Abstract
Background
Medication discrepancies may occur at transitions in care and negatively impact patient outcomes.
Objective
To determine if involving clinical pharmacists in hospital care, medication reconciliation and discharge medication plan communication can reduce medication discrepancies with a prospective, randomized, blinded, controlled trial.
Setting
A large, tertiary care, academic medical center.
Method
The intervention consisted of clinical pharmacist medication reconciliation, patient education and improved communication of the discharge medication plan, as devised by the hospital physician and care team, to primary care physicians and community pharmacists. Medication discrepancies were identified by blinded research pharmacists who reviewed primary care physician and pharmacy records at discharge through 90 days post-discharge to create 30-day and 90-day medication lists. Main outcome measure: Rate of medication discrepancies compared across groups.
Results
A total of 592 subjects from internal medicine, family medicine, cardiology and orthopedic services were evaluated for this study. Clinically important medication discrepancies in the primary care physician record were different between groups 30 days after hospital discharge following a clinical pharmacist's intervention. The mean number of medication discrepancies per patient for the enhanced group being nearly half the number in the control group. However, this effect did not persist to 90 days post-discharge and did not extend to community pharmacy records.
Conclusion
The present study demonstrates the involvement of pharmacists in hospital care, medication reconciliation and discharge medication plan communication may affect the quality of the outpatient medical record.
Keywords: medication discrepancies, Pharmacist Case Manager, Medication reconciliation, Transitions of Care, Medical Record, Pharmacy Record, Continuity of Care, hospital discharge, United States
INTRODUCTION
Medication discrepancies may occur at any transition of care, when individuals are admitted or discharged from a hospital or nursing home [1, 2]. Discrepancies may include omitted medications, out-of-date dosages, and non-active medications, among other inaccuracies. Poor communication at transition points has been reported as a major source of medication errors and adverse drug events [1]. Admission and discharge medication lists in institutions, medication lists in physician offices and in community pharmacies may all contain different information. Medication list errors are common with reported rates in hospitalized patients nearing 70% of patient lists in some reports [3, 4]. Community pharmacy records may be especially problematic in terms of medication discrepancies, as pharmacists may not be made aware of medication changes that occur at hospital discharge and/or in physician offices, particularly discontinued medications.
Medication discrepancies appear to have a negative effect on morbidity. Coleman et al. observed that among patients 65 years and older, 14.3% of those who had a medication discrepancy were re-hospitalized at 30 days compared to 6.1% of the patients who did not experience a discrepancy [5]. Medication discrepancies can lead to adverse events upon admission to the hospital and at discharge from the hospital to the patient's home community [5, 6]. Avoiding medication discrepancies at transitions of care is a National Patient Safety Goal (NPSG) in the United States (U.S.) [7]. The 2012 Joint Commission National Patient Safety Goals state that “medication discrepancies can affect patient outcomes,” and require medication reconciliation efforts be undertaken [7]. Review of a patient's medication information in the context of the current clinical situation should be completed at each of the aforementioned care transitions, a process called medication reconciliation [8]. The World Health Organization (WHO) describes medication reconciliation as obtaining, at the time of admission, a complete and accurate list of each patient's current home medications—including name, dosage, frequency and route; using that list when writing admission, transfer and/or discharge medication orders; and comparing the list against the patient's admission, transfer, and discharge orders, identifying and bringing any discrepancies to the attention of the prescriber and, if appropriate, making changes to the orders [9]. Optimal approaches to avoiding medication errors are not clear. Investigation into improved communication of the discharge medication plan and the subsequent impact on medication discrepancies may inform best practices. This is a sub-study from the Iowa Continuity of Care (ICOC) study, funded by the National Institutes of Health (NIH). The study was a randomized, controlled trial to determine if introducing clinical pharmacist case managers (PCMs) into the inpatient care team could reduce medication underutilization, adverse drug events, and readmissions [10]. The final results of the ICOC are due in 2014. As we work to evaluate the primary outcome measure we were interested in exploring the effect of our intervention on medication discrepancies and the outpatient medical record in this sub-study.
AIM OF THE STUDY
The objective of the present study was to determine if involving clinical pharmacist case managers in hospital care and discharge medication plan communication could reduce medication discrepancies after hospital discharge.
