Abstract
Significance: There is increasing evidence that the generation of reactive oxygen species (ROS) in the central nervous system (CNS) involves the NOX family of nicotinamide adenine dinucleotide phosphate oxidases. Controlled ROS generation appears necessary for optimal functioning of the CNS through fine-tuning of redox-sensitive signaling pathways, while overshooting ROS generation will lead to oxidative stress and CNS disease. Recent Advances: NOX enzymes are not only restricted to microglia (i.e. brain phagocytes) but also expressed in neurons, astrocytes, and the neurovascular system. NOX enzymes are involved in CNS development, neural stem cell biology, and the function of mature neurons. While NOX2 appears to be a major source of pathological oxidative stress in the CNS, other NOX isoforms might also be of importance, for example, NOX4 in stroke. Globally speaking, there is now convincing evidence for a role of NOX enzymes in various neurodegenerative diseases, cerebrovascular diseases, and psychosis-related disorders. Critical Issues: The relative importance of specific ROS sources (e.g., NOX enzymes vs. mitochondria; NOX2 vs. NOX4) in different pathological processes needs further investigation. The absence of specific inhibitors limits the possibility to investigate specific therapeutic strategies. The uncritical use of non-specific inhibitors (e.g., apocynin, diphenylene iodonium) and poorly validated antibodies may lead to misleading conclusions. Future Directions: Physiological and pathophysiological studies with cell-type-specific knock-out mice will be necessary to delineate the precise functions of NOX enzymes and their implications in pathomechanisms. The development of CNS-permeant, specific NOX inhibitors will be necessary to advance toward therapeutic applications. Antioxid. Redox Signal. 20: 2815–2837.
Introduction
Reactive oxygen species (ROS) are oxygen-derived small molecules that readily react with a variety of chemical structures, from other small molecules (such as nitric oxide) to large molecules, including proteins, lipids, sugars, and nucleic acids. ROS are often referred to as free radicals, which is mostly correct, except for hydrogen peroxide (H2O2), which is a non-radical ROS. The existence of free radicals and their high reactivity was first known to chemists. Biologists, however, realized that free radicals may impact biological systems. The toxic effect of hyperoxia and H2O2 on central nervous system (CNS) tissue has been recognized by Mann in 1946 (116). The radiation biologist Harman, observing aging-like phenotypes on irradiation-induced free radical generation, proposed the so-called “Free radicals Theory of Aging.” In this theory, he proposed that free radicals could be generated during normal cellular respiration and lead to tissue damage (67). It was only much later that the novel quantitative method permitted to measure the presence of oxidative modifications in aging brain tissue and in affected regions of Alzheimer brain (154).
Since ROS lead to modifications of biomolecules, they have long been considered exclusively as harmful elements within biological systems. This concept was also corroborated by the discovery of ROS detoxifying enzymes (e.g., superoxide dismutase [SOD], catalase, etc.) and scavengers (e.g., vitamin C and E) and the ROS-dependent bactericidal activity of neutrophils. However, at the dawn of the 21st century, this paradigm was strongly challenged by the discovery of a family of enzymes, namely nicotinamide adenine dinucleotide phosphate (NADPH) oxidases (NOX), which generate ROS and consume oxygen. This led to a new paradigm: Only uncontrolled levels of ROS are deleterious, while regulated ROS production has key functions in physiology and cell signaling. This is true for most organ systems (25), including the CNS (123). Therefore, excessive ROS generation leading to tissue damage should be referred to as “oxidative stress,” while controlled ROS generation, involved in the regulation of physiological cellular processes, should be referred to as “redox regulation.”
Sources of ROS in CNS
Earlier, activity of the mitochondrial respiratory chain was considered the most relevant cellular source of ROS. Indeed, it is assumed that a fraction of the oxygen consumed by mitochondria escapes the physiological default pathway, namely the generation of adenosine triphosphate (ATP) and water. This escape fraction of oxygen forms oxygen radicals, thereby leading to the damage of biological molecules. Since the brain is a major consumer of oxygen (it accounts for 20% of total body oxygen consumption), mitochondria are often considered the prime source of ROS in brain aging and, by extension, in age-related neurodegenerative diseases (53, 63).
However, NOX enzymes (in particular, NOX4) might be expressed in mitochondria, and the mitochondrial respiratory chain might be the victim rather than the source of cellular ROS generation (29, 96).
Indeed, as opposed to chromosomal DNA, mitochondrial DNA lacks crucial elements of DNA repair and is, therefore, particularly vulnerable to double-strand breaks that are induced by ROS. The concept that mitochondria might be downstream targets of cellular ROS generation is supported by observations in the mitochondrial mutator mice. These mice express a proof-reading-deficient version of DNA polymerase subunit gamma, the nucleus-encoded catalytic subunit of mitochondrial deoxyribonucleic acid polymerase. These animals are prone to increased accumulation of mitochondrial mutations and to increased senescence (169), but neither increased ROS production nor oxidative damage was detected (168). An external source of ROS upstream of mitochondria was, therefore, postulated.
In addition to the mitochondrial electron transport, numerous other cellular systems generate ROS as by-products of their reactions. These include the peroxisome, cytochrome P450, xanthine oxidase, nitric oxide synthases, heme oxygenase, cyclooxygenases, lipoxygenases, myeloperoxidase, and monoamine oxidases, among others (21). Only in a few instances, the role of these ROS-generating systems in CNS physiology and pathology has been well documented.
NADPH oxidases
The NOX NADPH oxidases are a family of ROS-producing enzymes (12). They are multi-subunit membrane-bound enzymes that catalyze the reduction of oxygen into superoxide (O2−•) by using NADPH as an electron donor and oxygen as an electron acceptor (Fig. 1). In most mammals, there are seven NOX isoforms: NOX1, NOX2, NOX3, NOX4, NOX5, dual oxidase (DUOX)1, and DUOX2 (Table 1). Most NOX isoforms have a characteristic tissue distribution and distinct activation mechanisms: NOX1, NOX2, and NOX3 require cytosolic factors for full activation; NOX5 and DUOX enzymes contain N-terminal EF-hand domains and require elevation in cellular Ca2+ concentrations; and NOX4 appears to generate ROS constitutively and might be regulated at the transcriptional level (149).
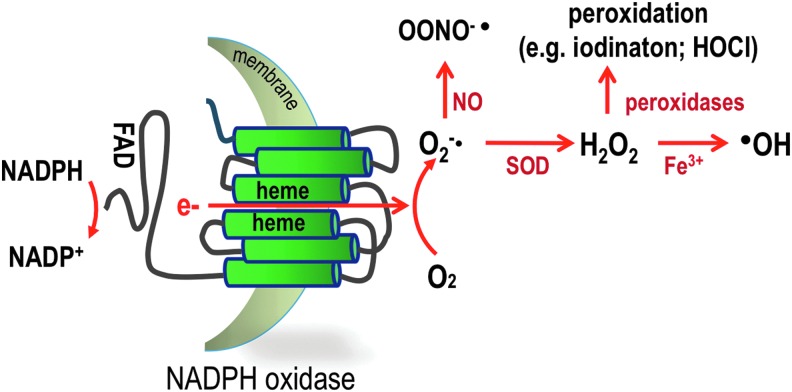
FIG. 1.
Schematic representation of NOX NADPH oxidases. NOX enzymes are transmembrane proteins that catalyze the transfer of two electrons through biological membranes using intracellular NADPH as an electron donor and extracellular molecular oxygen as an acceptor. The primary product of this reaction is the superoxide anion O2−•. From this primary product and depending on the systems, numerous other ROS can be generated. In the presence of NO, O2−• rapidly reacts and forms the highly toxic peroxynitrite or it can be dismutated either by a superoxide dismutase or spontaneously to form hydrogen peroxide, which displays numerous actions as a second messenger and reacts in the presence of peroxidase to form the microbicidal hypochlorous acid in the phagosome or in the presence of Fe3+, the highly reactive hydroxyl radical, which is known to modify lipids and proteins. NADPH, nicotinamide adenine dinucleotide phosphate; NO, nitric oxide; NOX, nicotinamide adenine dinucleotide phosphate-dependent oxidase; ROS, reactive oxygen species. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
Table 1.
Nicotinamide Adenine Dinucleotide Phosphate Oxidase Isoforms, Subunits, and Expression
| Enzyme | Subunits/motifs | Detected product | Activity | High-level tissue expression | Putative involvement in CNS function and disease |
|---|---|---|---|---|---|
| NOX1 | p22phox (M) | O2−• | Low basal activity phosphorylation-dependent translocation of cytosolic subunits | Colon | Cell death of dopaminergic neurons in PD |
| NOXO1 (C) | Vascular smooth muscle cells Endothelial cells | ||||
| NOXA1 (C) | |||||
| RAC1 (C) | |||||
| TKS4/5 (C) | |||||
| PDI | |||||
| NOX2 | p22phox (M) | O2−• | Low basal activity phosphorylation-dependent translocation of cytosolic subunits | Phagocytes | Synaptic connectivity |
| p47phox (C) | Vascular/endothelial cells | Microglia proliferation and function | |||
| p67phox (C) | Neural stem cell proliferation | ||||
| p40phox (C) | Neurotransmitter release | ||||
| RAC1/2 (C) | |||||
| 6-Phosphogluconate dehydrogenase (6GPH) | |||||
| S100A8 | |||||
| PDI | |||||
| Peroxiredoxin 6 | |||||
| NOX3 | p22phox (M) | O2−• | Unclear | Inner ear | Otoconia formation |
| NOXO1 (C) | |||||
| RAC1 (C) | |||||
| NOX4 | p22phox (M) | H2O2 | Constitutive activity | Kidney | Neuronal expression increased after stroke |
| POLDIP2 (C) | Endothelial cells | ||||
| TKS4/5 (C) | Fibroblast | ||||
| PDI | Cardiac myocytes | ||||
| Vascular smooth muscle cells | |||||
| NOX5 | EF-hand domains | O2−• | Intracellular Ca2+ phosphorylation | Testis | Unknown |
| Calmodulin | Lymph node | ||||
| Hsp90 | |||||
| DUOX1 | DUOXA1(M) | H2O2 | Intracellular Ca2+ phosphorylation | Thyroid gland | Unknown |
| DUOXA2(M) | Respiratory tract | ||||
| EF-hand domains | |||||
| DUOX2 | DUOXA1(M) | H2O2 | Intracellular Ca2+ phosphorylation | Thyroid gland | Unknown |
| DUOXA2 (M) | |||||
| EF-hand domains |
C, cytosol; M, membrane.
CNS, central nervous system; DUOX, dual oxidase; NOX, nicotinamide adenine dinucleotide phosphate-dependent oxidase; PD, Parkinson disease; PDI, protein disulfide isomerase.
The NADPH oxidase activity was originally discovered in neutrophils, as a source of O2−• formed during phagocytosis. The phagocyte NADPH oxidase NOX2 plays a vital role in innate immunity. It is highly expressed in neutrophils, and on activation, it is the key enzyme which is responsible for the oxidative burst (or respiratory burst) that leads to microbicidal activity. Loss of function of NOX2 results in chronic granulomatous disease (CGD), a hereditary disease that is characterized not only by a high susceptibility to certain fungal and bacterial infections due to impaired killing of these microorganisms, but also by the development of granulomas, which are due to sterile hyperinflammation (144).
Many NOX enzymes have high-level expression in a limited number of tissues; for example, NOX1 in the colon, NOX2 in phagocytes, NOX3 in the inner ear, NOX4 in the kidney, NOX5 in the testis and lymphoid tissues, and DUOX1 and 2 in the thyroid. However, data from knock-out animals suggest that high-level expression does not always provide information about the functional relevance of NOX isoforms in different tissues. For example, although NOX1 is mostly expressed in the colon, no readily detectable phenotype was found in NOX1-deficient mice. In contrast, NOX1-deficient vascular smooth muscle cells clearly show an altered angiotensin II (Ang II) response, despite the fact that they express only a moderate level of the enzyme (57).
Most mammals have seven NOX isoforms, but NOX5 has been lost in mice and rats. The reason for this loss of NOX5 in some animal species is not understood. However, it is interesting to note that the human NOX5 gene is more polymorphic than other NOX genes and that in some human populations, there is an NOX5 polymorphism leading to a truncation (and presumable loss of function) of the NOX5 protein (11). Thus, possibly the evolutionary requirement for a functional NOX5 gene is less stringent than the one for other NOX genes (56).
From a biochemical point of view, it is likely that the primary product of all NOX enzymes is O2−•. However, for several isoforms, in particular NOX4, DUOX1, and DUOX2, H2O2 is predominantly detected. A rapid dismutation of O2−• may account for this apparent H2O2 production. For NOX4, it has been shown that the third extracellular loop of the enzyme is crucially involved in this H2O2 generation (166).
This article reviews the physiology and pathophysiology of NOX enzymes in the CNS and represents a follow up and an update of a previous review by our lab (157).
Physiological Role of NOX in CNS
Cellular and subcellular localization of NOX enzymes in the CNS
Most of the currently available data on NOX enzymes in the CNS focus on NOX1-4. Little is known about NOX5 and DUOX1, 2, but currently available data do not exclude the fact that these NOX isoforms might be expressed in the CNS under certain circumstances.
Details on currently available localization data on NOX enzymes will be discussed next; however, the available information should be taken with caution:
(i) Only a few reliable antibodies against NOX enzymes are available and in most studies performing CNS immunolocalization of NOX enzymes, no stringent controls have been documented. In addition, only a few in-situ hybridization data are available, which would provide relevant controls with regard to antibody specificity (4).
(ii) Gene expression within the CNS shows strong regionalization and important species differences. Thus, the CNS expression of NOX isoforms cannot be extrapolated from a limited data set and requires experimental verification both in different brain regions and in different species.
Subcellular distribution of NOX enzymes
NOX enzymes have been described to be expressed at the cell surface as well as in intracellular organelles. At the cell surface, NOX enzymes may be concentrated in microdomains. With regard to intracellular organelles, the expression of NOX enzymes in a large number of structures has been described, including endosomes, granules, endoplasmic reticulum, and nuclear envelope, as well as in mitochondria. NOX2 is mainly found within intracellular vesicles, but it translocates to the phagosome and/or the plasma membrane on cell activation.
NOX2 cytosolic subunits are typically found in the cytoplasm in resting cells, but they translocate to NOX2-containing membranes in response to cellular activation. NOX4 appears mostly expressed in the endoplasmic reticulum (32, 187) and the nuclear envelope (4), but a mitochondrial localization has also been suggested (20). NOX1 and NOX5 might be present preferentially at the plasma membrane, possibly in specific membrane domains (157). DUOX enzymes are localized at the apical membranes of epithelial cells (95). The diverse subcellular localization of NOX enzymes serves a variety of essential roles in both physiological and pathophysiological conditions and may be an essential component of their function [for a comprehensive review on this subject, see ref. (105)]. For instance, in endothelial cells, NOX2 translocate, produce ROS, and facilitate migration toward the injury side (78). Similarly, localization of NOX4 (47), Nox1, and Nox3 in specific cellular membraneous compartments (59) regulates migration of cancer cells by ROS-dependent formation of invadopodia.
Partly due to the lack of connective tissue in the brain, cells of the CNS are generally much closer to each other than cells in peripheral tissues. Therefore, extracellular generation of ROS by NOX-expressing cells may have direct effects on neighboring cells. ROS can be toxic for neighboring cells as in the N-methyl-d-aspartate (NMDA)-mediated extracellular superoxide generation by neurons (140). However, it is tempting to hypothesize that paracrine regulation may occur as has been proposed for T-cell activation at the immunological synapse by NOX2-derived ROS (72).
In neurons, NOX1, NOX2, NOX3, and NOX4 have been documented (157). It should be noted that in many instances this expression is not constitutive, but rather induced in pathological states. For most parts, there is no detailed knowledge with regard to the expression of NOX isoforms in neuronal subtypes. Possible exceptions to this are the relatively well-documented expression of NOX1 in dopaminergic (DA) neurons (37), NOX2 expression in CA1 hippocampal neurons of old mice (50), the NOX2 subunit p47phox in hippocampal neurons (22) as well as in pyramidal neurons of socially isolated rats (145), and NOX4 expression in dorsal root ganglion (DRG) neurons (87) and in basal ganglion and cortical neurons after stroke (94). The subcellular localization of NOX enzymes in neurons is still relatively unexplored; however, there is some evidence for a synaptic localization of NOX2 (157). It should be noted that most reports detect intracellular, rather than extracellular ROS generation in neurons. Indeed, to the best of our knowledge, there are no reports on the release of superoxide (cytochrome C reduction) or H2O2 (Amplex Red etc.) by neurons. If confirmed in further studies, this would suggest that in neurons, NOX enzymes are predominantly localized intracellularly. Such localization would rather argue in favor of a signaling role.
In astrocytes, mostly NOX4, but also NOX1 and NOX2 have been described. In glioblastoma (a tumor probably derived from astrocyte precursors), NOX4 appears to be, by far, the most highly expressed isoform (see next), although NOX5 presence can be detected in a high proportion of glioblastoma (7). Subcellular localization of NOX enzymes in astrocytes has not been investigated in detail.
Oligodendrocytes are the only CNS cells with no clear documentation of NOX expression.
