Abstract
Background: Disruptive effects of caffeine on sleep have previously been reported, although measures of next-day mood and performance have rarely been included. The present study aims to evaluate the effects of caffeine on sleep and associated next-day effects in a naturalistic field setting.
Methods: Nineteen participants (daily caffeine intake 0–141 mg), assessed as good sleepers, took part in a randomized, placebo-controlled, double-blind, 2-week crossover study to assess the effects of bedtime caffeine use (250 mg) on sleep and next-day cognitive performance and mood, which were assessed on a mobile phone in the morning and afternoon. Sleep was assessed objectively (actiwatch) and subjectively (sleep diary).
Results: Caffeine's effects on sleep were largely restricted to the first day of administration, with actigraphically measured reduced sleep efficiency, increased activity score and fragmentation index, decreased self-rated sleep quality, and an increased occurrence of participants waking early; only decreased sleep efficiency remained over the week. Effects on next-day performance and mood were evident over the whole week, although despite disrupting sleep, accuracy on a working memory task was higher after caffeine than placebo administration.
Conclusions: Caffeine disrupted sleep, although when assessing next-day performance, which may have been affected by the presence of residual caffeine, performance appeared better after caffeine compared to placebo, although this was most likely due to prevention of the effects of overnight withdrawal from caffeine rather than representing a net benefit. Furthermore, partial tolerance developed to the effects of caffeine on sleep.
Introduction
In the United Kingdom, about 37% of adults have trouble falling or staying asleep, waking up during the night, or feeling tired and worn out the following morning on most nights.1 The consequences of sleep loss for daytime functioning range from an increase in human errors due to impairments in cognitive performance,2 negative social and economic effects,3 through to mood disorders, with over 90% of patients with major depression also reporting sleep problems.4
Secondary outcome measures in clinical trials of pharmacological treatments to improve sleep are often incorporated to assess undesirable residual effects of the medication, rather than as improvements secondary to improved sleep. In these cases, a satisfactory outcome is that participants perform no differently from the placebo level, and this seems to be a common finding for newer pharmacological treatments such as the non-benzodiazepines.5 However, a result of no change in performance in these studies that use a placebo-treated patient population for comparison suggests that even though parameters of sleep may be improved, daytime performance is not. This highlights a problem when using a poor sleeper population to assess the efficacy of treatments on next-day functioning, as even though previous research has found that poor sleep is associated with poor next-day mood, alertness, and cognitive function, this has only reliably been shown using healthy individuals in sleep-restriction studies6–8 and in insomniac patients using constant routine protocols.9 However, when not under these tightly controlled conditions, as with a field study in a naturalistic setting, the effects of poor sleep on next-day performance and mood are unlikely to be apparent, partly due to coping mechanisms employed by poor sleepers to overcome their sleepiness.10,11 Furthermore, the sleep of a poor sleeper may have to be consistently improved for a substantial amount of time before any positive changes to cognitive performance become evident. In sleep-restriction studies, where normal healthy individuals are deprived of sleep and then allowed to sleep freely, more than one night of extended sleep has been necessary to restore daytime impairments to prerestriction levels.6,8 Field studies obviously lack the control of laboratory studies, and one alternative method for use in the field may be to induce short-term poor sleep in otherwise healthy sleepers, rather than using a poor sleeper population that would likely be adept at using coping mechanisms to overcome and/or mask the negative daytime effects of poor sleep. A further method of elucidating the effects of sleep on performance is to use tasks that are uninteresting and simple. Wilkinson12 suggested that more complex tasks are less affected by sleep deprivation, because the challenge involved causes participants to employ compensatory efforts to overcome their sleepiness. Deficits in tests of working memory and attention have both been found using relatively short-duration tasks,13 with simple cognitive tasks of sustained attention showing the most consistent results.14
Caffeine is a widely consumed psychoactive substance, and its primary action is the antagonism of adenosine receptors, leading to increased wakefulness.15 Caffeine has previously been reported to disrupt sleep by extending wakefulness.16–20 Additionally, other parameters of sleep quality such as sleep efficiency17–19,21,22 and total sleep time22 are affected by administration of caffeine. In the current study, caffeine administered shortly before bedtime was used to disrupt sleep. To maximize the effects of caffeine, low-caffeine consumers (no more than two cups coffee or tea per day) were recruited, as Hindmarch et al.23 found greater disruptive effects of caffeine in low consumers. Furthermore, as administration of caffeine has been found to increase jitteriness scores in both nonconsumers24 and low consumers,25 it was predicted that this effect may also lead to increased restlessness scores during the night, possibly reflecting a psychomotor component of caffeine's mechanism of action. Finally, a decrease in sleepiness is a consistent finding in nonconsumers and low consumers even when increases in mental alertness have not been found, and sleep interference was one of the top four reasons cited for nonconsumption of caffeine-containing products in non- and extremely low-caffeine (<15 mg) consumers.26
The main aim was to assess the effects of bedtime caffeine use on sleep and subsequent daytime performance and mood. A secondary aim was to assess effects of caffeine on sleep over time, that is, tolerance.21 A further aim was to assess aspects of caffeine use as a model of short-term poor sleep and associated daytime consequences.
