Abstract
Three-dimensional (3D) cell culture systems have gained increasing interest in drug discovery and tissue engineering due to their evident advantages in providing more physiologically relevant information and more predictive data for in vivo tests. In this review, we discuss the characteristics of 3D cell culture systems in comparison to the two-dimensional (2D) monolayer culture, focusing on cell growth conditions, cell proliferation, population, and gene and protein expression profiles. The innovations and development in 3D culture systems for drug discovery over the past 5 years are also reviewed in the article, emphasizing the cellular response to different classes of anticancer drugs, focusing particularly on similarities and differences between 3D and 2D models across the field. The progression and advancement in the application of 3D cell cultures in cell-based biosensors is another focal point of this review.
Introduction
Cell-based assays have been an important pillar of the drug discovery process to provide a simple, fast, and cost-effective tool to avoid large-scale and cost-intensive animal testing. The key element—cultured cells—is the most critical part of such technique, since results are based on the cellular responses to drugs/compounds/external stimuli. To date, the majority of cell-based assays use traditional two-dimensional (2D) monolayer cells cultured on flat and rigid substrates. Although the time-honored 2D cell culture has proven to be a valuable method for cell-based studies, its limitations have been increasingly recognized. Since almost all cells in the in vivo environment are surrounded by other cells and extracellular matrix (ECM) in a three-dimensional (3D) fashion, 2D cell culture does not adequately take into account the natural 3D environment of cells. As a result, 2D cell culture tests sometimes provide misleading and nonpredictive data for in vivo responses.1–3 Currently, in drug discovery, the standard procedure of screening compounds starts with the 2D cell culture-based tests, followed by animal model tests, to clinical trials. Only about 10% of the compounds progress successfully through clinical development. Many of the drugs fail during clinical trials, especially during phase III, which is the most expensive phase of clinical development,4,5 largely due to the lack of clinical efficacy and/or unacceptable toxicity.6,7 A portion of these failures is attributed to data collected from the 2D monolayer culture tests in which the cellular response to drug(s) is altered due to their unnatural microenvironment. To lower the cost of failed compounds/molecules, the dismissal of ineffective and/or unacceptable toxic compounds should happen as early as possible, ideally before animal tests. Therefore, it is imperative to develop/establish in vitro cell-based systems that can more realistically mimic the in vivo cell behaviors and provide more predictable results to in vivo tests.
Recently, a growing body of evidence has suggested that 3D cell culture systems, in contrast to the 2D culture system, represent more accurately the actual microenvironment where cells reside in tissues. Thus, the behavior of 3D-cultured cells is more reflective of in vivo cellular responses. In fact, research has found that cells in the 3D culture environment differ morphologically and physiologically from cells in the 2D culture environment.8–10 It is the additional dimensionality of 3D cultures that is the crucial feature leading to the differences in cellular responses because not only does it influence the spatial organization of the cell surface receptors engaged in interactions with surrounding cells, but it also induces physical constraints to cells. These spatial and physical aspects in 3D cultures affect the signal transduction from the outside to the inside of cells, and ultimately influence gene expression and cellular behavior. It has been demonstrated that cell responses in 3D cultures are more similar to in vivo behavior compared to 2D culture.11–13 In the past several years, tremendous effort has been put into the development of a variety of 3D culture systems, as well as the adoption of 3D cell culture systems in drug discovery, cancer cell biology, stem cell study, engineered functional tissues for implantation, and other cell-based analysis. Such 3D culture systems provide excellent in vitro models, allowing the study of cellular responses in a setting that resembles in vivo environments.1,14–16
This article aims to review the following aspects of 3D cell culture systems primarily based on the literature published over the past 5 years: (1) the characteristics of 3D cultures from cell morphology, population, and proliferation, to gene and protein expression profiles of cells in 3D cultures in comparison to the 2D monolayer culture; (2) the cytotoxicity testing of different classes of anticancer drugs comparing 2D and 3D culture systems concentrating on the similarities and differences across the field, with a focus on the application in drug discovery; (3) the progress made in the development of 3D cell culture-based biosensors.
Discussion
Characteristics of 3D Cell Cultures Versus the Traditional 2D Cell Culture
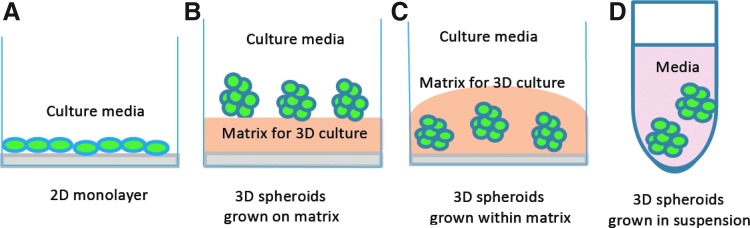
Figure 1 shows the schematic diagrams of the traditional 2D cell culture and three typical 3D cell cultures. While the traditional 2D culture usually grows cells into a monolayer on glass or, more commonly, tissue culture polystyrene plastic flasks (Fig. 1A), 3D cell cultures grow cells into 3D aggregates/spheroids using a scaffold/matrix (Fig. 1B, C) or in a scaffold-free manner (Fig. 1D). Scaffold/matrix-based 3D cultures can be generated by seeding cells on an acellular 3D matrix or by dispersing cells in a liquid matrix followed by solidification or polymerization. Commonly used scaffold/matrix materials include biologically derived scaffold systems and synthetic-based materials. Commercially available products such as BD Matrigel™ basement membrane matrix (BD Sciences), Cultrex® basement membrane extract (BME; Trevigen), and hyaluronic acid are commonly used biologically derived matrixes. Polyethylene glycol (PEG), polyvinyl alcohol (PVA), polylactide-co-glycolide (PLG), and polycaprolactone (PLA) are common materials used to form synthetic scaffolds.5,17–19 Scaffold-free 3D cell spheroids can be generated in suspensions by the forced floating method, the hanging drop method, or agitation-based approaches.5 More detailed information about a number of commercially available 3D cell culture systems, their specific features, as well as advantages and disadvantages of each type of the above-mentioned methods can be found in two of the most recent reviews.5,19 With each of these methods, cells grow naturally in a 3D environment, allowing cells to interact with each other, the ECM, and their microenvironment. In turn, these interactions in such 3D spatial arrangement affect a range of cellular functions, including cell proliferation, differentiation, morphology, gene and protein expression, and cellular responses to external stimuli. The following section reviews the characteristics of cells in 3D cultures in comparison to cells in traditional 2D culture.
Fig. 1.
Schematic diagrams of the traditional two-dimensional (2D) monolayer cell culture (A) and three typical three-dimensional (3D) cell culture systems: cell spheroids/aggregates grown on matrix (B), cells embedded within matrix (C), or scaffold-free cell spheroids in suspension (D).
Growth conditions, cell morphology, and population in 2D and 3D cultures
In traditional 2D monolayer culture, cells adhere and grow on a flat surface. Such monolayer setting allows all of the cells to receive a homogenous amount of nutrients and growth factors from the medium during growth.20 The monolayer is mainly composed of proliferating cells, since necrotic cells are usually detached from the surfaces and easily removed during medium change. Cells grown in 2D culture are usually more flat and stretched than they would appear in vivo. The abnormal cell morphology in 2D culture influences many cellular processes including cell proliferation, differentiation, apoptosis, and gene and protein expression.18 As a result, 2D-cultured cells may not behave as they would in the body because this model does not adequately mimic the in vivo microenvironment.21 Technologies, such as nano-patterning, which mimics the topographical features of the ECM, have been investigated to improve cellular function and behavior in 2D cell culture.22,23 However, whether or not these changes in cell function better emulate in vivo behaviors is still under investigation. The traditional 2D cell culture is still the most common in vitro test platform in drug screening.
