Abstract
Significance: Reactive oxygen species (ROS) play a critical role in vascular disease. While there are many possible sources of ROS, nicotinamide adenine dinucleotide phosphate (NADPH) oxidases play a central role. They are a source of “kindling radicals,” which affect other enzymes, such as nitric oxide synthase endothelial nitric oxide synthase or xanthine oxidase. This is important, as risk factors for atherosclerosis (hypertension, diabetes, hypercholesterolemia, and smoking) regulate the expression and activity of NADPH oxidases in the vessel wall. Recent Advances: There are seven isoforms in mammals: Nox1, Nox2, Nox3, Nox4, Nox5, Duox1 and Duox2. Nox1, Nox2, Nox4, and Nox5 are expressed in endothelium, vascular smooth muscle cells, fibroblasts, or perivascular adipocytes. Other homologues have not been found or are expressed at very low levels; their roles have not been established. Nox1/Nox2 promote the development of endothelial dysfunction, hypertension, and inflammation. Nox4 may have a role in protecting the vasculature during stress; however, when its activity is increased, it may be detrimental. Calcium-dependent Nox5 has been implicated in oxidative damage in human atherosclerosis. Critical Issues: NADPH oxidase-derived ROS play a role in vascular pathology as well as in the maintenance of normal physiological vascular function. We also discuss recently elucidated mechanisms such as the role of NADPH oxidases in vascular protection, vascular inflammation, pulmonary hypertension, tumor angiogenesis, and central nervous system regulation of vascular function and hypertension. Future Directions: Understanding the role of individual oxidases and interactions between homologues in vascular disease is critical for efficient pharmacological regulation of vascular NADPH oxidases in both the laboratory and clinical practice. Antioxid. Redox Signal. 20, 2794–2814.
Introduction
Reactive oxygen species (ROS) play an important role in the development of cardiovascular disease, including hypertension, atherosclerosis, diabetes, cardiac hypertrophy, and heart failure. Vascular ROS production is essential in all of these conditions as well as in the maintenance of normal vascular homeostasis (76, 164). In the vasculature, several differentially localized and expressed enzyme systems contribute to ROS formation. These include the nicotinamide adenine dinucleotide phosphate (NADPH) oxidases, endothelial nitric oxide (NO) synthases, enzymes of the respiratory chain, cytochrome P450 monoxygenases, and xanthine oxidase. While all of these systems are important in various disease states, NADPH oxidases seem to play the central role in orchestrating the activation and dysfunction of other enzymes. Initial generation of ROS by NADPH oxidases triggers the release of ROS from other sources (109). NADPH oxidase homologues are differentially expressed in the vascular wall, including endothelial cells, smooth muscle cells (SMCs), fibroblasts, and infiltrating immune cells (110). The expression profile of NADPH oxidases varies not only between different disease states, but also at various stages of the disease such as atherosclerosis. In general, it is accepted that under physiologic conditions, vascular NADPH oxidases have a relatively low level of constitutive activity. However, enzyme activity can be increased both acutely and chronically in response to stimuli such as cytokines (38), growth factors (23), hyperlipidemia, and high glucose (94), which disrupts vascular homeostasis and results in pathology.
While the role of vascular NADPH oxidases has been well described in pathology, their physiological functions remain less clear. We have recently gained substantial insight into the contribution of individual NADPH oxidase homologues in the maintenance of normal vascular function. In particular, the role of Nox4 in the regulation of endothelial function was clearly defined (166). This review focuses on the role of vascular NADPH oxidases in physiological and pathological processes in the vasculature, with particular emphasis on recently elucidated mechanisms such as the role of NADPH oxidases in vascular protection, vascular inflammation, pulmonary hypertension, and tumor angiogenesis. Finally, we briefly discuss the possibilities of pharmacological regulation of vascular NADPH oxidases and inhibitors being developed, in both the laboratory and clinical wards.
Localization, Structure, and Basic Functions of Major Nox Isoforms in Vasculature
Vascular Nox isoforms have six transmembrane domains, including alpha helices with cytosolic N- and C-termini, which participate in electron transfer, leading to the reduction of molecular oxygen to superoxide anion. Electron flow and thus ROS production is tightly controlled by the interactions of Nox subunits with other proteins, subunit phosphorylation, or elevation of intracellular calcium (15). There are seven isoforms of NADPH oxidases expressed in mammals: Nox1, Nox2, Nox3, Nox4, Nox5, Duox1, and Duox2. Four (Nox1, Nox2, Nox4, and Nox5) are most commonly expressed in vascular cells, while other homologues have not been found or are expressed at very low levels; thus, their role has not been established so far.
Nox2
Initially termed gp91phox, it has been cloned and identified as a phagocytic respiratory burst oxidase, critical for the initial nonspecific host defense. In addition to phagocytes, it is the most widely expressed vascular NADPH oxidase isoform. It is expressed in vascular smooth muscle cells (VSMCs), adventitial fibroblasts, endothelial cells, and perivascular adipocytes (92, 149, 188). This NADPH oxidase homologue has been characterized in detail and consists of the following subunits: gp91phox (glycoprotein-91 kDa phagocytic-oxidase, newly termed Nox2), p22phox, p47phox, p67phox, p40phox, and the GTPase Rac1. The gp91phox and p22phox subunits are membrane bound and together form cytochrome b558, located in cytoplasmic vesicles and the plasma membrane (20). The structure of this oxidase in vascular cells is similar to that found in phagocytes, although it may have additional or different regulatory subunits in selected conditions. In particular, Nox organizer protein 1 (NoxO1) and Nox activator protein 1 (NoxA1), initially discovered as Nox1 regulators (in place of p47phox and p67phox, respectively), may also have modest activating properties toward Nox2 (100). In endothelial cells, Nox2-derived ROS are important for p38 MAP-kinase-mediated proliferation and vascular endothelial growth factor (VEGF)-induced migration (187). Nox2 that is expressed in endothelial cells is involved in the regulation of numerous functions of the endothelial cell. For example, Nox2 activation affects NO bioavailability, and modulates expression of adhesion molecules during inflammation and angiogenesis. These will be further discussed next.
Nox1
Nox1 (Mox1, NOH1) has been identified as the first homologue of Nox2 (7). It shares a 60% amino-acid sequence identity with Nox2. Similar to its phagocytic homolog, Nox1 contains six transmembrane domains and conserved motifs corresponding to binding sites of heme, flavin, and NADPH. Nox1 is expressed in endothelial, smooth muscle, and adventitial cells of the vasculature. Most of the studies using recombinant Nox1 protein show localization in cell membranes, particularly in the plasma membrane (85). Using immunofluorescence microscopy, endogenous Nox1 protein displayed surface distribution along the cellular margins where it co-localized with caveolin-1 (33, 87). Other studies showed endogenous or overexpressed Nox1 protein localized to the nucleus, cytosol (28), endoplasmic reticulum (ER) (2), and mitochondria (40). Nox1 activity requires p22phox, NoxO1 (or possibly p47phox in some cases), and NoxA1, and the small GTPase Rac. Nox1-dependent ROS generation has been shown to play a pivotal role in cell signaling, cell growth, angiogenesis, and cell motility (6). Interestingly, ROS-generated via Nox1 have been reported to contribute to a growing number of diseases involving vasculature, including atherosclerosis, hypertension, neurological disorders, inflammation, and cancer (35, 142), which will be further discussed next. However, while highly expressed in animal models of vascular disease, Nox1 appears to be only expressed at low levels in human peripheral or coronary vessels (79, 80).
Nox4
This Nox isoform is a 67 kDa protein sharing a 39% amino-acid sequence identity with Nox2. It was initially detected in the kidney; therefore, it was termed Renox or kidney oxidase (KOX) (62). However, it was soon identified in vascular walls, particularly VSMCs, fibroblasts (15), and endothelial cells (64, 65). Studies of human coronary arteries have shown Nox4 to be expressed predominantly in the media (170). It has been suggested to be involved in stress signal transduction in the kidney (67) and SMCs (151). Nox4 mediates transforming growth factor β (TGF-β)-induced differentiation (173), insulin signaling (130), oxygen sensing (115), cardiac differentiation (116), and transcriptional regulation (107). Nox4 is predominantly localized at the ER (142, 151) and in the nucleus (40, 166). In addition, co-localization of Nox4 with the cytoskeleton and focal adhesions has been demonstrated (36, 87, 128). Enzymatic activity of Nox4 mainly depends on the expression level of Nox4 and p22phox and likely does not need any further activation (131). Currently, it is unclear which type of ROS is predominantly generated by Nox4. Unlike Nox1 or Nox2 that primarily produce O2−•, Nox4 has been shown to produce hydrogen peroxide (H2O2) (43, 85). Production of H2O2, rather than O2−•, by Nox4 is possible due to a highly conserved histidine residue in the E-loop of Nox4 that promotes rapid dismutation of O2−• before it leaves the enzyme (177). This aspect of Nox4 biology needs to be further clarified. Physiological roles of Nox4 differ depending on cell type and stimulus. Nox4 might have an antagonistic function to Nox1 and Nox2 (166). Nox4 is implicated in both pro- and anti-apoptotic pathways. It is needed for 7-ketocholesterol- induced apoptosis in SMCs (151), while silencing of the protein induces apoptosis in pancreatic cancer cells (136). Nox4 is important for the differentiation of cardiac cells from mouse embryonic stem cells (116) and myofibroblasts from cardiac fibroblasts (37). Differentiated VSMC require Nox4 to maintain expression of differentiation markers, smooth muscle major histocompatibility complex (MHC), alpha -actin, and calponin (36). Recent studies raise important questions regarding the major physiologic and pathologic roles of Nox4. While Nox4 contributes to oxidative stress, compelling evidence from Nox4−/− mice indicates that endogenous Nox4 protects the vasculature during ischemic or inflammatory stress (166). Interestingly, we have recently found that Nox4 expression is decreased in human abdominal aortic aneurysm (AAA) in spite of largely increased oxidative stress in the walls of AAAs (74). Moreover, Nox4 immunoreactivity has been demonstrated in the nucleus and nucleolus of VSMCs by confocal microscopy (87). A recent study has identified a novel nuclear-localized Nox4 splice variant with a size of 28-kDa, Nox4D, which lacks putative transmembrane domains (3). The possible functional role of nuclear Nox4 has been previously described (87). Interestingly, Nox4D overexpression results in increased NADPH-dependent ROS production and causes increased phosphorylation of extracellular signal-regulated kinase1/2 and the nuclear transcription factor Elk-1 (3). Thus, Nox4D may have important pathophysiologic effects through the modulation of nuclear signaling and DNA damage; however, its role in vascular biology should be further elucidated.
Nox5
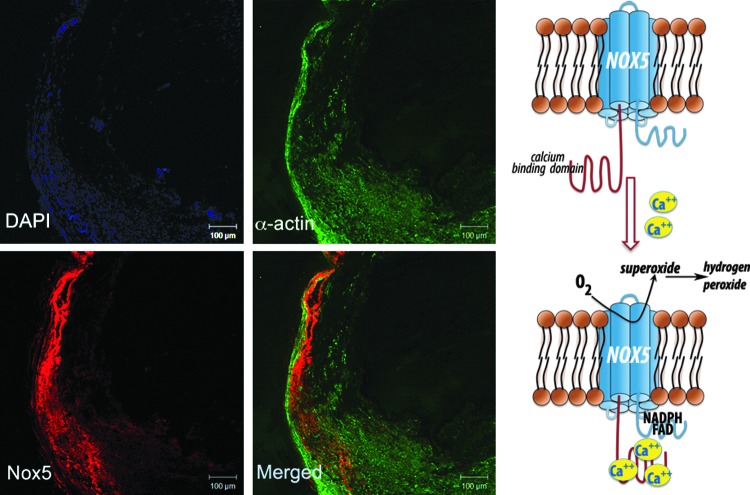
Nox5 is expressed in primates but does not naturally occur in rodents. It has been found in leukocytes, in the testis (during development), and in lymphatic tissue. Expression of lesser extent has been reported in ovaries, pancreas, and placenta (8, 32). Recent studies have also identified the presence of Nox5 in vasculature, namely in endothelial cells and VSMCs, where it is localized in the ER and the plasma membrane (16). Nox5 has been reported to produce both superoxide and H2O2; Nox5 is activated by Ca2+ and does not appear to require other subunits, although it may associate with p22phox. The basic transmembrane structure of Nox5 is similar to that described for other Nox isoforms, but what distinguishes Nox5 is a unique N-terminus which encodes four calcium-binding EF hands (Fig. 1) (8). We have identified a calcium-dependent Nox5 protein and mRNA in human coronary vasculature, in both endothelium and vascular media, which was functionally linked to calcium-dependent NADPH-oxidase activity (75).
FIG. 1.
Nox5 expression and function in human atherosclerosis. Based on Guzik et al. (75).
Diverse Mechanisms of Activation and Regulation of Vascular NADPH Oxidases
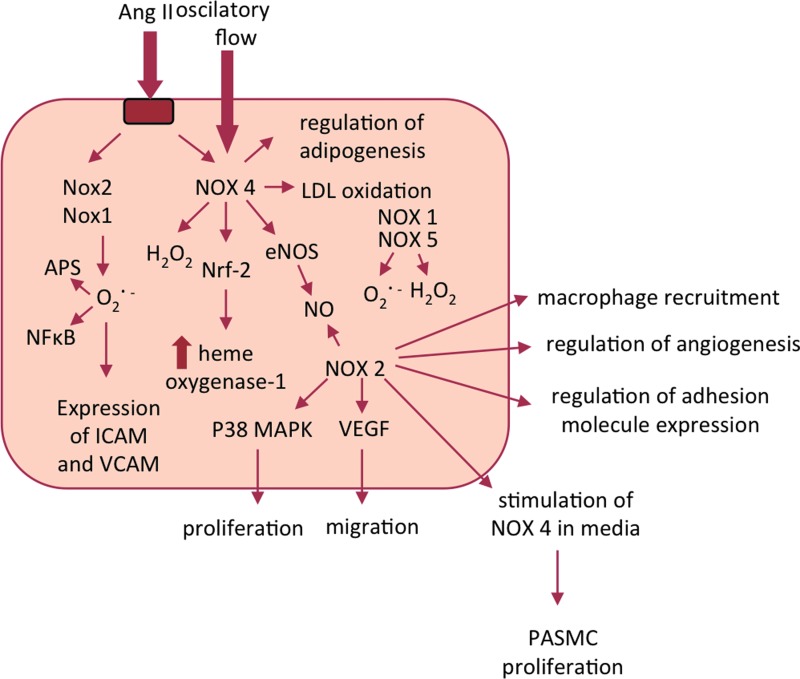
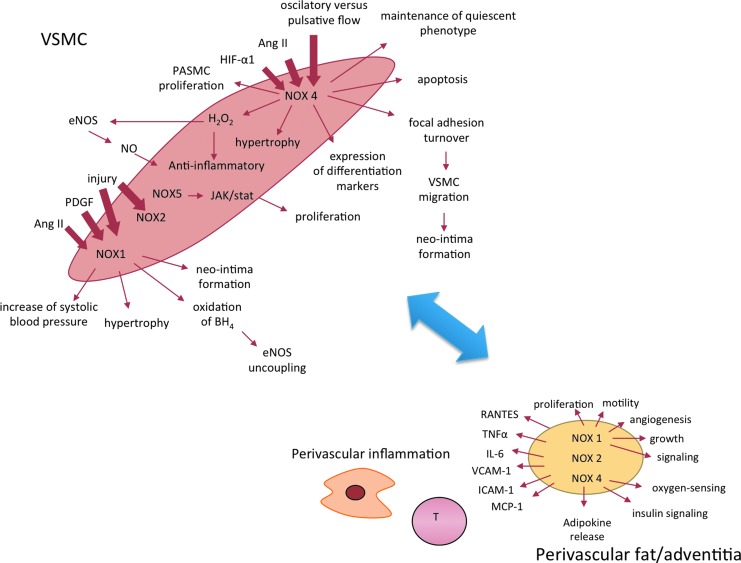
Despite similarities in core structures, vascular Nox homologues have different mechanisms of activation. Activation as well as its consequences may be different in the endothelium (Fig. 2) or VSMCs (Fig. 3) or other cells such as perivascular adventitial fibroblasts or adipocytes Nox1 and 2 require association with cytosolic components (p47phox, p67phox or NoxO1 and NoxA1), Nox4 is constitutively active, and Nox5 is activated by an elevation in intracellular Ca2+. The activation mechanism of Nox2 is most clearly defined and similar to that described in phagocytes. On activation, p47phox is phosphorylated and translocates to the membrane, through the formation of a complex with p67phox and p40phox. Phosphorylation of p47phox induces a conformational change in a tandem SRC Homology 3 (SH3) domain that enables binding to a proline-rich region in the cytosolic C-terminus of the transmembrane subunit p22phox. Independently, the GTP-binding protein Rac also moves to the membrane and activation occurs (15). In contrast to Nox2, the Nox4-based oxidase appears to be constitutively active and does not require p47phox, p67phox, or Rac.
