Abstract
Background
Radical resection is the primary treatment for rectal cancer. When anastomosis is possible, a temporary ileostomy is used to decrease morbidity from a poorly healed anastomosis. However, ileostomies are associated with complications, dehydration, and need for a second operation. Our purpose was to evaluate the impact of ileostomy related complications on the treatment of rectal cancer.
Methods
A retrospective cohort study of patients who underwent sphincter preserving surgery between January 2005 and December 2010 at a tertiary cancer center. The primary outcome was the overall rate of ileostomy related complications. Secondary outcomes included complications related to ileostomy status, ileostomy closure, anastomotic complications at primary resection, rate of stoma closure, and completion of adjuvant chemotherapy. Statistical analyses were performed with STATA 12.
Results
A total of 294 patients were analyzed, 32% (n=95) were women. Two hundred seventy-one (92%) received neoadjuvant chemoradiation. The median tumor distance from the anal verge was 7 centimeters (interquartile range 5-10). Two hundred eighty-one (96%) underwent stoma closure at a median 7 months (interquartile range 5.4 – 8.3). The most common complication related to readmission was dehydration (n=32, 11%). Readmission within 60 days of primary resection was associated with delay in initiating adjuvant chemotherapy (OR 3.01, 95% CI 1.42-6.38, p=0.004).
Conclusion
Diverting ileostomies created during surgical treatment of rectal cancers are associated with morbidity; however this is balanced against the risk of anastomosis-related morbidity at rectal resection. Given the potential benefit of fecal diversion, patient-oriented interventions to improve ostomy management, particularly during adjuvant chemotherapy, can be expected to yield marked benefits.
Introduction
Anastomotic leakage is one of the most significant complications after resection with anastomosis for rectal cancer. While temporary ileostomies may decrease morbidity associated with anastomotic leaks, they are themselves not risk free. A recent retrospective review of 603 patients with diverting ileostomies identified a 16.9% readmission rate within 60 days post operatively, with dehydration being the most common cause.[1] In addition, a prospective cohort study demonstrated that all patients with diverting ileostomies had a significant decrease in their glomerular filtration rates measured just prior to ileostomy closure compared to just after ileostomy creation.[2] Other ileostomy-related complications such as small bowel obstruction, stoma necrosis, prolapse, or retraction may not only result in readmissions but may also require reoperation. Other less clinically serious complications such as leakage from the stoma appliance and skin irritation may lead to a decrease in the patient's quality of life.
Patients with temporary ileostomies are also at risk for complications associated with a second operation for ileostomy closure. A systematic review of over 6000 patients reported an overall morbidity associated with ileostomy closure of 17.3%.[3] Moreover, while the diverting ileostomy is meant to be temporary, not all patients receive closure; the incidence of a permanent stoma has been reported to be as high as 20%.[4-16] Although much has been published regarding complications associated with ileostomies, data regarding the impact of ileostomies on the multidisciplinary care of the rectal cancer patient are lacking. The purpose of this study is to evaluate the prevalence of ileostomy related complications during treatment of rectal cancer at a comprehensive cancer center and to describe its impact on multidisciplinary care.
Materials and Methods
An institutional review board approved retrospective analysis of prospectively collected data supplemented by chart review was conducted of patients treated for rectal cancer with sphincter preservation from January 2005 to December 2010 at MD Anderson Cancer Center in Houston, TX. All patients who underwent rectal resection with anastomosis and received a planned diverting ileostomy were included. Patients with recurrent rectal tumors, synchronous tumors, non-adenocarcinomatous rectal masses, or multi-visceral resections were excluded. Diverting ileostomies were used in all patients who received rectal resection with anastomosis following neoadjuvant therapy and in the absence of neoadjuvant therapy at the discretion of the operating surgeon. Prior to ileostomy closure, all patients underwent routine imaging of the anastomotic area with Gastrograffin enema and some also had direct endoluminal visualization of the anastomosis. At the time of stoma closure, patients either had closure of skin with staples, closure over a Penrose drain, closure with wicks, or loose interrupted primary closure.
