Abstract
MPS1 protein kinases are found widely, but not ubiquitously, in eukaryotes. This family of potentially dual-specific protein kinases is among several that regulate a number of steps of mitosis. The most widely conserved MPS1 kinase functions involve activities at the kinetochore in both the chromosome attachment and the spindle checkpoint. MPS1 kinases also function at centrosomes. Beyond mitosis, MPS1 kinases have been implicated in development, cytokinesis, and several different signaling pathways. Family members are identified by virtue of a conserved C-terminal kinase domain, though the N-terminal domain is quite divergent. The kinase domain of the human enzyme has been crystallized, revealing an unusual ATP-binding pocket. The activity, level, and subcellular localization of Mps1 family members are tightly regulated during cell-cycle progression. The mitotic functions of Mps1 kinases and their overexpression in some tumors have prompted the identification of Mps1 inhibitors and their active development as anticancer drugs.
Keywords: TTK, spindle checkpoint, mitosis, kinetochore, cell cycle
1. INTRODUCTION
Protein kinases are critical regulators of cell division. Apart from the cyclin-dependent kinases (CDKs), which are considered the master regulators, a suite of additional conserved kinases control progression through mitosis, including Polo, Aurora, Bub, NEK/NimA, and MPS1 kinases. Collectively, these have been called the mitotic kinases because of the widely conserved nature of their functions in mitosis. Here, we review the MPS1 family of protein kinases, which are still being discovered, dissected, and potentially exploited for therapeutics owing to their critical functions in the control of mitosis.
1.1. Discovery and Initial Characterization
The MPS1 gene (monopolar spindle) was first identified in the budding yeast, Saccharomyces cerevisiase. The original mutant allele, mps1-1, causes yeast cells to fail at a restrictive temperature in spindle pole body (SPB) assembly (the yeast centrosome) (1), a critical cell-cycle event that is necessary to form a bipolar spindle. The MPS1 gene was first cloned as an essential gene encoding an apparent protein kinase by Poch et al. (2). It was named RPK1, but MPS1 is used because it was the first published name. Lauze et al. (3) demonstrated that glutathione S-transferase (GST)-tagged Mps1 was indeed a protein kinase. GST-Mps1 exhibited robust autophosphorylation, as well as substrate phosphorylation of several common in vitro kinase substrates. Mps1 was able to phosphorylate serines/threonines and tyrosines, suggesting that it is a dual-specificity protein kinase, but thus far, no biologically relevant substrate is known to be phosphorylated on tyrosine by Mps1.
Phenotypic analysis of the original yeast MPS1 mutants identified key functions of the kinase. As noted above, mps1-1 was first discovered because of a defect in yeast SPB duplication, leading to an aberrant monopolar spindle. It was also observed that mps1-1 mutant cells failed to arrest in mitosis with the monopolar spindle defect unlike other mutants defective in SPB duplication [kar1 (4), cdc31 and mps2 (1)]. A subsequent study demonstrated that this phenotype was because of the role of Mps1 in the spindle checkpoint (5). In addition, Hardwick et al. (6) showed that Mps1 overexpression caused mitotic arrest by triggering the spindle checkpoint and identified the checkpoint protein, Mad1, as the first Mps1 substrate. All of the original MPS1 alleles caused defects in both SPB duplication and in the spindle assembly checkpoint at a restrictive temperature (7). Interestingly, electron microscopic examination of the SPBs in these various strains revealed that Mps1 is required for multiple steps of SPB assembly.
Prior to cloning of the yeast MPS1 gene, Mps1 orthologs from humans [phosphotyrosine-picked threonine kinase/threonine and tyrosine kinase (PYT/TTK) aka hMPS1] (8, 9) and mice [esk (EC STY kinase aka MmMps1)] (10) had been discovered. Intriguingly, these kinase genes were identified in screens using antiphosphotyrosine antibodies that tested expression libraries for protein kinases that autophosphorylate on tyrosine residues. Indeed, these kinases phosphorylate serine, threonine, and tyrosine residues in vitro, offering the initial evidence that this is a family of dual-specificity protein kinases. Also, it was observed that both mRNA and protein levels of Mps1/TTK are readily detectable in all proliferating human cells and tissues but are markedly reduced or absent in resting cells and in tissues with a low proliferative index (11).
1.2. Mps1 Kinase Features
MPS1 kinase family members are ~85–95 kDa and have conserved C-terminal kinase domains. The N-terminal domains of the family members appear unrelated, and they lack any unifying motif [such as the Polo box observed in the Polo family of kinases (12)]. Nonetheless, the kinase domain is distinctive enough to identify family members (http://www.signaling-gateway.org/molecule/query?afcsid=A000882&type=blast&adv=latest). The crystal structure of the kinase domain reveals interesting features that are discussed below.
1.3. MPS1 Distribution and Diversity
MPS1 kinase genes are found in most eukaryotes. Interestingly, there is no well-documented case of a genome containing paralogs of an MPS1 gene. However, MPS1 isoforms generated by alternative splicing have been predicted in humans and observed in mice (10). Orthologs are easily identified in fungi, vertebrates, and invertebrates, like Drosophila, as well as in plants, including the ancient plant lineage of lycophytes (Selaginella moellendorffii), diatoms (Phaeodactylum tricornutum), and alga (Chlamydomonas) (13). Although no validated MPS1 has been identified in the nematode Caenorhabditis elegans, there are orthologs in the pathogenic nematode (Globodera), as well in flat and round worms.
2. MPS1 FUNCTIONS
MPS1 kinases have multiple roles in mitosis that we briefly survey here. The most widely conserved and prominent function of these kinases is to ensure proper biorientation of sister chromatids on the mitotic spindle at kinetochores, and this function involves the spindle checkpoint. Along with being implicated in other cellular processes, MPS1 kinases also function from the earliest steps of mitosis, including spindle pole duplication, to the latest steps of mitotic exit and cytokinesis.
2.1. Spindle Pole Assembly
As described above, the budding yeast MPS1 gene was identified by a mutation that is defective in SPB (centrosome) duplication. A collection of MPS1 alleles revealed that the kinase acts in multiple steps of the duplication pathway (7), and the kinase has been shown to phosphorylate numerous SPB components. These include Spc29 and Spc42, which are fungus specific, whose assembly and stability are controlled by Mps1 phosphorylation (14–16). The more widely conserved SPB components that are Mps1 substrates include centrin (Cdc31) (14), the γ-tubulin complex component Spc98 (17), and the Spc110 tether that holds the γ-tubulin complex (Tub4, Spc98, Spc97) to the SPB (18). Centrin (Cdc31) is a small, EF-hand calcium-binding protein (19), and its phosphorylation by Mps1 influences its interaction with a binding partner (14). Phosphorylation of Spc98 is only found on the nuclear pool of the γ-tubulin complex, possibly influencing its interaction with Spc110 (17). Likewise, Mps1 phosphorylation of Spc110 (in conjunction with phosphorylation by Cdc28; the yeast CDK) is required for interaction with Spc97 (18). Interestingly, both Mps1 and Cdk phosphorylate most of these substrates such that combinatorial control appears to be the rule (20, 21).
Mps1 is localized to SPBs in yeast (15), and the mammalian enzymes are localized to centrosomes (Figure 1). Overexpression of mammalian Mps1 leads to overduplication of the centrosomes, and overexpression of a kinase-inactive allele blocks centrosome duplication (reviewed in Reference 22). Despite these results, RNAi experiments have produced contradictory results concerning a requirement for Mps1 in centrosome duplication (22). Recently, Mps1 was deleted from human cell lines using cre-lox, and these cells were capable of centrosome duplication (23). Similarly, the fission yeast ortholog, Mph1 (the Schizosaccharomyces pombe Mps1 homolog), is not required for SPB duplication (24), nor is the Drosophila ortholog, Ald, required for centrosome duplication (25). Finally, C. elegans appears to lack an MPS1 ortholog and can execute centriole and centrosome duplication.
Figure 1.
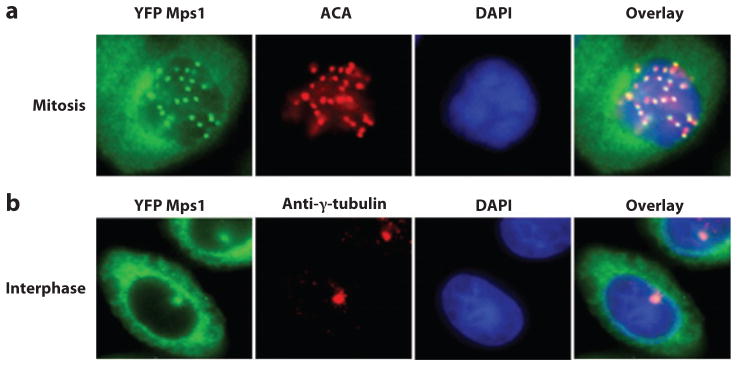
Localization of Mps1 in vertebrate mitotic and interphase cells. (a) Kinetochore localization of yellow fluorescent protein (YFP) Mps1 in mitotic SW480 cells. Anti-centromere antibodies (ACA) and 4′,6-diamidino-2-phenylindole (DAPI) were used to stain kinetochores and chromosomes. (b) YFP Mps1 is localized to centrosomes and the cytosol during interphase. Centrosomes and nuclei were stained by anti-γ-tubulin and DAPI, respectively.
Nonetheless, Mps1 influences centrosome duplication in human cells (reviewed by Reference 22). Furthermore, the centrosomal levels of hMps1 are exquisitely controlled, separately from other pools of the kinase (discussed below). Additionally, hMps1 centrosomal substrates, such as mortalin (26) and centrin 2 (27), have been identified. The phosphorylation of centrin 2 is required for its ability to stimulate centriole (the microtubule-based structural core of the centrosome) assembly (27). Remarkably, the major site of centrin 2 phosphorylation by hMps1 is T118 (27), which is the site analogous to the yMps1-phosphorylated T110 on the yeast centrin Cdc31 (14). These results suggest a deeply conserved regulatory event.
2.2. Kinetochores and the Spindle Assembly Checkpoint
MPS1 kinases have universally conserved functions at kinetochores. In yeast, Mps1 was implicated in the spindle checkpoint (5, 6) and was later shown to be localized to kinetochores (15). The spindle checkpoint monitors the correct bipolar attachment and tension of all chromosomes to the mitotic spindle. The cells are held at metaphase until every chromosome is properly attached; then the cells can proceed into anaphase. The molecular target of the checkpoint is the anaphase-promoting complex (APC), a ubiquitin ligase that targets mitotic cyclins and other proteins for destruction, allowing the cells to segregate their chromosomes. The APC is controlled by the Cdc20 activator, which in turn can be inactivated by the checkpoint protein Mad2. Mad2 is activated in the course of cycling on and off unattached kinetochores. A variety of other checkpoint proteins act with Mad2, both on and off of the kinetochore, to inhibit Cdc20 activity and therefore inhibit APC.
Xenopus Mps1, XlMps1, was the first vertebrate ortholog implicated in the spindle checkpoint and localized to kinetochores (28). Xenopus oocyte extracts require XlMps1 for mitotic arrest and for the recruitment of Mad2 and other checkpoint proteins to the kinetochore. Human Mps1 is also found at kinetochores and is required for the activation and maintenance of the spindle checkpoint (29, 30). These results have been repeated in several recent studies using a variety of tools, including depletion of hMps1, conditional deletion of the hMps1 gene, and small-molecule inhibition of the native or engineered forms of the kinase (reviewed in Reference 31). In most systems, kinetochore localization of Mad2 also requires active hMps1, although Mad2 appears not to be a hMps1 substrate.
