Abstract
Objective: The purpose of this study was to evaluate the efficacy and safety of duloxetine fixed dose in the treatment of children (7–11 years) and adolescents (12–17 years) with major depressive disorder (MDD).
Methods: Patients (n=463) in this 36 week study (10 week acute and 26 week extension treatment) received duloxetine 60 mg QD (n=108), duloxetine 30 mg QD (n=116), fluoxetine 20 mg QD (n=117, active control), or placebo (n=122). Measures included: Children's Depression Rating Scale-Revised (CDRS-R), treatment-emergent adverse events (TEAEs), and Columbia-Suicide Severity Rating Scale (C-SSRS).
Results: Neither active drug (duloxetine or fluoxetine) separated significantly (p<0.05) from placebo on mean change from baseline to end-point (10 weeks) on the CDRS-R total score. Total TEAEs and discontinuation for AEs were significantly (p<0.05) higher only for the duloxetine 60 mg group versus the placebo group during acute treatment. No clinically significant electrocardiogram (ECG) or laboratory abnormalities were observed, and no completed suicides or deaths occurred during the study. A total of 7 (6.7%) duloxetine 60 mg, 6 (5.2%) duloxetine 30 mg, 9 (8.0%) fluoxetine, and 11 (9.4%) placebo patients had worsening of suicidal ideation from baseline during acute treatment. Of the patients with suicidal ideation at baseline, 13/16 (81%) duloxetine 60 mg, 16/17 (94%) duloxetine 30 mg, 11/16 (69%) fluoxetine, and 13/15 (87%) placebo had improvement in suicidal ideation at end-point during acute treatment. One fluoxetine, one placebo, and six duloxetine patients had treatment-emergent suicidal behavior during the 36 week study.
Conclusions: Trial results were inconclusive, as neither the investigational drug (duloxetine) nor the active control (fluoxetine) separated from placebo on the CDRS-R at 10 weeks. No new duloxetine safety signals were identified relative to those seen in adults.
Clinical Trial Registry Number (www.ClinicalTrials.gov): NCT00849693
Introduction
Although several acute treatment studies of antidepressants (tricyclics, selective serotonin reuptake inhibitors [SSRIs], and serotonin and norepinephrine reuptake inhibitors [SNRIs]) have been completed in the pediatric major depressive disorder (MDD) patient population, only two antidepressants have shown replicate efficacy in two or more trials, and have received regulatory approval for the treatment of MDD in pediatric patients. Fluoxetine has shown replicate efficacy in the treatment of children and adolescents with MDD (Emslie et al. 1997, 2002) and escitalopram has shown replicate efficacy in adolescents (Wagner et al. 2006; Emslie et al. 2009).
Duloxetine, a dual SNRI, is approved for the treatment of MDD in adults (Cymbalta Full Prescribing Information); however, it has not been systematically evaluated for use in pediatric MDD patients until now. One previous open label study (n=72) assessed the safety, tolerability, and pharmacokinetics of duloxetine over a wide range of doses (20–120 mg QD) in pediatric MDD patients (7–17 years), and results from that study (Prakash et al. 2012) supported dosing aspects of the design of two larger scale randomized controlled trials to investigate the efficacy and safety of duloxetine in pediatric MDD patients. Fluoxetine was included in both larger scale studies as an active control in order to test the assay sensitivity of the studies. That is, the purpose of including a fluoxetine treatment arm was to determine if the studies could detect a difference between an active control with known efficacy (fluoxetine) and placebo.
This study is one of the two (the second being Atkinson et al. 2014) phase 3, placebo-controlled studies (sponsored by Eli Lilly and Company) to investigate the efficacy and safety of duloxetine in the treatment of children and adolescents with MDD. The majority of patients (∼80%) in this study were enrolled in the United States. This study incorporated a fixed dose scheme for both duloxetine and fluoxetine during acute treatment, whereas the sister study used flexible doses of both drugs. Both studies also incorporated double-blind, flexible dosing, extended treatment periods to further characterize the longer-term efficacy and safety profile of duloxetine.
Patients and Methods
Study design
The protocol for this study (F1J-MC-HMCL) was filed with the United States Food and Drug Administration prior to study initiation, and included all of the methodology presented here, in addition to a complete statistical analysis plan. This study was approved by the ethics review boards for each study site and conducted in accordance with good clinical practice guidelines. In accordance with the principles of the Declaration of Helsinki, a parent/legal representative of each study patient provided written informed consent prior to administration of any study drug or study procedures, and patients (children and adolescents) provided assent as appropriate to participate in the study. Patients participated in the study at 60 psychiatric clinical sites in four countries (United States, Canada, Mexico, and Argentina) from March 2009 to September 2011.
The primary objective of the study was to assess the efficacy of duloxetine 60 mg once daily (QD) compared with placebo in the acute treatment of children (7–11 years) and adolescents (12–17 years) who met criteria for MDD without psychotic features, single or recurrent episode, as defined in the Diagnostic and Statistical Manual of Mental Disorders, 4th ed., Text Revision (DSM-IV-TR) (American Psychiatric Association 2000). This objective was evaluated by assessing the mean change from baseline to end-point (10 weeks) on the Children's Depression Rating Scale-Revised (CDRS-R) total score, between duloxetine 60 mg and placebo.
This was a phase 3, randomized, double-blind, placebo- and fluoxetine-controlled study. Patients meeting entry criteria were randomly assigned 1:1:1:1 to duloxetine (60 mg QD), duloxetine (30 mg QD), fluoxetine (20 mg QD), or placebo, via interactive voice response system (IVRS). The duloxetine doses used in this study were based on results from a previous safety, tolerability, and pharmacokinetics study of duloxetine (Prakash et al. 2012), which suggested that adjustment of the duloxetine total daily dose based on body weight or age is not warranted for pediatric patients, and that different total daily doses may not be warranted for pediatric patients relative to adults. The target dose of duloxetine for adults is 60 mg daily (Cymbalta Full Prescribing Information). The duloxetine low-dose arm (30 mg) was incorporated into this study in order to further investigate results from a previous study (Prakash et al. 2012), which suggested that 30 mg QD may provide some clinical benefit for pediatric patients. The dose range for fluoxetine used in this study was based on its approved dose in the treatment of pediatric MDD (20 mg/day).
The study design incorporated a 2–4 week screening period, a 10 week double-blind (fixed dose) placebo-controlled acute treatment period, a 26 week double-blind (flexible dose) long-term treatment period in which all patients received either duloxetine (60–120 mg QD) or fluoxetine (20–40 mg QD), and a 2 week double-blind dose tapering period. Clinic visits were scheduled weekly during the screening period, at weeks 1, 2, 4, 7, and 10 during the placebo-controlled acute treatment period, and at weeks 12, 14, 16, 20, 24, 28, 32, and 36 during the double-blind long-term treatment period.
The study drug was provided as 30 mg duloxetine capsules, 10 or 20 mg fluoxetine capsules, or matching placebo. All patients received six capsules of the study drug to be taken once daily throughout the study. Patients randomized to duloxetine 30 mg initiated duloxetine at the 30 mg QD dose and maintained that dose through the 10 week acute treatment period. Patients randomized to duloxetine 60 mg or fluoxetine 20 mg initiated treatment with a low dose (duloxetine 30 mg QD or fluoxetine 10 mg QD) for 2 weeks and were then automatically increased to duloxetine 60 mg QD or fluoxetine 20 mg QD for the remaining 8 weeks of the acute treatment period.
