Abstract
Significance: Understanding isoform- and context-specific subcellular Nox reduced nicotinamide adenine dinucleotide phosphate (NADPH) oxidase compartmentalization allows relevant functional inferences. This review addresses the interplay between Nox NADPH oxidases and the endoplasmic reticulum (ER), an increasingly evident player in redox pathophysiology given its role in redox protein folding and stress responses. Recent Advances: Catalytic/regulatory transmembrane subunits are synthesized in the ER and their processing includes folding, N-glycosylation, heme insertion, p22phox heterodimerization, as shown for phagocyte Nox2. Dual oxidase (Duox) maturation also involves the regulation by ER-resident Duoxa2. The ER is the activation site for some isoforms, typically Nox4, but potentially other isoforms. Such location influences redox/Nox-mediated calcium signaling regulation via ER targets, such as sarcoendoplasmic reticulum calcium ATPase (SERCA). Growing evidence suggests that Noxes are integral signaling elements of the unfolded protein response during ER stress, with Nox4 playing a dual prosurvival/proapoptotic role in this setting, whereas Nox2 enhances proapoptotic signaling. ER chaperones such as protein disulfide isomerase (PDI) closely interact with Noxes. PDI supports growth factor-dependent Nox1 activation and mRNA expression, as well as migration in smooth muscle cells, and PDI overexpression induces acute spontaneous Nox activation. Critical Issues: Mechanisms of PDI effects include possible support of complex formation and RhoGTPase activation. In phagocytes, PDI supports phagocytosis, Nox activation, and redox-dependent interactions with p47phox. Together, the results implicate PDI as possible Nox organizer. Future Directions: We propose that convergence between Noxes and ER may have evolutive roots given ER-related functional contexts, which paved Nox evolution, namely calcium signaling and pathogen killing. Overall, the interplay between Noxes and the ER may provide relevant insights in Nox-related (patho)physiology. Antioxid. Redox Signal. 20, 2755–2775.
Introduction
Working paradigms of cellular redox signaling increasingly suggest compartmentalization of transduction pathways, in which oxidative stress can be modeled as a loss of such modular architecture (51). This pattern of organization assumes that reactive oxygen species (ROS) generation is highly regulated on a basis of space, time, and type of species being produced (27, 126, 167, 178), thus implying an important role of enzymatic ROS sources in this process. Nox family reduced nicotinamide adenine dinucleotide phosphate (NADPH) oxidases are the prototypical source of signaling ROS in most cells [reviewed in Refs. (12, 58, 96, 97)], even though in quantitative terms, mitochondria can predictably surpass Noxes as ROS sources in most, although likely not in all, cell types (10, 25). While in mitochondria, the modular architecture is provided by the organellar structure itself, in the case of Nox NADPH oxidases, this is achieved through a regulated set of multiple protein interactions and/or post-translational modifications associated with Nox complex assembling and traffic to specific subcellular locations, which vary according to the type of Nox isoform [reviewed in Refs. (12, 58, 96, 97)]. Thus, understanding isoform- and context-specific subcellular location of Nox(es) is relevant to allow inferences on their functional pathways.
The endoplasmic reticulum (ER) has emerged as an increasingly evident player in redox physiology and pathophysiology (110, 145). This is due to at least three facts, the first of which being the ER-dependent production of ROS via enzymes, such as ER oxidoreductin 1 (Ero1) and its thiol redox partner(s) protein disulfide isomerase(s) (PDI). Such ER oxidoreductases engage in thiol-disulfide exchange reactions that culminate with the downstream insertion by oxidized PDI of disulfides into nascent proteins, coupled at the upstream end to electron transfer from reduced Ero1 to oxygen, thus generating hydrogen peroxide and oxidized Ero1—the latter step re-enabling the redox protein folding cycle (14, 68, 159). The significance of this potentially enormous source of hydrogen peroxide is not yet clear, but peroxide can be used at least in part for productive protein folding by promoting parallel oxidations of ER-resident peroxiredoxin IV (199) or glutathione peroxidase 7/8 (122), in addition to glutathione (158)—all converging toward PDI oxidation. These processes have been previously reviewed in detail (99). Second, the ER closely communicates and interacts with mitochondria via specific membrane tethering structures composing the “mitochondria-associated membrane”, a subcompartment involved in calcium exchange, Ero1-mediated protein folding, and apoptosis regulation (21). Finally, the ER is closely involved in multiple aspects with Nox NADPH oxidases, in a way that extrapolates its expected role as the site of synthesis and protein processing of the transmembrane catalytic subunits. Increasing evidence points to the ER or ER-associated structures as an activation compartment for some isoforms, a pattern bearing possible ancestral connections and evolutionary relevance. Furthermore, ER chaperones, such as PDI, have been shown to display functionally relevant interactions with Nox(es) in distinct cell types (99). The aim of the present review is to discuss in more detail the mechanisms and implications of such interactions between the ER and Nox NADPH oxidases, an exercise we believe is likely to shed light on important aspects of Nox (patho)physiology.
Synthesis and Processing of Nox NADPH Oxidases at the ER
Excellent previous reviews (8, 9, 12, 58, 96, 97) have addressed in-depth the structural and functional aspects of Nox NADPH oxidases, which will not be covered here. As transmembrane proteins, the prototypical catalytic Nox isoforms are synthesized and processed within the ER, similar to the regulatory transmembrane subunit p22phox. Contrarily, the canonical cytosolic regulatory subunits p47phox, p67phox, p40phox, and Rac1 or Rac2 are likely synthesized at the cytosol. Full maturation of the Nox complex includes several steps: glycosylation (and putatively other post-translational modifications), p22phox heterodimerization, insertion of heme and flavin adenine dinucleotide (FAD), and traffic to relevant locations within the distal secretory pathway up to membrane-containing subcompartments. Distinct functions of the ER are involved to a variable extent in each of these steps. In addition, these steps are isoform-specific: for example, p22phox incorporation is not known to occur with Nox5, and traffic distal to the ER, as well as post-translational modifications, may be restricted in some aspects for isoforms active at ER membranes, such as Nox4. In addition, as discussed below, the incorporation of cytosolic subunits seems to occur as a process concomitant to the traffic of nonphagocytic Noxes toward membrane(s) (162), as opposed to a clear activation step for the phagocytic complexes [reviewed in Refs. (8, 9)].
The most well-known NADPH oxidase complex regarding synthesis and processing is the phagocytic Nox2 (8, 9). Flavocytochrome b558 contains two integral membrane proteins: gp91phox (a synonym for Nox2) and p22phox (127). The former is the redox center of the electron transfer, which binds heme and FAD, whereas the latter provides stability to gp91phox and fulfills the role of an adaptor protein working as an activation platform able to mediate interactions with the cytosolic regulatory subunits p67/p47 phox (35). The 91 kDa glycoprotein gp91phox weighs 50–55 kDa when fully deglycosylated (64, 127), whereas a 65 kDa precursor is most often detectable, even as the only form in cells, such as B cells (132) and PLB-985 human promyelocytic leukemia cells (190). Such gp65 precursor cosediments with ER, but not with Golgi, marker-containing fractions (188) and is susceptible to digestion with endoglycosidase H, suggesting that it contains ER-related high mannose-type carbohydrates (188, 190). Therefore, the synthesis of the larger subunit of flavocytocrhome b558 occurs in the ER, where initial glycosylation takes place (40, 188, 190). Surprisingly, less than one third of newly synthesized gp65 pool appears to be processed to gp91phox and a significant fraction is degraded by the proteasome (40). The functional importance of ER-dependent gp91phox N-glycosylation is supported by the fact that patients with congenital deficiency of ER membrane-located glucose-incorporating enzymes have neutrophil dysfunction in connection with impaired NADPH oxidase activity. Such Nox dysfunction is associated with normal protein expression of all subunits but decreased gp91phox molecular weight, indicating a failure of glycosylation, and in fact the N- and O-glycomes of such neutrophils exhibit a failure of galactosylation (69). In addition to ER-dependent glycosylation, gp91phox presents complex carbohydrates, likely added at the Golgi, in line with the fact that, contrarily to gp65 precursor, endoglycosidase H is unable to digest gp91phox carbohydrate chains (40).
Full processing of mature stable gp91phox involves, besides glycosylation, the interrelated events of heme addition and heterodimerization with p22phox. Coexpression of gp91 phox and p22phox is important for gp91phox maturation and increases its cell surface expression without influencing gp65 expression in Chinese hamster ovary cells (197). Gp91phox binds two heme groups by itself (189). Accordingly, proteolysis study of purified neutrophil plasma membrane reveals that the portion of cytochrome b bound to heme groups is located within the lipid bilayer (135) and critical histidines at the N-terminal region are involved in the coordination of the bis-heme structure (53, 189). Importantly, these histidine residues are conserved across the Nox family. Cells expressing gp91phox mutated in critical histidines (H101L, H115L, H209C, or H222L) do not process gp65 to gp91phox nor do they achieve plasma membrane expression of flavocytochrome b, whereas their NADPH oxidase activity is completely abolished. (18). Relevant to the present discussion, in these mutated cells, not only gp65 but also p22phox were normally synthesized, but degraded with time, suggesting that sustained binding to the latter requires heme insertion and that heme insertion is a post-translational process (18). Such post-translational nature is further suggested by experiments with the heme synthesis inhibitor succinyl acetone, which abolishes superoxide generation (72), but does not affect the expressions of gp65 protein or gp91phox and p22phox mRNA, while decreasing p22phox and mature gp91phox protein expression. This indicates a fundamental heme function in heterodimer assembly, which occurs in the ER with the gp65 precursor (188, 190). Golgi disruption or inhibition of N-linked glycosylation, respectively, did not impair the associations between p22phox and the precursors gp65 or gp58, although preventing gp91phox maturation (40). Induction or superexpression of heme oxygenase in murine macrophages significantly decreases superoxide production, in connection with enhanced proteasomal degradation of gp91phox and p22phox, indicating that post-translational heme incorporation is not only essential for adequate electron transfer by cytochrome b558 but also for its stability (157). After ER synthesis and Golgi processing, human flavocytochrome b558 is constitutively expressed on monocyte and neutrophil plasma membranes, as well as neutrophil secondary granules (54), and Nox activation at either or both locations depends on the stimulus [reviewed in Ref. (87)].
The neutrophil NADPH oxidase cell-free system requires FAD to be active and cytochrome b558 is the FAD-binding site (140). Neutrophil membranes from X-linked CGD patients have FAD content below the normal FAD/heme 1:2 ratio (146). FAD replenishment to FAD-depleted cytochrome b558 promotes 10-fold increase in the NADPH oxidase activity in the cell-free system, whereas FAD binding to cytochrome b558 increases in phorbol ester-stimulated whole neutrophils. Probably, in resting cells, FAD is loosely bound to cytochrome b558 and their interaction enhanced upon activation (66). The H338Y gp91phox mutation described in a patient (186) promotes FAD depletion as well as decreased gp91phox expression. His338 is located in the HPFT motif (His338ProPheThr), which is involved in FAD binding in a ferredoxin-NADP+ reductase. FAD replenishment restored phorbol myristate acetate (PMA) stimulation of the NADPH oxidase activity in such H338Y CGD granulocytes. Retention of nascent mutant H338Y gp91phox by the ER glycoprotein-processing chaperone calnexin prevents mutated gp91phox to be detected at the cell surface, implicating FAD binding in calnexin dissociation from gp91phox. In normal cells, gp65 interaction with calnexin is detectable 1 h after its synthesis and totally abolished within 5 h, whereas gp65 from H338Y mutant cells remains bound to calnexin up to 5 h after synthesis. Surprisingly, calnexin also associates with p22phox even in the absence of gp91phox, probably via an unknown glycoprotein (106). Interestingly, NADPH oxidase as well as in vitro and in vivo bactericidal activities were further restored in H338Y mutants in the concomitant presence of FAD and thapsigargin, which efficiently redistribute ER-retained gp91phox to plasma membranes (77). Thapsigargin effect was apparently unrelated to increases in cytosolic calcium levels, but rather on its action on ER calcium-dependent protein quality control, allowing relief of calnexin-mediated ER retention of mutant gp91phox (33). On the other hand, FAD presence was not detected in resting or stimulated T341K, C369R, G408E, and E568K gp91phox mutants displaying X+-CGD phenotype with loss in the NADPH oxidase activity. Surprisingly, the NADPH oxidase assembly is normal in T341K, although not in the other mutants, indicating that FAD binding is dispensable for the translocation of cytosolic factors. Of note, Thr-341, located at the predicted FAD-binding site, and Glu-568 are conserved in Nox1, Nox3, and Nox4 (39). Overall, these data indicate that FAD is not strictly required for gp91phox maturation and cell surface expression on the cell surface, although probably contributing in yet unclear aspects to sustain gp91phox expression. FAD binding to gp91phox likely occurs concomitantly or upon activation of NADPH oxidase and consequently not within the ER.
Lipid metabolism is an important ER-related function, and the association with lipid compartments appears important for the distribution and assembly of active Nox2 complex since mature gp91phox has been documented in lipid rafts, as opposed to gp65 precursor (170). Moreover, lipid raft disruption impairs NADPH oxidase assembly, as seen in the cell-free system, in which cholesterol is required for efficient p67phox and p47 phox translocation. Cholesterol depletion in Ra2 microglia and HL60 cell membranes changes the distribution of gp91phox and p22phox in detergent-resistant membranes from low- to high-density fraction, in association with the decrease in stimulated superoxide production (170).
Therefore, there are many levels of organization to be reached until NADPH oxidase becomes a functional enzymatic complex (48). These include (i) incorporation of redox components like heme and FAD, which are essential to proteins stability and heterodimer formation; (ii) correct localization on the membrane; and (iii) assembly of cytosolic components, which includes translocation and association of factors: p40phox, p47phox, p67phox, and Rac with flavocytochrome b558.
Little is known about the synthesis and early processing of the other Nox isoforms within the ER (the Duoxes will be discussed in the next section). There is, however, no apparent reason why Nox1, Nox3, and in some aspects Nox4 processing and assembling should be significantly distinct from that of Nox2. Nox4 location at the ER and the lack of downstream interaction with cytosolic subunits, in addition to the lack of Nox5 heterodimerization with p22phox, are, of course, obvious differences. Since Nox4 does not require cytosolic subunits for activation, the role of heterodimerized p22phox is likely of stabilization rather than activation. Of note, the mutation of Nox4 histidine 115 abolishes its interaction with p22phox, similar to Nox1 and Nox2 (4). In addition, there is no reason why these isoforms are not glycosylated in a manner analogous to that of Nox2, although objective evidence for this is presently lacking.
Nox NADPH Oxidase Localization and Activation Within the ER
A good deal of research in the Nox field has focused on understanding isoform localization as a conceivable window to reveal their possible functions. This approach, somewhat disappointingly, has proven so far only partially successful. No Nox isoform has a clear localization signal in its structure, and their subcellular location appears quite dynamic and stimulus-related, consistent with its preferential dependency on post-translational processes, such as protein modifications and protein–protein interactions, which remain to be better understood. In addition, most Nox isoform antibodies have questionable specificity, rendering localization studies a challenge. Few studies report controls with inhibitory peptides and Nox silencing to probe antibody specificity. In the case of the ER, the issue of location is further limited because, as the place of synthesis, all isoforms have at least a transient maturation stage within the ER, whereas in many cases, they do not become fully active at the ER. Importantly, many studies addressing Nox location make use of transfection of tagged isoforms, whereas overexpression of any protein can lead to their artifactual retention in the ER as they may not be correctly folded and/or they can overload the ER folding machinery.