Microglia is the phagocyte of the CNS. However, as opposed to most other phagocytes, recent data suggest that microglia is not bone marrow derived, but is rather of yolk sac origin (3, 61, 88). Thus, NOX2 (phagocyte NADPH oxidase) expression in microglia is a pertinent concept; however, some differences exist with other phagocytes. NOX2 expression is very low at a resting stage, but convincing evidence exists for increased expression of NOX2 and subunits in the activated state of microglia in both humans (55) and mice (185). However, at least in rodents, several studies have shown NOX2 (92, 150) or its subunit p47phox (145) in neurons without detecting it in microglia. Thus, most likely, NOX2 requires the induction of expression by specific stimuli in the mouse brain. In addition to NOX2, there is now also evidence for expression of NOX1 (34) and NOX4 (106) in microglial cells. Whether the different NOX isoforms are expressed in the same cell or whether there are microglia subpopulations expressing different NOX isoforms is not yet clear.
ROS, cell fate, and cellular signaling in the CNS
Under physiological circumstances, NOX enzymes likely generate only low levels of ROS in the CNS. The most likely function of such a low-level ROS generation is cellular signaling. However, ROS production leading to cell death might also be of physiological importance for apoptosis during fetal development (see next). It should, however, be underlined that cell fate decision in response to ROS exposure is not simply determined by the amplitude of the ROS generation. It is likely that sensitivity of CNS cells to ROS (through regulation of the antioxidant response) is a key factor for the signaling versus cell death decision. It is also likely that spatiotemporal aspects of ROS release play a role in cell fate decisions: (i) independently of the amplitude of the ROS signal, continuous ROS generation may impact cell fate decisions differently than short intermittent ROS generation, and (ii) high ROS levels in the submembraneous space might have a different impact on cell fate decisions than do high ROS levels which are close to mitochondria or to nuclei. The induction of cell death by ROS has traditionally been considered to be simply due to cell damage. This cell damage theory might be true under certain circumstances. However, the modulation of redox-sensitive signaling cascades contributes to cell death. Deregulation of ROS generation (increased amplitude; increased duration) will likely lead to a deregulated signaling. The emergence of novel imaging probes enabling the detection and quantification of cellular ROS in live cells will hopefully help in addressing the fine-tuning of ROS signaling (114).
Signal transduction through ROS
ROS impact cellular signaling through a variety of pathways. Among the most important is the alteration of protein function by its redox state. From a molecular point of view, this has been most extensively studied for protein tyrosine phosphatases, which are reversibly inactivated through the oxidation of cysteines in their catalytic domain (35). Redox regulation of tyrosine phosphatases is most likely also important in the CNS, but this question has so far not been studied in detail. However, it is most likely that redox-sensitive amino acids, in particular cysteine, within relevant signaling proteins within the CNS are of major importance to translate the redox potential into a cellular response. Redox-response proteins of relevance for the CNS include transcription factors and ion channels.
Redox regulation of cellular transcription factors is an important aspect of ROS signaling in the CNS. Hypoxia inducible factor (HIF)1α is an interesting example. Under normoxic conditions, HIF1α is degraded by the ubiquitin pathway through its interaction with the E3 ubiquitin ligase van Hippel-Lindau (VHL). Hypoxia leads to dissociation of VHL from HIF1α and, therefore, to expression of the protein. Interestingly, ROS can lead to VHL inhibition and HIF1α up-regulation in a hypoxia-independent manner (12). Such a redox-dependent HIF1α activation has, for example, been observed in the rat social isolation model (see Psychiatric disorders). Nuclear respiratory factor 2 (Nrf2), another transcription factor of relevance in the CNS (192), follows a similar pattern of activation: ROS lead to a dissociation of Nfr2 from the E3 ubiquitin ligase Kelch-like ECH-associated protein 1 (Keap1) through Keap1 oxidation. This dissociation leads to decreased degradation of Nrf2 and, hence, an increase in protein levels of the transcription factor. ROS may also lead to activation of the transcription factor nuclear factor kappa B (NFkappaB). However, the underlying mechanisms are only partially understood. Indeed, NFkappaB activation by ROS might be linked to activation of I Kappa B Kinase kinases and/or through inhibition of tyrosine phosphatases. Importantly, NFkappaB may have an anti-apoptotic function in the CNS (143). Thus, depending on the specificity of the ROS signal and on the cell type, ROS in the CNS may not only be uniformly pro-apoptotic, but also be involved in anti-apoptotic signaling.
The redox-regulated transcription factors described earlier are mainly involved in stress responses, rather than being involved in CNS-specific pathways. This makes sense given the role of ROS in the responses to stress; however, a possible role of redox regulation in CNS-specific transcriptional processes is not excluded and requires further investigation.
Numerous receptors of the CNS are regulated by redox modulation of cysteine residues, including NMDA (190), opioid (141), and gamma-aminobutyric acid receptors (26). Emerging evidence indicates a key role of a ROS-sensitive Ca2+ channel (e.g., transient receptor potential 2 channels) in neuronal Ca2+ signaling (103, 129).
NOX and ROS in CNS development and neural cell differentiation
There are arguments that the redox state might play a role during development. The shaping of the CNS is a result of a coordinated process involving not only cell proliferation and differentiation/migration but also cell death. Apoptosis, occurring at the right time and at the right place, is an important physiological regulator of brain development.
Cerebellar development: a role for microglia-derived ROS?
The best arguments in favor of a role of NOX enzymes in CNS development come from the cerebellum. The role of microglia-derived ROS in the regulation of programmed cell death during development has been observed in the cerebellum. Microglial cells play an important role in the programmed loss of neurons, necessary for brain morphogenesis, a prominent feature of the developing CNS. Microglia-induced apoptosis has been shown to occur during development in Purkinje cells of the cerebellum (120). NOX2-dependent ROS generation probably contributes to apoptosis induction in this process.
A recent study suggests that NOX2 also plays a role in microglial chemotaxis, thereby contributing to microglial infiltration of the developing CNS (104). This developmental mechanism of microglia migration and induction of neuronal apoptosis is reminiscent of microgliosis in neuroinflammatory CNS pathologies (see section “Pathological role of NOX in the CNS”). A recent publication of Coyoy et al identified specific patterns of ROS generation and expression of NOX enzymes (NOX1, NOX2 and NOX4) during various stages of rat cerebellum development and described distinct levels of ROS in the different cerebellar layers during development (43). Interestingly, this study was initiated because when pregnant mothers were treated with apocynin, the size of the cerebellum of mouse embryos was affected. Despite its designation as an NOX inhibitor, the pharmacological properties of apocynin are poorly understood and the compound may even increase oxidative stress under certain conditions (175). Although this study shows concomitant expression of NOX enzymes with ROS generation, the fact that this ROS generation is inhibited by apocynin does not prove that NOX are the source of ROS in cerebellar development.
NOX and ROS in neural stem cells and progenitors
Throughout adult life, tissue-specific stem cells can be found in the CNS, in a similar manner to many other tissues. In the adult mammalian brain, neural stem cells (NSCs) are defined by their ability to self-renew and to differentiate into neural cells such as neurons, astrocytes, and oligodendrocytes. Note that microglia is not a neural cell of ectodermal origin and, therefore, cannot be generated from NSC. During adult life, NSCs are considered as having a capacity to replace damaged neurons and to contribute to astrogliosis, the abnormal increase in astrocyte number, observed in various brain pathologies.
Stem cells are found in a so-called “niche,” a delimited region that contributes to tissue homeostasis via cell-to-cell interaction, extracellular matrix contact, as well as release of neurotrophic factors, growth factors, and neurotransmitters. Two areas of the CNS have been well documented as stem cell niches: the dentate gyrus of the hippocampus and the sub-ventricular zone (109). The sub-ventricular zone has a highly organized cytoarchitecture enabling anchorage of NSC in the ependymal cells by a signaling molecule called vascular cell adhesion molecule 1 (VCAM1) on their endfeet. NOX2 and VCAM1 co-localized in NSC, and ROS generation was increased after activation of VCAM1, suggesting a role for NOX2 in NSC maintenance (95). Decreased ROS generation was shown in the sub-ventricular zone of NOX2-deficient mice (102). Other studies showed decreased neurogenesis and a decreased number of mature neurons in the hippocampus of NOX2-deficient mice (28, 48). This is the first evidence for a slightly altered morphology in NOX2-deficient CNS, which may explain impaired synaptic plasticity and memory formation previously shown in these mice (93).
Studies with isolated NSCs in vitro have provided arguments in favor of a functional role of NOX2 in these cells: (i) NSCs express NOX2 and produce ROS; (ii) treatment of NSCs with growth factors increases NOX2 activity (48); (iii) ROS scavengers and NOX inhibitors decrease in parallel ROS levels and NSC proliferation (191); and (iv) hippocampal NSCs derived from NOX2-deficient mice show decreased proliferation (48). Thus, it is likely that NOX2 has a functional role in the organization of stem cell niches in the CNS; however, further studies will be necessary to fully understand its role in this context (Fig. 2).
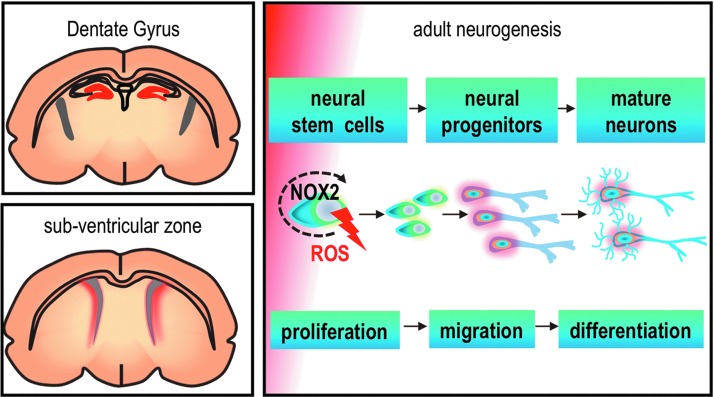
FIG. 2.
NOX2 controls neurogenesis of adult neural stem cells. Adult neural stem cells reside in two main regions of the brain: dentate gyrus and sub ventricular zone (regions shown with red color). A controlled level of ROS generation by NOX2 at this early stage of adult neurogenesis is involved with the proliferation and survival of neural stem cells.
NOX2 deficiency and cognitive function
NOX2-deficient mice kept under specific pathogen-free conditions present impaired memory and synaptic deficit (93). More recently, a comprehensive behavioral study, of mice with deletion in p47phox and neuronal nitric oxide synthase (nNOS) was performed. Both p47phox- and nNOS-deficient mice showed impaired learning and memory, increased locomotor activity, and reduced anxiety. Interestingly, in contrast to single knock-out mice, the double knock-out mice (p47phox and nNOS) showed a synergistic effect, resulting in marked elevation of locomotor activity and impaired learning and memory (177). This suggests a potential role of the oxidant peroxynitrite, which is quickly formed in vivo through a high-affinity chemical reaction between nitric oxide and superoxide. In humans, one study in transplanted and non-transplanted pediatric CGD patients found that cognitive performance was within the normal range (40). However, another study in pediatric CGD patients found a mild decrease in cognitive function (132). Thus, most likely, NOX2 plays a role in the optimization of CNS function. At this point, it is not clear whether this is due to a role of NOX2 in NSC proliferation, or rather due to NOX2-dependent signaling in mature neurons. NOX-deficient mouse models provide a relevant experimental system to investigate greater in detail NOX2-related neuronal connectivity and biochemical substrates in cognitive function.
Pathological Role of NOX in the CNS
Neurodegenerative disorders
Protein aggregation is a hallmark of most neurodegenerative diseases. Aggregates can be intracellular within neurons or in the extracellular space. For example, Parkinson disease (PD) is characterized by intracellular protein aggregates called Lewy bodies, while Alzheimer disease (AD) is characterized by extracellular protein aggregates called senile plaques. Neurodegenerative diseases are generally associated with neuroinflammation and oxidative stress. In many cases, there are good arguments for an involvement of ROS produced by NOX enzymes in pathogenesis. There is emerging evidence that the neurodegenerative process is a combination of cell-autonomous (i.e., a pathological protein expressed in neurons leads to neuronal damage) and non-cell autonomous mechanisms in which other cells are an important part of the disease process, in particular, the activation of microglia and astrogliosis (Fig. 3).
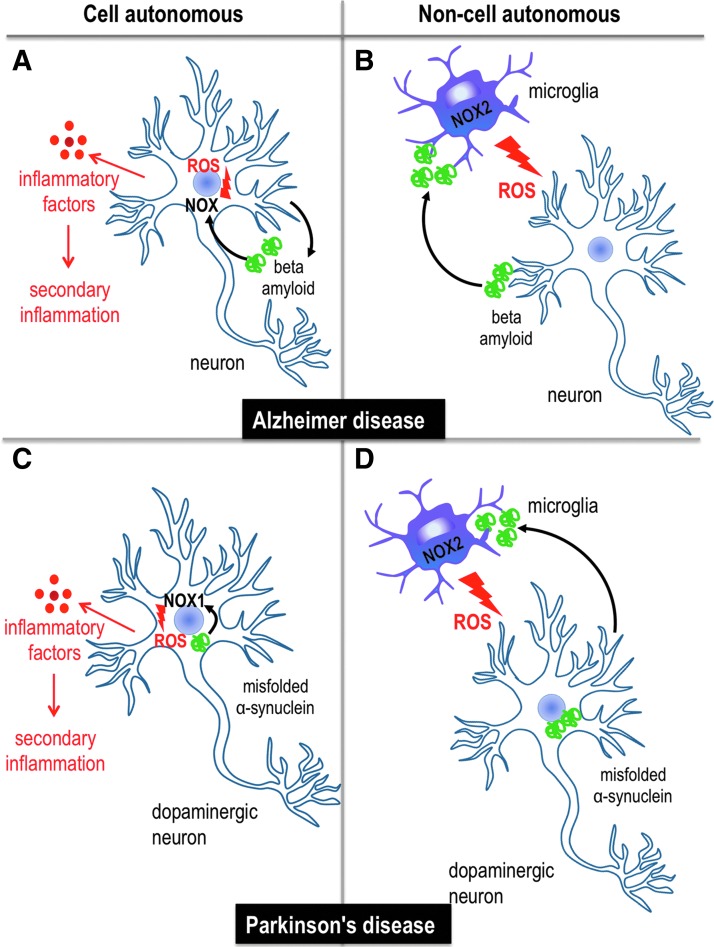
FIG. 3.
Neuroinflammation and oxidative stress in cell autonomous and/or non-cell autonomous models of neurodegenerative diseases. Both mechanisms are described in models of Alzheimer and Parkinson disease. (A) In Alzheimer diseases, amyloid fragments generated through proteolytic cleavage of the APP are released to the extracellular space. This pathological protein expressed in neurons leads to neuronal damage by ROS produced by NOX and release of inflammatory factors, leading to secondary inflammation. (B) Non-cell autonomous mechanisms involve cells other than neurons, in particular the activation of microglia and ROS production generated by NOX2. In Alzheimer disease, the activation of microglia cells and the generation of ROS are considered to be mediated by beta amyloid fragments that are released extracellularly. (C) In Parkinson disease, intracellular protein aggregates might lead to ROS production via NOX1 and cellular stress, which leads to the generation of inflammatory cytokines. (D) In the non-cell autonomous state, misfolded α-synuclein protein fragments are secreted from neurons and may, therefore, activate microglia cells, which leads to ROS generation by NOX2 and oxidative stress. APP, amyloid precursor protein. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
In the case of AD, the connection between protein aggregates, microglia activation, and generation of oxidative stress is relatively obvious. Beta amyloid fragments generated through proteolytic cleavage of the amyloid precursor protein (APP) are released to the extracellular space. The activation of microglia and its phagocyte NADPH oxidase may occur directly either through specific activation of microglial receptors by the amyloid fragments or through aggregates thereof. In the case of PD, recent studies have shown that α-synuclein is secreted from nerve cells in both monomer and aggregated forms, and that aggregated oligomeric and fibrillar forms are capable of activating microglia through the Toll-like receptor (TLR) 4 (54, 161) and TLR 2 (89).
It is possible that NOX-derived ROS act in a similar way: cell autonomously, that is, up-regulation of NOX in neurons leads to neurodegeneration, or non-cell autonomously, that is, activation of NOX enzymes in a different cell leads to neurodegeneration. In particular, the role of NOX2 in microglia leads to ROS generation, which, ultimately, damages neurons. Both mechanisms were described in models of PD (45, 164). As of today, sequences of events that are driven by NOX-derived ROS, leading to neurodegeneration, are mostly hypothetical. ROS by themselves are toxic chemical entities, which can lead to neuronal death through radical formation. However, NOX-derived ROS are also important in triggering cytokine expression in phagocytes and non-phagocytic cells, which, in turn, can lead to microglia activation and/or neurotoxicity. Conversely, cytokines such as interleukin (IL)-1β (31) and IL-6 (50) have been shown to activate NOX2.
