Abstract
Racial/ethnic minorities are underrepresented in current biobanking programs. The current study utilized community-based participatory research to identify motivating factors and barriers that affect older African Americans’ willingness to donate biospecimens. The standardized phone survey was administered to 78 African Americans who are 55 years old or older and live in the metropolitan Detroit area to assess their overall willingness to donate biospecimens and what factors were associated with it. The majority of the participants were willing to donate biospecimens, along with their personal information, for medical research and indicated that they did donate biospecimens when they were asked. However, African Americans were rarely asked to participate in biobanking programs. Furthermore, African Americans were not as concerned with research exploitation or as mistrusting of medical researchers as previously thought by the medical researchers. Even if African Americans were concerned over potential research exploitation or mistrust of medical researchers, these concerns or mistrust did not translate into an actual unwillingness to participate in biobanking programs. Rather, transparency in medical research and biobanking programs was more important when predicting African Americans’ willingness to donate biospecimens for medical research. The findings suggest that underrepresentation of African Americans in current biobanking programs may not be due to their willingness/unwillingness to participate in such programs, but rather due to a failure of medical researchers to approach them. Additionally, researchers and clinicians should focus on increasing the transparency of medical research and biobanking programs rather than changing African Americans’ potential negative attitudes toward them.
Keywords: African American underrepresentation, Biospecimen collection, Biobanks, Community-based participatory research, Motivating factors, Barriers
Use of biospecimens, such as tumors, blood, and saliva, donated by patients with cancer and population-based controls is critical to the advancement of cancer research and the development of new treatments [1–4]. However, it requires cancer researchers to spend tremendous time, effort, and money to collect and store biospecimens from a large enough sample of individuals to be sufficiently powered for rigorous analysis. In order to facilitate researchers’ access to large samples of biospecimens, many medical research institutions in the US, including the National Cancer Institute (NCI) and the National Human Genome Research Institute (NHGRI), have been making an effort to establish large-scale biobanking programs [5, 6]. Biobanks store biospecimens along with electronic data containing demographic, personal, and medical information that are relevant to cancer research; thus allowing cancer researchers to efficiently access large numbers of existing biospecimens and data [7, 8].
One important area of cancer research, among many, is the examination of biological and tumor variations by race/ethnicity and how they contribute to cancer disparities. In such research, it is critical for researchers to have access to large samples of biospecimens donated by individuals from a variety of racial/ethnic groups [9]. It has been documented, however, that racial/ethnic minorities are underrepresented in current biobanking programs. For instance, according to the March, 2012 report of The Cancer Genome Atlas (TCGA), which is a biobanking program developed and supported by NCI/NHGRI, only 9.94% of the 4959 cases in TCGA were collected from racial/ethnic minorities (American Indian/Alaska Native .18%, Asian 3.35%, Black/African American 6.25%, Native Hawaiian/Other Pacific Islander .16%) [5]. Thus, it appears that access to adequate numbers of biospecimens from racial/ethnic minorities is an ongoing problem for cancer researchers.
Addressing the underrepresentation of racial/ethnic minorities in biobanking programs is critical because lack of representation can contribute to the development and maintenance of cancer disparities. Thus, in order to reduce cancer disparities, it is essential to understand factors that either encourage (i.e., motivating factors) or discourage (i.e., barriers) racial/ethnic minorities to donate biospecimens to biobanks. Once motivators and barriers are identified, this information can be used to develop potential interventions aimed at increasing racial/ethnic minorities’ willingness to donate biospecimens to biobanks. The goal of the current study was to conduct a community-based participatory research study to identify motivating factors and barriers that affect older African Americans’ willingness to donate biospecimens. The study focused exclusively on older African Americans given their disproportionate cancer burden [10–13].
The development of biobanking programs is relatively new in the US [14–16], and research on the underrepresentation of African Americans in biobanks is still limited. Consequently, some studies looking at potential factors that may affect African Americans’ willingness to participate in biobanking programs have drawn upon the literature of underrepresentation of African Americans in clinical trials [17–19], which has a longer research history and shares some common characteristics with research related to biobanking programs. One important factor that has been consistently shown to affect African Americans’ willingness to participate in clinical trials is fear of research exploitation, which is rooted in past unethical medical research involving African Americans [20–26]. Fear of potential research exploitation (e.g., being guinea pigs) has also been found to be a major barrier in participation in biobanking programs among African Americans in recent studies [18, 19, 27].
