Abstract
Objectives: The purpose of this study was to assess long-term improvement in quality of life (QOL) in adolescents with attention-deficit/hyperactivity disorder (ADHD) treated with lisdexamfetamine dimesylate (LDX).
Methods: Adolescents with ADHD treated for ≥3 weeks in a 4 week, placebo-controlled study entered a 1 year, open-label study. After the 4 week dose optimization (30, 50, and 70 mg/day LDX) period, treatment was maintained for 48 additional weeks. Change from baseline (of prior study) to week 52/early termination (ET) (of open-label study) in ADHD Rating Scale IV (ADHD-RS-IV) assessed effectiveness, and the Youth QOL-Research Version (YQOL-R) assessed participant-perceived QOL. Post-hoc analyses described effectiveness and QOL for participants with self-perceived poor QOL at baseline (≥1 SD below the mean) versus all others, and for study completers versus study noncompleters.
Results: These post-hoc analyses included 265 participants. Participants with baseline self-perceived poor QOL (n=32) versus all others (n=232) exhibited robust YQOL-R perceptual score changes (improvement) with LDX, emerging by week 28 and maintained to week 52/ET. Week 52/ET mean change score ranged from +9.8 to +17.6 for participants with baseline self-perceived poor QOL and +0.4 to +5.1 for all others; week 52/ET improvements in ADHD-RS-IV total scores were similar, regardless of baseline YQOL-R total score. At week 52/ET, study completers had greater YQOL-R improvements than did noncompleters; ADHD-RS-IV total score changes were also numerically larger at week 52/ET for completers than for noncompleters.
Conclusion: Participant-perceived QOL and ADHD symptoms improved from baseline with LDX in adolescents with ADHD; greatest improvements occurred among participants with baseline self-perceived poor QOL.
Introduction
Attention-deficit/hyperactivity disorder (ADHD) is a childhood-onset neurobehavioral disorder with symptoms often continuing into adolescence and beyond (Pliszka 2007). In 2010, prevalence in the United States of parent-reported ADHD diagnosis was 11.2% for adolescents 11–14 years of age and 13.6% for those 15–17 years of age (Centers for Disease Control and Prevention 2010). Adolescents with ADHD are at risk for poor life outcomes, such as academic failure, emotional problems, delinquency, teenage pregnancy, sexually transmitted infections, early onset of substance abuse, and poor driving records (Schubiner and Katragadda 2008).
Although it is well established that psychostimulant and nonpsychostimulant therapy effectively improve core symptoms of hyperactivity/impulsivity and inattention (Greenhill et al. 2002; Pliszka 2007), detailed information about the impact of ADHD treatment on adolescent life outcomes and quality of life (QOL) is limited. With the growing recognition that ADHD often persists into adulthood, researchers have begun to examine the effects of ADHD and its treatment on broader life outcomes, beyond core symptoms. Decreased QOL among individuals with ADHD is well established (Klassen et al. 2004; Topolski et al. 2004; Zambrano-Sanchez et al. 2012), with QOL negatively correlated with symptom severity (Klassen et al. 2004; Matza et al. 2004). A meta-analysis of five clinical trials (three randomized, double-blind, placebo-controlled trials; two open-label trials) of the nonstimulant medication atomoxetine (all treatment durations: 8–12 weeks) showed large baseline impairments in QOL among adolescents with ADHD when only placebo-controlled trials were assessed and when all trials were assessed (placebo-controlled+open-label); impairment was particularly evident in the areas of self-esteem, family involvement, and academic achievement (Wehmeier et al. 2010). Following 8–12 weeks of treatment with atomoxetine, moderate correlations were detected between improvements in core ADHD symptoms and QOL scores, suggesting that treatment-related amelioration of core symptoms may contribute, over time, to broader improvements in patient QOL (Wehmeier et al. 2010); in particular, adolescents in the three placebo-controlled trials exhibited significant improvements in risk avoidance and threats to achievement. Similarly, pooled analyses from two published 12 week open-label trials examining psychostimulant medications in children and adolescents (Berek et al. 2011) and a 4 week trial in adults (Brams et al. 2011) reported improved QOL after treatment. In the 12 week pooled analyses, the largest changes reported by adolescents were improved school performance and decreased burden of disease (Berek et al. 2011).
Patients with ADHD may experience serious and long-lasting effects of functional problems, typically emerging during the adolescent years (Barkley et al. 2002; Schubiner and Katragadda 2008); therefore, it is important to determine the potential impact of treatment on QOL in adolescents with ADHD. A previously reported 4 week, randomized, double-blind, placebo-controlled trial (Findling et al. 2011) of lisdexamfetamine dimesylate (LDX), a long-acting prodrug stimulant indicated for ADHD in children (ages 6–12 years), adolescents (ages 13–17 years), and adults, reported greater improvement in ADHD symptoms from baseline on Attention-Deficit/Hyperactivity Disorder Rating Scale IV (ADHD-RS-IV) total score at the end of the study, versus placebo. However, greater improvements were not detected in participant-perceived QOL based on the Youth QOL-Research Version (YQOL-R) for any LDX dose (mean [SD] baseline value across doses: 79.5 [12.24]; mean end-point value across doses: 81.2 [12.53]) versus placebo (mean baseline value: 79.2 [11.08]; mean end-point value: 81.3 [12.16]). The reason for failure to observe QOL improvements despite improvements in symptoms with LDX treatment in this trial is unclear, although the short study duration (4 weeks) may not have permitted enough time for improvements in core symptoms to have an effect on broader functioning.