As opposed to 2D monolayer culture, when grown in 3D culture systems, cells form aggregates or spheroids within a matrix, on a matrix, or in a suspension medium. In cell aggregates/spheroids, cell–cell interactions and cell–ECM interactions more closely mimic the natural environment found in vivo, so that the cell morphology closely resembles its natural shape in the body. In addition, 3D spheroids are comprised of cells in various stages, usually including proliferating, quiescent, apoptotic, hypoxic, and necrotic cells.24,25 The outer layers of a spheroid, which is highly exposed to the medium, are mainly comprised of viable, proliferating cells.25 The core cells receive less oxygen, growth factors, and nutrients from the medium, and tend to be in a quiescent or hypoxic state.26 Such cellular heterogeneity is very similar to in vivo tissues, particularly in tumors. Since the morphology and the interactions of cells grown in 3D culture is more similar to what occurs in vivo, the cellular processes of these cells also closely emulate what is seen in vivo.17
The proliferation rates of cells cultured in 3D and 2D are usually different, and are cell line and matrix dependent. A variety of cell lines showed reduced proliferation rate in 3D cultures compared to those cultured in 2D.17,27–31 For example, endometrial cancer cell lines Ishikawa, RL95-2, KLE, and EN-1078D in 3D reconstituted basement membrane (3D rBM) had reduced proliferation compared to cells in the 2D monolayer culture, which was detected by decreased expression of the proliferating cell nuclear antigen (PCNA) protein marker, and reduced total cell number in 3D rBM after 8 days of growth.27 Many other cell lines have also been shown to proliferate more slowly in 3D cultures than in 2D culture, such as colorectal cancer (CRC) cell lines CACO-2, DLD-1, HT-29, SW480, LOVO, COLO-206F on Laminin-rich-extracellular matrix (IrECM),29 human submandibular salivary gland (HSG) cell line on Matrigel,31 human embryonic kidney (HEK) 293 cell line on microspheres of cell-rat-tail collagen type I,30 and the human mammary epithelial cell line MCF10A on a complex 3D culture system based on stromal cells, silk scaffolds, and Matrigel.32 However, some cell lines showed an opposite proliferation rate, growing faster in 3D than 2D. For example, JIMT1 breast cancer cells grew 1.86-fold faster in Matrigel than in 2D culture.33 But interestingly, the same cell line cultured on the synthetic poly(2-hydroxyethylmethacrylate) (polyHEMA) 3D scaffold, exhibited a proliferation rate that was 7.2-fold slower than in 2D culture,33 which suggests that the cell proliferation rate is also related to the type of 3D model in which the cells are cultured. In general, the proliferation rate of cells grown in 3D culture better represent the growth of tumors in vivo compared to those cultured in an unnatural 2D environment.17
Most studies have shown that the cell viability in 3D cultures during the first few days (1–5 days) was not significantly different when compared to that in 2D culture.30,34 In some cases, 3D cultures showed slightly reduced cell viability29 when the culture time prolonged, and this was related to the structure of spheroids, which may result in the lack of oxygen and nutrients, and the accumulation of waste at the core of the spheroid as they grow larger.25
The structures of 3D spheroids
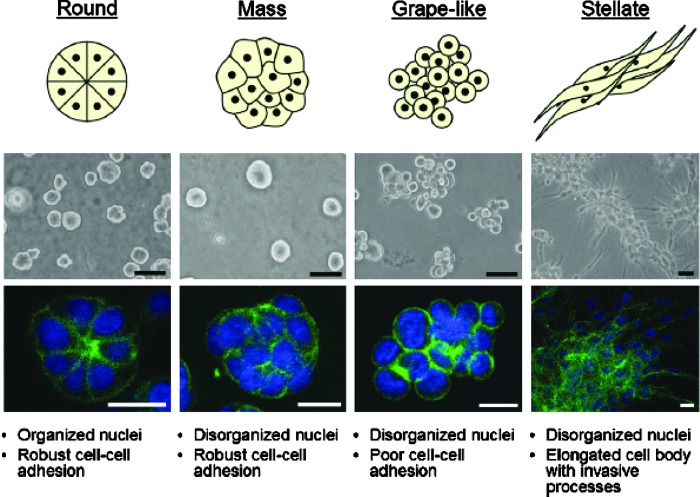
While various cell lines form nondistinct monolayers in 2D cell culture, distinctive differences in the structure of spheroids emerge as each cell line is cultured in 3D. Kenny et al.35 classified the structures of 3D spheroids formed by a panel of 25 breast cancer cell lines into four groups: round, mass, grape-like, and stellate structures. Figure 2 shows the four distinct structures of 3D spheroids formed by breast cancer cell lines.35 The round-type spheroid exhibits strong cell–cell adhesion, and the nuclei are regularly organized around the center of the colony. The round spheroids usually express tight cell junction proteins such as ZO-1. Colonies of round spheroids sometimes undergo lumen formation in the center,36 which usually occurs in a time-dependent manner.37 The mass-type spheroids are characterized by round colony outlines, disorganized nuclei, filled colony centers, and strong cellular communication. The mass spheroids are usually larger in diameter than round spheroids and overexpress luminal keratin 8 (KRT8) and keratin 18 (KRT18), yet lack a lumen. The grape-like spheroids display a distinguished grape-like appearance and usually have poor cell–cell interactions. The stellate-type spheroids are characterized by their invasive phenotype with stellate projections that often bridge multiple colonies and/or invade the matrixes.38
Fig. 2.
Four types of the structure of 3D spheroids based on the 3D cultures formed by a panel of 25 breast cancer cell lines. Reprinted from Kenny et al.35
Overall, the morphological appearance of 3D spheroids is primarily cell line dependent.29,35,37 However, the type of 3D model utilized may also affect cellular morphology.33 For example, Luca et al. noted that among the CRC cell lines they tested, those derived from metastatic cells formed grape-like morphology in lrECM matrix, whereas CRC cell lines established from primary tumor tissue formed mass spheroid morphology.29 They also found that spheroid morphology did not correlate with the migratory, invasive, or proliferative capacity of CRC cell lines. Hongisto et al.33 reported that the JIMT1 breast cancer cell line formed mass-shaped spheroids on both Matrigel and polyHEMA (poly(2-hydroxyethyl methacrylate)) matrix, but more uniformly shaped and sized spheroids on Matrigel were observed, while more single cells mixed with larger structures appeared on polyHEMA.
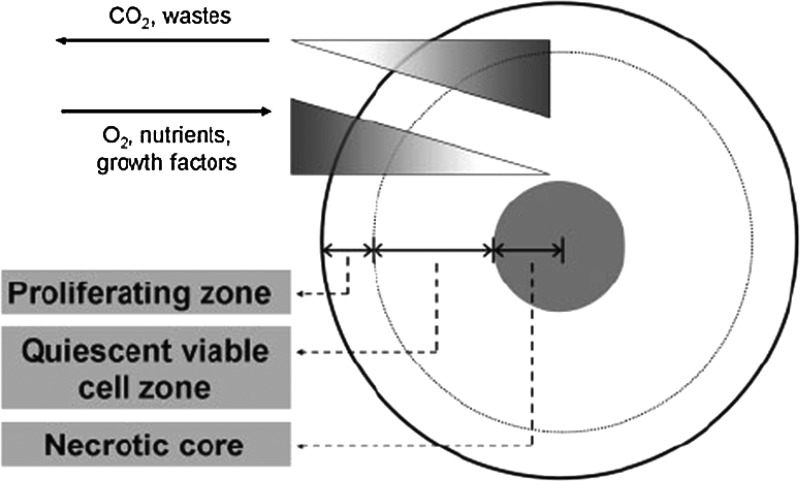
Although 3D culture systems provide a model that better mimics cell–cell interactions and cell–ECM interactions compared to the traditional 2D monolayer, the current 3D systems still lack the complex vascular systems that support tissues in vivo for oxygenation, nutrients, and waste removal. Cells grown in 3D culture perform these functions only by diffusion. For small spheroids, this system is not a problem, but for larger spheroids, this model still presents challenges. Figure 3 shows the schematic diagram of typical zones of cell proliferation and the distribution of diffused oxygen, CO2, and nutrition in a 3D cell spheroid.39 Such diffusion mode causes cells at different depths from the surface of the spheroid/aggregate to be in different nutritional states and, thus, at different stages of the cell cycle. Compared to 2D monolayer culture where uniformly rich oxygenation and nutrition are provided to all cells, the restricted oxygenation and nutrition environment in 3D spheroids/aggregates actually mimics the microenvironment of in vivo tissues to a certain extent.
Fig. 3.
