Abstract
Background
Current guidelines for prescribing antihypertensive medications focus on reaching specific blood pressure targets. We sought to determine if antihypertensive medications could be used more effectively by a treatment strategy based on tailored estimates of cardiovascular disease (CVD) events prevented.
Methods and Results
We developed a nationally representative sample of American adults aged 30 to 85 years with no history of myocardial infarction, stroke, or severe congestive heart failure using the National Health and Nutrition Examination Survey III. We then created a simulation model to estimate the effects of 5 years of treatment with a treat-to-target (TTT, treatment to specific blood pressure goals) and benefit-based tailored treatment (BTT, treatment based on estimated CVD event reduction) approaches to antihypertensive medication management. All effect size estimates were directly derived from meta-analyses of randomized trials. We found that 55% of the overall population of 176 million Americans would be treated identically under the two treatment approaches. BTT would prevent 900,000 more CVD events and save 2.8 million more QALYs, despite using 6% fewer medications over 5 years. In the 45% of the population treated differently by the strategies, BTT saves 159 QALYs per 1000 treated vs. 74 QALYs per 1000 treated by TTT. The findings were robust to sensitivity analyses.
Conclusions
We found that benefit-based tailored treatment was both more effective and required less antihypertensive medication than current guidelines based on treating to specific blood pressure goals.
Keywords: Hypertension, Prevention, Epidemiology
The purpose of prescribing blood pressure therapy is not to treat hypertension itself but to reduce the risk of clinical outcomes associated with hypertension, primarily cardiovascular disease. Most current blood pressure guidelines advocate a treat-to-target (TTT) strategy, which titrates treatment towards intermediate outcomes, notably a blood pressure goal. While blood pressure is central to CVD prevention, many factors beyond blood pressure influence a patient's benefit from a blood pressure medication, including the patient's overall untreated CVD risk (RiskUnRx, as determined by risk factors including age, smoking, and blood pressure), the medication's relative risk reduction (RRRRx) and the adverse effects of the specific medication (HarmRx) used for treatment. Benefit-based tailored treatment (BTT) strategies estimate an individual patient's net absolute benefit from treatment (Net Benefit = [RiskUnRx * RRRRx] - HarmRx), developed from all the best evidence for these. By basing decision-making on an individual's estimated absolute risk reduction from treatment, and by explicitly including estimates of treatment harm, treatment decisions can be made based on the net benefit of treatment, which is the factor that is mostly likely to matter most to patients.1-3
There is mounting evidence that BTT strategies are a more effective and efficient approach than TTT strategies.4, 5 For example, previous work has shown that guiding statin use with BTT strategies instead of using the LDL cholesterol based National Cholesterol Education Panel guideline's TTT approach could save more lives while treating fewer people intensively.6 Similarly, a tailored approach can better target patients who benefit most from aspirin therapy3, 7 and improve treatment decisions in patients with diabetes.8, 9
Although American guidelines still largely focus on the TTT strategy of achieving specific BP goals, there is some consideration of overall CVD risk when making decisions on treatment targets.10 For example, the JNC 7 report recommended more intensive blood pressure (BP) control for people with a history of CVD disease or diabetes than those without. In some European guidelines CVD risk plays a more central role in decision-making,11 but even these guidelines emphasize treating to a pre-specified BP value rather than directly basing decisions on estimates of CVD event reduction.
Here, we examine whether a BTT strategy for hypertension would prove superior to a traditional TTT strategy for hypertension treatment. We constructed a probability model, based on the best available evidence, to examine how many CVD events would be prevented and how many quality-adjusted life years would be saved with each of the two treatment strategies. We also assessed the implications of these strategies for individual patients and across a wide range of data assumptions.
Methods
Overview
We constructed a large simulated population with the distribution of CVD risk factors observed in the U.S., derived from the nationally representative National Health and Nutrition Examination Survey III. We then estimated event rates of CHD (coronary heart disease) and stroke (RiskUnRx) using the Framingham Heart Score,12 and derived the effects of hypertension treatments(RRRRx and HarmRx) from a large meta-analysis of randomized controlled trials.13 We chose the Framingham Heart Score because it is the most established, validated, and commonly used. We then developed a Markov simulation model that compared a BTT strategy to a JNC-style TTT strategy. The model estimates the impact of each BP treatment strategy on CVD events and Quality Adjusted Life-Years (QALYs) for each patient in the simulated population. This approach to using the best-available evidence to estimate the population and individual net benefits to different treatment strategies has been used and described previously.3, 6, 14, 15 We then estimated the lifetime effects of following either of the treatment strategies for five years on every patient in the population. A 5-year interval was chosen since starting BP treatment should be reassessed at least every 5 years, and probably considerably more often. Although presented in brief below, all methods are described in greater depth in the Supplemental Material, Supplementary Figure 1, and Supplementary Tables 1-4.
Population
We developed our simulated population from NHANES III (National Health and Nutrition Examination Survey), which includes a large, nationally representative probability sample of the U.S. population sample with detailed clinical information.6, 14, 15 We used NHANES III (conducted from1988 to 1994)16 because blood pressure treatment was much less common in that era, creating a more accurate sample population for comparing blood pressure treatment strategies. Since 1994, the population has gotten slightly older, diabetes prevalence has increased, and cardiovascular morbidity and mortality have declined.17, 18
We restricted our analyses to persons aged 30 to 85 years with no history of a myocardial infarction, stroke, or congestive heart failure (i.e., primary prevention) because FHS estimates are most accurate in this population and few individuals outside of this age range have been included in clinical trials. We created a large, robust simulated population of 167,000 people (0.1% of the eligible US population) using the method of imputation of chained equations. 19 This technique accounts for the observed risk factor distributions, correlations, and survey weights of in the 8291 eligible participants in NHANES. As in traditional survey weighting, these 167,000 people represent the 167 million people who meet the eligibility criteria nationwide. The imputation technique assures that the benefits of survey weighting are retained while providing a more robust representative database.
