Abstract
Mutant huntingtin (HTT) protein is the cause of Huntington's disease (HD), an incurable neurological disorder. Almost all patients are heterozygous for mutant HTT and approaches that reduce levels of mutant HTT while leaving expression of wild-type HTT intact might be ideal options for therapeutic development. We have developed several allele-selective strategies for silencing HTT, including single-stranded silencing RNAs (ss-siRNAs). ss-siRNAs are oligonucleotides containing chemical modifications that permit action through the RNA interference (RNAi) pathway. Modified ss-siRNAs chosen to test the effects of varying oligomer length, lipid modification, the introduction of mismatched bases, and variation of chemical modification. We find that several modified ss-siRNA are potent and allele-selective inhibitors of HTT expression. An ss-siRNA with three mismatched bases relative to the CAG repeat was an allele-selective inhibitor of HTT expression in the HdhQ175 mouse model. Multiple allele-selective ss-siRNAs provide a wide platform of modifications to draw on for further optimization and therapeutic development. Our data provide insights into how ss-siRNAs can be modified to improve their properties and facilitate the discovery of the lead compounds necessary for further development.
Introduction
Huntington's disease (HD) is a severe neurological disorder that affects 5–10 people per 100,000 worldwide (WALKER, 2007). Symptoms characterized by chorea, behavioral difficulties, and cognitive decline are usually noticed in middle age and progressively worsen over time. There are currently no curative treatments for HD and therapies that can slow the course of the disease or alleviate symptoms are urgently needed (Sah and Aronin, 2011; Matsui and Corey, 2012).
HD is caused by a trinucleotide expansion in the gene-encoding huntingtin (HTT) protein (MacDonald, et al., 1993). Individuals with fewer than 35 CAG repeats are not affected, while individuals with greater than 35–39 repeats are at risk of developing the disease. Those with more than 40 repeats are likely to be diagnosed with HD (DUYAO, 1993; KREMER, 1994). In general, there is an inverse correlation between disease onset and length of CAG expansion, with seven percent of patients developing juvenile HD prior to age 20 (Nance and Myers, 2001).
Unlike many other neurological diseases where several genes probably contribute to the conditions, the only cause of HD is expression of mutant HTT containing an expanded CAG repeat. Inhibition of mutant HTT expression, therefore, would be expected to delay the onset of symptoms or slow disease progression. This realization led to the use of duplex RNAs or antisense oligonucleotides to block expression of both mutant and wild-type HTT (Sah and Aronin, 2011). Animal studies with a non-allele-selective antisense oligonucleotide administered by intracerebroventricular infusion have shown that inhibition of HTT expression can alleviate disease pathology in HD mouse models and have the potential to reverse some symptoms (Kordasiewicz et al., 2012).
While non-allele-selective approaches to gene silencing are advancing towards clinical application, it is possible that chronic inhibition of wild-type HTT expression in humans might have detrimental consequences. To avoid potential problems associated with non-allele selective inhibition of HTT, strategies have been developed to preferentially inhibit expression of the disease-causing mutant allele. These strategies include the use of duplex RNAs (Schwartz et al., 2006; Difiglia et al., 2007; Boudreau et al., 2009; Pfister et al., 2009) or gapmer antisense oligonucleotides (Carroll et al., 2011; Ostergaard et al., 2013) designed to recognize single nucleotide polymorphisms (SNPs) within mutant HTT pre-mRNA. While impressive selectivities can be achieved, the HD population possesses varied SNPs and multiple drugs would need to be developed to treat a majority of patients (Pfister et al., 2009).
We have developed an approach using nucleic acids to target the only difference between the mutant and wild-type alleles common to all HD patients—the expanded CAG repeat. We, and others, have shown that both duplex RNAs (Hu et al., 2010; Fiszer et al., 2011; Hu et al., 2012) and antisense oligonucleotides (Hu et al., 2009; Gagnon et al., 2010; Evers et al., 2011) that are complementary to the CAG repeat can achieve allele-selective inhibition.
Recently, we have also shown that single-stranded small interfering RNAs (ss-siRNAs) (Fig. 1) are effective allele-selective agents (Yu et al., 2012). ss-siRNAs are chemically modified RNAs that can silence gene expression through the RNA interference pathway (Lima et al., 2012). They combine the favorable pharmacological properties of single stranded oligonucleotides, such as in vivo uptake upon administration in saline formulations, with the robust silencing produced by RNA interference (RNAi). For inhibition of HTT, our initial results showed that silencing was both potent and allele selective (Yu et al., 2012). Potencies were similar to those produced by analogous duplex RNAs and the proteins of the RNAi machinery were necessary for activity (Yu et al., 2012; Liu et al., 2013a).
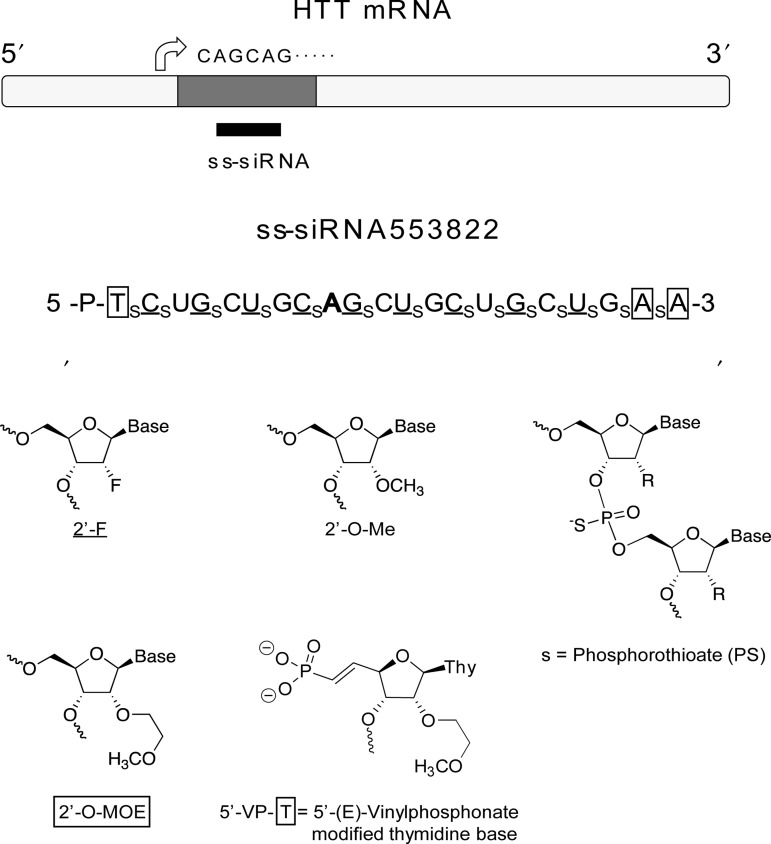
FIG. 1.
