Abstract
Objective: The purpose of this study was to assess the efficacy and safety of edivoxetine (LY2216684), a selective norepinephrine reuptake inhibitor, in pediatric patients with attention-deficit/hyperactivity disorder (ADHD).
Method: A fixed-dose, randomized, double-blind, 8 week study was conducted in patients 6–17 years of age, who were randomized by two strata: 1) Patients with prior stimulant use randomized to placebo, edivoxetine 0.1 mg/kg/day, 0.2 mg/kg/day, or 0.3 mg/kg/day arms in a 1:1:1:1 ratio; 2) Stimulant-naïve patients randomized to placebo, edivoxetine 0.1mg/kg/day, 0.2 mg/kg/day, 0.3 mg/kg/day, or osmotic-release oral system methylphenidate (OROS MPH) (18–54 mg/day based on body weight) arms in a 1:1:1:1:1 ratio. The primary efficacy measure was baseline-to-week 8 change of ADHD Rating Scale (ADHD-RS) total score for edivoxetine 0.2 mg/kg/day and 0.3 mg/kg/day.
Results: A total of 340 patients were randomized to placebo (n=78); edivoxetine 0.1 mg/kg/day (n=76), 0.2 mg/kg/day (n=75), or 0.3 mg/kg/day (n=75); or OROS MPH (n=36). In the stimulant-naïve stratum, the positive control, OROS MPH, was significantly superior to placebo in mean ADHD-RS total score change at end-point (−19.46, p=0.015). The edivoxetine 0.2 mg/kg/day and 0.3 mg/kg/day arms had statistically significantly greater improvement than the placebo arm in mean ADHD-RS total score change at end-point (placebo −10.35; edivoxetine 0.2 mg/kg/day −16.09, p<0.010; edivoxetine 0.3 mg/kg/day −16.39, p<0.010) and Clinical Global Impressions-Improvement score (placebo 3.05; edivoxetine 0.1 mg/kg/day 3.01, p=0.860; edivoxetine 0.2 mg/kg/day 2.54, p=0.013; edivoxetine 0.3 mg/kg/day 2.53, p=0.013). In the overall efficacy-analyses data set (n=270), the effect size estimates for edivoxetine doses 0.1 mg/kg/day, 0.2 mg/kg/day and 0.3 mg/kg/day at the week 8 time point were 0.17, 0.51, and 0.54, respectively (for the stimulant-naïve stratum, the effect size estimate for OROS MPH was 0.69). Compared with placebo, edivoxetine treatment was associated with statistically significant increases in blood pressure and pulse (p<0.050), and a smaller increase or slight decrease in weight.
Conclusions: Edivoxetine at doses of 0.2 mg/kg/day and 0.3 mg/kg/day demonstrated efficacy in ADHD treatment, despite the presence of a sizeable placebo response. No unexpected adverse events were identified.
Clinical Trial Registry identifier: NCT00922636
Introduction
Attention-deficit/hyperactivity disorder (ADHD) is one of the most frequently diagnosed neuropsychological disorders in children and adolescents, leading to a developmental impairment of executive functions in the brain. Noradrenergic and dopaminergic neurotransmitter systems have been implicated in the pathophysiology of ADHD (Pliszka 1998). These neurotransmitters and their effects on fronto-subcortical pathways are believed to play an important role in high-level executive functions, which tend to be impaired in patients with ADHD (Del Campo et al. 2011).
Psychostimulants are widely used drugs that treat ADHD (Culpepper 2006; Wilens 2006); however, up to 30% of patients with ADHD treated with psychostimulants discontinue treatment because of intolerance or inadequate response to these medications (Quintero et al. 2010). Nonstimulant medications such as the norepinephrine reuptake inhibitor (NERI) atomoxetine, the long-acting α-2 agonists, and other pharmacotherapies have been identified as alternative treatments for ADHD.
Edivoxetine – LY2216684; 2-morpholinemethanol, α-([5-fluoro-2-methoxyphenyl] methyl)-α-[tetrahydro-2H-pyran-4-yl]-, hydrochloride, (αR, 2S) – is a selective and potent NERI that has been evaluated in pediatric patients with ADHD. In a previous open-label study of edivoxetine at targeted doses of 0.05 mg/kg/day, 0.1 mg/kg/day, 0.2 mg/kg/day, and 0.3 mg/kg/day in pediatric patients with ADHD, the Attention-Deficit/Hyperactivity Disorder Rating Scale-IV-Parent Version: Investigator Administered and Scored (ADHD-RS-IV-Parent:Inv) total score, inattention and hyperactivity/impulsivity subscores, and Clinical Global Impressions-Attention-Deficit/Hyperactivity Disorder-Severity (CGI-ADHD-S) scores were statistically significantly improved at end-point compared with baseline (Jin et al. 2013). From that same study, the pharmacokinetic (PK) and pharmacodynamic (PD) characteristics of edivoxetine were also examined using measurement of the intraneuronal metabolite of norepinephrine, 3,4-dihydroxyphenylglycol (DHPG), as a functional measure of norepinephrine transporter (NET) activity (Kielbasa et al. 2012). Overall, the edivoxetine data were used to provide evidence for target pharmacology, inform the safety and effectiveness, and guide dose selection for larger scale efficacy and safety trials.
The purpose of the study reported herein was to evaluate the efficacy and safety of edivoxetine in pediatric patients with ADHD. The targeted dose levels in this study were 0.1 mg/kg/day, 0.2 mg/kg/day, and 0.3 mg/kg/day, administered as a once daily (QD) dose. The primary objective was to assess if edivoxetine at 0.2 mg/kg/day or 0.3 mg/kg/day could reduce ADHD symptom severity to a greater degree than placebo. An osmotic-release oral system formulation of methylphenidate (OROS MPH) was included as a positive control for study validation.
Method
Overview
This multicenter study was conducted by 31 principal investigators at 31 investigative sites in five countries: United States (23 sites), Canada (3 sites), Taiwan (3 sites), Mexico (1 site), and Puerto Rico (1 site). Principal investigators were licensed physicians specializing in psychiatry, pediatrics, or neurology, or were licensed clinical psychologists with a physician available for consultations. Enrollment began in June 2009, and the study was completed in October 2010. Ethical review boards representing each site approved the protocol, parents/caregivers provided informed consent, and patients provided assent after the study was explained and their questions were answered, before study procedures were initiated. This study was conducted in accordance with ethical principles of Good Clinical Practice and the Declaration of Helsinki and its guidelines.
Patient selection
The study included female and male patients ≥6 years and <17 years and 9 months of age at the time of informed consent. Study participants had to meet Diagnostic and Statistical Manual of Mental Disorders, 4th ed., Text Revision (DSM-IV-TR) (American Psychiatric Association 2000) criteria for ADHD, based on a clinician interview, and confirmed using the Kiddie Schedule for Affective Disorders and Schizophrenia for School Aged Children-Present and Lifetime version (K-SADS-PL) (Kaufman et al. 1997) (at screening), have an ADHD-RS-IV-Parent:Inv (DuPaul et al. 1998; Faries et al. 2001) total score ≥1.5 standard deviations above the age and gender norms (at screening and week 0), and have a CGI-ADHD-S (Guy 1976) score ≥4 (at screening and week 0). Patients' present or lifetime diagnosis of ADHD and other psychiatric disorders were evaluated with the K-SADS-PL. The following were primary exclusion criteria: Body weight <18 kg or >75 kg; history of bipolar I or II disorder, or psychosis; any seizure disorder or pervasive developmental disorder; presence of motor tics or a diagnosis of Tourette's syndrome; marked anxiety, tension, or agitation sufficient to contraindicate treatment with OROS MPH; history of electroencephalographic abnormalities; clinically significant abnormal electrocardiogram; serious or unstable medical illness; any medical condition that would increase sympathetic nervous system activity markedly (e.g., catecholamine-secreting neural tumor); requiring the daily use of medications with sympathomimetic activity (e.g., albuterol, pseudoephedrine); any medical condition that would be exacerbated by an increase in norepinephrine tone; or current or past history of clinically significant hypertension.