A role for NOX2 in microglia activation
Emerging evidence shows that NOX2 expression and ROS generation parallels different levels of microglia activation (Fig. 4). On activation, microglia display a wide range of functions and are characterized by morphological changes, ranging from a ramified “surveying” stage in which microglia “scan” the CNS parenchyma and help maintaining synaptic function to an “amoeboid” morphology with overshooting ROS generation, which may contribute to neurodegeneration. In surveying microglia, NOX2 expression is barely detectable, while high levels of NOX2 expression are detected in the proinflammatory and neurotoxic phenotype (151). In a somewhat reductionist fashion, activated microglia are divided into two phenotypes: alternatively activated M2 microglia and classically activated M1 microglia. When CNS homeostasis is disrupted, surveying microglia may switch to the alternative activation stage, which is characterized by intermediate NOX2 levels. Alternatively, activated microglia are probably not neurotoxic, might be associated with health, rather than disease, and might even be anti-inflammatory (151). This nomenclature was originally developed to describe the different activation of peripheral macrophages: M1 represents the classical, lipopolysaccharides (LPS), anti-bacterial and inflammatory activation while M2 is an alternative, IL-4/IL-10-dependent, tissue-regenerative, and inflammatory/anti-inflammatory activation stage. In microglia, as is the case for macrophages, numerous intermediate activation stages can be simultaneously found in inflamed tissue [for review, see ref. (167)]. Under aggravated pathological conditions, strongly activated M1 microglia is observed. This is typically the case in chronic neurodegenerative disease with a high load of protein aggregates. It is thought that M1 microglia is neurotoxic and may contribute to disease progression (138). There is some discussion in the literature as to what extent M1 microglia are simply derived from resident microglia, or whether they are also formed from bone marrow-derived microglia precursors that invade the CNS (58). Obviously, NOX-derived ROS are important for the neurotoxicity of M1 microglia. However, the absence of NOX2 might also promote the M2 microglia phenotype (6, 108). Given recent observations that during development, CNS migration of microglia precursors are NOX2 dependent (104), one may also consider the possibility that in the absence of NOX2, there is a decreased migration of blood-derived microglia precursors into the CNS.
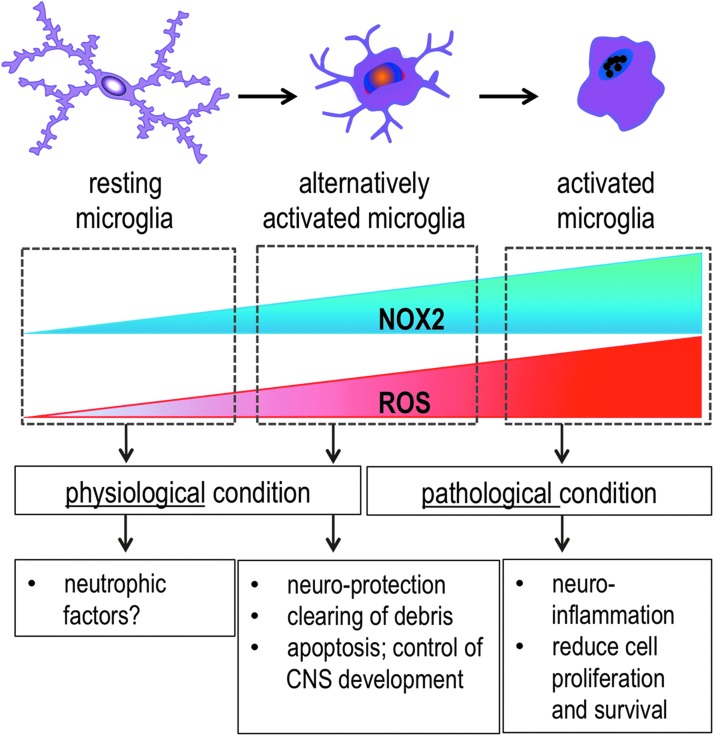
FIG. 4.
The dual function of microglia NOX2 in the CNS: physiology and pathology. At a resting stage, microglia is highly branched, generates little ROS, and shows very low NOX2 expression. An elevated and controlled level of ROS generated by microglial NOX2 is involved in the physiological condition and healthy non-inflammatory state of the CNS, which plays a role in programmed cell death of cells in the brain during CNS development. After CNS insult, microglial NOX2 is induced. Microglia develop into an active state in which they display a protective phenotype (alternative activation) in order to clear debris and support neuronal survival. In some instances, the inflammatory signals such as environmental toxins and fragments of proteins are not removed from the CNS. In response, microglia become amoeboid and enter an over-activated and neurotoxic stage that is characterized by high NOX2 expression and ROS generation. CNS, central nervous system. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
In the adult CNS, a wide range of microglial activation stages are most likely present. Typically, clusters of activated microglia accumulate at the location of injury, plaque deposition, or inflammation; therefore, the question of whether, how, and when the activation of microglia is beneficial (e.g., by removing debris from the CNS) or deleterious for the CNS (e.g., when generating too much ROS) is abundantly debated (1, 69).
Microglia activation and, in consequence, increased NOX2 expression is a hallmark of virtually all CNS insults, including stroke, chronic progressive neurodegeneration due to aggregation or autoimmune disorders and psychiatric disorders. However, the role of NOX enzymes is not limited to the control of microglia regulation. The next few chapters describe the current knowledge on NOX enzymes in different CNS pathologies.
Alzheimer: activation of microglia by amyloid peptides?
AD is a relatively common form of dementia, leading to cognitive impairment and memory loss. Key histological features in the brain of AD patients are senile plaques (due to extracellular accumulation of amyloid β [Aβ] peptide) and neurofibrillary tangles (due to intracellular accumulation of hyperphosphorylated Tau). Activation of microglia, astrogliosis, decrease of synaptic density, and loss of neurons are common features of AD. Most cases of AD are “late-onset sporadic AD” without strong familiar transmission, but the apolipoprotein E4 polymorphism is a well-documented risk factor. The pathogenesis of late-onset AD is poorly understood, but lifestyle risk factors are shared with cardiovascular diseases (high fat diet, sedentary lifestyle etc.) (97).
Histologically, neuroinflammation and oxidative stress are consistently observed in late-onset AD (91, 117). “Early-onset, inherited AD” cases are rare, but the identification of causative mutations has made an important contribution to the understanding of the disease. Mutations leading to early-onset inherited AD were identified in the genes for APP, and presenilin 1 (PS1) and 2. At present, there is no curative treatment for AD. Interestingly, despite the fact that accumulation of hyperphosphorylated Tau is a prominent histological feature in all forms of AD (late and early onset), no Tau mutations leading to AD have been described. Thus, the accumulation of hyperphosphorylated Tau appears to be a common downstream pathway in AD.
Cellular models
Co-culture systems of Alzheimer-type neurons (e.g., neurons expressing APP mutants) with microglial cells can be useful for the understanding of the neuroinflammatory basis of AD. In one of these models, a key role for NOX2 in APP-dependent killing of neurons by activated microglia has been described (135). In another cellular model, ROS were shown to induce pathognomonic feature of AD, namely tau hyperphosphorylation (188).
Rodent models
Most AD models in rodents are either transgenic mouse expressing AD-typical mutants (APP, presenilin) or a combination thereof, which lead to a high production of β-amyloid in the CNS, or a direct injection of β-amyloid into the rodent brain. These models are relevant for a general understanding of AD pathology and will be discussed next; however, they do not reproduce the frequent late-onset AD.
Some recent studies investigating the mechanisms of brain aging in rodent models might provide relevant information about the mechanisms of late-onset AD. One study investigating the impact of a high fat diet found increased NOX expression and increased ROS production in the aging mouse brain (24, 136). Another study investigating the impact of a single LPS injection found an NOX2-dependent activation of microglia and brain oxidative stress. Amazingly, this impact of a single time point LPS injection lasted for approximately 20 months, that is, essentially for the lifetime of a mouse (136).
Several studies using NOX2-deficient mice bred with different models of AD, suggested that NOX2 is involved in neurotoxic insult downstream from senile plaques [reviewed in ref. (157)]. No novel studies were published to describe NOX2 knock-out mice that were bred with other AD models, but a study used APP×PS1 knock-in mice and showed that the age-dependent cognitive deficit was associated with increased NOX4 and p47phox (a NOX2 subunit) expression and NADPH-dependent lucigenin chemiluminescence in cell membranes isolated from frontal cortex (23). A strong increase of synaptic ROS production was described in old mice showing cognitive impairment (2). Note that in both studies, ROS measurements were done in isolated membranes after the addition of NADPH. However, such assays do not represent conclusive evidence for NOX activity. Indeed, although lucigenin chemiluminescence increases after NADPH addition and is inhibited by diphenylene iodonium (DPI), such NADPH-dependent chemiluminescence in homogenates has never been unambiguously attributed to ROS generated by any isoform of NOX (114). Since specific methods that measure ROS in isolated tissues are still lacking, an alternative approach is the quantification of NOX2 activation as membrane translocation of the cytosolic subunit p47phox (130).
Choi et al. (38) described a key role of NOX2 in microglia activation. After an intracerebrovascular injection of LPS or Aβ1–42 oligomers, a strong increase of microglial inflammatory markers is observed. This increase is reduced in p47phox-deficient, NOX2-deficient, as well as apocynin-treated mice. Interestingly, gene deletion of p47phox and NOX2 promotes an alternative activation state (M2) of microglia activation as assessed by the increased expression of macrophage receptor with collagenous domain (MARCO) and chitinase 3-like 3 (YM1), which are markers of a neuroprotective and anti-inflammatory status of microglia (38). This study provides a strong rationale for inhibiting NOX2 inhibition in M1 microglial cells in order not only to decrease excess neurotoxic ROS generation but also to promote a neuroprotective stage of microglia.
Based on the previous proof-of-principle studies for NOX2 inhibition, several groups attempted a pharmacological approach using the antioxidant/NOX inhibitor apocynin. Apocynin did not improve cognitive impairment, synaptic deficit, or Aβ aggregation in the mouse model of Alzheimer Tg19959, which express human APP695 with two familial AD mutations (KM670/671NL and V717F), under the control of the hamster prion protein promoter (52), but reduced plaque size and cortical microgliosis in the hAPP (751)(SL) mice (113). Thus, the use of apocynin did not yield unequivocal results in mouse models of AD. Nevertheless, since apocynin is not a bonafide NOX inhibitor, but acts rather as an antioxidant, this does not preclude the conceptual validity of targeting NOX enzymes in AD (153).
Studies in humans
A study using postmortem human material showed increased NADPH-dependent lucigenin chemiluminescence in material from early stage (mild cognitive impairment) and AD compared with controls, which correlated with cognitive status (disease progression). Interestingly, cytosolic subunits (p67phox, p47phox, and p40phox) were increased, but not NOX2 (6). Similarly, another study showed increased p47phox, but not NOX2 in the AD brain; however, the messenger ribonucleic acid (mRNA) levels from other subunits were not evaluated (38).
About 40% of AD patients present a capillary cerebral amyloid angiopathy (CCAA), a condition in which Aβ accumulates in cortical capillaries. Immunohistochemistry was performed on postmortem brain from six subjects with CCAA and two age-matched controls. Strong NOX2 immunoreactivity was shown in microglia and endothelial cells of CCAA patients, while only low and microglial-specific NOX2 staining was detected in control brains (27).Thus, ROS generated by the phagocyte NADPH oxidase NOX2 play a role in the progression of AD. However, whether this NOX2 comes mostly from microglia, or also from blood-derived inflammatory cells is currently not known.
PD: a cross-talk between neuronal NOX1 and microglial NOX2?
PD is a neurodegenerative disease of the elderly, leading to rest tremor, impaired coordination, as well as postural and gait instability. PD is characterized by the degeneration of DA neurons, in particular in the substantia nigra. In most instances, the cause of PD is unknown. However, for the relatively rare familiar forms of PD (∼5%), defined mutations have been described; for example, mutations in alpha-synuclein, parkin, leucine-rich repeat kinase 2(LRRK2), PTEN-induced putative kinase 1 (PINK1), DJ-1, and ATP13A2. In addition, there are some forms of parkinsonergic disease due to toxins, such as the synthetic drug 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine or pesticides such as rotenone or paraquat (PQ).
ROS and microglia activation are known to contribute to DA neuron death (134). A comprehensive review describing the key role of NOX2 and microglia activation in the neurodegeneration of DA neurons of the substantia nigra was recently published (164).
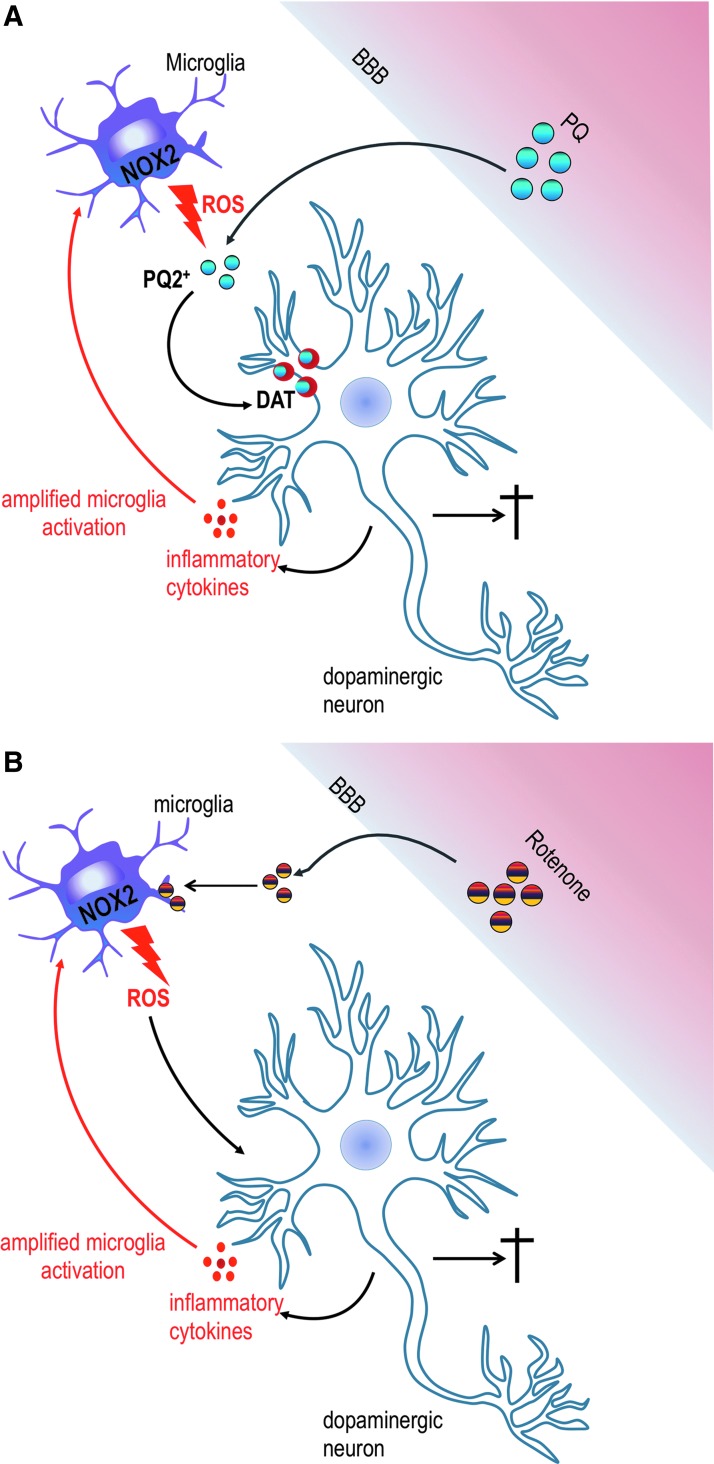
Different modes of action involving NOX2 have been described for toxin-induced DA neurodegeneration. PQ is one of the most used herbicides worldwide. PQ acts as a spontaneous ROS generator by redox cycling, which is usually initiated by cellular diaphorases. A study by Rappold et al. has shown that on reduction by NOX2, PQ becomes a substrate for the dopamine transporter, which actively imports it into DA neurons where it accumulates and induces oxidative stress and cytotoxicity (Fig. 5A) (138). Another mode of action was described for rotenone, a broad spectrum insecticide known to induce PD symptoms. Rotenone was suggested to bind directly to NOX2 on the membrane of microglia and to activate its ROS generating activity (Fig. 5B) (194).
FIG. 5.
Activation of microglia NOX2 via environmental toxics through two unique mechanisms in Parkinson disease. (A) If PQ is oxidized (PQ2+) by ROS generated by NOX2, it becomes a substrate for the DAT, which actively transports it into dopaminergic neurons. Oxidized PQ might be directly neurotoxic and also lead to a release of inflammatory factors by neurons. This eventually leads to amplification of the signal (red arrow) and death of dopaminergic neurons. (B) Rotenone directly interacts with microglia NOX2 and elicits ROS production and damage of dopaminergic neurons. This leads to a release of inflammatory factors, the recruitment of more microglia cells, and, eventually, dopaminergic neuron death. DAT, dopamine transporter; PQ, paraquat. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
There is also increasing evidence for a role of NOX1 in DA neurons and pathophysiology of PD. Adenoviral particles encoding NOX1-specific short hairpin ribonucleic acid (shRNA) significantly reduced α-synuclein expression in DA neurons as well as typical features of PD, such as α-synuclein aggregation and α-synuclein ubiquitin expression levels in a PQ-mediated model of PD (44, 45). By targeting NOX1 and the Rac1 subunit with adenovectors expressing shRNAs, DA neuronal death was prevented (37). Taken together, there is increasing evidence that two NOX enzymes play a role in Parkinon's disease. It is likely that NOX2 is expressed in microglia and NOX1 is expressed in DA neurons. Thus, NOX1 might be the mediator of cell autonomous pathology of DA neurons, while NOX2 might be involved in the inflammatory cascade through microgliosis. Based on these considerations, it would be of interest to evaluate the impact of NOX1/NOX2 double knock-out mice, as well as the efficacy of NOX1/NOX2 inhibitors in the Parkinson model.