After the 10 week time point, all patients were allowed to continue in the study for an additional 26 weeks of double-blind, flexible dose, extension treatment with duloxetine or fluoxetine. Patients initially randomized to duloxetine or fluoxetine were continued on flexible dose duloxetine (60, 90, 120 mg QD) or fluoxetine (20, 40 mg QD). Patients initially randomized to placebo were transitioned to duloxetine 30 mg QD for 2 weeks with subsequent automatic escalation to 60 mg QD and flexible dosing (60, 90, 120 mg QD) thereafter. Dose decreases were permitted during the extension treatment period; however, the lowest dose allowed was 60 mg QD for duloxetine and 20 mg QD for fluoxetine. Dose adjustments for all patients were based on investigators' assessment of tolerability as well as inadequate clinical response (Clinical Global Impressions-Severity [CGI-S] ≥3), and were implemented in a blinded fashion via IVRS.
Selection of patients
Study participants were outpatient children (7–11 years) and adolescents (12–17 years), who met DSM-IV-TR criteria for MDD without psychotic features, had a CDRS-R (Poznanski et al. 1979, 1984) total score ≥40 and a CGI-S (Guy 1976) score ≥4 at the three screening visits. An MDD diagnosis was supported by the Mini International Neurospychiatric Interview for children and adolescents (MINI-Kid) (Sheehan et al. 2010) and was conducted by two independent evaluators, with at least one evaluator being a psychiatrist. Patients were required to be medically stable based on the physical examination, laboratory tests, and electrocardiogram (ECG) completed at the screening visits. Female patients were required to have a negative serum pregnancy test at screening. Patients were excluded from the study if they were pregnant or lactating, had a <20 kg baseline weight, current Axis I disorder (other than MDD) requiring pharmacotherapy, first-degree relative with bipolar I disorder, serious or unstable medical illness, serious suicide risk, history of substance abuse/dependence within the last year, or an unexplained positive urine drug screen. In general, use of concomitant medications having primarily central nervous system activity was not permitted during the study. Use of antidepressants, antipsychotics, anticonvulsants, anorexics, benzodiazepines, psychostimulants (excluding caffeine), and herbal preparations was strictly prohibited during the study. Episodic use of diphenhydramine and antiemetics was allowed. Limited use of narcotic analgesics to relieve pain from surgical procedures or acute injury was allowed, and patients could continue on stable doses of hormones, antihypertensives, oral hypoglycemics, and diuretics to treat stable medical conditions, provided dosing had been stable 3 months prior to enrollment in the study.
Efficacy measures
The clinician-rated CDRS-R total score (sum of all 17 items) (Poznanski et al. 1979, 1984) and CGI-S (Guy 1976) efficacy measures were collected at every visit. The CDRS-R was administered only by site personnel who underwent training on the use of the instrument and met predetermined inter-rater reliability criteria, which were evaluated during rater training sessions at the study startup meetings.
Safety assessments
Spontaneously reported adverse events (AEs), pulse, blood pressure, and weight were recorded at every visit. Pharmacokinetic samples were collected at various time points and results will be presented elsewhere. Chemistry and hematology blood samples were collected at baseline and weeks 4, 10, 14, 20, 24, 36, or early termination with ECGs collected at baseline and weeks 10, 24, 36, or early termination. Prespecified definitions of potentially clinically significant (PCS) changes in vital signs and weight are shown in Table 2.
Table 2.
Vital Signs and Weight During Acute Treatment
| Acute period vital signs and weight | Duloxetine (60 mg QD) n=105 | Duloxetine (30 mg QD) n=114 | Fluoxetine (20 mg QD) n=112 | Placebo n=117 |
|---|---|---|---|---|
| Vital signs and weight mean change (MMRM)a | ||||
| Weight, kg (mean[SE]) | −0.5 (0.2)*§β | 0.2 (0.2) | 0.5 (0.2) | 0.7 (0.2) |
| Sitting pulse, bpm (mean[SE]) | −0.3 (1.0) | −1.3 (1.0) | −1.7 (1.0) | −0.5 (1.0) |
| Sitting systolic blood pressure, mm Hg (mean[SE]) | 1.2 (0.9) | 0.1 (0.9) | 0.3 (0.9) | −0.5 (0.9) |
| Sitting diastolic blood pressure, mm Hg (mean[SE]) | 2.8 (0.8)β | 0.6 (0.8) | 1.7 (0.8) | 0.7 (0.8) |
| PCS vital signs and weight at any time | ||||
| PCS decrease in weight (n/N, %)b | 14/105 (13.3)* | 10/114 (8.8) | 13/112 (11.6) | 6/117 (5.1) |
| PCS increase in pulse (n/N, %)c | 0/105 (0.0) | 0/114 (0.0) | 0/112 (0.0) | 0/117 (0.0) |
| PCS decrease in pulse (n/N, %)b | 0/100 (0.0) | 0/108 (0.0) | 0/108 (0.0) | 0/112 (0.0) |
| PCS systolic blood pressure (n/N, %)c | 8/88 (9.1) | 12/95 (12.6) | 12/93 (12.9) | 10/98 (10.2) |
| PCS diastolic blood pressure (n/N, %)c | 11/93 (11.8) | 7/100 (7.0) | 10/99 (10.1) | 5/110 (4.5) |
| PCS vital signs and weight at end-point | ||||
| PCS decrease in weight (n/N, %)b | 8/105 (7.6) | 4/114 (3.5) | 8/112 (7.1) | 3/117 (2.6) |
| PCS increase in pulse (n/N, %)c | 0/105 (0.0) | 0/114 (0.0) | 0/112 (0.0) | 0/117 (0.0) |
| PCS decrease in pulse (n/N, %)b | 0/100 (0.0) | 0/108 (0.0) | 0/108 (0.0) | 0/112 (0.0) |
| PCS systolic blood pressure (n/N, %)c | 4/88 (4.5) | 4/95 (4.2) | 2/93 (2.2) | 1/98 (1.0) |
| PCS diastolic blood pressure (n/N, %)c | 4/93 (4.3) | 2/100 (2.0) | 2/99 (2.0) | 2/110 (1.8) |
| Sustained elevation in blood pressure | ||||
| Sustained elevation in systolic blood pressure (n/N, %)c | 0/88 (0.0) | 0/95 (0.0) | 1/93 (1.1) | 1/98 (1.0) |
| Sustained elevation in diastolic blood pressure (n/N, %)c | 0/93 (0.0) | 1/100 (1.0) | 1/99 (1.0) | 0/110 (0.0) |
Baseline was defined as last observation during the screening period, and end-point was the 10 week end-point of the acute treatment period.
N=number of patients with high or normal values at baseline and with at least one nonmissing postbaseline measure; n=number of patients with a potentially clinically significant (PCS) postbaseline measurement.
N=number of patients with low or normal values at baseline and with at least one nonmissing postbaseline measure; n=number of patients with a PCS postbaseline measurement.
p<0.05 vs. placebo; βp<0.05 vs. duloxetine 30 mg; §p<0.05 vs. fluoxetine
PCS increase in blood pressure=increase ≥5 mm Hg from baseline high to a value above the 95th percentile based on age, height, and sex (National High Blood Pressure Education Program Working Group 2004).
PCS decrease in pulse=decrease ≥25 bpm from baseline low to a value of <60 bpm for children or decrease ≥15 bpm from baseline low to a value <50 bpm for adolescents.