FIG. 2.
Selected interactions between NADPH oxidases in the endothelium. While certain functions were demonstrated for selected homologues, high redundancy of functions is expected. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
FIG. 3.
Diversity of functional consequences of the activation of NADPH oxidases in vascular smooth muscle cells and perivascular tissues. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
Sustained activation of vascular NADPH oxidases occurs in response to numerous agonists. Vascular NADPH oxidases are responsive to several growth (platelet-derived growth factor [PDGF], epidermal growth factor [EGF], and TGF-β), cytokines (e.g., tumor necrosis factor, interleukin-1 [IL-1], and platelet aggregation factor), mechanical forces (cyclic stretch, laminar, and oscillatory shear stress), metabolic factors (hyperglycemia, hyperinsulinemia, free fatty acids, and advanced glycation end products), and G protein–coupled receptor agonists (serotonin, thrombin, bradykinin, endothelin, and angiotensin II [Ang II]) (71, 152, 154). In addition, c-Src, p21Ras, protein kinase C (PKC), phospholipase D, and phospholipase A2 (PLA2) have been demonstrated to play key roles in signaling involved in vascular NADPH oxidase activation (1, 12, 39).
Ang II is one of the key agonists stimulating Nox1 and Nox2 oxidase subunit expression as well as oxidase activation in vascular cells and neutrophils (148, 156, 187). However, its effects on Nox4 and Nox5 are much less pronounced and perhaps indirect, which will be further discussed next (37, 111, 139). Ang II-induced production of ROS by VSMCs is initially PKC-dependent (early phase), whereas the prolonged phase (>30 min) is dependent on Rac, Src, and phosphatidylinositol 3-kinase (167). Ang II increases mRNA levels not only of Nox proteins but also of p22phox and p67phox (55, 148). Ang II-dependent or H2O2-induced activation of Nox4 in mesangial cells or cardiac fibroblasts has been reported to be dependent on PLA2 and release of arachidonic acid (37, 86).
Cytokines have also been shown to regulate vascular NADPH oxidases, which links inflammation with oxidative stress. In particular, tumor necrosis factor-α (TNF-α) stimulates NADPH oxidase Nox1, Nox2, and Nox4 expression and activation in a variety of vascular cells (4, 11, 138). Other stimuli that induce vascular NADPH oxidases include ER stress (151), shear stress (91), or an elevation of intracellular Ca2+, which could act as an upstream signal to activate Nox during cellular stress. The Nox5 isoform contains multiple EF-hand Ca2+-binding domains in the N-terminal region, allowing its activation through calcium sensing (8). Calcium binding induces a conformational change in the N terminus of Nox5, resulting in the exposure of a hydrophobic motif, which leads to a direct interaction between the regulatory N-terminus and the catalytic C-terminus. This intra-molecular interaction is likely to be responsible for Ca2+-induced Nox5 activation (9), as it enables electron flow to the heme moieties and, consequently, drives superoxide production.
Redox regulation of vascular NADPH oxidase activation provides both negative feedback and feed-forward regulation mechanisms. For example, Rac1 protein turnover is strongly dependent on the redox status of the cell, creating a negative feedback mechanism (106). At the same time, a feed-forward mechanism exists (118), by which exogenous exposure of SMC or fibroblasts to H2O2-activated NADPH oxidase stimulates them to produce endogenous O2−•, thereby amplifying the vascular injury process. The self-limiting mechanism could predominate during physiologic conditions, and be involved in maintaining low output of the nonphagocyte NADPH oxidase, whereas the feed-forward mechanism may have a role in NADPH oxidase-dependent oxidative stress in a variety of diseases, including atherosclerosis and inflammation.
NADPH Oxidases in Vascular Pathobiology
The complexity of vascular NADPH oxidase systems makes it difficult to unequivocally define the role of individual Nox oxidases in vascular physiology and pathology. Several genetically modified mouse strains with either removed or overexpressed Nox proteins have been used to investigate the roles of NADPH oxidases in the vasculature (Table 1). Such studies have clearly linked Nox1, Nox2, and Nox4 to the pathogenesis of vascular diseases, while other Nox isoforms showed little to no vascular expression or involvement. Obviously, this approach is not optimal for studies involving Nox5, which would require a more complex humanized model approach. Numerous studies in animal models and in humans have also characterized the role of Nox proteins in the most common vascular disease states such as atherosclerosis, hypertension, and diabetes, which will be discussed next.
Table 1.
Selected Mouse Models Used to Study the Importance of Vascular NADPH Oxidases and Their Functional Effects
| Disease | Mouse model | Effect on disease | References |
|---|---|---|---|
| Atherosclerosis | Double knockout Nox2−/−/ApoE−/− | No change in lesion area in the aortic sinus | Kirk et al. (102) |
| Double knockout p47−/−/ApoE−/− | No change in lesion area in the aortic sinus; | Hsich et al. (90) | |
| Decreased total aortic lesion area | Barry-Lane et al. (10) | ||
| Nox2 endothelium-specific overexpression in ApoE−/− | Increased, vascular inflammation, and oxidation but not atherosclerosis. | Douglas et al. (50) | |
| Nox1 VSMC overexpression | Increased neointima on wire injury | Lee et al. (114) | |
| Hypertension | Nox1−/− | Decreased BP and O2−• levels | Matsuno et al. (132), Gavazzi et al. (60) |
| Nox1 VSMC overexpression | Increased BP | Dikalova et al. (44) | |
| Nox2−/− | No change in BP | Touyz et al. (182) | |
| Decreased BP | Jung et al. (98), Wang et al. (193) | ||
| Nox4−/− | No change in BP | Kleinschnitz et al. (103) | |
| p47−/− | Decreased BP and O2− levels | Landmesser et al. (108), Lavigne et al. (113) | |
| Pulmonary hypertension | Nox4−/− | No change in pulmonary blood pressure but prevention of poststroke neurodegeneration | Kleinschnitz et al. (103) |
| Cardiac failure | Nox1−/−; Nox2−/− | Effect on infarct size in myocardial ischemia/reperfusion; Decreased hypertrophy; Increased cardiac hypertrophy | Braunersrenther V et al. (23a) |
| Bendall et al. (17) | |||
| Byrne et al. (25) | |||
| Nox4−/− | Decreased cardiac hypertrophy and failure | Kuroda et al. (107) |
ApoE, apolipoprotein E; NADPH, nicotinamide adenine dinucleotide phosphate; VSMC, vascular smooth muscle cell.
Atherosclerosis
A number of studies have investigated the role of individual NADPH oxidases in atherosclerosis in animal models. Nox2 levels were increased in the aortas of apolipoprotein E (ApoE)−/− atherosclerotic mice, while Nox4 levels were not significantly different between ApoE−/− and wild type (97). However, studies using deletion of the Nox2 gene in ApoE−/− mice have not been fully consistent. Nox2/ApoE double knockout mice, which had been fed a high fat diet, were found to have no obvious protection against atherosclerosis when examining aortic sinus sections, in spite of decreased vascular superoxide formation (102). Similar surprising results were reported in global p47phox knockout mice crossed with ApoE−/− mice (90). Endothelial-targeted Nox2 overexpression in ApoE−/− mice was sufficient to increase vascular superoxide production, endothelial cell activation, and subsequently increased macrophage recruitment. This initial increase in macrophage recruitment did not alter the progression of atherosclerosis (50). In contrast to these studies, Barry-Lane et al. found that total aortic lesion area (from arch to bifurcation) was reduced in p47phox knockout mice crossed with ApoE−/− mice, suggesting that Nox1 and/or Nox2 may be involved in atherogenesis (10). Such discrepancies in the findings regarding the vascular consequences of p47phox−/− could be due to several reasons. An example is the inclusion of assessments of different areas from aortic lesions (sinus vs. whole aorta). Another reason for the differences may also be related to the removal of p47phox, which causes immunodeficiency. These mice are prone to sub-clinical infections that are characterized by splenomegaly and inflammation which could counteract the anti-atherosclerotic effect of the loss of NADPH oxidase. While studies in animal models are conflicting, a genetic hereditary deficiency of NADPH oxidases, particularly Nox2 (gp91phox), occurs in humans with chronic granulomatous disease (CGD). In humans, the genetic deficiency of Nox2 is associated with enhanced endothelium-dependent flow-mediated vasorelaxation, and decreased markers of vascular aging and oxidative stress (192). Importantly, diminished atherosclerosis burden has been described in these patients, which emphasizes the possible role of Nox2 oxidase in atherosclerosis (191). Although promising, these observations should be interpreted carefully, as CGD is a serious disease leading to important changes in the immune system, which could affect vasculature in a number of additional ways.
Nox4 may also participate in atherogenesis, although much less is known in this regard. It has been shown, however, that Nox4 regulates VSMC migration and differentiation, which is critical for neo-intima formation (128). In human atherosclerosis, Nox4 expression is increased in intimal lesions of coronary arteries (170). Similarly, Nox1 overexpression in the media results in increased neo-intima formation on vascular injury (114).
Numerous studies have looked at vascular NADPH oxidases in human atherosclerosis. Our group as well as others have characterized NADPH oxidases in human coronary arteries with evident atherosclerotic plaques (75, 79, 170), as well as in peripheral arteries and veins without plaques but characterized by systemic endothelial dysfunction (73, 74, 80–82). The NADPH oxidase is predominantly Nox2-based in veins, whereas an Nox4-based oxidase appears proportionately more important in human mammary or radial arteries (80). In veins, the predominance of Nox2 expression may also suggest the major contribution of endothelium and adventitia to total vascular ROS production, as these contain Nox2-based oxidases (14), potentially contributing to endothelial dysfunction (160, 170, 175). However, the cellular expression of individual oxidases has not been clearly established. While initial studies in human vessels suggest that Nox2 is not usually present in medial VSMCs, it has been demonstrated that human microvascular SMCs can express Nox2 in response to Ang II (182). Meanwhile, Nox4 expression is increased by oscillatory versus pulsatile flow, which could be particularly relevant to the development of atherosclerotic plaques in the regions of turbulent flow (91). This clearly illustrates that Nox isoform expression in human vascular cells is regulated in a complex manner that depends on the cell type predominating in different vessels and on the nature of pathophysiologic stimuli.
Still, it is vital to emphasize that overall, enzymatic sources of O2−• in coronary arteries are similar to those of peripheral arteries (18, 78). In summary, Nox2, Nox4, Nox5, p22phox, and, to a lesser extent, Nox1 are expressed in human peripheral and coronary vessels, which may contribute to atherosclerosis (80, 170). Nox1 expression directly alters cell proliferation (174), and treatment of VSMC with Ang II or PDGF up-regulates Nox1, while down-regulating Nox4 (111). Vascular injury increases expression of Nox1, Nox2, and p22phox, while Nox4 increases later (175), coinciding with a reduction in the rate of VSMC proliferation. Taken together, these findings suggest that while Nox1 and Nox2 are involved in acute response to injury or to Ang II stimulation, Nox4 is involved in maintaining the quiescent VSMC phenotype (175). Thus, a high Nox2/Nox4 ratio in veins could partially account for the susceptibility to intimal hyperplasia, remodeling, and accelerated atherosclerosis in vein grafts (197); whereas a lower Nox2/Nox4 ratio in mammary artery grafts could convey less susceptibility to atherosclerosis. Functional consequences of increased NADPH oxidase activity also include the effects on lipid oxidation, which is a critical step in the initiation of atherosclerotic plaque development. For instance, Nox4 expression elevates in relation to oscillatory flow and coincides with increased oxidative stress and low-density lipoprotein (LDL) oxidation (91), while NADPH oxidase activity is correlated with oxidized-LDL in carotid plaques. Despite the differences in the expression levels between individual Nox homologues, the expression of both p22phox and Nox4 mRNA is strikingly correlated in human arteries and veins (80). This indicates that risk factors for atherosclerosis may be critical for the systemic regulation of NADPH oxidase expression (80). Indeed, activity and expression of major NADPH oxidase components is correlated to the number of major risk factors for atherosclerosis in humans (82).
Finally, Nox5 is an important source of ROS in atherosclerosis (75). Nox5 mRNA and Nox5 protein are dramatically increased in human coronary arteries obtained from patients with coronary artery disease. These correlate with the Ca2+-dependent NADPH oxidase activity in arteries. Nox5 was expressed in the endothelium of early-stage lesions and in VSMCs in the intima of advanced coronary lesions (Fig. 1) (75).
In summary, differential generation of ROS by functionally distinct NADPH oxidase isoforms expressed in different vascular cell types in atherosclerosis may be used as a therapeutic advantage. For example, Nox2-based production of ROS predominates in the endothelium and adventitia, whereas Nox1 and/or Nox4 could be much more important in VSMC (158), as their expression and activity differs depending on the stage of the disease. Thus, one could postulate, that if we were able to specifically inhibit these individual oxidases, we would be able to affect distinct stages in vascular disease processes.
Hypertension
Vascular NADPH oxidases are probably characterized in most detail in relation to hypertension. This was one of the first pathologies in which NADPH oxidases were clearly implicated (156). Ang II plays an important role in the development of hypertension and is one of the most important inducers of increased NADPH oxidase-dependent superoxide production in VSMCs (48) and throughout the vascular wall (141). The effects of Ang II on NADPH oxidases are mediated primarily via the angiotensin receptor type 1 (AT1) receptor (79). NADPH oxidases also participate in Ang II-stimulated intracellular H2O2 production, which mediates vascular hypertrophy (205). Ang II increases the expression of several NADPH oxidase homologues (Nox1, Nox2, Nox4, and p22phox) (156), all of which may to some extent participate in the pathogenesis of hypertension and associated vascular dysfunction. Nox1 is increased in the aortas of aged spontaneously hypertensive rats (SHRs) in parallel with increased NADPH oxidase activity. While Nox2 was also up-regulated, Nox4 protein levels were unchanged or even decreased in the aortas of SHRs (198). Overexpression of Nox1 in mouse VSMCs caused a marked increase in systolic blood pressure and hypertrophy in response to Ang II (44). In contrast, deletion of Nox1 in mice results in a blunted hypertensive response to Ang II and protects from development of endothelial dysfunction, oxidative stress, vascular hypertrophy, and aortic dissection (60, 61, 132). Overexpression of p22phox in VSMCs leads not only to up-regulation of Nox1-based oxidase in the vessel wall, but also to increased hypertensive responses to Ang II (112, 195). At the same time, silencing of p22phox in rats prevents a slow pressor response to Ang II (137). Similarly, p47phox knockout mice, which have deficient Nox1 (and Nox2) activation, exhibit reduced hypertension and preserved endothelial function after chronic Ang II treatment (108, 109). VSMCs from p47phox-deficient mice do not produce superoxide (113). Moreover, local overexpression of Nox1 in VSMCs has profound effects on other cell types in the vessel wall. For example, this can lead to the oxidation of tetrahydrobiopterin (BH4) in endothelial cells and endothelial nitric oxide synthase (eNOS) uncoupling, resulting in a decrease of NO bioavailability and causing endothelial dysfunction (45). In contrast, Nox2−/− mice were reported to have only modestly altered (193) or even unaltered (183) blood pressure responses to Ang II, suggesting that this isoform may be less important in blood pressure regulation, but still showing effects on vascular hypertrophy (193) and endothelial dysfunction during experimental hypertension (98). In addition, Nox2 was critical for cardiac hypertrophy in Ang II-dependent hypertension (17), but not in a pressure-overload-dependent model, where hypertrophy might be related to other homologues such as Nox4 (25). While gene-specific deletion or overexpression approaches have proved useful in defining the role of Nox1and Nox2 in the vascular system, the functional importance of Nox4, while undeniable, has been raising the most controversy and discussion. Endothelium-targeted Nox4 overexpression causes enhanced vasodilation and reduced basal blood pressure, suggesting a protective role for Nox4 that has been linked to increased H2O2 production by Nox4 (157). In the absence of pathogenic stimuli, Nox4 knockout mice do not have an obvious phenotype and are normotensive (176). However, more recent data have convincingly shown that in vascular pathologies, Nox4 may serve as a protective oxidase (22, 166). In the conditions associated with stress induced by ischemia or by Ang II, loss of Nox4 resulted in reduction of eNOS expression, NO production, and heme oxygenase-1 (HO-1) expression. These changes were associated with apoptosis and inflammatory activation in Nox4−/− mice (166). These effects were partially related to Nrf-2 induction. Consequently, there is now clear evidence that endogenous Nox4, in contrast to Nox1 and Nox2, may protect the vasculature during ischemic or hypertensive stress (166). However, this may not be the case in the brain, where Nox4 has been shown to contribute to oxidative stress related to stroke and other pathologic conditions (103). Hence, further studies are needed to determine whether Nox4 is always protective or whether it can become dysfunctional.