An emerging discussion in this context is the role of dehydrogenase domains, which are common to all Noxes. Such domains canonically mediate electron transfer from NADPH to FAD, that is, diaphorase activity. Normally, electron transfer from FAD to transmembrane heme groups is optimized by changes in protein conformation, as suggested for Nox2 (37). However, electron transfer from FAD to artificial substrates has been reported for Nox2 (62) and Nox4 (80, 121, 124). Whether in vivo the corruption of such dehydrogenase activity can generate ROS if these electrons are transferred to oxygen is interesting but yet speculative. However, the demonstration of cellular ROS generation for short alternatively spliced Nox4 isoforms lacking the transmembrane domain, such as Nox4D (56), which has recently been shown to display nuclear location (6), is consistent with such possibility. Similar findings have been shown for short alternatively spliced Nox2 beta isoform, which has only two transmembrane domains and lacks FAD-binding as well as all heme-binding domains (65). These facts allow some speculations. Since some of these short isoforms may lack domains responsible for traffic and membrane anchoring, they could behave as immature Noxes, being retained at the ER. An analogous mechanism could account for ROS generation by immature and/or slightly misfolded Noxes retained at the ER, even for otherwise normally membrane-located isoforms.
Nox4
Nox4 is the isoform most consistently associated with the ER according to many studies, some of them detailed in Table 1. However, even in the case of Nox4, several other locations have been described: mitochondria, focal adhesions and cytoskeleton, and nucleus, the latter associated to one of its short spliced isoforms (6). In addition, recently developed monoclonal antibodies have detected Nox4 localization also at the plasma membrane by total internal reflection fluorescence (TIRF) microscopy (194) (Table 1). High glucose and palmitate induce Nox4 translocation to lipid rafts in adipocytes (63). Overall, a seemingly emerging consensus about the yet controversial topic of Nox4 location is that this isoform is primarily an ER-targeted Nox but undergoes traffic to many other compartments depending on unknown factors that likely include cell type, triggering stimuli, proliferative status, and probably culture conditions. Each of these Nox4 locations, including the ER itself, may require specific protein–protein associations or post-translational modifications since Nox4 does not depict a known ER retention signal, whereas a software prediction algorithm yielded high score for mitochondrial location (19). Studies with chimeric Nox1 and Nox4 indicate that location, as well as type of produced ROS, are determined by the N-terminal portion of each Nox, whereas constitutive activity is regulated by cytosolic tail (70).
Table 1.
Selected Studies on Nox4 Subcellular Localization
| Cell type | Model | Detection method | Subcellular localization | Obs. | Ref. |
|---|---|---|---|---|---|
| Epithelial cells | Nox4 overexpression | Confocal IF, colocalization with p22phox | ER | Nox4 is constitutively activated. | 114 |
| HEK293, COS7 | |||||
| HEK293 | Nox4 inducible with tetracyclin | TIRF microscopy | Plasma membrane | 194 | |
| HEK293 T-Rex™ (tet-inducible Nox4) | Confocal microscopy | ER | |||
| HEK293 T-Rex (tet-inducible Nox4) | Nox4 overexpression | Light microscopy superoxide detection by NBT | Perinuclear | Detection of superoxide production in inducible Nox4-overexpressing cells. | 147 |
| Nuclear envelope | |||||
| HUVEC | Nox4-GFP overexpression | TIRF microscopy, Hyper sensor | ER | Nox4 mediates UPR and ER signaling through local Ras activation. | 181 |
| HEK293 | Nox4 overexpression | Confocal IF, colocalization with p22phox | Cytoskeleton | Nox4 heterodimerizes with p22phox in the ER. | 4 |
| Human ASM | ER | ||||
| HAEC | Overexpression of AdNox4-V5 | Confocal IF, colocalization with GRP78 | ER | Nox4 in the ER regulates oxidation and inactivation of PTP1B and ER resident proteins. | 28 |
| Electron microscopy, immunogold labeling | |||||
| Nicodenz gradient | |||||
| Human monocyte-derived macrophage) | oxLDL-stimulated macrophages | Immunohistochemistry, confocal IF, colocalization with p22phox and PDI | ER | Nox4 increases oxidized LDL-induced macrophage cytotoxicity and apoptosis. Nox4 is increased with oxLDL stimulus. | 100 |
| Nucleus | |||||
| Adult and neonatal cardiomyocytes | Endogenous and overexpressed Nox4 | Confocal IF, colocalization with PDI | Perinuclear region, probably ER | Nox4 is a regulator of myocardial angiogenesis. | 195 |
| Rat primary VSMC | Primary culture, endogenous Nox4 | Confocal IF, colocalization of endogenous Nox4 with vinculin | Focal adhesions, stress fibers, nucleus | Colocalization of Nox4 and p22phox | 74 |
| Compartmentation of ROS production | |||||
| Redox regulation of focal adhesion proteins | |||||
| Rat VSMC | Endogenous Nox4 | Endogenous Nox4 in confocal IF | Focal adhesions | Nox4 is activated by p22phox/Poldip2 recruitment | 109 |
| Human VSMC, HEK293 | Stress fibers, nucleus | ||||
| Primary cardiomyocytes | Nox4 Tg mice endogenous Nox4 | Confocal IF, colocalization of Nox4 with F1F0 ATP synthase (complex V) and mitotracker | Mitochondria | Nox4 promotes apoptosis and mitochondrial dysfunction when upregulated under hypertrophic stimuli. | 1 |
| Truncated Nox4 without mitochondria localization signal does not colocalize with mitochondria. | |||||
| Rat kidney cortex and mesangial cells | Rat model of diabetes induced by streptozocin | Mitochondria purification | Mitochondria | Nox4 is an important source of ROS in diabetes. | 19 |
| Confocal IF | ER | ||||
| Cardiomyocytes | Tg mouse with cardiac-specific Nox4 overexpression×Nox4 KO | SOD-inhibitable ROS production by lucigenin chemiluminescence in purified mitochondria | Mitochondria | Nox4 is the major source of superoxide and/or hydrogen peroxide in the heart, mediating mitochondrial dysfunction during pressure overload. | 93 |
| HUVEC | Endogenous Nox4 | Immunocytochemistry | Nucleus | Nox4 activity is enhanced in the nuclear fraction after PMA. | 94 |
| Electron microscopy with 3 antibodies | |||||
| Nuclei fractionation | |||||
| Adult | Nox4 KO mice and Tg mice with cardiac specific overexpression of Nox4 | Nuclei fractionation | Nucleus | Nox4 is involved in cysteine oxidation and nuclear exit of HDAC4, mediating cardiac hypertrophy in response to phenylephrine and pressure overload. | 115 |
| Cardiomyocytes | WB, confocal IF | Mitochondria | |||
| Microsome | |||||
| Mice hepatocytes | shRNA for Nox4 silencing | Nuclei fractionation, WB, electron microscopy, confocal IF | Nuclear envelope—inner and outer membranes | Nox4 most significantly contributes to NADPH-dependent hepatic nuclear superoxide production. | 151 |
| THP-1 monocytes | LDL and high glucose-induced metabolic stress in vitro | Confocal IF, colocalization with phalloidin | F actin | Nox4-derived H2O2 remodels actin, leading to chemotactic activity of monocytes. | 166 |
| Human airway smooth muscle cells | Primary culture | Confocal IF with dye that shifts to red in the ER | ER | No colocalization with mitochondria or focal adhesions. Nox4 induced by TGF-β mediates airway smooth muscle cell proliferation and asthmatic airway remodeling. | 153 |
| Nucleus | |||||
| Rabbit/mouse hindlimb skeletal muscle | Primary culture | Subcellular fractionation of sarcoplasmic reticulum with sucrose gradient and WB | Microsome | SR Nox4-derived H2O2 regulates O2-coupled RyR1, promoting changes in pO2. | 154 |
| Sarcoplasmic reticulum | |||||
| HUVEC | Nox4-GFP overexpression by microinjection | Confocal IF, colocalization with calreticulin | ER | No Nox4 localization in cytoskeleton, lisosomes or mitochondria. Noxes play a role in EC cell–cell adhesion/motility | 168 |
| HMEC (microvascular) |
ASM, aortic smooth muscle; ER, endoplasmic reticulum; GFP, green fluorescent protein; HAEC, human aortic endothelial cell; HUVEC, human umbilical vein endothelial cell; IF, immunofluorescence; KO, knockout; oxLDL, oxidized low-density lipoprotein; NADPH, reduced nicotinamide adenine dinucleotide phosphate; PDI, protein disulfide isomerase; PMA, phorbol myristate acetate; ROS, reactive oxygen species; SOD, superoxide dismutase; Tg, transgenic; TGF-β, transforming growth factor beta; TIRF, total internal reflection fluorescence; UPR, unfolded protein response; VSMC, vascular smooth muscle cells; WB, western blot.
Since Nox4 is constitutively active, evidence for Nox4 location at the ER implicates that Nox4 is active within this organelle. However, there is not a wealth of data reporting direct evidence for this fact. This is suggested by Nox4 loss- or gain-of-function experiments utilizing ER-localized sensors, such as Hyper (175, 181). Although an important advance, studies with redox sensors should yet be analyzed with caution, as their precise in vivo reaction mechanisms and limitations are not understood (116). Table 1 summarizes some specific aspects addressed in studies focusing on Nox4 within the ER. Since the ER lumen is topologically analogous to the extracellular milieu, it is expected that Nox4 is oriented at the ER membranes to release ROS toward the ER lumen. Interestingly, the opposite orientation cannot in principle be ruled out since the NADPH pool is preserved and reportedly thiol-independent at the ER lumen (130). Whether Nox4-derived ROS contribute to protein folding is possible but unknown.
The implications of ER location regarding Nox4 functions are not clear-cut, similar to Nox4 functions themselves. However, some specific ER-related targets have been identified, apart from Nox4 involvement with ER stress responses, discussed in the next section. One such target is sarcoendoplasmic reticulum calcium ATPase (SERCA), an ER membrane-located enzyme, which upon nitric oxide (NO)-stimulated cys674 glutathiolation increases calcium uptake into the ER, thereby impairing cytosolic calcium-dependent cell migration. Nox4 upregulation in vascular smooth muscle cells (VSMC) of prediabetic rats promotes irreversible SERCA cys674 oxidation after transforming growth factor-β stimulation, preventing NO-mediated inhibition of cell migration. Knockdown of Nox4 prevented SERCA cys674 oxidation and neointima formation after carotid artery injury (161). In endothelial cells, Nox4-derived hydrogen peroxide synergizes with NO to promote SERCA glutathiolation and increased vascular endothelial growth factor-dependent migration. Interestingly, SERCA glutathiolation also required Nox4-induced generation of Nox2-derived superoxide (50). Another potential Nox4 target is the ryanodine receptor. In striated muscle myocytes, a p(O2)-dependent generation of Nox4-derived oxidants targets ryanodine receptor cysteines to promote calcium release and muscle contraction. These data place Nox4 as a novel oxygen sensor, at least in skeletal muscle (154). Other observations in coronary artery myocytes, however, place the ryanodine receptor upstream of Nox4 ROS generation (192). Protein-tyrosine phosphatase-1B (PTP-1B), another ER-located enzyme, is oxidatively inactivated by Nox4. Through this effect, Nox4 is able to sustain epidermal growth factor (EGF) receptor signaling by negating PTP-1B-mediated inhibition of this tyrosine kinase, which occurs through its dephosphorylation at the ER (28). PTP-1B can also potentiate IRE1-dependent signaling during ER stress (59) and thus can be a potential target of Nox4 in this condition (see below).
Nox1
The fact that Nox1 colocalizes with PDI in smooth muscle cells (82) has been sometimes taken to implicate an ER location for this isoform. The evidences for Nox1 location at plasma membrane, endosomes, and caveolae are compelling, however, and the ER pool of Nox1 may represent to some extent immature enzyme—in addition to the fact that not all PDI may localize at the ER lumen (164). In gastrointestinal cancer cells, a major Golgi-located pool of Nox1 and its regulatory subunits has been described (160). Nevertheless, in lipopolysaccharide (LPS)-stimulated human colon cancer cells, diphenylene iodonium-sensitive oxidant generation displays a pattern suggestive of ER location, on the basis of colocalization between oxidant-sensitive 2′,7′-dichlorofluorescein (DCF) and ER-tracker dyes. Although both Nox1 and Nox2 are expressed in these cells, oxidant signals and increased cell adhesion were completely abrogated by Nox1 siRNA (125).
Rac1 and p47phox
Although Rac1 and p47phox are cytosolic subunits, they have been associated (probably indirectly) with dysfunction of calcium regulation. A Rac1-dependent NADPH oxidase controls intracellular calcium oscillations in histamine-simulated endothelial cells, via possible ER sensitization to inositol 1,4,5-trisphosphate (76). Also, the oxidation of calcium/calmodulin-dependent protein kinase II (CAMKII) has been associated with cardiac sinus node dysfunction, an effect significantly inhibited upon genetic deletion of p47phox (155). These results implicate a role for distinct Noxes, in addition to Nox4, at several levels of calcium signaling regulation.
Nox2
Phagocytic Nox2 is clearly functional at the plasma membrane, phagosomes, and specific granules (8). In macrophages, LPS stimulation promotes internalization of Nox2 via clathrin-coated pits for recycling within the endosomal secretory compartment (46). In contrast, nonphagocytic Nox2 displays locations that resemble the ER, for example, in endothelial cells (102, 168). It is unclear, however, whether the enzyme can be active at this location or whether this represents just a maturation stage of the active complex. In the cardiomyocyte, Nox2 localizes at the sarcolemma, as well at transverse T-tubules, which are deep sarcolemmal invaginations toward the cytosol juxtaposed to terminal cysternae of the smooth ER. Physiological stretch activates, via microtubules, Nox2 at those locations, promoting a flash of oxidants that act locally at the ryanodine receptor from junctional ER, releasing a calcium spark for contraction. This circuit integrates and tunes excitation–contraction coupling (133, 134) in cardiac as well as skeletal muscles.
p22phox
Recent evidence indicates close correlation between p22phox and ROS generation detectable by an ER-specific aryl-boronate probe in a human acute myeloid leukemia cell line, for which Nox2 may contribute at least partially to the phenotype. ROS generation is inhibitable by the Nox inhibitor VAS2870 or siRNA against p22phox. The expression of p22phox is reduced, through proteasome degradation, upon tyrosine kinase inhibition, thus providing a connection between p22phox and the leukemic phenotype, further reinforced by demonstration of p22phox-related downtream signal transducer and activator of transcription 5 (STAT5) signaling (179).
Nox5
The location of Nox5 seems variable and dynamic, similar to other Noxes, but there are some evidences for an ER location. Importantly, Nox5 has many distinct variants with potential location-related functions (13). Within the cell, either endogenous or overexpressed Nox5 displays an ER-resembling perinuclear distribution pattern, colocalizes with calreticulin in endothelial cells (11, 13), and has been reported at the detergent-resistant microdomains of the ER (81). When overexpressed in HEK293 cells, Nox5 seems located not only at the plasma membrane but also at the perinuclear region, generating superoxide in response to protein kinase C activation (148). Despite the evidences for Nox5 in the ER, the strategies of Nox5 to bind to the plasma membrane indicate the functional relevance of such location. One such strategy is the binding to p22phox: Nox5 coimmunoprecipitates with p22phox when both are overexpressed, but not with endogenous p22phox, whereas p22phox is not essentially required for Nox5β-dependent ROS production (13, 88). Also, Nox5 has two polybasic regions, one N-terminal and another C-terminal. Extracellular production of ROS by Nox5 is modulated by PtdIns4,5P2, which targets Nox5 location at the plasma membrane through its N-terminal polybasic region (89). The nonreceptor tyrosine kinase c-Abl comigrates with Nox5 to the plasma membrane in a calcium-mediated process (47). Finally, some studies indicate a cytosol location for Nox5 in myocardium (61), myometrium (34), and esophageal carcinoma cells (75). In the latter case, colocalization and functional interplay between the short Nox5S isoform and Rac1 has been reported.
