Abstract
Recent, studies have shown that Tcf7l2, an important transcription factor in Wnt pathway, plays critical roles in oligodendrocyte development. In this article we report a study showing that Tcf7l2 is under tight regulation during myelin formation. We have found that during early development, Tcf7l2 mRNA appears much earlier than the protein, suggesting a regulation at the translational level. We induced demyelination in a mouse model by a dietary toxin, where remyelination followed after a few weeks, and found that Tcf7l2 protein was expressed specifically during the active remyelination phase. Similarly, in human patients with demyelination diseases, Tcf7l2 protein expression was specifically promoted in regions undergoing active remyelination. During remyelination, Tcf7l2 was only expressed in non-dividing oligodendrocyte precursors and was associated with modest levels of nuclear beta-catenin. We also documented that Tcf7l2 could form protein complex with Olig2, but not with Olig1. Our data showed that during myelin formation, Tcf7l2/beta-catenin is regulated temporally, spatially, and also at levels of expression. These data suggest a key role for Tcf7l2 in myelination/remyelination processes via a tightly controlled activation of Wnt/beta-catenin pathway and the interaction with Olig2.
Keywords: Tcf7l2, Myelin formation, Demyelinating diseases, Wnt, Beta-catenin, Olig2
Introduction
In the mammalian central nervous system, the myelin sheath formed by oligodendrocytes wraps around the axons and plays critical roles in signal transduction and in axon maintenance (Miller 2002). However, oligodendrocytes are very vulnerable to damages from trauma or autoimmune attack. The resulting demyelination condition leads to neurological diseases like multiple sclerosis, which can be profoundly debilitating and severely impact the patient's quality of life. In normal adult brain, oligodendrocyte progenitors have the capacity to replenish demyelinated regions. However, in some cases, pathological conditions can impede this process: progenitors are successfully recruited to the demyelinated area, but they fail to differentiate into mature oligodendrocytes (Chang et al. 2002; Kuhlmann et al. 2008). Many details about the process of remyelination are unclear, and limited therapeutic resources are available for treating demyelinating diseases.
Oligodendrocytes originate from multifunctional neural stem cells. During the last decade, considerable research effort has been aimed at understanding the transcriptional control of oligodendrocyte development. A number of transcription factors, including Olig1/2, Sox10, Nkx2.2, Nkx6.2, Yy1, Zfp488, Zfp191,and Mrf, are expressed by cells of the oligodendrocyte lineage: some of these factors (e.g., Olig2 and Sox10) regulate the specification of oligodendrocytes, others (e.g., Olig1, Sox10, Nkx2.2, and Yy1) play key functional roles in the differentiation of oligodendrocytes, while still others (e.g., Nkx6.2, Zfp488, Zfp191, and Mrf) are expressed at later stages to affect the myelination process (Arnett et al. 2004; Cai et al. 2010; He et al. 2007; Howng et al. 2010; Lu et al. 2002; Qi et al. 2001; Southwood et al. 2004; Stolt et al. 2002; Takebayashi et al. 2002; Wang et al. 2006; Xin et al. 2005; Zhou and Anderson 2002; Emery et al. 2009).
In an earlier study, we reported the results of a genome-wide screen for transcription factors that regulate the development of astrocytes and/or oligodendrocytes, and identified Tcf7l2, a transcription factor involved in the Wnt-signaling pathway, as an important regulator for the maturation of oligodendrocytes (Gray et al. 2004; Fu et al. 2009). Our studies showed that Tcf7l2 is specifically expressed in oligodendrocytes during the time window that is critical for myelin formation, i.e., right before the formation of the myelin sheaths. Functionally, Tcf7l2 likely regulates the expression of several myelin genes. In fact, the expression of CNPase, MBP, and PLP is drastically reduced in Tcf7l2 null mice (Fu et al. 2009). Two other groups have also reported the importance of beta-catenin/ Tcf7l2 pathway for myelin genes (Fancy et al. 2009; Ye et al. 2009). However, there are some discrepancies among the results from different groups. Studies with over-activating beta-catenin mouse models showed that activation of Wnt/beta-catenin inhibits myelin formation (Fancy et al. 2009; Ye et al. 2009), while Tcf7l2 knockout mice showed myelin defect phenotype (Fu et al. 2009; Ye et al. 2009), suggesting Tcf7l2/beta-catenin signaling promotes myelin formation. Our study in this case found that the previous reports actually might not contradict each other. Wnt-signaling is under tight regulation during myelin formation: (1) Tcf7l2 is associated with only moderate levels of betacat-enin to promote myelin formation. High levels or very low levels of Wnt signal activation would inhibit the process. (2) Tcf7l2 might also be under translational control during the early development of oligodendrocytes.
Methods
Human Tissue
Collection and research-related use of discarded human specimens stored in the department of pathology were approved by the Brigham and Women's Hospital Institutional Review Board (protocol #2002-P-000579: Histological studies of progressive multifocal leukoencephalopathy (PML)). Since these were de-identified, discarded specimens, informed consent was waived by the Institutional Review Board.