Materials and Methods
Participants
Nineteen participants (14 women and 5 men aged 32±12.9 years) assessed as good sleepers using the statements “I am satisfied with my sleep”, “I feel very tired in the morning”, “My sleep is refreshing,” and “I feel very tired during the day” from the Bristol Sleep Questionnaire (BSQ)27 were recruited from the Bristol area. Additional criteria included habitual consumption of no more than two cups of caffeinated drink per day, the last one taken at least 4 hours before bedtime. Participants spent 0.47±0.57 hours in bed before falling asleep and slept for 7.75±1.17 hours and habitually consumed 65±53.7 mg/day caffeine (range 0–141 mg/day). In an attempt to classify habitual caffeine consumption, 37% habitually consumed <15 mg/day and could be classed as nonconsumers,28 and 63% consumed between 15 and 141 mg/day and could be classed as low-to-moderate caffeine consumers. However, it is noted that widely different classifications have been used in the literature. Participants were screened to make sure that they were not taking any medication for sleep or any other significant medical condition (specifically antidepressants) that they had a regular weekday routine, and did not consume more than 4 U of alcohol per weekday. Those with extreme Depression, Anxiety and Stress Scale (DASS) scores,29 or who worked with machinery, drove a long distance on a regular basis, were pregnant, breastfeeding, or looking after children aged 1 or younger were not eligible to take part. All participants gave signed consent before starting the study and received £40 on satisfactory completion. Ethics approval for this study was obtained from the Faculty of Science Human Research Ethics Committee, University of Bristol.
Study design and procedure
The study was carried out in the field (participants' normal everyday environment). Participants took part in a randomized, double-blind 2-week counterbalanced crossover study. Caffeine was administered for five nights consecutively for one week and placebo for five nights consecutively the other week, although participants were informed that it was random as to whether they would receive caffeine or placebo on any particular night. Each morning, participants were required to provide a saliva sample, which was not analyzed, but taken to encourage compliance with the study requirements. The weekend acted as a washout when there was no drug administration or testing. There was one practice day before the study started where participants familiarized themselves with the equipment. Participants completed the cognitive tasks three times before the study began, once during an initial training session and twice during the practice day. Half of the volunteers were instructed to take their caffeine capsule one hour before their usual bedtime, and the other half to take their capsule at lights out. Participants were asked to keep a regular routine throughout the study and to refrain from daytime naps. They were required to complete a Food and Activity Diary before the initial training session to allow discussion of the importance of keeping a regular routine for the duration of the study.
Each testing day (Monday–Friday), participants completed tasks assessing mood and cognitive performance (see below) in the morning before showering or eating breakfast and again in the afternoon. They were allowed to continue their usual habitual caffeine intake after they had completed the morning tests to prevent any potential effects of caffeine withdrawal later on.30,31
Drug administration
Participants were provided with ten 250 mg capsules, five of which contained caffeine BP (caffeine anhydrous powder; Courtin and Warner) and five contained placebo (corn flour). Capsules were a white vegetarian format and were purchased from Capsuline® with a disintegration time of <15 minutes.
Sleep analysis
Actiwatch Actigraphy Monitors (AW4; Cambridge Neurotechnology) were worn on the nondominant wrist and measured several sleep parameters (sleep efficiency, sleep latency, total activity score, and fragmentation index) by recording the number of changes in state per epoch (30 seconds). Participants were also provided with a sleep diary that contained the items “difficulty getting to sleep”, “quality of sleep”, “ease of waking”, how many times participants woke up restless during the night, and if they were troubled by waking early and not being able to get back to sleep again. Participants also rated how clear-headed/alert/refreshed/awake they felt upon awakening, how they performed, and how tired and alert they were during the day.
Neurobehavioral performance
Mobile phones (LG B2100) were set up with cognitive tasks and mood adjectives in Java®.32 Ten mood adjectives (see below) were presented using 26-point scales anchored “not at all” and “extremely”. Alarms were set according to times provided by participants for getting up (5–10 minutes after) and lunch time (1.5 hours after lunch) to coincide with the postlunch dip. Data were transmitted via a general packet radio service to a remote Web server at the end of each test battery, and a mean score was automatically created for each dependent variable. Questions assessing alertness and subjective performance over the day were included in the evening section of the sleep diary.
The Arrow Flankers task33 consisted of five stimuli presented in a row in the center of the screen and lasted 3 minutes. Participants were instructed to press left [4] or right [6] keys on the keypad corresponding to the direction of the central arrow as quickly as possible while ignoring the flankers, unless the flankers were crosses in which case participants were instructed not to respond. Ninety-five stimulus presentations were made in a random order of which 10 were crosses, and the remaining were split between congruent, incongruent, and neutral flankers (squares). Each stimulus was displayed for a maximum of 1.8 seconds with a stimulus being presented every 2 seconds. Mean scores and counts of incorrect and false responses were automatically generated by the program and resulted in dependent variables of mean reaction time, number incorrect, and response inhibition.
The n-back task, a two-back working memory task,34 lasted 3 minutes and consisted of a series of 180 letters presented on the screen one at a time. Participants were instructed to press “yes” [4] or “no” [6] depending on whether the letter presented was the same as the last, but one letter. The stimulus remained on the screen until a response occurred, and percentage accuracy and mean reaction time were taken as dependent variables.
The simple reaction time (SRT) task with variable delay lasted 5 minutes, and participants were instructed to press the middle key [5] as quickly as possible upon detection of a stimulus, a small star in the center of the screen. The stimulus was presented 15 times for each varying delay of 1, 2, 3, 5, and 7 seconds in a randomized order. The dependent variable was the overall mean reaction time and was created automatically by the program.
