Abstract
Significance: Numerous studies in animal models and human subjects corroborate that elevated levels of reactive oxygen species (ROS) play a pivotal role in the progression of multiple diseases. As a major source of ROS in many organ systems, the NADPH oxidase (Nox) has become a prime target for therapeutic development. Recent Advances: In recent years, intense efforts have been dedicated to the development of pan- and isoform-specific Nox inhibitors as opposed to antioxidants that proved ineffective in clinical trials. Over the past decade, an array of compounds has been proposed in an attempt to fill this void. Critical Issues: Although many of these compounds have proven effective as Nox enzyme family inhibitors, isoform specificity has posed a formidable challenge to the scientific community. This review surveys the most prominent Nox inhibitors, and discusses potential isoform specificity, known mechanisms of action, and shortcomings. Some of these inhibitors hold substantial promise as targeted therapeutics. Future Directions: Increased insight into the mechanisms of action and regulation of this family of enzymes as well as atomic structures of key Nox subunits are expected to give way to a broader spectrum of more potent, efficacious, and specific molecules. These lead molecules will assuredly serve as a basis for drug development aimed at treating a wide array of diseases associated with increased Nox activity. Antioxid. Redox Signal. 20, 2741–2754.
Introduction
Oxidative stress is manifested by a shift in the steady-state balance between the production of reactive oxygen or nitrogen species (ROS/RNS) and the antioxidant reserves of a biological system. When the proper cellular redox homeostasis is maintained, low levels of ROS evidently play an essential role as second messengers in myriad inter- and intracellular signaling cascades regulating neuronal signaling, blood pressure, and balance (52, 133). However, following a shift toward an increasingly pro-oxidant state, cells may succumb to an inexorable impairment of function and damage as a consequence of excessive protein and lipid oxidation, and DNA damage. Among the leading causes of death that afflict the U.S. population (72), cardiovascular diseases, neurodegenerative disorders, and cancer appear to share oxidative stress as a common nexus (18).
Excessive and unabated levels of ROS have been shown to play a key role in the pathophysiology of cardiovascular diseases, such as hypertension (6, 53, 99, 115, 160), atherosclerosis (149), cardiac hypertrophy (14), stroke (82), and conditions including ischemia reperfusion (110), and restenosis (76, 104). Moreover, the neurodegenerative Huntington's (164), Alzheimer's (9), and Parkinson's diseases have augmented ROS implicated in their etiology (27, 154). Further, evidence for the involvement of ROS in the progression of carcinogenesis is also demonstrated (172). Indeed, ROS can lead to oxidation of DNA resulting in gene mutations, duplication, and activation of oncogenes (87).
However, physiological ROS levels are demonstrated to regulate signaling pathways (52, 133) via thiol modification of redox-sensitive proteins, resulting in conformational changes that alter enzymatic activity (kinases and phosphatases involved in growth factor signaling) or DNA binding of activated transcription factors, such as NFκB and AP-1 (129, 176). The main cellular defense mechanisms that protect against increased ROS levels are antioxidant enzymes, such as superoxide dismutase, catalase, glutathione peroxidases, and thioredoxin as well as dietary scavengers, including α-tocopherol and ascorbic acid (52). Importantly, their roles in the neutralization of ROS derived from cellular respiration and other enzymatic sources, such as xanthine oxidase, uncoupled NO synthase, and, most important to this review, NADPH oxidase (Nox) are well established (47). Under normal homeostatic conditions, organ systems utilize these antioxidant systems to maintain the redox balance. Increasing evidence demonstrates Nox as a main cellular source of ROS, playing an important role in ROS-dependent signaling cascades (20, 98). Moreover, it is becoming increasingly evident that augmented ROS production by the Nox family of proteins promotes activation and upregulation of Nox isoforms in a “feed-forward” mechanism further contributing to oxidative stress and disease progression (40, 98).
Nox Family of Proteins
Nox enzymes belong to a closely related family of membrane proteins that catalyze the production of superoxide anion and/or hydrogen peroxide by electron transfer from NADPH to molecular oxygen via heme groups in their transmembrane domains, utilizing FAD as a cofactor. To date, seven members of the Nox family have been identified, namely, Nox1, Nox2, Nox3, Nox4, Nox5, Duox1, and Duox2. The isoforms differ in their subunit composition, activation, physiological and pathophysiological functions, and in their subcellular and tissue expression (25, 92) (Table 1). For more extensive details on the structure and activation of Nox isoforms, please refer to previous reviews (2, 13, 59, 66, 103, 152). The most well-studied Nox isozyme to date is the respiratory burst enzyme Nox2 (aka gp91phox) originally identified in phagocytes (141) where it has been well characterized (33) and subsequently discovered in a wide range of other cell types (65, 114, 122, 123, 131). The catalytic core of Nox2 consists of two membrane-spanning subunits that together form the cytochrome b558: one being the Nox2 subunit, a six transmembrane spanning protein containing NADPH- and FAD-binding sites on its C-terminal tail, and the other p22phox, its stabilizing cohort. The activity of Nox2 is regulated by the cytosolic subunits p47phox (organizer subunit), p67phox (activator subunit), and p40phox, and the small Rho-family GTP-binding protein Rac2 or Rac1. Beyond its major role in innate immune defense mechanisms, Nox2 is also involved in a wide array of well-regulated physiological processes in the cardiovascular system (139) and in the brain (148). Increased expression or deregulation of Nox2 in those tissues elicits profound health implications (18). The Nox1 system is comprised of membrane subunits Nox1 and p22phox and in its canonical conformation is regulated by cytosolic NOXO1 organizer (homologous to p47phox), NOXA1 activator (homologous to p67phox), and Rac1. However, increasing evidence implicates p47phox-induced activation of the hybrid-Nox1 system in a variety of disease settings (35, 101, 120). As Nox1, Nox3 requires p22phox and can be regulated by NOXA1 and NOXO1 but also by p47phox and p67phox (26, 163). Nox4, on the other hand, does require p22phox but the only other known regulator described for it is Poldip2. Nox5, distinct from Nox1–4, does not require p22phox and is regulated by calcium through EF-hand motifs present in its N-terminal region (11). Interestingly, Nox5 is the only isoform that is not expressed universally across mammalian species; that is, the Nox5 gene is absent in mouse and rat genome (12). Importantly, Nox5 is expressed in human vasculature and is abundant in lymphoid tissue and testes where it may be involved in spermatogenesis (11, 12, 25, 54, 142). Like Nox5, Duox1 and Duox2 do not require p22phox for activity and they also possess EF-hands, which render them Ca2+-dependent enzymes (7). Distinguishing characteristics in Duox1 and 2 versus other members of the Nox family are that they contain seven membrane-spanning domains and a peroxidase-like domain in their extracellular N-terminal region. At this juncture, it is important to assert that an individual Nox isoform could have unique and opposing effects in a species-, cell-, and tissue-dependent manner. For a comprehensive review of the role each Nox isoform plays in cellular physiology and signaling cascades, the readers are directed to some recent reviews (93, 101) and other articles in this issue.
Table 1.
Nox Family of Proteins
| Nox isozyme | Subunits/regulators | Tissue expression/ROS type | Main features |
|---|---|---|---|
| Nox1 | p22phox, NOXO1, NOXA1, Rac1 (canonical), or p22phox, p47phox, NOXA1, Rac1 (hybrid) | Heart, lungs, blood vessels, brain. | Depending on the cell type can utilize varied cytosolic subunits (canonical or hybrid). The canonical system is constitutively active. Involved in necrosis, hypertrophy, migration, and tissue growth. |
| Superoxide anion. | |||
| Nox2 | p22phox, p47phox, p67phox, p40phox, Rac1, or Rac2 | Innate immune cells, heart, lungs, blood vessels, brain. | Prototype Nox. Tightly regulated. Involved in immune defense, angiogenesis, neurodegenerative disorders, and necrosis. |
| Superoxide anion. | |||
| Nox3 | p22phox, NOXO1, NOXA1 (p47/p67), Rac1 | Inner ear (cochlear and vestibular sensory epithelia). | Constitutively active. Involved in balance, otoconia biosynthesis, and potentially hearing loss. |
| Superoxide anion. | |||
| Nox4 | p22phox, Poldip2 | Heart, lungs, blood vessels, kidney, prostate. | Involved in differentiation, migration, growth, and survival. |
| Hydrogen peroxide (?). | |||
| Nox5 | No subunit identified to date. Regulated by Ca2+ | Cardiovascular system, spleen lymph nodes, testes. | EF hand domains. Involved in inflammatory gene expression, growth, and proliferation. |
| Superoxide anion. | |||
| Duox1 | DUOXA1 (for maturation) | Thyroid gland, airway epithelium, cerebellum, testis, prostate. | EF hand domains and N-terminal peroxidase-like domain. Involved in thyroid function. |
| Hydrogen peroxide. | |||
| Duox2 | DUOXA2 (for maturation) | Thyroid gland, airway epithelium, gastrointestinal epithelia, uterus, gallbladder. | EF hand domains and N-terminal peroxidase-like domain. Involved in thyroid hormone synthesis. |
| Hydrogen peroxide. |
(?)=noted controversy as to whether H2O2 or superoxide is the primary metabolite.
Nox, NADPH oxidase; ROS, reactive oxygen species.