Animals
Animal husbandry was performed according to the approved protocol 03-131 by Institutional Animal Care and Use Committee (IACUC) at Dana-Farber Cancer Institute for all experiments reported. The maintenance and genotyping of Olig1 null mice have been reported previously (Arnett et al. 2004). Time-pregnant CD-1 mice were ordered from Charles River (Wilmington, MA). The Cuprizone-animal model has been reported previously (Arnett et al. 2004). In brief, C57BL/6 J mice of age 8–10 weeks were fed with a mixture of 0.2% cuprizone (Sigma-Aldrich, St. Louis, MO) in ground chow for up to six weeks. Cuprizone is a copper chelator that induces demyelination of the corpus callosum if administered orally to adult mice. Normal diet was given after six weeks of cuprizone treatment. The demyelination reached the peak in the fifth week. The remyelination occurred thereafter and was allowed to develop for two weeks in this study.
In Situ Hybridization and Immunostaining
In situ hybridization (ISH) was performed as described previously (Gray et al. 2004). Immunofluorescence (IF) procedures have also been published (Fu et al. 2002). Antibodies used in this study include anti-Tcf7l2 (1:200, Millipore, Billerica, MA), Olig2 (1:10,000, Chemicon, Billerica, MA), beta-Catenin (1:200, BD, San Jose, CA), and Ki67 (1:200, Novocastra, Buffalo Grove, IL). Vector NovaRED peroxidase substrate kit (Vector Laboratories, Burlingame, CA) was used for immunocytochemistry according to the manufacturer's protocol. Sections were then counter stained with H&E.
Immunoprecipitation
293T cells were grown to about 60% confluence and transfected with pcDNA3.1/V5:Tcf7l2 and pcDNA3.1/ myc:Olig2 or pcDNA3.1/myc:Olig1 by Lipofectamine 2000 according to the manufacture's protocol (Invitrogen, Grand Island, NY). Whole cell lysates were collected 48 h after transfection by using RIPA buffer supplemented with a Protease Inhibitor Complete Mini (Roche, Branchburg, NJ). For immunoprecipitation, cell lysate proteins were incubated with mouse anti-myc or anti-V5 mAb (Invitrogen) in RIPA buffer at 4°C for 2 h. The antigen–antibody complex was collected by adding protein-G Sepharose (GE healthcare, Piscataway, NJ) at 4°C for 1 h. After three washes with RIPA buffer, the complex-bound resin was suspended in SDS sample buffer, boiled, and resolved on a 12.5% SDS-PAGE gel. After western blotting, proteins were detected with mouse anti-Myc or V5 mAb conjugated with HRP (Invitrogen, Grand Island, NY) by using chemiluminescence with the ECL kit (Pierce, Rockford, IL), according to the manufacturer's instructions.
Image Analysis
ImageJ program (NIH) was used for quantitative analysis of the levels of nuclear beta-catenin. We used three cuprizone-fed mice with 6 week treatment for this study. The corpus callosum regions were double-stained with Tcf7l2 and beta-catenin antibodies. The average levels of nuclear beta-catenin in cells were measured by ImageJ program. For each section, we picked up more than 10 cells (n>[ 10) with the highest nuclear beta-catenin levels as reference cells. The average beta-catenin levels from those cells were arbitrarily designated as 100. Then, we calculated the relative strength of the average nuclear beta-catenin levels of all Tcf7l2 positive cells (n > [ 12) in the same section. For each animal, the data were collected from at least three sections.
Results
Tcf7l2 Transcripts are Expressed in Neural Stem Cells/ Progenitors at Early Stages and in Oligodendrocyte Lineage Cells at Later Stages
We have built a genome-wide transcription factor expression atlas in developing mouse CNS to systematically study the transcriptional control during neural development (Gray et al. 2004). We applied this atlas to screen for transcription factors that are involved in glial development and identified 12 transcription factors that are expressed in cells of the glial lineage. From these genes, we singled out Tcf7l2, an important Wnt-signaling molecule, for further study (Fu et al. 2009).
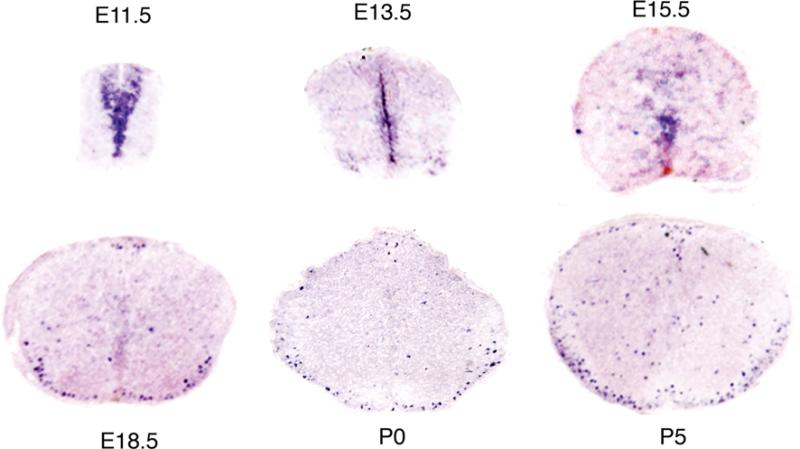
With the use of ISH, Tcf7l2 mRNA was detected in the mouse spinal cord as early as E11.5. From E11.5 to E15.5, Tcf7l2 mRNA was localized exclusively in the ventricular zone (Fig. 1). Its levels decreased during the developmental progress. At E15.5, very low levels of Tcf7l2 mRNA were detected. From E18.5 on, Tcf7l2 mRNA was no longer expressed in the ventricular zone, but was now visualized in the parenchyma of the spinal cord, predominantly in the future white matter (Fig. 1). In the adult mouse spinal cord, Tcf7l2 mRNA could not be detected with the use of ISH (data not shown). Our data suggest that Tcf7l2 developmental expression pattern has two stages: the early stage, the developmental period before E18.5, when Tcf7l2 mRNA is exclusively expressed in the ventricular zone; and the later stage, after E18.5, when Tcf7l2 is expressed mostly in the future white matter region of the spinal cord.