Questionnaires
The BSQ27 is a 23-item questionnaire that assesses the aspects of quality of sleep, including sleep initiation, maintenance, troubled sleep, and dreams, consequences associated with sleep such as feeling tired the next day and daytime naps, items that can be used to identify possible sleep disorders such as movement disorders, sleep apnea, parasomnias, and possible reasons for poor sleep such as partner's snoring and young children.
The DASS-2129 is a 21-item questionnaire assessing depression, anxiety, and stress, which also provides a total mood score. It has internal consistencies for each factor (Depression [α=0.91], Anxiety [α=0.84], Stress [α=0.90]), which correlate well with other similar measures.
Data editing and analysis
Determination of the appropriate sample size was difficult due to lack of effect sizes reported in similar previous studies, although three studies most similar to the current one that found significant disruptive effects of caffeine used sample sizes of 6,20 12,16 and 1719 participants. Mood scales presented on the mobile phones were combined, and the resulting components labeled energetic arousal (energetic and alert vs. tired and sleepy) and tense arousal (tense, stressed, miserable, and irritable vs. relaxed) based on Thayer.35 The scale item cheerful was analyzed separately. Three factors were identified using principal component analysis on sleep diary questions: daytime performance and energy, quality of sleep, and awakening. The remaining variables were analyzed separately. Three actiwatches failed to record any data. The questions from the BSQ were grouped into four factors: daytime sleep, troubled sleep (dreams), disturbed sleep, and morning tiredness, based on a factor analysis from a larger study.27 The scores from the DASS-21 were grouped into three factors (stress, anxiety, and depression) based on previous research from a large nonclinical sample.36
Data were averaged over the week, as reduced performance has also been found after recovery sleep.6 To evaluate differences between caffeine and placebo administration, mixed analyses of variance with within-subject factors of day (1–5) and week (caffeine or placebo) and a between-subject factor of order (placebo first or caffeine first) were conducted. The data from the first day only were also analyzed. For analysis of cognitive performance and mood, the time of testing session (morning or afternoon) was also included as a within-subject factor, and the Huynh-Feldt correction was applied when the sphericity assumption was violated. To compare the number of times participants were troubled by waking up early over the week, a McNemar exact test for paired samples using the binomial distribution was used for data at day 1, and t-tests were employed to compare the number of times participants were troubled by waking early over the week.
Results
During the study, five participants withdrew (one for personal reasons). The other reasons given were complaints of sleep loss and the effects on performance and mood the following day. The four who pulled out were all administered caffeine in the first week, taken one hour before bedtime and had an average habitual caffeine intake of 63±47 mg caffeine per day.
Participants turned the lights out to go to sleep during the caffeine week at 23:37±01:02 and during the placebo week at 23:20±00:48. There were main effects of time (p<0.05) for cognitive tasks and mood between morning and afternoon sessions, but no time by group interactions, so morning and afternoon results were combined for ease of discussion of group effects. Mood and performance were significantly (p<0.05) better in the afternoon than the morning with the exception of the SRT task and accuracy on the n-back task.
Sleep analyses
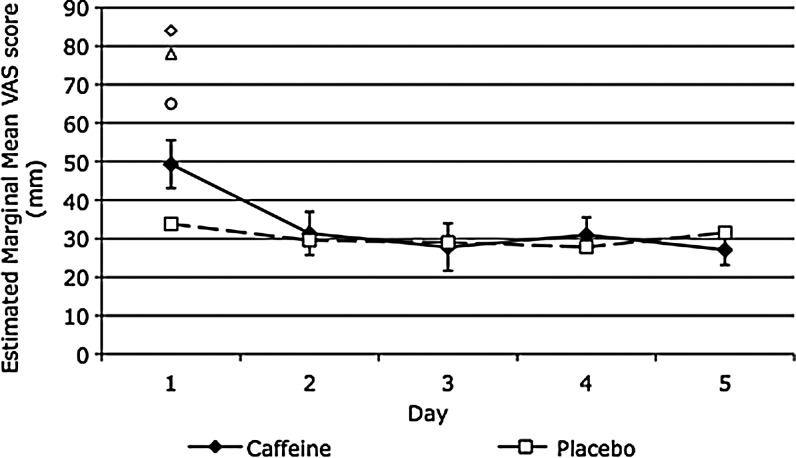
Table 1 shows that after the first night of administration, caffeine resulted in higher total activity and fragmentation index scores, and marginally insignificant lower sleep efficiency scores. There was a trend for subjective quality of sleep to be rated as worse after caffeine (see also Fig. 1), and significantly more participants were troubled by waking early after they had consumed caffeine. Figure 1 shows that caffeine had a particularly striking effect on sleep quality of three participants who subsequently withdrew from the study.
Table 1.
