Abstract
The serum and glucocorticoid inducible-kinase-1 (SGK1) is expressed following cell stress and exposure to a variety of hormones including gluco- and mineralocorticoids. It is activated by insulin and growth factors via phosphatidylinositol-3-kinase and the 3-phosphoinositide dependent kinase PDK1. SGK1 enhances the activity of a variety of ion channels, such as ENaC, TRPV5, ROMK, KCNE1/KCNQ1 and ClCKb, carriers, such as NHE3, NKCC2, NCC and SGLT1, as well as the Na+/K+-ATPase. SGK1 contributes to Na+ retention and K+ elimination of the kidney as well as mineralocorticoid stimulation of salt appetite. A certain SGK1 gene variant (combined polymorphisms in intron 6 [I6CC] and in exon 8 [E8CC/CT]) is associated with moderately enhanced blood pressure. The SGK1 gene variant has been shown to affect 3–5 % of Caucasians and some 10% of Africans. The gene variant sensitizes the carriers to the hypertensive effects of hyperinsulinemia. Moreover, the SGK1 gene variant is associated with increased body mass index, presumably a result of enhanced SGLT1 activity with accelerated intestinal glucose absorption. Obesity predisposes the carriers of the gene variant to development of type II diabetes. Moreover, SGK1 stimulates coagulation. Thus, SGK1 may participate in the pathogenesis of metabolic syndrome or syndrome X, a condition characterized by the coincidence of essential hypertension, procoagulant state, obesity, insulin resistance and hyperinsulinemia.
Keywords: blood pressure, obesity, fibrosis, inflammation, coagulation
Introduction
The serum- and glucocorticoid-inducible kinase 1 (SGK1) was originally cloned as an immediate early gene transcriptionally stimulated by serum and glucocorticoids in rat mammary tumor cells (1). The human isoform has been discovered as a gene upregulated by cell shrinkage (2). Compelling evidence points to a significant role of SGK1 in the pathophysiology of hypertension. The present brief review thus describes the physiological and pathophysiological impact of SGK1. Due to limited space, the citations had to be restricted and the reader may study a more comprehensive review (3) for more detailed information and earlier publications.
Regulation of SGK1 transcription and activity
As reviewed elsewhere (3, 4), SGK1 transcription is stimulated by a wide variety of factors (4) including glucocorticoids, mineralocorticoids. 1,25-dyhydroxyvitamin D3 (1,25(OH)2D3), transforming growth factor β (TGFβ), interleukin 6 fibroblast and platelet-derived growth factor, thrombin, endothelin, advanced glycation end products (AGE) and activation of peroxisome proliferator-activated receptor PPARγ. Distinct translational SGK1 isoforms differ in regulation of expression, subcellular localization and function (3, 4).
SGK1 is expressed in a wide variety of organs (3) including the kidney. The subcellular localisation of SGK1 presumably depends on the functional state. Cellular exposure to serum triggers importin-alpha mediated entry of SGK1 into the nucleus (3) whereas activation by hyperosmotic shock or glucocorticoids increases the cytosolic fraction of the kinase (1). SGK1 has further been localized to the mitochondrial membrane (3, 4). Expressed SGK1 is activated by the phosphatidylinositol-3-kinase (PI3-kinase) pathway involving 3-phosphoinositide (PIP3)-dependent kinase PDK1 and the mammalian target of rapamycin complex 2 TORC2, as well as the serine/threonine kinase WNK1 (with no lysine kinase 1) (3, 4). The PI3-kinase pathway and thus SGK1 is activated by insulin and several growth factors including IGF1 and hepatic growth factor (HGF) (3). Signaling involved in activation of SGK1 further includes bone marrow kinase/extracellular signal-regulated kinase 5 (BK/ERK5) or p38α, increase of cytosolic Ca2+ activity with subsequent activation of calmodulin-dependent protein kinase kinase (CaMKK) and the small G-protein Rac1 (3). SGK1 is ubiquitinated by the ubiquitin ligase Nedd4-2 (neuronal precursor cells expressed developmentally downregulated) (3, 4).