Fig 1.
Expression pattern of Tcf7l2 mRNA in the developing mouse spinal cord. Before E18.5, Tcf7l2 mRNA is restricted in the ventricular zone. After E18.5, Tcf7l2 mRNA expression spreads out, mostly into the (future) white matter
Tcf7l2 is Expressed During Remyelination Stages, in Human Patients, as well as in Animal Models of Demyelination
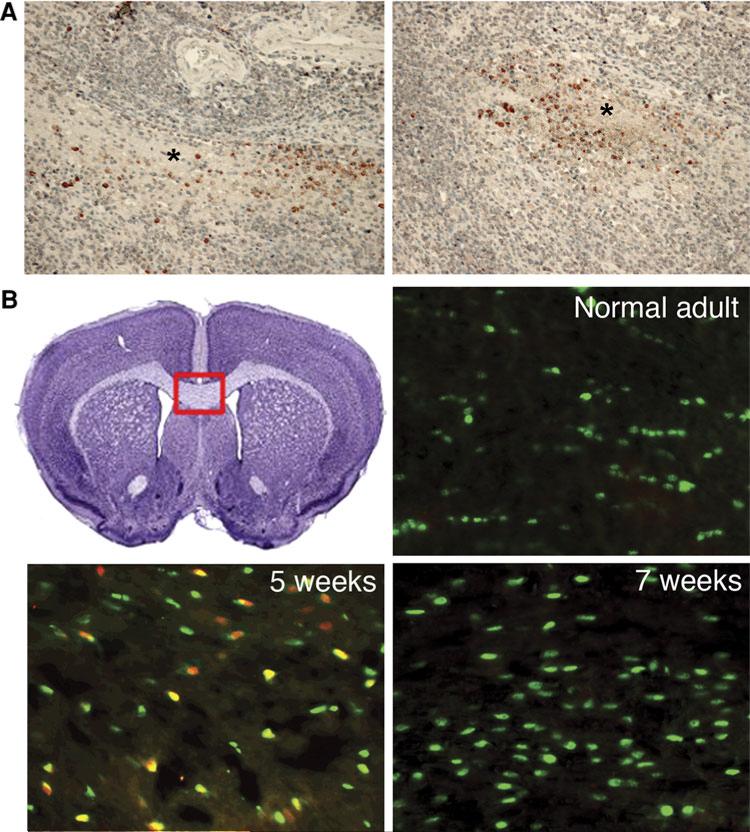
To explore the role of Tcf7l2 in the remyelination process, we studied its expression in sections from human brains with demyelination diseases. Tcf7l2 protein was not found in the normal brain tissue. However, in four cases of PML caused by JC virus, we detected Tcf7l2 protein specifically expressed at the edge of the demyelination plaque, where the process of remyelination was likely to occur (Fig. 2a). This differential expression of Tcf7l2 was also recapitulated in brains of patients with multiple sclerosis (MS) (Fancy et al. 2009, data not shown). For both diseases, Tcf7l2 protein was only found around demyelination lesions, presumably where the axons were actively being remyelinated by oligodendrocyte progenitors.
Fig 2.
Tcf7l2 protein is expressed in the brains of patients with PML and in the brains of the cuprizone-treated mice. a Brain sections of PML patients. Tcf7l2 protein (reddish brown) is expressed around the demyelination plaque (indicated by * symbol). The section is counter-stained with H&E. b Tcf7l2 protein (red) is expressed in the mouse corpus callosum (CC) after 5 weeks of cuprizone treatment. Before the treatment, only Olig2-positive cells (green) are seen in the CC region. After 5 week treatment, Tcf7l2 and Olig2 doublepositive cells (orange and yellow) are visible in the CC region. After 7 weeks, Tcf7l2 is no longer expressed, but Olig2-positive cells (green) remain in the CC region. The red frame indicates the region where the pictures are taken
Since Tcf7l2 expression showed spatial preference for the remyelinating regions in human demyelinating diseases, we proceeded to test the time preference of Tcf7l2 during the process of remyelination. To do this, we used cuprizone, a toxin known to cause demyelinating lesion followed by the process of remyelination. Cuprizone and other toxin-induced demyelination/remyelination animal models have been widely used to study the mechanism of remyelination (Arnett et al. 2004; Fancy et al. 2009). We found that Tcf7l2 is not expressed in the adult corpus callosum, where one would expect to find only mature oligodendrocytes and early oligodendrocyte progenitors. However, after five weeks of cuprizone treatment, the remyelination starts (Arnett et al. 2004) and Tcf7l2 protein is widely expressed in the Olig2 positive oligodendrocytes at the corpus callosum. After 7 weeks, when the remyelination process is almost completed, Tcf7l2 protein expression drops back to non-detectable levels as revealed by immunostaining (Fig. 2b).
