Abstract
Background
The progression of atrial fibrillation (AF) from paroxysmal to persistent forms remains a major clinical challenge. Abnormal sarcoplasmic reticulum (SR) Ca2+-leak via the ryanodine receptor (RyR2) has been observed as a source of ectopic activity in various AF models. However, its potential role in progression to long-lasting spontaneous AF (sAF) has never been tested. This study tested the hypothesis that enhanced RyR2-mediated Ca2+-release underlies the development of a substrate for sAF and to understand the underlying mechanisms.
Methods and Results
CREM-IbΔC-X transgenic (CREM)-mice developed age-dependent progression from spontaneous atrial ectopy to paroxysmal and eventually long-lasting AF. The development of sAF in CREM-mice was preceded by enhanced diastolic Ca2+-release, atrial enlargement and marked conduction abnormalities. Genetic inhibition of CaMKII-mediated RyR2-S2814 phosphorylation in CREM-mice normalized open probability of RyR2-channels and SR Ca2+-release, delayed the development of spontaneous atrial ectopy, fully prevented sAF, suppressed atrial dilation and forestalled atrial conduction-abnormalities. Hyperactive RyR2-channels directly stimulated the Ca2+-dependent hypertrophic pathway NFAT/Rcan1-4, suggesting a role for the NFAT/Rcan1-4 system in the development of a substrate for long-lasting AF in CREM mice.
Conclusions
RyR2-mediated SR Ca2+-leak directly underlies the development of a substrate for sAF in CREM-mice, the first demonstration of a molecular mechanism underlying AF-progression and sAF substrate development in an experimental model. Our work demonstrates that the role of abnormal diastolic Ca2+ release in AF may not be restricted to the generation of atrial ectopy, but extends to the development of atrial remodeling underlying the AF substrate.
Keywords: atrial fibrillation, ryanodine receptor 2, CREM-IbΔC-X, calcium
Introduction
Atrial fibrillation (AF) represents the most common sustained cardiac arrhythmia and is projected to increase considerably by 2050.1 One of the most striking yet least understood characteristics of AF is its progressive nature. Patients often show spontaneous atrial ectopy before manifesting clinical AF, which may progress from a paroxysmal form to chronic and persistent states associated with significant morbidity and mortality.2 Clinically, the risk factors for AF-progression remain elusive, although the ‘Cardiac failure, Hypertension, Age, Diabetes, Stroke [Doubled]’ (CHADS(2)) score appears to be useful in predicting which patients progress to more advanced persistent AF (perAF) forms during antiarrhythmic drug therapy.3 However, despite advances in AF treatment, there is still no effective therapeutic strategy for the prevention of AF recurrences and progression.
While there is hope that improved understanding of the molecular mechanisms underlying AF-progression can lead to more effective therapeutic strategies,2 the mechanisms underlying the progression from spontaneous atrial ectopy to spontaneous AF (sAF) remain poorly understood. The transgenic mouse model of cardiac overexpression of transcriptional repressor CREM-IbΔC-X (CREM) is the first experimental model of which we are aware that recapitulates the typical pattern of AF-progression seen in patients.4 Telemetry ECG recordings demonstrated that CREM-mice first exhibit spontaneous atrial ectopy by the age of 3 months, followed by the development of paroxysmal AF (pAF) and eventually perAF.
Enhanced activity of the sarcoplasmic reticulum (SR) Ca2+-release channel, also known as the type-2 ryanodine receptor channel (RyR2), has been implicated as one of the major mechanisms of cellular afterdepolarizations and triggered activity, an important mechanism underlying proarrhythmic ectopic activity in the heart.2, 5, 6 An important cause of enhanced RyR2 Ca2+-leak is RyR2-hyperphosphorylation.2, 5, 7 Increased CaMKII-activity and enhanced serine-2814 (S2814) phosphorylation of RyR2 are detected in patients with long-lasting perAF.5, 7, 8 Moreover, increased S2814 phosphorylation on RyR2 increases the susceptibility to AF-induction by programmed electrical stimulation in mutant mouse models.7 However, it is not known whether RyR2 dysfunction due to channel hyperphosphorylation contributes to AF-progression and whether this represents an important contributor to the development of sAF. Here, we tested the hypothesis that enhanced SR Ca2+-release due to increased phosphorylation of S2814 on RyR2 promotes the progression from atrial ectopy to pAF and perAF in CREM-mice. Our data revealed that 1) phosphorylation of S2814 on RyR2 is augmented prior to the onset of sAF, 2) genetic inhibition of S2814 phosphorylation of RyR2 delays the onset of atrial ectopy and prevents the development of sAF in CREM-mice, 3) normalization of RyR2-mediated SR Ca2+-release prevents atrial dilatation and atrial conduction abnormalities associated with the development of AF-maintenance. Together these findings suggest that the phosphorylation state of RyR2 may be a central motif in AF-progression and that preventing RyR2-hyperphosphorylation may be a novel and effective strategy for the prevention of more advanced forms of AF.
Methods
Experimental animals
Mouse studies were performed according to IACUC-approved protocols. CREM-Tg mice (FVB/N background) and RyR2-S2814A mice (C57Bl/6 background) were interbred, and mice on a mixed background were used.5
Telemetry ECG
Mice were implanted with telemeters as previously described.9
Intracardiac electrophysiology in mice
In-vivo electrophysiology studies were performed in mice at the age of 4-5months, as described.7
Myocyte Ca imaging
Atrial myocytes were isolated by a modified collagenase method as described.7 Fluo-4-AM-loaded myocytes were imaged using confocal microscopy, and SR Ca-content measured using caffeine application.7
Single-RyR2 recordings
Single-channel recordings were obtained under voltage-clamp conditions, as described.8
Optical mapping
Optical mapping of action potentials in the mouse atria was performed in mice at the age of 3-5months as described.5
Western blotting
Western blot analysis was performed in atrial samples of study animals at the ages of 1month (no atrial arrhythmias), 3months (atrial ectopy prior to the onset of spontaneous AF), and 7 months (long-lasting spontaneous AF).5
Human atrial samples were obtained with patients’ consent and approval of the IRB of Medical Faculty Mannheim-Heidelberg University. Patient characteristics are provided in Supplemental Table S1.
