Abstract
Significance: Hepatic fibrosis is the common pathophysiologic process resulting from chronic liver injury, characterized by the accumulation of an excessive extracellular matrix. Multiple lines of evidence indicate that oxidative stress plays a pivotal role in the pathogenesis of liver fibrosis. Nicotinamide adenine dinucleotide phosphate (NADPH) oxidase (NOX) is a multicomponent enzyme complex that generates reactive oxygen species (ROS) in response to a wide range of stimuli. In addition to phagocytic NOX2, there are six nonphagocytic NOX proteins. Recent Advances: In the liver, NOX is functionally expressed both in the phagocytic form and in the nonphagocytic form. NOX-derived ROS contributes to various kinds of liver disease caused by alcohol, hepatitis C virus, and toxic bile acids. Recent evidence indicates that both phagocytic NOX2 and nonphagocytic NOX isoforms, including NOX1 and NOX4, mediate distinct profibrogenic actions in hepatic stellate cells, the main fibrogenic cell type in the liver. The critical role of NOX in hepatic fibrogenesis provides a rationale to assess pharmacological NOX inhibitors that treat hepatic fibrosis in patients with chronic liver disease. Critical Issues: Although there is compelling evidence indicating a crucial role for NOX-mediated ROS generation in hepatic fibrogenesis, little is known about the expression, subcellular localization, regulation, and redox signaling of NOX isoforms in specific cell types in the liver. Moreover, the exact mechanism of NOX-mediated fibrogenic signaling is still largely unknown. Future Directions: A better understanding through further research about NOX-mediated fibrogenic signaling may enable the development of novel anti-fibrotic therapy using NOX inhibition strategy. Antioxid. Redox Signal. 20, 2854–2872.
Introduction
Liver fibrosis is the accumulation of extracellular matrix (ECM) proteins, mainly type I collagen, which occurs in most types of chronic liver disease. The main causes of liver fibrosis in industrialized countries include chronic hepatitis C virus (HCV) or hepatitis B virus (HBV) infection, alcohol abuse, and nonalcoholic steatohepatitis (NASH) (14). The accumulation of ECM proteins distorts the hepatic architecture by forming a fibrous scar, and the subsequent development of nodules of regenerating hepatocytes defines cirrhosis (70). Major complications of cirrhosis are portal hypertension and liver failure. Portal hypertension can lead to serious complications such as variceal bleeding, ascites, spontaneous bacterial peritonitis, and hepatic encephalopathy that are the major causes of mortality in cirrhotic patients. Cirrhosis also leads to the development of hepatocellular carcinoma (HCC) (204).
Liver fibrosis is a model of the wound-healing response to chronic liver injury. Hepatic stellate cells (HSCs), formerly known as lipocytes, Ito cells, or perisinusoidal cells, have been identified as the main collagen-producing cells in the liver (72). This cell type undergoes a dramatic phenotypic change in chronic liver diseases with the acquisition of fibrogenic properties. Quiescent HSCs are desmin-positive, perisinusoidal cells that are the primary cells in the body which are responsible for vitamin A storage (14). On activation by liver injury, quiescent HSCs transdifferentiate into myofibroblasts that produce inflammatory cytokines and several ECM proteins, including at least five collagen types, fibronectin, undulin, elastin, laminin, entactin, tenascin, and several proteoglycans (71). The molecular mechanisms leading to HSCs activation and increased ECM synthesis in liver fibrosis have been extensively investigated by using cultured HSCs and experimental models of chronic liver injury in rodents (14, 70, 153, 161). Besides HSCs, portal fibroblasts and cells of bone marrow origin have fibrogenic potential (106, 155, 167). Recent knowledge indicates that most fibrogenic myofibroblasts are endogenous to the liver, and activated HSCs and fibroblasts are the major endogenous fibrogenic cells in the liver (26).
The demonstration that even advanced liver fibrosis may be reversible has stimulated researchers to investigate anti-fibrotic therapies (83, 111). The only effective, available therapy to treat hepatic fibrosis to date is to remove the causative agent (13). For example, long-term administration of oral nucleos(t)ide analogues demonstrate the regression of liver fibrosis in patients with advanced liver fibrosis or even cirrhosis caused by chronic HBV infection (38, 126). Similarly, peginterferon α significantly reduces hepatic fibrosis in patients with chronic hepatitis C with or without cirrhosis in whom sustained virologic response occurs (32). However, a number of drugs are able to reduce liver fibrosis in experimental models without affecting the etiological agent causing the injury. Several candidate drugs, including antioxidants and renin-angiotensin system (RAS) blockers, have been evaluated for their anti-fibrotic efficacy and safety; however, evidence-based therapies are not yet available (17).
Reactive oxygen species (ROS) are involved as key secondary messengers in numerous signaling pathways, including transcriptional regulation, differentiation, proliferation, oncogenic transformation, and activation of programmed cell death (28). ROS contributes to the hepatic fibrosis from various kinds of liver injuries, including alcohol abuse, HCV infection, iron overload, and chronic cholestasis (150, 154). ROS can stimulate the production of the type I collagen, and may act as an intracellular signaling mediator of the fibrogenic action of TGF-β (56, 124, 136). ROS may be generated in the liver by multiple sources, including the mitochondrial respiratory chain, cytochrome P450 (CYP) family members, peroxisomes, xanthine oxidase, and nicotinamide adenine dinucleotide phosphate (NADPH) oxidases (55, 150). Accumulating evidence indicates that NADPH oxidases (NOXs)-mediated ROS has a critical role in HSCs activation and hepatic fibrogenesis. This review summarizes the current knowledge on the profibrogenic mechanisms of various NOX isoforms expressed in specific cell types in the liver, with particular focus on HSCs and discusses NOX isoforms as potential therapeutic targets for hepatic fibrosis.
NADPH Oxidases
NOX is a multimeric transmembrane enzyme complex that generates superoxide (O2•−) and hydrogen peroxide (H2O2) from molecular oxygen using NADPH as an electron donor (21). The mammalian NOX family comprises seven isoforms: NOX1, NOX2, NOX3, NOX4, NOX5, DUOX1, and DUOX2 (Table 1) (21, 113). Classic NOX is the phagocytic NOX found in neutrophils. The phagocytic NOX contains a heterodymeric membrane-bound flavocytochrome b558 complex, which consists of the catalytic subunit gp91phox (renamed NOX2) and the regulatory subunit p22phox located in the membrane (21, 162). Rac is a component required for the activation of NOX2. Rac is a member of the Rho family of small GTPase proteins that regulates cell proliferation and dynamic reorganization of the actin cytoskeleton (44). The NOX2 regulatory components, p47phox, p40phox, p67phox, and Rac, are usually located in the cytoplasm (21). On stimulation with agonists, the cytosolic subunits translocate to the membrane-bound flavocytochrome complex, leading to enzymatic activity.
Table 1.
The Characteristics of Selected NOX Isoforms
| Catalytic subunit | Components | Major tissue/cell distribution | Major agonists | Subcellular localization |
|---|---|---|---|---|
| NOX1 | NOX1, p22phox, NOXO1/p47phox, NOXA1/p67phox, Rac1 | Colon, vessel, heart, uterus, prostate, liver | Ang II, TNF-α, IL-1β, LPS, PDGF/PGF2α, EGF, bFGF IFN-γ, ET-1 | Plasma membrane, caveolae, perinuclear endosome |
| NOX2 | NOX2, p22phox, p40phox, p47phox, p67pho, Rac1/Rac2 | Neutrophil, phagocyte, vessel, heart, liver | LPS, IFN-γ, Ang II | Plasma membrane, perinuclear, nucleus |
| NOX3 | NOX3, p22phox, NOXO1/p47phox, NOXA1/p67phox, Rac1 | Fetal tissue, inner ear | Plasma membrane | |
| NOX4 | NOX4, p22phox, POLDIP2 | Kidney, vessel, heart, lung, liver | TGF-β, TNF-α, IFN-γ | Endoplasmic reticulum, focal adhesion |
| NOX5 | NOX5 | Testis, spleen, lymph node, heart, vessel | Ang II, ET-1, TNF-α | Plasma membrane |
| DUOX1/DUOX2 | DUOX1/DUOX2 | Thyroid tissue |
Ang II, angiotensin II; EGF, epidermal growth factor; NOX, NADPH oxidase; IFN, interferon.
NOX2-derived ROS acts in immune defense in neutrophils and phagocytes (21). The genetic defect of phagocytic NOX (NOX2) activity called chronic granulomatous disease (CGD) results in an inability to produce the superoxide anion that is necessary for killing bacteria and fungi by neutrophils and phagocytes. Patients with CGD are predisposed to serious bacterial and fungal infections as well as granulomatous complications (170). In contrast with CGD that are defective in NOX-mediated ROS generation, hereditary amyotrophic lateral sclerosis (ALS), a progressive motor neuron degenerative disease, may result from excessive ROS production by an overactive NOX (36).
Compared with NOX2 that is expressed in phagocytes, the nonphagocytic NOXs are expressed at low levels in complex tissue compartments composed of multiple cell types and different NOX isoforms (135). Among the seven isoforms of the NOX family, NOX1, NOX2, NOX3, and NOX4 form heterodimers with p22phox, whereas NOX5, DUOX1, and DUOX2 function without an association with p22phox. Instead, NOX5, DUOX1, and DUOX2 contain EF-hand calcium-binding motif (135). While NOX1 is also p22phox dependent, NOX1 may use NOXO1 (homologue of p47phox, an organizing cofactor) to organize the enzyme assembly and NOXA1 (homologue of p67phox, an activating cofactor) for enzyme activation (12). In many cases, neither NOXO1 nor NOXA1 has been identified in tissues where the implicated NOX protein will require these co-factors for activity (135). In contrast to other NOX isoforms, NOX4 requires only p22phox without recruitment of cytosolic regulatory components to exert its enzymatic activity (21, 76). All NOX enzymes are able to catalyze the reduction of molecular oxygen to superoxide, but there are key differences in their expression, subcellular localization, activation, and intracellular signaling.
Cellular distribution of NOXs in the liver
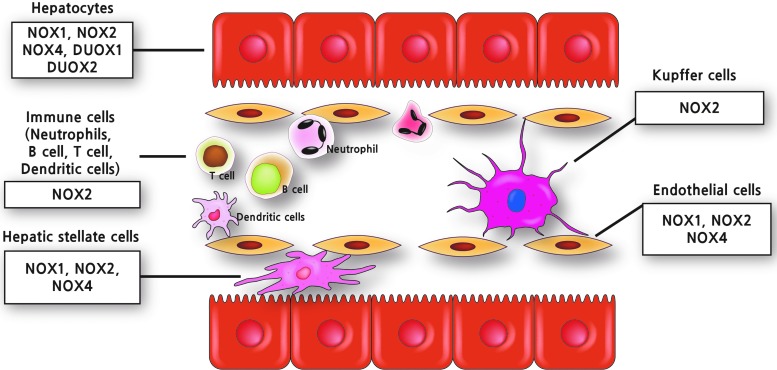
In the liver, NOX is functionally expressed both in the phagocytic form and in the nonphagocytic form (18, 192). Various NOX isoforms such as NOX1, NOX2, and NOX4 are distinctively expressed in the specific cell types, including Kupffer cells (KCs), HSCs, endothelial cells (ECs), hepatocytes, and infiltrating leukocytes in the liver (Fig. 1) (59, 143). KCs, the resident macrophage in the liver, express phagocytic NOX2 and its regulatory components such as p40phox, p47phox, and p67phox. There is no detectable mRNA expression for other NOX forms such as NOX1, NOX4 in KCs (143). In HSCs, both phagocytic NOX2 and nonphagocytic NOXs such as NOX1 and NOX4 are expressed (143). In ECs, NOX1, NOX2, and NOX4 are expressed (99, 134, 143). NOXO1 and NOXA1 are predominant regulatory components expressed in ECs and HSCs (143). Hepatocytes express mRNAs for NOX1, NOX2, NOX4, DUOX1, and DUOX2 as well as regulatory subunit p47phox (35, 60, 143, 157).
FIG. 1.
Cellular distribution of NOX isoforms in the liver. Various kinds of cell types in the liver, including hepatocytes, KCs, ECs, HSCs, and infiltrating leukocytes express multiple NOX isoforms, including both phagocytic and nonphagocytic NOX. ECs, endothelial cells; HSCs, hepatic stellate cells; KCs, Kupffer cells; NOX, NADPH oxidase. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
Subcellular localization of NOX characterizes signal transduction
NOX-generated superoxide anion has a short half life and is rapidly dismutated to H2O2 at a rate constant of 8×104 M−1s−1 spontaneously and 2×109 M−1s−1 by superoxide dismutase (SOD) (69). H2O2 is more stable than superoxide and is able to cross the cell membrane. ROS can react with nearby cellular proteins reversibly (40, 193). These properties of diffusibility, short half life, and reversible reactivity make ROS function as signal messengers. Complexity of NOX signaling arises from the multiple NOX isoforms that are expressed within the same cell and mediate distinctive signaling pathways in response to diverse stimuli. The specific subcellular localization of NOX isoforms and their regulatory subunits within specific subcellular compartments may modulate the specificity of ROS-mediated signal transduction (152, 186).
NOX2 is predominantly expressed in the plasma membrane. Stimulation of ECs with Fas ligand or TNF-α induces translocation of NOX2, p47phox, and Rac1 to the lamellipodial leading edge of the cell, where the focal complexes undergo rapid turnover and ROS production promotes cytoskeleton rearrangement for directional cell migration (94, 133, 194, 196). NOX2 also targets to phagosomes in neutrophils and to endosomes in nonphagocytic cells, dubbed as “redoxosomes” (119, 138, 139). Redoxosomal NOX2 transduces several stimuli, such as angiopoietin-1, vascular endothelial growth factor, thrombin, and TNF-α, into necrosis and angiogenesis via ROS production (63, 125, 187). p22phox is essential for optimal activity of NOX2. In myeloid cells, coexpression and heterodimerization of NOX2 and p22phox are required for the migration from the endoplasmic reticulum (ER) and proper localization in the plasma membrane (61). A mouse model of hepatic fibrosis shows that NOX2 level is elevated in activated HSCs (98, 143). However, the subcellular localization of NOX2 in HSCs has not been identified.
NOX1 has been found at the plasma membrane, in early endosomes, and in the nucleus in vascular smooth muscle cells (VSMCs) (92, 131). Recently, it was reported that NOX1 is also found in caveolin 1-containing lipid rafts in VSMCs (92, 186, 206). Binding of angiotensin II (Ang II) stimulates the angiotensin type I receptor (AT1R) to translocate to caveola, where Rac1 is also recruited to locally produce ROS with subsequent activation of epidermal growth factor receptor (EGFR). NOX1 is also involved in endosomal ROS production after hypoxia-reoxygenation injury, which requires both Rac-1 and c-Src recruitment (120). In addition, chloride channel 3 is co-localized for TNF-α-induced NOX1-mediated ROS production, leading to NF-κB activation, at early endosomes in vascular cells (131). In Huh7 cells, a human hepatoma cell line, NOX1 predominantly localizes in the cytoplasm, with apparent punctuate staining at the plasma membrane, even though the identity of these punctuate stains was not specifically addressed (60). Although NOX1 is expressed in HSCs, ECs, and hepatocytes in the liver (143, 157), its subcellular localization in these cells has not yet been investigated.