ETHICAL APPROVAL
The study was approved by the University of Iowa Institutional Review Board for Human Subjects and all subjects signed informed consent.
METHOD
The background and methods of this study have been previously published in addition to other data on acceptance of inpatient recommendations [10,11]. Briefly, the primary purpose of the ICOC study was to improve communication between the tertiary health center, primary care physicians and community pharmacists. A population-based approach was used and the PCMs were located centrally outside the hospital services and housed within the research unit in the College of Pharmacy, which was next to the hospital. This strategy was used so that the PCMS could cover multiple inpatient services. One or two PCMs participated in the study at any given time. Four PCMs participated over the course of the present study; all were Doctor of Pharmacy (Pharm.D.) graduates who had completed at least one year of post-graduate pharmacy residency training accredited by the American Society of Health-System Pharmacists.
Setting, Inclusion and Exclusion Criteria
This study was conducted at the University of Iowa Hospitals and Clinics (UIHC), a large, tertiary care, academic medical center. The ICOC study enrolled patients that were admitted to the cardiology, internal medicine, family medicine or orthopedic services at the UIHC. A total of 592 subjects signed consent and had complete data for the present sub-study. Subjects were randomized to the enhanced intervention (n=195), minimal intervention (n= 199) or control (n=198) groups. Subjects were at least 18 years old, spoke English or Spanish and had at least 1 of the following diagnoses: hypertension, hyperlipidemia, heart failure, coronary artery disease, myocardial infarction, transient ischemic attack, stroke, diabetes, asthma, chronic obstructive pulmonary disease or require anticoagulation. Subjects could not have hearing impairments, life expectancy of less than six months, cognitive impairments, substance abuse problems or severe psychiatric conditions.
Study Intervention
The control group received only usual hospital care without any involvement of the PCM. All of the patients in the study received the exposure to the usual hospital medication list collection process, which was most often done by the patient's floor nurse on admission. They also received the typical discharge summaries from the University of Iowa Hospitals and Clinics sent to primary care physicians for their records.
Patients in the minimal intervention group received a visit from the PCM to counsel them on their medications after admission to the hospital. The PCM took a detailed medication history including interviewing the patient, calling the patient's pharmacy and updating the medical record. This was followed by medication reconciliation where the PCM compared the inpatient medications to the patent's home medication list to identify and bring any discrepancies to the attention of the prescriber. This medication reconciliation process was repeated at discharge and a discharge medication teaching session covering important aspects of the patient's current medications and making sure new medications were fully understood by the patient. The discharge medication reconciliation focused on comparing the medications a patient was currently taking in the hospital with the patient's prior to admission (home) medication list and making sure all medications were addressed and active medications were appropriate for the patient and consistent with practice guidelines. The patient also received a discharge medication list listing all discharge medications and their purpose.
Patients in the enhanced intervention group received all the previously mentioned care as well as having a discharge care plan prepared and sent via fax to their community physician and community pharmacy. This discharge care plan focused on medication issues and changes that occurred during the hospitalization and highlighted which medications had been added, changed or stopped. Patients in the enhanced intervention group also received a follow-up phone call from the PCM 3-5 days after discharge to address any medication related issues that had developed since the discharge.
Data Collection
At the time of enrollment a baseline interview was conducted by a blinded research assistant. The research assistant administered the baseline surveys including Pfeiffer mental status questionnaire [12], Katz index of activities of daily living [13, 14], self-reported social support [15], self-reported adverse drug events [10,16], self-reported efficacy [17] and self-reported adherence [18, 19]. For the primary outcome measure, blinded, clinical research pharmacists (CRP) evaluated and compared the discharge medication lists from the hospital (updated to reflect intended changes since discharge) to 30-day and 90-day post-discharge medication lists found in the community physician and community pharmacy records evaluating the lists for medication discrepancies. This evaluation included a full review of the entire community physician's medical record (chart) and community pharmacy records. The CRPs were given all available information from all sources and dates to evaluate the active medications lists for discrepancies. The hospital discharge list was evaluated based on intended post-discharge changes documented in healthcare records to reflect what the CRP believed to be the intended list at each time point (30 and 90 days post-discharge) based on a thorough review of the patient's medical records (both in hospital and community physician) and community pharmacy records collected through requests for medical records at each time point from each source (hospital, physician medical office and community pharmacy). As an example: if a patient had low blood pressure at a follow-up appointment 14 days post discharge and the provider discontinued a medication on the discharge list this would not be scored as a discrepancy at the 30 or 90 day time points. A discrepancy was deemed present if a) medications that documentation indicated should be active were not on the list (unintended omission), b) medications were on list without documentation (unintended addition) or c) medications were found with different dose or frequency. The CRP determined the clinical significance of each discrepancy by giving a low, moderate or high designation based on the potential for patient harm. The following definitions were used by clinical research pharmacists in the evaluation of medication discrepancy significance:
Low: unlikely to impact any therapeutic outcome, little/no risk of harm to patient, most over the counter medication discrepancies.