To effectively assess the benefit of different blood pressure strategies, we estimated the untreated blood pressure of every person in the cohort. To do that, we used the measured blood pressure and average effectiveness of blood pressure medications to “back out” estimated untreated blood pressure for each individual, accounting for the variability in BP treatment response.
The Effect of Treatment on BP and Cardiovascular Risk
We separately assessed each patient's untreated 5-year risk of cardiovascular disease (CVD), including coronary heart disease (CHD) and stroke using equations from the Framingham Heart Study.12 We chose these particular Framingham models because they permit separate evaluations of CHD and stroke and include patients with and without diabetes. This is necessary because each medication reduces the risk of CHD and stroke by different amounts.
We then used data from a large meta-analysis by Law and colleagues to estimate the expected BP reduction and relative and absolute reduction in CHD and stroke risk for each of 4 possible treatment steps of increasing treatment intensity for each individual (Table 1).13 This meta-analysis produced estimates for treatments on systolic and diastolic blood pressure by pre-treatment blood pressure and for CHD and stroke rates by CHD and stroke risk, and age. The 4 treatment steps represent each of the 4 common, well-studied blood pressure classes (thiazides, angiotensin converting enzymes, beta-blockers, and calcium channel blockers) used in the order recommended in the JNC7 guidelines,10 but the medication order was varied in sensitivity analyses. We used a single standard dose for each medication to simplify presentation and since these studies showed that dosage adjustment has only small effects on outcomes. CHD risks for people on BP treatment were developed from this meta-analysis using a multifactorial model of pre-treatment risk, age, and pre-treatment SBP. (See supplementary Material)
Table 1.
Effect of treatments on blood pressure
| Treatment class and order of use | Example Medication (High dose/Low dose)* | Mean relative change, regular dose (%) | Mean relative change, low dose (%) | ||
|---|---|---|---|---|---|
| SBP | DBP | SBP | DBP | ||
| THI | Hydrochlorothiazide (25/12.5 mg) | 6.6 | 5.1 | 5.8 | 4.6 |
| ACE | Lisinopril (10/5 mg) | 6.4 | 5.8 | 5.6 | 4.9 |
| BBL | Atenolol (50/25 mg) | 7.2 | 8.0 | 5.1 | 6.9 |
| CCB | Amlodipine (5/2.5 mg) | 7.6 | 8.2 | 4.8 | 6.2 |
Abbreviations: THI, thiazide; ACE, angiotensin converting enzyme inhibitor; BBL, beta-blocker; CCB, calcium channel blocker; SBP, systolic blood pressure; DBP, diastolic blood pressure; CV, coefficient of variation
Based on meta-analysis by Law et al.13 The low dose is used only in the sensitivity analysis
To simulate more realistic clinical circumstances, we created values for blood pressure and CHD and stroke risk that accounted for clinical BP measurement uncertainty and variability in treatment response. Clinical BP measurement uncertainty results from daily variations in an individual's blood pressure, inconsistent measurement equipment or technique, and random measurement error. Parameters for these estimations were obtained from published data.20 Variability in treatment response represents true variation in blood pressure reduction from each treatment, caused by patient biologic variability and also was derived from published data.13-15 All treatment decisions were based on the “observed” BP (i.e., a clinic blood pressure) which includes measurement uncertainty and true variation in response) but all estimates of treatment benefit are based on the patient's “true” BP, which is known by the model but not the treating provider. These variations are fully described in the appendix and the implications of varying these factors (such as improving clinical BP measurement) are examined in sensitivity analyses. For the base case we selected what Law et al call a “standard” dose, but in sensitivity analyses we examined lower dosages for each medication.
Other sequelae of CVD were not included in the model because they would be very unlikely to alter the results, for multiple reasons. First, CHD and stroke are the cause of over 80% of hypertension-related QALY loss in developed countries.21 Second, most of the other sequelae of hypertension have risk factors that closely parallel CHD and stroke, including congestive heart failure,22 claudication,23 and chronic kidney disease.24 Third, the role of hypertension treatment in preventing these conditions is much less clearly estimated from current trials.
The Treat-to-Target (TTT) and Benefit-Based Tailored Treatment (BTT) Strategies
The number of medications that each patient received for each treatment strategy was determined sequentially so that the post-treatment observed BP and CVD risk were reassessed after each treatment step.
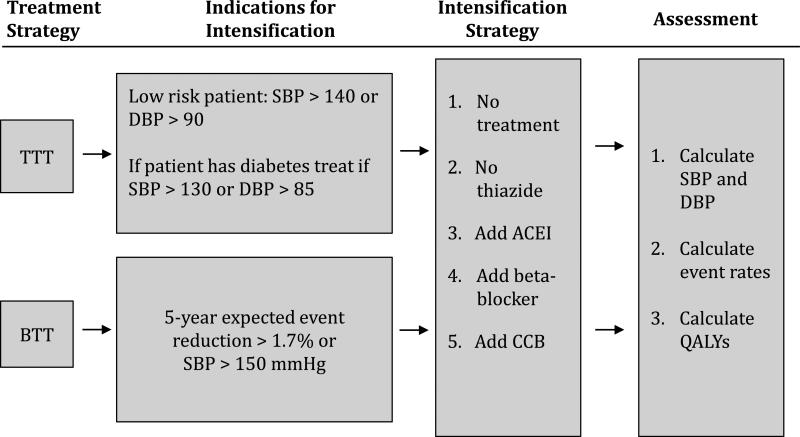
The base case treat-to-target (TTT) strategy was based on JNC 7 guidelines (Figure 1).10 In this strategy a patient's treatment was advanced with a new BP medication if his or her observed blood pressure was ≥140/90 or ≥130/85 for patients with diabetes. Medications were added sequentially until the target blood pressure was reached or until the patient was on four medications.