Molecular targets of SGK1
SGK1 and related kinases (3) phosphorylate target proteins at the consensus sequence R-X-R-X-X-(S/T)-phi (X = any amino acid, R = arginine, phi = hydrophobic amino acid). The only exclusive targets of SGK1 thus far identified are the N-myc downregulated genes NDRG1 and NDRG2 (3, 4).
SGK1 regulates a wide variety of channels, including the renal epithelial Na+ channel ENaC (Fig. 1), the renal outer medullary K+ channel ROMK1, the Ca2+ channels TRPV5,6, the Cl− channels ClCKa/barttin, ClC2, CFTR, the volume-sensitive osmolyte and anion channel VSOAC, the Na+ channel SCN5A, the K+ channels KCNE1/KCNQ1, KCNQ4, Kv1.3, Kv1.5, Kv4.3, the acid sensing ion channel ASIC1, the glutamate receptor GluR6, and the cation channel 4F2/LAT (3–6). SGK1 stimulates the NaCl cotransporter NCC, the Na+,K+,2Cl− cotransporter NKCC2, the Na+/H+ exchanger NHE3, the glucose transporters SGLT1 (Fig. 1), GLUT1, GLUT4, the amino acid transporters ASCT2, SN1, EAAT1, EAAT2, EAAT3, EAAT4, EAAT5, the peptide transporters PepT1/2, the Na+, dicarboxylate cotransporter NaDC-1, the creatine transporter CreaT, the Na+, myoinositol cotransporter SMIT, the phosphate transporter NaPiIIb, and the Na+/K+-ATPase (3, 4, 7–9).
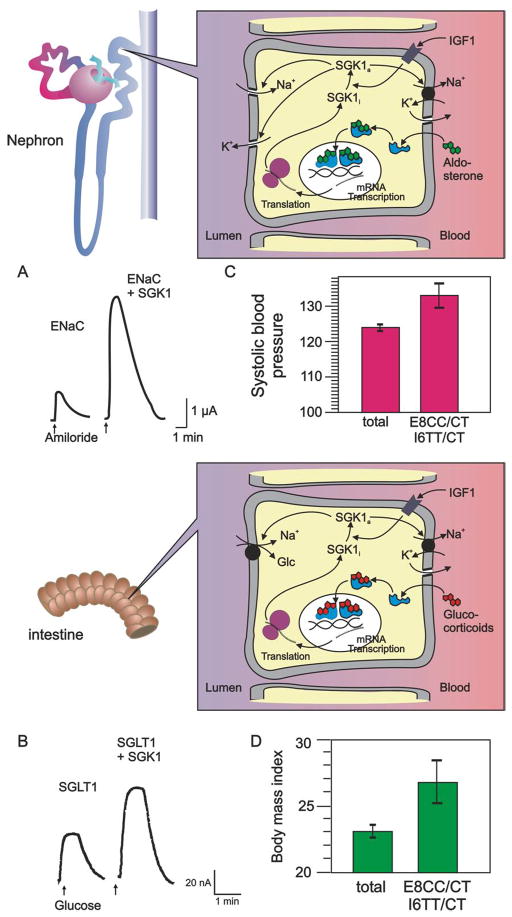
Fig. 1. Role of SGK1 in the stimulation of renal epithelial Na+ channel ENaC by aldosterone and intestinal glucose transporter SGLT1 by glucocorticoids.
Aldosterone and glucocorticoids stimulate transcription of SGK1. The kinase is activated by insulin like growth factor IGF1. Active SGK1 stimulates ENaC in the kidney and SGLT1 in intestine. In both tissues it stimulates the Na+/K+-ATPase. Pertinent data are shown in A–D. Coexpression of SGK1 stimulates amiloride sensitive currents, reflecting ENaC activity (A) and glucose induced currents reflecting SGLT1 activity (B) following heterologous expression. Individuals carrying certain polymorphisms of the SGK1 gene (E8CC/CT I6TT/CT) show significantly higher blood pressure (C) and body mass index (D) than the unselected population (total).