Drawing upon these findings, Kiviniemi and colleagues (2013) have developed an intervention in which one of the aims was to reduce negative affect potentially associated with biobanking programs, and as a result, they have successfully increased participant’s willingness to participate in biobanking programs [27]. As mentioned earlier, however, research on the motivating factors and barriers that affect African Americans in biobanking programs is still in its infancy, and automatically generalizing the findings from the dominant research areas to the new area of research without careful empirical scrutiny can sometimes lead to bias and inaccuracies. For instance, how the topics for focus groups were selected and/or how these topics were framed and asked would likely have been influenced by the literature of underrepresentation of African Americans in clinical trials.
The present study systematically examined whether fear of potential research exploitation is indeed associated with African Americans’ willingness to participate in biobanking programs. The study also explored other motivating factors and barriers that were found to influence African Americans’ willingness to participate in biobanking programs in previous focus group studies [18, 19].
Method
Participants
Seventy-eight participants, who are 55 years old or older and live in the metropolitan Detroit area, participated in this study from April to December, 2012. The participants were recruited via a research registry developed and maintained by the Karmanos Cancer Institute’s community research collaborative, Southeast Michigan Partners Against Cancer (SEMPAC) community partner, the Wayne State University Institute of Gerontology’s Healthier Black Elders Center (HBEC).[28] Because we were particularly interested in African Americans’ willingness to participate in biobanking programs, we excluded five participants who did not self-identify as African American (3 American Indian/Alaskan Native, 1 Multiracial, 1 unspecified) from the analyses, resulting in 73 analyzable cases. Table 1 displays demographic characteristics of the participants included in the final analyses.
Table 1.
Participant characteristics
| M ± SD or No. (%) | |
|---|---|
| Age (Missing = 1) | 71.82 ± 8.34 |
| Gender | |
| Female | 42 (57.5) |
| Male | 31 (42.5) |
| Education | |
| ≤ High School | 3 (4.1) |
| Completed High School | 15 (20.5) |
| Attended some college | 21 (28.8) |
| Completed college | 21 (28.8) |
| Completed a post graduate degree | 13 (17.8) |
| Income (Missing = 13) | |
| ≤ $20,000 | 14 (19.2) |
| $20,000 – $39,999 | 24 (32.9) |
| $40,000 – $59,999 | 13 (17.8) |
| ≥ $60,000 | 9 (12.2) |
| Marital Status | |
| Single | 4 (5.5) |
| Married | 31 (42.5) |
| Separated | 3 (4.4) |
| Divorced | 14 (19.2) |
| Widowed | 21 (28.8) |
Standardized Survey
The standardized survey was designed to assess individual’s overall willingness to donate biospecimens for medical research and what factors were associated with it. More specifically, the survey included demographic information, personal and family health history, and measures of perceived potential motivators and barriers. A total of 95 items were included in the survey. Because we were concerned about the generalizability of the findings, the majority of the items were close-ended questions. The survey was developed with the guidance of a community-based research advisory committee. The committee members ensured that the questions asked as well as the language used in the survey were relevant and meaningful to community members.
Procedure
The study procedures and the standardized survey were reviewed and approved by Karmanos Cancer Institute and Wayne State University Institutional Review Boards. Letters were sent to all potential participants on a randomly generated list provided by the HBEC research registry. These letters briefly described the project and informed potential participants that a research assistant would be calling to determine if they were interested in participating in the study. The letter also provided telephone numbers to opt in or opt out. Five interviewers (4 African Americans, 1 White American) called individuals on the list. If an individual expressed an interest in participating in the standardized phone survey, the interviewer read the information sheet that fully explained the purpose and procedures of the study. Once the individual verbally consented to participate in the study, the interviewer began the survey by first providing the participant with brief definitions of biospecimens, biospecimen collection, and biospecimen banking. In the survey, participants were asked to respond to each item by using multiple choice or Likert-type scales provided by the interviewer. The majority of the participants took approximately 45 minutes to complete the entire standardized phone survey. They received a $10 gift card for participating in the survey.
Measures
Although the standardized survey included a number of measures, we will only report the measures that were included in the present analyses (see Table 2). Participants’ willingness was assessed with a single item asking them to indicate how willing they were to donate their biospecimens for medical research, using a five-point Likert scale ranging from 1 (very unwilling) to 5 (very willing). If participants indicated that they were willing to donate biospecimens, they were further asked to indicate (1) what type of biospecimen (e.g., blood, hair, and nails) they were willing to donate, and (2) what personal information they were willing to provide along with their biospecimen (e.g., age, health history, and home address). They were also asked to report whether they have ever been asked to donate a biospecimen for medical research, and if so, whether or not they chose to donate.