The schematic diagram of typical zones of cell proliferation in a 3D spheroid, with the models of oxygenation, nutrition, and CO2 removal. Reprinted from Lin and Chang.39
Cell receptor, protein, and gene expression in 3D cultures versus 2D culture
In addition to the previously mentioned differences in physical and physiological properties, researchers have also found that cells in a 3D culture environment differ in gene, protein, and cell receptor expression from 2D-cultured cells.8–10 Various cancer cell lines grown in 2D and 3D culture often display different gene expression profiles, especially many genes that play a role in proliferation, angiogenesis, migration, invasion, and chemosensitivity.17,40 For example, Loessner et al. (2010) reported that ovarian cancer cells in 3D culture had significantly increased levels of mRNA expression of the cell surface receptors α3/α5/β1 integrins and the protease MMP9 compared to cells in 2D culture.41 In prostate cancer cells, the chemokine derived factor 1-alpha (SDF-1α), its primary receptor CXCR4, and the alternative SDF-1α–binding receptor CXCR7 highly regulate the metastatic process of prostate cancer, cell survival, and invasion. Kiss et al. (2013) recently demonstrated that cell–ECM interactions in 3D culture (on Matrigel) can modulate cell morphology and upregulate CXCR4 and CXCR7 expression in prostate cancer cell lines PC3, DU145, and LNCaP.42 Luca et al.29 reported the first systematic analysis of how the ECM in 3D cultures influence the phenotype and genotype of commonly used colorectal cancer (CRC) cell lines, including CACO-2, DLD-1, HT-29, SW480, LOVO, and COLO-206F. They investigated the expression levels of the epidermal growth factor receptor (EGFR) and the downstream activated kinases AKT (also known as protein kinase B) and p42/44 MAPK (mitogen-activated protein kinases), since EGFR stimulates proliferation via MAP-kinases, which has been established as a therapeutic target in the treatment of advanced CRC. The results showed not only the gene expression patterns in CRC cells growing in the lrECM 3D cell model was altered, but EGFR protein expression, phosphor-AKT, and phosphor-MAPK protein levels were altered as well compared to those in 2D culture. Decreased EGFR expression in lrECM-cultured cells seemed to be associated with an altered proliferative response to anti-EGFR therapy.29
Altered expression of proteins in 2D and 3D cultures has also been observed in cell lines other than cancer cell lines. For example, human submandibular salivary gland (HSG) cell line has been cultured in 3D models and evaluated for the development of salivary gland regeneration repair and tissue engineering treatments. Maria et al.31 found that HSG cells in 3D culture had an increase in the production/secretion of acinar proteins that were not associated with increases in mRNA transcription and a decrease in vimentin expression in comparison to those in 2D cells. They also found that the protein expression pattern associated with HSG in 3D culture was achieved through regulation of translation rather than the transcription of these relevant genes, while HSG cells in 2D might experience a protein translation defect.31
The differential gene and/or protein expression in 2D and 3D cell cultures is often the reason that the cells cultured in 3D systems behave differently in many cellular processes, including growth and proliferation, migration and invasion, morphology, and drug sensitivity in comparison to the cells cultured in 2D.17 Research has shown that transcriptional and translational changes have been associated with cell line adaptation.1 When tumor cells were removed out of their natural environment, the cells that continued to grow in vitro adapted to their new environment by changes at the transcriptional and translational levels. The comparison of gene expression levels between cell lines, including breast, colon, prostate, and lung cancer, and their tissue origins indicated that approximately 30% of genes are differently expressed in in vitro cell lines.1 Many genes that promote rapid growth and proliferation, as well as those that allow the cells to respond to factors in the culture medium, are often upregulated in cell lines in the 2D culture environment. Interestingly, the expression of genes that limit growth and proliferation are repressed in 2D-adapted cell lines compared to their corresponding tissue origins.1 This supports the observation that cells cultured in 2D culture models often proliferate more rapidly compared to cells cultured in 3D models. Although cells lose many of their native in vivo characteristics after being removed from the primary tumor and cultured in a 2D system, when the cells are placed back into an in vivo environment (i.e., an animal model) or grown in a reconstituted basement membrane that resembles the ECM, these differences in morphology, proliferation, and gene/protein expression are largely restored.1,35 This observation indicates that the 3D environment for cell adaption is critical in maintaining the transcriptional and translational functions and thus the gene and/or protein expression profiles to the most in vivo-like level, and ultimately making cells more likely to behave as they would in vivo.
3D multicellular culture systems
The 3D culture systems not only provide the spatial cell–cell interactions and cell–ECM interactions in monoculture for studying cell behaviors that mimic in vivo conditions, but also provide an opportunity for the co-culture of multiple types of cells to more closely mimic the in vivo conditions. Other types of cells interacting with the cells of interest play important roles in cell functions. Particularly, such multicellular systems provide a model for studying the role of stromal cells in tumors. The stromal cells of the tumor microenvironment play a major part in cancer, and their communication with cancer cells has been associated with the regulation of tumor growth, metastasis, and even treatment outcome, making them attractive targets for cancer treatment.43 For example, Wang et al.32 demonstrated that stromal cells played a critical role in regulating the functionality of mammary epithelial cells. MCF10A cells tricultured with human mammary fibroblasts (HMF) and human adipose-derived stem cells (hASCs) in 3D expressed the highest level of α-casein mRNA, which is a critical indicator for functional differentiation of mammary epithelial cells, than that in monoculture or co-culture with either HMF or hASCs. β-Casein mRNA expression was only detected in MCF10A cell triculture but not detectable in monoculture and either co-cultures. Pageau et al.44 generated a 3D multicellular model to study the effect of stromal components on the modulation of the phenotype of human bronchial epithelial cells, and found that primary human adult lung cancer-associated fibroblasts (LuCAFs) may alter the human bronchial epithelial cells (HBECs) by modifying biomechanical signals conveyed through the ECM, and altered the expression of six genes associated with immune responses, apoptosis, mitosis, cell survival, and differentiation. Shen et al. (2013) reported the first study to create a multicellular 3D culture of human adipose-derived stromal (ADS) cells and to investigate the capability of ADS cells in the 3D culture to undergo osteogenic differentiation. Their results indicated that ADS cells in the multicellular 3D culture exhibited increased differentiation potential and extracellular matrix production in comparison to the same cells cultured in 2D.45
Another possible approach to produce co-culture of different cell types is the use of cell sheet technology although this technique is mainly used in tissue engineering.46,47 Cell sheets are thin layers of cells connected to each other in a flat, sheet-like manner. They are produced by culturing cells on temperature responsive polymers in culture dishes. Overlaying of cell sheets has the potential to create thicker 3D structures. Studies have demonstrated a co-cultured sheet of hepatocyte and endothelial cells using patterned dual thermo-responsive surfaces48 and a double-layered co-culture overlaying an endothelial cell sheet onto a hepatocyte sheet.49 In these settings, hepatic cellular functions such as ammonium metabolism were proved to be enhanced by the hepatocyte–endothelial cell interactions.
Table 1 summarizes some of the key differences in 2D and 3D cell culture systems. Numerous studies have shown that the differential gene expression between cells cultured in 2D and 3D plays a major role in the differences in morphology, proliferation, and drug sensitivity observed in cells cultured in 2D and 3D.
Table 1.
Key Differences in Cellular Characteristics and Processes in Two-Dimensional and Three-Dimensional Culture Systems
| Cellular characteristics | 2D | 3D | Refs. |
|---|---|---|---|
| Morphology | Sheet-like flat and stretched cells in monolayer | Natural shape in spheroid/aggregate structures | 20,24,50 |
| Proliferation | Often proliferate at a faster rate than in vivo | May proliferate at a faster/slower rate compared to 2D-cultured cells depending on cell type and/or type of 3D model system | 17,51 |
| Exposure to medium/drugs | Cells in monolayer are equally exposed to nutrients/growth factors/drugs that are distributed in growth medium | Nutrients and growth factors or drugs may not be able to fully penetrate the spheroid, reaching cells near the core | 24,52 |
| Stage of cell cycle | More cells are likely to be in the same stage of cell cycle due to being equally exposed to medium | Spheroids contain proliferating, quiescent, hypoxic and necrotic cells | 18,24,53 |
| Gene/protein expression | Often display differential gene and protein expression levels compared to in vivo models | Cells often exhibit gene/protein expression profiles more similar to those in vivo tissue origins | 17,40,54 |
| Drug sensitivity | Cells often succumb to treatment and drugs appear to be very effective | Cells are often more resistant to treatment compared to those in 2D culture system, often being better predictors of in vivo drug responses | 17,33 |
2D, two-dimensional; 3D, three-dimensional.
3D Cell Cultures in Drug Discovery
Cell-based assays are the key tool used to assess the potential efficacy of a new compound in drug discovery. Capital investment in new pharmaceuticals to get from bench to clinical trial is estimated to be $1.3 billion,55 with only 21% of new compounds/molecules making it to phase I clinical trials. To get the most reliable results, the culture model used as the testing platform needs to perform similarly to the cells in vivo. The additional dimensionality of 3D cultures compared to 2D cultures not only influences the spatial organization of the cell surface receptors engaged in interactions with surrounding cells, but also induces physical constraints to the cells. These spatial and physical aspects in 3D cultures affect the signal transduction from the outside to the inside of cells, and ultimately influence gene expression and cellular behaviors.
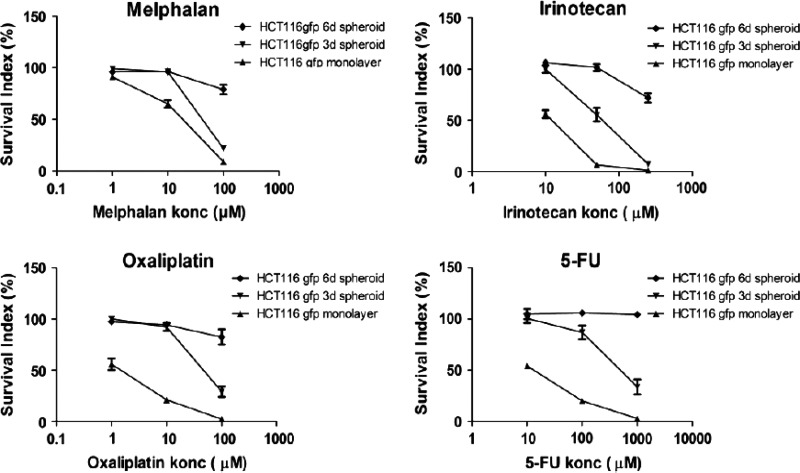
Cellular responses to drug treatments in 3D cultures have been shown to be more similar to what occurs in vivo compared to 2D culture.11–13 A number of studies have found that cells cultured in 3D models are more resistant to anticancer drugs than 2D cultures.41,56 For example, ovarian cancer cell survival and proliferation in 3D cultures after paclitaxel treatment was reduced by 40% or 60% in 3D cell spheroids, while the same treatment led to 80% reduced cell viability in the 2D cell monolayer.41 Karlsson et al.56 studied the drug sensitivity of colon cancer HCT-116 cells in a 3D spheroid and 2D monolayer model in response to four standard anticancer drugs (melphalan, 5-FU, oxaliplatin, and irinotecan) and two promising investigational cancer drugs (acriflavine and VLX50; Fig. 4). The results indicated that all drugs were highly active in 2D monolayer culture but generally less active and gradually lost their activity in 3D spheroids (6 day spheroids were more resistant than 3 day spheroids in Fig. 4), irrespective of different action mechanisms, suggesting that the geno- and phenotypical changes induced by 3D spheroids formation were associated with the increased drug resistance. The stronger drug resistance in 3D culture results primarily from signals from dynamic cellular interactions between neighboring cells and ECM input into the cellular decision-making process.57 The increased drug resistance in 3D culture can also be attributed to limited diffusion through the spheroid and to hypoxia, which has been shown to lead to the activation of genes involved in cell survival and drug sensitivity.53 Such chemoresistance developed in 3D spheroids is observed in vivo as well.58 A study using multicellular 3D culture of liver tumor cells as an in vitro model to test anticancer drugs further found that stromal cells also played a role in drug resistance of cancer cells.52
Fig. 4.