This short-term, randomized, placebo-controlled trial was followed by an additional 52 week open-label investigation. Primary safety and effectiveness findings from this open-label trial have been previously reported (Findling et al. 2013). In brief, the safety and tolerability profile of LDX in adolescents was found to be comparable to that in other long-term studies of LDX in children and adults (Findling et al. 2008; Weisler et al. 2009). Treatment-emergent adverse events (AEs) occurring in >10% of study participants included upper respiratory tract infection, decreased appetite, headache, decreased weight, irritability, and insomnia; vital sign assessment revealed modest increases in systolic blood pressure, diastolic blood pressure, and pulse rate at study end-point (Findling et al. 2013). In terms of effectiveness, mean change from baseline on ADHD-RS-IV total score with LDX at the end of the study was significant, as was the mean change from baseline on the transformed YQOL-R perceptual total score. However, because mean baseline YQOL-R scores for the participant sample as a whole did not indicate overall self-perceived poor QOL, a potential ceiling effect may have made it difficult to detect more robust treatment-related improvements in QOL. We therefore conducted a post-hoc analysis of this 52 week open-label study to explore QOL improvements in those participants who perceived their QOL as being poor (i.e., self-perceived poor QOL) at baseline.
Methods
Study design and overview
This 52 week, open-label, multicenter, single-arm extension investigation was designed to assess effectiveness and safety of clinically optimized doses of LDX (30, 50, or 70 mg/day) in adolescents with ADHD who had just completed a 4 week, randomized controlled trial. The preceding forced-dose-titration trial randomized participants to treatment with placebo or one of the three LDX doses. Baseline effectiveness and safety parameters were those from the baseline of the preceding 4 week study, and the enrollment visit of the current study was the final visit of the 4 week study. Participants who completed 3 weeks of the 4 week study and did not discontinue because of AEs or nonadherence were recruited at their week 3 visit. During the follow-up study, weeks 1–4 were for dose optimization, with each participant initiated at LDX 30 mg/day and having weekly (7±2 days) visits for dose titration in 20 mg/day increments, based on investigators' judgment of clinical response and tolerability. During the maintenance phase (48 weeks), evaluations occurred every 28±5 days, and the optimized LDX dose was either continued or adjusted.
Signed informed consent was provided by a parent or guardian, and participants signed documentation of assent to indicate that they were aware of the study procedures and restrictions. This study was conducted in accordance with the Declaration of Helsinki and International Conference on Harmonization Good Clinical Practice Guideline E6. In addition, the institutional review board of each institution reviewed and approved the consent form and the protocol.
Key inclusion and exclusion criteria
Participant selection criteria for the short-term antecedent trial have been described in detail previously (Findling et al. 2011). Briefly, eligible participants were 13–17 years of age, with a baseline ADHD-RS-IV total score ≥28 (DuPaul et al. 1998). To participate in the 52 week extension trial, participants were required to have reached visit 3 in the antecedent study without experiencing clinically significant AEs that would preclude further exposure to LDX. Key exclusion criteria included major psychiatric disorders as a comorbidity (with the exception of oppositional defiant disorder); concurrent chronic or acute, or unstable general medical condition; serious cardiac abnormalities; seizure or tic disorder; and hypersensitivity, intolerance, or prior history of nonresponse to amphetamine (Findling et al. 2011).
QOL assessment
The participant-reported YQOL-R, an additional secondary effectiveness measure, is a participant-completed 56 item generic instrument for adolescents, including those with physical/psychiatric disabilities (Edwards et al. 2002). Although not specifically designed for assessing QOL in adolescents diagnosed with ADHD (Edwards et al. 2002), the YQOL-R has been validated for this population (Patrick et al. 2002). This instrument consists of contextual (potentially verifiable by others) and perceptual (known only to participants) questions (Topolski et al. 2004). The YQOL-R was completed by participants at baseline and week 4 (of the antecedent study) and was reassessed at week 28 and at the final study visit (week 52 for study completers; the last available assessment/early termination [ET] for those not completing the study) in the current study; YQOL-R score at baseline of the antecedent study served as the baseline score for the current study. Although the contextual items may be useful in assessing the impact of ADHD and changes in QOL with intervention, the authors of the instrument describe the 41 perceptual items as the “heart of the YQOL-R” (Topolski et al. 2004). Validation of the instrument also focused on the perceptual domains (Patrick et al. 2002). Hence, the current analysis presents data from only YQOL-R perceptual items (Edwards et al. 2002; Patrick et al. 2002). Perceptual items comprise four domain scores that include self (adolescents' sense of self ), relationships (family and peer relationships), environment (engagement and participation in life activities), and general QOL (overall enjoyment and satisfaction with life), as well as a total perceptual score (Edwards et al. 2002; Patrick et al. 2002). YQOL-R raw scores are each transformed to a 100 point scale to aid interpretation. Higher scores indicate better QOL (Patrick et al. 2002).
Post-hoc statistical analyses
Analyses were performed on the full analysis set, which consisted of all participants who had taken at least one dose of study medication and had had at least one valid post-visit 1 ADHD-RS-IV assessment. Based on a two sided, one sample t test, week 52/ET changes from baseline ADHD-RS-IV total and YQOL-R scores were assessed. The post-hoc analyses were also conducted on the full analysis set. The study was not powered to detect statistically significant differences among subgroups; therefore, only descriptive statistics (e.g., mean, median, SD, standard error, 95% confidence interval [CI]) for the YQOL-R total score and its domains and for the ADHD-RS-IV were calculated post hoc.
Among participants stratified by baseline QOL score (participants with baseline YQOL-R scores ≤1 SD from the overall mean vs. all others) results by baseline QOL might reveal a potential ceiling effect of those with normal or near-normal QOL at baseline. The final study visit value included week 52 assessments for completers and ET assessments for noncompleters. Because QOL, at baseline and during the study, might affect the decision to continue participation, ADHD-RS-IV total scores and YQOL-R scores at baseline and change from baseline at week 52/ET were calculated for study completers versus noncompleters.
Results
Demographics and baseline characteristics
Detailed participant demographics and disposition for this study population, as well as primary safety and effectiveness findings for both the antecedent study and the current long-term study have been reported previously (Findling et al. 2011, 2013). A total of 265 participants were included in these post-hoc analyses. According to post-hoc stratification of participants by perceived QOL at baseline, 32 had a YQOL-R total perceptual score ≤1 SD of the mean (self-perceived poor QOL), and 232 had a YQOL-R total perceptual score above that level; stratification of participants by trial completion status yielded 156 study completers and 109 study noncompleters.