Table 2.
Items used to assess willingness, experience with biospecimen donation, fear of research exploitation, and trust
Willingness
|
Experience with biospecimen donation
|
Fear of research exploitation
|
Trust in medical researchers
|
Trust in biobanks
|
Willingness after barriers are addressed
|
Fear of potential research exploitations were assessed with three items. Specifically, participants were asked to indicate whether they were concerned that: (1) they would be treated like guinea pigs in medical research; (2) medical researchers would use contaminated equipment to collect biospecimens; and (3) if something like the Tuskegee Trial could happen again, using a five-point Likert scale ranging from 1 (strongly disagree) to 5 (strongly agree). Higher scores indicated greater fear of research exploitation.
Trust in medical researchers was assessed with a 5-item modified version of a medical trust measure developed by Hall and colleagues (α = .61) [29]. Trust in biobanks was also assessed with a modified version of Hall et al.’s (2002) medical trust measure, but only with four items (α = .67). The scale ranged from 1 (strongly disagree) to 5 (strongly agree), with higher scores indicating more trust.
Finally, participants were asked to indicate whether or not they would be willing to donate biospecimens if some of the barriers associated with biobanking programs were adequately addressed. These barriers included lack of transparency of information, privacy concerns, and lack of incentives. Some items were assessed on a four-point Likert scale ranging from 1 (very unlikely) to 4 (very likely), whereas others were assessed on a five-point Likert scale ranging from 1 (strongly disagree) to 5 (strongly agree). Regardless of the scale, higher scores indicated more willingness to donate biospecimens for medical research.
Analysis
First, we conducted descriptive statistics to examine how many participants expressed willingness to donate biospecimens for medical research as well as their past experience with biospecimen donation. In the next set of analyses, we conducted one-sample t-tests, with a midpoint on the 5-point Likert-scale as a reference point (i.e., score 3), to assess participants’ levels of concerns over research exploitation and mistrust. Because multiple comparisons were conducted (i.e., five separate t-tests), Bonferroni correction was used to control for the familywise error rate [30]. More specifically, for one-sample t-tests, we set the significance level at α = .01. Following the one-sample t-tests, we computed simple product-moment correlations between participant willingness, concerns over research exploitation, and mistrust to examine whether or not concerns over research exploitation and mistrust would predict African Americans’ willingness to donate biospecimens for medical research. Finally, one-sample t-tests, with midpoints on either the four-point or the five-point Likert scale as reference points (i.e., 2.5 and 3.0, respectively), were conducted to examine participants’ willingness to donate biospecimens once some previously identified barriers have been addressed. Again, in order to control for the familywise error rate due to multiple comparisons (i.e., seven separate t-tests), we set the significance level at α = .0071.
Results
Willingness and Past Experience with Biospecimen Donation
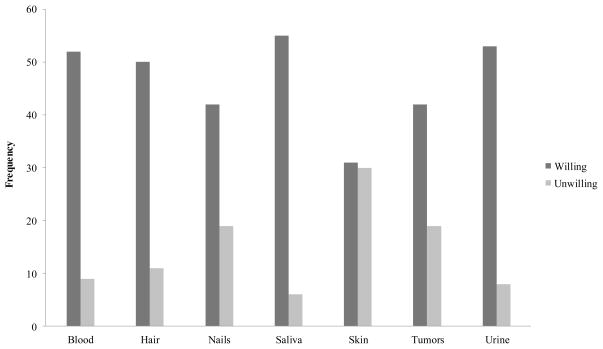
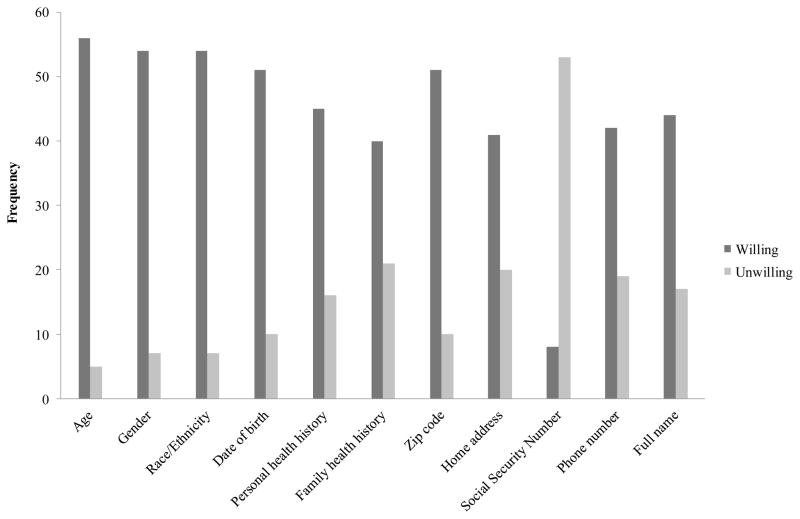