SGK1 regulates channels and carriers in part by direct phosphorylation of the target proteins (3). SGK1 further phosphorylates the ubiquitin ligase Nedd4-2 (3), which otherwise ubiquitinates channels and transport proteins leading to their subsequent clearance from the cell membrane (3). Phosphorylated Nedd4-2 binds to 14-3-3, and thus cannot ubiquitinate its target proteins (3, 4). SGK1 further phosphorylates WNK4, a kinase inhibiting ENaC activity (3, 4). SGK1 inhibits inducible nitric oxide synthase, thus abrogating the inhibitory effect of NO on ENaC (3, 4). SGK1 is further effective through activation of the phosphatidylinositol-3-phosphate-5-kinase PIKfyve and subsequent formation of PIP2 (3, 4). SGK1 may also influence channel or carrier expression (3, 4). The SGK1 dependent regulation of ENaC is in part due to impaired SGK1-mediated phosphorylation of Af9, which upregulates transcription and expression of ENaCα and thus ENaC activity (3, 4).
SGK1 dependent regulation of extrarenal functions
As reviewed elsewhere (3, 4), a wide variety of SGK1 sensitive functions have been described, including cell volume regulation, cell survival, cell proliferation, coagulation, insulin dependent cellular K+ uptake, glucose metabolism, aldosterone release, insulin release, mast cell degranulation, cardiac excitation, function of decidualizing cells, gastric acid secretion, intestinal transport, memory consolidation and pain perception. SGK1 participates in the regulation of salt intake (10). Accordingly, mineralocorticoids stimulate salt intake in sgk1+/+ but not in sgk1−/− mice (10). SGK1 participates in the pathophysiology of metabolic syndrome (see below), allergy, pulmonary hypertension, cardiac hypertrophy, tumor growth and metastasis as well as inflammation and fibrosis (3, 4).
SGK1 dependent regulation of renal function
SGK1 contributes to the stimulation of renal Na+ excretion by aldosterone, insulin and IGF1 (3, 4, 11). Part of the effect of aldosterone on ENaC is independent of SGK1 and effects of aldosterone and SGK1 are in part additive. In contrast, the effects of antidiuretic hormone (ADH) or insulin are fully dependent on SGK1 (12). In SGK1-knockout (sgk1−/−) mice the plasma aldosterone levels are enhanced and the enhanced mineralocorticoid action compensates for the lack of SGK1 leading to seemingly normal renal salt reabsorption during salt replete diet (8, 13). However, in sgk1−/− mice renal salt excretion cannot be sufficiently decreased during salt depleted diet: Renal salt excretion remains inadequately high in sgk1−/− mice, despite an increase of plasma aldosterone concentration, decrease of arterial blood pressure, decrease of glomerular filtration rate and enhanced proximal tubular Na+ reabsorption (3, 13). The renal salt loss of sgk1−/− mice is presumably at least in part due to decreased upregulation and phosphorylation of NCC and thus distal tubular NaCl cotransport (8, 9, 14). The hyperaldosteronism in sgk1−/− mice may under some conditions and possibly due to SGK1-independent effects of aldosterone, result in even enhanced ENaC activity in sgk1−/− mice (8). Similarly, despite the lack of SGK1 the colonic ENaC activity is enhanced in sgk1−/− mice, which is again thought to result from hyperaldosteronism (15). Inhibition of ENaC by triamterene leads to eventually lethal salt loss in sgk1−/− but not in sgk1+/+ mice, an observation again pointing to a renal transport defect other than ENaC in sgk1−/− mice (16). The presence of SGK1 is a prerequisite for the antinatriuretic effect of insulin, which is significantly blunted in sgk1−/− mice (17).
SGK1 further participates in the regulation of renal K+ excretion. The sgk1−/− mice do not adequately increase the renal K+ excretion following an acute K+ load (18) and develop excessive hyperkalemia following a chronic K+ load (18).
Despite decreased TRPV5 expression, the renal Ca2+ excretion is decreased in sgk1−/− mice (19). The anticalciuria is presumably the result of extracellular volume contraction following renal salt loss due to impaired stimulation of NaCl cotransport and ENaC (3). The volume contraction enhances proximal tubular Na+ and Ca2+ reabsorption, thus decreasing renal Ca2+ excretion. Pharmacological inhibition of NaCl cotransport by thiazide diuretics similarly lead to anticalciuria (20), an effect involving upregulation of proximal tubular Ca2+ reabsorption (21).