Huntington disease: a role of synaptic NOX2?
Huntington disease (HD) is a genetic disorder leading to motor, cognitive, and psychiatric impairment. The physical symptoms of HD typically occur in middle age and progressively lead to multi-system dysfunction, and patients often die from heart failure. HD is caused by an expansion of a CAG triplet repeat (above 35) within the Huntingtin gene, leading to polyglutamine (polyQ)-expanded proteins, which aggregate and are toxic for neurons. Medium spiny neurons of the caudate and subpopulations of cortical neurons are the most sensitive to Huntingtin toxicity, although neurons are affected in many brain areas. Interestingly, although neuroinflammation in HD is present, little is known about its contribution to disease progression and severity (124). However, increased oxidative stress is a known feature of this disease (160). Previous studies have shown increased oxidation, which was attributed to NADPH oxidase in a model of intrastriatal quinolic acid injection (115) and in vitro using the PC12 cell line overexpressing expanded polyQ proteins (17). It was not until very recently that the role of NOX2 was studied in a mouse model of HD consisting of a knock-in introducing 140 glutamine residues in the N-terminal part of endogenous Huntingtin (HD140Q/140Q mice). This study by Valencia et al. (172) used a panel of different experiments to understand the role of NOX2 in HD. Significant cytochrome C reduction was shown in homogenates of human postmortem cortex after the addition of NADPH. Convincingly, a correlation with NOX2 staining and increased ROS production was shown in primary cortical neurons and was abrogated in HD140Q/140Q mice that were bred with NOX2-deficient mice. However, an effect on disease progression in HD140Q/140Q mice that were bred with NOX2-deficient mice was not documented. The presence of NOX2 in microglia was not evaluated, but strong enrichment of NOX2 staining was shown in isolated synaptosomes of HD140Q/140Q mice by immunoblotting although NOX2-deficient synaptosomes were not tested for this antibody (172). Taken together, there are arguments for a role of NOX2 in generating oxidative stress in HD; however, more studies are needed to understand whether the enzyme is really an important player in the disease.
Amyotrophic lateral sclerosis: a key role of microglial NOX2
Amyotrophic lateral sclerosis (ALS) is a group of incurable diseases affecting motor neurons, and its characteristic symptoms are muscle weakness and complete paralysis. The disease onset is usually around 40–50 years, and its course is usually fatal within 5–10 years after diagnosis. ALS is a non-cell autonomous disease and neuroinflammation and microglia activation are key components for the progression of the disease (79). Etiology is most of the time unknown, but familial forms exist, and numerous ALS-causing genes have been identified, including SOD1, TAR DNA binding protein (TARDBP), fused in sarcoma (FUS), ubiquilin2 (UBQLN2), and the recently identified C9ORF72 (abbreviation for the open reading frame 72 on chromosome 9). Interestingly, in addition to spinal motor neuron degeneration, many of these mutations lead to frontotemporal degeneration (112).
Previous studies have shown the role of NOX2 in the progression of ALS, as mice overexpressing a mutant form of human SOD1 (SOD1G93A) cross-bred with NOX2-deficient mice have increased survival (185). There is also potential indication that NOX1 deficiency might slow the course of the ALS mouse model (118). One pharmacological study using the antioxidant/NOX inhibitor apocynin suggested a strong increase in the survival of ALS mice (68); however, this was not confirmed by other studies (110, 170).
In mouse models, NOX2 expression in the lumbar spinal cord can also be used as a marker of neuroinflammation and microgliosis (15, 20). Indeed, when comparing microglia purified from SOD1G93A mice at different stages of disease, Liao et al. (108) observed that microglia at an early stage presented a neuroprotective activity while it became neurotoxic for primary motoneurons at an end stage. The protective stage (M2, alternatively activated) was characterized by low NOX2 expression, while the injurious stage (M1, classically activated) was characterized by high NOX2 expression and a decrease in markers of alternative macrophage activation YM1, brain-derived neurotrophic factor, and CD163 (108).
A recent study identified protein disulfide isomerase (PDI) as a key regulator of NOX2 activation in microglia and its increased expression in glial cells present in the spinal cord of SOD1G93A mice (83). PDI is an oxidoreductase that is involved in oxidative protein folding in the endoplasmic reticulum with an emerging role in aggregation neurodegenerative diseases (5).
The role of NOX2 expression in microglia was studied using the SOD1G93A mice, but nowadays, novel rodent models of ALS expressing mutated TDP43 and FUS are being developed (171, 182) and novel studies on the role of NOX isoforms in these new models are nowadays needed (107).
Taken together, evidence from NOX2- (and, to a lesser extent, NOX1-) deficient mice suggest that NOX activation is causally involved in the progression of ALS. No reliable pharmacological data are available. Thus, NOX enzymes are probably not causative of ALS, but likely contribute to disease progression and, therefore, remain a valid target to improve ALS symptoms; however, more work is needed to definitively prove this point.
Multiple sclerosis: increase in microglial NOX2 and oxidation in myelin lesions
Multiple sclerosis (MS) is a chronic inflammatory disease, leading to demyelination, impaired motor function, and neurodegeneration. It is an autoimmune disease affecting the myelin sheath of the CNS. Polymorphisms and mutations affecting the neutrophil cytosolic factor 1 (Ncf1) gene (coding for p47phox subunit) were shown to significantly increase susceptibility for autoimmune disease in rodents, including experimental autoimmune encephalomyelitis, a model of MS (9, 76). This aspect of MS will not be discussed here, as the role of Ncf1 and NOX2-mediated oxidative burst in autoimmune disease by inhibiting T-cell activation has been extensively reviewed elsewhere (72, 142). Indeed, once the disease is declared, a strong increase of NOX in MS lesions likely governs the oxidative damage of the myelin sheath.
Two recent elegant studies used carefully dissected white matter lesions from MS patient's material to study oxidative stress markers and their connection with NOX enzymes. In active demyelinating lesions, the presence of lipid and DNA oxidation products not only in oligodendrocytes but also in neurons significantly correlated with inflammation (66). In subsequent studies, increased expression of NOX2 and subunits at both mRNA and protein levels was observed in dissected regions of active inflammatory foci from human brain material. Immunostaining showed up-regulation and co-localization of NOX2 and its subunits in activated microglia, correlating with markers of oxidative stress. NOX2 and p22phox were constitutively expressed in microglia in the initial lesion and were abundantly expressed in microglial clusters even in normal appearing lesions. In addition to NOX2 and subunits, NOX1 and NOXO1 staining was seen in activated microglia, as well as in astrocytes and endothelial cells. Interestingly, p47phox, NOX1, and NOXO1 expression was more restricted to the zone of initial damage, including astrocytes, or to lesions from patients with acute or early relapsing/remitting MS, suggesting a key role of NOX-derived ROS in acute MS lesions (55, 174). The presently available data suggest that microglia are the predominant NOX2-expressing cell in MS lesions; however, a role of other types of inflammatory cells is not excluded.
Taken together, NOX2 appears to be a double-edged sword in MS. While low NOX2 activity may contribute to the autoimmune pathogenesis, high NOX2 (and possibly also NOX1) activity in MS lesions might contribute toward enhancing immune-mediated neuronal damage.
NOX/ROS and neurovascular disease
ROS are involved in the development of vascular diseases (165), and the CNS is no exception in this context. Interestingly, however, NOX-derived ROS generation in the CNS is not only exclusively linked to vascular disease within the CNS, but may also—mostly through blood pressure regulation—influence vascular disease in the entire body. Reciprocally, vascular disease may trigger symptoms of neurodegenerative diseases, such as AD (148), as well as ischemic and hemorrhagic stroke. Increased ROS are observed in these pathologies, and the deletion of different NOX isoforms can be protective in animal models. This protection might be due to the regulation of cerebral blood flow by neurovascular cells (62, 91), invading immune cells, and/or the brain parenchyma itself (31) is a matter of debate. Cell-specific deletion of NOX isoform through the use of conditional knock-out mice will help understanding the respective roles of NOX-expressing cells in neurovascular function. Here, we will focus on ischemic stroke and the CNS renin angiotensin system (RAS) for which NOX enzymes play essential roles.
Ischemic stroke
Ischemic stroke results from the obstruction of blood supply to the brain usually due to atherosclerosis and thrombosis. Stroke can cause transient damage, permanent brain damage leading to life-long neurological symptoms, or death. Treatment options for stroke are limited. Thrombolytic agents can be useful; however, this treatment is efficacious only during the first hours after stroke and associated with a considerable bleeding risk. While a part of neuronal cell death during stroke is simply explained by hypoxia and nutrient deprivation, it has become clear that oxidative stress adds to the severity of the pathology. There are two situations in which oxidative stress is likely to be of importance: (i) reperfusion after ischemic obstruction and (ii) neuroinflammation in the penumbra of the stroke area (100). The role of oxidative stress has been known for a long time, and was the rationale for numerous clinical trials using antioxidants. However, not unexpectedly, antioxidant treatment did not result in therapeutic improvements (see CNS-Permeant NOX Inhibitors).
A first proof of concept for NOX2 as a principal source of ROS in stroke was already shown in 1997, when Walder et al. showed strong protection in transient middle cerebral artery occlusion in NOX2-deficient mice (176), raising strong interest in targeting NOX2 as potential therapy for stroke. Since then, this paradigm has been tested in numerous models using small molecules and NOX1-, NOX2-, and NOX4-deficient mice, resulting in different outcomes and leading to disputes as to whether NOX2 [comprehensively reviewed in ref. (86)] or NOX4 [comprehensively reviewed in ref. (137)] isoforms are the key pharmacological target for stroke. Interestingly, a recent report showed that deletion of the proton channel Hv1 significantly protected from brain damage caused by ischemic stroke (186). Since the Hv1 proton channel is considered crucial to extrude protons left behind by NOX activity and hence necessary for NOX function, this observation adds weight to the concept that NOX enzymes are deleterious in stroke. However, several points remain unclear: Is NOX2-derived ROS generation mainly due to microglia activation or do infiltrating blood-derived inflammatory cells play a major role? Does a single NOX isoform (e.g., NOX2 vs. NOX4) determine the outcome of stroke, or is there a complex pattern with different NOX isoforms intervening at different time points and in different pathological situations?
NOX and the brain RAS
The RAS has a vital role in regulating physiological processes of the cardiovascular system, including blood flow and cardiac homeostasis. Ang II binds to its receptors (angiotensin II receptor [ATR]1, ATR2) to mediate physiological responses in many organs, including vasculature, liver, kidney, adrenal gland, brain, lung, and heart. In the vasculature, activation of AT1R leads to direct NOX1- and NOX2-dependent ROS generation and NOX4-dependent vascular hypertrophy. The physiological and pathophysiological connections between NOX enzymes and Ang II in the cardiovascular system have been extensively described (101) and are beyond the scope of this review.
Of interest for this review, however, is emerging evidence for an intrinsic RAS in the CNS. This system acts as a regulator of fluid homeostasis, blood pressure, sexual behavior, and stress responses (184). The CNS RAS might be involved not only in the development of cardiovascular disease (30) but also in neuroinflammation, in particular in PD (99, 121).
Ang II treatment activates NOX-dependent ROS generation in neurons from regions of the brain that regulate fluid and electrolyte balance, such as the lamina terminalis (133), the dorsomedial portion of the nucleus tractus solitaries (178), and the hypothalamic paraventricular nucleus (179). In vivo, numerous studies describe a role of NOX-dependent ROS generation in the CNS that regulates peripheral renovascular effects of angiotensin. An intracerebral injection of adenoviral vectors siRNA targeting NOX2 and NOX4 in the subfornical organ (a key region for the control of fluid balance) showed that both NOX2 and NOX4 are required for blood pressure increase after central Ang II infusion, but only NOX2 regulates Ang II-dependent water intake (133).
Lob et al. (111) developed a mouse with a locus of cross-over in P1 sites flanking the coding region of p22phox (a NOX subunit necessary for membrane stability and function of NOX1, 2, 3, and 4). This approach enabled them to test region-specific effects of NOX deletion by injecting adenovectors encoding cre-recombinase in the subfornical organ. The specific deletion of p22phox eliminated the hypertensive response after peripheral chronic Ang II infusion, demonstrating the central control by NOX enzymes in the subfornical organ (111). Interestingly, the p22phox deletion diminished not only p22phox mRNA levels but also NOX2 and NOX4 transcripts.
Taken together, there is now good evidence that NOX enzymes, in particular NOX2 and NOX4, are involved in the regulation of blood pressure through the brain RAS.
Pain
Neuropathic pain refers to a pain that is caused by damage, injury, or dysfunction of nerves, leading to hypersensitization of a painful feeling in response to a normally innocuous stimulus. ROS are known to contribute to pain sensitization (75). Animal models of neuropathic pain consist of evaluating pain sensitization after peripheral injury. A number of studies suggested a role of NOX-derived ROS in this process of nociceptive hypersensitization (39, 80), but two studies addressed this question using genetically modified mice.
Kim et al. (90) showed a transient increase of NOX2 expression in microglia with a peak at 12 h after L5 spinal nerve transection. This was followed by increased 8-hydroxydeoxyguanosine (a marker of DNA oxidation), microglia activation, as well as mechanical and thermal hypersensitivity. All these parameters, including pain hypersensitivity, were reduced in NOX2-deficient mice. Interestingly, microglia activation and increased pain were reduced by sulforaphane (an inducer of the cellular antioxidant response, the Nrf2 pathway) only when administered before or 1 h after nerve injury (90). A role of neuronal NOX4 present in DRGs has also emerged. In two different models of neuropathic pain, the so-called spared nerve injury, and a modified version of the chronic constriction injury, basal pain sensitivity was normal in NOX4-deficient mice, while neuropathic pain was markedly reduced. In the spared nerve injury, NOX4-dependent H2O2 was measured in sciatic nerve while a decrease in axon size peripheral dismyelination of the sciatic nerve was observed. All these features were partially reduced in NOX4-deficient mice, although microglia activation was comparable between NOX4-deficient and control littermates 14 days after spared nerve injury (87). Thus, both microglial NOX2 and neuronal NOX4 might be involved in the development of neuropathic pain. However, the field is complicated by an excessive number of models of neuropathic pain [more than 40 described by Jaggi et al. (81)]. A study involving NOX2, NOX4, and NOX2/NOX4 double-deficient mice in carefully selected models may help resolving the exact role of each NOX isoform.
NOX enzymes might also be involved in morphine-induced analgesia and tolerance. Indeed, in NOX1-deficient mice, the magnitude of analgesia induced by morphine was enhanced, and tolerance development was decreased (77).
Taken together, there is good evidence that NOX enzymes may enhance pain and decrease the activity of pain medication. In many instances, the effect of NOX enzymes is considered as occurring in the CNS; however, effects on peripheral nerve and pain sensation also remain a possibility.
Traumatic brain injury and chronic traumatic encephalopathy
Traumatic injury, in particular, when occurring several times in the same person, may lead to a condition called chronic traumatic encephalopathy. This has been best studied in individuals practicing contact sports that often lead to head injury, as, for example, boxers and ice hockey players (8, 84). Patients with chronic traumatic encephalopathy show signs of CNS dysfunction, including memory impairment, depression, motoneuron disease, and dementia. Postmortem neuropathological examination shows that chronic traumatic encephalopathy is frequent in populations at risk and may include histopathological signs of classical neurodegenerative disorders, such as AD (173).
There is increasing evidence for a role of oxidative stress in the neurodegeneration after traumatic brain injury. Already 24 h after traumatic brain injury, microglia become activated and express NOX2 (98). In addition, it is possible that blood-derived inflammatory cells enter the CNS after injury. In response to traumatic brain injury, wild-type mice show substantial cortical areas of necrosis and apoptosis, while NOX2-deficient mice are at least partially protected (49). Interestingly, wild-type mice showed induction of β-amyloid production within a few days after traumatic brain injury; this β-amyloid induction was markedly diminished by treatment of mice with the antioxidant/NOX inhibitor apocynin (193). Loss of spatial learning in this model could also be attenuated by the administration of apocynin (156).
Taken together, there is good evidence for a role of NOX-dependent oxidative stress in post-traumatic brain injury and the subsequent neuronal damage. Probably, the phagocyte NADPH oxidase NOX2 is an important NOX isoform in traumatic brain injury. Future studies should focus on the role of NOX enzymes in the transition from posttraumatic brain injury to chronic traumatic encephalopathy and on the question as to whether specific NOX inhibitors might provide a preventive treatment after brain injury.
Sleep apnea
Sleep apnea is a condition in which sleep is disturbed by pauses in breathing. It is most commonly due to obstruction of the airways during sleep, but it also sometimes has a central origin. Other factors such as obesity are highly predictive of risk of obstructive sleep apnea (OSA). Typically, sleep apnea not only results in daytime fatigue, but also induces neuronal death, leading to symptoms such as attention deficit and memory impairment. ROS are considered as playing a role in this neuropathology, with NOX enzymes as a potential source (181).