Study design
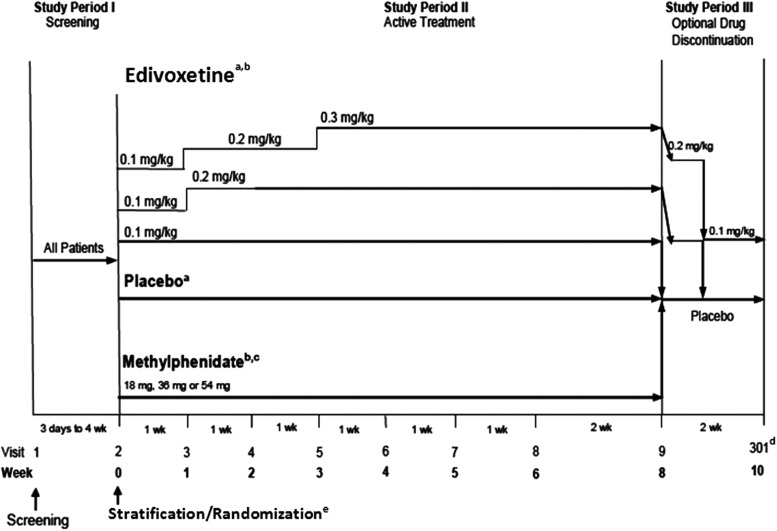
This was a fixed-dose, randomized, double-blind, parallel-arm, placebo-controlled study of edivoxetine in pediatric patients who met DSM-IV-TR diagnostic criteria for ADHD. The screening phase (Study Period I) (Fig. 1) was intended to diagnose and assess the patients for possible inclusion in the study and provide an adequate washout period for excluded medications. During the screening period, patients underwent a full clinical assessment, including a comprehensive medical and psychiatric evaluation, evaluation of baseline symptom severity, physical examination, and laboratory tests. Study Period II was an 8 week, double-blind, acute treatment period (weeks 0–8). Prior to randomization, patients were stratified into two strata based on their history of previous psychostimulant treatment. The rationale for this stratification was based on previous findings in which stimulant-naïve patients showed better response rates than those with prior experience of stimulant use when treated with either OROS MPH or atomoxetine (Newcorn et al. 2008). Patients with a prior history of psychostimulant treatment (stimulant-prior stratum) were randomized in a 1:1:1:1 ratio to placebo or one of the three fixed-dose treatment arms of edivoxetine at targeted doses of 0.1 mg/kg, 0.2 mg/kg, or 0.3 mg/kg administered daily. Patients with no history of treatment with psychostimulants (stimulant-naïve stratum) were randomized in a 1:1:1:1:1 ratio to placebo, OROS MPH, or one of the three fixed-dose treatment arms of edivoxetine. The OROS MPH arm was used as a positive control for study validation, in order to allow for a qualitative assessment of efficacy and not as a direct comparison. The OROS MPH was administered at the label-recommended doses and at a dose demonstrated to be safe and efficacious in a previous study of patients with ADHD who were treated with OROS MPH or atomoxetine. Sample sizes in this study were not adequately powered for direct comparisons between OROS MPH and any of the fixed-dose edivoxetine treatment arms.
FIG. 1.
Study design. aIncludes patients with prior stimulant treatment and patients with no prior stimulant treatment. bTitration to target dose was based on patient's weight (methylphenidate dose=18 mg [18.0–23.9 kg], 36 mg [24.0–41.9 kg], 54 mg [42.0–≤75 kg]). cIncludes only patients with no prior stimulant treatment. dPatients unable to tolerate the assigned dose during Study Period II were discontinued from the study and returned for a safety follow-up visit at visit 301 (end-point for Study Period III). ePatients were first stratified based on previous stimulant treatment history, then randomized; stimulant-naïve patients were randomized at a ratio of 1:1:1:1:1 to placebo, extended-release methylphenidate, or one of three fixed-dose arms of edivoxetine (targeted doses of 0.1, 0.2, or 0.3 mg/kg); stimulant-prior patients were randomized at a ratio of 1:1:1:1 to placebo or one of three fixed-dose arms of edivoxetine. wk=week.
The dose levels of edivoxetine selected for this study were based on results obtained from a previous open-label study investigating the safety, effectiveness, PK, and PD of edivoxetine (Kielbasa et al. 2012; Jin et al. 2013). The actual amount of edivoxetine (in milligrams) administered to patients in this study was based on an approximation of the median body weight within the range and the fixed-dose treatment arms to which they were randomized. The minimum and maximum doses of edivoxetine that could be administered were 2 mg and 18 mg, respectively. All patients randomized to edivoxetine began treatment at 0.1 mg/kg/day after week 0. Those patients randomized to the 0.1 mg/kg/day (dose range: 0.08–0.11 mg/kg) edivoxetine treatment arm continued with this treatment through week 8. Patients randomized to the 0.2 mg/kg/day (dose range: 0.16–0.22 mg/kg) or the 0.3 mg/kg/day (dose range: 0.24–0.33 mg/kg) edivoxetine treatment arms had doses titrated upward in a stepwise fashion from the 0.1 mg/kg/day dose. Patients randomized to the OROS MPH arm began treatment at 18 mg (weight range: 18.0–23.9 kg) daily after week 0. Based on patient weight, the daily dose of extended-release MPH was titrated to a target dose of 36 mg (weight range: 24.0–41.9 kg) or 54 mg (weight range: 42.0 to ≤75 kg). Patients who were unable to tolerate the assigned doses of the study drug were discontinued from the study. An interactive voice-response system was used for randomization and to determine which study drug to dispense. Patients returned for monitoring at weekly intervals from weeks 1–6, and at a 2 week interval between weeks 6 and 8. Study Period III was the drug discontinuation phase (2 weeks) in which all patients received study drug (at the reduced dose) or placebo for 2 weeks. This study period was designed to taper dosages for patients in the 0.2 mg/kg/day or 0.3 mg/kg/day edivoxetine treatment arms and to provide a discontinuation visit for patients who chose not to participate in a longer open-label extension study of edivoxetine. Patients randomized to the 0.3 mg/kg/day edivoxetine treatment arm had doses tapered to 0.2 mg/kg/day for the first week, and to 0.1 mg/kg/day the second week. Patients randomized to the 0.2 mg/kg/day edivoxetine treatment had doses tapered to 0.1mg/kg/day for the first week, and received placebo the second week. Patients randomized to other study arms received placebo during the 2 weeks of Study Period III.
Outcome measures and assessments
The primary efficacy measure was the ADHDRS-IV-Parent:Inv, which is an 18 item scale with one item for each of the 18 symptoms contained in the DSM-IV-TR diagnosis of ADHD (DuPaul et al. 1998; Faries et al. 2001). Each item was scored on a 0–3 scale (in which 0=never or rarely, 1=sometimes, 2=often, and 3=very often) and assessed symptom severity over the previous week. The scale was administered and scored by qualified personnel at the investigative site based on an interview with the parent and the patient. The total score was computed as the sum of the scores for each of the 18 items. The inattention subscale score was the sum of the scores for the odd numbered items, and the hyperactivity/impulsivity subscale score was the sum of the scores for the even numbered items.