Effect of Caffeine on Measures of Sleep and Daytime Performance
| Day 1 | ANOVA (treatment effect) | Days 2–5 | ANOVA (treatment effect) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Measure | Caffeine | Placebo | F | p | d | Caffeine | Placebo | F | p | d |
| Actiwatcha | ||||||||||
| Sleep efficiency (%) | 80.9±1.9 | 86.1±1.3 | 7.39 | 0.058 | 0.81 | 80.9±2.7 | 85.8±1.3 | 6.76 | 0.023 | 0.61 |
| Sleep-onset latency (minutes) | 10.7±3.4 | 15.6±5.8 | <1 | — | 0.27 | 20.5±9.0 | 13.3±5.3 | 1.7 | 0.216 | 0.25 |
| Total activity score (count) | 16,327±2646 | 7898±1160 | 9.94 | 0.008 | 1.11 | 13,023±2748 | 9224±1077 | 2.91 | 0.114 | 0.50 |
| Fragmentation index | 39.0±2.9 | 27.7±3.1 | 5.22 | 0.041 | 0.94 | 36.6±4.3 | 30.2±2.5 | 3.65 | 0.08 | 0.47 |
| Sleep diaryb | ||||||||||
| Quality of sleep (mm)c | 49.3±0.6 | 33.8±0.5 | 4.28 | 0.056 | 6.46 | 29.3±0.4 | 29.5±0.3 | <1 | — | 0.13 |
| Awakening (mm) | 46.2±0.5 | 48.0±0.5 | <1 | — | 0.83 | 50.1±0.3 | 52.8±0.3 | 1.46 | 0.246 | 2.06 |
| Daytime performance and energy (mm) | 51.0±0.5 | 45.5±0.5 | <1 | — | 2.52 | 48.7±0.2 | 43.8±0.3 | 1.63 | 0.221 | 4.50 |
| Times woke up restless | 1.9±0.3 | 1.6±0.3 | <1 | — | 0.23 | 1.1±0.2 | 1.4±0.2 | 2.68 | 0.122 | 0.34 |
| Troubled by waking early (no. of pp's) | 9 | 1 | 0.008d | 12 | 15 | <1e | — | |||
| Cognitive performancef | ||||||||||
| Psychomotor vigilance RT (ms) | 601±11 | 639±32 | <1 | — | 0.43 | 637±18 | 645±17 | <1 | — | 0.11 |
| Arrow flankers RT (ms) | 682±32 | 705±36 | 1.1 | 0.313 | 0.16 | 675±28 | 675±25 | <1 | — | 0.00 |
| Arrow flankers number incorrect | 2.6±0.7 | 5.9±1.9 | 2.64 | 0.128 | 0.62 | 2.6±0.4 | 2.4±0.5 | <1 | — | 0.11 |
| Response inhibition | 1.16±0.2 | 1.01±0.2 | <1 | — | 0.18 | 0.73±0.1 | 0.83±0.2 | <1 | — | 0.16 |
| n-Back accuracy (%)g | 84.6±2.4 | 78.7±3.1 | 7.97 | 0.017 | 0.14 | 83.2±2.6 | 77.6±3.2 | 20.1 | 0.001 | 0.50 |
| n-Back RT (ms) | 1000±33 | 1107±98 | 1.48 | 0.249 | 0.11 | 967±43 | 994±52 | <1 | — | 0.15 |
| Daily moodh | ||||||||||
| Energetic arousal | 50.4±4.2 | 52.0±3.0 | <1 | — | 0.10 | 51.2±1.8 | 51.1±2.7 | <1 | — | 0.01 |
| Tense arousal | 39.1±4.0 | 35.9±2.9 | <1 | — | 0.22 | 35.6±2.2 | 33.1±2.1 | 1.69 | 0.226 | 0.27 |
| Cheerful | 51.7±4.7 | 55.2±3.4 | <1 | — | 0.20 | 55.5±2.7 | 59.6±2.8 | 5.19 | 0.039 | 0.35 |
Values are mean±SE.
N=16, df=1, 12.
N=19, df=1, 15.
Data here also presented visually in Figure 1.
McNemar exact test for paired samples using binomial distribution (N=19).
Paired samples t-test; d=Cohen's d.
N=17 (n-back task, N=15), df=1, 11.
Data here also presented visually in Figure 2.
N=18, df=1, 14.
ANOVA, analysis of variance; SE, standard error; RT, reaction time.
FIG. 1.
Quality of sleep factor. A higher score represents worse quality of sleep. Error bars represent standard error. Individual markers are participants' scores who withdrew from the study after the first night (data for one unavailable). These participants are not included in the group mean.
Over days 2–5, caffeine administration resulted in significantly lower sleep efficiency scores compared to placebo. There were no other effects of caffeine on days 2–5, including no effect of caffeine on sleep quality (Fig. 1).
Cognitive performance
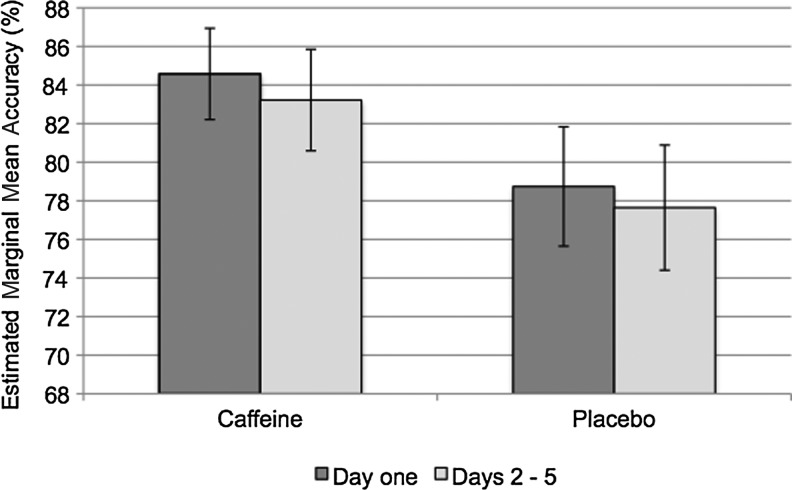
Table 1 and Figure 2 show that caffeine resulted in significantly higher accuracy scores on the n-back task the morning after the first night and over days 2–5 compared with placebo. For three participants who pulled out of the study after the first night of caffeine administration (data for one unavailable), accuracy scores were above average (87.6%±5.3%).