Data were collected regarding baseline demographics, tumor characteristics, operative techniques, and complications associated with primary surgery, stoma closure, and the interval period. Anastomotic leak was defined as any of the following: extravasation of contrast from the anastomosis, presence of a pelvic fluid collection requiring antibiotic therapy with or without percutaneous drainage, signs of peritonitis, or leakage of pus or feces from a pelvic drain. Placement of pelvic drains at the time of primary resection was based on surgeon discretion. Comorbidities were defined as follows: cardiovascular disease including history of myocardial infarction, congestive heart failure, coronary artery bypass or coronary stent placement; cerebrovascular disease including history of transient ischemia or stroke; pulmonary disease including chronic obstructive pulmonary disease; rheumatologic disease including systemic lupus erythematosus, rheumatoid arthritis, polymyositis, systemic sclerosis, and polymyalgia rheumatic; peptic ulcer disease including history of gastric, duodenal, or gastrojejunal ulcers; liver disease including cirrhosis and chronic hepatitis; diabetes; paraplegia/hemiplegia; renal disease including chronic renal failure, nephritis, nephropathy; malignancy included a second primary; history of HIV; any history of tobacco or steroid use at presentation. Patients were classified according to the World Health Organization definition of obesity if their BMI was ≥ 30 kg/m2.
Ileostomy related complications were separated into two categories: complications related to having an ileostomy (or ileostomy status) and complications of ileostomy closure. Complications of ileostomy status included readmission for dehydration and need for stoma revision. Other outcomes that were evaluated included delayed initiation of adjuvant therapy (> 8 weeks after primary resection), failure to complete adjuvant therapy (receiving less than 3 months), and stoma related problems (bleeding, prolapse, etc.). Additionally we collected data regarding complications at primary resection (e.g. anastomotic leak) in order to determine the effect of the ileostomy on primary surgery related complications. Surgical site infections were defined using the Centers for Disease Control definitions.[17]
Statistical analyses were performed using STATA 12 (College Station, TX). Student's t-test was used to evaluate continuous variables, and logistic regression was performed to evaluate categorical variables.
Results
There were a total of 372 patients who underwent rectal resections with anastomosis and ileostomy during the given time period. Of those, 294 patients met our inclusion criteria. Baseline demographic data are presented in Table 1. Most tumors were located in the mid or distal rectum and more than two-thirds of the patients were male. The majority of the patients received neoadjuvant therapy. The median length of stay after resection with anastomosis was 6 days (IQR, 5-8). The median length of follow up was 44.5 months (IQR, 29 – 63). The description of operative approaches is described in Table 2.
Table 1. Baseline patient characteristics.
| Characteristics | N = 294 (%) |
|---|---|
| Age (years ± SD) | 55.7 ± 13.2 |
| Female | 95 (32) |
| Body Mass Index (BMI) | 28 (IQR, 24.6-31) |
| Race | |
| Caucasian | 215 (73) |
| African-American | 19 (6.5) |
| Asian | 21 (7.1) |
| Other | 40 (14) |
| Median length of follow up (months) | 44.5 (IQR, 29-63) |
| Number of Comorbidities | |
| 0 | 72 (24) |
| 1 | 135 (46) |
| 2 | 66 (22) |
| >2 | 17 (5.8) |
| Patient Residence | |
| Harris County (Location of MDACC) | 57 (19) |
| Other Texas Counties | 139 (47) |
| Other US states | 81 (28) |
| International | 17 (6) |
| Median Distance of Tumor from Anal Verge (cm), (inter-quartile range) | 7, (5-10) |
| Proximal (≥10 cm) | 75 (26) |
| Mid (5-9 cm) | 146 (50) |
| Distal (<5 cm) | 62 (21) |
| Stage at presentation | N =262 |
| Stage I | 14 (5.3) |
| Stage II | 86 (29) |
| Stage III | 146 (50) |
| Stage IV | 16 (5.4) |
| Received Neoadjuvant Therapy (%) | 272 (92.5) |
Table 2. Operative characteristics.
