Abstract
Activation of the MAPK cascade in mammalian cell lines positively regulates the G2/M transition. The molecular mechanism underlying this biological phenomenon remains poorly understood. RSK is a key downstream element of the MAPK cascade. Our previous studies established roles of RSK2 in Cdc25C activation during progesterone-induced meiotic maturation of Xenopus oocytes. In this study, we demonstrate that both recombinant RSK and endogenous RSK in Xenopus egg extracts phosphorylate all three isoforms of human Cdc25 at a conserved motif near the catalytic domain. In human HEK293 and PC-3mm2 cell lines, RSK preferentially phosphorylates Cdc25A and Cdc25B in mitotic cells. Phosphorylation of the RSK sites in these Cdc25 isoforms increases their M phase-inducing activities. Inhibition of RSK-mediated phosphorylation of Cdc25 inhibits G2/M transition. Moreover, RSK is likely to be more active in mitotic cells than in interphase cells, as evidenced by the phosphorylation status of T359/S363 in RSK. Together, these findings indicate that RSK promotes G2/M transition in mammalian cells through activating phosphorylation of Cdc25A and Cdc25B.
Keywords: RSK, Cdc25, activating phosphorylation, mitosis, G2/M transition, cell cycle
INTRODUCTION
Proliferation of somatic cells in higher eukaryotes depends on the presence of external mitogens, which activate corresponding receptors present on the plasma membrane. A common downstream event of these receptor activations is the activation of the mitogen-activated protein kinase (MAPK) cascade by Ras (1–4). A typical MAPK cascade comprises three sequential protein kinases RAF, MEK and ERK. A critical mediator of ERK functions is the 90-kDa ribosomal S6 kinase (RSK) (5–7). Previous studies demonstrated that both ERK and RSK promote cell cycle entry of quiescent cells and G1/S transition of cycling cells through transcriptional activation of cyclin D expression and inactivation of the RB family pocket proteins by G1-Cdk (8–15). In addition to these well-understood G1 phase functions of the MAPK cascade, accumulating evidence indicates that activation of the MAPK cascade also positively regulates the G2/M transition in somatic cell cycles. For examples, cycling cells were found to activate the MAPK cascade in a biphasic manner, first in G1/S and then in G2/M phases (16–18). Inhibiting this cascade inhibited or delayed the G2/M transition in cycling cells (19–21), and the G2-checkpoint recovery in DNA-damaged cells (22). Increased activation of the MAPK cascade by ectopic expression of an oncogenic RAS produced opposite effects (23). In RB family proteins (RB, p107 and p130) triple knockout mouse embryonic fibroblasts, which no longer requires growth factors for passing the G1 phase restriction point, serum-starvation activates the secondary G2 phase restriction point. In these cells, serum stimulation of cell cycle reentry from G2 arrest requires activation of the MAPK cascade (24). These findings predict that the MAPK pathway targets key components in the regulatory network that controls G2/M transition.
Similar to the G2-restriction point-arrested mammalian cells, Xenopus oocytes (stage VI) are naturally arrested at the G2/M boundary of the first meiotic division, and resumption of meiotic cell cycles requires mitogen stimulation and activation of the MAPK cascade. Under physiological conditions, progesterone stimulation of fully grown Xenopus oocytes releases the G2 phase arrest by activating mitotic Cdk (25), and the process involves activation of the MAPK cascade by newly synthesized protein kinase MOS, which, in turn, activates MEK (26–29). Due to the similarity in the linkage of the MAPK cascade to the regulation of G2/M transition, delineating the molecular mechanism by which the MAPK cascade promotes the G2/M transition in progesterone-stimulated Xenopus oocytes may provide novel mechanistic insights into how this cascade positively regulates the G2/M transition in somatic cell cycles.
Three molecular mechanisms have been unraveled through which activation of the MAPK cascade positively regulates the G2/M transition in progesterone-stimulated Xenopus oocytes. The first involves RSK-mediated phosphorylation and inactivation of Myt1 (30), the protein kinase in Xenopus oocytes that inactivates the Cdk1/cyclin B complex by catalyzing the inhibitory phosphorylations on Cdk1 (31). The second involves ERK1/2-mediated phosphorylation of Cdc25C (32), the protein phosphatase in Xenopus oocytes that activates the Cdk1/cyclin B complex through removing the inhibitory phosphorylations on Cdk1 (33–35). The third involves activation of Cdc25C by RSK2 (36); RSK2 is the major RSK isoform in Xenopus oocytes (37). Since many of the biochemical regulations regulating meiotic cycles of Xenopus oocytes also occur in mitotic cycles of mammalian cells (38), the three mechanisms described above suggest the possibility that activation of the MAPK cascade positively regulates G2/M transition in mammalian cells through phosphorylation of Cdc25 and Myt 1 by ERK and/or RSK family members. However, only the ERK-mediated phosphorylation of Cdc25C has been demonstrated to promote G2/M transition in mitotic cycles of mammalian cells (32). Whether RSK phosphorylates Cdc25 and/or Myt 1 in mammalian cells has not been determined. The previous finding that mouse oocyte maturation does not require RSK function (39) makes it possible that RSK is not involved in Cdc25 and Myt1 regulations in mammalian cells.
In this study, we characterized the role of RSK in the phosphorylation and activation of human Cdc25 (hCdc25) isoforms in human cell lines. Our results provide evidence that RSK plays an important role in the phosphorylation and activation of hCdc25A and hCdc25B in the process of G2/M transition.
RESULTS
Recombinant RSK phosphorylates A, B and C isoforms of hCdc25 in a conserved motif near the catalytic domain
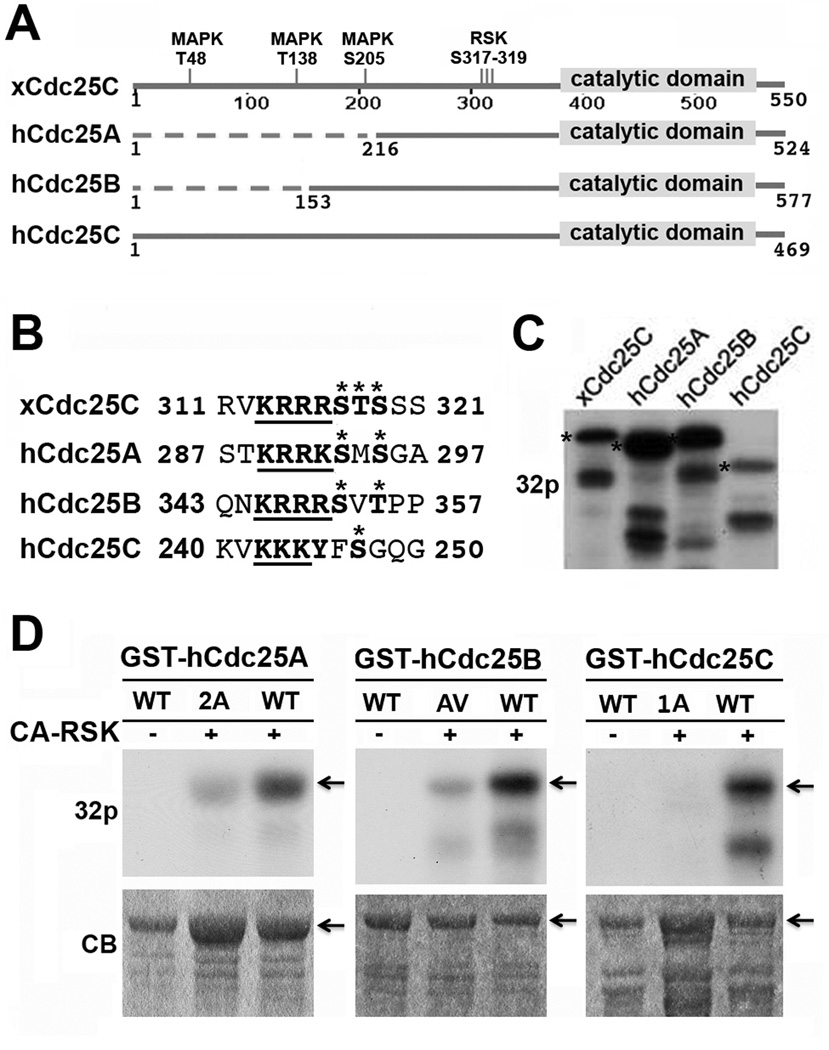
Among the numerous potential RSK phosphorylation sites in xCdc25C, RSK2 predominantly phosphorylates S317, T318 and/or S319 in the motif 313KRRRSTS319 (36). Pairwise alignment of hCdc25A, hCdc25B and hCdc25C with xCdc25C demonstrated that RSK2 phosphorylation sites in xCdc25C localize in a conserved region near the catalytic domain (Fig. 1A). Within this conserved region, all three hCdc25 isoforms contain a string of basic residues that align with the string of basic residues in xCdc25C. Following the basic residue string, there are two Ser residues in hCdc25A (S293 and S295), one Ser residue and one Thr residue in hCdc25B (S353 and T355), and one Ser residue in hCdc25C (S247). These Ser/Thr residues align with the identified RSK2 phosphorylation sites in xCdc25C (Fig. 1B). The sequence conservation suggests that RSK phosphorylates multiple isoforms of hCdc25 at this conserved motif.
Figure 1. CA-RSK phosphorylates recombinant hCdc25 isoforms at a conserved motif near the catalytic domain.
(A) Sequence alignment of hCdc25A, B, and C with xCdc25C. Conserved and non-conserved regions are schematically illustrated as solid and dotted lines, respectively. (B) Sequence alignment of the region of xCdc25C containing the three RSK2 phosphorylation sites (317–319) with hCdc25A, B, and C. Asterisks indicate the RSK2 phosphorylation sites in xCdc25C and the putative phosphorylation sties in hCdc25 isoforms. The underlined bold types indicate the string of basic residues on the N-terminal side of the potential RSK phosphorylation sites. (C) GST-tagged xCdc25C, hCdc25A, hCdc25B or hCdc25C was incubated at room temperature for 30 min with CA-RSK in the presence of γ-32P-ATP. The products were separated by SDS-PAGE and subjected to autoradiography. Stars indicate full-length proteins. (D) The immobilized wild type (WT) and indicated mutant form of GST-hCdc25A, GST-hCdc25B or GST-hCdc25C were incubated with CA-RSK in the presence of γ-32P-ATP for 30 min at room temperature. Proteins eluted from washed beads were separated by SDS-PAGE and subjected to autoradiography. Arrows indicate full-length proteins.