Results
Properties of the Model
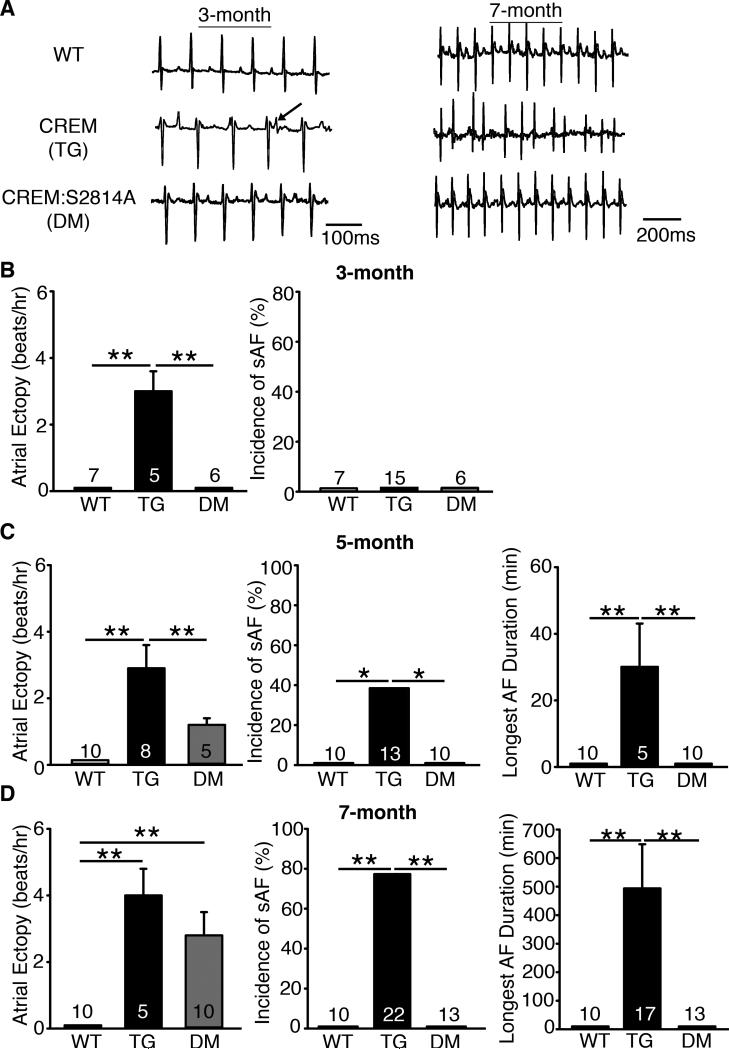
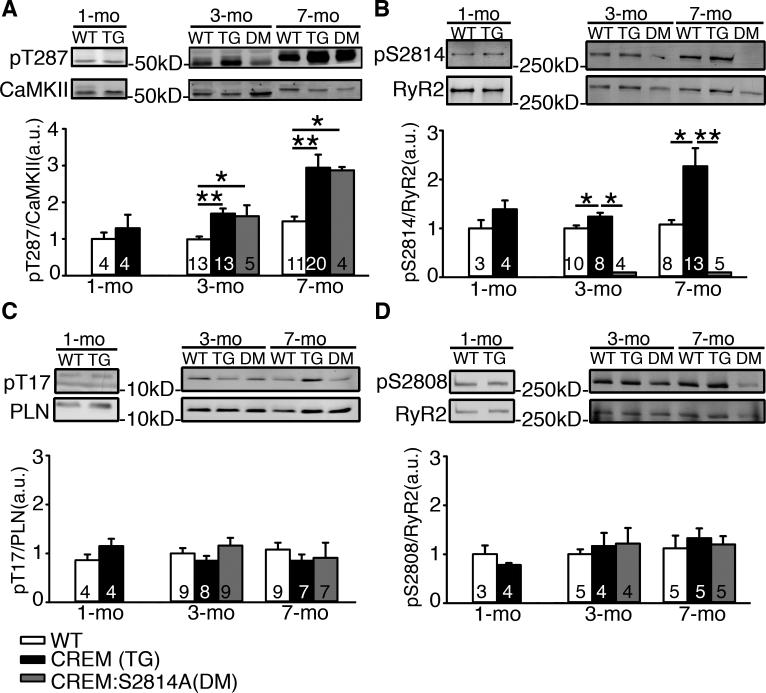
CREM-mice began to exhibit atrial ectopy around 3 months of age (Figure 1A). Quantification of atrial ectopic activity revealed a higher incidence of atrial ectopic beats (3.0±0.7 beats/hr) in 3-month-old CREM-mice compared to WT-mice, none of which exhibited atrial ectopic beats (Figure 1B). By 5 months of age, all of the CREM-mice showed ectopy (2.9±0.7 beats/hr) and 38% of CREM-mice showed at least one episode of sAF (5/13) at this age. The average duration of the longest sAF episode was 32.6±14.4 minutes (Figure 1C). By 7 months, over 77% of CREM-mice (17/22) had developed many and long-lasting episodes of sAF (Figure 1D). CREM-mice were in sAF for 52±10% of the recording time, much more than 5-month-old CREM-mice (9.0±5.3%, P<0.05). More strikingly, the average duration of the longest episode of sAF in each animal was greatly prolonged, to 493.6±154.9 minutes (P<0.05 vs.5-month-old CREM-mice). Importantly, none of the WT-mice developed sAF by 5 or 7-months of age. Based on previous work, we anticipated that CaMKII-activity might be enhanced in CREM-mice.4, 5 Figure 2A shows that CaMKII auto-phosphorylation at T287 indeed progressively increased with age (Figure 2A). Total CaMKII protein levels in CREM-mice, however, were reduced to 32±6% of those in WT-mice at 7 months of age. These changes in CaMKII-expression and auto-phosphorylation levels were associated with a progressive increase in RyR2-phosphorylation at the CaMKII-phosphorylation site S2814, starting at 3 months of age and further increasing by 7 months of age (Figure 2B). Another downstream target of CaMKII in the SR is threonine 17 (T17) of phospholamban (PLN), which was unchanged in CREM-mice (Figure 2C). This suggests that RyR2-hyperphosphorylation at S2814 might not result only from increased global CaMKII auto-phosphorylation. On the other hand, phosphorylation of RyR2-S2808, which is primarily regulated by protein kinase A (PKA), was also unaltered in CREM-mice (Figure 2D).
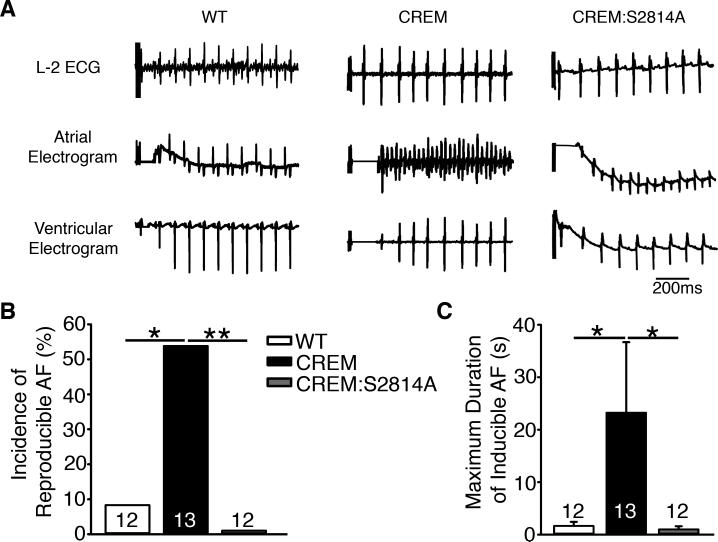
Figure 1.
Progression of atrial arrhythmia in CREM and CREM:S2814A mice. (A) Representative telemetry ECG recordings revealed the presence of spontaneous atrial ectopy at the age of 3 months and the presence of spontaneous AF (sAF) at the age of 7 months in CREM-mice, whereas no AF was observed in WT or CREM:S2814A mice. (B) left: The number of atrial ectopic events in mice that exhibited sinus rhythm at the age of 3 months. right: The incidence of sAF at the age of 3 months. (C-D) left: The number of atrial ectopic events in mice that exhibited sinus rhythm at the age of 5 and 7 months. middle: The incidence of sAF at the age of 5 and 7 months. right: The duration of longest episodes of sAF at the age of 5 and 7 months. Number in the bars indicated the number of animals studied. *P<0.05, **P<0.01.
Figure 2.
CaMKII-phosphorylation of RyR2 in CREM-mice increases prior to the onset of spontaneous AF. (A) Western blots showing age-dependent increase in levels of T287 auto-phosphorylation normalized to total CaMKII levels. (B) Western blots showing progressive increase in the level of S2814-phosphorylation normalized to total RyR2 levels. (C) Western blots showing the level of T17-phosphorylation normalized to total PLN levels. (D) Western blots showing the level of S2808-phosphorylation normalized to total RyR2 levels. Number in the bars indicated the number of animals studied *P<0.05, **P<0.01
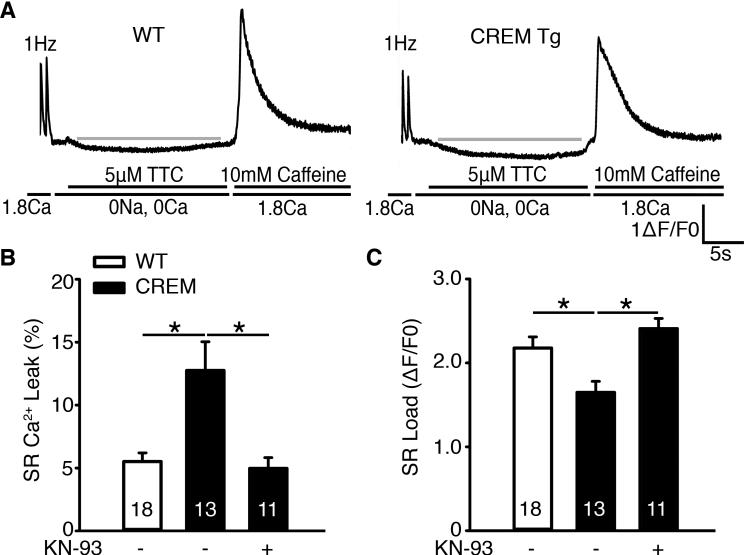
CaMKII-hyperphosphorylation of RyR2 can enhance SR Ca2+-release in cardiomyocytes. Therefore, we measured diastolic SR Ca2+-leak, with the use of standard methodology,10 as illustrated in Figure 3A. SR Ca2+-leak was enhanced in CREM-mice at the age of 7 months (Figure 3A-B). Enhanced SR Ca2+-leak can reduce SR Ca2+-content, which was shown in CREM-mouse atrial cardiomyocytes (Figure 3C). To determine whether increased CaMKII-activity contributes to abnormal RyR2-mediated SR Ca2+ release, SR Ca2+-leak was evaluated in cells pretreated with CaMKII inhibitor KN-93 for 30 min. Inhibition of CaMKII normalized both SR Ca2+-leak and SR Ca2+-load in CREM-mice to levels similar to those seen in WT-mice (Figure 3B-C). While these data point to a role for CaMKII hyperphosphorylation of RyR2 at S2814, the pharmacological probe KN-93 inhibits CaMKII generally. To better pinpoint the mechanism of action, we employed a genetic model in which S2814-phosphorylation is prevented by a serine-to-alanine mutation (S2814A) in order to assess specifically the role of this CaMKII-phosphorylation site.