Moreover, while the role of Nox1 in the pathogenesis of hypertension appears to be relatively well documented, chronic models of high renin transgenic overexpression do not show significant effects of Nox1 on blood pressure in this model of hypertension (204).
Diabetes
Diabetes is associated with a variety of metabolic abnormalities, such as insulin resistance and hyperglycemia. However, cardiovascular complications are the major cause of mortality in diabetic patients. ROS generated during hyperglycemia are implicated in the development of endothelial dysfunction and progression of diabetic vascular complications. Endothelial dysfunction characterized by increased NADPH oxidase-dependent ROS generation has been demonstrated in animal models of diabetes (88), and in patients with diabetes (82). In aortas from the streptozotocin (STZ)-induced diabetic ApoE−/− mice, levels of Nox2 and Nox4 are increased (47). Similarly, in db/db mice, Nox1 or Nox4 are up-regulated, which is associated with increased ROS production and inflammation, indicating a potential role of Nox1 and Nox4 in diabetic macrovascular disease (119). Another recent study has elegantly addressed the role of Nox1 in diabetic vasculopathy using the specific Nox inhibitor GKT137831, as well as Nox1−/− mice on an ApoE−/− background in which diabetes has been induced (69). Deletion of Nox1, but not Nox4, had a profound anti-atherosclerotic effect correlating with reduced ROS formation, diminished chemokine expression, reduced vascular adhesion of leukocytes, reduced macrophage infiltration, and reduced expression of proinflammatory and profibrotic markers (69). Similarly, treatment of diabetic ApoE-deficient mice with GKT137831 attenuated atherosclerosis development (69).
Hyperglycemia itself can induce vascular NADPH oxidases. Incubation of human endothelial cells with red blood cells isolated from patients with type 1 diabetes, but not from normal volunteers, activated endothelial NADPH oxidase and increased endothelial ROS generation, which was mediated by advanced glycation end products on the surface of red blood cells from diabetic patients (194). Hyperglycemia increases NADPH oxidase expression, levels of oxidative stress markers, and apoptosis (155) of human endothelial cells in relation to increased NADPH oxidase subunit expression (for example, p22phox and p47phox) (46). Similarly, in arteries and veins, superoxide anion production is strongly increased in diabetic patients, most prominently in the endothelium (78). Superoxide anion produced in large quantities by NADPH oxidases reduce NO bioactivity by direct scavenging.
While NADPH oxidases play a key role, uncoupled eNOS has also been shown to be a particularly important source of ROS in diabetic blood vessels (88). Importantly, superoxide derived from vascular NADPH oxidases leads to eNOS uncoupling through oxidation of BH4 (88). Ang II is also involved in the activation of vascular NADPH oxidases in diabetes. AT1 receptor-mediated NADPH oxidase activation appears to contribute to vascular insulin resistance, endothelial dysfunction, apoptosis, and inflammation (196). While vascular Nox4 does not appear to have a direct role in diabetic vasculopathy (69), it may have indirect effects through the regulation of adipogenesis (preadipocyte differentiation in response to insulin) in metabolic syndrome (165).
Consequently, vascular NADPH oxidases have emerged as potentially important targets contributing to the pathogenesis of long-term cardiovascular complications of diabetes. Of the numerous pathologies and vascular disease states, diabetic vasculopathy may prove be the most interesting area for the future application of NADPH oxidase inhibitors.
In summary, genetically manipulated mouse models as well as studies in human vasculature have been very useful in defining the functional importance of individual NADPH oxidases. Further studies characterizing individual knockout and overexpression of Nox isoforms, specifically in endothelial cells, VSMCs, and fibroblasts will undoubtedly provide a better understanding of the complex mechanisms involved in NADPH oxidase activation and regulation. Studies of Nox5 expression in various models are now warranted to further understand its role in the vasculature using these valuable molecular tools.
Vascular NADPH Oxidases in Pulmonary Hypertension
Recently, significant progress has been made in our understanding of the role of vascular NADPH oxidases in pulmonary hypertension. This is crucial, considering the clinical consequences of pulmonary hypertension and a lack of successful therapies. The major pathologic process in pulmonary hypertension is chronic hypoxia-induced vascular remodeling. It is characterized by malignant SMC hypertrophy and proliferation within the pulmonary vessel media (123). This leads to a decrease in vascular luminal area, increased vascular resistance, and thus development of pulmonary hypertension and increased right ventricular pressure. A role for Nox2-based NADPH oxidase in this process has been suggested, as all of these phenotypes are attenuated in Nox2−/− mice (123). This is consistent with the role of this oxidase in VSMC hypertrophy, particularly pulmonary artery smooth muscle cells (PASMCs) (93, 173).
However, the role of NADPH oxidases in pulmonary hypertension appears to be more complex than simply stimulating VSMC hypertrophy and proliferation. They may be involved at very early stages of disease pathogenesis. ROS derived from vascular Nox isoforms, in particular Nox2 and Nox4, are involved in long-term responses of pulmonary vasculature to hypoxia (52, 135). Thus, NADPH oxidases have been suggested to serve as oxygen sensors in the lung. Interestingly, Nox4 has been reported to be the predominant homolog in human airways and in PASMCs (49). PASMCs are particularly sensitive to oxygen availability and are responsible for acute hypoxic vasoconstriction and the development of pulmonary hypertension in response to chronic hypoxia (72). Nox4 expression level has been increased in mice exposed to chronic hypoxia and in isolated PASMCs exposed to hypoxia. Diebold et al. have described a putative hypoxia responsive element in the human Nox4 promoter and demonstrated that this sequence is indispensable for the binding of hypoxia-inducible factor-1α (HIF-1α) (42). In fact, RNA interference against HIF-1α suppressed the hypoxia-induced expression of Nox4 (42). In patients with idiopathic pulmonary arterial hypertension, the level of Nox4 expression has been up-regulated in the lung. Treatment of PASMCs with an anti-Nox4 siRNA significantly reduced their proliferation.
Interestingly, it appears that Nox2 and Nox4 play coordinated roles in the development of hypoxia-induced pulmonary hypertension. Theoretically, endothelial ROS generation by Nox2 may stimulate Nox4 up-regulation in the vessel media, which would be important for hypoxia-dependent PASMC proliferation (135). Hypoxia also increases the level of TGF-β (97), which has been shown to induce Nox4 expression. Inflammatory processes can also contribute to the induction of Nox4 in pulmonary hypertension. In particular, nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) is involved in the hypoxia-induced expression of Nox4 in PASMC, in that hypoxia increases the binding of NF-κB p65 to the putative NF-κB binding site adjacent to the putative hypoxia-responsive element. Furthermore, activation of the nuclear hormone receptor peroxisome proliferator-activated receptor gamma (PPARγ) attenuated hypoxia-induced Nox4 expression, pulmonary hypertension, and pulmonary vascular remodeling (143). Rosiglitazone, a synthetic agonist of PPARγ, attenuated hypoxia-induced pulmonary hypertension and vascular remodeling in the mouse and reduced hypoxic Nox4 induction and ROS generation in the lung (127). Thus, while both Nox2 and Nox4 seem to be involved in pulmonary hypertension, it appears that Nox4 may be involved in responses to hypoxia as well as inflammation, and may be an important target for novel pulmonary hypertension therapies.
Vascular NADPH Oxidases in Tumor and Ischemic Angiogenesis
Another field in which vascular NADPH oxidases (mostly endothelial and endothelial progenitor cells) seem to be gaining importance is angiogenesis. It encompasses a wide range of conditions ranging from physiological situations such as embryogenesis and wound repair, to pathological conditions such as diabetes, arthritis, and cancer. In endothelial cells within the tumor, ROS have a critical role in neovascularization during tumor growth (140), while the thiol-antioxidant, N-acetylcysteine (NAC), attenuates endothelium cells invasion and angiogenesis in an in vivo tumor model (26). Vascular NADPH oxidases respond to various pro-angiogenic factors such as VEGF, making them prime targets in anti-angiogenic therapies. For example, Nox2-derived ROS are important in signaling for cytoskeletal organization in endothelial cell migration (186). Similarly, Nox1, Nox4, and Nox5 expression is increased in melanoma cells, and affects neovascularization and angiogenesis in the tumor (68). While being targets for pro-angiogenic factors, vascular NADPH oxidases are important modulators of the release of various angiogenesis-related factors, which are redox sensitive. These include VEGF-A, HIF-1α, p53, and matrix metalloproteinases (MMPs). Nox1-induced H2O2 increases VEGF and VEGF receptor expression, and MMP activity, markers of the angiogenic switch, thereby promoting vascularization and rapid expansion of the tumors (6). Increased VEGF expression and enhanced angiogenesis in experimental atheromas have been demonstrated in transgenic mice overexpressing Nox1 and Nox2 (101). Implantation of tumorigenic B16F0 melanoma cells subcutaneously in wild-type mice has resulted in vascularized tumors after 10 days. In Nox1−/− mice, the tumors that have developed have been smaller and less vascularized. This reduction in angiogenesis was associated with a reduction in the expression of several genes, including VEGF, MMP-2, MMP-9, and a reduction in NF-kB activity (58).
In a similar fashion, NADPH oxidases participate in ischemic revascularization. Nox2 knockout mice showed reduced VEGF-induced new vessel formation (186) and delayed the blood flow recovery in ischemic hind limbs (181). It is noteworthy that Nox2 deficiency-induced impairment of ischemic angiogenesis was associated with reduced endothelial progenitor mobilization from the bone marrow in response to ischemic insults (185). Impaired blood flow recovery in Nox2−/− mice has been rescued by transplantation of wild-type bone marrow cells into Nox2-deficient animals. This indicates the therapeutic potential of targeting Nox in endothelial progenitors in the treatment of ischemic cardiovascular diseases. The role of Nox5 in angiogenesis has also been recently described. In human aortic SMCs, silencing of Nox5 abrogated PDGF-induced proliferation via a JAK/STAT signaling pathway (96). Nox5 also mediates thrombin-induced formation of capillary-like structures in endothelial cells, suggesting an additional role in angiogenesis (16).
While mechanisms of angiogenesis are well defined in both ischemia and cancer, the role of vascular NADPH oxidases is becoming clearer. Use of novel Nox inhibitors may turn out to be a valuable strategy in future anti-angiogenic therapies, although possible adverse effects on ischemic angiogenesis should also be taken into consideration.
Seasonal Variation of Vascular NADPH Oxidase Activity
A recent study has reported an interesting phenomenon that could have important implications for studies of vascular NADPH oxidases (105). Activity and expression of vascular NADPH oxidases may vary seasonally in both rats and mice (and possibly in humans). Seasonal variation in endothelial dysfunction and oxidative stress has been noted in humans and rats, suggesting it to be a common phenomenon of potential clinical relevance (89). The mechanism is still not fully understood and has been related to changes in physical activity. Interestingly, Langendorff-perfused guinea-pig and rat heart preparations generated close to 100% more O2−•, while human subjects excreted 65% more 8-isoprostane in the summer versus other seasons (105). Inhibitors of NADPH oxidase, xanthine oxidase, and NO synthase prevented seasonal O2−• overproduction (105). In the summer, NADPH oxidase and xanthine oxidase activity, and protein expression were increased. Meanwhile, endothelial NO synthase and superoxide dismutases were down-regulated, which was accompanied by adhesion molecule up-regulation and the endothelial glycocalyx destruction associated with these changes (105). Moreover, during the summer, STZ-induced diabetes was associated with greater vascular disturbance, in spite of similar hyperglycemia (105). These findings are intriguing, as they may indicate rhythmic regulation of Nox expression and activity, which should be further investigated and verified.
NADPH Oxidases in the Regulation of Vascular Inflammation
During the past several years, it has become apparent that inflammation and oxidation are two basic processes underlying the pathogenesis of most disease states in humans. Furthermore, it is now evident that these two distinct mechanisms are in a constant interplay, with interactions particularly evident in the vessel wall (121, 134, 176). Vascular oxidative stress regulates the development of vascular and renal inflammation that has been recently implicated in the pathogenesis not only of atherosclerosis but also of hypertension and hypercholesterolemia (84).
The role of vascular NADPH oxidases in the regulation of vascular inflammation has been initially defined in the pathogenesis of atherosclerosis. The expression of endothelial adhesion molecules such as vascular cell adhesion molecule 1 (VCAM-1), intercellular adhesion molecule 1 (ICAM-1), and P- and E-selectins on large vessel arterial lumen endothelial cells is redox sensitive and dependent on Nox1, Nox2, and Nox5 NADPH oxidases. This is in part, because they contain an NFκB site within the promoter region that is known to be regulated in a redox-sensitive fashion (169). It is the earliest and most critical step in the inflammatory process, which leads to transmigration of leukocytes into the subendothelial space (120, 144). This occurs not only in large vessels during atherosclerotic lesion formation, but also in the endothelium of the vasa vasorum, leading to accumulation of leukocytes in the adventitia and outer media. It appears that perivascular inflammation characterized by macrophage, dendritic cell, and T- and B-cell accumulation, in the form of tertiary lymphoid structures, is a key feature of early atherosclerosis (56, 99, 104) or hypertensive vascular dysfunction (77, 190). Although the precise mechanism underlying this early process is not entirely clear, NADPH oxidase-derived ROS are increasingly being recognized as important players (117, 150). Studies of coronary arteries from diabetic pigs have shown that diabetes-induced increases in NADPH oxidase activity are accompanied by up-regulation of inflammatory cytokines (IL-6 and TNF-α), chemokines (monocyte chemoattractant protein-1 [MCP-1]), and vascular cell adhesive molecules (VCAM-1), providing further support for the role of NADPH oxidase in vascular inflammation (206). In addition, NADPH oxidases, via overproduction of O2−•, mediate expression and release of chemokines such as regulated on activation, normal T cell expressed and secreted (RANTES), or chemokine (C-C motif) ligand 2 (CCL2) from endothelium or VSMCs, essential for leukocyte recruitment to the vascular wall (29). ROS derived from adventitial fibroblasts may also serve as a direct chemotactic signal for leukocytes (122) and increase another chemotactic molecule, MCP-1 (201). Scavenging of ROS by SOD and catalase suppresses the induction of MCP-1 mRNA by TNF-α (31). Ang II, endothelin 1 (ET-1), and inflammatory mediators can modulate basal NADPH oxidase-induced O2−• production by affecting both activation of the enzyme and expression of NADPH oxidase subunits (55). Liu et al. have shown that Ang II infusion for 1 week increased NADPH oxidase-derived ROS production in rat aortas, and enhanced ICAM-1 expression and subsequent adventitial macrophage infiltration (122). Similarly, Ang II infusion in mice induced a convergence of macrophages as well as T cells to the adventitia (24). Finally, recent mechanistic studies have shown a particular importance of Nox5 in the regulation of vascular inflammation, which has been linked to Fraktalkine gene expression (161). These effects are particularly important in the light of recent observations, showing that inflammatory cell activation is critical for the development of hypertension (190) and vascular dysfunction (83). However, these are not limited to the vasculature, as NADPH oxidases and ROS are essential in regulating inflammation in other organs such as the lungs (66).
While vascular NADPH oxidases are important in the regulation of inflammatory responses, NADPH oxidases are also expressed in immune cells. Ang II-induced hypertension is associated with increased Nox2-mediated superoxide production and Nox2 expression in T cells and monocytes in peripheral blood. This has been linked to the activation of these cells and may be important in the pathogenesis of Ang II-mediated hypertension. Furthermore, Nox4 is critical in monocyte priming for chemotaxis and recruitment to the vessel wall (184).