Moderate: may impact therapeutic outcome and/or possibility of harm to patient.
High: likely to adversely affect outcome, medications with narrow therapeutic index, medications on Institute for Safe Medication Practices (ISMP) high alert list and/or impending risk to patient.
Analysis
Differences in demographics between study subgroups groups were compared using ANOVA and Chi square. The dependent variable for the primary analysis was the number of medication discrepancies per patient at each level of discrepancy significance. Since the data were not normally distributed a non-parametric Kruskal Wallis ANOVA was used to test for differences between study groups.
RESULTS
Demographics
The three study arms had participants with comparable demographics and medical conditions (Table 1). The mean age of subjects was approximately 60 years old and nearly two thirds were married. Educational levels and incomes appeared normally distributed. No significant differences were found when self-rated social support, self-rated adherence, self-rated efficacy and self-rated health were evaluated.
Table 1.
Baseline Demographics.
| Control N=198 | Minimal N =199 | Enhanced N=195 | P value | |
|---|---|---|---|---|
| Age | 60.0±12.7 | 59.8±12.8 | 61.1±12.8 | 0.592 |
| Gender (% female) | 44.9% | 51.7% | 49.2% | 0.390 |
| Marital Status | 0.468 | |||
| Married or Partner | 63.6% | 67.2% | 64.9% | |
| Single or divorced | 36.4% | 32.8% | 35.1% | |
| Income | 0.409 | |||
| <$10,000 | 14.7% | 13.2% | 12.4% | |
| $10,000-24,999 | 23.4% | 21.8% | 21.2% | |
| $25,000-39,999 | 13.7% | 17.3% | 23.3% | |
| $40,000-54,999 | 12.7% | 11.7% | 13.0% | |
| $55,000-79,999 | 10.7% | 9.6% | 13.0% | |
| $80,000-99,999 | 7.1% | 7.1% | 5.7% | |
| >$100,000 | 9.1% | 7.1% | 6.7% | |
| Declined to answer | 8.6% | 12.2% | 4.7% | |
| Education | 0.437 | |||
| 8th grade or less | 6.6% | 7.0% | 5.7% | |
| High School | 51.5% | 48.7% | 42.8% | |
| Some college | 16.7% | 20.6% | 21.1% | |
| College graduate | 17.7% | 14.1% | 16.5% | |
| Professional or graduate degree | 7.6% | 9.5% | 13.9% | |
| Conditions | ||||
| Hypertension | 75.3% | 73.9% | 81.0% | 0.205 |
| Hyperlipidemia | 61.1% | 64.8% | 63.6% | 0.737 |
| Heart failure | 27.3% | 32.7% | 28.7% | 0.476 |
| Coronary artery disease | 30.3% | 36.2% | 36.4% | 0.350 |
| Myocardial Infarction | 19.2% | 23.6% | 25.1% | 0.343 |
| Diabetes | 36.4% | 41.2% | 37.4% | 0.581 |
| Cancer | 17.2% | 14.6% | 17.9% | 0.639 |
| Depression or anxiety | 50.5% | 54.3% | 52.3% | 0.754 |
| Asthma or COPD | 27.3% | 29.1% | 27.2% | 0.886 |
| Number of Conditions | 5.6±2.5 | 6.0±2.3 | 5.9±2.0 | 0.141 |
| Self-Rated Social Support | 6.2±1.0 | 6.1±1.0 | 6.1±1.1 | 0.363 |
| Self-Rated Adherence | 1.48±1.8 | 1.67±1.9 | 1.94±1.9 | 0.058 |
| Self-Rated Efficacy | 122.7±10.4 | 121.6±13.1 | 119.9±13.2 | 0.072 |
| Self-Rated Health | 0.736 | |||
| Excellent | 4.5% | 4.5% | 3.6% | |
| Very good | 14.1% | 13.6% | 19.1% | |
| Good | 39.2% | 35.2% | 30.9% | |
| Fair | 27.1% | 30.2% | 30.9% | |
| Poor | 15.1% | 16.5% | 15.5% |
Self-rated social support: possible range 0-9; higher valves represent more (better) social support. Self-rated adherence: possible range 0-16; higher values represent lower (worse) adherence. Self-rated self efficacy: possible range 28-140; higher values represent higher confidence in taking medications effectively (better self-efficacy).