Duox1 and Duox2
While mature Duoxes clearly locate at plasma membranes, maturation and processing of these glycoproteins involve a tight connection with the ER. Oxidative folding is the rate-limiting step for Duox2 maturation and exit from the ER, which is facilitated by the specific maturation factor DuoxA2 (57). Interestingly, the partially glycosylated form of Duox2 is located at the ER and generates, in a calcium-dependent way, superoxide detectable with electron paramagnetic resonance techniques, contrarily to the mature enzyme, which releases mainly hydrogen peroxide (5). Duox2 targeting to plasma membrane is preceded by its exit from the ER, which is regulated by an N-terminal ectodomain segment including the first transmembrane domain. Such exit is completely inhibited by mutation of four specific cysteine residues (119).
Overall, the data discussed in this section indicate that Nox4 (constitutively) and possibly other isoforms (circumstantially) do exhibit ER localization, and there is emerging evidence that at least Nox4 in fact becomes active at this organelle. At the same time, this discussion indicates that, for each isoform, focusing on location as a window to define function is a limited strategy in the absence of a specific (patho)physiological context in which this is inquired. In fact, Noxes appear to have evolved to display varied subcellular locations, which are not only isoform-specific but also stimulus-specific, likely in connection with acquisition of many specialized functions.
ER Stress and Nox Activation
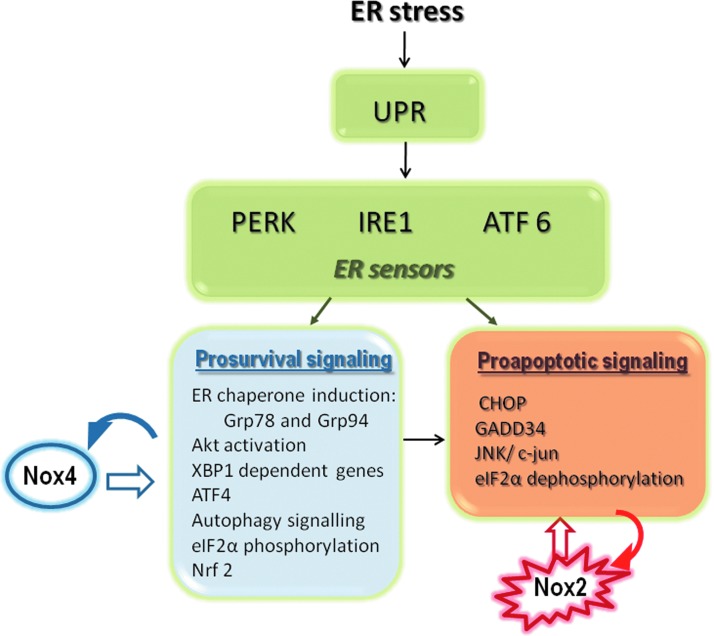
Adequate protein folding and processing is likely to be one of the most fail-safe and conserved cell functions, as judged by the number and nature of known defensive strategies to assist, preserve, correct, or balance it (23, 150). Protein processing within the ER lumen includes three major general activities: folding, disulfide introduction, and N-glycosyl residue insertion (23, 33). Failure at any of these steps will promote an imbalance between protein load and the ER capacity to process such cargo, leading to ER stress. ER stress counteractivates homeostatic signaling known as the “unfolded protein response (UPR),” a complex network of signals arising from ER membrane sensors toward the nucleus and back to the ER and several other cell compartments. Details of UPR signaling have been reviewed elsewhere (139, 143, 156, 180) and are briefly summarized in Figure 1. The UPR aims (i) to prevent, at least temporarily, protein synthesis, (ii) to improve the ER folding capacity, (iii) to remodel the ER, and (iv) to adjust cell metabolism to a defensive mode. Some features of the UPR recapitulate general cell stress responses, such as the arrest in protein synthesis, some metabolic adjustments, and enhanced protein degradation, whereas other aspects are more specific, for example, increased expression of ER chaperones, such as Grp78, Grp94, and calreticulin (139). Although such responses are primarily homeostatic, they can, as with any stress response, lead to cell damage and apoptosis if ER stress is sufficiently sustained or strong (143). Experimental studies often address the acute UPR (145). In many disease states, however, there is a state of chronic protracted UPR activation, in which sustained or oscillating ER stress coexists with variable combinations of adaptive as well as apoptotic signaling. Such state, which is so far not well understood, is a hallmark of many disease states, including atherosclerosis, diabetes, obesity, and cancer (112). Oxidative stress and ROS generation is an integral component of the acute as well as some chronic states of UPR signaling, whereas ROS generation by itself can apparently trigger the UPR in some, but not every case (145). Antioxidant compounds have been repeatedly shown to protect against apoptosis, especially during the acute UPR, in many distinct circumstances (110, 145). This, however, should not be taken as a rule, and protective effects of ROS during the UPR have been reported as well (15, 181, 182). Overall, it is increasingly clear that the UPR is a complex response activated in highly specific patterns, often partially, and depends highly on the cell type and context. Clearly, however, ER stress is a major context in which transient or sustained oxidative stress arises in many disease states.
FIG. 1.
Organization of the unfolded protein response (UPR) signaling and interplay with Nox NADPH oxidases. ER stress triggers UPR signaling from three main arms based on distinct ER transmembrane sensors: the endonuclease/kinase IRE1, the kinase PERK, and transcription factor ATF6. Each one triggers signals emerging from the cytosolic surface of ER, which integrate pathways for decreasing global protein synthesis load, improving protein folding, adjusting metabolism and amino acid sufficiency, and inducing misfolded protein degradation. Such responses can be classified as either prosurvival or proapoptotic, although both types are triggered simultaneously. Among several UPR-responsive homeostatic genes, the chaperones Grp78 (BiP), Grp94, and calreticulin are regulators, as well as operational markers, of the UPR. The most specific apoptosis pathway is the transcription factor CHOP (GADD 153). Nox4 is induced early during the UPR and is upstream to several signaling events that may induce autophagy, survival, or eventually apoptosis. Nox2 is induced through a CHOP/CAMKII pathway to mediate apoptosis. CAMKII, calcium/calmodulin-dependent protein kinase II; CHOP, CCAT/enhancer binding protein homologous protein; ER, endoplasmic reticulum; Grp, glucose-regulated protein; JNK, c-Jun N-terminal kinases; NADPH, reduced nicotinamide adenine dinucleotide phosphate. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
The mechanisms accounting for ROS generation during the UPR appear to be multiple [reviewed in Ref. (145)]. Prior suggestive evidence has been published with respect to ER folding-related oxidoreductase cycles and mitochondria. This is, however, not without criticism since compelling evidence that the ER is underoxidized during the UPR in yeast (117) or quite resistant to redox imbalances in mammalian cells (7a) argues against a predominant luminal ER source of oxidants during ER stress. More recently, increasing evidence suggests that Nox NADPH oxidases are important ROS sources during the UPR in distinct cell types (Fig. 1). Nox4 has been the most studied isoform in this context, with consistent evidence for increase in its gene and protein expression during ER stress induced by distinct stimuli. In VSMC, incubation with the oxidized cholesterol product 7-ketocholesterol induced 3-fold increase in Nox4 mRNA and protein expression (128), similar to the classical ER stressor tunicamycin, which increases Nox4 mRNA levels about 10-fold (145). Neither stimulus significantly affects Nox1 expression. In this scenario, ROS generation is virtually abolished by Nox4 knockdown, implicating this isoform as “a” and eventually “the” major ROS source in this circumstance. Intriguing evidence indicates that Nox4 is a proximal trigger of the UPR in endothelial cells exposed to tunicamycin or the ER stressor protein HIV-tat (181). In this case, the ER surface constitutes a platform for spatial organization of Nox4 signaling and downstream K-Ras activation, both as upstream UPR triggers (182). Nox4-dependent signals activate autophagy as a protective mechanism since autophagy inhibition promotes apoptosis. Transfection with an ER-located Hyper plasmid allowed detection of hydrogen peroxide production within the ER triggered by tunicamycin or HIV-tat, in a Nox4-dependent way, even though Nox4 could still affect hydrogen peroxide production indirectly. Moreover, the Hyper probe can be influenced by pH changes and is heavily affected by thiol-disulfide redox (116). Intriguingly, hydrogen peroxide production was not locally detected after thapsigargin or DTT-induced stress, even though shRNA for Nox4 decreased UPR signaling triggered by them (181). Thus, Nox4 can have a central role as upstream regulator of the UPR, although the mechanisms of Nox4 effects appear to be dependent on the nature of the UPR trigger. The distinct outcomes—survival versus apoptosis—regarding Nox4 within the UPR context is in line both with the conflicting evidences for protective versus damaging effects of Nox4 (1, 195), discussed above, as well as with the known dichotomy of ER stress endpoints. To what extent Nox4 itself is at the crossroads of UPR-related cell decisions is unknown.
Nox2 has also been implicated in the UPR (Fig. 1). ER stress induction with cholesterol or 7-ketocholesterol promoted apoptosis and oxidative shift in macrophages, which were abolished by Nox2 siRNA (101). Such apoptosis signaling involved a CCAT/enhancer binding protein homologous protein (CHOP)-CaMKII-c-Jun N-terminal kinases (JNK) pathway. Remarkably, the expression of CHOP (the canonical effector of UPR-related apoptosis) was dependent on double-strand RNA-dependent protein kinase (PKR) and was decreased in stressed macrophages from Nox2−/− mice. Mitochondrial pathway of apoptosis was also diminished in Nox2−/− macrophages. Nox2−/− mice also displayed kidney protection upon an in vivo challenge with tunicamycin, in connection with abrogated CHOP induction (101). Close convergence between ER stress signaling and Nox subunits also occurs in central nervous system regulation of hypertension. Loss-of-function of p22phox in the subfornical organ eliminated hypertensive responses to angiotensin II (AngII) (107) in association with decrease in UPR marker expression (187).
In addition to the above-discussed investigations, a relatively large body of studies report coincidences between Nox or Nox subunit effects and the occurrence ER stress, without showing clear evidence that such Nox plays a direct role in UPR signaling. In most such studies, loss-of-function of Nox promotes decrease in some ER stress-associated targets or another pathway promotes both Nox activation and ER stress. Such results should be interpreted bearing in mind the complex pathways connecting Nox to ER stress-dependent target responses, which can be indirect and involve collateral signaling, such as inflammatory mediators (193), metabolic adaptations, cell adhesion processes, and many others. A few examples can be listed regarding these studies: Nox2 in atherosclerosis (104); Nox2 in neurodegenerative diseases (16); p47phox in impaired cardiac contractility (196); p47phox in cytokine-induced inflammatory angiogenesis and autophagy (141); Nox4 in glycation product-induced fibroblast apoptosis (108); Rac1 in saturated fat-induced JNK activation in hepatocytes (149); Rac1 in diabetes-induced cardiac hypertrophy, collagen deposition, and contractility (103); Rac1 in high glucose-induced ER stress in culture cardiomyocytes (103); Nox activity in vascular cell calcification (105). ER stress plays a prominent role in Toxoplasma gondii-induced trophoblast apoptosis, whereas an increase in Nox1 expression and oxidant generation preceded UPR signaling and downstream activation of caspase 12, and UPR-related apoptotic signaling was abolished by N-acetylcysteine (183).
The UPR closely converges with the “endoplasmic reticulum-associated degradation (ERAD)” to promote enhanced degradation of un/misfolded proteins in the cytosol by the proteasome (22). ERAD is the executive arm of essential protein quality control mechanisms that can exert control on Nox subunit expression, although information for this is incipient. Such is the case not only of defective forms, for example, mutated Nox2 (106), but also of normal regulation of p22phox (20) or Rac1 (92), both cases seemingly involving redox-dependent steps (42, 92). The proteasome also helps sustain Nox4 mRNA expression during the UPR (3).
In summary, evidence for the involvement of Noxes in UPR signaling, as well as in protein quality control, is growing, but the mechanistical links are yet fragile. As judged from the convergence between Noxes and the ER discussed in this review, it can be speculated that more chapters of this story are to be written.
PDI Interaction with Nox NADPH Oxidase
Another point of convergence between the ER and Nox NADPH oxidases is the interaction between Noxes and ER chaperones from the PDI family. There are more than 20 PDI isoforms, with PDI itself, also called PDIA1 or P4HB gene product, as the founding member. These proteins belong to the thioredoxin superfamily and have, as hallmarks, a thioredoxin fold and in most cases dithiol groups. Most studies addressing PDI convergence with Noxes concentrate on PDIA1 (or simply PDI), which has two dithiols with the typical –CXXC– sequence. PDI displays oxidase, reductase, and—as the hallmark of this family—thiol isomerase activity (7, 68, 78, 99, 176). In addition, PDI has a chaperone effect—preventing protein aggregation and sustaining their folding—which in itself is independent on its thiol groups, although cooperative with thiol oxidoreductase activity. Such chaperone effect is mostly attributable to a hydrophobic pocket within the main protein core (67, 68). Access to this core is flanked by a mobile arm, which opens when PDI is oxidized and closes upon its reduction (172, 173). The canonical function of PDI in the ER lumen is to introduce and/or reshuffle disulfide bonds into nascent proteins destined to membrane insertion or secretion (176). PDI composes with other isoforms or related chaperones to integrate protein folding, disulfide introduction, and N-glycosyl residue attachment, the three main steps of protein processing in the ER. All such PDI effects provide it with the intrinsic function of being the convergent hub of ER redox homeostasis and, consequently, an important player in ER-dependent redox signaling processes (99). The role of PDI in redox signaling, as well as ER and cellular redox homeostasis, was reviewed in detail recently (99).
In addition to its canonical ER location, PDI, similar to some other ER chaperones, is also present at the cell surface and pericellular compartments (“peri/epicellular PDI” or pecPDI), where it mediates a number of thiol-disulfide exchange events (reductase and possibly oxidase and isomerase activities) (86, 99, 164). Such redox effects mediate a number of physiological or pathophysiological processes related to thrombus formation (30, 31, 84, 137), tissue factor functional regulation (2, 137, 169), phospholipid asymetry (131), platelet adhesion, and aggregation [reviewed in Ref. (49)], ADAM activity (177), citoxicity of diphtheria toxin (111), uptake of viral particles (55, 113, 171), and galectin regulation (17), among others. The putative route through which PDI gains access to its epi/pericellular location remains elusive (71, 86). This remains a crucial question since it is possible that PDI effects on Nox regulation outside the ER take place at the physical route of PDI externalization.