The secondary efficacy measures included the Clinical Global Impressions-Attention-Deficit/Hyperactivity Disorder-Improvement (CGI-ADHD-I) (Guy 1976), CGI-ADHD-S, Swanson Nolan, and Pelham Rating Scale-Revised (SNAP-IV) (Swanson 1992), and the Conners Comprehensive Behavior Rating Scales (CBRS) (Conners 1997). The SNAP-IV is a teacher-rated ADHD subscale, and patients were not excluded from the study if teachers or schools did not agree to provide ratings, or patients and/or parent or legal guardian declined to have a teacher rate SNAP-IV. The numbers of responses for the SNAP-IV that were provided by the institutions were ≤10 per arm, and, consequently, these numbers were insufficient to allow for a meaningful analysis.
Response was defined as 1) a final ADHDRS-IV-Parent:Inv total score ≤60% of baseline ADHDRS-IV-Parent:Inv total score (i.e., 40% reduction from baseline) or 2) an end-point CGI-ADHD-I score ≤2. The time-to-response analyses were based on the abovementioned response criteria.
Safety assessments
Safety assessments included adverse events, vital signs (National High Blood Pressure Education Program Working Group [NHBPEPWG] 2004), clinical laboratory tests (e.g., chemistry, hematology, and urinalysis), physical examination, and electrocardiograms. The occurrence, severity, and frequency of suicide-related thoughts and behaviors were evaluated using the Columbia Suicide Severity Rating Scale (C-SSRS) (Posner et al. 2007).
Data analysis
Safety analyses were conducted on an intent-to-treat basis (ITT), meaning that data were analyzed by the treatment arms to which patients were randomly assigned, even if the patient did not take the assigned treatment, did not receive the correct treatment, or did not comply with the protocol. This is referred to as the safety analyses data set. The ITT database included data from all randomized patients. Efficacy analyses were conducted on a per protocol basis. The per protocol database (n=270) was defined as a subset of the ITT database (n=340), excluding patients from sites that inadvertently received potentially identifying laboratory data from the central laboratory vendor. This is referred to as the efficacy analyses data set. Analyses of mean change from baseline to end-point included only patients who had a baseline and a postbaseline measurement.
The primary efficacy variable was the change from baseline to each visit in ADHD-RS-IV-Parent:Inv total score. The primary analysis was a restricted maximum likelihood-based, mixed-model repeated measures analysis of change from baseline in ADHD total score. The model included fixed class effects of treatment, investigative site, prior stimulant use status, visit, and treatment-by-visit interaction, as well as continuous, fixed covariates of baseline ADHD-RS-IV-Parent: Inv total score and baseline score-by-visit interaction. Dunnett's adjustment was used to control the overall type I error rate when comparing each of the two dose arms (edivoxetine 0.2 mg/kg/day and edivoxetine 0.3 mg/kg/day) with placebo. For the secondary analyses, the same mixed-model repeated measures analysis model was used to analyze continuous variables.
Response rates were analyzed using a Cochran–Mantel–Haenszel test controlling for investigator. The log rank test was used to compare the time-to-response between each treatment arm with placebo. Fisher's exact test was used to analyze categorical changes in safety measures.
Edivoxetine pharmacokinetic sampling and analysis
Blood samples were collected from each patient at week 2 and weeks 4–8. The time of the blood sample collection was recorded along with the time the dose was taken on that day, which could be obtained from an appropriate source, such as a parent or guardian. Edivoxetine plasma concentration data were statistically summarized and illustrated graphically.
Edivoxetine plasma sample bioanalysis
Plasma samples were analyzed at Covance Laboratories Inc. (Madison, WI) for edivoxetine using a validated liquid chromatography with tandem mass spectrometric detection method. The lower limit of quantification was 0.20 ng/mL, and the upper limit of quantification was 100.00 ng/mL. Samples above the limit of quantification were diluted and reanalyzed to yield results within the calibrated range. The interassay accuracy during validation ranged from -3.5% to 2.7%. The interassay precision (% relative standard deviation) during validation ranged from 1.4% to 4.5%. Edivoxetine was stable for up to 364 days when stored at ∼ -20°C and -70°C. Samples collected from patients receiving placebo or OROS MPH were not analyzed to determine OROS MPH or edivoxetine plasma concentrations.
Results
Patients
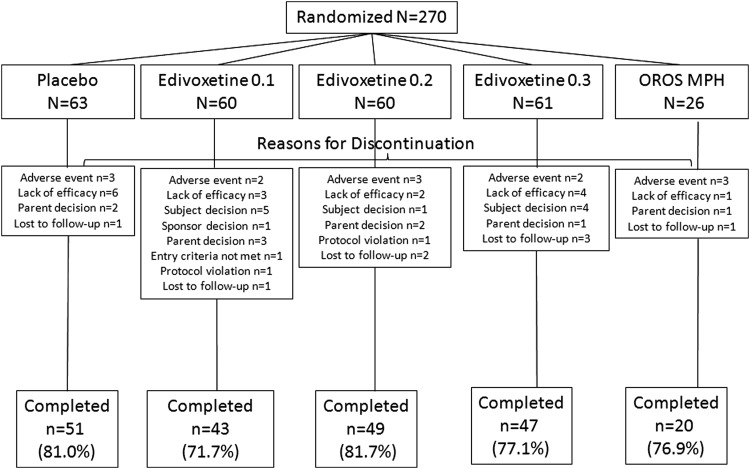
A total of 448 patients were screened for this study, and 340 patients who met the inclusion criteria were randomized to placebo (n=78), edivoxetine 0.1 mg/kg/day (n=76), edivoxetine 0.2 mg/kg/day (n=75), edivoxetine 0.3 mg/kg/day (n=75), and OROS MPH (n=36). A total of 268 patients completed the acute phase of the study (placebo=80.8%, edivoxetine 0.1 mg/kg/day=75.0%, edivoxetine 0.2 mg/kg/day=78.7%, edivoxetine 0.3 mg/kg/day=80.0%, OROS MPH=80.6%), and 72 patients discontinued the study (reasons for discontinuation are shown in Fig. S1) (see online supplementary material at http://www.liebertonline.com/jcap). A significantly greater number of patients discontinued because of subject decision in the edivoxetine 0.1 mg/kg/day arm than in the placebo arm (p=0.027). The patient disposition in the efficacy analyses data set is shown in Figure 2.
FIG. 2.
Patient disposition in the efficacy analyses data set (potentially unblinded patients excluded). 0.1=0.1 mg/kg/day; 0.2=0.2 mg/kg/day; 0.3=0.3 mg/kg/day; OROS MPH=osmotic-release oral system methylphenidate; n/N=number of patients.
Table 1 summarizes patient demographics and baseline characteristics for all randomized patients. Overall, 70.6% of randomized patients were male, and the majority of patients were Caucasian (72.6%). The mean age of randomized patients was 11.6 years and the mean age at first episode of ADHD was 4.73 years. Of the 340 enrolled patients, 70.9% of patients reported a current episode of ADHD combined subtype, 4.1% reported hyperactive/impulsive subtype, and 25% reported inattentive subtype. Less than 20% of patients presented with a current episode of oppositional defiant disorder; 44% of placebo-treated patients, 47% of edivoxetine-treated patients in the 0.1 mg/kg/day arm, and 49% of edivoxetine-treated patients in each of the 0.2 mg/kg/day and 0.3 mg/kg/day arms had used stimulants previously.
Table 1.