NOX4 is ubiquitously expressed and inducible (27). Unlike other NOX, NOX4 does not require regulatory cellular binding partners and is constitutively active. However, p22phox is required for the enzymatic activity (5, 21, 39, 123). NOX4 is not only mainly localized in the perinuclear, ER area, but it is also detected at the plasma membrane, at focal adhesions, and within the nucleus (21). NOX4 is localized to the ER in VSMCs and ECs (41, 89, 195), at both ER and nucleus in renal cortical cells (151, 164), in the nucleus in monocytes and ECs, at the plasma membrane of lung epithelial cells (188, 189), and only in mitochondria in breast tumors and adipose-derived stem cells (78, 103). There are several possibilities for these conflicting reports for NOX4 localization. Different cell types with various functions might require local ROS production at various subcellular locations. It is also possible that NOX4 localization might be transient; different stimulation might lead NOX4 to translocate from one place to others for the necessary local ROS. Indeed, HCV infection in a hepatocyte cell line increases NOX4 expression level and also increases nuclear localization (60). In experiments with primary VSMCs, NOX4 relocates from actin-based stress fibers to focal adhesions on de-differentiation (47). NOX4 has several splicing variants, which might determine localization: In the A549 lung cell line, some splicing variants are detected in the ER and others are detected at the plasma membrane (77). Alternatively, different antibodies might recognize different isoforms of the NOX4. For example, two antibodies discovered through phage-display screening share similar epitopes and neutralize NOX4 enzymatic activity, but only one antibody detects NOX4 at the plasma membrane (205).
Role of NOX-Derived ROS in Liver Diseases
Oxidative stress contributes to a variety of liver diseases, including alcohol abuse, acetaminophen, HCV, iron overload, chronic cholestasis, and ischemia-reperfusion (55). Small amounts of ROS are usually released by the mitochondrial respiratory chain in the liver. Liver injury can develop when excessive amounts of ROS are generated from multiple potential sources, including the mitochondrial respiratory chain, CYP family members, peroxisomes, xanthine oxidase, and NOX (55, 150). It has been reported that NOX-mediated ROS contributes to various liver diseases, including alcoholic hepatitis (107, 109), chronic hepatitis C (24, 29, 60, 183), cholestatic liver injury (157, 158, 177, 180), and hepatic fibrosis (16, 18, 58, 59, 98, 143).
The phagocytic NOX2 releases large amounts of superoxide anion in bursts in response to agonists, exhibiting no detectable constitutive activity. While the nonphagocytic NOX produce low levels of superoxide intracellularly in the basal state, which can be further stimulated by various agonists (68). NOX generates ROS in response to a wide range of stimuli, including TNF-α, IL-1β, Ang II, and leptin (42, 57). All NOX isoforms generate superoxide from molecular oxygen, and superoxide is converted to H2O2 by SOD. However, the kind of ROS may vary according to specific NOX isoforms that are expressed in specific cells. For example, NOX4 generates predominantly H2O2 instead of superoxide (128, 173). ROS derived from specific NOX isoforms in specific cell types in the liver may serve as diverse signaling function by inducing specific biochemical changes in their molecular targets.
Alcoholic hepatitis
There is strong evidence that ROS play a pivotal role in alcohol-induced liver injury. Ethanol-induced oxidative stress results in a hepatocellular injury by promoting hepatocyte apoptosis or necrosis and also contributes to hepatic fibrosis by triggering the release of profibrogenic cytokines and HSCs activation (3). Ethanol induces ROS production by several enzymatic systems, including the CYP2E microsomal monooxygenase system, mitochondrial respiratory chain, and NOX (9). Both intracellular and extracellular sources of ROS contribute to alcohol-induced oxidative stress (3). Oxidative DNA damage as an index of intracellular oxidative stress is decreased in CYP2E1-deficient mice but not in p47phox-deficient mice (25). However, CYP2E1-deficient mice are not protected from early alcohol-induced liver injury (107). Four-week intragastric alcohol administration to p47phox-deficient mice results in less hepatic-free radical production and liver pathology compared with wild-type (WT) mice (109). KCs highly express phagocytic NOX2 and generate ROS during early ethanol-induced liver injury (192). Collectively, these data indicate that ROS from NOX in hepatic KCs plays a critical role in the pathogenesis of early alcohol-induced hepatitis.
The important role of NOX in the pathogenesis of alcoholic liver disease is corroborated by microarray analysis of hepatic gene expression in patients with alcoholic hepatitis (48). As expected, the expression of genes encoding ECMs such as procollagen α1(I), α4(I), fibrogenesis mediators, and inflammatory cytokines are markedly elevated in human livers with alcoholic hepatitis. In addition, various NOX components, including DUOX2 (23-fold), NOX4 (12.7-fold), DUOX1 (6.7-fold), p22phox (2.0-fold), and Rac1 (4.1-fold), are upregulated in the livers with alcoholic hepatitis compared with normal livers (48). An important finding to note is that nonphagocytic NOX components (NOX4, DUOX1, and DUOX2) are barely detected in normal livers but are de novo expressed in alcoholic hepatitis (48). Expression of NOX2, NOXO1, NOXA1 are unchanged and other NOX isoforms such as NOX1, NOX3, and NOX5 are undetected. By contrast, another important ROS generator CYP2E1 is down-regulated in the livers with alcoholic hepatitis (48). Taken together, these results suggest that nonphagocytic NOX isoforms such as NOX4 and DUOXs as well as phagocytic NOX2 mediate alcohol-induced liver injury.
HCV-induced hepatocellular damage
HCV is the leading cause of mortality from chronic liver disease, including cirrhosis and HCC in many countries including Japan, Southern Europe, and the United States (66). Accumulating evidence suggests that ROS play an important role in the development and progression of chronic hepatitis C (43). HCV is a 9.6-kb positive strand RNA virus consisting of 10 genes that encode 4 structural and 6 nonstructural proteins (24). Among 10 HCV proteins, the core, NS3, and NS5a protein are associated with increased oxidative stress (16, 29, 74, 141). Recombinant NS3 protein phosphorylates p47phox and activates NOX to produce ROS, mainly superoxide, in human monocytes (29). A strong HCV-specific T-cell response, especially CD4+ T-cell response, is critical for the clearance of HCV in patients with acute HCV infection (101, 182). NS3 protein induces a prolonged release of ROS via NOX2 in mononuclear and polymorphonuclear phagocytes, which, in turn, induces dysfunction and/or apoptosis of lymphocytes of relevance (183).
Expression of HCV proteins in hepatocytes results in increased ROS generation, steatosis, and cell transformation developing liver cancer (118, 141). HCV NS5A and core protein induce ROS and reactive nitrogen species in a human hepatocyte-derived cell line (74). Expression of HCV (genotype Ia) cDNA constructs, core protein, viral RNA, or replicating HCV (JFH-AM2) induces NOX4 mRNA expression and ROS production in human hepatocytes cell lines (24). Dominant-negative NOX4 or knockdown of NOX4 using NOX4 short hairpin RNA decreases HCV-induced superoxide production in a human hepatocyte cell line. Full-length HCV as well as structural and nonstructural HCV constructs increases ROS generation in an NOX4-dependent manner. Human NOX4 promoter/reporter assay demonstrates that HCV positively regulates both human and murine NOX4 at the transcriptional level (24). TGF-β1 is a major fibrogenic cytokine that activates HSCs. TGF-β induces NOX4 in various cell types, including HSCs, hepatocytes, cardiac fibroblasts, and VSMCs (35, 53, 97, 163, 179). HCV core protein up-regulates TGF-β mRNA in HepG2 cells, and treatment of cells with TGF-β blocking antibody prevents the HCV core-mediated induction of NOX4 and reduces superoxide production (24). Taken together, these data indicate that HCV induces NOX4-mediated ROS generation via an autocrine TGF-β dependent mechanism (24).
Huh7 cells transfected with virus producing JFH1 HCV RNA induces extracellular H2O2 secretion and intracellular superoxide production (60). Both genotype 2a and Ib HCV induce NOX1 and its regulators such as NOXO1 and NOXA1 as well as NOX4 in the Huh7 human hepatocyte cell line (60). Furthermore, the increased expression of NOX1 and NOX4 proteins is demonstrated in HCV-infected human liver (60). Knockdown of either NOX1 or NOX4 using siRNA significantly decreases H2O2 and intracellular superoxide production in a hepatocyte cell line producing JFH1 HCV RNA (60). Interestingly, subcellular localization of NOX1 and NOX4 varies. NOX1 is predominantly located in the cytoplasm, and this location does not change significantly with HCV expression in Huh7 cells. While NOX4 is localized in the cytoplasm as well as in the nucleus in control Huh7 cells, HCV increases localization of NOX4 in the ER and nuclear membrane (60). Further investigation is required to understand the distinct contributory roles of NOX1 and NOX4 in HCV-induced hepatocyte injury that may result from different compartmentalization and signaling.
HCV core and NS3 proteins induce a marked increase in ROS production in human HSCs (16). These effects are attenuated by the nonspecific NOX inhibitor diphenylene iodonium (DPI). HCV core protein increases HSC proliferation in an Ras/extracellular signal-regulated kinase (ERK) and PI3K/protein kinase B (Akt)-dependent manner. In contrast, HCV NS3-NS5 protein preferentially induces proinflammatory actions such as chemokine secretion and expression of cell adhesion molecules through NF-κB and c-Jun-N-terminal kinase (JNK) pathways. Both HCV core and NS3–NS5 proteins increase TGF-β1 secretion and type I collagen expression in HSCs (16). Therefore, HCV induces liver inflammation and fibrosis, at least in part, via NOX-generated ROS. The NOX isoforms that are involved in HCV-induced fibrogenic responses in HSCs are not yet identified.
Hydrophobic bile salts-induced liver injury
Hydrophobic bile salts can induce hepatocytes apoptosis through a ligand-independent activation of the CD95 death receptor (132, 176). Taurolithocholate-3-sulfate, a hydrophobic proapoptotic bile salt, stimulates ceramide formation through chloride-dependent acidification of endosomes, with subsequent activation of acidic sphingomyelinase, which, in turn, activates protein kinase C (PKC)ζ (19). Activated PKCζ rapidly induces serine phosphorylation of p47phox, which is followed by ROS generation in hepatocytes (158). NOX-derived ROS activates JNK and an Src family kinase Yes, which then associates with and activates EGFR, followed by EGFR-catalyzed CD95 tyrosine phosphorylation, formation of the death-inducing signaling complex, and apoptosis of hepatocytes (157, 158).
Bile acids also activate EGFR in activated HSCs. In contrast to hepatocytes, bile acid-induced EGFR activation in HSCs does not induce apoptosis, but leads to cell proliferation (180). Hydrophobic bile acids induce phosphorylation of p47phox and ROS generation followed by a Yes-mediated EGFR activation, which subsequently phosphorylates ERK1/2 but not JNK and stimulates cell proliferation in quiescent HSCs (177). Treatment of cycloheximide or H2O2 in addition to hydrophobic bile acid was required for the activation of JNK signal and for the shift from proliferation to apoptosis in HSCs (177).
NOX-Derived ROS Play a Key Role in Hepatic Fibrogenesis
Liver fibrosis is a common pathway resulting from chronic liver injury. Although the pathogenesis of liver fibrosis varies according to etiology of the injury, oxidative stress seems to play a crucial role in most types of hepatic fibrogenesis (14). ROS has diverse effects with regard to different kinds, concentrations, and cell types. ROS tend to be compartmentalized in the cell and form a concentration gradient that originates from their sites of generation (20). H2O2 can diffuse across plasma membrane and throughout the cell; however, superoxide diffuses poorly across cell membranes (30). High concentrations of ROS can be cytotoxic, whereas low concentrations of ROS may serve as second messengers during cellular responses to a variety of physiologic stimuli. A low concentration of H2O2 has mitogenic effects and can mimic the function of growth factors (159). Regarding the effects of ROS on HSCs, contradictory results have been reported: both mitogenic and cell-death inducing properties. Nontoxic levels of ROS or lipid peroxidation products stimulate the activation, proliferation, and collagen production of HSCs, but high concentrations of ROS induce HSCs death (117, 136, 137).
Expression of various NOX isoforms is increased in patients with heart (91), lung (4), kidney (64), and liver fibrosis (48, 60). Moreover, NOX-mediated ROS is required for experimental animal models of fibrosis in various organs, including heart (53), lung (87), kidney (169), and liver (143). In HSCs, NOX mediates the fibrogenic responses in response to various agonists, including Ang II (18), PDGF (2), leptin (58), TGF-β (97), phagocytosis of apoptotic body (203), and advanced glycation end products (81). Taken together, NOX-derived ROS is a key mediator in hepatic fibrogenesis as well as in other organs such as the heart, lung, and kidney.
Upregulation of NOX components in activated HSCs
HSCs, the major fibrogenic cell type in the liver, express multiple NOX isoforms and their regulatory components. Analysis of mRNA expression of NOX components in isolated liver cell fractions from WT C57BL/6 mice demonstrated that WT HSCs express the mRNAs for both phagocytic NOX2 and nonphagocytic NOX, including NOX1 and NOX4. HSCs also express other NOX regulatory components, including p22phox, p40phox, p47phox, p67phox, NOXO1, NOXA1, and Rac1 (143). These NOX components increase when quiescent HSCs activate into myofibroblasts. After bile duct ligation (BDL)-induced fibrosis, the mRNA expression of p47phox is strongly upregulated in HSCs to a similar degree as in KCs, whereas less upregulation occurs in other cell types such as ECs, hepatocytes in the liver (59). The mRNAs for both phagocytic and nonphagocytic NOX catalytic subunits, including NOX1, NOX2, and NOX4, are upregulated in activated HSCs compared with quiescent HSCs (7, 143).
In human quiescent HSCs, the mRNA for the cytosolic regulatory factor p47phox and the catalytic subunits NOX2 and NOX1 at cell membrane are detected at low levels, while they are highly upregulated in culture-activated HSCs and in vivo-activated HSCs isolated from patients with liver fibrosis (18). Although NOX plays an important role in HSCs activation, the exact structure of this complex is still unknown. All components and functionalities of the phagocytic NOX are already well established, but the interactions between the nonphagocytic NOX components in HSCs are unknown.
Contribution of various NOX isoforms in hepatic fibrogenesis
Expression of both NOX2 and NOX1 proteins is increased in the fibrotic liver in mice (143). BDL-induced liver fibrosis is attenuated in p47phox-deficient mice compared with WT mice (18). Although the degree of protection was less, metabolically induced hepatic fibrosis by the methionine choline-deficient diet is also reduced in p47phox-deficient mice (59). Direct evidence of the contribution of NOX2 in hepatic fibrogenesis is demonstrated by the attenuated hepatic fibrosis in NOX2-deficient mice compared with WT mice after CCl4 treatment or BDL (8, 98). BDL-induced hepatic fibrosis is attenuated in NOX2-deficient mice regardless of eradication of NOX2 expressing KCs by GdCl3, demonstrating that NOX2 activity in HSCs contributes to hepatic fibrosis (98). Furthermore, NOX2 is involved in the activation of HSCs by phagocytosis of apoptotic hepatocytes (33, 203). Phagocytosis of apoptotic hepatocytes induces HSC activation and collagen production via NOX2, and NOX2-derived ROS induces collagen α1 promoter activity in HSCs (98). In addition, impaired translocation of Rac1 to the cell membrane is observed in NOX2-deficient HSCs (98). Collectively, these data indicate that phagocytic NOX2 is an important mediator of HSCs activation and hepatic fibrosis.
It is important to note that nonphagocytic NOXs, in addition to phagocytic NOX2, contributes to hepatic fibrogenesis. Mice deficient for either NOX1 or NOX2 display significantly less hepatic fibrosis compared with WT mice after CCl4 injection or BDL (143). Experiments of hepatic fibrosis induction in NOX1 or NOX2 bone marrow chimeric mice demonstrated that both NOX1 and NOX2 mediate hepatic fibrosis in endogenous liver cells, including HSCs, whereas NOX2 in KCs has a lesser contribution (143). Moreover, Ang II-induced ROS generation is more severely attenuated in NOX1-deficient HSCs than in NOX2-deficient HSCs. The contribution of NOX1 in hepatic fibrogenesis was corroborated by Cui et al. (54). Cell proliferation is attenuated in HSCs isolated from NOX1-deficient mice. NOX1-derived ROS oxidize and inactivate phosphatase and tensin homologue (PTEN), which, in turn, phosphorylates Akt and forkhead box O 4, resulting in down-regulation of p27kip1, a cell cycle suppressor, to promote HSCs proliferation (54).