Proximal tubular SGK1 expression is low in normal kidneys (3) and SGK1 presumably does not participate in the regulation of proximal tubular transport. Hyperglycemia, however, enhances proximal tubular expression of SGK1, which in turn stimulates renal tubular glucose transport (22).
SGK1 expression in glomerular podocytes (23, 24) is stimulated by aldosterone and oxidative stress (24). The proteinuria following DOCA treatment is significantly blunted in SGK1 knockout mice (25). However, the sgk1−/− mice are not protected against doxorubicin induced glomerular injury (26). SGK1-dependent renal salt retention contributes to the development of edema during treatment with PPARγ agonists (27) or in nephrotic syndrome (26). Moreover, enhanced SGK1 expression was found during ascites formation in cirrhotic rats (28).
SGK1 sensitive hypertension and metabolic syndrome
Owing to its influence on renal salt excretion and salt intake SGK1 is expected to participate in blood pressure control. Hyperinsulinemia by pretreatment with a high-fructose diet (17) or a high fat diet (29) indeed sensitizes arterial blood pressure to high-salt intake in sgk1+/+ but not in sgk1−/− mice. In accordance, the antinatriuretic effect of insulin observed in sgk1+/+ is blunted in sgk1−/− mice (17). Moreover, SGK1 plays a critical role in the hypertensive effects of glucocorticoids (30). In humans a certain variant of the SGK1 gene (the combined presence of distinct polymorphisms in intron 6 [I6CC] and in exon 8 [E8CC/CT]) is associated with moderately enhanced blood pressure (3). The gene variant affects some 3–5 % of Caucasians (3) and some 10% of Africans (31). The gene variant is particularly associated with insulin-sensitivity of blood pressure increase (32).
The same SGK1 gene variant is associated with increased body mass index (3). Accordingly, the SGK1 gene variant is more prevalent in patients with type 2 diabetes than in individuals without family history of diabetes (31). The impact of SGK1 on body weight results presumably from the stimulating effect of SGK1 on intestinal glucose absorption, which predisposes to obesity (3).
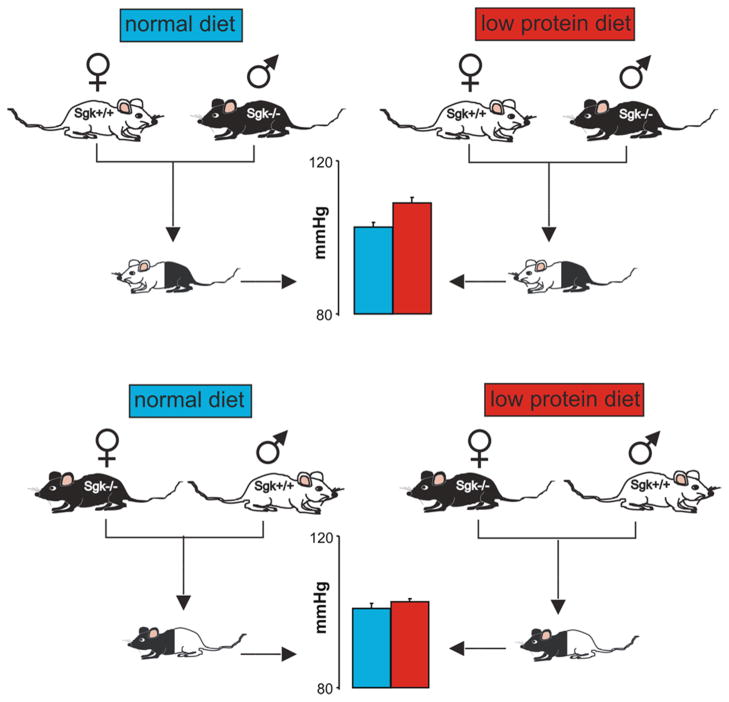
Hypertension, obesity and susceptibility to develop type II diabetes are hallmarks of metabolic syndrome, a condition associated with enhanced morbidity and mortality from cardiovascular disease (3). Metabolic syndrome is associated with enhanced coagulation (3), which is again stimulated by SGK1 (33). Accordingly, SGK1 presumably participates in the pathophysiology of metabolic syndrome. Moreover, maternal SGK1 contributes to fetal programming of hypertension (Fig 2). Protein deficient diet during pregnancy leads to increased blood pressure in offspring only, if the mother expresses SGK1 (34).
