Abstract
Firefly luciferase is the most widely used optical reporter for noninvasive bioluminescence imaging (BLI) in rodents. BLI relies on the ability of the injected luciferase substrate D-luciferin to access luciferase-expressing cells and tissues within the animal. Here we show that injection of mice with a synthetic luciferin, CycLuc1, improves BLI from existing luciferase reporters and enables imaging in the brain that could not be achieved with D-luciferin.
Bioluminescence imaging (BLI) with firefly luciferase is a powerful and popular method to noninvasively visualize molecular and cellular features in live mice1–3. Firefly luciferase chemically produces light from its small molecule substrate, D-luciferin. Introduction of these components into cells or whole animals produces light that can be captured by sensitive detectors. The inherent simplicity of this imaging method has led to its ubiquitous adoption for in vivo monitoring of numerous disease states and cellular functions1–3. Improvements to the sensitivity and dynamic range of BLI are therefore of immediate and general utility for a wide variety of applications.
Here we show that BLI with an alternative luciferase substrate, CycLuc1 (Figure 1), greatly improves the sensitivity of this optical imaging technique. We find that CycLuc1 exhibits superior properties to D-luciferin in vivo, requiring less substrate for imaging and providing more intense and persistent light output. Moreover, this substrate enabled imaging of luciferase-expressing cells in the brains of live mice that could not be observed with D-luciferin. Thus, using CycLuc1 in place of D-luciferin expands the scope of bioluminescence imaging with existing luciferase-expressing reporters.
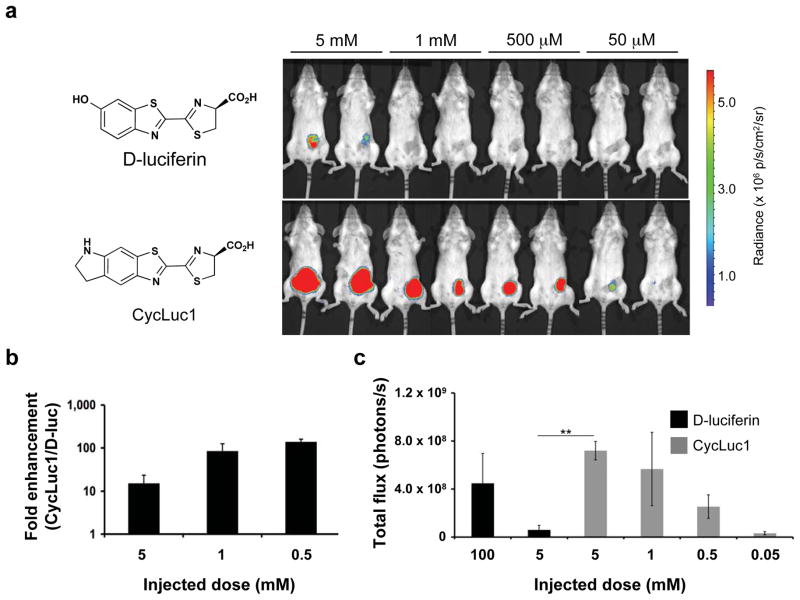
Figure 1.
Comparative BLI of tumor xenografts with D-luciferin and CycLuc1. (a) Photon flux from BALB/c mice harboring 4T1-luc2 tumors. Mice were injected i.p. with the indicated dose of D-luciferin or CycLuc1 and imaged 10 minutes post-injection. All images are plotted on the same scale. Photon intensities are shown in units of photons/s/cm2/steradian and quantified in (b). (c) Quantified light output from 4T1-luc2 cells in BALB/c mice (n = 3) injected i.p. with 100 μl of 100 mM D-luciferin (standard BLI conditions), 5 mM D-luciferin, or the indicated dose of CycLuc1. **P <0.01, (t-test).