Convergence between PDI and the NADPH oxidase complex was described by our group in the context of addressing mechanisms underlying thiol sensitivity of nonphagocytic NADPH oxidase (82). Evidences suggesting such an interaction have been obtained in both the context of functional interdependence and physical association, with the functional data showing the most clear results so far. In VSMC, antagonism of PDI by pharmacological compounds, as well as loss-of-function experiments with specific protein knockdown induces loss of NADPH oxidase activation and superoxide production in response to AngII (52, 82). Moreover, silencing PDI expression even partially is sufficient to prevent the enhanced expression of Nox1 due to AngII (52) or platelet-derived growth factor (129), whereas in both cases, the expression of Nox4 remains unaltered. Importantly, gain-of-function experiments in which PDI was overexpressed about 2–2.5 times depicted an enhanced spontaneous baseline activation of NADPH oxidase, reflected by superoxide and hydrogen peroxide detection, in a preemptive way, that is, further incubation with AngII did not enhance the NADPH oxidase activity. Such Nox activation was associated with enhanced expression of Nox1, but not Nox4 at baseline, whereas after AngII incubation, Nox4 expression was also enhanced (52). PDI requirement for NADPH activation was also observed in endothelial cells challenged with AngII (98), as well as PMA-activated macrophages (144) and neutrophils (38) and in glial cells (83). This indicates that the functional support of the NADPH oxidase activity by PDI occurs in distinct cell types and with nonphagocytic as well as phagocytic Noxes.
Importantly, PDI-dependent support of the Nox activity was shown to have functional implications. In response to AngII incubation in VSMC, PDI loss-of-function abrogates Akt phosphorylation (82), a response known to be associated with Nox1. Accordingly, PDI silencing with siRNA significantly impairs Nox1-dependent VSMC migration in response to PDGF (129). In macrophages, lowering PDI expression inhibits Leishmania chagasi phagocytosis (144).
Of note, the partial PDI knockdown in these experiments in VSMC and macrophages was unassociated with evidence of UPR activation, a result in line with other studies indicating that PDI loss-of-function does not necessarily trigger the UPR (120, 142) and that PDI is generally not considered an ER stress-responsive gene (68, 145, 176). However, in some cell types such as mouse embryonic fibroblasts (unpublished observations from our laboratory), PDI silencing associates with UPR marker expression and, indeed, PDI silencing can possibly sensitize most cells to other ER stressors. In fact, in neuronal cells (165) and oxidized low-density lipoprotein-challenged macrophages and endothelial cells (120), loss of PDI function promotes or amplifies the UPR, leading, respectively, to the formation of insoluble protein aggregates and CHOP expression/apoptosis. The extent to which PDI down-expression induces that UPR is likely dependent on cell type, secretory status, ER redox state, and concomitant expression of other PDI family members (99). PDI has a possible role to couple ER stress to Nox-dependent ROS generation (99). Evidence for this logical hypothesis is however poor, even though preliminary (unpublished observations) data from our group indicate that PDI silencing, while increasing apoptosis signaling, prevents ROS generation in tunicamycin-challenged VSMC, a situation in which Nox4 expression accounts for essentially all detectable ROS (145). Interestingly, UPR-induced increase in mRNA levels of both PDI and Nox4 are essentially abrogated by nonlethal concentrations of proteasome inhibitors, which nonetheless increase UPR-induced loss of VSMC (3).
The above results suggest a clear functional interplay between PDI and either Nox1 or Nox2, in distinct cell types, whereas the functional interplay between PDI and Nox4 is unclear at present. Evidences for physical interaction between PDI and Nox subunits further reinforce these functional data. A word of caution is important here since PDI is highly expressed in cells and displays a client-type interaction with a large number of proteins, although such interactions tend to be quite rapid and generally do not yield detectable protein complexes unless PDI is artificially mutated to trap substrates (85). Also, the large PDI hydrophobic core may interact nonspecifically with unfolded proteins (67) and even small substrates, such as estrogens, bisphenol A, insulin [reviewed in Ref. (78)], antigen peptides (32), and somatostatin (91). With these caveats in mind, there is substantial evidence for confocal colocalization and/or coimmunoprecipitation between PDI and many Nox subunits, including Nox1, Nox2, Nox4, p22phox, p47phox, and Rac1 or GTPase regulator RhoGDI (38, 82, 99, 144). Since these associations do not imply functional relevance, they may implicate a similar share of location at the ER or downstream ER-derived compartments. Such may be the case of Nox4, which clearly associates with PDI (82, 195), but does not show expression modulation in response to PDI loss- or gain-of-function, as discussed above. Another important point is that none of these associations has shown a clear pattern of modulation in response to Nox activation. Overall, the mode of PDI association is likely to be transient and possibly multiple, more akin to that expected for a peripheral regulatory protein rather than a more stable subunit of the Nox NADPH oxidase complex.
The mechanisms whereby PDI affects Nox NADPH oxidases are yet unclear and likely to be multiple, reflecting the diverse functions of PDI and its versatile array of interactions. Within contexts in which PDI exerts the regulation of AngII-mediated Nox1 activity, overall PDI expression exhibits little or no change, whereas there is a translocation of PDI toward particulate fraction containing membrane subcompartments (82). In such homogenates obtained after AngII stimulation, incubation with the thiol isomerase inhibitor bacitracin or a neutralizing antibody against PDI induces decrease in the NADPH oxidase activity, indicating that PDI segregates into locations close to the oxidase complex milieu and exerts Nox control via thiol redox mechanisms (82). Given PDI role as chaperone and folding catalyst, a role for PDI in complex stabilization and/or organization is possible, but remains to be proven. In addition, nonredox mechanisms also play a role in PDI effects since overexpression of cysteine-less PDI in VSMC induces acute activation of the NADPH oxidase activity and Nox1 expression similar to the wild-type counterpart (52). This indicates that at least acutely a PDI-mediated chaperone effect, known to be thiol-independent, may contribute to NADPH oxidase complex organization. In fact, it is well known that PDI binding to most client proteins occurs through a chaperone-like mechanism involving the hydrophobic PDI groove, whereas thiol redox pathways subsequently stabilize the interaction (67, 68, 99). Of note, redox sensitivity of any PDI interaction does not implicate that binding to target necessarily occurs via PDI thiols, as this may simply reflect the profound redox-dependent plasticity of PDI. When its thiols are oxidized, PDI is in open configuration, exposing its hydrophobic core and enhancing its chaperone activity. As PDI thiols are reduced, for example, following disulfide introduction into a client protein, the hydrophobic PDI groove shifts to closed configuration. These configurations are in line with PDI functions. Although oxidized PDI is expected to accomodate variable client proteins, reduced PDI configuration is consistent with an impaired binding to complex proteins, while allowing exposure of aromatic residues that bind the oxidase Ero1, thus potentially allowing PDI reoxidation (99, 173).
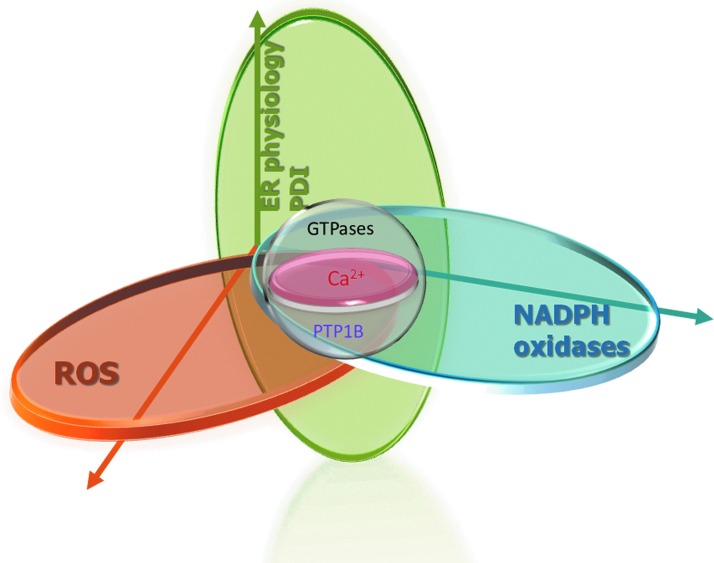
Surprisingly, not much is known about the existence and possible function of reactive thiols within Nox subunits. Classical thioredoxin-like dithiol motifs are absent in their structure, although a dithiol specific for Nox2 has been reported in its cytosolic domain (36, 37, 43). In addition, up to four cysteines may contribute to modulate p47phox function (79). Mutations of two Nox4 E-loop cysteines switch the ROS product from hydrogen peroxide to superoxide, with no change in its expression or subcellular localization (158). The absence of a clear Nox dithiol target, plus the somewhat protean nature of PDI association with Nox subunits discussed above, do not allow at present to highlight any particular catalytic or regulatory subunit as a specific PDI target, although possible candidates have recently emerged. We detected in human neutrophils a thiol redox-dependent association between PDI and p47phox in pull-down assays, whereas in the cytosol fraction during neutrophil activation, PDI shows a reductive shift, contrarily to the oxidation shift depicted for p47phox (38). In addition, we recently identified RhoGTPases (Rac1 and RhoA) and their regulator RhoGDI as possible mechanisms of PDI-mediated support of platelet-derived growth factor-induced, Nox1-dependent, VSMC migration (129). Such interaction was proposed from physical protein–protein interaction system biology maps, which identified high bottleneck score values for GTPases and GDI in PDI interactome. In fact, impaired cell migration after PDI silencing closely correlated with decreases in Rac1 or RhoA activity and profound disruption of cytoskeletal structures, such as stress fibers, focal adhesions, and adhesion vesicles. Importantly, Rac1 and RhoA can be strongly regulated by redox mechanisms, although it is yet unclear to what extent this occurs in vivo (118). Overall, these emerging findings allow the proposal that PDI may act as novel redox-related organizer of the NADPH oxidase complex. Together, PDI and ER physiology confer an additional dimension to Nox/redox-dependent signaling (Fig. 2).
FIG. 2.
PDI and ER (patho)physiology confer a novel dimension to Nox NADPH oxidase/ROS signaling. The mechanisms involved in these connections have been discussed throughout the text, whereas here we highlight: calcium signaling convergence with Nox(es), RhoGTPase convergence with PDI, and Nox(es) and PTP1B convergence with Nox4. PDI, protein disulfide isomerase; ROS, reactive oxygen species. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
The interaction between PDI and Noxes is not an isolated example, and other PDIs may also be involved in distinct ways. Erp72 associates with Nox1 at the plasma membrane and is oxidized by it upon EGF exposure, leading to decreased Erp72 reductase activity (29). The thioredoxin superfamily protein EFP1 interacts with Duoxes as a component of the assembled membrane complex (174). Endothelial cell incubation with atorvastatin promotes enhanced migration of endoplasmic reticulum protein 46 (Erp46, also known as endoPDI) to lipid rafts, the same location of Nox2 (60). In addition, Noxes may interact with other chaperone families, such as heat shock protein 90 (Hsp90), which binds to the C-terminal region of Nox1–3 and 5, though not of Nox4, to enhance their stability (26).
Evolutive Perspectives of ER-Nox Convergence: Implications for Calcium Signaling
Evolutive analysis provides a quantum leap in the quality of biological inferences. It is thus opportune to raise some evolutive perspectives about Nox convergence with the ER, even though evidences for such correlations are indirect. The major ER-related function connected to Nox NADPH oxidases is calcium handling. Calcium flashes are ancestral early injury signals that converge with Duox-type Noxes in zebrafish to trigger hydrogen peroxide generation after mechanical injury as a required step for leukocyte recruitment (123), which is dependent on a specific cysteine from the leukocyte Src-family kinase Lyn (185). The early calcium response as well as the ensuing Duox/redox-dependent activation of the epithelial Src-family kinase FynB are essential for adequate injury response since ablation of either prevents late epimorphic fin regeneration (184). Of note, in the same zebrafish model, epithelial Duox-derived hydrogen peroxide is not required for leukocyte recruitment after infection with Pseudomonas aeruginosa (41), thus suggesting some degree of specificity to this highly conserved stress response. In Drosophila embryos, calcium flashes are the earliest upstream response to laser injury, triggering Duox-dependent hydrogen peroxide generation (136). In filamentous fungi, mechanical injury promotes calcium signals associated with Nox1/NoxR activation and production of oxylipin metabolites (73)—with lipid metabolism being another important ER-related function. The high conservation of such Nox-dependent responses, likely to have strong evolutive pressure, is in line with evolutive analyses identifying calcium-sensitive Nox NADPH oxidases (Duoxes, Nox5) as the most ancestral Noxes in eukaryotes (90). Ancestral convergence between Nox regulation and calcium-dependent signaling is indicated by the fact that, in Arabidopsis, phosphorylation of a calcineurin B-like protein, physically associated with the Nox NADPH oxidase RBOHF, and its coexpression with the interacting protein CIPK26 strongly enhance ROS generation (44). The convergence between Noxes and calcium signaling in upper eukaryotes, regarding SERCA function, ryanodine receptor, muscle contraction, cell migration, oxygen sensing, and ER stress signaling, discussed throughout this article and summarized in Table 2, provides further links between Noxes and calcium signaling. Moreover, the roles of N-glycosylation, which is predicted or demonstrated for most Noxes, interactions between Noxes and PDI, as well as subcellular localizations of Noxes, all discussed previously, further corroborate those functional interplays.
Table 2.
Nox NADPH Oxidases and Calcium Regulation: A Summary of Discussed Evidences (References)
| Nox5 and Duoxes exhibit calcium-binding EF hands involved in enzyme activation and required for ROS generation (5, 8, 9, 12, 58, 81, 96, 97). |
| Calcium flashes are ancestral signals triggering activation of specific Noxes (36, 44, 73, 123, 136, 185). |
| ER calcium levels and related chaperones (ex. calnexin) control Nox processing in the ER (33). |
| ER calcium uptake by the ER via SERCA is redox/Nox-regulated (161). |
| Nox4 can affect calcium release via ryanodine receptor (154). |
| Nox2 at transverse T-tubules regulates ryanodine receptor and calcium-mediated muscle contraction (133, 134). |
| Rac1 interplays with calcium oscillations (76). |
| p47phox-related CAMKII oxidation impairs cardiac function (155). |
| Nox2 supports apoptosis during ER stress in macrophages via CHOP/CAMKII/JNK pathway (101). |
| Intracellular calcium levels mediate effects of Nox activation and can to some extent substitute for its effects: p47phox-related, VEGF-dependent c-Src, Akt and FAK phosphorylation in endothelial cells (100a); Nox1-dependent, thrombin-mediated VSMC migration (198). |
Akt, protein kinase B; CAMKII, calcium/calmodulin-dependent protein kinase II; CHOP, CCAT/enhancer binding protein homologous protein; Duox, dual oxidase; JNK, c-Jun N-terminal kinases; SERCA, sarcoendoplasmic reticulum calcium ATPase; VEGF, vascular endothelial growth factor.
Whether ancestral Nox NADPH oxidases from lower eukaryotes localize at the ER is yet poorly known, although emerging evidence indicates an ER location for Nox-related enzymes from yeast (138) and many fungi (163). On the other hand, a membrane location has been suggested for Arabidopsis (44, 191), Aspergillus (95), and Caenorhabditis elegans (45) NADPH oxidases, in association with specific functions, such as immune regulation, sexual differentiation, and cuticle strengthening, respectively. These considerations allow one to speculate that the ER has been a default subcellular localization of Noxes, whereas compartmentalization to other locations was associated with acquisition of specialized functions, in some cases involving alternative splicing (6). An additional arguable context around which Noxes evolved is pathogen killing, another function in which, among other organelles, the ER has a prominent role as a site for intracellular pathogen targeting and chaperone-mediated host–pathogen interaction (152), antigen presentation (32), and particularly as a possible but much-debated source of the Nox2-containing phagosome (24). Overall, Noxes appear to have evolved around prominent ER-related functions and conversely the ER itself has played a possible role in contexts associated with Nox evolution.