PCS increase in pulse=increase ≥15 bpm from baseline high to a value >140 bpm for children or increase ≥15 bpm from baseline high to a value >120 bpm for adolescents.
PCS decrease in weight=decrease ≥3.5% from baseline low.
Sustained elevation in blood pressure=PCS blood pressure at three consecutive post-baseline visits.
MMRM, mixed effects model repeated measures.
The Columbia-Suicide Severity Rating Scale (C-SSRS) (Posner et al. 2007, 2011) was used in this study to prospectively capture the occurrence, severity, and frequency of suicide-related thoughts and behaviors. Nonserious AEs obtained through the C-SSRS were recorded and analyzed separately and were not recorded as AEs via the case report form unless the nonserious AE was spontaneously reported by the patient or the parent/legal guardian. Only serious AEs (SAEs) elicited through the C-SSRS were recorded as AEs via the study case report forms. All occurrences of suicidal ideation, behaviors, or acts, or nonsuicidal self-injurious behavior during treatment were tabulated. These outcomes were also analyzed as treatment emergent (new occurrence or worsening of pre-existing events) based on lead-in baseline (defined for acute and extension treatment in Table 3), or improved from lead-in baseline.
Table 3.
C-SSRS Suicidal Ideation and Behavior During Acute and Extension Treatment
| Acute treatment CSSRS outcome, n/N (%) | Duloxetine (60 mg QD) | Duloxetine (30 mg QD) | Fluoxetine (20 mg QD) | Placebo |
|---|---|---|---|---|
| Any occurrence during treatment | ||||
| Suicidal ideation | 17/105 (16.2) | 11/115 (9.6) | 13/112 (11.6) | 15/117 (12.8) |
| Suicidal behaviors | 0/105 (0.0) | 0/115 (0.0) | 1/112 (0.9) | 1/117 (0.9) |
| Suicidal acts | 0/105 (0.0) | 0/115 (0.0) | 1/112 (0.9) | 1/117 (0.9) |
| Nonsuicidal self-injurious behavior | 3/105 (2.9) | 6/115 (5.2) | 2/112 (1.8) | 5/116 (4.3) |
| Treatment-emergent from lead-in baselinea | ||||
| Suicidal ideationb | 7/105 (6.7) | 6/115 (5.2) | 9/112 (8.0) | 11/117 (9.4) |
| Improvement in suicidal ideationc | 13/16 (81.3) | 16/17 (94.1) | 11/16 (68.8) | 13/15 (86.7) |
| Suicidal behaviors | 0/105 (0.0) | 0/115 (0.0) | 1/112 (0.9) | 1/117 (0.9) |
| Non-suicidal self-injurious behaviord | 3/104 (2.9) | 3/112 (2.7) | 2/112 (1.8) | 5/115 (4.3) |
| Extension treatment CSSRS outcome, n/N (%) | Duloxetine (60-120 mg QD) transitioned from 60 mg | Duloxetine (60-120 mg QD) transitioned from 30 mg | Fluoxetine (20-40 mg QD) transitioned from 20 mg | Duloxetine (60-120 mg QD) transitioned from placebo |
|---|---|---|---|---|
| Any occurrence during treatment | ||||
| Suicidal ideation | 6/71 (8.5) | 12/78 (15.4) | 8/80 (10.0) | 8/79 (10.1) |
| Suicidal behaviors | 2/71 (2.8) | 3/78 (3.8) | 0/80 (0.0) | 1/79 (1.3) |
| Suicidal acts | 1/71 (1.4) | 1/78 (1.3) | 0/80 (0.0) | 1/79 (1.3) |
| Non-suicidal self-injurious behavior | 4/71 (5.6) | 3/78 (3.8) | 1/80 (1.3) | 1/79 (1.3) |
| Treatment-emergent from lead-in baselinee | ||||
| Suicidal ideationb | 5/71 (7.0) | 8/78 (10.3) | 7/80 (8.8) | 6/79 (7.6) |
| Improvement in suicidal ideationc | 2/3 (66.7) | 4/6 (66.7) | 4/5 (80.0) | 2/4 (50.0) |
| Suicidal behaviors | 2/71 (2.8) | 3/78 (3.8) | 0/80 (0.0) | 1/79 (1.3) |
| Non-suicidal self-injurious behaviord | 3/70 (4.3) | 2/75 (2.7) | 1/80 (1.3) | 1/77 (1.3) |
Patients are counted once in each category.
N=patients with baseline and ≥1 postbaseline observation; n=patients with event.
Lead-in baseline for acute treatment includes 2 screening visits (study baseline).
N=number of enrolled patients with ≥1 postbaseline suicidal ideation score and whose maximum Columbia-Suicide Severity Rating Scale (C-SSRS) suicidal ideation score during the lead-in baseline period is not missing and less than the maximum severity category.
N=number of enrolled patients whose suicidal ideation score is not missing and >0 during lead-in baseline.
N=number of enrolled patients without nonsuicidal self-injurious behavior at lead-in baseline visits and with a nonmissing postbaseline observation.
Lead-in baseline for extension treatment includes the last 2 visits of acute treatment.
Suicidal ideation: wish to be dead, nonspecific active suicidal thoughts, active suicidal ideation with any methods (not plan) without intent to act, active suicidal ideation with some intent to act, without specific plan, and active suicidal ideation with specific plan and intent.
Suicidal behavior: preparatory acts or behavior, aborted attempt, interrupted attempt, nonfatal suicide attempt, and completed suicide.
Suicidal acts: nonfatal suicide attempt, and completed suicide.
Sample size
The study was powered to address the primary objective (contrast between duloxetine 60 mg and placebo on the CDRS-R total score mean change from baseline to 10 weeks). The powering of the study was based on an anticipated enrollment of 448 patients, randomized in a 1:1:1:1 ratio to duloxetine 60 mg, duloxetine 30 mg, fluoxetine 20 mg, and placebo. It was assumed that ∼10% of patients would discontinue the trial prior to providing postbaseline data on the primary efficacy outcome, leaving ∼100 patients per treatment arm with postbaseline data. This sample size was estimated to have 80% power to detect an effect size of 0.40 (duloxetine 60 mg efficacy relative to placebo on the CDRS-R total score) using a two group t test with a 0.05 two sided significance level. The effect size of 0.4 was determined based on historical data for the effect size of duloxetine 60 mg QD in adult patients with MDD (Pritchett et al. 2007).
Statistical methods
All analyses were completed on an intent-to-treat (ITT) basis unless otherwise specified. An ITT analysis is an analysis of data by the groups to which patients are assigned by random allocation, even if the patient did not take the assigned treatment, did not receive the correct treatment, or otherwise did not follow the protocol. All analyses of continuous measures included randomized patients with both a baseline and at least one postbaseline value for the variable being analyzed.
Unless otherwise specified, for analyses of continuous measures, baseline was defined as the last measurement taken at, or prior to, the visit when the study period (acute or long-term treatment period) began; end-point was defined as the last nonmissing measurement for the study period of interest.
