Abstract
Background
Focal segmental glomerulosclerosis (FSGS) recurs after kidney transplantation in more than 30 % of cases and can lead to allograft loss. Serum soluble urokinase -type plasminogen activator receptor (suPAR) is implicated in the pathogenesis of native and recurrent FSGS.
Methods
We conducted a retrospective study of 25 adults with post-transplant FSGS. We investigated the relationship between suPAR levels and podocyte changes and the impact of therapy on podocyte structure. We assessed response to therapy by improvement in proteinuria, allograft function and resolution of histologic changes.
Results
A median of 15 (interquartile range: 10–23) plasmapheresis sessions was administered; 13 of the subjects also received rituximab. Median pre-treatment suPAR levels were higher among those with severe (≥75%) versus those with mild (≤25%) podocyte foot process effacement (13,030 vs. 4,806 pg/mL; P=0.02). Overall, mean ± standard deviation of proteinuria improved from 5.1 ± 3.8 to 2.1 ± 2.8 mg/dL (P=0.003), mean podocyte effacement decreased from 57 ± 33% to 22 ± 22 % (P=0.0001), estimated glomerular filtration rates increased from median (interquartile range) of 32.9 (20.6 – 44.2) to 39.3 (28.8 – 63.4) (P<0.0001) and suPAR levels decreased from a median of 6,781 pg/ml to 4,129 pg/ml (P=0.02) with therapy.
Conclusions
Podocyte effacement is the first pathological manifestation of FSGS post-transplant. The degree of podocyte effacement correlates with suPAR levels at time of diagnosis. Response to therapy results in significant reduction of suPAR levels and complete or significant improvement of podocyte effacement.
Keywords: kidney transplant, podocyte effacement, FSGS, suPAR, rituximab
Introduction
Primary focal segmental glomerulosclerosis (FSGS) is a common cause of end-stage renal disease (ESRD) in the U.S (1) and recurs after kidney transplantation in approximately one-third of cases (2) and up to 64% of high-risk patients (3). Recurrence of FSGS is associated with increased risk for renal allograft failure (4).
Loss of podocyte structural integrity and function is thought to be the central abnormality leading to FSGS. Circulating permeability factors likely play a major role in the pathogenesis of primary and recurrent FSGS (5). Recently, compelling evidence suggests that the soluble urokinase -type plasminogen activator receptor (suPAR) is one of these causative permeability factors (6). In two studies by Wei and colleagues (6, 7), the majority of participants with primary FSGS had significantly higher levels of suPAR compared to those with other glomerular diseases. Furthermore, patients with recurrent FSGS had higher levels of suPAR pre-transplantation and during the course of FSGS recurrence post transplant (6). In animal models, circulating two domain suPAR (D1–D2) activated podocyte β3 integrin leading to podocyte foot process effacement, proteinuria and FSGS-like histopathology (6).
Prior case series implicated that minimal change-like histopathological changes may precede classic FSGS lesions in native and transplanted kidneys (8, 9). Moreover, foot process effacement can be observed as early as one hour after reperfusion in those who ultimately developed recurrent FSGS (10). While successful management of FSGS recurrence using plasmapheresis with or without adjunctive rituximab is assumed to be associated with rapid resolution of podocyte foot process effacement (11), there have been only limited data to support that (12). Thus, the purpose of this study was to detail the evolution of renal histopathological and ultrastructural changes and evaluate renal parameters, including serum suPAR levels, in 25 individuals who received plasmapheresis with or without rituximab for FSGS following kidney transplantation.
RESULTS
Clinical Characteristics
Table 1 displays the sociodemographic and clinical characteristics of the study population with post transplant recurrent and de novo FSGS (n=25). Mean follow up duration is 15.6 ± 10.6 months. Recurrent FSGS patients (n=21) were drawn from a population of 105 adult renal transplant recipients aged 18 years or older with FSGS in the native kidney (93 biopsy-proven and 12 with probable FSGS); with approximate recurrence rate of 20%. Among the seven patients with previous kidney transplantation, five lost their previous allografts due to recurrent FSGS and two due to other causes.
Table 1.
Demographic and Clinical Characteristics of Participants (n=25)
| Male, n (%) | 13 (52) |
| Black, n (%) | 13 (52) |
| Mean Age at Transplant, y ± SD | 43 ± 12 |
| Mean Age at FSGS Diagnosis, y ± SD | 33 ± 11 |
| Median Duration on Dialysis, y (IQR) | 2 (1 – 6) |
| Pre-Transplant Urine, n (%) | 16 (64) |
| Median Pre-Transplant Proteinuria, g/g (IQR) (n=8) | 2.3 (1.1 – 10.0) |
| Primary Pre-Transplant Diagnosis, n (%) | |
| FSGS | 21 (84) |
| Other* | 2 (8) |
| Unknown | 2 (8) |
| No. of Transplants at Time of FSGS Recurrence, n (%) | |
| 1 | 18 (72) |
| 2 | 4 (16) |
| 3 | 3 (12) |
| Living Donor, n (%) | 15 (60) |
| Related | 5 (33) |
| Unrelated | 10 (67) |
| ABO-Incompatible Transplant, n (%) | 5 (20) |
| Median Time to Treatment Post-transplant, days (IQR) | 39 (4 – 287) |
| Pre-Treatment Laboratory and Renal Pathology Data | |
| Mean Proteinuria, g/g ± SD (n=23) | 5.04 ± 3.6 |
| Median Serum Creatinine, mg/dL (IQR) | 2.2 (1.8 – 3.2) |
| Median eGFR, mL/min/1.73 m2 (IQR) | 31.8 (21.0 – 43.8) |
| Mean Foot Process Effacement, % ± SD (n=24) | 58 ± 34 |
| Median suPAR, pg/mL (IQR) (n=12)‡ | 5460 (4269 – 6172) |
| Mean CRP, mg/L ± SD (n=12) (Ref. range 0–20) | 9.8 ± 4.3 |
Abbreviations: y, years; SD, standard deviation; IQR, interquartile range; g, gram
Among individuals who had pre-transplant urination and had no urine quantification data
One patient with IgA nephropathy and the other with lupus nephritis.