Heretofore, efforts to improve the sensitivity of in vivo BLI have largely focused on improving the expression levels of firefly luciferase4,5 or identifying mutations that red-shift the emitted light to wavelengths that more readily penetrate through tissues6. Much less attention has been focused on modulating the properties of the requisite small molecule luciferin, despite the importance of its cell permeability and pharmacokinetic properties in vivo. In standard BLI, relatively high concentrations of D-luciferin are required: 150 mg/kg is the typical dose for routine i.p. injections, which equates to 0.1 ml of 100 mM D-luciferin for an average 20 g mouse. D-Luciferin is also only modestly cell-permeable7,8 and experiments with radiolabeled substrate have shown that its tissue distribution is not homogeneous and that uptake into some organs, such as the brain, is low9. Thus, while D-luciferin yields high light output in vitro and performs well in vivo, it may not be the optimal substrate for imaging in the mouse.
No synthetic luciferin has yet shown improvement over the standard imaging conditions with D-luciferin in live mice, which likely reflects issues of cell permeability7,8,10,11, poor biodistribution10,11, reduced efficiency of light emission, and/or no net increase in red light (tissue-penetrating) emission over D-luciferin12. Recently, a variety of new substrates for firefly luciferase have been reported13,14, including substrates with alkylated 6′-amino groups.15,16 All of these luciferin analogs yield substantially lower levels of light emission than D-luciferin with the wild-type firefly luciferase in vitro10,15,16. However, some substrates, such as the cyclic alkylaminoluciferin CycLuc1, exhibit improved light output in live cells relative to D-luciferin when compared at low substrate concentrations (e.g., < 10 μM)7. Although D-luciferin yields superior photon flux from live cells when supplied at higher concentrations, we hypothesized that CycLuc1 could perform relatively well in vivo if the actual intracellular concentration of luciferin achieved in the mouse is limiting.
To evaluate CycLuc1 in vivo, we first tested its ability to image well-established mouse xenograft tumor models. We implanted 4T1-luc2 luciferase-expressing breast cancer cells into the right mammary fat pads of BALB/c mice, and imaged the mice eight days post-engraftment. Intraperitoneal injection of CycLuc1 yielded a >10-fold higher bioluminescent signal than could be obtained from D-luciferin injection at equivalent doses (Fig. 1 and Supplementary Fig. 1). Surprisingly, a 20–200 fold lower dose of CycLuc1 yielded the same peak photon flux as the standard imaging conditions of 150 mg/kg D-luciferin (Fig. 1c and Supplementary Fig. 2). CycLuc1 doses up to 2000-fold lower than the standard D-luciferin imaging conditions could be imaged, while equivalent concentrations of D-luciferin provided either weak or no signal (Fig. 1). One hour after injection, the relative light emission from CycLuc1 was even more pronounced, primarily due to a ten-fold drop in D-luciferin light emission over this period (Supplementary Fig. 1). The improved performance of CycLuc1 also translated across different cell types, mouse strains, and implantation sites. Photon flux from luciferase-expressing DB7 breast cancer cells (DB7-luc) implanted in the hind flanks of FVB mice was up to 40-fold higher with CycLuc1 versus D-luciferin administration at an equivalent dose (Supplementary Fig. 3). CycLuc1 also enabled detection of luciferase-expressing CMT-64 lung cancer cells implanted into the hind flank of C57BL/6 mice, one of the most widely used mouse strains, providing increased signal intensity at all doses examined (Supplementary Fig. 4).