Figure 3.
Inhibition of CaMKII reduces SR Ca2+-leak in CREM-mice. (A) Representative [Ca2+]i tracings from atrial myocytes paced at 1 Hz followed by rapid switch to Tyrode solution containing 0 Na+, 0 Ca2+, and 5 μmol/L tetracaine (TTC) to block RyR2-mediated SR Ca2+-leak. 10 mM caffeine was added to measure SR Ca2+-content. At the age of 7 months, CREM-mice exhibited increased SR Ca2+-leak, measured as the curve below the red baseline. (B) Quantification of SR Ca2+-leak normalized to SR Ca2+-load revealed that increased leak in CREM-mice was normalized by CaMKII inhibition using KN-93. (C) Bar graph showing reduced SR Ca2+-load in CREM-mice, which was normalized using KN-93. Number in the bars indicated the number of cells studied from 3-4 animals. *P<0.05.
Genetic inhibition of CaMKII-phosphorylation of RyR2 reduces SR Ca2+-leak in CREM-mice
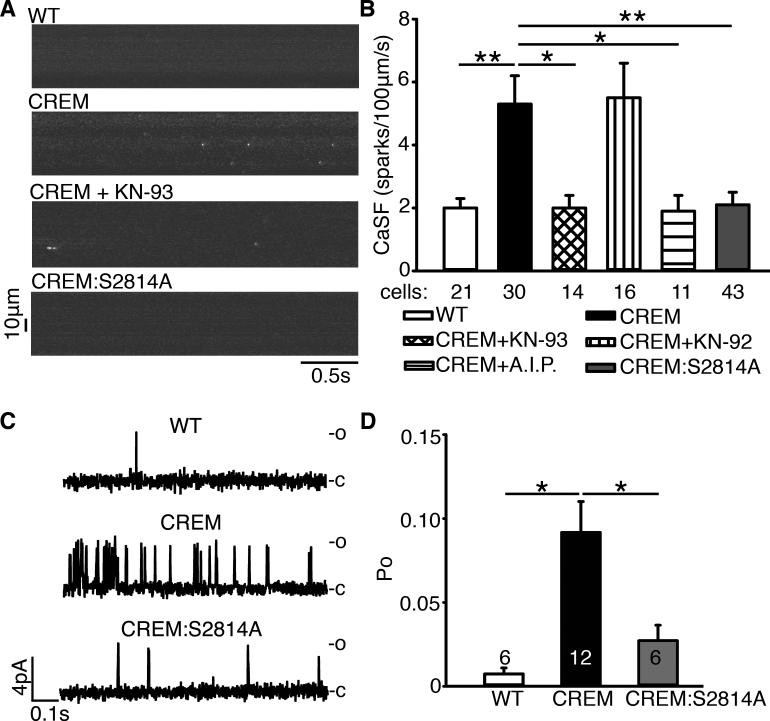
First, we tested whether inhibition of RyR2-phosphorylation at S2814 could normalize SR Ca2+-release defects caused by CREM-overexpression. Ca2+ spark frequency (CaSF) was measured in atrial cardiomyocytes from WT, CREM and CREM:S2814A mice. Consistent with previous studies,4 CaSF was significantly higher in CREM-mice (5.3±0.9 sparks/100μm/s) than in WT littermates (2.0±0.3 sparks/100 μm/s, P<0.05) (Figure 4A-B). Pretreating cells with the CaMKII-inhibitor KN-93 (1μmol/L) reduced CaSF (Figure 4A-B), consistent with its effects on cell Ca2+-leak (Figure 3), whereas the inactive control KN-92 (1μmol/L) was without effect (Figure 4B). Consistently, autocamtide-2-related inhibitory peptide (AIP, 1μmol/L), a CaMKII inhibitor with better specificity than KN-93, also reduced CaSF in CREM-mice (P<0.05 vs.CREM, Figure 4B). Moreover, genetic inhibition of RyR2-phosphorylation at S2814 in the CREM:S2814A mice also reduced CaSF (2.1±0.4 sparks/100μm/s, P<0.05 vs.CREM, Figure 4A, B) as well as reversed other Ca2+ spark abnormalities observed in CREM-mice, such as prolonged duration and slowed decay times (i.e., prolonged tau) (Supplemental Table S2). Together, these findings suggest that CaMKII-phosphorylation of RyR2 is a critical mechanism in terms of causing SR Ca2+-release defects.
Figure 4.
Genetic inhibition of S2814-phosphorylation on RyR2 normalizes RyR2 activity in CREM-mice. (A) Line-scan confocal images of atrial myocytes revealed more spontaneous Ca2+-sparks in CREM-mice. (B) Ca2+-spark frequency (CaSF). (C) RyR2 single channels recordings revealed more openings (o) as shown upward deflections from the closed (c) level in CREM-mice. (D) Open probability (Po) of RyR2-channels. Number in the bars indicated the number of cells/channels studied from 3-4 mice. *P<0.05.
RyR2-channels are hyperactive in CREM-mice before the onset of sAF
To determine the function of RyR2-channels in CREM-mice before the onset of sAF, we studied the single channel properties of RyR2-channels obtained from atrial tissues of WT, CREM and CREM:S2814A mice at the age of 3-months (Figure 4C). The RyR2-channels exhibited higher open probability (Po) in CREM-mice (0.092±0.018) than WT-mice (0.0074±0.0036, P<0.05). Genetic inhibition of RyR2-phosphorylation in CREM:S2814A mice reduced Po to 0.027±0.009 (P<0.05 vs. CREM;Figure 4D). These findings suggest that RyR2-channels are hyperactive in CREM-mice; hyperactivity was prevented by the S2814A mutation.
Inhibition of RyR2 Ca2+-leak prevents progression to sAF in CREM-mice
To evaluate the impact of RyR2-mediated Ca2+-release on spontaneous atrial arrhythmogenesis in CREM-mice, we performed 24-hour telemetry recordings in CREM:S2814A mice. Unlike CREM-mice, none of the CREM:S2814A mice exhibited atrial ectopy at 3 months of age (Figure 1B). By 5 months, none of the CREM:S2814A mice had developed sAF, though a few did exhibit spontaneous atrial ectopic events (Figure 1C). Moreover, by 7 months, none of the CREM:S2814A mice had progressed to sAF, whereas 77% of the CREM-mice exhibited prolonged of sAF-episodes (Figure 1D). The bar graph in Supplemental Figure S1 summarizes the incidence of spontaneous atrial ectopy and sAF in WT, CREM, and CREM:S2814A mice at 3 different ages, suggesting that inhibition of S2814 phosphorylation on RyR2 delays the onset of spontaneous atrial ectopy and, more importantly, prevents its progression to sAF.
Inhibition of RyR2 Ca2+-leak suppresses the inducibility and maintenance of AF in CREM-mice
By 5 months of age, all CREM-mice (n=13) exhibited atrial ectopic activity and 38.5% (5/13) also showed episodes of sAF. To evaluate whether S2814-phosphorylation plays a critical role in the substrate for AF, we performed programmed electrical stimulation (PES) at this age (Figure 5). PES was performed at a time when the CREM-mice did not yet exhibit sAF, in order to be sure that any AF-episodes were indeed induced and not a spontaneous occurrence or continuation of pre-existing AF. Whereas 54% (7/13) of the CREM-mice developed reproducible AF following atrial burst pacing (defined as at least 2 of the 3 burst pacing protocols resulting in AF-episodes lasting >1 sec), only 1/12 WT (8.3%) and none of the 12 CREM:S2814A (0%) mice developed pacing-induced AF (Figure 5B). In addition, the average duration of the longest inducible AF-episodes was greater in CREM-mice compared to WT (P<0.05) and CREM:S2814A (P<0.05) mice (Figure 5C), suggesting that the S2814A mutation in RyR2 prevents the perpetuation of AF in CREM-mice.