Excludes individuals who underwent ABO-incompatible renal transplantation
The median time to recurrent or de novo FSGS was 31 days (interquartile range (IQR): 5 – 225 days) after transplantation. Nine of recurrent FSGS patients had histopathological data available on their primary FSGS: three had collapsing, two had tip, and four had classic (NOS) FSGS variant (13). Of these individuals, four had histopathological data also available at the time of recurrence: three had concordant FSGS variants, and one subject who had primary classic FSGS had recurrent collapsing FSGS post transplant.
Prior to treatment, twenty-three (92%) subjects of our cohort had estimated glomerular filtration rates (eGFR) below 60 mL/min per 1.73 m2. Fourteen subjects had 3 g/g or greater of proteinuria, five of them had serum albumin levels below 3.5 g/dL. Data on serum lipid levels and presence of edema were not available to ascertain whether nephrotic syndrome was present in these patients.
Baseline pathological changes and correlation with suPAR Levels
Twenty-four subjects had renal biopsies available at time of post-transplant FSGS diagnosis; of these, six were obtained shortly after the initiation of therapy (mean of 5 days after treatment initiation, range: 3–9 days). On baseline renal biopsy, six of the subjects had histopathological changes consistent with FSGS on light microscopy (two with classic FSGS, one with tip and collapsing variant, two with collapsing variant and one with perihilar variants) while eighteen did not have any FSGS changes on light microscopy. Overall foot process effacement ranged from 13% to 100%.
Among subjects with renal biopsies available at diagnosis, pre-treatment suPAR levels were measured in 16 patients; five of them had ≤25% and four had ≥75% foot process effacement. suPAR levels significantly correlated with the severity of foot process effacement at baseline (r2=0.70, p=0.01). The median pre-treatment suPAR levels were more than two-fold higher among those with severe (≥75%) versus those with mild (≤25%) foot process effacement 13,030 (IQR: 7,679 – 17,858) vs. 4,806 (IQR: 4,450 – 5,961) pg/mL, respectively; P=0.02), although their median eGFR levels were similar; 22.3 mL/min/1.73 m2 (IQR: 15.7 – 51.9 mL/min/1.73 m2) vs. 37.9 mL/min/1.73 m2 (28.0 – 43.8 mL/min/1.73 m2), respectively; p=0.62.
Treatment outcomes
Twenty-four subjects underwent plasmapheresis with a median of 15 sessions (IQR: 10–23) received. One patient received only intensification of maintenance immunosuppression. Thirteen subjects were refractory to plasmapheresis and received adjunctive rituximab infusion. Nine (36%) subjects achieved complete remission, and ten (40%) individuals achieved partial remission.
Twenty patients had at least one follow-up biopsy, of which 15 were after full completion of the therapy. On follow-up biopsy, five of the subjects who had baseline light microscopic FSGS changes continued to have the same changes. Seven additional patients developed light microscopic FSGS changes on follow-up biopsies (four collapsing variant, one tip variant and two classic FSGS). Four of these seven patients were refractory to therapy and three had only partial response to therapy. Of the four refractory cases, three had allograft loss shortly after the FSGS recurrence. Of the three subjects who had partial response and subsequent FSGS changes on light microscopy, one had allograft failure after one year of the diagnosis.
The median time (Interquartile range (IQR)) between the end of treatment and post-treatment suPAR, serum creatinine, proteinuria and foot process assessments were 8 (2–52) days, 0 (0–1) day, 2 (0–19) days, and 25 (10–33) days, respectively. The median time (IQR) between the end of treatment and most recent available proteinuria measurement was 233 (127–381) days.
Although, the median eGFR improved significantly from 32.9 to 39.3 mL/min/1.73 m2 (p<0.0001) with treatment, the overall median (IQR) serum creatinine (SCr), although improved from 2.3 (1.7 – 3.3) to 1.9 (1.5 – 2.3), it did not reach statistical significance, p=0.08 (Table 2 and Figure 1a).
Table 2.