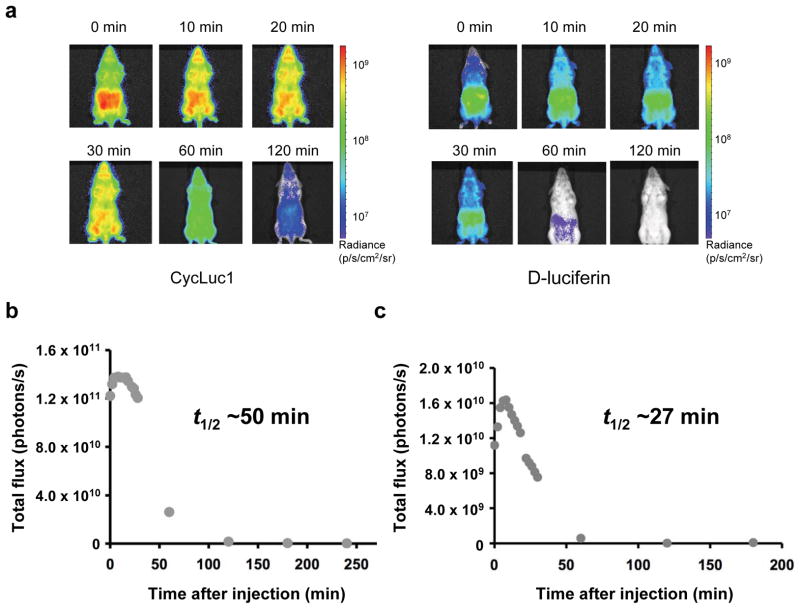
Next, to compare the distribution and trafficking of CycLuc1 and D-luciferin in vivo, we utilized a transgenic mouse that expresses luciferase in most tissues (L2G85-FVB)17. Consistent with previous studies, intraperitoneal injection of D-luciferin revealed access to tissues throughout the mouse, albeit with the highest photon flux confined to the abdominal cavity near the site of injection (Fig. 2)8. In contrast, i.p. injection of CycLuc1 revealed a bioluminescent signal that was ten-fold higher in intensity and escaped from the abdominal cavity to give a broad distribution that was maintained for up to two hours (Fig. 2). Comparison of intravenous injections was even more striking: CycLuc1 signal peaked at 4–5 min post-injection and then yielded a steady signal that was up to 100-fold greater than that of D-luciferin, and persisted for more than 60 min (Supplementary Figs. 5–6). By contrast, with D-luciferin the signal peaked immediately after injection, and dissipated over 20 min (Supplementary Figs. 5–6).
Figure 2.
Comparison of D-luciferin and CycLuc1 in luciferase-expressing transgenic mice. (a) Photon flux from L2G85-FVB mice injected i.p. with 100 μl of 5 mM CycLuc1 (left) or D-luciferin (right) and imaged over time. Photon intensities are shown in units of photons/s/cm2/steradian and plotted on logarithmic scales. (b)-(c) Total photon output from L2G85-FVB luc mice treated with (b) CycLuc1 or (c) D-luciferin from above. The apparent bioluminescent half-lives are shown. Data are representative of three independent experiments.
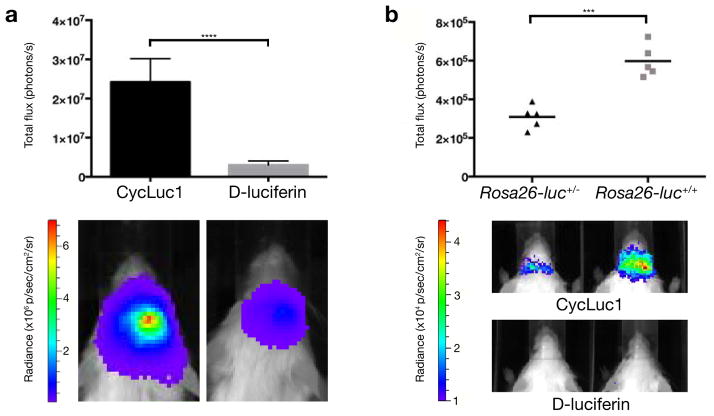
In light of these exciting results, we next asked whether CycLuc1 could improve bioluminescent signals in the brain. The blood-brain barrier limits the access of many small molecules to this tissue, and thus imaging in the brain is particularly challenging. To evaluate the ability of CycLuc1 to access brain tissue, we imaged mice that had been treated with adeno-associated virus 9 (AAV9)18 to express codon-optimized luc2 luciferase in the brain striatum. We found that i.p. injection of CycLuc1 provided an 8.1 ± 1.5 fold higher signal than the standard 150 mg/kg D-luciferin, despite a 20-fold lower imaging dose of CycLuc1 (Fig. 3 and Supplementary Fig. 7). Thus, CycLuc1 can readily cross the blood-brain barrier and access deep brain tissues. Next, we crossed Dat (also known as Slc6a3) Cre driver mice with Rosa26 floxed-stop luciferase reporter mice19. Dat-Cre drives reporter expression at low levels from the endogenous Rosa26 promoter in dopaminergic neurons, primarily in the substantia nigra, one of the deepest brain tissues20. Injection of these mice with D-luciferin failed to yield any measurable brain signal in vivo (Fig. 3 and Supplementary Fig. 8). In sharp contrast, we found that CycLuc1 not only enabled imaging in these same mice but that homozygous mice with two copies of the Rosa26-luc allele could be readily distinguished from heterozygous mice by the 1.9 ± 0.2 fold higher photon flux (Fig. 3 and Supplementary Fig. 8).