Kinetochore localization of checkpoint proteins is important for their function. In Xenopus, Cenp-E localization to kinetochores requires XlMps1 (28), and CENP-E has been identified as an Mps1 substrate in vitro (32). However, this result, and similar dependencies on Mps1 for kinetochore localization of various checkpoint proteins, has not been universally observed. Lan & Cleveland (31) carefully document the various discrepancies and propose that they arise from the use of various Mps1-inactivating methods and different cell types. Some of these studies, but not all, show the loss of several checkpoint proteins from the kinetochore when Mps1 is inactivated, consistent with work in Xenopus. Similarly, the Mps1 overexpression-induced arrest in yeast is dependent on the function of each of the checkpoint proteins. Collectively, these results suggest Mps1 is near the top of the checkpoint-signaling pathway. However, the complexity of the data indicates that a linear pathway may be too simple a model for the checkpoint. An alternative view is that hMps1 is a linchpin in a checkpoint network such that the absence of Mps1 activity disrupts several checkpoint activities, leading to catastrophic failure of the network and other spindle-related functions (31). These predictions are complicated by the fact that Mps1 likely has several substrates in this pathway or network. Already known Mps1 checkpoint substrates are Mad1 (in yeast), Cenp-E, and Mps1 itself (6, 32, 33).
Interestingly, Mps1 can act in the checkpoint without being present at the kinetochore. The overexpression of yMps1 is capable of imposing a mitotic arrest in ndc10-1 strains (34), which normally do not arrest because the mutation destroys the kinetochore and obviates its ability to act in checkpoint signaling. A similar phenomenon has been observed in human cells using a truncated allele of hMps1 that does not localize to kinetochores but retains the catalytic domain (23). This allele can still activate the mitotic checkpoint complex (35), which inhibits Cdc20 during mitosis. Mps1 also contributes to the formation of an interphase APC inhibitor that shares components with the mitotic checkpoint complex, such as Mad2 and BubR1 (23). Although these proteins can inhibit Cdc20 in vitro without Mps1 (36), their association in vivo is dependent on Mps1 activity (23). Indeed, Mps1 is so critical to controlling normal mitotic progression that cells lacking Mps1 activity transit mitosis faster than cells with the activity (reviewed in Reference 31).
Prior to checkpoint signaling, both the Aurora B and Mps1 kinases are required for the proper bipolar spindle attachment of chromosomes. These kinases are involved in resolving syntelic attachments in which both kinetochores of replicated sister chromatids are attached to the same pole instead of their correct bipolar attachment (23, 37–41). The dependency relationship between Aurora B and Mps1 in vertebrates is a point of contention. In some studies, Aurora B activity is reduced upon reduction of Mps1 activity (42, 43), placing Aurora B downstream of Mps1. One reported mechanism for this dependency is via phosphorylation of the chromosomal passenger protein Borealin, which influences Aurora B activity (42, 44). However, other studies, using various hMps1 inhibitors or inhibitable hMps1 alleles, found that Aurora B activity was unchanged by reducing Mps1 activity (23, 39, 40). Indeed, two of these studies (39, 40) have shown instead that reducing Aurora B activity results in reduced hMps1 at kinetochores, similar to findings in Xenopus extracts (45). Saurin et al. (46) report that Aurora B and the kinetochore component Hec1/hNdc80 are both required for Mps1 recruitment to the kinetochore, a precondition that can be circumvented by tethering Mps1 to the kinetochore. Furthermore, the kinetochore recruitment of Mps1 is necessary for timely activation of Mps1 in mitosis. These results place Aurora B upstream of hMps1 in the spindle checkpoint pathway, as seen in yeast (37). However, the yeast kinetochore protein Dam1 is a substrate of both Ipl1 [the yeast Aurora B kinase (47)] and yMps1 (48), suggesting that the kinases collaborate in controlling kinetochore attachment. Similarly, in reviewing recent findings, Lan & Cleveland (31) also suggest that shared substrates may explain some of the complexity in Aurora B and Mps1 interactions. Their candidate substrate for analysis is CENP-E, which is phosphorylated by both Aurora B (49) and Mps1 (32). Ndc80/Hec1 should be considered as well, as the yeast Ndc80 protein is an Mps1 substrate (50), and Hec1/hNdc80 is a substrate of Aurora B (51). Finally, Ipl1 and the chromosomal passenger complex in yeast can act in a pathway distinct from yMps1, Sgo1 (shugoshin), and Bub1 in processing syntelic attachments (52).
The collective Mps1 functions in chromosome segregation are sufficient to make the enzyme essential in most organisms [though S. pombe Mps1 (Mph1) is not essential (24)]. The use of inhibitable alleles in budding yeast (53) and in human cells (reviewed in Reference 31) reveals that cells die without Mps1 function, likely because of severe aneuploidy. In zebrafish, Mps1 (called nightcap) has also been found to be especially crucial for the very rapid and extensive cell proliferation during tissue regeneration, presumably because it prevents excess aneuploidy (54–56).
2.3. Other Signaling Pathways
Several lines of evidence implicate Mps1 in the genotoxic stress response. Genotoxic stress, such as DNA damage, causes tumor cells to arrest at G2/M or G1, or to commit apoptosis depending on the status of p53. Mps1 influences these responses through multiple mechanisms. Upon exposure to X-ray or UV irradiation, robust G2/M arrest of HeLa cells requires the activity of Mps1, which has been attributed to direct interaction between Mps1 and CHK2/Rad53/Cds1. Mps1 has been shown to phosphorylate CHK2 at Thr68 (57). CHK2 reciprocates Mps1’s action by phosphorylating Mps1 on Thr288 and increasing its stability, thereby creating a positive feedback loop for CHK2 Thr68 phosphorylation (58). Disruption of this positive feedback attenuates the DNA damage checkpoint at G2/M arrest (57). The Bloom syndrome protein (BLM) is another Mps1 target in the DNA damage pathway (59). BLM phosphorylation at Ser144 by Mps1 promotes its association with and phosphorylation by Polo-like kinase. Ser144 phosphorylation is important for sustained mitotic arrest in response to microtubule poisons and for accurate chromosome segregation (59).
Mps1 may also be involved in another facet of genotoxic stress response by regulating phosphorylation and subcellular localization of c-Abl. c-Abl is phosphorylated at Thr735, and pThr735-c-Abl normally localizes in cytosol, but it enters the nucleus upon exposure to oxidative stress (60). Mps1 has been identified as the Thr735 kinase, and phosphorylation of c-Abl at Thr735 enhances its association with 14-3-3 protein and cytoplasmic sequestration (60). It has been proposed that Mps1 phosphorylates c-Abl under normal and oxidative stress conditions.
The function of Mps1 in genotoxic response depends on the status of p53. Mps1 is required for the apoptotic response in p53 null cells exposed to the topoisomerase I inhibitor (CPT-11) (61), and Mps1 suppression partially overrides CPT-11-induced cell death. In the presence of functional p53, however, CPT-11 treatment leads to growth arrest without mitotic entry (61). This result suggests that cancer cells with high levels of Mps1 but defective p53 checkpoint pathways are susceptible to DNA damage–induced cell death. There is also a direct link between Mps1 and p53. In response to microtubule poisons, p53 is stabilized and phosphorylated at Thr18, which has been attributed to Mps1 (62). Phosphorylation of p53 by Mps1 may contribute to the postmitotic checkpoint, which arrests cells in G1, thereby preventing further increase in DNA content and genome polyploidization (62).
Mps1 has also been implicated in modulating other cellular signaling responses. Depolymerization of microtubules by nocodazole results in phosphorylation of Smad2 and Smad3 proteins at the C-terminal SSXS motif, a site normally targeted by TGF-β type I receptor (63, 64). Interestingly, this event requires Mps1 and is independent of TGF-β type I receptor (64). Mps1 interacts with Smad4 and phosphorylates Smad2/3 in vitro. Phosphorylation of Smad2 at the C-terminal SSXS motif and at the linker region increases significantly during mitosis. However, the significance of Smad2 mitotic phosphorylation remains unclear. Also, Mps1 has been shown to be a negative regulator of the MAP kinase (MAPK) pathway in yeast (65), although the specific Mps1 target(s) have not been identified.
2.4. Cytokinesis
Mps1 RNAi in human cells led to the appearance of multinucleated cells and to the proposal that Mps1 is involved in the exit from mitosis and/or cytokinesis (66). Hints of this from budding yeast include localization of Mps1 to the bud neck (67) and interaction with a Mob1 (Mps1 one binder) protein (68). Mob1 binds and activates the Dbf2 protein kinase, and the complex acts in the mitotic exit network (69). Although the interaction of yMps1 and Mob1 is not understood, Mps1 is inactivated as cells exit mitosis (70). A more direct link between Mps1 and cytokinesis comes from the discovery of an hMps1-binding partner and substrate, MIP1 (Mps1 interacting protein 1), which is a component of the actin cytoskeleton (71). MIP1 RNAi led to the accumulation of multinucleate cells and disorganization of the actin cytoskeleton. Live-cell recordings revealed a spindle rocking phenotype indicative of difficulties in organizing the cytokinetic furrow. It is not known how MIP1’s interaction with, or phosphorylation by, hMPS1 affects its function.
2.5. Meiosis
Disruption of MPS1 function in meiotic yeast cells (72), during meiosis I in mouse oocytes (73), female meiosis in Drosophila melanogaster (74, 75), and germ cell production in zebrafish (76), leads to severe chromosome missegregation and aneuploidy. These defects may all arise from the Mps1 mutants’ failing to maintain the spindle checkpoint and/or to properly attach chromosomes on the meiotic spindle, suggesting similar Mps1 functions in meiosis as detailed above in mitosis. In fact, Straight et al. (72) were able to demonstrate defects in both meiosis I and meiosis II segregation. Much of this work was done with hypomorphic alleles (25, 72, 76), suggesting that meiotic chromosome segregation is particularly sensitive to disruption in Mps1 activity. For instance, during meiosis I, hypomorphic alleles of the Drosophila Mps1 gene, Ald, destroy the metaphase pause, which normally leaves the spindles ample time to segregate the nonexchange chromosomes, leading to their loss in ald mutant flies (25).
Separate from spindle-related functions, Mps1 has been implicated in other meiotic and germ cell formation functions. Yeast Mps1 is required for both rounds of meiotic SPB duplication (72). Also in yeast, Mps1 is required for the postmeiotic event of spore formation. This developmental pathway depends on a transcriptional regulatory network and the function of a MAPK cascade. Mutant strains in MPS1 retain the normal function of the transcription regulatory network, but the mps1− phenotypes resemble defects in the Ste20-family kinase Sps1, such that Mps1 may function with this kinase to control specific aspects of the spore formation program (72).
XlMps1 has been implicated in CSF (cytostatic factor) function, which causes meiosis II metaphase arrest in eggs by inhibiting APC. Two distinct pathways, Mos dependent and CDK2/cyclin E dependent, contribute to the CSF arrest. XlMps1 is required for the CDK2/cyclin E-mediated arrest of cycling extracts (77). Paradoxically, XlMps1 activity is reduced at CSF arrest and must be restrained for extracts to exit CSF arrest. This regulation, as well as Mps1 synthesis, is dependent on CDK2/cyclin E and is associated with different electrophoretic versions of XlMps1, suggesting control by phosphorylation (77). Finally, Drosophila Ald has been implicated in hypoxia-induced arrest and the arrests of polar bodies, both of which may reflect a checkpoint function (74). Interestingly, Drosophila Ald, along with Polo kinase, is found in novel filamentous structures in oocytes that appear at the end of prophase and are maintained until egg activation (25, 78).