Change in Renal Parameters with Treatment in All Subjects †
| suPAR, pg/mL* | Median Pre (IQR) | Median Post (IQR) | P-value |
|---|---|---|---|
| Overall (n=7) | 6781 (5821 – 8557) | 4129 (3270 – 5949) | 0.02 |
| PXP alone (n=1) | 5821 | 3173 | na |
| PXP + Rituximab (n=6) | 7264 (6022 – 11416) | 4223 (3592 – 5949) | 0.03 |
| Foot Process Effacement, % | Mean Pre ± SD | Mean Post ± SD | P-value |
| Overall (n=16) | 57 ± 33 | 22 ± 22 | 0.0001 |
| PXP alone (n=6) | 76 ± 31 | 37 ± 29 | 0.01 |
| PXP + Rituximab (n=9) | 42 ± 30 | 14 ± 11 | 0.01 |
| Serum Creatinine, mg/dL | Median Pre (IQR) | Median Post (IQR) | P-value |
| Overall (n=24) | 2.3 (1.7 – 3.3) | 1.9 (1.5 – 2.3) | 0.08 |
| PXP alone (n=12) | 2.2 (1.9 – 4.2) | 1.8 (1.6 – 2.2) | 0.33 |
| PXP + Rituximab (n=12) | 2.3 (1.5 – 3.0) | 1.9 (1.3 – 3.0) | 0.25 |
| eGFR, mL/min per 1.73 m2 | Median Pre (IQR) | Median Post (IQR) | P-value |
| Overall (n=24) | 32.9 (20.6 – 44.2) | 39.3 (28.8 – 63.4) | <0.0001 |
| PXP alone (n=12) | 33.7 (17.5 – 43.4) | 42.4 (31.7 – 63.4) | 0.33 |
| PXP + Rituximab (n=12) | 32.9 (24.1 – 49.2) | 36.3 (27.1 – 65.5) | 0.14 |
| Proteinuria, g/g | Mean Pre ± SD | Mean Post ± SD | P-value |
| Overall (n=21) | 5.1 ± 3.8 | 2.1 ± 2.8 | 0.003 |
| PXP alone (n=10) | 4.3 ± 4.1 | 2.0 ± 3.5 | 0.15 |
| PXP + Rituximab (n=10) | 5.8 ± 3.8 | 2.3 ± 2.2 | 0.01 |
| Mean Peak ± SD | Mean Post ± SD | P-value | |
| Overall (n=22) | 11.0 ± 11.4 | 4.7 ± 10.2 | 0.0001 |
| PXP alone (n=9) | 4.5 ± 4.3 | 2.0 ± 3.8 | 0.17 |
| PXP + Rituximab (n=12) | 12.2 ± 13.3 | 6.8 ± 13.3 | 0.0002 |
SD, standard deviation; PXP, plasmapheresis, eGFR: estimated glomerular filtration rate.
Overall results include data from one subject who only received intensification of immunosuppression but not plasmapheresis or rituximab for FSGS treatment.
Excludes individuals who underwent ABO-incompatible renal transplantation
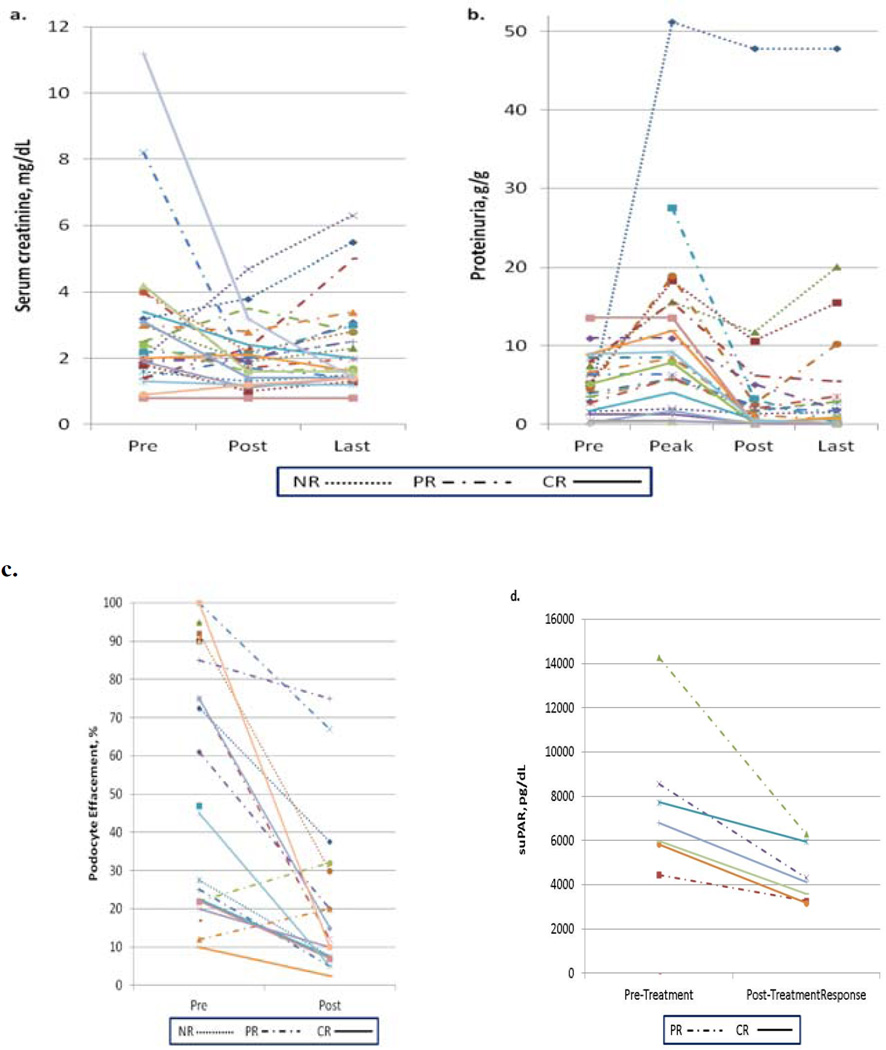
Figure 1. Changes in clinical parameters with therapy.
