Abstract
Hypoxia is an important factor that contributes to the development of drug-resistant cancer, yet few non-perturbative tools exist for studying oxygen in tissue. While progress has been made in the development of chemical probes for optical oxygen mapping, penetration into poorly perfused or avascular tumor regions remains problematic. Here we report a Click-Assembled Oxygen Sensing (CAOS) nanoconjugate and demonstrate its properties in an in vitro 3D spheroid cancer model. Our synthesis relies on sequential click-based ligation of poly(amidoamine)-like subunits for rapid assembly. Using near-infrared confocal phosphorescence microscopy, we demonstrate the ability of CAOS nanoconjugates to penetrate hundreds of microns into spheroids within hours and show their sensitivity to oxygen changes throughout the nodule. This proof-of-concept study demonstrates a modular approach that is readily extensible to a wide variety of oxygen and cellular sensors for depth-resolved imaging in tissue and tissue models.
Keywords: Cancer, Click chemistry, Dendrimers, Imaging agents, Nanotechnology
Individual lesions are host to heterogeneous microenvironments that vary in their properties and response to drugs. [1, 2] In many cancers, including ovarian cancer (OvCa), drug resistance has been linked to the presence of hypoxia, which has been shown to reduce therapeutic efficacy through a variety of mechanisms. [1]
Overcoming this hypoxia-induced treatment resistance requires model systems capable of recapitulating the complex tumor environment observed in human disease. In vitro tumor models, such as OvCa 3D spheroid cultures, exhibit many of the features of in vivo metastatic lesions, such as the presence of a hypoxic core, while also providing an environment that allows for consistent imaging at the same location before, during, and after therapy. [2–4] This approach enables a cell-by-cell correlation between microenvironmental factors and therapeutic response. The ability to incorporate hypoxia imaging into this platform, however, has been hampered by the absence of cellular-resolution oxygen sensing methods capable of performing depth-resolved imaging studies in in vitro model systems. Phosphorescence lifetime imaging is based on oxygen-dependent quenching of phosphorescence, and is a technique well-suited for the study of spheroid oxygenation. [5] In this approach, the phosphorescence decay time of an exogenous chemical oxygen sensor is measured and related to oxygen concentration through a Stern-Volmer-type relationship. [6] This technique, developed in parallel with novel molecular oxygen sensors such as Oxyphor G2, has been used to quantify in vivo oxygenation in a variety of tissue types, including tumor, brain, retina, and heart, and has been deployed using numerous modalities. [6–8] However, non-perturbative measurements of intracellular oxygen tension deep in tissue and methods for delivering oxygen sensors through hundreds of microns of cellular layers to hypoxic tumor regions have not yet been achieved. Moreover, the molecular oxygen probe Oxyphor G2, while well-characterized and commercially available, is incapable of spontaneously penetrating into cells or multicellular structures (Figure S1). [9]
This work describes the development of oxygen-sensitive CAOS nanoconjugates comprising novel poly(amidoamine)-like dendrons and demonstrates their spheroid-penetrating abilities. [3,4] Our nanoconjugate’s combination of NIR emissivity, lack of toxicity at functional doses, and ability to spontaneously penetrate hundreds of microns into 3D tumor models within hours renders it unique among oxygen sensors, enabling oxygen-sensitive imaging at >100 μm depths using NIR-optimized confocal microscopy. Furthermore, our modular synthetic approach enables the synthesis of higher generation dendrons in days instead of weeks. The CAOS nanoconjugate’s ability to spontaneously penetrate multiple cellular layers overcomes a key barrier to the use of in vitro tumor models as a hypoxia model system, while also enabling a host of other applications such as oxygen-sensitive imaging in poorly- or non-vascularized tissue regions.