Design of single-stranded silencing RNAs (ss-siRNAs) and chemical structure of modified bases and internucleotide linkages. Subscript “s” indicates phosphorothioate (PS) linkage; 2′-O-methyl (2′-O-Me) modified base is shown in uppercase; 2′-fluoro (2′-F) in underlined uppercase; 2′-methoxyethyl (2′-MOE) in enclosed box. The terminal thymidine has a 5′ phosphate or vinyl phosphonate. All other linkages are phosphate. A mismatched base is shown in bold face. HTT, huntingtin.
We now report structure activity relationships for inhibition of HTT expression by ss-siRNAs that vary in length and chemical modification. Our data demonstrate that a diverse group of ss-siRNAs can be potent and allele-selective inhibitors of mutant HTT expression and that the ss-siRNA platform can provide many choices for future development and optimization in vivo.
Materials and Methods
Cell culture and transfection
Single-stranded siRNAs were synthesized on an Applied Biosystems (ABI) 394 synthesizer (1–2 μmol scale) using phosphoramidite coupling and purified as described (Lima et al., 2012; Liu et al., 2013a). Prior to use, the ss-siRNAs were reconstituted in nuclease-free water. Patient-derived fibroblast cell lines GM04281 cells (69 mutant CAG repeats/17 wild-type repeats) or GM04869 (47 mutant CAG repeats/15 wild-type repeats) were obtained from the Coriell Institute. The fibroblasts were maintained at 37°C and 5% CO2 in minimal essential media Eagle (MEM) (Sigma, M4655) supplemented with 10% heat inactivated fetal bovine serum (Sigma) and 0.5% MEM nonessential amino acids (Sigma). Cells were plated at a density of 70,000 per well of a 6-well plate 48 hours before transfection. ss-siRNAs were transfected into cells with lipid RNAiMAX (Invitrogen) as previously described (Yu et al., 2012). Cells were typically harvested 4 days after transfection for analysis of protein levels.
Western blot analysis of HTT
Mutant and wild-type HTT proteins from GM04281 cells were separated by polyacrylamide gel electrophoresis (SDS-PAGE) as previously described (Hu et al., 2009). The primary antibodies used were anti-HTT (MAB2166. 1:10000; Chemicon) and anti-ß-actin (1:10,000; Sigma). Protein bands were quantified using ImageJ software. The percentage of inhibition was calculated as a relative value to a control sample. The program GraphPad Prism 4 was used for the fitting curves of dose response experiments for HTT inhibition. The following equation was used for fitting: y=100[1 – xm/(nm+xm)], where y is percentage of inhibition and x is the siRNA concentration, m and n are fitting parameters, where n is taken as the IC50 value. At least three experiment data sets were used for fitting the curves. The error bar is standard deviation.
HTT proteins from GM04869 cells are more difficult to separate than HTT proteins from GM04281 cells because the difference in repeat number between wild-type and mutant is only thirty-two. For SDS-PAGE to analyze HTT protein in GM04869 cells we used Tris·HCl SDS-PAGE [separating gel: 5% acrylamide-bisacrylamide (49:1), 450 mM Tris·HCl pH 8.8, 0.1% SDS; stacking gel 4% acrylamide-bisacrylamide (49:1), 150 mM Tris·HCl pH 6.8] and Tris/Glycine/SDS (BioRad) running buffer. Gels were run at 75 V for 15 minutes, then 110 V for 6 hours. The electrophoresis apparatus was placed in ice-water bath (keeping temperature around 15°C) to prevent overheating of the running buffer (keeping inner temperature around 25–30°C). Buffer was manually recycled periodically between the top and bottom reservoir to maintain temperature and salt concentrations.
In vivo study
Single-stranded siRNAs were delivered into the brain of HdhQ175 (Heikkinen et al., 2012; Menalled et al., 2012) mice using osmotic pumps as previously described (Yu et al., 2012). Three hundred micrograms per day of ss-siRNA or phosphate-buffered saline were infused for 28 days before sacrificing the animals. Brains were cut into 1- to 2-mm coronal sections and frozen on dry ice immediately and stored at −80°C. Brain samples were homogenized thoroughly in RIPA buffer [150 mM NaCl, 50 mM Tris-HCl PH7.4, 1% NP40, 0.25% Na-deoxycholate, proteinase inhibitor cocktail (Roche)]. After spinning at 12,000 rpm for 15 minutes, supernatant was collected and protein concentration was measured using Micro BCA Protein Assay Kit (Thermo Scientific). Fifteen micrograms of protein was applied for western blot analysis.
Results
Design of ss-siRNAs
The purpose of this study was to design series of ss-siRNAs to examine how changing specific variables would affect potency and allele selectivity. The variables changed included (1) ss-siRNA length to identify the minimum length compatible with activity; (2) lipid conjugates that might be useful alternatives for in vivo studies; (3) the exact position of mismatched bases; and (4) the number of chemically modified bases. The position of mismatches or the number of chemical modifications may improve potency and allele selectivity. A broader pool of ss-siRNAs would also provide more candidates for optimizing biodistribution and other properties in vivo.
Chemical modifications within ss-siRNAs stabilize the RNA strands from digestion while maintaining ability to function through RNAi and achieve allele-selective depletion of mutant HTT. Chemical modifications incorporated into the RNA strands included 2′-O-methyl (2′-O-me), 2′-fluoro (2′-F), and 2′-methoxyethyl (2′-MOE) nucleosides (Fig. 1). Internucleotide linkages were either phosphodiester or phosphorothioate. Some ss-siRNAs (ISIS 573303, ISIS 573312; ISIS 573310) also had lipid modifications. ss-siRNAs (ISIS 573303, ISIS 573312; ISIS 573310; ISIS 537775) contained a 5′-vinyl phosphonate necessary for activity in vivo, while all others contained a 5′-phosphate. The 5′-phosphate is active in cell culture and easier to synthesize, facilitating the testing of multiple compounds.
We had previously observed that introducing mismatched bases into the central region of ss-siRNAs or duplex RNAs can increase allele-selectivity by reducing inhibitory activity towards the non-expanded wild-type allele (Hu et al., 2010; Yu et al., 2012). All compounds used in these studies contained at least one mismatch at position 9 and some contained multiple mismatches. The ss-siRNAs were transfected into patient-derived GM04281 fibroblast cells (mutant allele/69 CAG repeats, wild-type allele/17 CAG repeats). Inhibition of HTT protein expression was evaluated by western analysis using protocols that allowed discrimination between the mutant (353 kD) and wild-type (347 kD) proteins.