Figure 1.
Overview of the treatment and analysis strategies.
In the BTT strategy treatment was advanced to the next step of therapy in two circumstances. First, treatment was advanced in any patient whose observed systolic BP was greater than 150 mmHg. This was selected because adverse effects from BP can multiply even as an isolated risk factor at very high levels and because elevations to this degree are less likely to be false positive measurements. Second, treatment was advanced in any patient whose rate of CVD events would be predicted to decrease by greater than a 1.7% chance of event averted for 5-years of therapy. By this standard, patients receive a medication if it has a sufficiently high likelihood of preventing a CVD event. This threshold was chosen empirically because it leads to roughly the same number of patients being treated as does the TTT strategy, facilitating direct comparison (see discussion section for comments on setting optimal BTT thresholds).
Assessing the clinical benefits of treatment
Using these treatment strategies, the population was then assessed in a Markov Model. Each patient began in the “healthy” state. During the 5 years of follow-up, they could develop CVD. CVD could constitute CHD or stroke and could be fatal or nonfatal. For each patient we estimated the clinical implications of each strategy (TTT vs. BTT) on patient systolic and diastolic blood pressure, event rates for CHD and stroke, and disease-specific and overall life years using the methods described above. This information was then used to estimate Quality-Adjusted Life-Years (QALYs) as outlined below.
QALY loss per event is based on our previously described method.3 In brief, we calculated a QALY loss for the year of a CVD event, a smaller QALY loss for each year of life after an event, a rate of fatality per event, and a reduction in life expectancy for each nonfatal event. Each of these estimates was obtained from published literature. Non-cardiovascular mortality (competing risk) was obtained from Centers for Disease Control and Prevention Life Tables.25, 26 To assess the fraction of events that would be fatal, we calibrated our nationally representative population event and fatality rates to high-quality sex, race, and age-specific literature to ensure reliability and population-level accuracy, similar to other policy models.3,27, 28
As a conservative estimate of the nuisance, side effects, and potential adverse effects of treatment, we applied a disutility of 0.001 for each blood pressure medication used.3, 6 Other parameters are summarized in Supplemental Tables 3 and 4.
Analysis
The primary analysis compared the effects of a TTT strategy with those of BTT. These were evaluated for the clinical implications of each strategy, including examining who would receive treatment by each strategy and the implications on the entire population. We then specifically focused on the ‘marginal’ patients6 – those patients who are treated substantially differently by one guideline than another, to examine how many are treated differently and what the clinical implications are of that treatment difference. Multiple sensitivity analyses were performed to assess the reliability of these outcomes (Table 2).29-32
Table 2.
Parameters used in model and sensitivity analyses
| Value | Baseline | Range |
|
|---|---|---|---|
| Low | High | ||
| BP measurement uncertainty (CV)20 | 0.09 per measurement, assume two measurements* | 0 | 0.09, assume one measurement CV in response |
| Variation in response to medication29 | 3% | 0 | equal to SD of drug response |
| Treatment-related disutility (QALYs per medication per year of treatment)30 | 0.001 | 0 | 0.02 |
| High risk value for JNC 7 (10-year FHS score)12 | 20% | None | 10% |
| Decremental treatment benefit† for 3rd and 4th medication14, 31 | 16% | None | 16% |
| Low dose BP medication | Low dose, per Law et al13, 32 | See Table 1 | |
Abbreviations: BP, blood pressure; CV, coefficient of variation; SD, standard deviation; QALY, quality-adjusted life-year; JNC, Joint National Committee
BP measurement uncertainty has a coefficient of variation of 0.09. We varied from one measurement to complete certainty, with the baseline value being two measurements.
Compared to when this treatment is the first or second BP medication in the patient's BP treatment regimen.
Results
Overall outcomes for Treat-to-Target (TTT) and Benefit-Based Tailored Treatment (BTT)
The TTT strategy would recommend use of 1 or more blood pressure medications for 79.0 million people, or about 44.6% of the 176 million adult Americans ages 35-85 with no history of heart failure, heart attack, or stroke: 33.0% of the population (58.4 million people) would receive 1-2 medications and 11.6% would receive 3-4 medications (20.4 million people) (Table 3) for an average of 1.9 medications per person treated. Compared with no treatment, 100% compliance with the TTT approach would save an estimated 19.3 million total QALYs nationally per 5 years of use.
Table 3.
CV events prevented with the treat-to-target (TTT) strategy versus the benefit-based tailored treatment (BTT) Strategy (treatment threshold 1.7% event reduction), in 177 million US patients
| TTT | BTT | |
|---|---|---|
| Medications used: | ||
| per 100 persons age 35-85 | 84.6 | 79.5 |
| per persons treated | 1.9 | 2.2 |
| Adults Who Receive Treatment, % (million n) | ||
| 1-2 medications | 33.0 (58.4) | 22.4 (39.6) |
| 3-4 medications | 11.6 (20.4) | 9.0 (23.0) |
| Initial SBP among treated, in mmHg | 145.3 | 148.2 |
| Final SBP among treated, in mmHg | 124.0 | 128.5 |
| Pre-treatment 5-year CVD risk among treated, mean % | 5.5 | 8.0 |
| Post-treatment 5-year CVD risk among treated, mean % | 3.0 | 3.8 |
| CAD Events prevented per 5 years | ||
| Total, millions | 3.3 | 4.2 |
| CHD, millions | 2.0 | 2.7 |
| Stroke, millions | 1.3 | 1.5 |
| Total QALYs saved, millions | 19.3 | 22.2 |
Abbreviations: SBP, systolic blood pressure; DBP, diastolic blood pressure; CVD, cardiovascular disease; CHD, coronary heart disease; QALYs, quality-adjusted life-years.