Panel a. Serum creatinine in mg/dL prior to treatment and upon completion of treatment. Panel b. Proteinuria in g/g prior to treatment, at peak level, upon completion of treatment, and last available level. Abbreviations: CR, complete remission; PR, partial remission; NR, no response. Panel c. Podocyte foot process effacement changes with therapy
Overall mean proteinuria decreased significantly from pre-treatment of 5.1 ± 3.8 g/g to post treatment of 2.1 ± 2.8 g/g, (P=0.003); this decrease was observed primarily among subjects who received rituximab in addition to plasmapheresis (Table 2). Among those who attained complete or partial remission (Table 3), mean pre- to post-treatment proteinuria declined significantly from 5.3 ± 3.9 to 1.5 ± 1.7 g/g (P=0.0005) (Figure 1b). This improvement persisted on the last available proteinuria measurements (mean peak to last available: 8.8 ± 7.0 g/g to 1.7 ± 2.6 g/g, p=0.0002) (Table 3). In contrast, individuals who did not respond to treatment (n=6) had persistent proteinuria, with a post-treatment mean proteinuria of 15.1 ± 18.7 g/g.
Table 3.
Change in Renal Parameters with Treatment Among Responders†
| Foot Process Effacement, % | Mean Pre ± SD | Mean Post ± SD | P-value |
|---|---|---|---|
| Overall (n=13) | 55 ± 34 | 22 ± 23 | 0.0009 |
| PXP alone (n=5) | 73 ± 34 | 38 ± 32 | 0.03 |
| PXP + Rituximab (n=7) | 40 ± 31 | 11 ± 6 | 0.05 |
| Serum Creatinine, mg/dL | Median Pre (IQR) | Median Post (IQR) | P-value |
| Overall (n=19) | 2.2 (1.4 – 3.4) | 1.9 (1.4 – 2.3) | 0.07 |
| PXP alone (n=9) | 2.0 (1.9 – 3.4) | 1.7 (1.6 – 2.1) | 0.21 |
| PXP + Rituximab (n=9) | 2.2 (1.4 – 3.0) | 2.0 (1.4 – 2.8) | 0.28 |
| Estimated GFR, mL/min per 1.73 m2 | Median Pre (IQR) | Median Post (IQR) | P-value |
| Overall (n=19) | 35.0 (27.3 – 44.6) | 42.1 (34.2 – 64.9) | 0.09 |
| PXP alone (n=9) | 37.9 (28.0 – 44.6) | 46.3 (42.1 – 64.9) | 0.26 |
| PXP + Rituximab (n=9) | 34.1 (27.8 – 43.8) | 36.1 (28.5 – 60.0) | 0.28 |
| Proteinuria, g/g | Mean Pre ± SD | Mean Post ± SD | P-value |
| Overall (n=18) | 5.3 ± 3.9 | 1.5 ± 1.7 | 0.0005 |
| PXP alone (n=8) | 4.3 ± 4.4 | 0.9 ± 1.1 | 0.07 |
| PXP + Rituximab (n=9) | 6.1 ± 3.7 | 2.1 ± 2.2 | 0.007 |
| Mean Peak ± SD | Mean Post ± SD | P-value | |
| Overall (n=18) | 8.8 ± 7.0 | 1.6 ± 1.8 | 0.0001 |
| PXP alone (n=8) | 9.7 ± 9.6 | 1.1 ± 1.3 | 0.02 |
| PXP + Rituximab (n=9) | 8.0 ± 4.6 | 2.1 ± 2.2 | 0.001 |
| Mean Peak ± SD |
Last Available Post Treatment ± SD |
P-value | |
| Overall (n=18) | 8.8 ± 7.0 | 1.7 ± 2.6 | 0.0002 |
| PXP alone (n=8) | 9.3 ± 9.1 | 1.8 ± 3.4 | 0.03 |
| PXP + Rituximab (n=8) | 8.3 ± 4.8 | 1.8 ± 1.9 | 0.002 |
SD, standard deviation; PXP, plasmapheresis
Overall results include data from one subject who only received intensification of immunosuppression but not plasmapheresis or rituximab for FSGS treatment.
The overall suPAR levels decreased from a median of 6,781 pg/ml to 4,129 pg/ml (P=0.02) with treatment (Table 2) and (Figure 1).
Improvements in these clinical parameters correlated with remarkable improvement in foot process effacement, which decreased from 55 ± 34% to 22 ± 23% (P=0.0009) (Figure 1c) in subjects who achieved complete or partial remission. Furthermore, the improvement in podocyte foot process effacements involved even patients who failed to respond to therapy in respect to proteinuria (Figure 2).