Conclusions and Perspectives
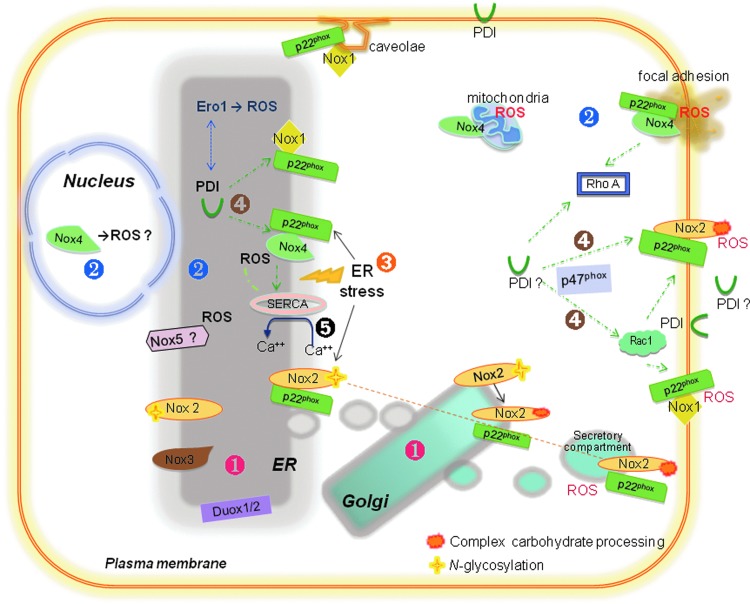
The data reviewed here place the ER at an important position of Nox physiology and pathophysiology (Figs. 2 and 3). This organelle is the site of synthesis and processing of all catalytic subunits, as well as p22phox. Several processing steps crucial for further Nox maturation, traffic, and regulation also occur at the ER, such as heme insertion, p22phox heterodimerization and, particularly, N-glycosyl residue attachment. On a second realm, some Noxes, such as Nox4—but maybe also other Nox isoforms in specific circumstances—are active at the ER. This location exposes functionally relevant ER-resident proteins to Nox-derived ROS: such is the case of calcium-related proteins such as SERCA and signaling effectors such as PTP-1B. In addition, Noxes interplay with ER stress responses and are integral components of the UPR, with documented roles for Nox4 and Nox2. In line with these events, ER chaperones such as PDI associate with and are involved in Nox regulation and can possibly further connect Noxes to ER-dependent functions (Fig. 3).
FIG. 3.
NADPH oxidase synthesis/processing, trafficking, and activation are connected to the ER. (1) Transmembrane catalytic Nox subunits, as well as p22phox, are synthesized in the ER. Nox2 is folded, N-glycosylated (p65), and heterodimerizes with p22phox in the ER and then migrates to Golgi apparatus, in which processing mannosidases add complex side chain carbohydrates. Mature gp91phox moves to secretory vesicles and/or to plasma membrane. The lumen of Nox2-containing vesicles is topologically analogous to the extracellular milieu regarding superoxide production. It is likely that other isoforms share at least to some extent the same sequence of processing, except for Nox4, which often seems to become active at the ER membrane. (2) Some Noxes become active at the ER, such as Nox4, and may be in specific cases other isoforms (Nox5 and other immature Noxes?). Nox1 can translocate to caveolae and plasma membrane, whereas Nox4 can also be found at locations other than ER, such as nucleus, focal adhesions, and mitochondria. (3) ER stress leads to the UPR, which associates with oxidative stress, reportedly related to Nox4 or Nox2. (4) ER chaperones such as PDI associate with and functionally regulate Nox activation. Although PDI is canonically involved in redox protein processing at the ER, PDI interaction with Noxes likely occurs at other cell locations and even at the cell surface. Mechanisms of PDI effects may include its association/interaction with p47phox and RhoGTPases Rac1 and RhoA. (5) Noxes converge with calcium level regulation at several levels, here exemplified by the effects of Nox4 on the oxidation of sarcoendoplasmic reticulum calcium ATPase (SERCA). Please see text for further details.
Much emphasis in previous years has been placed on Nox “localization.” Although this review further highlights the importance of understanding accurate Nox subcellular localization, it also raises the limitations of a monolithic approach to this question, indicating that such localization is highly dynamic and influenced by cell type, specific stimuli, protein and lipid interactions, and the occurrence of alternative splicing isoforms. In this context, the ER emerges as an important structural and functional reference to help understand the complex regulation of Nox NADPH oxidases.
Abbreviations Used
- Akt
protein kinase B
- AngII
angiotensin II
- c-Abl
Abelson murine leukemia viral oncogene homolog
- CAMKII
calcium/calmodulin-dependent protein kinase II
- CHOP
CCAT/enhancer binding protein homologous protein
- cys
cysteine
- DCF
2′,7′-dichlorofluorescein
- Duox
dual oxidase
- EGF
epidermal growth factor
- ER
endoplasmic reticulum
- ERAD
ER-associated degradation
- Ero-1
ER oxidoreductin 1
- Erp46
endoplasmic reticulum protein 46
- FAD
flavin adenine dinucleotide
- GFP
green fluorescent protein
- Grp
glucose-regulated protein
- GTPase
guanosine triphosphate hidrolase enzyme
- HAEC
human aortic endothelial cell
- Hsp90
heat shock protein 90
- HUVEC
human umbilical vein endothelial cell
- IF
immunofluorescence
- JNK
c-Jun N-terminal kinases
- KO
knockout
- LPS
lipopolysaccharide
- NADPH
reduced nicotinamide adenine dinucleotide phosphate
- NO
nitric oxide
- PDI
protein disulfide isomerase
- PMA
phorbol myristate acetate
- PTP-1B
protein-tyrosine phosphatase-1B
- ROS
reactive oxygen species
- SERCA
sarcoendoplasmic reticulum calcium ATPase
- STAT5
signal transducer and activator of transcription 5
- Tg
transgenic
- TGF-β
transforming growth factor beta
- TIRF
total internal reflection fluorescence
- UPR
unfolded protein response
- VEGF
vascular endothelial growth factor
- VSMC
vascular smooth muscle cells
Acknowledgments
Work from the author's group supported by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP, Grant number 09/54764-6), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), and Instituto Nacional de Ciência e Tecnologia Redoxoma.
References
- 1.Ago T, Kuroda J, Pain J, Fu C, Li H, and Sadoshima J. Upregulation of Nox4 by hypertrophic stimuli promotes apoptosis and mitochondrial dysfunction in cardiac myocytes. Circ Res 106: 1253–1264, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahamed J, Versteeg HH, Kerver M, Chen VM, Mueller BM, Hogg PJ, and Ruf W. Disulfide isomerization switches tissue factor from coagulation to cell signaling. Proc Natl Acad Sci U S A 103: 13932–13937, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amanso AM, Debbas V, and Laurindo FR. Proteasome inhibition represses unfolded protein response and Nox4, sensitizing vascular cells to endoplasmic reticulum stress-induced death. PLoS One 6: e14591, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ambasta RK, Kumar P, Griendling KK, Schmidt HH, Busse R, and Brandes RP. Direct interaction of the novel Nox proteins with p22phox is required for the formation of a functionally active NADPH oxidase. J Biol Chem 279: 45935–45941, 2004 [DOI] [PubMed] [Google Scholar]
- 5.Ameziane-El-Hassani R, Morand S, Boucher JL, Frapart YM, Apostolou D, Agnandji D, Gnidehou S, Ohayon R, Noël-Hudson MS, Francon J, Lalaoui K, Virion A, and Dupuy C. Dual oxidase-2 has an intrinsic Ca2+-dependent H2O2-generating activity. J Biol Chem 280: 30046–30054, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Anilkumar N, Jose GS, Sawyer I, Santos CX, Sand C, Brewer AC, Warren D, and Shah AM. A 28-kDa splice variant of NADPH Oxidase-4 is nuclear-localized and involved in redox signaling in vascular cells. Arterioscler Thromb Vasc Biol 33: e104–112, 2013 [DOI] [PubMed] [Google Scholar]
- 7.Appenzeller-Herzog C. and Ellgaard L. The human PDI family: versatility packed into a single fold. Biochim Biophys Acta 1783: 535–548, 2008 [DOI] [PubMed] [Google Scholar]
- 7a.Avezov E, Cross BC, Kaminski Schierle GS, Winters M, Harding HP, Melo EP, Kaminski CF, and Ron D. Lifetime imaging of a fluorescent protein sensor reveals surprising stability of ER thiol redox. J Cell Biol 15: 337–349, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Babior BM. NADPH oxidase: an update. Blood 93: 1464–1476, 1999 [PubMed] [Google Scholar]
- 9.Babior BM. NADPH oxidase. Curr Opin Immunol 16: 42–47, 2004 [DOI] [PubMed] [Google Scholar]
- 10.Balaban RS, Nemoto S, and Finkel T. Mitochondria, oxidants, and aging. Cell 120: 483–495, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Bedard K, Jaquet V, and Krause KH. NOX5: from basic biology to signaling and disease. Free Radic Biol Med 52: 725–734, 2012 [DOI] [PubMed] [Google Scholar]
- 12.Bedard K. and Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev 87: 245–313, 2007 [DOI] [PubMed] [Google Scholar]
- 13.BelAiba RS, Djordjevic T, Petry A, Diemer K, Bonello S, Banfi B, Hess J, Pogrebniak A, Bickel C, and Görlach A. NOX5 variants are functionally active in endothelial cells. Free Radic Biol Med 42: 446–459, 2007 [DOI] [PubMed] [Google Scholar]
- 14.Benham AM, van Lith M, Sitia R, and Braakman I. Ero1-PDI interactions, the response to redox flux and the implications for disulfide bond formation in the mammalian endoplasmic reticulum. Philos Trans R Soc Lond B Biol Sci 368: 20110403, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bernales S, McDonald KL, and Walter P. Autophagy counterbalances endoplasmic reticulum expansion during the unfolded protein response. PLoS Biol 4: e423, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bertoni A, Giuliano P, Galgani M, Rotoli D, Ulianich L, Adornetto A, Santillo MR, Porcellini A, and Avvedimento VE. Early and late events induced by polyQ-expanded proteins: identification of a common pathogenic property of polYQ-expanded proteins. J Biol Chem 286: 4727–4741, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bi S, Hong PW, Lee B, and Baum LG. Galectin-9 binding to cell surface protein disulfide isomerase regulates the redox environment to enhance T-cell migration and HIV entry. Proc Natl Acad Sci U S A 108: 10650–10655, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Biberstine-Kinkade KJ, DeLeo FR, Epstein RI, LeRoy BA, Nauseef WM, and Dinauer MC. Heme-ligating histidines in flavocytochrome b(558): identification of specific histidines in gp91(phox). J Biol Chem 276: 31105–31112, 2001 [DOI] [PubMed] [Google Scholar]
- 19.Block K, Gorin Y, and Abboud HE. Subcellular localization of Nox4 and regulation in diabetes. Proc Natl Acad Sci U S A 106: 14385–14390, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Block K, Gorin Y, Hoover P, Williams P, Chelmicki T, Clark RA, Yoneda T, and Abboud HE. NAD(P)H oxidases regulate HIF-2alpha protein expression. J Biol Chem 282: 8019–8026, 2007 [DOI] [PubMed] [Google Scholar]
- 21.Bravo-Sagua R, Rodriguez AE, Kuzmicic J, Gutierrez T, Lopez-Crisosto C, Quiroga C, Diaz-Elizondo J, Chiong M, Gillette TG, Rothermel BA, and Lavandero S. Cell death and survival through the endoplasmic reticulum- mitochondrial axis. Curr Mol Med 13: 317–329, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brodsky JL. Cleaning up: ER-associated degradation to the rescue. Cell 151: 1163–1167, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Buchberger A, Bukau B, and Sommer T. Protein quality control in the cytosol and the endoplasmic reticulum: brothers in arms. Mol Cell 40: 238–252, 2010 [DOI] [PubMed] [Google Scholar]
- 24.Campbell-Valois FX, Trost M, Chemali M, Dill BD, Laplante A, Duclos S, Sadeghi S, Rondeau C, Morrow IC, Bell C, Gagnon E, Hatsuzawa K, Thibault P, and Desjardins M. Quantitative proteomics reveals that only a subset of the endoplasmic reticulum contributes to the phagosome. Mol Cell Proteomics 11: M111.016378, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cardoso AR, Chausse B, da Cunha FM, Luévano-Martínez LA, Marazzi TB, Pessoa PS, Queliconi BB, and Kowaltowski AJ. Mitochondrial compartmentalization of redox processes. Free Radic Biol Med 52: 2201–2208, 2012 [DOI] [PubMed] [Google Scholar]
- 26.Chen F, Pandey D, Chadli A, Catravas JD, Chen T, and Fulton DJ. Hsp90 regulates NADPH oxidase activity and is necessary for superoxide but not hydrogen peroxide production. Antioxid Redox Signal 14: 2107–2119, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen K, Craige SE, and Keaney JF. Downstream targets and intracellular compartmentalization in Nox signaling. Antioxid Redox Signal 11: 2467–2480, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen K, Kirber MT, Xiao H, Yang Y, and Keaney JF. Regulation of ROS signal transduction by NADPH oxidase 4 localization. J Cell Biol 181: 1129–1139, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen W, Shang WH, Adachi Y, Hirose K, Ferrari DM, and Kamata T. A possible biochemical link between NADPH oxidase (Nox) 1 redox-signalling and ERp72. Biochem J 416: 55–63, 2008 [DOI] [PubMed] [Google Scholar]
- 30.Cho J, Furie BC, Coughlin SR, and Furie B. A critical role for extracellular protein disulfide isomerase during thrombus formation in mice. J Clin Invest 118: 1123–1131, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cho J, Kennedy DR, Lin L, Huang M, Merrill-Skoloff G, Furie BC, and Furie B. Protein disulfide isomerase capture during thrombus formation in vivo depends on the presence of β3 integrins. Blood 120: 647–655, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cho K, Cho S, Lee SO, Oh C, Kang K, Ryoo J, Lee S, Kang S, and Ahn K. Redox-regulated peptide transfer from the transporter associated with antigen processing to major histocompatibility complex class I molecules by protein disulfide isomerase. Antioxid Redox Signal 15: 621–633, 2011 [DOI] [PubMed] [Google Scholar]
- 33.Csala M, Kereszturi É, Mandl J, and Bánhegyi G. The endoplasmic reticulum as the extracellular space inside the cell: role in protein folding and glycosylation. Antioxid Redox Signal 16: 1100–1108, 2012 [DOI] [PubMed] [Google Scholar]
- 34.Cui XL, Chang B, and Myatt L. Expression and distribution of NADPH oxidase isoforms in human myometrium—role in angiotensin II-induced hypertrophy. Biol Reprod 82: 305–312, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dahan I, Issaeva I, Gorzalczany Y, Sigal N, Hirshberg M, and Pick E. Mapping of functional domains in the p22(phox) subunit of flavocytochrome b(559) participating in the assembly of the NADPH oxidase complex by “peptide walking”. J Biol Chem 277: 8421–8432, 2002 [DOI] [PubMed] [Google Scholar]
- 36.Dahan I, Molshanski-Mor S, and Pick E. Inhibition of NADPH oxidase activation by peptides mapping within the dehydrogenase region of Nox2-A “peptide walking” study. J Leukoc Biol 91: 501–515, 2012 [DOI] [PubMed] [Google Scholar]