In comparison, the BTT approach would result in fewer people being treated at all, but somewhat more intensive treatment in those treated. BTT would recommend treatment for 35.5% of the target population (62.7 million people), averaging 2.2 medications per person treated. Compared with no treatment, the BTT approach would save 22.2 million QALYs nationally per 5 years, about 3 million (13%) more QALYs than TTT, in spite of using 6% less medication.
Incremental (marginal) benefits of Treat-to-Target (TTT) vs. Benefit-Based Tailored Treatment (BTT)
About 97 million people (55% of the total population) would be recommended the same medications using either of the competing strategies, with 82 million of these people (46% of the total population) not being treated under either strategy. With so many people being treated similarly, the most informative analysis is to examine the differential benefits in the estimated 45% (79.9 million people) who would be treated differently under the two strategies (Table 4).33 26.5% of the total population (46.8 million people) are treated more intensively with TTT than BTT; in these people, TTT would recommend an additional 1.9 medications per person and save 204 QALYs per 1000 treated more intensively for 5 years. In contrast, the 18.7% of the population (33.0 million people) treated more intensively with BTT than TTT would receive an additional 2.5 medications per person and save 487 more QALYs per 1000 people treated more intensively, more than twice the benefit as those treated more intensively by TTT (Table 4). Because BTT is based on overall CV risk, people with more risk factors, especially older men who smoke, are treated more intensively with BTT. In contrast, people with only high blood pressure, but who have low overall CV risk, are treated more intensively with TTT.
Table 4.
Comparison of the incremental gains of the intensive treat-to-target (TTT) approach versus the benefit-based tailored treatment (BTT) approach in 177 million US patients
| People treated identically by both strategies | People treated more intensively by TTT | People treated more intensively by BTT | |
|---|---|---|---|
| Proportion treated, % (million n) | 54.8 (96.8) | 26.5 (46.8) | 18.7 (33.0) |
| Age | 52.0 | 55.8 | 68.8 |
| Women, % | 63 | 63 | 19 |
| Tobacco use, % | 24 | 18 | 39 |
| Diabetes, % | 5 | 8 | 13 |
| Mean initial SBP, mmHg | 124.1 | 141.6 | 140.4 |
| Mean initial DBP, mmHg | 74.2 | 84.6 | 76.2 |
| Mean final SBP, mmHg | 119.6 | 120.4 | 118.3 |
| Mean initial 5-y CVD risk, % | 2.6 | 2.7 | 9.5 |
| Mean final 5-y CVD risk, % | 1.9 | 1.4 | 4.3 |
| CVD events prevented, per 1000 treated more intensively | 12.2 | 22.5 | 74.7 |
| QALYs saved, per 1000 treated more intensively | 62 | 74 | 159 |
| Events prevented per medication used, per 1000 persons treated more intensively | 77.9 | 204 | 486 |
Abbreviations: SBP, systolic blood pressure; DBP, diastolic blood pressure; CVD, cardiovascular disease; QALYs, quality-adjusted life-years.
*A patient was considered to be treated more intensively if treatment by that strategy would lead to more BP medications being used.
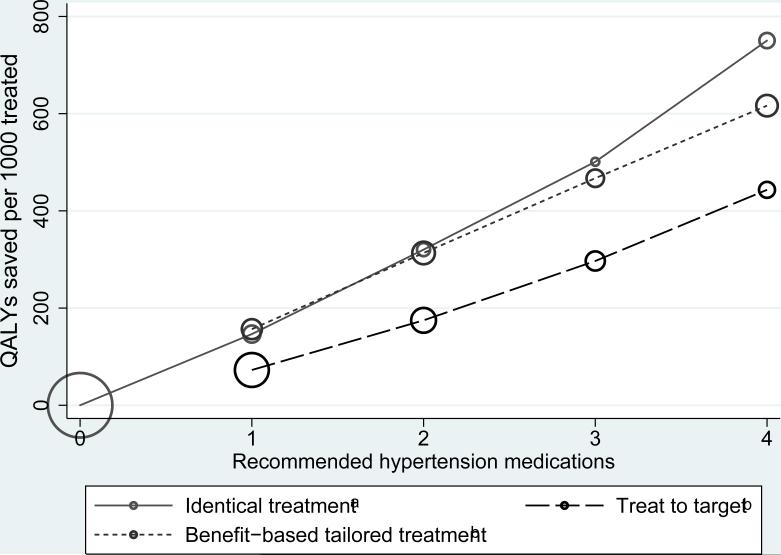
As shown in Figure 2, people who are recommended similar therapy by both strategies and those recommended more intensive treatment by BTT than TTT received roughly twice the benefit than those recommended treated more intensively by TTT (Figure 2 and Table 4).
Figure 2.
Average benefit per recommended medication. aCircle sizes are proportionate to the number of people who meet that criterion. For example, for the“identical treatment” group, 81.8 million people (largest green circle) would receive no BP treatment in either the benefit-based tailored treatment or the treat to target strategy, and 3.6 million people (smallest green circle) would receive 3 BP medications using either strategy. b At each level of treatment, the people who are treated by the TTT model only save about ½ as many QALYs as those recommended that level of treatment by BTT only or by both models.
To provide concrete examples of the clinical implications of treatment with BTT vs. TTT, 3 example patients are described in Appendix B and Supplemental Table 5.