Understanding the complex interactions between vascular oxidative stress and vascular inflammation has become possible, thanks to the development of novel strategies for quantitative assessment of vascular inflammation in relation to Nox activity and expression. Nevertheless, a further understanding of pro- and anti-inflammatory roles for vascular oxidases is now necessary (176).
NOX-Derived ROS and Central Regulation of Blood Pressure
Blood pressure regulation is a complex process involving renal, vascular, and central mechanisms. The brain is essential for processing and integrating neurohumoral signals from the periphery to maintain pressure and fluid homeostasis. NADPH oxidases have been identified as major producers of ROS in the brain and are expressed in numerous brain structures (19). Increased ROS production is seen in several regions of the brain in hypertension, including the circumventricular organs (CVOs) (210), hypothalamic centers, and the brainstem (146). Nox2 as well as Nox4 are the predominant homologues expressed in the fore-, mid-, and hindbrain of mice, while Nox1 is detectable at very low levels (92). However, Ang II induces expression of individual NADPH oxidase homologues, particularly Nox2 in the brain, which may be related to increased sympathetic nervous system activation (92). Taken together, it is possible that an increase in NADPH oxidase-derived ROS in CVOs, hypothalamic nuclei, and brainstem sites plays a central role in the neurocardiovascular dysfunction observed in hypertension (21). Vascular NADPH oxidases could also be important, as Nox2 appears to be a prominent mediator of the harmful effects of Ang II in the cerebral circulation during hypertension (34). In addition, knock down of Nox2 and Nox4 proteins in the paraventricular nucleus attenuated the development of Aldosterone/NaCl-induced hypertension (202). Silencing Nox2 or Nox4 alone in the subfornical organ (SFO) significantly attenuated the central Ang II-induced pressor response, while silencing both Nox2 and Nox4 together abolished this response. In contrast, silencing Nox2, but not Nox4, significantly attenuated the central Ang II-induced dipsogenic response, showing the different roles of Nox2 and Nox4 in the regulation of hypertension and the dipsogenic response (153). Nox2 and its cytosolic components (p40phox, p47phox, and p67phox) are expressed in the rostral ventrolateral medulla (RVLM) of rabbits, and are increased by intracerebroventricular Ang II administration (57). Similar findings were described in rat RVLM (30), additionally showing that Ang II increased the serine phosphorylation of p47phox, suggesting NADPH oxidase assembly and activation. Lob et al. showed, in turn, that deletion of p22phox from the SFO brain region diminished the hypertensive response and eliminated peripheral vascular inflammation caused by Ang II (126). Inhibition of NADPH oxidase in the SFO repressed the cardiovascular and dipsogenic effects to intracerebroventricular administration of Ang II (208). In addition, there is compelling evidence that superoxide anion is necessary to elicit the vasopressor, bradycardic, and dipsogenic responses produced by the intracerebroventricular administration of Ang II (209). Selective deletion of superoxide dismutase 3 (SOD3) in the SFO specifically increases Ang II-induced vascular T-cell and leukocyte infiltration in addition to increasing sympathetic modulation of heart rate, blood pressure, and vascular O2− (125). These studies provide compelling evidence for the role of ROS in the central regulation, not only of hypertension but also of vascular inflammation. However, we now need to clearly identify whether oxidative stress leads to increased sympathetic nervous system activation or whether it is the latter that controls induction of oxidative stress in hypertension, atherosclerosis, or other cardiovascular disorders.
NADPH Oxidase Inhibitors—Vascular Effects
Several classes of drugs currently used in clinical practice, such as medications affecting the renin-angiotensin-aldosterone system (133), statins (5, 162), and calcium channel blockers (203), have been shown to decrease NADPH oxidase activity and/or expression (76, 164). Moreover, some naturally occurring polyphenols may effectively inhibit NADPH oxidases in the vasculature or platelets, apart from simple scavenging of ROS (163). Understanding the importance of vascular NADPH oxidases and their potential value as therapeutic targets has sparked a search for specific and efficient Nox enzyme inhibitors. This is reviewed in depth elsewhere in this Forum. We have summarized selected vascular effects of NADPH oxidase inhibitors in Table 2. However, it should be noted that the specificity and selectivity of many of these compounds is under constant revision, and this aspect should be very carefully considered when designing new studies and trials.
Table 2.
Approaches to Inhibit Vascular NADPH Oxidases
| Inhibitor | Basic mechanisms of action | Examples of vascular effects |
|---|---|---|
| Apocynin | Inhibition of binding p47phox subunit to membrane complex (172). Unspecific scavenging of ROS reported in cells without MPO (86) | Dose-dependent decrease of P-selectin, VCAM-1, platelet adhesion, monocyte accumulation in LDL-R−/− (124) |
| Attenuation of ROS and hypertension in rats (13) | ||
| Prevention of NADPH oxidase expression and activation in fructose-fed animals (168) and streptozotocin diabetes (147) | ||
| DPI | Unspecific inhibition of flavin containing oxidases. | Considering its very broad spectrum of metabolic actions and toxicity, it is impossible to use it as a good vascular Nox inhibitor in either cellular or whole organism systems (145, 179) |
| Formation of covalent adducts Inhibition of NF-κB Inhibition of cholinesterase activity and internal Ca2+ pump | ||
| gp91ds-tat | Inhibition of the interaction of gp91phox and p47phox | Potent in inhibiting superoxide generation in the vascular system (160) |
| Tat peptide of the HIV virus, allows uptake into the cell | Blocking Ang II-induced O2− mouse aorta and attenuation of Ang II-induced hypertension (159) | |
| Lack of activity in potential oral administration limits its utility | Amelioration of endothelial dysfunction by reducing vascular superoxide and peroxynitrite in the Dahl salt-sensitive hypertension, but no significant effect on blood pressure in this model (207) | |
| VAS2870 | Triazolopyrimidine inhibiting ROS production, initially thought to be Nox specific. Recent reports suggest that it may not inhibit Nox-dependent ROS production (59) | Inhibition of ROS in aortas of aged SHRs (199). Inhibition of PDGF-stimulated NADPH oxidase activity in rat primary VSMCs (180), and oxidized LDL-mediated ROS formation in HUVECs (171) |
| Effects on NADPH oxidases have been recently questioned (59) | ||
| S17834 | Synthetic polyphenol, 6,8-diallyl 5,7-dihydroxy 2-(2-allyl 3-hydroxy 4methoxyphenyl)1-H benzo(b)pyran-4-one, Mechanism of inhibition—unclear | Regulator of adhesion molecule expression for treating chronic venous insufficiency (189) |
| Reduced TNF-α-stimulated VCAM, ICAM-1 and E-selectin expression, and reduced aortic atherosclerosis by 60% in ApoE−/− (27) | ||
| AEBSF | Interference with the interaction of p47phox and/or p67phox with cytochrome b559 probably by chemical modification of cyt (41) | Prevents activation of the O2- generating NADPH oxidase in both intact stimulated macrophages and in cell-free systems |
| Lack of specificity in studies of vascular NADPH oxidases (199) | ||
| Celastrol | Interference with interactions between the tandem SH3 domain of p47phox and NOXO1 and the proline-rich region of p22phox extracted from Tripterygium wilfordii, non specific | Inhibition of ROS production by Nox isoforms (Nox1 and 2 vs. Nox4 and 5) (95). In mouse microvascular endothelial cells, Celastrol has been reported to decrease ROS generation by Nox1 inhibition (200) |
| GKT137831 | Inhibition of Nox1- and Nox4-derived ROS production | Reduction of PPARγ expression and H202 generation as well as cell proliferation in human pulmonary artery endothelial and smooth muscle cells in pulmonary hypertension (70) |
| Prevention of hyperglycemia-induced endothelial cell oxidative stress | ||
| Prevention of atherosclerosis in diabetic ApoE−/− (69) | ||
| Dual Nox4-/Nox1-mediated effects should be carefully observed | ||
| ML171 | Phenothiazine Nox1 inhibitor that needs further specification | Potential use in Nox1 inhibition in colon cancer and vascular dysfunction(63) |
| 17DMAG | hsp90 inhibitor | Decreased expression of Nox1 and NoxO1 and reduction of NADPH-oxidase activity in VSMCs and monocytes(129) |
| DGLA | Cyclooxygenase substrate metabolized in macrophages to prostaglandin | Oral supplementation decreases aortic levels of p22phox and gp91phox mRNA in ApoE−/− mice (178) |
| Tyrphostin AG490 | Oral Jak2 inhibitor, | Diminishes activity and expression of aortic Nox1, Nox2, and Nox4 mRNA and protein in ApoE-deficient mice (51) |
| XJP-1, | Isolated from banana peel | Attenuation of p22phox and p47phox expression in HUVECs (54) |
| Modulation of the PI3K/Akt/eNOS pathway | ||
| EXP3179 | Inhibition of | Potential inhibition of NADPH oxidase in hypertension (53) |
| Losartan metabolite | Inhibition of protein kinase C-dependent p47phox subunit phosphorylation |
17DMAG, 17-dimethylaminoethylamino-17-demethoxygeldanamycin; AEBSF, 4-(2-aminoethyl)-benzenesulfonylfluoride; Ang II, angiotensin II; DGLA, dihomo-gamma-linolenic acid; DPI, diphenylene iodonium; eNOS, endothelial nitric oxide synthase; HUVECs, human umbilical vein endothelial cells; ICAM-1, intercellular adhesion molecule 1; LDL, low density lipoprotein; MPO, myeloperoxidase; NF-κB, nuclear factor kappa-light-chain-enhancer of activated B cells; NoXO1, Nox organizer protein 1; PDGF, platelet-derived growth factor; PPARγ, peroxisome proliferator-activated receptor gamma; ROS, reactive oxygen species; S17834, 6,8-diallyl 5,7-dihydroxy 2-(2-allyl 3-hydroxy 4-methoxyphenyl)1-H benzo(b)pyran-4-one; SH3, SRC Homology 3; SHR, spontaneously hypertensive rat; TNF-α, tumor necrosis factor-α; VCAM, vascular cell adhesion molecule; XJP-1, 7,8-Dihydroxy-3-methyl-isochromanone-4.
Conclusion
NADPH oxidase-derived ROS can act as vital signaling molecules or can cause toxicity depending on the time and cellular localization of Nox enzyme expression. This is particularly evident in the cardiovascular system. Therefore, a systematic and detailed characterization of NADPH oxidases in relation to the complex setting of cardiovascular diseases is warranted. Within recent years, we have further understood the complexity of this problem. For example, it is very difficult to draw a clear line between the protective and damaging effects of Nox4-derived ROS. In addition, discrepancies between studies looking at the consequences of Nox4 inhibition or Nox4 knockout should be further explained. We have also understood that expression and activity of NADPH oxidases should be very closely regulated, as either too much or too little of NADPH oxidase-derived ROS is detrimental. Thus, we need to learn how to tightly control/carefully affect their activity. Particularly, we need development of Nox oxidase-specific inhibitors, which would enable us to differentiate between various Nox homologues. Fortunately, much progress has been made in this field, and we are getting closer to this important goal.
Abbreviations Used
- 17DMAG
17-dimethylaminoethylamino-17-demethoxygeldanamycin
- AAA
abdominal aortic aneurysm
- AEBSF
4-(2-aminoethyl)-benzenesulfonylfluoride
- Ang II
angiotensin II
- ApoE
apolipoprotein E
- AT1
angiotensin receptor type 1
- BH4
tetrahydrobiopterin
- Ca2+
calcium
- CCL2
chemokine (C-C motif) ligand 2
- CGD
chronic granulomatous disease
- CVOs
circumventricular organs
- DGLA
dihomo-gamma-linolenic acid
- DPI
diphenylene iodonium
- Duox1
dual oxidase1
- EGF
epidermal growth factor
- eNOS
endothelial nitric oxide synthase
- ER
endoplasmic reticulum
- ET-1
endothelin 1
- H2O2
hydrogen peroxide
- HIF-1α
hypoxia-inducible factor-1α
- HO-1
heme oxygenase-1
- HUVECs
human umbilical vein endothelial cells
- ICAM-1
intercellular adhesion molecule 1
- IL-6
interleukin 6
- KOX
kidney oxidase
- LDL
low density lipoprotein
- MAP kinase
mitogen activated protein kinase
- MCP-1
monocyte chemoattractant protein-1
- MHC
major histocompatibility complex
- MMP-2
matrix metalloproteinase 2
- MMP-9
matrix metalloproteinase 9
- MPO
myeloperoxidase
- NAC
N-acetylcysteine
- NADPH
nicotinamide adenine dinucleotide phosphate
- NF-κB
nuclear factor kappa-light-chain-enhancer of activated B cells
- NO
nitric oxide
- NoXA1
Nox activator protein 1
- NoXO1
Nox organizer protein 1
- O2−
superoxide anion
- PASMCs
pulmonary artery smooth muscle cells
- PDGF
platelet-derived growth factor
- PKC
protein kinase C
- PLA2
phospholipase A2
- PPARγ
peroxisome proliferator-activated receptor gamma
- RANTES
regulated on activation, normal T cell expressed and secreted
- ROS
reactive oxygen species
- RVLM
rostral ventrolateral medulla
- S17834
6,8-diallyl 5,7-dihydroxy 2-(2-allyl 3-hydroxy 4-methoxyphenyl)1-H benzo(b)pyran-4-one
- SFO
subfornical organ
- SHR
spontaneously hypertensive rat
- SMC
smooth muscle cell
- SOD3
superoxide dismutase 3
- STZ
streptozotocin
- TGF-α
transforming growth factor α
- TGF-β
transforming growth factor β
- TNF-α
tumor necrosis factor-α
- VCAM-1
vascular cell adhesion molecule 1
- VEGF
vascular endothelial growth factor
- VSMC
vascular smooth muscle cell
- XJP-1
7,8-Dihydroxy-3-methyl-isochromanone-4
Acknowledgments
This work has been supported by the National Science Foundation of Poland Grant No 2997/B/P01/2009/36 and the Foundation for Polish Science Grant for T.J.G. and A.S. (Welcome02/09). T.J.G. is supported by The Wellcome Trust Senior Research Fellowship.