The role of NOX2 in CNS alterations has been evaluated in different mouse models of sleep apnea. As compared with wild type, NOX2-deficient mice showed less cognitive deficit in response to intermittent hypoxia, as well as less signs of lipid and DNA oxidation in the hippocampus (127). Interestingly, cognitive deficit induced by intermittent hypoxia was aggravated if animals were fed with a high fat diet (128). A clinical study addressing the role of NOX2 activity in OSA was performed using 244 children affected by OSA and 370 healthy controls (healthy subject). Leukocyte NOX2 activity was higher in OSA children than in healthy controls and among OSA children, NOX2 activity was higher in children with cognitive deficit. A similar pattern was observed in 8-hydroxydeoxyguanosine levels in morning urine. Interestingly, an analysis of single nucleotide polymorphisms (SNPs) of CYBA, the gene coding for p22phox, revealed that the polymorphism rs4673 (242C>T) was significantly more frequent among OSA children without cognitive deficits and was associated with lower NOX2 activity from leukocyte extracted from the blood (64). Indeed, SNP rs4673 was previously shown to be a part of a haplotype that was associated with decreased ROS generation (10). Unfortunately, in the study of Gozal et al., (64), only a single SNP was investigated and no haplotype analysis was performed. However, with this limitation in mind, it appears that a polymorphism in an NOX subunit is associated with the risk of developing cognitive deficit in sleep apnea. This concept is clearly supported by results derived from NOX2-deficient animals. Thus, NOX2 inhibition might become a concept of interest for patients at risk for cognitive deterioration because of sleep apnea (51).
NOX in CNS tumors
Primary malignant tumors of CNS account for 2% of all cancers. However, this number probably underestimates the importance of CNS tumors, because of the poor survival of patients suffering from this type of cancer. The most common and probably most deadly malignant tumor of the brain is glioblastoma. Other common brain tumors, such as astrocytoma, meningiomas and pituitary tumors, generally have better prognosis, however obviously threatening to the patient because of their CNS localization.
The generation of ROS by NOX enzymes is implicated in cancer pathophysiology, through mechanisms including ROS-dependent cell fate determination (proliferation vs. cell death), angiogenesis, genomic instability, and metastasis (19). The literature on NOX and tumor cell fate provides evidence for two apparently contradictory effects: NOX-dependent ROS may cause cell proliferation in certain instances, but cell death under other circumstances. Most likely, the set of redox-sensitive transcription factors and signaling proteins expressed in a given cell explains these very different types of responses. There is abundant evidence for a role of ROS in angiogenesis, and recent evidence points toward a role of NOX1 and NOX4 in tumor angiogenesis (42). In terms of mechanism, it has been proposed that NOX activity promotes cell migration and invasion in cancer cells by redox regulation of actin cytoskeleton (46) and/or by NOX-derived invadopodia formation (47, 60) as well as by direct activation of matrix metalloproteinases (MMPs) (163).
Genomic instability is due to double-strand breaks that are induced by ROS; although such breaks are readily repaired under normal circumstances, tumor cells may lack certain repair mechanisms due to somatic mutations or lack of mechanisms to detect genotoxicity, for example, p53 mutations (41). Finally, the role of NOX enzymes in metastasis is multifactorial, including, for example, a role of ROS in cell migration and activation of MMP.
Expression of NOX isoforms is highly dependent on tumor cell types and not necessarily correlated with NOX expression in the adjacent normal tissue (85). Already early after the discovery of NOX enzymes, it was suggested that NOX4 is highly expressed in glioblastoma cell lines (33). One study found that globally, NOX4 was highly expressed in human glioblastoma, as compared with astrocytoma, a related glia-derived tumor of lower malignancy (152). However, analyses of individual tumor samples rather suggests relatively important differences in NOX4 expression: Out of five glioblastoma samples studied, two showed very high levels, but three showed only low to intermediate NOX4 levels (85). The latter study also suggested that other NOX isoforms, such as DUOX1, may occasionally be highly expressed in glioblastoma.
ROS generated by NOX4 (or maybe other NOX enzymes) are considered to be involved in the oncogenic transformation and in the clinical course of glioblastoma.
Glioblastoma invasion
NOX4 might be involved in the invasion and infiltration of glioblastoma cells. Indeed, RNAi-knock-down of NOX4 reduces the invasion of glioblastoma cells in comparison to non-transfected cells (74). Evidence for a role of NOX enzymes was also provided by an in vitro assay of glioblastoma invasion (36): Tumor cell invasion was accompanied by ROS generation and could be blocked by the non-specific NOX inhibitor DPI. This study also provided a mechanistic hypothesis: DPI inhibition of tumor cell invasion was accompanied by inhibition of the MMP-9, which is considered required for tumor migration. Targeting NOX directly or targeting the pathways leading to NOX inhibition, such as NF-kappaB and Akt, may represent a novel approach for glioblastoma therapy (126).
Hypoxia
In response to hypoxia, cells activate the transcription factor HIF1α, which activates the cellular hypoxia response, such as release of angiogenic factors. This response can be elicited by hypoxia itself; however, there is increasing evidence that ROS are able to potentiate the response. Increased ROS level and increased NOX4 expression have been observed in glioblastoma cell lines under hypoxic condition, and it has been suggested that NOX4-generated ROS promote the activation of HIF-1 and of glioblastoma progression (73).
Tumor cell growth and apoptosis
There is some indication that NOX4 might be involved in glioblastoma cell growth. Indeed, knock-down of NOX4 by RNAi reduced cell growth (152). NOX4 might also be involved in treatment resistance of glioblastoma: NOX4 knock-down increased glioblastoma apoptosis in response to cisplatin and to radiotherapy (74, 152).
Taken together, there is evidence that NOX4 is highly expressed at least in some glioblastoma samples and that this expression might be relevant for tumor invasion, hypoxia response, tumor cell growth, and response to therapy. However, further investigations will be necessary to see whether NOX4 is, indeed, an interesting therapeutic target for glioblastoma treatment.
Psychiatric disorders
Redox-dependent processes have been implicated in a variety of psychiatric diseases, from major depression to autism. Here, we will only review evidence for psychosis and schizophrenia, in particular because a more thorough investigation of sources of oxidative stress has been performed for these pathologies.
The concept that schizophrenia might be linked to oxidative stress goes back to the 1950s (71). The concept was based on observations during World War II: Due to supply problems, there was a need to use epinephrine from expired stocks, which not only had changed color to pink, but also led to hallucinations in the patients who received the drug (http://drugabuse.ca/drug-file-adrenochromes). Subsequent chemical analysis demonstrated that the pink coloration is due to oxidation of epinephrin to adrenochrome (3-hydroxy-1-methyl-2, 3-dihydro-1H-indole-5, 6-dione). Subsequently, adrenochrome has been used as a recreational drug under the street name “pink adrenaline” (Fig. 6). The observation that oxidation of a natural neurotransmitter leads to generation of a hallucinogenic compound led Hoffer et al. (71) to develop the first hypothesis for a link between schizophrenia and oxidative stress. Since then, numerous investigations into the relationship between psychiatric diseases and oxidative stress have been performed (139). While initial investigations were rather of a descriptive, observational type, more recently, progress in the mechanistic understanding has been achieved. Indeed, there is increasing evidence that in schizophrenia, there is a disappearance of parvalbumin-positive inhibitory interneurons (119). Most evidence suggests that these neurons do not die, but rather lose their inhibitory phenotype, in particular, the expression of parvalbumin. The mechanism of this loss of inhibitory phenotype is not fully understood, but a key to the understanding of the phenomenon comes from animal studies. In a milestone paper, the Behrens group showed that in ketamine-induced psychosis a widely used mouse model of schizophrenia (65), loss of parvalbumin-positive neurons is driven by NOX2-derived ROS (13). Subsequent publications demonstrated that also early responses to ketamine, such as increases in glutamate levels, are NOX2 dependent (159) and that the cytokine IL-6 mediates NOX2 activation (14). In another animal model of psychosis, induced by social isolation of adolescent rats, both behavioral abnormalities and neurochemical alterations (including high glutamate and loss of parvalbumin-positive neurons) were prevented by apocynin treatment and—importantly—in rats with a loss-of-function mutation in an NOX2 subunit (145, 147). There are also indications that severe life stress (possibly contributing to psychosis) leads to oxidative stress in the CNS of humans (146). Further studies will be necessary to understand the relative importance of the NOX/ROS axis in human psychotic disease.
FIG. 6.
Hoffer and Osmond hypothesis for oxidative stress-mediated psychosis. Ex vivo oxidation of epinephrine (also known as adrenaline) generates adrenochrome, a potent psychotropic compound that is characterized by its pink color in the solution. This oxidized epinephrine is consumed as a recreational drug and its street name is “pink adrenaline.” A speculative, but interesting hypothesis suggests that increased oxidation in psychotic patients may lead to the generation of adrenochrome, which might contribute to characteristic symptoms of schizophrenia, such as hallucinations and delusion.
ROS-dependent signaling within mature post-mitotic neuron
NOX2-derived ROS can also be pathological, as they contribute to excitotoxicity, one of the main causes of neuronal death due to over-activation of glutamate receptors (such as in experimental models by using NMDA receptor agonists).
The activation of NMDA receptor by NMDA leads to a direct and fast superoxide generation by NOX2 in cortical neurons and hippocampus (22), leading to death of neighboring neurons (140). However, interestingly, blockade of NMDA receptor by antagonists, such as ketamine, can also activate NOX2-dependent ROS in cortical neurons and lead to parvalbumin neuron depletion, a hallmark of psychiatric disease (180).
This apparent paradox is most likely explained by the fact that NMDA receptor antagonists block the activation of inhibitory neurons, which, in turn, leads to an increased release of glutamate through glutamatergic neurons (159).
CNS-Permeant NOX Inhibitors
Antioxidants and scavengers
Before the emergence of NOX-based strategies, the only concept to target oxidative stress in the CNS was the use of antioxidant supplementation. However, almost invariably, clinical studies with antioxidants failed. The reason for this failure can be summarized as “too late, too little, and too non-specific.” Indeed, antioxidants do not block the overshooting production of ROS, but rather are supposed to capture ROS once they are already produced. Thus, given their high affinity, ROS will oxidize biomolecules of the organism before antioxidants can exert their activity. To make things worse, antioxidants can become pro-oxidants on interaction with ROS (122). Another limiting factor in the use of antioxidants is their bioavailability. This is particularly relevant for the CNS, where antioxidant concentrations rarely reach potentially therapeutic levels. Finally, there is a problem with the specificity of antioxidants. This is best illustrated by the example of the generation of thyroid hormones. The iodination of thyroglobulin, a key step in thyroid hormone production, requires ROS for the chemical peroxidation reaction that enables a covalent link between the protein and iodide. Thus, if an antioxidant that is sufficiently powerful to block in-vivo oxidation reactions could be developed, it may lead to hypothyroidism (158).
N-acetyl cysteine
N-acetyl cysteine is often also considered an antioxidant, but its mechanism of action is more complex. Indeed, it acts as a cysteine donor for glutathione synthesis and, therefore, replenishes the cellular antioxidant defense machinery. As opposed to the inefficiency of classical antioxidant scavengers in clinical trials, there are some indications that N-acetyl cysteine might, indeed, have an (albeit limited) use in the treatment of oxidative stress-related CNS diseases. The best documented example is probably the supplementation of schizophrenic patients with high-dose N-acetyl cysteine, which led to statistically significant improvements of symptoms (16).
Non-specific NOX inhibitors
The two most widely used compounds that act as non-specific NOX inhibitors are DPI and apocynin. Both compounds can be useful tools if used with caution, but can also lead to misleading conclusions if their limitations and mechanisms of action are not understood.
DPI is an entirely non-specific inhibitor of electron transporters. Its mechanism of action involves the formation of an iodonium radical through an interaction with an electron transport protein. The iodonium radical, in turn, will interact with and irreversibly block the electron transporter (131). Electron transporters that have been shown to be sensitive to irreversible inhibition by DPI include NADPH oxidase, nitric oxide synthase, xanthine oxidase, the mitochondrial electron transport system, and probably many others. Thus, albeit DPI remains one of the most potent inhibitors of NADPH oxidases and is useful in vitro, the interpretation of results obtained with this compound needs a very careful analysis as well as an abundant control experiment. DPI has been used in vivo, but it is highly insoluble and toxic; therefore, such results are virtually impossible to interpret.
Apocynin is probably the most widely used putative NADPH oxidase inhibitor that is used for in vivo studies. Indeed, relatively low amounts of the compounds may lead to marked inhibition of signs of oxidative stress in a variety of in vivo models [e.g., ref. (147)]. The compound, however, is remarkably inefficient in vitro. Only exceedingly high concentrations of the compound inhibit ROS generation in vitro, and this inhibition is most likely explained by an antioxidant effect that occurs at high concentrations (70). For this reason, it has been proposed that apocynin needs to be metabolized in order to become an NOX inhibitor. However, so far, there is no convincing demonstration of a metabolite of apocynin that is able to efficiently block NOX enzymes in a well-controlled experimental system. Thus, in our opinion, apocynin data might be useful when combined with other experimental evidence (e.g., NOX-deficient mice); however, they should not be considered a self-sufficient tool to prove (or to disprove) the role of NOX enzymes in a given biological process.
Specific NOX inhibitors
Several academic researcher groups and drug development companies have started working on the generation of specific NOX inhibitors (82). The currently best-documented compounds are GKT136901 and its close analogue GKT137831. Both compounds display NOX1/NOX4 inhibitory activity and only a weak activity with regard to NOX2. A compound of potential interest is VAS2870, which is considered a pan-NOX inhibitor (183). This compound showed a strong protective effect in a mouse model of stroke, which has been attributed to NOX4 inhibition (94). However, it required intrathecal administration, which makes its clinical use unlikely for CNS indications. Ebselen, a selenium compound known for a long time for its antioxidant and neuroprotective properties, was recently shown to be an NOX2 inhibitor (155). A beneficial effect has been described in multiple CNS disorders, including PD in primates (125) and stroke in humans (189); however, ebselen is rather unspecific and displays numerous modes of action, including glutathione mimetic (18) among others (188). As of today, in spite of the huge therapeutic potential of NOX inhibition in the CNS (158), no NOX inhibitor is available for CNS indications.
Conclusion
Research on NOX enzymes in the CNS has exploded over the previous century. A search in the Web of Knowledge database using the keyword “NADPH oxidase” and “central nervous system” shows that the first two articles including the two key words were published in 1991. Between 1991 and 2001, 339 articles on the topic were published. This number skyrocketed to 5659 in the time period from 2002 to 2012.
Key findings of this expanding field are, on the one hand, not only in the field of redox physiology in the CNS, but also in the field of disease development. The fact that apparently unrelated CNS disorders seem to benefit from NOX inhibition strongly suggests that NOX enzymes regulate central features of the pathological CNS. With the emergence of NOX inhibitors in clinical settings, the identification of the molecular mechanism controlling the protective action of NOX deficiency is fundamental. In fact, a direct neurotoxic effect of NOX-derived ROS cannot be ruled out, and it is most likely that NOX activity contributes to CNS physiopathology, via regulation of glutamatergic signaling (140, 159), and/or glia physiology (162).
The classical phagocyte NADPH oxidase remains a key player in the CNS, in terms of both physiology and pathology. However, the other NOX isoforms also become increasingly important. NOX enzymes are most likely involved in CNS development, NSC biology, and functioning of mature neurons. However, the CNS phenotype of NOX deficiency is relatively mild, suggesting a modulatory rather than an essential role of NOX enzymes. NOX-deficient mice are protected in several CNS disease models, particularly neurodegeneration, stroke, and psychosis. Taken together, these points suggest that NOX inhibition in oxidative stress-related CNS diseases is a reasonable concept. Thus, the development of CNS-permeant NOX inhibitors will be of interest for future medical developments in the NOX field.
Abbreviations Used
- Aβ
amyloid β
- AD
Alzheimer disease
- ALS
amyotrophic lateral sclerosis
- Ang II
angiotensin II
- APP
amyloid precursor protein
- ATP
adenosine triphosphate
- ATR
angiotensin II receptor
- CCAA
capillary cerebral amyloid angiopathy
- CGD
chronic granulomatous disease
- CNS
central nervous system
- DA
dopaminergic
- DAT
dopamine transporter
- DPI
diphenylene iodonium
- DRG
dorsal root ganglion
- DUOX
dual oxidase
- FUS
fused in sarcoma
- H2O2
hydrogen peroxide
- HD
Huntington disease
- HIF
hypoxia inducible factor
- IL
interleukin
- Keap1
Kelch-like ECH-associated protein 1
- LPS
lipopolysaccharides
- MMP
matrix metalloproteinase
- mRNA
messenger ribonucleic acid
- MS
multiple sclerosis
- NADPH
nicotinamide adenine dinucleotide phosphate
- Ncf1
neutrophil cytosolic factor 1
- NFkappaB
nuclear factor kappa B
- NMDA
N-methyl-d-aspartate
- nNOS
neuronal nitric oxide synthase
- NO
nitric oxide
- NOX
nicotinamide adenine dinucleotide phosphate-dependent oxidase
- Nrf2
nuclear respiratory factor 2
- NSC
neural stem cell
- OSA
obstructive sleep apnea
- PD
Parkinson disease
- PDI
protein disulfide isomerase
- polyQ
polyglutamine
- PQ
paraquat
- PS1
presenilin 1
- RAS
renin angiotensin system
- ROS
reactive oxygen species
- shRNA
short hairpin ribonucleic acid
- SNP
single nucleotide polymorphism
- SOD
superoxide dismutase
- TLR
Toll-like receptor
- VCAM-1
vascular cell adhesion molecule 1
- VHL
von Hippel-Lindau
- YM1
chitinase 3-like 3
Acknowledgments
The author Zeynab Nayernia is funded by the Swiss National Science Foundation (SNF) under grant number 310030B-141182, and Vincent Jaquet is funded by the European Community's Framework Program (FP7/2007–2013) under Grant 278611 (Neurinox). The authors would like to thank Tamara Seredenina for the editing of this article.