Cell survival obtained by fluorescent microculture cytotoxicity assay (FMCA), upon the treatment of four standard anticancer drugs to HCT-116 cells in 2D monolayer and 3 day and 6 day old 3D spheroids. Reprinted from Karlsson et al.56
Each year, a number of drug candidates fail in clinic trials due to low efficacy, adverse events, and other reasons. The Genetic Engineering and Biotechnology News reported a list of the top 10 biopharma clinical trial failures of 2013.59 Ziopharm Oncology announced that Palifosfamide, a DNA alkylating agent used to treat metastatic soft tissue sarcoma, failed Phase III clinical trials due to the drug being unable to provide patients with progression-free survival (PFS).59 The drug has shown cytotoxicity in all osteosarcoma (OS) cell lines tested in 2D cultures with IC50s in the low μM range except for OS222, which had an IC50 of 31.5 μM.60 The failure in this clinical trial illustrates that the results from 2D culture tests may sometimes be nonpredictive for in vivo tests. Genmab, an international biotechnology company specializing in the development of differentiated human antibody therapeutics for the treatment of cancer, has conducted a Phase III pivotal study of Zalutumumab. Zalutumumab is a fully human epidermal growth factor receptor (EGFR) monoclonal antibody, which has demonstrated clinical benefit in refractory head and neck cancer. The study failed to meet its primary endpoint.61 In an unrelated patent application,62 a new mechanism for resistance to anti-EGFR antibody was found. It involved a small cytosolic protein, fatty acid binding protein (FABP-3), which is expressed in a large proportion of clinical breast and colorectal carcinomas, but not in mammary epithelial cells in culture. FABP-3 induces a relocalization of EGFR to an intracellular compartment, which renders FABP-3 expressing cells resistant to anti-EGRF antibody.62 This resistance mechanism is not active in 2D cultures but is seen in 3D cell cultures and in-vivo in a xenograft model. Zalutumumab resistance may be or may not be related to FABP-3 mechanism. However, in some cases, if not all, earlier dismissal of drug candidates could be possible if better predictive in vitro models are available.
The differences in cellular responses between 2D and 3D cultures are possibly due to, but not limited to, the following aspects. First, the difference in physical and physiological properties between 3D and 2D cultures. While the 2D-cultured cells are stretched out in an unnatural state on a flat substrate, cells cultured in 3D on a biological or synthetic scaffold material maintain a normal morphology. Gurski et al. attributed this morphological spread for the differences in response to drug between 2D and 3D cultures.17 Second, the difference in the expression and the spatial organization of surface receptors in 3D and 2D culture. Many drugs are designed to target specific receptors on cell surfaces. The expression level of receptors and the binding efficiency of a drug to these receptors may be different in 3D and 2D cultures due to the differences in structure, localization, and spatial arrangement of these receptors on cell surfaces.29 Third, the difference in cancer gene expression levels. Cells growing in 2D monolayer are under stress and therefore some genes and proteins being expressed are altered as a result of this unnatural state. These genes and proteins may be engaged in drug actions and thus affect the effectiveness of a drug to the cells. Fourth, the difference in cell stages. While cells in 2D culture are mostly proliferating cells, cells in 3D cultures usually are a mixture of cells at different stages. Wen et al. indicated that larger spheroids are likely to be heterogeneous, having proliferating cells on the outer region and quiescent cells in the inner region due to lack of nutrients and gas exchange. Active cell proliferation is sometimes required for some drugs to be effective.63 Examples of drugs that require active proliferation to be effective are 5-fluorouracil (5-FU) and doxorubicin. Tung et al.64 showed that the lack proliferating cells in A431.H9 3D cultures resulted in 100-fold increase in resistance to 5-FU by culture method. Chitcholtan et al. observed the same pattern to a lesser degree with doxorubicin and KLE endometrial cells.27 Fifth, the difference in drug accessibility to cells and local pH. While drugs diffuse to cells in the 2D monolayer equally, drug diffusion to cells in a spheroid may be at variable concentrations depending on the depth to the surface where the cells located. The depth of cell local is also related to the local pH of the cells. Chitcholtan et al. found that regions of hypoxia may exist due to lack of a transport system to remove waste from the center of the spheroid.27 Swietach et al. highlighted the importance of intracellular pH on determining the efficacy of weakly basic chemotherapeutic drugs such as doxorubicin, by showing that a lower pH reduces drug uptake, contributing to drug resistance.65
As cancer therapeutics are moving toward targeted therapy, it is noted that 3D culture holds the promise in finding new targets that were not apparent from traditional 2D culture studies. Nam et al. demonstrated that human breast cancer cells in 3D culture overexpressed a cell adhesion molecule α5β1-integrin compared to nonmalignant cells, which can be specifically targeted for therapy.66 They then found a small peptide inhibitor that can prevent α5β1-integrin from binding to fibronectin, thus increases the cell killing effect of radiation. In another study, Michaylira et al.67 used 3D cell culture techniques to find that another cell adhesion molecule, periostin, which boosts the invasiveness of esophageal tumors, could be useful in search for new therapeutic targets for esophageal cancer. These studies highlight the value of 3D cell culture techniques in revealing new targets for cancer therapeutics.
While many studies on 3D cultures used established cancer cell lines, more recently, the use of patient-derived primary tumor cells in 3D cultures has been reported.68,69 Although this technology has not been optimized, it is recognized that its major advantage is the ability to use the same tumor model in vitro and in vivo. Praveen et al. (2012) used Cell-able plates (COSMO Bio) to culture patient-derived primary tumor cells, and evaluated cellular responses to antiproliferative cytotoxic and targeted agents.69 Oncotest, a spin-off company founded in 1993 by Prof. Fiebig in Germany, is a pioneer in the field of patient-derived tumor xenografts (PDX). The company has developed more than 200 of their PDX models available for tests in 3D assay systems.70 A research team from Osaka Medical Center for Cancer and Cardiovacular Disease in Japan reported a successful method for 3D culture of patient-derived colorectal cancer cells68 and the use of such 3D culture for the evaluation of chemosensitivity and signal pathway activation in cancer cells from individual patients. These studies showed the potential of 3D culture of patient-derived primary tumor cells in studying cancer biology and the development of personalized medicine.
Obviously, 3D cell culture has gained increasing attention in drug screening. However, many currently available 3D cell culture techniques are time consuming, expensive, and lack reproducibility. Scientists have been making an effort to develop standard and rapid protocols for using 3D cultures in drug screening.71 Others seek simply to develop a reproducible 3D cell culture platform for high throughput screening through the identification and validation of ubiquitous three-dimensionality biomarkers, such as the cytokine family.72 Table 2 summaries the key advantages and disadvantages of 3D cell culture systems for applications in drug discovery.
Table 2.