The protocol-specified primary analytic approach for assessing mean changes for all efficacy measures was the recommended (Lieberman et al. 2005; National Research Council 2010) restricted maximum likelihood (REML)-based mixed effects model repeated measures (MMRM) approach using all the longitudinal observations at each postbaseline visit. The MMRM model included the fixed categorical effects of treatment, pooled investigative site, visit, treatment-by-visit interaction, age category (children 7–11, adolescents 12–17), and age category-by-visit interaction, as well as the continuous, fixed covariates of the baseline value being analyzed and the baseline value of the variable being analyzed-by-visit interaction. The baseline value of the variable being analyzed and baseline-by-visit interaction was included to account for the differing influence over time of the baseline score on the postbaseline scores. An unstructured covariance structure was used to model the within-patient errors. A Kenward–Roger correction (Kenward and Roger 1997) was used to estimate denominator degrees of freedom. Significance tests were based on least-squares means (LS means) using a two sided α=0.05.
Additional analyses of continuous efficacy and safety measures were also performed using an analysis of variance (ANOVA) or an analysis of covariance (ANCOVA) model. When an ANOVA model was used, the model contained the main effects of treatment and pooled investigative site. An analysis of covariance (ANCOVA) model, in general, refers to the ANOVA model with baseline values and age category (children 7–11, adolescents 12–17) added as covariates. Type III sum-of-squares for the LS mean was used for the statistical comparison of main effects using ANOVA or ANCOVA. Statistical inference for ANOVA or ANCOVA interaction terms was based on type II sum-of-squares for the LS mean. A last observation carried forward (LOCF) method was used for these analyses.
Response was defined as a 50% improvement on the CDRS-R total score after subtracting the 17 item base score (Emslie et al. 2002), and remission was defined as a CDRS-R total score ≤28. Probabilities of response and remission were estimated using a categorical MMRM approach in which a marginal model based on a pseudolikelihood method was utilized and implemented in SAS PROC GLIMMIX. The model included the fixed, categorical effects of treatment, pooled investigator, visit, and treatment-by-visit interaction, age category (7–11 vs. 12–17 years), age category-by-treatment interaction, as well as the continuous, fixed covariate of baseline score and baseline-by-visit interaction. Categorical safety measures were analyzed using Fisher's exact test.
“Mean change” refers to adjusted LS mean change from MMRM, ANOVA, or ANCOVA model. Statistical comparisons between patients initially randomized to duloxetine or fluoxetine over the 36 week combined acute/extension periods were conducted. Statistical comparisons among treatment groups were not conducted for the extension period analyses, because of selection bias. In other words, only patients who completed the acute period of the study were included in the extension period analyses; therefore, patient characteristics at the beginning of the extension period were expected to be different among treatment groups, because of lack of randomization.
Results
Patients
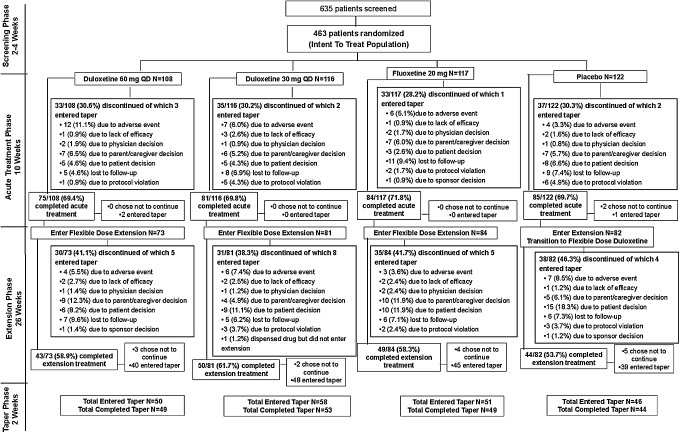
The flow of patients through the study is shown in Figure 1. The overall patient cohort was comprised of 41.5% children (7–11 years) and 58.5% adolescents (12–17 years) with approximately equal proportions of males (48.8%) and females (51.2%). The median age of randomized patients was 13.2 years. Among children (7–11 years), 55.2% were male and 44.8% were female. Among adolescents (12–17 years), 44.3% were male and 55.7% were female. Among female patients, 64.1% had reached menarche at study baseline. The study population was relatively diverse, ∼55% white (Table 1). This was an international multicenter study with the large majority of patients enrolled from the United States (78.6%), followed by Mexico (16%), Canada (5.2%), and Argentina (0.2%).
FIG. 1.
Patient flow.
Table 1.
Baseline Characteristics
| Baseline characteristics | Duloxetine (60 mg QD) n=108 | Duloxetine (30 mg QD) n=116 | Fluoxetine (20 mg QD) n=117 | Placebo n=122 |
|---|---|---|---|---|
| Mean age, y(SD) | 12.9(2.9) | 12.9(2.9) | 13.0(3.2) | 13.1(2.9) |
| Age category, n(%) | ||||
| 7–11 years | 44(40.7) | 49(42.2) | 50(42.7) | 49(40.2) |
| 12–17 years | 64(59.3) | 67(57.8) | 67(57.3) | 73(59.8) |
| Sex, n(%) | ||||
| Female | 60(55.6) | 47(40.5)* | 61(52.1) | 69(56.6) |
| Male | 48(44.4) | 69(59.5)* | 56(47.9) | 53(43.4) |
| BMI | ||||
| Mean(SD) | 23.7(7.2) | 22.5(5.2) | 23.2(6.7) | 24.0(6.8) |
| Median | 21.6 | 21.8 | 21.0 | 22.3 |
| Range (minimum-maximum) | 13.7–50.9 | 14.2–37.7 | 13.4–45.0 | 14.3–48.6 |
| Race, n(%) | ||||
| White | 54(52.9) | 61(54.0) | 67(58.8) | 62(52.1) |
| Native American or Alaska Native | 15(14.7) | 23(20.4) | 16(14.0) | 19(16.0) |
| Black or African American | 27(26.5) | 21(18.6) | 21(18.4) | 24(20.2) |
| Asian | 0(0.0) | 1(0.9) | 1(0.9) | 1(0.8) |
| Native Hawaiian or other Pacific Islander | 0(0.0) | 1(0.9) | 0(0.0) | 0(0.0) |
| Multiracial | 6(5.9) | 6(5.3) | 9(7.9) | 13(10.9) |
| Not provided (n) | 6 | 3 | 3 | 3 |
| Ethnicity, n(%) | ||||
| Hispanic or Latino | 34(31.8) | 38(33.6) | 39(33.6) | 46(38.0) |
| Non-Hispanic or Latino | 73(68.2) | 75(66.4) | 77(66.4) | 75(62.0) |
| Not answered (n) | 1 | 3 | 1 | 1 |
| CDRS-R total score | ||||
| Mean(SD) | 59.3(10.9) | 59.8(11.0) | 57.9(10.1) | 58.2(9.4) |
| CGI-Severity score | ||||
| Mean(SD) | 4.6(0.7) | 4.6(0.7) | 4.6(0.6) | 4.5(0.6) |
p<0.05 for gender distribution in the duloxetine 30 mg treatment arm vs. duloxetine 60 mg and placebo (greater proportion of males in the duloxetine 30 mg arm vs. duloxetine 60 mg and placebo).
BMI, body mass index; CDRS-R, Children's Depression Rating Scale-Revised; CGI, Clinical Global Impressions.
Among randomized patients, the mean age of first episode of MDD was 10.2 years, mean number of previous episodes was 1.6 (median 1.0), and 42% of patients were experiencing a first episode of MDD. The mean CDRS-R total score at baseline was 58.8 (median 58.0) indicating moderately severe depression consistent with the mean baseline CGI-S score of 4.6 (median 4.0). With the exception of the proportion of males to females in the duloxetine 30 mg treatment arm, there were no significant between-group differences in baseline demographics or psychiatric profile (Table 1). More than half of the study patients had a first-degree relative with diagnosed depression (59.2%), and a total of three patients had first-degree relatives with diagnosed bipolar disorder (all cases bipolar II).
