Abstract
Cardiovascular disease (CVD) is the leading cause of mortality in patients with type 2 diabetes mellitus (T2DM). Vitamin D deficiency is not only more prevalent in diabetics but also doubles the risk of developing CVD. However, it is unknown whether 25-hydroxy vitamin D [25(OH)D3] replacement slows monocyte adhesion and migration, critical mechanisms involved in atherosclerosis progression. In this study, monocytes from vitamin D-deficient diabetic patients were cultured either in the patient’s serum or in vitamin D-deficient media with or without 25(OH)D3 treatment. Adding 25(OH)D3 to monocytes cultured in vitamin D-deficient serum or media decreased monocyte adhesion to fibronectin and migration stimulated by monocyte chemotactic protein 1 (MCP-1). Accordingly, 25(OH)D3 decreased adhesion marker β1- and β2-integrin expression and migration receptor chemokine (C-C motif) receptor 2 (CCR2) expression. 25(OH)D3 treatment downregulated monocyte endoplasmic reticulum (ER) stress and scavenger receptor class A, type 1 (SR-A1) expression. The absence of SR-A1 prevented the increased macrophage adhesion and migration induced by vitamin D deficiency. Moreover, the absence of SR-A1 prevented the induction of adhesion and migration and expression of their associated membrane receptors by Thapsigargin, an ER stress inducer. These results identify cellular activation of monocyte/macrophage vitamin D signaling through 25(OH)D3 as a potential mechanism that could modulate adhesion and migration in diabetic subjects.
Keywords: Vitamin D, macrophage, adhesion, migration, SR-A1, diabetes
1. Introduction
Approximately 20 million Americans suffer from type 2 diabetes mellitus, a disease frequently associated with elevated blood pressure and characterized by an increased risk of cardiovascular disease [1]. The combination of these metabolic abnormalities is the most common cause of morbidity and mortality in Western populations [2]. Despite the notion that insulin resistance and chronic inflammation lead to accelerated vascular disease in patients with T2DM, very little is known about the mechanisms by which these risk factors promote vascular complications. Vascular inflammation is recognized as a major contributor to atherosclerotic plaque development [3–5]. Monocytes migrate from the circulation into the intima of the arterial wall where they differentiate into macrophages, which then take up modified lipoproteins thereby transforming into foam cells [6]. Monocyte-derived macrophages are abundantly present at all stages of the disease process and play a pivotal role in the development and progression of the disease [5, 7, 8]. Decreased circulating monocytes and reduced tissue macrophages resulting from the absence of macrophage colony-stimulating factor (M-CSF) in mice reduces high-fat-diet-induced atherosclerosis [9, 10]. Moreover, multiple genetically modified mouse models with the absence of monocyte chemokine receptors (CX3C chemokine receptor 1, CCR2, or CCR5) or deletion of adhesion molecules such as selectins or integrins are associated with decreased atherosclerosis development, suggesting that understanding monocyte behavior will be key to decreasing atherosclerosis progression [6].
The class A, type 1 scavenger receptor (SR-A1) belongs to a large family of scavenger receptors expressed primarily by monocytes/macrophages with critical roles in vascular inflammation and atherosclerosis progression. In early atherosclerosis, SR-A1 mediates macrophage adhesion [11], while SR-A1−/− peritoneal macrophages display reduced adhesion and spreading in the first 24 hours after isolation [12]. Interestingly, SR-A1–dependent macrophage adhesion may only be of significance under environmental conditions such as diabetes when SR-A1 expression is increased or when the SR-A1 ligands are glycated. Macrophages with absence of the insulin receptor show increased ER stress and SR-A1 expression, suggesting a link between insulin signaling and macrophage stress signaling pathways [13]. In vitro, macrophages can adhere to surfaces coated with glucose-modified basement membrane collagen IV through their SR-As, highlighting the role of SR-A1 in the accelerated atherosclerosis in diabetes [14]. SR-A1 facilitates modified-cholesterol uptake in macrophages and works as an immune pattern recognition receptor, triggering apoptosis in endoplasmic-reticulum-stressed macrophages, promoting advancement of lesions and plaque necrosis [15]. Thus, identifying environmental conditions that modulate vascular SR-A ligands and monocyte SR-A expression could be key to the development of novel therapies to slow the accelerated atherosclerosis seen in patients with diabetes.
Vitamin D deficiency is a largely unacknowledged epidemic associated with incident T2DM and CVD [16–18]. Deficiency of 25(OH)D, the principal storage form of vitamin D, is 30% more prevalent in diabetics than in control subjects and nearly doubles the relative risk of developing CVD compared to diabetic patients with normal 25(OH)D levels [19, 20]. The vitamin D receptor (VDR) and its converting enzyme, 25(OH)D3-1α-hydroxylase, are present in monocytes and macrophages, demonstrating the capability of these cells to generate active vitamin D and induce vitamin D cell signaling locally. 1,25-dihydroxy vitamin D [1,25(OH)2D3], the active form of vitamin D, facilitates adhesion in monocytic cell lines and stimulates differentiation of myeloid progenitors into macrophages in vitro through the VDR [21, 22]. During monocyte differentiation, the ER reorganizes both structurally and functionally in order to carry out new cell functions, leading to ER stress [23, 24]. We have shown that 1,25(OH)2D3 suppresses ER stress in monocytes and macrophages from diabetic patients, along with preventing monocyte adhesion and migration and macrophage cholesterol deposition, all indicating that active vitamin D modulates properties contributing to atherosclerosis progression [25–27]. Furthermore, cross-sectional analysis of T2DM patients with 25(OH)D >30 ng/mL demonstrated decreased monocyte adhesion and migration compared to those with 25OHD <20 ng/mL [26]. However, it is unknown whether 25(OH)D supplementation, more consistent with common clinical practice, maysuppress monocyte adhesion and migration.