Figure 5.
Genetic inhibition of S2814-phosphorylation on RyR2 suppresses the maintenance of AF in CREM-mice. (A) Electrograms of pacing-induced AF in CREM-mice at 5 months of age. (B) Increased incidence of pacing-induced AF in CREM-mice (n=12) at 5 months of age, compared with WT (n=10) and CREM:S2814A mice (n=12). (C) The average duration of the longest AF-episode in each animal induced by pacing. *P<0.05.
The increased likelihood of AF induction was not secondary to global changes in cardiac electrophysiology, since there were no significant differences in cardiac electrophysiological parameters such as heart rate, QRS, or QTc intervals among the different genotypes of mice (Supplemental Table S3). However, the PR interval was significantly prolonged in CREM compared to WT but normalized in the CREM:S2814A mice (Supplemental Table S3). Other parameters such as the corrected sinus node recovery time (cSNRT), atrial effective refractory period (AERP) and atrioventricular node effective refractory period (AVNERP) were all unchanged in CREM-mice compared to WT-mice (Supplemental Table S3). Finally, there were no significant changes in ventricular contractility and dimensions in 5-month-old CREM-mice compared with WT (Supplemental Table S4), suggesting that the development of persistent AF is not caused by ventricular remodeling.
Inhibition of RyR2 Ca2+-leak improves atrial conduction and excitability
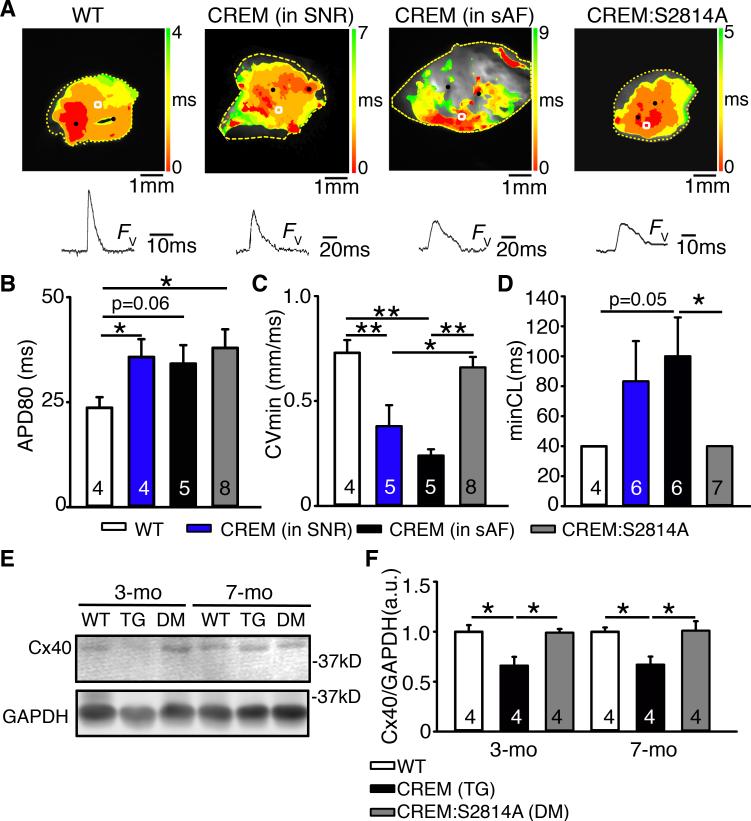
In order to understand the PR prolongation and the AF substrate demonstrated by prolonged sAF and pacing-induced AF in CREM-mice, we performed optical mapping on atrial tissue preparations isolated from 5 month-old mice. Surprisingly, we found that regardless of whether or not the CREM-mice were in sinus rhythm (SNR) or in sAF, all of them had developed electrical remodeling at this age (Figure 6). First, atrial preparations from all CREM-mice, both in SNR and with sAF displayed biatrial enlargement with apparent loss of viable cardiomyocytes, as evidenced by large inexcitable areas in their atrial tissue (Figure 6A). Second, during 5-Hz pacing, CREM-mice both in SNR (35.8±4.2 ms) or sAF (34.2±4.4 ms) exhibited prolonged atrial action potential-duration at 80% repolarization (APD80) compared with WT-mice (23.7±2.5 ms; P<0.05 vs. CREM in SNR, P=0.06 vs. CREM in sAF) (Figure 6B). Moreover, the conduction velocity (CV) was significantly reduced in both CREM-mice in SNR (0.38±0.10 mm/ms) and with sAF (0.24±0.03 mm/ms) compared with WT-mice (0.73±0.06 mm/ms; P<0.01) (Figure 6C). CREM-mice also had a decreased ability to sustain rapid pacing rates, and the minimum pacing cycle-length (minCL) at which they captured was longer (83.3±26.8 ms in SNR, 100±25.9 ms in sAF) than in WT-mice (40.0±0.0 ms; P=0.05;Figure 6D). Although atrial preparations from CREM:S2814A mice still exhibited prolonged APD80 (38.0±4.4 ms, P<0.05 vs.WT), they showed normalized atrial dimensions, improved CV (0.66±0.05 mm/ms, P<0.05 vs.CREM in SNR, P<0.01 vs.CREM in sAF), and normalized minCL (40.0±0.0 ms, P<0.05 vs.CREM in SNR) (Figure 6A-D). Since CREM-mice developed clear electrical remodeling event without AF at a time when a minority of mice displayed sAF, the remodeling is clearly not secondary to AF.
Figure 6.
Improved conduction velocity and excitability in CREM:S2814A mice. (A) Top: Activation map of isolated atria preparations obtained from 5-month-old mice. Bottom: Voltage fluorescence (Fv) traces obtained from the indicated regions (white squares). (B) APD80, (C) minimum conduction velocity, and (D) minimum cycle length that can pace the atria. (E) Western blots showing reduction in the level of Cx40 protein in CREM-mice before and after the onset of sAF. (F) Quantification of Cx40 protein level normalized to GAPDH. Numbers in the bars indicate the number of animals studied. *P<0.05, **P<0.01.
To address the mechanism of APD prolongation in both CREM and CREM:S2814A mice, we measured L-type Ca2+-current (ICa,L) in isolated atrial myocytes. Our data revealed that ICa,L was enhanced in both CREM and CREM:S2814A mice versus WT-mice (supplemental Figure S2), suggesting that ICa,L gain-of-function results from CREM-overexpression independent of RyR2-mediated Ca2+-release.
Previously, we showed that the reduced CV in CREM-mice is associated with a downregulation of connexin-40 (Cx40),4 a major component of atrial gap-junctions, while Cx43-expression is unaltered. To evaluate whether the improvement in CV in CREM:S2814A mice is related to a change in Cx40-expression, we quantified Cx40-protein by Western blotting. Consistent with optical mapping results, Cx40-expression was downregulated in CREM-mice as early as 3 months of age (P<0.05 vs.WT), before the onset of sAF (Figure 6E-F). Cx40 remained downregulated by 30% in CREM-mice as they developed long-lasting persistent sAF (P<0.05 vs.WT), consistent with previous observation.4 However, Cx40-expression was restored in CREM:S2814A mice at both 3 and 7 months of age (P<0.05 vs.CREM) (Figure 6E-F). These findings suggest that RyR2-mediated SR Ca2+-leak may be involved in the downregulation of Cx40.