Free-Radical “Scavengers” Versus Nox Inhibitors
Given the plethora of diseases involving oxidative stress and Nox, in particular, the need for delicate maintenance of cellular redox balance and prevention of harmful overproduction of ROS has become increasingly clear (165). Initial attempts to achieve this goal and prevent or reverse the damaging effects of ROS involved the use of antioxidants, such as vitamins E, C, and A, which in vitro and in animal studies proved beneficial (8, 162). Moreover, many observational studies over the years have propounded the benefits of an antioxidant-rich, for example, Mediterranean diet (83). However, data from randomized clinical trials using these vitamins reported no effects in improving cardiovascular disease outcomes and in some cases have paradoxically indicated deleterious effects. Two of the most notorious of these include reports of elevated heart failure rates in patients who took high dose of vitamin E (108) and an increased incidence of cancer in smokers supplemented with beta-carotene (68). The reason for the failure of these clinical trials is not entirely clear, but a general consensus in the field points to multiple flaws in study design (28). Moreover, scavenging a particular ROS moiety without regard for the potential consequence of generating another reactive species metabolite is seriously misguided (36, 159), and perhaps the most obvious of these flaws is our contention of an inadvertent disruption of salutary ROS signaling in clinical studies (28). In that regard, the popular mantra of “more is better” should be treated with healthy skepticism when it comes to both antioxidants and Nox inhibitors. Further, a growing body of evidence demonstrates a beneficial role for exogenously supplied catalase or the ROS scavenger Tempol in inhibiting the oxidative burden associated with “feed-forward” Nox activation and expression both in vitro and in vivo (42, 111, 112). This may include redox-sensitive pathways upstream or linking one Nox to another. For example, ROS are known to activate key kinases, that is, c-Src (61), and/or elicit the release of calcium (180) that is essential for Nox5 and Duox activities. However, the prudent use and extent of global ROS scavenger administration remains important as many ROS-generating enzymes contribute to salutary redox-signaling pathways under normal physiological conditions. Thus, it would appear that the preferred strategy to combat the deleterious consequences of oxidative stress is to directly assess the source of ROS generation, which in most of the systems involves one or more of the Nox isozymes (48). In fact, a quest for specific Nox inhibitors has been underway for more than two decades (31) and presently, it has intensified across scientific disciplines. Indeed, targeting Nox activity has great therapeutic potential for numerous disorders, including pancreatic cancer (166), hypertension (28, 124), cystic fibrosis (130), Parkinson's disease (27), acute lung injury (22), pulmonary fibrosis (71), heart failure (179), ischemia/reperfusion injury (88), chronic kidney disease (62), and stroke-related neurodegeneration (132) to name just a few. Since the regulation and structure of Nox isozymes is complex, there are many possible strategies to achieve inhibition (138). For example, one could target upstream regulators such as receptor modulators of Nox activity, like the angiotensin receptor (24, 158) and calcium channel blockers (51, 75), or inhibit kinases involved in the recruitment of cytosolic subunits to their respective Nox subunit (84, 102). Further, modification of mitochondrial-Nox crosstalk is another suggested mode of Nox activity modulation (91, 94). However, more studies are required to fully elucidate the dynamic nature surrounding this process, as well as the Nox isoforms involved. Also, inhibiting transcription processes that increase Nox expression or increase degradation of Nox subunits would lead to attenuated ROS production. All such tactics are expected to be problematic as they are involved in many other signaling cascades independent of Nox and their inhibition is likely to cause untoward effects. Similarly, compounds that antagonize key regulatory subunits, such as p47phox, unless specifically targeting their interaction with other key Nox components (e.g., Nox2) may have undesirable effects as it has become evident that individual Nox subunits may have Nox-independent functions (126). Thus, the need to directly target Nox-specific ROS-generating activity comes into greater focus.
Development of Nox Inhibitors (Peptidic Versus Small-Molecule Inhibitors)
An ideal Nox inhibitor would not inhibit other sources of ROS such as xanthine oxidase, would be devoid of ROS-scavenging or cytotoxic properties, would efficaciously and specifically target a unique Nox isoform, would be druggable, and would possess ideal pharmacokinetic characteristics. Despite the intense efforts dedicated to the identification of such an inhibitor, the task has proven extremely difficult. To date several molecules have been identified as Nox inhibitors; however, most of these are not specific. Nevertheless, a few hold significant promise. Many reviews have been dedicated to describing the available Nox inhibitors in recent years (29, 48, 78, 85, 89) and since then relatively few new compounds have come to the forefront. The following discussion is an effort to be as comprehensive and up-to-date on these developments.
Peptidic inhibitors
Attempts to develop Nox-specific peptidic inhibitors have been made since 1990 (136, 143). Peptide inhibitors of Nox have been extensively reviewed elsewhere (29, 37, 50). Interestingly, many examples of peptidic inhibitors that span regions of the Nox subunits themselves have been reported by the Quinn (41) and Pick (38, 39, 117) laboratories using phage display and peptide-walking techniques. Among these, the first peptide to be developed as a cell-permeant Nox-Specific inhibitor and potential therapeutic is Nox2ds-tat ([H]-R-K-K-R-R-Q-R-R-R-C-S-T-R-I-R-R-Q-L-[NH2], previously gp91ds-tat) (134).
Nox2ds-tat
More than 10 years after its development, Nox2ds-tat remains a widely utilized isoform-specific inhibitor of Nox2-derived ROS. Nox2ds-tat is a chimeric peptide that contains a nine-amino-acid sequence recapitulated from the cytosolic loop B of Nox2 (amino acids 86–94, underlined) and a nine-amino-acid subdomain of HIV-tat transport region that confers the peptide the ability to be internalized by cells (134, 135). Nox2ds-tat specifically blocks the interaction between Nox2 and p47phox and selectively blocks Nox2 activity with an IC50 of 0.7 μM (35). Nox2ds-tat does not inhibit Nox1- or Nox4-derived ROS generation; neither has it any effect on xanthine oxidase activity (35). Perhaps the best evidence of its specificity is that this inhibitor does not inhibit the canonical Nox1 oxidase that utilizes NOXO1 or the hybrid Nox1 oxidase that utilizes p47phox as its organizing subunit. The inhibitory effects of Nox2ds-tat have widely been demonstrated both in vitro as well as in vivo. In vitro assays indicate that Nox2ds-tat inhibits superoxide anion production in endothelial cells in response to various stimuli, including nutrient deprivation (109), hypoxia (4), atrial natriuretic peptide (55), angiopoietin-1 (70), interleukin-4 (170), shear stress (49), and calcineurin inhibitors (95). Nox2ds-tat also blocks angiotensin II-induced superoxide production in human resistance artery smooth muscle cells (161) and collagen-induced Nox activity in platelets (96). In vivo, subcutaneous infusion of Nox2ds-tat attenuated superoxide production in a variety of disease models (45, 76, 81, 105, 106, 173). More recently, Nox2ds-tat has been used to demonstrate the role of Nox2 in insulin-resistance-related endothelial cell dysfunction (151), triggering of inflammasome activation (1), and to study the interplay between platelets, TNFα, and Nox2 in heart failure (21).
Theoretically, by their nature, peptides may be rationally designed to better target protein–protein interactions within the enzyme complex important for Nox activity and thus, uniquely block only those sites that are involved in the assembly of the active complex. In practice, however, this may not be as straight forward. For example, a similar strategy that we applied to the design of Nox2ds-tat did not yield peptide inhibitors for Nox4. As we explain in that study, this could possibly be explained by a tightly assembled and active conformation of Nox4 that, unlike other Noxes, cannot be disrupted by conventional means (34). As potential drug therapies, peptidic inhibitors have historically been disregarded for their perceived intrinsic disadvantages; one of the most important of which is their suspected inability to penetrate the plasma membrane, a property dependent on sequence, charge distribution, and hydrophobic properties. Peptidic inhibitors are also judged at a disadvantage due to their poor oral bioavailability since peptides are readily degraded in the digestive system. However, great strides have been made in recent years to develop stabilization methodologies and alternative delivery systems that are expected to obviate these limitations (116, 169). Moreover, studies in our laboratory have indicated promising preliminary outcomes of right heart and left heart function preservation in two distinct models of pulmonary hypertension by aerosolization of Nox2ds-tat in mice (unpublished observations). It remains to be seen, therefore, whether these exciting and promising results hold true under scrutiny.
Small-molecule inhibitors
Diphenylene iodonium and apocynin
The traditionally used inhibitors of Nox activity are diphenylene iodonium (DPI) and apocynin (Fig. 1). Although widely utilized to investigate the role of the Nox family of proteins in a given pathway, their many limitations are increasingly becoming evident as their mechanisms of action are being appreciated (3).
FIG. 1.
Traditional inhibitors of Nox. Nox, NADPH oxidase.
DPI was first identified as a potent Nox inhibitor in a cell-free preparation of neutrophil membranes (32, 46) and was shown to form adducts with FAD to inhibit ROS formation (121). However, despite the fact that DPI is also an irreversible and nonselective inhibitor of flavin-dependent enzymes, including nitric oxide synthase and xanthine oxidase, its inhibitory effects continue to be inappropriately interpreted as evidence of Nox activity. These off-target effects nullify its potential as a therapeutic candidate. That said, in a recent study, DPI and its analog di-2-thienyliodonium (DTI) were shown, in the nanomolar range, to exhibit antineoplastic properties, decreasing colon cancer cell proliferation by blocking cell cycle progression at the G1/S interface and not just to decrease ROS levels but also Nox1 expression (44). Perhaps the emphasis here should be placed on the use of very low concentrations of the iodoniums that may prove relatively selective for Nox versus other flavoproteins. Nevertheless, it is difficult to fathom the systemic administration of these agents in vivo where many other enzymes of importance could be inhibited.
Perhaps the most controversial inhibitor of Nox activity to date is apocynin. Unlike DPI, many of apocynin's users have assumed that its effects are specific to Nox based on early reports in cell-free systems in which it was shown to inhibit the translocation of p47phox to plasma membranes (thereby inhibiting Nox2 activation). Apocynin is a natural methoxy-substituted catechol isolated from Picrorhiza kurroa that was shown to inhibit superoxide anion production in vitro and to have anti-inflammatory activity in vivo (155). This inhibitory effect required, however, activation by myeloperoxidase (146). Thus, apocynin has little or no effect in cells that do not express this peroxidase activity, which likely describes its widely variable activity across cells, tissues, and species. Further, apocynin can act as an antioxidant, per se (73), and in fact, it has notable capacity (>100 μM) as a scavenger of nonradical oxidant species, such as HOCl and H2O2 (128). Many of the nonspecific effects of apocynin have been well documented elsewhere (3), so it is somewhat surprising that the compound continues to be widely used to dissect the role of Nox both in vitro as well as in vivo studies. Without question, the results obtained utilizing apocynin require careful interpretation when implicating a role of Nox, per se, in experimental studies. At minimum, its effects should be validated with a more specific Nox2 inhibitor or using cells genetically void of Nox. On a positive note, however, the knowledge that as a result of peroxidase metabolism, apocynin yields reactive quinones that bind cysteine residues in p47phox and block its translocation to the membrane has generated a model of interaction between apocynin analogs and p47phox using computational analysis (79). This model and computational screening could lead to the identification of new analogs that inhibit the hybrid Nox1 and canonical Nox2 (isoforms activated by p47phox) and do not exhibit the undesirable properties described for apocynin. Until that time, apocynin should not be classified as a Nox-specific inhibitor (73). In summary, this discussion is not intended to dismiss the many important studies that employ apocynin and implicate Nox involvement in a variety of cell-signaling pathways and pathologies. However, with the advent of more specific Nox isoform inhibitors, the use of apocynin should likely be avoided.