Key Strengths and Weaknesses of the Currently Available Three-Dimensional Cell Culture Systems for Drug Discovery Applications
| Strengths |
|---|
| • Matrices contain ECM components that lead to increased cell–cell contact, communication, and signaling pathway activation17 |
| • Cell functional and morphological differentiation can be largely restored to what is seen in vivo35,73 |
| • Culture condition can be modified to include factors/proteins found in particular tumor microenvironment17 |
| • Heterogeneous cell population are similar to cells of tumor that are in different phases of cell cycle including proliferating, hypoxic, and necrotic cells24,53 |
| • The gene and protein expression levels of cells and thus the cellular behaviors are similar to in vivo levels17,27–31,40 |
| • Provide in vitro models for including different types of cells to build multicellular systems52 |
| • Bridges the gap between in vitro and in vivo drug screening, possibly decreasing the use of animal models5 |
| Weaknesses |
|---|
| • Variability in biologically derived matrices may cause nonreproducible experimental results18 |
| • Much more expensive for large-scale studies and high throughput assays than traditional 2D culture17 |
| • Some models produce spheroids that vary greatly in size, resulting in high variability within the same well/flask17 |
| • 3D models still lack vasculature which plays a vital role in tumor growth/survival and drug delivery74,75 |
3D Cell Cultures in Cell-Based Biosensors
Sensing element—2D cell culture versus 3D cell cultures
Cell-based biosensors have become an important pillar of the drug discovery process by serving as a simple, fast, and cost-effective alternative to large-scale and cost-intensive animal testing. The sensing element—cultured cells—is the most critical part of a cell-based biosensor for detecting analyte fluctuations in the local environment and relaying these changes to a signal transducer interfaced with the element.76 Although 2D and 3D cell cultures are suitable for exploring cellular responses to drugs, toxins, and biomaterials,77 only 3D cultures exhibit a hierarchical structure and cellular heterogeneity that can closely mimic in vivo cell morphology and function (proliferation, differentiation, gene expression, etc.),78,79 cell–cell interactions, and diffusion barriers. Therefore, 3D models can better replicate intrinsic physiological conditions and in vivo cellular responses to external stimuli compared to the 2D monolayer.77,78,80–82 As such, 3D-cultured cells can be the ideal sensing element in cell-based sensors to provide the most biologically relevant information and predictive data for in vivo tests.
Cells grown in 3D culture can be incorporated into a biosensor either by direct attachment onto a biotic or abiotic substrate surface or by indirect attachment via entrapment in a biocompatible biopolymer.83,84 Substrate selection depends on both the cell line and the sensor's application; most biosensors use silicon, glass, or plastic surfaces. Silicon offers advantageous electrical and mechanical properties but lacks optical transparency, thus limiting the utility of luminescent and fluorescent analytical techniques.83 Glass offers optical transparency, and commercial wafers are available in a wide variety of compositions and sizes,83 allowing for facile substrate selection and visual observation of cellular responses via fluorescent reporters and high resolution imaging systems.84 Plastics, although inexpensive, are autofluorescent and have a tendency to take up water and other solvents—attributes that can skew fluorescence signals from the sensing element.83
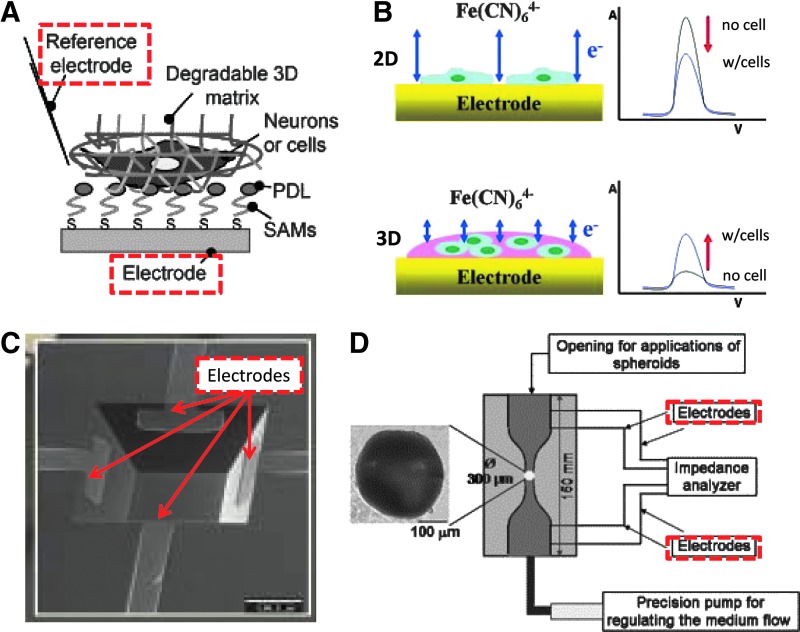
The two main 3D culture models—3D spheroids and cells embedded in different types of 3D matrixes—have been incorporated into 3D cell culture biosensors as the sensing element using specifically designed devices. One of most commonly used transduction techniques for 3D cell-based biosensors is the electrical/electrochemical biosensors. Figure 5 shows three typical designs that have been successfully used for developing 3D cell culture-based electrical/electrochemical biosensors. Figure 5A shows an electrochemical biosensor that incorporates the 3D culture in a synthetic hydrogel on a flat electrode surface.85 Figure 5B shows the transduction principle and the resulting signals of the 2D and 3D culture-based electrochemical biosensors (Fig. 5B).78 In the 2D culture-based sensor, as cells proliferate on the electrode, the electrical current decreased due to the increasing coverage of cells on the electrode, compared to the electrode without cells. On the other hand, the resultant change in electrical current signal in 3D culture-based sensor is opposite, signal increases with cell proliferation. Since hydrogels are weakly conductive or nonconductive, the electrical current of the sensor with hydrogels is weak or small. When cells are cultured in hydrogels, the gap junctions of cells possibly act as channels through the cell membrane, allowing electrical connections among neighboring cells. The other possibility is that cell growth in hydrogels leads to hydrogel degradation, making them less resistant, thus resulting in an increased electric current. Lin et al. used a flat microelectrode array to monitor the impedance change during the growth of NIH 3T3 fibroblasts and cortical neurons in a 3D matrix in a label-free manner.85 Jeong et al. reported a similar design of an electrochemical biosensor using several types of sol-gels (alginate, collagen, Matrigel) to grow A549 lung cancer cells in 3D on flat electrodes.78 Cellular responses to anticancer drugs were successfully monitored.
Fig. 5.
(A) Schematic diagram of an electrochemical biosensor with 3D cell culture in degradable matrix on a flat electrode. Reprinted from Lin et al.85 (B) The comparison of the principle and the typical signals of the electrochemical biosensors with 2D and 3D cell cultures. Reprinted from Jeong et al.78 (C) A cavity-based electric impedance biosensor for 3D cell spheroids. Reprinted from Kloss et al.88 (D) A capillary-based electric impedance biosensor for 3D cell spheroids. Reprinted from Hildebrandt et al.90
Many 3D cell culture-based biosensors use the similar design as Figure 5A using natural or synthetic hydrogels to create 3D cell structures, since such systems can better represent the in vivo distribution of metabolites, nutrients, oxygen, and signaling molecules,80 and more accurately mimic 3D tissue architecture, cell proliferation, motility, and migration via an artificial extracellular matrix.86 The wide variety of commercially available hydrogels promotes facile selection of one or more suitable matrices for each cell line and/or biosensor application and helps overcome the disadvantageous features of some polymer matrices, such as variable compositions and properties (animal-based hydrogels) and requisite cytotoxic pretreatment steps (some synthetic hydrogels).84 Collagen, collagen-chitosan, Matrigel, and fibrinogen matrices, alginate, Puramatrix scaffolds, and polyethylene glycol matrices have been used to fabricate 3D cell culture biosensors using various cell lines for drug screening, toxicology assays, and cell-biomaterial interaction screening, and for the identification of unknown pathogens and toxins.80,86,87
An alternative to the hydrogel matrix for cell lines that reach confluence quickly or that naturally have little to no extracellular matrix is a gel-free system that uses an intercellular polymeric linker to create 3D cellular aggregates supported by neighboring cells.84 Ong et al. produced the gel-free 3D system using two carcinoma cell lines and a primary bone marrow mesenchymal stem line. These 3D cell aggregates not only preserved cell viability and 3D morphology but also exhibited greater cell function when compared to similar 2D monolayer.84
Others biosensors are designed to incorporate 3D cell spheroids directly as the sensing element. Figure 5C shows a microcavity equipped with four electrodes each at one side of the cavity for impedance measuring of 3D spheroids.88 Figure 5D shows a microcapillary system with electrodes at both opening ends that can measure the electrical impedance of spheroids.15,89,90 In both the microcavity and the microcapillary designs, it is important that the size of the cavity or capillary match with the size of spheroids, allowing the spheroids to be adequately analyzed by maintaining their shape and properties during measurements.88 In these designs, the electrical behavior of the spheroid includes the effective extracellular resistance of the spheroid, Rext; the effective intracellular resistance of the spheroid, Rint; the effective cell membrane capacitance of the spheroid, Cmem; the electrical behavior of the detection system, which includes the stray capacitance of the system, Cs; and the resistance of the culture medium, Rmed.89 Kloss et al. demonstrated that drug-induced apoptosis in spheroids displayed increased impedance magnitudes, resulting from an altered morphology of the outer cells, using the microcavity system.88 Hildebrandt et al. used the capillary system to measure the impedance of mesenchymal stem cell (MSC) spheroids at three different conditions.90
Applications of 3D cell culture-based biosensors
Although the versatility of 3D cell-based biosensors gives them a plethora of biomedical and bioanalytical applications,91 including early detection and chronic management of illness92 and environmental monitoring,93 biosensors are prolific in pathogen testing, toxicology assays, and drug screening.