Inhibition of RyR2 Ca2+-leak attenuates atrial enlargement in CREM-mice
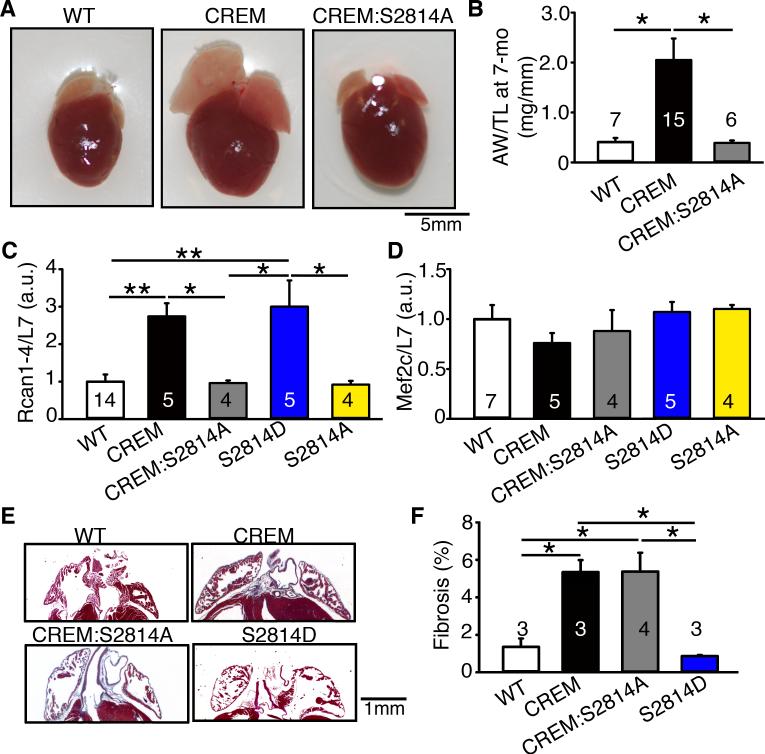
Continued presence of AF has been shown to induce atrial structural remodeling that further perpetuates AF in various large animal models.11, 12 To determine whether inhibition of RyR2 Ca2+-leak alters structural remodeling associated with cAF, hearts were collected from 7-month-old mice. There was clear biatrial enlargement in CREM-mice, compared to WT and CREM:S2814A mice (Figure 7A). Quantification of the atrial weight normalized to tibia length (AW/TL) revealed that the atrial mass was increased by 162% in CREM-mice (2.05±0.43 mg/mm) compared with WT mice (0.41±0.08 mg/mm; P<0.05) (Figure 7B). In contrast, AW/TL was normalized in CREM:S2814A mice (0.39±0.05mg/mm, P<0.05 vs.CREM). Quantification of the total heart weight normalized to tibia length did not reveal a significant increase in CREM-mice (7.9±0.4 mg/mm) compared to WT (6.7±1.0 mg/mm) and CREM:S2814A mice (7.5±0.3 mg/mm). To evaluate whether the structural remodeling in the atria preceded the onset of sAF, we quantified the AW/TL in 3-month-old mice. At this age, AW/TL was already significantly increased in CREM-mice (1.5±0.3 mg/mm) compared with WT (0.26±0.03, P<0.01), but was unchanged in CREM:S2814A mice (0.28±0.03 mg/mm, P<0.01 vs.CREM) (Supplemental Figure S3). These data suggest that hypertrophic pathways are activated directly by RyR2-mediated Ca2+-leak, and that atrial structural remodeling is not secondary to AF.
Figure 7.
Reversal of atrial hypertrophy but maintenance of atrial fibrosis in CREM:S2814A mice. (A) Whole mount photographs of hearts from 7-month-old WT, CREM, and CREM:S2814A mice. (B) The atrial weight-to-tibia length ratio (AW/TL of 7-months-old mice. (C) The level of Rcan1-4 mRNA normalized to L7. (D) The level of Mef2c mRNA normalized to L7. (E) Masson-Trichrome staining of fibrosis in atrial sections. (F) Quantification of atrial fibrosis. Numbers in the bars indicate the number of animals studied. *P<0.05, **P<0.01.
To determine whether known Ca2+-dependent hypertrophic signaling-systems, namely the calcineurin-NFAT-Rcan and CaMKII-HDAC-Mef2c pathways, are activated before the onset of sAF, we evaluated expression of Rcan1-4 and Mef2c mRNA in atrial tissues from 3-month-old mice. We found that Rcan1-4, but not Mef2c, was upregulated in CREM-mice compared with WT (P<0.01) (Figure 7C-D). Consistent with a causative role of Ca2+-leak in NFAT/Rcan1-4 activation, Rcan1-4 expression was normalized in CREM:S2814A mice (P<0.05 vs.CREM) (Figure 7C-D). To determine whether Rcan1-4 is directly activated by RyR2 Ca2+-leak, we studied RyR2-S2814D (S2814D) mice, in which the S2814D mutation mimics hyperphosphorylation and predisposes mice to pacing-induced AF.8 Rcan1-4 is also upregulated in S2814D mice, supporting the idea of direct RyR2-mediated activation of Rcan1-4 (Figure 7C). Similar to findings in CREM-mice, CaMKII-dependent activation of the hypertrophic molecule Mef2c was not altered in either S2814D or S2814A mice (Figure 7D). Together, these data indicate that RyR2-mediated Ca2+-release results in activation of the calcineurin-NFAT-Rcan pathway and promotes structural remodeling.
Finally, the level of atrial fibrosis was examined using Masson's trichrome (MT) staining of longitudinal cardiac sections (Figure 7E). Quantification of fibrosis revealed enhanced atrial fibrous-tissue content in both CREM (5.5±0.6%) and CREM:S2814A (5.4±1.0%) mice compared with WT-mice (1.4±0.4%; both P<0.05) (Figure 7F). These data suggest that the prevention of cAF development in CREM:S2814A did not result from inhibition of atrial fibrosis. In addition, both CREM (6.0±0.9%) and CREM:S2814A (7.2±1.7%) mice had more ventricular fibrosis than WT mice (1.1± 0.3%; both P<0.05) (Supplemental Figure S4). Consistent with the idea that fibrosis in CREM-mice does not result from RyR2-hyperphosphorylation/Ca2+-leak, RyR2-phosphomimetic S2814D mice did not exhibit fibrosis in either atria (0.87±0.06%, Figure 7E-F) or ventricles (0.45±0.09%, Figure S4). Therefore, it is likely that fibrosis observed in CREM-mice is a direct consequence of cardiac CREM-overexpression and not a consequence of AF-associated remodeling or a RyR2 Ca2+-release dependent process.
Increased level of CREM-IbΔC-X in AF patients
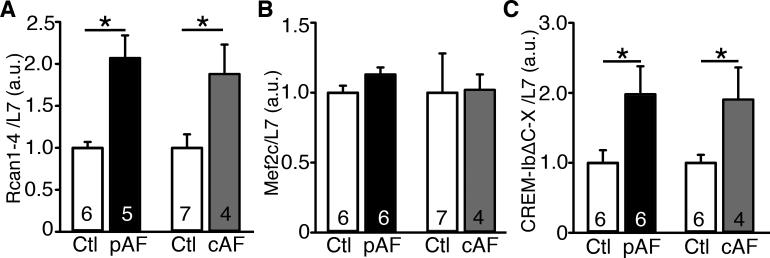
Consistent with the results we observed in CREM-mice, Rcan1-4 mRNA was increased 2-fold compared in atrial samples from pAF patients compared with patients with sinus rhythm (Ctl) (P<0.05) (Figure 8A). We also observed similar amount of Rcan1-4 upregulation in atrial samples from cAF patients (P<0.05 vs.Ctl) (Figure 8B). Unlike Rcan1-4, the level of Mef2c was unaltered in atrial samples from pAF and cAF patients (Figure 8B). These results further confirmed our findings regarding the hypertrophic signaling pathway involved in atrial remodeling in CREM-mice. Finally, we evaluated the mRNA-expression of CREM-IbΔC-X in atrial biopsies from patients in Ctl, pAF, and cAF. The expression of this transcriptional repressor CREM-IbΔC-X is increased almost 2-fold in atrial samples from pAF and cAF patients, compared with separate sets of randomly chosen Ctl patients (P<0.05) (Figure 8C), positioning CREM as a potential contributor to human AF-pathophysiology.
Figure 8.
Upregulation of Rcan1-4 and CREM-IbΔC-X mRNA in AF patients. The level of Rcan1-4 (A), Mef2c (B), and CREM-IbΔC-X (C) mRNA normalized to L7 in atrial samples from patients. Independent experiments using separate sets of randomly chosen sinus rhythm (Ctl) samples were used to compare with paroxysmal AF (pAF) and chronic AF (cAF) samples. Numbers in the bars indicate the number of patients studied. *P<0.05.