Headache (22.5%), seasonal allergy (12.1%), and attention-deficit/hyperactivity disorder (10.8%) were the most frequently reported preexisting conditions and the only preexisting conditions reported by ≥10% of the patient population. Preexisting conditions reported by ≥2% and <10% of the patient population were asthma (8.2%), dysmenorrhea (5.1% of female patients), insomnia (4.5%), oppositional defiant disorder (4.1%), abdominal pain upper (4.1%), migraine (3.9%), acne (2.8%), obesity (2.4%), generalized anxiety disorder (2.2%), constipation (2.2%), and hypersensitivity (2.2%). At baseline, headache was reported with statistically significantly greater incidence in the placebo group compared with the fluoxetine group, dysmenorrhea was reported with statistically significantly greater incidence in the duloxetine 60 mg female group and the fluoxetine female group compared with the placebo female group, and attention-deficit/hyperactivity disorder was reported with statistically significantly greater incidence in the fluoxetine group compared with both the placebo group and the duloxetine 60 mg group. There were no other statistically significant differences in the frequency of preexisting conditions at baseline between the active drug groups (duloxetine or fluoxetine) and the placebo group.
Acute efficacy
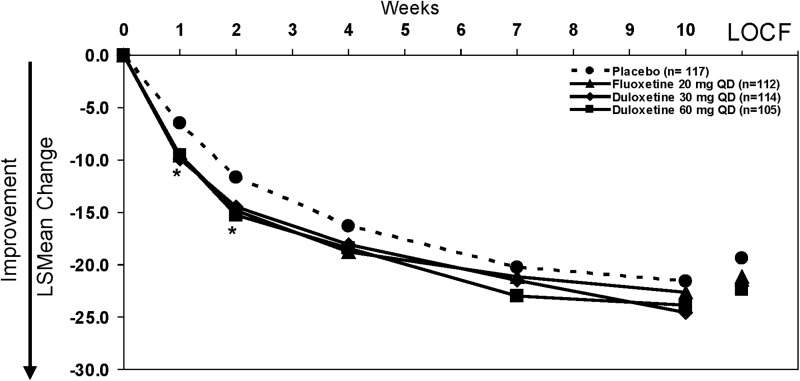
Results from the primary efficacy measure of the study showed that the active drugs (duloxetine and fluoxetine) both separated from placebo at early time points (weeks 1 and 2); however, the difference in the mean change (baseline to primary end-point week 10) between the active drug arms and placebo was not statistically significant (Fig. 2). Mean CDRS-R total scores at the 10 week time point (MMRM) were: 35.0, 34.4, 36.4, and 37.4 for duloxetine 60 mg, duloxetine 30 mg, fluoxetine 20 mg, and placebo, respectively. Mean CGI-S scores at the 10 week time point (MMRM) did not statistically differ among treatment groups (3.1 for all treatment groups).
FIG. 2.
Primary outcome: Mean change on the Children's Depression Rating Scale-Revised (CDRS-R) total score from baseline to 10 weeks (mixed effects model repeated measures [MMRM]). Mean changes at 10-weeks for duloxetine 60 mg-, duloxetine 30 mg-, fluoxetine-, and placebo-treated patients were −23.9, −24.6, −22.6, and −21.6, respectively. Mean changes to last observation carried forward (LOCF) end-point for duloxetine 60 mg-, duloxetine 30 mg-, fluoxetine-, and placebo-treated patients were −22.4, −22.0, −21.1, and −19.4, respectively. *p<.05 for all active drugs vs. placebo.
In subgroup analyses of children (7–11 years) and adolescents (12–17 years), there were no statistically significant differences in the mean change from baseline to last observation on the CDRS-R total score for duloxetine-treated children (mean change, −23.0 [60 mg], −19.0 [30 mg]) compared with placebo-treated children (mean change, −18.5) or for duloxetine-treated adolescents (mean change, −22.2 [60 mg], −24.2 [30 mg]) compared with placebo-treated adolescents (mean change, −19.8). There were no statistically significant differences between fluoxetine and placebo on the CDRS-R total score within either age group (mean change fluoxetine: −20.1 [children], −21.5 [adolescents]).
With regard to treatment by gender, a statistically significantly greater improvement from baseline to end-point (LOCF) in CDRS-R total score was observed for duloxetine 60 mg-treated females (mean change, −24.4) compared with placebo-treated females (mean change, −19.3) and for duloxetine 30 mg treated females (mean change, −25.6) compared with placebo-treated females. No statistically significant differences were observed for duloxetine-treated males compared with placebo-treated males or for fluoxetine-treated males or females compared with the respective placebo-treated gender group.
A comparison by geographic region (North America [United States and Canada] and Central/South America [Mexico and Argentina]) was conducted to assess the consistency of the results between regions. Consistent with the primary study results, the treatment-by-region interaction was not statistically significant (p=0.8), suggesting consistency in the treatment response (to both drug and placebo) across both regions.
Probabilities (MMRM) of treatment response (50% improvement in CDRS-R total score from baseline) at week 10 for patients receiving duloxetine 60 mg (69%), duloxetine 30 mg (69%), or fluoxetine 20 mg (61%) did not differ significantly from that for placebo-treated patients (60%). There were no significant between-group differences in the probabilities of remission (CDRS-R total score ≤28) at week 10 for duloxetine 60 mg (40%) or fluoxetine (32%) compared with placebo (30%). The probability of remission for duloxetine 30 mg (46%) was statistically higher (p<0.05) than that for placebo.
Remission rates at the last two nonmissing acute treatment visits was significantly (p<0.05) greater for duloxetine 60 mg (26%) than for placebo (14%).
Longer-term dosing
During extension treatment, the last prescribed dose of duloxetine was 60 mg, 90 mg, and 120 mg for 30.8%, 20.9%, and 46.2% of patients, respectively. The last prescribed dose of fluoxetine was 20 mg and 40 mg for 29.8% and 70.2% of patients, respectively.
Longer-term effectiveness
Mean CDRS-R total scores at the 36 week time point (MMRM) were: 24.3, 25.1, 25.0, and 25.8 for patients initially randomized to duloxetine 60 mg, duloxetine 30 mg, and fluoxetine, and patients transitioned from placebo to duloxetine, respectively. Mean CGI-S scores at the 36 week time point (MMRM) were: 1.8, 2.0, 1.8, and 1.9 for patients initially randomized to duloxetine 60 mg, duloxetine 30 mg, and fluoxetine, and patients transitioned from placebo to duloxetine, respectively.
For patients initially randomized to duloxetine 60 mg, the probability (MMRM) of remission at 36 weeks was 81%, and for patients initially randomized to fluoxetine it was 74%, with no statistically significant differences between groups at any time in the probability of remission during the 36 week study.
Safety: Patient disposition
Rates and reasons for early discontinuation during the study are shown in Figure 1. Discontinuation for AEs was significantly higher (p<0.05) for the duloxetine 60 mg group than for the placebo group during acute treatment, with no other statistically significant differences between treatment groups during acute treatment.