To test this possibility, we first incubated comparable amounts of GST-tagged hCdc25A, hCdc25B or hCdc25C (1–2 µg) with a constitutively active murine RSK (CA-RSK) for 30 min in the presence of γ-32P-ATP. GST-xCdc25C was used as a positive control. As shown in Fig. 1C, CA-RSK catalyzed similar or higher levels of 32P-incorportation into GST-hCdc25A, GST-hCdc25B and GST-hCdc25C as compared to the positive control. We then mutated Ser293 and Ser295 in hCdc25A to Ala293/Ala295 (2A), Ser353 and Thr355 in hCdc25B to Ala353/Val355 (AV), and Ser247 in hCdc25C to Ala247 (1A), and determined the effect on CA-RSK-catalyzed phosphorylations. As shown in Fig. 1D, these mutations greatly reduced the CA-RSK-mediated phosphorylation of hCdc25 isoforms. Together, these results demonstrate that purified RSK is able to phosphorylate the conserved motif in all three isoforms of hCdc25.
Recombinant RSK and endogenous RSK in Xenopus egg extracts phosphorylates all of the predicted RSK sites in hCdc25 isoforms
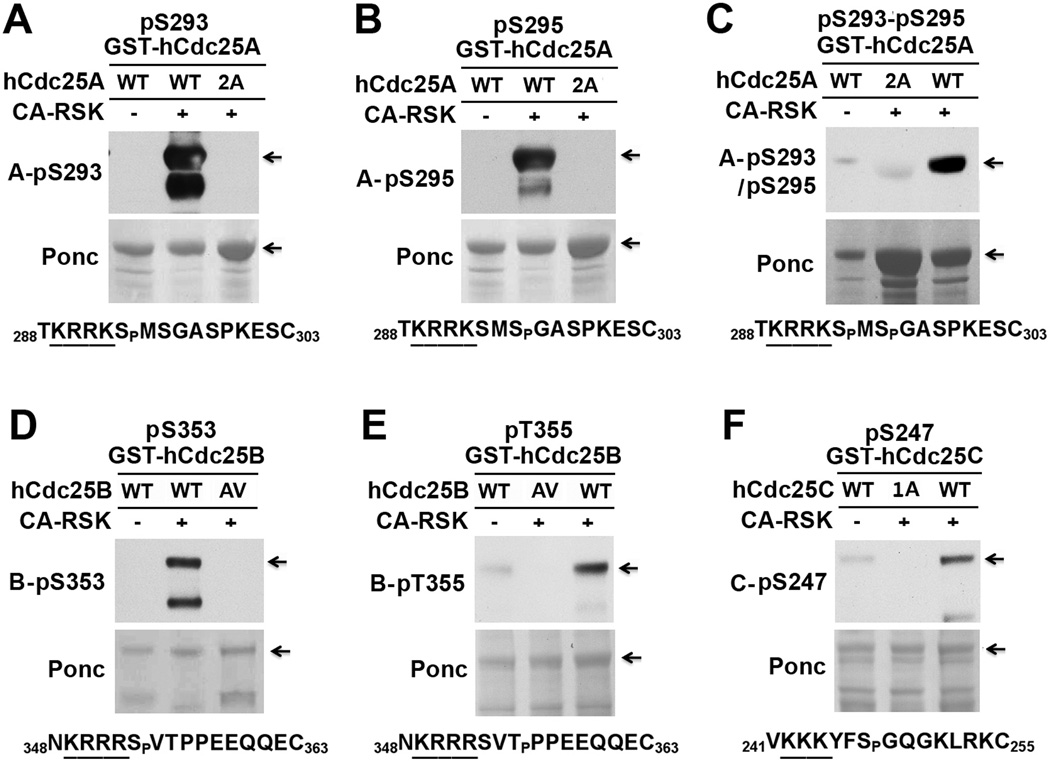
To further characterize the RSK phosphorylation sites in hCdc25 isoforms, we generated five phospho-specific antibodies (Table S1) and immunoblotted the wild type and phospho-defective mutant forms of GST-Cdc25 proteins that had been phosphorylated with CA-RSK. For GST-hCdc25A, the antibody for phosphorylated S293 (A-pS293 antibody) specifically reacted with the wild type protein phosphorylated by CA-RSK (Fig. 2A). Similar results were obtained when the antibody for phosphorylated S295 (A-pS295 antibody) or for phosphorylated S293-S295 (A-pS293-pS295 antibody) was used in immunoblotting (Fig. 2B&C). For GST-hCdc25B, the antibody for phosphorylated S353 (B-pS353 antibody) specifically reacted with the wild type protein phosphorylated by CA-RSK (Fig. 2D). Similar results were obtained when the antibody for phosphorylated T355 (B-pT355 antibody) was used in immunoblotting (Fig. 2E). For GST-hCdc25C, the antibody for phosphorylated S247 (C-pS247 antibody) preferentially reacted with the wild type protein phosphorylated by CA-RSK (Fig. 2F). In addition, we phosphorylated the wild GST-Cdc25 proteins with M phase-arrested Xenopus egg extracts (MEE, 10 mg/ml), which contain high levels of activated RSK1/2 (37). By immunoblotting phosphorylated substrates with above-described phospho-specific antibodies, 1:5-diluted MEE readily phosphorylated hCdc25 isoforms at all of the characterized RSK sites in a 30-min incubation (Fig. S1). These results demonstrate that RSK phosphorylates all of the putative RSK sites in hCdc25 isoforms.
Figure 2. CA-RSK phosphorylates all of the putative RSK sites in hCdc25 isoforms.
Immobilized GST-hCdc25 proteins were mock-treated or phosphorylated with CA-RSK. Proteins eluted from washed beads were immunoblotted with each of the indicated phospho-specific antibodies after Ponceau S (Ponc) staining. The sequence context of the phosphorylation site recognized by each of the phospho-specific antibodies is indicated below immunoblots. (A–C) Phosphorylation of the wild type (WT) and S293A-S295A mutant form (2A) of GST-Cdc25A. (D&E) Phosphorylation of the wild type (WT) and S353A-T355V mutant form (AV) of GST-Cdc25B. (F) Phosphorylation of the wild type (WT) and S247A mutant form (1A) of GST-Cdc25C.
The RSK sites in hCdc25A and hCdc25B are preferentially phosphorylated in mitotic cells
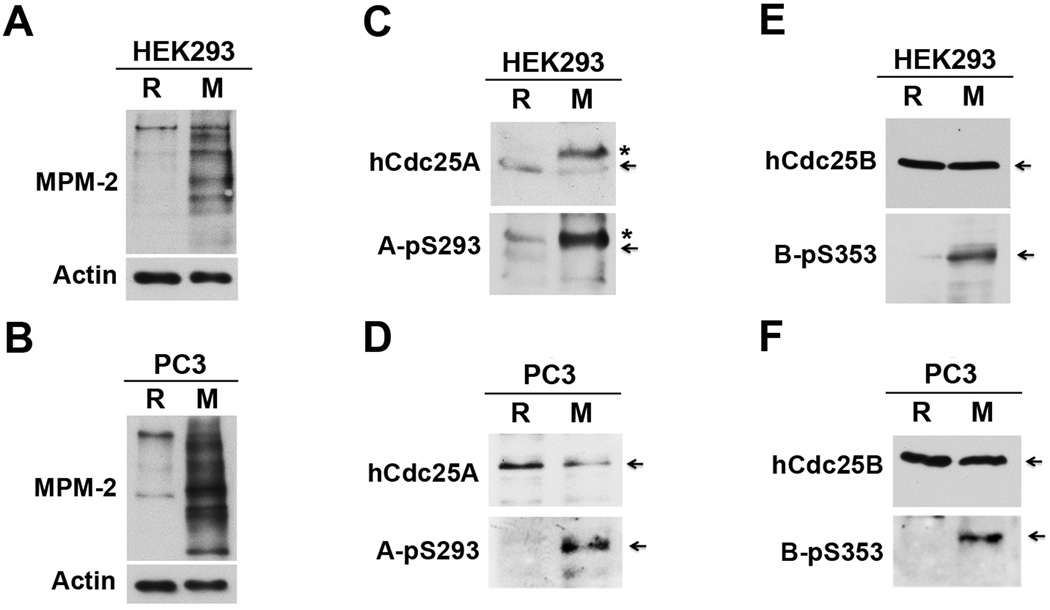
To characterize RSK-mediated phosphorylation of Cdc25 in mammalian cells, we first determined whether phosphorylation of the RSK sites in hCdc25 isoforms is an M phase associated event. Human embryonic kidney HEK293 cells (transformed) and human prostate cancer PC-3mm2 cells (metastatic) were either growing asynchronously (~5% cells in mitosis) or being synchronized in mitosis by a thymidine (TDR) block followed by a nocodazole (NC) block (>80% cells in mitosis). After extracts of random (R) and mitotically synchronized (M) cells were biochemically verified by immunoblotting with the mitotic phospho-protein monoclonal antibody MPM-2 (Fig. 3A&B)(40), phosphorylation of hCdc25 isoforms at the RSK sites was determined by immunoblotting with phospho-specific antibodies. We should note that HEK293 cells expressed all three isoforms of hCdc25, whereas PC-3mm2 cells only expressed detectable levels of hCdc25A and hCdc25B (data not shown).
Figure 3. The RSK sites in hCdc25A and hCdc25B are preferentially phosphorylated in mitotic cells.
