Abstract
The androgen receptor (AR) has re-emerged as a potential therapeutic target in breast cancer. This stems from recent progress made in preclinical models, that have recognized important differences in the effect of AR expression on patient outcomes among different breast cancer subtypes. In parallel, the clinical development of new generations of AR directed therapies for prostate cancer has begun to mature. The availability of these new agents has translated into new trials to treat breast cancer. It is critical that studies of the effect of AR expression and signaling in breast cancer be context and subtype specific in order to successfully target AR signalling as a therapeutic strategy for breast cancer. We will review developments in preclinical studies, and recent clinical trials targeting AR in breast cancer.
Keywords: Androgen receptor, Breast cancer, Breast cancer subtype, Androgen receptor directed therapy
Introduction
Sex steroid hormone receptors, including AR, estrogen receptor (ER) and progesterone receptor (PR), are critical in both the development and progression of breast cancer. Of the three receptors, ER has been most extensively studied. It is expressed in 70% of all breast cancers, the ER signaling pathway is a key determinant in the molecular subtyping of breast cancer (1), and most importantly, effective ER pathway antagonists have been used effectively to treat patients with early and advanced breast cancers (2). On the other hand, an important role for androgens in breast cancer biology has long been hypothesized but remains to be fully elucidated. In spite of the availability of AR and PR targeted therapies, these are not widely used currently for patients with breast cancer.
AR is expressed in 50–80% of invasive breast cancers and in approximately 85% of ductal carcinoma in situ (DCIS) lesions in large cohort studies (3–5), however, it is not routinely measured in the clinical context. AR expression varies across the different molecular subtypes. In a large study of over 2,000 invasive breast cancer samples obtained from the Nurses’ Health Study (NHS), AR was most highly co-expressed in luminal A and luminal B cancers (91% and 68%, respectively), and lowest in of HER2+ and triple-negative tumors (59% and 32%, respectively) (3). Breast cancer subtypes were defined immunohistochemically in this study.
While androgen is often thought of as a male-selective steroid, androgens and AR play important physiologic roles in females as well. Studies of AR knockout mice suggest a key role for androgens in breast development and normal female reproduction (6, 7). In women, approximately 25% of testosterone is ovarian in origin, 25% adrenal, and the remainder is derived from the peripheral conversion of androgen precursors (8). The androgen dehydroepiandrosterone sulphate (DHEAS) is produced primarily by the adrenal gland (9), and can be converted either to androstenedione and then to estrone, or to 5-androstenediol and then to testosterone. Androgenic effects in tissue are mediated primarily through the binding of testosterone or 5α-dihydrotestosterone (DHT) to AR (8, 10). Ligand binding of AR results in receptor dimerization and translocation to the nucleus which, following interactions with multiple, modulatory co-regulators, leads to expression of AR target genes.
A recent pooled analysis of seven prospective studies in premenopausal women comprising 767 patients with breast cancer and 1,699 controls found that breast cancer risk was positively associated with a doubling in concentrations of androstenedione (odds ratio (OR) 1·30, 95% confidence interval [CI] 1.10–1.55), DHEAS (OR 1·17, 95% CI 1.04–1.32), testosterone (OR 1·18, 1.03–1.35), and calculated free testosterone (OR 1·08, 95% CI 0.97–1.21) (11). Circulating estradiol and estrone also had similar positive associations with breast cancer risk, whilst luteal phase progesterone and sex hormone binding globulin (SHBG) were not. These results mirror those reported in postmenopausal women, but are lower in magnitude (12–14). Among postmenopausal women, the best summary of evidence on circulating androgens and breast cancer risk is from a pooled analysis of nine prospective studies (14). In this study, testosterone was positively associated with breast cancer risk: the relative risks (95% CI) for increasing quintile category (all relative to the lowest quintile of levels) were 1.3 (1.0–1.9), 1.6 (1.2–2.2), 1.6 (1.1–2.2) and 2.2 (1.6–3.1). Findings were generally similar for several other androgens measured. In a recent nested case-control study within the NHS, prospectively collected serum hormonal levels were compared for 707 postmenopausal breast cancer cases and 1,414 matched controls (12). Women in the top quartile of testosterone, free testosterone, DHEAS, serum estradiol and free estradiol levels were at a 50–110% higher risk of breast cancer compared to women in the lowest quartile. Interestingly, the relative risk of breast cancer extended out to 20 years from when the serum levels were measured, and the association was strongest for ER+/PR+ cancers, while there was no association for ER−/PR− cancers.
The prognostic effect of AR expression in breast cancer is dependent on breast cancer subtype. In a meta-analysis of nineteen studies of early stage breast cancer, AR expression was associated better overall survival and disease-free survival (15). In a subset of postmenopausal patients with early stage breast cancer from the NHS, investigators found in multivariate analysis that AR expression was associated with a 30% reduction in breast cancer mortality in the ER+ subgroup (n=1,164; 88% AR+; hazard ratio (HR): 0.68; p=0.03), and a non-significant association of AR and poorer overall survival in the ER− subgroup (n=303; 43% AR+; HR: 1.59; p=0.08) (4). A similar association was also found between AR expression and patient outcomes in early stage ER+ breast cancer following adjuvant endocrine or chemoendocrine therapy (16, 17). Another study of 215 patients demonstrated a positive prognostic effect of the degree of AR expression in ER+ breast cancer, whereby multivariate Cox regression analysis indicated a 3.0-fold increased risk of relapse and a 4.6-fold increased risk of cancer-related death for patients with tumors which had AR expression that was less than the median level, which was 75% in this patient cohort (18).
In summary, current epidemiological evidence suggest that circulating androgen levels have a positive association with breast cancer risk, with a greater relative risk of developing ER+ compared to ER− cancers. AR expression has an effect on breast cancer progression, with differential effects on patient outcomes according to the ER status of the breast tumor. It is critical therefore, that studies of the effect of AR expression and signaling in breast cancer be context and subtype specific in order to successfully translate AR modulation into a successful clinical strategy for breast cancer (19).