| Type of Primary Resection | N = 294 (%) |
|---|---|
| LAR/uLAR | 202 (69) |
| Coloanal | 92 (31) |
| Reservoirs | 24 (8.2) |
| Open | 264 (89) |
| Minimally Invasive | 28 (9.5) |
| Converted | 3 (1) |
| Ileostomy Closure | N = 281 (%) |
| Through ileostomy site | 270 (95) |
| Midline incision | 11 (3.8) |
| Hand-sewn | 21 (7.4) |
| Stapled | 260 (92) |
LAR, Low Anterior Resection; uLAR, Ultralow Anterior Resection
Interval Complications of Ileostomy Status
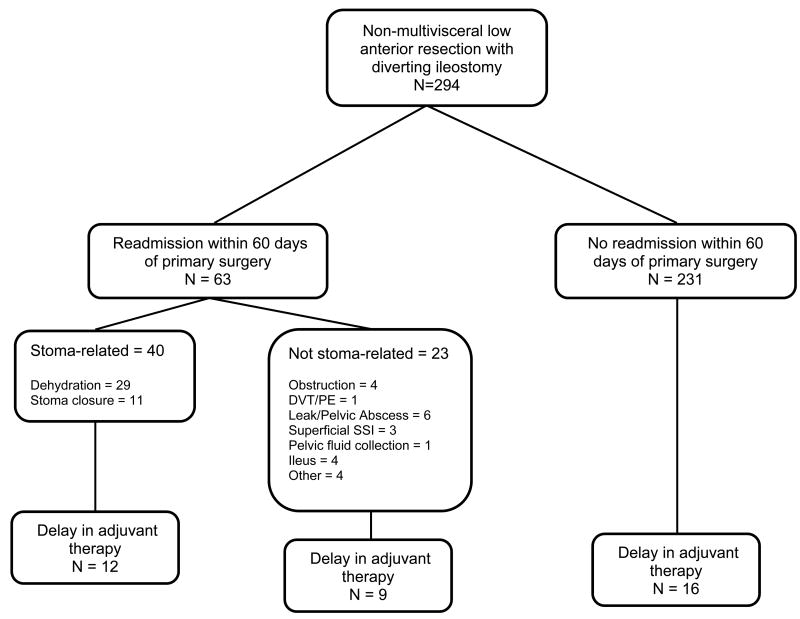
There were 63 patients (21%) with 75 readmissions within 60 days after primary resection prior to stoma closure (see Figure). The most common reason for readmission was dehydration (35 readmissions in 32 patients), and 53% (n = 17) of those patients had at least a 50% increase in their creatinine levels at readmission compared to their pre-operative values. Mean initial LOS (days) after primary resection was not significantly greater in those who were readmitted within 60 days compared to those who were not (7.3 ± 3.3 versus 8.0 ± 5.8, p=0.37).
Figure. Patient flow diagram.
*Delay defined as > 8 weeks after primary surgery
Complications at Ileostomy Closure
Two hundred eighty-one (96%) patients eventually underwent stoma closure at a median 7 months (interquartile range 5.4 – 8.3) after primary tumor resection. The median length of stay (days) at the time of closure was 4 (IQR 3-5). Eleven patients (4%) required laparotomy for closure of the ileostomy. The most common complications were ileus (n=31, 11%) followed by surgical site infection (n=23, 8.2%) and small bowel obstruction (n=10, 5.6%). Five patients (1.8%) required subsequent colostomy due to bowel dysfunction (increased frequency, increased urgency, anorectal pain, perianal excoriation, inability to completely evacuate). The most common wound related reason for late readmission (>60 days) was herniorrhaphy (parastomal n=18, ventral n=12).
Impact on Adjuvant Chemotherapy
Two hundred seventy-two (92.5%) patients received neoadjuvant chemoradiation and 245 of those went on to receive adjuvant chemotherapy. Two hundred sixty-six patients (90.5%) received some adjuvant chemotherapy with 191 (65%) completing at least 3 months. There were 37 (12.5%) patients who had a delay in starting adjuvant chemotherapy (adjuvant therapy started >8 weeks after primary resection). Readmission within 60 days was significantly associated with delay in initiating adjuvant chemotherapy (OR 3.01, 95% CI 1.42-6.38, p=0.004). In fact, one in three readmitted patients incurred a delay and the most common reasons for readmissions were stoma related (63%, n=40). Surgical site infections were the cause of readmission in 9 (14%) and itself did not reach statistical significance as a predictor of delay (OR 1.98, 95% CI 0.95-4.12, p=0.07). Thirty-three patients (13%) had 40 readmissions during their course of adjuvant chemotherapy. There were 36 patients (14%) who failed to complete at least 3 months of adjuvant chemotherapy.