Figure 3.
Comparison of D-luciferin and CycLuc1 in the brain. (a) Photon flux from mice expressing AAV9-CMV-luc2 in the brain striatum ten minutes after i.p. injection with 100 μl of 5 mM CycLuc1 or 100 mM D-luciferin (n = 5). Error bars are S.E.M. ****P < 0.0001 (t-test). (b) Photon flux from Dat-Cre+/− Rosa26-luc+/− (n = 5) and Dat-Cre+/− Rosa26-luc+/+ (n = 5) mice after i.p. injection with 100 μl of 5 mM CycLuc1. ***P < 0.001 (t-test). No quantifiable photon flux was observed from the brain after i.p. injection with 100 μl of 100 mM D-luciferin.
The improved sensitivity of BLI with CycLuc1 has immediate ramifications for biological studies in mice. Simply replacing the obligatory injection of D-luciferin with CycLuc1 improves the sensitivity of bioluminescent detection, while retaining the use of existing luciferase reporters. CycLuc1 reduces the amount of substrate required for BLI and allows imaging at low doses where D-luciferin provides weak or no signal. Furthermore, CycLuc1 allows detection of low-level luciferase expression in deep brain tissues that cannot be detected with D-luciferin, and thus opens up new applications for noninvasive imaging in the brain. One potential contributor to the improved in vivo performance of CycLuc1 is a red-shift in the emitted photons to more tissue-penetrating wavelengths (Supplementary Fig. 9)7,15. However, the percentage of Cy5.5-filtered flux from the brains of live AAV-treated mice is actually slightly higher for D-luciferin than CycLuc1 (9.4% vs. 8.1%; Supplementary Fig. 10), perhaps reflecting the red-shift in luciferase emission previously reported at 37 °C in vivo12. Moreover, a change in emission wavelength alone cannot explain the differences we observed in tissue distribution, signal persistence, or the ability to image at very low substrate concentrations. In cell culture, the luciferase-expressing tumor cells 4T1, DB7, and CMT-64 yield higher bioluminescent signals with D-luciferin, except at low substrate concentrations where CycLuc1 is favored (Supplementary Fig. 9). In vivo these same cells yield greater photon flux with CycLuc1, even in tumors that are located near the surface and/or proximal to the site of substrate injection. This suggests that the delivery of D-luciferin to luciferase-expressing cells in vivo is limiting, and that the cell permeability, lower Km7, and bioavailability of CycLuc1 play important roles in its superior in vivo performance.
In conclusion, CycLuc1 improves in vivo BLI using existing luciferase reporters, yet requires much less substrate for imaging. Transgenic luciferase-expressing mice treated with CycLuc1 demonstrated that the analog has broad access to mouse tissues, and more persistent light emission than with D-luciferin by either i.p. or i.v. injection. In the brain, CycLuc1 provided stronger BLI signals than D-luciferin, and even enabled detection of luciferase expression that could not be imaged with D-luciferin. Based on these results, CycLuc1 can be recommended for immediate use in BLI, while future adaptation of related synthetic luciferins and mutant luciferases7 is expected to allow even greater improvements in the sensitivity, selectivity, and scope of in vivo bioluminescent reporters.
ONLINE METHODS
General methods
D-Luciferin and CycLuc1 were synthesized as previously described13,15. Luciferase-expressing 4T1 cells, CMT-64 cells, and DB7 cells were provided by the Contag laboratory (Stanford University).
