Abstract
An allogeneic hematopoietic cell transplant (HCT) from an HLA-identical donor after high-dose (myeloablative) pre-transplant conditioning, is an effective therapy for some people with chronic lymphocytic leukemia (CLL). Because CLL is a highly radiosensitive cancer, we hypothesized total body irradiation (TBI) conditioning regimens may be associated with better outcomes than those without TBI. To answer this we analyzed data from 180 subjects with CLL receiving myeloablative doses of TBI (N=126) or not (N=54), transplanted from an HLA-identical sibling donor, between 1995 and 2007 and reported to the Center for International Blood & Marrow Transplant Research (CIBMTR). At 5 years, treatment-related mortality was 48% (95% CI, 39–57%) vs. 50% (95% CI, 36–64%); p=NS. Relapse rates were 17% (95% CI, 11–25%) vs. 22% (95% CI, 11–35%); p=NS. Five-year progression-free survival and overall survival was 34% (95% CI, 26–43%) vs. 28% (95% CI, 15–42%); p=NS and 42% (95% CI, 33–51%) vs. 33% (95% CI, 19–48%); p=NS, respectively. The single most common cause of death in both cohorts was recurrent/progressive CLL. No variable tested in the multivariate analysis was found to significantly affect these outcomes including having failed fludarabine. Within the limitations of this study we found no difference in HLA-identical sibling transplant outcomes between myeloablative TBI and chemotherapy pre-transplant conditioning in persons with CLL.
Introduction
Hematopoietic cell transplants from a human leukocyte antigen (HLA)-identical sibling are effective therapy for selected persons with chronic lymphocytic leukemia (CLL)[1–8]. Myeloablative conditioning regimens, with or without total body irradiation (TBI), were commonly used, in the past. Although reduced-intensity regimens are increasingly-used, data from transplants using myeloablative conditioning are mature for analysis. Most TBI regimens also contain cyclophosphamide [9–11]. Myeloablative regimens without TBI (referred to herein as chemotherapy (CT)) typically include busulfan, often, but not always with cyclophosphamide [12,13]. Two small retrospective studies comparing these conditioning regimens showed no difference or favored a TBI-based conditioning regimen [12,14].
TBI may be especially effective in highly radio-sensitive cancers such as CLL [15–17]. Consequently, we hypothesized that TBI-containing conditioning regimens may have better outcomes than CT conditioning regimens. We compared transplant outcomes of these two conditioning regimens in subjects reported to the CIBMTR.
Methods
Data Sources
The CIBMTR is a combined research program of the Medical College of Wisconsin and the National Marrow Donor Program (NMDP). CIBMTR comprises a voluntary network of more than 450 transplantation centers worldwide that contribute detailed data on consecutive allogeneic and autologous transplants to a centralized Statistical Center. Observational studies conducted by the CIBMTR are performed in compliance with all applicable federal regulations pertaining to the protection of human research participants. Protected Health Information used in the performance of such research is collected and maintained in CIBMTR’s capacity as a Public Health Authority under the HIPAA Privacy Rule. Additional details regarding the data source are described elsewhere [18].
Inclusion Criteria
180 patients with CLL (Richter’s transformation and pro-lymphocytic leukemia were excluded) who received a conventional myeloablative (no reduced-intensity) allogeneic transplant from an HLA-identical sibling between 1995 and 2007 were included. This population was extracted from an initially larger cohort of 1,260 subjects reported to the CIBMTR. Unrelated donor transplants were excluded because of too many missing pieces of data, leaving us with 619 subjects. Further exclusions included twin and other related donors (N=42), cord blood donors (N=31), subjects with missing survival data (N=1), subjects with missing data on regimen intensity (N=25), lack of informed consent (N=68), subjects with ex vivo T-cell depleted grafts (N=62) and less intensive conditioning (N=210). Completeness index was 77% overall with good follow-up in both cohorts of 91% at 3 years and 84% at 5 years post-transplant[19].
Definitions of variables and outcomes
Rai stage and Karnofsky Performance score were categorized as previously described [20,21]. Constitutional symptoms included unexplained weight loss of >10% of body weight within 6 months, fever (>38°C) or night sweats. Refractoriness to fludarabine was defined as having stable or progressive disease after fludarabine-based therapy at any stage of treatment, as reported by the participating centers. Refractoriness to the prior therapy was defined as stable or progressive disease after the most recent therapy as reported by the participating centers. Myeloablative pre-transplant conditioning regimens are defined according to the CIBMTR Reduced-Intensity Conditioning Regimen Workshop[22,23].