References
- 1.Adachi Y, Shibai Y, Mitsushita J, Shang WH, Hirose K, and Kamata T. Oncogenic Ras upregulates NADPH oxidase 1 gene expression through MEK-ERK-dependent phosphorylation of GATA-6. Oncogene 27: 4921–4932, 2008 [DOI] [PubMed] [Google Scholar]
- 2.Ambasta RK, Kumar P, Griendling KK, Schmidt HH, Busse R, and Brandes RP. Direct interaction of the novel Nox proteins with p22phox is required for the formation of a functionally active NADPH oxidase. J Biol Chem 279: 45935–45941, 2004 [DOI] [PubMed] [Google Scholar]
- 3.Anilkumar N, Jose GS, Sawyer I, Santos CX, Sand C, Brewer AC, Warren D, and Shah AM. A 28-kDa splice variant of NADPH oxidase-4 is nuclear-localized and involved in redox signaling in vascular cells. Arterioscler Thromb Vasc Biol 33: e104–e112, 2013 [DOI] [PubMed] [Google Scholar]
- 4.Anilkumar N, Weber R, Zhang M, Brewer A, and Shah AM. Nox4 and nox2 NADPH oxidases mediate distinct cellular redox signaling responses to agonist stimulation. Arterioscler Thromb Vasc Biol 28: 1347–1354, 2008 [DOI] [PubMed] [Google Scholar]
- 5.Antoniades C, Bakogiannis C, Tousoulis D, Reilly S, Zhang MH, Paschalis A, Antonopoulos AS, Demosthenous M, Miliou A, Psarros C, Marinou K, Sfyras N, Economopoulos G, Casadei B, Channon KM, and Stefanadis C. Preoperative atorvastatin treatment in CABG patients rapidly improves vein graft redox state by inhibition of Rac1 and NADPH-oxidase activity. Circulation 122: S66–S73, 2010 [DOI] [PubMed] [Google Scholar]
- 6.Arbiser JL, Petros J, Klafter R, Govindajaran B, McLaughlin ER, Brown LF, Cohen C, Moses M, Kilroy S, Arnold RS, and Lambeth JD. Reactive oxygen generated by Nox1 triggers the angiogenic switch. Proc Natl Acad Sci U S A 99: 715–720, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Banfi B, Maturana A, Jaconi S, Arnaudeau S, Laforge T, Sinha B, Ligeti E, Demaurex N, and Krause KH. A mammalian H+ channel generated through alternative splicing of the NADPH oxidase homolog NOH-1. Science 287: 138–142, 2000 [DOI] [PubMed] [Google Scholar]
- 8.Banfi B, Molnar G, Maturana A, Steger K, Hegedus B, Demaurex N, and Krause KH. A Ca(2+)-activated NADPH oxidase in testis, spleen, and lymph nodes. J Biol Chem 276: 37594–37601, 2001 [DOI] [PubMed] [Google Scholar]
- 9.Banfi B, Tirone F, Durussel I, Knisz J, Moskwa P, Molnar GZ, Krause KH, and Cox JA. Mechanism of Ca2+ activation of the NADPH oxidase 5 (NOX5). J Biol Chem 279: 18583–18591, 2004 [DOI] [PubMed] [Google Scholar]
- 10.Barry-Lane PA, Patterson C, van der Merwe M, Hu Z, Holland SM, Yeh ET, and Runge MS. p47phox is required for atherosclerotic lesion progression in ApoE(−/−) mice. J Clin Invest 108: 1513–1522, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Basuroy S, Bhattacharya S, Leffler CW, and Parfenova H. Nox4 NADPH oxidase mediates oxidative stress and apoptosis caused by TNF-alpha in cerebral vascular endothelial cells. Am J Physiol Cell Physiol 296: C422–C432, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bauldry SA, Elsey KL, and Bass DA. Activation of NADPH oxidase and phospholipase D in permeabilized human neutrophils. Correlation between oxidase activation and phosphatidic acid production. J Biol Chem 267: 25141–25152, 1992 [PubMed] [Google Scholar]
- 13.Baumer AT, Kruger CA, Falkenberg J, Freyhaus HT, Rosen R, Fink K, and Rosenkranz S. The NAD(P)H oxidase inhibitor apocynin improves endothelial NO/superoxide balance and lowers effectively blood pressure in spontaneously hypertensive rats: comparison to calcium channel blockade. Clin Exp Hypertens 29: 287–299, 2007 [DOI] [PubMed] [Google Scholar]
- 14.Bayraktutan U, Blayney L, and Shah AM. Molecular characterization and localization of the NAD(P)H oxidase components gp91-phox and p22-phox in endothelial cells. Arterioscler Thromb Vasc Biol 20: 1903–1911, 2000 [DOI] [PubMed] [Google Scholar]
- 15.Bedard K. and Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev 87: 245–313, 2007 [DOI] [PubMed] [Google Scholar]
- 16.BelAiba RS, Djordjevic T, Petry A, Diemer K, Bonello S, Banfi B, Hess J, Pogrebniak A, Bickel C, and Gorlach A. NOX5 variants are functionally active in endothelial cells. Free Radic Biol Med 42: 446–459, 2007 [DOI] [PubMed] [Google Scholar]
- 17.Bendall JK, Cave AC, Heymes C, Gall N, and Shah AM. Pivotal role of a gp91(phox)-containing NADPH oxidase in angiotensin II-induced cardiac hypertrophy in mice. Circulation 105: 293–296, 2002 [DOI] [PubMed] [Google Scholar]
- 18.Berry C, Hamilton CA, Brosnan MJ, Magill FG, Berg GA, McMurray JJ, and Dominiczak AF. Investigation into the sources of superoxide in human blood vessels: angiotensin II increases superoxide production in human internal mammary arteries. Circulation 101: 2206–2212, 2000 [DOI] [PubMed] [Google Scholar]
- 19.Bokoch GM. and Knaus UG. NADPH oxidases: not just for leukocytes anymore! Trends Biochem Sci 28: 502–508, 2003 [DOI] [PubMed] [Google Scholar]
- 20.Borregaard N, Heiple JM, Simons ER, and Clark RA. Subcellular localization of the b-cytochrome component of the human neutrophil microbicidal oxidase: translocation during activation. J Cell Biol 97: 52–61, 1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Braga VA, Colombari E, and Jovita MG. Angiotensin II-derived reactive oxygen species underpinning the processing of the cardiovascular reflexes in the medulla oblongata. Neurosci Bull 27: 269–274, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brandes RP, Takac I, and Schroder K. No superoxide—no stress?: Nox4, the good NADPH oxidase! Arterioscler Thromb Vasc Biol 31: 1255–1257, 2011 [DOI] [PubMed] [Google Scholar]
- 23.Brandes RP, Viedt C, Nguyen K, Beer S, Kreuzer J, Busse R, and Gorlach A. Thrombin-induced MCP-1 expression involves activation of the p22phox-containing NADPH oxidase in human vascular smooth muscle cells. Thromb Haemost 85: 1104–1110, 2001 [PubMed] [Google Scholar]
- 23a.Braunersreuther V, Montecucco F, Ashri M, Pelli G, Galan K, Frias M, Burger F, Quinderé AL, Montessuit C, Krause KH, Mach F, and Jaquet V. Role of NADPH oxidase isoforms NOX1, NOX2 and NOX4 in myocardial ischemia/reperfusion injury. J Mol Cell Cardiol. 64: 99–107, 2013 [DOI] [PubMed] [Google Scholar]
- 24.Bush E, Maeda N, Kuziel WA, Dawson TC, Wilcox JN, DeLeon H, and Taylor WR. CC chemokine receptor 2 is required for macrophage infiltration and vascular hypertrophy in angiotensin II-induced hypertension. Hypertension 36: 360–363, 2000 [DOI] [PubMed] [Google Scholar]
- 25.Byrne JA, Grieve DJ, Bendall JK, Li JM, Gove C, Lambeth JD, Cave AC, and Shah AM. Contrasting roles of NADPH oxidase isoforms in pressure-overload versus angiotensin II-induced cardiac hypertrophy. Circ Res 93: 802–805, 2003 [DOI] [PubMed] [Google Scholar]
- 26.Cai T, Fassina G, Morini M, Aluigi MG, Masiello L, Fontanini G, D'Agostini F, De Flora S, Noonan DM, and Albini A. N-acetylcysteine inhibits endothelial cell invasion and angiogenesis. Lab Invest 79: 1151–1159, 1999 [PubMed] [Google Scholar]
- 27.Cayatte AJ, Rupin A, Oliver-Krasinski J, Maitland K, Sansilvestri-Morel P, Boussard MF, Wierzbicki M, Verbeuren TJ, and Cohen RA. S17834, a new inhibitor of cell adhesion and atherosclerosis that targets nadph oxidase. Arterioscler Thromb Vasc Biol 21: 1577–1584, 2001 [DOI] [PubMed] [Google Scholar]
- 28.Chamulitrat W, Schmidt R, Tomakidi P, Stremmel W, Chunglok W, Kawahara T, and Rokutan K. Association of gp91phox homolog Nox1 with anchorage-independent growth and MAP kinase-activation of transformed human keratinocytes. Oncogene 22: 6045–6053, 2003 [DOI] [PubMed] [Google Scholar]
- 29.Chan CT, Moore JP, Budzyn K, Guida E, Diep H, Vinh A, Jones ES, Widdop RE, Armitage JA, Sakkal S, Ricardo SD, Sobey CG, and Drummond GR. Reversal of vascular macrophage accumulation and hypertension by a CCR2 antagonist in deoxycorticosterone/salt-treated mice. Hypertension 60: 1207–1212, 2012 [DOI] [PubMed] [Google Scholar]
- 30.Chan SH, Hsu KS, Huang CC, Wang LL, Ou CC, and Chan JY. NADPH oxidase-derived superoxide anion mediates angiotensin II-induced pressor effect via activation of p38 mitogen-activated protein kinase in the rostral ventrolateral medulla. Circ Res 97: 772–780, 2005 [DOI] [PubMed] [Google Scholar]
- 31.Chen XL, Zhang Q, Zhao R, and Medford RM. Superoxide, H2O2, and iron are required for TNF-alpha-induced MCP-1 gene expression in endothelial cells: role of Rac1 and NADPH oxidase. Am J Physiol Heart Circ Physiol 286: H1001–H1007, 2004 [DOI] [PubMed] [Google Scholar]
- 32.Cheng G, Cao Z, Xu X, van Meir EG, and Lambeth JD. Homologs of gp91phox: cloning and tissue expression of Nox3, Nox4, and Nox5. Gene 269: 131–140, 2001 [DOI] [PubMed] [Google Scholar]
- 33.Cheng G. and Lambeth JD. NOXO1, regulation of lipid binding, localization, and activation of Nox1 by the Phox homology (PX) domain. J Biol Chem 279: 4737–4742, 2004 [DOI] [PubMed] [Google Scholar]
- 34.Chrissobolis S, Banfi B, Sobey CG, and Faraci FM. Role of Nox isoforms in angiotensin II-induced oxidative stress and endothelial dysfunction in brain. J Appl Physiol 113: 184–191, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cifuentes ME. and Pagano PJ. Targeting reactive oxygen species in hypertension. Curr Opin Nephrol Hypertens 15: 179–186, 2006 [DOI] [PubMed] [Google Scholar]
- 36.Clempus RE, Sorescu D, Dikalova AE, Pounkova L, Jo P, Sorescu GP, Schmidt HH, Lassegue B, and Griendling KK. Nox4 is required for maintenance of the differentiated vascular smooth muscle cell phenotype. Arterioscler Thromb Vasc Biol 27: 42–48, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cucoranu I, Clempus R, Dikalova A, Phelan PJ, Ariyan S, Dikalov S, and Sorescu D. NAD(P)H oxidase 4 mediates transforming growth factor-beta1-induced differentiation of cardiac fibroblasts into myofibroblasts. Circ Res 97: 900–907, 2005 [DOI] [PubMed] [Google Scholar]
- 38.De Keulenaer GW, Alexander RW, Ushio-Fukai M, Ishizaka N, and Griendling KK. Tumour necrosis factor alpha activates a p22phox-based NADH oxidase in vascular smooth muscle. Biochem J 329 (Pt 3): 653–657, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dekker LV, Leitges M, Altschuler G, Mistry N, McDermott A, Roes J, and Segal AW. Protein kinase C-beta contributes to NADPH oxidase activation in neutrophils. Biochem J 347Pt 1: 285–289, 2000 [PMC free article] [PubMed] [Google Scholar]
- 40.Desouki MM, Kulawiec M, Bansal S, Das GM, and Singh KK. Cross talk between mitochondria and superoxide generating NADPH oxidase in breast and ovarian tumors. Cancer Biol Ther 4: 1367–1373, 2005 [DOI] [PubMed] [Google Scholar]
- 41.Diatchuk V, Lotan O, Koshkin V, Wikstroem P, and Pick E. Inhibition of NADPH oxidase activation by 4-(2-aminoethyl)-benzenesulfonyl fluoride and related compounds. J Biol Chem 272: 13292–13301, 1997 [DOI] [PubMed] [Google Scholar]
- 42.Diebold I, Petry A, Hess J, and Gorlach A. The NADPH oxidase subunit NOX4 is a new target gene of the hypoxia-inducible factor-1. Mol Biol Cell 21: 2087–2096, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dikalov SI, Dikalova AE, Bikineyeva AT, Schmidt HH, Harrison DG, and Griendling KK. Distinct roles of Nox1 and Nox4 in basal and angiotensin II-stimulated superoxide and hydrogen peroxide production. Free Radic Biol Med 45: 1340–1351, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dikalova A, Clempus R, Lassegue B, Cheng G, McCoy J, Dikalov S, San Martin A, Lyle A, Weber DS, Weiss D, Taylor WR, Schmidt HH, Owens GK, Lambeth JD, and Griendling KK. Nox1 overexpression potentiates angiotensin II-induced hypertension and vascular smooth muscle hypertrophy in transgenic mice. Circulation 112: 2668–2676, 2005 [DOI] [PubMed] [Google Scholar]
- 45.Dikalova AE, Gongora MC, Harrison DG, Lambeth JD, Dikalov S, and Griendling KK. Upregulation of Nox1 in vascular smooth muscle leads to impaired endothelium-dependent relaxation via eNOS uncoupling. Am J Physiol Heart Circ Physiol 299: H673–H679, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ding H, Aljofan M, and Triggle CR. Oxidative stress and increased eNOS and NADPH oxidase expression in mouse microvessel endothelial cells. J Cell Physiol 212: 682–689, 2007 [DOI] [PubMed] [Google Scholar]
- 47.Ding H, Hashem M, and Triggle C. Increased oxidative stress in the streptozotocin-induced diabetic apoE-deficient mouse: changes in expression of NADPH oxidase subunits and eNOS. Eur J Pharmacol 561: 121–128, 2007 [DOI] [PubMed] [Google Scholar]
- 48.Ding L, Chapman A, Boyd R, and Wang HD. ERK activation contributes to regulation of spontaneous contractile tone via superoxide anion in isolated rat aorta of angiotensin II-induced hypertension. Am J Physiol Heart Circ Physiol 292: H2997–H3005, 2007 [DOI] [PubMed] [Google Scholar]
- 49.Djordjevic T, BelAiba RS, Bonello S, Pfeilschifter J, Hess J, and Gorlach A. Human urotensin II is a novel activator of NADPH oxidase in human pulmonary artery smooth muscle cells. Arterioscler Thromb Vasc Biol 25: 519–525, 2005 [DOI] [PubMed] [Google Scholar]
- 50.Douglas G, Bendall JK, Crabtree MJ, Tatham AL, Carter EE, Hale AB, and Channon KM. Endothelial-specific Nox2 overexpression increases vascular superoxide and macrophage recruitment in ApoE(−)/(−) mice. Cardiovasc Res 94: 20–29, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fenyo IM, Florea IC, Raicu M, and Manea A. Tyrphostin AG490 reduces NAPDH oxidase activity and expression in the aorta of hypercholesterolemic apolipoprotein E-deficient mice. Vascul Pharmacol 54: 100–106, 2011 [DOI] [PubMed] [Google Scholar]
- 52.Fike CD, Slaughter JC, Kaplowitz MR, Zhang Y, and Aschner JL. Reactive oxygen species from NADPH oxidase contribute to altered pulmonary vascular responses in piglets with chronic hypoxia-induced pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 295: L881–L888, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fortuno A, Bidegain J, Robador PA, Hermida J, Lopez-Sagaseta J, Beloqui O, Diez J, and Zalba G. Losartan metabolite EXP3179 blocks NADPH oxidase-mediated superoxide production by inhibiting protein kinase C: potential clinical implications in hypertension. Hypertension 54: 744–750, 2009 [DOI] [PubMed] [Google Scholar]
- 54.Fu R, Wang Q, Guo Q, Xu J, and Wu X. XJP-1 protects endothelial cells from oxidized low-density lipoprotein-induced apoptosis by inhibiting NADPH oxidase subunit expression and modulating the PI3K/Akt/eNOS pathway. Vascul Pharmacol 58: 78–86, 2013 [DOI] [PubMed] [Google Scholar]