SOD1 is an enzyme that converts superoxide to H2O2. SOD1 also interacts with Rac1 in the active NOX complex to stimulate NOX activity. Specifically, SOD1 binds to Rac1 and stabilizes the GTP-bound active form of Rac1 (85). Mutations in SOD1, such as G93A and G37R, that are associated with hereditary ALS enhance Rac1 activation and NOX-dependent superoxide production in the spinal cord as well as in the liver (85, 129). Hepatic ROS production and Rac1 activity are increased in SOD1 G37R-mutant mice. CCl4-induced liver fibrosis is more pronounced in SOD1 G37R-mutant mice compared with WT mice (7).
GKT137831 is a potent dual NOX1/NOX4 inhibitor with an affinity similar to the irreversible, unspecific flavocytochrome inhibitor, DPI. GKT137831 has 15-fold less potency in NOX2 and 3-fold less potency in NOX5, and it has no significant inhibition in the NOX2-driven oxidative burst in neutrophils (7, 112). Inhibition of NOX1 and NOX4 by GKT137831 attenuates both CCl4-induced and BDL-induced ROS production and hepatic fibrosis in WT and SOD1 G37R-mutant mice, as well as mRNA expression of fibrogenic and NOX genes (7). GKT137831 inhibits collagen α1(I) promoter activity and ROS production induced by Ang II in WT and SOD1 G37R-mutant HSCs. The specific contribution of NOX4 in hepatic fibrogenesis is demonstrated by the attenuation of hepatic fibrosis in NOX4-deficient mice compared with WT mice (97). Taken together, nonphagocytic NOX, including NOX1 and NOX4 as well as phagocytic NOX2, represent fundamental mediators in experimental hepatic fibrogenesis.
NOX-mediated redox signaling contributing to hepatic fibrosis
Angiotensin II
The RAS plays a key role in maintaining blood pressure homeostasis, as well as fluid and salt balance through coordinated effects on the heart, blood vessels, and kidneys (95). In the classic pathway of RAS, renin cleaves the liver-derived precursor peptide angiotensinogen into the decapeptide Ang I. Ang I is further hydrolyzed into the octapeptide Ang II by the angiotensin-converting enzyme (ACE), which represents the main bioactive peptide of the RAS. Angiotensinogen is the only component of the RAS that is expressed in the normal liver. However, RAS, including ACE and AT1R, are markedly upregulated in the fibrotic liver (17, 146). Inhibition of Ang II synthesis and/or the blockade of AT1R decreases hepatic inflammation and fibrosis in experimental models of chronic liver injury (52, 147, 191). Consistently CCl4- or BDL-induced hepatic fibrosis is reduced in AT1R-deficient mice compared with WT mice (100, 199). These data strongly indicate that RAS is an important mediator of hepatic fibrosis. Many of the biological effects of Ang II involve the NOX-mediated production of ROS (17, 80). Ang II is functionally linked to NOX1, NOX2, and variably to NOX4 in the vasculature, NOX2 and NOX4 in the kidney, and NOX2 in the brain (75). p47phox−/− mice were the first used as a genetic model to study NOX inhibition (73, 109, 114), and p47phox−/− mice show attenuated liver injury and fibrosis compared with WT counterparts after BDL (18).
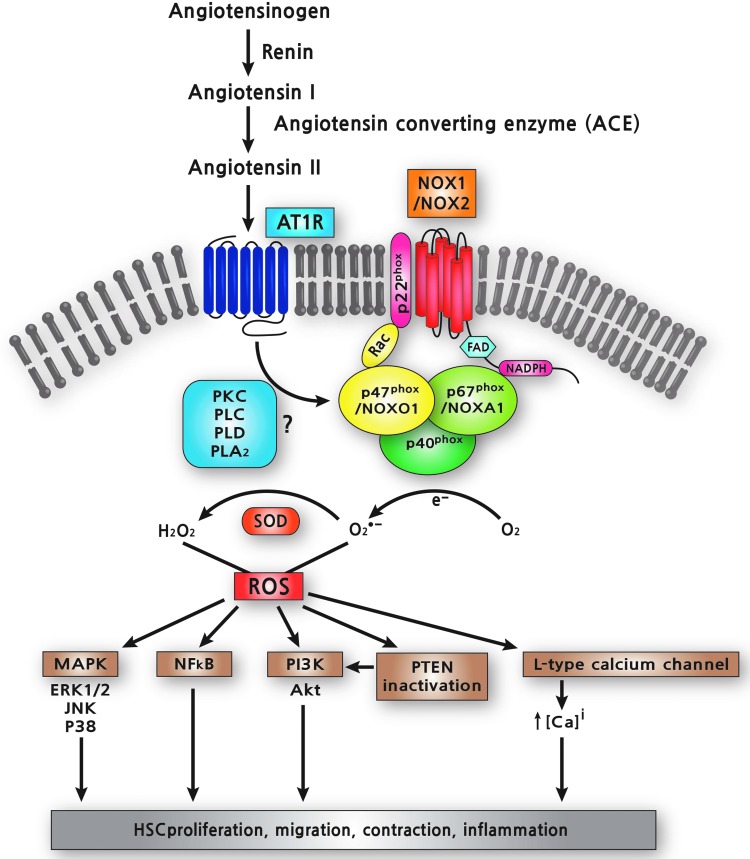
Activated HSCs highly express AT1R, which mediates cell proliferation, migration, and cell contraction (15). Stimulation of HSCs with Ang II results in TGF-β and type I collagen expression (18, 201). Ang II increases the intracellular calcium concentration and induces production of ROS, stimulating several key intracellular pathways, including PKC, PI3K/Akt, and MAPKs (18). NOX mediates the intracellular pathways induced by Ang II in HSCs (17, 18). Ang II-induced ROS formation in HSCs is inhibited by treatment with either an NOX inhibitor, DPI (108) or with the AT1R antagonist, losartan (52). Furthermore, Ang II induces the phosphorylation of p47phox through AT1R in activated HSCs. In particular, Ang II leads to an increase of intracellular ROS in HSCs isolated from WT mice, but not in p47phox−/− HSCs. In addition, Ang II induces DNA synthesis and cell migration in WT HSCs, as well as phosphorylation of ERK and c-Jun, while these effects are blunted in p47phox-deficient HSCs (18). Taken together, Ang II exerts direct profibrogenic actions on HSCs through the activation of the NOX complex and the subsequent production of ROS (Fig. 2).
FIG. 2.
Ang II/AT1R/NOX signaling in HSCs. Ang II binds to AT1R, which stimulates NOX-mediated ROS generation. The molecular mechanism of NOX activation by Ang II on HSCs is still poorly understood. Ang II may stimulate PKC-dependent NOX activity through three different PLs such as PLC, PLD, and PLA2. NOX-derived ROS activate MAPK, NF-κB, and PI3K, and increase intracellular calcium levels via L-type calcium channel in HSCs. ROS also inactivates PTEN, which, in turn, further activates PI3K/Akt pathway. As a result, NOX-derived ROS mediates cell proliferation, migration, contraction, and inflammatory response in HSCs. PLs, phospholipases; Akt, protein kinase B; Ang II, angiotensin II; AT1R, angiotensin type I receptor; PKC, protein kinase C; PTEN, phosphatase and tensin homologue; ROS, reactive oxygen species. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
The molecular mechanisms of NOX activation by Ang II are complex and still incompletely understood. In VSMCs, Ang II-stimulated ROS generation is biphasic (174). The first phase occurs rapidly (peak at 30 s) and is dependent on PKC activation, which phosphorylates p47phox, and then phosphorylated p47phox translocates to the membrane where it activates the NOX catalytic subunit NOX1 and/or NOX2. The larger second phase of ROS generation (peak at 30 min) requires Rac activation by upstream c-Src, EGFR transactivation, and PI3K activation (174). Ang II may stimulate PKC-dependent NOX activity through three different phospholipases (PLs), PLC, PLD, and PLA2 (75). The prolonged production of NOX-mediated ROS by Ang II requires upregulation of NOX catalytic subunits and components (184). Indeed, Ang II induces upregulation of both NOX1 and NOX4 mRNA in HSCs (7).
PDGF and leptin
PDGF, the most potent mitogen of HSCs (153), exerts its activity, in part, through NOX (2). PDGF-induced increase in DNA synthesis of HSC is reduced by pre-treatment with the anti-oxidant Mn-TBAP, and also with two different inhibitors of the NOX complex, DPI or apocynin. PDGF-induced HSCs proliferation is suppressed by an inhibitor of MEK or p38 MAPK, but the extent of suppression is greater with the p38 MAPK inhibitor (2). The experimental model of liver fibrosis induced in mice by parenteral DMN provides additional in vivo evidence for the role of NOX in liver fibrosis. The use of the NADPH inhibitor DPI or antioxidant drug Mn-TBAP reduces collagen deposition and liver fibrosis in DMN-treated mice (2). Similarly, PDGF increases NOX activity and DNA synthesis in pancreatic stellate cells (93). PDGF causes hypertrophy of VSMCs by induction of NOX1 through activation of PKCδ and activating transcription factor (ATF)-1 (67, 102). PDGF upregulates NOX1 in VSMCs, and an adenovirus expressing antisense NOX1 mRNA completely inhibits the early phase of superoxide production (115). A consensus activator protein (AP)-1 site was found at −98/−92 in the 5′-flanking region of the rat NOX1 gene (37). The NOX isoform that is responsible for PDGF-induced mitogenic signaling in HSCs is not yet identified. NOX is also a crucial mediator of the profibrogenic action of leptin (2, 58). Leptin-induced NOX acts downstream of Janus kinase activation but is independent of STAT3 (58). Thus, NOX is a core mediator of the fibrogenic signaling response in HSCs and demonstrates its potential as a pharmacological target for antifibrotic therapy.
Interaction of TGF-β/Smads signaling with NOX4
TGF-β1 induces the conversion of fibroblasts to α-SMA-expressing myofibroblasts in multiple organs, including the heart, lung, kidney, and liver (62, 84, 110). TGF-β1 is the strongest agonist for HSCs activation, and it is released from KCs and recruited macrophages with hepatic injury (14, 88). After being modified post-translationally, TGF-β1 binds to and activates TGF-β1 receptor (TGF-βR I and II), transmembrane receptor serine/threonine kinases (50, 175). Phosphorylation of the receptors is accompanied by phosphorylation of Smad2 and Smad3 that are then complexed with Smad4. Then, activated Smad complexes enter the nucleus and initiate the transcription of TGF-β1 target genes. Liver fibrosis is strongly reduced in TGF-β1-deficient and Smad3-deficient mice, while overexpression of TGF-β1 induces fibrosis in several organs, including the liver (14, 88, 116, 166, 185).
A study using NOX4-deficient HSCs shows that TGF-β1-mediated HSCs activation is dependent on NOX4-generating ROS (97). NOX4 siRNA inhibits ROS production in HSCs. Moreover, the activation of cultured HSCs is inhibited in NOX4-deficient HSCs compared with WT HSCs (97). TGF-β1 increases the level of NOX4 mRNA and protein in an Smad3-dependent manner, thereby increasing NOX4-derived ROS (87, 97). NOX4 knockdown decreases HSCs activation without affecting the expression of TGF-β1 and its receptor and phosphorylation of Smad2, suggesting that NOX4-mediated ROS is downstream of both TGF-β1 and the Smad complex (163). It has been reported that NOX4 acts downstream of Smad3 in kidney and lung myofibroblasts (23, 87), but upstream of Smad2/3 in cardiac myofibroblasts (53). Ang II induces upregulation of both NOX1 and NOX4 mRNA in HSCs (7). Interestingly, Ang-II-induced NOX4 mRNA up-regulation is suppressed in NOX1KO HSCs; however, NOX4 induction by TGF-β is not suppressed in NOX1 KO HSCs. This finding suggests that NOX1 is required for NOX4 up-regulation in HSCs stimulated with Ang II, but that TGF-β induces NOX4 independent of NOX1 in HSCs (7).
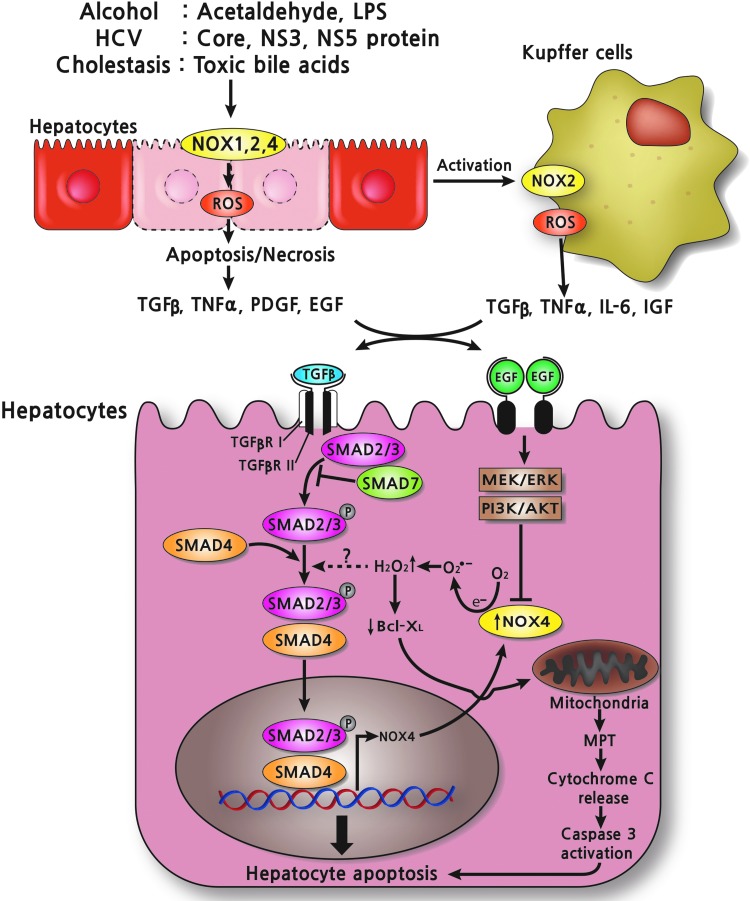
TGF-β1 induces apoptosis of hepatocytes (140). TGF-β binds to and activates the TGF-β receptor (TGF-βR I and II), which induces phosphorylation of Smad2 and Smad3 that are then complexed with Smad4. Activated Smad complexes enter the nucleus and initiate the transcription of TGF-β target genes, including NOX4 (35). TGF-β induces NOX4 expression in rat and human hepatocytes. Knockdown of NOX4 using siRNA attenuates NOX activity, caspase activation, and cell death in hepatocytes, indicating that NOX4 mediates TGF-β-induced hepatocyte apoptosis (35). NOX4-generated H2O2 down-regulates Bcl-XL, a member of the Bcl-2 family of pro-survival proteins, resulting in mitochondrial permeability transition followed by cytochrome C release, caspase 3 activation, and apoptosis of hepatocytes (90). In addition, NOX4-derived ROS upregulates two pro-apoptotic genes, Bmf and Bim (31, 156). Counteracting this effect, EGF binds to the EGFR, which, in turn, activates MEK/ERK and PI3K/Akt, which prevents TGF-β-induced NOX4 upregulation and hepatocyte death (34) (Fig 3.). Furthermore, FasL or TNFα/actinomycin D-induced apoptosis is significantly reduced in NOX4-deficient hepatocytes, suggesting that NOX4 contributes to death ligand-induced apoptosis of hepatocytes (97). Since hepatocytes apoptosis may trigger KCs and HSCs activation by secreting cytokines, chemokines, and microparticles, NOX4-mediated apoptosis of hepatocytes is another mechanism contributing to hepatic fibrosis. Taken together, NOX4 contributes to hepatic fibrogenesis by the mechanism of inducing HSCs activation and mediating TGF-β- or death ligand-induced hepatocytes apoptosis (7, 97).