To determine whether 25(OH)D3 decreases monocyte adhesion and migration and expression of membrane receptors implicated in these processes, we obtained monocytes from T2DM patients with vitamin D deficiency and incubated them briefly in culture in the original patient’s serum with or without 25(OH)D3 supplementation to mimic the normal in vivo environment. We also explored the effects of 25(OH)D3 on ER homeostasis in the regulation of monocyte/macrophage adhesion and migration and assessed whether these effects are SR-A1- dependent.
2. Materials and Methods
2.1 Study design
We performed a cross-sectional analysis of 12 obese patients with T2DM with vitamin D deficiency [25(OH)D <30 ng/mL; mean 17±7 ng/mL, BMI 36 ± 2.6]. Subjects were voluntarily recruited from the outpatient clinics at Washington University School of Medicine and Barnes-Jewish Hospital, St. Louis. Subjects underwent a single fasting venous blood draw for monocyte isolation and 25(OH)D level measured by liquid chromatography-tandem mass spectrometry (Mayo Medical Laboratories, Rochester, MN).
2.2 Isolation and preparation of humanmonocytes
Peripheral monocytes were isolated by standard Ficoll isolation techniques and selected by cluster of differentiation 14 (CD14) marker positivity (Miltenyi Biotec, Auburn, CA). To closely mimic in vivo conditions, monocytes were maintained in (vitamin D-deficient) 100% serum from the original patient with or without 25(OH)D3 incubation at 150 nM (60 ng/mL) for 24 hours [28]. Monocytes were then also maintained in vitamin D-deficient media [deficient in both 25(OH)D and 1,25(OH)2D; obtained by DMEM plus 10% charcoal/dextran-treated FBS] incubated with or without 25(OH)D3 at 150 nM for 24 hours [26, 29]. In both monocyte conditions 25(OH)D3 induced VDR signaling activation (Supplemental Figure 1).
2.3 Isolation of murine macrophages
Macrophages were isolated after peritoneal normal saline lavage of 16-week-old C57BL/6 mice lacking scavenger receptor A-1 (SR-A1−/−) and WT C57BL/6 mice fed a chow diet. Macrophages were cultured in vitamin D-deficient media incubated with or without 25(OH)D3 at 60ng/mL for 1 week. ER stress was induced by adding Thapsigargin (Sigma, St. Louis, MO) to macrophages for the final 18 hours of culture incubated in 25(OH)D3 media [29, 30].
2.4 Adhesion assays
96-well-plates were coated with fibronectin (Sigma) overnight. Macrophages (0.1 × 105 cells/plate) were added to fibronectin-coated plates and incubated for 4 hours at 37°C. Adhered cells were then washed, fixed in formaldehyde, and stained with Crystal violet. Well absorbance was read at 585 nm [26].
2.5 Migration assays
Transwell migration assays were performed (Costar polycarbonate filters, 5 μm pore size) as we previously described [27]. Membranes and 12-well plates were coated with fibronectin (5μl/mL; Life Technologies, Grand Island, NY) overnight at 4 degrees. Macrophages (0.5 ×105 cells/well) were added to the upper chamber, and MCP-1 (100 ng/well; Sigma) in 0.8% agarose solution was added to the lower chamber to stimulate migration. Cells migrating into the lower chamber after 8 hours of incubation were manually counted.
2.6 Gene expressionand western blot analysis
Quantitative reverse-transcription polymerase chain reaction (qPCR) analyses were performed by Sybrgreen methodologies. Results were normalized to the housekeeping gene L32. Western blot analyses from macrophage protein extracts were normalized to β-actin expression.
2.7 Statistical Analysis
GraphPad Prism software was used for statistical calculations. Descriptive variables were expressed as mean ± SD for continuous data. Experiments were carried out with duplicate or triplicate samples, and all experiments were replicated in multiple sets of samples. Analytic data were expressed as mean ± SEM for continuous variables. Normal distribution of continuous variables was verified graphically. Statistical significance of differences was calculated using t-test for parametric data involving two groups or one-way analysis of variance (ANOVA) followed by Tukey’s post-test for parametric data involving three or more groups. Differences were considered statistically significant if p ≤ 0.05.