- 55.Fukui T, Ishizaka N, Rajagopalan S, Laursen JB, Capers Qt, Taylor WR, Harrison DG, de Leon H, Wilcox JN, and Griendling KK. p22phox mRNA expression and NADPH oxidase activity are increased in aortas from hypertensive rats. Circ Res 80: 45–51, 1997 [DOI] [PubMed] [Google Scholar]
- 56.Galkina E, Kadl A, Sanders J, Varughese D, Sarembock IJ, and Ley K. Lymphocyte recruitment into the aortic wall before and during development of atherosclerosis is partially L-selectin dependent. J Exp Med 203: 1273–1282, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gao L, Wang W, Li YL, Schultz HD, Liu D, Cornish KG, and Zucker IH. Superoxide mediates sympathoexcitation in heart failure: roles of angiotensin II and NAD(P)H oxidase. Circ Res 95: 937–944, 2004 [DOI] [PubMed] [Google Scholar]
- 58.Garrido-Urbani S, Jemelin S, Deffert C, Carnesecchi S, Basset O, Szyndralewiez C, Heitz F, Page P, Montet X, Michalik L, Arbiser J, Ruegg C, Krause KH, and Imhof BA. Targeting vascular NADPH oxidase 1 blocks tumor angiogenesis through a PPARalpha mediated mechanism. PLoS One 6: e14665, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gatto GJ, Jr, Ao Z, Kearse MG, Zhou M, Morales CR, Daniels E, Bradley BT, Goserud MT, Goodman KB, Douglas SA, Harpel MR, and Johns DG. NADPH oxidase-dependent and -independent mechanisms of reported inhibitors of reactive oxygen generation. J Enzyme Inhib Med Chem 28: 95–104, 2013 [DOI] [PubMed] [Google Scholar]
- 60.Gavazzi G, Banfi B, Deffert C, Fiette L, Schappi M, Herrmann F, and Krause KH. Decreased blood pressure in NOX1-deficient mice. FEBS Lett 580: 497–504, 2006 [DOI] [PubMed] [Google Scholar]
- 61.Gavazzi G, Deffert C, Trocme C, Schappi M, Herrmann FR, and Krause KH. NOX1 deficiency protects from aortic dissection in response to angiotensin II. Hypertension 50: 189–196, 2007 [DOI] [PubMed] [Google Scholar]
- 62.Geiszt M, Kopp JB, Varnai P, and Leto TL. Identification of Renox, an NAD(P)H oxidase in kidney. Proc Natl Acad Sci U S A 97: 8010–8014, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gianni D, Nicolas N, Zhang H, Der Mardirossian C, Kister J, Martinez L, Ferguson J, Roush WR, Brown SJ, Bokoch GM, Hodder P, and Rosen H. Optimization and characterization of an inhibitor for NADPH oxidase 1 (NOX-1). In: Probe Reports from the NIH Molecular Libraries Program. Bethesda, MD, 2010 [PubMed] [Google Scholar]
- 64.Goettsch C, Goettsch W, Arsov A, Hofbauer LC, Bornstein SR, and Morawietz H. Long-term cyclic strain downregulates endothelial Nox4. Antioxid Redox Signal 11: 2385–2397, 2009 [DOI] [PubMed] [Google Scholar]
- 65.Goettsch C, Goettsch W, Muller G, Seebach J, Schnittler HJ, and Morawietz H. Nox4 overexpression activates reactive oxygen species and p38 MAPK in human endothelial cells. Biochem Biophys Res Commun 380: 355–360, 2009 [DOI] [PubMed] [Google Scholar]
- 66.Gongora MC, Lob HE, Landmesser U, Guzik TJ, Martin WD, Ozumi K, Wall SM, Wilson DS, Murthy N, Gravanis M, Fukai T, and Harrison DG. Loss of extracellular superoxide dismutase leads to acute lung damage in the presence of ambient air: a potential mechanism underlying adult respiratory distress syndrome. Am J Pathol 173: 915–926, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gorin Y, Ricono JM, Kim NH, Bhandari B, Choudhury GG, and Abboud HE. Nox4 mediates angiotensin II-induced activation of Akt/protein kinase B in mesangial cells. Am J Physiol Renal Physiol 285: F219–F229, 2003 [DOI] [PubMed] [Google Scholar]
- 68.Govindarajan B, Sligh JE, Vincent BJ, Li M, Canter JA, Nickoloff BJ, Rodenburg RJ, Smeitink JA, Oberley L, Zhang Y, Slingerland J, Arnold RS, Lambeth JD, Cohen C, Hilenski L, Griendling K, Martinez-Diez M, Cuezva JM, and Arbiser JL. Overexpression of Akt converts radial growth melanoma to vertical growth melanoma. J Clin Invest 117: 719–729, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gray SP, Di Marco E, Okabe J, Szyndralewiez C, Heitz F, Montezano AC, de Haan JB, Koulis C, El-Osta A, Andrews KL, Chin-Dusting JP, Touyz RM, Wingler K, Cooper ME, Schmidt HH, and Jandeleit-Dahm KA. NADPH oxidase 1 plays a key role in diabetes mellitus-accelerated atherosclerosis. Circulation 127: 1888–1902, 2013 [DOI] [PubMed] [Google Scholar]
- 70.Green DE, Murphy TC, Kang BY, Kleinhenz JM, Szyndralewiez C, Page P, Sutliff RL, and Hart CM. The Nox4 inhibitor GKT137831 attenuates hypoxia-induced pulmonary vascular cell proliferation. Am J Respir Cell Mol Biol 47: 718–726, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Griendling KK, Minieri CA, Ollerenshaw JD, and Alexander RW. Angiotensin II stimulates NADH and NADPH oxidase activity in cultured vascular smooth muscle cells. Circ Res 74: 1141–1148, 1994 [DOI] [PubMed] [Google Scholar]
- 72.Gupte SA. and Wolin MS. Oxidant and redox signaling in vascular oxygen sensing: implications for systemic and pulmonary hypertension. Antioxid Redox Signal 10: 1137–1152, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Guzik B, Chwala M, Matusik P, Ludew D, Skiba D, Wilk G, Mrowiecki W, Batko B, Cencora A, Kapelak B, Sadowski J, Korbut R, and Guzik TJ. Mechanisms of increased vascular superoxide production in human varicose veins. Pol Arch Med Wewn 121: 279–286, 2011 [PMC free article] [PubMed] [Google Scholar]
- 74.Guzik B, Sagan A, Ludew D, Mrowiecki W, Chwala M, Bujak-Gizycka B, Filip G, Grudzien G, Kapelak B, Zmudka K, Mrowiecki T, Sadowski J, Korbut R, and Guzik TJ. Mechanisms of oxidative stress in human aortic aneurysms—association with clinical risk factors for atherosclerosis and disease severity. Int J Cardiol 2013. [Epub ahead of print]; DOI: 10.1016/j.ijcard.2013.01.278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Guzik TJ, Chen W, Gongora MC, Guzik B, Lob HE, Mangalat D, Hoch N, Dikalov S, Rudzinski P, Kapelak B, Sadowski J, and Harrison DG. Calcium-dependent NOX5 nicotinamide adenine dinucleotide phosphate oxidase contributes to vascular oxidative stress in human coronary artery disease. J Am Coll Cardiol 52: 1803–1809, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Guzik TJ. and Harrison DG. Vascular NADPH oxidases as drug targets for novel antioxidant strategies. Drug Discov Today 11: 524–533, 2006 [DOI] [PubMed] [Google Scholar]
- 77.Guzik TJ, Hoch NE, Brown KA, McCann LA, Rahman A, Dikalov S, Goronzy J, Weyand C, and Harrison DG. Role of the T cell in the genesis of angiotensin II induced hypertension and vascular dysfunction. J Exp Med 204: 2449–2460, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Guzik TJ, Mussa S, Gastaldi D, Sadowski J, Ratnatunga C, Pillai R, and Channon KM. Mechanisms of increased vascular superoxide production in human diabetes mellitus: role of NAD(P)H oxidase and endothelial nitric oxide synthase. Circulation 105: 1656–1662, 2002 [DOI] [PubMed] [Google Scholar]
- 79.Guzik TJ, Sadowski J, Guzik B, Jopek A, Kapelak B, Przybylowski P, Wierzbicki K, Korbut R, Harrison DG, and Channon KM. Coronary artery superoxide production and nox isoform expression in human coronary artery disease. Arterioscler Thromb Vasc Biol 26: 333–339, 2006 [DOI] [PubMed] [Google Scholar]
- 80.Guzik TJ, Sadowski J, Kapelak B, Jopek A, Rudzinski P, Pillai R, Korbut R, and Channon KM. Systemic regulation of vascular NAD(P)H oxidase activity and nox isoform expression in human arteries and veins. Arterioscler Thromb Vasc Biol 24: 1614–1620, 2004 [DOI] [PubMed] [Google Scholar]
- 81.Guzik TJ, West NE, Black E, McDonald D, Ratnatunga C, Pillai R, and Channon KM. Functional effect of the C242T polymorphism in the NAD(P)H oxidase p22phox gene on vascular superoxide production in atherosclerosis. Circulation 102: 1744–1747, 2000 [DOI] [PubMed] [Google Scholar]
- 82.Guzik TJ, West NE, Black E, McDonald D, Ratnatunga C, Pillai R, and Channon KM. Vascular superoxide production by NAD(P)H oxidase: association with endothelial dysfunction and clinical risk factors. Circ Res 86: E85–E90, 2000 [DOI] [PubMed] [Google Scholar]
- 83.Harrison DG, Gongora MC, Guzik TJ, and Widder J. Oxidative stress and hypertension. J Am Soc Hypertens 1: 30–44, 2007 [DOI] [PubMed] [Google Scholar]
- 84.Harrison DG, Guzik TJ, Lob HE, Madhur MS, Marvar PJ, Thabet SR, Vinh A, and Weyand CM. Inflammation, immunity, and hypertension. Hypertension 57: 132–140, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Helmcke I, Heumuller S, Tikkanen R, Schroder K, and Brandes RP. Identification of structural elements in Nox1 and Nox4 controlling localization and activity. Antioxid Redox Signal 11: 1279–1287, 2009 [DOI] [PubMed] [Google Scholar]
- 86.Heumuller S, Wind S, Barbosa-Sicard E, Schmidt HH, Busse R, Schroder K, and Brandes RP. Apocynin is not an inhibitor of vascular NADPH oxidases but an antioxidant. Hypertension 51: 211–217, 2008 [DOI] [PubMed] [Google Scholar]
- 87.Hilenski LL, Clempus RE, Quinn MT, Lambeth JD, and Griendling KK. Distinct subcellular localizations of Nox1 and Nox4 in vascular smooth muscle cells. Arterioscler Thromb Vasc Biol 24: 677–683, 2004 [DOI] [PubMed] [Google Scholar]
- 88.Hink U, Li H, Mollnau H, Oelze M, Matheis E, Hartmann M, Skatchkov M, Thaiss F, Stahl RA, Warnholtz A, Meinertz T, Griendling K, Harrison DG, Forstermann U, and Munzel T. Mechanisms underlying endothelial dysfunction in diabetes mellitus. Circ Res 88: E14–E22, 2001 [DOI] [PubMed] [Google Scholar]
- 89.Hopkins ND, Stratton G, Tinken TM, Ridgers ND, Graves LE, McWhannell N, Cable NT, and Green DJ. Seasonal reduction in physical activity and flow-mediated dilation in children. Med Sci Sports Exerc 43: 232–238, 2011 [DOI] [PubMed] [Google Scholar]
- 90.Hsich E, Segal BH, Pagano PJ, Rey FE, Paigen B, Deleonardis J, Hoyt RF, Holland SM, and Finkel T. Vascular effects following homozygous disruption of p47(phox): an essential component of NADPH oxidase. Circulation 101: 1234–1236, 2000 [DOI] [PubMed] [Google Scholar]
- 91.Hwang J, Ing MH, Salazar A, Lassegue B, Griendling K, Navab M, Sevanian A, and Hsiai TK. Pulsatile versus oscillatory shear stress regulates NADPH oxidase subunit expression: implication for native LDL oxidation. Circ Res 93: 1225–1232, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Infanger DW, Sharma RV, and Davisson RL. NADPH oxidases of the brain: distribution, regulation, and function. Antioxid Redox Signal 8: 1583–1596, 2006 [DOI] [PubMed] [Google Scholar]
- 93.Ismail S, Sturrock A, Wu P, Cahill B, Norman K, Huecksteadt T, Sanders K, Kennedy T, and Hoidal J. NOX4 mediates hypoxia-induced proliferation of human pulmonary artery smooth muscle cells: the role of autocrine production of transforming growth factor-{beta}1 and insulin-like growth factor binding protein-3. Am J Physiol Lung Cell Mol Physiol 296: L489–L499, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Jansen F, Yang X, Franklin BS, Hoelscher M, Schmitz T, Bedorf J, Nickenig G, and Werner N. High glucose condition increases NADPH oxidase activity in endothelial microparticles that promote vascular inflammation. Cardiovasc Res 98: 94–106, 2013 [DOI] [PubMed] [Google Scholar]
- 95.Jaquet V, Marcoux J, Forest E, Leidal KG, McCormick S, Westermaier Y, Perozzo R, Plastre O, Fioraso-Cartier L, Diebold B, Scapozza L, Nauseef WM, Fieschi F, Krause KH, and Bedard K. NADPH oxidase (NOX) isoforms are inhibited by celastrol with a dual mode of action. Br J Pharmacol 164: 507–520, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Jay DB, Papaharalambus CA, Seidel-Rogol B, Dikalova AE, Lassegue B, and Griendling KK. Nox5 mediates PDGF-induced proliferation in human aortic smooth muscle cells. Free Radic Biol Med 45: 329–335, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Judkins CP, Diep H, Broughton BR, Mast AE, Hooker EU, Miller AA, Selemidis S, Dusting GJ, Sobey CG, and Drummond GR. Direct evidence of a role for Nox2 in superoxide production, reduced nitric oxide bioavailability, and early atherosclerotic plaque formation in ApoE−/− mice. Am J Physiol Heart Circ Physiol 298: H24–H32, 2010 [DOI] [PubMed] [Google Scholar]
- 98.Jung O, Schreiber JG, Geiger H, Pedrazzini T, Busse R, and Brandes RP. gp91phox-containing NADPH oxidase mediates endothelial dysfunction in renovascular hypertension. Circulation 109: 1795–1801, 2004 [DOI] [PubMed] [Google Scholar]
- 99.Kadl A, Galkina E, and Leitinger N. Induction of CCR2-dependent macrophage accumulation by oxidized phospholipids in the air-pouch model of inflammation. Arthritis Rheum 60: 1362–1371, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kawano M, Miyamoto K, Kaito Y, Sumimoto H, and Tamura M. Noxa1 as a moderate activator of Nox2-based NADPH oxidase. Arch Biochem Biophys 519: 1–7, 2012 [DOI] [PubMed] [Google Scholar]
- 101.Khatri JJ, Johnson C, Magid R, Lessner SM, Laude KM, Dikalov SI, Harrison DG, Sung HJ, Rong Y, and Galis ZS. Vascular oxidant stress enhances progression and angiogenesis of experimental atheroma. Circulation 109: 520–525, 2004 [DOI] [PubMed] [Google Scholar]
- 102.Kirk EA, Dinauer MC, Rosen H, Chait A, Heinecke JW, and LeBoeuf RC. Impaired superoxide production due to a deficiency in phagocyte NADPH oxidase fails to inhibit atherosclerosis in mice. Arterioscler Thromb Vasc Biol 20: 1529–1535, 2000 [DOI] [PubMed] [Google Scholar]
- 103.Kleinschnitz C, Grund H, Wingler K, Armitage ME, Jones E, Mittal M, Barit D, Schwarz T, Geis C, Kraft P, Barthel K, Schuhmann MK, Herrmann AM, Meuth SG, Stoll G, Meurer S, Schrewe A, Becker L, Gailus-Durner V, Fuchs H, Klopstock T, de Angelis MH, Jandeleit-Dahm K, Shah AM, Weissmann N, and Schmidt HH. Post-stroke inhibition of induced NADPH oxidase type 4 prevents oxidative stress and neurodegeneration. PLoS Biol 8: pii:, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Koltsova EK, Garcia Z, Chodaczek G, Landau M, McArdle S, Scott SR, von Vietinghoff S, Galkina E, Miller YI, Acton ST, and Ley K. Dynamic T cell-APC interactions sustain chronic inflammation in atherosclerosis. J Clin Invest 122: 3114–3126, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Konior A, Klemenska E, Brudek M, Podolecka E, Czarnowska E, and Beresewicz A. Seasonal superoxide overproduction and endothelial activation in guinea-pig heart; seasonal oxidative stress in rats and humans. J Mol Cell Cardiol 50: 686–694, 2011 [DOI] [PubMed] [Google Scholar]