FIG. 2.
Mean accuracy score over the week on the n-back task. Error bars represent standard error of the mean. See Table 1 for statistics.
There was a statistically significant main effect of the covariate, age, for the reaction time part of the Arrow Flankers test [F(1, 31)=17.18, p<0.0001, r=0.60] that showed that as age increased reaction time slowed.
Mood
Cheerfulness was significantly lower over days 2–5 of the caffeine week, but not on the first day (Table 1).
Effect of habitual caffeine intake
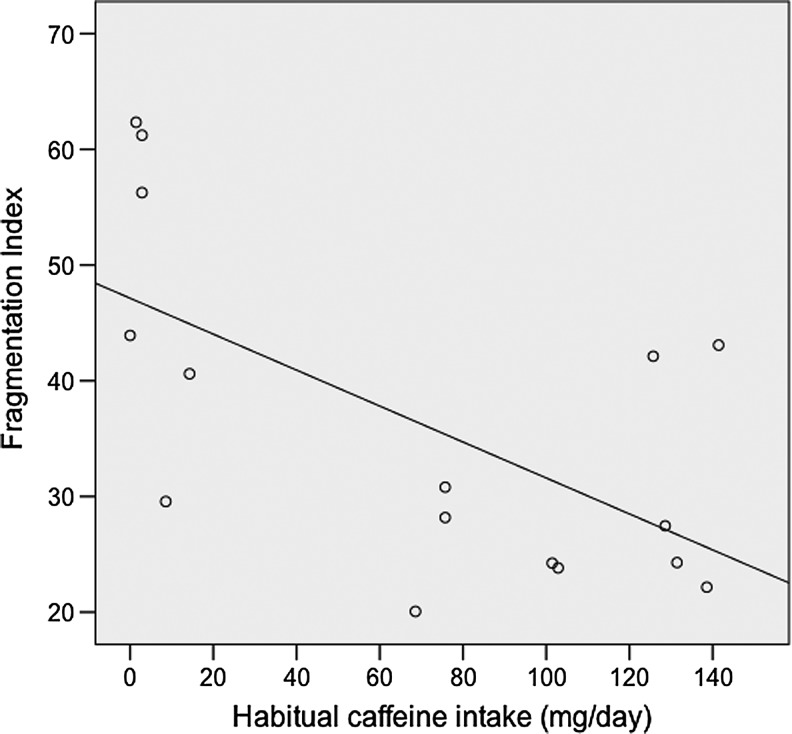
Habitual caffeine intake was significantly negatively correlated with fragmentation index scores averaged over days 1–5 that participants were administered caffeine before bedtime r=− 0.658, p=0.008, n=13 (with age as covariate), higher restlessness scores being associated with lower habitual caffeine intake (Fig. 3).
FIG. 3.
Association of habitual caffeine intake with restlessness scores (averaged over days 1–5) when participants were administered caffeine (250 mg) before bedtime (n=16).
Discussion
Factors encompassing disturbed sleep and daytime tiredness from the BSQ were used to identify good sleepers. Caffeine (250 mg) was administered before bedtime with the aim of inducing poor sleep, and has previously been observed to disrupt sleep. After the first night, caffeine reduced sleep efficiency and increased activity score and fragmentation index as measured by actiwatch. Additionally, there was an increase in the number of times participants woke early, and a decrease in self-rated sleep quality that would have likely reached significance if it were not for the four participants who withdrew due to the effects of caffeine on their sleep and subsequent daytime functioning. Only decreased sleep efficiency remained significant for the remainder of the week. Accuracy scores on the n-back task however remained significantly higher after caffeine than placebo administration throughout the week.
Although caffeine was observed to disrupt sleep, there were two main findings worthy of further discussion: that disrupted sleep was not associated with worse next-day performance, and that the majority of effects were restricted to the first day.