Anastomotic Complications at Primary Resection
Data regarding complications at primary resection were also collected to evaluate the effect of the ileostomy on primary tumor related complications. Perioperative wound complications at primary resection are listed in Table 3. A total of 86 patients (28%) had some type of wound related infectious complication. After primary resection, there were 41 (13.9%) patients with anastomotic leak or pelvic abscess, 16 (39%) of which were asymptomatic leaks identified only on routine imaging prior to ileostomy closure. Of those 41, 7 (17%) required reoperation and 16 (39%) were managed with percutaneous drainage. Male gender was associated with increased risk of anastomotic leak or pelvic abscess (OR 3.9, 95% CI 1.5-10.3, p=0.006). Obesity (body mass index ≥ 30 kg/m2) was significantly associated with development of superficial surgical site infection (OR 2.5, 95% CI 1.34-4.72, p=0.004).The mean LOS (days) was significantly greater for patients who had any surgical site infection compared to those who did not (9.4 ± 7.4 versus 7.3 ± 4.3, p =0.003). Furthermore, having any type of surgical site infection was associated with readmission within 60 days (OR 2.14, 95% CI 1.15-3.98, p=0.016).
Table 3. Perioperative Complications.
| Primary Resection N = 294 (%) | Ileostomy Closure N = 281 (%) | |
|---|---|---|
| Mortality | 1 (0.3) | 1 (0.4) |
| Wound-related | ||
| Superficial/Deep Surgical site infection | 48 (16.3) | 23 (7.8) |
| Enterocutaneous fistula | 1 (0.3) | 2 (0.7) |
| Organ space abscess (excluding pelvic abscess) | 1 (0.3) | 2 (0.7) |
| Anastomosis Related | ||
| Anastomotic Leak (Including Pelvic Abscess) | 41 (13.9) | 4 (1.4) |
| Reoperation due to leak | 7 (2.3) | 3 (1) |
| Percutaneous drainage for leak | 16 (5.4) | 2 (0.7) |
| Anastomotic Stricture/Stenosis | 18 (6.1) | 0 |
| Rectovaginal/Pouch-vaginal fistula | 2 (0.6) | 0 |
Discussion
In this study we evaluated the impact of diverting ileostomies on the multidisciplinary treatment of rectal cancer and observed that stoma-related complications were a major factor with respect to delays in adjuvant chemotherapy initiation. However, while the risk for ileostomy-related morbidity was remarkable, in this high-risk rectal cancer population, fecal diversion was also associated with approximately 40% of anastomosis related complications being asymptomatic, and identified only during routine radiographic interrogation prior to ileostomy reversal. In addition, those patients whose leaks were clinically apparent could typically be managed by relatively low-risk interventions such as percutaneous intervention. These findings suggest that fecal diversion with ileostomy plays an important role in rectal cancer management but additional interventions should be directed at reducing the risk for ileostomy-related morbidity.
In addition to anastomotic complications, this study also evaluated wound-related complications at primary tumor resection and ileostomy reversal. The relatively high rate of superficial surgical site infections at primary resection was unexpected. Among patients who underwent minimally invasive resections, further investigation demonstrated that infectious complications were often associated with the wound in proximity to the ileostomy. In fact among patients who underwent laparoscopy and developed a surgical site infection, at least half were manifest as erythema at the right lateral port site (adjacent to ileostomy). Together the wound and ileostomy related complications had a significant impact on the receipt of adjuvant chemotherapy primarily associated with a delay to initiation. This is a particular concern as delaying initiation of adjuvant chemotherapy by more than two months in Stage III colon cancer patients is associated with a higher mortality.[18] A retrospective review of Stage II and III rectal cancer patients using the Surveillance, Epidemiology, and End Results database used postoperative hospital stay as a surrogate for post-operative complications and found it to be an independent predictor for delay in beginning adjuvant chemotherapy.[19] We found that readmission within 60 days after primary resection was significantly associated with delay in initiation of adjuvant chemotherapy. Since dehydration was the most common reason for readmission, preventing readmissions due to dehydration should be an important priority.