Fig. 2. Role of maternal SGK1 in fetal programming of blood pressure.
To test for the role of maternal SGK1 in fetal programming of blood pressure, female SGK1 deficient (sgk1−/−) mice were mated with male wild type (sgk1+/+) mice and female sgk1+/+ mice were mated with male sgk1−/− mice. Accordingly, in both cases the offspring was heterocygous. The offspring of sgk1+/+ mothers (closed bars), but not the offspring of sgk1−/− mothers (open bars) developped hypertension, if the mothers were exposed to low protein diet. Thus maternal lack of SGK1 protected against the fetal programming by maternal low protein diet (34).
Conclusions
SGK1 participates in the regulation of renal tubular Na+ reabsorption and intestinal glucose absorption. The kinase thus contributes to blood pressure and body weight control. Accordingly, deranged SGK1 activity could lead to metabolic syndrome.
Footnotes
Disclosure:
The study in the laboratories of the authors is funded by the Deutsche Forschungsgemeinschaft (DFG to F.L.) and National Institutes of Health (NIH-RO1HL, Paul, Roland, NIH-R01DK56248 and NIH-P30DK079337 to V.V.). The authors declare that they have no conflict of interest and has been seen and approved by all authors and that it is not under consideration for publication elsewhere.
References
- 1.Firestone GL, Giampaolo JR, O’Keeffe BA. Stimulus-dependent regulation of the serum and glucocorticoid inducible protein kinase (Sgk) transcription, subcellular localization and enzymatic activity. Cell Physiol Biochem. 2003;13:1–12. doi: 10.1159/000070244. [DOI] [PubMed] [Google Scholar]
- 2.Waldegger S, Barth P, Raber G, et al. Cloning and characterization of a putative human serine/threonine protein kinase transcriptionally modified during anisotonic and isotonic alterations of cell volume. Proc Natl Acad Sci U S A. 1997;94:4440–4445. doi: 10.1073/pnas.94.9.4440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lang F, Bohmer C, Palmada M, et al. (Patho)physiological significance of the serum- and glucocorticoid-inducible kinase isoforms. Physiol Rev. 2006;86:1151–1178. doi: 10.1152/physrev.00050.2005. [DOI] [PubMed] [Google Scholar]
- 4.Lang F, Artunc F, Vallon V. The physiological impact of the serum and glucocorticoid-inducible kinase SGK1. Curr Opin Nephrol Hypertens. 2009;18:439–448. doi: 10.1097/MNH.0b013e32832f125e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boehmer C, Laufer J, Jeyaraj S, et al. Modulation of the voltage-gated potassium channel Kv1.5 by the SGK1 protein kinase involves inhibition of channel ubiquitination. Cell Physiol Biochem. 2008;22:591–600. doi: 10.1159/000185543. [DOI] [PubMed] [Google Scholar]
- 6.Shaw JR, Sato JD, VanderHeide J, et al. The role of SGK and CFTR in acute adaptation to seawater in Fundulus heteroclitus. Cell Physiol Biochem. 2008;22:69–78. doi: 10.1159/000149784. [DOI] [PubMed] [Google Scholar]
- 7.Boehmer C, Palmada M, Klaus F, et al. The peptide transporter PEPT2 is targeted by the protein kinase SGK1 and the scaffold protein NHERF2. Cell Physiol Biochem. 2008;22:705–714. doi: 10.1159/000185554. [DOI] [PubMed] [Google Scholar]
- 8.Fejes-Toth G, Frindt G, Naray-Fejes-Toth A, et al. Epithelial Na+ channel activation and processing in mice lacking SGK1. Am J Physiol Renal Physiol. 2008;294:F1298–F1305. doi: 10.1152/ajprenal.00579.2007. [DOI] [PubMed] [Google Scholar]