In spite of the fact that African Americans are grossly underrepresented in the current biobanking programs, the majority (n = 58, 80.6%) of African American participants expressed their willingness to donate biospecimens for medical research (Table 3). Of those who expressed willingness, the majority of participants were willing to donate a variety of biospecimens from blood to tumor, with the exception of skin. About half of participants expressed unwillingness to donate a skin sample (see Figure 1). Additionally, the majority of participants were willing to provide a wide range of personal information, including identifiable information, such as home address, phone number, and even full name, but most were unwilling to share their social security numbers (Figure 2). Taken together, it appears that, in general, older African Americans are willing to provide many types of biospecimens and personal information along with their biospecimens for the purposes of medical research. However, further analysis showed that, despite their willingness, only 13 participants (17.8%) had ever been asked to donate biospecimens. Furthermore, of those 13 participants who had been asked to donate biospecimens, everyone except one participant actually donated a biospecimen for medical research, reflecting a high level of general acceptance of biobanking programs among African Americans.
Table 3.
Willingness to donate biospecimens for medical research
| Frequency | Percent | |
|---|---|---|
| Very unwilling | 6 | 8.3 |
| Somewhat unwilling | 5 | 6.9 |
| Neither unwilling nor willing | 3 | 4.2 |
| Somewhat willing | 30 | 41.7 |
| Very willing | 28 | 38.9 |
Note. M = 4.00, SD = 1.22, and missing = 1.
Fig 1.
Type of biospecimens older African Americans are willing to donate
Fig 2.
Type of personal information older African Americans are willing to provide along with their biospecimen
Fear of Research Exploitation, Mistrust, and Willingness
One-sample t-tests revealed that African American participants were concerned that something like the Tuskegee Trial could happen again (M = 3.79, SD = 1.44, t(67) = 4.54, SEM = .18, p < .0001). There was also a trend related to concerns about being treated as guinea pigs in medical research (M = 2.66, SD = 1.58, t(70) = −1.81, SEM = .19, p = .08) or that medical researchers would use contaminated equipment to collect biospecimens (M = 2.62, SD = 1.53, t(70) = −2.09, SEM = .18, p = .04). However, it should be noted that these results are not statistically significant once we controlled for the familywise error rate by setting the significance level at α = .0071. Overall, these findings suggest that African Americans do not appear as concerned with potential research exploitation as previously reported in the context of biobanking programs. In terms of mistrust, participants tended to express trust in both medical researchers and biobanks, M = 3.23, SD = .79, t(72) = 2.55, SEM = .09, p = .01 and M = 3.43, SD = .87, t(72) = 4.23, SEM = .10, p < .0001, respectively. Again, these findings suggest that African Americans may be more trusting of medical researchers and biobanks than previously speculated.
Our analysis of correlations revealed that none of the concerns over research exploitation were associated with participant willingness to donate biospecimens for medical research (r’s < .14, p’s > .23), nor was trust in medical researchers associated with willingness, (r = .18, p = .13). However, there was a marginally significant correlation between trust in biobanks and willingness, such that those who expressed more trust in biobanks tended to express higher willingness to donate biospecimens for medical research (r = .17, p = .01). The summary of these findings is presented in Table 4.
Table 4.
Descriptive statistics of and correlations among concerns over research exploitation, mistrust, and willingness.
| 1 | 2 | 3 | 4 | 5 | 6 | |
|---|---|---|---|---|---|---|
|
|
||||||
| M (SD) | 4.00 (1.22) | 2.66 (1.58) | 2.62 (1.53) | 3.79 (1.44) | 3.23 (.79) | 3.43 (.87) |
|
|
||||||
| 1. Willingness | -- | |||||
| 2. Guinea pigs | .06 (70) | -- | ||||
| 3. Contaminated equipment | −.03 (70) | .29* (70) | -- | |||
| 4. Tuskegee | .12 (67) | .15 (67) | .45*** (67) | -- | ||
| 5. Trust_medical researchers | .18 (72) | −.42*** (71) | −.36** (71) | −.45*** (68) | -- | |
| 6. Trust_biobanks | .20† (72) | −.37*** (71) | −.30* (71) | −.43*** (68) | .69*** (73) | -- |
Note. Values in the parentheses indicate the sample sizes on which correlations were computed.
indicates p < .01,
indicates p < .05,
indicates p < .01, and
indicates p < .001.