Endpoints were measured from the time of transplant. For survival, subjects were considered to have an event at time of death from any cause. Survivors were censored at last contact. Relapse was defined by standard criteria and treatment-related mortality (TRM) was considered a competing event. TRM was defined as death without leukemia recurrence. Relapse was considered a competing event. PFS was defined as time to treatment-failure (death or relapse). Overall survival (OS) was defined as time to death from any cause. For relapse, TRM, and PFS, subjects alive in continuous complete remission were censored at last follow-up. Neutrophil recovery was defined as the first day of neutrophils ≥0.5 × 109/L for 3 consecutive days. Platelet recovery was defined as achieving platelets>20 × 109/L without platelet transfusions for 7 days. Acute and chronic graft vs. host disease (GvHD) were graded as described[24,25]. For engraftment and GvHD, death without the event was considered a competing event.
Data Analysis
Subject-, disease- and transplant-related variables of the TBI and CT cohorts were compared using the Chi-square test for categorical variables and the Kruskal-Wallis test for continuous variables. TRM, relapse, engraftment, acute and chronic GvHD were estimated as cumulative incidences taking into account competing risks. Probabilities of PFS and survival were calculated using the Kaplan-Meier estimator with variance estimated by the Greenwood formula. Survival curve estimates were compared using the log-rank test. Multivariate analyses were conducted to identify significant predictors of hematopoietic recovery, acute and chronic GvHD, TRM, relapse, PFS, and OS.
The proportional hazards model was built by forcing the main effect variable (TBI vs. CT) into the model. Backward elimination with a criterion of p<0.05 for retention was used to select a final model. The following variables were analyzed for their prognostic value on each of the outcomes: subject-related variables (age, gender and Karnofsky performance score at transplant), disease-related variables (Rai stage at diagnosis, Rai stage at transplant, constitutional symptoms at diagnosis, elevated LDH at transplant, splenectomy, refractoriness to fludarabine, and disease state at transplant) and transplant-related variables (time from diagnosis to transplant, donor age, donor-recipient gender and CMV serology, year of transplant and GvHD prophylaxis). None of the variables with >20% missing information were included in the model.
Results and Discussion
Demographic features of the cohorts are shown in Table 1. Median ages were 48 and 49 years with a male predominance, in both groups. Most subjects had early Rai stage at diagnosis (66% vs. 54%) without B-symptoms (60% vs. 63%). Both cohorts had a median of 3 prior therapies and most were resistant to their last therapy (75% vs. 77%), did not have a splenectomy (91% for both) and a similar proportion had failed fludarabine (45% vs. 50%, respectively). The proportions of subjects in complete or partial remission pre-transplant were similar, 46% and 44%.
Table 1.
Subject-, Disease- and Transplant-Related Variables
| Variable | TBI | CT | p-value |
|---|---|---|---|
| Subject-related | |||
| N subjects | 126 | 54 | |
| N centers | 65 | 24 | |
| Age, median(range), years | 48 (24–64) | 49 (27–62) | 0.38 |
| Gender | |||
| Male | 86 | 41 | 0.30 |