- 37.Dahan I. and Pick E. Strategies for identifying synthetic peptides to act as inhibitors of NADPH oxidases, or “all that you did and did not want to know about Nox inhibitory peptides”. Cell Mol Life Sci 69: 2283–2305, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.de A Paes AM, Veríssimo-Filho S, Guimarães LL, Silva AC, Takiuti JT, Santos CX, Janiszewski M, Laurindo FR, and Lopes LR. Protein disulfide isomerase redox-dependent association with p47(phox): evidence for an organizer role in leukocyte NADPH oxidase activation. J Leukoc Biol 90: 799–810, 2011 [DOI] [PubMed] [Google Scholar]
- 39.Debeurme F, Picciocchi A, Dagher MC, Grunwald D, Beaumel S, Fieschi F, and Stasia MJ. Regulation of NADPH oxidase activity in phagocytes: relationship between FAD/NADPH binding and oxidase complex assembly. J Biol Chem 285: 33197–33208, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.DeLeo FR, Burritt JB, Yu L, Jesaitis AJ, Dinauer MC, and Nauseef WM. Processing and maturation of flavocytochrome b558 include incorporation of heme as a prerequisite for heterodimer assembly. J Biol Chem 275: 13986–13993, 2000 [DOI] [PubMed] [Google Scholar]
- 41.Deng Q, Harvie EA, and Huttenlocher A. Distinct signalling mechanisms mediate neutrophil attraction to bacterial infection and tissue injury. Cell Microbiol 14: 517–528, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Djordjevic T, Pogrebniak A, BelAiba RS, Bonello S, Wotzlaw C, Acker H, Hess J, and Görlach A. The expression of the NADPH oxidase subunit p22phox is regulated by a redox-sensitive pathway in endothelial cells. Free Radic Biol Med 38: 616–630, 2005 [DOI] [PubMed] [Google Scholar]
- 43.Doussiere J, Poinas A, Blais C, and Vignais PV. Phenylarsine oxide as an inhibitor of the activation of the neutrophil NADPH oxidase—identification of the beta subunit of the flavocytochrome b component of the NADPH oxidase as a target site for phenylarsine oxide by photoaffinity labeling and photoinactivation. Eur J Biochem 251: 649–658, 1998 [DOI] [PubMed] [Google Scholar]
- 44.Drerup MM, Schlücking K, Hashimoto K, Manishankar P, Steinhorst L, Kuchitsu K, and Kudla J. The calcineurin B-like calcium sensors CBL1 and CBL9 together with their interacting protein kinase CIPK26 regulate the arabidopsis NADPH oxidase RBOHF. Mol Plant 6: 559–569, 2013 [DOI] [PubMed] [Google Scholar]
- 45.Edens WA, Sharling L, Cheng G, Shapira R, Kinkade JM, Lee T, Edens HA, Tang X, Sullards C, Flaherty DB, Benian GM, and Lambeth JD. Tyrosine cross-linking of extracellular matrix is catalyzed by Duox, a multidomain oxidase/peroxidase with homology to the phagocyte oxidase subunit gp91phox. J Cell Biol 154: 879–891, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ejlerskov P, Christensen DP, Beyaie D, Burritt JB, Paclet MH, Gorlach A, van Deurs B, and Vilhardt F. NADPH oxidase is internalized by clathrin-coated pits and localizes to a Rab27A/B GTPase-regulated secretory compartment in activated macrophages. J Biol Chem 287: 4835–4852, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.El Jamali A, Valente AJ, Lechleiter JD, Gamez MJ, Pearson DW, Nauseef WM, and Clark RA. Novel redox-dependent regulation of NOX5 by the tyrosine kinase c-Abl. Free Radic Biol Med 44: 868–881, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.El-Benna J, Dang PM, and Gougerot-Pocidalo MA. Priming of the neutrophil NADPH oxidase activation: role of p47phox phosphorylation and NOX2 mobilization to the plasma membrane. Semin Immunopathol 30: 279–289, 2008 [DOI] [PubMed] [Google Scholar]
- 49.Essex DW. Redox control of platelet function. Antioxid Redox Signal 11: 1191–1225, 2009 [DOI] [PubMed] [Google Scholar]
- 50.Evangelista AM, Thompson MD, Bolotina VM, Tong X, and Cohen RA. Nox4- and Nox2-dependent oxidant production is required for VEGF-induced SERCA cysteine-674 S-glutathiolation and endothelial cell migration. Free Radic Biol Med 53: 2327–2334, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fernandes DC, Bonatto D, and Laurindo FRM. The evolving concepto f oxidative stress. In: Studies on Cardiovascular Disorders, edited by Sauer H, Shah AM, and Laurindo FRM. New York, NY: Humana Press, 2010, pp. 1–41 [Google Scholar]
- 52.Fernandes DC, Manoel AH, Wosniak J, and Laurindo FR. Protein disulfide isomerase overexpression in vascular smooth muscle cells induces spontaneous preemptive NADPH oxidase activation and Nox1 mRNA expression: effects of nitrosothiol exposure. Arch Biochem Biophys 484: 197–204, 2009 [DOI] [PubMed] [Google Scholar]
- 53.Finegold AA, Shatwell KP, Segal AW, Klausner RD, and Dancis A. Intramembrane bis-heme motif for transmembrane electron transport conserved in a yeast iron reductase and the human NADPH oxidase. J Biol Chem 271: 31021–31024, 1996 [DOI] [PubMed] [Google Scholar]
- 54.Garcia RC. and Segal AW. Changes in the subcellular distribution of the cytochrome b-245 on stimulation of human neutrophils. Biochem J 219: 233–242, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gilbert J, Ou W, Silver J, and Benjamin T. Downregulation of protein disulfide isomerase inhibits infection by the mouse polyomavirus. J Virol 80: 10868–10870, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Goyal P, Weissmann N, Rose F, Grimminger F, Schäfers HJ, Seeger W, and Hänze J. Identification of novel Nox4 splice variants with impact on ROS levels in A549 cells. Biochem Biophys Res Commun 329: 32–39, 2005 [DOI] [PubMed] [Google Scholar]
- 57.Grasberger H, De Deken X, Miot F, Pohlenz J, and Refetoff S. Missense mutations of dual oxidase 2 (DUOX2) implicated in congenital hypothyroidism have impaired trafficking in cells reconstituted with DUOX2 maturation factor. Mol Endocrinol 21: 1408–1421, 2007 [DOI] [PubMed] [Google Scholar]
- 58.Griendling KK, Sorescu D, and Ushio-Fukai M. NAD(P)H oxidase: role in cardiovascular biology and disease. Circ Res 86: 494–501, 2000 [DOI] [PubMed] [Google Scholar]
- 59.Gu F, Nguyên DT, Stuible M, Dubé N, Tremblay ML, and Chevet E. Protein-tyrosine phosphatase 1B potentiates IRE1 signaling during endoplasmic reticulum stress. J Biol Chem 279: 49689–49693, 2004 [DOI] [PubMed] [Google Scholar]
- 60.Gu MX, Fu Y, Sun XL, Ding YZ, Li CH, Pang W, Pan S, and Zhu Y. Proteomic analysis of endothelial lipid rafts reveals a novel role of statins in antioxidation. J Proteome Res 11: 2365–2373, 2012 [DOI] [PubMed] [Google Scholar]
- 61.Hahn NE, Meischl C, Kawahara T, Musters RJ, Verhoef VM, van der Velden J, Vonk AB, Paulus WJ, van Rossum AC, Niessen HW, and Krijnen PA. NOX5 expression is increased in intramyocardial blood vessels and cardiomyocytes after acute myocardial infarction in humans. Am J Pathol 180: 2222–2229, 2012 [DOI] [PubMed] [Google Scholar]
- 62.Han CH, Nisimoto Y, Lee SH, Kim ET, and Lambeth JD. Characterization of the flavoprotein domain of gp91phox which has NADPH diaphorase activity. J Biochem 129: 513–520, 2001 [DOI] [PubMed] [Google Scholar]
- 63.Han CY, Umemoto T, Omer M, Den Hartigh LJ, Chiba T, LeBoeuf R, Buller CL, Sweet IR, Pennathur S, Abel ED, and Chait A. NADPH oxidase-derived reactive oxygen species increases expression of monocyte chemotactic factor genes in cultured adipocytes. J Biol Chem 287: 10379–10393, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Harper AM, Chaplin MF, and Segal AW. Cytochrome b-245 from human neutrophils is a glycoprotein. Biochem J 227: 783–788, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Harrison CB, Selemidis S, Guida E, King PT, Sobey CG, and Drummond GR. NOX2β: A novel splice variant of NOX2 that regulates NADPH oxidase activity in macrophages. PLoS One 7: e48326, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hashida S, Yuzawa S, Suzuki NN, Fujioka Y, Takikawa T, Sumimoto H, Inagaki F, and Fujii H. Binding of FAD to cytochrome b558 is facilitated during activation of the phagocyte NADPH oxidase, leading to superoxide production. J Biol Chem 279: 26378–26386, 2004 [DOI] [PubMed] [Google Scholar]
- 67.Hatahet F. and Ruddock LW. Substrate recognition by the protein disulfide isomerases. FEBS J 274: 5223–5234, 2007 [DOI] [PubMed] [Google Scholar]
- 68.Hatahet F. and Ruddock LW. Protein disulfide isomerase: a critical evaluation of its function in disulfide bond formation. Antioxid Redox Signal 11: 2807–2850, 2009 [DOI] [PubMed] [Google Scholar]
- 69.Hayee B, Antonopoulos A, Murphy EJ, Rahman FZ, Sewell G, Smith BN, McCartney S, Furman M, Hall G, Bloom SL, Haslam SM, Morris HR, Boztug K, Klein C, Winchester B, Pick E, Linch DC, Gale RE, Smith AM, Dell A, and Segal AW. G6PC3 mutations are associated with a major defect of glycosylation: a novel mechanism for neutrophil dysfunction. Glycobiology 21: 914–924, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Helmcke I, Heumüller S, Tikkanen R, Schröder K, and Brandes RP. Identification of structural elements in Nox1 and Nox4 controlling localization and activity. Antioxid Redox Signal 11: 1279–1287, 2009 [DOI] [PubMed] [Google Scholar]
- 71.Henderson B. and Pockley AG. Proteotoxic stress and circulating cell stress proteins in the cardiovascular diseases. Cell Stress Chaperones 17: 303–311, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Henderson LM, Banting G, and Chappell JB. The arachidonate-activable, NADPH oxidase-associated H+ channel. Evidence that gp91-phox functions as an essential part of the channel. J Biol Chem 270: 5909–5916, 1995 [PubMed] [Google Scholar]
- 73.Hernández-Oñate MA, Esquivel-Naranjo EU, Mendoza-Mendoza A, Stewart A, and Herrera-Estrella AH. An injury-response mechanism conserved across kingdoms determines entry of the fungus Trichoderma atroviride into development. Proc Natl Acad Sci U S A 109: 14918–14923, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hilenski LL, Clempus RE, Quinn MT, Lambeth JD, and Griendling KK. Distinct subcellular localizations of Nox1 and Nox4 in vascular smooth muscle cells. Arterioscler Thromb Vasc Biol 24: 677–683, 2004 [DOI] [PubMed] [Google Scholar]
- 75.Hong J, Resnick M, Behar J, Wands J, DeLellis RA, and Cao W. Role of Rac1 in regulation of NOX5-S function in Barrett's esophageal adenocarcinoma cells. Am J Physiol Cell Physiol 301: C413–C420, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hu Q, Yu ZX, Ferrans VJ, Takeda K, Irani K, and Ziegelstein RC. Critical role of NADPH oxidase-derived reactive oxygen species in generating Ca2+ oscillations in human aortic endothelial cells stimulated by histamine. J Biol Chem 277: 32546–32551, 2002 [DOI] [PubMed] [Google Scholar]
- 77.Huang YF, Liu SY, Yen CL, Yang PW, and Shieh CC. Thapsigargin and flavin adenine dinucleotide ex vivo treatment rescues trafficking-defective gp91phox in chronic granulomatous disease leukocytes. Free Radic Biol Med 47: 932–940, 2009 [DOI] [PubMed] [Google Scholar]
- 78.Imaoka S. Chemical stress on protein disulfide isomerases and inhibition of their functions. Int Rev Cell Mol Biol 290: 121–166, 2011 [DOI] [PubMed] [Google Scholar]
- 79.Inanami O, Johnson JL, and Babior BM. The leukocyte NADPH oxidase subunit p47PHOX: the role of the cysteine residues. Arch Biochem Biophys 350: 36–40, 1998 [DOI] [PubMed] [Google Scholar]
- 80.Jackson HM, Kawahara T, Nisimoto Y, Smith SM, and Lambeth JD. Nox4 B-loop creates an interface between the transmembrane and dehydrogenase domains. J Biol Chem 285: 10281–10290, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Jagnandan D, Church JE, Banfi B, Stuehr DJ, Marrero MB, and Fulton DJ. Novel mechanism of activation of NADPH oxidase 5. calcium sensitization via phosphorylation. J Biol Chem 282: 6494–6507, 2007 [DOI] [PubMed] [Google Scholar]
- 82.Janiszewski M, Lopes LR, Carmo AO, Pedro MA, Brandes RP, Santos CX, and Laurindo FR. Regulation of NAD(P)H oxidase by associated protein disulfide isomerase in vascular smooth muscle cells. J Biol Chem 280: 40813–40819, 2005 [DOI] [PubMed] [Google Scholar]
- 83.Jaronen M, Vehviläinen P, Malm T, Keksa-Goldsteine V, Pollari E, Valonen P, Koistinaho J, and Goldsteins G. Protein disulfide isomerase in ALS mouse glia links protein misfolding with NADPH oxidase-catalyzed superoxide production. Hum Mol Genet 22: 646–655, 2013 [DOI] [PubMed] [Google Scholar]
- 84.Jasuja R, Passam FH, Kennedy DR, Kim SH, van Hessem L, Lin L, Bowley SR, Joshi SS, Dilks JR, Furie B, Furie BC, and Flaumenhaft R. Protein disulfide isomerase inhibitors constitute a new class of antithrombotic agents. J Clin Invest 122: 2104–2113, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jessop CE, Watkins RH, Simmons JJ, Tasab M, and Bulleid NJ. Protein disulphide isomerase family members show distinct substrate specificity: P5 is targeted to BiP client proteins. J Cell Sci 122: 4287–4295, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Jordan PA. and Gibbins JM. Extracellular disulfide exchange and the regulation of cellular function. Antioxid Redox Signal 8: 312–324, 2006 [DOI] [PubMed] [Google Scholar]
- 87.Karlsson A. and Dahlgren C. Assembly and activation of the neutrophil NADPH oxidase in granule membranes. Antioxid Redox Signal 4: 49–60, 2002 [DOI] [PubMed] [Google Scholar]
- 88.Kawahara T, Jackson HM, Smith SM, Simpson PD, and Lambeth JD. Nox5 forms a functional oligomer mediated by self-association of its dehydrogenase domain. Biochemistry 50: 2013–2025, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kawahara T. and Lambeth JD. Phosphatidylinositol (4,5)-bisphosphate modulates Nox5 localization via an N-terminal polybasic region. Mol Biol Cell 19: 4020–4031, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kawahara T, Quinn MT, and Lambeth JD. Molecular evolution of the reactive oxygen-generating NADPH oxidase (Nox/Duox) family of enzymes. BMC Evol Biol 7: 109, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Klappa P, Ruddock LW, Darby NJ, and Freedman RB. The b′ domain provides the principal peptide-binding site of protein disulfide isomerase but all domains contribute to binding of misfolded proteins. EMBO J 17: 927–935, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kovacic HN, Irani K, and Goldschmidt-Clermont PJ. Redox regulation of human Rac1 stability by the proteasome in human aortic endothelial cells. J Biol Chem 276: 45856–45861, 2001 [DOI] [PubMed] [Google Scholar]