Protein extracts of R and M HEK293 cells or PC-3mm2 cells were immunoblotted with MPM-2 or an anti-actin antibody (A&B), with an anti-hCdc25A antibody or the A-pS293 antibody (C&D) or with an anti-hCdc25B antibody or the B-pS353 antibody (E&F). Arrows indicate full-length proteins, and stars indicate shifted hCdc25A.
For hCdc25A, S293 phosphorylation was readily detectable only in M cell extracts (Fig. 3C&D). In contrast, the hCdc25A protein level was similar in between R and M extracts of PC-3mm2 cells. Although the level of hCdc25A protein was 2–3 fold higher in M than in R extracts of HEK293 cells, this difference could not account for a ~10 fold difference in the level of S293-phosphorylated hCdc25A. For hCdc25B, S353 phosphorylation was also readily detectable only in M cell extracts. In this case, the level of hCdc25B protein was similar in between R and M cell extracts for both cell lines (Fig. 3E&F). For hCdc25C, S247 phosphorylation was detected at borderline levels in both R and M extracts of HEK293 cells despite the presence of readily detectable levels of hCdc25C protein (data not shown). These results indicate that the RSK sites in hCdc25A and hCdc25B but not in hCdc25C are preferentially phosphorylated in mitotic cells.
To further examine this possibility, we incubated GST-hCdc25A, GST-hCdc25B, or GST-hCdc25C with different dilutions of mitotic extracts (ME) for 30 min, and determined the RSK site phosphorylation by immunoblotting with phospho-specific antibodies (Fig. S2). For both HEK293 and PC-3mm2 cells, even 1:64-diluted ME readily phosphorylated GST-hCdc25A and GST-hCdc25B at the RSK sites. In contrast, only undiluted ME from HEK293 cells phosphorylated S247 in GST-hCdc25C, and no phosphorylation of GST-hCdc25C at S247 was detected with undiluted ME from PC-3mm2 cells. These results support the preferential phosphorylation of the RSK sites in hCdc25A and hCdc25B in mitotic cells.
Phosphorylation of the RSK sites in hCdc25A and hCdc25B increases their M phase inducing activities
To determine the functional impact of RSK-mediated phosphorylation of hCdc25A and hCdc25B, we injected the wild type and RSK site-mutant forms of myc-tagged hCdc25A or hCdc25B and compared their abilities to induce Xenopus oocyte maturation (32, 41, 42). For myc-hCdc25A, the phospho-defective mutant induced ~50% oocyte maturation with a 1-hr delay as compared to the wild type molecule (Fig. S3A). In contrast, the phospho-mimetic mutant induced oocyte maturation at similar rates as the wild type molecule (Fig. S3B). For myc-hCdc25B, the wild type protein induced ~50% oocyte maturation at 4 hr, whereas the phospho-defective mutant induced ~50% oocyte maturation at 8 hr (Fig. S3C). Again, the phospho-mimetic mutant acted similarly to the wild type protein (Fig. S3D). These results demonstrate that phosphorylation of the RSK sites in hCdc25A and hCdc25B increases their M phase inducing activities.
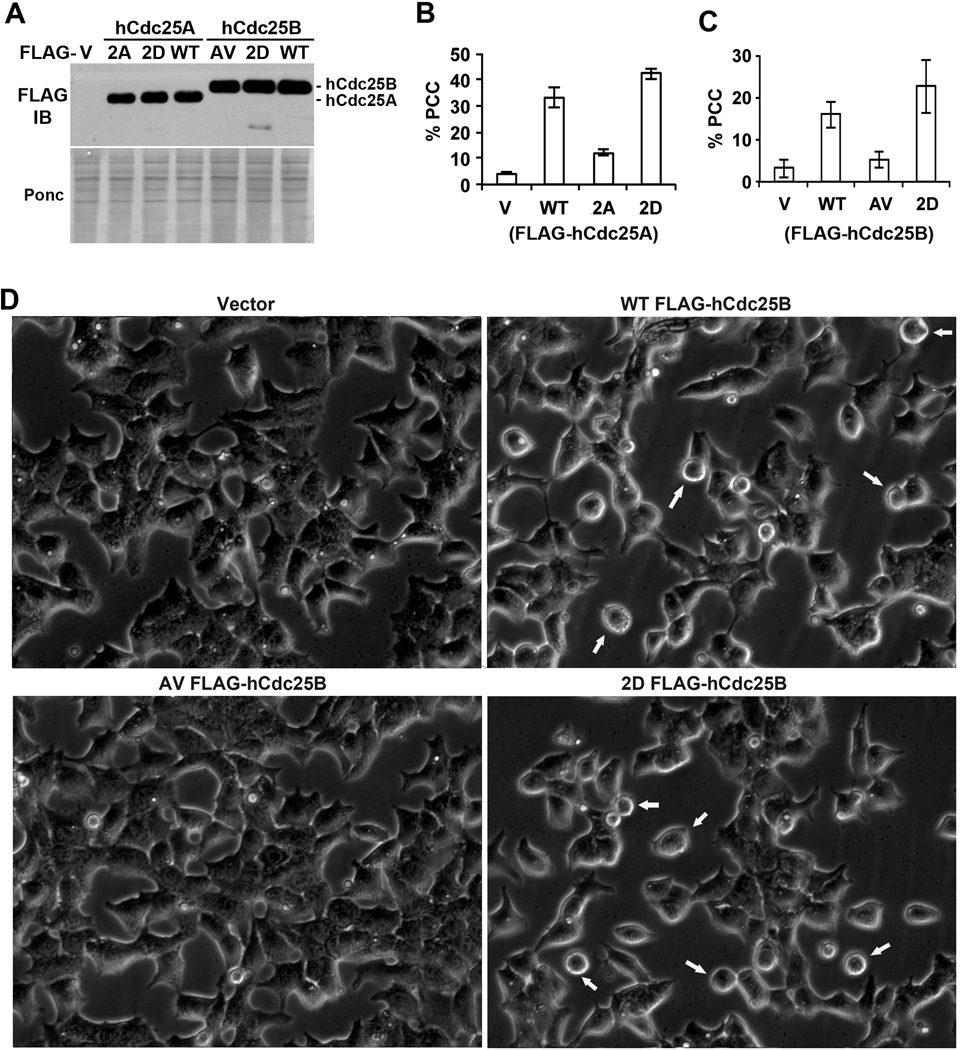
To further examine this observation, we ectopically expressed similar levels of the wild type and RSK-site mutant forms of hCdc25A or hCdc25B in S phase-arrested HEK293T cells (Fig. 4A) and compared their abilities to induce unscheduled mitotic entry, also called PCC (premature chromosome condensation) (43). HEK293T cells were chosen for this experiment because ~90% of transfected cells expressed transgenes. Cells in the PCC state were identified by their round cell morphology, which had been pre-determined by nuclear staining to closely correlate with chromosome condensation (data not shown). Fig. 4B shows representative results from ectopic expression of different forms of FLAG-hCdc25A. Vector-transfected cells had ~4% in the PCC state. This population increased by >8 fold in cells expressing FLAG-hCdc25A and by >10 fold in cells expressing the phospho-mimetic form of FLAG-hCdc25A. However, in cells expressing the phospho-defective form of FLAG-hCdc25A, the PCC population only increased by 3 fold. Fig. 4C shows representative results from ectopic expression of different forms of FLAG-hCdc25B. Again, the wild type or phospho-mimetic form of the protein was much more effective than the phosphor-defective form of the protein in inducing PCC in S-phase arrested cells. Fig. 4D shows representative phase contrast images of live cells expressing different forms of FLAG-hCdc25B. These results support the conclusion from oocyte maturation assays that phosphorylation of the RSK sites in hCdc25A and hCdc25B increases their M phase inducing activities.
Figure 4. Phosphorylation of the RSK sites in hCdc25A and hCdc25B increases their PCC inducing activities.
Exponentially growing HEK293T cells were first treated with thymidine (TDR) for ~12 hrs to accumulate cells at the beginning of and within S phase and then transfected with expression vectors for the wild type or RSK-site mutant forms of FLAG-hCdc25A or FLAG-hCdc25B. (A) After these cells were cultured further for ~15 hrs in the continued presence of TDR to allow the transgene expression, cell lysates were separated by SDS-PAGE and immunoblotted with an anti-FLAG antibody after Ponceau S (Ponc) staining. (B&C) Three random selected fields from FLAG-hCdc25A (B) or FLAG-hCdc25B (C) transfected cells as well as from empty vector-transfected cells (V) were photographed under phase contrast microscopy, and percentages of cells with round cell morphology (indicative of PCC state) were determined manually on captured images. Error bars indicate standard deviations. (D) Representative images from phase contrast microscopy of live cells from (C). Arrows indicate cells with round cell morphology (PCC state).