3. Results
3.1 25(OH) vitamin D decreases monocyte adhesion to fibronectin and adhesion receptor expression
To determine whether 25(OH) vitamin D regulates monocyte adhesion, we performed functional adhesion assays in monocytes from vitamin D-deficient diabetic patients. Monocytes from vitamin D-deficient diabetics were isolated and cultured for 24 hours in 100% serum from the original patient with or without incubation with 25(OH)D3. Incubation with 25(OH)D3 significantly decreased the adhesion to fibronectin compared to monocytes maintained in vitamin D-deficient patient serum, (Figure 1A, p<0.05). Accordingly, incubation with 25(OH)D3 also decreased mRNA expression levels of adhesion markers β1-integrin and β2-integrin by more than 50% (Figure 1B–C, p<0.001 for both) compared to vitamin D-deficient cells. To confirm the influence of 25(OH)D3 on monocyte adhesion, we also cultured monocytes for 24 hours in vitamin D-deficient media with or without incubation with 25(OH)D3. Similarly to culture conditions with patient serum, 25(OH)D3 significantly suppressed monocyte adhesion to fibronectin, and decreased β1-integrin and β2-integrin expression compared to cells cultured in vitamin D-deficient media (Figure 1D–F, p<0.01 for all). In both monocyte models (incubated in serum or media) 25(OH)D3 suppressed β1-integrin protein expression (Supplemental Figure 2). However, the difference between β1-integrin gene expression in serum monocyte models and the protein expression suggests a posttranslational defect induced by the serum of vitamin D deficient patients (supplemental Figure 2). These data suggest that monocytes from patients with diabetes exhibit increased atherogenic behaviors under vitamin D-deficient conditions that are reversible with 25(OH)D3 supplementation.
Figure 1. 25(OH)D3 suppresses monocyte adhesion in type 2 diabetics.
For A–C, monocytes from vitamin D-deficient type 2 diabetics were cultured in vitamin D-deficient or 25(OH)D3-treated patient serum. A. Adhesion assay showing absorbance of cells adhered to fibronectin (n=12, *p<0.05 vs. vitamin D-deficient). B–C. qPCR for mRNA of adhesion receptors B. β1-integrin and C. β2-integrin (n=6, *p<0.001 for both vs. vitamin D-deficient). For D–F, monocytes from vitamin D-deficient type 2 diabetics were cultured in vitamin D-deficient or 25(OH)D3-treated media. D. Adhesion assay showing absorbance of cells adhered to fibronectin (n=12, *p<0.001 vs. vitamin D-deficient). E–F. qPCR for mRNA of adhesion receptors E. β1-integrin and F. β2-integrin (n=6, *p<0.01 for both vs. vitamin D-deficient).
3.2 25(OH) vitamin D decreases monocyte migration and migration receptor expression
To determine whether 25(OH)D3 regulates monocyte migration, we performed functional assays in monocytes from T2DM cultured one of four conditions: 100% serum from the original patient or vitamin D-deficient media, both with and without incubation with 25(OH)D3. Transwell assays demonstrated at least a 20% reduction in monocyte migration in response to MCP-1 in cells incubated with 25(OH)D3 compared to vitamin D-deficient conditions, regardless of whether cells were cultured in media or patient serum (Figure 2A–B, p<0.005 for both). To further investigate the correlations between vitamin D and monocyte migration, we assessed expression of cell surface migration molecules in monocytes from a subset of cells under each of the four conditions. We found that the mRNA and protein expression of migration receptor CCR2 was significantly suppressed in cells incubated with 25(OH)D3 when compared to vitamin D-deficient conditions for both patient serum and media (Figure 2C–D, p<0.001 for both, and Supplemental Figure 2), supporting our findings of suppression of monocyte adhesion and migration by 25(OH)D3.
Figure 2. 25(OH)D3 suppresses monocyte migration in type 2 diabetics.
Transwell migration assays in monocytes from vitamin D-deficient type 2 diabetics cultured in A. vitamin D-deficient or 25(OH)D3-treated patient serum (n=12, *p<0.001 vs. vitamin D-deficient) and B. vitamin D-deficient or 25(OH)D3-treated media (n=12, *p<0.005 vs. vitamin D-deficient). C–D. qPCR for mRNA of CCR2 from monocytes from vitamin D-deficient type 2 diabetics cultured inC. vitamin D-deficient or 25(OH)D3-treated patient serum and D. vitamin D-deficient or 25(OH)D3-treated media (n=6, *p<0.001 for both vs. vitamin D-deficient).
3.3 25(OH) vitamin D decreases monocyte ER stress and SR-A1 expression
We previously demonstrated that active vitamin D suppresses monocyte adhesion and migration by downregulation of monocyte ER stress [26, 27]. We also found that phenylbutyric acid, a chaperone that inhibits ER stress, suppresses adhesion and migration induced by vitamin D deficiency. To further characterize the mechanisms involved in the regulation of adhesion and migration by 25(OH)D3, we evaluated protein expression of SR-A1, a downstream target of ER stress involved in monocyte adhesion, and ER stress proteins in cultured monocytes under the same four conditions: 100% serum from the original patient or vitamin D-deficient media, both incubated with or without 25(OH)D3. We found that incubation with 25(OH)D3 was sufficient to suppress expression of SR-A1, as well as ER stress proteins phospho-protein kinase RNA-like endoplasmic reticulum kinase (p-PERK), phospho-inositol-requiring enzyme 1 alpha (p-IRE1α), and CEBP homologous protein (CHOP), compared to vitamin D-deficient conditions for both patient serum and media, suggesting that brief incubation with 25(OH)D3 induces monocyte cellular vitamin D signaling and suppresses ER stress (Figure 3).
Figure 3. 25(OH)D3 suppresses monocyte ER stress in type 2 diabetics.

Monocytes from vitamin D-deficient type 2 diabetics were cultured in vitamin D-deficient or 25(OH)D3-treated patient serum or media. Western blot for protein expression of scavenger receptor SR-A1 and ER stress proteins p-PERK, p-IRE1α, and CHOP.