Effectiveness: Post-hoc analyses
Participants with self-perceived poor QOL at baseline (n=32) had a mean (SD) transformed YQOL-R total perceptual score of 57.6 (9.84) at baseline versus 82.8 (7.38) for the others (n=232). With LDX treatment, those with self-perceived poor QOL exhibited numerically greater change in transformed YQOL-R total perceptual score at all time points assessed (ranging from 12.5 to 17.6) than those categorized as others (2.9–3.8) (Table 1). Numerically greater mean YQOL-R improvements at week 52/ET were also seen in YQOL-R domain scores for those with self-perceived poor QOL (9.8–17.6) versus the others (0.4–5.1).
Table 1.
Transformeda YQOL-R Perceptual Scores and Change from Baselineb Scores Stratified by Participant-Perceived QOL at Baseline or by Study Completion Status
| Stratified by participant-perceived QOL at baseline | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Poorc QOL (n=32) | Othersd (n=232) | ||||||||
| Observed value | Change from baselineb score | Observed value | Change from baselineb score | ||||||
| Variables | Visit | n | Mean (SD) | n | Mean (SD) | n | Mean (SD) | n | Mean (SD) |
| Transformeda YQOL-R total perceptual score | Baselineb | 32 | 57.6 (9.84) | - | - | 232 | 82.8 (7.38) | - | - |
| Week 28 | 20 | 75.2 (10.63) | 20 | 17.6 (9.51) | 160 | 85.9 (8.49) | 160 | 3.0 (7.93) | |
| Week 52 | 17 | 70.9 (14.24) | 17 | 14.7 (11.16) | 136 | 86.7 (7.89) | 136 | 3.8 (8.59) | |
| Week 52/ET | 26 | 70.0 (15.29) | 26 | 12.5 (13.02) | 211 | 85.6 (9.03) | 211 | 2.9 (8.72) | |
| Transformeda YQOL-R domain scores | |||||||||
| Self | Baselineb | 32 | 53.6 (10.96) | - | - | 232 | 70.4 (9.05) | - | - |
| Week 52/ET | 26 | 63.7 (11.77) | 26 | 9.8 (13.55) | 211 | 75.5 (8.72) | 211 | 5.1 (9.83) | |
| Relationships | Baselineb | 32 | 58.0 (16.11) | - | - | 232 | 84.2 (9.89) | - | - |
| Week 52/ET | 26 | 69.0 (17.73) | 26 | 11.4 (16.43) | 211 | 86.8 (11.16) | 211 | 2.7 (10.83) | |
| Environment | Baselineb | 32 | 62.2 (14.15) | - | - | 232 | 85.5 (9.44) | - | - |
| Week 52/ET | 26 | 72.8 (16.62) | 26 | 11.4 (12.63) | 211 | 88.9 (10.33) | 211 | 3.3 (10.09) | |
| General | Baselineb | 32 | 56.6 (17.79) | - | - | 232 | 91.3 (10.10) | - | - |
| Week 52/ET | 26 | 74.4 (22.45) | 26 | 17.6 (22.26) | 211 | 91.4 (11.73) | 211 | 0.4 (13.48) | |
| Stratified by completerse vs. noncompletersf of current study | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Study completerse (n=156) | Study noncompletersf (n=109) | ||||||||
| Observed value | Change from baselineb score | Observed value | Change from baselineb score | ||||||
| Variables | Visit | n | Mean (SD) | n | Mean (SD) | n | Mean (SD) | n | Mean (SD) |
| Transformeda YQOL-R Total Perceptual Score | Baselineb | 155 | 79.9 (11.66) | - | - | 109 | 79.6 (10.76) | - | - |
| Week 52/ET | 154 | 84.9 (10.04) | 153 | 5.0 (9.52)g | 82 | 82.1 (12.55) | 82 | 1.9 (9.64) | |
| Transformeda YQOL-R domain scores | |||||||||
| Self | Baselineb | 155 | 68.4 (11.24) | - | - | 109 | 68.3 (10.12) | - | - |
| Week 52/ET | 154 | 74.6 (9.45) | 153 | 6.2 (10.38)g | 82 | 73.6 (10.32) | 82 | 4.9 (10.18) | |
| Relationships | Baselineb | 155 | 81.1 (14.45) | - | - | 109 | 80.9 (12.79) | - | - |
| Week 52/ET | 154 | 85.8 (12.59) | 153 | 4.7 (11.58)g | 82 | 83.0 (14.27) | 82 | 1.8 (12.03) | |
| Environment | Baselineb | 155 | 83.3 (12.93) | - | - | 109 | 81.8 (12.22) | - | - |
| Week 52/ET | 154 | 88.4 (11.03) | 153 | 5.1 (10.42)g | 82 | 84.9 (14.02) | 82 | 2.4 (10.86) | |
| General | Baselineb | 155 | 86.8 (16.06) | - | - | 109 | 87.5 (15.95) | - | - |
| Week 52/ET | 154 | 90.9 (12.00) | 153 | 4.0 (14.47)g | 82 | 86.9 (17.65) | 82 | –1.4 (16.64) | |
The YQOL-R raw score is transformed to a 0–100 point scale to assist in result interpretation; higher scores indicate improvement in QOL.
Baseline is based on the baseline of the antecedent 4 week study.
Poor=participants with YQOL-R scores ≤(mean−1 SD).
Others=participants with YQOL-R scores >(mean−1 SD).
Study completers=participants who completed the current study.
Study noncompleters=participants who did not complete the current study.
p≤0.001 based on a two sided one sample t test.
ET, early termination; QOL, quality of life; YQOL-R, Youth Quality of Life-Research Version.