Discussion
Our data show for the first time a direct causal role of RyR2-mediated SR Ca2+-leak in atrial structural remodeling, which is required for the development of the substrate underlying spontaneous AF in CREM-Tg mice. Specifically, increased CaMKII-mediated phosphorylation of RyR2 preceded the transition from atrial ectopy to spontaneous AF in CREM-mice, a transition that mimics the progressive nature of AF observed in humans. Suppression of SR Ca2+-leak by genetic inhibition of RyR2-phosphorylation at S2814 completely prevented spontaneous AF. Moreover, our study demonstrated that normalization of RyR2-mediated Ca2+-leak prevented slowed atrial conduction and atrial dilatation in CREM-mice. Thus, our findings uncovered an unanticipated role for RyR2-mediated Ca2+-leak in the progression to persistent AF forms.
New insights from the CREM-Tg mouse model of persistent AF
Mouse models represent an important animal system in which molecular signaling events involved in the pathogenesis of cardiac disease can be elucidated. However, to date, few mouse models of spontaneous AF have been reported. Prior studies demonstrated that CREM-transgenic mice not only exhibit spontaneous AF, but also show a gradual, age-dependent transition from atrial ectopy to paroxysmal AF and long-lasting AF, thus mimicking disease progression often seen in AF patients.4 Indeed, CREM-mice at 7-months were in AF over 50% of the time, with longest episodes averaging almost 10-hours in each mouse. Although it is clearly difficult to know what AF duration in man would correspond to 10 hours of continuous AF in the mouse, correction by life-expectancy suggests this may be equivalent to a few weeks of continuous AF in man.13 Similar to classic large animal models of AF induced by tachycardiac pacing,14, 15 CREM-mice recapitulate key features of the persistent AF substrate such as slowed conduction, atrial dilatation and increased fibrous-tissue content, hallmarks of structural remodeling that correlate with the severity and persistence of AF in patients.2 Therefore, we took advantage of this mouse model to gain new insights into the molecular pathogenesis of AF.
CaMKII-mediated activation of RyR2 in AF
Previous studies have demonstrated that expression-levels and activity of cytosolic CaMKII are upregulated in long-lasting persistent AF patients and in experimental AF models, including CREM-mice.5, 8, 16 CaMKII may be activated by the increased atrial rate or via enhanced oxidation of CaMKII.17 CaMKII-phosphorylation of RyR2 increases channel activity,18 associated with increased SR Ca2+-leak, enhanced Ca2+-spark generation, and an increased susceptibility to AF-induction.8 Conversely, genetic inhibition of RyR2-phosphorylation at S2814 prevents pacing-induced AF in mouse models with RyR2 defects.5, 7, 8 Moreover, enhanced SR Ca2+-leak via defective RyR2-channels promotes atrial ectopic activity and promotes AF inducibility in several mouse models, including FKBP12.6-deficient mice,7, 19 and mice heterozygous for catecholaminergic polymorphic ventricular tachycardia (CPVT)-associated RyR2 mutations.5, 20 These studies in mice and more recent work in atrial myocytes from patients with persistent AF demonstrated a mechanistic link between abnormal SR Ca2+-release events, activation of the Na+/Ca2+ exchanger (NCX), and an increased likelihood of DADs and triggered activity that underlie atrial ectopic-impulse formation.8
A previous study revealed that CREM-Tg mice exhibit increased SR Ca2+-leak as evidenced by an increased frequency of Ca2+-sparks and SR Ca2+-leak revealed by tetracaine exposure,4 but the mechanisms underlying RyR2-mediated Ca2+-leak and its role in AF substrate development were not investigated. Here we show that RyR2-hyperphosphorylation is clearly responsible for the hyperactive RyR2-channels in CREM-Tg mice, since enhanced open probability of RyR2-channels and the higher frequency of Ca2+-sparks were suppressed by inhibition of RyR2-phosphorylation in CREM:S2814A mice. Moreover, RyR2 hyperactivity is not due to enhanced phosphorylation-levels of RyR2 at S2808, an important target for PKA-phosphorylation. In contrast, CaMKII-phosphorylation of another key Ca2+-handling protein, PLN (at T17), was also unchanged in CREM-mice. A prior study by Greiser et al.21 also reported that only phosphorylation of S2814 on RyR2 is enhanced in two goat models of AF, whereas levels of S2808 phosphorylation and PLN-T17 phosphorylation were unaltered. These findings suggest that phosphorylation by CaMKII, but not PKA, is responsible for RyR2 dysfunction in CREM-mice. Since PLN was also not hyperphosphorylated by CaMKII, it is possible that altered regulation by protein phosphatases within the RyR2-complex may contribute to its differential phosphorylation.22
Mechanistic link between RyR2-mediated SR Ca2+-leak and atrial remodeling
Our data reveal that key components of atrial remodeling in CREM-mice, in particular atrial dilatation and conduction slowing, are mediated by increased diastolic SR Ca2+-leak via RyR2. This conclusion is based on the observation that RyR2-activity is increased in CREM-mice prior to the onset of spontaneous AF, and that RyR2 single-channel activity is reduced to WT levels in CREM:S2814A mice. Moreover, CREM:S2814A were protected from developing atrial dilatation, conduction velocity slowing, and spontaneous AF. In contrast, other major contributors to atrial remodeling, such as atrial fibrosis and altered APD were not normalized in CREM:S2814 mice, suggesting that these aspects of atrial remodeling occur independently of RyR2-mediated Ca2+-leak.
Our data suggest that the conduction-slowing in CREM-mice is caused at least in part by downregulation of Cx40, an important protein in atrial gap junctions.4 Normalization of the Cx40-expression in CREM:S2814A mice suggests that the Cx40 downregulation may also be driven by RyR2-mediated Ca2+-leak. A recent study by King et al.23 also demonstrated slower interatrial CVs in a mouse model of CPVT, with a gain-of-function RyR2-mutation causing SR Ca2+-leak. Taken together, these findings suggest that RyR2 dysfunction may promote reentrant arrhythmogenesis by promoting the development of a susceptible substrate involving structural remodeling and Cx40-downregulation mediated conduction slowing.23
Regarding potential mechanisms underlying structural remodeling, our data revealed that RyR2-mediated SR Ca2+-leak dependent activation of the calcineurin-NFAT-Rcan pathway in both CREM-Tg mice and AF patients, whereas the CaMKII-HDAC-Mef2c pathway was not affected. Interestingly, constitutive CaMKII-phosphorylation of S2814, which is associated with enhanced RyR2 single channel activity, was sufficient to activate the calcineurin-NFAT-Rcan pathway, suggesting a direct causal link between RyR2-mediated SR Ca2+-leak and structural remodeling. Enhanced activation of calcineurin-NFAT-Rcan pathway has been implicated in humans with AF and animal models of AF.24 NFAT translocation regulates mRNA transcription, leading to remodeling of 2 key atrial ion channels, i.e. downregulation of α-subunit of ICa,L25 and microRNA-26 mediated upregulation of Kir2.1 subunit of the inward-rectifier K+ channel (IK1).26 Augmentation of IK1 is an important AF-maintenance factor, due to its APD shortening effect leading to stabilization of rotors.27 Our results suggest that the RyR2-mediated SR Ca2+-leak may be the key process that initiates atrial remodeling both electrical and structural, promoting the transition from paroxysmal to persistent AF.
Frequent atrial ectopy is not sufficient to cause spontaneous AF in CREM-mice
It is often assumed that ectopic complexes precede AF because they manifest as a sustained tachyarrhythmic “driver” or trigger reentrant arrhythmia in a vulnerable substrate. Another potential idea is that atrial ectopy can by itself induce atrial remodeling. The present findings suggest, however, that atrial ectopy is not sufficient to cause atrial remodeling associated with substrate development for sAF in CREM-mice. Although CREM:S2814A eventually exhibited substantial atrial ectopy, they never developed spontaneous episodes of AF. The S2814A mutation in RyR2 did not normalize APD-prolongation in CREM-mice. Our data also revealed that both CREM and CREM:S2814A mice exhibit enhanced ICa,L, which could underlie APD-prolongation.28 Increased ICa,L and APD-prolongation increase the propensity to early afterdepolarizations (EADs), which can induce triggered activity and spontaneous ectopy.29 Thus, EAD-mediated processes may underlie the eventual development of atrial ectopy in CREM:S2814A mice, with the protection against ectopy at 3 and 5 months related to suppressed DAD-generation, but further work is needed to directly test this notion. Another candidate mechanism for atrial ectopy in CREM:S2814A mice could be abnormal cardiomyocytefibroblast interactions. Fibroblasts can affect electrical properties of cardiomyocytes and induce spontaneous automaticity.30 CREM:S2814A mice developed atrial fibrosis, possibly causing atrial ectopy due to abnormal atrial myocyte-fibroblast interactions.