The role of AR in breast cancer development
AR is expressed in approximately 20% of normal mammary epithelium and primarily in luminal cells (20). The effect of AR expression on breast cancer risk is unclear. Several breast cancer risk factors, including physical activity and alcohol intake, have also been hypothesized to operate, in part, by altering the androgen milieu. In order to gain a better understanding of the oncogenic effects of AR in mammary epithelial cells, one may gain considerable insight by exploring the expression and roles of AR signaling in normal mammary epithelial cells. Significant progress has been made in the delineation of the normal epithelial hierarchy with the identification of cell surface markers that could be used to fractionate subpopulations of cells by flow-assisted cytometry. Antibodies against CD49f and EpCAM reproducibly fractionated lineage-negative mammary epithelial cells into four subpopulations (21). These have been defined functionally through in vivo cleared mammary fat pad transplantation studies and in vitro culture experiments and include mammary stem cell-enriched (CD49hiEpCAM−), luminal progenitor (CD49+EpCAM+), mature luminal (CD49−EpCAM+), and stromal (CD49−EpCAM−) subpopulations. In this study, AR mRNA was most abundantly expressed in the mature luminal subpopulation, followed by the luminal progenitor cells. These finding were confirmed and extended upon in a recent paper whereby double immunofluorescent staining demonstrated that in sections of normal mammary lobules, all AR+ cells were luminal in origin, as defined by the expression of keratins 7 and 18, and claudin-4 (22). Interestingly, there was only a 44% overlap of AR+ cells with ER+ cells (n=429), and no overlap with proliferating Ki-67+ cells. These studies have shed light on the lineage and distribution of AR+ cells in the normal mammary gland and serve as a foundation to study the effect of AR on breast cancer risk. The AR signaling program in these AR+ normal mammary epithelial cells have not yet been defined. By comparing the AR target genes, collaborating transcription factors and androgen stimulated gene expression profile in AR+ normal cells and breast cancer cells, it should be possible to tease apart those genes involved in AR signaling that are required for normal mammary epithelial function and those that are aberrant in breast cancer.
AR biology in ER+ breast cancer
AR is present in the majority of and often co-expressed with ER and PR in primary and metastatic invasive breast tumors (3, 4, 23). AR expression has been shown to be a positive prognostic factor in ER+ breast cancer in a number of studies (4, 16–18). This clinical observation has been corroborated by laboratory studies that suggest that androgens may influence tumor growth differentially in ER+ compared to ER− tumors, with AR decreasing proliferation in ER+ tumors by antagonizing ER (18), and AR stimulating tumor growth in ER− tumors (24) (Figure 1). Though androgen metabolites of DHEAS can bind either to AR or to ER (25, 26), and have been hypothesized to be partial ER antagonists (25, 27), the role of androgens in ER signaling remains to be fully elucidated.
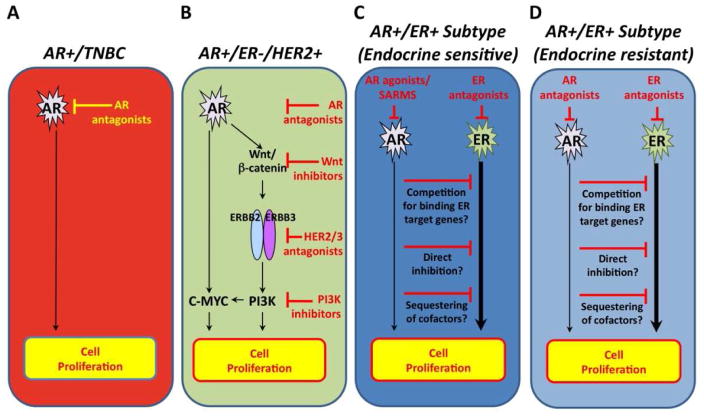
Figure 1. Preclinical insights of AR signaling according to breast cancer subtype.
AR antagonists have been shown to inhibit cell proliferation in A) TNBC AR+ and B) ER-HER2+AR+ breast cancer subtypes, the latter through its interaction with the HER2/3, Wnt/β-Catenin and c-MYC pathways. On the other hand, AR antagonists results in increased cell proliferation in C) ER+AR+ breast cancer cells through its interaction with the ER signaling pathway, and AR agonists may therefore be of benefit in this setting. D) AR overexpression may be a mechanism of Tamoxifen resistance, and inhibition of AR may be of benefit in this setting.
The physiological interplay of AR and ER signaling is complex. In ER+ breast cancer cell lines, AR signaling inhibits ERα transactivation activity and thereby 17β-estradiol mediated growth (18). Several potential mechanisms have been proposed to explain these effects. Estrogen has been suggested to induce a physical association between the AR amino-terminal domain and the ERα ligand-binding domain (28), and it has been hypothesized that AR competes with ER for chromatin binding to a subset of estrogen-response elements (EREs), subsequently repressing the activation of specific ER target genes that mediate the proliferative effect of estradiol on ER+ luminal breast cancer cells (18). An alternative hypothesis is that ligand-bound AR and ER compete for common coregulators, but bind to independent sites in genome, following which AR exerts its inhibitory effects on ER signaling (Figure 1C) (19).
There is an added level of complexity in the interplay between AR and ER signaling in the context of anti-estrogen therapy. Retrospective studies have suggested that high AR expression was a significant predictor for responsiveness to endocrine therapy in ER+ breast cancers (4, 17, 18). In contrast, AR is highly expressed in tamoxifen-resistant MCF-7 breast cancer cells. Recently, investigators have shown that the exogenous overexpression of AR rendered ERα-positive MCF-7 breast cancer cells resistant to the growth-inhibitory effects of tamoxifen by enhancing tamoxifen agonist activity on ERα at ERE sites (29). Treatment with the AR antagonist bicalutamide restored tamoxifen sensitivity in these cells. Potential mechanisms in which AR may interact with ER signaling include the displacement of corepressor proteins, the recruitment of coactivators, or acting as a coactivator in ER signaling (Figure 1D) (19, 29).