The Potential Interaction of Tcf7l2 with Beta-Catenin and Olig2
Tcf7l2 is an important transcription factor involved in the Wnt-signaling pathway. Tcf7l2 alone can bind to the DNA to inhibit the transcription of downstream target genes. When the canonical Wnt pathway is activated, beta-catenin translocates from the cytoplasm into the nucleus, where it binds to Tcf transcription factors, including Tcf7l2. The beta-catenin binding switches the Tcf7l2 from a transcription inhibitor to an activator for downstream targets (Clevers 2006).
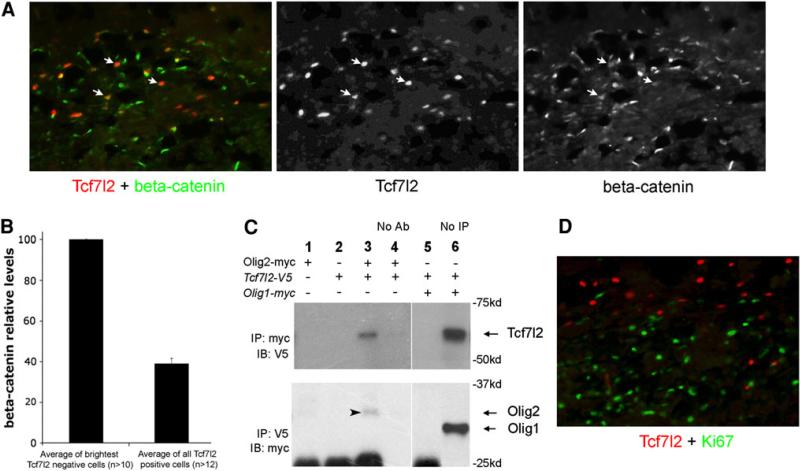
Co-immunolabeling experiments showed that during remyelination, Tcf7l2 was coexpressed with beta-catenin in the nucleus, suggesting that the canonical Wnt-signaling is activated and that Tcf7l2 is functioning as a transcription activator (Fig. 3a). However, further analysis reveals that Tcf7l2 only associates with moderate levels of nuclear beta-catenin, less than 40% to the highest nuclear beta-catenin levels in the same section (Fig. 3b), suggesting that during remyelination, the activation level of Wnt-signaling is under tight control. Double-labeling experiments showed that Tcf7l2 and Ki67, a cell division marker, are not co-localized during the remyelination (Fig. 3d), indicating that Tcf7l2 is expressed in post-mitotic oligodendrocytes. This result is similar to that reported for oligodendrocyte development (Fu et al. 2009).
Fig 3.
Tcf7l2 associates with nuclear beta-catenin and with Olig2. a Tcf7l2 (red) and beta-catenin (green) double staining in the mouse corpus callosum after 5 weeks of cuprizone treatment. Arrows point to the cells that are positive for Tcf7l2 and also exhibit weak nuclear beta-catenin expression. b The quantitative analysis of nuclear beta-catenin levels in Tcf7l2 positive cells. Comparing to the average of the highest nuclear beta-catenin levels in the same section, Tcf7l2-positive cells only associate with moderate levels of nuclear betacatenin (<40%) during the remyelination process. n, the total number of cells measured in each brain section. The error bar shows the value of the standard deviation. c Tcf7l2 protein can form protein complex with Olig2, but not with Olig1, in 293T cells. Cells are only transfected with Olig2-myc plasmid (Lane 1); only Tcf7l2-V5 plasmid (Lane 2); both Olig2-myc and Tcf7l2-V5 plasmids (Lane 3); both Olig2-myc and Tcf7l2-V5 plasmids with no antibodies used to pull down myc or V5 antigens (Lane 4, control); both Olig1-myc and Tcf7l2-V5 plasmids (Lane 5); both Olig1-myc and Tcf7l2-V5 plasmids with myc or V5 immunoblotting only (Lane 6). Arrowhead points to the Olig2 protein in the immunoblotting. Arrows indicate the positions of Tcf7l2, Olig1, and Olig2 proteins in the immunoblotting determined by molecular weight. d Tcf7l2 (red) is not expressed with Ki67 (green) in the mouse corpus callosum after 5 week cuprizone treatment
During the process of remyelination, the expression pattern of Tcf7l2 protein is very similar to that reported for the nuclear Olig1 protein, both in brains of human patients and in animal models (Arnett et al. 2004). We thus decided to examine whether Tcf7l2 could potentially interact with Olig1/2. Expression vectors carrying Tcf7l2, Olig1, and Olig2 were transfected into 293T cells. Tcf7l2 was tagged with V5 peptide, and Olig1/2 was tagged with myc peptide. Cell lysates were collected 48 h after transfection and used in Co-immunoprecipitation (CoIP) experiments. We found that Tcf7l2 did not exist in an immunoprecipitated protein complex with Olig1, but did exist in a complex with Olig2 (Fig. 3c).