Sensitivity analyses
Multiple sensitivity analyses were examined on a wide array of parameters in the model (Table 5). Changing model assumptions had some effect on treatment intensity (ie, how many medications individuals were recommended), although BTT almost always led to fewer people being treated at the population-level. BTT always had a substantially larger total population benefit. The relative benefit of BTT vs. TTT per medication used was insensitive to variations in model assumptions. The most dramatic change was BTT having an even larger relative benefit at higher levels of treatment disutility (the amount that a patient dislikes taking a medication, which includes costs, patient dislike of taking medications, side effects and adverse events), because fewer people are treated and thus experience treatment burdens (Supplemental Figure 2). Improving the accuracy of BP measurement only resulted in a modest improvement in the efficiency of a TTT strategy. Similarly, assumptions that lead to greater treatment by the TTT strategy, such as creating a high-risk group based around cardiac risk (such as currently exists in cholesterol guidelines), and assuming that people will use low doses of blood pressure medications (which is recommended in the United Kingdom) did not substantially increase the efficiency of TTT (see Table 5). Altering the order in which blood pressure medications are used also did not substantially change our results. Even changing an assumption that substantially reduced absolute benefit of treatment, such as assuming a 40% treatment nonadherence (we assumed 100% adherence in the base estimates), did not substantially alter the relative benefit of BTT over TTT.
Table 5.
Sensitivity analyses of benefit-based tailored treatment (BTT) vs. treat-to-target (TTT)*
| Treatment intensity (BTT/TTT ratio of medications used per 100 people) | Total Population Benefit (BTT/TTT of QALYs saved per 1000 treated) | Treatment Efficiency, (BTT/TTT of QALYs saved per 1000 treated) | |
|---|---|---|---|
| Baseline | 0.94 | 1.15 | 1.23 |
| True variation in medication response | |||
| None | 0.91 | 1.12 | 1.23 |
| Large: Same size as treatment response | 0.98 | 1.19 | 1.21 |
| Treatment-related disutility (0 and 0.02) | |||
| None | 0.94 | 1.14 | 1.21 |
| High (0.02 QALYs/medication*year) | 0.94 | 1.46 | 1.56 |
| Double if age ≥ 70 | 0.94 | 1.15 | 1.22 |
| High risk treatments – goal BP < 130/80 if: | |||
| Diabetes or 10-year CVD risk > 20% | 0.86 | 1.04 | 1.21 |
| No circumstances | 1.01 | 1.24 | 1.23 |
| Decline in treatment benefit for additional medications | |||
| No decline for additional medications | 0.91 | 1.12 | 1.24 |
| 16% decline per medication14 | 1.01 | 1.21 | 1.20 |
| 40% nonadherence | 0.84 | 1.15 | 1.23 |
| Low dose BP medications (per Law et al.)13 | 0.79 | 1.02 | 1.28 |
Abbreviations: QALYs, quality-adjusted life-years; BP, blood pressure; CVD, cardiovascular disease
All values are BTT/TTT ratios. For example, the first column shows that in most circumstances, treatment is more intense by TTT, the second column shows that in all circumstances net population benefit is greater with BTT, and third column shows that in all circumstances treatment is more efficient with BTT.
Discussion
Clinicians and policy-makers have long recognized that blood pressure is not the only predictor of treatment benefit from anti-hypertensive medications. The larger absolute benefit in patients at high risk (e.g., CVD, diabetes) has led to lower blood pressure targets in these highest-risk patients.34, 35 Our findings suggest there could be major benefit in taking an additional step in this progression: to base BP management decisions on estimates of individual-patient benefit, instead of focusing primarily on treating based only on the intermediate risk factor of blood pressure.
In this study, we found that tailoring hypertension management by estimating an individual's expected net benefit from additional BP treatment (benefit-based tailored treatment [BTT]) has the potential to be a more efficient and effective strategy for improving patient outcomes than current treat-to-target (TTT) guidelines. We have also shown that net benefit can be appropriately estimated with a patient's untreated CVD risk, blood pressure, and current treatment regimen. The important finding in this study is not simply that the BTT approach was better than the TTT approach, since that is true almost by definition, but that just as we found in past research on lipid therapy,6 BTT is much better. For lipid therapy, we found that BTT saved more than 3 times more QALYs than TTT in those treated differently by the two approaches, and in the current study we found that BTT produced about twice as much benefit for those treated differentially for blood pressure management.
We see BTT as a natural evolution of CVD prevention guidelines, not a divergence from them. Recent guidelines have recognized that people with elevated risk, such as a history of CVD or diabetes, are much more likely to benefit from treatment than those with lower CVD risk. TTT guidelines begin to account for this by recommending a lower blood pressure goal in those at high risk, 34, 36 and our results suggest that BTT can further improve treatment effectiveness and efficiency and prevent many more CVD events by considering all the patient and treatment factors that clinical trials have found to influence absolute risk reduction.
Our findings are consistent with our prior work examining a BTT approach.3, 6, 14, 15 Our group has already demonstrated how baseline-risk, and not LDL cholesterol concentration, is central to decisions regarding statin use6 and how concentrating on overall microvascular and macrovascular risk can improve decision-making over current guidelines for diabetes treatments.14, 15 Our findings are consistent with another study that found that model-based hypertensive management could be more cost-effective than current care.37 Unlike that study, however, our work considers adverse effects of BP treatments, uses a model that can be directly inspected by other researchers, and demonstrates that purchase and use of a proprietary product is not necessarily required to achieve the benefit of BTT.
The primary limitation of our study's main conclusion, that BTT has higher efficacy than a TTT approach, is that we are limited by the current evidence in the medical literature. Caution is always advisable when interpreting simulation models, since a model is only as good as its inputs and assumptions; however, our study is based on very strong evidence. Most of the key parameters are drawn directly from a large meta-analysis of randomized trials,13 and our study population was derived from a nationally representative sample of the U.S. population.
Further, we made several assumptions that are favorable to a TTT approach. First, the real-world accuracy of BP measurement is lower than that seen in the studies we used.38 Second, we assumed that the benefits of antihypertensive medications are directly related to their impact on an individual's resting BP, rather than other effects of the medications themselves, including patient's BP levels when active.6 Third, we used a very small treatment disutility, which our sensitivity analyses found greatly favors a TTT approach. Finally, improved CVD risk prevention can make BTT even more valuable. For example, if new risk prediction tools (such as the Reynolds Score or coronary artery calcium score)39 improve our ability to risk-stratify patients beyond the Framingham score, the benefit of BTT over TTT would become even larger.