Willingness Once Barriers Are Addressed
One-sample t-tests revealed that transparency of information was an important factor that appears to influence African American participants’ willingness to donate biospecimens. More specifically, researchers should fully explain who they are, why they are asking people to donate biospecimens, how they intend to use biospecimens in their research, how they protect donors’ privacy, and the anticipated results of the research in order to increase African Americans’ willingness to participate. Contrary to previous research, addressing the lack of monetary incentives did not appear to increase African Americans’ willingness to donate biospecimens for medical research. Interestingly, however, participants reported that they would be willing to donate biospecimens in return for a health service, such as a physical checkup and dental work. Table 5 presents a summary of these findings.
Table 5.
Willingness after barriers are addressed
| M | SD | Reference Point | t | df | SEM | Sig. | |
|---|---|---|---|---|---|---|---|
| Lack of Transparency | |||||||
| Who medical researchers are | 3.21 | .86 | 2.5 | 6.73 | 66 | .11 | < .001 |
| Why being asked to donate | 3.42 | .78 | 2.5 | 9.94 | 71 | .09 | < .001 |
| How biospecimens are used | 3.34 | .91 | 2.5 | 7.77 | 70 | .11 | < .001 |
| How personal information is protected | 3.34 | .84 | 2.5 | 8.37 | 70 | .10 | < .001 |
| What the research results are | 4.14 | 1.38 | 3.0 | 7.05 | 72 | .16 | < .001 |
| Incentives | |||||||
| Money | 3.32 | 1.52 | 3.0 | 1.79 | 71 | .18 | .078 |
| Health service | 3.93 | 1.54 | 3.0 | 5.06 | 69 | .18 | < .001 |
Discussion
The primary goal of the present study was to systematically examine factors that influence older African Americans’ willingness to participate in biospecimen collection and biobanking by using a standardized phone survey. In doing so, we specifically focused on several factors that were found to play an important role in determining people’s attitudes toward the biobanking programs in previous research, including concerns over research exploitation, mistrust, lack of transparency, privacy concerns, and lack of incentives. Our findings suggest that the majority of African Americans are willing to donate different types of biospecimens for medical research as well as sharing personal information along with their biospecimens and that they do donate biospecimens when they are asked. However, it appears that African Americans are rarely asked to participate in biobanking programs. These findings allude to a possibility that underrepresentation of African Americans in current biobanking programs may not be due to their willingness/unwillingness to participate in such programs, but rather due to a failure of medical researchers to approach them. Researchers often assume that there is hesitancy and resistance among African Americans to participate in medical research due to fear or anger of past research exploitation [27, 31]. For instance, Simmonds (2008) claimed that “[a] common complaint of health researchers is that minorities and specifically African Americans are unwilling to participate in health research therefore there is lack of information regarding disease processes and progression in this segment of the population” (p. 69) [26]. The expectation that African Americans would refuse to participate in biobanking programs even when they are asked may discourage medical researchers from approaching them, resulting in a self-fulfilling prophecy.
The present study provides two important pieces of evidence that discredit the negative presumptions about African Americans commonly held by medical researchers. First, our findings suggest that African Americans may not be as concerned with research exploitation or as mistrusting of medical researchers as previously thought by the medical researchers [18, 19, 27, 31]. We had three items assessing African Americans’ concerns over potential research exploitation and a measure assessing their trust of medical researchers, but only one item, the concern that something like the Tuskegee Trial could happen again, came out to be statistically significant. Concern about being treated as guinea pigs was not statistically significant, even though this concern has been consistently found to be one of the major barriers discouraging African Americans from participating in biobanking programs in previous focus group studies [18, 19]. The discrepancy between the previous findings and the current findings may be due to the limited generalizability of focus group findings. Our findings also suggest that even if African Americans were concerned over potential research exploitation or mistrust of medical researchers, these concerns or mistrust do not translate into an actual unwillingness to participate in biobanking programs. We speculate that fear of research exploitation or mistrust of medical researchers may be more influential factors in deciding whether to participate in clinical trials [20, 21, 32, 33] than in biobanking programs. This is because donating biospecimens for biobanks is often a single event. In contrast, in clinical trials, participants are often required to take part in repeated medical procedures and/or receive non-standard treatment. In these situations, it is conceivable that African Americans may feel like they could be “experimented on” if they do not trust medical researchers. We are not suggesting that it is unimportant to address African Americans’ concerns about trust when recruiting African Americans into biobanking programs, but rather we are arguing that just because some African Americans express concerns or are less trusting of medical researchers does not mean they are unwilling to participate.