Medication discrepancies
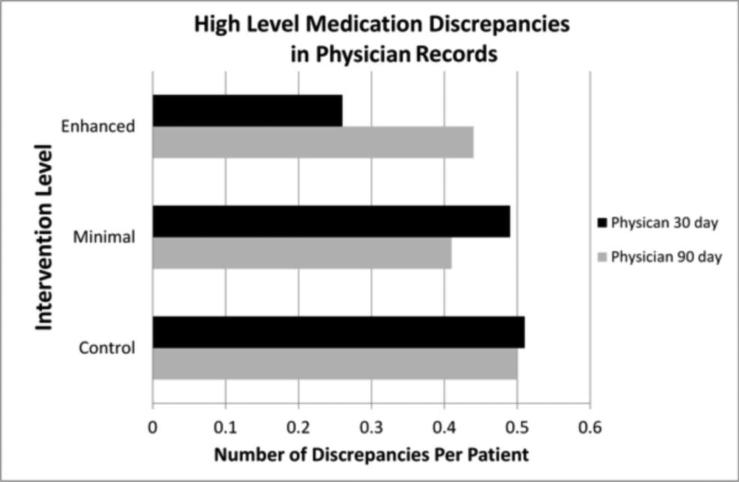
The number of medication discrepancies of high significance was lower in physician records at 30 days in the enhanced intervention group (p=0.013, Figure 1). A trend of decreasing discrepancy rates was seen with increased pharmacist intervention at this time point (control >minimal pharmacist intervention > enhanced pharmacist intervention). Additionally, the mean number of medication discrepancies per patient for high level discrepancies for both the minimal and enhanced pharmacist intervention were below the mean for the control group at 90 days in the physician record, although this did not reach statistical significance (Figure 1).
Figure 1.
Average high level medication discrepancies in physician medical record at 30 and 90 days post discharge. Black bars represent the physician data at 30 days. A statistically significant difference was found between treatment groups at 30 days (p= 0.013). Gray bars represent the physician data at 90 days.
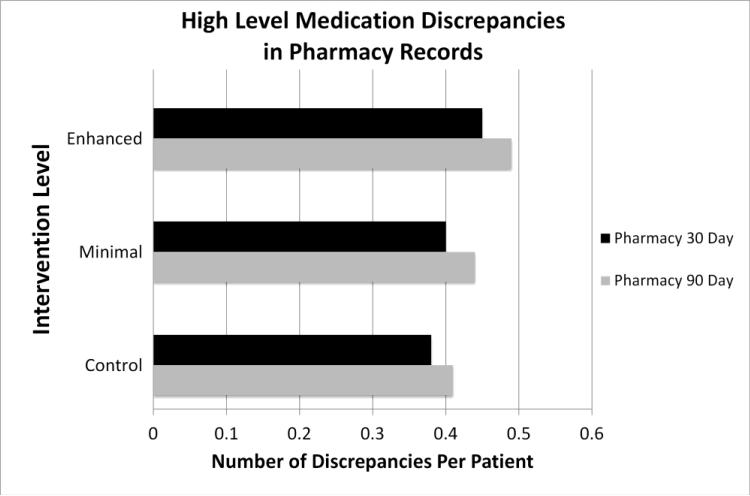
In the pharmacy records, the trend was predominantly reversed with greater PCM involvement associated with increased discrepancies of nearly all severity levels in the community pharmacy record (figure 2). In fact, low level discrepancies were found to be significantly increased with PCM involvement in the community pharmacy record 90 days after the intervention (p=0.03, Table 3).
