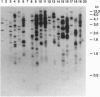
Abstract
DNA molecules undergoing transformation into yeast are highly recombinogenic, even when diverged. We reasoned that transformation-associated recombination (TAR) could be employed to clone large DNAs containing repeat sequences, thereby eliminating the need for in vitro enzymatic reactions such as restriction and ligation and reducing the amount of DNA handling. Gently isolated human DNA was transformed directly into yeast spheroplasts along with two genetically marked (M1 and M2) linearized vectors that contained a human Alu sequence at one end and a telomere sequence at the other end (Alu-CEN-M1-TEL and Alu-M2-TEL). Nearly all the M1-selected transformants had yeast artificial chromosomes (YACs) containing human DNA inserts that varied in size from 70 kb to > 600 kb. Approximately half of these had also acquired the unselected M2 marker. The mitotic segregational stability of YACs generated from one (M1) or two (M1 and M2) vector(s) was comparable, suggesting de novo generation of telomeric ends. Since no YACs were isolated when rodent DNAs or a vector lacking an Alu sequence was used, the YACs were most likely the consequence of TAR between the repeat elements on the vector(s) and the human DNA. Using the BLUR13 Alu-containing vector, we demonstrated that human DNA could be efficiently cloned from mouse cells that contained a single human chromosome 16. The distribution of cloned DNAs on chromosome 16 was determined by fluorescence in situ hybridization. We propose that TAR cloning can provide an efficient means for generating YACs from specific chromosomes and subchromosome fragments and that TAR cloning may be useful for isolating families of genes and specific genes from total genome DNA.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abidi F. E., Wada M., Little R. D., Schlessinger D. Yeast artificial chromosomes containing human Xq24-Xq28 DNA: library construction and representation of probe sequences. Genomics. 1990 Jul;7(3):363–376. doi: 10.1016/0888-7543(90)90170-y. [DOI] [PubMed] [Google Scholar]
- Aguinaga M. P., Kiper C. E., Valenzuela M. S. Enriched autonomously replicating sequences in a nuclear matrix-DNA complex isolated from synchronized HeLa cells. Biochem Biophys Res Commun. 1987 Apr 29;144(2):1018–1024. doi: 10.1016/s0006-291x(87)80065-7. [DOI] [PubMed] [Google Scholar]
- Burke D. T., Carle G. F., Olson M. V. Cloning of large segments of exogenous DNA into yeast by means of artificial chromosome vectors. Science. 1987 May 15;236(4803):806–812. doi: 10.1126/science.3033825. [DOI] [PubMed] [Google Scholar]
- Carle G. F., Olson M. V. Separation of chromosomal DNA molecules from yeast by orthogonal-field-alternation gel electrophoresis. Nucleic Acids Res. 1984 Jul 25;12(14):5647–5664. doi: 10.1093/nar/12.14.5647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson J. R., Johnston M. Direct cloning of yeast genes from an ordered set of lambda clones in Saccharomyces cerevisiae by recombination in vivo. Genetics. 1993 May;134(1):151–157. doi: 10.1093/genetics/134.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke Y., Zimmer M. Construction of a human MluI YAC library. Genomics. 1994 May 1;21(1):58–62. doi: 10.1006/geno.1994.1224. [DOI] [PubMed] [Google Scholar]