S17834 and AEBSF
Another compound potentially displaying a broad profile of effects is S17834 (23), a polyphenol inhibitor of superoxide formation by Nox with an IC50>25 μM, which also increases SIRT1 deacetylase activity, LKB1 phosphorylation at Ser428, and AMPK activity (74, 177, 178). Yet, despite its protective role in a range of vascular disorders, its precise mechanism of Nox modulation remains unknown. Additionally, AEBSF inhibits binding of cytochrome b558 to p47phox, but it is a known serine protease inhibitor (43). Resultantly, its use as a tool to delineate the precise role of Nox in disease models is limited due to its multiple effects in cells (175).
As the demand for Nox inhibitors has increased dramatically in recent years, major efforts have been dedicated to high-throughput screening (HTS) and development of orally bioavailable lead molecules both in academia and the pharmaceutical industry. From these laborious endeavors, a few compounds have emerged with therapeutic potential.
Triazolo pyrimidine derivatives: VAS2870 and VAS3947
Two triazolo pyrimidine derivatives VAS2870 and VAS3947 have been identified by Vasopharm as Nox inhibitors and at least VAS2870 has been made commercially available (Fig. 2). VAS2870 [3-benzyl-7-(2-benzoxazolyl)thio-1,2,3-triazolo[4,5-d]pyrimidine] was first characterized as a Nox inhibitor, without antioxidant properties or attenuation of xanthine oxidase activity, and was shown to effectively suppress growth factor-mediated ROS generation and vascular smooth muscle cell migration (156). In a neutrophil-cell-free system, VAS2870 inhibited superoxide production with an IC50 of 10.6 μM. These results appear inconsistent with findings from Gatto et al. who reported lack of inhibition of Nox2 by VAS2870 in a semipurified enzyme preparation (58). This difference may be a consequence of the dynamics of complex assembly that is dependent on the order of addition of the stimulus. VAS2870 (50 μM) also inhibits the stimulation of vasculogenesis by PDGF-BB of mouse embryonic stem cells (100), and it inhibits wound-margin H2O2 production without obvious toxicity in zebrafish larvae (119). At 10 μM it inhibits Nox activity in ox-LDL-exposed human umbilical vein endothelial cells (150). VAS2870 was shown to inhibit Nox4-derived ROS production in the sarcoplasmic reticulum of mammalian skeletal muscle and it abolished O2-coupled redox regulation of the ryanodine receptor-Ca2+ channel (RyR1). It was also found that VAS2870 directly caused thiol alkylation modification of RyR1 (153). Thus, the off-target effects of VAS2870, such as the direct effect on the cellular thiol redox status, must be taken into consideration when assessing the agent's utility. Nevertheless, VAS2870 should be considered a pan-Nox inhibitor, which in its own right may prove very useful in certain disease states. Indeed, it was shown to inhibit Nox1-, preassembled Nox2-, as well as Nox4- and Nox5-dependent ROS production (5). Similarly, VAS3947, a VAS2870 analog with improved solubility, is also considered a pan-Nox inhibitor as it inhibited ROS generation in three cellular models with varied expression patterns of all known Nox isoforms (175).
FIG. 2.
Triazolo pyrimidines.
Pyrazolopyridine derivatives: GK-136901 and GKT137831
HTS of small-molecule libraries and subsequent optimization of lead compounds has identified dual Nox1/4 inhibitors (56, 97). Among these are GKT136901 [2-(2-chlorophenyl)-4-methyl-5-(pyridin-2-ylmethyl)-1H-pyrazolo [4,3-c]pyridine-3,5(2H,5H)-dione] and GKT137831, which now represent the first orally-active dual Nox1 and 4 inhibitors (Fig. 3) (10, 63, 80). GKT137831 is a candidate drug currently being developed as a new therapy for diabetic nephropathy and reportedly undergoing phase I clinical testing (174). As the first pyrazolopyridine derivative described, GKT136901 was found to be a Nox inhibitor with high degree of potency for Nox4 (inhibitory constant [Ki]=165±5 nM) and Nox1 (Ki=160±10 nM) and with a 10-fold selectivity over Nox2 (Ki=1530±90 nM) while exhibiting no inhibitory effects on other ROS-producing enzymes, redox-sensitive enzymes, or other proteins (140). CD44–hyaluronic acid-dependent gene regulation role in atherosclerosis via Nox1/4 activation was first demonstrated by the attenuation of ROS generation and atherosclerosis by GKT136901 (168). Moreover, administration of GKT136901 reduced angiogenesis and tumor growth in vivo in a PPARα-dependent manner, confirming the role of Nox1 in endothelial cell migration and angiogenesis (57). GKT136901 has also been shown to inhibit Nox5 (Ki=∼450 nM) in a recent study by Musset et al. (118). The study used GKT136901 to investigate whether Nox5 accounts for Ca2+-dependent superoxide production in spermatozoa, where Nox1 or Nox4 is not detectable. GKT136901 completely inhibited sperm cell motility induced by hydrogen peroxide and other important cell functions mediated by Nox5 (118).
FIG. 3.
Pyrazolopyridine derivatives.
The second pyrazolopyridine derivative GKT137831 (Fig. 3) has been used to demonstrate the role of Nox in liver fibrosis and hepatocyte apoptosis (10, 80). Similar to GKT136901, GKT137831 was shown to be a highly potent inhibitor of human Nox4 (Ki=140±40 nM) and human Nox1 (Ki=110±30 nM) and was found to be at least 10-fold less potent on Nox2 (Ki=1750±700 nM) and 3-fold less potent on Nox5 (Ki=410±100 nM) (10). GKT137831 was also used to study the role of Nox4 in hypoxia-induced pulmonary vascular cell proliferation (64) where it attenuated hypoxia-induced H2O2 production, proliferation, and TGF-β1 expression. In the same study, it was shown in vitro to blunt reductions in PPARγ in HPAECs and HPASMCs, while in vivo, it inhibited hypoxia-induced increases in TGF-β1, reduced PPARγ expression, and attenuated right ventricular hypertrophy and pulmonary artery wall thickness (64). GKT137831 has also been used to investigate the role of Nox1 in diabetes-accelerated atherosclerosis as it prevented oxidative stress in response to hyperglycemia in human aortic endothelial cells (63). However, while these models of Nox-associated disease show a protective role of GKT136901 and GKT137831 by modulating ROS levels, their precise mode of inhibition remains to be demonstrated. A new report that addresses the pharmacology of this compound provides evidence of its property as a selective scavenger of peroxynitrite (137).
ML171
To our knowledge, only one small-molecule isoform-specific Nox inhibitor has been reported so far, namely, ML171 (2-acetylphenothiazine) (Fig. 4). This compound belongs to a subset of phenothiazines and is identified as a selective Nox1 inhibitor. ML171 has an IC50 for Nox1 in the submicromolar range, that is, 0.129 μM in HT29 cells and 0.25 μM in a HEK293-Nox1 reconstituted cell system, ∼20-fold higher for Nox-2, −3, and −4 as well as for xanthine oxidase (60). ML171's mechanism of action may involve interaction with the Nox1 catalytic subunit since only overexpression of this subunit overcomes the ML171-dependent inhibition of ROS generation in heterologous Nox1-expressing HEK293 cells (60). Further, ML171 blocked Nox1-dependent extracellular matrix-degrading, actin-rich cellular structures (invadopodia) in colon cancer cells, providing support for the potential therapeutic use of this drug (60). Despite the commercial availability of this compound, only two studies to our knowledge have been published using ML171 (112). In that study, ML171 was utilized to investigate the reciprocal relationship between mitochondrial- and/or Nox-derived ROS and cyclooxygenase-2 in vascular dysfunction and hypertension. Another report utilized ML171 to inhibit collagen- and fibrinogen-dependent ROS production in platelets that express both Nox1 and Nox2 (167). In this study, 0.5 μM of ML171 totally abolished intracellular ROS production in response to platelet adhesion to either collagen or fibrinogen.
FIG. 4.
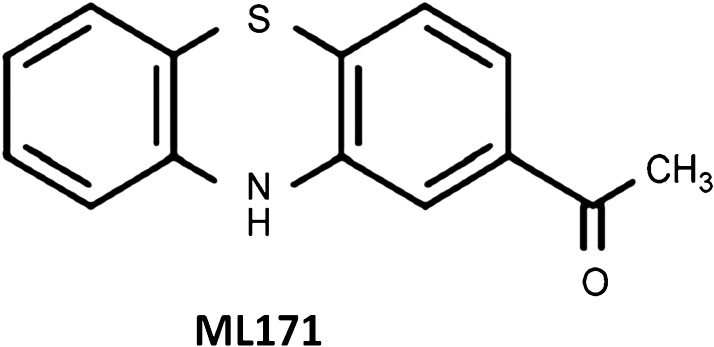
Phenothiazine derivative.
Fulvene-5
Triphenylmethane dyes, such as brilliant green and gentian violet that have chemical similarity to DPI were shown to be potent and efficacious inhibitors of Nox2 and Nox4 activity (127). Development of fulvene derivatives by a structure-based approach generated a new class of inhibitors. One of these derivatives is fulvene-5 (Fig. 5) that at 5 μM equally inhibited Nox2 and Nox4 in vitro, and when applied in vivo, successfully blocked the growth of endothelial tumors of mice (15). Reportedly, this was most likely through its ability to inhibit Nox4, since the effect of fulvene treatment was consistent with the results obtained using Nox4 shRNA (15). Since then, no further reports have been published; information about the specificity of fulvene-5, mechanism of action, and full pharmacological profile for potency, efficacy, and cytotoxicity are lacking.
FIG. 5.
Other Nox-inhibiting compounds.
Naloxone
Naloxone (Fig. 5), a commonly used antagonist of opioid receptors, was found to be highly effective in preventing dopaminergic degeneration in different models of rodent Parkinson's disease by inhibiting inflammatory responses (107). In search for the potential neuroprotective action of naloxone, it was found that it binds to Nox2 and blocks translocation of p47phox to the plasma membrane, leading to inhibition of ROS production (171). In fact, the neuroprotective effect of this drug is dependent on Nox2 but independent of opioid receptors since (+)-naloxone, the inactive isomer for the activation of opioid receptors, is as potent as (−)-naloxone at inhibiting ROS production and binding to Nox2. In fact, the IC50 for both isomers was closed to 2 μM. It is interesting to note that naloxone was also effective at inhibiting the activity of preassembled enzyme (171). That said, no reports could be found that compare its binding or inhibition of other Nox subunits.