ss-siRNA length influences allele-selective silencing of mutant HTT
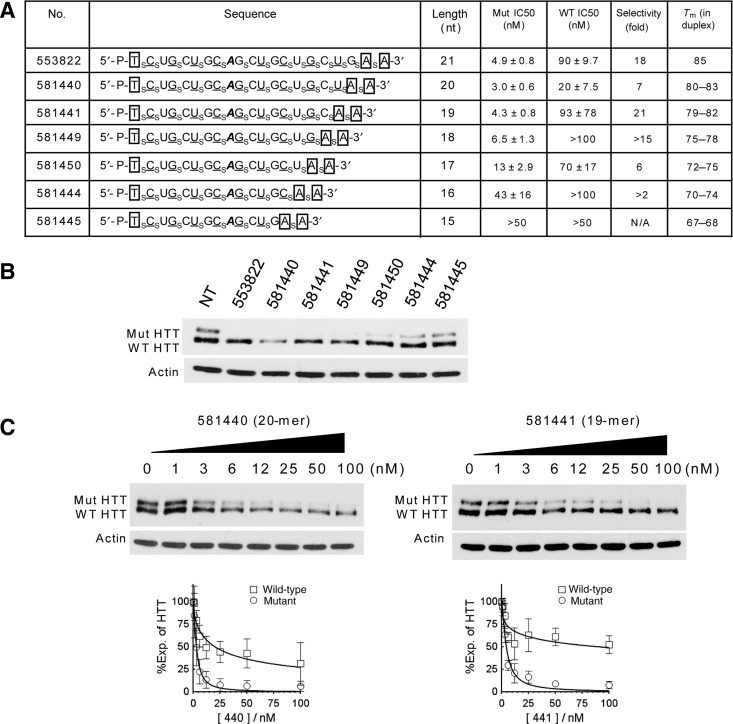
We investigated the impact of altering ss-siRNA structure by varying length (Fig. 2A). Our benchmark ss-siRNA was ISIS 553822 (Yu et al., 2012). This twenty-one base ss-siRNA had been characterized previously and contained a single mismatched base at position 9. We synthesized six other compounds with decreasing lengths that showed some potential for allele-selective silencing when tested at 25 nM (Fig. 2B).
FIG. 2.
Effect of ss-siRNA length on inhibition of HTT expression. (A) List of ss-siRNAs. Subscript “s” indicates PS linkage; 2′-O-Me modified base is shown in uppercase; 2′-F in underlined uppercase; 2′-MOE in enclosed box. Mismatched base is shown in bold and italic. (B) Allele-selective depletion of mutant HTT in patient fibroblasts (GM 04281) treated with ss-siRNAs tested at 25 nM. (C) Effect on HTT expression of increasing concentrations of ISIS 581440 and ISIS 581441. Western analysis data is representative of triplicate experiments that were averaged to yield IC50 and selectivity values. Mut, mutant; nt, nucleotides; Tm, melting temperature with a complementary 22-mer RNA; WT, wild type.
We evaluated the potencies of each ss-siRNA. For parent ss-siRNA ISIS 553822, we measured a potency of 4.9 nM and an allele selectivity for inhibiting mutant HTT expression of 18-fold, with allele selectivity being the ratio of the IC50 value for inhibition of wild-type HTT expression relative to the inhibition of mutant HTT expression. ss-siRNAs with 20 or 19 bases behaved similarly, with potencies of 3.0 nM and 4.3 nM and selectivities of 7-fold and 21-fold respectively (Fig. 2A, C). As lengths dropped from 17 to 15 bases, potencies decreased (Fig. 2A, B), consistent with a reduced ability to function in conjunction with the protein factors involved in RNAi. These results demonstrate that there is flexibility when choosing the length of ss-siRNA used for allele-selective silencing of HTT, with a two-base deletion permitting 7-fold selectivity.
Effect of lipid conjugation on allele-selectivity of ss-siRNAs
Lipid modifications are one strategy for improving biodistribution, cellular uptake, and in vivo gene inhibition. For example, one lipid modified antisense oligonucleotide (GRN163L, Imetelstat) is currently in phase 2 trials for treatment of essential thrombocythemia. It is also possible that the attachment of lipid moieties to the ss-siRNA may increase selectivity by forming more favorable interactions with mutant HTT mRNA relative to the wild-type mRNA.
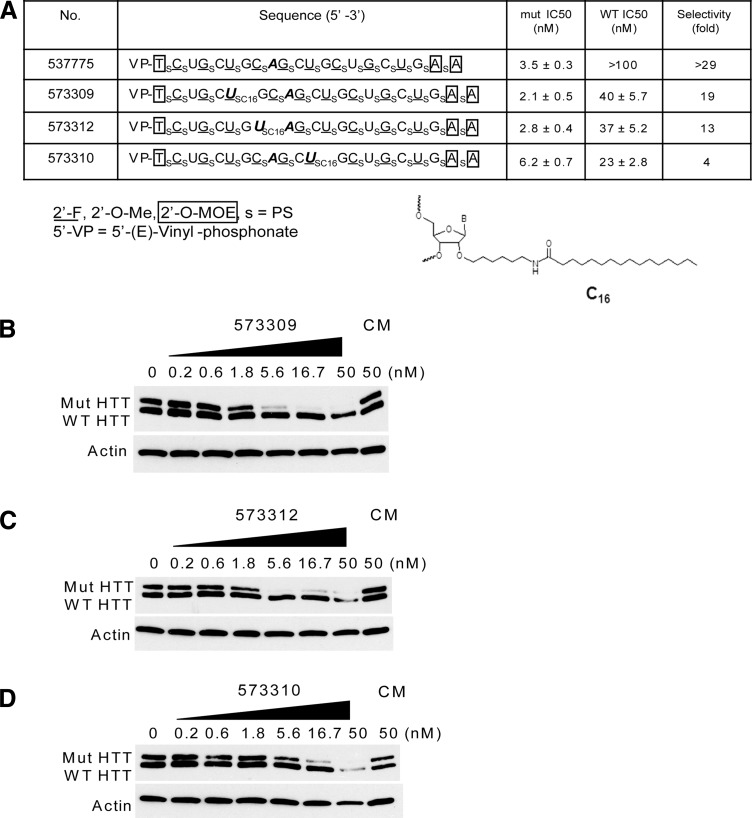
To test whether lipid modifications could have favorable impact on inhibition of mutant HTT expression in cell culture we evaluated the effects of attaching linear sixteen carbon (C16) chains at three different positions within an ss-siRNA (Fig. 3A). The ss-siRNAs were based on the sequence of allele-selective ss-siRNA ISIS 537775 that contains a 5′-(E)vinyl phosphonate and a mismatch at position 9. We had previously shown that ISIS 537775 could reduce mutant HTT expression throughout the brain in an HD mouse model (Yu et al., 2012). The lipid moieties were introduced during synthesis using uridine monomers modified with C16.
FIG. 3.
Effect of lipid modification on inhibition of HTT expression by ss-siRNAs. (A) List of ss-siRNAs. Subscript “s” indicates PS linkage; 2′-O-Me modified base is shown in uppercase; 2′-F in underlined uppercase; 2′-MOE in enclosed box. Mismatched base is shown in bold and italic. The terminal T has a 5′-(E)vinyl phosphonate. All other linkages are phosphate. (B-D) Effect of increasing concentrations of ISIS 573309, ISIS 573312, and ISIS 573310 respectively. CM: non complementary duplex RNA. C16: location of lipid modification.
All three lipid-modified ss-siRNAs were inhibitors of mutant HTT expression with IC50 values ranging from 2.1 to 6.2 nM and selectivities of 4 to 19-fold (Fig. 3). The most efficient ss-siRNA, ISIS 573309, possessed a lipid modification at position 6. Its IC50 value for inhibition of mutant HTT expression by ISIS 573309 was similar with the parent compound ISIS 537775 although it was also less selective (Yu et al., 2012). The two other positions for lipid modifications led to compounds (ISIS 573312 and ISIS 5733310) that were less allele selective than ISIS 573309. These data show that lipid modifications are compatible with highly allele-selective compounds, but that placement at certain locations can reduce allele selectivity.