Since there is evidence that diverting ileostomies decrease complications relating to anastomotic leak after rectal resection,[20-22] it is important to evaluate the ways in which ileostomy related morbidity may be reduced. Our findings of risk for dehydration and need for readmission and impact on renal function are consistent with prior reports from diverse reporting centers demonstrating that the challenges are universal and still need further study.[1,2] Perhaps the greatest opportunity for improvement is through interventions to manage the volume of ileostomy output. There are existing guidelines and algorithms regarding treatment of high output stomas. [23,24] For example, one algorithm for treatment of high output stomas (greater than 2000ml/24 hours for 3 or more consecutive days) is broken into 3 stages: 1) identify causes of high output (sepsis, SBO, medications, infectious colitis, etc.); 2) progression through use of medications to decrease output; 3) intravenous fluids to rehydrate the patient. Unfortunately, this algorithm is geared toward treating patients who are already symptomatic, have been admitted, and have been referred to the hospital's “Nutrition Support Team”— earlier intervention would be more optimal. Another obstacle to more widespread use of protocols for treatment of high output stomas is the varying definition of what constitutes “high output,” that ranges from 1,000 ml/24 hours to more than 2,000 mL/24 hours.[25,26] Establishing a clinically meaningful definition of high output may be an important first step towards identifying patients most at risk for dehydration, and designing interventions to prevent and identify dehydration before it progresses to require hospital intervention.
There are several limitations to this study. First, this was a retrospective review at a comprehensive cancer center that draws patients from around the world. In our study 33% of patients were either from another state or from another country. Therefore this may under-represent stoma-related complications that occur at home or at outside hospitals. In addition we defined dehydration as having been admitted for ostomy related dehydration and we were therefore not able to measure the impact of ostomy related complications that did not require readmission. As this was a single center review which may limit the generalizability of the findings. Although imaging prior to ileostomy takedown is routine, it is possible that we did not capture additional subclinical anastomotic leaks that healed prior to planning for stoma closure. However, factors such as immediate complications and their effect on initiation and completion of adjuvant therapy are universal and generalizable. Finally, in this study we did not evaluate long term outcomes data so the effects of readmission, delays in adjuvant chemotherapy, and ileostomy-related morbidity on recurrence and survival cannot be determined at this time.
Diverting ileostomies created during surgical treatment of rectal cancers are associated with a significant risk for ileostomy-related morbidity including dehydration and perioperative complications of stoma closure. However this morbidity may be balanced by the benefit of decreasing anastomosis-related morbidity at rectal resection. Given the potential benefit of fecal diversion, future studies of patient-oriented interventions to improve stoma management, particularly during adjuvant chemotherapy could improve overall outcomes.
Table 4. Interval Ileostomy Related Complications.
| N = 294 (%) | |
|---|---|
| Dehydration | 57 (19.3) |
| Readmissions | 48* (14) |
| Creatinine > =1.5x pre-op Cr | 20 (6.8) |
| Stoma prolapse | 3 (1) |
| Bleeding from stoma | 4 (1.3) |
| Symptomatic Parastomal/Stoma Site hernia | 27 (9.2) |
| Need for stoma revision | 1 (0.3) |
48 separate admissions in 41 patients; Cr, serum creatinine
Synopsis.
We conducted a retrospective cohort study of patients who received sphincter preserving surgery with diverting ileostomy to assess the impact of stoma-related morbidity on treatment of rectal cancer. Stoma-related morbidity was not a significantly associated with delays in adjuvant therapy.
Acknowledgments
Funded in part through an ASCRS Research Foundation Resident Initiation Grant (URP) and National Cancer Institute Research Grant K07-CA133187 (GJC) and Core Grant CA16672 (MDACC).
Footnotes
Presented at the American Society of Colon and Rectal Surgeons meeting San Antonio, TX June 2 -6, 2012
The authors have no relevant commercial disclosures.