Previous research has demonstrated a relationship between poor sleep quality and impaired performance,6–8,37–39 yet here, caffeine resulted in higher accuracy scores on the n-back task relative to placebo. The most likely explanation for this unexpected finding is that residual caffeine may have directly ameliorated some of the effects of poor sleep. Between 0.5 and 2 μg/mL residual caffeine has been found in saliva after a similar dose and sleep opportunity as in this study,19,22,40 and Landolt et al.22 found as little as 0.58 μg/mL residual caffeine present in saliva to be associated with reduced sleep efficiency (16 hours after 200 mg). Effects of caffeine on cognitive performance have been found after as little as 12.5 mg caffeine,41 related to reversal of withdrawal symptoms seen in overnight-abstained caffeine consumers. Indeed, as at least some of the participants habitually consumed moderate amounts of caffeine daily, and even individuals consuming 100 mg/day can be physically dependent on caffeine31; this effect of caffeine on next-day performance could be explained by withdrawal reversal.42,43 Administration of caffeine at bedtime may have prevented overnight caffeine withdrawal that is likely to have occurred in the habitual consumers during the placebo week. Furthermore, as the order of administration of caffeine or placebo was counterbalanced over the 2 weeks, even the nonconsumers may have suffered from withdrawal during the placebo week if preceded by a week of caffeine administration that would have induced caffeine dependence.31 However, the present study did not control for caffeine withdrawal and withdrawal reversal,42 and therefore no firm conclusions can be made as to whether next-day performance represented a net benefit of caffeine or was due to reversal of negative caffeine-withdrawal effects. Residual caffeine is also a valid explanation for why participants were troubled by waking early more often when they received caffeine. However, it is also a possible explanation that the extent of sleep disruption was not great enough to elicit deficits in next-day performance, or that any deficits were masked by participants' use of coping mechanisms commonly employed by poor sleepers to overcome negative effects of poor sleep.
It was additionally predicted that the effects of caffeine would change over time as tolerance may develop,21 and various results, including sleep quality, supported the development of at least a partial tolerance. Alford et al.20 reported subjective effects of caffeine on sleep quality (averaged over the first two nights) when giving a much larger dose (400–600 mg). Sleep efficiency was significantly reduced by caffeine in the present study, which is consistent with previous studies,17–19,21,22 and restlessness scores were increased. Even though sleep efficiency scores remained reasonably constant over the week, other parameters of sleep were only significantly affected by caffeine on the first night, and not for the remainder of the week. The development of tolerance was perhaps most striking for self-rated sleep quality, whereas only a partial tolerance was evident for objectively measured sleep. The significant relationship between restlessness scores and the level of habitual caffeine intake shows that caffeine exerted a greater effect on those who habitually consumed smaller amounts of caffeine. Together, these results support the hypothesis that at least a partial tolerance develops rapidly to the effects of caffeine on sleep.
In the present study, it seems likely that the effects of caffeine on sleep would have been larger if the apparently most reactive individuals had continued for the full 5 days. This highlights a limitation of using caffeine (or other similar interventions) as an experimental model for poor sleep, as from an ethics standpoint, participants must be allowed to withdraw if they wish, but this will reduce the impact of the intervention. A further limitation is evident when repeated testing is involved due to the development of partial tolerance to the effects of caffeine on sleep. Furthermore, it was found not to be a good model for the purposes of assessing daytime consequences of poor sleep. Indeed, caffeine (vs. placebo) before bedtime resulted in a better next-day performance (or prevented deterioration of performance), presumably due to the direct effects of caffeine still present in the morning and beyond, regardless of whether this is due to a net benefit or prevention of overnight withdrawal.
The lack of an observed effect of caffeine on sleep onset latency is perhaps surprising, but has been seen before.44 This effect could be the result of various factors: first, the actiwatch has been found to be unreliable when measuring sleep-onset latency.45 Secondly, since half the participants took their caffeine capsule at lights out, they may have fallen asleep before there was a substantial rise in the plasma caffeine concentration. Third, after the first night of sleep disrupted by caffeine administration, participants may be in need of recovery sleep on subsequent nights. In situations such as this, it has been reported that sleep-onset latency was unaffected by caffeine.46 However, the first night of caffeine administration also revealed no significant effects on sleep-onset latency, suggesting that the lack of effect found may be due to a combination of the above reasons.
Finally, ratings of cheerfulness were reduced during the caffeine week, in particular over days 2–5. This adverse effect of caffeine could represent a consequence of reduced sleep efficiency, as poor mood is common in those who report poor sleep,4 or it might be due to a direct effect of caffeine or caffeine withdrawal.
Conclusions
Although caffeine disrupted sleep objectively and somewhat subjectively in habitually low- and noncaffeine consumers, on the surface, the next-day performance appeared better after bedtime consumption of caffeine as compared to placebo, presumably due to the presence of residual caffeine still present in the morning and beyond. Even though this study was not designed to assess whether effects of caffeine are related to a net benefit or reversal of withdrawal effects, the most plausible explanation here is that bedtime caffeine use was preventing the adverse effects of overnight caffeine withdrawal that would likely have occurred during placebo administration. Furthermore, partial tolerance developed to the effects of caffeine on sleep. Due to this tolerance over repeated testing, withdrawal of participants most affected by caffeine, and residual effects of caffeine the following morning, the use of caffeine in low- and noncaffeine consumers in a naturalistic field setting is not recommended for modeling the effects of poor sleep on the next-day performance and mood.
Acknowledgments
We thank Barry O'Neill, Sophie Putnam (GlaxoSmithKline), and anonymous reviewers for their comments on the manuscript.
Author Disclosure Statement
This research was supported jointly by the Biotechnology and Biological Sciences Research Council (student grant reference number BBSRC DTC/PSYC SG1421.6525) and GlaxoSmithKline.