- Green E. D., Riethman H. C., Dutchik J. E., Olson M. V. Detection and characterization of chimeric yeast artificial-chromosome clones. Genomics. 1991 Nov;11(3):658–669. doi: 10.1016/0888-7543(91)90073-n. [DOI] [PubMed] [Google Scholar]
- Hamada H., Petrino M. G., Kakunaga T. A novel repeated element with Z-DNA-forming potential is widely found in evolutionarily diverse eukaryotic genomes. Proc Natl Acad Sci U S A. 1982 Nov;79(21):6465–6469. doi: 10.1073/pnas.79.21.6465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ketner G., Spencer F., Tugendreich S., Connelly C., Hieter P. Efficient manipulation of the human adenovirus genome as an infectious yeast artificial chromosome clone. Proc Natl Acad Sci U S A. 1994 Jun 21;91(13):6186–6190. doi: 10.1073/pnas.91.13.6186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korenberg J. R., Chen X. N. Human cDNA mapping using a high-resolution R-banding technique and fluorescence in situ hybridization. Cytogenet Cell Genet. 1995;69(3-4):196–200. doi: 10.1159/000133962. [DOI] [PubMed] [Google Scholar]
- Korenberg J. R., Rykowski M. C. Human genome organization: Alu, lines, and the molecular structure of metaphase chromosome bands. Cell. 1988 May 6;53(3):391–400. doi: 10.1016/0092-8674(88)90159-6. [DOI] [PubMed] [Google Scholar]
- Kouprina N., Eldarov M., Moyzis R., Resnick M., Larionov V. A model system to assess the integrity of mammalian YACs during transformation and propagation in yeast. Genomics. 1994 May 1;21(1):7–17. doi: 10.1006/geno.1994.1218. [DOI] [PubMed] [Google Scholar]
- Larionov V., Kouprina N., Nikolaishvili N., Resnick M. A. Recombination during transformation as a source of chimeric mammalian artificial chromosomes in yeast (YACs). Nucleic Acids Res. 1994 Oct 11;22(20):4154–4162. doi: 10.1093/nar/22.20.4154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick M. K., Campbell E., Deaven L., Moyzis R. Low-frequency chimeric yeast artificial chromosome libraries from flow-sorted human chromosomes 16 and 21. Proc Natl Acad Sci U S A. 1993 Feb 1;90(3):1063–1067. doi: 10.1073/pnas.90.3.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mézard C., Pompon D., Nicolas A. Recombination between similar but not identical DNA sequences during yeast transformation occurs within short stretches of identity. Cell. 1992 Aug 21;70(4):659–670. doi: 10.1016/0092-8674(92)90434-e. [DOI] [PubMed] [Google Scholar]
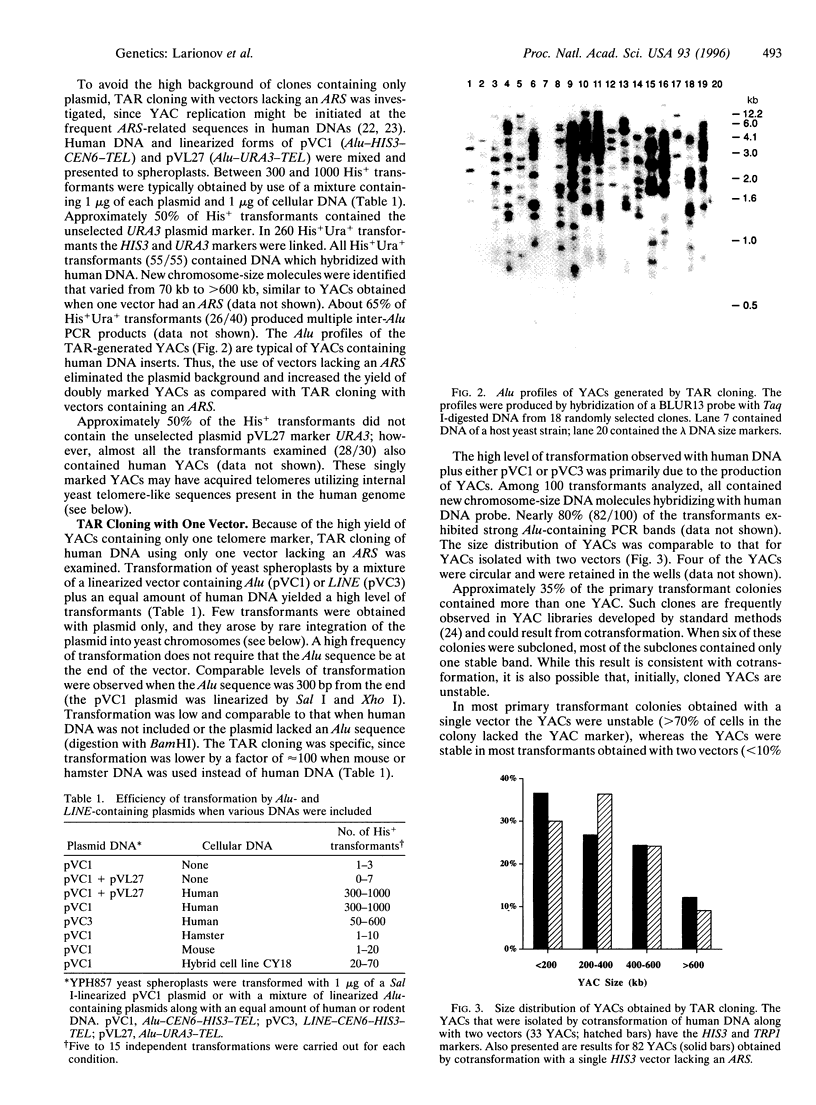