Finally, the present study provides further information as to what factors are associated with African Americans’ willingness to participate in the biobanking programs. Our findings suggest that transparency in medical research and biobanking programs is important when predicting African Americans’ willingness to donate biospecimens for medical research. More specifically, medical researchers should fully explain who they are (e.g., education background, medical training, research expertise/records), why they are asking African Americans to donate, how the collected biospecimens will be used in their research, and how biobanks ensure the privacy of personal information associated with the biospecimens, all at the time of recruitment. Researchers should also express full commitment to sharing the results of the project after the research study is complete. These findings are consistent with previous studies suggesting the importance of participant education [18, 27] and further support the NCI’s emphasis and efforts on the development of educational communication priming tools [19, 34]. Finally, the present study suggests that providing health services (e.g., physical check-ups, dental work) may be more effective at increasing African Americans’ willingness to donate biospecimens for medical research than providing standard monetary incentives.
Although the present study greatly contributes to the literature on underrepresentation of African Americans in biobanking programs, it is not without certain limitations. One important limitation is that it assessed African Americans’ willingness to donate biospecimens for medical research, but not whether or not they were actually going to donate biospecimens for medical research when asked to do so. Research has shown that intention and behavior are not always consistent and that people often do not have insights into how they actually behave in given situations [35, 36]. Thus, it is critical for future research to examine whether the findings from the present study can be used to predict the actual donating behavior of African Americans. Another potential limitation is a self-selection bias. The participants in the current study were all recruited through our community partner HBEC’s research registry. HBEC aims to improve the health of older African Americans in Detroit, and provides a wide range of educational health workshops. Thus, individuals who signed up for this research registry tended to have relatively high levels of education and were likely to be inherently more interested in health and medical research than the average population. Although this bias cannot account for the association between willingness and several factors we found in the present study and also although the generalizability of this particular registry for minority health research has been tested [37], it may have resulted in an overestimation of the number of African Americans who are willing to participate in biobanking programs. Future research should include multiple samples of African Americans from different community organizations.
Notwithstanding these potential limitations, the current findings have important implications for the development of effective interventions that aim to further encourage African Americans to participate in biobanking programs. Interventions should focus on increasing transparency of medical research and biobanking programs rather than changing African Americans’ potential negative attitudes toward them (e.g., fear of research exploitation or mistrust). This is not only more effective as our findings suggest, but also more practical because changing negative attitudes to positive attitudes is rather difficult [38, 39], and even if it was possible it happens over time [40, 41]. Protocols should be developed that minimize potential researchers’ bias against African Americans (or any racial/ethnic minorities) and ensure that all eligible individuals are uniformly and systematically approached and asked to participate in the biobanking programs regardless of their racial/ethnic background. The findings from this study indicate that, given African American’s willingness to provide biospecimens, it may be possible to increase African American’s participation in biobanking simply by making a greater effort to ask them.
Acknowledgments
We would like to thank the members of our community-based Research Advisory Committee, Detroit Area Agency on Aging, Interfaith Health and Hope Coalition, and Pro-Literacy Detroit, for their strong commitment and significant contribution to this project. This project was supported by NCI center grants (U54 CA153606-03, U01CA114583, P30CA022453) to the Karmanos Cancer Institute/Wayne State University and NIH grant (5P30 AG015281) to the Michigan Center for Urban African American Aging Research
References
- 1.Khoury MJ, Millikan R, Little J, Gwinn M. The emergence of epidemiology in the genomics age. Int J Epidemiol. 2004;33:936–944. doi: 10.1093/ije/dyh278. [DOI] [PubMed] [Google Scholar]
- 2.Morente MM, Fernandez PL, de Alava E. Biobanking: old activity or young discipline? Semin Diagn Pathol. 2008;25:317–322. doi: 10.1053/j.semdp.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 3.Oosterhuis JW, Coebergh JW, van Veen EB. Tumour banks: well-guarded treasures in the interest of patients. Nat Rev Cancer. 2003;3:73–77. doi: 10.1038/nrc973. [DOI] [PubMed] [Google Scholar]
- 4.Scharff DP, Mathews KJ, Jackson P, et al. More than Tuskegee: understanding mistrust about research participation. J Health Care Poor Underserved. 2010;21:879–897. doi: 10.1353/hpu.0.0323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.National Cancer Institute. [Accesseded 02/25/2013];The cancer genome atlas. 2012 http://cancergenome.nih.gov/
- 6.National Human Genome Research Institute. [Accesseded 05/10/2013.];Building a biobank to explore mysteries of the genome. http://www.genome.gov/27550100.