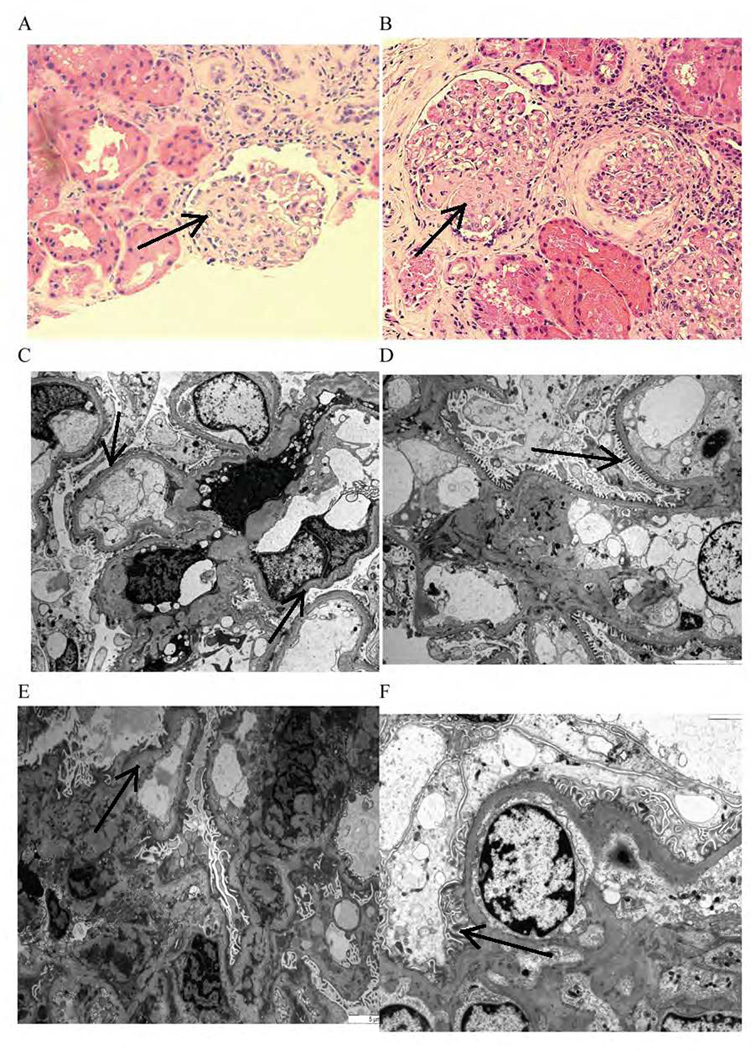
Figure 2. Histopathologic changes before and after therapy.
Biopsy taken 10 months after kidney transplantation from a patient with recurrent FSGS shows segmental glomerular sclerosis changes on light microscopy (arrow) before treatment (A) that did not change (arrow) after therapy (B). Electron microscopy (power of 3000) showed moderate (arrow) podocyte foot process effacement (C) that recovered (arrow) after therapy (D). Biopsy taken from another patient 3 months after transplantation; electron microscopy (scale of 5 µm) shows severe (arrow) podocyte foot process effacement (E) that recovered (arrow) after therapy (F); no light microscopic changes
C-Reactive Protein Levels in Individuals with Post-Transplant FSGS
To evaluate the association between suPAR and inflammation in post- transplant FSGS subjects, C-reactive protein (CRP) levels were measured in 15 individuals with post-transplant FSGS at time of diagnosis. Mean CRP levels were low (9.8 ± 4.3) (Ref. range 0–20) in these patients (Table 1).
DISCUSSION
Our study confirmed that the first pathological finding of recurrent renal allografts is podocyte foot process effacement. We demonstrated that circulating suPAR levels at time of diagnosis correlate with the severity of effacement. Furthermore, a complete or even partial response to therapy results in significant resolution of foot process effacement and improvement in proteinuria. In the seven patients with suPAR measured before and after therapy, we noted a significant decrease in the levels after therapy.
Early reports implicated the importance of podocytes in the recurrence of FSGS post-transplant. Harrison et al. reported a case of a 62 year old woman who suffered recurrent FSGS (14). Biopsies of native kidney and at the time of post-transplant recurrence showed similar alterations in the podocyte junctions. Hoyer and colleagues reported in 1972 that minimal change disease preceded FSGS recurrence in two cases (15). In a report of a patient with immediate recurrent FSGS, the allograft biopsy at one hour following transplantation showed minor glomerular abnormalities with partial foot process effacement on electron microscopy (16). We also recently reported on nineteen pairs of pre- and post-reperfusion biopsy results obtained from patients with FSGS undergoing renal transplantation (10). The mean number of effaced foot processes on the post-reperfusion biopsy was higher among the seven subjects who developed recurrent FSGS within 30 days compared to those who did not. Cheong and colleagues also reported on six children with early FSGS recurrence, five of them had only foot process effacement initially. However, three of them later developed segmental sclerosis on subsequent renal biopsies (17). Our study, which represents the largest sample to date, confirms that the earliest detectable ultrastructural change in recurrent FSGS after renal transplantation is podocyte foot process effacement.