- 9.Vallon V, Schroth J, Lang F, et al. Expression and phosphorylation of the Na+-Cl− cotransporter NCC in vivo is regulated by dietary salt, potassium, and SGK1. Am J Physiol Renal Physiol. 2009;297:F704–F712. doi: 10.1152/ajprenal.00030.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vallon V, Huang DY, Grahammer F, et al. SGK1 as a determinant of kidney function and salt intake in response to mineralocorticoid excess. Am J Physiol Regul Integr Comp Physiol. 2005;289:R395–R401. doi: 10.1152/ajpregu.00731.2004. [DOI] [PubMed] [Google Scholar]
- 11.Gonzalez-Rodriguez E, Gaeggeler HP, Rossier BC. IGF-1 vs insulin: respective roles in modulating sodium transport via the PI-3 kinase/Sgk1 pathway in a cortical collecting duct cell line. Kidney Int. 2007;71:116–125. doi: 10.1038/sj.ki.5002018. [DOI] [PubMed] [Google Scholar]
- 12.Arteaga MF, Canessa CM. Functional specificity of Sgk1 and Akt1 on ENaC activity. Am J Physiol Renal Physiol. 2005;289:F90–F96. doi: 10.1152/ajprenal.00390.2004. [DOI] [PubMed] [Google Scholar]
- 13.Wulff P, Vallon V, Huang DY, et al. Impaired renal Na(+) retention in the sgk1- knockout mouse. J Clin Invest. 2002;110:1263–1268. doi: 10.1172/JCI15696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ellison DH. The voltage-gated K+ channel subunit Kv1.1 links kidney and brain. J Clin Invest. 2009;119:763–766. doi: 10.1172/JCI38835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rexhepaj R, Artunc F, Grahammer F, et al. SGK1 is not required for regulation of colonic ENaC activity. Pflugers Arch. 2006;453:97–105. doi: 10.1007/s00424-006-0111-4. [DOI] [PubMed] [Google Scholar]
- 16.Artunc F, Ebrahim A, Siraskar B, et al. Responses to diuretic treatment in gene-targeted mice lacking serum- and glucocorticoid-inducible kinase 1 (SGK1) Kidney & Blood Press Res. 2009 doi: 10.1159/000214439. in press. [DOI] [PubMed] [Google Scholar]
- 17.Huang DY, Boini KM, Friedrich B, et al. Blunted hypertensive effect of combined fructose and high-salt diet in gene-targeted mice lacking functional serum- and glucocorticoid-inducible kinase SGK1. Am J Physiol Regul Integr Comp Physiol. 2006;290:R935–R944. doi: 10.1152/ajpregu.00382.2005. [DOI] [PubMed] [Google Scholar]
- 18.Huang DY, Wulff P, Volkl H, et al. Impaired regulation of renal K+ elimination in the sgk1-knockout mouse. J Am Soc Nephrol. 2004;15:885–891. doi: 10.1097/01.asn.0000120368.59693.a8. [DOI] [PubMed] [Google Scholar]
- 19.Sandulache D, Grahammer F, Artunc F, et al. Renal Ca2+ handling in sgk1 knockout mice. Pflugers Arch. 2006;452:444–452. doi: 10.1007/s00424-005-0021-x. [DOI] [PubMed] [Google Scholar]
- 20.Stier CT, Jr, Itskovitz HD. Renal calcium metabolism and diuretics. Annu Rev Pharmacol Toxicol. 1986;26:101–116. doi: 10.1146/annurev.pa.26.040186.000533. [DOI] [PubMed] [Google Scholar]
- 21.Nijenhuis T, Vallon V, van der Kemp AW, et al. Enhanced passive Ca2+ reabsorption and reduced Mg2+ channel abundance explains thiazide-induced hypocalciuria and hypomagnesemia. J Clin Invest. 2005;115:1651–1658. doi: 10.1172/JCI24134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ackermann TF, Boini KM, Volkl H, et al. SGK1-sensitive renal tubular glucose reabsorption in diabetes. Am J Physiol Renal Physiol. 2009;296:F859–F866. doi: 10.1152/ajprenal.90238.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nagase M, Fujita T. Aldosterone and glomerular podocyte injury. Clin Exp Nephrol. 2008;12:233–242. doi: 10.1007/s10157-008-0034-9. [DOI] [PubMed] [Google Scholar]