3. MPS1 STRUCTURE, ENZYMOLOGY AND INHIBITORS
3.1. Structure of the Mps1 Catalytic Domain
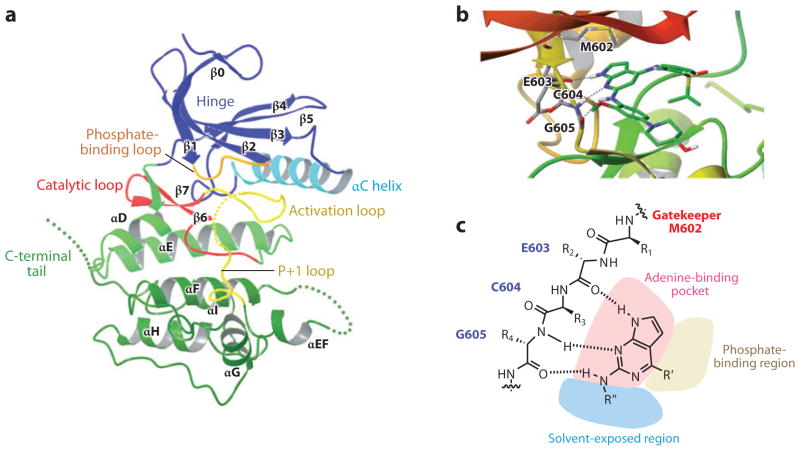
Several groups have solved the structure of the hMps1 kinase domain with very good agreement (79–82). The Mps1 kinase domain adopts the typical protein kinase bilobe architecture. The N-terminal small lobe consists of a standard five-stranded β-sheet and an αC helix, a canonical feature seen in many protein kinases (Figures 2a and 3). In addition, Mps1 contains an extra β-strand (β0) at the N terminus of the small lobe, which, together with part of β1, covers the twisted β-sheet (Figure 2a). The two lobes are joined by a hinge loop (Glu603-Gly605) at the back of the active-site cleft. The C-terminal large lobe shows a standard kinase structure, composed of a two-stranded β-sheet (β6 and β7) adjacent to the small lobe, seven α-helices, the catalytic loop, and the activation loop. The loop between helices αEF and αF (700–708) and the C-terminal tail are disordered (795–857). All of the reported Mps1 catalytic domain structures adopt an inactive conformation, as indicated by incorrect positioning of the αC helix, which prevents ion pairing between the conserved αC glutamate (Glu571) and the active-site lysine (Lys553), the unstructured activation loop, and the inactive conformation of the P+1 loop (684–688). In two structures, a polyethylene glycol molecule, which is a widely used precipitant in protein crystallization, is present as a ring surrounding the catalytic lysine (Lys553). Even though polyethylene glycol is artificially introduced into Mps1 by the crystallization conditions, its presence created a secondary pocket unseen in other kinases, a feature that could be exploited for inhibitor design. The Mps1 kinase domain has been cocrystalized with ATP (83). However, the ATP did not significantly alter the kinase domain conformation in that the Mps1-ATP structure is indistinguishable from the apo or inhibitor-bound conformations. This result suggests that ATP binding is insufficient for switching the kinase to an active conformation, raising tantalizing questions about the active kinase conformation (83).
Figure 2.
(a) Ribbon representation of the structure of the Mps1 catalytic domain. Key structural elements are labeled. The structure has been rendered from the Protein Data Bank (PDB) entry 3DBQ, using the Maestro interface from Schrödinger. The dotted lines represent the disordered regions in the activation loop, the loop between αEF and αF and also at the C-terminal tail. (b) Ribbon representation of the structure of Mps1 in complex with a small-molecule inhibitor, Mps1-IN-1. The structure has been rendered from the PDB entry 3GFW using the Maestro interface. The residues in the hinge region are shown. (c) Illustration of the inhibitor-binding mode. The gatekeeper residue M602, hinge region residues that interact with the inhibitor and the ATP-binding pocket are shown. The dotted lines represent hydrogen bonds.
3.2. Regulation of Mps1 Activity by Phosphorylation
The Mps1 C-terminal catalytic domain undergoes autophosphorylation and is active toward exogenous substrates (33, 80, 81, 84, 85). Initial structure characterization efforts focused on either dephosphorylated, kinase-defective mutants or the normal kinase complexed with small-molecule inhibitors (79, 80). These structures reveal the expected inactive kinase domain conformation, indicated by an unstructured activation, a P+1 loop, and an incorrect αC helix position (Figures 2a and 3) (79, 80). Surprisingly, structures of the wild-type kinase reveal that the catalytic domain still adopts an inactivate conformation, despite extensive autophosphorylation at nine different sites (81). The active catalytic domain may be quite heterogeneous owing to extensive posttranslational modifications; this makes it challenging to obtain crystals of the highly active enzyme. Although dephosphorylated enzymes can be prepared, the enzyme reactivates when ATP is introduced (33, 79). The puzzle remains as to why the active catalytic domain alone or in complex with ATP does not lead to an active enzyme conformation.
Figure 3.
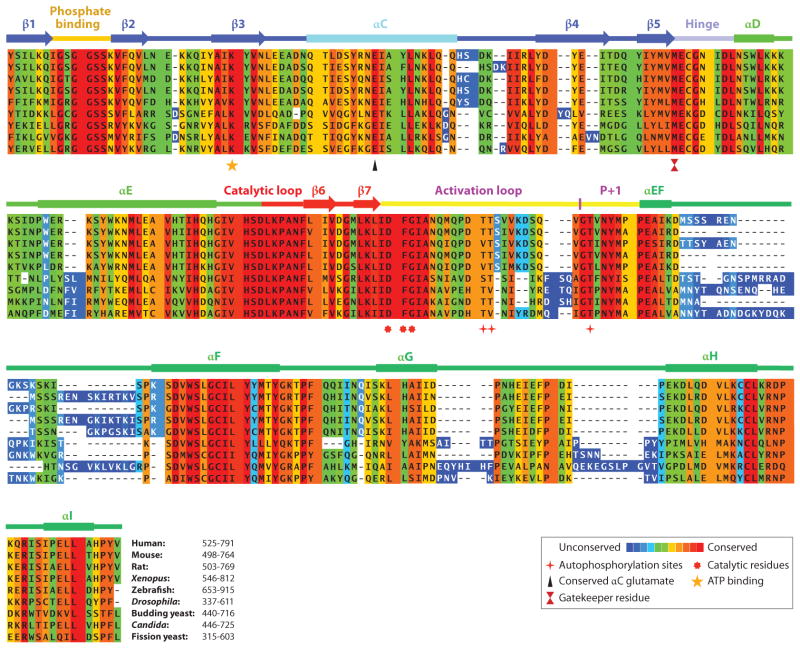
Multiple sequence alignment of the kinase domain of Mps1 from representative species using PRALINE multiple sequence alignment (http://www.ibi.vu.nl/programs/pralinewww/). Amino acid conservation is shown by a color-coded heat map from unconserved to conserved. The secondary structure of Mps1 is shown above the Mps1. Key amino acid residues are shown below the alignment.
Mps1 undergoes extensive autophosphorylation in vitro. Mps1 isolated from mitotic HeLa cells or insect cells were also phosphorylated at numerous sites (84–88). Phosphorylation occurs predominantly at Ser/Thr sites, although Tyr phosphorylation is also observed in vitro (33, 79). Among a myriad of phosphorylation sites, Thr676 and Thr686 are observed to have significant effects on kinase activity (33, 79, 80, 84, 85, 89). Thr676 lies in the activation loop, whereas Thr686 is on the P+1 loop. Mutation of the Thr676 residue to Ala reduces Mps1 transphosphorylation kinase activity by sevenfold. Interestingly, this mutation causes only a 1.4-fold reduction in autophosphorylation (33). Nevertheless, Thr676 phosphorylation is required for Mps1 to function optimally in yeast and in human cells (33, 84, 85). Supporting the theory that autophosphorylation increases kinase activity, kinetic analysis of Mps1 phosphorylation revealed a lag phase in product formation that is eliminated by preincubation with cold ATP (80, 89). Therefore, Thr676 phosphorylation is likely a priming event for kinase activation. Without an active Mps1 structure, we can only speculate about how phosphorylation stabilizes the activation loop. Mps1 lacks the basic RD pocket, which is referred to as a catalytic loop between the β6 and β7 strands featuring highly conserved Arg (R) and Asp (D) residues. The basic RD pocket, present in many protein kinases regulated through activation loop phosphorylation, directly interacts with the phospho residue in the activation loop, causing a switch to an active conformation. In the place of the RD pocket, it has been proposed that the Mps1 pThr676 phospho group may interact with one of the three lysine residues in the disordered loop between αEF and αF (700–708) (84). Confirmation or repudiation of this hypothesis awaits the availability of an active Mps1 catalytic domain structure.
The Thr residue at the beginning of the P+1 loop is invariant in numerous Ser/Thr kinases, including the AGC, CAMK, and CMGC group of kinases (90). Thr686 is the corresponding residue in Mps1 and, unlike many other Ser/Thr kinases, is autophosphorylated both in vitro and in vivo (33, 79, 80, 84, 85, 87, 88). Mutation of Thr686 reduces the kinase activity by at least 40-fold in vitro and inactivates Mps1 function in vivo (33). Phosphorylated Thr686 is likely a feature of active Mps1 kinase as a phospho-T686 antibody can deplete the active kinase (80, 87). What is unique about Thr686 phosphorylation in Mps1 is that the hydroxyl groups of this residue in other Ser/Thr kinases (e.g., Cdk2) form hydrogen bonds with conserved catalytic residues of the active kinases. The equivalent residue in the P+1 loop of tyrosine kinases is a proline, which is involved in substrate binding. Phosphorylation of the P+1 loop is unique to Mps1, and it is tempting to speculate that modulating the P+1 loop conformation via phosphorylation could be a novel mechanism for kinase activation and that this difference in the P+1 loop is associated with the dual specificity of Mps1 (80).
3.3. Diversity in Phosphorylation Site Selection and Substrate Recognition
Many Mps1 substrates have been identified. Surveying a variety of Mps1 auto- and transphosphorylation sites makes clear that there is no strict consensus phosphorylation sequence associated with the Mps1 kinase. A recent study reported a preference for D/E/N/Q at the −2 position (88), a recognition feature that is also associated with Plk1. This finding suggests the interesting possibilities that Mps1 and Plk1 may share some common physiological substrates and that some of the Mps1 in vitro autophosphorylation sites could be targeted by Plk1 in vivo (88). Despite these notable preferences, the sites targeted by Mps1 are highly diverse, and it is impossible to predict the authentic Mps1 phosphorylation sites in vitro and in vivo.
How Mps1 recognizes diverse substrates remains a mystery, although there are some hints that there may be different requirements for Mps1 autophosphorylation and transphosphorylation. As mentioned above, Thr676 mutation affects transphosphorylation more than autophosphorylation (33). Another unexpected finding came from deletion analysis of the C-terminal region of MPS1, a 65-amino-acid region that is disordered in all known Mps1 structures. This region is susceptible to proteolysis, which may explain the disagreement in abundance measurements using N-terminal or C-terminal antibodies (89). Removal of the 65-amino acid (noncatalytic) tail reduces Mps1 transphosphorylation by about sixfold but has little impact on Mps1 autophosphorylation (89). The most straightforward interpretation of this result is that this region of Mps1 is involved in exogenous substrate recognition. The significance of this region is underscored by the fact that, without it, Mps1 is defective in the spindle assembly checkpoint response, demonstrating that the presence of a kinase domain alone is insufficient for Mps1 function in vivo (89).
3.4. Dimerization
Kinase autophosphorylation can occur through intermolecular or intramolecular mechanisms. Mps1 transphosphorylation was first shown in vitro (33, 80, 84). Induced dimerization of Mps1 is sufficient to activate its kinase activity in cells (84). Mps1 dimerization and transphosphorylation have also been demonstrated using differentially tagged Mps1 constructs in a coimmunoprecipitation assay (39). Finally, kinetic studies of Mps1 phosphorylation in vitro support the notion that Mps1 undergoes intermolecular autophosphorylation in vitro, as the rate of autophosphorylation increases with increasing concentrations of Mps1 (89). Dimerization of Mps1 may have implications about where Mps1 is initially activated during cell-cycle progression. One proposal is that the kinetochore localization of Mps1 could raise its local concentration, leading to its activation during mitosis via more efficient intermolecular autophosphorylation (84, 91, 92). Although this may be the case for elevated Mps1 activity during spindle assembly checkpoint signaling, Mps1 activity also needs to increase prior to its relocalization to kinetochores to control centrosome duplication. It is equally possible that the initial activation of Mps1 occurs at the centrosome, where Mps1 is also highly concentrated prior to mitotic entry (Figure 1).