- 106.Kovacic HN, Irani K, and Goldschmidt-Clermont PJ. Redox regulation of human Rac1 stability by the proteasome in human aortic endothelial cells. J Biol Chem 276: 45856–45861, 2001 [DOI] [PubMed] [Google Scholar]
- 107.Kuroda J, Nakagawa K, Yamasaki T, Nakamura K, Takeya R, Kuribayashi F, Imajoh-Ohmi S, Igarashi K, Shibata Y, Sueishi K, and Sumimoto H. The superoxide-producing NAD(P)H oxidase Nox4 in the nucleus of human vascular endothelial cells. Genes Cells 10: 1139–1151, 2005 [DOI] [PubMed] [Google Scholar]
- 108.Landmesser U, Cai H, Dikalov S, McCann L, Hwang J, Jo H, Holland SM, and Harrison DG. Role of p47(phox) in vascular oxidative stress and hypertension caused by angiotensin II. Hypertension 40: 511–515, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Landmesser U, Dikalov S, Price SR, McCann L, Fukai T, Holland SM, Mitch WE, and Harrison DG. Oxidation of tetrahydrobiopterin leads to uncoupling of endothelial cell nitric oxide synthase in hypertension. J Clin Invest 111: 1201–1209, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lassegue B. and Clempus RE. Vascular NAD(P)H oxidases: specific features, expression, and regulation. Am J Physiol Regul Integr Comp Physiol 285: R277–R297, 2003 [DOI] [PubMed] [Google Scholar]
- 111.Lassegue B, Sorescu D, Szocs K, Yin Q, Akers M, Zhang Y, Grant SL, Lambeth JD, and Griendling KK. Novel gp91(phox) homologues in vascular smooth muscle cells: nox1 mediates angiotensin II-induced superoxide formation and redox-sensitive signaling pathways. Circ Res 88: 888–894, 2001 [DOI] [PubMed] [Google Scholar]
- 112.Laude K, Cai H, Fink B, Hoch N, Weber DS, McCann L, Kojda G, Fukai T, Schmidt HH, Dikalov S, Ramasamy S, Gamez G, Griendling KK, and Harrison DG. Hemodynamic and biochemical adaptations to vascular smooth muscle overexpression of p22phox in mice. Am J Physiol Heart Circ Physiol 288: H7–H12, 2005 [DOI] [PubMed] [Google Scholar]
- 113.Lavigne MC, Malech HL, Holland SM, and Leto TL. Genetic demonstration of p47phox-dependent superoxide anion production in murine vascular smooth muscle cells. Circulation 104: 79–84, 2001 [DOI] [PubMed] [Google Scholar]
- 114.Lee MY, San Martin A, Mehta PK, Dikalova AE, Garrido AM, Datla SR, Lyons E, Krause KH, Banfi B, Lambeth JD, Lassegue B, and Griendling KK. Mechanisms of vascular smooth muscle NADPH oxidase 1 (Nox1) contribution to injury-induced neointimal formation. Arterioscler Thromb Vasc Biol 29: 480–487, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lee YM, Kim BJ, Chun YS, So I, Choi H, Kim MS, and Park JW. NOX4 as an oxygen sensor to regulate TASK-1 activity. Cell Signal 18: 499–507, 2006 [DOI] [PubMed] [Google Scholar]
- 116.Li J, Stouffs M, Serrander L, Banfi B, Bettiol E, Charnay Y, Steger K, Krause KH, and Jaconi ME. The NADPH oxidase NOX4 drives cardiac differentiation: Role in regulating cardiac transcription factors and MAP kinase activation. Mol Biol Cell 17: 3978–3988, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Li JM. and Shah AM. Endothelial cell superoxide generation: regulation and relevance for cardiovascular pathophysiology. Am J Physiol Regul Integr Comp Physiol 287: R1014–R1030, 2004 [DOI] [PubMed] [Google Scholar]
- 118.Li WG, Miller FJ, Jr, Zhang HJ, Spitz DR, Oberley LW, and Weintraub NL. H(2)O(2)-induced O(2) production by a non-phagocytic NAD(P)H oxidase causes oxidant injury. J Biol Chem 276: 29251–29256, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Liang CF, Liu JT, Wang Y, Xu A, and Vanhoutte PM. Toll-like receptor 4 mutation protects obese mice against endothelial dysfunction by decreasing NADPH oxidase isoforms 1 and 4. Arterioscler Thromb Vasc Biol 33: 777–784, 2013 [DOI] [PubMed] [Google Scholar]
- 120.Libby P, Ridker PM, and Maseri A. Inflammation and atherosclerosis. Circulation 105: 1135–1143, 2002 [DOI] [PubMed] [Google Scholar]
- 121.Lichtman AH, Binder CJ, Tsimikas S, and Witztum JL. Adaptive immunity in atherogenesis: new insights and therapeutic approaches. J Clin Invest 123: 27–36, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Liu J, Yang F, Yang XP, Jankowski M, and Pagano PJ. NAD(P)H oxidase mediates angiotensin II-induced vascular macrophage infiltration and medial hypertrophy. Arterioscler Thromb Vasc Biol 23: 776–782, 2003 [DOI] [PubMed] [Google Scholar]
- 123.Liu JQ, Zelko IN, Erbynn EM, Sham JS, and Folz RJ. Hypoxic pulmonary hypertension: role of superoxide and NADPH oxidase (gp91phox). Am J Physiol Lung Cell Mol Physiol 290: L2–L10, 2006 [DOI] [PubMed] [Google Scholar]
- 124.Liu Y, Davidson BP, Yue Q, Belcik T, Xie A, Inaba Y, McCarty OJ, Tormoen GW, Zhao Y, Ruggeri ZM, Kaufmann BA, and Lindner JR. Molecular imaging of inflammation and platelet adhesion in advanced atherosclerosis effects of antioxidant therapy with NADPH oxidase inhibition. Circ Cardiovasc Imaging 6: 74–82, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Lob HE, Marvar PJ, Guzik TJ, Sharma S, McCann LA, Weyand C, Gordon FJ, and Harrison DG. Induction of hypertension and peripheral inflammation by reduction of extracellular superoxide dismutase in the central nervous system. Hypertension 55: 277–283, 6p following 283, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Lob HE, Schultz D, Marvar PJ, Davisson RL, and Harrison DG. Role of the NADPH oxidases in the subfornical organ in angiotensin II-induced hypertension. Hypertension 61: 382–387, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Lu X, Murphy TC, Nanes MS, and Hart CM. PPAR{gamma} regulates hypoxia-induced Nox4 expression in human pulmonary artery smooth muscle cells through NF-{kappa}B. Am J Physiol Lung Cell Mol Physiol 299: L559–L566, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Lyle AN, Deshpande NN, Taniyama Y, Seidel-Rogol B, Pounkova L, Du P, Papaharalambus C, Lassegue B, and Griendling KK. Poldip2, a novel regulator of Nox4 and cytoskeletal integrity in vascular smooth muscle cells. Circ Res 105: 249–259, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Madrigal-Matute J, Fernandez-Garcia CE, Gomez-Guerrero C, Lopez-Franco O, Munoz-Garcia B, Egido J, Blanco-Colio LM, and Martin-Ventura JL. HSP90 inhibition by 17-DMAG attenuates oxidative stress in experimental atherosclerosis. Cardiovasc Res 95: 116–123, 2012 [DOI] [PubMed] [Google Scholar]
- 130.Mahadev K, Motoshima H, Wu X, Ruddy JM, Arnold RS, Cheng G, Lambeth JD, and Goldstein BJ. The NAD(P)H oxidase homolog Nox4 modulates insulin-stimulated generation of H2O2 and plays an integral role in insulin signal transduction. Mol Cell Biol 24: 1844–1854, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Martyn KD, Frederick LM, von Loehneysen K, Dinauer MC, and Knaus UG. Functional analysis of Nox4 reveals unique characteristics compared to other NADPH oxidases. Cell Signal 18: 69–82, 2006 [DOI] [PubMed] [Google Scholar]
- 132.Matsuno K, Yamada H, Iwata K, Jin D, Katsuyama M, Matsuki M, Takai S, Yamanishi K, Miyazaki M, Matsubara H, and Yabe-Nishimura C. Nox1 is involved in angiotensin II-mediated hypertension: a study in Nox1-deficient mice. Circulation 112: 2677–2685, 2005 [DOI] [PubMed] [Google Scholar]
- 133.Miguel-Carrasco JL, Zambrano S, Blanca AJ, Mate A, and Vazquez CM. Captopril reduces cardiac inflammatory markers in spontaneously hypertensive rats by inactivation of NF-kB. J Inflamm (Lond) 7: 21, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Miller YI, Choi SH, Wiesner P, Fang L, Harkewicz R, Hartvigsen K, Boullier A, Gonen A, Diehl CJ, Que X, Montano E, Shaw PX, Tsimikas S, Binder CJ, and Witztum JL. Oxidation-specific epitopes are danger-associated molecular patterns recognized by pattern recognition receptors of innate immunity. Circ Res 108: 235–248, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Mittal M, Roth M, Konig P, Hofmann S, Dony E, Goyal P, Selbitz AC, Schermuly RT, Ghofrani HA, Kwapiszewska G, Kummer W, Klepetko W, Hoda MA, Fink L, Hanze J, Seeger W, Grimminger F, Schmidt HH, and Weissmann N. Hypoxia-dependent regulation of nonphagocytic NADPH oxidase subunit NOX4 in the pulmonary vasculature. Circ Res 101: 258–267, 2007 [DOI] [PubMed] [Google Scholar]
- 136.Mochizuki T, Furuta S, Mitsushita J, Shang WH, Ito M, Yokoo Y, Yamaura M, Ishizone S, Nakayama J, Konagai A, Hirose K, Kiyosawa K, and Kamata T. Inhibition of NADPH oxidase 4 activates apoptosis via the AKT/apoptosis signal-regulating kinase 1 pathway in pancreatic cancer PANC-1 cells. Oncogene 25: 3699–3707, 2006 [DOI] [PubMed] [Google Scholar]
- 137.Modlinger P, Chabrashvili T, Gill PS, Mendonca M, Harrison DG, Griendling KK, Li M, Raggio J, Wellstein A, Chen Y, Welch WJ, and Wilcox CS. RNA silencing in vivo reveals role of p22phox in rat angiotensin slow pressor response. Hypertension 47: 238–244, 2006 [DOI] [PubMed] [Google Scholar]
- 138.Moe KT, Yin NO, Naylynn TM, Khairunnisa K, Wutyi MA, Gu Y, Atan MS, Wong MC, Koh TH, and Wong P. Nox2 and Nox4 mediate tumour necrosis factor-alpha-induced ventricular remodelling in mice. J Cell Mol Med 15: 2601–2613, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Mollnau H, Wendt M, Szocs K, Lassegue B, Schulz E, Oelze M, Li H, Bodenschatz M, August M, Kleschyov AL, Tsilimingas N, Walter U, Forstermann U, Meinertz T, Griendling K, and Munzel T. Effects of angiotensin II infusion on the expression and function of NAD(P)H oxidase and components of nitric oxide/cGMP signaling. Circ Res 90: E58–E65, 2002 [DOI] [PubMed] [Google Scholar]
- 140.Monte M, Davel LE, and Sacerdote de Lustig E. Hydrogen peroxide is involved in lymphocyte activation mechanisms to induce angiogenesis. Eur J Cancer 33: 676–682, 1997 [DOI] [PubMed] [Google Scholar]
- 141.Nakane H, Miller FJ, Jr, Faraci FM, Toyoda K, and Heistad DD. Gene transfer of endothelial nitric oxide synthase reduces angiotensin II-induced endothelial dysfunction. Hypertension 35: 595–601, 2000 [DOI] [PubMed] [Google Scholar]
- 142.Nauseef WM. Nox enzymes in immune cells. Semin Immunopathol 30: 195–208, 2008 [DOI] [PubMed] [Google Scholar]
- 143.Nisbet RE, Bland JM, Kleinhenz DJ, Mitchell PO, Walp ER, Sutliff RL, and Hart CM. Rosiglitazone attenuates chronic hypoxia-induced pulmonary hypertension in a mouse model. Am J Respir Cell Mol Biol 42: 482–490, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.O'Brien KD, McDonald TO, Chait A, Allen MD, and Alpers CE. Neovascular expression of E-selectin, intercellular adhesion molecule-1, and vascular cell adhesion molecule-1 in human atherosclerosis and their relation to intimal leukocyte content. Circulation 93: 672–682, 1996 [DOI] [PubMed] [Google Scholar]
- 145.O'Donnell BV, Tew DG, Jones OT, and England PJ. Studies on the inhibitory mechanism of iodonium compounds with special reference to neutrophil NADPH oxidase. Biochem J 290 (Pt 1): 41–49, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Oliveira-Sales EB, Nishi EE, Carillo BA, Boim MA, Dolnikoff MS, Bergamaschi CT, and Campos RR. Oxidative stress in the sympathetic premotor neurons contributes to sympathetic activation in renovascular hypertension. Am J Hypertens 22: 484–492, 2009 [DOI] [PubMed] [Google Scholar]
- 147.Olukman M, Orhan CE, Celenk FG, and Ulker S. Apocynin restores endothelial dysfunction in streptozotocin diabetic rats through regulation of nitric oxide synthase and NADPH oxidase expressions. J Diabetes Complications 24: 415–423, 2010 [DOI] [PubMed] [Google Scholar]
- 148.Pagano PJ, Chanock SJ, Siwik DA, Colucci WS, and Clark JK. Angiotensin II induces p67phox mRNA expression and NADPH oxidase superoxide generation in rabbit aortic adventitial fibroblasts. Hypertension 32: 331–337, 1998 [DOI] [PubMed] [Google Scholar]
- 149.Paravicini TM. and Touyz RM. NADPH oxidases, reactive oxygen species, and hypertension: clinical implications and therapeutic possibilities. Diabetes Care 31Suppl 2: S170–S180, 2008 [DOI] [PubMed] [Google Scholar]
- 150.Park HS, Chun JN, Jung HY, Choi C, and Bae YS. Role of NADPH oxidase 4 in lipopolysaccharide-induced proinflammatory responses by human aortic endothelial cells. Cardiovasc Res 72: 447–455, 2006 [DOI] [PubMed] [Google Scholar]
- 151.Pedruzzi E, Guichard C, Ollivier V, Driss F, Fay M, Prunet C, Marie JC, Pouzet C, Samadi M, Elbim C, O'Dowd Y, Bens M, Vandewalle A, Gougerot-Pocidalo MA, Lizard G, and Ogier-Denis E. NAD(P)H oxidase Nox-4 mediates 7-ketocholesterol-induced endoplasmic reticulum stress and apoptosis in human aortic smooth muscle cells. Mol Cell Biol 24: 10703–10717, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Pendyala S, Usatyuk PV, Gorshkova IA, Garcia JG, and Natarajan V. Regulation of NADPH oxidase in vascular endothelium: the role of phospholipases, protein kinases, and cytoskeletal proteins. Antioxid Redox Signal 11: 841–860, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Peterson JR, Burmeister MA, Tian X, Zhou Y, Guruju MR, Stupinski JA, Sharma RV, and Davisson RL. Genetic silencing of Nox2 and Nox4 reveals differential roles of these NADPH oxidase homologues in the vasopressor and dipsogenic effects of brain angiotensin II. Hypertension 54: 1106–1114, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Purushothaman D. and Sarin A. Cytokine-dependent regulation of NADPH oxidase activity and the consequences for activated T cell homeostasis. J Exp Med 206: 1515–1523, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Quagliaro L, Piconi L, Assaloni R, Da Ros R, Maier A, Zuodar G, and Ceriello A. Intermittent high glucose enhances ICAM-1, VCAM-1 and E-selectin expression in human umbilical vein endothelial cells in culture: the distinct role of protein kinase C and mitochondrial superoxide production. Atherosclerosis 183: 259–267, 2005 [DOI] [PubMed] [Google Scholar]
- 156.Rajagopalan S, Kurz S, Munzel T, Tarpey M, Freeman BA, Griendling KK, and Harrison DG. Angiotensin II-mediated hypertension in the rat increases vascular superoxide production via membrane NADH/NADPH oxidase activation. Contribution to alterations of vasomotor tone. J Clin Invest 97: 1916–1923, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Ray R, Murdoch CE, Wang M, Santos CX, Zhang M, Alom-Ruiz S, Anilkumar N, Ouattara A, Cave AC, Walker SJ, Grieve DJ, Charles RL, Eaton P, Brewer AC, and Shah AM. Endothelial Nox4 NADPH oxidase enhances vasodilatation and reduces blood pressure in vivo. Arterioscler Thromb Vasc Biol 31: 1368–1376, 2011 [DOI] [PubMed] [Google Scholar]