Discussion
Tcf7l2 is Likely Under Translational Control During Early Development of Oligodendrocytes
We have previously shown that Tcf7l2 protein is expressed in post-mitotic, pre-myelinating oligodendrocytes (Fu et al. 2009). In the mouse spinal cord, it is expressed not earlier than E18.5 (Fu et al. 2009), a result that differed from our earlier findings from the ISH study done in the genomewide screening, where we reported that Tcf7l2 mRNA was expressed in the ventricular zone at E13.5 and in the white matter at P0 (Gray et al. 2004). To resolve this discrepancy in the timing of initial expression of Tcf712, we undertook the current, more detailed study of Tcf7l2 mRNA expression in the developing mouse spinal cord. We reported that Tcf7l2 mRNA expression has two spatially and temporally distinct patterns: at an early stage (before E18.5), Tcf7l2 is specifically in the ventricular zone, while at a later stage (after E18.5), Tcf7l2 is expressed mostly in the white matter. The spatiotemporal expression pattern of the protein only matches the late stage of the mRNA expression.
In general, protein expression occurs later than the transcription of mRNA. However, in this study, the time difference between the mRNA and protein expression of Tcf7l2 is more than 5 days. The time disparity could be due to a number of other causes: 1. Technical artifact: We think this is not likely, since we can detect the Tcf7l2 protein as early as E13.5 in neurons of the brain. 2. Alternative splicing: Since many alternatively spliced forms of Tcf7l2 have been detected in both mouse and human cells (Weise et al. 2010), it is possible that different isoforms were expressed at different developmental stages of oligodendrocytes, and were differentially recognized by our in situ probe and antibody. We found that both the probe used for ISH and the antibody recognize the similar region in the N-terminus of Tcf7l2, while the major difference among splicing isoforms is located in the C-terminus (Fu et al. 2009). It was determined that both the in situ probe and the antibody recognize most of the Tcf7l2 isoforms. 3. Tcf7l2 is under translation control, such as miRNA regulation, during the early stages of brain development when oligodendrocytes are starting to mature. We think this is the situation most likely to occur. We speculate that Tcf7l2 mRNA is under the control of miRNA activity, as several possible miRNA-binding sites have been predicted on Tcf7l2 mRNA: TargetScan predicts miR-212 and miR-132-binding sites at the 3-UTP region, while PicTar provides a list of 18 potential binding sites, including those two sites predicted by TargetScan.
The studies from the laboratories of Dr. Ben Barres and Dr. Richard Lu report that miRNA plays important roles in oligodendrocyte development (Zhao et al. 2010; Dugas et al. 2010). Note that Olig1, another transcription factor involved in myelin formation, also shows a mismatch between the protein and mRNA expression. Our unpublished data shows that mRNA for Olig1 can be detected as early as E9.5. However, the protein can only be detected after E18.5. These data suggest that both Olig1 and Tcf7l2 are under similar translational regulations, and this regulation might be important for oligodendrocyte development.
Tcf7l2 Expression During the Process of Remyelination
Previous studies showed that the expression of Tcf7l2 protein is closely coupled with the timing of myelin formation during oligodendrocyte development (Fu et al. 2009). After oligodendrocyte precursors exit the cell cycle, Tcf7l2 protein starts to be expressed– this is the phase that occurs right before the myelin formation. Once the oligodendrocytes are matured, Tcf7l2 protein expression is turned off. In other words, Tcf7l2 expression and myelin formation are temporally correlated. Further studies on Tcf7l2 knockout mice have since confirmed that Tcf7l2 is functionally important for myelin formation (Fu et al. 2009).
In this study, we have demonstrated that Tcf7l2 is also re-expressed during the remyelination process, underscoring its requirement for the process of myelin formation. We have examined several demyelination/remyelination models. In the dietary cuprizone mouse model, Tcf7l2 was specifically expressed during the remyelination period—no Tcf7l2 protein was detected before or after remyelination. Since the loss of myelin is almost completely recovered in this mouse model, it is very likely that Tcf7l2 has a role in promoting remyelination. Evidence from Olig1 null mice also supports the pro-remyelinating role of Tcf7l2: Olig1 null mice were not able to remyelinate their axons and died after the cuprizone treatment, and we could barely detect Tcf7l2 protein in the corpus callosum of the treated mice (data not shown). The study of human patients with demyelinating diseases is also consistent with mouse data: in patients with MS or PML, Tcf7l2 protein is expressed at the edges of the demyelination region, the area where active remyelination is taking place (Arnett et al. 2004). And finally, data from other labs are also consistent with our findings, in documenting that Tcf7l2 is activated during the remyelinating process in several animal models of demyelination, and in the brains of MS patients (Fancy et al. 2009).