FIG. 3.
TGF-β mediates apoptosis of hepatocytes via NOX4 upregulation. After various kinds of liver injury, including alcohol, HCV, and toxic bile acids, ROS is produced via NOXs in hepatocytes, some hepatocytes undergo apoptosis/necrosis, and KCs get activated, releasing proinflammatory and profibrogenic cytokines such as TGF-β. TGF-β binds to and activates TGF-β receptor (TGF-βR I and II), which is accompanied by phosphorylation of Smad2 and Smad3 that are then complexed with Smad4. Then, activated Smad complexes enter the nucleus and initiate the transcription of TGF-β target genes, including NOX4. NOX4-generated H2O2 down-regulates Bcl-XL, a Bcl-2 family of pro-survival protein, resulting in mitochondrial permeability transition in mitochondria followed by cytochrome C release, caspase 3 activation, and apoptosis of hepatocytes. However, EGF binds to EGF receptor, which, in turn, activates MEK/ERK and PI3K/Akt and blocks TGF-β-induced NOX4 upregulation and hepatocytes cell death. EGF, epidermal growth factor; ERK, extracellular signal-regulated kinase; H2O2, hydrogen peroxide; HCV, hepatitis C virus. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
NOX4 generates predominantly H2O2 instead of superoxide (128, 173). Since H2O2 activates protein tyrosine phosphorylation by inhibiting phosphatases and by activating kinases, regulation of H2O2-generating NOX4 might play a central role in mediating several TGF-β1 signaling events (11). However, the exact mechanisms of NOX4/TGF-β-mediated fibrogenic signaling, especially what are the targets of NOX4-generating H2O2, are largely unknown.
Inflammation, LPS, and toll-like receptor signaling in hepatic fibrosis
Patients with liver fibrosis have increased intestinal permeability, which allows entry of bacterial LPS and CpG-containing DNAs into the liver where they stimulate inflammatory responses through toll-like receptors (TLRs) (171, 198). All TLRs consist of an N-terminal extracellular domain containing leucine-rich repeats and a C-terminal conserved cytosolic domain. While the extracellular domain of TLRs recognizes the corresponding ligands having either pathogen-associated molecular pattern or damage-associated molecular pattern, the cytosolic domain mediates downstream signal transduction to produce interferon (IFN)-α/-β or proinflammatory cytokines (6, 171). The inflammatory cytokines recruit and activate KCs to release TGF-β1, which activate HSCs.
Both quiescent and activated HSCs express the mRNAs of multiple TLRs (190). However, HSCs respond weakly to TLR2 ligands (144, 172). TLR3 ligands are able to stimulate HSCs for IFN-β production, but not TLR4 ligands, while macrophages are responsive to both ligands for IFN-β production (190). Instead, TLR4 ligands on HSCs induce NF-κB and JNK/AP-1 activation to produce proinflammatory cytokines (145). On binding of LPS to TLR4 with the aid of CD14 and MD2, TLR4 homodimerizes and transmits signals through MyD88 and TIRAP. Then, MyD88 recruits IL-1R-associated kinase (IRAK)-1/-4, and activates NF-κB through the TRAF6/TAK1/TAB2 complex (6, 171). Activated NF-κB enters the nucleus and drives the transcription of various chemokines (MCP-1, MIP-1α, RANTES, and IL-8) and adhesion molecules (ICAM-1 and VCAM-1). Then, those molecules facilitate the recruitment of KCs and circulating macrophages to the site of HSCs, where KCs and recruited macrophages release TGF-β1 to activate HSCs (6, 198). In fact, antibiotic treatment to reduce the gut microflora attenuates liver fibrosis, indicating the essential role of bacterial products in hepatic fibrogenesis, and TLR4-mutant mice and MyD88-deficient mice are resistant to fibrosis (172).
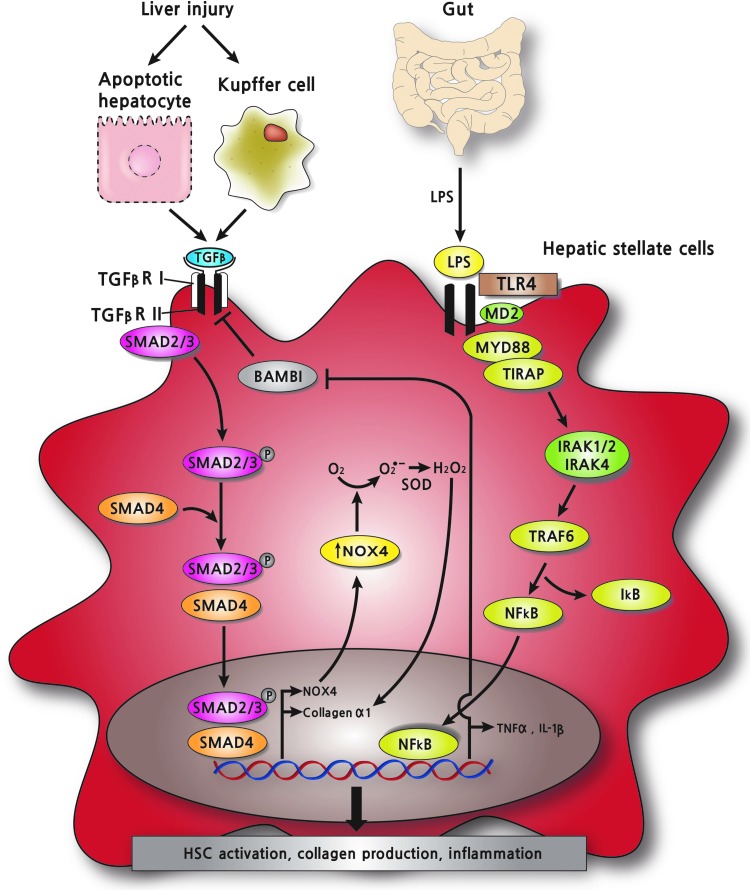
HSCs containing a collagen promoter-driven GFP reporter (Col1-GFP) show weak reporter activity on TGF-β1 stimulation, but much better reporter activity on pretreatment with LPS, suggesting that LPS-TLR4 signaling primes HSCs for TGF-β1 signaling (172). In addition, coculture studies of TLR4-mutant KCs with Col1-GFP containing HSCs show that LPS stimulation does not enhance TGF-β1-mediated reporter activity, suggesting that KCs are not responsible for LPS-TLR4-mediated HSCs activation. Indeed, two TLR4 SNP mutants, T399I and D299G, reduce not only NF-κB activity and chemokine production, but also HSCs activation (82). Gene expression analysis identified bone morphogenetic protein and activin membrane-bound inhibitor (Bambi) as a key factor for LPS-TLR4-mediated enhancement of HSCs activation by TGF-β1 (172). HSCs, but not KCs and hepatocytes, express high levels of Bambi that binds TGF-β1 receptor and inhibits Smad3 phosphorylation on TGF-β1 stimuli (142, 172, 197). LPS-TLR4 in HSCs activates NF-κB in an MyD88-TIRAP-dependent manner, activates NF-κB, and down-regulates Bambi, thereby rendering HSCs ready for TGF-β1-mediated activation (Fig. 4) (172).
FIG. 4.
Interactions between TGFβ-NOX4 and LPS-TLR4-mediated profibrogenic signaling in HSCs. TGF-β released from injured hepatocytes and activated KCs binds to TGF-β receptor, which, in turn, upregulates NOX4 in an Smad2/3-dependent manner in HSCs. NOX4-generated H2O2 activates collagen α1 promoter to produce type I collagen in HSCs promoting hepatic fibrosis. Gut-derived LPS binds TLR4 and activates NF-κB via MyD88/TIRAP/IRAKs/TRAF6 pathway, which, in turn, suppresses BAMBI, a TGF-β inhibitor, in HSCs. LPS/TLR4/MyD88/NF-κB signaling further propagates TGF-β/NOX4-mediated profibrogenic redox signaling via down-regulation of BAMBI in HSCs. TLR, toll-like receptor; IRAK, IL-1R-associated kinase. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
Little work has been reported on the role that NOX or ROS might play in LPS-TLR4 mediated inflammatory or fibrogenic signaling in HSCs. LPS induces the expression of NOX1 through TLR4 in macrophages, suggesting that the interaction between LPS/TLR4 signaling and NOX1 might modulate fibrogenic response in the liver (104). In neutrophils, antioxidant treatment reduces LPS-stimulated nuclear localization of NF-κB (10). In macrophages, NOX inhibitors (DPI and apocynin) or siRNA against p22phox greatly diminish LPS-TLR4-mediated X-box-binding protein 1 splicing, a marker of ER stress, suggesting that ROS plays an important role in LPS-TLR4 signaling (127). Moreover, yeast-two hybrid assay and GST-pull down assay in HEK 293 cells demonstrates that the C-terminal domain of NOX4 interacts with the C-terminal cytosolic domain of TLR4 (149). In human aortic ECs, overexpression of the C-terminal region of NOX4 inhibits LPS-mediated NF-κB activation, via reduced ROS production (148). Moreover, siRNA of NOX4 decreases adhesion molecule (ICAM-1) and chemokines (IL-8 and MCP-1) by LPS stimulation, suggesting that LPS-TLR4 signaling induces NF-κB activation and inflammatory responses through NOX4-derived ROS in human aortic ECs (148). Interestingly, mouse renal tubule epithelial cells express a splicing variant of NOX4 (28 kDa NOX4) more than full-length 65 kDa NOX4, and the 28-kDa NOX4 interacts with TLR4 in co-immunoprecipitation (22). Therefore, future studies are needed to determine the role of NOX4-derived ROS in mediating the LPS-TLR4-NF-κB cascade in HSCs activation, perhaps through a direct interaction between NOX4 and TLR4.
Therapeutic strategies and future direction of NOX-mediated hepatic fibrosis
Based on the knowledge of the pathogenic role of ROS in hepatic fibrogenesis, clinical trials of antifibrotic therapy targeting oxidative stress using antioxidants have been attempted in several kinds of chronic liver disease. However, treatment using agents with nonspecific antioxidant actions such as vitamin E, vitamin C, polyenyl phosphatidyl choline, or UDCA failed to demonstrate antifibrotic efficacy in patients with various kinds of chronic liver disease, including alcoholic hepatitis (121), NASH (122, 165), and chronic hepatitis C (202). Moreover, high doses of vitamin E supplementation (≥400 IU/day) may increase all-cause mortality (130). Perhaps more potent and specific inhibitors of oxidative stress may be required for the development of antifibrotic agents.
The multiple lines of evidence demonstrating the critical role of NOX in hepatic fibrogenesis provide a strong rationale for the use of pharmacological inhibitors of NOX to treat patients with chronic liver disease. Ang II blocking agents are the first such drugs that have been assessed by several investigators. Antifibrotic effects of angiotensin type 1 receptor (AT1-R) blocker (ARB) are reported in several retrospective or small pilot studies in patients with chronic hepatitis C (51, 160, 178) and NASH (200), The effects of losartan, an ARB, on liver fibrosis in patients with chronic hepatitis C were prospectively investigated (49). Baseline biopsies showed that the degree of fibrosis was F2 in seven patients (50%), F3 in four patients (29%), and F4 in three patients (21%). After 18 months of oral losartan 50 mg/day treatment, the degree of fibrosis decreased in seven patients (50%), remained unchanged in three patients (21%), and progressed in four patients (29%). Furthermore, losartan treatment was associated with a significant decrease in the expression of several fibrogenic genes, including procollagen α1(I) and α1(IV), urokinase type plasminogen activator, metalloproteinase type 2, and NOX genes, including NOXO1, NOXA1, and Rac1 (49). The demonstration that AT1R blockade is associated with a decrease in procollagen α1(I) suggests that losartan inhibits collagen synthesis in patients with chronic hepatitis C. Since prolonged hepatic necroinflammation results in fibrosis, the anti-inflammatory effect of losartan may also contribute to the reduction of hepatic fibrosis. Recently, a randomized controlled study by Kim et al. reported that candesartan, an ARB, decreases hepatic fibrosis as well as hepatic expression of NOX components such as p22phox and Rac1 in patients with alcoholic liver disease (105).
In contrast, long-term administration of angiotensin-converting enzyme inhibitor (ACEi) or ARB did not retard the progression of hepatic fibrosis in patients with chronic hepatitis C who were enrolled in the HALT-C cohort (1). Specifically, fibrosis progression occurred in 33.3% of the ACEi/ARB group, 32.5% of the other antihypertensive medications group, and in 25.7% of subjects taking no antihypertensive medications among the HALT-C cohort. Cholongitas et al. investigated the effect of ARB use in a cohort of 102 consecutive hypertensive patients with recurrent HCV after liver transplantation. Administration of an ARB was associated with less progression in inflammation but not in fibrosis (45).
These conflicting results suggest the possibility that ARB's antifibrotic efficacy may vary according to the etiology of the chronic liver disease or that alone are insufficient to block the multiple fibrogenic pathways. The extent of hepatic fibrosis may be an important factor that affects anti-fibrotic effects of ARB. ARB may exert the maximal anti-fibrogenic effects early in the fibrogenic process, and this effect may diminish at a later stage of fibrosis (1, 181). Further well-designed, large-scale clinical studies are required to determine the antifibrotic efficacy of ARB in the specific setting of chronic liver disease. A clinical study is underway to examine the efficacy of irbesartan on the progression of liver fibrosis in adult patients with chronic hepatitis C (ClinicalTrials.gov NCT00265642).
The development of novel pharmacological NOX inhibitors is being assessed as potential anti-fibrotic therapeutics for hepatic fibrosis. Detailed information of these NOX inhibitors has been provided in comprehensive review articles (46, 65, 96). Recently, a small molecule NOX1/NOX4 dual inhibitor was developed by GenKyoTex (Geneva, Switzerland) (112). As previously mentioned, the inhibition of both NOX1 and NOX4 by GKT137831 attenuates CCl4- or BDL-induced ROS production and hepatic fibrosis in mice (7, 97). GKT137831 inhibits collagen α1(I) promoter activity, and it suppresses TGF-β, αSMA, and ROS production in HSCs. NOX4 also mediates lung myofibroblasts activation and fibrogenic responses in lung (87). GKT137831 attenuates hypoxia-induced H2O2 release, proliferation, and TGF-β1 expression in human pulmonary artery endothelial or smooth muscle cells (79). In 2010, GKT137831 was granted orphan drug status for the treatment of idiopathic pulmonary fibrosis by the European Commission (86). In phase I clinical study, GKT137831 was found to be safe and well tolerated when administered orally to a total of 72 healthy adult men, at single doses of approximately 1800 mg OD, and at multiple doses of approximately 900 mg OD for 10 days. The results of animal study and phase I clinical study warrant a clinical trial of GKT137831 to investigate antifibrotic efficacy in the treatment of hepatic fibrosis.
When considering NOX inhibition as a therapeutic strategy for hepatic fibrosis, the collateral inhibition of normal physiological function of NOX should be also considered. For example, the protective role of NOX4 in vasculature from ischemic or inflammatory injury was reported (168). Loss of NOX4 results in the reduction of endothelial nitric oxide synthase expression, nitric oxide production, and heme oxygenase-1 expression, which was associated with apoptosis and inflammatory activation in vasculature (168). Since the liver comprises multiple cell types that express multiple NOX isoforms, inhibition of specific NOX isoforms may disturb the normal physiological role. A better understanding of the components and functions of specific NOX isoforms in specific cell types and interactions of NOX signaling between cell types in the liver may answer these unresolved questions.