We did not include an SBP and/or DBP at which further treatment is contraindicated for either strategy because the possible clinical harms of treatment towards low blood pressure are unclear.40 We do feel that caution about over-treatment in older patients is particularly important. One advantage of a BTT approach is that it can easily incorporate stopping rules and any other complex assumptions or new research findings. Such modifications and updates simply need to be added to the risk/benefit estimations. We used a 5-year treatment window because longer time frames are inconsistent with appropriate clinical decision-making, in that re-evaluation of the need for blood pressure treatment should occur at least every 3-5 years. Furthermore, using a five-year window, as opposed to a lifetime window, would only alter the relative benefits of BTT vs. TTT if there are delayed benefits from initiating BP treatment earlier. We found no evidence that this occurs for blood pressures in the range evaluated in our study (ie, SBP < 150mmHg).36
This study demonstrates how when high quality evidence is available, simulation models can help make the results of randomized trials more clinically useful. Although, a trial directly comparing BTT vs. TTT would take many years to conduct and may prove prohibitively expensive, the simulation approach used in this study merely interprets the best-available data, mainly using the results of randomized controlled trials. Of note, the BTT approach examined in this study uses results of trials more directly than current TTT guidelines, which make many more untested assumptions, such as assuming that an individual's degree of blood pressure reduction as measured in routine clinical practice is accurate and that treatment disutilities are negligible.
Our study shows that benefit-based tailored treatment is more efficient than current guidelines. In this paper, we chose a benefit cut-point – the 1.7% absolute event reduction per year above which we would recommend treatment – so that population-level treatment would be comparable between BTT and TTT, ensuring a clearer comparison to current TTT guidelines. We are not making global recommendations about how intensive treatment should be. It is reasonable to assume that individual patients will have different thresholds at which they would consider treatment beneficial, so that a shared decision making approach should be adopted. More development of tools and clinical policy to support this approach is necessary.
This work also is not relevant to the importance of non-medical prevention of CVD. For example, the value of smoking cessation likely outweighs all of these decisions and effective changes to diet and exercise change risk factors and, therefore, estimated benefit.
This study is a proof of principle that BBT could prevent more CVD events than current TTT guidelines. However, one practical limitation of our strategy's potential effectiveness is that clinicians cannot be expected to do the necessary algebraic calculations in their head. The model can, however, be easily placed on a computer, website, or handheld device and could potentially be integrated with a facility's electronic health record, limiting the need for data entry. With appropriate tools, this could be time-efficient and quite simple.
While technically fairly easy, wide-spread adoption of a BTT approach will most likely require the support of guideline organizations, changes in quality measures, and the development of easy-to-use decision support tools (preferably EHR-based). While ambitious, similar changes are already occurring in treatment of hypercholesterolemia, where the ACC-AHA guidelines for treatment of patients with stable ischemic heart disease has recently removed its TTT component,41 the VA system has changed its quality measures to be consistent with BTT,42 and treatment decision support systems (though not BTT-based) already exist.43 Before creating a public access decision tool for BTT, we feel it appropriate to await public vetting of our findings and perhaps await changes in formal guidelines and quality measures. Implementation of this work would be a major challenge, but given the importance of blood pressure treatment we feel that such discussions should be made a priority. In the interim, even a qualitative understanding that clinicians pay more attention to overall CVD risk when making BP treatment decisions is important and valuable.
In summary, our results suggest that CVD events can be prevented more effectively with a more comprehensive accounting for all available factors that contribute to net patient benefit, such as other clinical risk factors and polypharmacy, rather than chiefly basing decisions on whether the observed blood pressure level is above or below a prespecified BP target. The next wave of clinical treatment strategies may be more efficient, effective, and transparent with a full assessment of risk and benefit and the use of benefit-base tailored treatment.
Supplementary Material
Acknowledgments
We thank HwaJung Choi, PhD for her contributions to the simulation model and John Yudkin, MD for his helpful comments on an earlier draft on the manuscript.
Funding Sources: This work was supported in part by the U.S. Department of Veterans Affairs Quality Enhancement Research Initiative (QUERI DIB 98-001), and the Methods Core of the Michigan Center for Diabetes Translational Research (NIDDK of The National Institutes of Health [P60 DK-20572]). The authors report no conflicts of interest with this work and the funding agencies had no direct role in the analysis or reporting of the results.
Footnotes
Conflict of Interest Disclosures: None.