| Karnofsky score pre-transplant | 0.41 | ||
| <90% | 41 | 19 | |
| >=90% | 81 | 35 | |
| Missing | 4 | 0 | |
| Disease-related | |||
| Rai stage at diagnosis | 0.19 | ||
| Early Rai stages | 83 | 29 | |
| Late Rai stages | 24 | 11 | |
| Missing | 19 | 14 | |
| Rai stage pre-transplant | 0.46 | ||
| Early | 73 | 29 | |
| Advanced | 41 | 22 | |
| Missing | 12 | 3 | |
| Constitutional-symptoms at diagnosis | 0.63 | ||
| Absent | 76 | 34 | |
| Present | 26 | 8 | |
| Unknown | 24 | 12 | |
| Elevated LDH at transplant | 0.69 | ||
| No | 69 | 26 | |
| Yes | 37 | 19 | |
| Unknown | 20 | 9 | |
| Splenectomy | 0.42 | ||
| No | 115 | 49 | |
| Yes | 8 | 5 | |
| Missing | 3 | 0 | |
| N lines therapy pre-transplant, median(range) | 3 (1–5) | 3 (1–5) | 0.98 |
| Disease status at transplant | 0.96 | ||
| CR/PR/nPR | 58 | 24 | |
| Stable/progressive | 62 | 27 | |
| Unknown/untreated/not evaluable | 6 | 3 | |
| Refractory to prior therapy | 0.74 | ||
| No | 21 | 10 | |
| Yes | 79 | 36 | |
| Unknown/missing | 5 | 1 | |
| Fludarabine refractory | 0.80 | ||
| No | 57 | 23 | |
| Yes | 57 | 27 | |
| Missing | 12 | 4 | |
| Transplant related | |||
| Interval from diagnosis to transplant, median (range), months | 42 (2–223) | 41 (4–198) | 0.47 |
| Donor-recipient sex-match | 0.48 | ||
| M-M | 42 | 25 | |
| F-F | 13 | 3 | |
| M-F | 26 | 10 | |
| F-M | 44 | 16 | |
| Missing | 1 | 0 | |
| Donor-recipient CMV match | 0.57 | ||
| D(−)/R(−) | 31 | 15 | |
| D(+)/R(+) | 57 | 25 | |
| D(+)/R(−) | 13 | 5 | |
| D(−)/R(+) | 24 | 7 | |
| Missing | 1 | 2 | |
| Graft source | 0.006 | ||
| Bone marrow | 63 | 15 | |
| Blood | 63 | 39 | |
| Donor age, median(range), years | 47 (13–66) | 45 (24–67) | 0.70 |
| ATG | <0.001 | ||
| Yes | 0 | 8 | |
| No | 125 | 46 | |
| Missing | 1 | 0 | |
| GvHD prophylaxis | 0.24 | ||
| Tacrolimus + methotrexate +/− other | 13 | 11 | |
| Tacrolimus +/− other | 8 | 5 | |
| Cyclosporine + methotrexate +/− other | 80 | 32 | |
| Cyclosporine +/− other | 20 | 4 | |
| Missing | 5 | 2 | |
| Year of transplant | 0.02 | ||
| 1995–2000 | 100 | 34 | |
| 2001–2007 | 26 | 20 | |
| Median follow-up of survivors, range, months | 130 (3–175) | 56 (3–135) |
Most subjects in the TBI cohort received cyclophosphamide. Ninety-six percent of subjects in the CT cohort received a busulfan-based regimen which was given orally in 48%, intravenously in 28% and not reported in 24%. Fifty percent of TBI subjects received blood cell grafts vs. 72% of CT subjects (p=0.006). Fifteen percent of subjects in the CT cohort received anti-thymocyte globulin (ATG) pre-transplant vs. none in the TBI cohort (p<0.001).
One hundred day cumulative incidences of neutrophil recovery in the TBI and CT cohorts were similar, (98% (95% CI, 95–100%) and 96% (95% CI, 90–100%); p=0.45). Corresponding 100-day cumulative incidences of platelet recovery were 82% (95% CI, 74–88%) and 83% (95% CI, 72–91%); p=0.86. Five-year TRM rates were 48% (95% CI, 39–57%) vs. 50% (95% CI, 36–64%); p=NS. One hundred day rates of ≥grade-2 acute GvHD were similar, 49% (95% CI, 41–58%) vs. 43% (95% CI, 30–57%); p=0.47. One-year incidence of chronic GVHD was 45% (95%, CI 36–54%) vs. 37% (95% CI, 24–51%); p=0.14. Five-year relapse rates were 17% (95% CI, 11–25%) vs. 22% (95% CI, 11–35%); p=NS. Five-year PFS was 34% (95% CI, 26–43%) vs. 28% (95% CI, 15–42%); p=NS. Five-year OS was 42% (95% CI, 33–51%) vs. 33% (95% CI, 19–48%); p=NS (see Figure).
Figure.