Conclusions
NOX-generated ROS is implicated in the pathogenesis of various kinds of chronic liver disease. A growing body of literature indicates that the excessive amount of ROS generated by NOX plays a pivotal role in hepatic fibrogenesis. Importantly, nonphagocytic NOXs, including NOX1 and NOX4 as well as phagocytic NOX2 in HSCs, mediate hepatic fibrosis. Of note, NOX4-mediated ROS generation is related to TGF-β/Smads signaling, critical profibrogenic signaling pathways in the liver. Various NOX isoforms expressed in cell types comprising the liver such as KCs, ECs, hepatocytes, and infiltrating leukocytes also seem to be involved in hepatic fibrogenesis. However, identification and regulation of the NOX isoforms in specific cell types in the liver are still largely unknown. Targeting specific NOX isoforms such as NOX1 and/or NOX4 may be promising to treat hepatic fibrosis, while escaping the collateral suppression of host defence caused by NOX2 inhibition. A better understanding about NOX-mediated fibrogenic signaling may enable the development of novel anti-fibrotic therapy using NOX inhibition strategy.
Abbreviations Used
- ACE
angiotensin converting enzyme
- ACEi
angiotensin-converting enzyme inhibitor
- Akt
protein kinase B
- ALS
amyotrophic lateral sclerosis
- Ang II
angiotensin II
- AP
activator protein
- ARB
angiotensin receptor blocker
- AT1R
angiotensin type I receptor
- ATF
activating transcription factor
- BDL
bile duct ligation
- CGD
chronic granulomatous disease
- CYP
cytochrome p450
- DPI
diphenylene iodonium
- ECM
extracellular matrix
- ECs
endothelial cells
- EGF
epidermal growth factor
- EGFR
epidermal growth factor receptor
- ER
endoplasmic reticulum
- ERK
extracellular signal-regulated kinase
- H2O2
hydrogen peroxide
- HBV
hepatitis B virus
- HCC
hepatocellular carcinoma
- HCV
hepatitis C virus
- HSCs
hepatic stellate cells
- IFN
interferon
- IRAK
IL-1R-associated kinase
- JNK
c-Jun-N-terminal kinase
- KCs
Kupffer cells
- NADPH
nicotinamide adenine dinucleotide phosphate
- NASH
nonalcoholic steatohepatitis
- NOX
NADPH oxidase
- PKC
protein kinase C
- PLs
phospholipases
- PTEN
phosphatase and tensin homologue
- RAS
renin-angiotensin system
- ROS
reactive oxygen species
- SOD
superoxide dismutase
- TLR
toll-like receptor
- VSMCs
vascular smooth muscle cells
- WT
wild-type
Acknowledgments
This work was supported by funding from the National Research Foundation of Korea (NRF) grant funded by the Korea government (MEST) (no. 2011-0029328), (no. 2012R1A1A2041980) (to Y.H.P.) and by NIH R01 GM041804, P50 AA011999, P42 ES010337, and U01 AA021856 (to D.A.B.). We express special thanks to SungKab Kim, a senior manager of multimedia part at Samsung Medical Center, for illustrating figures.
References
- 1.Abu Dayyeh BK, Yang M, Dienstag JL, and Chung RT. The effects of angiotensin blocking agents on the progression of liver fibrosis in the HALT-C Trial cohort. Dig Dis Sci 56: 564–568, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adachi T, Togashi H, Suzuki A, Kasai S, Ito J, Sugahara K, and Kawata S. NAD(P)H oxidase plays a crucial role in PDGF-induced proliferation of hepatic stellate cells. Hepatology 41: 1272–1281, 2005 [DOI] [PubMed] [Google Scholar]
- 3.Albano E. Alcohol, oxidative stress and free radical damage. Proc Nutr Soc 65: 278–290, 2006 [DOI] [PubMed] [Google Scholar]
- 4.Amara N, Goven D, Prost F, Muloway R, Crestani B, and Boczkowski J. NOX4/NADPH oxidase expression is increased in pulmonary fibroblasts from patients with idiopathic pulmonary fibrosis and mediates TGFbeta1-induced fibroblast differentiation into myofibroblasts. Thorax 65: 733–738, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ambasta RK, Kumar P, Griendling KK, Schmidt HH, Busse R, and Brandes RP. Direct interaction of the novel Nox proteins with p22phox is required for the formation of a functionally active NADPH oxidase. J Biol Chem 279: 45935–45941, 2004 [DOI] [PubMed] [Google Scholar]
- 6.Aoyama T, Paik YH, and Seki E. Toll-like receptor signaling and liver fibrosis. Gastroenterol Res Pract 2010, 2010 DOI: 10.1155/2010/192543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aoyama T, Paik YH, Watanabe S, Laleu B, Gaggini F, Fioraso-Cartier L, Molango S, Heitz F, Merlot C, Szyndralewiez C, Page P, and Brenner DA. Nicotinamide adenine dinucleotide phosphate oxidase in experimental liver fibrosis: GKT137831 as a novel potential therapeutic agent. Hepatology 56: 2316–2327, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aram G, Potter JJ, Liu X, Wang L, Torbenson MS, and Mezey E. Deficiency of nicotinamide adenine dinucleotide phosphate, reduced form oxidase enhances hepatocellular injury but attenuates fibrosis after chronic carbon tetrachloride administration. Hepatology 49: 911–919, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arteel GE. Oxidants and antioxidants in alcohol-induced liver disease. Gastroenterology 124: 778–790, 2003 [DOI] [PubMed] [Google Scholar]
- 10.Asehnoune K, Strassheim D, Mitra S, Kim JY, and Abraham E. Involvement of reactive oxygen species in Toll-like receptor 4-dependent activation of NF-kappa B. J Immunol 172: 2522–2529, 2004 [DOI] [PubMed] [Google Scholar]
- 11.Bae YS, Oh H, Rhee SG, and Yoo YD. Regulation of reactive oxygen species generation in cell signaling. Mol Cells 32: 491–509, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Banfi B, Clark RA, Steger K, and Krause KH. Two novel proteins activate superoxide generation by the NADPH oxidase NOX1. J Biol Chem 278: 3510–3513, 2003 [DOI] [PubMed] [Google Scholar]
- 13.Bataller R. and Brenner DA. Hepatic stellate cells as a target for the treatment of liver fibrosis. Semin Liver Dis 21: 437–451, 2001 [DOI] [PubMed] [Google Scholar]
- 14.Bataller R. and Brenner DA. Liver fibrosis. J Clin Invest 115: 209–218, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bataller R, Gines P, Nicolas JM, Gorbig MN, Garcia-Ramallo E, Gasull X, Bosch J, Arroyo V, and Rodes J. Angiotensin II induces contraction and proliferation of human hepatic stellate cells. Gastroenterology 118: 1149–1156, 2000 [DOI] [PubMed] [Google Scholar]
- 16.Bataller R, Paik YH, Lindquist JN, Lemasters JJ, and Brenner DA. Hepatitis C virus core and nonstructural proteins induce fibrogenic effects in hepatic stellate cells. Gastroenterology 126: 529–540, 2004 [DOI] [PubMed] [Google Scholar]
- 17.Bataller R, Sancho-Bru P, Gines P, and Brenner DA. Liver fibrogenesis: a new role for the renin-angiotensin system. Antioxid Redox Signal 7: 1346–1355, 2005 [DOI] [PubMed] [Google Scholar]
- 18.Bataller R, Schwabe RF, Choi YH, Yang L, Paik YH, Lindquist J, Qian T, Schoonhoven R, Hagedorn CH, Lemasters JJ, and Brenner DA. NADPH oxidase signal transduces angiotensin II in hepatic stellate cells and is critical in hepatic fibrosis. J Clin Invest 112: 1383–1394, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Becker S, Reinehr R, Grether-Beck S, Eberle A, and Haussinger D. Hydrophobic bile salts trigger ceramide formation through endosomal acidification. Biol Chem 388: 185–196, 2007 [DOI] [PubMed] [Google Scholar]
- 20.Beckman JS. and Koppenol WH. Nitric oxide, superoxide, and peroxynitrite: the good, the bad, and ugly. Am J Physiol 271: C142 4–C1437, 1996 [DOI] [PubMed] [Google Scholar]
- 21.Bedard K. and Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev 87: 245–313, 2007 [DOI] [PubMed] [Google Scholar]
- 22.Ben Mkaddem S, Pedruzzi E, Werts C, Coant N, Bens M, Cluzeaud F, Goujon JM, Ogier-Denis E, and Vandewalle A. Heat shock protein gp96 and NAD(P)H oxidase 4 play key roles in Toll-like receptor 4-activated apoptosis during renal ischemia/reperfusion injury. Cell Death Differ 17: 1474–1485, 2010 [DOI] [PubMed] [Google Scholar]
- 23.Bondi CD, Manickam N, Lee DY, Block K, Gorin Y, Abboud HE, and Barnes JL. NAD(P)H oxidase mediates TGF-beta1-induced activation of kidney myofibroblasts. J Am Soc Nephrol 21: 93–102, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boudreau HE, Emerson SU, Korzeniowska A, Jendrysik MA, and Leto TL. Hepatitis C virus (HCV) proteins induce NADPH oxidase 4 expression in a transforming growth factor beta-dependent manner: a new contributor to HCV-induced oxidative stress. J Virol 83: 12934–12946, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bradford BU, Kono H, Isayama F, Kosyk O, Wheeler MD, Akiyama TE, Bleye L, Krausz KW, Gonzalez FJ, Koop DR, and Rusyn I. Cytochrome P450 CYP2E1, but not nicotinamide adenine dinucleotide phosphate oxidase, is required for ethanol-induced oxidative DNA damage in rodent liver. Hepatology 41: 336–344, 2005 [DOI] [PubMed] [Google Scholar]
- 26.Brenner DA, Kisseleva T, Scholten D, Paik YH, Iwaisako K, Inokuchi S, Schnabl B, Seki E, De Minicis S, Oesterreicher C, and Taura K. Origin of myofibroblasts in liver fibrosis. Fibrogenesis Tissue Repair 5(Suppl 1): S17, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brown DI. and Griendling KK. Nox proteins in signal transduction. Free Radic Biol Med 47: 1239–1253, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bubici C, Papa S, Dean K, and Franzoso G. Mutual cross-talk between reactive oxygen species and nuclear factor-kappa B: molecular basis and biological significance. Oncogene 25: 6731–6748, 2006 [DOI] [PubMed] [Google Scholar]
- 29.Bureau C, Bernad J, Chaouche N, Orfila C, Beraud M, Gonindard C, Alric L, Vinel JP, and Pipy B. Nonstructural 3 protein of hepatitis C virus triggers an oxidative burst in human monocytes via activation of NADPH oxidase. J Biol Chem 276: 23077–23083, 2001 [DOI] [PubMed] [Google Scholar]
- 30.Cadenas E. Biochemistry of oxygen toxicity. Annu Rev Biochem 58: 79–110, 1989 [DOI] [PubMed] [Google Scholar]
- 31.Caja L, Sancho P, Bertran E, Iglesias-Serret D, Gil J, and Fabregat I. Overactivation of the MEK/ERK pathway in liver tumor cells confers resistance to TGF-{beta}-induced cell death through impairing up-regulation of the NADPH oxidase NOX4. Cancer Res 69: 7595–7602, 2009 [DOI] [PubMed] [Google Scholar]
- 32.Camma C, Di Bona D, Schepis F, Heathcote EJ, Zeuzem S, Pockros PJ, Marcellin P, Balart L, Alberti A, and Craxi A. Effect of peginterferon alfa-2a on liver histology in chronic hepatitis C: a meta-analysis of individual patient data. Hepatology 39: 333–342, 2004 [DOI] [PubMed] [Google Scholar]
- 33.Canbay A, Taimr P, Torok N, Higuchi H, Friedman S, and Gores GJ. Apoptotic body engulfment by a human stellate cell line is profibrogenic. Lab Invest 83: 655–663, 2003 [DOI] [PubMed] [Google Scholar]
- 34.Carmona-Cuenca I, Herrera B, Ventura JJ, Roncero C, Fernandez M, and Fabregat I. EGF blocks NADPH oxidase activation by TGF-beta in fetal rat hepatocytes, impairing oxidative stress, and cell death. J Cell Physiol 207: 322–330, 2006 [DOI] [PubMed] [Google Scholar]
- 35.Carmona-Cuenca I, Roncero C, Sancho P, Caja L, Fausto N, Fernandez M, and Fabregat I. Upregulation of the NADPH oxidase NOX4 by TGF-beta in hepatocytes is required for its pro-apoptotic activity. J Hepatol 49: 965–976, 2008 [DOI] [PubMed] [Google Scholar]
- 36.Carter BJ, Anklesaria P, Choi S, and Engelhardt JF. Redox modifier genes and pathways in amyotrophic lateral sclerosis. Antioxid Redox Signal 11: 1569–1586, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cevik MO, Katsuyama M, Kanda S, Kaneko T, Iwata K, Ibi M, Matsuno K, Kakehi T, Cui W, Sasaki M, and Yabe-Nishimura C. The AP-1 site is essential for the promoter activity of NOX1/NADPH oxidase, a vascular superoxide-producing enzyme: possible involvement of the ERK1/2-JunB pathway. Biochem Biophys Res Commun 374: 351–355, 2008 [DOI] [PubMed] [Google Scholar]
- 38.Chang TT, Liaw YF, Wu SS, Schiff E, Han KH, Lai CL, Safadi R, Lee SS, Halota W, Goodman Z, Chi YC, Zhang H, Hindes R, Iloeje U, Beebe S, and Kreter B. Long-term entecavir therapy results in the reversal of fibrosis/cirrhosis and continued histological improvement in patients with chronic hepatitis B. Hepatology 52: 886–893, 2010 [DOI] [PubMed] [Google Scholar]
- 39.Chen F, Haigh S, Barman S, and Fulton DJ. From form to function: the role of Nox4 in the cardiovascular system. Front Physiol 3: 412, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen K, Craige SE, and Keaney JF., Jr.Downstream targets and intracellular compartmentalization in Nox signaling. Antioxid Redox Signal 11: 2467–2480, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen K, Kirber MT, Xiao H, Yang Y, and Keaney JF., Jr.Regulation of ROS signal transduction by NADPH oxidase 4 localization. J Cell Biol 181: 1129–1139, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cheng G, Cao Z, Xu X, van Meir EG, and Lambeth JD. Homologs of gp91phox: cloning and tissue expression of Nox3, Nox4, and Nox5. Gene 269: 131–140, 2001 [DOI] [PubMed] [Google Scholar]
- 43.Choi J. and Ou JH. Mechanisms of liver injury. III. Oxidative stress in the pathogenesis of hepatitis C virus. Am J Physiol Gastrointest Liver Physiol 290: G847–G851, 2006 [DOI] [PubMed] [Google Scholar]
- 44.Choi SS, Sicklick JK, Ma Q, Yang L, Huang J, Qi Y, Chen W, Li YX, Goldschmidt-Clermont PJ, and Diehl AM. Sustained activation of Rac1 in hepatic stellate cells promotes liver injury and fibrosis in mice. Hepatology 44: 1267–1277, 2006 [DOI] [PubMed] [Google Scholar]
- 45.Cholongitas E, Vibhakorn S, Lodato F, and Burroughs AK. Angiotensin II antagonists in patients with recurrent hepatitis C virus infection after liver transplantation. Liver Int 30: 334–335, 2010 [DOI] [PubMed] [Google Scholar]