Clinical Relevance
There are presently two major challenges for AF-treatment. First, AF-recurrence is very common; it remains challenging to maintain sinus rhythm in AF-patients who undergo cardioversion or AF-ablation. Second, up to 15% of initially-diagnosed paroxysmal AF patients will progress to persistent or permanent forms each year. 31
CaMKII-blockers are being developed for clinical use,32 with the goal of suppressing ectopic atrial activity related to RyR2-hyperphosphorylation that may initiate AF.2,19 The present findings raise the exciting possibility that such agents may act against AF not only by attacking arrhythmic triggers, but also by preventing the Ca2+-leak dependent development of the reentry substrate that underlies progression to more persistent forms. The promise of this approach is underscored by evidence that clinical AF leads to CaMKII-hyperphosphorylation of RyR2 and consequent SR Ca2+-leak, 10 and by our finding in the present study that CREM-expression is enhanced in clinical AF (Figure 8), positioning Ca2+-leak induced remodeling as a candidate mechanism for AF-progression in man.
Potential Limitations
The clinical pathophysiology of AF is very complex,33 and our findings likely don't apply to all clinical forms of AF, nor to all aspects of AF pathophysiology in any individual patient. We studied a mouse model that has tantalizing similarities to clinical AF in many patients, with the initial presentation of frequent atrial ectopy followed by spontaneous AF of progressively longer duration. To our knowledge, this is the only animal model presenting spontaneous atrial arrhythmias and progression of this type. However, any animal model is limited in the extent to which it mimics clinical AF, and therefore extrapolation of our findings to man should be very cautious. Moreover, the mechanistic link between the CREM-overexpression and CaMKII- activation is unknown and requires extensive work in subsequent studies. Nevertheless, our finding that CREM is overexpressed in AF-patients and the well-known increase in RyR2 Ca2+-leak in clinical AF 7, 8, 11, 34 argue for the potential clinical relevance of our work. These findings provide a potentially important new concept that needs to be evaluated in further experimental and translational studies: that SR Ca2+-leak may contribute not only to the spontaneous generation of local triggered activity, but also to the development and progression of the AF-maintaining substrate.
Conclusions
RyR2-hyperphosphorylation and SR Ca2+-leak is an essential mechanism for the development of spontaneous AF in CREM-mice. Thus, RyR2-dysfunction can play a role in AF not only as a cause of ectopic trigger-activity but also by promote the AF-maintaining substrate. These findings constitute the first demonstration of molecular mechanisms underlying progression to spontaneous AF-development in an experimental model, with potentially important pathophysiological and therapeutic implications.
Supplementary Material
Acknowledgments
Funding Sources: This study is supported by grants 09POST2260300 and 12BGIA12050207 (to N.L.), 12PRE11700012 (to D.Y.C.), and 13EIA14560061 (to X.H.T.W.) from the American Heart Association. This work was supported in part by NHLBI grants R01-HL089598 and R01-HL091947, the Muscular Dystrophy Association, Fondation Leducq (‘Alliance for CaMKII Signaling in Heart’ to X.H.T.W. and ‘European North-American Atrial Fibrillation Research Alliance’ to D.D. and S.N.), DZHK (German Center for Cardiovascular Research to D.D.), and the Deutsche Forschungsgemeinschaft (DFG MU 1376/11-1 to F.U.M. and W.S.), by the IZKF Münster (Mü1/014/11 to F.U.M. and W.S.), by the Canadian Institutes of Health Research (MGP6957 and MOP44365 to S.N.) and the Heart and Stroke Foundation of Canada (to S.N.). D.Y.C. was also supported by the Medical Scientist Training Program Caskey Scholarship.
Footnotes
Conflict of Interest Disclosures: None.
References
- 1.Naccarelli GV, Varker H, Lin J, Schulman KL. Increasing prevalence of atrial fibrillation and flutter in the United States. Am J Cardiol. 2009;104:1534–1539. doi: 10.1016/j.amjcard.2009.07.022. [DOI] [PubMed] [Google Scholar]
- 2.Dobrev D, Nattel S. New antiarrhythmic drugs for treatment of atrial fibrillation. Lancet. 2010;375:1212–1223. doi: 10.1016/S0140-6736(10)60096-7. [DOI] [PubMed] [Google Scholar]
- 3.Komatsu T, Sato Y, Ozawa M, Kunugita F, Ueda H, Tachibana H, Morino Y, Nakamura M. Relationship between CHADS2 score and efficacy of antiarrhythmic drug therapy in patients with paroxysmal atrial fibrillation. Circ J. 2013;77:639–645. doi: 10.1253/circj.cj-12-0854. [DOI] [PubMed] [Google Scholar]
- 4.Kirchhof P, Marijon E, Fabritz L, Li N, Wang W, Wang T, Schulte K, Hanstein J, Schulte JS, Vogel M, Mougenot N, Laakmann S, Fortmueller L, Eckstein J, Verheule S, Kaese S, Staab A, Grote-Wessels S, Schotten U, Moubarak G, Wehrens XH, Schmitz W, Hatem S, Muller FU. Overexpression of cAMP-response element modulator causes abnormal growth and development of the atrial myocardium resulting in a substrate for sustained atrial fibrillation in mice. Int J Cardiol. 2013;166:366–374. doi: 10.1016/j.ijcard.2011.10.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chelu MG, Sarma S, Sood S, Wang S, van Oort RJ, Skapura DG, Li N, Santonastasi M, Muller FU, Schmitz W, Schotten U, Anderson ME, Valderrabano M, Dobrev D, Wehrens XH. Calmodulin kinase II-mediated sarcoplasmic reticulum Ca2+ leak promotes atrial fibrillation in mice. J Clin Invest. 2009;119:1940–1951. doi: 10.1172/JCI37059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vest JA, Wehrens XH, Reiken SR, Lehnart SE, Dobrev D, Chandra P, Danilo P, Ravens U, Rosen MR, Marks AR. Defective cardiac ryanodine receptor regulation during atrial fibrillation. Circulation. 2005;111:2025–2032. doi: 10.1161/01.CIR.0000162461.67140.4C. [DOI] [PubMed] [Google Scholar]
- 7.Li N, Wang T, Wang W, Cutler MJ, Wang Q, Voigt N, Rosenbaum DS, Dobrev D, Wehrens XH. Inhibition of CaMKII phosphorylation of RyR2 prevents induction of atrial fibrillation in FKBP12.6 knockout mice. Circ Res. 2012;110:465–470. doi: 10.1161/CIRCRESAHA.111.253229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Voigt N, Li N, Wang Q, Wang W, Trafford AW, Abu-Taha I, Sun Q, Wieland T, Ravens U, Nattel S, Wehrens XH, Dobrev D. Enhanced sarcoplasmic reticulum Ca2+ leak and increased Na+-Ca2+ exchanger function underlie delayed afterdepolarizations in patients with chronic atrial fibrillation. Circulation. 2012;125:2059–2070. doi: 10.1161/CIRCULATIONAHA.111.067306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van Oort RJ, McCauley MD, Dixit SS, Pereira L, Yang Y, Respress JL, Wang Q, De Almeida AC, Skapura DG, Anderson ME, Bers DM, Wehrens XH. Ryanodine receptor phosphorylation by calcium/calmodulin-dependent protein kinase II promotes life-threatening ventricular arrhythmias in mice with heart failure. Circulation. 2010;122:2669–2679. doi: 10.1161/CIRCULATIONAHA.110.982298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shannon TR, Ginsburg KS, Bers DM. Quantitative assessment of the SR Ca2+ leak-load relationship. Circ Res. 2002;91:594–600. doi: 10.1161/01.res.0000036914.12686.28. [DOI] [PubMed] [Google Scholar]