3.5. Mutant and Analog-Sensitive Alleles
Several mutant MPS1 alleles have been discovered in yeast, fly, and zebrafish. The original yeast temperature-sensitive-for-growth mps1-1 allele (C696Y/αH/domain XI), as well as five additional temperature-sensitive alleles all arose from point mutations in the kinase domain (7). The mutations varied in their effect on in vitro kinase activity from very severe (e.g., mps1-1) to rather mild (e.g., mps1-6 C642Y/αF/domain IX). The importance of kinase activity for Mps1 function is reinforced by the finding that the hypomorphic nightcap mutation in zebrafish is an Ile843Lys mutation in subdomain VI of the kinase domain, which is conserved in Mps1 kinases as a hydrophobic Ile or Leu residue (655 in hMps1/β6/catalytic loop/domain VI) (54). Finally, a null allele of the Drosophila Mps1 ortholog, aldC3, was found to contain a nine-amino acid deletion in the kinase domain (codon 369–377 between the β3 and αC/domain III) (25).
The N-terminal, noncatalytic region of Mps1 kinases is also mutated in some alleles of MPS1 genes. The original hypomorphic Ald mutation in Drosophila, ald1, is an Arg to His substitution at amino acid 7. Similarly, hypomorphic alleles of yeast MPS1, pac8-1 and pac8-2, identified as synthetically lethal with the deletion allele of the kinesin Cin8 (93), are point mutations in the N terminus (M. Winey, unpublished observation). These alleles are likely defective in a kinetochore function of Mps1, as is mps1-7, which contains a point mutation in the N terminus and is defective in the spindle checkpoint but competent for SPB duplication (94). The mps1-8 allele is a temperature-sensitive allele that contains several mutations in the N terminus, which do not affect the kinase activity but are defective in SPB duplication (15).
Analog-sensitive alleles have been a valuable tool in probing kinase function and identifying authentic kinase substrates. The gatekeeper residue of Mps1 is a Met at positions 516 and 602 in yeast and human enzymes, respectively. The yeast Mps1-as1 allele was first created by changing the bulky gatekeeper residue Met to a smaller Gly, which makes the kinase specifically sensitive to a cell-permeable ATP analog inhibitor, 1-NM-PP1 (53). The Mps1-as1 allele not only reaffirmed the function of Mps1 in SPB duplication and the spindle checkpoint but was also used to show that Mps1 acts in bipolar chromosome attachment (38, 53) and to show that Mps1 must be inactivated to exit the cell cycle (70). Mps1-as alleles have also been used to resolve some of the controversies surrounding Mad1 kinetochore recruitment and Aurora B activation by Mps1, which is discussed below (23, 43, 95).
3.6. Small-Molecule Mps1 Inhibitors
The Mps1 kinase has emerged as a novel drug target for cancer therapy. Cincreasin was the first reported small inhibitor of Mps1, though it is not particularly potent with 50% inhibitory concentration (IC50) = 700 μM (96). SP600125, a JNK inhibitor, was found to inhibit Mps1 off target (97). In recent years, a variety of structurally diverse Mps1 inhibitors have been described (Tables 1 and 2). Several Mps1 kinase catalytic domain crystal structures, apo or complexed with an inhibitor, were solved recently (79, 81–83). These structures helped shed light on how a small-molecule inhibitor binds with the Mps1 kinase and on how to design selective Mps1 inhibitors. Shown in Figure 2b is the crystal structure of the Mps1 kinase domain in complex with Mps1-IN-1 to illustrate kinase inhibitor-binding modes (81). As shown in Figure 2, the pyrrolopyrimidine scaffold forms the anchor of the inhibitor. It sits in the adenine-binding pocket, making hydrogen bond interactions between the substitutions on the scaffold and the protein inside the adenosine-binding pocket; these substitutions could also extend out into the solvent-exposed region and phosphate-binding region (Figure 2c). Cys604, a hinge residue that varies between kinases, has been explored in designing more selective Mps1 inhibitors (82).
Table 1.
Summary of published potent hMps1 inhibitors
| Inhibitor | Structure | IC50 (nM) | References (patent number) | Protein Data Bank crystal structure |
|---|---|---|---|---|
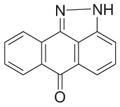
| SP600125 |
|
692 | 78, 83, 97 | 2ZMC |
| AZ3146 |
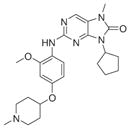
|
35 | 39 (WO2009024824) | |
| Mps1-IN-1 |
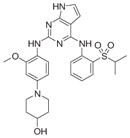
|
370 | 81 (WO2009032694) | 3GFW |
| Mps1-IN-2 |
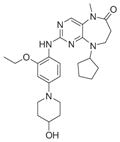
|
145 | 81 (WO2010080712) | 3H9F |
| NMS-P715 |
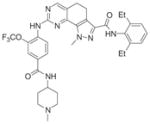
|
8 | 82 (WO2009156315) | 2X9E |
| MPI-0479605 |
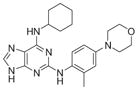
|
3.5 | 106, 132 | |
| Reversine |
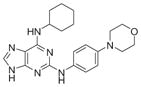
|
3/6 | 40 | |
| Staurosporine |
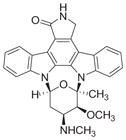
|
102 | 83 | 3HMO |
| Cpd4 |
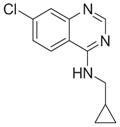
|
38,000 | 83 | 3HMP |
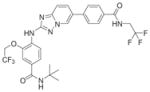
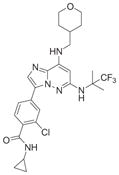
Table 2.
Additional patented hMps1 inhibitors
| Claimed structure | Example | IC50(nM) | Patent number (company) |
|---|---|---|---|
|
|
3 | JP2010111624 (Shionogi & Co., Ltd.) |
|
|
2.6 | WO2010124826 (Bayer Pharma) |
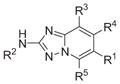
|
|
1 | WO2011063907 WO2011063908 WO2011064328 (Bayer Pharma) |
|
|
3.9 | WO2011013729 (OncoTherapy Science, Inc.) |
|
|
4 | WO2011016472 (OncoTherapy Science, Inc.) |
Because Mps1 is an essential gene in the pathogenic fungi Candida albicans and significant sequence divergence exists between human and Candida Mps1, species-specific inhibitors of Mps1 kinase could be employed as antifungal agents. In an effort to identify novel antifungal chemotherapeutics, the guanylate cyclase inhibitor LY83583 was found to inhibit Candida Mps1 without affecting hMps1 activity (98). Further advancing the feasibility of species-specific targeting of Mps1, SP600125, which inactivates human Mps1, has only modest inhibitory effects on Candida Mps1 and is nontoxic to Candida (98). Thus, sequence variations in Mps1 may offer a window of opportunity for new therapeutics in combating human pathogens.
4. REGULATION OF MPS1 KINASES
To accomplish a myriad of functions, MPS1 kinases must be exquisitely regulated. Indeed, experimental changes in the expression levels of MPS1 kinases (active and inactive) are detrimental in a variety of cell types. In general, MPS1 kinases are expressed at low levels, and the most important regulatory mechanisms operate via the posttranslational mechanisms of phosphorylation (discussed above with reference to the catalytic domain structure) and degradation.
4.1. Transcription
Many mammalian proteins that function in mitosis and mitotic checkpoint signaling, including Mps1, are controlled at the transcriptional level by the E2F family of transcription factors (99–103). Mps1 mRNA peaks in mitosis (11), and E2F4 and, to a lesser degree, E2F1 bind the MPS1 promoter region (103). In mouse embryonic fibroblasts lacking p107 and p130, which are two Retinoblastoma family transcriptional repressors of E2F transcription, MPS1 transcription is derepressed, and the mRNA transcribed at a higher level than in the wild-type control. These data suggest that the retinoblastoma E2F complex may be directly involved in repressing MPS1 transcription in interphase cells (103). Whether MPS1 transcription is directly regulated by E2F4 family transcription factors remains to be investigated. Finally, MPS1 mRNA levels are elevated in freshly isolated peripheral blood lymphocyte or T cell blasts (104). IL-2 incubation also induces Mps1 expression in proliferating peripheral blood lymphocyte blasts (104). Thus, transcription of Mps1 is upregulated when cells enter the cell cycle and transit through mitosis.
4.2. Localization
Subcellular localization of Mps1 is both spatially and temporally regulated during cell-cycle progression (29, 30, 66, 86). In mammalian cells, Mps1 primarily resides within the cytosol during G1. In late G2, Mps1 accumulates on centrosomes and the nuclear envelope (29, 66, 86, 105). At the G2/M boundary, Mps1 abruptly enters into the nucleus prior to nuclear membrane breakdown (106). Nuclear import of Mps1 requires two LXXLL motifs in the N terminus of Mps1. In interphase cells, Mps1 likely shuttles between nucleus and cytosol constantly, as leptomycin B treatment can lead to redistribution of Mps1 into the nucleus (106). As cells move into prophase, Mps1 preferentially associates with kinetochores and is slowly lost until the onset of anaphase, when Mps1 disassociates from kinetochores (91, 92, 105). The noncatalytic N-terminal domain is necessary and sufficient for localization to kinetochores in isolation, whereas the C-terminal domain by itself cannot locate hMps1 to kinetochores (23, 29, 86, 107). However, the function of the kinetochore-targeting signal in the N terminus could be masked by the sequence in the C-terminal region of Mps1. For example, without phosphorylation of Ser844 of XIMps1 by MAPK, XIMps1 cannot relocate to kinetochores even though the N-terminal-targeting signal is intact (108). Similar observations were made with hMps1 (86, 109). These results imply that the C-terminal region of Mps1 may regulate access to the kinetochore-targeting signal that resides in the N terminus of Mps1.
Besides MAPK, two other kinases, PRP4 (premessenger RNA processing 4) and Aurora B, have been implicated in the regulation of Mps1 kinetochore localization. PRP4 protein kinase associates with kinetochores during mitosis and is required for efficient Mps1 kinetochore targeting (110). Depletion of PRP4 induces mitotic acceleration, chromosome misalignment, and defects in Mad2 localization, which are phenotypes observed with inactivation of Mps1. The mechanism by which PRP4 regulates Mps1 remains to be determined. Similarly, as mentioned above, inhibition of Aurora B by various methods reduces Mps1 localization to unattached kinetochores throughout mitosis (46). However, Mad2 recruitment to kinetochores, which requires Mps1 activity, is significantly affected only in early mitosis, suggesting that Aurora B may regulate the timing or amplitude of Mps1 activation. Delayed Mps1 activation caused by Aurora B inhibition also causes a delay in establishment of the spindle checkpoint. The defects in Mps1 kinetochore targeting in early mitosis and spindle checkpoint delay can be rescued by tethering Mps1 to the kinetochore (46). This result suggests that Aurora B acts upstream in promoting early mitotic Mps1 kinetochore targeting. The effects of Aurora B on Mps1 have also been investigated in conjunction with Hec1/Ndc80, a core component of the kinetochore essential for organizing microtubule attachment sites (51). Hec1 is required for the recruitment of Mps1 kinase and Mad1/Mad2 complexes to kinetochores (111). Although there has been no report of direct interaction between Hec1 and Mps1, the budding yeast Hec1 ortholog, Ndc80, directly interacts with yeast Mps1 (50). Hec1 may well be the kinetochore-bound acceptor for Mps1 in mammalian cells. Consistent with a direct role of Hec1 on Mps1 targeting, depletion of Hec1 results in more dramatic effects on Mps1 targeting than Aurora B inactivation. However, one intriguing possibility is that Aurora B may act by phosphorylating the N terminus of Hec1 to regulate Mps1 kinetochore localization (51). It will be interesting to investigate whether Hec1 phosphorylation by Aurora B creates a docking site for Mps1 to bind kinetochores.
Mps1 centrosome localization is mediated by its N-terminal domain, but the precise motifs have not been characterized. Nonetheless, distinct regions of the N terminus of the yeast and human enzymes have been implicated in its centrosome function. In yeast, deletion analysis with the clever use of analog-sensitive alleles revealed that amino acids 201–300 are required for SPB duplication and are distinct from amino acids 151–200, which are required for chromosome biorientation (14). Similarly, a region internal to the N terminus of hMps1, amino acids 420–507, called the MDS (Mps1 degradation signal), is critical in controlling centrosomal levels of hMps1 (112). Deletion of this region stabilizes the protein and localizes it to centrosomes, driving excess centrosome production. The MDS is recognized by centrosome-localized OAZ (antizyme), which is responsible for the degradation of centrosomal Mps1 (113). The level of the centrosomal Mps1 is also regulated by its phosphorylation on Thr468 within the MDS by Cdk2 (112), which stabilizes the protein, opposing OAZ-mediated degradation to create a regulatory circuit that controls centrosomal hMps1 levels (22).