Figure 2.
Average high level medication discrepancies in outpatient pharmacy record at 30 and 90 days post discharge. Black bars represent the pharmacy data at 30 days. Gray bars represent the pharmacy data at 90 days.
Table 3.
Number of Medication Discrepancies per Patient at 90 Days
| SOURCE | SIGNIFICANCE OF DISCREPANCY | CONTROL | MINIMAL | ENHANCED | P-VALUE |
|---|---|---|---|---|---|
| Physician Records | High-Level Discrepancies | 0.50 | 0.41 | 0.44 | 0.656 |
| Mid-Level Discrepancies | 3.03 | 2.56 | 2.83 | 0.568 | |
| Low-Level Discrepancies | 2.78 | 2.50 | 2.78 | 0.217 | |
| Pharmacy Records | High-Level Discrepancies | 0.41 | 0.44 | 0.49 | 0.954 |
| Mid-Level Discrepancies | 3.25 | 3.44 | 3.62 | 0.688 | |
| Low-Level Discrepancies | 4.12 | 4.60 | 5.04 | 0.030 |
DISCUSSION
This study found that clinically important medication discrepancies in the primary care physician record were different between groups 30 days after hospital discharge following a clinical pharmacist's intervention. The mean number of medication discrepancies per patient for the enhanced group being nearly half the number in the control group This intervention included medication reconciliation, communication of a medication focused discharge care plan to outpatient healthcare providers, patient education and a follow-up phone call with discharged patients.
However, this effect was not sustained at 90 days post hospital discharge. This may be due to the fact that our intervention to limit medication discrepancies was primarily targeted to the inpatient admission without a continued outpatient effort post-discharge. Additionally, poorly documented but intended medication changes initiated by outpatient providers may have occurred over time and increased the number of identified discrepancies by 90 days post hospital discharge.
Our observation that high level medication discrepancies in the physician record at 30 days were affected by the pharmacist intervention supports observations by Schnipper et al. [20] who found that pharmacists can impact medication specific outcomes following hospital discharge. Schnipper found that pharmacist involvement was associated with a lower rate of preventable adverse drug events 30 days after hospital discharge [20]. Pharmacists in that study also reconciled patient's medication regimens, counseled patients before discharge and followed-up with a patient phone call. Additionally, a recent multifaceted intervention comprising pharmacist-led medication reconciliation and tailoring, patient education, collaborative care between pharmacist and patients’ primary care clinician and/or cardiologist and voice messaging demonstrated improved cardiovascular medication adherence after hospital discharge but did not affect clinical end points such as mortality [21].
Medication discrepancies can have a negative effect on hospital readmission as seen in data by Coleman et al. who noted that those who had a medication discrepancy were more than twice as likely to be re-hospitalized at 30 days when compared with patients that did not experience a discrepancy [5]. In the present study the decreased rate of medication discrepancies measured at 30 days post-discharge was not maintained at 90 days post-discharge. The study is limited by the fact that our intervention was focused around the hospital admission and discharge period and did not provide continued evaluation of the patient's medications and communication with outpatient providers after 5 days post discharge. This may explain why the effect was limited to 30 days post-discharge. These findings may be important given the recent national payer focus in the United States (U.S.) that penalizes health systems for 30 day readmissions. This finding may reflect the need for continued long-term surveillance and monitoring of the medication list after discharge to minimize discrepancies.
In contrast to the physician records, there was a significant increase in the number of low-level medication discrepancies in pharmacy records at 90 days post-discharge and no statistical difference in high level discrepancies between groups in the pharmacy record data. The trend observed in the pharmacy prescription records seems to be counter-intuitive at first glance. However, since most pharmacy medication records are created for the sole purpose of dispensing prescriptions and are not often used for therapeutic decision making, community pharmacists have little incentive to remove outdated medications. This appears to produce a “building” effect in many outpatient pharmacy records, where new prescriptions are added with old prescriptions often remaining active until expiring (1 year from issue in most cases in the U.S.). Since the intervention did not formally cancel any prescriptions it was not able to have a major effect on the general building trend of these pharmacy databases. In fact, since pharmacy case managers often recommended new medications to the physicians of patients in the minimal and enhanced groups, this may have increased the potential of some (particularly low level) discrepancies to occur in the pharmacy databases, for example, if a new mediation was intended to replace old medication they might both be found in the pharmacy database.