RSK is one of the major kinases in mitotic cell lysates that phosphorylate the RSK sites in hCdc25A and hCdc25B
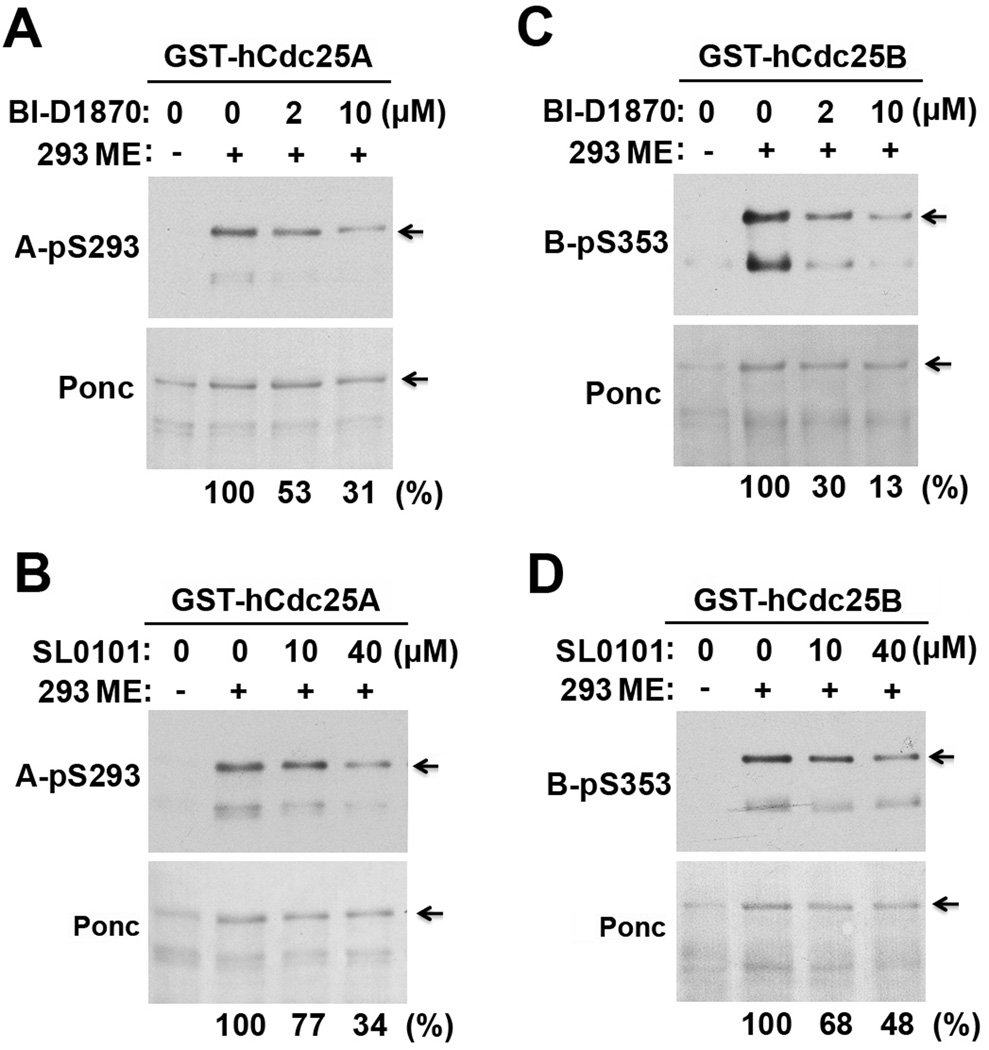
Aurora kinase A was previously shown to phosphorylate S353 of hCdc25B at centrosomes at the onset of mitosis (44, 45). Other AGC family protein kinases in mitotic cells may also phosphorylate the RSK sites in hCdc25 isoforms due to their similar phosphorylation acceptor sites (46, 47). To characterize the contribution of RSK to the mitotic phosphorylation of hCdc25A and hCdc25B at the RSK sites, we phosphorylated GST-hCdc25A and GST-hCdc25B with M extracts (ME) from HEK293 or PC-3mm2 cells in the presence or absence of the RSK2 inhibitor BI-D1870 or SL0101 and immunoblotted the substrates with phospho-specific antibodies; phosphorylation of the same substrates with Xenopus MEE served as a positive control.
In 1:30-diluted ME from HEK293 cells, 2 µM of BI-D1870 or 40 µM of SL0101 caused a ~50% reduction in the phosphorylation of GST-hCdc25A at S293 (Fig. 5A&B) and of GST-hCdc25B at S353 (Fig. 5C&D). Similar results were obtained when the phosphorylation was performed with 1:60 diluted ME from PC-3mm2 cells or 1:20-diluted Xenopus MEE (data not shown). However, no inhibition was observed when the phosphorylation was performed with undiluted ME or MEE (data not shown). These results indicate that RSK is one of the major kinases in mitotic and meiotic cell lysates that phosphorylate the RSK sites in hCdc25A and hCdc25B.
Figure 5. RSK inhibitors partially inhibit phosphorylation of the RSK sites in hCdc25A and hCdc25B in mitotic cell lysates.
The immobilized GST-hCdc25A (A&B) and GST-hCdc25B (C&D) were phosphorylated with 1:30-diluted ME from HEK293 cells in the presence or absence of indicated concentrations of BI-D1870 (A&C) or SL0101 (B&D) at room temperature for 30 min. Proteins eluted from washed beads were immunoblotted with indicated phospho-specific antibodies.
RSK-mediated phosphorylation of hCdc25 plays a positive role in G2/M transition
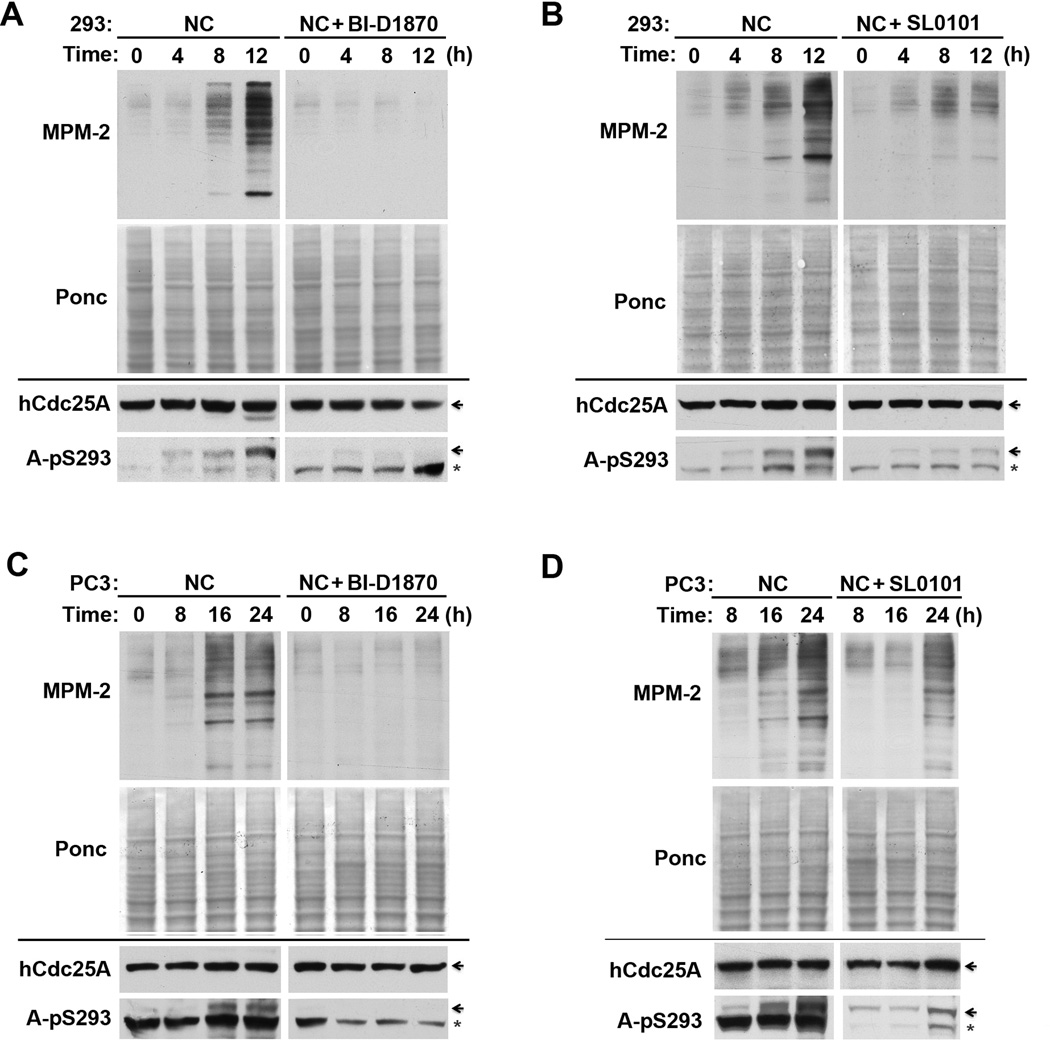
In our initial effort to determine the role of RSK-mediated phosphorylation of Cdc25 in the regulation of G2/M transition, we characterized the effect of the two RSK inhibitors on G2/M transition and hCdc25A phosphorylation in HEK293 and PC-3mm2 cells. Cells were first arrested in S phase by TDR and then released in the presence of the mitotic blocker NC. By examination of cell morphology (data not shown) and by MPM-2 immunoblotting over time, both BI-D1870 and SL0101 inhibited G2/M transition in both HEK293 and PC-3mm2 cells (upper panels of Fig. 6A–D). This result coincided with inhibition of the phosphorylation of hCdc25A at S293 (lower panels of Fig. 6A–D). The effect of BI-D1870 on the G2/M transition was also examined in HEK293 or PC-3mm2 cells without a prior TDR block. Again, by both MPM-2 immunoblotting and phase contrast microscopy, BI-D1870 inhibited mitotic entry in both HEK293 and PC-3mm2 cells (data not shown), indicating that the inhibitory effect of RSK inhibitors on G2/M transition did not depend on a prior S phase block. To complement the inhibitor studies, we then transfected PC-3mm2 cells with four different combinations of RSK1 and RSK2 siRNAs and determined the effect on G2/M transition after releasing the S phase block. Since RSK3 is also widely expressed in human cell lines (5, 7, 48), this RNAi approach should cause only a partial RSK inhibition. For each combination of siRNAs used, both RSK1 and RSK2 expressions were knockdown by >80%, and the mitotic entry at 24 hr was partially inhibited as determined by MPM-2 immunoblotting (Fig. S4). Consistent with each other, these results demonstrate that RSK plays a positive role in G2/M transition.
Figure 6. Inhibition of RSK activity by RSK inhibitors inhibits G2/M transition.
(A&B) HEK293 cells released from a single TDR block were cultured in the presence of either nochodazole (NC) alone or NC plus BI-D1870 (A) or SL0101 (B) for indicated hrs, and extracted proteins were immunoblotted with MPM-2 after Ponceau S (Ponc) staining (upper panels) or immunoblotted with anti-hCdc25A and A-pS293 antibodies (lower panels). Arrows indicate the position of hCdc25A and stars indicate cross-reactive polypeptides. (C&D) PC-3mm2 cells released from a single TDR block were treated as described for Fig. 6A and Fig. 6B.