Patient Demographics and Baseline Illness Characteristics (All Intent-to-Treat Patients)
| Placebo n=78 | Edivoxetine 0.1 n=76 | Edivoxetine 0.2 n=75 | Edivoxetine 0.3 n=75 | OROS MPHa n=36 | |
|---|---|---|---|---|---|
| Age (years) mean | 11.4 | 11.9 | 12.6 | 11.5 | 9.9 |
| Sex (% male) | 67.9 | 68.4 | 70.7 | 73.3 | 75.0 |
| Race (% white) | 76.9 | 72.4 | 76.0 | 66.7 | 69.4 |
| Prior psychostimulant use (% yes) | 43.6 | 47.4 | 49.3 | 49.3 | 0.0 |
| ADHD subtype (%) | |||||
| Combined | 71.8 | 65.8 | 66.7 | 73.3 | 83.3 |
| Hyperactive/Impulsive | 7.7 | 5.3 | 2.7 | 2.7 | 0 |
| Inattentive | 20.5 | 28.9 | 30.7 | 24.0 | 16.7 |
| Age of onset ADHD symptoms (years) mean | 4.8 | 4.8 | 4.9 | 4.7 | 4.4 |
| Age of initial ADHD diagnosis (years) mean | 8.9 | 9.2 | 9.5 | 9.1 | 8.9 |
| ODD present (% yes)b | 20.5 | 17.1 | 16.0 | 20.0 | 27.8 |
| Conduct disorder present (% yes)b | 2.6 | 2.6 | 1.3 | 2.7 | 0 |
| MDD present (% yes)b | 0 | 1.3 | 0 | 0 | 2.8 |
| GAD present (% yes)b | 1.3 | 2.6 | 1.3 | 1.3 | 0 |
Included only stimulant-naïve patients.
From K-SADS-PL.
0.1=0.1 mg/kg/day; 0.2=0.2 mg/kg/day; 0.3=0.3 mg/kg/day.
ADHD, attention deficit/hyperactivity disorder; GAD, generalized anxiety disorder; K-SADS-PL, Kiddie Schedule for Affective Disorders and Schizophrenia for School Aged Children– Present and Lifetime; ODD, oppositional defiant disorder; MDD, major depressive disorder; OROS MPH, osmotic-release oral system methylphenidate.
Efficacy
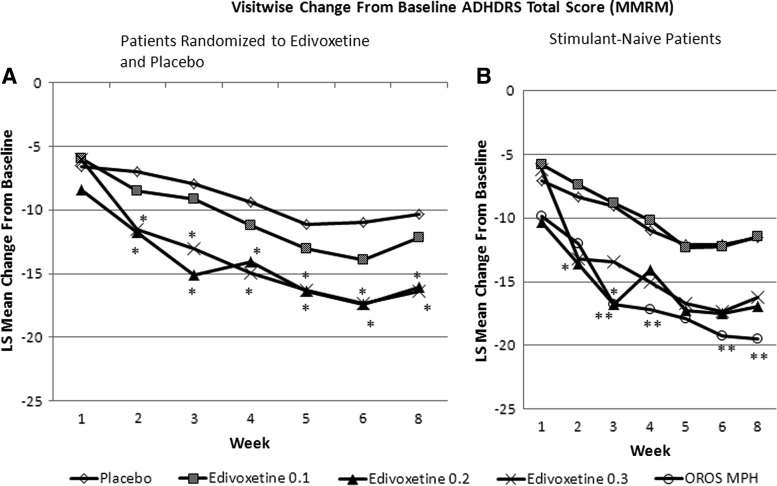
In the overall efficacy analyses data set, edivoxetine at both 0.2 mg/kg/day and 0.3 mg/kg/day demonstrated statistically significantly greater mean reductions from baseline ADHD-RS-IV-Parent:Inv total score relative to placebo at week 8 (Fig. 3A). Both the edivoxetine 0.2 mg/kg/day and 0.3 mg/kg/day dose groups demonstrated a statistically significantly greater mean reduction than did the placebo group at week 2, and these differences were maintained at all subsequent visits. In the stimulant-naïve group of patients, the OROS MPH arm demonstrated a statistically significantly greater mean reduction from baseline ADHDRS-IV-Parent:Inv total score compared with the placebo arm at week 3, week 4, week 6, and week 8 (Fig. 3B). In the stimulant-naïve stratum, the effect size estimates for edivoxetine doses 0.1 mg/kg/day, 0.2 mg/kg/day and 0.3 mg/kg/day, and OROS MPH at the week 8 time point were −0.02, 0.47, 0.41, and 0.69, respectively. In the overall efficacy analyses data set, the effect size estimates for edivoxetine doses 0.1 mg/kg/day, 0.2 mg/kg/day, and 0.3 mg/kg/day at the week 8 time point were 0.17, 0.51, and 0.54, respectively.
FIG. 3.
Primary efficacy measure in the efficacy analyses data set: Baseline-to-endpoint change in ADHD-RS-IV-Parent:Inv total score according to per-protocol analysis of patients with previous stimulant exposure (A: left panel) and stimulant-naive patients (B: right panel). *p<0.05 edivoxetine vs. placebo; **p<0.05 methylphenidate (MPH) vs. placebo. 0.1=0.1 mg/kg/day; 0.2=0.2 mg/kg/day; 0.3=0.3 mg/kg/day; ADHDRS-IV-Parent:Inv=Attention-Deficit/Hyperactivity Disorder Rating Scale-IV-Parent Version: Investigator Administered and Scored; LS=least squares; MMRM=mixed model repeated measures; OROS MPH=osmotic-release oral system methylphenidate.
Mean change from baseline to each visit ADHD-RS-IV-Parent:Inv total score was conducted by age subgroup (age<12 years vs. age ≥12 years). There was no significant age subgroup-by-treatment interaction for the ADHD-RS-IV-Parent:Inv total score.
In the efficacy analyses data set, response rates using a criterion of ADHD-RS-IV-Parent:Inv total score ≤60% of baseline (i.e., 40% reduction from the baseline score), were statistically significantly greater in the edivoxetine 0.2 mg/kg/day arm than in the placebo arm (edivoxetine 0.2 mg/kg/day: 56.67%; placebo: 34.92%; p=0.007), whereas there were no significant differences between the 0.3 mg/kg/day arm and the placebo arm. In the stimulant-naïve group of patients, none of the active treatment arms, including the OROS MPH arm, were statistically significantly different from the placebo arm.
The median time-to-response for the edivoxetine 0.2 mg/kg/day and 0.3 mg/kg/day treatment arms were significantly shorter relative to the placebo arm (time [days] for 50% of patients to respond [reach 40% reduction in symptoms]: placebo=59 days, edivoxetine 0.2 mg/kg/day=29 days, edivoxetine 0.3 mg/kg/day=28 days). The p value for the overall comparison of the three survival curves was 0.012. In the stimulant-naïve group of patients, median times to reach response using the 40% reduction from baseline on the ADHD-RS-IV-Parent:Inv total score response criterion were numerically shorter for the edivoxetine 0.2 mg/kg/day, edivoxetine 0.3 mg/kg/day, and OROS MPH treatment arms than for the placebo arm (time [days] for 50% of patients to respond [reach 40% reduction in symptoms]: placebo=46 days, edivoxetine 0.2 mg/kg/day=22 days, edivoxetine 0.3 mg/kg/day=23 days, OROS MPH=22 days). These differences approached but did not reach statistical significance (p=0.089, p=0.059 and p=0.050, respectively).