Phosphorylation of Mps1 is also important for its localization. Mutating the nine autophosphorylation sites in the N terminus of Mps1 causes a significant decrease in kinetochore targeting of Mps1 without affecting centrosomal localization in SW480 cells (86). This result suggests that the kinetochore-targeting signal is independent of the centrosome-localization signal. Among these sites, T12 and S15 appear to be critical in mediating Mps1 accumulation on kinetochores (86). Consistent with these results, kinase-inactive Mps1, expressed in SW480 cells, exhibits reduced kinetochore relocalization upon depletion of endogenous Mps1 (86). Reduced kinetochore localization of endogenous Mps1 was also observed when US2OS cells were treated with the inhibitor NMS-P715 (82). However, kinetochore accumulation of transiently transfected Mps1 or Mps1KD in HeLa increases when cells are treated with the inhibitor AZ3146, suggesting that kinase activity inhibits kinetochore recruitment in HeLa cells (39). It is interesting to note that the results of AZ3146 on recruitment of spindle checkpoint proteins to kinetochores depend on whether the inhibitor is administrated before or after mitotic entry. To reconcile the timing effect, it was proposed that there are two phases of checkpoint protein recruitment to kinetochores: an initial phase prior to mitotic entry and a subsequent maintenance phase during mitosis (39). Increased Mps1 accumulation on kinetochores in the presence of AZ3146 may be a result of reduced release from kinetochores. In this way, the Mps1 kinase activity may be required for targeting to kinetochores and also for release from kinetochores. The details about these requirements remain unsettled and may, like the role of Mps1 at the centrosome, depend on the cell type and experimental conditions.
4.3. Degradation and Inactivation
The major route of Mps1 inactivation is degradation. The expression and activity of Mps1 are cell cycle dependent in both yeast and mammalian cells (70, 89, 107). Expression peaks in metaphase and declines when cells enter anaphase. Timely inactivation of Mps1 is required for proper cell-cycle progression and termination of spindle checkpoint signaling. During normal cell-cycle progression, Mps1 is partially degraded in anaphase by the ubiquitin E3 ligase APCCdc20. Overexpression of Mps1 in anaphase can activate the checkpoint by inhibiting APCCdc20 and blocks mitotic exit in yeast (70). There are three D-boxes in the N terminus of yeast Mps1, which are required for proteolysis by APCCdc20 (70). Therefore, APCCdc20 and Mps1 are mutually inhibitory, forming a double negative feedback loop. This circuit may enable the metaphase to anaphase transition to be switch like and irreversible. Human Mps1 contains only one canonical D-box, and it is sequentially degraded by APCCdc20 and APCCdh1 in a D-box-dependent manner (114). A D-box-deficient hMps1 perturbs normal mitosis and causes centrosome overreplication in human cells. Efficient degradation of Mps1 is also aided by Ufd2, a U-box-containing ubiquitin-protein ligase, in both yeast and mammalian cells (115). Hence, proteolysis regulates temporal expression and activity of Mps1.
Degradation of Mps1 also occurs spatially. Centrosome accumulation of hMps1 is greatly enhanced by phosphorylation at Thr468 by Cdk2 (112). Phosphomimetic mutations at Thr468 or deletion of the region surrounding Thr468 protects Mps1 from degradation at centrosomes (112). Yeast Mps1 is stabilized by CDK phosphorylation of Thr29, but the mechanism is unknown (20). Kinetochore-associated Mps1 also may be regulated by proteolysis. The retention time for Mps1 on unattached kinetochores in checkpoint-arrested cells is about 10 s (91). Treatment with both MG132 and Mps1 inhibitors enhances its accumulation at kinetochores (39), suggesting a role for proteolysis. Because Mps1 has a distinct subcellular localization during cell-cycle progression, it is possible that different pools of Mps1 are differentially regulated by proteolysis.
Another possible mechanism of Mps1 inactivation is dephosphorylation. Mps1 is hyperphosphorylated in mitosis (29, 30) and rapidly dephosphorylated upon anaphase entry (29). To date, phosphatases that specifically act on MPS1 family kinases have not been identified. Early in vitro studies show that PTP1B can remove the phospho-Tyr epitope produced by mouse Mps1 autophosphorylation (10). PP1γ has also been shown to dephosphorylate Mps1 in vitro (77). Whether any of these phosphatases inactivate Mps1 in vivo remains unknown.
4.4. Misregulation in Tumor Cells
Like many cell-cycle regulators, Mps1 transcription is deregulated in a variety of human tumors. Elevated Mps1 mRNA levels are found in several human cancers, including thyroid papillary carcinoma, breast cancer, gastric cancer tissue, bronchogenic carcinoma, and lung cancers (8, 116–120). Furthermore, high levels of Mps1 correlate with a high histological grade in breast cancers (119). Conversely, Mps1 mRNA is markedly reduced or absent in resting cells and in tissues with a low proliferative index (11). Thus, there is a correlation between elevated Mps1 levels and cell proliferation as well as with tumor aggressiveness. Consistent with the notion that oncogenic signaling promotes Mps1 expression, the levels and activity of Mps1 are increased by 3- and 10-fold, respectively, in human melanoma cell lines containing the B-RafV600E mutant (121). Inhibition of B-Raf or MEK1 reduces Mps1 expression (109, 121).
The observation that tumor cells frequently overexpress spindle checkpoint proteins is perplexing as the conventional wisdom would postulate that tumor cells would have a weakened checkpoint, contributing to chromosome missegregation and aneuploidy. Indeed, significant evidence from yeast to mice supports the notion that a weakened checkpoint leads to chromosome instability (122). However, mutations in key checkpoint proteins are rare in human tumors, and correlative evidence showing that compromised checkpoint signaling directly contributes to the development of human tumors has been elusive. MPS1 missense mutations have been found in the noncatalytic N terminus in bladder (123) and lung cancers (124), as well as in the kinase domain in pancreatic (125) and lung cancers (124). Interestingly, frameshift mutations that truncate the protein arise from microsatellite instability in the hMps1 gene in gastric (126) and colorectal cancers (127). Thus, mutations in hMPS1 have been detected in tumor-derived cells; however, their influence on tumorigenesis is not known.
The prevalence of high levels of checkpoint protein expression, such as Mps1, in human tumors prompts an alternative hypothesis regarding the potential role of checkpoint proteins in cancer cells, i.e., overexpression of these proteins may promote either cancer initiation or survival of aneuploid cancer cells (119, 128). Accordingly, reductions in key checkpoint proteins should severely decrease human cancer cell viability. This prediction is confirmed for several checkpoint proteins, including Mps1 (66, 119), BubRI (129), and Mad2 (130, 131). Suppression of Mps1 expression in Hs578T breast cancer cells also reduces the tumorigenicity of these cells in xenografts. Cancer cell death is likely the result of severe chromosome segregation errors when the checkpoint is disabled. Interestingly, cells that survived reduced Mps1 levels often display lower levels of aneuploidy, suggesting that lower levels of Mps1 potentially inactivating the checkpoint are incompatible with aneuploidy (119). This concept is in excellent agreement with the observation that reduction in checkpoint proteins makes tumor cells more sensitive than untransformed human fibroblasts to low doses of spindle poisons (129). Differential cellular responses to checkpoint inhibition between normal and tumor cells could be key in developing new anticancer drugs targeting hMps1. Recent results from at least one hMps1 inhibitor, NMS-P715, show great promise in preclinical cancer models (82). We anxiously await the determination of whether inhibitors of Mps1 are efficacious and safe, either as single agents or in combination, in clinically relevant settings.
SUMMARY POINTS.
Mps1 kinases with their conserved, C-terminal kinase domains are widely, but not ubiquitously, distributed among eukaryotes.
Mps1 kinases are localized at kinetochores, where they function with Aurora B kinases to ensure proper bipolar attachment.
Mps1 kinases act at an early step in the spindle checkpoint, and the functions of most of the checkpoint proteins are dependent, directly or indirectly, on Mps1 activity.
Mps1 kinases are found in centrosomes, are required for SPB (centrosome) assembly in yeast, and influence centrosome assembly in mammals.
Mps1 kinases exhibit significant levels of autophosphorylation, which is essential for its activation and subcellular localization.
Mps1 kinases are inactivated by APC-dependent degradation, which is necessary for cells to exit mitosis correctly.
Mps1 kinase genes are misregulated in tumors, supporting the hypothesis that the checkpoint is necessary for the viability of aneuploid tumor cells.
Mps1 kinases have become of interest for the development of small-molecule inhibitors. It is anticipated that some of the inhibitors discovered will be tested in clinical trials.
FUTURE ISSUES.
Since the discovery of the first Mps1 allele, there has been tremendous progress in understanding the biological function and underlying mechanisms of this protein kinase. However, many important questions regarding Mps1 function remain. For example:
What are the molecular mechanisms of Mps1 in its known functions in kinetochore attachment, the spindle checkpoint, and centrosome assembly? Particularly, what are the pertinent Mps1 substrates for these various functions?
What are all of the Mps1 kinase functions? Mps1 kinases function in genotoxic stress, the actin cytoskeleton, and likely in other contexts that remain to be identified.
Is the lack of Mps1 paralogs functionally significant? Could the myriad and complex functions of these kinases require a single isoform for correct regulation?
What protein kinases carry out the various functions of Mps1 kinases in organisms lacking this kinase?
What governs Mps1 subcellular localization and its changes during the cell cycle?
Are Mps1 kinases inactivated by reversible mechanisms, such as dephosphorylation? A biosensor assay for active Mps1 would be critical for this work, and it would be useful in examining Mps1 at its various cellular locations.
What is the active conformation of Mps1, and what can it tell us about the mechanisms of Mps1 activation and substrate recognition?
Will Mps1 be found to be a good drug target for antitumor therapy?
Answers to these questions will undoubtedly provide a more lucid and exciting picture of how Mps1 orchestrates normal cell-cycle progression and its deviation in tumorigenesis.
Acknowledgments
We are indebted to Quanbin Xu for the images in Figure 1. We thank Harold Fisk and Shelly Jones for critically reading the manuscript. We also thank Gan Zhang and Robert Holton-Burke for preparing the inhibitor tables and structure figures. X.L. is supported by the National Institutes of Health (NIH) grants CA107089 and GM083172. M.W.’s work on Mps1 is supported by NIH grant GM51312.
Glossary
- MPS1
monopolar spindle 1
- SPB
spindle pole body
- Centrosome
the cellular structure that contains centrioles, nucleates microtubule formation, and organizes mitotic spindles
- Autophosphorylation
the action of a kinase adding one or more phosphate groups to itself
- Dual-specificity protein kinase
a protein kinase that exhibits Ser/Thr and Tyr phosphorylation activities
- Spindle checkpoint
this mechanism ensures proper chromosome attachment to microtubules prior to chromosome segregation (aka spindle assembly checkpoint or mitotic checkpoint)
- Kinetochore
this structure, assembled at centromeres, captures spindle microtubules and serves as the signaling platform for the spindle checkpoint
- APC
anaphase-promoting complex
- Syntelic attachments
an aberrant chromosome attachment, where both sister chromosomes are attached to a single spindle pole instead of a bipolar attachment
- Ald
Drosophila Mps1 homolog
- Transphosphorylation
the action of a kinase mediating transfer of phosphate to its cognate substrates
- Analog-sensitive allele
a variant protein kinase carrying a mutation at the gatekeeper residue, allowing it to accept a bulky analog of ATP for inhibition
- Gatekeeper residue
the residue located in the ATP-binding pocket of a protein kinase that controls the selectivity and sensitivity to small-molecule inhibitors
Footnotes
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
Contributor Information
Xuedong Liu, Email: xuedong.liu@colorado.edu.
Mark Winey, Email: mark.winey@colorado.edu.