Celastrol
A triterpenoid antioxidant compound celastrol (Fig. 5), isolated from the Chinese Thunder of God vine (Tripterygium wilfordi), has been used in traditional Chinese medicine for its beneficial and curative effects of various inflammatory diseases and cancer (19). Recently, it has been shown that celastrol is, in fact, a potent Nox inhibitor in general but with preference against Nox1 and Nox2 (Nox1 IC50=0.41±0.20 μM, Nox2 IC50=0.59±0.34 μM, Nox4 IC50=2.79±0.79 μM, and Nox5 IC50=3.13±0.85 μM) (77). Analysis of enzyme kinetics showed positive cooperativity in its inhibition of Nox1 and 2 activity (77). Paradoxically, celastrol was found to suppress the viability of breast cancer MCF-7 cells while stimulating ROS production (86). Further, Hansen et al. demonstrated that celastrol is able to act on several distinct stress response pathways and identified numerous cellular effects of celastrol (69), thus, questioning the value of this compound as a Nox-specific inhibitor. Nonetheless, it is possible that structural activity relationship (SAR) studies may identify analogs of celastrol that selectively inhibit Nox.
Ebselen
Ebselen is yet another compound that has been described as a Nox inhibitor but has previously been characterized as having unrelated effects (Fig. 5). Identified as a glutathione peroxidase mimetic (145), ebselen and its selenium- (but not sulfur-) containing analogs are able to consume hydrogen peroxide in a catalytic cycle that utilizes thiol-containing compounds, such as glutathione, as a substrate (125, 145). Recently however, using an in vitro fluorescence polarization Nox2 assay, Smith et al. identified ebselen and some of its analogs as potent Nox2 inhibitors that, depending on the congener, inhibited Nox1, 4, and 5 activity at substantially lower potency (147). One of these compounds, JM-77b, displays an especially attractive profile of selectivity for Nox2 (IC50=0.4 μM) compared with Nox1 (IC50=6.3 μM), Nox5 (IC50=17 μM), and Nox4 (no significant inhibition) (147). Smith et al. also showed that ebselen blocked the translocation of p47phox to neutrophil membranes, thus, interfering with the assembly of Nox2 and suggesting that ebselen and its congeners could represent a class of compounds with significant therapeutic potential (147). However, further studies are required to demonstrate the mechanism of action of ebselen, or its analogs, on p47phox activation due to the recent demonstration that p47phox may still translocate to the plasma membrane independently of the indicated ebselen site of inhibition (157). Moreover, until which time a congener can be found that is devoid of peroxidase mimetic activity, some caution should be applied when interpreting its effects.
Tetrahydroquinolines
Recently, work published by our group demonstrated the effect of bridged tetrahydroquinolines as selective Nox2 inhibitors (30). In this report, (±)-(1S,4R,9S)-5-bromo-3,3-dimethyl‐9-(2-methylallyl)-10-pentyl‐1,2,3,4-tetrahydro-1,4‐(epiminomethano)naphthalene (compound 11g) and (±)-(1S,4R,9S)-5-bromo-3,3-dimethyl-9-(2-methylallyl)-10-(thiophen-2-ylmethyl)-1,2,3,4-tetrahydro-1,4-(epiminomethano)naphthalene (compound 11h) (Fig. 6) exhibited selective inhibition of Nox2 in intact Cos-Nox2 cells stimulated with phorbol-12-myristate-13-acetate (IC50 20±1.9 and 32±1.9 μM, respectively). Importantly, these compounds were unable to inhibit ROS production from Nox1-, Nox4-, and Nox5-expressing cells and displayed no free-radical scavenging effect or cytotoxicity in preliminary screens. These early data suggest that bridged tetrahydroquinolines represent a new group of compounds with potential to serve as a platform for developing therapeutic agents for the treatment of Nox2-dependent oxidative stress disorders. Thus, full assessment of specificity, in vivo efficacy, and pharmacokinetic properties are warranted.
FIG. 6.
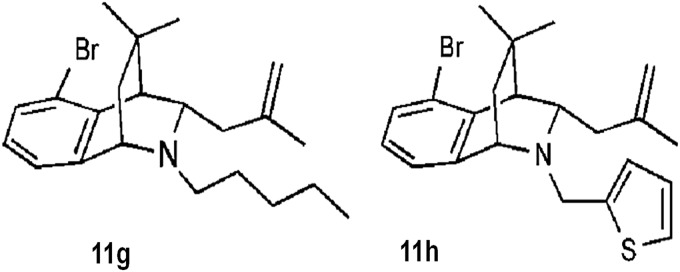
Tetrahydroquinolines.
Other Nox inhibitors
In a recent study, Borbely et al. (16) showed the methodical development of derivatives of primary hits obtained by an HTS campaign that searched for small-molecule inhibitors of Nox4. The most potent compounds belong to the following core structures: oxalyl hydrazides, flavonoids, oxindoles, benzoquinolines, and benzothiophenes. The best hit molecules identified by SAR analysis shared a 3D structure consisting of four pharmacophore points that feature hydrogen bond donors, acceptors, and two aromatic rings independent of the core structure. Although these studies shed light on the structural requirements for inhibitors under the conditions tested, there is no information to our knowledge on the specificity of these compounds for Nox4 as compared with other Noxes or other ROS-generating enzymes.
One of the major complications of HTS of small-molecule libraries is that once a hit is identified, it is necessary to rule out cell/organ toxicity before it can be deemed useful as a drug. In an attempt to avert this problem, screening a subset of natural compounds derived from edible plants that per se may not be toxic has been an alternative strategy. This strategy has been used to characterize inhibitors of Nox4 (90); as such, several diarylheptanoids and lignans were identified with relative specificity toward Nox4 and with potency comparable to that of GKT137831. One caveat, however, is that one compound isolated from or mimicking a naturally occurring moiety from an edible source may not, on its own, be nontoxic. More broadly speaking, simple designation as “naturally occurring” does not necessarily deem a compound safe. If that were the case, venoms and plant-derived toxins would be considered intrinsically safe for humans. Therefore, while the concept of such exploration from natural products is rational, exciting, and potentially fruitful, it does not come without risk.
The last compound to be discussed here, although yet to be referred to as a Nox inhibitor, is 3-amino-3-(4-fluoro-phenyl)-1H-quinoline-2,4-dione; a novel synthetic compound that inhibits cisplatin-induced hearing loss by suppression of ROS, implicating perhaps inhibition of Nox3 function (144). Further analysis of this compound in terms of its direct effect on Nox3 versus other Nox isoforms is necessary.
Future Considerations for Specific Nox Inhibitors
Current Nox inhibitor strategies look to characterize inhibition as a read-out of modulated Nox-derived ROS in a variety of cell types and cellular systems that overexpress a single Nox isoform, followed by cytotoxicity analysis (56, 60, 156). However, little mechanistic data are demonstrated for their mode of inhibition and therefore their characterization remains incomplete. To date, many Nox inhibitors display a lack of specificity for a single Nox isoform (48). Interestingly, of the developed Nox inhibitors, many compounds share structural similarities to nucleotides; therefore, from the interaction with Nox-nucleotide domains, we may potentially infer their inhibitory mechanisms and explain the lack of isoform specificity. However, this remains to be supported through binding analysis that requires improved investigatory tools. Importantly, work by Smith et al. details the first technological advance for the development of p47phox-dependent Nox inhibitors independent of ROS analysis alone (147), and provides a reliable assay to infer potential modes of inhibition. As our understanding of the Nox family of enzymes improves, we anticipate the development of similar tools delineating chemical interaction, which, when used in conjunction with “activity” assays, will overcome the limitations of current screening programs that solely investigate ROS detection.
With the technological developments and growing knowledge of structure and key interactions between subunits and regulators, a new approach to target these interaction mechanisms is gaining traction. This is the rational design and use of computational capabilities for virtual screenings of thousands of compounds; the only limiting factor of this strategy is the availability of NMR or X-ray crystal-based structures for the targeted proteins or protein–protein interactions. An exciting example of such a strategy was reported by Bosco et al. on an in silico screen to identify inhibitors of the Rac1–p67phox interaction (17). The analysis was based on the X-ray crystal structure of the Rac1 and p67phox complex, and using docking simulations the authors were able to virtually screen over 300,000 compounds. Subsequent biochemical assays and SAR analysis of initial hits allowed the identification of Phox-I1 class of inhibitors of Nox2. Virtual screening to identify small molecules that target the catalytic core of the enzyme, although elegant and highly efficient, is not feasible at this time as membrane-spanning subunits of Nox have not been crystallized. However, many core protein interactions between the cytosolic Nox subunits have been elucidated (66, 67, 113); therefore, we anticipate increased interest in specific inhibitors of Nox subunit interactions in the near future using a similar technology. Importantly, this could be one strategy to develop isoform-specific inhibitors as many Nox systems have distinct activation mechanisms.
Conclusions
Despite the great efforts by many laboratories dedicated to the development of isoform-specific inhibitors of Nox, the task has proven very challenging as only a handful of lead compounds described actually target Nox activity specifically. Much work is still necessary to delineate the mechanism of action of the most promising inhibitors and to optimize their pharmacological and pharmacodynamics properties for their use as viable therapeutic tools for the treatment of oxidative-stress-associated disorders. That notwithstanding, all of the compounds discussed here have contributed to a better understanding of the regulation and function of the Nox family of proteins, both in their roles in signal transduction as well as disease. From that perspective, we remain hopeful that Nox-based therapies will be available in the near future and will have a profound impact in alleviating human disease.
Abbreviations Used
- DPI
diphenylene iodonium
- DTI
di-2-thienyliodonium
- HTS
high-throughput screening
- Ki
inhibitory constant
- Nox
NADPH oxidase
- RNS
reactive nitrogen species
- ROS
reactive oxygen species
- RyR1
ryanodine receptor-Ca2+ channel
- SAR
structural activity relationship
Acknowledgments
This work was supported by the National Institutes of Health (RO1HL079207 and PO1HL103455-02 to P.J.P.), the Institute for Transfusion Medicine, and the Hemophilia Center of Western Pennsylvania (to the Vascular Medicine Institute, University of Pittsburgh). The authors wish to thank Dr. Gabor Csanyi for his helpful suggestions in editing this review.