Mice
Pathogen-free BALB/c, FVB/N, C57BL/6, and luciferase-expressing transgenic mice (FVB-Tg(CAG-luc,-GFP)L2G85Chco/FathJ)17 were obtained from either Charles River or the Jackson Laboratory and housed in UC Irvine’s AAALAC-accredited animal-care facility. All mice used were littermates or age-matched (6–12 weeks of age) females, were provided access to food and water ad libitum, and housed in the animal facilities at UC Irvine. All procedures with these mice were approved by the Animal Care and Use Committee at UC Irvine (protocol #2011-2987 to J.A.P.). FVB/NJ, B6.SJL-Slc6a3tm1.1(cre)Bkmn/J (“Dat-Cre”)21, and FVB.129S6(B6)-Gt (ROSA)26Sortm1(Luc)Kael/J (“Rosa26-luc”)19 mice were purchased from the Jackson Laboratory and were maintained and used according to the guidelines of the Institutional Animal Care and Use Committee of the University of Massachusetts Medical School (docket #A978-12 to N.A.). Dat-Cre mice were mated with floxed-stop Rosa26-luc mice to generate white Dat-Cre+/− Rosa26-luc+/− and Dat-Cre+/− Rosa26-luc+/+ mice. Offspring were genotyped by PCR.
Tumor cell inoculations
Mice were inoculated subcutaneously with 1 × 106 luciferase-expressing CMT-64 or DB7 cells in serum-free RPMI media. Tumors were allowed to establish for 1–2 weeks before imaging. To establish 4T1 tumors, aliquots of the cells were mixed 1:1 by volume with Matrigel (usually 5 × 105 cells in 50 μl serum-free DMEM media plus 50 μl Matrigel per injection) and transplanted into the orthotopic second or fourth mammary fat pads (left or right side) of mice. Eight days post-engraftment, the mice were injected i.p. with 100 μl of either D-luciferin or CycLuc1 (50 μM – 5 mM), and imaged ten minutes post-injection.
Bioluminescence imaging
To image tumor cells in vivo, mice were injected i.p. with 100 μl of luciferin stocks (in PBS). The animals were anesthetized with isoflurane (2% in 1 l/min oxygen), and bioluminescence images were acquired using the IVIS Lumina® system (a Xenogen Product from Caliper Life Sciences, now Perkin-Elmer). Images were acquired every 2 min for 30 min (10 s exposure/image). The mice were also imaged 1 h post-injection and 2 h post-injection (10 s exposure/image). Images were analyzed using Living Image software. Regions of interest (ROIs) were drawn around each cell mass, and the total number of photons within each ROI were recorded. ROI size was held constant across all images. Prior to imaging with CycLuc1, the mice were stratified using standard BLI conditions (100 μl i.p. injection of 100 mM D-luciferin in PBS).
To analyze the biodistribution of D-luciferin and CycLuc1 in vivo, the compounds were injected (100 μl of 5 mM solutions in PBS) i.p. or i.v. into luciferase-expressing FVB transgenic mice. The mice were imaged over time as described above, and quantitative analyses of the light emission were performed using Living Image software as above.
Construction of AAV-CMV-luc2
Codon-optimized luc2 luciferase from pGL4 (Promega) was cloned into the EcoRI-SalI sites of a pAAV-CMV vector (gift of Guangping Gao). The plasmid was packaged into AAV serotype 9 by the University of Massachusetts Medical School Viral Vector Core.
AAV9-CMV-luc2 vector striatal injections
FVB/NJ mice (5 females, 6 weeks old, Jackson Laboratory, Bar Harbor, ME) were anesthetized with 250 mg/kg tribromoethanol prior to surgery, placed on a stereotactic frame and injected with 0.25 μL 1e13 GC AAV9-CMV-Luc2 by micropump syringe (NanoFil, World Precision Instruments) at the lateral edge of the striatum/cortex border (anterior 1 mm, lateral 3 mm and ventral 2 mm from bregma). Imaging was performed at least two weeks after AAV injection.
Brain imaging
Bioluminescence imaging luciferase-expressing mice was performed as described above in the University of Massachusetts Medical School Small Animal Imaging Core using an IVIS 100 imaging system and analyzed with Living Image software and GraphPad Prism 6.