References
- 1.Morphy H, Dunn KM, Lewis M, Boardman HF, Croft PR. Epidemiology of insomnia: a longitudinal study in a UK population. Sleep 2007;30:274–280 [PubMed] [Google Scholar]
- 2.Williamson AM, Feyer AM. Moderate sleep deprivation produces impairments in cognitive and motor performance equivalent to legally prescribed levels of alcohol intoxication. Occup. Environ. Med. 2000;57:649–655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Metlaine A, Leger D, Choudat D. Socioeconomic impact of insomnia in working populations. Ind. Health 2005;43:11–19 [DOI] [PubMed] [Google Scholar]
- 4.Wilson SJ, Argyropoulos S. Antidepressants and sleep: a qualitative review of the literature. Drugs 2005;65:927–947 [DOI] [PubMed] [Google Scholar]
- 5.Bogan RK. Treatment options for insomnia—pharmacodynamics of zolpidem extended-release to benefit next-day performance. Postgrad. Med. 2008;120:161–71 [DOI] [PubMed] [Google Scholar]
- 6.Belenky G, Wesensten NJ, Thorne DR, et al. Patterns of performance degradation and restoration during sleep restriction and subsequent recovery: a sleep dose-response study. J. Sleep Res. 2003;12:1–12 [DOI] [PubMed] [Google Scholar]
- 7.Van Dongen HP, Maislin G, Mullington JM, Dinges DF. The cumulative cost of additional wakefulness: dose-response effects on neurobehavioral functions and sleep physiology from chronic sleep restriction and total sleep deprivation. Sleep 2003;26:117–126 [DOI] [PubMed] [Google Scholar]
- 8.Dinges DF, Pack F, Williams K, et al. Cumulative sleepiness, mood disturbance, and psychomotor vigilance performance decrements during a week of sleep restricted to 4–5 hours per night. Sleep 1997;20:267–277 [PubMed] [Google Scholar]
- 9.Varkevisser M, Kerkhof GA. Chronic insomnia and performance in a 24-h constant routine study. J. Sleep Res. 2005;14:49–59 [DOI] [PubMed] [Google Scholar]
- 10.Varkevisser M, Van Dongen HP, Van Amsterdam JG, Kerkhof GA. Chronic insomnia and daytime functioning: an ambulatory assessment. Behav. Sleep Med. 2007;5:279–296 [DOI] [PubMed] [Google Scholar]
- 11.Kloss JD. Daytime sequalae of insomnia. In: Insomnia: Principles and Management. Szuba M.P, Kloss J.D, Dinges D.F. (Eds). Cambridge: Cambridge University Press; 2003: pp. 23–42 [Google Scholar]
- 12.Wilkinson RT. The measurement of sleepiness. In: Sleep, Arousal, and Performance. Broughton R.J, Ogilvie R.D. (Eds). Boston: Birkhauser; 1992 [Google Scholar]
- 13.Dinges DF. Probing the limits of functional capacity: the effects of sleep loss on short-duration tasks. In: Sleep, Arousal, and Performance. Broughton R.J, Ogilvie R.D. (Eds). Boston: Birkhauser; 1992 [Google Scholar]
- 14.Durmer JS, Dinges DF. Neurocognitive consequences of sleep deprivation. Semin. Neurol. 2005;25:117–129 [DOI] [PubMed] [Google Scholar]
- 15.Roehrs T, Roth T. Caffeine: sleep and daytime sleepiness. Sleep Med. Rev. 2008;12:153–162 [DOI] [PubMed] [Google Scholar]
- 16.Paterson LM, Wilson SJ, Nutt DJ, Hutson PH, Ivarsson M. A translational, caffeine-induced model of onset insomnia in rats and healthy volunteers. Psychopharmacology (Berl.) 2007;191:943–950 [DOI] [PubMed] [Google Scholar]
- 17.Landolt HP, Dijk DJ, Gaus SE, Borbely AA. Caffeine reduces low-frequency delta activity in the human sleep EEG. Neuropsychopharmacology 1995;12:229–238 [DOI] [PubMed] [Google Scholar]
- 18.Drapeau C, Hamel-Hebert I, Robillard R, et al. Challenging sleep in aging: the effects of 200 mg of caffeine during the evening in young and middle-aged moderate caffeine consumers. J. Sleep Res. 2006;15:133–141 [DOI] [PubMed] [Google Scholar]
- 19.Carrier J, Fernandez-Bolanos M, Robillard R, et al. Effects of caffeine are more marked on daytime recovery sleep than on nocturnal sleep. Neuropsychopharmacology 2007;32:964–972 [DOI] [PubMed] [Google Scholar]
- 20.Alford C, Bhatti J, Leigh T, Jamieson A, Hindmarch I. Caffeine-induced sleep disruption: effects on waking the following day and its reversal with an hypnotic. Hum Psychopharmacol Clin Exp 1996;11:185–198 [Google Scholar]
- 21.Bonnet MH, Arand DL. Caffeine use as a model of acute and chronic insomnia. Sleep 1992;15:526–536 [PubMed] [Google Scholar]
- 22.Landolt HP, Werth E, Borbely AA, Dijk DJ. Caffeine intake (200 mg) in the morning affects human sleep and EEG power spectra at night. Brain Res. 1995;675:67–74 [DOI] [PubMed] [Google Scholar]
- 23.Hindmarch I, Rigney U, Stanley N, et al. A naturalistic investigation of the effects of day-long consumption of tea, coffee and water on alertness, sleep onset and sleep quality. Psychopharmacology (Berl.) 2000;149:203–216 [DOI] [PubMed] [Google Scholar]