- 7.Bauer K, Taub S, Parsi K. Ethical issues in tissue banking for research: a brief review of existing organizational policies. Theor Med Bioeth. 2004;25:113–142. doi: 10.1023/b:meta.0000033772.84738.ad. [DOI] [PubMed] [Google Scholar]
- 8.Hewitt RE. Biobanking: the foundation of personalized medicine. Curr Opin Oncol. 2011;23:112–119. doi: 10.1097/CCO.0b013e32834161b8. [DOI] [PubMed] [Google Scholar]
- 9.Albain KS, Unger JM, Crowley JJ, Coltman CA, Jr, Hershman DL. Racial disparities in cancer survival among randomized clinical trials patients of the Southwest Oncology Group. J Natl Cancer Inst. 2009;101:984–992. doi: 10.1093/jnci/djp175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Crawley LM, Ahn DK, Winkleby MA. Perceived medical discrimination and cancer screening behaviors of racial and ethnic minority adults. Cancer Epidemiol Biomarkers Prev. 2008;17:1937–1944. doi: 10.1158/1055-9965.EPI-08-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Amirikia KC, Mills P, Bush J, Newman LA. Higher population-based incidence rates of triple-negative breast cancer among young African-American women: Implications for breast cancer screening recommendations. Cancer. 2011;117:2747–2753. doi: 10.1002/cncr.25862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.American Cancer Society. [Accesseded 05/15/2013.];Cancer facts and figures 2013. 2013 http://www.cancer.org/research/cancerfactsstatistics/cancerfactsfigures2013/index.
- 13.Howlader N, Noone AM, Krapcho M, Garshell J, Neyman N, Altekruse SF, Kosary CL, Yu M, Ruhl J, Tatalovich Z, Cho H, Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA. SEER Cancer Statistics Review, 1975–2010. National Cancer Institute; Bethesda, MD: 2013. [Google Scholar]
- 14.McCarty CA, Chapman-Stone D, Derfus T, Giampietro PF, Fost N. Community consultation and communication for a population-based DNA biobank: the Marshfield clinic personalized medicine research project. Am J Med Genet A. 2008;146A:3026–3033. doi: 10.1002/ajmg.a.32559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ormond KE, Cirino AL, Helenowski IB, Chisholm RL, Wolf WA. Assessing the understanding of biobank participants. Am J Med Genet A. 2009;149A:188–198. doi: 10.1002/ajmg.a.32635. [DOI] [PubMed] [Google Scholar]
- 16.Roden DM, Pulley JM, Basford MA, et al. Development of a large-scale de-identified DNA biobank to enable personalized medicine. Clin Pharmacol Ther. 2008;84:362–369. doi: 10.1038/clpt.2008.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Axler RE, Irvine R, Lipworth W, Morrell B, Kerridge IH. Why might people donate tissue for cancer research? Insights from organ/tissue/blood donation and clinical research. Pathobiology. 2008;75:323–329. doi: 10.1159/000164216. [DOI] [PubMed] [Google Scholar]
- 18.Erwin DO, Moysich K, Kiviniemi MT, et al. Community-based partnership to identify keys to biospecimen research participation. J Cancer Educ. 2013;28:43–51. doi: 10.1007/s13187-012-0421-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Luque JS, Quinn GP, Montel-Ishino FA, et al. Formative research on perceptions of biobanking: what community members think. J Cancer Educ. 2012;27:91–99. doi: 10.1007/s13187-011-0275-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Byrd GS, Edwards CL, Kelkar VA, et al. Recruiting intergenerational African American males for biomedical research Studies: a major research challenge. J Natl Med Assoc. 2011;103:480–487. doi: 10.1016/s0027-9684(15)30361-8. [DOI] [PubMed] [Google Scholar]
- 21.Corbie-Smith G, Thomas SB, St George DM. Distrust, race, and research. Arch Intern Med. 2002;162:2458–2463. doi: 10.1001/archinte.162.21.2458. [DOI] [PubMed] [Google Scholar]
- 22.Gamble VN. A legacy of distrust: African Americans and medical research. Am J Prev Med. 1993;9:35–38. [PubMed] [Google Scholar]