Whether these podocyte changes resolve with treatment, however, has not been well delineated in the current literature. Artero and colleagues reported on their 10-year experience of recurrent FSGS in adults and children (12). They noted one individual whose foot process effacement resolved with plasmapheresis accompanied by decline in proteinuria and three who achieved remission with treatment, had only podocyte changes; whereas two who did not respond to therapy already had sclerosis prior to plasmapheresis. The recent case report by Gallon and colleagues in which an allograft from a recipient with early FSGS recurrence was subsequently re-transplanted to a different patient without primary FSGS supports the reversibility of foot process effacement in FSGS. These changes resolved on post-operative day 14 (18). In our study, we found that our therapy resulted in significant improvement in podocyte effacement decreasing from a mean of 55% to 22%. The decrease in podocyte effacement involved not only responders but also patients who did not respond to therapy by way of a significant decrease in proteinuria. These improvements may be due to the early recognition of FSGS recurrence and institution of therapy.
Furthermore, we found that suPAR levels correlated significantly with the degree of the ultrastructural changes in podocytes. In line with such a quick correction of podocyte ultrastructure are observations from a mother with FSGS who gave birth to a child with transient proteinuria (19). Both mother and child had high suPAR blood levels; the baby’s resolution of proteinuria is likely due to the decrease of suPAR serum levels and correction of podocyte injury.
In our study, post-transplant CRP levels were normal, arguing against the contribution of inflammation to the high suPAR levels observed among our post-transplant FSGS subjects. However, CRP represents only one marker of the activity of inflammatory pathways, and further analysis is needed.
While clinical improvement among recipients with post-transplant FSGS associated with treatment may have been mediated by plasmapheresis-related declines in suPAR, rituximab was found by Fornoni and colleagues to have direct stabilizing effects on podocyte function (3). Taken in the context of variable response to plasmapheresis with or without rituximab and the fact that suPAR binds and activate β3 integrin expressed on podocytes (6), prospective studies are needed to determine whether the gene expression that encodes for β3 integrin (ITGB3) in the recipient or allograft kidney (20) also has prognostic bearing on recurrent FSGS and response to treatment.
In spite of these significant findings of our study, it has a few limitations. Our main limitation was the retrospective design of our study, which may have led to missing some important cofounders that could have influenced our results. Although a prospective study in this field will be of great importance, our overall findings will have significant clinical application in kidney transplant recipients with post transplant FSGS. In addition, our study size was small, and findings reflect the clinical practice of a single center; therefore, our results may not be generalizable to other renal transplant centers. Furthermore, our study lacks control groups with non-recurrent FSGS or non-FSGS patients. This was in most part due to the limited available sera obtained at the same time points (as per our study methods) for suPAR measurements, in addition to incomplete clinical data on these individuals. Finally, while median suPAR levels exceeded 3000 pg/mL, reported as the threshold to discriminate between FSGS cases and other nephrotic syndromes, these data were reported in a population comprised primarily of individuals with primary FSGS (6, 7). Thus, this threshold may not be applicable for post transplant FSGS.
In summary, early post transplant recurrent or de novo FSGS manifests histologically by podocyte foot processes effacement. Response to therapy with plasmapheresis with or without rituximab may prevent light microscopic changes of FSGS from developing. suPAR may be one the main circulating permeability factors that causes recurrent FSGS via binding to the podocytes. However, larger and multi-centers prospective studies are needed to clarify the utility of suPAR measurements in predicting FSGS recurrence after transplantation and in monitoring response to therapy.
MATERIALS AND METHODS
The study was approved by the Johns Hopkins Medicine Institutional Review Board (Baltimore, MD). This is a retrospective case series study of all adult renal transplant recipients who underwent renal transplantation between January 1, 2003 to December 31, 2011 in our center and developed recurrent or de novo FSGS after kidney transplantation. Twenty-five individuals included in this study developed FSGS post-transplantation.
All subjects received thymoglobulin and high-dose steroid for induction, and a three-drug regimen consisting of mycophenolate mofetil, tacrolimus, and prednisone for maintenance immunosuppressions. Four patients with recurrent FSGS were switched from tacrolimus to cyclosporine during their treatment course for FSGS. None of the subjects received sirolimus.
Recurrent FSGS was defined by the presence of proteinuria as measured by urine protein-to-creatinine ratio and confirmed by 24-hour urine collection. A ratio of greater than 1 g/g in individuals who were anuric prior to renal transplantation (n=8) or a persistent increase in urine protein-to-creatinine ratio by greater than 1 g/g from baseline proteinuria among those who made urine prior to renal transplantation (n=16) was consistent with the diagnosis (data of urine before transplant was not available on one patient). The diagnosis was confirmed by histologic findings of FSGS on biopsies obtained prior to or within 10 days of treatment commencement. On the other hand, de novo FSGS post transplant was defined by: 1) new onset of more than 1 g/g proteinuria (or +2 on urinalysis in one patient with unavailable urine protein-to-creatinine ratio at time of diagnosis) in recipients whose primary cause of ESRD was not attributed to FSGS (n=2, IgA nephropathy and lupus nephritis) or was unknown (n=2, native kidney biopsy was not performed), in addition to 2) allograft biopsy with light microscopic changes consistent with FSGS; which is not explained by other pathologic findings.