Effect of varying number and placement of mismatched bases
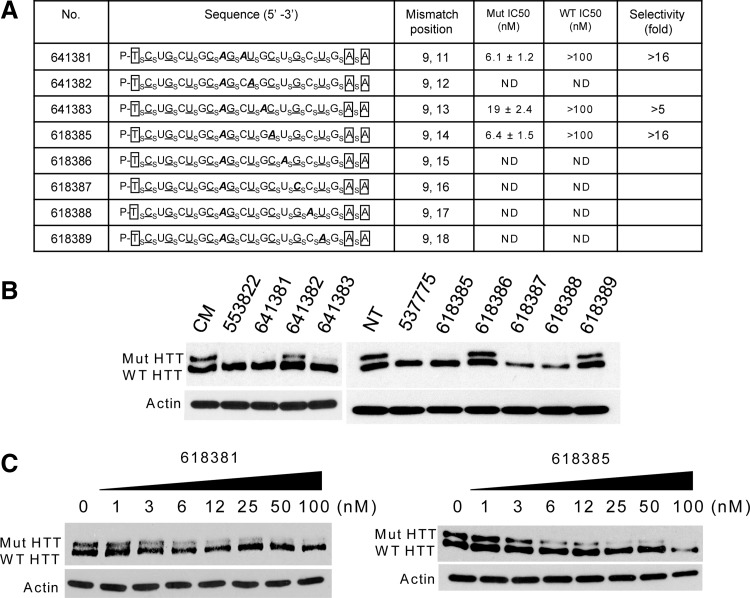
Changing the complementarity of an ss-siRNA relative to its target mRNA can modulate recognition, potency of inhibition, and allele selectivity. All ss-siRNAs used in this study contained a mismatch at position 9 relative to the poly-CAG target region. Mismatches at position 9 disrupt the potential for cleavage of substrate by argonaute 2 and shift the mechanism of gene silencing towards that used by micro RNAs (Hu et al., 2010; Yu et al., 2012). For this series of ss-siRNAs we retained the mismatch at position 9 and added secondary mismatches systematically at other positions (Fig. 4A).
FIG. 4.
Effect of changing the position of a second mismatched base on allele-selective inhibition of HTT. (A) Table of ss-siRNAs. Subscript “s” indicates PS linkage; 2′-O-Me modified base is shown in uppercase; 2′-F in underlined uppercase; 2′-MOE in enclosed box. Mismatched base is shown in bold and italic. The terminal T has a 5′ phosphate. ND: not determined. (B) Inhibition of HTT expression by ss-siRNAs tested at 25 nM concentrations. (C) Effect of increasing concentrations of ss-siRNAs ISIS 618381 and ISIS 618385, representative data from triplicate experiments. CM: non complementary duplex RNA. NT: no treatment.
We observed a variety of outcomes (Fig. 4B). Adding a second mismatch at positions 12 (ISIS 641382), 15 (ISIS 618386), or 18 (ISIS 618389) led to reduced inhibition of mutant HTT expression. Duplexes with a second mismatch at positions 16 (ISIS 618387) or 17 (ISIS 618388) are selective inhibitors but were observed to reduce viability of cultured cells. Examination of the sequences for these two relatively toxic ss-siRNAs does not reveal a reason for their effects on cell proliferation but does reinforce the recognition that individual compounds can behave unpredictably and the importance of testing multiple compounds when constructing trends.
Duplexes ISIS 618381 (mismatched at 9 and 11) and ISIS 618385 (mismatched at 9 and 14) showed the most promising activities and were selected for further characterization (Fig. 4C). Both had potencies of approximately 6 nM and selectivities of >16-fold. These results show that it was possible to introduce secondary mismatches without greatly affecting the inhibitory properties of the doubly substituted ss-siRNAs, thereby expanding the pool of ss-siRNAs that can be used for further development.
Effect of manipulating the potential for internal ss-siRNA structure
When testing the effect of doubly-mismatched ss-siRNAs we noted that ISIS 618386 and ISIS 618389 were relatively inactive (Fig. 4B). Inspection of their sequences revealed the potential for secondary structure within the two ss-siRNAs. We reasoned that altering the nature of the second mismatch to reduce structure formation would lead to higher potencies and allele selectivities.
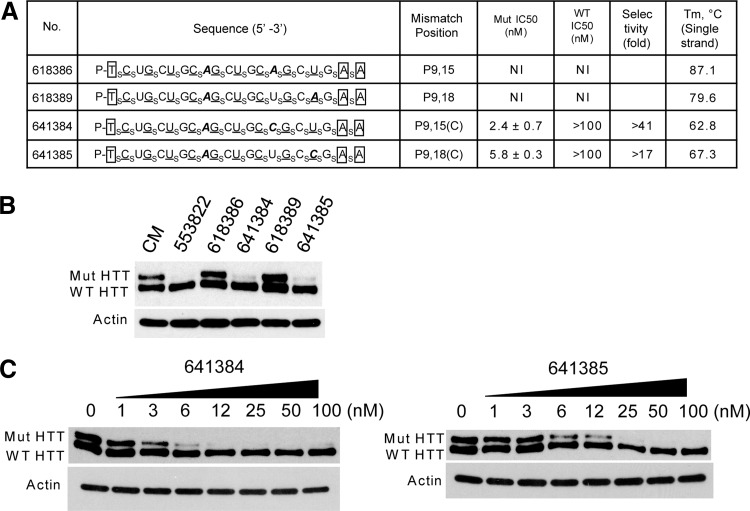
To test this hypothesis we synthesized ss-siRNAs ISIS 641384 and ISIS 641385 (Fig. 5A). The position of mismatches is the same. The only difference is that we used cytosine instead of adenosine for the second mismatch. Their melting temperatures (Tm) values for intramolecular association of single strands were reduced compared with Tm values for ISIS 618386 and ISIS 618389.
FIG. 5.
Altering intra-molecular structure of ss-siRNAs to improve allele-selective inhibition of HTT expression. (A) List of ss-siRNAs. Subscript “s” indicates PS linkage; 2′-O-Me modified base is shown in uppercase; 2′-F in underlined uppercase; 2′-MOE in enclosed box. Mismatched base is shown in bold and italic. The terminal T has a 5′ phosphate. All other linkages are phosphate. (B) Effects of ss-siRNAs tested at 25 nM. (C) Effect of increasing concentration of ISIS 641384 and ISIS 641385, representative data from triplicate experiments. CM: non complementary duplex RNA.
Screening revealed that both ss-siRNAs were more allele selective than their parent compounds (Fig. 5B). We observed IC50/selectivity values for ISIS 641384 and ISIS 641385 of 2.4 nM/>41-fold and 5.8 nM/>17 fold respectively (Fig. 5C). These data show that manipulation of internal structure is a viable strategy for improving the efficiency and allele-selectivity for inhibiting mutant HTT expression. A similar result was previously achieved for inhibition of mutant ataxin-3 (ATX-3) expression (Liu et al., 2013a).