Many oxygen sensors are dendrimeric structures, in which an oxygen-sensitive porphyrin core is surrounded by dendritic branches, or dendrons. In protein-free solutions, these dendrons serve to both solubilize the central porphyrin and modulate its dynamic range of oxygen sensitivity. [6] Importantly, in protein-rich environments such as the bloodstream, non-PEGylated dendrimers have been shown to interact extensively with endogenous proteins; this interaction can decrease the rate of oxygen quenching by an order of magnitude or more. [6] We have observed that fourth generation, dye-tagged poly(amido) amine (G4 PAMAM) “starburst” den-drimers were taken up by monolayer OvCa cultures after 40 minutes (Figure S2); the same molecule penetrated throughout >250 μm diameter OvCa 3D spheroids after 3.5 hours at low concentrations (500 nM) with no observable toxicity (Figure S3, Movie S1). PAMAM dendrimers are known to form complexes with proteins such as bovine serum albumin (BSA) and are readily endocytosed. [10,11] We therefore reasoned that a phosphorescent porphyrin coupled to one or more higher-generation (≥G2) PAMAM dendrons would benefit from the cell- and spheroid-penetrating properties of PAMAM while also interacting with cellular proteins, thereby creating an oxygen sensor with a useful dynamic range for biological experiments. [10,11] For this work, we selected Pd-tetracarboxytetrabenzoporphyrin (PdTCTBP) as our phosphorescent porphyrin core because its lifetime properties have been characterized in the literature, its synthesis is well-known, and its near-infrared emission (810 nm) lies in the “optical window” ideal for tissue imaging. Additionally, its spectral profile minimizes overlap with known regions of intense autofluorescence, and it can be excited by readily available laser lines (445 nm and 635 nm). [6]
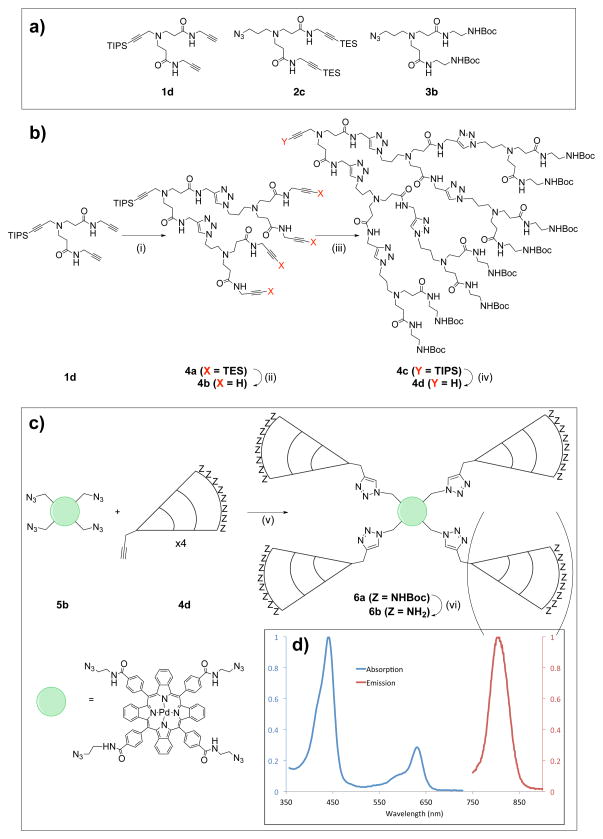
To create a spheroid-permeable oxygen sensor, we developed a click-based strategy to rapidly assemble PAMAM-like subunits and couple them to an oxygen-sensitive porphyrin core, as prior synthetic schemes involving commercially available PAMAM dendrons were found to result in low yields. PAMAM dendrons with azide/alkyne functionality have been reported in the literature, but they problematically make use of extremely slow reactions and require months to synthesize. [12] In contrast, our scheme enables rapid assembly of PAMAM-like dendrons from three branched subunits (Figure 2 and Figure S5). Briefly, it relies on the differing lability of triethylsilyl (TES) and triisopropylsilyl (TIPS) alkyne protecting groups, allowing for sequential subunit ligations while avoiding polymerization (Figure 2, Figure S6): First, the TIPS-protected “starting” subunit 1d is reacted with 2 equivalents of the “intermediate” subunit 2c and catalytic quantities of sodium ascorbate and CuSO4. Following isolation of the reaction product 4a, the TES protecting groups are removed using mild conditions that leave the focal-point TIPS protecting group intact, yielding 4b and preparing the growing dendron for another ligation round. Primary amine functionality can then be added by ligation of “final” subunit 3b to create 4c, the focal group alkyne of which is then deprotected using tetrabutylammonium fluoride to yield 4d. [13] Each dendron growth phase can be conducted overnight, and the product can be isolated without the use of chromatography. This allows higher-generation, triazole-bridged, PAMAM-like dendrons to be synthesized in a matter of days as opposed to months. Once complete, the dendrons can be conjugated to any central moiety in a single convergent step. In this study, we coupled the dendrons to Pd-tetraazidotetrabenzoporphyrin, an azido-functionalized PdTCTBP derivative.