Data Collection
Sociodemographic and clinical data were abstracted from patient medical records from the time of renal transplantation to 3 years following renal transplantation or the latest available clinical follow-up. Donor clinical characteristics included donor vital status, relatedness to the recipient and ABO-compatibility with the recipient. Recipient clinical characteristics included age at FSGS diagnosis, number of prior renal transplantations, duration of dialysis, serum creatinine, and proteinuria estimated by urine protein-to-creatinine ratio. Estimated glomerular filtration rate (eGFR) was measured using the CKD-Epi equation which adjusts for the variation in serum creatinine associated with age, gender and race (21).
Treatment with plasmapheresis was initiated at the time of diagnosis. A 100% albumin replacement fluid was routinely used. We used fresh frozen plasma when patients were to undergo a renal allograft biopsy within 48 hours or in cases of daily plasmapheresis. One or two rituximab infusions (dose of 375 mg/m2 of body surface area) were administered to patients who failed to achieve complete or partial remission with plasmapheresis therapy alone. Complete remission was defined by a decrease in proteinuria to below 1 g/g upon completion of the treatment course. Partial remission was defined as a decline in proteinuria by 50% from the peak proteinuria level, but remaining 1 g/g or greater at the end of therapy.
Renal histopathology was assessed by a renal pathologist using light microscopy, immunofluorescence and electron microscopy. The degree of podocyte foot process effacement was based upon biopsy reports as well as second assessments of the electron microscopy by another renal pathologist blinded to the original biopsy report and the recipient's outcome. These two assessments were then averaged to obtain the mean podocyte foot process effacement, which was then categorized into mild (≤25%), moderate (26–74%) and severe (≥75%). Baseline electron microscopy results were obtained from renal biopsies performed prior to or within 10 days of initiating therapy. Post-treatment foot process effacement was assessed from renal biopsies performed following the completion of the plasmapheresis sessions and rituximab infusion in refractory cases. A minimum of 10 capillaries was used to determine podocyte effacement.
Among recipients with stored sera available prior to and after treatment, suPAR levels and CRP levels were measured. suPAR levels were measured by using Quantikine Human uPAR immunoassay (R&D Systems) while CRP was measured by using Quantikine ELISA kit (R & D Systems). Individuals who underwent ABO-incompatible transplantation were excluded from the suPAR and CRP analyses.
Statistical Analysis
We performed descriptive analyses to evaluate the distribution of the recipients' baseline sociodemographic and clinical characteristics. The Kruskal-Wallis test was used to compare medians of non-normal continuous variables across categories while the Mann-Whitney U test was used to compare means of normally distributed variables across categories. Pre-treatment and post-treatment comparisons were performed using paired t-test and the Wilcoxon sign-rank test, as appropriate. Comparisons of pre- and post-treatment clinical parameters were conducted using the overall study population and then restricted only to recipients who attained complete or partial response to treatment. All statistical analyses were performed using Stata/ MP version 11.2 (StataCorp LP, College Station, TX, USA).
Acknowledgements
This work was supported in part by grants from the NIH (DK073495 and DK089394 to JR). AF is supported by NIH-NIDDK Grant R01DK090316 and by the Nephcure Foundation. MME is supported by NIH-NIDDK grant 1K23DK081317. We like to acknowledge Rachel Marino, NP for her help in collecting some of the clinical data.
Abbreviations
- CKD-Epi
Chronic kidney disease-epidemiology Collaboration
- eGFR
estimated glomerular filtration rate
- ESRD
End-stage renal disease
- FSGS
Focal segmental glomerulosclerosis
- IQR
Interquartile range
- SD
Standard deviation
- suPAR
Soluble urokinase-type plasminogen activator receptor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
1NA: participated in research design, writing the paper, performance of the research and data analysis. The author has no conflict of interest.
2CW: participated in suPAR measurements and analysis, writing the paper. The author is an inventor on pending or issued patents related to novel anti-proteinuric strategies and therapeutics.
3LJA: participated in providing the pathology images and writing the paper. The author has no conflict of interest.
1AMJ: participated in providing patients sera for suPAR measurements and wiring the paper. The author has no conflict in interest.
3LCR: participated in writing of the text and reviewing the pathologic data. The author has no conflict of interest.
4AF: participated in writing the paper. The author is supported by NIH-NIDDK Grant R01DK090316 and by the Nephcure Foundation. The author has no conflict of interest.
5GB: participated in writing the paper. The author has no conflict of interest.
1HR: participated in writing the paper. The author has no conflict of interest.
6KK: participated in collecting the clinical data. The author has no conflict on interest.
2JR: participated in research design, writing the paper, performance of the research and data analysis. The author is supported in part by grants from the NIH (DK073495 and DK089394) and is an inventor on pending or issued patents related to novel anti-proteinuric strategies and therapeutics.
1MME: participated in research design, writing the paper, performance of the research and data analysis. The author is supported by NIH-NIDDK grant 1K23DK081317. The author has no conflict on interest.