Allele selective inhibition in a mutant cell line with fewer CAG repeats
Most of the experiments to evaluate allele-selective inhibition of HTT were performed in GM04281 patient-derived fibroblast cells containing 69 CAG repeats in the mutant allele while the median repeat number in HD patients is 45 CAG repeats (Duyao et al., 1993; MacDonald et al., 1993). HTT is a large protein, with a wild-type molecular weight of approximately 345–347 kDa. The expanded repeat encodes a long poly-glutamine region that increases the molecular weight relatively little, making it difficult to separate mutant and wild-type proteins.
We developed protocols that allow separation of mutant and wild-type HTT (Hu et al., 2009) and we routinely use GM04281 cells because the relatively large difference in CAG repeat number between the 69 repeat mutant allele (353.7 kDA) and the 17 repeat wild-type allele (347 kDA) facilitate separation of the two HTT variants by SDS-PAGE, allowing accurate the quantitative analysis of large numbers of compounds. However, while more difficult, it is also possible to analyze mutant HTT variants with fewer repeats. We have previously shown that antisense oligonucleotides, duplex RNAs, and ss-siRNAs that are allele selective for inhibition of mutant HTT expression in GM04281 cells are also allele selective in cells with fewer repeats (Hu et al., 2009; Hu et al., 2010; Yu et al., 2012).
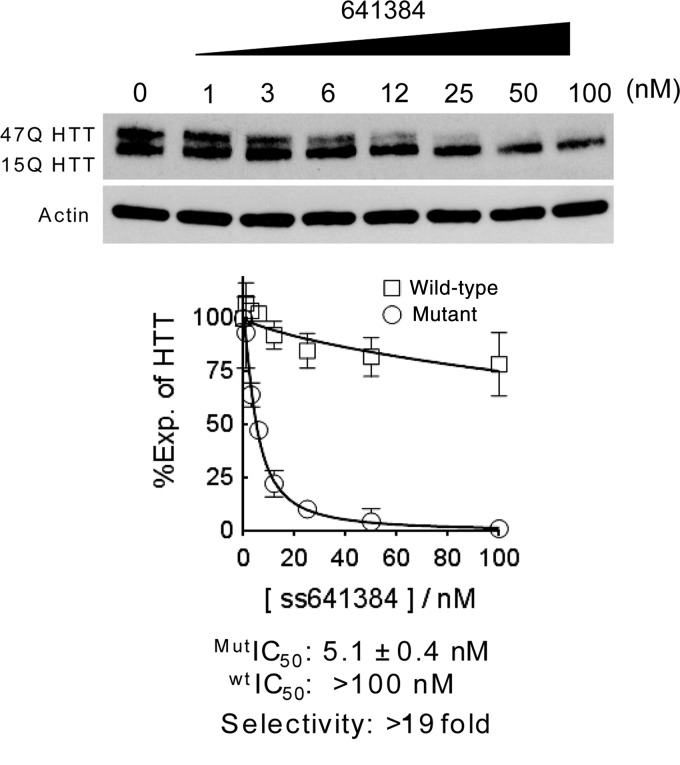
To confirm that allele selectivity in shorter repeat cells could also be achieved in this study we transfected ISIS 641384 into patient derived fibroblast line GM04869. GM04869 has 47 repeats in the mutant allele (350.9 kDA) and 15 repeats in the wild-type allele (346.8 kDA) and we developed a new electrophoresis protocol that separates the variants more reliably. We observed an IC50 value for inhibition of mutant HTT expression by ISIS 641384 of 5.1 nM and a selectivity of >19-fold (Fig. 6).
FIG. 6.
Inhibition of HTT expression in a cell line with fewer mutant repeats. Effect on expression of HTT from adding ISIS 641384 to GM04869 patient-derived fibroblast cells.
The allele selectivity of >19 can be compared to the allele-selectivity of >41 in the benchmark GM04281 cells. This decrease is due to a less potent IC50 (5.1 nM relative to 2.4 nM), and the less potent IC50 may be related to the lower mutant repeat number. It seems reasonable to believe that that potency will decrease as the number of mutant repeat decreases. Complicating this interpretation, however, in our previous report (Yu et al., 2012) we found that ISIS 553822 in 44 repeat cell line GM04719 has a greater >100-fold selectivity. More experiments will be needed to establish the impact shorter mutant repeats on inhibition of mutant HTT throughout the range of HD patients. Experiments in cells with fewer repeats, however, are complicated by the much greater difficulty involved in separating mutant from wild-type HTT in HD cell lines with mutant repeat numbers near or below the median number of forty-five.
Effect of altering chemical modifications
Chemical modifications to nucleic acids are important for conferring stability and optimizing biodistribution in animals. For ss-siRNAs, modifications also affect the ability to enter the RNA-induced silencing complex (RISC). As a result, a balance must be struck between the need for modifications to achieve biological activity, the constraints of RISC loading, and the need to achieve allele-selective inhibition.
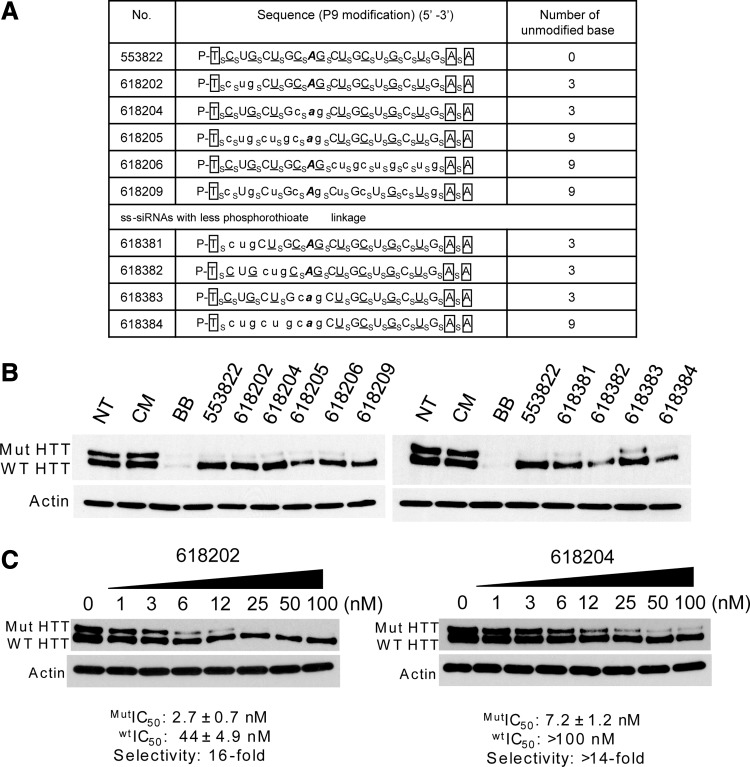
To investigate the impact of chemical modification on ss-siRNA mediated inhibition of HTT expression we synthesized a series of ss-siRNAs containing varying numbers of unmodified bases relative to 2′-O-methyl or 2′-fluoro substitutions and by lowering the number of phosphorothioate linkages (Fig. 7A). A wide variety of different modification patterns were compatible with allele-selective inhibition when compounds were added to cells at 25 nM (Fig. 7B). Two of these were examined more closely, ISIS 618202 and ISIS 618204 (Fig. 7C). Both compounds contained three unmodified bases, a significant reduction from parent compound ISIS 553822 that was modified at every position. ISIS 618202 possessed an IC50 value for mutant HTT of 2.7 nM and a selectivity of 16-fold, while ISIS 618204 possessed and IC50 value of 7.2 and a selectivity of >14 fold. These results indicate that altering the level of chemical modification can widen the pool of allele-selective inhibitors of HTT expression.
