Epub 1999/10/12.eng. [DOI] [PubMed] [Google Scholar]
- 59.Tomita Y, Himeno K, Nomoto K, Endo H, Hirohata T. Augmentation of tumor immunity against syngeneic tumors in mice by beta-carotene. Journal of the National Cancer Institute. 1987 Apr;78(4):679–81. Epub 1987/04/01.eng. [PubMed] [Google Scholar]
- 60.Glatthaar BE, Hornig DH, Moser U. The role of ascorbic acid in carcinogenesis. Adv Exp Med Biol. 1986;206:357–77. doi: 10.1007/978-1-4613-1835-4_27. Epub 1986/01/01.eng. [DOI] [PubMed] [Google Scholar]
- 61.Sandhu JK, Haqqani AS, Birnboim HC. Effect of dietary vitamin E on spontaneous or nitric oxide donor-induced mutations in a mouse tumor model. Journal of the National Cancer Institute. 2000 Sep 6;92(17):1429–33. doi: 10.1093/jnci/92.17.1429. Epub 2000/09/07.eng. [DOI] [PubMed] [Google Scholar]
- 62.Myung SK, Kim Y, Ju W, Choi HJ, Bae WK. Effects of antioxidant supplements on cancer prevention: meta-analysis of randomized controlled trials. Ann Oncol. 2010 Jan;21(1):166–79. doi: 10.1093/annonc/mdp286. Epub 2009/07/23.eng. [DOI] [PubMed] [Google Scholar]
- 63.Morris MC, Tangney CC. A potential design flaw of randomized trials of vitamin supplements. JAMA: the journal of the American Medical Association. 2011 Apr 6;305(13):1348–9. doi: 10.1001/jama.2011.383. Epub 2011/04/07.eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mayne ST, Ferrucci LM, Cartmel B. Lessons learned from randomized clinical trials of micronutrient supplementation for cancer prevention. Annual review of nutrition. 2012 Aug 21;32:369–90. doi: 10.1146/annurev-nutr-071811-150659. Epub 2012/04/25.eng. [DOI] [PubMed] [Google Scholar]
- 65.Blot WJ, Li JY, Taylor PR, Guo W, Dawsey S, Wang GQ, et al. Nutrition intervention trials in Linxian, China: supplementation with specific vitamin/mineral combinations, cancer incidence, and disease-specific mortality in the general population. Journal of the National Cancer Institute. 1993 Sep 15;85(18):1483–92. doi: 10.1093/jnci/85.18.1483. Epub 1993/09/15.eng. [DOI] [PubMed] [Google Scholar]
- 66.Myung SK, Ju W, Cho B, Oh SW, Park SM, Koo BK, et al. Efficacy of vitamin and antioxidant supplements in prevention of cardiovascular disease: systematic review and meta-analysis of randomised controlled trials. Bmj. 2013;346:f10. doi: 10.1136/bmj.f10. Epub 2013/01/22.eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dietary Supplement Health and Education Act of 1994. Public Law 103–417; 103rd Congress; http://ods.od.nih.gov/About/DSHEA_Wording.aspx. [Google Scholar]
- 68.Institute of Medicine. Nutrient Recommendations: Dietary Reference Intakes. www.nap.edu/catalog/9810.htmlhttp://ods.od.nih.gov/Health_Information/Dietary_Reference_Intakes.aspx.
- 69.Mayne ST. Antioxidant nutrients and chronic disease: use of biomarkers of exposure and oxidative stress status in epidemiologic research. J Nutr. 2003 Mar;133(Suppl 3):933S–40S. doi: 10.1093/jn/133.3.933S. Epub 2003/03/04.eng. [DOI] [PubMed] [Google Scholar]
- 70.Therond P, Bonnefont-Rousselot D, Davit-Spraul A, Conti M, Legrand A. Biomarkers of oxidative stress: an analytical approach. Curr Opin Clin Nutr Metab Care. 2000 Sep;3(5):373–84. doi: 10.1097/00075197-200009000-00009. Epub 2001/01/11.eng. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