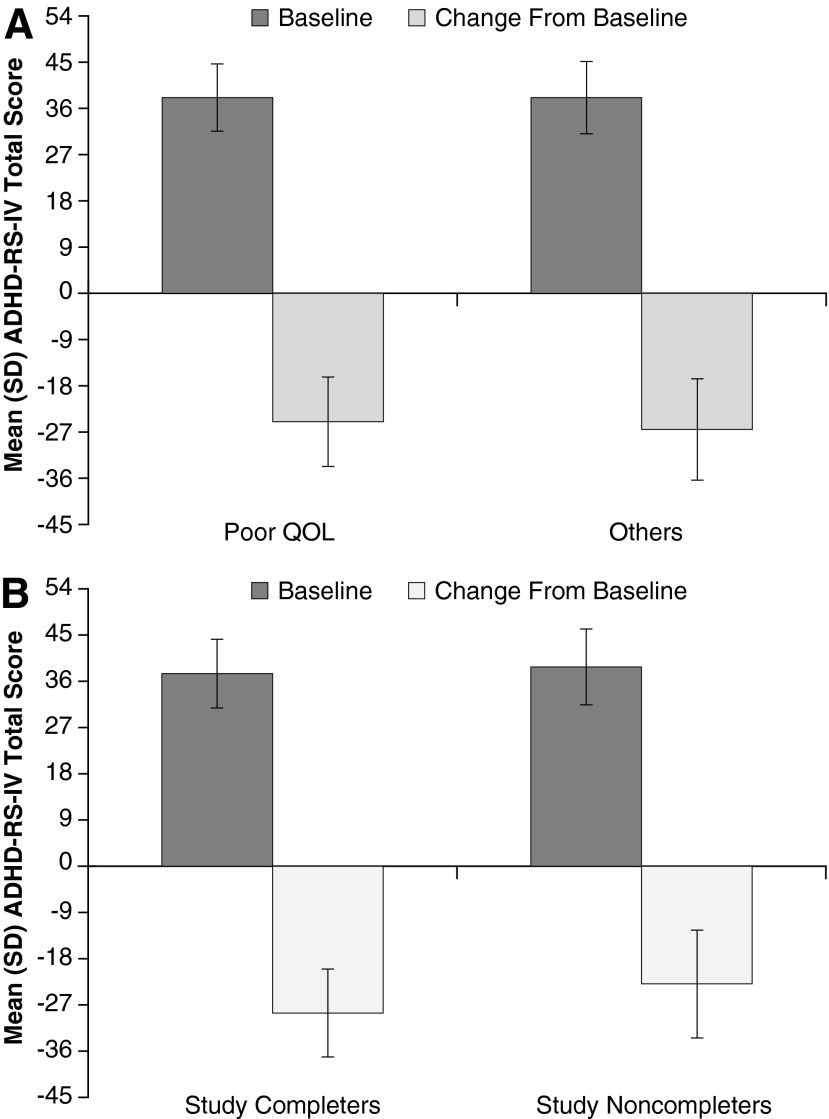
Mean ADHD-RS-IV total score at baseline and change scores at week 52/ET, as stratified by baseline participant-perceived QOL, were similar for participants with poor baseline YQOL-R scores and others (Fig. 1A).
FIG. 1.
Mean (SD) ADHD-RS-IV total score in the full analysis set at baselinea and change from baseline scoreb stratified by (A) transformed participant-perceived QOL at baseline or (B) completersc versus noncompletersd of the current study. aBaseline score is based on the baseline of the antecedent 4 week study. bChange from baseline for study completers was assessed at week 52 and for noncompleters at ET. cStudy completers=participants who completed the current study. dStudy noncompleters=participants who did not complete the current study. ADHD-RS-IV, Attention-Deficit/Hyperactivity Disorder Rating Scale IV; ET, early termination; QOL, quality of life.
Study completers (n=156) and noncompleters (n=109) showed similar baseline transformed YQOL-R perceptual total scores. At week 52/ET, completers were noted to have numerically greater improvements from baseline in transformed YQOL-R scores than did noncompleters (Table 1). Also, baseline mean ADHD-RS-IV total scores for study completers were similar to the scores of study noncompleters; however, changes in ADHD-RS-IV total scores were numerically larger at week 52/ET for study completers than for study noncompleters (Fig. 1B).
Discussion
Findings overview
In a previous publication (Findling et al. 2013), the primary safety and efficacy data from this 52 week open-label extension trial were reported. Overall, the safety and tolerability profile of LDX in adolescents was found to be comparable with that seen in other long-term studies of LDX in children and adults (Findling et al. 2008; Weisler et al. 2009). Furthermore, in the overall study population, LDX demonstrated effectiveness, as measured by significant reductions from baseline on ADHD-RS-IV total score and by small reductions from baseline on the transformed YQOL-R perceptual total score (Findling et al. 2013).
In the current study, the mean (SD) baseline transformed YQOL-R perceptual total score in the overall population was 79.8 (11.28) (Findling et al. 2013), which is numerically higher than the estimated marginal mean (SE) scores observed in individuals with ADHD (75.19 [1.48]) in the initial validation study of the YQOL-R, but numerically lower than was observed in individuals with no self-reported chronic condition (82.2 [1.14]) (Patrick et al. 2002). This suggests that the overall population in the current study exhibited slightly impaired QOL at baseline. At week 52/ET of the current study, the mean (SD) transformed YQOL-R perceptual total score of 83.9 (11.00) for the overall population (Findling et al. 2013) was somewhat higher than, but generally comparable with, the estimated marginal mean score in individuals without a self-reported chronic condition in the validation study (Patrick et al. 2002). The clinical significance of changes in YQOL-R scores has not been fully established; however, a systematic literature review reported that a change of 0.5 SD from the mean was indicative of a minimally important difference in health-related QOL measures, regardless of the scale response type or length, or whether the fact that the scale was generic versus disease-specific was clinically relevant (Norman et al. 2003). Therefore, the previously reported YQOL-R change in the overall population approached, but did not reach, a clinically meaningful improvement during open-label LDX treatment.