FIG. 7.
Effect of varying chemical modifications on inhibition of HTT expression. (A) Table of ss-siRNAs. Subscript “s” indicates PS linkage; 2′-O-Me modified base is shown in uppercase; 2′-F in underlined uppercase; 2′-MOE in enclosed box; Unmodified RNA base is in lowercase. Mismatched base is shown in bold and italic. The terminal T has a 5′ phosphate. All other sugars are ribose and all other linkages are phosphate. (B) Western blot images of ss-siRNAs tested at 25 nM. (C) Effect of increasing concentrations of ISIS 618202 and ISIS 618204, representative data from triplicate experiments. CM: non complementary duplex RNA. NT: No treatment. BB: a non-allele selective positive control RNA that targets outside the CAG repeat.
Effects of ss-siRNAs on expression of HTT in Hdh175Q/7Q mouse model
We previously showed that ss-siRNA ISIS 537775, an ss-siRNA containing one mismatched base, could achieve allele-selective inhibition of HTT expression in HdhQ150/7Q model mice (Yu et al., 2012). We now tested whether this inhibition could also be achieved by an ss-siRNA with three centrally located mismatches in a different mouse model. In our previous study in cell culture, ss-siRNA ISIS 557426 containing three central mismatches possessed outstanding potency (3.3 nM) and allele-selectivity (>30-fold), making it an excellent candidate for further examination in mice.
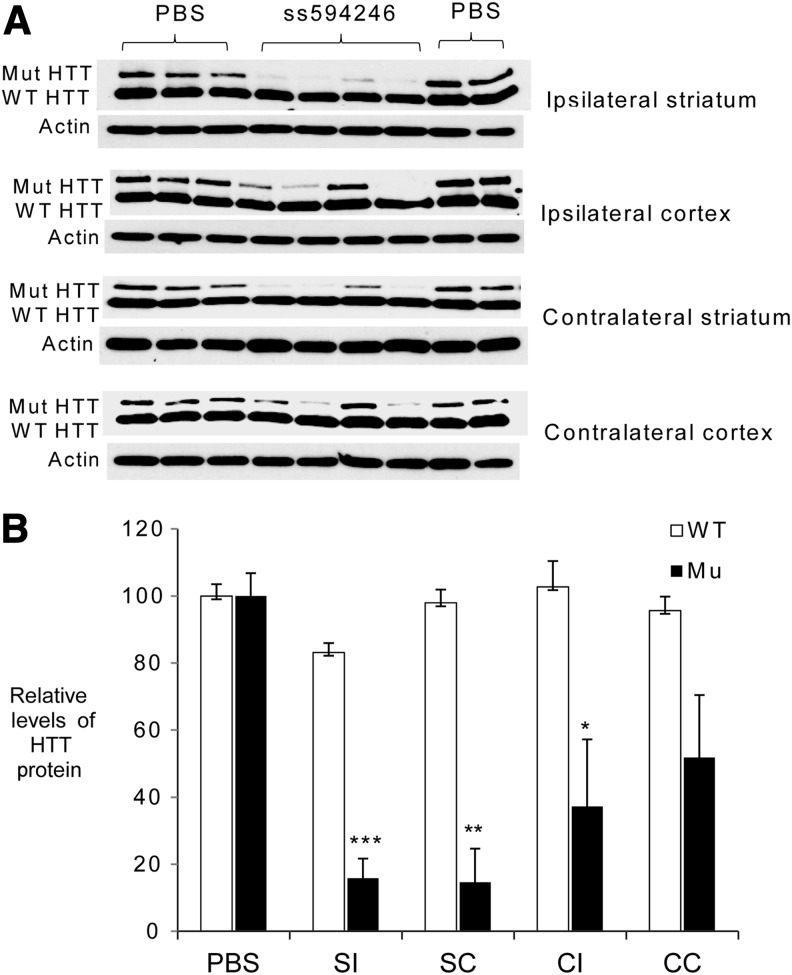
We synthesized the phosphonate version of ISIS 557426, ISIS 594246, and tested its activity after intraventricular infusion in HdhQ175 model mice (Heikkinen et al., 2012; Menalled et al., 2012). We administered 300 μg/day ss-siRNA or phosphate-buffered saline over 4 weeks by osmotic pump to achieve distribution throughout the central nervous system (Kordasiewicz et al., 2012; Yu et al., 2012; Lagier-Tourenne et al., 2013). The mice were monitored for survival during the treatment and no abnormal phenotype as observed, although we note that in vivo tolerability of the compound will require further study including the use of larger cohorts of animals. We observed that ISIS 594246 was a selective inhibitor of HTT expression in all brain sections tested, including ipsilateral and contralateral striatum and cortex (Fig. 8A, B), demonstrating that in vivo activity is compatible with different ss-siRNA designs.
FIG. 8.
In vivo inhibition of HTT expression by ss-siRNA ISIS 594246 in Hdh Q175/Q7 model mice. (A) Western blot images of different brain tissues form HdhQ175/Q7 mice after treating with 300 μg ss-siRNA 594246 or phosphate buffered saline (n = 4). (B) Quantification of HTT protein levels from (A). Error bars represent standard error of the mean. *p < 0.05; **p < 0.01; ***p < 0.001.
Discussion
Nucleic acids are a promising approach to controlling gene expression in the central nervous system (CNS) for several neurodegenerative diseases. This promise is accompanied by experimental challenges. Successful agents will need to be administered to the CNS, reach diseased tissues, enter cells, and silence gene expression. Side effects must be minimized and drugs should be well tolerated by patients. These challenges are similar to those faced by any drug during development. As with any other drug, one strategy for overcoming obstacles will involve chemical modifications to improve biological properties.
The purpose of our study was to examine how modifying RNA duplexes would affect potency and allele-selective inhibition of HTT expression and how broad the pool of active compounds might be. Having a broad pool of candidates is important because little information is available about how ss-siRNAs act in vivo and any drug designed to treat HD will need to be optimized for high potency and low toxicity. Having many choices for development will increase the likelihood that drug candidates will have efficacies that justify extensive preclinical characterization.
We find that there is considerable room for varying the chemical composition of ss-siRNAs. RNA strand length, location of mismatched bases, and the pattern of chemical modifications can all be changed while retaining favorable properties. Lipid groups can be added and the resulting compounds can be selective inhibitors of HTT expression. These results are consistent with parallel studies showing that allele-selective inhibition of HTT expression can also be achieved by many different duplexes containing abasic (Liu et al., 2013b) or unlocked nucleic acid (UNA) substitutions (Aiba et al., 2014). Our results suggest that there is a broad pool of compounds to draw from to further optimize ss-siRNAs or other compounds that function through the RNAi pathway for inhibition of HTT expression.