AR has been implicated as playing a role in the growth inhibitory effect of aromatase inhibitors (AIs) in ER+ tumors. During aromatase inhibition, androgen precursors can act directly on AR or be converted to DHT, subsequently exerting an antiproliferative effect on ER+ tumor cells by activating AR (30). Blockade of AR by bicalutamide, a non-steroidal AR antagonist, or RNA interference abolished the antiproliferative effects of both DHT and letrozole. These findings suggest that the antitumor action of AIs on ER+ breast tumors is partly mediated through increased androgen signaling in addition to a reduction in peripheral estrogen production, and may explain the lower relapse free survival in postmenopausal women with adjuvant AI therapy compared with tamoxifen.
In summary, the effect of AR and ER signaling is complex and needs to be assessed in a context dependent fashion including whether the tumor is ER therapy sensitive or resistant, whether concurrent ER-directed therapy is given, and if so, what type of therapy, and what the menopausal status of the patient is. It is likely that the interactions between these two pathways may have opposing effects in different clinical scenarios.
AR biology in HER2+ breast cancer
In contrast to ER, most studies have not examined the prognostic value of AR by HER2 status. In our analysis of published microarray datasets of breast tumors to gain an overall view on AR gene expression across different molecular subtypes in breast cancer (31–33), we found that high AR expression was correlated with HER2 amplification and overexpression (34, 35), and notably AR was not frequently expressed in the basal-like subtype. We also demonstrated that DHT, an androgen that cannot be converted by aromatase to estradiol, promoted growth in MDA-MB-453 (ER−/AR+/HER2+) breast cancer cells in an androgen dependent manner. Addition of bicalutamide abolished the DHT-stimulated growth of these cells (12). In contrast, DHT significantly inhibited the estradiol-induced growth of ER+ T47D and ZR75-1 breast cancer cells. To understand the oncogenic role of androgen and AR in ER− breast cancer, we sought to determine the AR target genes through genomic profiling of the chromatin occupancy of AR and the androgen-responsive genes. The integrated analysis revealed that AR signals to the HER2 pathway by inducing the expression of HER3, which is primarily mediated by the Wnt signaling pathway. AR induction of WNT7B activated the nuclear translocation of β-catenin, which in cooperation with AR to stimulate HER3 gene transcription, which represents a positive feedback loop between the AR and HER2/HER3 signaling pathways (34).
More recently, we also identified an additional positive feedback mechanism whereby AR signaling is amplified by c-MYC (36). AR directly activates c-MYC gene transcription and AR-mediated activation of HER2/HER3 signaling pathway increases the transcriptional activity of c-MYC by antagonizing MAD, the competitor of c-MYC binding to MAX. Consequently c-MYC enhances the AR-activated gene transcription through acting on the promoters of AR target genes. These findings revealed a complex regulatory network of AR pathway in ER−/HER2+ breast cancers in which AR cooperates with c-MYC and HER2 signaling pathways to drive oncogenic growth (Figure 1B). A positive feedback interaction between the AR and ERK signaling pathways has also been shown to promote androgen and HER2-mediated cell proliferation in molecular apocrine breast cancer (37). The combination of AR and MEK inhibitors not only resulted in synergistic therapeutic effects on MDA-MB-453 cells, but also had activity against trastuzumab-resistant MDA-MB-453 cells.
Taken together, these studies have further defined the complex regulatory mechanisms of AR function, and the crosstalk between AR, HER2 and other signaling pathways in ER−/HER2+/AR+ breast cancer. These insights provide preclinical rationale for exploring combinatorial therapies in this subset of breast cancer.
AR biology in TNBC
In contrast to ER+ breast cancers, the relation between AR expression and prognosis in ER− breast cancer less defined. Furthermore, as this is a less common subtype of breast cancer, most of the studies of AR in this subgroup are limited by small sample sizes. In the NHS, among women with ER− tumors (n=303, 42.9% AR+), there was a non-significant positive association between AR status and increased risk of breast cancer death (HR=1.59) (4). In contrast, another smaller study found that AR positivity was associated with improved survival in ER− breast cancers (49% AR +, n=69, HR=0.33) (38). It is important to note that the HER2 status was not factored into these analyses, and therefore these results are not representative of TNBC.
More recent studies have included HER2 into the analysis of AR expression in breast cancer. Most of the immunohistochemical studies have found the AR+ tumors represent a small subset within TNBCs, ranging from 12–23% (39–43). In a recent study, whereby 23% of tumors were AR+ (defined as ≥10 % nuclear staining, n=94), locoregional recurrence, overall and disease-specific survival were similar between patients with AR+ and AR− cancers, although AR-positivity was associated with more advanced disease (39).
A recent study differentiated molecular TNBC subtypes using a large collection of publically available gene expression profiles (44). One of the six TNBC subtypes (termed Luminal AR, representing 10–15% of TNBC analyzed) was characterized by ER-negativity, but had the highest expression of gene ontologies that were enriched in hormonally regulated pathways, including steroid synthesis, androgen and estrogen metabolism. AR mRNA was 9-fold greater than all other subtypes of TNBC, and it correlated with the highest AR expression by immunohistochemistry in a small sampling. The authors went on to identify basal breast cancer cell lines that had a similar gene expression profile to the luminal AR subtype. These included MDA-MB-453, SUM185PE, CAL-148, and MFM-223 cells. These cell lines were sensitive to AR antagonists and HSP90 inhibitors in vitro, which support the hypothesis that luminal AR tumors are driven by AR signaling (Figure 1A).
These results confirmed our studies in MDA-MB-453 cells, but this cell line were classified as HER2 amplified in our hands and are likely more representative of HER2+/AR+ breast cancers rather than TNBC (34, 36). Regardless, there is clearly a small subset of clinically defined TNBC tumors that are enriched for an AR signaling gene signature and AR represents a logical therapeutic target in this subset.