Our study confirms the importance of involving pharmacists in the medication reconciliation process. These efforts may improve the quality of the medical record, although this effect does not seem to persist to 90 days with a single, short-term intervention. Correct medication lists are important to avoid medication duplications, drug-drug interactions and drug-condition interactions. A team based approach to dealing with drug therapy problems utilizing physicians, pharmacists and nurses has been shown to decrease the cost of care and improve quality of care in a health care system [22]. Hospitals often contact outpatient pharmacies or medical clinics to obtain the patient's active medication list. Our study suggests these two sources of information differ in content and accuracy. Hospital physician and hospital pharmacist involvement in maintaining an accurate medication list is essential and future outpatient clinical information technology systems design should incorporate features to improve the accuracy of these medication lists. The differences in the two medication list sources highlights the importance of collecting information from multiple sources during medication reconciliation to avoid medication errors as advocated by the World Health Organization (WHO)'s “High 5s Tool for Medication Reconciliation” [9].
One limitation of this study is that the analysis did not evaluate readmissions directly but instead focused on mediation discrepancies. A link between discrepancies and poor patient outcomes has been previously published [5, 7, 23]. While re-admission data are not available for the present study, data on re-hospitalization and adverse events will be available with the final ICOC analyses when the main study is published. Additionally, our definition of medication discrepancies may in some cases reflect poor record keeping and not errors that reached the patient (i.e. the physician instructed the patient to stop a duplicate medication but did not document it). We feel that even if these documentation lapses do not currently translate into a clinical problem they at the very least represent the potential for an adverse drug event and as such are relevant to patient care. One can imagine if poorly documented medication lists are collected as part of a medication reconciliation this information may be propagated throughout the healthcare system with potentially serious consequences. The assessment of medication discrepancies clinical significance was limited in that no standardized validated tool exists for evaluating medication discrepancies. We used experienced clinical pharmacists with access to multiple data sources combined with defined discrepancy significance levels to improve the quality of our evaluation.
Finally, the non-integrated nature of the pharmacist involved in this intervention may be a weakness of the intervention model. An integrated pharmacist who rounds with the hospital team or works in the physician office is able to build relationships with providers in the hospital and community and may have a greater effect. Study pharmacists were located off site and had to do most communication with providers over the phone or via fax. However this model is more easily replicated in large populations at a lesser expense when compared with placing a clinical pharmacist on each hospital service and/or physician office. Continued involvement of clinical pharmacists in care transitions may reduce discrepancies and adverse drug events over the long term for hospitalized patients. Future research should evaluate greater collaborations and sustained efforts to maintain accurate medication lists. Additionally, hospitals should consider communicating medication changes that occur during hospitalization with the patient's community pharmacy. Communication of this plan may be most effectively done via a physician order to explicitly discontinue medications that have been stopped in hospital (a prescription to discontinue) and may represent a quality improvement and research opportunity.
Conclusion
This study found that involving clinical pharmacists in identifying medication discrepancies and communicating this information affected medication discrepancies in the medical record. No effect on discrepancies in pharmacy records was found. This study suggests that clinical pharmacists may fill an important role in identifying medication discrepancies and communicating this information to affect their impact.
IMPACT OF FINDINGS ON PRACTICE.
* Involving clinical pharmacists in hospital care, medication reconciliation and discharge medication plan communication can affect medication discrepancies in the outpatient medication record.
* Improved communication between providers in the hospital and providers in the community can play a role in identifying medication discrepancies and communicating this information may reduce their impact.
* Hospitals should consider improving the communication of medication changes that occur during hospitalization with the patient's primary care physician and community pharmacy. Continued efforts may be required to have a lasting impact.
Table 2.