REFERENCES
- 1.Kitiyakara C, Eggers P, Kopp JB. Twenty-one-year trend in ESRD due to focal segmental glomerulosclerosis in the United States. Am J Kidney Dis. 2004;44:815. [PubMed] [Google Scholar]
- 2.Hickson LJ, Gera M, Amer H, et al. Kidney transplantation for primary focal segmental glomerulosclerosis: outcomes and response to therapy for recurrence. Transplantation. 2009;87:1232. doi: 10.1097/TP.0b013e31819f12be. [DOI] [PubMed] [Google Scholar]
- 3.Fornoni A, Sageshima J, Wei C, et al. Rituximab targets podocytes in recurrent focal segmental glomerulosclerosis. Sci Transl Med. 2011;3:85. doi: 10.1126/scitranslmed.3002231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abbott KC, Sawyers ES, Oliver JD, 3rd, et al. Graft loss due to recurrent focal segmental glomerulosclerosis in renal transplant recipients in the United States. Am J Kidney Dis. 2001;37:366. doi: 10.1053/ajkd.2001.21311. [DOI] [PubMed] [Google Scholar]
- 5.Savin VJ, Sharma R, Sharma M, et al. Circulating factor associated with increased glomerular permeability to albumin in recurrent focal segmental glomerulosclerosis. N Engl J Med. 1996;334:878. doi: 10.1056/NEJM199604043341402. [DOI] [PubMed] [Google Scholar]
- 6.Wei C, El Hindi S, Li J, et al. Circulating urokinase receptor as a cause of focal segmental glomerulosclerosis. Nat Med. 2011;17:952. doi: 10.1038/nm.2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wei C, Trachtman H, Li J, et al. Circulating suPAR in two cohorts of primary FSGS. J Am Soc Nephrol. 2012;23:2051. doi: 10.1681/ASN.2012030302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fogo A, Hawkins EP, Berry PL, et al. Glomerular hypertrophy in minimal change disease predicts subsequent progression to focal glomerular sclerosis. Kidney Int. 1990;38:115. doi: 10.1038/ki.1990.175. [DOI] [PubMed] [Google Scholar]
- 9.IJpelaar DHT, Farris AB, Goemaere N, et al. Fidelity and evolution of recurrent FSGS in renal allografts. J Am Soc Nephrol. 2008;19:2219. doi: 10.1681/ASN.2007121365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chang J-W, Pardo V, Sageshima J, et al. Podocyte Foot Process Effacement in Postreperfusion Allograft Biopsies Correlates With Early Recurrence of Proteinuria in Focal Segmental Glomerulosclerosis. Transplantation. 2012;93:1238. doi: 10.1097/TP.0b013e318250234a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vincenti F, Ghiggeri GM. New insights into the pathogenesis and the therapy of recurrent focal glomerulosclerosis. Am J Transplant. 2005;5:1179. doi: 10.1111/j.1600-6143.2005.00968.x. [DOI] [PubMed] [Google Scholar]
- 12.Artero M, Biava C, Amend W, Tomlanovich S, Vincenti F. Recurrent focal glomerulosclerosis: natural history and response to therapy. Am J Med. 1992;92:375. doi: 10.1016/0002-9343(92)90267-f. [DOI] [PubMed] [Google Scholar]
- 13.D’Agati VD, Fogo AB, Bruijn JA, Jennette JC. Pathologic classification of focal segmental glomerulosclerosis: a working proposal. Am J Kidney Dis. 2004;43:368. doi: 10.1053/j.ajkd.2003.10.024. [DOI] [PubMed] [Google Scholar]
- 14.Harrison DJ, Jenkins D, Dick J. An unusual interpodocyte cell junction and its appearance in a transplant graft kidney. J Clin Pathol. 1988;41:155. doi: 10.1136/jcp.41.2.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoyer JR, Vernier RL, Najarian JS, Raij L, Simmons RL, Michael AF. Recurrence of idiopathic nephrotic syndrome after renal transplantation. Lancet. 1972;2:343. doi: 10.1016/s0140-6736(72)91734-5. [DOI] [PubMed] [Google Scholar]
- 16.Sakai K, Takasu J, Nihei H, et al. Protocol biopsies for focal segmental glomerulosclerosis treated with plasma exchange and rituximab in a renal transplant patient. Clin Transplant. 2010;22:60. doi: 10.1111/j.1399-0012.2010.01279.x. [DOI] [PubMed] [Google Scholar]
- 17.Cheong HI, Han HW, Park HW, et al. Early recurrent nephrotic syndrome after renal transplantation in children with focal segmental glomerulosclerosis. Nephrol Dial Transplant. 2000;15:78. doi: 10.1093/ndt/15.1.78. [DOI] [PubMed] [Google Scholar]
- 18.Gallon L, Leventhal J, Skaro A, Kanwar Y, Alvarado A. Resolution of recurrent focal segmental glomerulosclerosis after retransplantation. N Engl J Med. 2012;366:1648. doi: 10.1056/NEJMc1202500. [DOI] [PubMed] [Google Scholar]
- 19.Kemper MJ, Wei C, Reiser J. Transmission of Glomerular Permeability Factor Soluble Urokinase Plasminogen Activator Receptor (suPAR) From a Mother to Child. Am J Kidney Dis. 2013;61:352. doi: 10.1053/j.ajkd.2012.10.011. [DOI] [PubMed] [Google Scholar]
- 20.Vijayan KV, Goldschmidt-Clermont PJ, Roos C, Bray PF. The Pl(A2) polymorphism of integrin beta(3) enhances outside-in signaling and adhesive functions. J Clin Invest. 2000;105:793. doi: 10.1172/JCI6982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]