In the current post-hoc analysis, participants with self-perceived poor QOL at baseline (i.e., individuals with transformed YQOL-R perceptual total scores ≥1 SD below the overall mean) exhibited marked impairment compared with the study populations described in the initial validation study (Patrick et al. 2002), as evidenced by mean (SD) transformed YQOL-R perceptual total scores of 57.6 (9.84) at baseline. During open-label LDX treatment, substantial YQOL-R improvements were observed at week 28 (mean [SD] score, 75.2 [10.63]; mean [SD] change from baseline, 17.6 [9.51] points) and week 52/ET (mean [SD] score, 70.0 [15.29]; mean [SD] change from baseline, 12.5 [13.02] points). Based on a minimally important difference threshold of 0.5 SD, the improvements in QOL in those with self-perceived poor QOL at baseline would be considered to be clinically relevant. In contrast, impaired QOL was not observed in the other study participants at baseline (mean [SD] score, 82.8 [7.38]), and little or no change in transformed YQOL-R perceptual total scores was observed among other study participants (mean [SD] week 28 score, 85.9 [8.49], change from baseline, 3.8 [7.93] points; mean [SD] week 52/ET score, 85.6 [9.03], change from baseline, 2.9 [8.72] points).
Post-hoc analysis also showed that among participants who completed the 52 week trial, YQOL-R change scores at week 28 and week 52/ET were numerically greater among completers than among those who discontinued early, but the magnitude of improvements in both completers and noncompleters was low. ADHD symptom improvement at week 52/ET, based on the ADHD-RS-IV, was comparable regardless of baseline QOL, and was numerically greater among study completers than among noncompleters.
Interpretation and implications
The broad improvements in participant-perceived QOL observed currently are in line with those described previously with psychostimulant and nonstimulant treatment in children, adolescents, and adults with ADHD (Spencer et al. 2008; Cannon et al. 2009; Gerwe et al. 2009; Brown and Landgraf 2010; Wehmeier et al. 2010; Berek et al. 2011; Brams et al. 2011). The current investigation, however, differs from prior research on QOL in ADHD in several important aspects, adding to existing evidence and raising new questions. Most of these prior reports have been based on short-term or medium-term studies, ranging from 4 to 12 weeks in duration. To our knowledge, this is the first report to describe adolescent participant-perceived QOL following up to 1 year of psychostimulant treatment. For the overall study population, significant improvements in transformed YQOL-R total and domain scores were seen at week 52/ET, with the largest improvements reported for the self and environment domains (Findling et al. 2013). The self domain assesses individuals' perceptions about their own coping behaviors, inner strength, and feelings of self-esteem. The environment domain ascertains individuals' perceived safety and enjoyment within their daily milieu at home, at school, and in the neighborhood (Patrick et al. 2002). At week 52/ET in the current trial, mean (SD) improvement in self domain scores was 5.7 (10.37) and in the environment domain was 4.1 (10.68). This contrasts with QOL findings from the short-term antecedent trial, with 4 weeks of forced-dose titration LDX, in which YQOL-R total and domain scores at week 52/ET showed small improvements that were not statistically significantly different from those seen with placebo (Findling et al. 2011).
It seems likely that the longer duration of treatment and the use of clinically optimized LDX dosing accounted for the differences in QOL outcomes between the studies. QOL was only assessed after four time points across these two trials (baseline and week 4 of the antecedent trial, weeks 28 and 52/ET of this open-label trial). Therefore, the complete time course of QOL improvements with long-term LDX treatment is not known; assessment at earlier time points would have provided additional insight. However, it should be noted that discrepancies in the effects of treatment on ADHD symptomatology and QOL are not uncommon, most likely because of the multiple factors than can influence QOL. For example, in a 7 week open-label extension study of long-acting methylphenidate, improvements in hyperactivity and impulsivity were associated with improved QOL, but improvements in inattention were not (Buitelaar et al. 2012).
As noted, minimally important differences in health-related QOL measures are typically found at 0.5 SD from the mean (Norman et al. 2003). Therefore, when interpreting the YQOL-R changes observed in this study, a 0.5 SD change from the baseline mean transformed YQOL-R perceptual total score corresponds to ∼5.5 points (Findling et al. 2013). In the current post-hoc analysis, mean changes in individuals with poor self-perceived QOL substantially exceeded this threshold, mean changes in study completers were roughly comparable to this threshold, and mean changes in those without self-perceived poor QOL and in study noncompleters did not meet this threshold.
The current post-hoc analysis identified participant-perceived QOL at baseline as a potentially important moderator of QOL outcomes. An impetus for the current analysis was the failure in the antecedent trial to detect an improvement in QOL given short-term LDX treatment. Baseline YQOL-R scores from the antecedent trial suggested limited QOL impairment perceived among participants. This represented a potential ceiling effect and, therefore, a limited ability to observe QOL improvements, particularly over the short duration of the antecedent study. To address this challenge, post-hoc analyses were conducted based on stratified subgroups: participants with baseline self-perceived poor QOL, and those with average baseline perceived QOL. As expected, those with average perceived QOL (others group), who comprised the large majority of the sample (n=232), showed small improvements (range, 0.4–5.1 at week 52/ET) in QOL with up to 52 weeks of LDX treatment. In sharp contrast, among the subgroup of participants with self-perceived poor QOL at baseline (n=32), large improvements in YQOL-R total and domain scores were seen (range, 9.8–17.6 at week 52/ET); these improvements are approximately two- to threefold larger than those observed based on analysis of the sample overall. Such observations support the notion that ceiling effects in the short-term study related to biased self-perceived symptomatology, as has previously been described in adolescents (Wolraich et al. 2005), artificially elevated QOL scores and likely limited the ability to detect QOL improvements. The current findings also suggest that baseline patient-perceived QOL can strongly influence outcomes, and is an important factor that should be accounted for in future investigations.