References
- 1.Messaris E, Sehgal R, Deiling S, et al. Dehydration is the most common indication for readmission after diverting ileostomy creation. Diseases of the colon and rectum. 2012 Feb;55(2):175–180. doi: 10.1097/DCR.0b013e31823d0ec5. [DOI] [PubMed] [Google Scholar]
- 2.Beck-Kaltenbach N, Voigt K, Rumstadt B. Renal impairment caused by temporary loop ileostomy. International journal of colorectal disease. 2011 May;26(5):623–626. doi: 10.1007/s00384-010-1086-3. [DOI] [PubMed] [Google Scholar]
- 3.Chow A, Tilney HS, Paraskeva P, Jeyarajah S, Zacharakis E, Purkayastha S. The morbidity surrounding reversal of defunctioning ileostomies: a systematic review of 48 studies including 6,107 cases. International journal of colorectal disease. 2009 Jun;24(6):711–723. doi: 10.1007/s00384-009-0660-z. [DOI] [PubMed] [Google Scholar]
- 4.Bakx R, Busch OR, Bemelman WA, Veldink GJ, Slors JF, van Lanschot JJ. Morbidity of temporary loop ileostomies. Digestive surgery. 2004;21(4):277–281. doi: 10.1159/000080201. [DOI] [PubMed] [Google Scholar]
- 5.Carlsen E, Bergan AB. Loop ileostomy: technical aspects and complications. The European journal of surgery = Acta chirurgica. 1999 Feb;165(2):140–143. doi: 10.1080/110241599750007324. discussion 144. [DOI] [PubMed] [Google Scholar]
- 6.Edwards DP, Leppington-Clarke A, Sexton R, Heald RJ, Moran BJ. Stoma-related complications are more frequent after transverse colostomy than loop ileostomy: a prospective randomized clinical trial. The British journal of surgery. 2001 Mar;88(3):360–363. doi: 10.1046/j.1365-2168.2001.01727.x. [DOI] [PubMed] [Google Scholar]
- 7.Fonkalsrud EW, Thakur A, Roof L. Comparison of loop versus end ileostomy for fecal diversion after restorative proctocolectomy for ulcerative colitis. Journal of the American College of Surgeons. 2000 Apr;190(4):418–422. doi: 10.1016/s1072-7515(99)00295-1. [DOI] [PubMed] [Google Scholar]
- 8.Gooszen AW, Geelkerken RH, Hermans J, Lagaay MB, Gooszen HG. Temporary decompression after colorectal surgery: randomized comparison of loop ileostomy and loop colostomy. The British journal of surgery. 1998 Jan;85(1):76–79. doi: 10.1046/j.1365-2168.1998.00526.x. [DOI] [PubMed] [Google Scholar]
- 9.Hallbook O, Matthiessen P, Leinskold T, Nystrom PO, Sjodahl R. Safety of the temporary loop ileostomy. Colorectal disease : the official journal of the Association of Coloproctology of Great Britain and Ireland. 2002 Sep;4(5):361–364. doi: 10.1046/j.1463-1318.2002.00398.x. [DOI] [PubMed] [Google Scholar]
- 10.Khoury GA, Lewis MC, Meleagros L, Lewis AA. Colostomy or ileostomy after colorectal anastomosis?: a randomised trial. Annals of the Royal College of Surgeons of England. 1987 Jan;69(1):5–7. [PMC free article] [PubMed] [Google Scholar]
- 11.Law WL, Chu KW, Choi HK. Randomized clinical trial comparing loop ileostomy and loop transverse colostomy for faecal diversion following total mesorectal excision. The British journal of surgery. 2002 Jun;89(6):704–708. doi: 10.1046/j.1365-2168.2002.02082.x. [DOI] [PubMed] [Google Scholar]
- 12.O'Toole GC, Hyland JM, Grant DC, Barry MK. Defunctioning loop ileostomy: a prospective audit. Journal of the American College of Surgeons. 1999 Jan;188(1):6–9. doi: 10.1016/s1072-7515(98)00267-1. [DOI] [PubMed] [Google Scholar]
- 13.Rullier E, Le Toux N, Laurent C, Garrelon JL, Parneix M, Saric J. Loop ileostomy versus loop colostomy for defunctioning low anastomoses during rectal cancer surgery. World journal of surgery. 2001 Mar;25(3):274–277. doi: 10.1007/s002680020091. discussion 277-278. [DOI] [PubMed] [Google Scholar]