The Role of Wnt-Signaling in Myelin Formation
Wnt-signaling pathway has key roles in the development of a number of tissues (Clevers 2006; Grigoryan et al. 2008), and increasing attention is now being focused on how this pathway might influence oligodendrocyte development. Wnt-signaling proteins are expressed during the early as well as the late stages of oligodendrocyte development (Kim et al. 2008), and functional studies show that over-activated Wnt-signaling inhibits the early phase of oligodendrocyte development either by pharmacological activation (Kim et al. 2008; Shimizu et al. 2005) or by genetic animal model (Fancy et al. 2009; Ye et al. 2009). As the cells are maturing, the exact function of Wnt-signaling is less clear: overactivating beta-catenin inhibits the development of oligodendrocyte precursors (Fancy et al. 2009; Ye et al. 2009), while conditional beta-catenin null mice display no obvious defects in oligodendrocyte development (Fancy et al. 2009). However, in Tcf7l2 null mice, the expression levels of myelin proteins are greatly reduced (Fu et al. 2009; Ye et al. 2009).
We have observed that Tcf7l2 positive oligodendrocytes coexpress moderate-to-low levels of nuclear beta-catenin, both during myelination and remyelination. At P5 spinal cord (Supplementary Fig. 1) or the corpus callosum after 5 weeks of cuprizone treatment (Fig. 2), Tcf7l2 is colocalized with weak nuclear beta-catenin. For reference, we observed that some of the neurons on the same section expressed much higher levels of nuclear beta-catenin. These data indicate that the level of Tcf7l2/beta-catenin signal activation is tightly controlled during the maturation of oligodendrocytes, which explains the reason that in studies of overactivating beta-catenin, the myelination process was inhibited (Fancy et al. 2009; Ye et al. 2009). Fancy et al. has recently found that Axin2 is important for remyelination, and pharmacological stabilization of Axin2 accelerates remyelination after injury (Fancy et al. 2011). The transcription of Axin2 is promoted by Tcf/beta-catenin, while the Axin2 protein promotes the degradation of beta-catenin. Therefore, Axin2 negatively feeds back on the Tcf/beta-catenin pathway and keeps the signal levels down. The importance of Axin2 in remyelination further supports the significance of low levels of Tcf7l2/beta-catenin during myelin formation.
Tcf7l2 is under the regulation of several transcription factors, such as Olig1/2, Nkx2.2, and Nkx6.1 (Fu et al. 2009). In this study, we further examined the protein– protein interaction between Tcf7l2 and Olig1/2. The relationship between Tcf7l2 and Olig1 is very interesting. Tcf7l2 expression is very similar to that of Olig1. Both have early expression of mRNA during early spinal cord development and the late protein expressions starting around E18.5. Tcf7l2 is coexpressed with Olig1 protein during development as well as during the remyelination process (Fancy et al. 2009), and both proteins are actively involved in myelin formation. However, under our experimental conditions, Olig1 did not form a protein complex with Tcf7l2, while Olig2 did—this finding suggests that Olig2 is also actively involved in myelin formation as a partner protein with other transcription factors, such as Tcf7l2 and/or Zfp488 (Wang et al. 2006). Studies from our lab and others have documented that Tcf7l2 maybe the converging point for transcriptional control of myelin formation (Supplementary Fig. 2) (Ye et al. 2009).
Till now, there are no effective therapies for remyelination in treating demyelinating diseases. The problem is not the recruitment of oligodendrocyte progenitors, but that the progenitors are unable to differentiate into myelinating oligodendrocytes. Our study showed that Wnt-signaling might be important for myelin formation. Specifically, the levels of Tcf7l2/beta-catenin could be critical. Our data showed that Tcf7l2 positive cells always express nuclear beta-catenin at low levels, suggesting that during myelin formation, Wnt-signaling pathway is activated, and the strength of the signal is tightly controlled. In pathological conditions, this balance and control mechanism could be easily broken and hard to recover, which could be one of the reasons that makes remyelination such a tough medical challenge.
In summary, our data support that Tcf7l2/beta-catenin plays critical roles during myelin formation, and the activation of this canonical Wnt pathway is tightly controlled during the process. Tcf7l2 is regulated by transcription factors expressed in oligodendrocyte progenitors and controls the downstream myelin genes. Tcf7l2 is expressed only in short time window—right before myelin formation, and probably interacts directly with Olig2 and beta-catenin to promote myelin formation. The levels of Tcf7l2/beta-catenin are tightly controlled during development and remyelination processes. At early developmental stages, Tcf7l2 is probably under translational control as its mRNA appears much earlier than its protein. During myelin formation, Tcf7l2 is only associated with moderate levels of beta-catenin. High levels or low levels of Tcf7l2/beta-catenin have been shown to be detrimental for myelin formation (Fu et al. 2009; Fancy et al. 2009; Ye et al. 2009). During pathological conditions, the control mechanism of levels of Tcf7l2/beta-catenin could be dysfunctional, which would result in unsuccessful remyelination. Since conventional Tcf7l2 null mice die at P0, we are currently working with inducible oligodendrocyte-specific Tcf7l2 knockout mice to study further details of Tcf7l2 function, which may provide direct evidence of its role during myelination and remyelination, as well as its interaction with beta-catenin/Wnt-signaling pathway.
Supplementary Material
Acknowledgments
This research was supported by National Natural Science Foundation of China Grant NO 30860131 and NIH grant R01NS059893, NIH 2P20RR017702-061A1.
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s10571-011-9778-y) contains supplementary material, which is available to authorized users.