- 46.Cifuentes-Pagano E, Csanyi G, and Pagano PJ. NADPH oxidase inhibitors: a decade of discovery from Nox2ds to HTS. Cell Mol Life Sci 69: 2315–2325, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Clempus RE, Sorescu D, Dikalova AE, Pounkova L, Jo P, Sorescu GP, Schmidt HH, Lassegue B, and Griendling KK. Nox4 is required for maintenance of the differentiated vascular smooth muscle cell phenotype. Arterioscler Thromb Vasc Biol 27: 42–48, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Colmenero J, Bataller R, Sancho-Bru P, Bellot P, Miquel R, Moreno M, Jares P, Bosch J, Arroyo V, Caballeria J, and Gines P. Hepatic expression of candidate genes in patients with alcoholic hepatitis: correlation with disease severity. Gastroenterology 132: 687–697, 2007 [DOI] [PubMed] [Google Scholar]
- 49.Colmenero J, Bataller R, Sancho-Bru P, Dominguez M, Moreno M, Forns X, Bruguera M, Arroyo V, Brenner DA, and Gines P. Effects of losartan on hepatic expression of nonphagocytic NADPH oxidase and fibrogenic genes in patients with chronic hepatitis C. Am J Physiol Gastrointest Liver Physiol 297: G72 6–G734, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cong M, Iwaisako K, Jiang C, and Kisseleva T. Cell signals influencing hepatic fibrosis. Int J Hepatol 2012: 158547, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Corey KE, Shah N, Misdraji J, Abu Dayyeh BK, Zheng H, Bhan AK, and Chung RT. The effect of angiotensin-blocking agents on liver fibrosis in patients with hepatitis C. Liver Int 29: 748–753, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Croquet V, Moal F, Veal N, Wang J, Oberti F, Roux J, Vuillemin E, Gallois Y, Douay O, Chappard D, and Cales P. Hemodynamic and antifibrotic effects of losartan in rats with liver fibrosis and/or portal hypertension. J Hepatol 37: 773–780, 2002 [DOI] [PubMed] [Google Scholar]
- 53.Cucoranu I, Clempus R, Dikalova A, Phelan PJ, Ariyan S, Dikalov S, and Sorescu D. NAD(P)H oxidase 4 mediates transforming growth factor-beta1-induced differentiation of cardiac fibroblasts into myofibroblasts. Circ Res 97: 900–907, 2005 [DOI] [PubMed] [Google Scholar]
- 54.Cui W, Matsuno K, Iwata K, Ibi M, Matsumoto M, Zhang J, Zhu K, Katsuyama M, Torok NJ, and Yabe-Nishimura C. NOX1/nicotinamide adenine dinucleotide phosphate, reduced form (NADPH) oxidase promotes proliferation of stellate cells and aggravates liver fibrosis induced by bile duct ligation. Hepatology 54: 949–958, 2011 [DOI] [PubMed] [Google Scholar]
- 55.Czaja MJ. Cell signaling in oxidative stress-induced liver injury. Semin Liver Dis 27: 378–389, 2007 [DOI] [PubMed] [Google Scholar]
- 56.De Bleser PJ, Xu G, Rombouts K, Rogiers V, and Geerts A. Glutathione levels discriminate between oxidative stress and transforming growth factor-beta signaling in activated rat hepatic stellate cells. J Biol Chem 274: 33881–33887, 1999 [DOI] [PubMed] [Google Scholar]
- 57.De Minicis S, Bataller R, and Brenner DA. NADPH oxidase in the liver: defensive, offensive, or fibrogenic? Gastroenterology 131: 272–275, 2006 [DOI] [PubMed] [Google Scholar]
- 58.De Minicis S, Seki E, Oesterreicher C, Schnabl B, Schwabe RF, and Brenner DA. Reduced nicotinamide adenine dinucleotide phosphate oxidase mediates fibrotic and inflammatory effects of leptin on hepatic stellate cells. Hepatology 48: 2016–2026, 2008 [DOI] [PubMed] [Google Scholar]
- 59.De Minicis S, Seki E, Paik YH, Osterreicher CH, Kodama Y, Kluwe J, Torozzi L, Miyai K, Benedetti A, Schwabe RF, and Brenner DA. Role and cellular source of nicotinamide adenine dinucleotide phosphate oxidase in hepatic fibrosis. Hepatology 52: 1420–1430, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.de Mochel NS, Seronello S, Wang SH, Ito C, Zheng JX, Liang TJ, Lambeth JD, and Choi J. Hepatocyte NAD(P)H oxidases as an endogenous source of reactive oxygen species during hepatitis C virus infection. Hepatology 52: 47–59, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.DeLeo FR, Burritt JB, Yu L, Jesaitis AJ, Dinauer MC, and Nauseef WM. Processing and maturation of flavocytochrome b558 include incorporation of heme as a prerequisite for heterodimer assembly. J Biol Chem 275: 13986–13993, 2000 [DOI] [PubMed] [Google Scholar]
- 62.Desmouliere A, Darby IA, and Gabbiani G. Normal and pathologic soft tissue remodeling: role of the myofibroblast, with special emphasis on liver and kidney fibrosis. Lab Invest 83: 1689–1707, 2003 [DOI] [PubMed] [Google Scholar]
- 63.Diebold I, Djordjevic T, Petry A, Hatzelmann A, Tenor H, Hess J, and Gorlach A. Phosphodiesterase 2 mediates redox-sensitive endothelial cell proliferation and angiogenesis by thrombin via Rac1 and NADPH oxidase 2. Circ Res 104: 1169–1177, 2009 [DOI] [PubMed] [Google Scholar]
- 64.Djamali A, Vidyasagar A, Adulla M, Hullett D, and Reese S. Nox-2 is a modulator of fibrogenesis in kidney allografts. Am J Transplant 9: 74–82, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Drummond GR, Selemidis S, Griendling KK, and Sobey CG. Combating oxidative stress in vascular disease: NADPH oxidases as therapeutic targets. Nat Rev Drug Discov 10: 453–471, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.El-Serag HB. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology 142: 1264–1273.e1261, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fan CY, Katsuyama M, and Yabe-Nishimura C. PKCdelta mediates up-regulation of NOX1, a catalytic subunit of NADPH oxidase, via transactivation of the EGF receptor: possible involvement of PKCdelta in vascular hypertrophy. Biochem J 390: 761–767, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Frey RS, Ushio-Fukai M, and Malik AB. NADPH oxidase-dependent signaling in endothelial cells: role in physiology and pathophysiology. Antioxid Redox Signal 11: 791–810, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fridovich I. Superoxide radical: an endogenous toxicant. Annu Rev Pharmacol Toxicol 23: 239–257, 1983 [DOI] [PubMed] [Google Scholar]
- 70.Friedman SL. Mechanisms of hepatic fibrogenesis. Gastroenterology 134: 1655–1669, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Friedman SL. Molecular regulation of hepatic fibrosis, an integrated cellular response to tissue injury. J Biol Chem 275: 2247–2250, 2000 [DOI] [PubMed] [Google Scholar]
- 72.Friedman SL, Roll FJ, Boyles J, and Bissell DM. Hepatic lipocytes: the principal collagen-producing cells of normal rat liver. Proc Natl Acad Sci USA 82: 8681–8685, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gao XP, Standiford TJ, Rahman A, Newstead M, Holland SM, Dinauer MC, Liu QH, and Malik AB. Role of NADPH oxidase in the mechanism of lung neutrophil sequestration and microvessel injury induced by Gram-negative sepsis: studies in p47phox-/- and gp91phox-/- mice. J Immunol 168: 3974–3982, 2002 [DOI] [PubMed] [Google Scholar]
- 74.Garcia-Mediavilla MV, Sanchez-Campos S, Gonzalez-Perez P, Gomez-Gonzalo M, Majano PL, Lopez-Cabrera M, Clemente G, Garcia-Monzon C, and Gonzalez-Gallego J. Differential contribution of hepatitis C virus NS5A and core proteins to the induction of oxidative and nitrosative stress in human hepatocyte-derived cells. J Hepatol 43: 606–613, 2005 [DOI] [PubMed] [Google Scholar]
- 75.Garrido AM. and Griendling KK. NADPH oxidases and angiotensin II receptor signaling. Mol Cell Endocrinol 302: 148–158, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Geiszt M, Kopp JB, Varnai P, and Leto TL. Identification of renox, an NAD(P)H oxidase in kidney. Proc Natl Acad Sci USA 97: 8010–8014, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Goyal P, Weissmann N, Rose F, Grimminger F, Schafers HJ, Seeger W, and Hanze J. Identification of novel Nox4 splice variants with impact on ROS levels in A549 cells. Biochem Biophys Res Commun 329: 32–39, 2005 [DOI] [PubMed] [Google Scholar]
- 78.Graham KA, Kulawiec M, Owens KM, Li X, Desouki MM, Chandra D, and Singh KK. NADPH oxidase 4 is an oncoprotein localized to mitochondria. Cancer Biol Ther 10: 223–231, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Green DE, Murphy TC, Kang BY, Kleinhenz JM, Szyndralewiez C, Page P, Sutliff RL, and Hart CM. The Nox4 inhibitor GKT137831 attenuates hypoxia-induced pulmonary vascular cell proliferation. Am J Respir Cell Mol Biol 47: 718–726, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Griendling KK. and Ushio-Fukai M. Reactive oxygen species as mediators of angiotensin II signaling. Regul Pept 91: 21–27, 2000 [DOI] [PubMed] [Google Scholar]
- 81.Guimaraes EL, Empsen C, Geerts A, and van Grunsven LA. Advanced glycation end products induce production of reactive oxygen species via the activation of NADPH oxidase in murine hepatic stellate cells. J Hepatol 52: 389–397, 2010 [DOI] [PubMed] [Google Scholar]
- 82.Guo J, Loke J, Zheng F, Hong F, Yea S, Fukata M, Tarocchi M, Abar OT, Huang H, Sninsky JJ, and Friedman SL. Functional linkage of cirrhosis-predictive single nucleotide polymorphisms of Toll-like receptor 4 to hepatic stellate cell responses. Hepatology 49: 960–968, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hammel P, Couvelard A, O'Toole D, Ratouis A, Sauvanet A, Flejou JF, Degott C, Belghiti J, Bernades P, Valla D, Ruszniewski P, and Levy P. Regression of liver fibrosis after biliary drainage in patients with chronic pancreatitis and stenosis of the common bile duct. N Engl J Med 344: 418–423, 2001 [DOI] [PubMed] [Google Scholar]
- 84.Hardie WD, Glasser SW, and Hagood JS. Emerging concepts in the pathogenesis of lung fibrosis. Am J Pathol 175: 3–16, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Harraz MM, Marden JJ, Zhou W, Zhang Y, Williams A, Sharov VS, Nelson K, Luo M, Paulson H, Schoneich C, and Engelhardt JF. SOD1 mutations disrupt redox-sensitive Rac regulation of NADPH oxidase in a familial ALS model. J Clin Invest 118: 659–670, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hecker L, Cheng J, and Thannickal VJ. Targeting NOX enzymes in pulmonary fibrosis. Cell Mol Life Sci 69: 2365–2371, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hecker L, Vittal R, Jones T, Jagirdar R, Luckhardt TR, Horowitz JC, Pennathur S, Martinez FJ, and Thannickal VJ. NADPH oxidase-4 mediates myofibroblast activation and fibrogenic responses to lung injury. Nat Med 15: 1077–1081, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hellerbrand C, Stefanovic B, Giordano F, Burchardt ER, and Brenner DA. The role of TGFbeta1 in initiating hepatic stellate cell activation in vivo. J Hepatol 30: 77–87, 1999 [DOI] [PubMed] [Google Scholar]
- 89.Helmcke I, Heumuller S, Tikkanen R, Schroder K, and Brandes RP. Identification of structural elements in Nox1 and Nox4 controlling localization and activity. Antioxid Redox Signal 11: 1279–1287, 2009 [DOI] [PubMed] [Google Scholar]
- 90.Herrera B, Fernandez M, Alvarez AM, Roncero C, Benito M, Gil J, and Fabregat I. Activation of caspases occurs downstream from radical oxygen species production, Bcl-xL down-regulation, and early cytochrome C release in apoptosis induced by transforming growth factor beta in rat fetal hepatocytes. Hepatology 34: 548–556, 2001 [DOI] [PubMed] [Google Scholar]
- 91.Heymes C, Bendall JK, Ratajczak P, Cave AC, Samuel JL, Hasenfuss G, and Shah AM. Increased myocardial NADPH oxidase activity in human heart failure. J Am Coll Cardiol 41: 2164–2171, 2003 [DOI] [PubMed] [Google Scholar]
- 92.Hilenski LL, Clempus RE, Quinn MT, Lambeth JD, and Griendling KK. Distinct subcellular localizations of Nox1 and Nox4 in vascular smooth muscle cells. Arterioscler Thromb Vasc Biol 24: 677–683, 2004 [DOI] [PubMed] [Google Scholar]
- 93.Hu R, Wang YL, Edderkaoui M, Lugea A, Apte MV, and Pandol SJ. Ethanol augments PDGF-induced NADPH oxidase activity and proliferation in rat pancreatic stellate cells. Pancreatology 7: 332–340, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ikeda S, Yamaoka-Tojo M, Hilenski L, Patrushev NA, Anwar GM, Quinn MT, and Ushio-Fukai M. IQGAP1 regulates reactive oxygen species-dependent endothelial cell migration through interacting with Nox2. Arterioscler Thromb Vasc Biol 25: 2295–2300, 2005 [DOI] [PubMed] [Google Scholar]
- 95.Inagami T. The renin-angiotensin system. Essays Biochem 28: 147–164, 1994 [PubMed] [Google Scholar]
- 96.Jaquet V, Scapozza L, Clark RA, Krause KH, and Lambeth JD. Small-molecule NOX inhibitors: ROS-generating NADPH oxidases as therapeutic targets. Antioxid Redox Signal 11: 2535–2552, 2009 [DOI] [PubMed] [Google Scholar]
- 97.Jiang JX, Chen X, Serizawa N, Szyndralewiez C, Page P, Schroder K, Brandes RP, Devaraj S, and Torok NJ. Liver fibrosis and hepatocyte apoptosis are attenuated by GKT137831, a novel NOX4/NOX1 inhibitor in vivo. Free Radic Biol Med 53: 289–296, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Jiang JX, Venugopal S, Serizawa N, Chen X, Scott F, Li Y, Adamson R, Devaraj S, Shah V, Gershwin ME, Friedman SL, and Torok NJ. Reduced nicotinamide adenine dinucleotide phosphate oxidase 2 plays a key role in stellate cell activation and liver fibrogenesis in vivo. Gastroenterology 139: 1375–1384, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Jones SA, O'Donnell VB, Wood JD, Broughton JP, Hughes EJ, and Jones OT. Expression of phagocyte NADPH oxidase components in human endothelial cells. Am J Physiol 271: H162 6–H1634, 1996 [DOI] [PubMed] [Google Scholar]
- 100.Kanno K, Tazuma S, and Chayama K. AT1A-deficient mice show less severe progression of liver fibrosis induced by CCl(4). Biochem Biophys Res Commun 308: 177–183, 2003 [DOI] [PubMed] [Google Scholar]