References
- 1.Hayward RA, Kent DM, Vijan S, Hofer TP. Multivariable risk prediction can greatly enhance the statistical power of clinical trial subgroup analysis. BMC Med Res Methodol. 2006;6:18. doi: 10.1186/1471-2288-6-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hayward RA, Krumholz HM. Three reasons to abandon low-density lipoprotein targets: An open letter to the adult treatment panel iv of the national institutes of health. Circ Cardiovasc Qual Outcomes. 2012;5:2–5. doi: 10.1161/CIRCOUTCOMES.111.964676. [DOI] [PubMed] [Google Scholar]
- 3.Sussman JB, Vijan S, Choi H, Hayward RA. Individual and population benefits of daily aspirin therapy: A proposal for personalizing national guidelines. Circ Cardiovasc Qual Outcomes. 2011;4:268–275. doi: 10.1161/CIRCOUTCOMES.110.959239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kent DM, Hayward RA, Griffith JL, Vijan S, Beshansky JR, Califf RM, Selker HP. An independently derived and validated predictive model for selecting patients with myocardial infarction who are likely to benefit from tissue plasminogen activator compared with streptokinase. Am J Med. 2002;113:104–111. doi: 10.1016/s0002-9343(02)01160-9. [DOI] [PubMed] [Google Scholar]
- 5.Rothwell PM, Warlow CP. Prediction of benefit from carotid endarterectomy in individual patients: A risk-modelling study. European carotid surgery trialists’ collaborative group. Lancet. 1999;353:2105–2110. doi: 10.1016/s0140-6736(98)11415-0. [DOI] [PubMed] [Google Scholar]
- 6.Hayward RA, Krumholz HM, Zulman DM, Timbie JW, Vijan S. Optimizing statin treatment for primary prevention of coronary artery disease. Ann Intern Med. 2010;152:69–77. doi: 10.7326/0003-4819-152-2-201001190-00004. [DOI] [PubMed] [Google Scholar]
- 7.Baigent C, Blackwell L, Collins R, Emberson J, Godwin J, Peto R, Buring J, Hennekens C, Kearney P, Meade T, Patrono C, Roncaglioni MC, Zanchetti A. Aspirin in the primary and secondary prevention of vascular disease: Collaborative meta-analysis of individual participant data from randomised trials. Lancet. 2009;373:1849–1860. doi: 10.1016/S0140-6736(09)60503-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Timbie JW, Hayward RA, Vijan S. Variation in the net benefit of aggressive cardiovascular risk factor control across the us population of patients with diabetes mellitus. Arch Intern Med. 2010;170:1037–1044. doi: 10.1001/archinternmed.2010.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Timbie JW, Hayward RA, Vijan S. Diminishing efficacy of combination therapy, response- heterogeneity, and treatment intolerance limit the attainability of tight risk factor control in patients with diabetes. Health Serv Res. 2010;45:437–456. doi: 10.1111/j.1475-6773.2009.01075.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr., Jones DW, Materson BJ, Oparil S, Wright JT, Jr., Roccella EJ. The seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure: The jnc 7 report. Jama. 2003;289:2560–2572. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 11.Jbs 2: Joint british societies' guidelines on prevention of cardiovascular disease in clinical practice. Heart. 2005;91(Suppl 5):v1–52. doi: 10.1136/hrt.2005.079988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Anderson KM, Odell PM, Wilson PW, Kannel WB. Cardiovascular disease risk profiles. Am Heart J. 1991;121:293–298. doi: 10.1016/0002-8703(91)90861-b. [DOI] [PubMed] [Google Scholar]
- 13.Law MR, Morris JK, Wald NJ. Use of blood pressure lowering drugs in the prevention of cardiovascular disease: Meta-analysis of 147 randomised trials in the context of expectations from prospective epidemiological studies. Bmj. 2009;338:b1665. doi: 10.1136/bmj.b1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Timbie JW, Hayward RA, Vijan S. Variation in the net benefit of aggressive cardiovascular risk factor control across the us population of patients with diabetes mellitus. Arch Intern Med. 170:1037–1044. doi: 10.1001/archinternmed.2010.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Timbie JW, Hayward RA, Vijan S. Diminishing efficacy of combination therapy, response- heterogeneity, and treatment intolerance limit the attainability of tight risk factor control in patients with diabetes. Health Serv Res. 45:437–456. doi: 10.1111/j.1475-6773.2009.01075.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.National center for health statistics Third national health and nutrition examination survey (nhanes iii), 1988–1994 public-use data files.
- 17.Gregg EW, Cheng YJ, Cadwell BL, Imperatore G, Williams DE, Flegal KM, Narayan KM, Williamson DF. Secular trends in cardiovascular disease risk factors according to body mass index in us adults. Jama. 2005;293:1868–1874. doi: 10.1001/jama.293.15.1868. [DOI] [PubMed] [Google Scholar]
- 18.Romero CX, Romero TE, Shlay JC, Ogden LG, Dabelea D. Changing trends in the prevalence and disparities of obesity and other cardiovascular disease risk factors in three racial/ethnic groups of USA adults. Adv Prev Med. 2012;2012:172423. doi: 10.1155/2012/172423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cameron AC, Trivedi PK. Microeconometrics using stata. Stata Press; 2009. [Google Scholar]
- 20.Powers BJ, Olsen MK, Smith VA, Woolson RF, Bosworth HB, Oddone EZ. Measuring blood pressure for decision making and quality reporting: Where and how many measures? Ann Intern Med. 2011;154:781–788. doi: 10.7326/0003-4819-154-12-201106210-00005. [DOI] [PubMed] [Google Scholar]
- 21.Lawes CM, Vander Hoorn S, Rodgers A. Global burden of blood-pressure-related disease, 2001. Lancet. 2008;371:1513–1518. doi: 10.1016/S0140-6736(08)60655-8. [DOI] [PubMed] [Google Scholar]
- 22.Kannel WB, D'Agostino RB, Silbershatz H, Belanger AJ, Wilson PW, Levy D. Profile for estimating risk of heart failure. Arch Intern Med. 1999;159:1197–1204. doi: 10.1001/archinte.159.11.1197. [DOI] [PubMed] [Google Scholar]