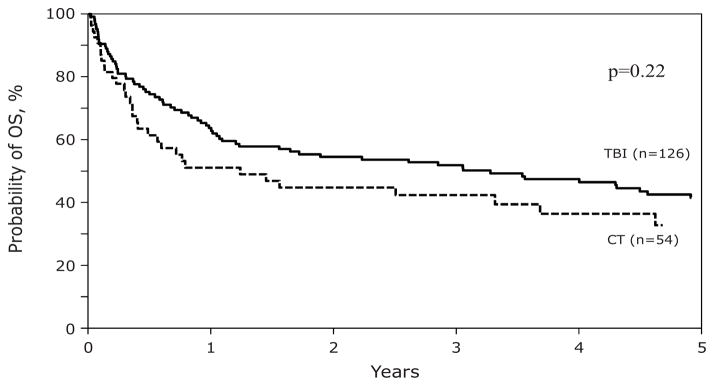
Adjusted Survival
The proportion of deaths in both cohorts was similar at 61% and 65% (see Table 2). The single most frequent cause of death was relapse. However, the pattern of other causes of deaths differed between the two groups: the TBI cohort had more deaths from infection, adult respiratory distress syndrome (ARDS) and GvHD whereas the CT cohort had more deaths from organ failure, hemorrhage and liver veno-occlusive disease. No factor tested significantly affected reported outcomes including having failed fludarabine (Table 3). New cancers occurred in both cohorts (TBI=11 vs. CT=3). Rates were not significantly different but this conclusion is limited by the small cohorts. A total of 4 deaths from new cancers occurred only in the TBI cohort (acute lymphoblastic leukemia [N=1], breast cancer [N=1], gastrointestinal cancer [N=1] and other cancer [N=1].
Table 2.
Causes of Death
| Variable | TBI | CT |
|---|---|---|
| N deaths | 81 | 33 |
| Causes | ||
| CLL | 21 | 9 |
| GvHD | 11 | 3 |
| ARDS | 4 | 0 |
| Infection | 18 | 3 |
| Organ failure | 11 | 9 |
| Graft-failure | 2 | 0 |
| Hemorrhage | 2 | 3 |
| Interstitial pneumonitis | 1 | 0 |
| Other | 2 | 3 |
| Secondary malignancy | 4 | 0 |
| Thromboembolic disease | 0 | 1 |
| Veno-occlusive disease | 2 | 2 |
| Missing | 3 | 0 |
Table 3.
Multivariate Analysis
| Outcomes | RR | p-value | 95% CI | N |
|---|---|---|---|---|
| Neutrophil recovery | ||||
| TBI | 1 | 123 | ||
| CT | 0.64 | 0.63 | 0.10–3.93 | 53 |
| Acute GvHD ≥grade-2 | ||||
| TBI | 1 | 123 | ||
| CT | 0.96 | 0.86 | 0.60–1.54 | 52 |
| Chronic GvHD | ||||
| TBI | 1 | 112 | ||
| CT | 0.88 | 0.65 | 0.52–1.51 | 52 |
| Relapse | ||||
| TBI | 1 | 123 | ||
| CT | 1.49 | 0.30 | 0.70–3.18 | 53 |
| TRM | ||||
| TBI | 1 | 123 | ||
| CT | 1.28 | 0.30 | 0.80–2.05 | 53 |
| PFS | ||||
| TBI | 1 | 123 | ||
| CT | 1.33 | 0.16 | 0.90–1.99 | 53 |
| Survival | ||||
| TBI | 1 | 126 | ||
| CT | 1.34 | 0.161 | 0.89–2.02 | 54 |
In summary, we found no significant differences in outcomes after myeloablative HLA-identical sibling transplants for CLL using TBI-containing or CT conditioning regimens. The strength of our conclusion is tempered by the small sample size, especially in the CT cohort with resultant low power to detect possible differences. A larger observational data set to address this question is unlikely to evolve because of a shift to less intensive conditioning and because no randomized study is likely to be done.