References
- 1.Abais JM, Zhang C, Xia M, Liu Q, Gehr TW, Boini KM, and Li PL. NADPH Oxidase-Mediated Triggering of Inflammasome in mouse podocytes and glomeruli during hyperhomocysteinemia. Antioxid Redox Signal 18: 1537–1548, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Al Ghouleh I, Khoo NK, Knaus UG, Griendling KK, Touyz RM, Thannickal VJ, Barchowsky A, Nauseef WM, Kelley EE, Bauer PM, Darley-Usmar V, Shiva S, Cifuentes-Pagano E, Freeman BA, Gladwin MT, and Pagano PJ. Oxidases and peroxidases in cardiovascular and lung disease: new concepts in reactive oxygen species signaling. Free Radic Biol Med 51: 1271–1288, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aldieri E, Riganti C, Polimeni M, Gazzano E, Lussiana C, Campia I, and Ghigo D. Classical inhibitors of NOX NAD(P)H oxidases are not specific. Curr Drug Metab 9: 686–696, 2008 [DOI] [PubMed] [Google Scholar]
- 4.Al-Shabrawey M, Bartoli M, El-Remessy AB, Platt DH, Matragoon S, Behzadian MA, Caldwell RW, and Caldwell RB. Inhibition of NAD(P)H oxidase activity blocks vascular endothelial growth factor overexpression and neovascularization during ischemic retinopathy. Am J Pathol 167: 599–607, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Altenhofer S, Kleikers PW, Radermacher KA, Scheurer P, Rob Hermans JJ, Schiffers P, Ho H, Wingler K, and Schmidt HH. The NOX toolbox: validating the role of NADPH oxidases in physiology and disease. Cell Mol Life Sci 69: 2327–2343, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Amanso AM. and Griendling KK. Differential roles of NADPH oxidases in vascular physiology and pathophysiology. Front Biosci (Schol Ed) 4: 1044–1064, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ameziane-El-Hassani R, Morand S, Boucher JL, Frapart YM, Apostolou D, Agnandji D, Gnidehou S, Ohayon R, Noel-Hudson MS, Francon J, Lalaoui K, Virion A, and Dupuy C. Dual oxidase-2 has an intrinsic Ca2+-dependent H2O2-generating activity. J Biol Chem 280: 30046–30054, 2005 [DOI] [PubMed] [Google Scholar]
- 8.Anderson D. and Phillips BJ. Comparative in vitro and in vivo effects of antioxidants. Food Chem Toxicol 37: 1015–1025, 1999 [DOI] [PubMed] [Google Scholar]
- 9.Ansari MA. and Scheff SW. NADPH-oxidase activation and cognition in Alzheimer disease progression. Free Radic Biol Med 51: 171–178, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aoyama T, Paik YH, Watanabe S, Laleu B, Gaggini F, Fioraso-Cartier L, Molango S, Heitz F, Merlot C, Szyndralewiez C, Page P, and Brenner DA. Nicotinamide adenine dinucleotide phosphate oxidase in experimental liver fibrosis: GKT137831 as a novel potential therapeutic agent. Hepatology 56: 2316–2327, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Banfi B, Molnar G, Maturana A, Steger K, Hegedus B, Demaurex N, and Krause KH. A Ca(2+)-activated NADPH oxidase in testis, spleen, and lymph nodes. J Biol Chem 276: 37594–37601, 2001 [DOI] [PubMed] [Google Scholar]
- 12.Bedard K, Jaquet V, and Krause KH. NOX5: from basic biology to signaling and disease. Free Radic Biol Med 52: 725–734, 2012 [DOI] [PubMed] [Google Scholar]
- 13.Bedard K. and Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev 87: 245–313, 2007 [DOI] [PubMed] [Google Scholar]
- 14.Bendall JK, Cave AC, Heymes C, Gall N, and Shah AM. Pivotal role of a gp91(phox)-containing NADPH oxidase in angiotensin II-induced cardiac hypertrophy in mice. Circulation 105: 293–296, 2002 [DOI] [PubMed] [Google Scholar]
- 15.Bhandarkar SS, Jaconi M, Fried LE, Bonner MY, Lefkove B, Govindarajan B, Perry BN, Parhar R, Mackelfresh J, Sohn A, Stouffs M, Knaus U, Yancopoulos G, Reiss Y, Benest AV, Augustin HG, and Arbiser JL. Fulvene-5 potently inhibits NADPH oxidase 4 and blocks the growth of endothelial tumors in mice. J Clin Invest 119: 2359–2365, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Borbely G, Szabadkai I, Horvath Z, Marko P, Varga Z, Breza N, Baska F, Vantus T, Huszar M, Geiszt M, Hunyady L, Buday L, Orfi L, and Keri G. Small-molecule inhibitors of NADPH oxidase 4. J Med Chem 53: 6758–6762, 2010 [DOI] [PubMed] [Google Scholar]
- 17.Bosco EE, Kumar S, Marchioni F, Biesiada J, Kordos M, Szczur K, Meller J, Seibel W, Mizrahi A, Pick E, Filippi MD, and Zheng Y. Rational design of small molecule inhibitors targeting the Rac GTPase-p67(phox) signaling axis in inflammation. Chem Biol 19: 228–242, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brieger K, Schiavone S, Miller FJ, Jr., and Krause KH. Reactive oxygen species: from health to disease. Swiss Med Wkly 142: w13659, 2012 [DOI] [PubMed] [Google Scholar]
- 19.Brinker AM, Ma J, Lipsky PE, and Raskin I. Medicinal chemistry and pharmacology of genus Tripterygium (Celastraceae). Phytochemistry 68: 732–766, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brown DI. and Griendling KK. Nox proteins in signal transduction. Free Radic Biol Med 47: 1239–1253, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cangemi R, Celestini A, Del Ben M, Pignatelli P, Carnevale R, Proietti M, Calabrese CM, Basili S, and Violi F. Role of platelets in NOX2 activation mediated by TNFalpha in heart failure. Intern Emerg Med 2012. [Epub ahead of print]; DOI: 10.1007/s11739-012-0837-2 [DOI] [PubMed]
- 22.Carnesecchi S, Pache JC, and Barazzone-Argiroffo C. NOX enzymes: potential target for the treatment of acute lung injury. Cell Mol Life Sci 69: 2373–2385, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cayatte AJ, Rupin A, Oliver-Krasinski J, Maitland K, Sansilvestri-Morel P, Boussard MF, Wierzbicki M, Verbeuren TJ, and Cohen RA. S17834, a new inhibitor of cell adhesion and atherosclerosis that targets nadph oxidase. Arterioscler Thromb Vasc Biol 21: 1577–1584, 2001 [DOI] [PubMed] [Google Scholar]
- 24.Chabrashvili T, Kitiyakara C, Blau J, Karber A, Aslam S, Welch WJ, and Wilcox CS. Effects of ANG II type 1 and 2 receptors on oxidative stress, renal NADPH oxidase, and SOD expression. Am J Physiol Regul Integr Comp Physiol 285: R117–R124, 2003 [DOI] [PubMed] [Google Scholar]
- 25.Cheng G, Cao Z, Xu X, van Meir EG, and Lambeth JD. Homologs of gp91phox: cloning and tissue expression of Nox3, Nox4, and Nox5. Gene 269: 131–140, 2001 [DOI] [PubMed] [Google Scholar]
- 26.Cheng G, Ritsick D, and Lambeth JD. Nox3 regulation by NOXO1, p47phox, and p67phox. J Biol Chem 279: 34250–34255, 2004 [DOI] [PubMed] [Google Scholar]
- 27.Choi DH, Cristovao AC, Guhathakurta S, Lee J, Joh TH, Beal MF, and Kim YS. NADPH oxidase 1-mediated oxidative stress leads to dopamine neuron death in Parkinson's disease. Antioxid Redox Signal 16: 1033–1045, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cifuentes ME. and Pagano PJ. Targeting reactive oxygen species in hypertension. Curr Opin Nephrol Hypertens 15: 179–186, 2006 [DOI] [PubMed] [Google Scholar]
- 29.Cifuentes-Pagano E, Csanyi G, and Pagano PJ. NADPH oxidase inhibitors: a decade of discovery from Nox2ds to HTS. Cell Mol Life Sci 69: 2315–2325, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cifuentes-Pagano E, Saha J, Csanyi G, Al Ghouleh I, Sahoo S, Rodriguez A, Wipf P, Pagano PJ, and Skoda EM. Bridged tetrahydroisoquinolines as selective NADPH oxidase 2 (Nox2) inhibitors. MedChemComm 4: 1085–1092, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cross AR. Inhibitors of the leukocyte superoxide generating oxidase: mechanisms of action and methods for their elucidation. Free Radic Biol Med 8: 71–93, 1990 [DOI] [PubMed] [Google Scholar]
- 32.Cross AR. and Jones OT. The effect of the inhibitor diphenylene iodonium on the superoxide-generating system of neutrophils. Specific labelling of a component polypeptide of the oxidase. Biochem J 237: 111–116, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cross AR. and Segal AW. The NADPH oxidase of professional phagocytes—prototype of the NOX electron transport chain systems. Biochim Biophys Acta 1657: 1–22, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Csanyi G. and Pagano PJ. Strategies aimed at Nox4 oxidase inhibition employing peptides from Nox4 B-loop and C-terminus and p22 (phox) N-terminus: an elusive target. Int J Hypertens 2013: 842827, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Csanyi G, Cifuentes-Pagano E, Al Ghouleh I, Ranayhossaini DJ, Egana L, Lopes LR, Jackson HM, Kelley EE, and Pagano PJ. Nox2 B-loop peptide, Nox2ds, specifically inhibits the NADPH oxidase Nox2. Free Radic Biol Med 51: 1116–1125, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Csanyi G, Taylor WR, and Pagano PJ. NOX and inflammation in the vascular adventitia. Free Radic Biol Med 47: 1254–1266, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dahan I. and Pick E. Strategies for identifying synthetic peptides to act as inhibitors of NADPH oxidases, or “all that you did and did not want to know about Nox inhibitory peptides”. Cell Mol Life Sci 69: 2283–2305, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dahan I, Issaeva I, Gorzalczany Y, Sigal N, Hirshberg M, and Pick E. Mapping of functional domains in the p22(phox) subunit of flavocytochrome b(559) participating in the assembly of the NADPH oxidase complex by “peptide walking”. J Biol Chem 277: 8421–8432, 2002 [DOI] [PubMed] [Google Scholar]