- 23.Murabito JM, D'Agostino RB, Silbershatz H, Wilson WF. Intermittent claudication. A risk profile from the framingham heart study. Circulation. 1997;96:44–49. doi: 10.1161/01.cir.96.1.44. [DOI] [PubMed] [Google Scholar]
- 24.Echouffo-Tcheugui JB, Kengne AP. Risk models to predict chronic kidney disease and its progression: A systematic review. PLoS medicine. 2012;9:e1001344. doi: 10.1371/journal.pmed.1001344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arias E. United states life tables, 2004. Natl Vital Stat Rep. 2007;56:1–39. [PubMed] [Google Scholar]
- 26.Heron M. Deaths: Leading causes for 2004. Natl Vital Stat Rep. 2007;56:1–95. [PubMed] [Google Scholar]
- 27.Pletcher MJ, Lazar L, Bibbins-Domingo K, Moran A, Rodondi N, Coxson P, Lightwood J, Williams L, Goldman L. Comparing impact and cost-effectiveness of primary prevention strategies for lipid-lowering. Ann Intern Med. 2009;150:243–254. doi: 10.7326/0003-4819-150-4-200902170-00005. [DOI] [PubMed] [Google Scholar]
- 28.Stern M, Williams K, Eddy D, Kahn R. Validation of prediction of diabetes by the archimedes model and comparison with other predicting models. Diabetes Care. 2008;31:1670–1671. doi: 10.2337/dc08-0521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bell KJ, Hayen A, Macaskill P, Craig JC, Neal BC, Irwig L. Mixed models showed no need for initial response monitoring after starting antihypertensive therapy. J Clin Epidemiol. 2009;62:650–659. doi: 10.1016/j.jclinepi.2008.07.018. [DOI] [PubMed] [Google Scholar]
- 30.Pignone M, Earnshaw S, Pletcher MJ, Tice JA. Aspirin for the primary prevention of cardiovascular disease in women: A cost-utility analysis. Arch Intern Med. 2007;167:290–295. doi: 10.1001/archinte.167.3.290. [DOI] [PubMed] [Google Scholar]
- 31.Wu J, Kraja AT, Oberman A, Lewis CE, Ellison RC, Arnett DK, Heiss G, Lalouel JM, Turner ST, Hunt SC, Province MA, Rao DC. A summary of the effects of antihypertensive medications on measured blood pressure. Am J Hypertens. 2005;18:935–942. doi: 10.1016/j.amjhyper.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 32.Law MR, Wald NJ, Morris JK, Jordan RE. Value of low dose combination treatment with blood pressure lowering drugs: Analysis of 354 randomised trials. Bmj. 2003;326:1427. doi: 10.1136/bmj.326.7404.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gold MR. Cost-effectiveness in health and medicine. Oxford University Press; New York: 1996. [Google Scholar]
- 34.Hansson L, Zanchetti A, Carruthers SG, Dahlof B, Elmfeldt D, Julius S, Menard J, Rahn KH, Wedel H, Westerling S. Effects of intensive blood-pressure lowering and low-dose aspirin in patients with hypertension: Principal results of the hypertension optimal treatment (hot) randomised trial. Hot study group. Lancet. 1998;351:1755–1762. doi: 10.1016/s0140-6736(98)04311-6. [DOI] [PubMed] [Google Scholar]
- 35.Vijan S, Hayward RA. Treatment of hypertension in type 2 diabetes mellitus: Blood pressure goals, choice of agents, and setting priorities in diabetes care. Ann Intern Med. 2003;138:593–602. doi: 10.7326/0003-4819-138-7-200304010-00018. [DOI] [PubMed] [Google Scholar]
- 36.Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: Ukpds 38. Uk prospective diabetes study group. Bmj. 1998;317:703–713. [PMC free article] [PubMed] [Google Scholar]
- 37.Eddy DM, Adler J, Patterson B, Lucas D, Smith KA, Morris M. Individualized guidelines: The potential for increasing quality and reducing costs. Ann Intern Med. 2011;154:627–634. doi: 10.7326/0003-4819-154-9-201105030-00008. [DOI] [PubMed] [Google Scholar]
- 38.Niiranen TJ, Hanninen MR, Johansson J, Reunanen A, Jula AM. Home-measured blood pressure is a stronger predictor of cardiovascular risk than office blood pressure: The finn-home study. Hypertension. 2010;55:1346–1351. doi: 10.1161/HYPERTENSIONAHA.109.149336. [DOI] [PubMed] [Google Scholar]
- 39.Blaha MJ, Budoff MJ, DeFilippis AP, Blankstein R, Rivera JJ, Agatston A, O'Leary DH, Lima J, Blumenthal RS, Nasir K. Associations between c-reactive protein, coronary artery calcium, and cardiovascular events: Implications for the jupiter population from mesa, a population-based cohort study. Lancet. 2011;378:684–692. doi: 10.1016/S0140-6736(11)60784-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thompson AM, Hu T, Eshelbrenner CL, Reynolds K, He J, Bazzano LA. Antihypertensive treatment and secondary prevention of cardiovascular disease events among persons without hypertension: A meta-analysis. JAMA. 2011;305:913–922. doi: 10.1001/jama.2011.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fihn SD, Gardin JM, Abrams J, Berra K, Blankenship JC, Dallas AP, Douglas PS, Foody JM, Gerber TC, Hinderliter AL, King SB, 3rd, Kligfield PD, Krumholz HM, Kwong RY, Lim MJ, Linderbaum JA, Mack MJ, Munger MA, Prager RL, Sabik JF, Shaw LJ, Sikkema JD, Smith CR, Jr., Smith SC, Jr., Spertus JA, Williams SV, Anderson JL. 2012 accf/aha/acp/aats/pcna/scai/sts guideline for the diagnosis and management of patients with stable ischemic heart disease: A report of the american college of cardiology foundation/american heart association task force on practice guidelines, and the american college of physicians, american association for thoracic surgery, preventive cardiovascular nurses association, society for cardiovascular angiography and interventions, and society of thoracic surgeons. Circulation. 2012;126:e354–471. doi: 10.1161/CIR.0b013e318277d6a0. [DOI] [PubMed] [Google Scholar]
- 42.Beard AJ, Hofer TP, Downs JR, Lucatorto M, Klamerus ML, Holleman R, Kerr EA. Assessing appropriateness of lipid management among patients with diabetes mellitus: Moving from target to treatment. Circ Cardiovasc Qual Outcomes. 2013;6:66–74. doi: 10.1161/CIRCOUTCOMES.112.966697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Goldstein MK. Using health information technology to improve hypertension management. Curr Hypertens Rep. 2008;10:201–207. doi: 10.1007/s11906-008-0038-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.