Anticancer drug screening has been an important application for 3D cell culture biosensors because the biosensor-based assays enable multiplex analysis of different drugs and/or cancer cells.94 Lee et al. created the DataChip, a miniaturized 3D array of alginate-encapsulated cells capable of high throughput drug screening, to measure the response of breast cancer cells and human liver hepaocellular carcinoma cells to 3 and 27 drug candidates respectively in as few as 6 h.13 In both cases, the data agreed with values obtained from 2D/3D cell cultures in 96-well plates or from the literature, demonstrating that the 3D array's miniaturization has no appreciable effect on either cell line's cytotoxicity responses.13 Other researchers have used 3D cell-based electrochemical biosensors to quantify cancer cell resistance to drug compounds78,95 and to study cancer cell proliferation in the presence of anticancer drugs.94
The application of 3D cell culture biosensors in drug screening can also be beneficial for studying cells other than cancer cells. Daus et al. studied action potential emissions from 2D and 3D cellular networks of primary cardiac myocytes and discovered that the 3D network continued emitting action potentials for multiple weeks, as opposed to the 2D network's cessation after 9–11 divisions, thus expanding the ability of cardioactive drug discovery studies to predict long-term in vivo responses.79
Three-dimensional cell-based biosensors have also been designed to detect pathogens or toxins. Banerjee et al.96 created a cell-based, colorimetric sensing system that uses collagen-encapsulated B-hybridoma cells to detect rapidly viable cells of pathogenic Listeria, toxin listeriolysin O, and enterotoxin from Bacillus species in less than 10 h via quantifiable alkaline phosphatase release. The biosensor both simplified the assay and showed no statistically significant difference in percent cytoxicity values from the 2D assay format, confirming that 3D hybridoma cell biosensors maintain cellular sensitivity to pathogens.96
Another application of 3D biosensors is the rapid screening of cell–biomaterial interactions since biomaterial properties can modify cell behavior by influencing cell proliferation, survival, shape, migration, differentiation, as well as gene expression.80 Wang et al. designed a 3D tricellular composite that more accurately replicates human breast tissue than both co-cultures and monocultures by encapsulating human mammary epithelial cells, human fibroblasts, and adipocytes in a Matrigel-collagen gel.32 This model is capable of bridging the gap between 2D cell culture models and whole-animal systems by helping clarify the role of cell–cell and cell–substrate interactions in neoplastic transformation and by better understanding how breast epithelium responds to ovarian hormones and induction of specific protein synthesis.32 Screening cell–biomaterial interactions also has immediate use in medical diagnostics. Nguyen et al.97 used electrical cell–substrate impedance sensing to investigate the migration of metastatic and nonmetastatic human mammary gland/breast cells through Matrigel. Each cell line showed a disparate impedance change, suggesting the biosensor can differentiate between metastatic and nonmetastatic cells. Also, because the sensor could track single cells in a 3D matrix, functions such as individual cell adhesion, spreading, and migration could be monitored.97
Conclusions
It is increasingly evident that 3D cell culture models are better models than the traditional 2D monolayer culture due to improved cell–cell interactions, cell–ECM interactions, and cell populations and structures that resemble in vivo architecture. In the past several years, a huge variety of 3D cell culture systems have been created as experimental tools for diverse research purposes. There is no doubt that 3D culture systems hold great promise for applications in drug discovery, cancer cell biology, stem cell research, and many other cell-based analyses and devices, by bridging the traditional 2D monolayer cell culture to animal models. While the 3D cell culture models are currently widely studied in academia with a focus on creation of 3D systems with excellent biological relevance, there are still many hurdles that must be overcome before these systems can be widely accepted in industry. Recent developments clearly indicate that the transition from 2D to 3D cell cultures for industry applications is promising, but the maturity of the technology and the cost are still the main concerns in making this transition possible. Much effort is still needed to assure reproducibility, high throughput analysis, compatible readout techniques, and automation in order to establish standardized and validated 3D cell culture models.
Abbreviations Used
- 2D
two-dimensional
- 3D
three-dimensional
- 5-FU
5-fluorouracil
- ADS
adipose-derived stromal (cells)
- Cmem
cell membrane capacitance
- CRC
colorectal cancer
- Cs
stray capacitance
- CXCR
chemokine receptor
- ECM
extracellular matrix
- EGFR
epidermal growth factor receptor
- FABP
fatty acid binding protein
- hASCs
human adipose-derived stem cells
- HBECs
human bronchial epithelial cells
- HEK
human embryonic kidney
- HMF
human mammary fibroblasts
- HSG
human submandibular gland
- KRT
keratin
- lrECM
laminin-rich extracellular matrix
- LuCAFs
lung cancer-associated fibroblasts
- MAPK
mitogen-activated protein kinase
- MMP9
matrix metalloproteinase 9
- MSC
mesenchymal stem cell
- PCNA
proliferating cell nuclear antigen
- PDX
patient-derived xenograft
- PEG
polyethylene glycol
- PLA
polycaprolactone
- PLG
polylactide-co-glycolide
- polyHEMA
poly-2-hydroxyethyl methacrylate
- PVA
polyvinyl alcohol
- rBM
reconstituted basement membrane
- Rext
extracellular resistance
- Rint
intracellular resistance
- Rmem
resistance of culture medium
- SDF-1
stromal cell-derived factor 1
Acknowledgment
This work was supported by the National Science Foundation (NSF), CBET #1159871.
Disclosure Statement
No competing financial interests exist.
References
- 1.Birgersdotter A, Sandberg R, Ernberg I: Gene expression perturbation in vitro—a growing case for three-dimensional (3D) culture systems. Semin Cancer Biol 2005;15:405–412 [DOI] [PubMed] [Google Scholar]
- 2.Weaver VM, Petersen OW, Wang F, et al. : Reversion of the malignant phenotype of human breast cells in three-dimensional culture and in vivo by integrin blocking antibodies. J Cell Biol 1997;137:231–245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bhadriraju K, Chen CS: Engineering cellular microenvironments to improve cell-based drug testing. Drug Discov Today 2002;7:612–620 [DOI] [PubMed] [Google Scholar]
- 4.DiMasi JA, Grabowski HG: Economics of new oncology drug development. J Clin Oncol 2007;25:209–216 [DOI] [PubMed] [Google Scholar]
- 5.Breslin S, O'Driscoll L: Three-dimensional cell culture: the missing link in drug discovery. Drug Discov Today 2013;18:240–249 [DOI] [PubMed] [Google Scholar]
- 6.Hopkins AL: Network pharmacology: the next paradigm in drug discovery. Nat Chem Biol 2008;4:682–690 [DOI] [PubMed] [Google Scholar]
- 7.Kola I: The state of innovation in drug development. Clin Pharmacol Ther 2008;83:227–230 [DOI] [PubMed] [Google Scholar]
- 8.Baharvand H, Hashemi SM, Kazemi Ashtiani S, Farrokhi A: Differentiation of human embryonic stem cells into hepatocytes in 2D and 3D culture systems in vitro. Int J Dev Biol 2006;50:645–652 [DOI] [PubMed] [Google Scholar]
- 9.Benya PD, Shaffer JD: Dedifferentiated chondrocytes reexpress the differentiated collagen phenotype when cultured in agarose gels. Cell 1982;30:215–224 [DOI] [PubMed] [Google Scholar]
- 10.Nelson CM, Bissell MJ: Modeling dynamic reciprocity: engineering three-dimensional culture models of breast architecture, function, and neoplastic transformation. Semin Cancer Bio. 2005;15:342–352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shield K, Ackland ML, Ahmed N, Rice GE: Multicellular spheroids in ovarian cancer metastases: biology and pathology. Gynecol Oncol 2009;113:143–148 [DOI] [PubMed] [Google Scholar]
- 12.Zietarska M, Maugard CM, Filali-Mouhim A, et al. : Molecular description of a 3D in vitro model for the study of epithelial ovarian cancer (EOC). Mol Carcinog 2007;46:872–885 [DOI] [PubMed] [Google Scholar]
- 13.Lee J, Cuddihy MJ, Kotov NA: Three-dimensional cell culture matrices: state of the art. Tissue Eng Part B Rev 2008;14:61–86 [DOI] [PubMed] [Google Scholar]
- 14.Justice BA, Badr NA, Felder RA: 3D cell culture opens new dimensions in cell-based assays. Drug Discov Today 2009;14:102–107 [DOI] [PubMed] [Google Scholar]
- 15.Reininger-Mack A, Thielecke H, Robitzki AA: 3D-biohybrid systems: applications in drug screening. Trends Biotechnol 2002;20:56–61 [DOI] [PubMed] [Google Scholar]