Figure 2.
Generalized overview of the synthetic scheme used in the synthesis of CAOS nanoconjugates. A: Dendron subunits used in the assembly of the PAMAM-like dendron. Numbering refers to the labeling scheme used in the supplemental information; importantly, the TES and TIPS protecting groups differ in their lability. B: Synthesis of G3 PAMAM-like dendrons through sequential click ligation of subunits. i) 1 eq. 1d, 2 eq. 2c, 0.2 eq. CuSO4, 0.4 eq. sodium ascorbate, DMF/H2O, r.t.; ii) 50 eq K2CO3, MeOH, 36h, r.t.; iii) 1 eq. 4b, 4 eq. 3b, 0.2 eq. CuSO4, 0.4 eq. sodium ascorbate, DMF/H2O, r.t.; iv) 5 eq. TBAF, THF, r.t.. C: Overview of the convergent nanoconjugate assembly step. v) 1 eq. 7b, 5 eq 4d, 12 eq. CuSO4, 24 eq. sodium ascorbate, DMF/H2O, r.t.; vi) TFA, DCM, r.t.. D, inset: Normalized absorption and emission spectra of the G3 CAOS nanoconjugate.
Using this sequential click method, we synthesized third-generation (G3) CAOS nanoconjugates from G2 PAMAM-like dendrons. The Pd-tetraazidotetrabenzoporphyrin core is capable of reacting with up to four equivalents of dendrons. In practice, a distribution of adducts was observed. These G3 CAOS nanoconjugates were characterized via NMR and MALDI-TOF MS as being composed of mono-, di- and tri-dendron adducts; NMR integration suggests that, on average, between two and three dendrons are attached to each nanoconjugate (Figures S8, S29–31).
G3 CAOS nanoconjugates were studied in a dense, protein-rich environment and were found to exhibit uniform oxygen sensitivity. To evaluate the photophysical properties of G3 CAOS, we dissolved the nanoconjugate in 2% bovine serum albumin (BSA) and monitored its phosphorescence lifetime as a function of pO2. [6] Oxygen quenching of phosphorescence lifetime is traditionally described using the Stern-Volmer relationship:
| (1) |
Where τ is the exponential lifetime of the phosphor, kq is the quenching coefficient, [pO2] is the partial pressure of dissolved oxygen, and τ0 is the exponential lifetime of the phosphor in the absence of oxygen. [6] kq is a function of the phosphor’s chemical environment and reflects the sensor’s dynamic range; in general, a lower kq is desirable. We compared the kq and τ0 of G3 CAOS to Oxyphor G2 (Figure Figure 3, Table Table 1), which possesses a PdTCTBP core surrounded by second generation glutamate dendrimers. [6, 7] The order of magnitude of the kq observed for G3 CAOS is consistent with that of Oxyphor G2, the calibration is linear, and the dynamic range is sufficient for oxygen measurement across physiological ranges. [6]
Figure 3.
Calibration data for G3 CAOS and the commercially available Oxyphor G2. τ = phosphorescence lifetime (s).
Table 1.
Calibration constants for G3 CAOS and Oxyphor G2. τ0 = phosphorescence lifetime in zero oxygen, kq = phosphorescence quenching constant.
| τ0(μs) | kq(mmHg−1s−1) | |
|---|---|---|
| G3 CAOS | 173 | 377 |
| Oxyphor G2 | 178 | 283 |
Having verified that G3 CAOS nanoconjugates exhibit the desired calibration properties, we next tested their uptake in cells. Given the low quantum yield (< 1%) of PdTCTBPs in ambient oxygen conditions and the low NIR throughput and sensitivity of commercially available microscopes, we first tagged G3 CAOS with AlexaFluor 488. Confocal imaging of OvCa cells treated for 40 minutes with 500 nM AlexaFluor 488-G3 CAOS confirmed uptake and a punctate subcellular localization pattern consistent with lysosomal uptake (Figure S35), as described by previous reports. [11] An MTT viability assay confirmed the lack of toxicity at all doses used (1 μM – 10 μM, Figure S4).