The edivoxetine 0.2 mg/kg/day and 0.3 mg/kg/day arms demonstrated a significantly greater reduction from baseline than did the placebo arm on the ADHD-RS-IV-Parent:Inv inattention and hyperactivity/impulsivity subscale scores (Table 2). The CGI-ADHD-I scores at end-point for the edivoxetine 0.2 mg/kg/day and 0.3 mg/kg/day arms were significantly lower relative to the placebo arm (lower score indicating greater clinical improvement). For the CGI-ADHD-S score analysis, the edivoxetine 0.2 mg/kg/day arm demonstrated a significantly greater mean reduction from baseline than did placebo. The edivoxetine 0.3 mg/kg/day arm demonstrated a significantly greater mean reduction from baseline to week 8 than the placebo arm in the Conners CBRS ADHD hyperactivity/impulsivity and inattentive subscale scores. The edivoxetine 0.3 mg/kg/day arm had a significantly greater mean reduction than did the placebo arm in the Conners CBRS academic difficulty total standard score. For response defined as an end-point CGI-ADHD-I score ≤2, the edivoxetine 0.2 mg/kg/day arm experienced a statistically significantly greater response rate than the placebo arm.
Table 2.
Secondary Efficacy Measures: Efficacy Analyses Data Seta
| Placebo n=63 | Edivoxetine 0.1 n=58 | Edivoxetine 0.2 n=60 | Edivoxetine 0.3 n=59 | OROS MPHb n=26 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | Change at end-point | Baseline | Change at end-point | Baseline | Change at end-point | Baseline | Change at end-point | Baseline | Change at end-point | |
| ADHD-RS-IV-Parent:Inv | ||||||||||
| Total score | 38.0 | −10.4 | 39.9 | −12.2 | 38.1 | −16.1* | 40.2 | −16.4* | 40.1 | −19.5† |
| Inattention | 20.7 | −5.6 | 22.8 | −6.2 | 21.8 | −8.6* | 22.0 | −9.1* | 21.9 | −10.5† |
| Hyperactivity-Impulsivity | 17.3 | −4.9 | 17.2 | −5.9 | 16.3 | −7.5* | 18.2 | −7.3* | 18.3 | −9.0† |
| CGI-ADHD-I | − | 3.1 | − | 3.0 | − | 2.5* | − | 2.5* | − | 2.3 |
| CGI-ADHD-S | 4.8 | −1.1 | 4.8 | −1.0 | 4.7 | −1.5* | 4.9 | −1.4 | 4.8 | −1.7 |
| CBRS | ||||||||||
| Hyperactive-Impulsive | 81.1 | −8.7 | 81.8 | −6.3 | 80.3 | −12.9 | 83.2 | −15.7* | 79.0 | −12.4 |
| Inattentive | 80.4 | −10.7 | 81.9 | −8.3 | 80.5 | −11.0 | 82.6 | −17.0* | 85.5 | −18.3 |
| Academic difficulty | 70.3 | −6.1 | 72.6 | −6.1 | 71.0 | −5.9 | 72.9 | −12.5* | 74.4 | −12.1 |
| Response: CGI-ADHD-I ≤2 (%) | 31.8 | 27.1 | 55.0* | 44.1 | 50.0 | |||||
| Response Rate (40% Reduction from baseline in ADHDRS-IV-Parent:Inv score) | 34.9 | 32.8 | 56.7* | 47.5 | 53.9 | |||||
Change scores expressed as least-squares means.
Patients who received OROS MPH were in the stimulant-naïve strata only.
p<0.05 vs. placebo; †p<0.05 vs. the stimulant-naïve placebo group.
0.1=0.1 mg/kg/day; 0.2=0.2 mg/kg/day; 0.3=0.3 mg/kg/day.
ADHD, attention deficit/hyperactivity disorder; ADHD-RS, ADHD rating scale; CBRS, Conners Comprehensive Behavior Rating Scales; CGI-ADHD-I, Clinical Global Impressions-Attention-Deficit/Hyperactivity Disorder-Improvement; CGI-ADHD-S, Clinical Global Impression-Attention-Deficit/Hyperactivity Disorder-Severity; OROS MPH, osmotic-release oral system methylphenidate.
In the stimulant-naïve group of patients, the OROS MPH arm demonstrated a statistically significantly greater mean reduction in the ADHD-RS-IV-Parent:Inv total score, hyperactivity/impulsivity subscale score, and the inattention subscale score (Table 2).
For the Conners CBRS ADHD symptom scores in the efficacy analyses data set, there was significant improvement in the Conners CBRS oppositional defiant disorder and generalized anxiety symptom scores for the edivoxetine 0.3 mg/kg/day arm compared with the placebo arm (Table 3).
Table 3.
| Placebo (n=63) | Edivoxetine 0.1 (n=58) | Edivoxetine 0.2 (n=60) | Edivoxetine 0.3 (n=59) | OROS MPHc (n=26) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Comorbid symptoms measure (CBRS) | Baseline | Change at end-point | Baseline | Change at end-point | Baseline | Change at end-point | Baseline | Change at end-point | Baseline | Change at end-point |
| ODD | 69.1 | −7.2 | 72.0 | −4.0 | 66.9 | −10.2 | 71.1 | −12.3* | 70.5 | −13.8 |
| Generalized anxiety | 71.9 | −9.1 | 73.8 | −5.3 | 71.4 | −11.1 | 73.3 | −15.4* | 70.4 | −8.8 |
| Separation anxiety | 55.9 | −6.7 | 58.7 | −1.1* | 60.5 | −5.9 | 57.8 | −8.6 | 59.4 | −2.9 |
| Conduct disorder | 61.1 | −4.6 | 65.8 | −2.2 | 58.3 | −6.3 | 62.7 | −7.6 | 58.8 | −7.1 |
| Manic episode | 76.1 | −9.4 | 77.3 | −5.0 | 73.6 | −12.6 | 78.4 | −12.4 | 75.8 | −11.9 |
| Social phobia | 58.3 | −6.8 | 59.8 | −2.2* | 58.8 | −6.1 | 60.1 | −10.0 | 61.3 | −3.1 |
| Major depressive episode | 69.6 | −10.3 | 71.9 | −5.4* | 69.4 | −9.6 | 69.8 | −13.7 | 67.0 | −1.1 |
Change scores expressed as least-squares means.
CBRS symptom scores are expressed as t scores, based on age and gender norms.
Patients who received OROS MPH were in the stimulant-naïve strata only.
p<0.05 vs. placebo
0.1=0.1 mg/kg/day; 0.2=0.2 mg/kg/day; 0.3=0.3 mg/kg/day.
CBRS, Conners Comprehensive Behavior Rating Scales; MMRM, mixed model repeated measures; ODD, oppositional defiance disorder; OROS MPH, osmotic-release oral system methylphenidate.
Safety and tolerability
In the safety analyses data set, among study patients who were exposed to edivoxetine, the overall mean duration of exposure was 50.66 days and total exposure to edivoxetine was 31.07 patient years. The edivoxetine and placebo treatment arms did not differ in the number of patients who reported at least one treatment-emergent adverse event (TEAE) (p>0.050). The TEAEs experienced by ≥5% of edivoxetine-treated patients and by at least twice that in placebo-treated patients are shown in Table 4. The majority of TEAEs were mild to moderate in severity. There were no serious adverse events or deaths reported in this trial. There were no significant differences between any of the edivoxetine treatment arms and placebo in the number of patients who discontinued the study because of an adverse event. TEAEs in the efficacy analyses data set are shown in Table S1(see online supplementary material at http://www.liebertonline.com/jcap).
Table 4.