- 16.Sun T, Jackson S, Haycock JW, MacNeil S: Culture of skin cells in 3D rather than 2D improves their ability to survive exposure to cytotoxic agents. J Biotechnol 2006;122:372–381 [DOI] [PubMed] [Google Scholar]
- 17.Gurski L, Petrelli N, Jia X, Farach-Carson M: Three-dimensional matrices for anti-cancer drug testing and development. Oncol Issues 2010;25:20–25 [Google Scholar]
- 18.Tibbitt MW, Anseth KS: Hydrogels as extracellular matrix mimics for 3D cell culture. Biotechnol Bioeng 2009;103:655–663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rimann M, Graf-Hausner U: Synthetic 3D multicellular systems for drug development. Curr Opin Biotechnol 2012;23:803–809 [DOI] [PubMed] [Google Scholar]
- 20.Huang H, Ding Y, Sun XS, Nguyen TA: Peptide hydrogelation and cell encapsulation for 3D culture of MCF-7 breast cancer cells. PLoS One 2013;8:e59482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huh D, Hamilton GA, Ingber DE: From 3D cell culture to organs-on-chips. Trends Cell Biol 2011;21:745–754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim D-H, Provenzano PP, Smith CL, Levchenko A: Matrix nanotopography as a regulator of cell function. J Cell Biol 2012;197:351–360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen W, Villa-Diaz LG, Sun Y, et al. : Nanotopography influences adhesion, spreading, and self-renewal of human embryonic stem cells. ACS Nano 2012;6:4094–4103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim JB: Three-dimensional tissue culture models in cancer biology. Semin Cancer Biol 2005;15:365–377 [DOI] [PubMed] [Google Scholar]
- 25.Khaitan D, Chandna S, Arya MB, Dwarakanath BS: Establishment and characterization of multicellular spheroids from a human glioma cell line; Implications for tumor therapy. J Transl Med 2006;4:1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mehta G, Hsiao AY, Ingram M, Luker GD, Takayama S: Opportunities and challenges for use of tumor spheroids as models to test drug delivery and efficacy. J Control Release 2012;164:192–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chitcholtan K, Sykes P, Evans J: The resistance of intracellular mediators to doxorubicin and cisplatin are distinct in 3D and 2D endometrial cancer. J Transl Med 2012;10:1–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fallica B, Maffei JS, Villa S, Makin G, Zaman M: Alteration of cellular behavior and response to PI3K pathway inhibition by culture in 3D collagen gels. PLoS One 2012;7:e48024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Luca AC, Mersch S, Deenen R, et al. : Impact of the 3D microenvironment on phenotype, gene expression, and EGFR inhibition of colorectal cancer cell lines. PLoS One 2013;8:e59689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wong S, El-Gamal A, Griffin P, Nishi Y, Pease F, Plummer J: Monolithic 3D integrated circuits. Paper presented at VLSI Technology, Systems and Applications 2007 (VLSI-TSA 2007), Hsinchu, Taiwan, April23–25, 2007 [Google Scholar]
- 31.Maria OM, Maria O, Liu Y, Komarova SV, Tran SD: Matrigel improves functional properties of human submandibular salivary gland cell line. Int J Biochem Cell Biol 2011;43:622–631 [DOI] [PubMed] [Google Scholar]
- 32.Wang X, Sun L, Maffini MV, Soto A, Sonnenschein C, Kaplan DL: A complex 3D human tissue culture system based on mammary stromal cells and silk scaffolds for modeling breast morphogenesis and function. Biomaterials 2010;31:3920–3929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hongisto V, Jernstrom S, Fey V, et al. : High-throughput 3D screening reveals differences in drug sensitivities between culture models of JIMT1 breast cancer cells. PLoS One 2013;8:e77232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gurski LA, Jha AK, Zhang C, Jia X, Farach-Carson MC: Hyaluronic acid-based hydrogels as 3D matrices for in vitro evaluation of chemotherapeutic drugs using poorly adherent prostate cancer cells. Biomaterials 2009;30:6076–6085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kenny PA, Lee GY, Myers CA, et al. : The morphologies of breast cancer cell lines in three-dimensional assays correlate with their profiles of gene expression. Mol Oncol 2007;1:84–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sodunke TR, Turner KK, Caldwell SA, McBride KW, Reginato MJ, Noh HM: Micropatterns of Matrigel for three-dimensional epithelial cultures. Biomaterials 2007;28:4006–4016 [DOI] [PubMed] [Google Scholar]
- 37.Chitcholtan K, Asselin E, Parent S, Sykes PH, Evans JJ: Differences in growth properties of endometrial cancer in three dimensional (3D) culture and 2D cell monolayer. Exper Cell Res 2013;319:75–87 [DOI] [PubMed] [Google Scholar]
- 38.Härmä V, Virtanen J, Mäkelä R, et al. : A comprehensive panel of three-dimensional models for studies of prostate cancer growth, invasion and drug responses. PLoS One 2010;5:e10431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lin RZ, Chang HY: Recent advances in three-dimensional multicellular spheroid culture for biomedical research. Biotechnol J 2008;3:1172–1184 [DOI] [PubMed] [Google Scholar]
- 40.Price KJ, Tsykin A, Giles KM, et al. : Matrigel basement membrane matrix influences expression of microRNAs in cancer cell lines. Biochem Biophys Res Commun 2012;427:343–348 [DOI] [PubMed] [Google Scholar]
- 41.Loessner D, Stok KS, Lutolf MP, Hutmacher DW, Clements JA, Rizzi SC: Bioengineered 3D platform to explore cell–ECM interactions and drug resistance of epithelial ovarian cancer cells. Biomaterials 2010;31:8494–8506 [DOI] [PubMed] [Google Scholar]
- 42.Kiss DL, Windus LCE, Avery VM: Chemokine receptor expression on integrin-mediated stellate projections of prostate cancer cells in 3D culture. Cytokine 2013;64:122–130 [DOI] [PubMed] [Google Scholar]
- 43.Li H, Fan X, Houghton J: Tumor microenvironment: the role of the tumor stroma in cancer. J Cell Biochem 2007;101:805–815 [DOI] [PubMed] [Google Scholar]
- 44.Pageau SC, Sazonova OV, Wong JY, Soto AM, Sonnenschein C: The effect of stromal components on the modulation of the phenotype of human bronchial epithelial cells in 3D culture. Biomaterials 2011;32:7169–7180 [DOI] [PubMed] [Google Scholar]
- 45.Shen FH, Werner BC, Liang H, et al. : Implications of adipose-derived stromal cells in a 3D culture system for osteogenic differentiation: an in vitro and in vivo investigation. Spine J 2013;13:32–43 [DOI] [PubMed] [Google Scholar]
- 46.Matsuda N, Shimizu T, Yamato M, Okano T: Tissue engineering based on cell sheet technology. Adv Mater 2007;19:3089–3099 [Google Scholar]
- 47.Kawaguchi N, Hatta K, Nakanishi T: 3D-culture system for heart regeneration and cardiac medicine. Biomed Res Int 2013;2013:895967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tsuda Y, Kikuchi A, Yamato M, Chen G, Okano T: Heterotypic cell interactions on a dually patterned surface. Biochem Biophys Res Commun 2006;348:937–944 [DOI] [PubMed] [Google Scholar]
- 49.Harimoto M, Yamato M, Hirose M, et al. : Novel approach for achieving double-layered cell sheets co-culture: overlaying endothelial cell sheets onto monolayer hepatocytes utilizing temperature-responsive culture dishes. J Biomed Mater Res 2002;62:464–470 [DOI] [PubMed] [Google Scholar]
- 50.Baker BM, Chen CS: Deconstructing the third dimension—how 3D culture microenvironments alter cellular cues. J Cell Sci 2012;125:3015–3024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xu X, Gurski LA, Zhang C, Harrington DA, Farach-Carson MC, Jia X: Recreating the tumor microenvironment in a bilayer, hyaluronic acid hydrogel construct for the growth of prostate cancer spheroids. Biomaterials 2012;33:9049–9060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yip D, Cho CH: A multicellular 3D heterospheroid model of liver tumor and stromal cells in collagen gel for anti-cancer drug testing. Biochem Biophys Res Commun 2013;433:327–332 [DOI] [PubMed] [Google Scholar]
- 53.Trédan O, Galmarini CM, Patel K, Tannock IF: Drug resistance and the solid tumor microenvironment. J Nat Cancer Inst 2007;99:1441–1454 [DOI] [PubMed] [Google Scholar]
- 54.Szot CS, Buchanan CF, Freeman JW, Rylander MN: 3D in vitro bioengineered tumors based on collagen I hydrogels. Biomaterials 2011;32:7905–7912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.DiMasi JA, Hansen RW, Grabowski HG: The price of innovation: new estimates of drug development costs. J Health Econ 2003;22:151–185 [DOI] [PubMed] [Google Scholar]
- 56.Karlsson H, Fryknäs M, Larsson R, Nygren P: Loss of cancer drug activity in colon cancer HCT-116 cells during spheroid formation in a new 3-D spheroid cell culture system. Exper Cell Res 2012;318:1577–1585 [DOI] [PubMed] [Google Scholar]