Safety: Deaths and SAEs
No deaths occurred during the study; however, 22 patients (14 duloxetine, 6 fluoxetine, 2 placebo) experienced 28 SAEs. Of these 28 SAEs, 9 were suicide-related and are discussed with C-SSRS results. All SAEs resulted in hospitalization of the patient.
Nonsuicide-related SAEs reported for duloxetine-treated patients were: Intentional overdose (two patients during acute treatment and in both cases, the investigator did not classify the overdose as a suicide attempt, as the overdose was taken without intent to die), nonsuicidal self-injurious behavior (cutting) and hallucination (two events in one patient during acute treatment), depression (two patients, one during acute treatment and one during extension), Stevens–Johnson syndrome (one patient, extension), and irritable bowel syndrome (one patient, extension). SAEs reported for duloxetine-treated patients after transitioning from placebo were: Intentional overdose (one patient, extension; not considered a suicide attempt or self-injurious behavior as the patient took an overdose of diphenhydramine without intent to die or harm self), benign rolandic epilepsy (one patient, extension), and road traffic accident (one patient, extension, and patient was a passenger in the vehicle). Nonsuicide-related SAEs reported for fluoxetine-treated patients were: Abnormal (destructive) behavior and aggression (two events in one patient during acute treatment), aggression (one patient, acute), somnolence (one patient, acute), and tuberculosis of peripheral lymph nodes (one patient, acute). Nonsuicide-related SAEs reported for placebo-treated patients were: Homicidal ideation (one patient, acute), and nonsuicidal self-injurious behavior (cutting) (one patient, acute).
Safety: AEs
During acute treatment, the proportion of patients who experienced at least one treatment-emergent adverse event (TEAE) was 73.1% (duloxetine 60 mg), 57.8% (duloxetine 30 mg), 61.5% (fluoxetine), and 58.2% (placebo) with significantly more duloxetine 60 mg treated patients experiencing at least one TEAE compared with placebo (p=0.02) and duloxetine 30 mg (p=0.02). During extension treatment, the proportion of patients who experienced at least one TEAE did was greater for patients initially randomized to duloxetine 60 mg (68.5%) and patients transitioned from placebo to duloxetine (67.1%) than for patients initially randomized to duloxetine 30 mg (56.8%) and fluoxetine (53.6%). The most frequently reported TEAEs (≥10%) during the study were: Nausea, vomiting, upper abdominal pain, headache, and somnolence for duloxetine, and only headache for fluoxetine.
A total of 49 patients discontinued because of an AE during the 36 week study (Fig. 1), and AEs leading to discontinuation in more than patient during the study were: Suicide attempt (two duloxetine, both SAEs), intentional overdose (two duloxetine, one fluoxetine, all SAEs), suicidal ideation (three duloxetine, one of which was an SAE, one fluoxetine, one placebo), self-injurious behavior (one duloxetine, one placebo, both SAEs), depression (four duloxetine, two of which were SAEs), hallucination (one duloxetine, one fluoxetine), irritability (five duloxetine, one fluoxetine), aggression (one duloxetine, two fluoxetine, both of which were SAEs), fatigue (two duloxetine), somnolence (two duloxetine), nausea (six duloxetine), and upper abdominal pain (one duloxetine, one placebo).
Safety: Vital signs, weight, and laboratory and ECG data
Table 2 shows mean changes in vital signs and weight from baseline to 10 weeks (MMRM) as well as the incidence of PCS changes in weight, pulse, and blood pressure at any time and at end-point during acute treatment. In general, the incidence of PCS changes in weight, pulse, and blood pressure were lower at end-point during acute treatment as compared with at any time during acute treatment (Table 2). With regard to longer-term results, there were no statistically significant differences in mean changes for weight or blood pressure between patients initially randomized to duloxetine 60 mg and those randomized to fluoxetine at 36 weeks, and similar mean increases in weight were observed at 36 weeks (MMRM) for patients initially randomized to duloxetine 60 mg (2.9 kg) and those initially randomized to fluoxetine (3.3 kg). Mean change pulse at 36 weeks was statistically greater (4.5 bpm) for patients initially randomized to duloxetine 60 mg than for those initially randomized to fluoxetine (-0.6 bpm, p<0.05).
There were no statistically significant differences between patients initially randomized to duloxetine 60 mg and those randomized to fluoxetine in the incidence of PCS changes in weight, pulse, or blood pressure at any time or at end-point (last observation) during the 36 week study. Among patients initially randomized to duloxetine 60 mg, no patients had sustained (three consecutive visits) elevation in systolic or diastolic blood pressure during the 36 week study. Among patients initially randomized to fluoxetine, a total of five patients experienced sustained elevation in blood pressure (three patients, systolic blood pressure and two patients, diastolic blood pressure) during the 36 week study.
No patients in any treatment group met PCS criteria for prolonged QTc Fridericia correction (>500 msec) at any time during the study.
No patient had an SAE related to laboratory results or was discontinued because of abnormal laboratory values, and no patients had a treatment-emergent alanine aminotransferase (ALT) equal to or more than three times the upper limit of normal at any time during the study.
Safety: Suicidal ideation and behavior
Table 3 shows outcomes for suicidal ideation and behavior as classified using the C-SSRS. There were no statistically significant differences between any treatment groups for any outcome on the C-SSRS during acute treatment. As assessed by the C-SSRS, suicidal ideation occurred in 9–15% of patients overall during acute treatment (that is, either preexisting or treatment emergent); however compared with study baseline (lead-in), the suicidal ideation was treatment emergent (that is, new occurrence or any increase in severity from baseline) in fewer patients (5–9%) during acute treatment. During the extension treatment period, suicidal ideation occurred in 8–15% of patients overall; and compared with the end of acute treatment (lead-in), the suicidal ideation was treatment emergent in 7–10% of patients.
Suicidal ideation was reported as an SAE in three patients (one duloxetine [adolescent male] and two fluoxetine [one child and one adolescent, both males]) during acute treatment. Also during the acute treatment period, two patients reported suicidal behavior (nonfatal suicide attempt) on the C-SSRS. Both nonfatal suicide attempts were reported as SAEs (intentional overdose [fluoxetine; adolescent, male] and suicide attempt by wrapping object around neck [placebo; child, male]).
During the extension treatment period, six patients (all duloxetine) engaged in suicidal behavior (three nonfatal, two interrupted, and one aborted suicide attempt). Of these six incidents of suicidal behavior, four were reported as SAEs (three nonfatal attempts [one stabbing puncture wound in an adolescent male, one tying object around neck in a male child, one overdose on antiinflammatory medications in an adolescent female], and one interrupted attempt to jump from a second story window in a male child). For the other two patients, the suicidal behavior was only reported on the C-SSRS (one male child made an interrupted suicide attempt and was discontinued from the study because of suicidal ideation, and another male child made an aborted attempt and was discontinued because of parent decision to seek alternative treatment for behavior problems).
Discussion
Efficacy results from this study indicated that duloxetine fixed doses (60 mg or 30 mg QD) were not statistically significantly different from placebo in the treatment of children and adolescents with MDD. In addition, the active control (fluoxetine), with known efficacy in children and adolescents with MDD, was not statistically significantly different from placebo on the primary outcome measure. Because neither the investigational drug (duloxetine), nor the active control (fluoxetine) separated from placebo on the primary outcome measure, the study is considered to be inconclusive.