Treatment-Emergent Adverse Events and Vital Signs: Safety Analyses Data Set
| Treatment-emergent adverse events (occurring in ≥5% of patients in any edivoxetine group or OROS MPH group and twice the rate in placebo group) | |||||
|---|---|---|---|---|---|
| Placebo (%) n=78 | Edivoxetine 0.1 (%) n=76 | Edivoxetine 0.2 (%) n=75 | Edivoxetine 0.3 (%) n=75 | OROS MPHa (%) n=36 | |
| Upper abdominal pain | 9.0 | 18.4 | 4.0 | 14.7 | 22.2 |
| Nausea | 5.1 | 6.6 | 17.3* | 13.3 | 8.3 |
| Vomiting | 3.8 | 9.2 | 12.0 | 13.3* | 2.8 |
| Decreased appetite | 3.8 | 7.9 | 13.3* | 10.7 | 47.2* |
| Irritability | 5.1 | 13.2 | 9.3 | 6.7 | 16.7 |
| Somnolence | 6.4 | 5.3 | 18.7* | 5.3 | 0.0 |
| Sedation | 1.3 | 5.3 | 9.3* | 5.3 | 8.3 |
| Increased heart rate | 0.0 | 2.6 | 0.0 | 5.3 | 2.8 |
| Diarrhea | 2.6 | 6.6 | 4.0 | 1.3 | 0.0 |
| Aggression | 1.3 | 1.3 | 2.7 | 2.7 | 5.6 |
| Altered mood | 1.3 | 1.3 | 4.0 | 2.7 | 5.6 |
| Affect lability | 0.0 | 0.0 | 0.0 | 1.3 | 5.6 |
| Cough | 5.1 | 3.9 | 1.3 | 2.7 | 11.1 |
| Fatigue | 3.8 | 3.9 | 4.0 | 4.0 | 11.1 |
| Initial insomnia | 2.6 | 2.6 | 1.3 | 1.3 | 5.6 |
| Insomnia | 6.4 | 6.6 | 5.3 | 2.7 | 19.4 |
| Depressed mood | 0.0 | 0.0 | 0.0 | 0.0 | 5.6 |
| Gastroenteritis | 0.0 | 0.0 | 0.0 | 0.0 | 5.6 |
| Oropharyngeal pain | 2.6 | 2.6 | 4.0 | 0.0 | 5.6 |
| Sleep disorder | 0.0 | 0.0 | 1.3 | 0.0 | 11.1* |
| Weight decreased | 0.0 | 0.0 | 1.3 | 0.0 | 11.1* |
| Vital signs, weight, and height | |||||
|---|---|---|---|---|---|
| LS mean change from baseline to week 8 | |||||
| Weight (kg) | 1.3 | −0.1* | 0.2* | −0.3* | −1.85† |
| Height (cm) | 0.7 | 0.8 | 0.7 | 0.6 | 0.9 |
| Pulse (bpm) sitting | −1.2 | 7.0* | 12.0* | 11.9* | 4.49† |
| Diastolic BP (mmHg) sitting | −1.1 | 5.8* | 7.0* | 5.2* | 2.93 |
| Systolic BP (mmHg) sitting | 0.7 | 4.6* | 5.7* | 5.1* | 2.75 |
| Percentage of patients with potentially clinically significant QTc changes at any time | |||||
|---|---|---|---|---|---|
| QTc Fridericia's >30 ms increase | 1.3 | 1.4 | 5.6 | 0.0 | 2.9 |
| QTc Fridericia's >450 ms increase | 0.0 | 1.4 | 1.4 | 0.0 | 0.0 |
| Percentage of patients with a categorical shift in BP | ||||||
|---|---|---|---|---|---|---|
| Sitting diastolic BP increase ≥5 mmHg and ≥95th percentile | At end-point | 1.3 | 8.1 | 9.6* | 4.1 | 5.7 |
| At any time | 10.3 | 18.9 | 26.0* | 23.3* | 11.4 | |
| Sitting systolic BP increase ≥5 mmHg and ≥95th percentile | At end-point | 6.4 | 8.1 | 9.6 | 2.7 | 2.9 |
| At any time | 16.7 | 21.6 | 30.1 | 17.8 | 25.7 | |
Patients who received OROS MPH were in the stimulant-naïve strata only.
p<0.05 vs. placebo; †p<0.05 vs. the stimulant-naïve placebo group.
0.1=0.1 mg/kg/day; 0.2=0.2 mg/kg/day; 0.3=0.3 mg/kg/day.
BP, blood pressure; LS, least squares; OROS MPH, osmotic-release oral system methylphenidate; QTc, corrected QT interval.
Statistically significant differences relative to placebo were observed for all edivoxetine dose arms with respect to changes in weight. Patients treated with placebo experienced a mean increase in weight from baseline to end-point, whereas those treated with edivoxetine experienced a smaller increase or slight decreases in weight (Table 4). No clinically meaningful differences between the edivoxetine and placebo treatment arms were observed with respect to changes in clinical laboratory measures. All the edivoxetine dose arms demonstrated statistically significantly greater mean increases in sitting heart rates, and sitting systolic and diastolic blood pressure, than the placebo arm (Table 4). A statistically significantly greater percentage of patients treated with edivoxetine 0.2 mg/kg/day or 0.3 mg/kg/day met criteria for a categorical shift to an increased diastolic blood pressure (increase of ≥5 mm Hg and were ≥95th percentile [NHBPEPWG 2004]) at any time relative to the placebo arm. For the edivoxetine 0.2 mg/kg/day arm, the percentage of patients meeting these criteria at end-point was also significantly greater than for placebo (Table 4). There were no statistically significant differences between the edivoxetine dose arms and the placebo arm in the frequency of potentially clinically significant changes in the QTc interval (Table 4). The TEAEs and vital signs data for the stimulant-naïve group of patients are shown in Table S2 (see online supplementary material at http://www.liebertonline.com/jcap).
In the safety analyses data set, for the C-SSRS, no patients in any group reported suicidal behavior. There were eight patients in the edivoxetine group who reported suicidal ideation, with no significant difference between any of the treatment groups, compared with placebo.
Plasma concentrations of edivoxetine
A summary of the edivoxetine plasma concentrations in children and adolescents is shown in Table 5. Included in these analyses are patients who had evaluable edivoxetine plasma concentration data and time-related dosing information. Across the targeted dose groups, individual patients received edivoxetine doses ranging from 2 to 18 mg. Patients randomized to 0.1 mg/kg/day could receive the lowest dose of 2 mg, and patients randomized to the 0.3 mg/kg/day arm could receive the highest dose of 18 mg, which depended upon their body weight. Pediatric blood collections ranged from about <1 hour to 36 hours after edivoxetine was administered, with a median time of 7.72–9.42 hours, depending upon the targeted dose group. The median edivoxetine plasma concentrations increased as the target dose level increased, indicating a relationship between dose administered and exposure; however, there was overlap of the plasma concentrations most notably between the 0.2 mg/kg/day and 0.3 mg/kg/day groups. No discernable difference in plasma concentrations was noted between children and adolescents.
Table 5.