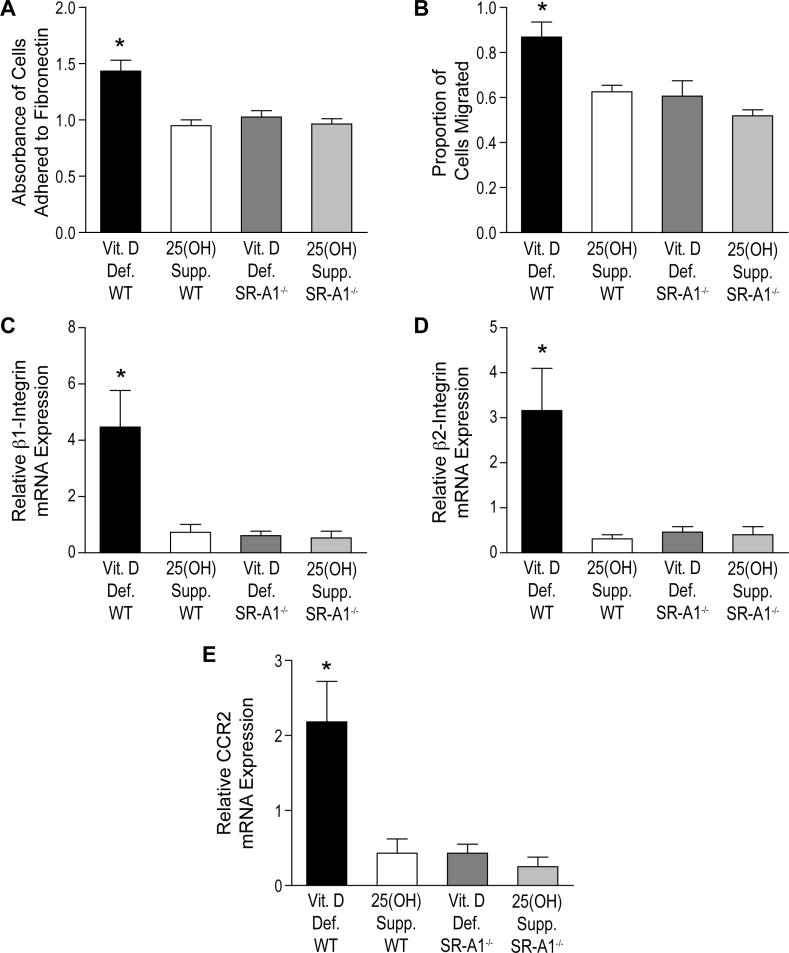
3.4 Absence of SR-A1 prevents increased monocyte adhesion and migration induced by ER stress
It is known that peritoneal macrophages from SR-A1−/− mice display reduced adhesion and migration in vivo [31]. Furthermore, macrophage ER stress regulates SR-A expression [29]. To determine whether 25(OH)D3 decreases monocyte adhesion and migration by downregulation of ER stress and SR-A1 expression, we obtained peritoneal macrophages from SR-A1−/− or WT mice and incubated them in vitamin D-deficient media with or without 25(OH)D3. One week of incubation with 25(OH)D3 suppressed macrophage adhesion to fibronectin by 33% and migration by 28% in WT cells, but had no effect in SR-A1−/− cells, which already showed low adhesion and migration (Figure 4A–B p<0.005 for both). To further determine whether the absence of SR-A1 prevents increased expression of adhesion and migration membrane receptors, we performed quantitative PCR of β1- and β2-integrin and CCR2, demonstrating that WT cells in vitamin D-deficient conditions showed at least 5-fold higher expression than WT cells in 25(OH)-treated conditions or SR-A1−/− cells regardless of the incubation with 25(OH)D3 (Figure 4C–E p<0.01 for all). To clarify whether ER stress stimulation of macrophage adhesion and migration is blunted by the absence of SR-A1, we obtained peritoneal macrophages from SR-A1−/− and WT mice, cultured in 25(OH)D3-treated media for one week, then induced ER stress by adding Thapsigargin for the final 18 hours of culture. Thapsigargin-treated WT macrophages had at least 49% increased adhesion (Figure 5A, p<0.001) and 25% increased migration (Figure 5B, p<0.001) when compared to non-Thapsigargin-treated WT and both groups of SR-A1−/− macrophages, suggesting that the absence of SR-A1 blunts the induction of adhesion and migration by ER stress. However, the absence of SR-A1 did not completely prevent the induction of macrophage migration by Thapsigargin when compared to non-Thapsigargin-treated cells, suggesting possible other contributing mechanisms. Accordingly, Thapsigargin induced expression of adhesion membrane markers β1- and β2-integrin by at least 4-fold and expression of migration membrane receptor CCR2 by at least 6-fold in macrophages from obtained from WT mice when compared to non-Thapsigargin-treated WT or both groups of SR-A1−/− macrophages (Figure 5C–E, p<0.005 for all). These data suggest that downregulation of ER stress by 25(OH)D3 reduced SR-A1 expression and suppressed monocyte adhesion and migration.
Figure 4. SR-A1 knockout suppresses macrophage adhesion and migration induced by vitamin D deficiency.