To image the weak, oxygen-dependent phosphorescence of the G3 CAOS nanoconjugates, we built a single-photon counting confocal microscope and optimized its performance for imaging in the 800–900 nm wavelength range corresponding to G3 CAOS’s emission (Figure S34). We used this system to confocally image non-labeled G3 CAOS in monolayer-plated OvCa cells, thereby demonstrating our successful creation of a cell-penetrating oxygen-sensitive nanoconjugate (Figure S35). We also observed the phosphorescence intensity’s inverse dependence on pO2, noting a two- to four-fold increase in signal following a purge of the cell culture dish with nitrogen.
Using this photon-counting NIR confocal microscope, we confirmed that G3 CAOS nanoconjugates penetrate 3D OvCa spheroids. Prior to imaging, spheroids were incubated with 2 μM G3 CAOS for four hours. 3D volume acquisition with confocal optical sectioning revealed that phosphorescence signal could be observed throughout each spheroid at depths exceeding 100 μm, whereas signal was not observed in untreated spheroids (Figure 4, Movie S2). To confirm that the probe is sensitive to oxygen levels, we purged the culture dish with nitrogen, and observed that spheroids exhibited an approximate doubling of phosphorescence signal. To avoid the influence of photobleaching, different spheroids were imaged before and after the nitrogen purge.
Figure 4.
NIR Phosphorescence confocal microscopy imaging reveals that G3 CAOS probes spontaneously penetrate 3D OvCa spheroids. A: 3D OvCa spheroids, no treatment control (bottom/grey: transmission channel). B: False-color image of spheroids treated for 4 hours with 2 μM G3 CAOS, imaged under room air. Legend bar: phosphorescence photon count C: Spheroids treated for 4 hours and imaged after a nitrogen purge. All images were acquired at the approximate midpoint along the z-axis of each spheroid, corresponding to an approximate depth of 100 μm.
In conclusion, we have developed a novel approach for the synthesis of oxygen-sensitive nanoconjugates and provided proof-of-concept that click-assembled nanoconjugates can be used as deep tissue imaging agents for 3D oxygen-sensitive imaging. Our novel assembly strategy does not require the long reaction times typically necessary to achieve higher generation dendrons. Future work in this area will involve the synthesis and testing of a ratiometric CAOS nanoconjugate, which would facilitate more straightforward calibration and quantitative oxygen mapping in the complex cellular environment. Additionally, we chose PdTCTBP for an initial demonstration of the CAOS approach due to the extensive body of work describing its use for quantitative oxygen measurements in biological systems; [6] future work will make use of phosphorescent porphyrins with a higher quantum yield, thereby enabling imaging on less specialized microscopes and reducing the barrier to the use of CAOS-type nanoconjugates in biological experiments.
Supplementary Material
Figure 1.

Acknowledgments
A. Nichols would like to acknowledge the support of an NSF Graduate Research Fellowship and an NDSEG Fellowship. This work was funded by the Air Force Office for Scientific Research (FA9550-13-1-0068) and the National Institutes of Health through the NIH Director’s New Innovator Award Program, Grant No. 1 DP2 OD007096. Information on the New Innovator Award Program can be found at http://nihroadmap.nih.gov/newinnovator/
Contributor Information
Alexander J. Nichols, Wellman Center for Photomedicine, Massachusetts General Hospital, CNY 149-3210, 13th Street, Charlestown, MA 02129 (USA). Harvard University Program in Biophysics, Building C2 Room 112, 240 Longwood Avenue, Boston, MA 02115 (USA). Harvard-MIT Division of Health Sciences and Technology, 77 Massachusetts Avenue E25-519, Cambridge, MA 02139 (USA)
Dr. Emmanuel Roussakis, Wellman Center for Photomedicine, Massachusetts General Hospital, CNY 149-3210, 13th Street, Charlestown, MA 02129 (USA)