A follow up study looked at the outcomes in patients diagnosed with TNBC who had residual tumor following neoadjuvant chemotherapy (45). The TNBC subgroup that had a relatively favorable prognosis was characterized by high expression of “luminal-like” genes such as AR and GATA3 in the residual tumor. These results suggests that there is heterogeneity in the tumors of patients who do not achieve a complete response to chemotherapy, and that that luminal AR is a favorable subtype in this context.
Clinical trials targeting AR in breast cancer
Early trials targeting AR in breast cancer were largely failures, suffered from poor accrual and did not adequately consider breast cancer subtype. In addition these trials tested less effective AR antagonists such as bicalutamide. Recent interest in targeting AR in breast cancer follows the successful development of next generation AR-directed therapies in prostate cancer (46–48). Of the breast cancer subtypes, AR-directed therapies are being clinically evaluated first in TNBC.
A phase II trial of bicalutamide (150mg daily PO) in metastatic breast cancer was recently completed (41). This trial involved a prospective screening step in which TNBC tumors were assessed for AR expression prior to being assigned to therapy. The frequency of AR positivity defined as >10% nuclear staining by immunohistochemistry was low 12% (n=51 of 424 screened). The primary end point of a clinical benefit rate, defined as the proportion of patients who had a clinical response or stable disease for >6 months duration, was 19%, and the median progression free survival was 12 weeks. Importantly, bicalutamide was well tolerated and the most common treatment-related adverse events included fatigue, hot flashes, limb edema, and transaminase elevations. Major limitations of bicalutamide are its partial agonist activity on AR, the low affinity that it binds to AR, and that it has been shown to induce escape mechanisms in prostate cancer (49). The clinical results have not paralleled the promise shown in vitro in AR+ breast cancer cell lines.
Enzalutamide has been developed as an AR antagonist for use in prostate cancer, and has a six-fold higher affinity to AR relative to bicalutamide. It targets multiple steps in the AR signaling pathway, including inhibition of AR nuclear translocation, DNA binding, and co-activator recruitment of the ligand–receptor complex (47). In contrast to bicalutamide, it has not been found to have agonist activity. This improvement in AR antagonistic activity has translated into improved survival of men with metastatic castration-resistant prostate cancer after chemotherapy in a phase III trial, and has led to its FDA approval in this clinical setting (46). Enzalutamide is currently being evaluated in a phase I safety study in women with advanced breast cancer (ClinicalTrials.gov Identifier: NCT01597193) and in a phase II safety and efficacy study in patients with advanced, AR+ TNBC at a dose of 160mg daily (ClinicalTrials.gov Identifier: NCT01889238) (Table 1). Another second generation AR antagonist ARN-509, which has activities very much like enzalutamide is in development for the treatment of castration-resistant prostate cancer (48). Interestingly, it has been suggested to have greater efficacy and a higher therapeutic index in preclinical models relative to enzalutamide. Trials of this agent in castration-resistant prostate cancer have just begun and there are no current trials of this agent in breast cancer.
Table 1.
Clinical trials of AR-directed therapy in breast cancer
| Therapy | Phase | Breast cancer Subtype | Site/Sponsor | Clinical Trials.gov | Enrolment |
|---|---|---|---|---|---|
| Letrozole 2.5mg PO daily + DHEA 500 mg/1000mg PO daily | 1 | ER-Advanced disease in postmenopausal patients | OHSU Knight Cancer Institute | NCT00516542 | Jul 2007–Dec 2010; Terminated due to poor accrual |
| Bicalutamide 150mg PO daily | 2 | AR+ TNBC, Advanced disease | Memorial Sloan-Kettering Cancer Center | NCT00468715 | May 2007–Oct 2013; Completed |
| Enzalutamide 80mg and 160mg PO daily | 1 | Advanced disease | Medivation | NCT01597193 | May 2012–current; ongoing |
| Enzalutamide 160mg PO daily | 2 | AR+ TNBC, Advanced disease | Medivation | NCT01889238 | Jun 2013–current; ongoing |
| Abiraterone Acetate | 1/2 | Advanced disease in postmenopausal patients | Cancer Research UK | NCT00755885 | Oct 2008–current, ongoing |
| Enobosarm 9mg PO daily | 2 | ER+ advanced disease, in patients who previously responded to hormone therapy | GTx | NCT01616758 | April 2012–current; ongoing |
Abbreviations: DHEA, Dehydroepiandrosterone; TNBC, Triple negative breast cancer.
The clinical development of AR-directed therapies in the ER+ and HER2+ subtypes requires more complex trial design, as there are other effective targeted therapies used to treat these subtypes, raising the possibility of combination therapies. In ER+ breast cancer, as summarized above, numerous studies have shown that AR positivity has been associated with improved breast cancer outcomes. Furthermore, androgens have been used in the treatment of ER+ breast cancers, alone or in combination with tamoxifen (50–52). These results have not been consistently positive, and androgens not frequently therapy used in this setting. Whilst there is evidence for AR signaling to inhibit ER activation of growth stimulatory genes in vitro (18), other studies have shown the antagonism of AR may restore tamoxifen sensitivity by inhibiting the ER agonist response of tamoxifen (29). Thus the use of AR antagonists in ER+/AR+ breast cancer needs to be carefully considered by the clinical context.
In HER2+/AR+ tumors, we and others have demonstrated in preclinical studies, that the AR and HER signaling pathways interact and that AR-directed therapies alone or in combination with HER2-directed therapies result in tumor responses (34, 36). The logical step forward is therefore to evaluate this combination in a clinical trial. This is not without challenges however as there has been a wealth of new and effective HER2-directed therapies that have been developed in recent years, representing a high bar to improve tumor response rates and patient outcomes with the addition of an AR antagonist.