LITERATURE CITED
- 1.Winey M, Goetsch L, Baum P, Byers B. MPS1 and MPS2: novel yeast genes defining distinct steps of spindle pole body duplication. J Cell Biol. 1991;114:745–54. doi: 10.1083/jcb.114.4.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Poch O, Schwob E, de Fraipont F, Camasses A, Bordonne R, Martin RP. RPK1, an essential yeast protein kinase involved in the regulation of the onset of mitosis, shows homology to mammalian dual-specificity kinases. Mol Gen Genet. 1994;243:641–53. doi: 10.1007/BF00279573. [DOI] [PubMed] [Google Scholar]
- 3.Lauze E, Stoelcker B, Luca FC, Weiss E, Schutz AR, Winey M. Yeast spindle pole body duplication gene MPS1 encodes an essential dual specificity protein kinase. EMBO J. 1995;14:1655–63. doi: 10.1002/j.1460-2075.1995.tb07154.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rose MD, Fink GR. KAR1, a gene required for function of both intranuclear and extranuclear microtubules in yeast. Cell. 1987;48:1047–60. doi: 10.1016/0092-8674(87)90712-4. [DOI] [PubMed] [Google Scholar]
- 5.Weiss E, Winey M. The Saccharomyces cerevisiae spindle pole body duplication gene MPS1 is part of a mitotic checkpoint. J Cell Biol. 1996;132:111–23. doi: 10.1083/jcb.132.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hardwick KG, Weiss E, Luca FC, Winey M, Murray AW. Activation of the budding yeast spindle assembly checkpoint without mitotic spindle disruption. Science. 1996;273:953–56. doi: 10.1126/science.273.5277.953. [DOI] [PubMed] [Google Scholar]
- 7.Schutz AR, Winey M. New alleles of the yeast MPS1 gene reveal multiple requirements in spindle pole body duplication. Mol Biol Cell. 1998;9:759–74. doi: 10.1091/mbc.9.4.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mills GB, Schmandt R, McGill M, Amendola A, Hill M, et al. Expression of TTK, a novel human protein kinase, is associated with cell proliferation. J Biol Chem. 1992;267:16000–6. [PubMed] [Google Scholar]
- 9.Lindberg RA, Fischer WH, Hunter T. Characterization of a human protein threonine kinase isolated by screening an expression library with antibodies to phosphotyrosine. Oncogene. 1993;8:351–59. [PubMed] [Google Scholar]
- 10.Douville EM, Afar DE, Howell BW, Letwin K, Tannock L, et al. Multiple cDNAs encoding the esk kinase predict transmembrane and intracellular enzyme isoforms. Mol Cell Biol. 1992;12:2681–89. doi: 10.1128/mcb.12.6.2681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hogg D, Guidos C, Bailey D, Amendola A, Groves T, et al. Cell cycle dependent regulation of the protein kinase TTK. Oncogene. 1994;9:89–96. [PubMed] [Google Scholar]
- 12.Lowery DM, Mohammad DH, Elia AE, Yaffe MB. The Polo-box domain: a molecular integrator of mitotic kinase cascades and Polo-like kinase function. Cell Cycle. 2004;3:128–31. [PubMed] [Google Scholar]
- 13.Chu M, Eyers P. UCSD-Nature Molecule Pages: MPS1. 2010 http://www.signaling-gateway.org/molecule/query?afcsid=A000882.
- 14.Araki Y, Gombos L, Migueleti SP, Sivashanmugam L, Antony C, Schiebel E. N-terminal regions of Mps1 kinase determine functional bifurcation. J Cell Biol. 2010;189:41–56. doi: 10.1083/jcb.200910027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Castillo AR, Meehl JB, Morgan G, Schutz-Geschwender A, Winey M. The yeast protein kinase Mps1p is required for assembly of the integral spindle pole body component Spc42p. J Cell Biol. 2002;156:453–65. doi: 10.1083/jcb.200111025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Holinger EP, Old WM, Giddings TH, Wong C, Yates JR, Winey M. Budding yeast centrosome duplication requires stabilization of Spc29 via Mps1-mediated phosphorylation. J Biol Chem. 2009;284:12949–55. doi: 10.1074/jbc.M900088200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pereira G, Knop M, Schiebel E. Spc98p directs the yeast gamma-tubulin complex into the nucleus and is subject to cell cycle–dependent phosphorylation on the nuclear side of the spindle pole body. Mol Biol Cell. 1998;9:775–93. doi: 10.1091/mbc.9.4.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Friedman DB, Kern JW, Huneycutt BJ, Vinh DB, Crawford DK, et al. Yeast Mps1p phosphorylates the spindle pole component Spc110p in the N-terminal domain. J Biol Chem. 2001;276:17958–67. doi: 10.1074/jbc.M010461200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schiebel E, Bornens M. In search of a function for centrins. Trends Cell Biol. 1995;5:197–201. doi: 10.1016/s0962-8924(00)88999-0. [DOI] [PubMed] [Google Scholar]
- 20.Jaspersen SL, Huneycutt BJ, Giddings TH, Resing KA, Ahn NG, Winey M. Cdc28/Cdk1 regulates spindle pole body duplication through phosphorylation of Spc42 and Mps1. Dev Cell. 2004;7:263–74. doi: 10.1016/j.devcel.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 21.Keck JM, Jones MH, Wong CC, Binkley J, Chen D, et al. A cell cycle phosphoproteome of the yeast centrosome. Science. 2011;332:1557–61. doi: 10.1126/science.1205193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pike AN, Fisk HA. Centriole assembly and the role of Mps1: defensible or dispensable? Cell Div. 2011;6:9. doi: 10.1186/1747-1028-6-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maciejowski J, George KA, Terret M-E, Zhang C, Shokat KM, Jallepalli PV. Mps1 directs the assembly of Cdc20 inhibitory complexes during interphase and mitosis to control M phase timing and spindle checkpoint signaling. J Cell Biol. 2010;190:89–100. doi: 10.1083/jcb.201001050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.He X, Jones MH, Winey M, Sazer S. Mph1, a member of the Mps1-like family of dual specificity protein kinases, is required for the spindle checkpoint in S. pombe. J Cell Sci. 1998;111(Part 12):1635–47. doi: 10.1242/jcs.111.12.1635. [DOI] [PubMed] [Google Scholar]
- 25.Gilliland WD, Hughes SE, Cotitta JL, Takeo S, Xiang Y, Hawley RS. The multiple roles of mps1 in Drosophila female meiosis. PLoS Genet. 2007;3:e113. doi: 10.1371/journal.pgen.0030113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kanai M, Ma Z, Izumi H, Kim S-H, Mattison CP, et al. Physical and functional interaction between mortalin and Mps1 kinase. Genes Cells. 2007;12:797–810. doi: 10.1111/j.1365-2443.2007.01091.x. [DOI] [PubMed] [Google Scholar]
- 27.Yang CH, Kasbek C, Majumder S, Yusof AM, Fisk HA. Mps1 phosphorylation sites regulate the function of centrin 2 in centriole assembly. Mol Biol Cell. 2010;21:4361–72. doi: 10.1091/mbc.E10-04-0298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Abrieu A, Magnaghi-Jaulin L, Kahana JA, Peter M, Castro A, et al. Mps1 is a kinetochore-associated kinase essential for the vertebrate mitotic checkpoint. Cell. 2001;106:83–93. doi: 10.1016/s0092-8674(01)00410-x. [DOI] [PubMed] [Google Scholar]
- 29.Liu S-T, Chan GKT, Hittle JC, Fujii G, Lees E, Yen TJ. Human MPS1 kinase is required for mitotic arrest induced by the loss of CENP-E from kinetochores. Mol Biol Cell. 2003;14:1638–51. doi: 10.1091/mbc.02-05-0074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stucke VM, Sillje HHW, Arnaud L, Nigg EA. Human Mps1 kinase is required for the spindle assembly checkpoint but not for centrosome duplication. EMBO J. 2002;21:1723–32. doi: 10.1093/emboj/21.7.1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lan W, Cleveland DW. A chemical tool box defines mitotic and interphase roles for Mps1 kinase. J Cell Biol. 2010;190:21–24. doi: 10.1083/jcb.201006080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Espeut J, Gaussen A, Bieling P, Morin V, Prieto S, et al. Phosphorylation relieves autoinhibition of the kinetochore motor Cenp-E. Mol Cell. 2008;29:637–43. doi: 10.1016/j.molcel.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 33.Mattison CP, Old WM, Steiner E, Huneycutt BJ, Resing KA, et al. Mps1 activation loop autophosphorylation enhances kinase activity. J Biol Chem. 2007;282:30553–61. doi: 10.1074/jbc.M707063200. [DOI] [PubMed] [Google Scholar]
- 34.Fraschini R, Beretta A, Lucchini G, Piatti S. Role of the kinetochore protein Ndc10 in mitotic checkpoint activation in Saccharomyces cerevisiae. Mol Genet Genomics. 2001;266:115–25. doi: 10.1007/s004380100533. [DOI] [PubMed] [Google Scholar]
- 35.Chan GK, Yen TJ. The mitotic checkpoint: a signaling pathway that allows a single unattached kinetochore to inhibit mitotic exit. Prog Cell Cycle Res. 2003;5:431–39. [PubMed] [Google Scholar]
- 36.Kulukian A, Han JS, Cleveland DW. Unattached kinetochores catalyze production of an anaphase inhibitor that requires a Mad2 template to prime Cdc20 for BubR1 binding. Dev Cell. 2009;16:105–17. doi: 10.1016/j.devcel.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Biggins S, Murray AW. The budding yeast protein kinase Ipl1/Aurora allows the absence of tension to activate the spindle checkpoint. Genes Dev. 2001;15:3118–29. doi: 10.1101/gad.934801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maure J-F, Kitamura E, Tanaka TU. Mps1 kinase promotes sister-kinetochore bi-orientation by a tension-dependent mechanism. Curr Biol. 2007;17:2175–82. doi: 10.1016/j.cub.2007.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hewitt L, Tighe A, Santaguida S, White AM, Jones CD, et al. Sustained Mps1 activity is required in mitosis to recruit O-Mad2 to the Mad1-C-Mad2 core complex. J Cell Biol. 2010;190:25–34. doi: 10.1083/jcb.201002133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Santaguida S, Tighe A, D’Alise AM, Taylor SS, Musacchio A. Dissecting the role of MPS1 in chromosome biorientation and the spindle checkpoint through the small molecule inhibitor reversine. J Cell Biol. 2010;190:73–87. doi: 10.1083/jcb.201001036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lampson MA, Renduchitala K, Khodjakov A, Kapoor TM. Correcting improper chromosome-spindle attachments during cell division. Nat Cell Biol. 2004;6:232–37. doi: 10.1038/ncb1102. [DOI] [PubMed] [Google Scholar]
- 42.Jelluma N, Brenkman AB, van den Broek NJF, Cruijsen CWA, van Osch MHJ, et al. Mps1 phosphorylates Borealin to control Aurora B activity and chromosome alignment. Cell. 2008;132:233–46. doi: 10.1016/j.cell.2007.11.046. [DOI] [PubMed] [Google Scholar]
- 43.Sliedrecht T, Zhang C, Shokat KM, Kops GJ. Chemical genetic inhibition of Mps1 in stable human cell lines reveals novel aspects of Mps1 function in mitosis. PLoS ONE. 2010;5:e10251. doi: 10.1371/journal.pone.0010251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bourhis E, Lingel A, Phung Q, Fairbrother WJ, Cochran AG. Phosphorylation of a Borealin dimerization domain is required for proper chromosome segregation. Biochemistry. 2009;48:6783–93. doi: 10.1021/bi900530v. [DOI] [PubMed] [Google Scholar]