Results during extended treatment suggested that improvement in MDD symptoms continued for all of the treatment groups based on the mean improvement on the CDRS-R total score and CGI-S score, with no statistically significant differences in probability of remission at any time during the 36 week study between patients initially randomized to duloxetine 60 mg and those initially randomized to fluoxetine. Consistent with the preliminary safety study in pediatric MDD (Prakash et al. 2012), the majority of duloxetine-treated patients in this study required dose escalation to higher doses during the extension period in order to optimize efficacy.
The profile and nature of TEAEs reported for duloxetine-treated children and adolescents with MDD in this study was generally similar to that reported in the preliminary open-label study in pediatric MDD patients (Prakash et al. 2012), and was also consistent with the TEAE profile seen in duloxetine-treated adult patients (Cymbalta Full Prescribing Information). In addition, no new safety signals were identified based on laboratory, ECG, or blood pressure results from this study.
Treatment-emergent suicidal ideation and behavior are areas of concern in pediatric MDD patients, and suicidal ideation and behavior were closely monitored and solicited at every visit using the C-SSRS in this study. All events related to suicidal thoughts, behaviors, or nonsuicidal self-injurious behavior that were collected by spontaneous report on the AE case report forms during the 36-week study were confirmed to be collected on the C-SSRS; however, not all events solicited on the C-SSRS were recorded on the AE case report forms. Therefore, with regard to suicidal ideation, behavior, and nonsuicidal self-injurious behavior, the results of the C-SSRS provide the most complete information from the study. There were no completed suicides or deaths during the 36 week study. During acute treatment, the incidence of treatment-emergent suicidal ideation was similar in all three drug treatment arms (5–8%) as compared with placebo (9%). These rates for suicidal ideation are more consistent with the findings for antidepressant treatment arms (0–8%) versus placebo treatment arms (0–5%) in pediatric studies reported by Hammad and colleagues (2006). This study was also one of the few studies to provide longer-term safety results with regard to suicidal ideation and behavior in the pediatric MDD population. Perhaps as expected because of the longer time frame, the frequency of suicidal behaviors (nonfatal, interrupted, and aborted attempts) was greater in the 26 week extension period than in the 10 week acute treatment period. All suicidal behaviors reported during the extension period occurred in duloxetine-treated patients; however, it should also be noted that the majority (74%) of patients contributing data in the extension period were taking duloxetine because of this study design (all placebo-treated patients were transitioned to duloxetine for extended treatment). The frequency of treatment-emergent suicidal ideation, however, was similar to the frequency reported during the acute treatment period.
Although this study was carefully designed, incorporating both a placebo- and an active-control to assess the acute treatment efficacy of duloxetine, the failure of this study to show assay sensitivity (a statistically significant separation of the active control from placebo on the primary outcome) deserves some discussion. First, it should be noted that studies of investigational antidepressants failing to separate from placebo (negative studies) are more common than not in the pediatric population (Bridge et al. 2009). This set of two studies, however, were the first randomized controlled trials to demonstrate that the studies failed to detect a statistical difference between placebo and an active control (fluoxetine) with known efficacy in the pediatric MDD population, rendering results with regard to the investigational drug (duloxetine) inconclusive as opposed to negative. The lack of separation of the active control from placebo appears to be the result of a high placebo response. Mean CDRS-R improvement (MMRM) during acute treatment for the duloxetine and fluoxetine treatment arms (22–25 point improvement for both drugs) was similar to that for fluoxetine (22 points) in published studies of fluoxetine in the treatment of children and adolescents with MDD (Emslie et al. 1997, 2002; March et al. 2004). In contrast, mean CDRS-R improvement (MMRM) during acute treatment for the placebo arm in this study (∼22 point improvement) was greater than mean improvements of 10–15-points for the placebo arms in published studies of fluoxetine in the treatment of children and adolescents with MDD (Emslie et al. 1997, 2002), in which statistically significant separation of fluoxetine from placebo was observed as well as the19 point placebo improvement in the Treatment for Adolescents With Depression Study (TADS) (March et al. 2004). This observed trend of increasing placebo response over time from 10 points (in 1997) to 22 points (in 2012) on the CDRS-R total score could be the result of several factors including increased sample size for studies, which in turn required an increased number of participating clinical sites and countries. In addition, whereas the treatment response was consistent across geographic regions (North America vs. Central/South America), the low enrollment by site and the subsequent large number of sites needed to fully enroll the study were likely key factors affecting variability and mediating the placebo response observed in this study. Such factors related to study size have been proposed as the better predictors of placebo response in studies of pediatric MDD patients (Bridge et al. 2009). In a recently published review, Rutherford and Roose (2013) summarized research findings in the area of placebo response and presented key study design features that influence placebo response based on the strength of supporting evidence from the body of published research. The four design features with the strongest evidence of influence on placebo response were: Number of sites, quality of rater blinding, number of treatment arms, and probability of receiving placebo. It is of note that the current study included four treatment arms with a 25% chance of receiving placebo, and as discussed in Rutherford and Roose (2013), designs with a single active treatment arm and a higher chance of receiving placebo (50%) are preferable, to reduce patient expectation and placebo response. However, given the positive results from the TADS, which included four treatment arms and showed a typical drug response rate, a low placebo response rate, and a strong drug–placebo difference (March et al. 2004), it is unclear if the number of treatment arms and chance of receiving placebo influence placebo response in pediatric studies to the same degree as in adult studies. To the extent possible, the study design incorporated other features such as minimal assessments at each visit, to reduce the time spent with study patients, and a slightly lower frequency of study visits (scheduled at weeks 1, 2, 4, 7, and 10, vs. every week or every other week) during the placebo-controlled acute treatment period, in order to address the possibility of increased placebo response; however, a substantial placebo response was still observed.
Limitations
Several limitations must be taken into account when interpreting the results of this study. Patients with significant suicidal risk, comorbid psychiatric conditions requiring medication to manage, or other significant or unstable medical conditions, were excluded from this study. Therefore, the safety results from these studies have limited generalizability to a broader pediatric MDD population with greater suicide risk as well as greater psychiatric or medical burden. Also, because of the need to blind multiple doses of two different drugs, all patients were required to take six capsules of study drug per day. Although the capsule size was small (size 3, ∼4×14 mm), swallowing capsules may be difficult for pediatric patients, especially in the younger age range. Therefore, only a subset of pediatric patients who could swallow capsules were enrolled in this study. Finally, although the inclusion of active and placebo-controlled arms helped in the interpretation of the study results, increasing the number of treatment arms, and, therefore, the likelihood of receiving active drug versus placebo, may have also contributed to the placebo response observed in these studies.
Conclusions and Clinical Significance
Efficacy was not demonstrated in this study, as the results were inconclusive because neither the investigational drug (duloxetine) nor the active control (fluoxetine) demonstrated a statistically significant separation from placebo on the primary efficacy analysis of mean change from baseline to week 10 on the CDRS-R total score.
Overall safety findings from this study were consistent with the known safety and tolerability profile for duloxetine.
Acknowledgments
The authors thank the principal investigators and their clinical staff. The authors also thank the many patients and their families who generously agreed to participate in this clinical trial, and the clinical operations staff and statistical analysts of the Duloxetine Antidepressant Team for their excellent implementation of the trial.