An alternative strategy to target AR is to inhibit the enzyme CYP17A1 that is required for androgen synthesis. Abiraterone works through this mechanism and has been FDA approved for the treatment of castration resistant prostate cancer (53, 54). This drug is currently being evaluated in a phase I/II trial in the UK, in postmenopausal women with advanced breast cancer of all three clinical subtypes (ClinicalTrials.gov Identifier: NCT00755885). Finally, enobosarm (GTx-024) is a selective AR modulator that induces conformational changes in AR upon binding, selectively altering the interaction of AR with coactivator and corepressor proteins, and resultant AR signaling. This novel therapy has been evaluated in the setting of cancer associated cachexia (55), and there is currently a phase I trial in patients with advanced ER+ breast cancer who have previously received and responded to up to three prior hormonal therapies (ClinicalTrials.gov Identifier: NCT01616758).
Finally, one needs to consider if AR expression alone will be sufficient to define the presence of AR signaling. One potential strategy to identify a more robust indicator of AR signaling is to define a breast cancer subtype specific AR-target gene signature that encapsulates other components of AR signaling, similar to the approach that was used to define the Luminal AR subtype in TNBC (44). There has been recent development of novel PET tracers developed for AR in the setting of prostate cancer. Preliminary clinical studies have shown that 18F-fluoro-5α-dihydrotestosterone (FDHT) localizes to prostate cancers, and the uptake of FDHT in tumors is reduced with administration of AR-directed therapies (56–58). In a recent study, FDHT-PET was also used to demonstrate the binding of enzalutamide to AR (59). All patients who underwent PET imaging (n=22) had a decrease in FDHT uptake after one month of enzalutamide treatment, and an association was found between a greater FDHT response and higher drug doses despite varying serum drug levels. FDHT has not yet been evaluated for imaging breast cancer, and may be potentially useful for noninvasive tumor characterization, patient stratification, and evaluating response to therapy to anti-androgens in breast cancer.
Concluding remarks
There is a renaissance in the field of clinically targeting AR in breast cancer, stemming from a greater understanding of AR signaling that is breast cancer subtype specific, coupled with the progress in the development of new AR-directed therapies in prostate cancer. We can now begin to translate observations from preclinical studies into rationally designed clinical trials. Fundamental insights have been made in the identification of a luminal AR subtype in TNBC, and the interaction between the AR and HER2 pathways. These have translated into the completion of the first wave of clinical trials in TNBC, and trials of the next generation of AR-directed therapies are current underway in this cancer subtype. The preclinical rationale is also present for targeting AR and HER2, and it is likely that these combinatorial strategies will be explored in clinical trials in the near future. The interactions of AR and ER signaling are complex, and whilst progress has been made in understanding how these pathways interact, targeting AR appropriately in ER+ breast cancer remains a challenge.
Footnotes
Conflict of Interest
Elgene Lim has received a DF/HCC SPORE grant (P50 CA168504) and a grant from the Breast Cancer Research Foundation.
Myles Brown has received grants from the Breast Cancer Research Foundation, National Cancer Institute, Novartis Pharmaceuticals, Medivation Inc. (pending), and has served on a scientific advisory board for Susan G. Komen for the Cure and is a consultant for Novartis.
Min Ni, Aditi Hazra, Shiliang Cao, Rulla M. Tamimi declare that they have no conflict of interest.
Compliance with Ethics Guidelines
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
References
- 1.Perou CM, Sørlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, et al. Molecular portraits of human breast tumours. Nature. 2000;17;406(6797):747–52. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- 2.Early Breast Cancer Trialists’ Collaborative G. Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;365(9472):1687–717. doi: 10.1016/S0140-6736(05)66544-0. [DOI] [PubMed] [Google Scholar]
- 3•.Collins LC, Cole KS, Marotti JD, Hu R, Schnitt SJ, Tamimi RM. Androgen receptor expression in breast cancer in relation to molecular phenotype: results from the Nurses’ Health Study. Mod Pathol. 2011;24(7):924–31. doi: 10.1038/modpathol.2011.54. One of the largest studies of androgen receptor (AR) expression in primary breast cancers using the Nurses Health Study tumor microarray resource, comparing AR expression according to the different breast cancer subtypes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4•.Hu R, Dawood S, Holmes MD, Collins LC, Schnitt SJ, Cole K, et al. Androgen receptor expression and breast cancer survival in postmenopausal women. Clin Cancer Res. 2011;17(7):1867–74. doi: 10.1158/1078-0432.CCR-10-2021. This is one of the first reports studying the impact of AR expression on breast cancer outcomes in post-menopausal women according to breast cancer subtype. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ogawa Y, Hai E, Matsumoto K, Ikeda K, Tokunaga S, Nagahara H, et al. Androgen receptor expression in breast cancer: relationship with clinicopathological factors and biomarkers. Int J Clin Oncol. 2008;13(5):431–5. doi: 10.1007/s10147-008-0770-6. [DOI] [PubMed] [Google Scholar]