- 39.Dahan I, Molshanski-Mor S, and Pick E. Inhibition of NADPH oxidase activation by peptides mapping within the dehydrogenase region of Nox2-A “peptide walking” study. J Leukoc Biol 91: 501–515, 2012 [DOI] [PubMed] [Google Scholar]
- 40.Datla SR. and Griendling KK. Reactive oxygen species, NADPH oxidases, and hypertension. Hypertension 56: 325–330, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.DeLeo FR, Yu L, Burritt JB, Loetterle LR, Bond CW, Jesaitis AJ, and Quinn MT. Mapping sites of interaction of p47-phox and flavocytochrome b with random-sequence peptide phage display libraries. Proc Natl Acad Sci USA 92: 7110–7114, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dennis KE, Aschner JL, Milatovic D, Schmidt JW, Aschner M, Kaplowitz MR, Zhang Y, and Fike CD. NADPH oxidases and reactive oxygen species at different stages of chronic hypoxia-induced pulmonary hypertension in newborn piglets. Am J Physiol Lung Cell Mol Physiol 297: L596–L607, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Diatchuk V, Lotan O, Koshkin V, Wikstroem P, and Pick E. Inhibition of NADPH oxidase activation by 4-(2-aminoethyl)-benzenesulfonyl fluoride and related compounds. J Biol Chem 272: 13292–13301, 1997 [DOI] [PubMed] [Google Scholar]
- 44.Doroshow JH, Gaur S, Markel S, Lu J, van Balgooy J, Synold TW, Xi B, Wu X, and Juhasz A. Effects of iodonium-class flavin dehydrogenase inhibitors on growth, reactive oxygen production, cell cycle progression, NADPH oxidase 1 levels, and gene expression in human colon cancer cells and xenografts. Free Radic Biol Med 57: 162–175, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dourron HM, Jacobson GM, Park JL, Liu J, Reddy DJ, Scheel ML, and Pagano PJ. Perivascular gene transfer of NADPH oxidase inhibitor suppresses angioplasty-induced neointimal proliferation of rat carotid artery. Am J Physiol Heart Circ Physiol 288: H946–H953, 2005 [DOI] [PubMed] [Google Scholar]
- 46.Doussiere J. and Vignais PV. Diphenylene iodonium as an inhibitor of the NADPH oxidase complex of bovine neutrophils. Factors controlling the inhibitory potency of diphenylene iodonium in a cell-free system of oxidase activation. Eur J Biochem 208: 61–71, 1992 [DOI] [PubMed] [Google Scholar]
- 47.Droge W. Free radicals in the physiological control of cell function. Physiol Rev 82: 47–95, 2002 [DOI] [PubMed] [Google Scholar]
- 48.Drummond GR, Selemidis S, Griendling KK, and Sobey CG. Combating oxidative stress in vascular disease: NADPH oxidases as therapeutic targets. Nat Rev Drug Discov 10: 453–471, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Duerrschmidt N, Stielow C, Muller G, Pagano PJ, and Morawietz H. NO-mediated regulation of NAD(P)H oxidase by laminar shear stress in human endothelial cells. J Physiol 576: 557–567, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.El-Benna J, Dang PM, and Perianin A. Towards specific NADPH oxidase inhibition by small synthetic peptides. Cell Mol Life Sci 69: 2307–2314, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fan YY, Kohno M, Nakano D, Hitomi H, Nagai Y, Fujisawa Y, Lu XM, Fu H, Du J, Ohmori K, Hosomi N, Kimura S, Kiyomoto H, and Nishiyama A. Inhibitory effects of a dihydropyridine calcium channel blocker on renal injury in aldosterone-infused rats. J Hypertens 27: 1855–1862, 2009 [DOI] [PubMed] [Google Scholar]
- 52.Finkel T. Signal transduction by reactive oxygen species. J Cell Biol 194: 7–15, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fujii A, Nakano D, Katsuragi M, Ohkita M, Takaoka M, Ohno Y, and Matsumura Y. Role of gp91phox-containing NADPH oxidase in the deoxycorticosterone acetate-salt-induced hypertension. Eur J Pharmacol 552: 131–134, 2006 [DOI] [PubMed] [Google Scholar]
- 54.Fulton DJ. Nox5 and the regulation of cellular function. Antioxid Redox Signal 11: 2443–2452, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Furst R, Brueckl C, Kuebler WM, Zahler S, Krotz F, Gorlach A, Vollmar AM, and Kiemer AK. Atrial natriuretic peptide induces mitogen-activated protein kinase phosphatase-1 in human endothelial cells via Rac1 and NAD(P)H oxidase/Nox2-activation. Circ Res 96: 43–53, 2005 [DOI] [PubMed] [Google Scholar]
- 56.Gaggini F, Laleu B, Orchard M, Fioraso-Cartier L, Cagnon L, Houngninou-Molango S, Gradia A, Duboux G, Merlot C, Heitz F, Szyndralewiez C, and Page P. Design, synthesis and biological activity of original pyrazolo-pyrido-diazepine, -pyrazine and -oxazine dione derivatives as novel dual Nox4/Nox1 inhibitors. Bioorg Med Chem 19: 6989–6999, 2011 [DOI] [PubMed] [Google Scholar]
- 57.Garrido-Urbani S, Jemelin S, Deffert C, Carnesecchi S, Basset O, Szyndralewiez C, Heitz F, Page P, Montet X, Michalik L, Arbiser J, Ruegg C, Krause KH, and Imhof B. Targeting vascular NADPH oxidase 1 blocks tumor angiogenesis through a PPARalpha mediated mechanism. PLoS One 6: e14665, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gatto GJ, Jr., Ao Z, Kearse MG, Zhou M, Morales CR, Daniels E, Bradley BT, Goserud MT, Goodman KB, Douglas SA, Harpel MR, and Johns DG. NADPH oxidase-dependent and -independent mechanisms of reported inhibitors of reactive oxygen generation. J Enzyme Inhib Med Chem 28: 95–104, 2013 [DOI] [PubMed] [Google Scholar]
- 59.Geiszt M. NADPH oxidases: new kids on the block. Cardiovasc Res 71: 289–299, 2006 [DOI] [PubMed] [Google Scholar]
- 60.Gianni D, Taulet N, Zhang H, DerMardirossian C, Kister J, Martinez L, Roush WR, Brown SJ, Bokoch GM, and Rosen H. A novel and specific NADPH oxidase-1 (Nox1) small-molecule inhibitor blocks the formation of functional invadopodia in human colon cancer cells. ACS Chem Biol 5: 981–993, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Giannoni E. and Chiarugi P. Redox circuitries driving Src regulation. Antioxid Redox Signal 2013. [Epub ahead of print]; DOI: 10.1089/ars.2013.5525 [DOI] [PubMed] [Google Scholar]
- 62.Gorin Y. Nox4 as a potential therapeutic target for treatment of uremic toxicity associated to chronic kidney disease. Kidney Int 83: 541–543, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gray SP, Di Marco E, Okabe J, Szyndralewiez C, Heitz F, Montezano AC, de Haan JB, Koulis C, El-Osta A, Andrews KL, Chin-Dusting JP, Touyz RM, Wingler K, Cooper ME, Schmidt HH, and Jandeleit-Dahm KA. Nox1 plays a key role in diabetes accelerated atherosclerosis. Circulation 127: 1888–1902, 2013 [DOI] [PubMed] [Google Scholar]
- 64.Green DE, Murphy TC, Kang BY, Kleinhenz JM, Szyndralewiez C, Page P, Sutliff RL, and Hart CM. The Nox4 inhibitor GKT137831 attenuates hypoxia-induced pulmonary vascular cell proliferation. Am J Respir Cell Mol Biol 47: 718–726, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Griendling KK, Minieri CA, Ollerenshaw JD, and Alexander RW. Angiotensin II stimulates NADH and NADPH oxidase activity in cultured vascular smooth muscle cells. Circ Res 74: 1141–1148, 1994 [DOI] [PubMed] [Google Scholar]
- 66.Groemping Y. and Rittinger K. Activation and assembly of the NADPH oxidase: a structural perspective. Biochem J 386: 401–416, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Groemping Y, Lapouge K, Smerdon SJ, and Rittinger K. Molecular basis of phosphorylation-induced activation of the NADPH oxidase. Cell 113: 343–355, 2003 [DOI] [PubMed] [Google Scholar]
- 68.Group TA-TBCCPS. The effect of vitamin E and beta carotene on the incidence of lung cancer and other cancers in male smokers. The Alpha-Tocopherol, Beta Carotene Cancer Prevention Study Group. N Engl J Med 330: 1029–1035, 1994 [DOI] [PubMed] [Google Scholar]
- 69.Hansen J, Palmfeldt J, Vang S, Corydon TJ, Gregersen N, and Bross P. Quantitative proteomics reveals cellular targets of celastrol. PLoS One 6: e26634, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Harfouche R, Malak NA, Brandes RP, Karsan A, Irani K, and Hussain SN. Roles of reactive oxygen species in angiopoietin-1/tie-2 receptor signaling. FASEB J 19: 1728–1730, 2005 [DOI] [PubMed] [Google Scholar]
- 71.Hecker L, Cheng J, and Thannickal VJ. Targeting NOX enzymes in pulmonary fibrosis. Cell Mol Life Sci 69: 2365–2371, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Heron M. Deaths: leading causes for 2008. Natl Vital Stat Rep 60: 1–94, 2012 [PubMed] [Google Scholar]
- 73.Heumuller S, Wind S, Barbosa-Sicard E, Schmidt HH, Busse R, Schroder K, and Brandes RP. Apocynin is not an inhibitor of vascular NADPH oxidases but an antioxidant. Hypertension 51: 211–217, 2008 [DOI] [PubMed] [Google Scholar]
- 74.Hou X, Xu S, Maitland-Toolan KA, Sato K, Jiang B, Ido Y, Lan F, Walsh K, Wierzbicki M, Verbeuren TJ, Cohen RA, and Zang M. SIRT1 regulates hepatocyte lipid metabolism through activating AMP-activated protein kinase. J Biol Chem 283: 20015–20026, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Iwai M, Kanno H, Inaba S, Senba I, Sone H, Nakaoka H, and Horiuchi M. Nifedipine, a calcium-channel blocker, attenuated glucose intolerance and white adipose tissue dysfunction in type 2 diabetic KK-A(y) mice. Am J Hypertens 24: 169–174, 2011 [DOI] [PubMed] [Google Scholar]