Disclosures
Graham J. Emslie has received research support from BioMarin, Duke University, Eli Lilly and Company, Forest Laboratories, GlaxoSmithKline, and Mylan, and has served as a consultant for Allergan, BioBehavioral Diagnostics Inc., Bristol Myers Squibb, Eli Lilly and Company, INC Research Inc., Lundbeck, Pfizer, Seaside Therapeutics, Shire, the Texas Department of State Health Services, Valeant, and Wyeth. Now an emeritus faculty member, John S. March has no established relationships with the pharmaceutical industry. Dr. March has served as a consultant or scientific advisor to Attention Therapeutics, Bristol Myers Squibb, Eli Lilly and Company, and Pfizer; received study drug for an National Institute of Mental Health (NIMH)-funded study from Eli Lilly and Company and Pfizer; is an equity holder in MedAvante; and receives royalties from Guilford Press, Multihealth Systems, and Oxford University Press. Dr. March has received research support from National Institute on Drug Abuse (NIDA), NIMH, and Pfizer. Dr. March has not engaged in promotional work, for example, serving on a speakers bureau or training, for >15 years. Apurva Prakash, Qi Zhang, Beth A. Pangallo, and Mark E. Bangs are employees of and own stock or equity in Eli Lilly and Company.
References
- American Psychiatric Association: Diagnostic and Statistical Manual of Mental Disorders, 4th ed., Text Revision. Washington, DC: American Psychiatric Association; 2000 [Google Scholar]
- Atkinson SD, Prakash A, Zhang Q, Pangallo BA, Bangs ME, Emslie GJ, March JS: A double-blind, efficacy and safety study of duloxetine flexible doses in children and adolescents with major depressive disorder. J Child Adolesc Psychopharmacol 24:180–189, 2014 [DOI] [PubMed] [Google Scholar]
- Bridge JA, Birmaher B, Iyengar S, Barbe RP, Brent DA: Placebo response in randomized controlled trials of antidepressants for pediatric major depressive disorder. Am J Psychiatry 166:42–49, 2009 [DOI] [PubMed] [Google Scholar]
- Cymbalta Full Prescribing Information. Available at http://pi.lilly.com/us/Cymbalta-pi.pdf Accessed March1, 2014
- Emslie GJ, Heiligenstein JH, Wagner KD, Hoog SL, Ernest DE, Brown E, Nilsson M, Jacobson JG: Fluoxetine for acute treatment of depression in children and adolescents: A placebo-controlled, randomized clinical trial. J Am Acad Child Adolesc Psychiatry 41:1205–1215, 2002 [DOI] [PubMed] [Google Scholar]
- Emslie GJ, Rush AJ, Weinberg WA, Kowatch RA, Hughes CW, Carmody T, Rintelmann J: A double-blind, randomized, placebo-controlled trial of fluoxetine in children and adolescents with depression. Arch Gen Psychiatry 54:1031–1037, 1997 [DOI] [PubMed] [Google Scholar]
- Emslie GJ, Ventura D, Korotzer A, Tourkodimitris S: Escitalopram in the treatment of adolescent depression: A randomized placebo-controlled multisite trial. J Am Acad Child Adolesc Psychiatry 48:721–729, 2009 [DOI] [PubMed] [Google Scholar]
- Guy W: ECDEU assessment manual for psychopharmacology. Rockville, MD: National Institute of Mental Health, Psychopharmacology Research Branch; 1976 [Google Scholar]
- Hammad TA, Laughren T, Racoosin J: suicidality in pediatric patients treated with antidepressant drugs. Arch Gen Psychiatry 63:332–339, 2006 [DOI] [PubMed] [Google Scholar]
- Kenward MG, Roger JH: Small sample inference for fixed effects from restricted maximum likelihood. Biometrics 53:983–997, 1997 [PubMed] [Google Scholar]
- Lieberman JA, Greenhouse J, Hamer RM, Krishnan KR, Nemeroff CB, Sheehan DV, Thase ME, Keller MB: Comparing the effects of antidepressants: Consensus guidelines for evaluating quantitative reviews of antidepressant efficacy. Neuropsychopharmacology 30:445–60, 2005 [DOI] [PubMed] [Google Scholar]
- March J, Silva S, Petrycki S, Curry J, Wells K, Fairbank J, Burns B, Domino M, McNulty S, Vitiello B, Severe J: Fluoxetine, cognitive-behavioral therapy, and their combination for adolescents with depression: Treatment for Adolescents With Depression Study (TADS) randomized controlled trial. JAMA 292:807–820, 2004 [DOI] [PubMed] [Google Scholar]
- National High Blood Pressure Education Program Working Group on Hypertension Control in Children and Adolescents: Update on the 1996 task force report on high blood pressure in children and adolescents: A working group report from the National High Blood Pressure Education Program. Pediatrics 114:555–576, 2004 [PubMed] [Google Scholar]
- National Research Council: The Prevention and Treatment of Missing Data in Clinical Trials. Panel on Handling Missing Data in Clinical Trials. Committee on National Statistics, Division of Behavioral and Social Sciences and Education. Washington, DC: The National Academies Press; 2010 [Google Scholar]
- Posner K, Brown GK, Stanley B, Brent DA, Yershova KV, Oquendo MA, Currier GW, Melvin GA, Greenhill L, Shen S, Mann JJ: The Columbia-Suicide Severity Rating Scale: Initial validity and internal consistency findings from three multisite studies with adolescents and adults. Am J Psychiatry 168:1266–1277, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner K, Oquendo MA, Gould M, Stanley B, Davies M: Columbia Classification Algorithm of Suicide Assessment (C-CASA): Classification of suicidal events in the FDA's pediatric suicidal risk analysis of antidepressants. Am J Psychiatry 164:1035–1043, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poznanski EO, Cook SC, Carroll BJ: A depression rating scale for children. Pediatrics 164:442–450, 1979 [PubMed] [Google Scholar]
- Poznanski EO, Grossman JA, Buchsbaum Y, Banegas M, Freeman L, Gibbons R: Preliminary studies of the reliability and validity of the Children's Depression Rating Scale. J Am Acad Child Psychiatry 23:191–197, 1984 [DOI] [PubMed] [Google Scholar]
- Prakash A, Lobo E, Kratochvil CJ, Tamura RN, Pangallo BA, Bullok KE, Quinlan T, Emslie GJ, March JS: An open-label safety and pharmacokinetics study of duloxetine in pediatric patients with major depression. J Child Adolesc Psychopharmacol 22:48–55, 2012 [DOI] [PubMed] [Google Scholar]
- Pritchett YL, Marciniak MD, Corey–Lisle PK, Berzon RA, Desaiah D, Detke MJ: Use of effect size to determine optimal dose of duloxetine in major depressive disorder. J Psychiatr Res 41:311–318, 2007 [DOI] [PubMed] [Google Scholar]
- Rutherford BR, Roose SP: A model of placebo response in antidepressant clinical trials. Am J Psychiatry 170:723–733, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan DV, Sheehan KH, Shytle RD, Janavs J, Bannon Y, Rogers JE, Milo KM, Stock SL, Wilkinson B: Reliability and validity of the Mini International Neuropsychiatric interview for Children and Adolescents (MINI-KID). J Clin Psychiatry 71:313–326, 2010 [DOI] [PubMed] [Google Scholar]
- Wagner KD, Jonas J, Findling RL, Ventura D, Saikali K: A double-blind, randomized, placebo-controlled trial of escitalopram in the treatment of pediatric depression. J Am Acad Child Adolesc Psychiatry 45:280–288, 2006 [DOI] [PubMed] [Google Scholar]