- 45.Vigneron S, Prieto S, Bernis C, Labbe JC, Castro A, Lorca T. Kinetochore localization of spindle checkpoint proteins: Who controls whom? Mol Biol Cell. 2004;15:4584–96. doi: 10.1091/mbc.E04-01-0051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Saurin AT, van der Waal MS, Medema RH, Lens SMA, Kops GJPL. Aurora B potentiates Mps1 activation to ensure rapid checkpoint establishment at the onset of mitosis. Nat Commun. 2011;2:316. doi: 10.1038/ncomms1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cheeseman IM, Anderson S, Jwa M, Green EM, Kang J, et al. Phospho-regulation of kinetochore-microtubule attachments by the Aurora kinase Ipl1p. Cell. 2002;111:163–72. doi: 10.1016/s0092-8674(02)00973-x. [DOI] [PubMed] [Google Scholar]
- 48.Shimogawa MM, Graczyk B, Gardner MK, Francis SE, White EA, et al. Mps1 phosphorylation of Dam1 couples kinetochores to microtubule plus ends at metaphase. Curr Biol. 2006;16:1489–501. doi: 10.1016/j.cub.2006.06.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kim Y, Holland AJ, Lan W, Cleveland DW. Aurora kinases and protein phosphatase 1 mediate chromosome congression through regulation of CENP-E. Cell. 2010;142:444–55. doi: 10.1016/j.cell.2010.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kemmler S, Stach M, Knapp M, Ortiz J, Pfannstiel J, et al. Mimicking Ndc80 phosphorylation triggers spindle assembly checkpoint signalling. EMBO J. 2009;28:1099–110. doi: 10.1038/emboj.2009.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.DeLuca JG, Gall WE, Ciferri C, Cimini D, Musacchio A, Salmon ED. Kinetochore microtubule dynamics and attachment stability are regulated by Hec1. Cell. 2006;127:969–82. doi: 10.1016/j.cell.2006.09.047. [DOI] [PubMed] [Google Scholar]
- 52.Storchova Z, Becker JS, Talarek N, Kogelsberger S, Pellman D. Bub1, Sgo1, and Mps1 mediate a distinct pathway for chromosome biorientation in budding yeast. Mol Biol Cell. 2011;22:1473–85. doi: 10.1091/mbc.E10-08-0673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jones MH, Huneycutt BJ, Pearson CG, Zhang C, Morgan G, et al. Chemical genetics reveals a role for Mps1 kinase in kinetochore attachment during mitosis. Curr Biol. 2005;15:160–65. doi: 10.1016/j.cub.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 54.Poss KD, Nechiporuk A, Hillam AM, Johnson SL, Keating MT. Mps1 defines a proximal blastemal proliferative compartment essential for zebrafish fin regeneration. Development. 2002;129:5141–49. doi: 10.1242/dev.129.22.5141. [DOI] [PubMed] [Google Scholar]
- 55.Poss KD, Wilson LG, Keating MT. Heart regeneration in zebrafish. Science. 2002;298:2188–90. doi: 10.1126/science.1077857. [DOI] [PubMed] [Google Scholar]
- 56.Wills AA, Kidd AR, Lepilina A, Poss KD. Fgfs control homeostatic regeneration in adult zebrafish fins. Development. 2008;135:3063–70. doi: 10.1242/dev.024588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wei JH, Chou YF, Ou YH, Yeh YH, Tyan SW, et al. TTK/hMps1 participates in the regulation of DNA damage checkpoint response by phosphorylating CHK2 on threonine 68. J Biol Chem. 2005;280:7748–57. doi: 10.1074/jbc.M410152200. [DOI] [PubMed] [Google Scholar]
- 58.Yeh YH, Huang YF, Lin TY, Shieh SY. The cell cycle checkpoint kinase CHK2 mediates DNA damage-induced stabilization of TTK/hMps1. Oncogene. 2009;28:1366–78. doi: 10.1038/onc.2008.477. [DOI] [PubMed] [Google Scholar]
- 59.Leng M, Chan DW, Luo H, Zhu C, Qin J, Wang Y. MPS1-dependent mitotic BLM phosphorylation is important for chromosome stability. Proc Natl Acad Sci USA. 2006;103:11485–90. doi: 10.1073/pnas.0601828103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nihira K, Taira N, Miki Y, Yoshida K. TTK/Mps1 controls nuclear targeting of c-Abl by 14-3-3-coupled phosphorylation in response to oxidative stress. Oncogene. 2008;27:7285–95. doi: 10.1038/onc.2008.334. [DOI] [PubMed] [Google Scholar]
- 61.Bhonde MR, Hanski ML, Budczies J, Cao M, Gillissen B, et al. DNA damage-induced expression of p53 suppresses mitotic checkpoint kinase hMps1: the lack of this suppression in p53MUT cells contributes to apoptosis. J Biol Chem. 2006;281:8675–85. doi: 10.1074/jbc.M511333200. [DOI] [PubMed] [Google Scholar]
- 62.Huang YF, Chang MD, Shieh SY. TTK/hMps1 mediates the p53-dependent postmitotic checkpoint by phosphorylating p53 at Thr18. Mol Cell Biol. 2009;29:2935–44. doi: 10.1128/MCB.01837-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dong C, Li Z, Alvarez R, Jr, Feng XH, Goldschmidt-Clermont PJ. Microtubule binding to Smads may regulate TGF beta activity. Mol Cell. 2000;5:27–34. doi: 10.1016/s1097-2765(00)80400-1. [DOI] [PubMed] [Google Scholar]
- 64.Zhu S, Wang W, Clarke DC, Liu X. Activation of Mps1 promotes transforming growth factor-beta-independent Smad signaling. J Biol Chem. 2007;282:18327–38. doi: 10.1074/jbc.M700636200. [DOI] [PubMed] [Google Scholar]
- 65.Cappell SD, Baker R, Skowyra D, Dohlman HG. Systematic analysis of essential genes reveals important regulators of G protein signaling. Mol Cell. 2010;38:746–57. doi: 10.1016/j.molcel.2010.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fisk HA, Mattison CP, Winey M. Human Mps1 protein kinase is required for centrosome duplication and normal mitotic progression. Proc Natl Acad Sci USA. 2003;100:14875–80. doi: 10.1073/pnas.2434156100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Huh WK, Falvo JV, Gerke LC, Carroll AS, Howson RW, et al. Global analysis of protein localization in budding yeast. Nature. 2003;425:686–91. doi: 10.1038/nature02026. [DOI] [PubMed] [Google Scholar]
- 68.Luca FC, Winey M. MOB1, an essential yeast gene required for completion of mitosis and maintenance of ploidy. Mol Biol Cell. 1998;9:29–46. doi: 10.1091/mbc.9.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mohl DA, Huddleston MJ, Collingwood TS, Annan RS, Deshaies RJ. Dbf2-Mob1 drives relocalization of protein phosphatase Cdc14 to the cytoplasm during exit from mitosis. J Cell Biol. 2009;184:527–39. doi: 10.1083/jcb.200812022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Palframan WJ, Meehl JB, Jaspersen SL, Winey M, Murray AW. Anaphase inactivation of the spindle checkpoint. Science. 2006;313:680–84. doi: 10.1126/science.1127205. [DOI] [PubMed] [Google Scholar]
- 71.Mattison CP, Stumpff J, Wordeman L, Winey M. Mip1 associates with both the Mps1 kinase and actin, and is required for cell cortex stability and anaphase spindle positioning. Cell Cycle. 2011;10:783–93. doi: 10.4161/cc.10.5.14955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Straight PD, Giddings TH, Winey M. Mps1p regulates meiotic spindle pole body duplication in addition to having novel roles during sporulation. Mol Biol Cell. 2000;11:3525–37. doi: 10.1091/mbc.11.10.3525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hached K, Xie SZ, Buffin E, Cladiere D, Rachez C, et al. Mps1 at kinetochores is essential for female mouse meiosis I. Development. 2011;138:2261–71. doi: 10.1242/dev.061317. [DOI] [PubMed] [Google Scholar]
- 74.Fischer MG, Heeger S, Hacker U, Lehner CF. The mitotic arrest in response to hypoxia and of polar bodies during early embryogenesis requires Drosophila Mps1. Curr Biol. 2004;14:2019–24. doi: 10.1016/j.cub.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 75.Gilliland WD, Wayson SM, Hawley RS. The meiotic defects of mutants in the Drosophila mps1 gene reveal a critical role of Mps1 in the segregation of achiasmate homologs. Curr Biol. 2005;15:672–77. doi: 10.1016/j.cub.2005.02.062. [DOI] [PubMed] [Google Scholar]
- 76.Poss KD, Nechiporuk A, Stringer KF, Lee C, Keating MT. Germ cell aneuploidy in zebrafish with mutations in the mitotic checkpoint gene mps1. Genes Dev. 2004;18:1527–32. doi: 10.1101/gad.1182604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Grimison B, Liu J, Lewellyn AL, Maller JL. Metaphase arrest by cyclin E-Cdk2 requires the spindle-checkpoint kinase Mps1. Curr Biol. 2006;16:1968–73. doi: 10.1016/j.cub.2006.08.055. [DOI] [PubMed] [Google Scholar]
- 78.Gilliland WD, Vietti DL, Schweppe NM, Guo F, Johnson TJ, Hawley RS. Hypoxia transiently sequesters Mps1 and Polo to collagenase-sensitive filaments in Drosophila prometaphase oocytes. PLoS ONE. 2009;4:e7544. doi: 10.1371/journal.pone.0007544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chu MLH, Chavas LMG, Douglas KT, Eyers PA, Tabernero L. Crystal structure of the catalytic domain of the mitotic checkpoint kinase Mps1 in complex with SP600125. J Biol Chem. 2008;283:21495–500. doi: 10.1074/jbc.M803026200. [DOI] [PubMed] [Google Scholar]
- 80.Wang W, Yang Y, Gao Y, Xu Q, Wang F, et al. Structural and mechanistic insights into Mps1 kinase activation. J Cell Mol Med. 2009;13:1679–94. doi: 10.1111/j.1582-4934.2008.00605.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kwiatkowski N, Jelluma N, Filippakopoulos P, Soundararajan M, Manak MS, et al. Small-molecule kinase inhibitors provide insight into Mps1 cell cycle function. Nat Chem Biol. 2010;6:359–68. doi: 10.1038/nchembio.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Colombo R, Caldarelli M, Mennecozzi M, Giorgini ML, Sola F, et al. Targeting the mitotic checkpoint for cancer therapy with NMS-P715, an inhibitor of MPS1 kinase. Cancer Res. 2010;70:10255–64. doi: 10.1158/0008-5472.CAN-10-2101. [DOI] [PubMed] [Google Scholar]
- 83.Chu ML, Lang Z, Chavas LM, Neres J, Fedorova OS, et al. Biophysical and X-ray crystallographic analysis of Mps1 kinase inhibitor complexes. Biochemistry. 2010;49:1689–701. doi: 10.1021/bi901970c. [DOI] [PubMed] [Google Scholar]
- 84.Kang J, Chen Y, Zhao Y, Yu H. Autophosphorylation-dependent activation of human Mps1 is required for the spindle checkpoint. Proc Natl Acad Sci USA. 2007;104:20232–37. doi: 10.1073/pnas.0710519105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jelluma N, Brenkman AB, McLeod I, Yates JR, Cleveland DW, et al. Chromosomal instability by inefficient Mps1 auto-activation due to a weakened mitotic checkpoint and lagging chromosomes. PLoS ONE. 2008;3:e2145. doi: 10.1371/journal.pone.0002415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Xu Q, Zhu S, Wang W, Zhang X, Old W, et al. Regulation of kinetochore recruitment of two essential mitotic spindle checkpoint proteins by Mps1 phosphorylation. Mol Biol Cell. 2009;20:10–20. doi: 10.1091/mbc.E08-03-0324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tyler RK, Chu MLH, Johnson H, McKenzie EA, Gaskell SJ, Eyers PA. Phosphoregulation of human Mps1 kinase. Biochem J. 2009;417:173–81. doi: 10.1042/BJ20081310. [DOI] [PubMed] [Google Scholar]
- 88.Dou Z, von Schubert C, Korner R, Santamaria A, Elowe S, Nigg EA. Quantitative mass spectrometry analysis reveals similar substrate consensus motif for human Mps1 kinase and Plk1. PLoS ONE. 2011;6:e18793. doi: 10.1371/journal.pone.0018793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sun T, Yang X, Wang W, Zhang X, Xu Q, et al. Cellular abundance of Mps1 and the role of its carboxyl terminal tail in substrate recruitment. J Biol Chem. 2010;285:38730–39. doi: 10.1074/jbc.M110.177642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Nolen B, Taylor S, Ghosh G. Regulation of protein kinases: controlling activity through activation segment conformation. Mol Cell. 2004;15:661–75. doi: 10.1016/j.molcel.2004.08.024. [DOI] [PubMed] [Google Scholar]