- 14.Senapati A, Nicholls RJ, Ritchie JK, Tibbs CJ, Hawley PR. Temporary loop ileostomy for restorative proctocolectomy. The British journal of surgery. 1993 May;80(5):628–630. doi: 10.1002/bjs.1800800529. [DOI] [PubMed] [Google Scholar]
- 15.Wexner SD, Taranow DA, Johansen OB, et al. Loop ileostomy is a safe option for fecal diversion. Diseases of the colon and rectum. 1993 Apr;36(4):349–354. doi: 10.1007/BF02053937. [DOI] [PubMed] [Google Scholar]
- 16.Williams NS, Nasmyth DG, Jones D, Smith AH. De-functioning stomas: a prospective controlled trial comparing loop ileostomy with loop transverse colostomy. The British journal of surgery. 1986 Jul;73(7):566–570. doi: 10.1002/bjs.1800730717. [DOI] [PubMed] [Google Scholar]
- 17.Horan TC, Gaynes RP, Martone WJ, Jarvis WR, Emori TG. CDC definitions of nosocomial surgical site infections, 1992: a modification of CDC definitions of surgical wound infections. Infection control and hospital epidemiology : the official journal of the Society of Hospital Epidemiologists of America. 1992 Oct;13(10):606–608. [PubMed] [Google Scholar]
- 18.Hershman D, Hall MJ, Wang X, et al. Timing of adjuvant chemotherapy initiation after surgery for stage III colon cancer. Cancer. 2006 Dec 1;107(11):2581–2588. doi: 10.1002/cncr.22316. [DOI] [PubMed] [Google Scholar]
- 19.Cheung WY, Neville BA, Earle CC. Etiology of delays in the initiation of adjuvant chemotherapy and their impact on outcomes for Stage II and III rectal cancer. Diseases of the colon and rectum. 2009 Jun;52(6):1054–1063. doi: 10.1007/DCR.0b013e3181a51173. discussion 1064. [DOI] [PubMed] [Google Scholar]
- 20.Guenaga KF, Lustosa SA, Saad SS, Saconato H, Matos D. Ileostomy or colostomy for temporary decompression of colorectal anastomosis. Cochrane Database Syst Rev. 2007;(1):CD004647. doi: 10.1002/14651858.CD004647.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Matthiessen P, Hallbook O, Rutegard J, Simert G, Sjodahl R. Defunctioning stoma reduces symptomatic anastomotic leakage after low anterior resection of the rectum for cancer: a randomized multicenter trial. Annals of surgery. 2007 Aug;246(2):207–214. doi: 10.1097/SLA.0b013e3180603024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Peeters KC, Tollenaar RA, Marijnen CA, et al. Risk factors for anastomotic failure after total mesorectal excision of rectal cancer. The British journal of surgery. 2005 Feb;92(2):211–216. doi: 10.1002/bjs.4806. [DOI] [PubMed] [Google Scholar]
- 23.Baker ML, Williams RN, Nightingale JM. Causes and management of a high-output stoma. Colorectal disease : the official journal of the Association of Coloproctology of Great Britain and Ireland. 2011 Feb;13(2):191–197. doi: 10.1111/j.1463-1318.2009.02107.x. [DOI] [PubMed] [Google Scholar]
- 24.Nightingale J, Woodward JM. Guidelines for management of patients with a short bowel. Gut. 2006 Aug;(55 Suppl 4):iv1–12. doi: 10.1136/gut.2006.091108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chun LJ, Haigh PI, Tam MS, Abbas MA. Defunctioning loop ileostomy for pelvic anastomoses: predictors of morbidity and nonclosure. Diseases of the colon and rectum. 2012 Feb;55(2):167–174. doi: 10.1097/DCR.0b013e31823a9761. [DOI] [PubMed] [Google Scholar]
- 26.Tilney HS, Sains PS, Lovegrove RE, Reese GE, Heriot AG, Tekkis PP. Comparison of outcomes following ileostomy versus colostomy for defunctioning colorectal anastomoses. World journal of surgery. 2007 May;31(5):1142–1151. doi: 10.1007/s00268-006-0218-y. [DOI] [PubMed] [Google Scholar]