- 57.Walker DM, Boey G, McDonald LA: The pathology of oral cancer. Pathology 2003;35:376–383 [DOI] [PubMed] [Google Scholar]
- 58.Sodek KL, Ringuette MJ, Brown TJ: Compact spheroid formation by ovarian cancer cells is associated with contractile behavior and an invasive phenotype. Int J Cancer 2009;124:2060–2070 [DOI] [PubMed] [Google Scholar]
- 59.News GEaB. Top 10 Clinical Trial Failures of 2013. Top 10 Clinical Trial Failures of 2013. www.genengnews.com/insight-and-intelligenceand153/top-10-clinical-trial-failures-of-2013/77900029/?page=1 (last accessed on February3, 2014)
- 60.Hingorani P, Zhang W, Piperdi S, et al. : Preclinical activity of palifosfamide lysine (ZIO-201) in pediatric sarcomas including oxazaphosphorine-resistant osteosarcoma. Cancer Chemother Pharmacol 2009;64:733–740 [DOI] [PubMed] [Google Scholar]
- 61.Genmab. Zalutumumab. www.genmab.com/partnering/licensing-opportunities/products (last accessed on April7, 2014)
- 62.Ivaska J, Nevo J: Assessing risk of metastases and/or ddfs of patients with neoplasms, screening of patients responding to cancer therapy and such therapy. WO Patent App. PCT/FI2009/050,555. December30, 2009 [Google Scholar]
- 63.Wen Z, Liao Q, Hu Y, You L, Zhou L, Zhao Y: A spheroid-based 3-D culture model for pancreatic cancer drug testing, using the acid phosphatase assay. Braz J Med Biol Res 2013;46:634–642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tung Y-C, Hsiao AY, Allen SG, Torisawa Y-s, Ho M, Takayama S: High-throughput 3D spheroid culture and drug testing using a 384 hanging drop array. Analyst 2011;136:473–478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Swietach P, Hulikova A, Patiar S, Vaughan-Jones RD, Harris AL: Importance of intracellular pH in determining the uptake and efficacy of the weakly basic chemotherapeutic drug, doxorubicin. PLoS One 2012;7:e35949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nam J-M, Onodera Y, Bissell MJ, Park CC: Breast cancer cells in three-dimensional culture display an enhanced radioresponse after coordinate targeting of integrin α5β1 and fibronectin. Cancer Res 2010;70:5238–5248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Michaylira CZ, Wong GS, Miller CG, et al. : Periostin, a cell adhesion molecule, facilitates invasion in the tumor microenvironment and annotates a novel tumor-invasive signature in esophageal cancer. Cancer Res 2010;70:5281–5292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kondo J, Endo H, Okuyama H, et al. : Retaining cell–cell contact enables preparation and culture of spheroids composed of pure primary cancer cells from colorectal cancer. Proc Natl Acad Sci USA 2011;108:6235–6240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Praveen K, Streiner N, Vo M, Anderes K, Yokota K, Ikeya T: Evaluation of Cell-able spheroid culture system for culturing patient derived primary tumor cells. Proceedings of the 103rd Annual Meeting of the American Association for Cancer Research. Cancer Res 2012;72(8 Suppl):5270 [Abstract] [Google Scholar]
- 70.Fiebig H: Oncotest. www.oncotest.com/id-3d-assays.html (last accessed on April11, 2014)
- 71.Vinci M, Gowan S, Boxall F, et al. : Advances in establishment and analysis of three-dimensional tumor spheroid-based functional assays for target validation and drug evaluation. BMC Biol 2012;10:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lai Y, Asthana A, Kisaalita WS: Biomarkers for simplifying HTS 3D cell culture platforms for drug discovery: the case for cytokines. Drug Discov Today 2011;16:293–297 [DOI] [PubMed] [Google Scholar]
- 73.Albrecht C, Helmreich M, Tichy B, et al. : Impact of 3D-culture on the expression of differentiation markers and hormone receptors in growth plate chondrocytes as compared to articular chondrocytes. Int J Mol Med 2009;23:347–355 [DOI] [PubMed] [Google Scholar]
- 74.Mishra DK, Sakamoto JH, Thrall MJ, et al. : Human lung cancer cells grown in an ex vivo 3D lung model produce matrix metalloproteinases not produced in 2D culture. PLoS One 2012;7:e45308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yamada KM, Cukierman E: Modeling tissue morphogenesis and cancer in 3D. Cell 2007;130:601–610 [DOI] [PubMed] [Google Scholar]
- 76.Bohunicky B, Mousa SA: Biosensors: the new wave in cancer diagnosis. Nanotechnol Sci Appl 2010;4:1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lei KF, Wu MH, Hsu CW, Chen YD: Real-time and non-invasive impedimetric monitoring of cell proliferation and chemosensitivity in a perfusion 3D cell culture microfluidic chip. Biosens Bioelectron 2014;51:16–21 [DOI] [PubMed] [Google Scholar]
- 78.Jeong SH, Lee DW, Kim S, Kim J, Ku B: A study of electrochemical biosensor for analysis of three-dimensional (3D) cell culture. Biosens Bioelectron 2012;35:128–133 [DOI] [PubMed] [Google Scholar]
- 79.Daus AW, Layer PG, Thielemann C: A spheroid-based biosensor for the label-free detection of drug-induced field potential alterations. Sensor Actuat B-Chem 2012;165:53–58 [Google Scholar]
- 80.Zhou L, Huang G, Wang S, et al. : Advances in cell-based biosensors using three-dimensional cell-encapsulating hydrogels. Biotechnol J 2011;6:1466–1476 [DOI] [PubMed] [Google Scholar]
- 81.Wu M-H, Chang Y-H, Liu Y-T, et al. : Development of high throughput microfluidic cell culture chip for perfusion 3-dimensional cell culture-based chemosensitivity assay. Sensor Actuat B-Chem 2011;155:397–407 [Google Scholar]
- 82.Zhang X, Yang ST: High-throughput 3-D cell-based proliferation and cytotoxicity assays for drug screening and bioprocess development. J Biotechnol 2011;151:186–193 [DOI] [PubMed] [Google Scholar]
- 83.Ben-Yoav H, Melamed S, Freeman A, Shacham-Diamand Y, Belkin S: Whole-cell biochips for bio-sensing: integration of live cells and inanimate surfaces. Crit Rev Biotechnol 2011;31:337–353 [DOI] [PubMed] [Google Scholar]
- 84.Ong S-M, Zhang C, Toh Y-C, et al. : A gel-free 3D microfluidic cell culture system. Biomaterials 2008;29:3237–3244 [DOI] [PubMed] [Google Scholar]
- 85.Lin SP, Kyriakides TR, Chen JJ: On-line observation of cell growth in a three-dimensional matrix on surface-modified microelectrode arrays. Biomaterials 2009;30:3110–3117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tan W, Desai TA: Layer-by-layer microfluidics for biomimetic three-dimensional structures. Biomaterials 2004;25:1355–1364 [DOI] [PubMed] [Google Scholar]
- 87.Kim MS, Yeon JH, Park JK: A microfluidic platform for 3-dimensional cell culture and cell-based assays. Biomed Microdevices 2007;9:25–34 [DOI] [PubMed] [Google Scholar]
- 88.Kloss D, Kurz R, Jahnke HG, et al. : Microcavity array (MCA)-based biosensor chip for functional drug screening of 3D tissue models. Biosens Bioelectron 2008;23:1473–1480 [DOI] [PubMed] [Google Scholar]
- 89.Thielecke H, Mack A, Robitzki A: A multicellular spheroid-based sensor for anti-cancer therapeutics. Biosens Bioelectron 2001;16:261–269 [DOI] [PubMed] [Google Scholar]
- 90.Hildebrandt C, Buth H, Cho S, Impidjati , Thielecke H: Detection of the osteogenic differentiation of mesenchymal stem cells in 2D and 3D cultures by electrochemical impedance spectroscopy. J Biotechnol 2010;148:83–90 [DOI] [PubMed] [Google Scholar]
- 91.Wang J, Wu C, Hu N, Zhou J, Du L, Wang P: Microfabricated electrochemical cell-based biosensors for analysis of living cells in vitro. Biosensors 2012;2:127–170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ngoepe M, Choonara Y, Tyagi C, et al. : Integration of biosensors and drug delivery technologies for early detection and chronic management of illness. Sensors 2013;13:7680–7713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Souiri M, Gammoudi I, Mora L, et al. : A novel 3-D nano-assembly bacteria based biosensor for enhanced detection of heavy metal pollutants. J Environ Sci Eng 2012;1:924–935 [Google Scholar]
- 94.Torisawa Y-s, Shiku H, Yasukawa T, Nishizawa M, Matsue T: Multi-channel 3-D cell culture device integrated on a silicon chip for anticancer drug sensitivity test. Biomaterials 2005;26:2165–2172 [DOI] [PubMed] [Google Scholar]
- 95.Lei KF, Wu MH, Hsu CW: Electrical impendance determination of cancer cell viability in a 3-dimensional cell culture microfluidic chip. Int J Electrochem Sci 2012;7:12817–12828 [Google Scholar]
- 96.Banerjee P, Lenz D, Robinson JP, Rickus JL, Bhunia AK: A novel and simple cell-based detection system with a collagen-encapsulated B-lymphocyte cell line as a biosensor for rapid detection of pathogens and toxins. Lab Invest 2008;88:196–206 [DOI] [PubMed] [Google Scholar]
- 97.Nguyen TA, Yin TI, Reyes D, Urban GA: Microfluidic chip with integrated electrical cell-impedance sensing for monitoring single cancer cell migration in three-dimensional matrixes. Anal Chem 2013;85:11068–11076 [DOI] [PubMed] [Google Scholar]