References
- 1.Aguzzi A, Barres BA, and Bennett ML. Microglia: scapegoat, saboteur, or something else? Science 339: 156–161, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ali SS, Young JW, Wallace CK, Gresack J, Jeste DV, Geyer MA, Dugan LL, and Risbrough VB. Initial evidence linking synaptic superoxide production with poor short-term memory in aged mice. Brain Res 1368: 65–70, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alliot F, Godin I, and Pessac B. Microglia derive from progenitors, originating from the yolk sac, and which proliferate in the brain. Brain Res Dev Brain Res 117: 145–152, 1999 [DOI] [PubMed] [Google Scholar]
- 4.Altenhofer S, Kleikers PW, Radermacher KA, Scheurer P, Rob Hermans JJ, Schiffers P, Ho H, Wingler K, and Schmidt HH. The NOX toolbox: validating the role of NADPH oxidases in physiology and disease. Cell Mol Life Sci 69: 2327–2343, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andreu CI, Woehlbier U, Torres M, and Hetz C. Protein disulfide isomerases in neurodegeneration: from disease mechanisms to biomedical applications. FEBS Lett 586: 2826–2834, 2012 [DOI] [PubMed] [Google Scholar]
- 6.Ansari MA. and Scheff SW. NADPH-oxidase activation and cognition in Alzheimer disease progression. Free Radic Biol Med 51: 171–178, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Antony S, Wu Y, Hewitt SM, Anver MR, Butcher D, Jiang G, Meitzler JL, Liu H, Juhasz A, Lu J, Roy KK, and Doroshow JH. Characterization of NADPH oxidase 5 expression in human tumors and tumor cell lines with a novel mouse monoclonal antibody. Free Radic Biol Med 65C: 497–508, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baugh CM, Stamm JM, Riley DO, Gavett BE, Shenton ME, Lin A, Nowinski CJ, Cantu RC, McKee AC, and Stern RA. Chronic traumatic encephalopathy: neurodegeneration following repetitive concussive and subconcussive brain trauma. Brain Imaging Behav 6: 244–254, 2012 [DOI] [PubMed] [Google Scholar]
- 9.Becanovic K, Jagodic M, Sheng JR, Dahlman I, Aboul-Enein F, Wallstrom E, Olofsson P, Holmdahl R, Lassmann H, and Olsson T. Advanced intercross line mapping of Eae5 reveals Ncf-1 and CLDN4 as candidate genes for experimental autoimmune encephalomyelitis. J Immunol 176: 6055–6064, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Bedard K, Attar H, Bonnefont J, Jaquet V, Borel C, Plastre O, Stasia MJ, Antonarakis SE, and Krause KH. Three common polymorphisms in the CYBA gene form a haplotype associated with decreased ROS generation. Hum Mutat 30: 1123–1133, 2009 [DOI] [PubMed] [Google Scholar]
- 11.Bedard K, Jaquet V, and Krause KH. NOX5: from basic biology to signaling and disease. Free Radic Biol Med 52: 725–734, 2012 [DOI] [PubMed] [Google Scholar]
- 12.Bedard K. and Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev 87: 245–313, 2007 [DOI] [PubMed] [Google Scholar]
- 13.Behrens MM, Ali SS, Dao DN, Lucero J, Shekhtman G, Quick KL, and Dugan LL. Ketamine-induced loss of phenotype of fast-spiking interneurons is mediated by NADPH-oxidase. Science 318: 1645–1647, 2007 [DOI] [PubMed] [Google Scholar]
- 14.Behrens MM, Ali SS, and Dugan LL. Interleukin-6 mediates the increase in NADPH-oxidase in the ketamine model of schizophrenia. J Neurosci 28: 13957–13966, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Berger JV, Deumens R, Goursaud S, Schafer S, Lavand'homme P, Joosten EA, and Hermans E. Enhanced neuroinflammation and pain hypersensitivity after peripheral nerve injury in rats expressing mutated superoxide dismutase 1. J Neuroinflamm 8: 33, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Berk M, Copolov D, Dean O, Lu K, Jeavons S, Schapkaitz I, Anderson-Hunt M, Judd F, Katz F, Katz P, Ording-Jespersen S, Little J, Conus P, Cuenod M, Do KQ, and Bush AI. N-acetyl cysteine as a glutathione precursor for schizophrenia—a double-blind, randomized, placebo-controlled trial. Biol Psychiatry 64: 361–368, 2008 [DOI] [PubMed] [Google Scholar]
- 17.Bertoni A, Giuliano P, Galgani M, Rotoli D, Ulianich L, Adornetto A, Santillo MR, Porcellini A, and Avvedimento VE. Early and late events induced by polyQ-expanded proteins: identification of a common pathogenic property of polYQ-expanded proteins. J Biol Chem 286: 4727–4741, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bhabak KP. and Mugesh G. Functional mimics of glutathione peroxidase: bioinspired synthetic antioxidants. Acc Chem Res 43: 1408–1419, 2010 [DOI] [PubMed] [Google Scholar]
- 19.Block K. and Gorin Y. Aiding and abetting roles of NOX oxidases in cellular transformation. Nat Rev Cancer 12: 627–637, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boucherie C, Schafer S, Lavand'homme P, Maloteaux JM, and Hermans E. Chimerization of astroglial population in the lumbar spinal cord after mesenchymal stem cell transplantation prolongs survival in a rat model of amyotrophic lateral sclerosis. J Neurosci Res 87: 2034–2046, 2009 [DOI] [PubMed] [Google Scholar]
- 21.Braunersreuther V. and Jaquet V. Reactive oxygen species in myocardial reperfusion injury: from physiopathology to therapeutic approaches. Curr Pharm Biotechnol 13: 97–114, 2012 [DOI] [PubMed] [Google Scholar]
- 22.Brennan AM, Suh SW, Won SJ, Narasimhan P, Kauppinen TM, Lee H, Edling Y, Chan PH, and Swanson RA. NADPH oxidase is the primary source of superoxide induced by NMDA receptor activation. Nat Neurosci 12: 857–863, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bruce-Keller AJ, Gupta S, Knight AG, Beckett TL, McMullen JM, Davis PR, Murphy MP, Van Eldik LJ, St. Clair D, and Keller JN. Cognitive impairment in humanized APPxPS1 mice is linked to Abeta(1–42) and NOX activation. Neurobiol Dis 44: 317–326, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bruce-Keller AJ, White CL, Gupta S, Knight AG, Pistell PJ, Ingram DK, Morrison CD, and Keller JN. NOX activity in brain aging: exacerbation by high fat diet. Free Radic Biol Med 49: 22–30, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Burgoyne JR, Mongue-Din H, Eaton P, and Shah AM. Redox signaling in cardiac physiology and pathology. Circ Res 111: 1091–1106, 2012 [DOI] [PubMed] [Google Scholar]
- 26.Calero CI, Vickers E, Moraga Cid G, Aguayo LG, von Gersdorff H, and Calvo DJ. Allosteric modulation of retinal GABA receptors by ascorbic acid. J Neurosci 31: 9672–9682, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carrano A, Hoozemans JJ, van der Vies SM, Rozemuller AJ, van Horssen J, and de Vries HE. Amyloid beta induces oxidative stress-mediated blood-brain barrier changes in capillary amyloid angiopathy. Antioxid Redox Signal 15: 1167–1178, 2011 [DOI] [PubMed] [Google Scholar]
- 28.Carroll KS. Metabolite imaging: knock, nox-ROS there? Nat Chem Biol 7: 71–72, 2011 [DOI] [PubMed] [Google Scholar]
- 29.Case AJ, Li S, Basu U, Tian J, and Zimmerman MC. Mitochondrial-localized NADPH oxidase 4 is a source of superoxide in angiotensin II-stimulated neurons. Am J Physiol Heart Circ Physiol 305: H19–H28, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chan SH. and Chan JY. Angiotensin-generated reactive oxygen species in brain and pathogenesis of cardiovascular diseases. Antioxid Redox Signal 19: 1074–1084, 2013 [DOI] [PubMed] [Google Scholar]
- 31.Chen H, Kim GS, Okami N, Narasimhan P, and Chan PH. NADPH oxidase is involved in post-ischemic brain inflammation. Neurobiol Dis 42: 341–348, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen K, Kirber MT, Xiao H, Yang Y, and Keaney JF, Jr., Regulation of ROS signal transduction by NADPH oxidase 4 localization. J Cell Biol 181: 1129–1139, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cheng G, Cao Z, Xu X, van Meir EG, and Lambeth JD. Homologs of gp91phox: cloning and tissue expression of Nox3, Nox4, and Nox5. Gene 269: 131–140, 2001 [DOI] [PubMed] [Google Scholar]
- 34.Cheret C, Gervais A, Lelli A, Colin C, Amar L, Ravassard P, Mallet J, Cumano A, Krause KH, and Mallat M. Neurotoxic activation of microglia is promoted by a nox1-dependent NADPH oxidase. J Neurosci 28: 12039–12051, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chiarugi P. PTPs versus PTKs: the redox side of the coin. Free Radic Res 39: 353–364, 2005 [DOI] [PubMed] [Google Scholar]
- 36.Chiu WT, Shen SC, Chow JM, Lin CW, Shia LT, and Chen YC. Contribution of reactive oxygen species to migration/invasion of human glioblastoma cells U87 via ERK-dependent COX-2/PGE(2) activation. Neurobiol Dis 37: 118–129, 2010 [DOI] [PubMed] [Google Scholar]
- 37.Choi DH, Cristovao AC, Guhathakurta S, Lee J, Joh TH, Beal MF, and Kim YS. NADPH oxidase 1-mediated oxidative stress leads to dopamine neuron death in Parkinson's disease. Antioxid Redox Signal 16: 1033–1045, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Choi SH, Aid S, Kim HW, Jackson SH, and Bosetti F. Inhibition of NADPH oxidase promotes alternative and anti-inflammatory microglial activation during neuroinflammation. J Neurochem 120: 292–301, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Choi SR, Roh DH, Yoon SY, Kang SY, Moon JY, Kwon SG, Choi HS, Han HJ, Beitz AJ, Oh SB, and Lee JH. Spinal sigma-1 receptors activate NADPH oxidase 2 leading to the induction of pain hypersensitivity in mice and mechanical allodynia in neuropathic rats. Pharmacol Res 74C: 56–67, 2013 [DOI] [PubMed] [Google Scholar]
- 40.Cole TS, McKendrick F, Cant AJ, Pearce MS, Cale CM, Goldblatt DR, Gennery AR, and Titman P. Cognitive ability in children with chronic granulomatous disease: a comparison of those managed conservatively with those who have undergone hematopoietic stem cell transplant. Neuropediatrics 44: 230–232, 2013 [DOI] [PubMed] [Google Scholar]
- 41.Colman MS, Afshari CA, and Barrett JC. Regulation of p53 stability and activity in response to genotoxic stress. Mutat Res 462: 179–188, 2000 [DOI] [PubMed] [Google Scholar]
- 42.Coso S, Harrison I, Harrison CB, Vinh A, Sobey CG, Drummond GR, Williams ED, and Selemidis S. NADPH oxidases as regulators of tumor angiogenesis: current and emerging concepts. Antioxid Redox Signal 16: 1229–1247, 2012 [DOI] [PubMed] [Google Scholar]
- 43.Coyoy A, Olguin-Albuerne M, Martinez-Briseno P, and Moran J. Role of reactive oxygen species and NADPH-oxidase in the development of rat cerebellum. Neurochem Int 62: 998–1011, 2013 [DOI] [PubMed] [Google Scholar]
- 44.Cristovao AC, Choi DH, Baltazar G, Beal MF, and Kim YS. The role of NADPH oxidase 1-derived reactive oxygen species in paraquat-mediated dopaminergic cell death. Antioxid Redox Signal 11: 2105–2118, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cristovao AC, Guhathakurta S, Bok E, Je G, Yoo SD, Choi DH, and Kim YS. NADPH oxidase 1 mediates alpha-synucleinopathy in Parkinson's disease. J Neurosci 32: 14465–14477, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Diaz B. and Courtneidge SA. Redox signaling at invasive microdomains in cancer cells. Free Radic Biol Med 52: 247–256, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Diaz B, Shani G, Pass I, Anderson D, Quintavalle M, and Courtneidge SA. Tks5-dependent, nox-mediated generation of reactive oxygen species is necessary for invadopodia formation. Sci Signal 2: ra53, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dickinson BC, Peltier J, Stone D, Schaffer DV, and Chang CJ. Nox2 redox signaling maintains essential cell populations in the brain. Nat Chem Biol 7: 106–112, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dohi K, Ohtaki H, Nakamachi T, Yofu S, Satoh K, Miyamoto K, Song D, Tsunawaki S, Shioda S, and Aruga T. Gp91phox (NOX2) in classically activated microglia exacerbates traumatic brain injury. J Neuroinflammation 7: 41, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dugan LL, Ali SS, Shekhtman G, Roberts AJ, Lucero J, Quick KL, and Behrens MM. IL-6 mediated degeneration of forebrain GABAergic interneurons and cognitive impairment in aged mice through activation of neuronal NADPH oxidase. PLoS One 4: e5518, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dumitrascu R, Heitmann J, Seeger W, Weissmann N, and Schulz R. Obstructive sleep apnea, oxidative stress and cardiovascular disease: lessons from animal studies. Oxid Med Cell Longev 2013: 234631, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dumont M, Stack C, Elipenhali C, Calingasan NY, Wille E, and Beal MF. Apocynin administration does not improve behavioral and neuropathological deficits in a transgenic mouse model of Alzheimer's disease. Neurosci Lett 492: 150–154, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Federico A, Cardaioli E, Da Pozzo P, Formichi P, Gallus GN, and Radi E. Mitochondria, oxidative stress and neurodegeneration. J Neurol Sci 322: 254–262, 2012 [DOI] [PubMed] [Google Scholar]
- 54.Fellner L, Irschick R, Schanda K, Reindl M, Klimaschewski L, Poewe W, Wenning GK, and Stefanova N. Toll-like receptor 4 is required for alpha-synuclein dependent activation of microglia and astroglia. Glia 61: 349–360, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fischer MT, Sharma R, Lim JL, Haider L, Frischer JM, Drexhage J, Mahad D, Bradl M, van Horssen J, and Lassmann H. NADPH oxidase expression in active multiple sclerosis lesions in relation to oxidative tissue damage and mitochondrial injury. Brain 135: 886–899, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fulton DJ. Nox5 and the regulation of cellular function. Antioxid Redox Signal 11: 2443–2452, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gavazzi G, Deffert C, Trocme C, Schappi M, Herrmann FR, and Krause KH. NOX1 deficiency protects from aortic dissection in response to angiotensin II. Hypertension 50: 189–196, 2007 [DOI] [PubMed] [Google Scholar]
- 58.Getts DR, Terry RL, Getts MT, Muller M, Rana S, Shrestha B, Radford J, Van Rooijen N, Campbell IL, and King NJ. Ly6c+ “inflammatory monocytes” are microglial precursors recruited in a pathogenic manner in West Nile virus encephalitis. J Exp Med 205: 2319–2337, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gianni D, Diaz B, Taulet N, Fowler B, Courtneidge SA, and Bokoch GM. Novel p47(phox)-related organizers regulate localized NADPH oxidase 1 (Nox1) activity. Sci Signal 2: ra54, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gianni D, Taulet N, DerMardirossian C, and Bokoch GM. c-Src-mediated phosphorylation of NoxA1 and Tks4 induces the reactive oxygen species (ROS)-dependent formation of functional invadopodia in human colon cancer cells. Mol Biol Cell 21: 4287–4298, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ginhoux F, Greter M, Leboeuf M, Nandi S, See P, Gokhan S, Mehler MF, Conway SJ, Ng LG, Stanley ER, Samokhvalov IM, and Merad M. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science 330: 841–845, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Girouard H. and Iadecola C. Neurovascular coupling in the normal brain and in hypertension, stroke, and Alzheimer disease. J Appl Physiol 100: 328–335, 2006 [DOI] [PubMed] [Google Scholar]
- 63.Gomez-Cabrera MC, Sanchis-Gomar F, Garcia-Valles R, Pareja-Galeano H, Gambini J, Borras C, and Vina J. Mitochondria as sources and targets of damage in cellular aging. Clin Chem Lab Med 50: 1287–1295, 2012 [DOI] [PubMed] [Google Scholar]
- 64.Gozal D, Khalyfa A, Capdevila OS, Kheirandish-Gozal L, Khalyfa AA, and Kim J. Cognitive function in prepubertal children with obstructive sleep apnea: a modifying role for NADPH oxidase p22 subunit gene polymorphisms? Antioxid Redox Signal 16: 171–177, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gunduz-Bruce H. The acute effects of NMDA antagonism: from the rodent to the human brain. Brain Res Rev 60: 279–286, 2009 [DOI] [PubMed] [Google Scholar]