Acknowledgments
The CIBMTR is supported by Public Health Service Grant/Cooperative Agreement U24 CA076518 from the National Cancer Institute (NCI), the National Heart, Lung and Blood Institute (NHLBI) and the National Institute of Allergy and Infectious Diseases (NIAID); a Grant/Cooperative Agreement U10 HL069294 from NHLBI and NCI; a contract HHSH250201200016C with Health Resources and Services Administration (HRSA/DHHS); two Grants N00014-12-1-0142 and N00014-13-1-0039 from the Office of Naval Research; and grants from Allos Therapeutics, Inc.; Amgen, Inc.; Anonymous donation to the Medical College of Wisconsin; Ariad; Be the Match Foundation; Blue Cross and Blue Shield Association; Celgene Corporation; Fresenius-Biotech North America, Inc.; Gamida Cell Teva Joint Venture Ltd.; Genentech, Inc.;Gentium SpA; Genzyme Corporation; GlaxoSmithKline; HistoGenetics, Inc.; Kiadis Pharma; The Leukemia & Lymphoma Society; The Medical College of Wisconsin; Merck & Co, Inc.; Millennium: The Takeda Oncology Co.; Milliman USA, Inc.; Miltenyi Biotec, Inc.; National Marrow Donor Program; Onyx Pharmaceuticals; Optum Healthcare Solutions, Inc.; Osiris Therapeutics, Inc.; Otsuka America Pharmaceutical, Inc.; Remedy Informatics; Sanofi US; Seattle Genetics; Sigma-Tau Pharmaceuticals; Soligenix, Inc.; StemCyte, A Global Cord Blood Therapeutics Co.; Stemsoft Software, Inc.; Swedish Orphan Biovitrum; Tarix Pharmaceuticals; TerumoBCT; Teva Neuroscience, Inc.; THERAKOS, Inc.; and Wellpoint, Inc. The views expressed in this article do not reflect the official policy or position of the National Institute of Health, the Department of the Navy, the Department of Defense, or any other agency of the U.S. Government.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Keating MJ, O’Brien S, Kontoyiannis D, et al. Results of first salvage therapy for patients refractory to a fludarabine regimen in chronic lymphocytic leukemia. Leuk Lymphoma. 2002;43:1755–1762. doi: 10.1080/1042819021000006547. [DOI] [PubMed] [Google Scholar]
- 2.Badoux XC, Keating MJ, Wang X, et al. Fludarabine, cyclophosphamide, and rituximab chemoimmunotherapy is highly effective treatment for relapsed patients with CLL. Blood. 2011;117:3016–3024. doi: 10.1182/blood-2010-08-304683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dohner H, Stilgenbauer S, Benner A, et al. Genomic aberrations and survival in chronic lymphocytic leukemia. N Engl J Med. 2000;343:1910–1916. doi: 10.1056/NEJM200012283432602. [DOI] [PubMed] [Google Scholar]
- 4.Oscier D, Wade R, Davis Z, et al. Prognostic factors identified three risk groups in the LRF CLL4 trial, independent of treatment allocation. Haematologica. 2010;95:1705–1712. doi: 10.3324/haematol.2010.025338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grever MR, Lucas DM, Dewald GW, et al. Comprehensive assessment of genetic and molecular features predicting outcome in patients with chronic lymphocytic leukemia: results from the US Intergroup Phase III Trial E2997. J Clin Oncol. 2007;25:799–804. doi: 10.1200/JCO.2006.08.3089. [DOI] [PubMed] [Google Scholar]
- 6.Austen B, Skowronska A, Baker C, et al. Mutation status of the residual ATM allele is an important determinant of the cellular response to chemotherapy and survival in patients with chronic lymphocytic leukemia containing an 11q deletion. J Clin Oncol. 2007;25:5448–5457. doi: 10.1200/JCO.2007.11.2649. [DOI] [PubMed] [Google Scholar]
- 7.Zenz T, Eichhorst B, Busch R, et al. TP53 mutation and survival in chronic lymphocytic leukemia. J Clin Oncol. 2010;28:4473–4479. doi: 10.1200/JCO.2009.27.8762. [DOI] [PubMed] [Google Scholar]
- 8.Dreger P European Group for Blood and Marrow Transplantation (EBMT) The evolving role of stem cell transplantation in chronic lymphocytic leukemia. Hematol Oncol Clin North Am. 2013;27:355–369. doi: 10.1016/j.hoc.2013.01.007. [DOI] [PubMed] [Google Scholar]
- 9.Pavletic ZS, Arrowsmith ER, Bierman PJ, et al. Outcome of allogeneic stem cell transplantation for B cell chronic lymphocytic leukemia. Bone Marrow Transplant. 2000;25:717–722. doi: 10.1038/sj.bmt.1702237. [DOI] [PubMed] [Google Scholar]