- Nelson D. L., Ledbetter S. A., Corbo L., Victoria M. F., Ramírez-Solis R., Webster T. D., Ledbetter D. H., Caskey C. T. Alu polymerase chain reaction: a method for rapid isolation of human-specific sequences from complex DNA sources. Proc Natl Acad Sci U S A. 1989 Sep;86(17):6686–6690. doi: 10.1073/pnas.86.17.6686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavan W. J., Hieter P., Reeves R. H. Generation of deletion derivatives by targeted transformation of human-derived yeast artificial chromosomes. Proc Natl Acad Sci U S A. 1990 Feb;87(4):1300–1304. doi: 10.1073/pnas.87.4.1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavan W. J., Hieter P., Sears D., Burkhoff A., Reeves R. H. High-efficiency yeast artificial chromosome fragmentation vectors. Gene. 1991 Sep 30;106(1):125–127. doi: 10.1016/0378-1119(91)90576-w. [DOI] [PubMed] [Google Scholar]
- Priebe S. D., Westmoreland J., Nilsson-Tillgren T., Resnick M. A. Induction of recombination between homologous and diverged DNAs by double-strand gaps and breaks and role of mismatch repair. Mol Cell Biol. 1994 Jul;14(7):4802–4814. doi: 10.1128/mcb.14.7.4802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandell L. L., Zakian V. A. Loss of a yeast telomere: arrest, recovery, and chromosome loss. Cell. 1993 Nov 19;75(4):729–739. doi: 10.1016/0092-8674(93)90493-a. [DOI] [PubMed] [Google Scholar]
- Schlessinger D., Little R. D., Freije D., Abidi F., Zucchi I., Porta G., Pilia G., Nagaraja R., Johnson S. K., Yoon J. Y. Yeast artificial chromosome-based genome mapping: some lessons from Xq24-q28. Genomics. 1991 Dec;11(4):783–793. doi: 10.1016/0888-7543(91)90001-u. [DOI] [PubMed] [Google Scholar]
- Shepherd N. S., Smoller D. The P1 vector system for the preparation and screening of genomic libraries. Genet Eng (N Y) 1994;16:213–228. [PubMed] [Google Scholar]
- Shero J. H., McCormick M. K., Antonarakis S. E., Hieter P. Yeast artificial chromosome vectors for efficient clone manipulation and mapping. Genomics. 1991 Jun;10(2):505–508. doi: 10.1016/0888-7543(91)90343-d. [DOI] [PubMed] [Google Scholar]
- Shizuya H., Birren B., Kim U. J., Mancino V., Slepak T., Tachiiri Y., Simon M. Cloning and stable maintenance of 300-kilobase-pair fragments of human DNA in Escherichia coli using an F-factor-based vector. Proc Natl Acad Sci U S A. 1992 Sep 15;89(18):8794–8797. doi: 10.1073/pnas.89.18.8794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikorski R. S., Hieter P. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics. 1989 May;122(1):19–27. doi: 10.1093/genetics/122.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stallings R. L., Doggett N. A., Okumura K., Ward D. C. Chromosome 16-specific repetitive DNA sequences that map to chromosomal regions known to undergo breakage/rearrangement in leukemia cells. Genomics. 1992 Jun;13(2):332–338. doi: 10.1016/0888-7543(92)90249-r. [DOI] [PubMed] [Google Scholar]
- Stinchcomb D. T., Thomas M., Kelly J., Selker E., Davis R. W. Eukaryotic DNA segments capable of autonomous replication in yeast. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4559–4563. doi: 10.1073/pnas.77.8.4559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada M., Abe K., Okumura K., Taguchi H., Kohno K., Imamoto F., Schlessinger D., Kuwano M. Chimeric YACs were generated at unreduced rates in conditions that suppress coligation. Nucleic Acids Res. 1994 May 11;22(9):1651–1654. doi: 10.1093/nar/22.9.1651. [DOI] [PMC free article] [PubMed] [Google Scholar]