- 101.Kaplan DE, Sugimoto K, Newton K, Valiga ME, Ikeda F, Aytaman A, Nunes FA, Lucey MR, Vance BA, Vonderheide RH, Reddy KR, McKeating JA, and Chang KM. Discordant role of CD4 T-cell response relative to neutralizing antibody and CD8 T-cell responses in acute hepatitis C. Gastroenterology 132: 654–666, 2007 [DOI] [PubMed] [Google Scholar]
- 102.Katsuyama M, Fan C, Arakawa N, Nishinaka T, Miyagishi M, Taira K, and Yabe-Nishimura C. Essential role of ATF-1 in induction of NOX1, a catalytic subunit of NADPH oxidase: involvement of mitochondrial respiratory chain. Biochem J 386: 255–261, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kim JH, Song SY, Park SG, Song SU, Xia Y, and Sung JH. Primary involvement of NADPH oxidase 4 in hypoxia-induced generation of reactive oxygen species in adipose-derived stem cells. Stem Cells Dev 21: 2212–2221, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kim JS, Yeo S, Shin DG, Bae YS, Lee JJ, Chin BR, Lee CH, and Baek SH. Glycogen synthase kinase 3beta and beta-catenin pathway is involved in toll-like receptor 4-mediated NADPH oxidase 1 expression in macrophages. FEBS J 277: 2830–2837, 2010 [DOI] [PubMed] [Google Scholar]
- 105.Kim MY, Cho MY, Baik SK, Jeong PH, Suk KT, Jang YO, Yea CJ, Kim JW, Kim HS, Kwon SO, Yoo BS, Kim JY, Eom MS, Cha SH, and Chang SJ. Beneficial effects of candesartan, an angiotensin-blocking agent, on compensated alcoholic liver fibrosis—a randomized open-label controlled study. Liver Int 32: 977–987, 2012 [DOI] [PubMed] [Google Scholar]
- 106.Kisseleva T, Uchinami H, Feirt N, Quintana-Bustamante O, Segovia JC, Schwabe RF, and Brenner DA. Bone marrow-derived fibrocytes participate in pathogenesis of liver fibrosis. J Hepatol 45: 429–438, 2006 [DOI] [PubMed] [Google Scholar]
- 107.Kono H, Bradford BU, Yin M, Sulik KK, Koop DR, Peters JM, Gonzalez FJ, McDonald T, Dikalova A, Kadiiska MB, Mason RP, and Thurman RG. CYP2E1 is not involved in early alcohol-induced liver injury. Am J Physiol 277: G125 9–G1267, 1999 [DOI] [PubMed] [Google Scholar]
- 108.Kono H, Rusyn I, Uesugi T, Yamashina S, Connor HD, Dikalova A, Mason RP, and Thurman RG. Diphenyleneiodonium sulfate, an NADPH oxidase inhibitor, prevents early alcohol-induced liver injury in the rat. Am J Physiol Gastrointest Liver Physiol 280: G100 5–G1012, 2001 [DOI] [PubMed] [Google Scholar]
- 109.Kono H, Rusyn I, Yin M, Gabele E, Yamashina S, Dikalova A, Kadiiska MB, Connor HD, Mason RP, Segal BH, Bradford BU, Holland SM, and Thurman RG. NADPH oxidase-derived free radicals are key oxidants in alcohol-induced liver disease. J Clin Invest 106: 867–872, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kuwahara F, Kai H, Tokuda K, Kai M, Takeshita A, Egashira K, and Imaizumi T. Transforming growth factor-beta function blocking prevents myocardial fibrosis and diastolic dysfunction in pressure-overloaded rats. Circulation 106: 130–135, 2002 [DOI] [PubMed] [Google Scholar]
- 111.Kweon YO, Goodman ZD, Dienstag JL, Schiff ER, Brown NA, Burchardt E, Schoonhoven R, Brenner DA, and Fried MW. Decreasing fibrogenesis: an immunohistochemical study of paired liver biopsies following lamivudine therapy for chronic hepatitis B. J Hepatol 35: 749–755, 2001 [DOI] [PubMed] [Google Scholar]
- 112.Laleu B, Gaggini F, Orchard M, Fioraso-Cartier L, Cagnon L, Houngninou-Molango S, Gradia A, Duboux G, Merlot C, Heitz F, Szyndralewiez C, and Page P. First in class, potent, and orally bioavailable NADPH oxidase isoform 4 (Nox4) inhibitors for the treatment of idiopathic pulmonary fibrosis. J Med Chem 53: 7715–7730, 2010 [DOI] [PubMed] [Google Scholar]
- 113.Lambeth JD. NOX enzymes and the biology of reactive oxygen. Nat Rev Immunol 4: 181–189, 2004 [DOI] [PubMed] [Google Scholar]
- 114.Landmesser U, Cai H, Dikalov S, McCann L, Hwang J, Jo H, Holland SM, and Harrison DG. Role of p47(phox) in vascular oxidative stress and hypertension caused by angiotensin II. Hypertension 40: 511–515, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lassegue B, Sorescu D, Szocs K, Yin Q, Akers M, Zhang Y, Grant SL, Lambeth JD, and Griendling KK. Novel gp91(phox) homologues in vascular smooth muscle cells: nox1 mediates angiotensin II-induced superoxide formation and redox-sensitive signaling pathways. Circ Res 88: 888–894, 2001 [DOI] [PubMed] [Google Scholar]
- 116.Latella G, Vetuschi A, Sferra R, Catitti V, D'Angelo A, Zanninelli G, Flanders KC, and Gaudio E. Targeted disruption of Smad3 confers resistance to the development of dimethylnitrosamine-induced hepatic fibrosis in mice. Liver Int 29: 997–1009, 2009 [DOI] [PubMed] [Google Scholar]
- 117.Lee KS, Buck M, Houglum K, and Chojkier M. Activation of hepatic stellate cells by TGF alpha and collagen type I is mediated by oxidative stress through c-myb expression. J Clin Invest 96: 2461–2468, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lerat H, Honda M, Beard MR, Loesch K, Sun J, Yang Y, Okuda M, Gosert R, Xiao SY, Weinman SA, and Lemon SM. Steatosis and liver cancer in transgenic mice expressing the structural and nonstructural proteins of hepatitis C virus. Gastroenterology 122: 352–365, 2002 [DOI] [PubMed] [Google Scholar]
- 119.Li Q, Harraz MM, Zhou W, Zhang LN, Ding W, Zhang Y, Eggleston T, Yeaman C, Banfi B, and Engelhardt JF. Nox2 and Rac1 regulate H2O2-dependent recruitment of TRAF6 to endosomal interleukin-1 receptor complexes. Mol Cell Biol 26: 140–154, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Li Q, Zhang Y, Marden JJ, Banfi B, and Engelhardt JF. Endosomal NADPH oxidase regulates c-Src activation following hypoxia/reoxygenation injury. Biochem J 411: 531–541, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Lieber CS, Weiss DG, Groszmann R, Paronetto F, and Schenker S. II. Veterans Affairs Cooperative Study of polyenylphosphatidylcholine in alcoholic liver disease. Alcohol Clin Exp Res 27: 1765–1772, 2003 [DOI] [PubMed] [Google Scholar]
- 122.Lindor KD, Kowdley KV, Heathcote EJ, Harrison ME, Jorgensen R, Angulo P, Lymp JF, Burgart L, and Colin P. Ursodeoxycholic acid for treatment of nonalcoholic steatohepatitis: results of a randomized trial. Hepatology 39: 770–778, 2004 [DOI] [PubMed] [Google Scholar]
- 123.Lyle AN, Deshpande NN, Taniyama Y, Seidel-Rogol B, Pounkova L, Du P, Papaharalambus C, Lassegue B, and Griendling KK. Poldip2, a novel regulator of Nox4 and cytoskeletal integrity in vascular smooth muscle cells. Circ Res 105: 249–259, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Maher JJ, Tzagarakis C, and Gimenez A. Malondialdehyde stimulates collagen production by hepatic lipocytes only upon activation in primary culture. Alcohol Alcohol 29: 605–610, 1994 [PubMed] [Google Scholar]
- 125.Maraldi T, Prata C, Caliceti C, Vieceli Dalla Sega F, Zambonin L, Fiorentini D, and Hakim G. VEGF-induced ROS generation from NAD(P)H oxidases protects human leukemic cells from apoptosis. Int J Oncol 36: 1581–1589, 2010 [DOI] [PubMed] [Google Scholar]
- 126.Marcellin P, Gane E, Buti M, Afdhal N, Sievert W, Jacobson IM, Washington MK, Germanidis G, Flaherty JF, Schall RA, Bornstein JD, Kitrinos KM, Subramanian GM, McHutchison JG, and Heathcote EJ. Regression of cirrhosis during treatment with tenofovir disoproxil fumarate for chronic hepatitis B: a 5-year open-label follow-up study. Lancet 381: 468–475, 2013 [DOI] [PubMed] [Google Scholar]
- 127.Martinon F, Chen X, Lee AH, and Glimcher LH. TLR activation of the transcription factor XBP1 regulates innate immune responses in macrophages. Nat Immunol 11: 411–418, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Martyn KD, Frederick LM, von Loehneysen K, Dinauer MC, and Knaus UG. Functional analysis of Nox4 reveals unique characteristics compared to other NADPH oxidases. Cell Signal 18: 69–82, 2006 [DOI] [PubMed] [Google Scholar]
- 129.Miana-Mena FJ, Gonzalez-Mingot C, Larrode P, Munoz MJ, Olivan S, Fuentes-Broto L, Martinez-Ballarin E, Reiter RJ, Osta R, and Garcia JJ. Monitoring systemic oxidative stress in an animal model of amyotrophic lateral sclerosis. J Neurol 258: 762–769, 2011 [DOI] [PubMed] [Google Scholar]
- 130.Miller ER, 3rd, Pastor-Barriuso R, Dalal D, Riemersma RA, Appel LJ, and Guallar E. Meta-analysis: high-dosage vitamin E supplementation may increase all-cause mortality. Ann Intern Med 142: 37–46, 2005 [DOI] [PubMed] [Google Scholar]
- 131.Miller FJ, Jr., Filali M, Huss GJ, Stanic B, Chamseddine A, Barna TJ, and Lamb FS. Cytokine activation of nuclear factor kappa B in vascular smooth muscle cells requires signaling endosomes containing Nox1 and ClC-3. Circ Res 101: 663–671, 2007 [DOI] [PubMed] [Google Scholar]
- 132.Miyoshi H, Rust C, Roberts PJ, Burgart LJ, and Gores GJ. Hepatocyte apoptosis after bile duct ligation in the mouse involves Fas. Gastroenterology 117: 669–677, 1999 [DOI] [PubMed] [Google Scholar]
- 133.Moldovan L, Moldovan NI, Sohn RH, Parikh SA, and Goldschmidt-Clermont PJ. Redox changes of cultured endothelial cells and actin dynamics. Circ Res 86: 549–557, 2000 [DOI] [PubMed] [Google Scholar]
- 134.Mollnau H, Wendt M, Szocs K, Lassegue B, Schulz E, Oelze M, Li H, Bodenschatz M, August M, Kleschyov AL, Tsilimingas N, Walter U, Forstermann U, Meinertz T, Griendling K, and Munzel T. Effects of angiotensin II infusion on the expression and function of NAD(P)H oxidase and components of nitric oxide/cGMP signaling. Circ Res 90: E5 8–E65, 2002 [DOI] [PubMed] [Google Scholar]
- 135.Nauseef WM. Biological roles for the NOX family NADPH oxidases. J Biol Chem 283: 16961–16965, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Nieto N, Greenwel P, Friedman SL, Zhang F, Dannenberg AJ, and Cederbaum AI. Ethanol and arachidonic acid increase alpha 2(I) collagen expression in rat hepatic stellate cells overexpressing cytochrome P450 2E1. Role of H2O2 and cyclooxygenase-2. J Biol Chem 275: 20136–20145, 2000 [DOI] [PubMed] [Google Scholar]
- 137.Novo E, Marra F, Zamara E, Valfre di Bonzo L, Caligiuri A, Cannito S, Antonaci C, Colombatto S, Pinzani M, and Parola M. Dose dependent and divergent effects of superoxide anion on cell death, proliferation, and migration of activated human hepatic stellate cells. Gut 55: 90–97, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Oakley FD, Abbott D, Li Q, and Engelhardt JF. Signaling components of redox active endosomes: the redoxosomes. Antioxid Redox Signal 11: 1313–1333, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Oakley FD, Smith RL, and Engelhardt JF. Lipid rafts and caveolin-1 coordinate interleukin-1beta (IL-1beta)-dependent activation of NFkappaB by controlling endocytosis of Nox2 and IL-1beta receptor 1 from the plasma membrane. J Biol Chem 284: 33255–33264, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Oberhammer FA, Pavelka M, Sharma S, Tiefenbacher R, Purchio AF, Bursch W, and Schulte-Hermann R. Induction of apoptosis in cultured hepatocytes and in regressing liver by transforming growth factor beta 1. Proc Natl Acad Sci USA 89: 5408–5412, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Okuda M, Li K, Beard MR, Showalter LA, Scholle F, Lemon SM, and Weinman SA. Mitochondrial injury, oxidative stress, and antioxidant gene expression are induced by hepatitis C virus core protein. Gastroenterology 122: 366–375, 2002 [DOI] [PubMed] [Google Scholar]
- 142.Onichtchouk D, Chen YG, Dosch R, Gawantka V, Delius H, Massague J, and Niehrs C. Silencing of TGF-beta signalling by the pseudoreceptor BAMBI. Nature 401: 480–485, 1999 [DOI] [PubMed] [Google Scholar]
- 143.Paik YH, Iwaisako K, Seki E, Inokuchi S, Schnabl B, Osterreicher CH, Kisseleva T, and Brenner DA. The nicotinamide adenine dinucleotide phosphate oxidase (NOX) homologues NOX1 and NOX2/gp91(phox) mediate hepatic fibrosis in mice. Hepatology 53: 1730–1741, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Paik YH, Lee KS, Lee HJ, Yang KM, Lee SJ, Lee DK, Han KH, Chon CY, Lee SI, Moon YM, and Brenner DA. Hepatic stellate cells primed with cytokines upregulate inflammation in response to peptidoglycan or lipoteichoic acid. Lab Invest 86: 676–686, 2006 [DOI] [PubMed] [Google Scholar]
- 145.Paik YH, Schwabe RF, Bataller R, Russo MP, Jobin C, and Brenner DA. Toll-like receptor 4 mediates inflammatory signaling by bacterial lipopolysaccharide in human hepatic stellate cells. Hepatology 37: 1043–1055, 2003 [DOI] [PubMed] [Google Scholar]
- 146.Paizis G, Cooper ME, Schembri JM, Tikellis C, Burrell LM, and Angus PW. Up-regulation of components of the renin-angiotensin system in the bile duct-ligated rat liver. Gastroenterology 123: 1667–1676, 2002 [DOI] [PubMed] [Google Scholar]
- 147.Paizis G, Gilbert RE, Cooper ME, Murthi P, Schembri JM, Wu LL, Rumble JR, Kelly DJ, Tikellis C, Cox A, Smallwood RA, and Angus PW. Effect of angiotensin II type 1 receptor blockade on experimental hepatic fibrogenesis. J Hepatol 35: 376–385, 2001 [DOI] [PubMed] [Google Scholar]
- 148.Park HS, Chun JN, Jung HY, Choi C, and Bae YS. Role of NADPH oxidase 4 in lipopolysaccharide-induced proinflammatory responses by human aortic endothelial cells. Cardiovasc Res 72: 447–455, 2006 [DOI] [PubMed] [Google Scholar]
- 149.Park HS, Jung HY, Park EY, Kim J, Lee WJ, and Bae YS. Cutting edge: direct interaction of TLR4 with NAD(P)H oxidase 4 isozyme is essential for lipopolysaccharide-induced production of reactive oxygen species and activation of NF-kappa B. J Immunol 173: 3589–3593, 2004 [DOI] [PubMed] [Google Scholar]
- 150.Parola M. and Robino G. Oxidative stress-related molecules and liver fibrosis. J Hepatol 35: 297–306, 2001 [DOI] [PubMed] [Google Scholar]