Supplementary Material
Acknowledgments
This work was supported by grants from the US National Institutes of Health (R01EB013270 to S.C.M. and NS38194 to N.A.), Cure Huntington’s Disease Initiative (CHDI) to N.A., American Cancer Society (IRG-98-279-07 to J.A.P.), and the University of California, Irvine School of Physical Sciences (J.A.P.).
Footnotes
Author Contributions
M.S.E. and J.A.P. imaged tumors and L2G85-FVB mice, G.R.R. synthesized CycLuc1, M.A.P. imaged cultured cells, J.P.C. bred Dat mice, S.T.A. and J.P.C. imaged AAV9 and Dat mice, N.A. contributed to the development of AAV9 and Dat mouse models and manuscript editing, S.C.M. designed CycLuc1, S.C.M. and J.A.P. led the study and wrote the manuscript.
Competing Financial Interests
UMass Medical School holds patents on luciferin substrates (US7910087 and US8216550).
Note: Supplementary material is available in the online version of the paper.
References
- 1.Contag CH, Bachmann MH. Annu Rev Biomed Eng. 2002;4:235–60. doi: 10.1146/annurev.bioeng.4.111901.093336. [DOI] [PubMed] [Google Scholar]
- 2.Dothager RS, et al. Curr Opin Biotechnol. 2009;20:45–53. doi: 10.1016/j.copbio.2009.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Prescher JA, Contag CH. Curr Op Chem Biol. 2010;14:80–89. doi: 10.1016/j.cbpa.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 4.Rabinovich BA, et al. Proc Natl Acad Sci USA. 2008;105:14342–14346. doi: 10.1073/pnas.0804105105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim JB, et al. PLoS ONE. 2010;5:e9364. doi: 10.1371/journal.pone.0009364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mezzanotte L, et al. Mol Imaging Biol. 2010;12:406–414. doi: 10.1007/s11307-009-0291-3. [DOI] [PubMed] [Google Scholar]
- 7.Harwood KR, Mofford DM, Reddy GR, Miller SC. Chem Biol. 2011;18:1649–1657. doi: 10.1016/j.chembiol.2011.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shinde R, Perkins J, Contag CH. Biochemistry. 2006;45:11103–12. doi: 10.1021/bi060475o. [DOI] [PubMed] [Google Scholar]
- 9.Berger F, Paulmurugan R, Bhaumik S, Gambhir SS. Eur J Nucl Med Mol Imaging. 2008;35:2275–2285. doi: 10.1007/s00259-008-0870-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kojima R, et al. Angew Chem Int Ed Engl. 2013;52:1175–1179. doi: 10.1002/anie.201205151. [DOI] [PubMed] [Google Scholar]
- 11.Conley NR, Dragulescu-Andrasi A, Rao J, Moerner WE. Angew Chem Int Ed. 2012;51:3350–3353. doi: 10.1002/anie.201105653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhao H, et al. J Biomed Opt. 2005;10:41210. doi: 10.1117/1.2032388. [DOI] [PubMed] [Google Scholar]
- 13.McCutcheon DC, Paley MA, Steinhardt RC, Prescher JA. J Am Chem Soc. 2012;134:7604–7607. doi: 10.1021/ja301493d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Woodroofe CC, et al. Biochemistry. 2012;51:9807–9813. doi: 10.1021/bi301411d. [DOI] [PubMed] [Google Scholar]
- 15.Reddy GR, Thompson WC, Miller SC. J Am Chem Soc. 2010;132:13586–13587. doi: 10.1021/ja104525m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Woodroofe CC, et al. Biochemistry. 2008;47:10383–10393. doi: 10.1021/bi800505u. [DOI] [PubMed] [Google Scholar]
- 17.Cao YA, et al. Proc Natl Acad Sci USA. 2004;101:221–226. [Google Scholar]
- 18.Zhang H, et al. Mol Ther. 2011;19:1440–1448. doi: 10.1038/mt.2011.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Safran M, et al. Mol Imaging. 2003;2:297–302. doi: 10.1162/15353500200303154. [DOI] [PubMed] [Google Scholar]
- 20.Madisen L, et al. Nat Neurosci. 2010;13:133–140. doi: 10.1038/nn.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bäckman CM, et al. genesis. 2006;44:383–390. doi: 10.1002/dvg.20228. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.