- 10.Pavletic SZ, Khouri IF, Haagenson M, et al. Unrelated donor marrow transplantation for B-cell chronic lymphocytic leukemia after using myeloablative conditioning: results from the Center for International Blood and Marrow Transplant research. J Clin Oncol. 2005;23:5788–5794. doi: 10.1200/JCO.2005.03.962. [DOI] [PubMed] [Google Scholar]
- 11.Khouri IF, Keating MJ, Saliba RM, Champlin RE. Long-term follow-up of patients with CLL treated with allogeneic hematopoietic transplantation. Cytotherapy. 2002;4:217–221. doi: 10.1080/146532402320219736. [DOI] [PubMed] [Google Scholar]
- 12.Toze CL, Galal A, Barnett MJ, et al. Myeloablative allografting for chronic lymphocytic leukemia: evidence for a potent graft-versus-leukemia effect associated with graft-versus-host disease. Bone Marrow Transplant. 2005;36:825–830. doi: 10.1038/sj.bmt.1705130. [DOI] [PubMed] [Google Scholar]
- 13.Michallet M, Archimbaud E, Bandini G, et al. HLA-identical sibling bone marrow transplantation in younger patients with chronic lymphocytic leukemia. European Group for Blood and Marrow Transplantation and the International Bone Marrow Transplant Registry. Ann Intern Med. 1996;124:311–315. doi: 10.7326/0003-4819-124-3-199602010-00005. [DOI] [PubMed] [Google Scholar]
- 14.Doney KC, Chauncey T, Appelbaum FR Seattle Bone Marrow Transplant Team. Allogeneic related donor hematopoietic stem cell transplantation for treatment of chronic lymphocytic leukemia. Bone Marrow Transplant. 2002;29:817–823. doi: 10.1038/sj.bmt.1703548. [DOI] [PubMed] [Google Scholar]
- 15.Rossier C, Schick U, Miralbell R, Mirimanoff RO, Weber DC, Ozsahin M. Low-dose radiotherapy in indolent lymphoma. Int J Radiat Oncol Biol Phys. 2011;81:e1–e6. doi: 10.1016/j.ijrobp.2010.12.062. [DOI] [PubMed] [Google Scholar]
- 16.Chan EK, Fung S, Gospodarowicz M, et al. Palliation by low-dose local radiation therapy for indolent non-Hodgkin lymphoma. Int J Radiat Oncol Biol Phys. 2011;81:e781–e786. doi: 10.1016/j.ijrobp.2010.10.013. [DOI] [PubMed] [Google Scholar]
- 17.Russo AL, Chen YH, Martin NE, et al. Low-dose involved-field radiation in the treatment of non-hodgkin lymphoma: predictors of response and treatment failure. Int J Radiat Oncol Biol Phys. 2013;86:121–127. doi: 10.1016/j.ijrobp.2012.12.024. [DOI] [PubMed] [Google Scholar]
- 18.Horowitz M. The role of registries in facilitating clinical research in BMT: examples from the Center for International Blood and Marrow Transplant Research. Bone Marrow Transplant. 2008;42(Suppl-S2) doi: 10.1038/bmt.2008.101. [DOI] [PubMed] [Google Scholar]
- 19.Clark TG, Altman DG, De Stavola BL. Quantification of the completeness of follow-up. Lancet. 2002;359:1309–1310. doi: 10.1016/s0140-6736(02)08272-7. [DOI] [PubMed] [Google Scholar]
- 20.Rai KR, Sawitsky A, Cronkite EP, Chanana AD, Levy RN, Pasternack BS. Clinical staging of chronic lymphocytic leukemia. Blood. 1975;46:219–234. doi: 10.1182/blood-2016-08-737650. [DOI] [PubMed] [Google Scholar]
- 21.Karnofsky DA, Burchenal JH. The clinical evaluation of chemotherapeutic agents in cancer. Columbia University Press; New York: 1949. p. 196. [Google Scholar]
- 22.Giralt S, Ballen K, Rizzo D, et al. Reduced-intensity conditioning regimen workshop: defining the dose spectrum. Report of a workshop convened by the center for international blood and marrow transplant research. Biol Blood Marrow Transplant. 2009;15:367–369. doi: 10.1016/j.bbmt.2008.12.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bacigalupo A, Ballen K, Rizzo D, et al. Defining the intensity of conditioning regimens: working definitions. Biol Blood Marrow Transplant. 2009;15:1628–1633. doi: 10.1016/j.bbmt.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Przepiorka D, Weisdorf D, Martin P, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. 1995;15:825–828. [PubMed] [Google Scholar]
- 25.Shulman HM, Sullivan KM, Weiden PL, et al. Chronic graft-versus-host syndrome in man. A long-term clinicopathologic study of 20 Seattle patients. Am J Med. 1980;69:204–217. doi: 10.1016/0002-9343(80)90380-0. [DOI] [PubMed] [Google Scholar]