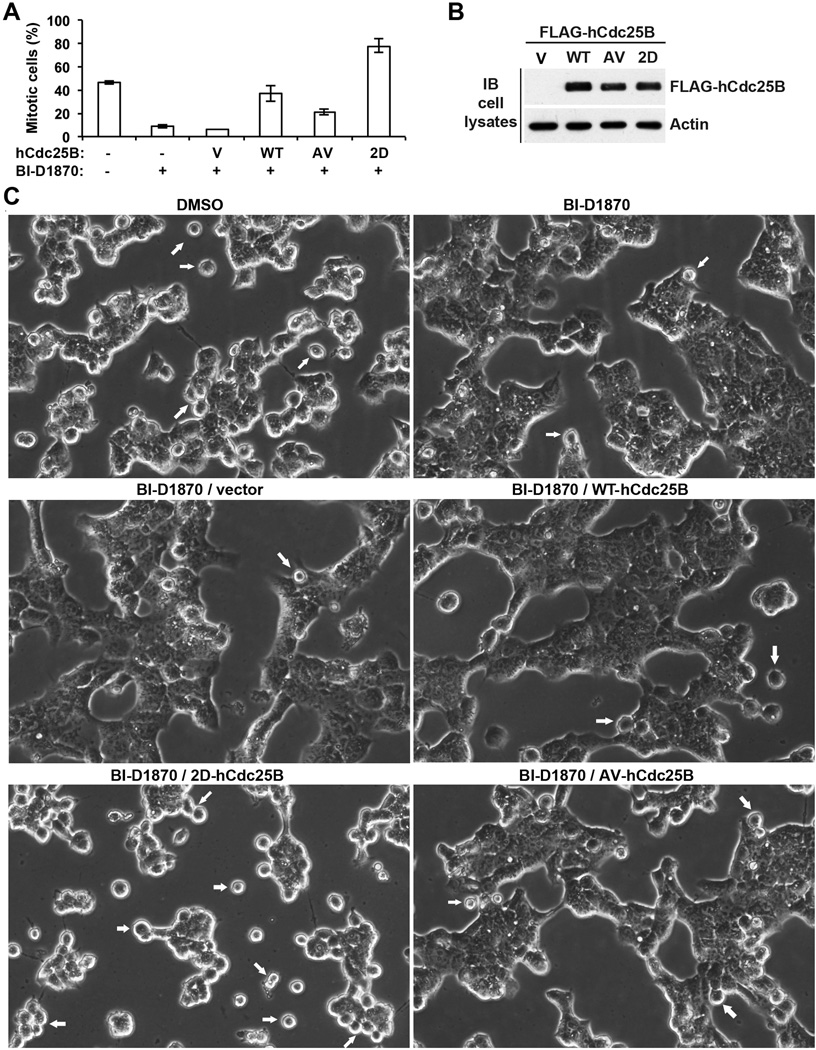
RSK is known to have a variety of biological functions. To determine whether promoting Cdc25 activation is at least one of the major mechanisms through which RSK promotes G2/M transition, we ectopically expressed the wild type or RSK-site defective or mimetic mutant forms of FLAG-hCdc25B in S phase-arrested HEK293T cells and compared their abilities to counteract the inhibitory effect of BI-D1870 on the subsequent G2/M transition. As non-phosphorylated Cdc25B has a basal level of the phosphatase activity, all three forms of FLAG-hCdc25B were expected to have some rescuing effects. However, if promoting Cdc25 activation is a major mechanism through which RSK promotes G2/M transition, the phospho-mimetic form of FLAG-hCdc25B should be more potent than the other two forms of the molecule in rescuing the inhibitory effect of BI-D1870.
We observed mitotic entry at ~7 hrs after releasing the S phase arrest in control cells. As shown in Fig. 7A, ~50% of non-transfected cells had entered mitosis without the BI-D1870 treatment, whereas <10% of non-transfected cells had entered mitosis with the BI-D1870 treatment, confirming the inhibitory effect of BI-D1870 on the G2/M transition. Strikingly, expression of the phospho-mimetic form of FLAG-hCdc25B led to ~80% of cells entering mitosis despite the presence of BI-D1870. In contrast, expression of the wild type or phospho-defective form of FLAG-hCdc25B led to <40% or ~20% of cells entering mitosis in the presence of BI-D1870. Immunoblot results presented in Fig. 7B verified that the three forms of FLAG-hCdc25B were expressed at similar levels. Fig. 7C shows representative images of live cells from phase contrast microscopy. These results support the notion that phosphorylating hCdc25A and hCdc25B at the RSK sites is at least one of the major mechanisms through which RSK promotes G2/M transition.
Figure 7. The phospho-mimetic mutant form of hCdc25B most effectively rescues the inhibitory effect of BI-D1870 on the G2/M transition.
(A) Exponentially growing HEK293T cells were treated with TDR for ~12 hrs and then transfected with the expression vector for the wild type (WT) or the RSK-site phospho-defective (AV) or phospho-mimetic (2D) mutant form of FLAG-hCdc25B or the parental empty vector (V). After transfected cells were cultured further for ~6 hrs in the continued presence of TDR, all cells were changed into the culture medium supplemented with NC plus BI-D1870 or DMSO. At ~7 hrs after the medium change, three randomly selected fields of cells were photographed under a phase contrast microscope, and the percentages of cells showing round mitotic morphology were manually determined on captured images. Errors indicate standard deviations. (B) Immunoblots of cell lysates with anti-FLAG and anti-actin antibodies. (C) Representative images from phase contrast microscopy. Arrows indicate cells with round mitotic morphology.
One of the key activating sites in RSK is preferentially phosphorylated in mitotic cells
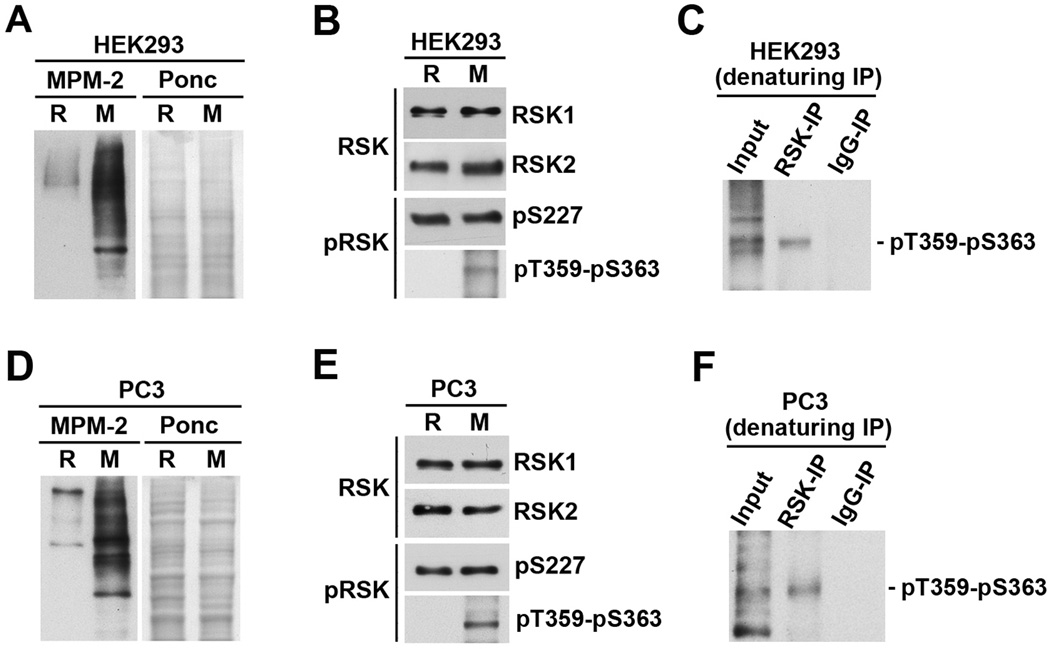
Full activation of RSK1/2/3 requires phosphorylation of both S227 (numbering by RSK2) and S363 (numbering by RSK1) residues (5, 7, 48). To examine the activation status of RSK in mitotic cells versus interphase cells, verified R and M extracts from HEK293 cells (Fig. 8A–C) or PC-3mm2 cells (Fig. 8D–F) were immunoblotted with antibodies that recognize either RSK1 and RSK2 proteins or their activating phosphorylations. For both HEK293 and PC-3mm2 cells, RSK1 and RSK2 proteins are expressed at similar levels in R and M cells. However, while the level of S227-phosphorylation was similar in R and M cells, the level of T359-S363 phosphorylation was greatly increased in M cells relative to R cells (Fig. 8B&E). The T359-S363 phosphorylation of RSK was further confirmed by denaturing immunoprecipitation of RSK1-3 proteins from M extracts followed by immunoblotting with the T359-S363 phospho-specific antibodies (Fig. 8C&F). Interestingly, the anti-RSK1-3 antibodies used did not immunoprecipitate T359-S363 phosphorylated RSK under non-denaturing conditions (data not shown). As a further support of mitotic specific phosphorylation of T359-S363 in RSK, the T359-S363 phospho-specific antibodies preferentially stained mitotic cells in paraffin-sections of PC-3mm2 cell xenograft tumors in nude mice (Fig. S5). The combined results demonstrate for the first time that RSK may be more active in mitotic cells than in interphase cells.
Figure 8. One of the activation sites in RSK is preferentially phosphorylated in mitotic cells.
(A–C) Denatured R and M extracts from HEK293 cells were immunoblotted with MPM-2 after Ponceau S (Ponc) staining (A) or immunoblotted with anti-RSK1 and anti-RSK2 antibodies or anti-phospho-S227 and anti-phospho-T359-S363 antibodies (B). The M extracts were immunoprecipitated with anti-RSK1/2/3 antibodies or rabbit IgG, and the precipitated proteins were immunoblotted with anti-phospho-T359-S363 antibodies (C). (D–F) Denatured R and M extracts from PC-3mm2 cells were processed as described for Fig. 8A–C.