Edivoxetine Dose and Plasma Concentration: Time Summary Statistics in Pediatric Patients
| Adolescents | Children | |||||
|---|---|---|---|---|---|---|
| Targeted dose group (mg/kg) | 0.1 | 0.2 | 0.3 | 0.1 | 0.2 | 0.3 |
| Actual dose range (mg/kg) | 0.080–0.105 | 0.0849–0.217 | 0.162–0.309 | 0.080–0.107 | 0.161–0.212 | 0.162–0.319 |
| Actual dose range (mg) | 3–6 | 5–12 | 6–18 | 2–6 | 4–12 | 4–18 |
| N | 30 | 37 | 30 | 35 | 31 | 34 |
| Plasma Concentration (ng/mL) | ||||||
| n | 119 | 146 | 124 | 133 | 125 | 143 |
| Mean | 6.90 | 19.3 | 25.8 | 7.07 | 15.3 | 22.3 |
| SD | 4.96 | 14.2 | 20.5 | 5.04 | 12.3 | 14.4 |
| Minimum | 0.24 | 0.46 | 0.41 | 0.23 | 0.37 | 0.46 |
| Median | 6.01 | 16.61 | 21.00 | 6.03 | 12.69 | 18.80 |
| Maximum | 23.56 | 97.97 | 113.18 | 21.15 | 63.48 | 67.86 |
| Blood sampling time (h)a | ||||||
| Minimum | 1.42 | 0.67 | 0.80 | 0.17 | 1.25 | 0.15 |
| Median | 9.25 | 7.72 | 8.48 | 7.75 | 9.42 | 8.00 |
| Maximum | 35.00 | 32.78 | 36.00 | 35.12 | 33.83 | 32.67 |
Blood sample collection time relative to edivoxetine dose administration.
0.1=0.1 mg/kg/day; 0.2=0.2 mg/kg/day; 0.3=0.3 mg/kg/day.
N, number of patients; n, number of plasma concentrations; SD, standard deviation.
Discussion
The results from this study demonstrate that edivoxetine at doses of 0.2 mg/kg/day and 0.3 mg/kg/day was effective in reducing ADHD symptom severity during the 8 week treatment period. Edivoxetine-treated patients given 0.2 mg/kg/day and 0.3 mg/kg/day had significantly greater improvement than did placebo-treated patients in the primary outcome measure; mean change in ADHD-RS-IV-Parent:Inv total score. This outcome was supported by positive findings on various secondary outcome measures. There was a significant improvement in edivoxetine- than in placebo-treated patients on both the hyperactivity/impulsiveness and inattentive subscale scores of the ADHD-RS-IV-Parent:Inv and on the CGI-ADHD-I score. The response rate was significantly higher for patients treated with edivoxetine 0.2 mg/kg/day than for placebo-treated patients. The edivoxetine 0.2 mg/kg/day and 0.3 mg/kg/day arms reached the 40% reduction in ADHD-RS-IV-Parent:Inv total score response criterion in a significantly shorter time than the placebo arm. The efficacy outcomes for the 0.2 mg/kg/day and 0.3 mg/kg/day edivoxetine arms varied depending on the measure, but in general they were similar in magnitude.
Baseline scores on the Conners CBRS indicated substantial comorbid symptomatology in multiple domains, such as oppositional defiant disorder, anxiety, and depression, which did not worsen with edivoxetine in this pediatric ADHD patient population. The most prevalent comorbid psychiatric diagnosis observed in this population was oppositional defiant disorder as diagnosed by investigators using K-SADS-PL, which is consistent with what has been reported in the literature (Hazell 2010). For the other comorbid symptom subscales of the Conners CBRS (i.e., separation anxiety, conduct disorder, manic episode, social phobia, and major depressive episode), there were numerical, although not statistically significant, differences in favor of the edivoxetine-treated arms relative to placebo.
To validate the sensitivity of this study, one treatment arm of the stimulant-naïve patient stratum received OROS MPH. The OROS MPH arm was used as a positive control, rather than a direct comparator. Given the fact that the OROS MPH-treated patients received a maximum dose of 54 mg, it is possible that a small proportion of patients may have been underdosed, because they were not allowed to receive a maximum dose of 72 mg. The OROS MPH-treated patients demonstrated a greater mean reduction in the ADHD-RS-IV-Parent:Inv total score than did the corresponding placebo-treated patients, thus confirming adequate assay sensitivity.
The PK and PD of edivoxetine in pediatric patients were described previously (Kielbasa et al. 2012). Additional PK information was collected in this larger-scale clinical trial to support our initial findings and to build a database that could assist in subsequent population analyses. The descriptive PK analysis contained herein serves as a summary of the exposure in patients from this study, and provides insight related to the safety and efficacy of edivoxetine. The data obtained in this study are in accordance with prior PK results, in which we generally observed exposures that were dose dependent and comparable in children and adolescents.
Clinical insight on the edivoxetine dose – plasma concentration – efficacy relationship was gained from this study. The doses of edivoxetine were based on an approximation of the median body weight within the predefined ranges, and the fixed-dose treatment groups to which the patients were randomized, to mitigate potential safety concerns in this initial larger-scale efficacy trial with pediatric patients. Using this weight-based dosing scheme, median plasma concentrations observed in the groups of patients who received edivoxetine at doses of 0.2 mg/kg/day and 0.3 mg/kg/day were two to three times greater than in those who received the 0.1 mg/kg/day dose (see Table 5). Edivoxetine at doses of 0.2 mg/kg/day and 0.3 mg/kg/day were shown to be efficacious based on the mean ADHD-RS-IV-Parent:Inv total score change, but not at the 0.1 mg/kg/day dose. However, this dosing paradigm contributed to an overlap of the actual dose (mg) administered across the treatment groups, most notably at 0.2 mg/kg/day and 0.3 mg/kg/day, resulting in an overlap of the range of edivoxetine plasma concentrations (see Table 5). A dosing paradigm that provides better separation across treatment groups may be beneficial in further understanding the exposure – efficacy – safety relationship of edivoxetine.
The safety profile of edivoxetine was similar to that seen in a previous study of edivoxetine (Jin et al. 2013), as well as to that seen in studies of drugs with a similar mechanism of action (i.e., inhibition or norepinephrine reuptake) such as atomoxetine, in pediatric patients with ADHD (Michelson et al. 2001). There were no significant differences between treatment arms in the number of patients who discontinued because of an adverse event, and there were no deaths or serious adverse events reported in this study. Although the outcomes for the two higher dose edivoxetine arms were similar in magnitude on various efficacy measures, the edivoxetine 0.2 mg/kg/day dose was also associated with a numerically higher incidence of some adverse events. Compared with placebo, a statistically significantly greater number of patients in the edivoxetine 0.2 mg/kg/day and 0.3-mg/kg/day arms experienced a categorical shift to an increased diastolic blood pressure at any time during the study.
Limitations
Some limitations to this study should be considered. Both continuous and categorical ADHD symptom measures of placebo response in the present study were higher than those observed in previous ADHD trials (Michelson et al. 2002; Matza et al. 2004). This study was conducted at 31 investigative sites. Investigative sites were pooled together for analyses if they had fewer than two patients per treatment arm in any of the three edivoxetine dose arms or the placebo arm with nonmissing change from baseline on ADHD-RS-IV-Parent:Inv total score. As the ADHD-RS-IV-Parent:Inv is an investigator-rated instrument, a large number of sites and raters (because there were fewer patients per site) might lead to greater variability in investigator rating, which could be a potential cause of a higher placebo response in this study. In addition, previous studies have shown a higher placebo response observed by parents than by clinicians or teachers for behavioral assessments in ADHD (Grizenko et al., 2004, 2013). As the investigator-rated ADHD-RS-IV-Parent:Inv instrument was scored based on an interview with the parent and patient, it is possible that this may have contributed to the higher placebo response in this trial. Sample sizes were calculated to provide adequate power for the primary efficacy analysis, but were not adequately powered for direct comparisons between OROS MPH and edivoxetine. The OROS MPH arm was included as a positive control for study validation.
Conclusions
In summary, the results of this study demonstrated that edivoxetine treatment with doses of 0.2 mg/kg/day and 0.3 mg/kg/day is efficacious in reducing ADHD symptom severity as determined by significantly greater reductions in the mean ADHD-RS-IV-Parent:Inv total score than for placebo. The safety profile for edivoxetine treatment in this study was consistent with that reported for other studies of edivoxetine and for drugs with a similar mechanism of action (i.e., inhibition or norepinephrine reuptake) such as atomoxetine, and no unexpected adverse events were identified in this pediatric patient population.