- 93.Kuroda J, Ago T, Matsushima S, Zhai P, Schneider MD, and Sadoshima J. NADPH oxidase 4 (Nox4) is a major source of oxidative stress in the failing heart. Proc Natl Acad Sci U S A 107: 15565–15570, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kuroda J, Nakagawa K, Yamasaki T, Nakamura K, Takeya R, Kuribayashi F, Imajoh-Ohmi S, Igarashi K, Shibata Y, Sueishi K, and Sumimoto H. The superoxide-producing NAD(P)H oxidase Nox4 in the nucleus of human vascular endothelial cells. Genes Cells 10: 1139–1151, 2005 [DOI] [PubMed] [Google Scholar]
- 95.Lara-Ortíz T, Riveros-Rosas H, and Aguirre J. Reactive oxygen species generated by microbial NADPH oxidase NoxA regulate sexual development in Aspergillus nidulans. Mol Microbiol 50: 1241–1255, 2003 [DOI] [PubMed] [Google Scholar]
- 96.Lassègue B. and Griendling KK. NADPH oxidases: functions and pathologies in the vasculature. Arterioscler Thromb Vasc Biol 30: 653–661, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lassègue B, San Martín A, and Griendling KK. Biochemistry, physiology, and pathophysiology of NADPH oxidases in the cardiovascular system. Circ Res 110: 1364–1390, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Laurindo FR, Fernandes DC, Amanso AM, Lopes LR, and Santos CX. Novel role of protein disulfide isomerase in the regulation of NADPH oxidase activity: pathophysiological implications in vascular diseases. Antioxid Redox Signal 10: 1101–1113, 2008 [DOI] [PubMed] [Google Scholar]
- 99.Laurindo FR, Pescatore LA, and Fernandes Dde C. Protein disulfide isomerase in redox cell signaling and homeostasis. Free Radic Biol Med 52: 1954–1969, 2012 [DOI] [PubMed] [Google Scholar]
- 100.Lee CF, Qiao M, Schröder K, Zhao Q, and Asmis R. Nox4 is a novel inducible source of reactive oxygen species in monocytes and macrophages and mediates oxidized low density lipoprotein-induced macrophage death. Circ Res 106: 1489–1497, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100a.Lee M, Spokes KC, Aird WC, and Abid MR. Intracellular Ca2+ can compensate for the lack of NADPH oxidase-derived ROS in endothelial cells. FEBS Lett 16: 3131–3136, 2010 [DOI] [PubMed] [Google Scholar]
- 101.Li G, Scull C, Ozcan L, and Tabas I. NADPH oxidase links endoplasmic reticulum stress, oxidative stress, and PKR activation to induce apoptosis. J Cell Biol 191: 1113–1125, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Li JM. and Shah AM. Intracellular localization and preassembly of the NADPH oxidase complex in cultured endothelial cells. J Biol Chem 277: 19952–19960, 2002 [DOI] [PubMed] [Google Scholar]
- 103.Li J, Zhu H, Shen E, Wan L, Arnold JM, and Peng T. Deficiency of rac1 blocks NADPH oxidase activation, inhibits endoplasmic reticulum stress, and reduces myocardial remodeling in a mouse model of type 1 diabetes. Diabetes 59: 2033–2042, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Liao X, Sluimer JC, Wang Y, Subramanian M, Brown K, Pattison JS, Robbins J, Martinez J, and Tabas I. Macrophage autophagy plays a protective role in advanced atherosclerosis. Cell Metab 15: 545–553, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Liberman M, Johnson RC, Handy DE, Loscalzo J, and Leopold JA. Bone morphogenetic protein-2 activates NADPH oxidase to increase endoplasmic reticulum stress and human coronary artery smooth muscle cell calcification. Biochem Biophys Res Commun 413: 436–441, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lin SJ, Huang YF, Chen JY, Heyworth PG, Noack D, Wang JY, Lin CY, Chiang BL, Yang CM, Liu CC, and Shieh CC. Molecular quality control machinery contributes to the leukocyte NADPH oxidase deficiency in chronic granulomatous disease. Biochim Biophys Acta 1586: 275–286, 2002 [DOI] [PubMed] [Google Scholar]
- 107.Lob HE, Schultz D, Marvar PJ, Davisson RL, and Harrison DG. Role of the NADPH oxidases in the subfornical organ in angiotensin II-induced hypertension. Hypertension 61: 382–387, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Loughlin DT. and Artlett CM. Precursor of advanced glycation end products mediates ER-stress-induced caspase-3 activation of human dermal fibroblasts through NAD(P)H oxidase 4. PLoS One 5: e11093, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lyle AN, Deshpande NN, Taniyama Y, Seidel-Rogol B, Pounkova L, Du P, Papaharalambus C, Lassègue B, and Griendling KK. Poldip2, a novel regulator of Nox4 and cytoskeletal integrity in vascular smooth muscle cells. Circ Res 105: 249–259, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Malhotra JD, Miao H, Zhang K, Wolfson A, Pennathur S, Pipe SW, and Kaufman RJ. Antioxidants reduce endoplasmic reticulum stress and improve protein secretion. Proc Natl Acad Sci U S A 105: 18525–18530, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Mandel R, Ryser HJ, Ghani F, Wu M, and Peak D. Inhibition of a reductive function of the plasma membrane by bacitracin and antibodies against protein disulfide-isomerase. Proc Natl Acad Sci U S A 90: 4112–4116, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Marciniak SJ. and Ron D. Endoplasmic reticulum stress signaling in disease. Physiol Rev 86: 1133–1149, 2006 [DOI] [PubMed] [Google Scholar]
- 113.Markovic I, Stantchev TS, Fields KH, Tiffany LJ, Tomiç M, Weiss CD, Broder CC, Strebel K, and Clouse KA. Thiol/disulfide exchange is a prerequisite for CXCR4-tropic HIV-1 envelope-mediated T-cell fusion during viral entry. Blood 103: 1586–1594, 2004 [DOI] [PubMed] [Google Scholar]
- 114.Martyn KD, Frederick LM, von Loehneysen K, Dinauer MC, and Knaus UG. Functional analysis of Nox4 reveals unique characteristics compared to other NADPH oxidases. Cell Signal 18: 69–82, 2006 [DOI] [PubMed] [Google Scholar]
- 115.Matsushima S, Kuroda J, Ago T, Zhai P, Park JY, Xie LH, Tian B, and Sadoshima J. Increased oxidative stress in the nucleus caused by Nox4 mediates oxidation of HDAC4 and cardiac hypertrophy. Circ Res 112: 651–663, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Mehmeti I, Lortz S, and Lenzen S. The H2O2-sensitive HyPer protein targeted to the endoplasmic reticulum as a mirror of the oxidizing thiol-disulfide milieu. Free Radic Biol Med 53: 1451–1458, 2012 [DOI] [PubMed] [Google Scholar]
- 117.Merksamer PI, Trusina A, and Papa FR. Real-time redox measurements during endoplasmic reticulum stress reveal interlinked protein folding functions. Cell 135: 933–947, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Mitchell L, Hobbs GA, Aghajanian A, and Campbell SL. Redox regulation of Ras and Rho GTPases: mechanism and function. Antioxid Redox Signal 18: 250–258, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Morand S, Agnandji D, Noel-Hudson MS, Nicolas V, Buisson S, and Macon-Lemaitre L, Gnidehou S, Kaniewski J, Ohayon R, Virion A, and Dupuy C. Targeting of the dual oxidase 2 N-terminal region to the plasma membrane. J Biol Chem 279: 30244–30251, 2004 [DOI] [PubMed] [Google Scholar]
- 120.Muller C, Bandemer J, Vindis C, Camaré C, Mucher E, Guéraud F, Larroque-Cardoso P, Bernis C, Auge N, Salvayre R, and Negre-Salvayre A. Protein disulfide isomerase modification and inhibition contribute to ER stress and apoptosis induced by oxidized low density lipoproteins. Antioxid Redox Signal 18: 731–742, 2013 [DOI] [PubMed] [Google Scholar]
- 121.Nguyen MV, Zhang L, Lhomme S, Mouz N, Lenormand JL, Lardy B, and Morel F. Recombinant Nox4 cytosolic domain produced by a cell or cell-free base systems exhibits constitutive diaphorase activity. Biochem Biophys Res Commun 419: 453–458, 2012 [DOI] [PubMed] [Google Scholar]
- 122.Nguyen VD, Saaranen MJ, Karala AR, Lappi AK, Wang L, Raykhel IB, Alanen HI, Salo KE, Wang CC, and Ruddock LW. Two endoplasmic reticulum PDI peroxidases increase the efficiency of the use of peroxide during disulfide bond formation. J Mol Biol 406: 503–515, 2011 [DOI] [PubMed] [Google Scholar]
- 123.Niethammer P, Grabher C, Look AT, and Mitchison TJ. A tissue-scale gradient of hydrogen peroxide mediates rapid wound detection in zebrafish. Nature 459: 996–999, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Nisimoto Y, Jackson HM, Ogawa H, Kawahara T, and Lambeth JD. Constitutive NADPH-dependent electron transferase activity of the Nox4 dehydrogenase domain. Biochemistry 49: 2433–2442, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.O'Leary DP, Bhatt L, Woolley JF, Gough DR, Wang JH, Cotter TG, and Redmond HP. TLR-4 signalling accelerates colon cancer cell adhesion via NF-κB mediated transcriptional up-regulation of Nox-1. PLoS One 7: e44176, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Oakley FD, Abbott D, Li Q, and Engelhardt JF. Signaling components of redox active endosomes: the redoxosomes. Antioxid Redox Signal 11: 1313–1333, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Parkos CA, Allen RA, Cochrane CG, and Jesaitis AJ. Purified cytochrome b from human granulocyte plasma membrane is comprised of two polypeptides with relative molecular weights of 91,000 and 22,000. J Clin Invest 80: 732–742, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Pedruzzi E, Guichard C, Ollivier V, Driss F, Fay M, Prunet C, Marie JC, Pouzet C, Samadi M, Elbim C, O'dowd Y, Bens M, Vandewalle A, Gougerot-Pocidalo MA, Lizard G, and Ogier-Denis E. NAD(P)H oxidase Nox-4 mediates 7-ketocholesterol-induced endoplasmic reticulum stress and apoptosis in human aortic smooth muscle cells. Mol Cell Biol 24: 10703–10717, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Pescatore LA, Bonatto D, Forti FL, Sadok A, Kovacic H, and Laurindo FR. Protein disulfide isomerase is required for platelet-derived growth factor-induced vascular smooth muscle cell migration, Nox1 NADPH oxidase expression, and RhoGTPase activation. J Biol Chem 287: 29290–29300, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Piccirella S, Czegle I, Lizák B, Margittai E, Senesi S, Papp E, Csala M, Fulceri R, Csermely P, Mandl J, Benedetti A, and Bánhegyi G. Uncoupled redox systems in the lumen of the endoplasmic reticulum. Pyridine nucleotides stay reduced in an oxidative environment. J Biol Chem 281: 4671–4677, 2006 [DOI] [PubMed] [Google Scholar]
- 131.Popescu NI, Lupu C, and Lupu F. Extracellular protein disulfide isomerase regulates coagulation on endothelial cells through modulation of phosphatidylserine exposure. Blood 116: 993–1001, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Porter CD, Parkar MH, Verhoeven AJ, Levinsky RJ, Collins MK, and Kinnon C. p22-phox-deficient chronic granulomatous disease: reconstitution by retrovirus-mediated expression and identification of a biosynthetic intermediate of gp91-phox. Blood 84: 2767–2775, 1994 [PubMed] [Google Scholar]
- 133.Prosser BL, Khairallah RJ, Ziman AP, Ward CW, and Lederer WJ. X-ROS signaling in the heart and skeletal muscle: Stretch-dependent local ROS regulates [Ca(2+)](i). J Mol Cell Cardiol 58: 172–181, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Prosser BL, Ward CW, and Lederer WJ. X-ROS signaling: rapid mechano-chemo transduction in heart. Science 333: 1440–1445, 2011 [DOI] [PubMed] [Google Scholar]
- 135.Quinn MT, Mullen ML, and Jesaitis AJ. Human neutrophil cytochrome b contains multiple hemes. Evidence for heme associated with both subunits. J Biol Chem 267: 7303–7309, 1992 [PubMed] [Google Scholar]
- 136.Razzell W, Evans IR, Martin P, and Wood W. Calcium flashes orchestrate the wound inflammatory response through DUOX activation and hydrogen peroxide release. Curr Biol 23: 424–429, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Reinhardt C, von Brühl ML, Manukyan D, Grahl L, Lorenz M, Altmann B, Dlugai S, Hess S, Konrad I, Orschiedt L, Mackman N, Ruddock L, Massberg S, and Engelmann B. Protein disulfide isomerase acts as an injury response signal that enhances fibrin generation via tissue factor activation. J Clin Invest 118: 1110–1122, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Rinnerthaler M, Büttner S, Laun P, Heeren G, Felder TK, Klinger H, Weinberger M, Stolze K, Grousl T, Hasek J, Benada O, Frydlova I, Klocker A, Simon-Nobbe B, Jansko B, Breitenbach-Koller H, Eisenberg T, Gourlay CW, Madeo F, Burhans W, and Breitenbach M. Yno1p/Aim14p, a NADPH-oxidase ortholog, controls extramitochondrial reactive oxygen species generation, apoptosis, and actin cable formation in yeast. Proc Natl Acad Sci U S A 109: 8658–8663, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Ron D. and Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol 8: 519–529, 2007 [DOI] [PubMed] [Google Scholar]
- 140.Rotrosen D, Yeung CL, Leto TL, Malech HL, and Kwong CH. Cytochrome b558: the flavin-binding component of the phagocyte NADPH oxidase. Science 256: 1459–1462, 1992 [DOI] [PubMed] [Google Scholar]
- 141.Roy A. and Kolattukudy PE. Monocyte chemotactic protein-induced protein (MCPIP) promotes inflammatory angiogenesis via sequential induction of oxidative stress, endoplasmic reticulum stress and autophagy. Cell Signal 24: 2123–2131, 2012 [DOI] [PubMed] [Google Scholar]
- 142.Rutkevich LA, Cohen-Doyle MF, Brockmeier U, and Williams DB. Functional relationship between protein disulfide isomerase family members during the oxidative folding of human secretory proteins. Mol Biol Cell 21: 3093–3105, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Rutkowski DT. and Kaufman RJ. That which does not kill me makes me stronger: adapting to chronic ER stress. Trends Biochem Sci 32: 469–476, 2007 [DOI] [PubMed] [Google Scholar]
- 144.Santos CX, Stolf BS, Takemoto PV, Amanso AM, Lopes LR, Souza EB, Goto H, and Laurindo FR. Protein disulfide isomerase (PDI) associates with NADPH oxidase and is required for phagocytosis of Leishmania chagasi promastigotes by macrophages. J Leukoc Biol 86: 989–998, 2009 [DOI] [PubMed] [Google Scholar]