Peritoneal macrophages were isolated from WT and SR-A1−/− mice after normal saline injection and cultured in vitamin D-deficient or 25(OH)D3-treated media. A. Adhesion assay showing absorbance of cells adhered to fibronectin (n=4 per group, p<0.001 by ANOVA, *p<0.05 vs. all other groups by Tukey’s post-test). B. Transwell migration assay (n=4 per group, p<0.005 by ANOVA, *p<0.05 vs. all other groups by Tukey’s post-test). C– E. qPCR for mRNA of adhesion receptors C. β1-integrin and D. β2-integrin and E. migration receptor CCR2 (n=3 per group, p<0.01 by ANOVA for each, *p<0.05 vs. all other groups by Tukey’s post-test).
Figure 5. SR-A1 knockout suppresses macrophage adhesion and migration induced by ER stress.
Peritoneal macrophages were isolated from WT and SR-A1−/− mice after normal saline injection and cultured in 25(OH)D3-treated media with or without Thapsigargin. A. Adhesion assay showing absorbance of cells adhered to fibronectin (n=4 per group, p<0.001 by ANOVA, *p<0.05 vs. all other groups by Tukey’s post-test). B. Transwell migration assay (n=4 per group, p<0.001 by ANOVA, *p<0.05 vs. all other groups by Tukey’s post-test, **p<0.05 between indicated groups by Tukey’s post-test). C–E. qPCR for mRNA of adhesion receptors C. β1-integrin and D. β2-integrin and E. migration receptor CCR2 (n=3 per group, p<0.005 by ANOVA for each, *p<0.05 vs. all other groups by Tukey’s post-test).
4. Discussion
Multiple observational studies support the potential role of vitamin D in cardiovascular disease. Immune cells express the VDR and are also able to transform 25(OH)D into its active form, which functions as an autocrine/paracrine factor [32]. In vitro studies suggest that active vitamin D decreases adhesion, migration, and cholesterol deposition in macrophages [25–27]. However, the effects of 25(OH)D replacement on atherogenic properties of circulating monocytes in patients with type 2 diabetes are unknown. In this study, we found that 25(OH)D3 treatment suppressed monocyte adhesion and migration by suppressing ER stress and SR-A1 in patients with diabetes. 25(OH)D3 replacement also decreased expression of monocyte adherence proteins β1- and β2-integrin and migration receptor CCR2. The absence of SR-A1 prevented macrophage adhesion and migration induced by vitamin D deficiency by suppressing β1- and β2-integrin and CCR2 expression. Moreover, increased macrophage adhesion and migration induced by ER stress were prevented by the absence of SR-A1. These data suggest that local cellular activation of vitamin D signaling in monocyte/macrophages decreases adhesion and migration by downregulation of ER stress and suppression of SR-A1 expression in patients with diabetes.
Downregulation of macrophage ER stress by 1,25(OH)2D exerts a pivotal role in atherosclerotic plaque development [30, 33]. The absence of vitamin D receptor signaling in mice increases blood pressure by increasing renal renin release and accelerates atherogenesis [33]. Peritoneal macrophages from these mice have higher expression of adhesion markers intercellular adhesion molecule 1 and P-selectin, suggesting potential effects in early atherosclerosis. In patients with diabetes, downregulation of ER stress by 1,25(OH)2D3 generates a monocyte/macrophage with anti-atherogenic properties, preventing monocyte adhesion and migration and suppressing macrophage modified cholesterol uptake and foam cell formation [25, 27, 29]. 25(OH)D deficiency has also been associated with monocyte/macrophage properties that contribute to vascular inflammation and atherosclerosis. In mice, diet-induced vitamin D deficiency increases macrophage ER stress and accelerates atherosclerosis [30]. Previously, we found that monocytes from T2DM patients with 25(OH)D levels below 20 ng/mL have increased adhesion when compared to those from diabetics with 25(OH)D levels >30 ng/mL [26]. However, this is the first study that has evaluated the effects of 25(OH)D3 treatment on atherogenic monocyte/macrophage functions. We have shown that in monocytes from patients with diabetes, brief incubation with 25(OH)D3 suppressed monocyte adhesion and migration and expression of their corresponding membrane receptors by downregulation of ER stress. Therefore, similarly to the effects of 1,25(OH)2D3 on monocyte/macrophage function, 25(OH)D3 seems to prevent early events in the development of atherosclerosis, potentially through local conversion to 1,25(OH)2D3 or independent activation of the VDR.
Diabetes is a major risk factor for atherosclerotic disease. Insulin resistance and the resultant hyperinsulinemia are considered independent risk factors for macrovascular atherosclerotic disease [34]. Macrophages with absence of the insulin receptor have lower insulin-stimulated AKT phosphorylation, increased induction of SR-A1 expression by ER stress, and larger, more complex atherosclerotic lesions [13]. However, the contributions of SR-A1 to mouse models of diet-induced insulin resistance and atherosclerosis have been difficult to reconcile. In the Apolipoprotein E (ApoE) −/− background, mice with the complete absence of SR-A1 show significantly reduced lesion formation compared with ApoE−/− controls [12], while in low density lipoprotein receptor-deficient (LDLR−/−) mice, the absence of SR-A1 only results in marginal lesion reduction, suggesting that SR-A1 may function differently depending on an animal’s genetic background [35]. However, transplant of hematopoietic progenitors from SR-A1−/− mice into lethally radiated C57BL/6 or LDLR−/− mice reduced atherosclerosis formation by 60%, suggesting that SR-A1 expression in macrophages is a key to atherosclerosis prevention [36]. In this study, we found that circulating monocytes from vitamin D-deficient patients with T2DM have increased induction of SR-A1 expression by ER stress and acceleration of monocyte adhesion and migration. The absence of macrophage SR-A1 prevented adhesion and migration and expression of their associated membrane receptors by vitamin D deficiency. Furthermore, SR-A1 knockout suppressed macrophage adhesion and migration by ER stress, suggesting that the downregulation of ER stress and SR-A1 expression by 25(OH)D3 may be a potential treatment to address the increased susceptibility to vascular inflammation seen in diabetics.