- 6.Walters KA, Simanainen U, Handelsman DJ. Molecular insights into androgen actions in male and female reproductive function from androgen receptor knockout models. Human reproduction update. 2010;16(5):543–58. doi: 10.1093/humupd/dmq003. [DOI] [PubMed] [Google Scholar]
- 7.Zhou X. Roles of androgen receptor in male and female reproduction: lessons from global and cell-specific androgen receptor knockout (ARKO) mice. Journal of andrology. 2010;31(3):235–43. doi: 10.2164/jandrol.109.009266. [DOI] [PubMed] [Google Scholar]
- 8.Nicolas Diaz-Chico B, German Rodriguez F, Gonzalez A, Ramirez R, Bilbao C, Cabrera de Leon A, et al. Androgens and androgen receptors in breast cancer. J Steroid Biochem Mol Biol. 2007;105(1–5):1–15. doi: 10.1016/j.jsbmb.2006.11.019. [DOI] [PubMed] [Google Scholar]
- 9.Labrie F, Luu-The V, Labrie C, Belanger A, Simard J, Lin SX, et al. Endocrine and intracrine sources of androgens in women: inhibition of breast cancer and other roles of androgens and their precursor dehydroepiandrosterone. Endocrine reviews. 2003;24(2):152–82. doi: 10.1210/er.2001-0031. [DOI] [PubMed] [Google Scholar]
- 10.Li J, Al-Azzawi F. Mechanism of androgen receptor action. Maturitas. 2009;63(2):142–8. doi: 10.1016/j.maturitas.2009.03.008. [DOI] [PubMed] [Google Scholar]
- 11•.Key TJ, Appleby PN, Reeves GK, Travis RC, et al. Sex hormones and risk of breast cancer in premenopausal women: a collaborative reanalysis of individual participant data from seven prospective studies. Lancet Oncol. 2013;14(10):1009–19. doi: 10.1016/S1470-2045(13)70301-2. A pooled analysis with data for 767 women with breast cancer and 1699 controls, that showed that circulating oestrogens and androgens are positively associated with the risk for breast cancer in premenopausal women. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang X, Tworoger SS, Eliassen AH, Hankinson SE. Postmenopausal plasma sex hormone levels and breast cancer risk over 20 years of follow-up. Breast Cancer Res Treat. 2013;137(3):883–92. doi: 10.1007/s10549-012-2391-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaaks R, Rinaldi S, Key TJ, Berrino F, Peeters PH, Biessy C, et al. Postmenopausal serum androgens, oestrogens and breast cancer risk: the European prospective investigation into cancer and nutrition. Endocr Relat Cancer. 2005;12(4):1071–82. doi: 10.1677/erc.1.01038. [DOI] [PubMed] [Google Scholar]
- 14.Key T, Appleby P, Barnes I, Reeves G, Endogenous H Breast Cancer Collaborative G. Endogenous sex hormones and breast cancer in postmenopausal women: reanalysis of nine prospective studies. J Natl Cancer Inst. 2002;94(8):606–16. doi: 10.1093/jnci/94.8.606. [DOI] [PubMed] [Google Scholar]
- 15•.Vera-Badillo FE, Templeton AJ, de Gouveia P, Diaz-Padilla I, Bedard PL, Al-Mubarak M, et al. Androgen Receptor Expression and Outcomes in Early Breast Cancer: A Systematic Review and Meta-Analysis. J Natl Cancer Inst. 2013 doi: 10.1093/jnci/djt319. A meta-analysis of 19 studies with a total of 7693 women with early breast cancer that demonstrated expression of AR in women with breast cancer is associated with better OS and DFS irrespective of coexpression of ER. [DOI] [PubMed] [Google Scholar]
- 16.Gonzalez-Angulo AM, Stemke-Hale K, Palla SL, Carey M, Agarwal R, Meric-Berstam F, et al. Androgen receptor levels and association with PIK3CA mutations and prognosis in breast cancer. Clin Cancer Res. 2009;15(7):2472–8. doi: 10.1158/1078-0432.CCR-08-1763. [DOI] [PubMed] [Google Scholar]
- 17.Castellano I, Allia E, Accortanzo V, Vandone AM, Chiusa L, Arisio R, et al. Androgen receptor expression is a significant prognostic factor in estrogen receptor positive breast cancers. Breast Cancer Res Treat. 2010;124(3):607–17. doi: 10.1007/s10549-010-0761-y. [DOI] [PubMed] [Google Scholar]
- 18.Peters AA, Buchanan G, Ricciardelli C, Bianco-Miotto T, Centenera MM, Harris JM, et al. Androgen receptor inhibits estrogen receptor-alpha activity and is prognostic in breast cancer. Cancer Res. 2009;69(15):6131–40. doi: 10.1158/0008-5472.CAN-09-0452. [DOI] [PubMed] [Google Scholar]
- 19.Lim E, Ni M, Hazra A, Tamimi RM, Brown M. Elucidating the role of the Androgen Receptor in breast cancer. Clinical Investigation. 2012;2(10):1003–11. [Google Scholar]
- 20.Li S, Han B, Liu G, Li S, Ouellet J, Labrie F, et al. Immunocytochemical localization of sex steroid hormone receptors in normal human mammary gland. The journal of histochemistry and cytochemistry: official journal of the Histochemistry Society. 2010;58(6):509–15. doi: 10.1369/jhc.2009.954644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lim E, Vaillant F, Wu D, Forrest NC, Pal B, Hart AH, et al. Aberrant luminal progenitors as the candidate target population for basal tumor development in BRCA1 mutation carriers. Nat Med. 2009;15(8):907–13. doi: 10.1038/nm.2000. [DOI] [PubMed] [Google Scholar]
- 22.Santagata S, Thakkar A, Ergonul A, Wang B, Woo T, Hu R, et al. Taxonomy of Breast Cancers Based on Normal Cell Phenotype. Journal of Clinical Investigation. 2013 doi: 10.1172/JCI70941. In press. • This study of the distribution of AR+ cells in normal mammary lobules demonstrated that AR+ cells were luminal in origin, as defined by the expression of keratins 7 and 18, and claudin-4. There was only a 44% overlap of AR+ cells with ER+ cells, and no overlap with proliferating Ki-67+ cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lea OA, Kvinnsland S, Thorsen T. Improved measurement of androgen receptors in human breast cancer. Cancer Res. 1989;49(24 Pt 1):7162–7. [PubMed] [Google Scholar]