Despite the relatively high level of perceived QOL among most current study participants, there are well-established functional impairments and poor life outcomes seen in adolescents with ADHD (Schubiner and Katragadda 2008). The high level of perceived QOL reported by most participants, despite all having moderate to marked ADHD symptoms at baseline, may be caused by lack of patient insight. As alluded to, previous research has found that adolescents with ADHD often underreport symptoms, suggesting a lack of insight into their condition; therefore, patient self-reported QOL in this population may not provide an accurate assessment (Wolraich et al. 2005), In one survey investigation, young adults with ADHD reported having a mean of only two core symptoms, whereas parents reported a mean of nine symptoms for their adult child (Barkley et al. 2002). The high level of QOL reported by participants currently may also be related to potential insensitivity of the YQOL-R for detecting self-perceived ADHD-related QOL impairments. The YQOL-R is designed to assess general QOL in adolescents and was validated in both normal probands in the community as well as in adolescents with a range of chronic disabilities, including ADHD. QOL instruments tailored to adolescents with ADHD are needed, similar to the ADHD Impact Module designed for adult patients (AIM-A) (Landgraf 2007).
There is increasing recognition of the importance of evaluating the impact of therapy on QOL (Spencer et al. 2008; Wehmeier et al. 2010); relief of core clinical symptoms may not necessarily lead to “normalized” functioning. It may be more clinically meaningful to understand how a patient perceives the effects of treatment, compared with parent/teacher-reported outcomes typically used in pediatric studies. Moreover, adolescent/young adult perspectives on QOL and the impact of treatment may play a role in decisions to continue treatment as well as the perceived value of such treatment (Spencer et al. 2008). The current findings highlight the fact that QOL impairments in adolescents with ADHD are not fully understood; limited baseline and treatment-related QOL data are available for this clinical population. To gain greater understanding of disease-specific and patient-relevant information on QOL in this group of patients in future investigations, it may also be useful to pair disease-specific QOL instruments, such as the AIM-A, which has frequently been used in adult patients with ADHD, with more general health-related QOL instruments, such as the Short-Form 36 (Ware 1994).
Limitations
Several study limitations should be considered when interpreting the current findings. This investigation employed an open-label treatment design, raising the possibility of positive results caused by expectation bias on the part of participants and investigators when reporting and/or assessing improvement. In addition, open-label extension studies present an additional potential bias because participants who advance to the open-label phase are not advancing at random. Unlike the antecedent short-term trial, the current investigation enrolled participants who had tolerated and/or responded to either placebo or LDX in the antecedent study. Those who had experienced intolerable side effects with placebo or LDX, or whose symptoms had responded poorly to study treatment were unlikely to have enrolled, creating a nonrandom sample. Moreover, a potential limitation of these data is the fact that parent report was not used to assess QOL. With regard to reporting of symptoms, discrepancies between participant self-report and parent-report data have been reported, with parent-reported data being considered to be more diagnostically sensitive than self-reports (Sibley et al. 2012). Because QOL was a secondary end-point, planned evaluation and statistical analyses were limited, and the post-hoc nature of this analysis indicates that these data should be interpreted with caution until they are confirmed in trials designed to prospectively assess these end-points. Moreover, the QOL assessment instrument used currently, the YQOL-R, is not ADHD-specific, although it has been validated in adolescents with ADHD (Patrick et al. 2002). Most participants reported only mild or moderate perceived QOL impairments at baseline; the current post-hoc analysis indicates that this may have created a ceiling effect, limiting our ability to detect improvements with LDX treatment in the overall sample. Also, the potential impact of regression to the mean contributing to the observed differences between subgroups cannot be discounted.
Conclusions
Adolescents with ADHD who received up to 52 weeks of treatment with clinically optimized doses of LDX exhibited statistically significant improvements in QOL. Post-hoc analysis showed that, at week 52/ET, those participants with baseline self-perceived poor QOL exhibited robust QOL improvements across all domains examined versus modest improvements among other participants who reported average to normal baseline perceived QOL. Future research regarding the impact of medication treatment on perceived QOL in patients with ADHD should take into account baseline perceived QOL as a critical moderating factor.
Clinical Significance
The current findings highlight the potential challenges faced by clinicians in raising the awareness of adolescent and young adult patients with ADHD about the impact of their symptoms on daily functioning and the value of comprehensive disease management. Most adolescents with ADHD in the current investigation did not perceive QOL impairments at baseline, and reported average/normal QOL throughout the 52 week trial. A small subgroup of participants, however, perceived poor QOL at baseline and reported large QOL improvements with LDX treatment, which were maintained throughout the trial. For adolescent and young adult patients with ADHD to begin to independently manage their ADHD, clinicians can play an active role in helping them gain insight into how core symptoms and ADHD treatment may affect their broader personal lives and daily functioning.
Acknowledgments
Under the direction of the authors, Dr. Michael Pucci, an employee of Scientific Communications & Information (SCI), Dr. Karen Dougherty, a former employee of SCI, and Dr. Craig Slawecki and Amanda Kelly, employees of Complete Healthcare Communications, Inc., provided writing assistance for this publication. Editorial assistance in the form of proofreading, copy editing, and fact checking was also provided by SCI. Dr. Thomas Babcock, from Shire Development LLC, also reviewed and edited the manuscript for scientific accuracy; Mohamed Hamdani, from Shire Development LLC, analyzed the data.