5. Conclusion
This study demonstrates that in monocytes from type 2 diabetics with vitamin D deficiency, 25(OH)D3 works as a monocyte/macrophage ER stress reliever, suppressing SR-A1 expression and decreasing adhesion and migration similarly to active vitamin D supplementation. This data suggest that at a cellular level, local hydroxylation of 25(OH)D3 to 1,25(OH)2D3 may be critical to the immunomodulatory and anti-atherogenic effects of vitamin D and lays the mechanistic groundwork for interventional trials of vitamin D replacement in patients with T2DM.
Supplementary Material
25(OH)D3 activates monocyte VDR signaling in type 2 diabetics qPCR for CYP24 mRNA (VDR target gene). Monocytes from vitamin D-deficient type 2 diabetics were cultured in A. patient serum with or without 25(OH)D3 treatment or B. media with or without 25(OH)D3 treatment. (n=4 per group, p<0.005 by t-test).
25(OH)D3 suppresses monocyte β1 integrin and CCR2 protein expression in type 2 diabetics. Monocytes from vitamin D-deficient type 2 diabetics were cultured in vitamin D-deficient or 25(OH)D3-treated patient serum or media. Western blot of β1integrin, CCR2 and β-actin.
Highlights.
25(OH)D suppresses monocyte/macrophage adhesion to fibronectin
25(OH)D reduces monocyte/macrophage migration induced by MCP-1
25(OH)D downregulates monocyte ER stress protein and SR-A1 expression
SR-A1 knockout prevents vitamin D deficiency-induced macrophage adhesion/migration
SR-A1 knockout suppresses ER-stress-induced macrophage adhesion/migration
Acknowledgments
This publication was made possible by the American Diabetes Association 1-12-CT-08, NIH R01HL094818-0, and UL1TR000448/Sub-Award KL2TR000450from the National Center for Advancing Translational Sciences (NCATS), Children Discovery Institute (CDI) CH-II-2012-209. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NIH.
Abbreviations: Abbreviations used include
- CVD
cardiovascular disease
- T2DM
type 2 diabetes mellitus
- 25(OH)D
25-hydroxy vitamin D
- MCP-1
monocyte chemotactic protein 1
- CCR2
chemokine (C-C motif) receptor 2
- ER
endoplasmic reticulum
- SR-A1
scavenger receptor class A, type 1
- M-CSF
macrophage colony-stimulating factor
- VDR
vitamin D receptor
- 1,25(OH)2D
1,25-dihydroxy vitamin D
- CD14
cluster of differentiation 14
- qPCR
quantitative RT-PCR
- p-PERK
phospho-protein kinase RNA-like endoplasmic reticulum kinase
- p-IRE1α
phospho-inositol-requiring enzyme 1 alpha
- CHOP
CEBP homologous protein
- ApoE
Apolipoprotein E
- LDLR
low density lipoprotein receptor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Amy E. Riek, Email: ariek@dom.wustl.edu.
Jisu Oh, Email: joh@dom.wustl.edu.
Isra Darwech, Email: idarwech@dom.wustl.edu.
Clare E. Moynihan, Email: cbernal@dom.wustl.edu.