- 24.Rogers PJ, Martin J, Smith C, Heatherley SV, Smit HJ. Absence of reinforcing, mood and psychomotor performance effects of caffeine in habitual non-consumers of caffeine. Psychopharmacology (Berl.) 2003;167:54–62 [DOI] [PubMed] [Google Scholar]
- 25.Rogers PJ, Smith JE, Heatherley SV, Pleydell-Pearce CW. Time for tea: mood, blood pressure and cognitive performance effects of caffeine and theanine administered alone and together. Psychopharmacology (Berl.) 2008;195:569–577 [DOI] [PubMed] [Google Scholar]
- 26.Rogers PJ, Smith JE. Caffeine, Mood and Cognition. Cambridge, UK: Woodhead Publishing Ltd.; 2011 [Google Scholar]
- 27.Smith A, Nutt D, Wilson S, et al. Noise and Insomnia: A Study of Community Noise Exposure, Sleep Disturbance, Noise Sensitivity and Subjective Reports of Health. London: Department of Health; 2002: pp. 217 [Google Scholar]
- 28.Richardson NJ, Rogers PJ, Elliman NA, O'Dell RJ. Mood and performance effects of caffeine in relation to acute and chronic caffeine deprivation. Pharmacol. Biochem. Behav. 1995;52:313–320 [DOI] [PubMed] [Google Scholar]
- 29.Henry JD, Crawford JR. The short-form version of the Depression Anxiety Stress Scales (DASS-21): construct validity and normative data in a large non-clinical sample. Br. J. Clin. Psychol. 2005;44:227–239 [DOI] [PubMed] [Google Scholar]
- 30.Rogers PJ, Heatherley SV, Hayward RC, et al. Effects of caffeine and caffeine withdrawal on mood and cognitive performance degraded by sleep restriction. Psychopharmacology (Berl.) 2005;179:742–752 [DOI] [PubMed] [Google Scholar]
- 31.Evans SM, Griffiths RR. Caffeine withdrawal: a parametric analysis of caffeine dosing conditions. J. Pharmacol. Exp. Ther. 1999;289:285–294 [PubMed] [Google Scholar]
- 32.Tiplady B, Oshinowo B, Thomson J, Drummond GB. Alcohol and cognitive function: assessment in everyday life and laboratory settings using mobile phones. Alcohol Clin. Exp. Res. 2009;33:2094–2102 [DOI] [PubMed] [Google Scholar]
- 33.Tiplady B, Bowness E, Stien L, Drummond G. Selective effects of clonidine and temazepam on attention and memory. J. Psychopharmacol. 2005;19:259–265 [DOI] [PubMed] [Google Scholar]
- 34.Kirchner WK. Age differences in short-term retention of rapidly changing information. J. Exp. Psychol. 1958;55:352–358 [DOI] [PubMed] [Google Scholar]
- 35.Thayer RE. The Biopsychology of Mood and Arousal. New York: Oxford University Press; 1989 [Google Scholar]
- 36.Crawford JR, Henry JD. The Depression Anxiety Stress Scales (DASS): normative data and latent structure in a large non-clinical sample. Br. J. Clin. Psychol. 2003;42:111–131 [DOI] [PubMed] [Google Scholar]
- 37.Edinger JD, Means MK, Carney CE, Krystal AD. Psychomotor performance deficits and their relation to prior nights' sleep among individuals with primary insomnia. Sleep 2008;31:599–607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Drake CL, Roehrs TA, Burduvali E, et al. Effects of rapid versus slow accumulation of eight hours of sleep loss. Psychophysiology 2001;38:979–987 [DOI] [PubMed] [Google Scholar]
- 39.Ferrara M, De Gennaro L, Casagrande M, Bertini M. Selective slow-wave sleep deprivation and time-of-night effects on cognitive performance upon awakening. Psychophysiology 2000;37:440–446 [PubMed] [Google Scholar]
- 40.Hashiguchi M, Fujimura A, Ohashi K, Ebihara A. Diurnal effect on caffeine clearance. J. Clin. Pharmacol. 1992;32:184–187 [DOI] [PubMed] [Google Scholar]
- 41.Smit HJ, Rogers PJ. Effects of low doses of caffeine on cognitive performance, mood and thirst in low and higher caffeine consumers. Psychopharmacology (Berl.) 2000;152:167–173 [DOI] [PubMed] [Google Scholar]
- 42.James JE, Rogers PJ. Effects of caffeine on performance and mood: withdrawal reversal is the most plausible explanation. Psychopharmacology (Berl.) 2005;182:1–8 [DOI] [PubMed] [Google Scholar]
- 43.Rogers PJ, Hohoff C, Heatherley SV, et al. Association of the anxiogenic and alerting effects of caffeine with ADORA2A and ADORA1 polymorphisms and habitual level of caffeine consumption. Neuropsychopharmacology 2010;35:1973–1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nicholson AN, Stone BM. Heterocyclic amphetamine derivatives and caffeine on sleep in man. Br J Clin Pharmacol 1980;9:195–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pollak CP, Tryon WW, Nagaraja H, Dzwonczyk R. How accurately does wrist actigraphy identify the states of sleep and wakefulness? Sleep 2001;24:957–965 [DOI] [PubMed] [Google Scholar]
- 46.LaJambe CM, Kamimori GH, Belenky G, Balkin TJ. Caffeine effects on recovery sleep following 27 h total sleep deprivation. Aviat. Space Environ. Med. 2005;76:108–113 [PubMed] [Google Scholar]