Departments and institutions where work was done: Clinical Study Centers, LLC, Little Rock, AR; Valley Clinical Research, Inc., El Centro, CA; Peninsula Research Associates, Inc., Rolling Hills Estates, CA; Psychiatric Centers at San Diego, San Diego, CA; Elite Clinical Trials, Inc., Wildomar, CA; Florida Clinical Research Center, LLC, Bradenton, FL; Sarkis Clinical Trials, Gainesville, FL; Amedica Research Institute, Inc., Hialeah, FL; Clinical Neuroscience Solutions, Inc., Jacksonville, FL; Clinical Neuroscience Solutions, Inc., Orlando, FL; Miami Research Associates, South Miami, FL; Janus Center for Psychiatric Research, West Palm Beach, FL; Atlanta Center for Medical Research, Atlanta, GA; Northwest Behavioral Research Center, Marietta, GA; Capstone Clinical Research, Libertyville, IL; Clinco Inc., Terre Haute, IN; Psychiatric Associates, Overland Park, KS; Vince and Associates Clinical Research, Inc., Overland Park, KS; Cientifica, Inc, Wichita, KS; Pedia Research LLC, Owensboro, KY; Four Rivers Clinical Research Inc., Paducah, KY; Louisiana Research Associates, Inc., New Orleans, LA; Bart Sangal, Sterling Heights, MI; Triangle Neuropsychiatry, PLLC, Durham, NC; Innovis Health/Odyssey Research, Fargo, ND; Children's Specialized Hospital, Toms River, NJ; Bioscience Research LLC, Mount Kisco, NY; Center for Psychiatry and Behavioral Medicine, Inc., Las Vegas, NV; University Hospitals of Cleveland, Division of Child and Adolescent Psychiatry, Cleveland, OH; IPS Research Company, Oklahoma City, OK; OCCI Inc. (Oregon Center for Clinical Investigations, Inc.), Eugene, OR; Summit Research Network, Portland, OR; OCCI Inc., Salem, OR; CRI Worldwide, Philadelphia, PA; Western Psychiatric Institute and Clinic, Pittsburgh, PA; CNS Healthcare, Memphis, TN; Futuresearch Trials, Austin, TX; Bayou City Research, Ltd, Houston, TX; Red Oak Psychiatry Associates P.A., Houston, TX; ADHD Clinic of San Diego, San Antonio, TX; Vermont Clinical Study Center, Burlington, VT; Neuropsychiatric Associates, Woodstock, VT; Neuroscience, Inc., Herndon, VA; Dominion Clinical Research, Midlothian, VA; Northwest Clinical Research Center, Bellevue, WA.
Disclosures
Dr. Childress receives or has received research support from and acted as a consultant and/or speaker for Abbott, Bristol-Myers Squibb, GlaxoSmithKline, Ironshore, Johnson & Johnson Pharmaceutical Research & Development, LLC, Lilly USA, LLC, NextWave, Novartis, Ortho-McNeil Janssen Scientific Affairs, Rhodes, Sepracor, Shire, Somerset, Sunovion, Theravance, and Pfizer. Dr. Cutler receives or has received research support from and acted as a consultant and/or speaker for Cephalon, Euthymics, Genomind, GlaxoSmithKline, Johnson & Johnson PRD, Lilly, Neo Therapeutics, NextWave, Novartis, Noven, Ortho-McNeil-Janssen, Otsuka, Pfizer, Rhodes, Shionogi, Shire, Sunovion, Supernus, Targacept, and Theravance. Dr. Saylor receives or has received research support from and acted as a consultant for Abbott, AstraZeneca, Bristol-Myers Squibb, Cephalon, Lilly, Johnson & Johnson, Merck, Novartis, Otsuka, Psychogenics, Shire, Sunovian, and Supernus. Dr. Gasior is a Shire employee and holds stock and/or stock options in Shire. Mr. Hamdani is a Shire employee and holds stock and/or stock options in Shire. Dr. Ferreira-Cornwell is a Shire employee and holds stock and/or stock options in Shire. Dr. Findling receives or has received research support from, and acted as a consultant and/or served on a speaker's bureau for Alexza Pharmaceuticals, American Psychiatric Press, AstraZeneca, Bracket, Bristol-Myers Squibb, Clinsys, Cognition Group, Dana Foundation, Forest, GlaxoSmithKline, Guilford Press, Johns Hopkins University Press, Johnson & Johnson, KemPharm, Lilly, Lundbeck, Merck, NIH, Novartis, Noven, Otsuka, Oxford University Press, Pfizer, Physicians Postgraduate Press, Rhodes Pharmaceuticals, Roche, Sage, Seaside Pharmaceuticals, Shire, Stanley Medical Research Institute, Sunovion, Supernus Pharmaceuticals, Transcept Pharmaceuticals, Validus, and WebMD.
Although the sponsor was involved in the design, collection, analysis, interpretation, and fact checking of information, the content of this manuscript, the ultimate interpretation, and the decision to submit it for publication in the Journal of Child and Adolescent Psychopharmacology were made by the authors independently.
References
- Barkley RA, Fischer M, Smallish L, Fletcher K: The persistence of attention-deficit/hyperactivity disorder into young adulthood as a function of reporting source and definition of disorder. J Abnorm Psychol 111:279–289, 2002 [PubMed] [Google Scholar]
- Berek M, Kordon A, Hargarter L, Mattejat F, Slawik L, Rettig K, Schauble B: Improved functionality, health related quality of life and decreased burden of disease in patients with ADHD treated with OROS® MPH: Is treatment response different between children and adolescents? Child Adolesc Psychiatry Ment Health 5: 26, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brams M, Giblin J, Gasior M, Gao J, Wigal T: Effects of open-label lisdexamfetamine dimesylate on self-reported quality of life in adults with ADHD. Postgrad Med 123:99–108, 2011 [DOI] [PubMed] [Google Scholar]
- Brown TE, Landgraf JM: Improvements in executive function correlate with enhanced performance and functioning and health-related quality of life: Evidence from 2 large, double-blind, randomized, placebo-controlled trials in ADHD. Postgrad Med 122: 42–51, 2010 [DOI] [PubMed] [Google Scholar]
- Buitelaar JK, Casas M, Philipsen A, Kooij JJ, Ramos–Quiroga JA, Dejonckheere J, van Oene JC, Schauble B: Functional improvement and correlations with symptomatic improvement in adults with attention deficit hyperactivity disorder receiving long-acting methylphenidate. Psychol Med 42:195–204, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon M, Pelham WH, Sallee FR, Palumbo DR, Bukstein O, Daviss WB: Effects of clonidine and methylphenidate on family quality of life in attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol 19:511–517, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention: Increasing prevalence of parent-reported attention-deficit/hyperactivity disorder among children—United States, 2003 and 2007. MMWR Morb Mortal Wkly Rep 59:1439–1443, 2010 [PubMed] [Google Scholar]