DISCUSSION
RSK is a widely expressed downstream effector of the MAPK cascade. In this study, we demonstrate that both recombinant RSK and RSK in Xenopus egg extracts phosphorylate all three isoforms of hCdc25 at a conserved motif near the catalytic domain. In human cell lines, RSK preferentially phosphorylates Cdc25A and Cdc25B in mitotic cells. Phosphorylation of the RSK sites in hCdc25A and hCdc25B increases their M phase inducing activities. RSK-mediated activation of Cdc25 plays a positive role in G2/M transition. Moreover, RSK is likely to be more active in mitotic cells than in interphase cells. These findings identify a novel link between activation of the MAPK cascade and regulation of the G2/M transition.
All three isoforms of Cdc25 can activate the mitotic Cdk complex during G2/M transition of mammalian cells. Previous studies have already identified multiple protein kinases that activate one or multiple isoforms of Cdc25 by phosphorylating unique sites (49, 50). However, most of the identified kinases activating Cdc25 in mitotic cells are either the substrates of Cdc25 (e.g., Cdk1 or Cdk2 complexes) or kinases that are activated downstream of Cdks (e.g., Plk1 or Aurora kinases). Although these kinases are able to enhance Cdc25 activation by a feed forward mechanism, they may not play important roles in initiating Cdc25 activation before Cdk activation. Our previous results demonstrated that ERK preferentially phosphorylates and activates hCdc25C in mitotic cells (32). As ERK activation does not depend on the activation of mitotic Cdk, we hypothesize that ERK is one of the initiating kinases for hCdc25C activation during G2/M transition (36). Different from ERK, the results in this study show that RSK preferentially activates hCdc25A and hCdc25B during G2/M transition. It is thus conceivable that RSK may complement ERK in initiating Cdc25 activation during G2/M transition of mammalian cells.
During G2 phase, cells are meant to fulfill various requirements for entry into mitosis, including repairing DNA damage accumulated in earlier phases of the cell cycle. Because inhibition of Cdc25 activation is the most critical event in maintaining cells in G2 phase before the requirements are properly fulfilled (51–54), abnormally high levels of Cdc25 expression or activation weaken the G2 checkpoint and promote abnormal mitosis and genetic instability in both cellular and animal models (55–57). Importantly, the MAPK cascade is frequently overexpressed or overactivated in tumor cells due to a variety of mechanisms (2). RSK1/2 protein is overexpressed in multiple types of human cancers (58–60). Since we now know that at least two components linked to the MAPK cascade directly activate Cdc25 during the G2/M transition, it is conceivable that hyperactivation of the MAPK cascade or RSK overexpression in tumor cells results in less controlled Cdc25 activation and, as a result, weakens the G2 checkpoint. This hypothesis may explain why oncogenic activation of RAS promotes abnormal mitosis and genetic instability within one cell cycle (61), and why this effect is greatly enhanced by overexpression of Cdc25A (62). This hypothesis also raises the possibility that attenuation of Cdc25 activation by RSK inhibitors or partial inhibition of Cdc25 activity by Cdc25 inhibitors counteracts the genetic instability in tumor cells.
RSK comprises a family of structurally related kinases, consisting of RSK1, RSK2, RSK3 and RSK4. Among the four isoforms, RSK1, RSK2 and RSK3 are widely expressed in human tissues and cell lines (5, 7, 48), and are likely to phosphorylate overlapping substrates. Thus RSK referred to in this study includes at least RSK1, RSK2 and RSK3. However, we are also aware that different isoforms of RSK may play different roles in certain aspects of cell regulation (59, 63). Thus we cannot exclude the possibility that different isoforms of RSK contribute differently to the phosphorylation of hCdc25A and/or hCdc25B during the G2/M transition. Clarification of this issue will require detailed analysis of different RSK isoforms in phosphorylating hCdc25A and hCdc25B in vitro and in vivo.
RSK contains an N-terminal kinase domain (NTK) responsible for the phosphorylation of substrates, a middle linker region, and a C-terminal kinase domain (CTK) that activates NTK. Previous studies have demonstrated that full activation of RSK1-3 by the ERK bound to the C-terminus of CTK requires two independent mechanisms (5, 7, 48). One mechanism involves ERK-mediated phosphorylation of two adjacent conserved residues (T359 and Ser363 in RSK1) in the linker region of RSK, in which S363 phosphorylation directly activates the NTK (64). In parallel, ERK-mediated phosphorylation of a conserved residue (Thr577 in RSK2) in the CTK activates the CTK, which in turn phosphorylates a conserved residue (Ser380 in RSK1) in the linker region. The S380 phosphorylation results in the recruitment of 3-phosphoinositide-dependent protein kinase 1 (PDK1), which then stimulates the NTK activity through phosphorylating a conserved residue (Ser227 in RSK2) in the NTK. Based on these previous studies, a fully activated RSK should have both S227 and S363 phosphorylated. Interestingly, while the level of the S227 phosphorylation is similar in between interphase and mitotic cells, the T359-Ser363 phosphorylation is readily detectable only in mitotic cells (Fig. 8B&F). These findings provide the first evidence that RSK1/2/3 may be more active in mitotic cells than in interphase cells. This possibility provides one plausible explanation as to why the RSK sites in hCdc25A and hCdc25B are preferentially phosphorylated in mitotic cells. We also observed that anti-RSK antibodies specifically could not immunoprecipitate T359-S363-phosphorylated RSK under nondenaturing conditions (data not shown), raising a possibility that the T359-S363 phosphorylation may induce a change in RSK conformation or partner protein interaction. Future studies will elucidate the mechanism by which the T359 and S363 are preferentially phosphorylated in mitotic cells and address the functional impact on the mitotic specific phosphorylation of the RSK sites in hCdc25A and hCdc25B.
MATERIALS AND METHODS
In vitro phosphorylation of Cdc25 recombinant proteins
Preparations of GST-tagged CA-RSK and various Cdc25 recombinant proteins were described previously (32, 36). RSK site mutant forms of GST tagged hCdc25 isoforms were produced by site-directed mutagenesis using ExSite™ PCR-based site-directed mutagenesis kit (Stratagene). The GST-tagged Cdc25 recombinant proteins were expressed in E. coli BL-21 (Invitrogen) or Rosseta strain (Novagen) and affinity absorbed onto glutathione-sepharose (GE Healthcare) (32). The immobilized Cdc25 recombinant proteins were stored in 20% glycerol at −80°C until use. GST-hCdc25C was incubated with 10 mM DTT on ice for 30 minutes before being used in phosphorylation reactions. Preparation of cell lysates and in vitro phosphorylation of Cdc25 recombinant proteins were performed as described in Supplemental Materials.
Cell culture, treatment of cells with RSK inhibitors and cDNA transfection
HEK293 or HEK293T cells were cultured in Dulbecco’s Modification of Eagle’s Medium (DMEM) (Mediatech Inc) supplemented with 2 mM L-glutamine, 1% penicillin and streptomycin, and 10% fetal bovine serum (Atlanta Biologicals). PC-3mm2 cells were cultured in RPMI-1640 supplemented with 2 mM L-glutamine, 1% penicillin and streptomycin, and 10% fetal bovine serum. Mitotic population of HEK293 and PC3-mm2 cells were obtained by a 12 to 16 hr block with 2 mM thymidine (CALBIOCHEM) followed by a 12 to 16-hr block with 0.05 g/ml nocodazole (CALBIOCHEM). Percentages of cells in mitosis were determined from 3–5 images taken under a phase contrast microscope. BI-D1870 was chemically synthesized by The Translational Chemistry Core Facility at M. D. Anderson Cancer Center or purchased from Exon Medchem and dissolved with DMSO. SL0101 was purchased from Toronto Research Chemicals Inc. These inhibitors were dissolved with DMSO to make a 1000× stock based on the final concentration needed, and the stock solutions were stored at −80°C in small aliquots until use.
pCMV-Tag-2B-based mammalian expression vectors for FLAG-hCdc25A and FLAG-hCdc25B were produced by PCR-amplification of the coding region for hCdc25A or hCdc25B, followed by subcloning of the coding region into pCMV-Tag-2B vector. These vectors were used as templates to generate expression vectors for the RSK-site mutant forms of FLAG-hCdc25A or FLAG-hCdc25B through site-directed mutagenesis. HEK293T cells were transfected with these expression constructs or the parental vector by calcium phosphatase transfection method.
Supplementary Material
ACKNOWLEDGMENT
This work was supported by the Department of Defense (DOD) Prostate Cancer Research Program (PCRP) grants W81XWH-09-1-0274 (J. Kuang) and W81XWH-09-1-0272 (S.H. Lin), by Grant 30300173 from National Natural Science Foundation of China (W. Zhang), and Grant 20071D0503100293 from Beijing Talents Foundation (W. Zhang), a PCF Challenge grant and NCI CA140388 (G.E. Gallick). DNA sequencing was performed by the DNA Analysis Facility of UT M. D. Anderson Cancer Center supported by NCI Grant CA 16672. The Translational Chemistry Core Facility at M. D. Anderson Cancer Center was supported by the Cancer Center support grant CA016672.