Oliver J. Klein, Wellman Center for Photomedicine, Massachusetts General Hospital, CNY 149-3210, 13th Street, Charlestown, MA 02129 (USA)
Prof. Dr. Conor L. Evans, Email: evans.conor@mgh.harvard.edu, Wellman Center for Photomedicine, Massachusetts General Hospital, CNY 149-3210, 13th Street, Charlestown, MA 02129 (USA). Harvard University Program in Biophysics, Building C2 Room 112, 240 Longwood Avenue, Boston, MA 02115 (USA)
References
- 1.Hanahan Douglas, Weinberg Robert A. Hallmarks of cancer: the next generation. Cell. 2011 Mar;144(5):646–74. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 2.Evans Conor L, Abu-Yousif Adnan O, Park Yong Jin, Klein Oliver J, Celli Jonathan P, Rizvi Imran, Zheng Xiang, Hasan Tayyaba. Killing Hypoxic Cell Populations in a 3D Tumor Model with EtNBS-PDT. PloS one. 2011 Jan;6(8):e23434. doi: 10.1371/journal.pone.0023434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roskelley Calvin D, Bissell Mina J. The dominance of the microenvironment in breast and ovarian cancer. Seminars in cancer biology. 2002 Apr;12(2):97–104. doi: 10.1006/scbi.2001.0417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Celli Jonathan P, Rizvi Imran, Evans Conor L, Abu-Yousif Adnan O, Hasan Tayyaba. Quantitative imaging reveals heterogeneous growth dynamics and treatment-dependent residual tumor distributions in a three-dimensional ovarian cancer model. Journal of biomedical optics. 2011;15(5):051603. doi: 10.1117/1.3483903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rumsey WL, Vanderkooi JM, Wilson DF. Imaging of phosphorescence: a novel method for measuring oxygen distribution in perfused tissue. Science. 1988 Sep;241(4873):1649–51. doi: 10.1126/science.241.4873.1649. [DOI] [PubMed] [Google Scholar]
- 6.Vinogradov Sergei A, Wilson David F. Porphyrin dendrimers as biological oxygen sensors. In: Campagna Sebastiano, Ceroni Paola, Puntoriero Fausto., editors. Designing Dendrimers. 1. chapter 12. Wiley; Hoboken, New Jersey: 2012. pp. 463–503. [Google Scholar]
- 7.Dunphy Isolde, Vinogradov Sergei A, Wilson David F. Oxyphor R2 and G2: phosphors for measuring oxygen by oxygen-dependent quenching of phosphorescence. Analytical biochemistry. 2002 Nov;310(2):191–8. doi: 10.1016/s0003-2697(02)00384-6. [DOI] [PubMed] [Google Scholar]
- 8.Babilas Philipp, Liebsch Gregor, Schacht Vivien, Klimant Ingo, Wolfbeis Otto S, Szeimies Rolf-Markus, Abels Christoph. In Vivo Phosphorescence Imaging of pO2 Using Planar Oxygen Sensors. Microcirculation. 2005;12(6):477–487. doi: 10.1080/10739680591003314. [DOI] [PubMed] [Google Scholar]
- 9.Dmitriev Ruslan I, Papkovsky Dmitri B. Optical probes and techniques for O2 measurement in live cells and tissue. Cellular and molecular life sciences: CMLS. 2012 Jun;69(12):2025–39. doi: 10.1007/s00018-011-0914-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Klajnert Barbara, Stanisawska Lidia, Bryszewska Maria, Paecz Bartomiej. Interactions between PAMAM dendrimers and bovine serum albumin. Biochimica et Biophysica Acta (BBA) - Proteins and Pro-teomics. 2003 May;1648(1–2):115–126. doi: 10.1016/s1570-9639(03)00117-1. [DOI] [PubMed] [Google Scholar]
- 11.Sadekar S, Ghandehari H. Transepithelial transport and toxicity of PAMAM dendrimers: implications for oral drug delivery. Advanced drug delivery reviews. 2012 May;64(6):571–88. doi: 10.1016/j.addr.2011.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee Jae Wook, Kim Hee Joo, Han Seung Choul, Kim Ji Hyeon, Jin Sung-Ho. Designing poly(amido amine) dendrimers containing core diversities by click chemistry of the propargyl focal point poly(amido amine) dendrons. Journal of Polymer Science Part A: Polymer Chemistry. 2008 Feb;46(3):1083–1097. [Google Scholar]
- 13.Valverde Ibai E, Delmas Agnès F, Aucagne Vincent. Click à la carte: robust semi-orthogonal alkyne protecting groups for multiple successive azide/alkyne cycloadditions. Tetrahedron. 2009 Sep;65(36):7597–7602. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.