- 151.Pendergrass KD, Gwathmey TM, Michalek RD, Grayson JM, and Chappell MC. The angiotensin II-AT1 receptor stimulates reactive oxygen species within the cell nucleus. Biochem Biophys Res Commun 384: 149–154, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Petry A, Weitnauer M, and Gorlach A. Receptor activation of NADPH oxidases. Antioxid Redox Signal 13: 467–487, 2010 [DOI] [PubMed] [Google Scholar]
- 153.Pinzani M, Gesualdo L, Sabbah GM, and Abboud HE. Effects of platelet-derived growth factor and other polypeptide mitogens on DNA synthesis and growth of cultured rat liver fat-storing cells. J Clin Invest 84: 1786–1793, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Poli G. and Parola M. Oxidative damage and fibrogenesis. Free Radic Biol Med 22: 287–305, 1997 [DOI] [PubMed] [Google Scholar]
- 155.Ramadori G. and Saile B. Portal tract fibrogenesis in the liver. Lab Invest 84: 153–159, 2004 [DOI] [PubMed] [Google Scholar]
- 156.Ramjaun AR, Tomlinson S, Eddaoudi A, and Downward J. Upregulation of two BH3-only proteins, Bmf and Bim, during TGF beta-induced apoptosis. Oncogene 26: 970–981, 2007 [DOI] [PubMed] [Google Scholar]
- 157.Reinehr R, Becker S, Eberle A, Grether-Beck S, and Haussinger D. Involvement of NADPH oxidase isoforms and Src family kinases in CD95-dependent hepatocyte apoptosis. J Biol Chem 280: 27179–27194, 2005 [DOI] [PubMed] [Google Scholar]
- 158.Reinehr R, Becker S, Keitel V, Eberle A, Grether-Beck S, and Haussinger D. Bile salt-induced apoptosis involves NADPH oxidase isoform activation. Gastroenterology 129: 2009–2031, 2005 [DOI] [PubMed] [Google Scholar]
- 159.Reth M. Hydrogen peroxide as second messenger in lymphocyte activation. Nat Immunol 3: 1129–1134, 2002 [DOI] [PubMed] [Google Scholar]
- 160.Rimola A, Londono MC, Guevara G, Bruguera M, Navasa M, Forns X, Garcia-Retortillo M, Garcia-Valdecasas JC, and Rodes J. Beneficial effect of angiotensin-blocking agents on graft fibrosis in hepatitis C recurrence after liver transplantation. Transplantation 78: 686–691, 2004 [DOI] [PubMed] [Google Scholar]
- 161.Rockey DC, Boyles JK, Gabbiani G, and Friedman SL. Rat hepatic lipocytes express smooth muscle actin upon activation in vivo and in culture. J Submicrosc Cytol Pathol 24: 193–203, 1992 [PubMed] [Google Scholar]
- 162.Rotrosen D, Yeung CL, Leto TL, Malech HL, and Kwong CH. Cytochrome b558: the flavin-binding component of the phagocyte NADPH oxidase. Science 256: 1459–1462, 1992 [DOI] [PubMed] [Google Scholar]
- 163.Sancho P, Mainez J, Crosas-Molist E, Roncero C, Fernandez-Rodriguez CM, Pinedo F, Huber H, Eferl R, Mikulits W, and Fabregat I. NADPH oxidase NOX4 mediates stellate cell activation and hepatocyte cell death during liver fibrosis development. PLoS One 7: e45285, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Santos CXC, Nabeebaccus AA, Shah AM, Camargo LL, Filho SV, and Lopes LR. Endoplasmic reticulum stress and Nox-mediated reactive oxygen species signaling in the peripheral vasculature: potential role in hypertension. Antioxid Redox Signal. 20: 121–134, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Sanyal AJ, Chalasani N, Kowdley KV, McCullough A, Diehl AM, Bass NM, Neuschwander-Tetri BA, Lavine JE, Tonascia J, Unalp A, Van Natta M, Clark J, Brunt EM, Kleiner DE, Hoofnagle JH, and Robuck PR. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med 362: 1675–1685, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Schnabl B, Kweon YO, Frederick JP, Wang XF, Rippe RA, and Brenner DA. The role of Smad3 in mediating mouse hepatic stellate cell activation. Hepatology 34: 89–100, 2001 [DOI] [PubMed] [Google Scholar]
- 167.Scholten D, Reichart D, Paik YH, Lindert J, Bhattacharya J, Glass CK, Brenner DA, and Kisseleva T. Migration of fibrocytes in fibrogenic liver injury. Am J Pathol 179: 189–198, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Schroder K, Zhang M, Benkhoff S, Mieth A, Pliquett R, Kosowski J, Kruse C, Luedike P, Michaelis UR, Weissmann N, Dimmeler S, Shah AM, and Brandes RP. Nox4 is a protective reactive oxygen species generating vascular NADPH oxidase. Circ Res 110: 1217–1225, 2012 [DOI] [PubMed] [Google Scholar]
- 169.Sedeek M, Callera G, Montezano A, Gutsol A, Heitz F, Szyndralewiez C, Page P, Kennedy CR, Burns KD, Touyz RM, and Hebert RL. Critical role of Nox4-based NADPH oxidase in glucose-induced oxidative stress in the kidney: implications in type 2 diabetic nephropathy. Am J Physiol Renal Physiol 299: F134 8–F1358, 2010 [DOI] [PubMed] [Google Scholar]
- 170.Segal BH, Leto TL, Gallin JI, Malech HL, and Holland SM. Genetic, biochemical, and clinical features of chronic granulomatous disease. Medicine (Baltimore) 79: 170–200, 2000 [DOI] [PubMed] [Google Scholar]
- 171.Seki E. and Brenner DA. Toll-like receptors and adaptor molecules in liver disease: update. Hepatology 48: 322–335, 2008 [DOI] [PubMed] [Google Scholar]
- 172.Seki E, De Minicis S, Osterreicher CH, Kluwe J, Osawa Y, Brenner DA, and Schwabe RF. TLR4 enhances TGF-beta signaling and hepatic fibrosis. Nat Med 13: 1324–1332, 2007 [DOI] [PubMed] [Google Scholar]
- 173.Serrander L, Cartier L, Bedard K, Banfi B, Lardy B, Plastre O, Sienkiewicz A, Forro L, Schlegel W, and Krause KH. NOX4 activity is determined by mRNA levels and reveals a unique pattern of ROS generation. Biochem J 406: 105–114, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Seshiah PN, Weber DS, Rocic P, Valppu L, Taniyama Y, and Griendling KK. Angiotensin II stimulation of NAD(P)H oxidase activity: upstream mediators. Circ Res 91: 406–413, 2002 [DOI] [PubMed] [Google Scholar]
- 175.Sheppard D. Transforming growth factor beta: a central modulator of pulmonary and airway inflammation and fibrosis. Proc Am Thorac Soc 3: 413–417, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Sodeman T, Bronk SF, Roberts PJ, Miyoshi H, and Gores GJ. Bile salts mediate hepatocyte apoptosis by increasing cell surface trafficking of Fas. Am J Physiol Gastrointest Liver Physiol 278: G99 2–G999, 2000 [DOI] [PubMed] [Google Scholar]
- 177.Sommerfeld A, Reinehr R, and Haussinger D. Bile acid-induced epidermal growth factor receptor activation in quiescent rat hepatic stellate cells can trigger both proliferation and apoptosis. J Biol Chem 284: 22173–22183, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Sookoian S, Fernandez MA, and Castano G. Effects of six months losartan administration on liver fibrosis in chronic hepatitis C patients: a pilot study. World J Gastroenterol 11: 7560–7563, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Sturrock A, Cahill B, Norman K, Huecksteadt TP, Hill K, Sanders K, Karwande SV, Stringham JC, Bull DA, Gleich M, Kennedy TP, and Hoidal JR. Transforming growth factor-beta1 induces Nox4 NAD(P)H oxidase and reactive oxygen species-dependent proliferation in human pulmonary artery smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 290: L66 1–L673, 2006 [DOI] [PubMed] [Google Scholar]
- 180.Svegliati-Baroni G, Ridolfi F, Hannivoort R, Saccomanno S, Homan M, De Minicis S, Jansen PL, Candelaresi C, Benedetti A, and Moshage H. Bile acids induce hepatic stellate cell proliferation via activation of the epidermal growth factor receptor. Gastroenterology 128: 1042–1055, 2005 [DOI] [PubMed] [Google Scholar]
- 181.Terui Y, Saito T, Watanabe H, Togashi H, Kawata S, Kamada Y, and Sakuta S. Effect of angiotensin receptor antagonist on liver fibrosis in early stages of chronic hepatitis C. Hepatology 36: 1022, 2002 [DOI] [PubMed] [Google Scholar]
- 182.Thimme R, Oldach D, Chang KM, Steiger C, Ray SC, and Chisari FV. Determinants of viral clearance and persistence during acute hepatitis C virus infection. J Exp Med 194: 1395–1406, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Thoren F, Romero A, Lindh M, Dahlgren C, and Hellstrand K. A hepatitis C virus-encoded, nonstructural protein (NS3) triggers dysfunction and apoptosis in lymphocytes: role of NADPH oxidase-derived oxygen radicals. J Leukoc Biol 76: 1180–1186, 2004 [DOI] [PubMed] [Google Scholar]
- 184.Touyz RM, Chen X, Tabet F, Yao G, He G, Quinn MT, Pagano PJ, and Schiffrin EL. Expression of a functionally active gp91phox-containing neutrophil-type NAD(P)H oxidase in smooth muscle cells from human resistance arteries: regulation by angiotensin II. Circ Res 90: 1205–1213, 2002 [DOI] [PubMed] [Google Scholar]
- 185.Ueberham E, Low R, Ueberham U, Schonig K, Bujard H, and Gebhardt R. Conditional tetracycline-regulated expression of TGF-beta1 in liver of transgenic mice leads to reversible intermediary fibrosis. Hepatology 37: 1067–1078, 2003 [DOI] [PubMed] [Google Scholar]
- 186.Ushio-Fukai M. Compartmentalization of redox signaling through NADPH oxidase-derived ROS. Antioxid Redox Signal 11: 1289–1299, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Ushio-Fukai M. Redox signaling in angiogenesis: role of NADPH oxidase. Cardiovasc Res 71: 226–235, 2006 [DOI] [PubMed] [Google Scholar]
- 188.von Lohneysen K, Noack D, Jesaitis AJ, Dinauer MC, and Knaus UG. Mutational analysis reveals distinct features of the Nox4-p22 phox complex. J Biol Chem 283: 35273–35282, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.von Lohneysen K, Noack D, Wood MR, Friedman JS, and Knaus UG. Structural insights into Nox4 and Nox2: motifs involved in function and cellular localization. Mol Cell Biol 30: 961–975, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Wang B, Trippler M, Pei R, Lu M, Broering R, Gerken G, and Schlaak JF. Toll-like receptor activated human and murine hepatic stellate cells are potent regulators of hepatitis C virus replication. J Hepatol 51: 1037–1045, 2009 [DOI] [PubMed] [Google Scholar]
- 191.Wei HS, Li DG, Lu HM, Zhan YT, Wang ZR, Huang X, Zhang J, Cheng JL, and Xu QF. Effects of AT1 receptor antagonist, losartan, on rat hepatic fibrosis induced by CCl(4). World J Gastroenterol 6: 540–545, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Wheeler MD, Kono H, Yin M, Nakagami M, Uesugi T, Arteel GE, Gabele E, Rusyn I, Yamashina S, Froh M, Adachi Y, Iimuro Y, Bradford BU, Smutney OM, Connor HD, Mason RP, Goyert SM, Peters JM, Gonzalez FJ, Samulski RJ, and Thurman RG. The role of Kupffer cell oxidant production in early ethanol-induced liver disease. Free Radic Biol Med 31: 1544–1549, 2001 [DOI] [PubMed] [Google Scholar]
- 193.Winterbourn CC. and Metodiewa D. Reactivity of biologically important thiol compounds with superoxide and hydrogen peroxide. Free Radic Biol Med 27: 322–328, 1999 [DOI] [PubMed] [Google Scholar]
- 194.Wu RF, Gu Y, Xu YC, Nwariaku FE, and Terada LS. Vascular endothelial growth factor causes translocation of p47phox to membrane ruffles through WAVE1. J Biol Chem 278: 36830–36840, 2003 [DOI] [PubMed] [Google Scholar]
- 195.Wu RF, Ma Z, Liu Z, and Terada LS. Nox4-derived H2O2 mediates endoplasmic reticulum signaling through local Ras activation. Mol Cell Biol 30: 3553–3568, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Wu RF, Xu YC, Ma Z, Nwariaku FE, Sarosi GA, Jr., and Terada LS. Subcellular targeting of oxidants during endothelial cell migration. J Cell Biol 171: 893–904, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Yan X, Lin Z, Chen F, Zhao X, Chen H, Ning Y, and Chen YG. Human BAMBI cooperates with Smad7 to inhibit transforming growth factor-beta signaling. J Biol Chem 284: 30097–30104, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Yang L. and Seki E. Toll-like receptors in liver fibrosis: cellular crosstalk and mechanisms. Front Physiol 3: 138, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Yang L, Bataller R, Dulyx J, Coffman TM, Gines P, Rippe RA, and Brenner DA. Attenuated hepatic inflammation and fibrosis in angiotensin type 1a receptor deficient mice. J Hepatol 43: 317–323, 2005 [DOI] [PubMed] [Google Scholar]
- 200.Yokohama S, Yoneda M, Haneda M, Okamoto S, Okada M, Aso K, Hasegawa T, Tokusashi Y, Miyokawa N, and Nakamura K. Therapeutic efficacy of an angiotensin II receptor antagonist in patients with nonalcoholic steatohepatitis. Hepatology 40: 1222–1225, 2004 [DOI] [PubMed] [Google Scholar]
- 201.Yoshiji H, Kuriyama S, Yoshii J, Ikenaka Y, Noguchi R, Nakatani T, Tsujinoue H, and Fukui H. Angiotensin-II type 1 receptor interaction is a major regulator for liver fibrosis development in rats. Hepatology 34: 745–750, 2001 [DOI] [PubMed] [Google Scholar]
- 202.Zarski JP, Sturm N, Desmorat H, Melin P, Raabe JJ, Bonny C, Sogni P, Pinta A, Rouanet S, Babany G, Cheveau A, and Chevallier M. Non-invasive assessment of liver fibrosis progression in hepatitis C patients retreated for 96 weeks with antiviral therapy: a randomized study. Liver Int 30: 1049–1058, 2010 [DOI] [PubMed] [Google Scholar]
- 203.Zhan SS, Jiang JX, Wu J, Halsted C, Friedman SL, Zern MA, and Torok NJ. Phagocytosis of apoptotic bodies by hepatic stellate cells induces NADPH oxidase and is associated with liver fibrosis in vivo. Hepatology 43: 435–443, 2006 [DOI] [PubMed] [Google Scholar]
- 204.Zhang DY. and Friedman SL. Fibrosis-dependent mechanisms of hepatocarcinogenesis. Hepatology 56: 769–775, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Zhang L, Nguyen MV, Lardy B, Jesaitis AJ, Grichine A, Rousset F, Talbot M, Paclet MH, Qian G, and Morel F. New insight into the Nox4 subcellular localization in HEK293 cells: first monoclonal antibodies against Nox4. Biochimie 93: 457–468, 2011 [DOI] [PubMed] [Google Scholar]
- 206.Zuo L, Ushio-Fukai M, Ikeda S, Hilenski L, Patrushev N, and Alexander RW. Caveolin-1 is essential for activation of Rac1 and NAD(P)H oxidase after angiotensin II type 1 receptor stimulation in vascular smooth muscle cells: role in redox signaling and vascular hypertrophy. Arterioscler Thromb Vasc Biol 25: 1824–1830, 2005 [DOI] [PubMed] [Google Scholar]