- 66.Haider L, Fischer MT, Frischer JM, Bauer J, Hoftberger R, Botond G, Esterbauer H, Binder CJ, Witztum JL, and Lassmann H. Oxidative damage in multiple sclerosis lesions. Brain 134: 1914–1924, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Harman D. Aging: a theory based on free radical and radiation chemistry. J Gerontol 11: 298–300, 1956 [DOI] [PubMed] [Google Scholar]
- 68.Harraz MM, Marden JJ, Zhou W, Zhang Y, Williams A, Sharov VS, Nelson K, Luo M, Paulson H, Schoneich C, and Engelhardt JF. SOD1 mutations disrupt redox-sensitive Rac regulation of NADPH oxidase in a familial ALS model. J Clin Invest 118: 659–670, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hellwig S, Heinrich A, and Biber K. The brain's best friend: microglial neurotoxicity revisited. Front Cell Neurosci 7: 71, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Heumuller S, Wind S, Barbosa-Sicard E, Schmidt HH, Busse R, Schroder K, and Brandes RP. Apocynin is not an inhibitor of vascular NADPH oxidases but an antioxidant. Hypertension 51: 211–217, 2008 [DOI] [PubMed] [Google Scholar]
- 71.Hoffer A, Osmond H, and Smythies J. Schizophrenia; a new approach. II. Result of a year's research. J Ment Sci 100: 29–45, 1954 [DOI] [PubMed] [Google Scholar]
- 72.Holmdahl R, Sareila O, Pizzolla A, Winter S, Hagert C, Jaakkola N, Kelkka T, Olsson LM, Wing K, and Backdahl L. Hydrogen peroxide as an immunological transmitter regulating autoreactive T cells. Antioxid Redox Signal 18: 1463–1474, 2013 [DOI] [PubMed] [Google Scholar]
- 73.Hsieh CH, Shyu WC, Chiang CY, Kuo JW, Shen WC, and Liu RS. NADPH oxidase subunit 4-mediated reactive oxygen species contribute to cycling hypoxia-promoted tumor progression in glioblastoma multiforme. PLoS One 6: e23945, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hsieh CH, Wu CP, Lee HT, Liang JA, Yu CY, and Lin YJ. NADPH oxidase subunit 4 mediates cycling hypoxia-promoted radiation resistance in glioblastoma multiforme. Free Radic Biol Med 53: 649–658, 2012 [DOI] [PubMed] [Google Scholar]
- 75.Hulsebosch CE, Hains BC, Crown ED, and Carlton SM. Mechanisms of chronic central neuropathic pain after spinal cord injury. Brain Res Rev 60: 202–213, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hultqvist M, Olofsson P, Holmberg J, Backstrom BT, Tordsson J, and Holmdahl R. Enhanced autoimmunity, arthritis, and encephalomyelitis in mice with a reduced oxidative burst due to a mutation in the Ncf1 gene. Proc Natl Acad Sci U S A 101: 12646–12651, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ibi M, Matsuno K, Matsumoto M, Sasaki M, Nakagawa T, Katsuyama M, Iwata K, Zhang J, Kaneko S, and Yabe-Nishimura C. Involvement of NOX1/NADPH oxidase in morphine-induced analgesia and tolerance. J Neurosci 31: 18094–18103, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ikeda S, Yamaoka-Tojo M, Hilenski L, Patrushev NA, Anwar GM, Quinn MT, and Ushio-Fukai M. IQGAP1 regulates reactive oxygen species-dependent endothelial cell migration through interacting with Nox2. Arterioscler Thromb Vasc Biol 25: 2295–2300, 2005 [DOI] [PubMed] [Google Scholar]
- 79.Ilieva H, Polymenidou M, and Cleveland DW. Non-cell autonomous toxicity in neurodegenerative disorders: ALS and beyond. J Cell Biol 187: 761–772, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Im YB, Jee MK, Choi JI, Cho HT, Kwon OH, and Kang SK. Molecular targeting of NOX4 for neuropathic pain after traumatic injury of the spinal cord. Cell Death Dis 3: e426, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Jaggi AS, Jain V, and Singh N. Animal models of neuropathic pain. Fundam Clin Pharmacol 25: 1–28, 2011 [DOI] [PubMed] [Google Scholar]
- 82.Jaquet V, Scapozza L, Clark RA, Krause KH, and Lambeth JD. Small-molecule NOX inhibitors: ROS-generating NADPH oxidases as therapeutic targets. Antioxid Redox Signal 11: 2535–2552, 2009 [DOI] [PubMed] [Google Scholar]
- 83.Jaronen M, Vehvilainen P, Malm T, Keksa-Goldsteine V, Pollari E, Valonen P, Koistinaho J, and Goldsteins G. Protein disulfide isomerase in ALS mouse glia links protein misfolding with NADPH oxidase-catalyzed superoxide production. Hum Mol Genet 22: 646–655, 2013 [DOI] [PubMed] [Google Scholar]
- 84.Jordan BD. The clinical spectrum of sport-related traumatic brain injury. Nat Rev Neurol 9: 222–230, 2013 [DOI] [PubMed] [Google Scholar]
- 85.Juhasz A, Ge Y, Markel S, Chiu A, Matsumoto L, van Balgooy J, Roy K, and Doroshow JH. Expression of NADPH oxidase homologues and accessory genes in human cancer cell lines, tumours and adjacent normal tissues. Free Radic Res 43: 523–532, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kahles T. and Brandes RP. Which NADPH oxidase isoform is relevant for ischemic stroke? The case for nox 2. Antioxid Redox Signal 18: 1400–1417, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kallenborn-Gerhardt W, Schroder K, Del Turco D, Lu R, Kynast K, Kosowski J, Niederberger E, Shah AM, Brandes RP, Geisslinger G, and Schmidtko A. NADPH oxidase-4 maintains neuropathic pain after peripheral nerve injury. J Neurosci 32: 10136–10145, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kierdorf K, Erny D, Goldmann T, Sander V, Schulz C, Perdiguero EG, Wieghofer P, Heinrich A, Riemke P, Holscher C, Muller DN, Luckow B, Brocker T, Debowski K, Fritz G, Opdenakker G, Diefenbach A, Biber K, Heikenwalder M, Geissmann F, Rosenbauer F, and Prinz M. Microglia emerge from erythromyeloid precursors via Pu.1- and Irf8-dependent pathways. Nat Neurosci 16: 273–280, 2013 [DOI] [PubMed] [Google Scholar]
- 89.Kim C, Ho DH, Suk JE, You S, Michael S, Kang J, Joong Lee S, Masliah E, Hwang D, Lee HJ, and Lee SJ. Neuron-released oligomeric alpha-synuclein is an endogenous agonist of TLR2 for paracrine activation of microglia. Nat Commun 4: 1562, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kim D, You B, Jo EK, Han SK, Simon MI, and Lee SJ. NADPH oxidase 2-derived reactive oxygen species in spinal cord microglia contribute to peripheral nerve injury-induced neuropathic pain. Proc Natl Acad Sci U S A 107: 14851–14856, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kim HA, Miller AA, Drummond GR, Thrift AG, Arumugam TV, Phan TG, Srikanth VK, and Sobey CG. Vascular cognitive impairment and Alzheimer's disease: role of cerebral hypoperfusion and oxidative stress. Naunyn Schmiedebergs Arch Pharmacol 385: 953–959, 2012 [DOI] [PubMed] [Google Scholar]
- 92.Kim MJ, Shin KS, Chung YB, Jung KW, Cha CI, and Shin DH. Immunohistochemical study of p47Phox and gp91Phox distributions in rat brain. Brain Res 1040: 178–186, 2005 [DOI] [PubMed] [Google Scholar]
- 93.Kishida KT, Hoeffer CA, Hu D, Pao M, Holland SM, and Klann E. Synaptic plasticity deficits and mild memory impairments in mouse models of chronic granulomatous disease. Mol Cell Biol 26: 5908–5920, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kleinschnitz C, Grund H, Wingler K, Armitage ME, Jones E, Mittal M, Barit D, Schwarz T, Geis C, Kraft P, Barthel K, Schuhmann MK, Herrmann AM, Meuth SG, Stoll G, Meurer S, Schrewe A, Becker L, Gailus-Durner V, Fuchs H, Klopstock T, de Angelis MH, Jandeleit-Dahm K, Shah AM, Weissmann N, and Schmidt HH. Post-stroke inhibition of induced NADPH oxidase type 4 prevents oxidative stress and neurodegeneration. PLoS Biol 8: e1000479, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kokovay E, Wang Y, Kusek G, Wurster R, Lederman P, Lowry N, Shen Q, and Temple S. VCAM1 is essential to maintain the structure of the SVZ niche and acts as an environmental sensor to regulate SVZ lineage progression. Cell Stem Cell 11: 220–230, 2012 [DOI] [PubMed] [Google Scholar]
- 96.Koziel R, Pircher H, Kratochwil M, Lener B, Hermann M, Dencher NA, and Jansen-Durr P. Mitochondrial respiratory chain complex I is inactivated by NADPH oxidase Nox4. Biochem J 452: 231–239, 2013 [DOI] [PubMed] [Google Scholar]
- 97.Krstic D. and Knuesel I. Deciphering the mechanism underlying late-onset Alzheimer disease. Nat Rev Neurol 9: 25–34, 2013 [DOI] [PubMed] [Google Scholar]
- 98.Kumar A, Stoica BA, Sabirzhanov B, Burns MP, Faden AI, and Loane DJ. Traumatic brain injury in aged animals increases lesion size and chronically alters microglial/macrophage classical and alternative activation states. Neurobiol Aging 34: 1397–1411, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Labandeira-Garcia JL, Rodriguez-Pallares J, Rodriguez-Perez AI, Garrido-Gil P, Villar-Cheda B, Valenzuela R, and Guerra MJ. Brain angiotensin and dopaminergic degeneration: relevance to Parkinson's disease. Am J Neurodegener Dis 1: 226–244, 2012 [PMC free article] [PubMed] [Google Scholar]
- 100.Lakhan SE, Kirchgessner A, and Hofer M. Inflammatory mechanisms in ischemic stroke: therapeutic approaches. J Transl Med 7: 97, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lassegue B, San Martin A, and Griendling KK. Biochemistry, physiology, and pathophysiology of NADPH oxidases in the cardiovascular system. Circ Res 110: 1364–1390, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Le Belle JE, Orozco NM, Paucar AA, Saxe JP, Mottahedeh J, Pyle AD, Wu H, and Kornblum HI. Proliferative neural stem cells have high endogenous ROS levels that regulate self-renewal and neurogenesis in a PI3K/Akt-dependant manner. Cell Stem Cell 8: 59–71, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lee CR, Machold RP, Witkovsky P, and Rice ME. TRPM2 channels are required for NMDA-induced burst firing and contribute to H(2)O(2)-dependent modulation in substantia nigra pars reticulata GABAergic neurons. J Neurosci 33: 1157–1168, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lelli A, Gervais A, Colin C, Cheret C, de Almodovar CR, Carmeliet P, Krause KH, Boillee S, and Mallat M. The NADPH oxidase Nox2 regulates VEGFR1/CSF-1R-mediated microglial chemotaxis and promotes early postnatal infiltration of phagocytes in the subventricular zone of the mouse cerebral cortex. Glia 61: 1542–1555, 2013 [DOI] [PubMed] [Google Scholar]
- 105.Leto TL, Morand S, Hurt D, and Ueyama T. Targeting and regulation of reactive oxygen species generation by Nox family NADPH oxidases. Antioxid Redox Signal 11: 2607–2619, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Li B, Bedard K, Sorce S, Hinz B, Dubois-Dauphin M, and Krause KH. NOX4 expression in human microglia leads to constitutive generation of reactive oxygen species and to constitutive IL-6 expression. J Innate Immun 1: 570–581, 2009 [DOI] [PubMed] [Google Scholar]
- 107.Li Q, Spencer NY, Pantazis NJ, and Engelhardt JF. Alsin and SOD1(G93A) proteins regulate endosomal reactive oxygen species production by glial cells and proinflammatory pathways responsible for neurotoxicity. J Biol Chem 286: 40151–40162, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Liao B, Zhao W, Beers DR, Henkel JS, and Appel SH. Transformation from a neuroprotective to a neurotoxic microglial phenotype in a mouse model of ALS. Exp Neurol 237: 147–152, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lim DA, Huang YC, and Alvarez-Buylla A. The adult neural stem cell niche: lessons for future neural cell replacement strategies. Neurosurg Clin N Am 18: 81–92, ix, 2007 [DOI] [PubMed] [Google Scholar]
- 110.Lincecum JM, Vieira FG, Wang MZ, Thompson K, De Zutter GS, Kidd J, Moreno A, Sanchez R, Carrion IJ, Levine BA, Al-Nakhala BM, Sullivan SM, Gill A, and Perrin S. From transcriptome analysis to therapeutic anti-CD40L treatment in the SOD1 model of amyotrophic lateral sclerosis. Nat Genet 42: 392–399, 2010 [DOI] [PubMed] [Google Scholar]
- 111.Lob HE, Schultz D, Marvar PJ, Davisson RL, and Harrison DG. Role of the NADPH oxidases in the subfornical organ in angiotensin II-induced hypertension. Hypertension 61: 382–387, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ludolph AC, Brettschneider J, and Weishaupt JH. Amyotrophic lateral sclerosis. Curr Opin Neurol 25: 530–535, 2012 [DOI] [PubMed] [Google Scholar]
- 113.Lull ME, Levesque S, Surace MJ, and Block ML. Chronic apocynin treatment attenuates beta amyloid plaque size and microglial number in hAPP(751)(SL) mice. PLoS One 6: e20153, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Maghzal GJ, Krause KH, Stocker R, and Jaquet V. Detection of reactive oxygen species derived from the family of NOX NADPH oxidases. Free Radic Biol Med 53: 1903–1918, 2012 [DOI] [PubMed] [Google Scholar]
- 115.Maldonado PD, Molina-Jijon E, Villeda-Hernandez J, Galvan-Arzate S, Santamaria A, and Pedraza-Chaverri J. NAD(P)H oxidase contributes to neurotoxicity in an excitotoxic/prooxidant model of Huntington's disease in rats: protective role of apocynin. J Neurosci Res 88: 620–629, 2010 [DOI] [PubMed] [Google Scholar]
- 116.Mann PJ. and Quastel JH. Toxic effects of oxygen and of hydrogen peroxide on brain metabolism. Biochem J 40: 139–144, 1946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Marchesi VT. Alzheimer's dementia begins as a disease of small blood vessels, damaged by oxidative-induced inflammation and dysregulated amyloid metabolism: implications for early detection and therapy. FASEB J 25: 5–13, 2011 [DOI] [PubMed] [Google Scholar]
- 118.Marden JJ, Harraz MM, Williams AJ, Nelson K, Luo M, Paulson H, and Engelhardt JF. Redox modifier genes in amyotrophic lateral sclerosis in mice. J Clin Invest 117: 2913–2919, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Marin O. Interneuron dysfunction in psychiatric disorders. Nat Rev Neurosci 13: 107–120, 2012 [DOI] [PubMed] [Google Scholar]
- 120.Marin-Teva JL, Dusart I, Colin C, Gervais A, van Rooijen N, and Mallat M. Microglia promote the death of developing Purkinje cells. Neuron 41: 535–547, 2004 [DOI] [PubMed] [Google Scholar]
- 121.Mertens B, Vanderheyden P, Michotte Y, and Sarre S. The role of the central renin-angiotensin system in Parkinson's disease. J Renin Angiotensin Aldosterone Syst 11: 49–56, 2010 [DOI] [PubMed] [Google Scholar]
- 122.Miller ER, 3rd, Pastor-Barriuso R, Dalal D, Riemersma RA, Appel LJ, and Guallar E. Meta-analysis: high-dosage vitamin E supplementation may increase all-cause mortality. Ann Intern Med 142: 37–46, 2005 [DOI] [PubMed] [Google Scholar]
- 123.Milton VJ. and Sweeney ST. Oxidative stress in synapse development and function. Dev Neurobiol 72: 100–110, 2012 [DOI] [PubMed] [Google Scholar]
- 124.Moller T. Neuroinflammation in Huntington's disease. J Neural Transm 117: 1001–1008, 2010 [DOI] [PubMed] [Google Scholar]
- 125.Moussaoui S, Obinu MC, Daniel N, Reibaud M, Blanchard V, and Imperato A. The antioxidant ebselen prevents neurotoxicity and clinical symptoms in a primate model of Parkinson's disease. Exp Neurol 166: 235–245, 2000 [DOI] [PubMed] [Google Scholar]
- 126.Munson J, Bonner M, Fried L, Hofmekler J, Arbiser J, and Bellamkonda R. Finding new small molecule anti-invasive compounds for glioma treatment. Cell Cycle 12: 2200–2209, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Nair D, Dayyat EA, Zhang SX, Wang Y, and Gozal D. Intermittent hypoxia-induced cognitive deficits are mediated by NADPH oxidase activity in a murine model of sleep apnea. PLoS One 6: e19847, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Nair D, Ramesh V, and Gozal D. Adverse cognitive effects of high-fat diet in a murine model of sleep apnea are mediated by NADPH oxidase activity. Neuroscience 227: 361–369, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Naziroglu M. TRPM2 cation channels, oxidative stress and neurological diseases: where are we now? Neurochem Res 36: 355–366, 2011 [DOI] [PubMed] [Google Scholar]