- 24.Doane AS, Danso M, Lal P, Donaton M, Zhang L, Hudis C, et al. An estrogen receptor-negative breast cancer subset characterized by a hormonally regulated transcriptional program and response to androgen. Oncogene. 2006;25(28):3994–4008. doi: 10.1038/sj.onc.1209415. [DOI] [PubMed] [Google Scholar]
- 25.Adams JB, Archibald L, Seymour-Munn K. Dehydroepiandrosterone and androst-5-ene-3 beta,17 beta-diol in human mammary cancer cytosolic and nuclear compartments and their relationship to estrogen receptor. Cancer Res. 1980;40(10):3815–20. [PubMed] [Google Scholar]
- 26.Boccuzzi G, Di Monaco M, Brignardello E, Leonardi L, Gatto V, Pizzini A, et al. Dehydroepiandrosterone antiestrogenic action through androgen receptor in MCF-7 human breast cancer cell line. Anticancer research. 1993;13(6A):2267–72. [PubMed] [Google Scholar]
- 27.Liao DJ, Dickson RB. Roles of androgens in the development, growth, and carcinogenesis of the mammary gland. J Steroid Biochem Mol Biol. 2002;80(2):175–89. doi: 10.1016/s0960-0760(01)00185-6. [DOI] [PubMed] [Google Scholar]
- 28.Panet-Raymond V, Gottlieb B, Beitel LK, Pinsky L, Trifiro MA. Interactions between androgen and estrogen receptors and the effects on their transactivational properties. Molecular and cellular endocrinology. 2000;167(1–2):139–50. doi: 10.1016/s0303-7207(00)00279-3. [DOI] [PubMed] [Google Scholar]
- 29.De Amicis F, Thirugnansampanthan J, Cui Y, Selever J, Beyer A, Parra I, et al. Androgen receptor overexpression induces tamoxifen resistance in human breast cancer cells. Breast Cancer Res Treat. 2010;121(1):1–11. doi: 10.1007/s10549-009-0436-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Macedo LF, Guo Z, Tilghman SL, Sabnis GJ, Qiu Y, Brodie A. Role of androgens on MCF-7 breast cancer cell growth and on the inhibitory effect of letrozole. Cancer Res. 2006;66(15):7775–82. doi: 10.1158/0008-5472.CAN-05-3984. [DOI] [PubMed] [Google Scholar]
- 31.Hess KR, Anderson K, Symmans WF, Valero V, Ibrahim N, Mejia JA, et al. Pharmacogenomic predictor of sensitivity to preoperative chemotherapy with paclitaxel and fluorouracil, doxorubicin, and cyclophosphamide in breast cancer. J Clin Oncol. 2006;24(26):4236–44. doi: 10.1200/JCO.2006.05.6861. [DOI] [PubMed] [Google Scholar]
- 32.Ivshina AV, George J, Senko O, Mow B, Putti TC, Smeds J, et al. Genetic reclassification of histologic grade delineates new clinical subtypes of breast cancer. Cancer Res. 2006;66(21):10292–301. doi: 10.1158/0008-5472.CAN-05-4414. [DOI] [PubMed] [Google Scholar]
- 33.Wang Y, Klijn JG, Zhang Y, Sieuwerts AM, Look MP, Yang F, et al. Geneexpression profiles to predict distant metastasis of lymph-node-negative primary breast cancer. Lancet. 2005;365(9460):671–9. doi: 10.1016/S0140-6736(05)17947-1. [DOI] [PubMed] [Google Scholar]
- 34••.Ni M, Chen Y, Lim E, Wimberly H, Bailey ST, Imai Y, et al. Targeting androgen receptor in estrogen receptor-negative breast cancer. Cancer Cell. 2011;20(1):119–31. doi: 10.1016/j.ccr.2011.05.026. This study provides evidence that AR is enriched in ER− breast tumors that overexpress HER2, and that AR mediates ligand-dependent activation of Wnt and HER2 signaling pathways through the direct transcriptional induction of WNT7B and HER3. Specific targeting of AR, Wnt or HER2 signaling impairs androgenstimulated tumor cell growth, suggesting potential therapeutic approaches for ER−/HER2+ breast cancers. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sanga S, Broom BM, Cristini V, Edgerton ME. Gene expression metaanalysis supports existence of molecular apocrine breast cancer with a role for androgen receptor and implies interactions with ErbB family. BMC medical genomics. 2009;2:59. doi: 10.1186/1755-8794-2-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ni M, Chen Y, Fei T, Li D, Lim E, Liu XS, et al. Amplitude modulation of androgen signaling by c-MYC. Genes Dev. 2013;27(7):734–48. doi: 10.1101/gad.209569.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chia KM, Liu J, Francis GD, Naderi A. A feedback loop between androgen receptor and ERK signaling in estrogen receptor-negative breast cancer. Neoplasia. 2011;13(2):154–66. doi: 10.1593/neo.101324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Agoff SN, Swanson PE, Linden H, Hawes SE, Lawton TJ. Androgen receptor expression in estrogen receptor-negative breast cancer. Immunohistochemical, clinical, and prognostic associations. Am J Clin Pathol. 2003;120(5):725–31. doi: 10.1309/42F0-0D0D-JD0J-5EDT. [DOI] [PubMed] [Google Scholar]
- 39.McGhan LJ, McCullough AE, Protheroe CA, Dueck AC, Lee JJ, Nunez-Nateras R, et al. Androgen Receptor-Positive Triple Negative Breast Cancer: A Unique Breast Cancer Subtype. Ann Surg Oncol. 2013 doi: 10.1245/s10434-013-3260-7. [DOI] [PubMed] [Google Scholar]
- 40.Rakha EA, El-Sayed ME, Green AR, Lee AH, Robertson JF, Ellis IO. Prognostic markers in triple-negative breast cancer. Cancer. 2007;109(1):25–32. doi: 10.1002/cncr.22381. [DOI] [PubMed] [Google Scholar]
- 41•.Gucalp A, Tolaney S, Isakoff SJ, Ingle JN, Liu MC, Carey LA, et al. Phase II Trial of Bicalutamide in Patients with Androgen Receptor-Positive, Estrogen Receptor-Negative Metastatic Breast Cancer. Clin Cancer Res. 2013;19(19):5505–12. doi: 10.1158/1078-0432.CCR-12-3327. This is a recently completed phase II trial of Bicalutamide in advanced TNBC which reporteded a clinical benefit rate of 19%, and a median progression free survival was 12 weeks. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Loibl S, Muller BM, von Minckwitz G, Schwabe M, Roller M, Darb-Esfahani S, et al. Androgen receptor expression in primary breast cancer and its predictive and prognostic value in patients treated with neoadjuvant chemotherapy. Breast Cancer Res Treat. 2011;130(2):477–87. doi: 10.1007/s10549-011-1715-8. [DOI] [PubMed] [Google Scholar]