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
REFERENCES
- 1.Yoon S, Seger R. The extracellular signal-regulated kinase: multiple substrates regulate diverse cellular functions. Growth Factors. 2006;24(1):21–44. doi: 10.1080/02699050500284218. Epub 2006/01/06. [DOI] [PubMed] [Google Scholar]
- 2.McCubrey JA, Steelman LS, Chappell WH, Abrams SL, Wong EW, Chang F, et al. Roles of the Raf/MEK/ERK pathway in cell growth, malignant transformation and drug resistance. Biochim Biophys Acta. 2007;1773(8):1263–1284. doi: 10.1016/j.bbamcr.2006.10.001. Epub 2006/11/28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roberts PJ, Der CJ. Targeting the Raf-MEK-ERK mitogen-activated protein kinase cascade for the treatment of cancer. Oncogene. 2007;26(22):3291–3310. doi: 10.1038/sj.onc.1210422. Epub 2007/05/15. [DOI] [PubMed] [Google Scholar]
- 4.Shaul YD, Seger R. The MEK/ERK cascade: from signaling specificity to diverse functions. Biochim Biophys Acta. 2007;1773(8):1213–1226. doi: 10.1016/j.bbamcr.2006.10.005. Epub 2006/11/23. [DOI] [PubMed] [Google Scholar]
- 5.Anjum R, Blenis J. The RSK family of kinases: emerging roles in cellular signalling. Nat Rev Mol Cell Biol. 2008;9(10):747–758. doi: 10.1038/nrm2509. Epub 2008/09/25. [DOI] [PubMed] [Google Scholar]
- 6.Carriere A, Ray H, Blenis J, Roux PP. The RSK factors of activating the Ras/MAPK signaling cascade. Front Biosci. 2008;13:4258–4275. doi: 10.2741/3003. Epub 2008/05/30. [DOI] [PubMed] [Google Scholar]
- 7.Romeo Y, Zhang X, Roux PP. Regulation and function of the RSK family of protein kinases. Biochem J. 2012;441(2):553–569. doi: 10.1042/BJ20110289. Epub 2011/12/23. [DOI] [PubMed] [Google Scholar]
- 8.Alvarez E, Northwood IC, Gonzalez FA, Latour DA, Seth A, Abate C, et al. Pro-Leu-Ser/Thr-Pro is a consensus primary sequence for substrate protein phosphorylation. Characterization of the phosphorylation of c-myc and c-jun proteins by an epidermal growth factor receptor threonine 669 protein kinase. J Biol Chem. 1991;266(23):15277–15285. Epub 1991/08/15. [PubMed] [Google Scholar]
- 9.Seth A, Gonzalez FA, Gupta S, Raden DL, Davis RJ. Signal transduction within the nucleus by mitogen-activated protein kinase. J Biol Chem. 1992;267(34):24796–24804. Epub 1992/12/05. [PubMed] [Google Scholar]
- 10.Daksis JI, Lu RY, Facchini LM, Marhin WW, Penn LJ. Myc induces cyclin D1 expression in the absence of de novo protein synthesis and links mitogen-stimulated signal transduction to the cell cycle. Oncogene. 1994;9(12):3635–3645. Epub 1994/12/01. [PubMed] [Google Scholar]
- 11.Bouchard C, Thieke K, Maier A, Saffrich R, Hanley-Hyde J, Ansorge W, et al. Direct induction of cyclin D2 by Myc contributes to cell cycle progression and sequestration of p27. EMBO J. 1999;18(19):5321–5333. doi: 10.1093/emboj/18.19.5321. Epub 1999/10/03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hermeking H, Rago C, Schuhmacher M, Li Q, Barrett JF, Obaya AJ, et al. Identification of CDK4 as a target of c-MYC. Proc Natl Acad Sci U S A. 2000;97(5):2229–2234. doi: 10.1073/pnas.050586197. Epub 2000/02/26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brown JR, Nigh E, Lee RJ, Ye H, Thompson MA, Saudou F, et al. Fos family members induce cell cycle entry by activating cyclin D1. Mol Cell Biol. 1998;18(9):5609–5619. doi: 10.1128/mcb.18.9.5609. Epub 1998/08/26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee RJ, Albanese C, Stenger RJ, Watanabe G, Inghirami G, Haines GK, 3rd, et al. pp60(v-src) induction of cyclin D1 requires collaborative interactions between the extracellular signal-regulated kinase, p38, and Jun kinase pathways. A role for cAMP response element-binding protein and activating transcription factor-2 in pp60(v-src) signaling in breast cancer cells. J Biol Chem. 1999;274(11):7341–7350. doi: 10.1074/jbc.274.11.7341. Epub 1999/03/06. [DOI] [PubMed] [Google Scholar]
- 15.Fox KE, Colton LA, Erickson PF, Friedman JE, Cha HC, Keller P, et al. Regulation of cyclin D1 and Wnt10b gene expression by cAMP-responsive element-binding protein during early adipogenesis involves differential promoter methylation. J Biol Chem. 2008;283(50):35096–35105. doi: 10.1074/jbc.M806423200. Epub 2008/10/30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tamemoto H, Kadowaki T, Tobe K, Ueki K, Izumi T, Chatani Y, et al. Biphasic activation of two mitogen-activated protein kinases during the cell cycle in mammalian cells. J Biol Chem. 1992;267(28):20293–20297. [PubMed] [Google Scholar]
- 17.Shapiro PS, Vaisberg E, Hunt AJ, Tolwinski NS, Whalen AM, McIntosh JR, et al. Activation of the MKK/ERK pathway during somatic cell mitosis: direct interactions of active ERK with kinetochores and regulation of the mitotic 3F3/2 phosphoantigen. J Cell Biol. 1998;142(6):1533–1545. doi: 10.1083/jcb.142.6.1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harding A, Giles N, Burgess A, Hancock JF, Gabrielli BG. Mechanism of mitosis-specific activation of MEK1. J Biol Chem. 2003;278(19):16747–16754. doi: 10.1074/jbc.M301015200. [DOI] [PubMed] [Google Scholar]
- 19.Roberts EC, Shapiro PS, Nahreini TS, Pages G, Pouyssegur J, Ahn NG. Distinct cell cycle timing requirements for extracellular signal-regulated kinase and phosphoinositide 3-kinase signaling pathways in somatic cell mitosis. Mol Cell Biol. 2002;22(20):7226–7241. doi: 10.1128/MCB.22.20.7226-7241.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu X, Yan S, Zhou T, Terada Y, Erikson RL. The MAP kinase pathway is required for entry into mitosis and cell survival. Oncogene. 2004;23(3):763–776. doi: 10.1038/sj.onc.1207188. Epub 2004/01/23. [DOI] [PubMed] [Google Scholar]
- 21.Wright JH, Munar E, Jameson DR, Andreassen PR, Margolis RL, Seger R, et al. Mitogen-activated protein kinase kinase activity is required for the G(2)/M transition of the cell cycle in mammalian fibroblasts. Proc Natl Acad Sci U S A. 1999;96(20):11335–11340. doi: 10.1073/pnas.96.20.11335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abbott DW, Holt JT. Mitogen-activated protein kinase kinase 2 activation is essential for progression through the G2/M checkpoint arrest in cells exposed to ionizing radiation. J Biol Chem. 1999;274(5):2732–2742. doi: 10.1074/jbc.274.5.2732. Epub 1999/01/23. [DOI] [PubMed] [Google Scholar]
- 23.Knauf JA, Ouyang B, Knudsen ES, Fukasawa K, Babcock G, Fagin JA. Oncogenic RAS induces accelerated transition through G2/M and promotes defects in the G2 DNA damage and mitotic spindle checkpoints. J Biol Chem. 2006;281(7):3800–3809. doi: 10.1074/jbc.M511690200. Epub 2005/12/01. [DOI] [PubMed] [Google Scholar]
- 24.Foijer F, Simonis M, van Vliet M, Wessels L, Kerkhoven R, Sorger PK, et al. Oncogenic pathways impinging on the G2-restriction point. Oncogene. 2008;27(8):1142–1154. doi: 10.1038/sj.onc.1210724. Epub 2007/08/19. [DOI] [PubMed] [Google Scholar]
- 25.Maller JL. OOcyte maturation in amphibians. Dev Biol (N Y 1985) 1985;1:289–311. doi: 10.1007/978-1-4615-6814-8_6. Epub 1985/01/01. [DOI] [PubMed] [Google Scholar]
- 26.Castro A, Peter M, Lorca T, Mandart E. c-Mos and cyclin B/cdc2 connections during Xenopus oocyte maturation. Biol Cell. 2001;93(1–2):15–25. doi: 10.1016/s0248-4900(01)01128-5. [DOI] [PubMed] [Google Scholar]
- 27.Palmer A, Nebreda AR. The activation of MAP kinase and p34cdc2/cyclin B during the meiotic maturation of Xenopus oocytes. Prog Cell Cycle Res. 2000;4:131–143. doi: 10.1007/978-1-4615-4253-7_12. [DOI] [PubMed] [Google Scholar]
- 28.Gebauer F, Richter JD. Synthesis and function of Mos: the control switch of vertebrate oocyte meiosis. Bioessays. 1997;19(1):23–28. doi: 10.1002/bies.950190106. Epub 1997/01/01. [DOI] [PubMed] [Google Scholar]
- 29.Gotoh Y, Nishida E. Activation mechanism and function of the MAP kinase cascade. Mol Reprod Dev. 1995;42(4):486–492. doi: 10.1002/mrd.1080420417. Epub 1995/12/01. [DOI] [PubMed] [Google Scholar]
- 30.Palmer A, Gavin AC, Nebreda AR. A link between MAP kinase and p34(cdc2)/cyclin B during oocyte maturation: p90(rsk) phosphorylates and inactivates the p34(cdc2) inhibitory kinase Myt1. Embo J. 1998;17(17):5037–5047. doi: 10.1093/emboj/17.17.5037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mueller PR, Coleman TR, Kumagai A, Dunphy WG. Myt1: a membrane-associated inhibitory kinase that phosphorylates Cdc2 on both threonine-14 and tyrosine-15. Science. 1995;270(5233):86–90. doi: 10.1126/science.270.5233.86. [DOI] [PubMed] [Google Scholar]