- 158.Ray R. and Shah AM. NADPH oxidase and endothelial cell function. Clin Sci (Lond) 109: 217–226, 2005 [DOI] [PubMed] [Google Scholar]
- 159.Rey FE, Cifuentes ME, Kiarash A, Quinn MT, and Pagano PJ. Novel competitive inhibitor of NAD(P)H oxidase assembly attenuates vascular O(2)(−) and systolic blood pressure in mice. Circ Res 89: 408–414, 2001 [DOI] [PubMed] [Google Scholar]
- 160.Rey FE, Li XC, Carretero OA, Garvin JL, and Pagano PJ. Perivascular superoxide anion contributes to impairment of endothelium-dependent relaxation: role of gp91(phox). Circulation 106: 2497–2502, 2002 [DOI] [PubMed] [Google Scholar]
- 161.Rius C, Piqueras L, Gonzalez-Navarro H, Albertos F, Company C, Lopez-Gines C, Ludwig A, Blanes JI, Morcillo EJ, and Sanz MJ. Arterial and venous endothelia display differential functional fractalkine (CX3CL1) expression by angiotensin-II. Arterioscler Thromb Vasc Biol 33: 96–104, 2013 [DOI] [PubMed] [Google Scholar]
- 162.Rueckschloss U, Galle J, Holtz J, Zerkowski HR, and Morawietz H. Induction of NAD(P)H oxidase by oxidized low-density lipoprotein in human endothelial cells: antioxidative potential of hydroxymethylglutaryl coenzyme A reductase inhibitor therapy. Circulation 104: 1767–1772, 2001 [DOI] [PubMed] [Google Scholar]
- 163.Ryszawa N, Kawczynska-Drozdz A, Pryjma J, Czesnikiewicz-Guzik M, Adamek-Guzik T, Naruszewicz M, Korbut R, and Guzik TJ. Effects of novel plant antioxidants on platelet superoxide production and aggregation in atherosclerosis. J Physiol Pharmacol 57: 611–626, 2006 [PubMed] [Google Scholar]
- 164.Schramm A, Matusik P, Osmenda G, and Guzik TJ. Targeting NADPH oxidases in vascular pharmacology. Vascul Pharmacol 56: 216–231, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Schroder K, Wandzioch K, Helmcke I, and Brandes RP. Nox4 acts as a switch between differentiation and proliferation in preadipocytes. Arterioscler Thromb Vasc Biol 29: 239–245, 2009 [DOI] [PubMed] [Google Scholar]
- 166.Schroder K, Zhang M, Benkhoff S, Mieth A, Pliquett R, Kosowski J, Kruse C, Luedike P, Michaelis UR, Weissmann N, Dimmeler S, Shah AM, and Brandes RP. Nox4 is a protective reactive oxygen species generating vascular NADPH oxidase. Circ Res 110: 1217–1225, 2012 [DOI] [PubMed] [Google Scholar]
- 167.Seshiah PN, Weber DS, Rocic P, Valppu L, Taniyama Y, and Griendling KK. Angiotensin II stimulation of NAD(P)H oxidase activity: upstream mediators. Circ Res 91: 406–413, 2002 [DOI] [PubMed] [Google Scholar]
- 168.Shinozaki K, Ayajiki K, Nishio Y, Sugaya T, Kashiwagi A, and Okamura T. Evidence for a causal role of the renin-angiotensin system in vascular dysfunction associated with insulin resistance. Hypertension 43: 255–262, 2004 [DOI] [PubMed] [Google Scholar]
- 169.Simpson CS. and Morris BJ. Regulation of neuronal cell adhesion molecule expression by NF-kappa B. J Biol Chem 275: 16879–16884, 2000 [DOI] [PubMed] [Google Scholar]
- 170.Sorescu D, Weiss D, Lassegue B, Clempus RE, Szocs K, Sorescu GP, Valppu L, Quinn MT, Lambeth JD, Vega JD, Taylor WR, and Griendling KK. Superoxide production and expression of nox family proteins in human atherosclerosis. Circulation 105: 1429–1435, 2002 [DOI] [PubMed] [Google Scholar]
- 171.Stielow C, Catar RA, Muller G, Wingler K, Scheurer P, Schmidt HH, and Morawietz H. Novel Nox inhibitor of oxLDL-induced reactive oxygen species formation in human endothelial cells. Biochem Biophys Res Commun 344: 200–205, 2006 [DOI] [PubMed] [Google Scholar]
- 172.Stolk J, Hiltermann TJ, Dijkman JH, and Verhoeven AJ. Characteristics of the inhibition of NADPH oxidase activation in neutrophils by apocynin, a methoxy-substituted catechol. Am J Respir Cell Mol Biol 11: 95–102, 1994 [DOI] [PubMed] [Google Scholar]
- 173.Sturrock A, Cahill B, Norman K, Huecksteadt TP, Hill K, Sanders K, Karwande SV, Stringham JC, Bull DA, Gleich M, Kennedy TP, and Hoidal JR. Transforming growth factor-beta1 induces Nox4 NAD(P)H oxidase and reactive oxygen species-dependent proliferation in human pulmonary artery smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 290: L661–L673, 2006 [DOI] [PubMed] [Google Scholar]
- 174.Suh YA, Arnold RS, Lassegue B, Shi J, Xu X, Sorescu D, Chung AB, Griendling KK, and Lambeth JD. Cell transformation by the superoxide-generating oxidase Mox1. Nature 401: 79–82, 1999 [DOI] [PubMed] [Google Scholar]
- 175.Szocs K, Lassegue B, Sorescu D, Hilenski LL, Valppu L, Couse TL, Wilcox JN, Quinn MT, Lambeth JD, and Griendling KK. Upregulation of Nox-based NAD(P)H oxidases in restenosis after carotid injury. Arterioscler Thromb Vasc Biol 22: 21–27, 2002 [DOI] [PubMed] [Google Scholar]
- 176.Takac I, Schroder K, and Brandes RP. The nox family of NADPH oxidases: friend or foe of the vascular system? Curr Hypertens Rep 14: 70–78, 2012 [DOI] [PubMed] [Google Scholar]
- 177.Takac I, Schroder K, Zhang L, Lardy B, Anilkumar N, Lambeth JD, Shah AM, Morel F, and Brandes RP. The E-loop is involved in hydrogen peroxide formation by the NADPH oxidase Nox4. J Biol Chem 286: 13304–13313, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Takai S, Jin D, Kawashima H, Kimura M, Shiraishi-Tateishi A, Tanaka T, Kakutani S, Tanaka K, Kiso Y, and Miyazaki M. Anti-atherosclerotic effects of dihomo-gamma-linolenic acid in ApoE-deficient mice. J Atheroscler Thromb 16: 480–489, 2009 [DOI] [PubMed] [Google Scholar]
- 179.Tazzeo T, Worek F, and Janssen L. The NADPH oxidase inhibitor diphenyleneiodonium is also a potent inhibitor of cholinesterases and the internal Ca(2+) pump. Br J Pharmacol 158: 790–796, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.ten Freyhaus H, Huntgeburth M, Wingler K, Schnitker J, Baumer AT, Vantler M, Bekhite MM, Wartenberg M, Sauer H, and Rosenkranz S. Novel Nox inhibitor VAS2870 attenuates PDGF-dependent smooth muscle cell chemotaxis, but not proliferation. Cardiovasc Res 71: 331–341, 2006 [DOI] [PubMed] [Google Scholar]
- 181.Tojo T, Ushio-Fukai M, Yamaoka-Tojo M, Ikeda S, Patrushev N, and Alexander RW. Role of gp91phox (Nox2)-containing NAD(P)H oxidase in angiogenesis in response to hindlimb ischemia. Circulation 111: 2347–2355, 2005 [DOI] [PubMed] [Google Scholar]
- 182.Touyz RM, Chen X, Tabet F, Yao G, He G, Quinn MT, Pagano PJ, and Schiffrin EL. Expression of a functionally active gp91phox-containing neutrophil-type NAD(P)H oxidase in smooth muscle cells from human resistance arteries: regulation by angiotensin II. Circ Res 90: 1205–1213, 2002 [DOI] [PubMed] [Google Scholar]
- 183.Touyz RM, Mercure C, He Y, Javeshghani D, Yao G, Callera GE, Yogi A, Lochard N, and Reudelhuber TL. Angiotensin II-dependent chronic hypertension and cardiac hypertrophy are unaffected by gp91phox-containing NADPH oxidase. Hypertension 45: 530–537, 2005 [DOI] [PubMed] [Google Scholar]
- 184.Ullevig S, Zhao Q, Lee CF, Seok Kim H, Zamora D, and Asmis R. NADPH oxidase 4 mediates monocyte priming and accelerated chemotaxis induced by metabolic stress. Arterioscler Thromb Vasc Biol 32: 415–426, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Urao N, Inomata H, Razvi M, Kim HW, Wary K, McKinney R, Fukai T, and Ushio-Fukai M. Role of nox2-based NADPH oxidase in bone marrow and progenitor cell function involved in neovascularization induced by hindlimb ischemia. Circ Res 103: 212–220, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Ushio-Fukai M, Tang Y, Fukai T, Dikalov SI, Ma Y, Fujimoto M, Quinn MT, Pagano PJ, Johnson C, and Alexander RW. Novel role of gp91(phox)-containing NAD(P)H oxidase in vascular endothelial growth factor-induced signaling and angiogenesis. Circ Res 91: 1160–1167, 2002 [DOI] [PubMed] [Google Scholar]
- 187.Ushio-Fukai M, Zafari AM, Fukui T, Ishizaka N, and Griendling KK. p22phox is a critical component of the superoxide-generating NADH/NADPH oxidase system and regulates angiotensin II-induced hypertrophy in vascular smooth muscle cells. J Biol Chem 271: 23317–23321, 1996 [DOI] [PubMed] [Google Scholar]
- 188.Van Buul JD, Fernandez-Borja M, Anthony EC, and Hordijk PL. Expression and localization of NOX2 and NOX4 in primary human endothelial cells. Antioxid Redox Signal 7: 308–317, 2005 [DOI] [PubMed] [Google Scholar]
- 189.Verbeuren TJ, Bouskela E, Cohen RA, and Vanhoutte PM. Regulation of adhesion molecules: a new target for the treatment of chronic venous insufficiency. Microcirculation 7: S41–S48, 2000 [PubMed] [Google Scholar]
- 190.Vinh A, Chen W, Blinder Y, Weiss D, Taylor WR, Goronzy JJ, Weyand CM, Harrison DG, and Guzik TJ. Inhibition and genetic ablation of the B7/CD28 T-cell costimulation axis prevents experimental hypertension. Circulation 122: 2529–2537, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Violi F, Pignatelli P, Pignata C, Plebani A, Rossi P, Sanguigni V, Carnevale R, Soresina A, Finocchi A, Cirillo E, Catasca E, Angelico F, and Loffredo L. Reduced atherosclerotic burden in subjects with genetically determined low oxidative stress. Arterioscler Thromb Vasc Biol 33: 406–412, 2013 [DOI] [PubMed] [Google Scholar]
- 192.Violi F, Sanguigni V, Carnevale R, Plebani A, Rossi P, Finocchi A, Pignata C, De Mattia D, Martire B, Pietrogrande MC, Martino S, Gambineri E, Soresina AR, Pignatelli P, Martino F, Basili S, and Loffredo L. Hereditary deficiency of gp91(phox) is associated with enhanced arterial dilatation: results of a multicenter study. Circulation 120: 1616–1622, 2009 [DOI] [PubMed] [Google Scholar]
- 193.Wang HD, Xu S, Johns DG, Du Y, Quinn MT, Cayatte AJ, and Cohen RA. Role of NADPH oxidase in the vascular hypertrophic and oxidative stress response to angiotensin II in mice. Circ Res 88: 947–953, 2001 [DOI] [PubMed] [Google Scholar]
- 194.Wautier MP, Chappey O, Corda S, Stern DM, Schmidt AM, and Wautier JL. Activation of NADPH oxidase by AGE links oxidant stress to altered gene expression via RAGE. Am J Physiol Endocrinol Metab 280: E685–E694, 2001 [DOI] [PubMed] [Google Scholar]
- 195.Weber DS, Rocic P, Mellis AM, Laude K, Lyle AN, Harrison DG, and Griendling KK. Angiotensin II-induced hypertrophy is potentiated in mice overexpressing p22phox in vascular smooth muscle. Am J Physiol Heart Circ Physiol 288: H37–H42, 2005 [DOI] [PubMed] [Google Scholar]
- 196.Wei Y, Whaley-Connell AT, Chen K, Habibi J, Uptergrove GM, Clark SE, Stump CS, Ferrario CM, and Sowers JR. NADPH oxidase contributes to vascular inflammation, insulin resistance, and remodeling in the transgenic (mRen2) rat. Hypertension 50: 384–391, 2007 [DOI] [PubMed] [Google Scholar]
- 197.West N, Guzik T, Black E, and Channon K. Enhanced superoxide production in experimental venous bypass graft intimal hyperplasia: role of NAD(P)H oxidase. Arterioscler Thromb Vasc Biol 21: 189–194, 2001 [DOI] [PubMed] [Google Scholar]
- 198.Wind S, Beuerlein K, Armitage ME, Taye A, Kumar AH, Janowitz D, Neff C, Shah AM, Wingler K, and Schmidt HH. Oxidative stress and endothelial dysfunction in aortas of aged spontaneously hypertensive rats by NOX1/2 is reversed by NADPH oxidase inhibition. Hypertension 56: 490–497, 2010 [DOI] [PubMed] [Google Scholar]
- 199.Wind S, Beuerlein K, Eucker T, Muller H, Scheurer P, Armitage ME, Ho H, Schmidt HH, and Wingler K. Comparative pharmacology of chemically distinct NADPH oxidase inhibitors. Br J Pharmacol 161: 885–898, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Wu F, Han M, and Wilson JX. Tripterine prevents endothelial barrier dysfunction by inhibiting endogenous peroxynitrite formation. Br J Pharmacol 157: 1014–1023, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Wung BS, Cheng JJ, Hsieh HJ, Shyy YJ, and Wang DL. Cyclic strain-induced monocyte chemotactic protein-1 gene expression in endothelial cells involves reactive oxygen species activation of activator protein 1. Circ Res 81: 1–7, 1997 [DOI] [PubMed] [Google Scholar]
- 202.Xue B, Beltz TG, Johnson RF, Guo F, Hay M, and Johnson AK. PVN adenovirus-siRNA injections silencing either NOX2 or NOX4 attenuate aldosterone/NaCl-induced hypertension in mice. Am J Physiol Heart Circ Physiol 302: H733–H741, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Yamamoto E, Kataoka K, Dong YF, Nakamura T, Fukuda M, Nako H, Ogawa H, and Kim-Mitsuyama S. Benidipine, a dihydropyridine L-type/T-type calcium channel blocker, affords additive benefits for prevention of cardiorenal injury in hypertensive rats. J Hypertens 28: 1321–1329, 2010 [DOI] [PubMed] [Google Scholar]
- 204.Yogi A, Mercure C, Touyz J, Callera GE, Montezano AC, Aranha AB, Tostes RC, Reudelhuber T, and Touyz RM. Renal redox-sensitive signaling, but not blood pressure, is attenuated by Nox1 knockout in angiotensin II-dependent chronic hypertension. Hypertension 51: 500–506, 2008 [DOI] [PubMed] [Google Scholar]
- 205.Zafari AM, Ushio-Fukai M, Akers M, Yin Q, Shah A, Harrison DG, Taylor WR, and Griendling KK. Role of NADH/NADPH oxidase-derived H2O2 in angiotensin II-induced vascular hypertrophy. Hypertension 32: 488–495, 1998 [DOI] [PubMed] [Google Scholar]
- 206.Zhang L, Zalewski A, Liu Y, Mazurek T, Cowan S, Martin JL, Hofmann SM, Vlassara H, and Shi Y. Diabetes-induced oxidative stress and low-grade inflammation in porcine coronary arteries. Circulation 108: 472–478, 2003 [DOI] [PubMed] [Google Scholar]
- 207.Zhou MS, Hernandez Schulman I, Pagano PJ, Jaimes EA, and Raij L. Reduced NAD(P)H oxidase in low renin hypertension: link among angiotensin II, atherogenesis, and blood pressure. Hypertension 47: 81–86, 2006 [DOI] [PubMed] [Google Scholar]
- 208.Zimmerman MC, Dunlay RP, Lazartigues E, Zhang Y, Sharma RV, Engelhardt JF, and Davisson RL. Requirement for Rac1-dependent NADPH oxidase in the cardiovascular and dipsogenic actions of angiotensin II in the brain. Circ Res 95: 532–539, 2004 [DOI] [PubMed] [Google Scholar]
- 209.Zimmerman MC, Lazartigues E, Lang JA, Sinnayah P, Ahmad IM, Spitz DR, and Davisson RL. Superoxide mediates the actions of angiotensin II in the central nervous system. Circ Res 91: 1038–1045, 2002 [DOI] [PubMed] [Google Scholar]
- 210.Zimmerman MC, Lazartigues E, Sharma RV, and Davisson RL. Hypertension caused by angiotensin II infusion involves increased superoxide production in the central nervous system. Circ Res 95: 210–216, 2004 [DOI] [PubMed] [Google Scholar]