- DuPaul GJ, Power TJ, Anastopoulos AD, Reid R: ADHD Rating Scale-IV: Checklists, Norms, and Clinical Interpretation. New York: Guilford Press; 1998 [Google Scholar]
- Edwards TC, Huebner CE, Connell FA, Patrick DL: Adolescent quality of life, part I: Conceptual and measurement model. J Adolesc 25:275–286, 2002 [DOI] [PubMed] [Google Scholar]
- Findling RL, Childress AC, Cutler AJ, Gasior M, Hamdani M, Ferreira–Cornwell MC, Squires L: Efficacy and safety of lisdexamfetamine dimesylate in adolescents with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry 50:395–405, 2011 [DOI] [PubMed] [Google Scholar]
- Findling RL, Childress AC, Krishnan S, McGough JJ: Long-term effectiveness and safety of lisdexamfetamine dimesylate in school-aged children with attention-deficit/hyperactivity disorder. CNS Spectr 13:614–620, 2008 [DOI] [PubMed] [Google Scholar]
- Findling RL, Cutler AJ, Saylor K, Gasior M, Hamdani M, Ferreira–Cornwell MC, Childress AC: A long-term open-label safety and effectiveness trial of lisdexamfetamine dimesylate in adolescents with attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol 23:11–21, 2013 [DOI] [PubMed] [Google Scholar]
- Gerwe M, Stollhoff K, Mossakowski J, Kuehle HJ, Goertz U, Schaefer C, Bogdanow M, Heger S: Tolerability and effects of OROS® MPH (Concerta®) on functioning, severity of disease and quality of life in children and adolescents with ADHD: Results from a prospective, non-interventional trial. Atten Defic Hyperact Disord 1:175–186, 2009 [DOI] [PubMed] [Google Scholar]
- Greenhill LL, Pliszka S, Dulcan MK, Bernet W, Arnold V, Beitchman J, Benson RS, Bukstein O, Kinlan J, McClellan J, Rue D, Shaw JA, Stock S: Practice parameter for the use of stimulant medications in the treatment of children, adolescents, and adults. J Am Acad Child Adolesc Psychiatry 41:26S–49S, 2002 [DOI] [PubMed] [Google Scholar]
- Klassen AF, Miller A, Fine S: Health-related quality of life in children and adolescents who have a diagnosis of attention-deficit/hyperactivity disorder. Pediatrics 114:e541–e547, 2004 [DOI] [PubMed] [Google Scholar]
- Landgraf JM: Monitoring quality of life in adults with ADHD: Reliability and validity of a new measure. J Atten Disord 11:351–362, 2007 [DOI] [PubMed] [Google Scholar]
- Matza LS, Rentz AM, Secnik K, Swensen AR, Revicki DA, Michelson D, Spencer T, Newcorn JH, Kratochvil CJ: The link between health-related quality of life and clinical symptoms among children with attention-deficit hyperactivity disorder. J Dev Behav Pediatr 25:166–174, 2004 [DOI] [PubMed] [Google Scholar]
- Norman GR, Sloan JA, Wyrwich KW: Interpretation of changes in health-related quality of life: The remarkable universality of half a standard deviation. Med Care 41:582–592, 2003 [DOI] [PubMed] [Google Scholar]
- Patrick DL, Edwards TC, Topolski TD: Adolescent quality of life, part II: Initial validation of a new instrument. J Adolesc 25:287–300, 2002 [DOI] [PubMed] [Google Scholar]
- Pliszka S: Practice parameter for the assessment and treatment of children and adolescents with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry 46:894–921, 2007 [DOI] [PubMed] [Google Scholar]
- Schubiner H, Katragadda S: Overview of epidemiology, clinical features, genetics, neurobiology, and prognosis of adolescent attention-deficit/hyperactivity disorder. Adolesc Med State Art Rev 19:209–215, vii, 2008 [PubMed] [Google Scholar]
- Sibley MH, Pelham WE, Molina BS, Gnagy EM, Waxmonsky JG, Waschbusch DA, Derefinko KJ, Wymbs BT, Garefino AC, Babinski DE, Kuriyan AB: When diagnosing ADHD in young adults emphasize informant reports, DSM items, and impairment. J Consult Clin Psychol 80:1052–1061, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer TJ, Landgraf JM, Adler LA, Weisler RH, Anderson CS, Youcha SH: Attention-deficit/hyperactivity disorder-specific quality of life with triple-bead mixed amphetamine salts (SPD465) in adults: Results of a randomized, double-blind, placebo-controlled study. J Clin Psychiatry 69:1766–1775, 2008 [DOI] [PubMed] [Google Scholar]
- Topolski TD, Edwards TC, Patrick DL, Varley P, Way ME, Buesching DP: Quality of life of adolescent males with attention-deficit hyperactivity disorder. J Atten Disord 7:163–173, 2004 [DOI] [PubMed] [Google Scholar]
- Ware JE: SF-36 Physical and Mental Health Summary Scales: A User's Manual. Boston: The Health Institute, New England Medical Center, 1994 [Google Scholar]
- Wehmeier PM, Schacht A, Escobar R, Savill N, Harpin V: Differences between children and adolescents in treatment response to atomoxetine and the correlation between health-related quality of life and attention deficit/hyperactivity disorder core symptoms: Meta-analysis of five atomoxetine trials. Child Adolesc Psychiatry Ment Health 4:30, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisler R, Young J, Mattingly G, Gao J, Squires L, Adler L, Study G: Long-term safety and effectiveness of lisdexamfetamine dimesylate in adults with attention-deficit/hyperactivity disorder. CNS Spectr 14:573–585, 2009 [DOI] [PubMed] [Google Scholar]
- Wolraich ML, Wibbelsman CJ, Brown TE, Evans SW, Gotlieb EM, Knight JR, Ross EC, Shubiner HH, Wender EH, Wilens T: Attention-deficit/hyperactivity disorder among adolescents: A review of the diagnosis, treatment, and clinical implications. Pediatrics 115:1734–1746, 2005 [DOI] [PubMed] [Google Scholar]
- Zambrano–Sanchez E, Martinez–Cortes JA, del Rio-Carlos Y, Dehesa–Moreno M, Poblano A: Low quality of life scores in school children with attention deficit-hyperactivity disorder related to anxiety. Arq Neuropsiquiatr 70:180–184, 2012 [DOI] [PubMed] [Google Scholar]