- 91.Howell BJ, Moree B, Farrar EM, Stewart S, Fang G, Salmon ED. Spindle checkpoint protein dynamics at kinetochores in living cells. Curr Biol. 2004;14:953–64. doi: 10.1016/j.cub.2004.05.053. [DOI] [PubMed] [Google Scholar]
- 92.Jelluma N, Dansen TB, Sliedrecht T, Kwiatkowski NP, Kops GJPL. Release of Mps1 from kinetochores is crucial for timely anaphase onset. J Cell Biol. 2010;191:281–90. doi: 10.1083/jcb.201003038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Geiser JR, Schott EJ, Kingsbury TJ, Cole NB, Totis LJ, et al. Saccharomyces cerevisiae genes required in the absence of the CIN8-encoded spindle motor act in functionally diverse mitotic pathways. Mol Biol Cell. 1997;8:1035–50. doi: 10.1091/mbc.8.6.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Castillo A. Analysis of MPS1 Separation-of-Function Alleles Reveals a Novel Interaction at the Spindle Pole Bodies in Yeast. Bolder, CO: Univ. Colorado; 2000. p. 392. [Google Scholar]
- 95.Tighe A, Staples O, Taylor S. Mps1 kinase activity restrains anaphase during an unperturbed mitosis and targets Mad2 to kinetochores. J Cell Biol. 2008;181:893–901. doi: 10.1083/jcb.200712028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Dorer RK, Zhong S, Tallarico JA, Wong WH, Mitchison TJ, Murray AW. A small-molecule inhibitor of Mps1 blocks the spindle-checkpoint response to a lack of tension on mitotic chromosomes. Curr Biol. 2005;15:1070–76. doi: 10.1016/j.cub.2005.05.020. [DOI] [PubMed] [Google Scholar]
- 97.Schmidt M, Budirahardja Y, Klompmaker R, Medema RH. Ablation of the spindle assembly checkpoint by a compound targeting Mps1. EMBO Rep. 2005;6:866–72. doi: 10.1038/sj.embor.7400483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Tsuda K, Nishiya N, Umeyama T, Uehara Y. Identification of LY83583 as a specific inhibitor of Candida albicans MPS1 protein kinase. Biochem Biophys Res Commun. 2011;409:418–23. doi: 10.1016/j.bbrc.2011.05.010. [DOI] [PubMed] [Google Scholar]
- 99.Ishida S, Huang E, Zuzan H, Spang R, Leone G, et al. Role for E2F in control of both DNA replication and mitotic functions as revealed from DNA microarray analysis. Mol Cell Biol. 2001;21:4684–99. doi: 10.1128/MCB.21.14.4684-4699.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Polager S, Kalma Y, Berkovich E, Ginsberg D. E2Fs up-regulate expression of genes involved in DNA replication, DNA repair and mitosis. Oncogene. 2002;21:437–46. doi: 10.1038/sj.onc.1205102. [DOI] [PubMed] [Google Scholar]
- 101.Zhu W, Giangrande PH, Nevins JR. Temporal control of cell cycle gene expression mediated by E2F transcription factors. Cell Cycle. 2005;4:633–36. doi: 10.4161/cc.4.5.1650. [DOI] [PubMed] [Google Scholar]
- 102.Zhu W, Giangrande PH, Nevins JR. E2Fs link the control of G1/S and G2/M transcription. EMBO J. 2004;23:4615–26. doi: 10.1038/sj.emboj.7600459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ren B, Cam H, Takahashi Y, Volkert T, Terragni J, et al. E2F integrates cell cycle progression with DNA repair, replication, and G(2)/M checkpoints. Genes Dev. 2002;16:245–56. doi: 10.1101/gad.949802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Schmandt R, Hill M, Amendola A, Mills GB, Hogg D. IL-2-induced expression of TTK, a serine, threonine, tyrosine kinase, correlates with cell cycle progression. J Immunol. 1994;152:96–105. [PubMed] [Google Scholar]
- 105.Dou Z, Sawagechi A, Zhang J, Luo H, Brako L, Yao XB. Dynamic distribution of TTK in HeLa cells: insights from an ultrastructural study. Cell Res. 2003;13:443–49. doi: 10.1038/sj.cr.7290186. [DOI] [PubMed] [Google Scholar]
- 106.Zhang X, Yin Q, Ling Y, Zhang Y, Ma R, et al. Two LXXLL motifs in the N terminus of Mps1 are required for Mps1 nuclear import during G 2/M transition and sustained spindle checkpoint responses. Cell Cycle. 2011;10:2742–50. doi: 10.4161/cc.10.16.15927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Stucke VM, Baumann C, Nigg EA. Kinetochore localization and microtubule interaction of the human spindle checkpoint kinase Mps1. Chromosoma. 2004;113:1–15. doi: 10.1007/s00412-004-0288-2. [DOI] [PubMed] [Google Scholar]
- 108.Zhao Y, Chen R-H. Mps1 phosphorylation by MAP kinase is required for kinetochore localization of spindle-checkpoint proteins. Curr Biol. 2006;16:1764–69. doi: 10.1016/j.cub.2006.07.058. [DOI] [PubMed] [Google Scholar]
- 109.Borysova MK, Cui Y, Snyder M, Guadagno TM. Knockdown of B-Raf impairs spindle formation and the mitotic checkpoint in human somatic cells. Cell Cycle. 2008;7:2894–901. doi: 10.4161/cc.7.18.6678. [DOI] [PubMed] [Google Scholar]
- 110.Montembault E, Dutertre S, Prigent C, Giet R. PRP4 is a spindle assembly checkpoint protein required for MPS1, MAD1, and MAD2 localization to the kinetochores. J Cell Biol. 2007;179:601–9. doi: 10.1083/jcb.200703133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Martin-Lluesma S, Stucke VM, Nigg EA. Role of Hec1 in spindle checkpoint signaling and kinetochore recruitment of Mad1/Mad2. Science. 2002;297:2267–70. doi: 10.1126/science.1075596. [DOI] [PubMed] [Google Scholar]
- 112.Kasbek C, Yang C-H, Yusof AM, Chapman HM, Winey M, Fisk HA. Preventing the degradation of Mps1 at centrosomes is sufficient to cause centrosome reduplication in human cells. Mol Biol Cell. 2007;18:4457–69. doi: 10.1091/mbc.E07-03-0283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kasbek C, Yang C-H, Fisk HA. Antizyme restrains centrosome amplification by regulating the accumulation of Mps1 at centrosomes. Mol Biol Cell. 2010;21:3878–89. doi: 10.1091/mbc.E10-04-0281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Cui Y, Cheng X, Zhang C, Zhang Y, Li S, et al. Degradation of the human mitotic checkpoint kinase Mps1 is cell cycle-regulated by APC-cCdc20 and APC-cCdh1 ubiquitin ligases. J Biol Chem. 2010;285:32988–98. doi: 10.1074/jbc.M110.140905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Liu C, van Dyk D, Choe V, Yan J, Majumder S, et al. Ubiquitin ligase Ufd2 is required for efficient degradation of Mps1 kinase. J Biol Chem. 2011;286:43660–67. doi: 10.1074/jbc.M111.286229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Salvatore G, Nappi TC, Salerno P, Jiang Y, Garbi C, et al. A cell proliferation and chromosomal instability signature in anaplastic thyroid carcinoma. Cancer Res. 2007;67:10148–58. doi: 10.1158/0008-5472.CAN-07-1887. [DOI] [PubMed] [Google Scholar]
- 117.Yuan B, Xu Y, Woo JH, Wang Y, Bae YK, et al. Increased expression of mitotic checkpoint genes in breast cancer cells with chromosomal instability. Clin Cancer Res. 2006;12:405–10. doi: 10.1158/1078-0432.CCR-05-0903. [DOI] [PubMed] [Google Scholar]
- 118.Kilpinen S, Ojala K, Kallioniemi O. Analysis of kinase gene expression patterns across 5681 human tissue samples reveals functional genomic taxonomy of the kinome. PLoS ONE. 2010;5:e15068. doi: 10.1371/journal.pone.0015068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Daniel J, Coulter J, Woo J-H, Wilsbach K, Gabrielson E. High levels of the Mps1 checkpoint protein are protective of aneuploidy in breast cancer cells. Proc Natl Acad Sci USA. 2010;108:5384–89. doi: 10.1073/pnas.1007645108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Landi MT, Dracheva T, Rotunno M, Figueroa JD, Liu H, et al. Gene expression signature of cigarette smoking and its role in lung adenocarcinoma development and survival. PLoS ONE. 2008;3:e1651. doi: 10.1371/journal.pone.0001651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Cui Y, Guadagno TM. B-RafV600E signaling deregulates the mitotic spindle checkpoint through stabilizing Mps1 levels in melanoma cells. Oncogene. 2008;27:3122–33. doi: 10.1038/sj.onc.1210972. [DOI] [PubMed] [Google Scholar]
- 122.Weaver BA, Cleveland DW. Decoding the links between mitosis, cancer, and chemotherapy: the mitotic checkpoint, adaptation, and cell death. Cancer Cell. 2005;8:7–12. doi: 10.1016/j.ccr.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 123.Olesen SH, Thykjaer T, Orntoft TF. Mitotic checkpoint genes hBUB1, hBUB1B, hBUB3 and TTK in human bladder cancer, screening for mutations and loss of heterozygosity. Carcinogenesis. 2001;22:813–15. doi: 10.1093/carcin/22.5.813. [DOI] [PubMed] [Google Scholar]
- 124.Nakagawa Y, Daigo Y, Nakatsuru S. Japan Patent No. WO 2008/072777 A2 2008
- 125.Carter H, Samayoa J, Hruban RH, Karchin R. Prioritization of driver mutations in pancreatic cancer using cancer-specific high-throughput annotation of somatic mutations (CHASM) Cancer Biol Ther. 2010;10:582–87. doi: 10.4161/cbt.10.6.12537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ahn CH, Kim YR, Kim SS, Yoo NJ, Lee SH. Mutational analysis of TTK gene in gastric and colorectal cancers with microsatellite instability. Cancer Res Treat. 2009;41:224–28. doi: 10.4143/crt.2009.41.4.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Niittymaki I, Gylfe A, Laine L, Laakso M, Lehtonen HJ, et al. High frequency of TTK mutations in microsatellite-unstable colorectal cancer and evaluation of their effect on spindle assembly checkpoint. Carcinogenesis. 2011;32:305–11. doi: 10.1093/carcin/bgq272. [DOI] [PubMed] [Google Scholar]
- 128.Sotillo R, Hernando E, Díaz-Rodríguez E, Teruya-Feldstein J, Cordón-Cardo C, et al. Mad2 overexpression promotes aneuploidy and tumorigenesis in mice. Cancer Cell. 2007;11:9–23. doi: 10.1016/j.ccr.2006.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Janssen A, Kops GJPL, Medema RH. Elevating the frequency of chromosome mis-segregation as a strategy to kill tumor cells. Proc Natl Acad Sci USA. 2009;106:19108–13. doi: 10.1073/pnas.0904343106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kops GJ, Foltz DR, Cleveland DW. Lethality to human cancer cells through massive chromosome loss by inhibition of the mitotic checkpoint. Proc Natl Acad Sci USA. 2004;101:8699–704. doi: 10.1073/pnas.0401142101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Michel L, Diaz-Rodriguez E, Narayan G, Hernando E, Murty VV, Benezra R. Complete loss of the tumor suppressor MAD2 causes premature cyclin B degradation and mitotic failure in human somatic cells. Proc Natl Acad Sci USA. 2004;101:4459–64. doi: 10.1073/pnas.0306069101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Tardif KD, Rogers A, Cassiano J, Roth BL, Cimbora DM, et al. Characterization of the cellular and antitumor effects of MPI-0479605, a small-molecule inhibitor of the mitotic kinase Mps1. Mol Cancer Ther. 2011;10:2267–75. doi: 10.1158/1535-7163.MCT-11-0453. [DOI] [PubMed] [Google Scholar]