- 24.Shibata S, Nagase M, Yoshida S, et al. Podocyte as the target for aldosterone: roles of oxidative stress and Sgk1. Hypertension. 2007;49:355–364. doi: 10.1161/01.HYP.0000255636.11931.a2. [DOI] [PubMed] [Google Scholar]
- 25.Artunc F, Amann K, Nasir O, et al. Blunted DOCA/high salt induced albuminuria and renal tubulointerstitial damage in gene-targeted mice lacking SGK1. J Mol Med. 2006;84:737–746. doi: 10.1007/s00109-006-0082-0. [DOI] [PubMed] [Google Scholar]
- 26.Artunc F, Nasir O, Amann K, et al. Serum- and glucocorticoid-inducible kinase 1 in doxorubicin-induced nephrotic syndrome. Am J Physiol Renal Physiol. 2008;295:F1624–F1634. doi: 10.1152/ajprenal.00032.2008. [DOI] [PubMed] [Google Scholar]
- 27.Artunc F, Sandulache D, Nasir O, et al. Lack of the serum and glucocorticoid-inducible kinase SGK1 attenuates the volume retention after treatment with the PPARgamma agonist pioglitazone. Pflugers Arch. 2008;456:425–436. doi: 10.1007/s00424-007-0401-5. [DOI] [PubMed] [Google Scholar]
- 28.Ackermann D, Mordasini D, Cheval L, et al. Sodium retention and ascites formation in a cholestatic mice model: role of aldosterone and mineralocorticoid receptor? Hepatology. 2007;46:173–179. doi: 10.1002/hep.21699. [DOI] [PubMed] [Google Scholar]
- 29.Huang DY, Boini KM, Osswald H, et al. Resistance of mice lacking the serum- and glucocorticoid-inducible kinase SGK1 against salt-sensitive hypertension induced by a high-fat diet. Am J Physiol Renal Physiol. 2006;291:F1264–F1273. doi: 10.1152/ajprenal.00299.2005. [DOI] [PubMed] [Google Scholar]
- 30.Boini KM, Nammi S, Grahammer F, et al. Role of serum- and glucocorticoid-inducible kinase SGK1 in glucocorticoid regulation of renal electrolyte excretion and blood pressure. Kidney Blood Press Res. 2008;31:280–289. doi: 10.1159/000151666. [DOI] [PubMed] [Google Scholar]
- 31.Schwab M, Lupescu A, Mota M, et al. Association of SGK1 gene polymorphisms with type 2 diabetes. Cell Physiol Biochem. 2008;21:151–160. doi: 10.1159/000113757. [DOI] [PubMed] [Google Scholar]
- 32.von Wowern F, Berglund G, Carlson J, et al. Genetic variance of SGK-1 is associated with blood pressure, blood pressure change over time and strength of the insulin-diastolic blood pressure relationship. Kidney Int. 2005;68:2164–2172. doi: 10.1111/j.1523-1755.2005.00672.x. [DOI] [PubMed] [Google Scholar]
- 33.Belaiba RS, Djordjevic T, Bonello S, et al. The serum- and glucocorticoid-inducible kinase Sgk-1 is involved in pulmonary vascular remodeling: role in redox-sensitive regulation of tissue factor by thrombin. Circ Res. 2006;98:828–836. doi: 10.1161/01.RES.0000210539.54861.27. [DOI] [PubMed] [Google Scholar]
- 34.Rexhepaj R, Boini KM, Huang DY, et al. Role of maternal glucocorticoid inducible kinase SGK1 in fetal programming of blood pressure in response to prenatal diet. Am J Physiol Regul Integr Comp Physiol. 2008;294:R2008–R2013. doi: 10.1152/ajpregu.00737.2007. [DOI] [PubMed] [Google Scholar]