- 145.Santos CX, Tanaka LY, Wosniak J, and Laurindo FR. Mechanisms and implications of reactive oxygen species generation during the unfolded protein response: roles of endoplasmic reticulum oxidoreductases, mitochondrial electron transport, and NADPH oxidase. Antioxid Redox Signal 11: 2409–2427, 2009 [DOI] [PubMed] [Google Scholar]
- 146.Segal AW, West I, Wientjes F, Nugent JH, Chavan AJ, Haley B, Garcia RC, Rosen H, and Scrace G. Cytochrome b-245 is a flavocytochrome containing FAD and the NADPH-binding site of the microbicidal oxidase of phagocytes. Biochem J 284 (Pt 3): 781–788, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Serrander L, Cartier L, Bedard K, Banfi B, Lardy B, Plastre O, Sienkiewicz A, Fórró L, Schlegel W, and Krause KH. NOX4 activity is determined by mRNA levels and reveals a unique pattern of ROS generation. Biochem J 406: 105–114, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Serrander L, Jaquet V, Bedard K, Plastre O, Hartley O, Arnaudeau S, Demaurex N, Schlegel W, and Krause KH. NOX5 is expressed at the plasma membrane and generates superoxide in response to protein kinase C activation. Biochimie 89: 1159–1167, 2007 [DOI] [PubMed] [Google Scholar]
- 149.Sharma M, Urano F, and Jaeschke A. Cdc42 and Rac1 are major contributors to the saturated fatty acid-stimulated JNK pathway in hepatocytes. J Hepatol 56: 192–198, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Sitia R. and Braakman I. Quality control in the endoplasmic reticulum protein factory. Nature 426: 891–894, 2003 [DOI] [PubMed] [Google Scholar]
- 151.Spencer NY, Yan Z, Boudreau RL, Zhang Y, Luo M, Li Q, Tian X, Shah AM, Davisson RL, Davidson B, Banfi B, and Engelhardt JF. Control of hepatic nuclear superoxide production by glucose 6-phosphate dehydrogenase and NADPH oxidase-4. J Biol Chem 286: 8977–8987, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Stolf BS, Smyrnias I, Lopes LR, Vendramin A, Goto H, Laurindo FR, Shah AM, and Santos CX. Protein disulfide isomerase and host-pathogen interaction. ScientificWorldJournal 11: 1749–1761, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Sturrock A, Huecksteadt TP, Norman K, Sanders K, Murphy TM, Chitano P, Wilson K, Hoidal JR, and Kennedy TP. Nox4 mediates TGF-beta1-induced retinoblastoma protein phosphorylation, proliferation, and hypertrophy in human airway smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 292: L1543–L1555, 2007 [DOI] [PubMed] [Google Scholar]
- 154.Sun QA, Hess DT, Nogueira L, Yong S, Bowles DE, Eu J, Laurita KR, Meissner G, and Stamler JS. Oxygen-coupled redox regulation of the skeletal muscle ryanodine receptor-Ca2+ release channel by NADPH oxidase 4. Proc Natl Acad Sci U S A 108: 16098–16103, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Swaminathan PD, Purohit A, Soni S, Voigt N, Singh MV, Glukhov AV, Gao Z, He BJ, Luczak ED, Joiner ML, Kutschke W, Yang J, Donahue JK, Weiss RM, Grumbach IM, Ogawa M, Chen PS, Efimov I, Dobrev D, Mohler PJ, Hund TJ, and Anderson ME. Oxidized CaMKII causes cardiac sinus node dysfunction in mice. J Clin Invest 121: 3277–3288, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Tabas I. and Ron D. Integrating the mechanisms of apoptosis induced by endoplasmic reticulum stress. Nat Cell Biol 13: 184–190, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Taillé C, El-Benna J, Lanone S, Dang MC, Ogier-Denis E, Aubier M, and Boczkowski J. Induction of heme oxygenase-1 inhibits NAD(P)H oxidase activity by down-regulating cytochrome b558 expression via the reduction of heme availability. J Biol Chem 279: 28681–28688, 2004 [DOI] [PubMed] [Google Scholar]
- 158.Takac I, Schröder K, Zhang L, Lardy B, Anilkumar N, Lambeth JD, Shah AM, Morel F, and Brandes RP. The E-loop is involved in hydrogen peroxide formation by the NADPH oxidase Nox4. J Biol Chem 286: 13304–13313, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Tavender TJ. and Bulleid NJ. Molecular mechanisms regulating oxidative activity of the Ero1 family in the endoplasmic reticulum. Antioxid Redox Signal 13: 1177–1187, 2010 [DOI] [PubMed] [Google Scholar]
- 160.Tominaga K, Kawahara T, Sano T, Toida K, Kuwano Y, Sasaki H, Kawai T, Teshima-Kondo S, and Rokutan K. Evidence for cancer-associated expression of NADPH oxidase 1 (Nox1)-based oxidase system in the human stomach. Free Radic Biol Med 43: 1627–1638, 2007 [DOI] [PubMed] [Google Scholar]
- 161.Tong X, Hou X, Jourd'heuil D, Weisbrod RM, and Cohen RA. Upregulation of Nox4 by TGF{beta}1 oxidizes SERCA and inhibits NO in arterial smooth muscle of the prediabetic Zucker rat. Circ Res 107: 975–983, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Touyz RM, Yao G, Quinn MT, Pagano PJ, and Schiffrin EL. p47phox associates with the cytoskeleton through cortactin in human vascular smooth muscle cells: role in NAD(P)H oxidase regulation by angiotensin II. Arterioscler Thromb Vasc Biol 25: 512–518, 2005 [DOI] [PubMed] [Google Scholar]
- 163.Tudzynski P, Heller J, and Siegmund U. Reactive oxygen species generation in fungal development and pathogenesis. Curr Opin Microbiol 15: 653–659, 2012 [DOI] [PubMed] [Google Scholar]
- 164.Turano C, Coppari S, Altieri F, and Ferraro A. Proteins of the PDI family: unpredicted non-ER locations and functions. J Cell Physiol 193: 154–163, 2002 [DOI] [PubMed] [Google Scholar]
- 165.Uehara T, Nakamura T, Yao D, Shi ZQ, Gu Z, Ma Y, Masliah E, Nomura Y, and Lipton SA. S-nitrosylated protein-disulphide isomerase links protein misfolding to neurodegeneration. Nature 441: 513–517, 2006 [DOI] [PubMed] [Google Scholar]
- 166.Ullevig S, Zhao Q, Lee CF, Seok Kim H, Zamora D, and Asmis R. NADPH oxidase 4 mediates monocyte priming and accelerated chemotaxis induced by metabolic stress. Arterioscler Thromb Vasc Biol 32: 415–426, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Ushio-Fukai M. Compartmentalization of redox signaling through NADPH oxidase-derived ROS. Antioxid Redox Signal 11: 1289–1299, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Van Buul JD, Fernandez-Borja M, Anthony EC, and Hordijk PL. Expression and localization of NOX2 and NOX4 in primary human endothelial cells. Antioxid Redox Signal 7: 308–317, 2005 [DOI] [PubMed] [Google Scholar]
- 169.Versteeg HH. and Ruf W. Tissue factor coagulant function is enhanced by protein-disulfide isomerase independent of oxidoreductase activity. J Biol Chem 282: 25416–25424, 2007 [DOI] [PubMed] [Google Scholar]
- 170.Vilhardt F and van Deurs B. The phagocyte NADPH oxidase depends on cholesterol-enriched membrane microdomains for assembly. EMBO J 23: 739–748, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Wan SW, Lin CF, Lu YT, Lei HY, Anderson R, and Lin YS. Endothelial cell surface expression of protein disulfide isomerase activates β1 and β3 integrins and facilitates dengue virus infection. J Cell Biochem 113: 1681–1691, 2012 [DOI] [PubMed] [Google Scholar]
- 172.Wang C, Li W, Ren J, Fang J, Ke H, Gong W, Feng W, and Wang CC. Structural insights into the redox-regulated dynamic conformations of human protein disulfide isomerase. Antioxid Redox Signal 19: 36–45, 2013 [DOI] [PubMed] [Google Scholar]
- 173.Wang C, Yu J, Huo L, Wang L, Feng W, and Wang CC. Human protein-disulfide isomerase is a redox-regulated chaperone activated by oxidation of domain a′. J Biol Chem 287: 1139–1149, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Wang D, De Deken X, Milenkovic M, Song Y, Pirson I, Dumont JE, and Miot F. Identification of a novel partner of duox: EFP1, a thioredoxin-related protein. J Biol Chem 280: 3096–3103, 2005 [DOI] [PubMed] [Google Scholar]
- 175.Weyemi U, Lagente-Chevallier O, Boufraqech M, Prenois F, Courtin F, Caillou B, Talbot M, Dardalhon M, Al Ghuzlan A, Bidart JM, Schlumberger M, and Dupuy C. ROS-generating NADPH oxidase NOX4 is a critical mediator in oncogenic H-Ras-induced DNA damage and subsequent senescence. Oncogene 31: 1117–1129, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Wilkinson B. and Gilbert HF. Protein disulfide isomerase. Biochim Biophys Acta 1699: 35–44, 2004 [DOI] [PubMed] [Google Scholar]
- 177.Willems SH, Tape CJ, Stanley PL, Taylor NA, Mills IG, Neal DE, McCafferty J, and Murphy G. Thiol isomerases negatively regulate the cellular shedding activity of ADAM17. Biochem J 428: 439–450, 2010 [DOI] [PubMed] [Google Scholar]
- 178.Winterbourn CC. Reconciling the chemistry and biology of reactive oxygen species. Nat Chem Biol 4: 278–286, 2008 [DOI] [PubMed] [Google Scholar]
- 179.Woolley JF, Naughton R, Stanicka J, Gough DR, Bhatt L, Dickinson BC, Chang CJ, and Cotter TG. H2O2 production downstream of FLT3 is mediated by p22phox in the endoplasmic reticulum and is required for STAT5 signalling. PLoS One 7: e34050, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Wu J. and Kaufman RJ. From acute ER stress to physiological roles of the unfolded protein response. Cell Death Differ 13: 374–384, 2006 [DOI] [PubMed] [Google Scholar]
- 181.Wu RF, Ma Z, Liu Z, and Terada LS. Nox4-derived H2O2 mediates endoplasmic reticulum signaling through local Ras activation. Mol Cell Biol 30: 3553–3568, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Wu RF. and Terada LS. Focal oxidant and Ras signaling on the ER surface activates autophagy. Autophagy 6: 828–829, 2010 [DOI] [PubMed] [Google Scholar]
- 183.Xu X, Liu T, Zhang A, Huo X, Luo Q, Chen Z, Yu L, Li Q, Liu L, Lun ZR, and Shen J. Reactive oxygen species-triggered trophoblast apoptosis is initiated by endoplasmic reticulum stress via activation of caspase-12, CHOP, and the JNK pathway in Toxoplasma gondii infection in mice. Infect Immun 80: 2121–2132, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 184.Yoo SK, Freisinger CM, LeBert DC, and Huttenlocher A. Early redox, Src family kinase, and calcium signaling integrate wound responses and tissue regeneration in zebrafish. J Cell Biol 199: 225–234, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Yoo SK, Starnes TW, Deng Q, and Huttenlocher A. Lyn is a redox sensor that mediates leukocyte wound attraction in vivo. Nature 480: 109–112, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Yoshida LS, Saruta F, Yoshikawa K, Tatsuzawa O, and Tsunawaki S. Mutation at histidine 338 of gp91(phox) depletes FAD and affects expression of cytochrome b558 of the human NADPH oxidase. J Biol Chem 273: 27879–27886, 1998 [DOI] [PubMed] [Google Scholar]
- 187.Young CN, Cao X, Guruju MR, Pierce JP, Morgan DA, Wang G, Iadecola C, Mark AL, and Davisson RL. ER stress in the brain subfornical organ mediates angiotensin-dependent hypertension. J Clin Invest 122: 3960–3964, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Yu L, DeLeo FR, Biberstine-Kinkade KJ, Renee J, Nauseef WM, and Dinauer MC. Biosynthesis of flavocytochrome b558. gp91(phox) is synthesized as a 65-kDa precursor (p65) in the endoplasmic reticulum. J Biol Chem 274: 4364–4369, 1999 [DOI] [PubMed] [Google Scholar]
- 189.Yu L, Quinn MT, Cross AR, and Dinauer MC. Gp91(phox) is the heme binding subunit of the superoxide-generating NADPH oxidase. Proc Natl Acad Sci U S A 95: 7993–7998, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Yu L, Zhen L, and Dinauer MC. Biosynthesis of the phagocyte NADPH oxidase cytochrome b558. Role of heme incorporation and heterodimer formation in maturation and stability of gp91phox and p22phox subunits. J Biol Chem 272: 27288–27294, 1997 [DOI] [PubMed] [Google Scholar]
- 191.Yun BW, Feechan A, Yin M, Saidi NB, Le Bihan T, Yu M, Moore JW, Kang JG, Kwon E, Spoel SH, Pallas JA, and Loake GJ. S-nitrosylation of NADPH oxidase regulates cell death in plant immunity. Nature 478: 264–268, 2011 [DOI] [PubMed] [Google Scholar]
- 192.Zhang F, Jin S, Yi F, Xia M, Dewey WL, and Li PL. Local production of O2- by NAD(P)H oxidase in the sarcoplasmic reticulum of coronary arterial myocytes: cADPR-mediated Ca2+ regulation. Cell Signal 20: 637–644, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Zhang K. and Kaufman RJ. From endoplasmic-reticulum stress to the inflammatory response. Nature 454: 455–462, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Zhang L, Nguyen MV, Lardy B, Jesaitis AJ, Grichine A, Rousset F, Talbot M, Paclet MH, Qian G, and Morel F. New insight into the Nox4 subcellular localization in HEK293 cells: first monoclonal antibodies against Nox4. Biochimie 93: 457–468, 2011 [DOI] [PubMed] [Google Scholar]
- 195.Zhang M, Brewer AC, Schröder K, Santos CX, Grieve DJ, Wang M, Anilkumar N, Yu B, Dong X, Walker SJ, Brandes RP, and Shah AM. NADPH oxidase-4 mediates protection against chronic load-induced stress in mouse hearts by enhancing angiogenesis. Proc Natl Acad Sci U S A 107: 18121–18126, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Zhang Y. and Ren J. Thapsigargin triggers cardiac contractile dysfunction via NADPH oxidase-mediated mitochondrial dysfunction: Role of Akt dephosphorylation. Free Radic Biol Med 51: 2172–2184, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 197.Zhu Y, Marchal CC, Casbon AJ, Stull N, von Löhneysen K, Knaus UG, Jesaitis AJ, McCormick S, Nauseef WM, and Dinauer MC. Deletion mutagenesis of p22phox subunit of flavocytochrome b558: identification of regions critical for gp91phox maturation and NADPH oxidase activity. J Biol Chem 281: 30336–30346, 2006 [DOI] [PubMed] [Google Scholar]
- 198.Zimmerman MC, Takapoo M, Jagadeesha DK, Stanic B, Banfi B, Bhalla RC, and Miller FJ., Jr.Activation of NADPH oxidase 1 increases intracellular calcium and migration of smooth muscle cells. Hypertension 58: 446–453, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Zito E, Melo EP, Yang Y, Wahlander Å, Neubert TA, and Ron D. Oxidative protein folding by an endoplasmic reticulum-localized peroxiredoxin. Mol Cell 40: 787–797, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]