- 32.Wang R, He G, Nelman-Gonzalez M, Ashorn CL, Gallick GE, Stukenberg PT, et al. Regulation of Cdc25C by ERK-MAP kinases during the G2/M transition. Cell. 2007;128(6):1119–1132. doi: 10.1016/j.cell.2006.11.053. [DOI] [PubMed] [Google Scholar]
- 33.Kumagai A, Dunphy WG. The cdc25 protein controls tyrosine dephosphorylation of the cdc2 protein in a cell-free system. Cell. 1991;64(5):903–914. doi: 10.1016/0092-8674(91)90315-p. Epub 1991/03/08. [DOI] [PubMed] [Google Scholar]
- 34.Gautier J, Solomon MJ, Booher RN, Bazan JF, Kirschner MW. cdc25 is a specific tyrosine phosphatase that directly activates p34cdc2. Cell. 1991;67(1):197–211. doi: 10.1016/0092-8674(91)90583-k. Epub 1991/10/04. [DOI] [PubMed] [Google Scholar]
- 35.Gabrielli BG, Lee MS, Walker DH, Piwnica-Worms H, Maller JL. Cdc25 regulates the phosphorylation and activity of the Xenopus cdk2 protein kinase complex. J Biol Chem. 1992;267(25):18040–18046. [PubMed] [Google Scholar]
- 36.Wang R, Jung SY, Wu CF, Qin J, Kobayashi R, Gallick GE, et al. Direct roles of the signaling kinase RSK2 in Cdc25C activation during Xenopus oocyte maturation. Proc Natl Acad Sci U S A. 2010;107(46):19885–19890. doi: 10.1073/pnas.1003528107. Epub 2010/11/03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bhatt RR, Ferrell JE., Jr Cloning and characterization of Xenopus Rsk2, the predominant p90 Rsk isozyme in oocytes and eggs. J Biol Chem. 2000;275(42):32983–32990. doi: 10.1074/jbc.M006386200. [DOI] [PubMed] [Google Scholar]
- 38.Nurse P. Universal control mechanism regulating onset of M-phase. Nature. 1990;344(6266):503–508. doi: 10.1038/344503a0. Epub 1990/04/05. [DOI] [PubMed] [Google Scholar]
- 39.Dumont J, Umbhauer M, Rassinier P, Hanauer A, Verlhac MH. p90Rsk is not involved in cytostatic factor arrest in mouse oocytes. J Cell Biol. 2005;169(2):227–231. doi: 10.1083/jcb.200501027. Epub 2005/04/20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Davis FM, Tsao TY, Fowler SK, Rao PN. Monoclonal antibodies to mitotic cells. Proc Natl Acad Sci U S A. 1983;80(10):2926–2930. doi: 10.1073/pnas.80.10.2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee MS, Ogg S, Xu M, Parker LL, Donoghue DJ, Maller JL, et al. cdc25+ encodes a protein phosphatase that dephosphorylates p34cdc2. Mol Biol Cell. 1992;3(1):73–84. doi: 10.1091/mbc.3.1.73. Epub 1992/01/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rime H, Huchon D, De Smedt V, Thibier C, Galaktionov K, Jessus C, et al. Microinjection of Cdc25 protein phosphatase into Xenopus prophase oocyte activates MPF and arrests meiosis at metaphase I. Biol Cell. 1994;82(1):11–22. doi: 10.1016/0248-4900(94)90061-2. Epub 1994/01/01. [DOI] [PubMed] [Google Scholar]
- 43.Karlsson C, Katich S, Hagting A, Hoffmann I, Pines J. Cdc25B and Cdc25C differ markedly in their properties as initiators of mitosis. J Cell Biol. 1999;146(3):573–584. doi: 10.1083/jcb.146.3.573. Epub 1999/08/12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cazales M, Schmitt E, Montembault E, Dozier C, Prigent C, Ducommun B. CDC25B phosphorylation by Aurora-A occurs at the G2/M transition and is inhibited by DNA damage. Cell Cycle. 2005;4(9):1233–1238. doi: 10.4161/cc.4.9.1964. Epub 2005/08/06. [DOI] [PubMed] [Google Scholar]
- 45.Dutertre S, Cazales M, Quaranta M, Froment C, Trabut V, Dozier C, et al. Phosphorylation of CDC25B by Aurora-A at the centrosome contributes to the G2-M transition. J Cell Sci. 2004;117(Pt 12):2523–2531. doi: 10.1242/jcs.01108. Epub 2004/05/07. [DOI] [PubMed] [Google Scholar]
- 46.Moritz A, Li Y, Guo A, Villen J, Wang Y, MacNeill J, et al. Akt-RSK-S6 kinase signaling networks activated by oncogenic receptor tyrosine kinases. Sci Signal. 2010;3(136):ra64. doi: 10.1126/scisignal.2000998. Epub 2010/08/26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pearce LR, Komander D, Alessi DR. The nuts and bolts of AGC protein kinases. Nat Rev Mol Cell Biol. 2010;11(1):9–22. doi: 10.1038/nrm2822. Epub 2009/12/23. [DOI] [PubMed] [Google Scholar]
- 48.Hauge C, Frodin M. RSK and MSK in MAP kinase signalling. J Cell Sci. 2006;119(Pt 15):3021–3023. doi: 10.1242/jcs.02950. Epub 2006/07/27. [DOI] [PubMed] [Google Scholar]
- 49.Boutros R, Dozier C, Ducommun B. The when and wheres of CDC25 phosphatases. Curr Opin Cell Biol. 2006;18(2):185–191. doi: 10.1016/j.ceb.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 50.Boutros R, Lobjois V, Ducommun B. CDC25 phosphatases in cancer cells: key players? Good targets? Nat Rev Cancer. 2007;7(7):495–507. doi: 10.1038/nrc2169. [DOI] [PubMed] [Google Scholar]
- 51.Aressy B, Ducommun B. Cell cycle control by the CDC25 phosphatases. Anticancer Agents Med Chem. 2008;8(8):818–824. doi: 10.2174/187152008786847756. Epub 2008/12/17. [DOI] [PubMed] [Google Scholar]
- 52.Chen Y, Poon RY. The multiple checkpoint functions of CHK1 and CHK2 in maintenance of genome stability. Front Biosci. 2008;13:5016–5029. doi: 10.2741/3060. Epub 2008/05/30. [DOI] [PubMed] [Google Scholar]
- 53.Reinhardt HC, Yaffe MB. Kinases that control the cell cycle in response to DNA damage: Chk1, Chk2, and MK2. Curr Opin Cell Biol. 2009;21(2):245–255. doi: 10.1016/j.ceb.2009.01.018. Epub 2009/02/24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Donzelli M, Draetta GF. Regulating mammalian checkpoints through Cdc25 inactivation. EMBO Rep. 2003;4(7):671–677. doi: 10.1038/sj.embor.embor887. Epub 2003/07/02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Aressy B, Bugler B, Valette A, Biard D, Ducommun B. Moderate variations in CDC25B protein levels modulate the response to DNA damaging agents. Cell Cycle. 2008;7(14):2234–2240. doi: 10.4161/cc.7.14.6305. Epub 2008/07/19. [DOI] [PubMed] [Google Scholar]
- 56.Cangi MG, Piccinin S, Pecciarini L, Talarico A, Dal Cin E, Grassi S, et al. Constitutive overexpression of CDC25A in primary human mammary epithelial cells results in both defective DNA damage response and chromosomal breaks at fragile sites. Int J Cancer. 2008;123(6):1466–1471. doi: 10.1002/ijc.23659. Epub 2008/06/21. [DOI] [PubMed] [Google Scholar]
- 57.Varmeh S, Manfredi JJ. Overexpression of the dual specificity phosphatase, Cdc25C, confers sensitivity on tumor cells to doxorubicin-induced cell death. Mol Cancer Ther. 2008;7(12):3789–3799. doi: 10.1158/1535-7163.MCT-08-0838. Epub 2008/12/17. [DOI] [PubMed] [Google Scholar]
- 58.Clark DE, Errington TM, Smith JA, Frierson HF, Jr, Weber MJ, Lannigan DA. The serine/threonine protein kinase, p90 ribosomal S6 kinase, is an important regulator of prostate cancer cell proliferation. Cancer Res. 2005;65(8):3108–3116. doi: 10.1158/0008-5472.CAN-04-3151. Epub 2005/04/19. [DOI] [PubMed] [Google Scholar]
- 59.Kang S, Elf S, Lythgoe K, Hitosugi T, Taunton J, Zhou W, et al. p90 ribosomal S6 kinase 2 promotes invasion and metastasis of human head and neck squamous cell carcinoma cells. J Clin Invest. 2010;120(4):1165–1177. doi: 10.1172/JCI40582. Epub 2010/03/18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Eisenmann KM, VanBrocklin MW, Staffend NA, Kitchen SM, Koo HM. Mitogen-activated protein kinase pathway-dependent tumor-specific survival signaling in melanoma cells through inactivation of the proapoptotic protein bad. Cancer Res. 2003;63(23):8330–8337. Epub 2003/12/18. [PubMed] [Google Scholar]
- 61.Denko NC, Giaccia AJ, Stringer JR, Stambrook PJ. The human Ha-ras oncogene induces genomic instability in murine fibroblasts within one cell cycle. Proc Natl Acad Sci U S A. 1994;91(11):5124–5128. doi: 10.1073/pnas.91.11.5124. Epub 1994/05/24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.LoPiccolo J, Blumenthal GM, Bernstein WB, Dennis PA. Targeting the PI3K/Akt/mTOR pathway: effective combinations and clinical considerations. Drug Resist Updat. 2008;11(1–2):32–50. doi: 10.1016/j.drup.2007.11.003. Epub 2008/01/02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nam HJ, Kim S, Lee MW, Lee BS, Hara T, Saya H, et al. The ERK-RSK1 activation by growth factors at G2 phase delays cell cycle progression and reduces mitotic aberrations. Cell Signal. 2008;20(7):1349–1358. doi: 10.1016/j.cellsig.2008.03.008. Epub 2008/05/03. [DOI] [PubMed] [Google Scholar]
- 64.Dalby KN, Morrice N, Caudwell FB, Avruch J, Cohen P. Identification of regulatory phosphorylation sites in mitogen-activated protein kinase (MAPK)-activated protein kinase-1a/p90rsk that are inducible by MAPK. J Biol Chem. 1998;273(3):1496–1505. doi: 10.1074/jbc.273.3.1496. Epub 1998/01/27. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.