- 23.Gorelick PB, Harris Y, Burnett B, Bonecutter FJ. The recruitment triangle: reasons why African Americans enroll, refuse to enroll, or voluntarily withdraw from a clinical trial. An interim report from the African-American Antiplatelet Stroke Prevention Study (AAASPS) J Natl Med Assoc. 1998;90:141–145. [PMC free article] [PubMed] [Google Scholar]
- 24.Shavers-Hornaday VL, Lynch CF, Burmeister LF, Torner JC. Why are African Americans under-represented in medical research studies? Impediments to participation. Ethn Health. 1997;2:31–45. doi: 10.1080/13557858.1997.9961813. [DOI] [PubMed] [Google Scholar]
- 25.Sheikh A. Why are ethnic minorities under-represented in US research studies? PLoS Med. 2006;3:e49. doi: 10.1371/journal.pmed.0030049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Simmonds G. African American participation in public health research. ABNF J. 2008;19:69–72. [PubMed] [Google Scholar]
- 27.Kiviniemi MT, Saad-Harfouche FG, Ciupak GL, et al. Pilot intervention outcomes of an educational program for biospecimen research participation. J Cancer Educ. 2013;28:52–59. doi: 10.1007/s13187-012-0434-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chadiha LA, Washington OG, Lichtenberg PA, et al. Building a registry of research volunteers among older urban African Americans: recruitment processes and outcomes from a community-based partnership. Gerontologist. 2011;51(Suppl 1):S106–115. doi: 10.1093/geront/gnr034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hall MA, Camacho F, Dugan E, Balkrishnan R. Trust in the medical profession: conceptual and measurement issues. Health Serv Res. 2002;37:1419–1439. doi: 10.1111/1475-6773.01070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shaffer JP. Multiple hypothesis testing. Annual Review of Psychology. 1995;46:561–584. [Google Scholar]
- 31.Goldman R, Kingdon C, Wasser J, et al. Rhode Islanders’ attitudes towards the development of a statewide genetic biobank. Personalized Medicine. 2008;5:339–359. doi: 10.2217/17410541.5.4.339. [DOI] [PubMed] [Google Scholar]
- 32.Kennedy BR, Mathis CC, Woods AK. African Americans and their distrust of the health care system: healthcare for diverse populations. J Cult Divers. 2007;14:56–60. [PubMed] [Google Scholar]
- 33.Brawley OW, Tejeda H. Minority inclusion in clinical trials issues and potential strategies. J Natl Cancer Inst Monogr. 1995:55–57. [PubMed] [Google Scholar]
- 34.Compton C. Cancer biobanking: The American perspective. European Journal of Cancer-Supplements. 2007;5:5–6. [Google Scholar]
- 35.Ajzen I. The theory of planned behavior. In: Lange PAMV, Kruglanski AW, Higgins ET, editors. Handbook of theories of social psychology. Vol. 1. Thousand Oaks, CA: Sage Publications Ltd; 2012. pp. 438–459. [Google Scholar]
- 36.Armitage CJ, Conner M. Social cognition models and health behaviour: A structured review. Psychology & Health. 2000;15:173–189. [Google Scholar]
- 37.Lichtenberg PA. The generalizability of a participant registry for minority health research. Gerontologist. 2011;51(Suppl 1):S116–124. doi: 10.1093/geront/gnr021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bohner G, Dickel N. Attitudes and attitude change. Annu Rev Psychol. 2011;62:391–417. doi: 10.1146/annurev.psych.121208.131609. [DOI] [PubMed] [Google Scholar]
- 39.Petty RE, Wegener DT. Attitude change: Multiple roles for persuasion variables. In: Gilbert DT, Fiske ST, Lindzey G, editors. The handbook of social psychology. 4. 1 and 2. New York, NY, US: McGraw-Hill; 1998. pp. 323–390. [Google Scholar]
- 40.Brewer MB, editor. A dual process model of impression formation. Mahwah, NJ: Erlbaum; 1988. [Google Scholar]
- 41.Fiske ST, Lin MH, Neuberg SL, editors. The continuum model: Ten years later. New York: Guilford; 1999. [Google Scholar]