The ss-siRNAs examined in this study for their inhibition of HTT expression were also examined for inhibition of ATX-3 expression (Liu et al., 2013a). Mutant ATX-3 also contains an expanded CAG repeat and is responsible for Machado Joseph disease, raising the possibility that a single anti-CAG ss-siRNA may be able to treat multiple diseases. IC50 values for inhibition of both ATX-3 and HTT expression were obtained for 10 compounds and some trends emerge from the results (Table 1). In five cases, selectivities were higher for inhibition of HTT; in two cases selectivity was better for ATX-3, while selectivity was similar for three compounds. The fact that trends for inhibition of ATX-3 and HTT expression are not identical demonstrates that the sequences surrounding the shared CAG repeat affects recognition. Effects are not always predictable and testing multiple compounds is necessary to accurately evaluate the potential for allele-selective inhibition of a target gene. ISIS 618385 and 641385 are potent and allele-selective inhibitors for both ATX-3 and HTT and might be good starting points for developing agents that might be used to treat multiple expanded repeat diseases.
Table 1.
IC50 Values for Inhibition of Ataxin-3 and Huntingtin Expression for Single-Stranded Silencing RNAs Examined in this Study
| Effect on HTT | Effect on ATX-3 | ||||||
|---|---|---|---|---|---|---|---|
| No. | Mismatch position | mut IC50 (nM) | wt IC50 (nM) | Selectivity (fold) | mut IC50 (nM) | wt IC50 (nM) | Selectivity (fold) |
| 537775 | 9 | 3.5±0.3 | >100 | >29 | 3.6±1.4 | 20±2.5 | 6 |
| 537787 | None | 8.0±1.7 | >100 | >13 | 28±6.4 | 52±12 | 2 |
| 553822 | 9 | 4.9±0.8 | 90±9.7 | 18 | 8.4±2.5 | 99±28 | 12 |
| 557409 | 9, 10 | 6.3±0.5 | >100 | >16 | 16±3.5 | 62±16 | 4 |
| 557426 | 9, 10, 11 | 3.3±0.5 | >100 | >30 | 30±6.5 | >100 | 3 |
| 581440 | 9 | 3.0±0.6 | 20±7.5 | 7 | 12±1.2 | 75±18 | 6 |
| 618385 | 9, 14 | 6.4±1.5 | >100 | >16 | 1.4±0.2 | >50 | >35 |
| 641385 | 9, 18 | 5.8±0.3 | >100 | >17 | 2.1±0.3 | >50 | >23 |
| 618202 | 9 | 2.7±0.7 | 44±4.9 | 16 | 7.9±1.4 | >50 | >6 |
| 618204 | 9 | 7.2±1.2 | >100 | >14 | 3.1±0.8 | >50 | >16 |
ATX-3, ataxin-3; HTT, huntingtin; IC50, half maximal inhibitory concentration; Mut, mutant; ss-siRNAs, single-stranded silencing RNA; WT, wild-type.
Other approaches to selective targeting of expanded trinucleotide repeats include rationally designed compounds (Disney, 2013) and peptoids identified by screening (Chen et al., 2011). The advantage of small molecule inhibitors is that they may have more potential for improved uptake through the blood-brain barrier or oral bioavailability. A disadvantage is that unlike nucleic acids, which can exploit complementary base pairing, it may be more difficult for small molecules to achieve potent and selective recognition of individual target RNA sequences. All approaches that target CAG repeats face challenges but much progress has been made and the urgent need for drugs to treat HD and other expanded repeat diseases will continue to encourage innovative approaches.
Acknowledgments
Work in the Corey Laboratory was supported by the National Institutes of Health (NIGMS 73042), an award from the McKnight Foundation for Neuroscience, and the Robert A. Welch Foundation (I-1244). DRC, CLT, JB, and JWA are supported by Cure Huntington's Disease Initiative (CHDI). JH was supported by a Young Investigator Award from the National Ataxia Foundation. We thank Matthew Hensley for his thoughtful comments on this manuscript.
Author Disclosure Statement
TP Prakash is employed by ISIS Pharmaceutical and a patent application related to ss-siRNAs has been filed.
References
- AIBA Y., HU J., LIU J., XIANG Q., MARTINEZ C., and COREY D.R. (2014). Effect of unlocked nucleic acids (UNA) modifications on RNAi and allele-selective inhibition of huntingtin and ataxin-3. Biochemistry 52,9329–9338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOUDREAU R.L., MCBRIDE J.L., MARTINS I., SHEN S., XING Y., CARTER B.J., and DAVIDSON B.L. (2009). Nonallele-specific silencing of mutant and wild-type huntingtin demonstrates therapeutic efficacy in Huntington's disease mice. Mol. Ther. 17,1053–1063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- CARROLL J.B., WARBY S.C., SOUTHWELL A.L., DOTY C.N., GREENLEE S., SKOTTE N., HUNG G., BENNETT C.F., FREIER S.M., and HAYDEN M.R. (2011). Potent and selective antisense oligonucleotides targeting single-nucleotide polymorphisms in the Huntington disease gene/allele specific silencing of mutant huntingtin. Mol. Ther. 19,2178–2185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHEN X., WU J., LUO X., SUPNET C., KIM M.W., LOTZ G.P., YANG G., MUCHOWSKI P.J., KODADEK T., and BEZPROZVANNY I. (2011). Expanded polyglutamine-binding peptoid as a novel therapeutic agent for treatment of huntington's disease. Chem. Biol. 18,1113–11125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DIFIGLIA M., SENA-ESTEVES M., CHASE K., SAPP E., PFISTER E., SASS M., YODER J., REEVES P., PANDEY R.K., RAJEEV K.G., et al. (2007). Therapeutic silencing of mutant huntingtin with siRNA attenuates striatal and cortical neuropathology and behavioral deficits. Proc. Natl. Acad. Sci. U. S. A. 104,17204–17209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DISNEY M.D. (2102). DISNEY, M.D. (2102). Rational design of chemical genetics probes of RNA function and lead therapeutics targeting repeating transcripts. Drug Discov. Today 18,1228–1236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUYAO M., AMBROSE C., MYERS R., NOVELLETTO A., PERSICHETTI F., FRONTALI M., FOLSTEIN S., ROSS C., FRANZ M., ABBOTT M., et al. (1993). Trinucleotide repeat length instability and age of onset in Huntington's disease. Nat. Genet. 4,387–392 [DOI] [PubMed] [Google Scholar]