- 43.He J, Peng R, Yuan Z, Wang S, Peng J, Lin G, et al. Prognostic value of androgen receptor expression in operable triple-negative breast cancer: a retrospective analysis based on a tissue microarray. Medical oncology. 2012;29(2):406–10. doi: 10.1007/s12032-011-9832-0. [DOI] [PubMed] [Google Scholar]
- 44••.Lehmann BD, Bauer JA, Chen X, Sanders ME, Chakravarthy AB, Shyr Y, et al. Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J Clin Invest. 2011;121(7):2750–67. doi: 10.1172/JCI45014. Describes six triple-negative breast cancer subtypes displaying unique gene expression and ontologies, including a luminal AR subtype characterized by AR signaling, PIK3CA mutations and sensitivity to bicalutamide. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yu KD, Zhu R, Zhan M, Rodriguez AA, Yang W, Wong S, et al. Identification of prognosis-relevant subgroups in patients with chemoresistant triple-negative breast cancer. Clin Cancer Res. 2013;19(10):2723–33. doi: 10.1158/1078-0432.CCR-12-2986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Scher HI, Fizazi K, Saad F, Taplin ME, Sternberg CN, Miller K, et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med. 2012;367(13):1187–97. doi: 10.1056/NEJMoa1207506. [DOI] [PubMed] [Google Scholar]
- 47.Tran C, Ouk S, Clegg NJ, Chen Y, Watson PA, Arora V, et al. Development of a second-generation antiandrogen for treatment of advanced prostate cancer. Science. 2009;324(5928):787–90. doi: 10.1126/science.1168175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Clegg NJ, Wongvipat J, Joseph JD, Tran C, Ouk S, Dilhas A, et al. ARN-509: a novel antiandrogen for prostate cancer treatment. Cancer Res. 2012;72(6):1494–503. doi: 10.1158/0008-5472.CAN-11-3948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Taplin ME, Bubley GJ, Ko YJ, Small EJ, Upton M, Rajeshkumar B, et al. Selection for androgen receptor mutations in prostate cancers treated with androgen antagonist. Cancer Res. 1999;59(11):2511–5. [PubMed] [Google Scholar]
- 50.Ingle JN, Suman VJ, Mailliard JA, Kugler JW, Krook JE, Michalak JC, et al. Randomized trial of tamoxifen alone or combined with fluoxymesterone as adjuvant therapy in postmenopausal women with resected estrogen receptor positive breast cancer. North Central Cancer Treatment Group Trial 89-30-52. Breast Cancer Res Treat. 2006;98(2):217–22. doi: 10.1007/s10549-005-9152-1. [DOI] [PubMed] [Google Scholar]
- 51.Ingle JN, Twito DI, Schaid DJ, Cullinan SA, Krook JE, Mailliard JA, et al. Combination hormonal therapy with tamoxifen plus fluoxymesterone versus tamoxifen alone in postmenopausal women with metastatic breast cancer. An updated analysis. Cancer. 1991;67(4):886–91. doi: 10.1002/1097-0142(19910215)67:4<886::aid-cncr2820670405>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 52.Rose C, Kamby C, Mouridsen HT, Andersson M, Bastholt L, Moller KA, et al. Combined endocrine treatment of elderly postmenopausal patients with metastatic breast cancer. A randomized trial of tamoxifen vs. tamoxifen + aminoglutethimide and hydrocortisone and tamoxifen + fluoxymesterone in women above 65 years of age. Breast Cancer Res Treat. 2000;61(2):103–10. doi: 10.1023/a:1006460925986. [DOI] [PubMed] [Google Scholar]
- 53.Ryan CJ, Molina A, Griffin T. Abiraterone in metastatic prostate cancer. N Engl J Med. 2013;368(15):1458–9. doi: 10.1056/NEJMc1301594. [DOI] [PubMed] [Google Scholar]
- 54.Ryan CJ, Smith MR, de Bono JS, Molina A, Logothetis CJ, de Souza P, et al. Abiraterone in metastatic prostate cancer without previous chemotherapy. N Engl J Med. 2013;368(2):138–48. doi: 10.1056/NEJMoa1209096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dobs AS, Boccia RV, Croot CC, Gabrail NY, Dalton JT, Hancock ML, et al. Effects of enobosarm on muscle wasting and physical function in patients with cancer: a double-blind, randomised controlled phase 2 trial. Lancet Oncol. 2013;14(4):335–45. doi: 10.1016/S1470-2045(13)70055-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Larson SM, Morris M, Gunther I, Beattie B, Humm JL, Akhurst TA, et al. Tumor localization of 16beta-18F-fluoro-5alpha-dihydrotestosterone versus 18F-FDG in patients with progressive, metastatic prostate cancer. Journal of nuclear medicine: official publication, Society of Nuclear Medicine. 2004;45(3):366–73. [PubMed] [Google Scholar]
- 57.Dehdashti F, Picus J, Michalski JM, Dence CS, Siegel BA, Katzenellenbogen JA, et al. Positron tomographic assessment of androgen receptors in prostatic carcinoma. European journal of nuclear medicine and molecular imaging. 2005;32(3):344–50. doi: 10.1007/s00259-005-1764-5. [DOI] [PubMed] [Google Scholar]
- 58.Beattie BJ, Smith-Jones PM, Jhanwar YS, Schoder H, Schmidtlein CR, Morris MJ, et al. Pharmacokinetic assessment of the uptake of 16beta-18F-fluoro-5alpha-dihydrotestosterone (FDHT) in prostate tumors as measured by PET. Journal of nuclear medicine: official publication, Society of Nuclear Medicine. 2010;51(2):183–92. doi: 10.2967/jnumed.109.066159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Scher HI, Beer TM, Higano CS, Anand A, Taplin ME, Efstathiou E, et al. Antitumour activity of MDV3100 in castration-resistant prostate cancer: a phase 1–2 study. Lancet. 2010;375(9724):1437–46. doi: 10.1016/S0140-6736(10)60172-9. [DOI] [PMC free article] [PubMed] [Google Scholar]