Conflict of interest None.
Contributor Information
Hui Fu, Department of Physiology, Basic Medical College of Nanchang University, Nanchang 330006, Jiangxi, China; Department of Cancer Biology, Dana-Farber Cancer Institute, Harvard Medical School, Boston, MA 02115, USA.
Santosh Kesari, Division of Neuro-Oncology, Department of Neurosciences, UC San Diego, Moores Cancer Center, La Jolla, CA 92093, USA skesari@ucsd.edu.
Jun Cai, Department of Pediatrics, Kosair Children's Hospital Research Institute, University of Louisville School of Medicine, Louisville, KY 40202, USA j0cai002@louisville.edu.
References
- Arnett HA, Fancy SP, Alberta JA, Zhao C, Plant SR, Kaing S, Raine CS, Rowitch DH, Franklin RJ, Stiles CD. bHLH transcription factor Olig1 is required to repair demyelinated lesions in the CNS. Science. 2004;306:2111–2115. doi: 10.1126/science.1103709. [DOI] [PubMed] [Google Scholar]
- Cai J, Zhu Q, Zheng K, Li H, Qi Y, Cao Q, Qiu M. Colocalization of Nkx6.2 and Nkx2.2 homeodomain proteins in differentiated myelinating oligodendrocytes. Glia. 2010;58(4):458–468. doi: 10.1002/glia.20937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang A, Tourtellotte WW, Rudick R, Trapp BD. Premyelinating oligodendrocytes in chronic lesions of multiple sclerosis. N Engl J Med. 2002;346:165–173. doi: 10.1056/NEJMoa010994. [DOI] [PubMed] [Google Scholar]
- Clevers H. Wnt/beta-catenin signaling in development and disease. Cell. 2006;127:469–480. doi: 10.1016/j.cell.2006.10.018. [DOI] [PubMed] [Google Scholar]
- Dugas JC, Cuellar TL, Scholze A, Ason B, Ibrahim A, Emery B, Zamanian JL, Foo LC, McManus MT, Barres BA. Dicer1 and miR-219 are required for normal oligodendrocyte differentiation and myelination. Neuron. 2010;65:597–611. doi: 10.1016/j.neuron.2010.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emery B, Agalliu D, Cahoy JD, Watkins TA, Dugas JC, Mulinyawe SB, Ibrahim A, Ligon KL, Rowitch DH, Barres BA. Myelin gene regulatory factor is a critical transcriptional regulator required for CNS myelination. Cell. 2009;138:172–185. doi: 10.1016/j.cell.2009.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fancy SP, Baranzini SE, Zhao C, Yuk DI, Irvine KA, Kaing S, Sanai N, Franklin RJ, Rowitch DH. Dysregulation of the Wnt pathway inhibits timely myelination and remyelination in the mammalian CNS. Genes Dev. 2009;23:1571–1585. doi: 10.1101/gad.1806309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fancy SP, Harrington EP, Yuen TJ, Silbereis JC, Zhao C, Baranzini SE, Bruce CC, Otero JJ, Huang EJ, Nusse R, Franklin RJ, Rowitch DH. Axin2 as regulatory and therapeutic target in newborn brain injury and remyelination. Nat Neurosci. 2011;14:1009–1016. doi: 10.1038/nn.2855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu H, Qi Y, Tan M, Cai J, Takebayashi H, Nakafuku M, Richardson W, Qiu M. Dual origin of spinal oligodendrocyte progenitors and evidence for the cooperative role of Olig2 and Nkx2.2 in the control of oligodendrocyte differentiation. Development. 2002;129:681–693. doi: 10.1242/dev.129.3.681. [DOI] [PubMed] [Google Scholar]
- Fu H, Cai J, Clevers H, Fast E, Gray S, Greenberg R, Jain MK, Ma Q, Qiu M, Rowitch DH, Taylor CM, Stiles CD. A genome-wide screen for spatially restricted expression patterns identifies transcription factors that regulate glial development. J Neurosci. 2009;29:11399–11408. doi: 10.1523/JNEUROSCI.0160-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray PA, Fu H, Luo P, Zhao Q, Yu J, Ferrari A, Tenzen T, Yuk DI, Tsung EF, Cai Z, Alberta JA, Cheng LP, Liu Y, Stenman JM, Valerius MT, Billings N, Kim HA, Greenberg ME, McMahon AP, Rowitch DH, Stiles CD, Ma Q. Mouse brain organization revealed through direct genome-scale TF expression analysis. Science. 2004;306:2255–2257. doi: 10.1126/science.1104935. [DOI] [PubMed] [Google Scholar]