Clinical Significance
This study assessed the efficacy and safety of edivoxetine, a selective norepinephrine reuptake inhibitor, in pediatric patients with ADHD. The findings of this study demonstrate that edivoxetine is efficacious in reducing ADHD symptom severity; the safety profile for edivoxetine treatment was consistent with its pharmacological action on norepinephrine transmission, and no unexpected adverse events were identified in this pediatric patient population.
Supplementary Material
Acknowledgment
The authors acknowledge Tonya Quinlan, B.S. (affiliation: Eli Lilly and Company, Indianapolis, IN) for providing PK analysis support for this manuscript.
Disclosures
Drs. Lin, Jin, Xu, Kielbasa, and Allen are employees and stockholders of Eli Lilly and Company. Dr. D'Souza is an employee of inVentiv Health Company, LLC. Dr. Kratochvil has received grant support from Eli Lilly, the National Institutes of Health (NIH), and Shire; and been a consultant for Quintiles, Theravance, and the United States Food and Drug Administation (FDA); served on data and safety monitoring boards for Seaside, Otsuka, Pfizer, and Neuren; and received royalties from Oxford Press. The author and study statistical expert, Dr. Wen Xu, analyzed the data, and all the authors (Drs. Lin, Kratochvil, Xu, Jin, D'Souza, Kielbasa, and Allen) contributed to the writing of the manuscript.
References
- American Psychiatric Association: Diagnostic and Statistical Manual of Mental Disorders, 4th ed., Text Revision. Washington, DC: American Psychiatric Association; 2000 [Google Scholar]
- Conners CK: Conners Rating Scales - Revised. North Tonawanda, New York: Multi-Health Systems, Inc.; 1997 [Google Scholar]
- Culpepper L: Primary care treatment of attention-deficit/hyperactivity disorder. J Clin Psychiatry 67Suppl 8:51–58, 2006 [PubMed] [Google Scholar]
- Del Campo N, Chamberlain SR, Sahakian BJ, Robbins TW: The roles of dopamine and noradrenaline in the pathophysiology and treatment of attention-deficit/hyperactivity disorder. Biol Psychiatry 69:e145–e157, 2011 [DOI] [PubMed] [Google Scholar]
- DuPaul GJ, Power TJ, Anastopoulos AD, Reid R: ADHD Rating Scale-IV: Checklists, norms, and clinical interpretations. New York, Guilford Press, 1998 [Google Scholar]
- Faries DE, Yalcin I, Harder D, Heiligenstein JH: Validation of the ADHD Rating Scale as a clinician administered and scored instrument. J Atten Disord 5:107–115, 2001 [Google Scholar]
- Grizenko N, Lachance M, Collard V, Lageix P, Baron C, Ben Amor L, Stepanian MT, Mbekou V, Schwartz G, Bellingham J, Joober R: Sensitivity of tests to assess improvement in ADHD symptomatology. Can Child Adolesc Psychiatr Rev 13:36–39, 2004 [PMC free article] [PubMed] [Google Scholar]
- Grizenko N, Rodrigues Pereira RM, Joober R: Sensitivity of scales to evaluate change in symptomatology with psychostimulants in different ADHD subtypes. J Can Acad Child Adolesc Psychiatry 22:153–158, 2013 [PMC free article] [PubMed] [Google Scholar]
- Guy W: ECDEU assessment manual for psychopharmacology, Revised. Rockville, MD: United States Department of Health, Education, and Welfare; 1976 [Google Scholar]
- Hazell P: Review of attention-deficit/hyperactivity disorder comorbid with oppositional defiant disorder. Australas Psychiatry 18:556–559, 2010 [DOI] [PubMed] [Google Scholar]
- Jin L, Xu W, Krefetz D, Gruener D, Kielbasa W, Tauscher–Wisniewski S, Allen AJ: Clinical outcomes from an open-label study of edivoxetine in pediatric patients with attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol 23:200–207, 2013 [DOI] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Williamson D, Ryan N: Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): Initial reliability and validity data. J Am Acad Child Adolesc Psychiatry 36:980–988, 1997 [DOI] [PubMed] [Google Scholar]
- Kielbasa W, Quinlan T, Jin L, Xu W, Lachno DR, Dean RA, Allen AJ: Pharmacokinetics and pharmacodynamics of edivoxetine (LY2216684), a norepinephrine reuptake inhibitor, in pediatric patients with attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol 22:269–276, 2012 [DOI] [PubMed] [Google Scholar]
- Matza LS, Rentz AM, Secnik K, Swensen AR, Revicki DA, Michelson D, Spencer T, Newcorn JH, Kratochvil CJ: The link between health-related quality of life and clinical symptoms among children with attention-deficit hyperactivity disorder. J Dev Behav Pediatr 25:166–174, 2004 [DOI] [PubMed] [Google Scholar]
- Michelson D, Allen AJ, Busner J, Casat C, Dunn D, Kratochvil C, Newcorn J, Sallee FR, Sangal RB, Saylor K, West S, Kelsey D, Wernicke J, Trapp NJ, Harder D: Once-daily atomoxetine treatment for children and adolescents with attention deficit hyperactivity disorder: a randomized, placebo-controlled study. Am J Psychiatry 159:1896–1901, 2002 [DOI] [PubMed] [Google Scholar]
- Michelson D, Faries D, Wernicke J, Kelsey D, Kendrick K, Sallee FR, Spencer T; Atomoxetine ADHD Study Group: Atomoxetine in the treatment of children and adolescents with attention-deficit/hyperactivity disorder: A randomized, placebo-controlled, dose-response study. Pediatrics 108:E83, 2001 [DOI] [PubMed] [Google Scholar]
- Newcorn JH, Kratochvil CJ, Allen AJ, Casat CD, Ruff DD, Moore RJ, Michelson D: Atomoxetine/Methylphenidate Comparative Study Group: Atomoxetine and osmotically released methylphenidate for the treatment of attention deficit hyperactivity disorder: acute comparison and differential response. Am J Psychiatry 165:721–730, 2008 [DOI] [PubMed] [Google Scholar]
- National High Blood Pressure Education Program Working Group (NHBPEPWG) on High Blood Pressure in Children and Adolescents: The Fourth Report on the Diagnosis, Evaluation, and Treatment of High Blood Pressure in Children and Adolescents. Pediatrics 114Suppl 2:555–576, 2004 [PubMed] [Google Scholar]
- Pliszka SR: Comorbidity of attention-deficit/hyperactivity disorder with psychiatric disorder: An overview. J Clin Psychiatry 59Suppl 7:50–58, 1998 [PubMed] [Google Scholar]
- Posner K, Oquendo MA, Gould M, Stanley B, Davies M: Columbia Classification Algorithm of Suicide Assessment (C-CASA): Classification of suicidal events in the FDA's pediatric suicidal risk analysis of antidepressants. Am J Psychiatry 164:1035–1043, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintero J, López–Muñoz F, Alamo C, Loro M, García–Campos N: Reboxetine for ADHD in children non-responders or with poor tolerance to methylphenidate: A prospective long-term open-label study. Atten Defic Hyperact Disord 2:107–113, 2010 [DOI] [PubMed] [Google Scholar]
- Swanson JM: School-based assessments and interventions for ADD students. Irvine, CA: K.C. Publishing, 1992 [Google Scholar]
- Wilens TE: Mechanism of action of agents used in attention-deficit/hyperactivity disorder. J Clin Psychiatry 67Suppl 8:32–38, 2006 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.