References
- 1.National diabetes fact sheet: general information and national estimates on diabetes in the United States. Centers for Disease Control and Prevention; 2011. pp. 1–12. [Google Scholar]
- 2.National Diabetes Statistics; N.I.o. Health, editor. NIH publication 08-3892. 2007. pp. 1–24. [Google Scholar]
- 3.Binder CJ, Chang MK, Shaw PX, Miller YI, Hartvigsen K, Dewan A, Witztum JL. Innate and acquired immunity in atherogenesis. Nat Med. 2002;8(11):1218–1226. doi: 10.1038/nm1102-1218. [DOI] [PubMed] [Google Scholar]
- 4.Lusis AJ. Atherosclerosis. Nature. 2000;407(6801):233–241. doi: 10.1038/35025203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moore KJ, Tabas I. Macrophages in the pathogenesis of atherosclerosis. Cell. 2011;145(3):341–355. doi: 10.1016/j.cell.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Woollard KJ, Geissmann F. Monocytes in atherosclerosis: subsets and functions. Nat Rev Cardiol. 2010;7(2):77–86. doi: 10.1038/nrcardio.2009.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tabas I. Macrophage death and defective inflammation resolution in atherosclerosis. Nature reviews Immunology. 2010;10(1):36–46. doi: 10.1038/nri2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hansson GK, Libby P. The immune response in atherosclerosis: a double-edged sword. Nature reviews Immunology. 2006;6(7):508–519. doi: 10.1038/nri1882. [DOI] [PubMed] [Google Scholar]
- 9.de Villiers WJ, Smith JD, Miyata M, Dansky HM, Darley E, Gordon S. Macrophage phenotype in mice deficient in both macrophage-colony-stimulating factor (op) and apolipoprotein E. Arterioscler Thromb Vasc Biol. 1998;18(4):631–640. doi: 10.1161/01.atv.18.4.631. [DOI] [PubMed] [Google Scholar]
- 10.Rajavashisth T, Qiao JH, Tripathi S, Tripathi J, Mishra N, Hua M, Wang XP, Loussararian A, Clinton S, Libby P, Lusis A. Heterozygous osteopetrotic (op) mutation reduces atherosclerosis in LDL receptor- deficient mice. J Clin Invest. 1998;101(12):2702–2710. doi: 10.1172/JCI119891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fraser I, Hughes D, Gordon S. Divalent cation-independent macrophage adhesion inhibited by monoclonal antibody to murine scavenger receptor. Nature. 1993;364(6435):343–346. doi: 10.1038/364343a0. [DOI] [PubMed] [Google Scholar]
- 12.Suzuki H, Kurihara Y, Takeya M, Kamada N, Kataoka M, Jishage K, Ueda O, Sakaguchi H, Higashi T, Suzuki T, Takashima Y, Kawabe Y, Cynshi O, Wada Y, Honda M, Kurihara H, Aburatani H, Doi T, Matsumoto A, Azuma S, Noda T, Toyoda Y, Itakura H, Yazaki Y, Kodama T, et al. A role for macrophage scavenger receptors in atherosclerosis and susceptibility to infection. Nature. 1997;386(6622):292–296. doi: 10.1038/386292a0. [DOI] [PubMed] [Google Scholar]
- 13.Han S, Liang CP, DeVries-Seimon T, Ranalletta M, Welch CL, Collins-Fletcher K, Accili D, Tabas I, Tall AR. Macrophage insulin receptor deficiency increases ER stress-induced apoptosis and necrotic core formation in advanced atherosclerotic lesions. Cell Metab. 2006;3(4):257–266. doi: 10.1016/j.cmet.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 14.el Khoury J, Thomas CA, Loike JD, Hickman SE, Cao L, Silverstein SC. Macrophages adhere to glucose-modified basement membrane collagen IV via their scavenger receptors. J Biol Chem. 1994;269(14):10197–10200. [PubMed] [Google Scholar]
- 15.Devries-Seimon T, Li Y, Yao PM, Stone E, Wang Y, Davis RJ, Flavell R, Tabas I. Cholesterol-induced macrophage apoptosis requires ER stress pathways and engagement of the type A scavenger receptor. J Cell Biol. 2005;171(1):61–73. doi: 10.1083/jcb.200502078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pittas AG, Chung M, Trikalinos T, Mitri J, Brendel M, Patel K, Lichtenstein AH, Lau J, Balk EM. Systematic review: Vitamin D and cardiometabolic outcomes. Ann Intern Med. 152(5):307–314. doi: 10.1059/0003-4819-152-5-201003020-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang L, Manson JE, Song Y, Sesso HD. Systematic review: Vitamin D and calcium supplementation in prevention of cardiovascular events. Ann Intern Med. 152(5):315–323. doi: 10.7326/0003-4819-152-5-201003020-00010. [DOI] [PubMed] [Google Scholar]
- 18.Melamed ML, Michos ED, Post W, Astor B. 25-hydroxyvitamin D levels and the risk of mortality in the general population. Arch Intern Med. 2008;168(15):1629–1637. doi: 10.1001/archinte.168.15.1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cigolini M, Iagulli MP, Miconi V, Galiotto M, Lombardi S, Targher G. Serum 25-hydroxyvitamin D3 concentrations and prevalence of cardiovascular disease among type 2 diabetic patients. Diabetes Care. 2006;29(3):722–724. doi: 10.2337/diacare.29.03.06.dc05-2148. [DOI] [PubMed] [Google Scholar]
- 20.Isaia G, Giorgino R, Adami S. High prevalence of hypovitaminosis D in female type 2 diabetic population. Diabetes Care. 2001;24(8):1496. doi: 10.2337/diacare.24.8.1496. [DOI] [PubMed] [Google Scholar]