Number of Medication Discrepancies per Patient at 30 Days
| SOURCE | SIGNIFICANCE OF DISCREPANCY | CONTROL | MINIMAL | ENHANCED | P-VALUE |
|---|---|---|---|---|---|
| Physician Records | High-Level Discrepancies | 0.51 | 0.49 | 0.26 | 0.013 |
| Mid-Level Discrepancies | 2.89 | 2.45 | 2.61 | 0.688 | |
| Low-Level Discrepancies | 2.31 | 2.14 | 2.31 | 0.429 | |
| Pharmacy Records | High-Level Discrepancies | 0.38 | 0.40 | 0.45 | 0.783 |
| Mid-Level Discrepancies | 3.36 | 3.68 | 3.42 | 0.655 | |
| Low-Level Discrepancies | 3.92 | 4.34 | 4.56 | 0.134 |
Acknowledgments
Funding
This study is supported, in part by the National Heart, Lung, and Blood Institute grant 1RO1 HL082711.
Footnotes
Conflicts of Interest
No conflicts of interest to report.
Contributor Information
T. Michael Farley, Department of Pharmacy Practice and Science University of Iowa College of Pharmacy and Clinical Pharmacist Specialist Mercy Hospital Iowa City 115 South Grand Avenue Iowa City, Iowa, 52242 No conflicts of interest.
Constance Shelsky, Department of Pharmacy Practice and Science University of Iowa College of Pharmacy No conflicts of interest.
Shanique Powell, Department of Pharmacy Practice and Science University of Iowa College of Pharmacy No conflicts of interest.
Karen B. Farris, Social and Administrative Sciences (Pharmacy) Graduate Program University of Michigan College of Pharmacy No conflicts of interest.
Barry L. Carter, Department of Pharmacy Practice and Science University of Iowa College of Pharmacy College of Pharmacy and Professor, Department of Family Medicine Roy J. and Lucille A. Carver College of Medicine No conflicts of interest.
REFERENCES
- 1.Rozich JD, Resar RK. Medication safety: one organization's approach to the challenge. J Clin Outcomes Manag. 2001;8(10):27–34. [Google Scholar]
- 2.Rozich JD, Howard RJ, Justeson JM, Macken PD, Lindsay ME, Resar RK. Standardization as a mechanism to improve safety in health care. Jt Comm J Qual Saf. 2004;30(1):5–14. doi: 10.1016/s1549-3741(04)30001-8. [DOI] [PubMed] [Google Scholar]
- 3.Gleason KM, McDaniel MR, Feinglass J, Baker DW, Lindquist L, Liss D, et al. Results of the Medications at Transitions and Clinical Handoffs (MATCH) study: an analysis of medication reconciliation errors and risk factors at hospital admission. J Gen Intern Med. 2010;25(5):441–7. doi: 10.1007/s11606-010-1256-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Green CF, Burgul K, Armstrong DJ. A study of the use of medicine lists in medicines reconciliation: please remember this, a list is just a list. Int J Pharm Pract. 2010;18(2):116–21. [PubMed] [Google Scholar]
- 5.Coleman EA, Smith JD, Raha D, Min SJ. Posthospital medication discrepancies: prevalence and contributing factors. Arch Intern Med. 2005;165(16):1842–7. doi: 10.1001/archinte.165.16.1842. [DOI] [PubMed] [Google Scholar]
- 6.Forster AJ, Murff HJ, Peterson JF, Gandhi TK, Bates DW. The incidence and severity of adverse events affecting patients after discharge from the hospital. Ann Intern Med. 2003;138(3):161–7. doi: 10.7326/0003-4819-138-3-200302040-00007. [DOI] [PubMed] [Google Scholar]
- 7.National Patient Safety Goals, Stat [5 May 2013]; NPSG.03.06.01 2013. Avaliable from: http://www.jointcommission.org/standards_information/npsgs.aspx.