- 76.Jacobson GM, Dourron HM, Liu J, Carretero OA, Reddy DJ, Andrzejewski T, and Pagano PJ. Novel NAD(P)H oxidase inhibitor suppresses angioplasty-induced superoxide and neointimal hyperplasia of rat carotid artery. Circ Res 92: 637–643, 2003 [DOI] [PubMed] [Google Scholar]
- 77.Jaquet V, Marcoux J, Forest E, Leidal KG, McCormick S, Westermaier Y, Perozzo R, Plastre O, Fioraso-Cartier L, Diebold B, Scapozza L, Nauseef WM, Fieschi F, Krause KH, and Bedard K. NOX NADPH oxidase isoforms are inhibited by celastrol with a dual mode of action. Br J Pharmacol 164: 507–520, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Jaquet V, Scapozza L, Clark R, Krause KH, and Lambeth JD. Small molecule NOX inhibitors: ROS-generating NADPH oxidases as therapeutic targets. Antioxid Redox Signal 11: 2535–2552, 2009 [DOI] [PubMed] [Google Scholar]
- 79.Jiang J, Kang H, Song X, Huang S, Li S, and Xu J. A model of interaction between nicotinamide adenine dinucleotide phosphate (NADPH) oxidase and apocynin analogues by docking method. Int J Mol Sci 14: 807–817, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jiang JX, Chen X, Serizawa N, Szyndralewiez C, Page P, Schroder K, Brandes RP, Devaraj S, and Torok NJ. Liver fibrosis and hepatocyte apoptosis are attenuated by GKT137831, a novel NOX4/NOX1 inhibitor in vivo. Free Radic Biol Med 53: 289–296, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Jung O, Schreiber JG, Geiger H, Pedrazzini T, Busse R, and Brandes RP. gp91phox-containing NADPH oxidase mediates endothelial dysfunction in renovascular hypertension. Circulation 109: 1795–1801, 2004 [DOI] [PubMed] [Google Scholar]
- 82.Kahles T, Luedike P, Endres M, Galla HJ, Steinmetz H, Busse R, Neumann-Haefelin T, and Brandes RP. NADPH oxidase plays a central role in blood-brain barrier damage in experimental stroke. Stroke 38: 3000–3006, 2007 [DOI] [PubMed] [Google Scholar]
- 83.Kay CD, Kris-Etherton PM, and West SG. Effects of antioxidant-rich foods on vascular reactivity: review of the clinical evidence. Curr Atheroscler Rep 8: 510–522, 2006 [DOI] [PubMed] [Google Scholar]
- 84.Kilpatrick LE, Sun S, Li H, Vary TC, and Korchak HM. Regulation of TNF-induced oxygen radical production in human neutrophils: role of delta-PKC. J Leukoc Biol 87: 153–164, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kim JA, Neupane GP, Lee ES, Jeong BS, Park BC, and Thapa P. NADPH oxidase inhibitors: a patent review. Expert Opin Ther Pat 21: 1147–1158, 2011 [DOI] [PubMed] [Google Scholar]
- 86.Kim JH, Lee JO, Lee SK, Kim N, You GY, Moon JW, Sha J, Kim SJ, Park SH, and Kim HS. Celastrol suppresses breast cancer MCF-7 cell viability via the AMP-activated protein kinase (AMPK)-induced p53-polo like kinase 2 (PLK-2) pathway. Cell Signal 25: 805–813, 2013 [DOI] [PubMed] [Google Scholar]
- 87.Klaunig JE, Kamendulis LM, and Hocevar BA. Oxidative stress and oxidative damage in carcinogenesis. Toxicol Pathol 38: 96–109, 2010 [DOI] [PubMed] [Google Scholar]
- 88.Kleikers PW, Wingler K, Hermans JJ, Diebold I, Altenhofer S, Radermacher KA, Janssen B, Gorlach A, and Schmidt HH. NADPH oxidases as a source of oxidative stress and molecular target in ischemia/reperfusion injury. J Mol Med (Berl) 90: 1391–1406, 2012 [DOI] [PubMed] [Google Scholar]
- 89.Kleniewska P, Piechota A, Skibska B, and Goraca A. The NADPH oxidase family and its inhibitors. Arch Immunol Ther Exp (Warsz) 60: 277–294, 2012 [DOI] [PubMed] [Google Scholar]
- 90.Kofler PA, Pircher H, von Grafenstein S, Diener T, Holl M, Liedl KR, Siems K, and Jansen-Durr P. Characterisation of nox4 inhibitors from edible plants. Planta Med 79: 244–252, 2013 [DOI] [PubMed] [Google Scholar]
- 91.Koziel R, Pircher H, Kratochwil M, Lener B, Hermann M, Dencher NA, and Jansen-Durr P. Mitochondrial respiratory chain complex I is inactivated by NADPH oxidase Nox4. Biochem J 452: 231–239, 2013 [DOI] [PubMed] [Google Scholar]
- 92.Krause KH. Tissue distribution and putative physiological function of NOX family NADPH oxidases. Jpn J Infect Dis 57: S28–S29, 2004 [PubMed] [Google Scholar]
- 93.Krause KH. and Bedard K. NOX enzymes in immuno-inflammatory pathologies. Semin Immunopathol 30: 193–194, 2008 [DOI] [PubMed] [Google Scholar]
- 94.Kroller-Schon S, Steven S, Kossmann S, Scholz A, Daub S, Oelze M, Xia N, Hausding M, Mikhed Y, Zinssius E, Mader M, Stamm P, Treiber N, Scharffetter-Kochanek K, Li H, Schulz E, Wenzel P, Munzel T, and Daiber A. Molecular mechanisms of the crosstalk between mitochondria and NADPH oxidase through reactive oxygen species—studies in white blood cells and in animal models. Antioxid Redox Signal 2013. [Epub ahead of print]; DOI: 10.1089/ars.2012.4953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Krotz F, Keller M, Derflinger S, Schmid H, Gloe T, Bassermann F, Duyster J, Cohen CD, Schuhmann C, Klauss V, Pohl U, Stempfle HU, and Sohn HY. Mycophenolate acid inhibits endothelial NAD(P)H oxidase activity and superoxide formation by a Rac1-dependent mechanism. Hypertension 49: 201–208, 2007 [DOI] [PubMed] [Google Scholar]
- 96.Krotz F, Sohn HY, Gloe T, Zahler S, Riexinger T, Schiele TM, Becker BF, Theisen K, Klauss V, and Pohl U. NAD(P)H oxidase-dependent platelet superoxide anion release increases platelet recruitment. Blood 100: 917–924, 2002 [DOI] [PubMed] [Google Scholar]
- 97.Laleu B, Gaggini F, Orchard M, Fioraso-Cartier L, Cagnon L, Houngninou-Molango S, Gradia A, Duboux G, Merlot C, Heitz F, Szyndralewiez C, and Page P. First in class, potent, and orally bioavailable NADPH oxidase isoform 4 (Nox4) inhibitors for the treatment of idiopathic pulmonary fibrosis. J Med Chem 53: 7715–7730, 2010 [DOI] [PubMed] [Google Scholar]
- 98.Lambeth JD, Krause KH, and Clark RA. NOX enzymes as novel targets for drug development. Semin Immunopathol 30: 339–363, 2008 [DOI] [PubMed] [Google Scholar]
- 99.Landmesser U, Cai H, Dikalov S, McCann L, Hwang J, Jo H, Holland SM, and Harrison DG. Role of p47 phox in vascular oxidative stress and hypertension caused by angiotensin II. Hypertension 40: 511–515, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lange S, Heger J, Euler G, Wartenberg M, Piper HM, and Sauer H. Platelet-derived growth factor BB stimulates vasculogenesis of embryonic stem cell-derived endothelial cells by calcium-mediated generation of reactive oxygen species. Cardiovasc Res 81: 159–168, 2009 [DOI] [PubMed] [Google Scholar]
- 101.Lassegue B, San Martin A, and Griendling KK. Biochemistry, physiology, and pathophysiology of NADPH oxidases in the cardiovascular system. Circ Res 110: 1364–1390, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lee I, Dodia C, Chatterjee S, Zagorski J, Mesaros C, Blair IA, Feinstein SI, Jain M, and Fisher AB. A novel nontoxic inhibitor of the activation of NADPH oxidase reduces reactive oxygen species production in mouse lung. J Pharmacol Exp Ther 345: 284–296, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Leto TL, Morand S, Hurt D, and Ueyama T. Targeting and regulation of reactive oxygen species generation by Nox family NADPH oxidases. Antioxid Redox Signal 11: 2607–2619, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Li JM, Newburger PE, Gounis MJ, Dargon P, Zhang X, and Messina LM. Local arterial nanoparticle delivery of siRNA for NOX2 knockdown to prevent restenosis in an atherosclerotic rat model. Gene Ther 17: 1279–1287, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Liu J, Ormsby A, Oja-Tebbe N, and Pagano PJ. Gene transfer of NAD(P)H oxidase inhibitor to the vascular adventitia attenuates medial smooth muscle hypertrophy. Circ Res 95: 587–594, 2004 [DOI] [PubMed] [Google Scholar]
- 106.Liu J, Yang F, Yang XP, Jankowski M, and Pagano PJ. NAD(P)H oxidase mediates angiotensin II-induced vascular macrophage infiltration and medial hypertrophy. Arterioscler Thromb Vasc Biol 23: 776–782, 2003 [DOI] [PubMed] [Google Scholar]
- 107.Liu Y, Qin L, Wilson BC, An L, Hong JS, and Liu B. Inhibition by naloxone stereoisomers of beta-amyloid peptide (1–42)-induced superoxide production in microglia and degeneration of cortical and mesencephalic neurons. J Pharmacol Exp Ther 302: 1212–1219, 2002 [DOI] [PubMed] [Google Scholar]
- 108.Lonn E, Bosch J, Yusuf S, Sheridan P, Pogue J, Arnold JM, Ross C, Arnold A, Sleight P, Probstfield J, Dagenais GR, Hope, and Investigators H-TT. Effects of long-term vitamin E supplementation on cardiovascular events and cancer: a randomized controlled trial. JAMA 293: 1338–1347, 2005 [DOI] [PubMed] [Google Scholar]
- 109.Lopes NHM, Vasudevan SS, Gregg D, Selvakumar B, Pagano PJ, Kovacic H, and Goldschmidt-Clermont PJ. Rac-dependent monocyte chemoattractant protein-1 production is induced by nutrient deprivation. Circ Res 91: 798–805, 2002 [DOI] [PubMed] [Google Scholar]