- 21.Polla BS, Healy AM, Amento EP, Krane SM. 1,25-Dihydroxyvitamin D3 maintains adherence of human monocytes and protects them from thermal injury. J Clin Invest. 1986;77(4):1332–1339. doi: 10.1172/JCI112438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hewison M, Dabrowski M, Faulkner L, Hughson E, Vadher S, Rut A, Brickell PM, O’Riordan JL, Katz DR. Transfection of vitamin D receptor cDNA into the monoblastoid cell line U937. The role of vitamin D3 in homotypic macrophage adhesion. J Immunol. 1994;153(12):5709–5719. [PubMed] [Google Scholar]
- 23.Dickhout JG, Brooke H, Basseri S, Lhotak S, SKS, Austin RC. Macrophage Differentiation Involves Activation of the Unfolded Protein Response. FASEB. 2008;22:924.911. [Google Scholar]
- 24.Schaefer A, Magocsi M, Stocker U, Kosa F, Marquardt H. Early transient suppression of c-myb mRNA levels and induction of differentiation in Friend erythroleukemia cells by the [Ca2+]i-increasing agents cyclopiazonic acid and thapsigargin. J Biol Chem. 1994;269(12):8786–8791. [PubMed] [Google Scholar]
- 25.Oh J, Weng S, Felton SK, Bhandare S, Riek A, Butler B, Proctor BM, Petty M, Chen Z, Schechtman KB, Bernal-Mizrachi L, Bernal-Mizrachi C. 1,25(OH)2 vitamin d inhibits foam cell formation and suppresses macrophage cholesterol uptake in patients with type 2 diabetes mellitus. Circulation. 2009;120(8):687–698. doi: 10.1161/CIRCULATIONAHA.109.856070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Riek AE, Oh J, Sprague JE, Timpson A, de las Fuentes L, Bernal-Mizrachi L, Schechtman KB, Bernal-Mizrachi C. Vitamin D suppression of endoplasmic reticulum stress promotes an antiatherogenic monocyte/macrophage phenotype in type 2 diabetic patients. J Biol Chem. 2012;287(46):38482–38494. doi: 10.1074/jbc.M112.386912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Riek AE, Oh J, Bernal-Mizrachi C. 1,25(OH)(2) vitamin D suppresses macrophage migration and reverses atherogenic cholesterol metabolism in type 2 diabetic patients. J Steroid Biochem Mol Biol. 2013 doi: 10.1016/j.jsbmb.2012.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hewison M, Burke F, Evans KN, Lammas DA, Sansom DM, Liu P, Modlin RL, Adams JS. Extra-renal 25-hydroxyvitamin D3-1alpha-hydroxylase in human health and disease. J Steroid Biochem Mol Biol. 2007;103(3–5):316–321. doi: 10.1016/j.jsbmb.2006.12.078. [DOI] [PubMed] [Google Scholar]
- 29.Oh J, Riek AE, Weng S, Petty M, Kim D, Colonna M, Cella M, Bernal-Mizrachi C. Endoplasmic reticulum stress controls M2 macrophage differentiation and foam cell formation. J Biol Chem. 2012;287(15):11629–11641. doi: 10.1074/jbc.M111.338673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weng S, Sprague JE, Oh J, Riek AE, Chin K, Garcia M, Bernal-Mizrachi C. Vitamin D deficiency induces high blood pressure and accelerates atherosclerosis in mice. PLoS One. 2013;8(1):e54625. doi: 10.1371/journal.pone.0054625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.de Winther MP, van Dijk KW, Havekes LM, Hofker MH. Macrophage scavenger receptor class A: A multifunctional receptor in atherosclerosis. Arterioscler Thromb Vasc Biol. 2000;20(2):290–297. doi: 10.1161/01.atv.20.2.290. [DOI] [PubMed] [Google Scholar]
- 32.Adams JS, Ren S, Liu PT, Chun RF, Lagishetty V, Gombart AF, Borregaard N, Modlin RL, Hewison M. Vitamin d-directed rheostatic regulation of monocyte antibacterial responses. J Immunol. 2009;182(7):4289–4295. doi: 10.4049/jimmunol.0803736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Szeto FL, Reardon CA, Yoon D, Wang Y, Wong KE, Chen Y, Kong J, Liu SQ, Thadhani R, Getz GS, Li YC. Vitamin d receptor signaling inhibits atherosclerosis in mice. Mol Endocrinol. 2012;26(7):1091–1101. doi: 10.1210/me.2011-1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pirro M, Mauriege P, Tchernof A, Cantin B, Dagenais GR, Despres JP, Lamarche B. Plasma free fatty acid levels and the risk of ischemic heart disease in men: prospective results from the Quebec Cardiovascular Study. Atherosclerosis. 2002;160(2):377–384. doi: 10.1016/s0021-9150(01)00588-3. [DOI] [PubMed] [Google Scholar]
- 35.Sakaguchi H, Takeya M, Suzuki H, Hakamata H, Kodama T, Horiuchi S, Gordon S, van der Laan LJ, Kraal G, Ishibashi S, Kitamura N, Takahashi K. Role of macrophage scavenger receptors in diet-induced atherosclerosis in mice. Lab Invest. 1998;78(4):423–434. [PubMed] [Google Scholar]
- 36.Babaev VR, Gleaves LA, Carter KJ, Suzuki H, Kodama T, Fazio S, Linton MF. Reduced atherosclerotic lesions in mice deficient for total or macrophage-specific expression of scavenger receptor-A. Arterioscler Thromb Vasc Biol. 2000;20(12):2593–2599. doi: 10.1161/01.atv.20.12.2593. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
25(OH)D3 activates monocyte VDR signaling in type 2 diabetics qPCR for CYP24 mRNA (VDR target gene). Monocytes from vitamin D-deficient type 2 diabetics were cultured in A. patient serum with or without 25(OH)D3 treatment or B. media with or without 25(OH)D3 treatment. (n=4 per group, p<0.005 by t-test).
25(OH)D3 suppresses monocyte β1 integrin and CCR2 protein expression in type 2 diabetics. Monocytes from vitamin D-deficient type 2 diabetics were cultured in vitamin D-deficient or 25(OH)D3-treated patient serum or media. Western blot of β1integrin, CCR2 and β-actin.