- Grigoryan T, Wend P, Klaus A, Birchmeier W. Deciphering the function of canonical Wnt signals in development and disease: conditional loss- and gain-of-function mutations of beta-catenin in mice. Genes Dev. 2008;22:2308–2341. doi: 10.1101/gad.1686208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y, Dupree J, Wang J, Sandoval J, Li J, Liu H, Shi Y, Nave KA, Casaccia-Bonnefil P. The transcription factor Yin Yang 1 is essential for oligodendrocyte progenitor differentiation. Neuron. 2007;55:217–230. doi: 10.1016/j.neuron.2007.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howng SY, Avila RL, Emery B, Traka M, Lin W, Watkins T, Cook S, Bronson R, Davisson M, Barres BA, Popko B. ZFP191 is required by oligodendrocytes for CNS myelination. Genes Dev. 2010;24:301–311. doi: 10.1101/gad.1864510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Kim SH, Kim H, Chung AY, Cha YI, Kim CH, Huh TL, Park HC. Frizzled 8a function is required for oligodendrocyte development in the zebrafish spinal cord. Dev Dyn. 2008;237:3324–3331. doi: 10.1002/dvdy.21739. [DOI] [PubMed] [Google Scholar]
- Kuhlmann T, Miron V, Cui Q, Wegner C, Antel J, Bruck W. Differentiation block of oligodendroglial progenitor cells as a cause for remyelination failure in chronic multiple sclerosis. Brain. 2008;131:1749–1758. doi: 10.1093/brain/awn096. [DOI] [PubMed] [Google Scholar]
- Lu QR, Sun T, Zhu Z, Ma N, Garcia M, Stiles CD, Rowitch DH. Common developmental requirement for Olig function indicates a motor neuron/oligodendrocyte connection. Cell. 2002;109:75–86. doi: 10.1016/s0092-8674(02)00678-5. [DOI] [PubMed] [Google Scholar]
- Miller RH. Regulation of oligodendrocyte development in the vertebrate CNS. Prog Neurobiol. 2002;67:451–467. doi: 10.1016/s0301-0082(02)00058-8. [DOI] [PubMed] [Google Scholar]
- Qi Y, Cai J, Wu Y, Wu R, Lee J, Fu H, Rao M, Sussel L, Rubenstein J, Qiu M. Control of oligodendrocyte differentiation by the Nkx2.2 homeodomain transcription factor. Development. 2001;128:2723–2733. doi: 10.1242/dev.128.14.2723. [DOI] [PubMed] [Google Scholar]
- Shimizu T, Kagawa T, Wada T, Muroyama Y, Takada S, Ikenaka K. Wnt signaling controls the timing of oligodendrocyte development in the spinal cord. Dev Biol. 2005;282:397–410. doi: 10.1016/j.ydbio.2005.03.020. [DOI] [PubMed] [Google Scholar]
- Southwood C, He C, Garbern J, Kamholz J, Arroyo E, Gow A. CNS myelin paranodes require Nkx6–2 homeoprotein transcriptional activity for normal structure. J Neurosci. 2004;24:11215–11225. doi: 10.1523/JNEUROSCI.3479-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolt CC, Rehberg S, Ader M, Lommes P, Riethmacher D, Schachner M, Bartsch U, Wegner M. Terminal differentiation of myelin-forming oligodendrocytes depends on the transcription factor Sox10. Genes Dev. 2002;16:165–170. doi: 10.1101/gad.215802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takebayashi H, Nabeshima Y, Yoshida S, Chisaka O, Ikenaka K, Nabeshima Y. The basic helix-loop-helix factor Olig2 is essential for the development of motoneuron and oligodendrocyte lineages. Curr Biol. 2002;12:1157–1163. doi: 10.1016/s0960-9822(02)00926-0. [DOI] [PubMed] [Google Scholar]
- Wang SZ, Dulin J, Wu H, Hurlock E, Lee SE, Jansson K, Lu QR. An oligodendrocyte-specific zinc-finger transcription regulator cooperates with Olig2 to promote oligodendrocyte differentiation. Development. 2006;133:3389–3398. doi: 10.1242/dev.02522. [DOI] [PubMed] [Google Scholar]
- Weise A, Bruser K, Elfert S, Wallmen B, Wittel Y, Wohrle S, Hecht A. Alternative splicing of Tcf7l2 transcripts generates protein variants with differential promoter-binding and transcriptional activation properties at Wnt/beta-catenin targets. Nucleic Acids Res. 2010;38:1964–1981. doi: 10.1093/nar/gkp1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin M, Yue T, Ma Z, Wu FF, Gow A, Lu QR. Myelinogenesis and axonal recognition by oligodendrocytes in brain are uncoupled in Olig1-null mice. J Neurosci. 2005;25:1354–1365. doi: 10.1523/JNEUROSCI.3034-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye F, Chen Y, Hoang T, Montgomery RL, Zhao XH, Bu H, Hu T, Taketo MM, van Es JH, Clevers H, Hsieh J, Bassel-Duby R, Olson EN, Lu QR. HDAC1 and HDAC2 regulate oligodendrocyte differentiation by disrupting the beta-catenin-TCF interaction. Nat Neurosci. 2009;12:829–838. doi: 10.1038/nn.2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X, He X, Han X, Yu Y, Ye F, Chen Y, Hoang T, Xu X, Mi QS, Xin M, Wang F, Appel B, Lu QR. MicroRNA-mediated control of oligodendrocyte differentiation. Neuron. 2010;65:612–626. doi: 10.1016/j.neuron.2010.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q, Anderson DJ. The bHLH transcription factors OLIG2 and OLIG1 couple neuronal and glial subtype specification. Cell. 2002;109:61–73. doi: 10.1016/s0092-8674(02)00677-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.