- 11.Gillis AM, Krahn AD, Skanes AC, Nattel S. Management of atrial fibrillation in the year 2033: new concepts, tools, and applications leading to personalized medicine. Can J Cardiol. 2013;29:1141–1146. doi: 10.1016/j.cjca.2013.07.006. [DOI] [PubMed] [Google Scholar]
- 12.Zou R, Kneller J, Leon LJ, Nattel S. Substrate size as a determinant of fibrillatory activity maintenance in a mathematical model of canine atrium. Am J Physiol Heart Circ Physiol. 2005;289:H1002–1012. doi: 10.1152/ajpheart.00252.2005. [DOI] [PubMed] [Google Scholar]
- 13.Demetrius L. Aging in mouse and human systems: a comparative study. Ann N Y Acad Sci. 2006;1067:66–82. doi: 10.1196/annals.1354.010. [DOI] [PubMed] [Google Scholar]
- 14.Morillo CA, Klein GJ, Jones DL, Guiraudon CM. Chronic rapid atrial pacing. Structural, functional, and electrophysiological characteristics of a new model of sustained atrial fibrillation. Circulation. 1995;91:1588–1595. doi: 10.1161/01.cir.91.5.1588. [DOI] [PubMed] [Google Scholar]
- 15.Willems R, Holemans P, Ector H, Sipido KR, Van de Werf F, Heidbuchel H. Mind the model: effect of instrumentation on inducibility of atrial fibrillation in a sheep model. J Cardiovasc Electrophysiol. 2002;13:62–67. doi: 10.1046/j.1540-8167.2002.00062.x. [DOI] [PubMed] [Google Scholar]
- 16.Neef S, Dybkova N, Sossalla S, Ort KR, Fluschnik N, Neumann K, Seipelt R, Schondube FA, Hasenfuss G, Maier LS. CaMKII-dependent diastolic SR Ca2+ leak and elevated diastolic Ca2+ levels in right atrial myocardium of patients with atrial fibrillation. Circ Res. 2010;106:1134–1144. doi: 10.1161/CIRCRESAHA.109.203836. [DOI] [PubMed] [Google Scholar]
- 17.Purohit A, Rokita AG, Guan X, Chen B, Koval OM, Voigt N, Neef S, Sowa T, Gao Z, Luczak ED, Stefansdottir H, Behunin AC, Li N, El Accaoui RN, Yang B, Swaminathan PD, Weiss RM, Wehrens XH, Song LS, Dobrev D, Maier LS, Anderson ME. Oxidized CaMKII Triggers Atrial Fibrillation. Circulation. 2013;128:1748–1757. doi: 10.1161/CIRCULATIONAHA.113.003313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wehrens XH, Lehnart SE, Reiken SR, Marks AR. Ca2+/calmodulin-dependent protein kinase II phosphorylation regulates the cardiac ryanodine receptor. Circ Res. 2004;94:e61–70. doi: 10.1161/01.RES.0000125626.33738.E2. [DOI] [PubMed] [Google Scholar]
- 19.Sood S, Chelu MG, van Oort RJ, Skapura D, Santonastasi M, Dobrev D, Wehrens XH. Intracellular calcium leak due to FKBP12.6 deficiency in mice facilitates the inducibility of atrial fibrillation. Heart Rhythm. 2008;5:1047–1054. doi: 10.1016/j.hrthm.2008.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shan J, Xie W, Betzenhauser M, Reiken S, Chen BX, Wronska A, Marks AR. Calcium leak through ryanodine receptors leads to atrial fibrillation in 3 mouse models of catecholaminergic polymorphic ventricular tachycardia. Circ Res. 2012;111:708–717. doi: 10.1161/CIRCRESAHA.112.273342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Greiser M, Neuberger HR, Harks E, El-Armouche A, Boknik P, de Haan S, Verheyen F, Verheule S, Schmitz W, Ravens U, Nattel S, Allessie MA, Dobrev D, Schotten U. Distinct contractile and molecular differences between two goat models of atrial dysfunction: AV block-induced atrial dilatation and atrial fibrillation. J Mol Cell Cardiol. 2009;46:385–394. doi: 10.1016/j.yjmcc.2008.11.012. [DOI] [PubMed] [Google Scholar]
- 22.El-Armouche A, Boknik P, Eschenhagen T, Carrier L, Knaut M, Ravens U, Dobrev D. Molecular determinants of altered Ca2+ handling in human chronic atrial fibrillation. Circulation. 2006;114:670–680. doi: 10.1161/CIRCULATIONAHA.106.636845. [DOI] [PubMed] [Google Scholar]
- 23.King JH, Zhang Y, Lei M, Grace AA, Huang CL, Fraser JA. Atrial arrhythmia, triggering events and conduction abnormalities in isolated murine RyR2-P2328S hearts. Acta Physiol (Oxf) 2013;207:308–323. doi: 10.1111/apha.12006. [DOI] [PubMed] [Google Scholar]
- 24.Nattel S, Dobrev D. The multidimensional role of calcium in atrial fibrillation pathophysiology: mechanistic insights and therapeutic opportunities. Eur Heart J. 2012;33:1870–1877. doi: 10.1093/eurheartj/ehs079. [DOI] [PubMed] [Google Scholar]
- 25.Qi XY, Yeh YH, Xiao L, Burstein B, Maguy A, Chartier D, Villeneuve LR, Brundel BJ, Dobrev D, Nattel S. Cellular signaling underlying atrial tachycardia remodeling of L-type calcium current. Circ Res. 2008;103:845–854. doi: 10.1161/CIRCRESAHA.108.175463. [DOI] [PubMed] [Google Scholar]
- 26.Luo X, Pan Z, Shan H, Xiao J, Sun X, Wang N, Lin H, Xiao L, Maguy A, Qi XY, Li Y, Gao X, Dong D, Zhang Y, Bai Y, Ai J, Sun L, Lu H, Luo XY, Wang Z, Lu Y, Yang B, Nattel S. MicroRNA-26 governs profibrillatory inward-rectifier potassium current changes in atrial fibrillation. J Clin Invest. 2013;123:1939–1951. doi: 10.1172/JCI62185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pandit SV, Berenfeld O, Anumonwo JM, Zaritski RM, Kneller J, Nattel S, Jalife J. Ionic determinants of functional reentry in a 2-D model of human atrial cells during simulated chronic atrial fibrillation. Biophys J. 2005;88:3806–3821. doi: 10.1529/biophysj.105.060459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wehrens XH, Abriel H, Cabo C, Benhorin J, Kass RS. Arrhythmogenic mechanism of an LQT-3 mutation of the human heart Na(+) channel alpha-subunit: A computational analysis. Circulation. 2000;102:584–590. doi: 10.1161/01.cir.102.5.584. [DOI] [PubMed] [Google Scholar]
- 29.Koval OM, Guan X, Wu Y, Joiner ML, Gao Z, Chen B, Grumbach IM, Luczak ED, Colbran RJ, Song LS, Hund TJ, Mohler PJ, Anderson ME. CaV1.2 beta-subunit coordinates CaMKII- triggered cardiomyocyte death and afterdepolarizations. Proc Natl Acad Sci U S A. 2010;107:4996–5000. doi: 10.1073/pnas.0913760107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yue L, Xie J, Nattel S. Molecular determinants of cardiac fibroblast electrical function and therapeutic implications for atrial fibrillation. Cardiovasc Res. 2011;89:744–753. doi: 10.1093/cvr/cvq329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Camm J. Antiarrhythmic drugs for the maintenance of sinus rhythm: risks and benefits. Int J Cardiol. 2012;155:362–371. doi: 10.1016/j.ijcard.2011.06.012. [DOI] [PubMed] [Google Scholar]
- 32.Savelieva I, Camm J. Anti-arrhythmic drug therapy for atrial fibrillation: current anti-arrhythmic drugs, investigational agents, and innovative approaches. Europace. 2008;10:647–665. doi: 10.1093/europace/eun130. [DOI] [PubMed] [Google Scholar]
- 33.Iwasaki YK, Nishida K, Kato T, Nattel S. Atrial fibrillation pathophysiology: implications for management. Circulation. 2011;124:2264–2274. doi: 10.1161/CIRCULATIONAHA.111.019893. [DOI] [PubMed] [Google Scholar]
- 34.Voigt N, Heijman J, Wang Q, Chiang DY, Li N, Karck M, Wehrens XH, Nattel S, Dobrev D. Cellular and Molecular Mechanisms of Atrial Arrhythmogenesis in Patients With Paroxysmal Atrial Fibrillation. Circulation. 2013 Nov 18; doi: 10.1161/CIRCULATIONAHA.113.006641. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.