- EVERS M.M., PEPERS B.A., VAN DEUTEKOM J.C., DEN DUNNEN J. T., VAN OMMEN G.J., and VAN ROON-MOM W. M. (2011). Targeting several cag expansion diseases with a single antisense oligonucleotide. PLoS One 6,e24308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FISZER A., MYKOWSKA A., and KRYZOSIAK W.J. (2011). Inhibition of mutant huntingtin expression by RNA duplex targeting expanded CAG repeats. Nucleic Acids Res. 39,5578–5585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- GAGNON K. T., PENDERGRAFF H., DELEAVEY G., SWAYZE E., POTIER P., RANDOLPH J., ROESCH E., CHATTOPADHYAYA J., DAMHA M., BENNETT C.F., et al. (2010). Allele-selective silencing of huntingtin expression with antisense oligonucleotides targeting the mRNA expanded CAG repeat. Biochemistry 49,10166–10178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- HEIKKINEN T., LEHTIMAKI K., VARTIANEN N., PUOLIVALI J., HENDRICKS S.J., GLASER J.R., BRADAIA A., WADEL K., TOULLER C., KONTKANANEN O., et al. (2102). Characterization of neurophysiologic and behavioral changes, MRI brain volumetry and 1H MRS in zQ175 knock-in mouse model of Huntington's disease. PLoS One 7,e50717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HU J., MATSUI M., GAGNON K.T., SCHWARTZ J.C., GABILLET S., ARAR K., WU J., BEZPROZVANNY I., and COREY D.R. (2009). Allele-specific silencing of mutant huntingtin and ataxin-3 genes by targeting expanded CAG repeats in mRNAs. Nat. Biotechnol. 27,478–484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- HU J., LIU J., and COREY D.R. (2010). Allele-selective inhibition of huntingtin expression by switching to an miRNA-like RNAi mechanism. Chem. Biol. 17,1183–1188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- HU J., LIU J., CHU Y., YU D., and COREY D. R. (2012). Mechanism of allele-selective inhibition of huntingtin expression by duplex RNAs that target CAG repeats. Nucleic Acids Res. 40,11270–11280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- KORDASIEWICZ H.B., STANEK L.M., WANCEWICZ E.V., MAZUR C., MCALONIS M.M., PYTEL K.A., ARTATES J.W., CHENG S.H., SHIHABUDDIN L.S., HUNG G., et al. (2012). Sustained therapeutic reversal of Huntington's disease by transient repression of mutant huntingtin synthesis. Neuron 74,1031–1044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- KREMER B., GOLDBERG P., ANDREW S.E., THEILMANN J., TELENIUS H., ZEISLER J., SQUITIERI F., LIN B., BASSETT A., ALMQUIST E., et al. (1994). A worldwide study of the Huntington's disease mutation. The sensitivity and specificity of measuring CAG repeats. N. Engl. J. Med. 330,1401–1406 [DOI] [PubMed] [Google Scholar]
- LAGIER-TOURENNE C., BAUGHN M., RIGO F., SUN S., LIU P., LI H.R., JIANG J., WATT A.T., CHUN S., KATZ M., et al. (2013). Targeted degradation of sense and antisense C9orf72 RNA foci as therapy for ALS and frontotemporal degeneration. Proc. Natl. Acad. Sci. U. S. A. 110,e4530–4539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIMA W.F., PRAKASH T.P., MURRAY H.M., KINBERGER G.A., LI W., CHAPPELL A.E., Li C.S., Murray S.F., GAUS H., SETH P.P., et al. (2012). Single-stranded siRNAs activate RNAi in animals. Cell 150,883–894 [DOI] [PubMed] [Google Scholar]
- LIU J., YU D., AIBA Y., PENDERGRAFF H., SWAYZE E.E., LIMA W.F., PRAKASH T.P., and COREY D.R. (2013a). ss-siRNAs allele-selectively inhibit ataxin-3 expression: Multiple mechanisms for an alternative gene silencing strategy. Nucl. Acids Res. 41,9570–9583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIU J., PENDERGRAFF H., NARAYANANNAIR K.J., LACKEY J.G., KUCHIMANCHI S., RAJEEV K.G., MANOHARAN M., HU J., COREY D.R. (2013b). RNA duplexes with abasic substitutions are potent and allele-selective inhibitors of huntingtin and ataxin-3 expression. Nucl. Acids Res. 41,8788–8801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MACDONALD M.E., AMBROSE C.M., DUYAO M.P., MYERS R.H., LIN C., SRINIDHI L., BARNES G., TAYLOR S.A., JAMES M., GROOT N., et al. . The Huntington's Disease Collaborative Research Group. (1993). A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington's disease chromosomes. Cell 72,971–983 [DOI] [PubMed] [Google Scholar]
- MATSUI M., and COREY D.R. (2012). Allele selective inhibition of trinucleotide repeats. Drug Discov. Today 17,443–450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MENALLED L.B., KUDWA A.E., MILLER S., FITZPATRICK J., WATSON-JOHNSON J., KEATING N., RUIZ M., MUSHLIN R., ALSOSIO W., MCCONNELL K., et al. (2012). Comprehensive behavioral and molecular characterization of a new knock-in mouse model of Huntington's disease: zQ175. PLoS One 7,e49838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NANCE M.A., and MYERS R.H. (2001). Juvenile onset Huntington's disease—clinical and research perspectives. MRDD Res. Rev. 7,153–157 [DOI] [PubMed] [Google Scholar]
- OSTERGAARD M.E., SOUTHWELL A.L., KORDASIEWICZ H., WATT A.T., SKOTTE N.H., DOTY C.N., VAID K., VILLANUEVA E.B., SWAYZE E.E., BENNETT C.F., et al. (2013). Rational design of antisense oligonucleotides targeting single nucleotide polymorphisms for potent and allele-selective suppression of mutant huntingtin in the CNS. Nucleic Acids Res. 41,9634–9650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- ORR H.T., and ZOGBHI H.Y. (2007). Trinucleotide repeat disorders. Ann. Rev. Neurosci. 30,575–621 [DOI] [PubMed] [Google Scholar]
- PFISTER E.L., KENNINGTON L., STRAUBHAAR J., WAGH S., LIU W., DIFIGLIA M., LANDWEHRMEYER B., VONSATTEL J.P., ZAMORE P.D., and ARONIN N. (2009). Five siRNAs targeting three SNPs may provide therapy for three-quarters of Huntington's disease patients. Curr. Biol. 19,774–778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAH D.W., and ARONIN N. (2011). Oligonucleotide therapeutic approaches for Huntington disease. J. Clin. Invest. 121,500–507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHWARZ D.S., DING H., KENNINGTON L., MOORE J.T., SCHELTER J., BURCHARD J., LINSLEY P.S., ARONIN N., XU Z., and ZAMORE P.D. (2006). Designing siRNA that distinguish between genes that differ by a single nucleotide. PLoS Genet. 2,e140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WALKER F.O. (2007). Huntington's disease. Lancet 369,218–228 [DOI] [PubMed] [Google Scholar]
- YU D., PENDERGRAFF H., LIU J., KORDASIEWICZ H.B., CLEVELAND D. W., SWAYZE E., LIMA W., CROOKE S. T., PRAKASH T., and COREY D. R. (2012). Single-stranded RNAs that function through RNAi are potent and allele-selective inhibitors of huntingtin expression. Cell 150,895–908 [DOI] [PMC free article] [PubMed] [Google Scholar]