- 8.Chen D, Burns A, editors. ASHP-APhA Medicati on Reconciliation Initiative Workgroup Meeting: Summary and Recommendations. American Society of Health-System Pharmacists; Bethesda, MD: 2007. [Google Scholar]
- 9.Leotsakos A, Caisley L, Karga M, Kelly E, O'Leary D, Timmons K. High 5s: addressing excellence in patient safety. World Hosp Health Serv. 2009;45(2):19–22. [PubMed] [Google Scholar]
- 10.Carter BL, Farris KB, Abramowitz PW, Weetman DB, Kaboli PJ, Dawson JD, et al. The Iowa Continuity of Care study: Background and methods. Am J Health Syst Pharm. 2008;65(17):1631–42. doi: 10.2146/ajhp070600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Anderegg SV, DeMik DE, Carter BL, Dawson JD, Farris K, Shelsky C, et al. Acceptance of recommendations by inpatient pharmacy case managers: unintended consequences of hospitalist and specialist care. Pharmacotherapy. 2013;33:11–21. doi: 10.1002/phar.1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pfeiffer E. A short portable mental status questionnaire for the assessment of organic brain deficit in elderly patients. J Am Geriatr Soc. 1975;(10):433–41. doi: 10.1111/j.1532-5415.1975.tb00927.x. [DOI] [PubMed] [Google Scholar]
- 13.Katz S, Akpom CA. A measure of primary sociobiological functions. Int J Health Serv. 1976;6(3):493–508. doi: 10.2190/UURL-2RYU-WRYD-EY3K. [DOI] [PubMed] [Google Scholar]
- 14.Katz S, Ford AB, Moskowitz RW, Jackson BA, Jaffe MW. Studies of Illness in the Aged. The Index of Adl: A Standardized Measure of Biological and Psychosocial Function. Jama. 1963;185:914–9. doi: 10.1001/jama.1963.03060120024016. [DOI] [PubMed] [Google Scholar]
- 15.Sarason IG, Sarason BR, Shearin EN. Social support as an individual difference variable: Its stability, origins, and relational aspects. J Personality and Social Psychology. 1982;50:845–55. [Google Scholar]
- 16.Carter BL, Ardery G, Dawson JD, James PA, Bergus GR, Doucette WR, et al. Physician and pharmacist collaboration to improve blood pressure control. Arch Intern Med. 2009;169(21):1996–2002. doi: 10.1001/archinternmed.2009.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bandura A. Self-efficacy: The Exercise of Control. W.H. Freeman and Company; New York, NY: 1997. [Google Scholar]
- 18.Morisky DE, Green LW, Levine DM. Concurrent and predictive validity of a self-reported measure of medication adherence. Med Care. 1986;24(1):67–74. doi: 10.1097/00005650-198601000-00007. [DOI] [PubMed] [Google Scholar]
- 19.Morisky DE, Levine DM, Green LW, Shapiro S, Russell RP, Smith CR. Five-year blood pressure control and mortality following health education for hypertensive patients. Am J Public Health. 1983;73(2):153–62. doi: 10.2105/ajph.73.2.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schnipper JL, Kirwin JL, Cotugno MC, Wahlstrom SA, Brown BA, Tarvin E, et al. Role of pharmacist counseling in preventing adverse drug events after hospitalization. Arch Intern Med. 2006;166(5):565–71. doi: 10.1001/archinte.166.5.565. [DOI] [PubMed] [Google Scholar]
- 21.Ho PM, Lambert-Kerzner A, Carey EP, Fahdi IE, Bryson CL, Melnyk SD, et al. Multifaceted Intervention to Improve Medication Adherence and Secondary Prevention Measures After Acute Coronary Syndrome Hospital Discharge: A Randomized Clinical Trial. JAMA Intern Med. Nov. 2013;18:1–8. doi: 10.1001/jamainternmed.2013.12944. [DOI] [PubMed] [Google Scholar]
- 22.Isetts BJ, Brummel AR, de Oliveira DR, Moen DW. Managing drug-related morbidity and mortality in the patient-centered medical home. Med Care. 2012;50(11):997–1001. doi: 10.1097/MLR.0b013e31826ecf9a. [DOI] [PubMed] [Google Scholar]
- 23.Cornish PL, Knowles SR, Marchesano R, Tam V, Shadowitz S, Juurlink DN, et al. Unintended medication discrepancies at the time of hospital admission. Arch Intern Med. 2005;165(4):424–9. doi: 10.1001/archinte.165.4.424. [DOI] [PubMed] [Google Scholar]