- 130.Noh KM. and Koh JY. Induction and activation by zinc of NADPH oxidase in cultured cortical neurons and astrocytes. J Neurosci 20: RC111, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.O'Donnell BV, Tew DG, Jones OT, and England PJ. Studies on the inhibitory mechanism of iodonium compounds with special reference to neutrophil NADPH oxidase. Biochem J 290 (Pt1): 41–49, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Pao M, Wiggs EA, Anastacio MM, Hyun J, DeCarlo ES, Miller JT, Anderson VL, Malech HL, Gallin JI, and Holland SM. Cognitive function in patients with chronic granulomatous disease: a preliminary report. Psychosomatics 45: 230–234, 2004 [DOI] [PubMed] [Google Scholar]
- 133.Peterson JR, Burmeister MA, Tian X, Zhou Y, Guruju MR, Stupinski JA, Sharma RV, and Davisson RL. Genetic silencing of Nox2 and Nox4 reveals differential roles of these NADPH oxidase homologues in the vasopressor and dipsogenic effects of brain angiotensin II. Hypertension 54: 1106–1114, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Peterson LJ. and Flood PM. Oxidative stress and microglial cells in Parkinson's disease. Mediators Inflamm 2012: 401264, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Qin B, Cartier L, Dubois-Dauphin M, Li B, Serrander L, and Krause KH. A key role for the microglial NADPH oxidase in APP-dependent killing of neurons. Neurobiol Aging 27: 1577–1587, 2006 [DOI] [PubMed] [Google Scholar]
- 136.Qin L, Liu Y, Hong JS, and Crews FT. NADPH oxidase and aging drive microglial activation, oxidative stress, and dopaminergic neurodegeneration following systemic LPS administration. Glia 61: 855–868, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Radermacher KA, Wingler K, Langhauser F, Altenhofer S, Kleikers P, Hermans JJ, Hrabe de Angelis M, Kleinschnitz C, and Schmidt HH. Neuroprotection after stroke by targeting NOX4 as a source of oxidative stress. Antioxid Redox Signal 18: 1418–1427, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Rappold PM, Cui M, Chesser AS, Tibbett J, Grima JC, Duan L, Sen N, Javitch JA, and Tieu K. Paraquat neurotoxicity is mediated by the dopamine transporter and organic cation transporter-3. Proc Natl Acad Sci U S A 108: 20766–20771, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Reddy RD. and Yao JK. Free radical pathology in schizophrenia: a review. Prostaglandins Leukot Essent Fatty Acids 55: 33–43, 1996 [DOI] [PubMed] [Google Scholar]
- 140.Reyes RC, Brennan AM, Shen Y, Baldwin Y, and Swanson RA. Activation of neuronal NMDA receptors induces superoxide-mediated oxidative stress in neighboring neurons and astrocytes. J Neurosci 32: 12973–12978, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Rodriguez-Munoz M. and Garzon J. Nitric oxide and zinc-mediated protein assemblies involved in Mu opioid receptor signaling. Mol Neurobiol 48: 769–782, 2013 [DOI] [PubMed] [Google Scholar]
- 142.Sareila O, Kelkka T, Pizzolla A, Hultqvist M, and Holmdahl R. NOX2 complex-derived ROS as immune regulators. Antioxid Redox Signal 15: 2197–2208, 2011 [DOI] [PubMed] [Google Scholar]
- 143.Sarnico I, Lanzillotta A, Benarese M, Alghisi M, Baiguera C, Battistin L, Spano P, and Pizzi M. NF-kappaB dimers in the regulation of neuronal survival. Int Rev Neurobiol 85: 351–362, 2009 [DOI] [PubMed] [Google Scholar]
- 144.Schappi MG, Jaquet V, Belli DC, and Krause KH. Hyperinflammation in chronic granulomatous disease and anti-inflammatory role of the phagocyte NADPH oxidase. Semin Immunopathol 30: 255–271, 2008 [DOI] [PubMed] [Google Scholar]
- 145.Schiavone S, Jaquet V, Sorce S, Dubois-Dauphin M, Hultqvist M, Backdahl L, Holmdahl R, Colaianna M, Cuomo V, Trabace L, and Krause KH. NADPH oxidase elevations in pyramidal neurons drive psychosocial stress-induced neuropathology. Transl Psychiatry 2: e111, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Schiavone S, Jaquet V, Trabace L, and Krause KH. Severe life stress and oxidative stress in the brain: from animal models to human pathology. Antioxid Redox Signal 18: 1475–1490, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Schiavone S, Sorce S, Dubois-Dauphin M, Jaquet V, Colaianna M, Zotti M, Cuomo V, Trabace L, and Krause KH. Involvement of NOX2 in the development of behavioral and pathologic alterations in isolated rats. Biol Psychiatry 66: 384–392, 2009 [DOI] [PubMed] [Google Scholar]
- 148.Schneider JA. and Bennett DA. Where vascular meets neurodegenerative disease. Stroke 41: S144–S146, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Serrander L, Cartier L, Bedard K, Banfi B, Lardy B, Plastre O, Sienkiewicz A, Forro L, Schlegel W, and Krause KH. NOX4 activity is determined by mRNA levels and reveals a unique pattern of ROS generation. Biochem J 406: 105–114, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Serrano F, Kolluri NS, Wientjes FB, Card JP, and Klann E. NADPH oxidase immunoreactivity in the mouse brain. Brain Res 988: 193–198, 2003 [DOI] [PubMed] [Google Scholar]
- 151.Shechter R. and Schwartz M. Harnessing monocyte-derived macrophages to control central nervous system pathologies: no longer ‘if’ but ‘how’. J Pathol 229: 332–346, 2013 [DOI] [PubMed] [Google Scholar]
- 152.Shono T, Yokoyama N, Uesaka T, Kuroda J, Takeya R, Yamasaki T, Amano T, Mizoguchi M, Suzuki SO, Niiro H, Miyamoto K, Akashi K, Iwaki T, Sumimoto H, and Sasaki T. Enhanced expression of NADPH oxidase Nox4 in human gliomas and its roles in cell proliferation and survival. Int J Cancer 123: 787–792, 2008 [DOI] [PubMed] [Google Scholar]
- 153.Simonyi A, He Y, Sheng W, Sun AY, Wood WG, Weisman GA, and Sun GY. Targeting NADPH oxidase and phospholipases A2 in Alzheimer's disease. Mol Neurobiol 41: 73–86, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Smith CD, Carney JM, Starke-Reed PE, Oliver CN, Stadtman ER, Floyd RA, and Markesbery WR. Excess brain protein oxidation and enzyme dysfunction in normal aging and in Alzheimer disease. Proc Natl Acad Sci U S A 88: 10540–10543, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Smith SM, Min J, Ganesh T, Diebold B, Kawahara T, Zhu Y, McCoy J, Sun A, Snyder JP, Fu H, Du Y, Lewis I, and Lambeth JD. Ebselen and congeners inhibit NADPH oxidase 2-dependent superoxide generation by interrupting the binding of regulatory subunits. Chem Biol 19: 752–763, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Song SX, Gao JL, Wang KJ, Li R, Tian YX, Wei JQ, and Cui JZ. Attenuation of brain edema and spatial learning de fi cits by the inhibition of NADPH oxidase activity using apocynin following diffuse traumatic brain injury in rats. Mol Med Rep 7: 327–331, 2012 [DOI] [PubMed] [Google Scholar]
- 157.Sorce S. and Krause KH. NOX enzymes in the central nervous system: from signaling to disease. Antioxid Redox Signal 11: 2481–2504, 2009 [DOI] [PubMed] [Google Scholar]
- 158.Sorce S, Krause KH, and Jaquet V. Targeting NOX enzymes in the central nervous system: therapeutic opportunities. Cell Mol Life Sci 69: 2387–2407, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Sorce S, Schiavone S, Tucci P, Colaianna M, Jaquet V, Cuomo V, Dubois-Dauphin M, Trabace L, and Krause KH. The NADPH oxidase NOX2 controls glutamate release: a novel mechanism involved in psychosis-like ketamine responses. J Neurosci 30: 11317–11325, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Stack EC, Matson WR, and Ferrante RJ. Evidence of oxidant damage in Huntington's disease: translational strategies using antioxidants. Ann N Y Acad Sci 1147: 79–92, 2008 [DOI] [PubMed] [Google Scholar]
- 161.Stefanova N, Fellner L, Reindl M, Masliah E, Poewe W, and Wenning GK. Toll-like receptor 4 promotes alpha-synuclein clearance and survival of nigral dopaminergic neurons. Am J Pathol 179: 954–963, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Surace MJ. and Block ML. Targeting microglia-mediated neurotoxicity: the potential of NOX2 inhibitors. Cell Mol Life Sci 69: 2409–2427, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Svineng G, Ravuri C, Rikardsen O, Huseby NE, and Winberg JO. The role of reactive oxygen species in integrin and matrix metalloproteinase expression and function. Connect Tissue Res 49: 197–202, 2008 [DOI] [PubMed] [Google Scholar]
- 164.Taetzsch T. and Block ML. Pesticides, microglial NOX2, and Parkinson's disease. J Biochem Mol Toxicol 27: 137–149, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Takac I, Schroder K, and Brandes RP. The Nox family of NADPH oxidases: friend or foe of the vascular system? Curr Hypertens Rep 14: 70–78, 2012 [DOI] [PubMed] [Google Scholar]
- 166.Takac I, Schroder K, Zhang L, Lardy B, Anilkumar N, Lambeth JD, Shah AM, Morel F, and Brandes RP. The E-loop is involved in hydrogen peroxide formation by the NADPH oxidase Nox4. J Biol Chem 286: 13304–13313, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Town T, Nikolic V, and Tan J. The microglial “activation” continuum: from innate to adaptive responses. J Neuroinflammation 2: 24, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Trifunovic A, Hansson A, Wredenberg A, Rovio AT, Dufour E, Khvorostov I, Spelbrink JN, Wibom R, Jacobs HT, and Larsson NG. Somatic mtDNA mutations cause aging phenotypes without affecting reactive oxygen species production. Proc Natl Acad Sci U S A 102: 17993–17998, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Trifunovic A, Wredenberg A, Falkenberg M, Spelbrink JN, Rovio AT, Bruder CE, Bohlooly YM, Gidlof S, Oldfors A, Wibom R, Tornell J, Jacobs HT, and Larsson NG. Premature ageing in mice expressing defective mitochondrial DNA polymerase. Nature 429: 417–423, 2004 [DOI] [PubMed] [Google Scholar]
- 170.Trumbull KA, McAllister D, Gandelman MM, Fung WY, Lew T, Brennan L, Lopez N, Morre J, Kalyanaraman B, and Beckman JS. Diapocynin and apocynin administration fails to significantly extend survival in G93A SOD1 ALS mice. Neurobiol Dis 45: 137–144, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Tsao W, Jeong YH, Lin S, Ling J, Price DL, Chiang PM, and Wong PC. Rodent models of TDP-43: recent advances. Brain Res 1462: 26–39, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Valencia A, Sapp E, Kimm JS, McClory H, Reeves PB, Alexander J, Ansong KA, Masso N, Frosch MP, Kegel KB, Li X, and DiFiglia M. Elevated NADPH oxidase activity contributes to oxidative stress and cell death in Huntington's disease. Hum Mol Genet 22: 1112–1131, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 173.Van Den Heuvel C, Thornton E, and Vink R. Traumatic brain injury and Alzheimer's disease: a review. Prog Brain Res 161: 303–316, 2007 [DOI] [PubMed] [Google Scholar]
- 174.van Horssen J, Singh S, van der Pol S, Kipp M, Lim JL, Peferoen L, Gerritsen W, Kooi EJ, Witte ME, Geurts JJ, de Vries HE, Peferoen-Baert R, van den Elsen PJ, van der Valk P, and Amor S. Clusters of activated microglia in normal-appearing white matter show signs of innate immune activation. J Neuroinflammation 9: 156, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Vejrazka M, Micek R, and Stipek S. Apocynin inhibits NADPH oxidase in phagocytes but stimulates ROS production in non-phagocytic cells. Biochim Biophys Acta 1722: 143–147, 2005 [DOI] [PubMed] [Google Scholar]
- 176.Walder CE, Green SP, Darbonne WC, Mathias J, Rae J, Dinauer MC, Curnutte JT, and Thomas GR. Ischemic stroke injury is reduced in mice lacking a functional NADPH oxidase. Stroke 28: 2252–2258, 1997 [DOI] [PubMed] [Google Scholar]
- 177.Walton JC, Selvakumar B, Weil ZM, Snyder SH, and Nelson RJ. Neuronal nitric oxide synthase and NADPH oxidase interact to affect cognitive, affective, and social behaviors in mice. Behav Brain Res 256: 320–327, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Wang G, Anrather J, Glass MJ, Tarsitano MJ, Zhou P, Frys KA, Pickel VM, and Iadecola C. Nox2, Ca2+, and protein kinase C play a role in angiotensin II-induced free radical production in nucleus tractus solitarius. Hypertension 48: 482–489, 2006 [DOI] [PubMed] [Google Scholar]
- 179.Wang G, Coleman CG, Chan J, Faraco G, Marques-Lopes J, Milner TA, Guruju MR, Anrather J, Davisson RL, Iadecola C, and Pickel VM. Angiotensin II slow-pressor hypertension enhances NMDA currents and NOX2-dependent superoxide production in hypothalamic paraventricular neurons. Am J Physiol Regul Integr Comp Physiol 304: R1096–R1106, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Wang X, Pinto-Duarte A, Sejnowski TJ, and Behrens MM. How Nox2-containing NADPH oxidase affects cortical circuits in the NMDA receptor antagonist model of schizophrenia. Antioxid Redox Signal 18: 1444–1462, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Wang Y, Zhang SX, and Gozal D. Reactive oxygen species and the brain in sleep apnea. Respir Physiol Neurobiol 174: 307–316, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Wegorzewska I. and Baloh RH. TDP-43-based animal models of neurodegeneration: new insights into ALS pathology and pathophysiology. Neurodegener Dis 8: 262–274, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Wingler K, Altenhoefer SA, Kleikers PW, Radermacher KA, Kleinschnitz C, and Schmidt HH. VAS2870 is a pan-NADPH oxidase inhibitor. Cell Mol Life Sci 69: 3159–3160, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Wright JW. and Harding JW. Brain renin-angiotensin—a new look at an old system. Prog Neurobiol 95: 49–67, 2011 [DOI] [PubMed] [Google Scholar]
- 185.Wu DC, Re DB, Nagai M, Ischiropoulos H, and Przedborski S. The inflammatory NADPH oxidase enzyme modulates motor neuron degeneration in amyotrophic lateral sclerosis mice. Proc Natl Acad Sci U S A 103: 12132–12137, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Wu LJ, Wu G, Akhavan Sharif MR, Baker A, Jia Y, Fahey FH, Luo HR, Feener EP, and Clapham DE. The voltage-gated proton channel Hv1 enhances brain damage from ischemic stroke. Nat Neurosci 15: 565–573, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Wu RF, Ma Z, Liu Z, and Terada LS. Nox4-derived H2O2 mediates endoplasmic reticulum signaling through local Ras activation. Mol Cell Biol 30: 3553–3568, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Xie L, Zheng W, Xin N, Xie JW, Wang T, and Wang ZY. Ebselen inhibits iron-induced tau phosphorylation by attenuating DMT1 up-regulation and cellular iron uptake. Neurochem Int 61: 334–340, 2012 [DOI] [PubMed] [Google Scholar]
- 189.Yamaguchi T, Sano K, Takakura K, Saito I, Shinohara Y, Asano T, and Yasuhara H. Ebselen in acute ischemic stroke: a placebo-controlled, double-blind clinical trial. Ebselen Study Group. Stroke 29: 12–17, 1998 [DOI] [PubMed] [Google Scholar]
- 190.Yang YJ, Wu PF, Long LH, Yu DF, Wu WN, Hu ZL, Fu H, Xie N, Jin Y, Ni L, Wang JZ, Wang F, and Chen JG. Reversal of aging-associated hippocampal synaptic plasticity deficits by reductants via regulation of thiol redox and NMDA receptor function. Aging Cell 9: 709–721, 2010 [DOI] [PubMed] [Google Scholar]
- 191.Yoneyama M, Kawada K, Gotoh Y, Shiba T, and Ogita K. Endogenous reactive oxygen species are essential for proliferation of neural stem/progenitor cells. Neurochem Int 56: 740–746, 2010 [DOI] [PubMed] [Google Scholar]
- 192.Zhang M, An C, Gao Y, Leak RK, Chen J, and Zhang F. Emerging roles of Nrf2 and phase II antioxidant enzymes in neuroprotection. Prog Neurobiol 100: 30–47, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Zhang QG, Laird MD, Han D, Nguyen K, Scott E, Dong Y, Dhandapani KM, and Brann DW. Critical role of NADPH oxidase in neuronal oxidative damage and microglia activation following traumatic brain injury. PLoS One 7: e34504, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Zhou H, Zhang F, Chen SH, Zhang D, Wilson B, Hong JS, and Gao HM. Rotenone activates phagocyte NADPH oxidase by binding to its membrane subunit gp91phox. Free Radic Biol Med 52: 303–313, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]