- 110.Loukogeorgakis SP, van den Berg MJ, Sofat R, Nitsch D, Charakida M, Haiyee B, de Groot E, MacAllister RJ, Kuijpers TW, and Deanfield JE. Role of NADPH oxidase in endothelial ischemia/reperfusion injury in humans. Circulation 121: 2310–2316, 2010 [DOI] [PubMed] [Google Scholar]
- 111.Martin A, Perez-Giron JV, Hernanz R, Palacios R, Briones AM, Fortuno A, Zalba G, Salaices M, and Alonso MJ. Peroxisome proliferator-activated receptor-gamma activation reduces cyclooxygenase-2 expression in vascular smooth muscle cells from hypertensive rats by interfering with oxidative stress. J Hypertens 30: 315–326, 2012 [DOI] [PubMed] [Google Scholar]
- 112.Martinez-Revelles S, Avendano MS, Garcia-Redondo AB, Alvarez Y, Aguado A, Perez-Giron JV, Garcia-Redondo L, Esteban V, Redondo JM, Alonso MJ, Briones AM, and Salaices M. Reciprocal relationship between reactive oxygen species and cyclooxygenase-2 and vascular dysfunction in hypertension. Antioxid Redox Signal 18: 51–65, 2013 [DOI] [PubMed] [Google Scholar]
- 113.Massenet C, Chenavas S, Cohen-Addad C, Dagher MC, Brandolin G, Pebay-Peyroula E, and Fieschi F. Effects of p47phox C terminus phosphorylations on binding interactions with p40phox and p67phox. Structural and functional comparison of p40phox and p67phox SH3 domains. J Biol Chem 280: 13752–13761, 2005 [DOI] [PubMed] [Google Scholar]
- 114.Mohazzab KM, Kaminski PM, and Wolin MS. NADH oxidoreductase is a major source of superoxide anion in bovine coronary artery endothelium. Am J Physiol 266: H2568–H2572, 1994 [DOI] [PubMed] [Google Scholar]
- 115.Montezano AC. and Touyz RM. Molecular mechanisms of hypertension—reactive oxygen species and antioxidants: a basic science update for the clinician. Can J Cardiol 28: 288–295, 2012 [DOI] [PubMed] [Google Scholar]
- 116.Morachis JM, Mahmoud EA, and Almutairi A. Physical and chemical strategies for therapeutic delivery by using polymeric nanoparticles. Pharmacol Rev 64: 505–519, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Morozov I, Lotan O, Joseph G, Gorzalczany Y, and Pick E. Mapping of functional domains in p47 phox involved in the activation of NADPH oxidase by “peptide walking”. J Biol Chem 273: 15435–15444, 1998 [DOI] [PubMed] [Google Scholar]
- 118.Musset B, Clark RA, DeCoursey TE, Petheo GL, Geiszt M, Chen Y, Cornell JE, Eddy CA, Brzyski RG, and El Jamali A. NOX5 in human spermatozoa: expression, function, and regulation. J Biol Chem 287: 9376–9388, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Niethammer P, Grabher C, Look AT, and Mitchison TJ. A tissue-scale gradient of hydrogen peroxide mediates rapid wound detection in zebrafish. Nature 459: 996–999, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Niu XL, Madamanchi NR, Vendrov AE, Tchivilev I, Rojas M, Madamanchi C, Brandes RP, Krause KH, Humphries J, Smith A, Burnand KG, and Runge MS. Nox activator 1: a potential target for modulation of vascular reactive oxygen species in atherosclerotic arteries. Circulation 121: 549–559, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.O'Donnell VB, Tew DG, Jones OTG, and England PJ. Studies on the inhibitory mechanism of iodonium compounds with special reference to neutrophil NADPH oxidase. Biochem J 290: 41–49, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Pagano PJ, Clark JK, Cifuentes-Pagano ME, Clark SM, Callis GM, and Quinn MT. Localization of a constitutively active, phagocyte-like NADPH oxidase in rabbit aortic adventitia: enhancement by angiotensin II. Proc Natl Acad Sci USA 94: 14483–14488, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Pagano PJ, Ito Y, Tornheim K, Gallop PM, Tauber AI, and Cohen RA. An NADPH oxidase superoxide-generating system in the rabbit aorta. Am J Physiol 268: H2274–H2280, 1995 [DOI] [PubMed] [Google Scholar]
- 124.Paravicini TM. and Touyz RM. NADPH oxidases, reactive oxygen species, and hypertension: clinical implications and therapeutic possibilities. Diabetes Care 31(Suppl 2): S170–S180, 2008 [DOI] [PubMed] [Google Scholar]
- 125.Parnham M. and Sies H. Ebselen: prospective therapy for cerebral ischaemia. Expert Opin Investig Drugs 9: 607–619, 2000 [DOI] [PubMed] [Google Scholar]
- 126.Patel VB, Wang Z, Fan D, Zhabyeyev P, Basu R, Das SK, Wang W, Desaulniers J, Holland SM, Kassiri Z, and Oudit GY. Loss of p47phox subunit enhances susceptibility to biomechanical stress and heart failure because of dysregulation of cortactin and actin filaments. Circ Res 112: 1542–1556, 2013 [DOI] [PubMed] [Google Scholar]
- 127.Perry BN, Govindarajan B, Bhandarkar SS, Knaus UG, Valo M, Sturk C, Carrillo CO, Sohn A, Cerimele F, Dumont D, Losken A, Williams J, Brown LF, Tan X, Ioffe E, Yancopoulos GD, and Arbiser JL. Pharmacologic blockade of angiopoietin-2 is efficacious against model hemangiomas in mice. J Invest Dermatol 126: 2316–2322, 2006 [DOI] [PubMed] [Google Scholar]
- 128.Petronio MS, Zeraik ML, Fonseca LM, and Ximenes VF. Apocynin: chemical and biophysical properties of a NADPH oxidase inhibitor. Molecules 18: 2821–2839, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Poli G, Leonarduzzi G, Biasi F, and Chiarpotto E. Oxidative stress and cell signalling. Curr Med Chem 11: 1163–1182, 2004 [DOI] [PubMed] [Google Scholar]
- 130.Pongnimitprasert N, El-Benna J, Foglietti MJ, Gougerot-Pocidalo MA, Bernard M, and Braut-Boucher F. Potential role of the “NADPH oxidases” (NOX/DUOX) family in cystic fibrosis. Ann Biol Clin (Paris) 66: 621–629, 2008 [DOI] [PubMed] [Google Scholar]
- 131.Quinn MT. and Gauss KA. Structure and regulation of the neutrophil respiratory burst oxidase: comparison with nonphagocyte oxidases. J Leukoc Biol 76: 760–781, 2004 [DOI] [PubMed] [Google Scholar]
- 132.Radermacher KA, Wingler K, Langhauser F, Altenhofer S, Kleikers P, Hermans JJ, Hrabe de Angelis M, Kleinschnitz C, and Schmidt HH. Neuroprotection after stroke by targeting NOX4 as a source of oxidative stress. Antioxid Redox Signal 18: 1418–1427, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ray PD, Huang BW, and Tsuji Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell Signal 24: 981–990, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Rey FE, Cifuentes ME, Kiarash A, Quinn MT, and Pagano PJ. Novel competitive inhibitor of NAD(P)H oxidase assembly attenuates vascular O(2)(-) and systolic blood pressure in mice. Circ Res 89: 408–414, 2001 [DOI] [PubMed] [Google Scholar]
- 135.Rey FE, Li XC, Carretero OA, Garvin JL, and Pagano PJ. Perivascular superoxide anion contributes to impairment of endothelium-dependent relaxation. Role of gp91 phox. Circulation 106: 2497–2502, 2002 [DOI] [PubMed] [Google Scholar]
- 136.Rotrosen D, Kleinberg ME, Nunoi H, Leto T, Gallin JI, and Malech HL. Evidence for a functional cytoplasmic domain of phagocyte oxidase cytochrome b558. J Biol Chem 265: 8745–8750, 1990 [PubMed] [Google Scholar]
- 137.Schildknecht S, Weber A, Gerding HR, Pape R, Robotta M, Drescher M, Marquardt A, Daiber A, Ferger B, and Leist M. The NOX1/4 inhibitor GKT136901 as selective and direct scavenger of peroxynitrite. Curr Med Chem 2013. [Epub ahead of print]; DOI: 10.2174/09298673113209990179 [DOI] [PubMed] [Google Scholar]
- 138.Schramm A, Matusik P, Osmenda G, and Guzik TJ. Targeting NADPH oxidases in vascular pharmacology. Vascul Pharmacol 56: 216–231, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Schroder K. Isoform specific functions of Nox protein-derived reactive oxygen species in the vasculature. Curr Opin Pharmacol 10: 122–126, 2010 [DOI] [PubMed] [Google Scholar]
- 140.Sedeek M, Callera G, Montezano A, Gutsol A, Heitz F, Szyndralewiez C, Page P, Kennedy CR, Burns KD, Touyz RM, and Hebert RL. Critical role of Nox4-based NADPH oxidase in glucose-induced oxidative stress in the kidney: implications in type 2 diabetic nephropathy. Am J Physiol Renal Physiol 299: F1348–F1358, 2010 [DOI] [PubMed] [Google Scholar]
- 141.Segal AW. and Jones OT. Novel cytochrome b system in phagocytic vacuoles of human granulocytes. Nature 276: 515–517, 1978 [DOI] [PubMed] [Google Scholar]
- 142.Serrander L, Jaquet V, Bedard K, Plastre O, Hartley O, Arnaudeau S, Demaurex N, Schlegel W, and Krause KH. NOX5 is expressed at the plasma membrane and generates superoxide in response to protein kinase C activation. Biochimie 89: 1159–1167, 2007 [DOI] [PubMed] [Google Scholar]
- 143.Shi J, Ross CR, Leto TL, and Blecha F. PR-39, a proline-rich antibacterial peptide that inhibits phagocyte NADPH oxidase activity by binding to Src homology 3 domains of p47 phox Proc Natl Acad Sci USA 93: 6014–6018, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Shin YS, Song SJ, Kang SU, Hwang HS, Choi JW, Lee BH, Jung YS, and Kim CH. A novel synthetic compound, 3-amino-3-(4-fluoro-phenyl)-1H-quinoline-2,4-dione, inhibits cisplatin-induced hearing loss by the suppression of reactive oxygen species: In vitro and in vivo study. Neuroscience 232C: 1–12, 2012 [DOI] [PubMed] [Google Scholar]
- 145.Sies H. Ebselen, a selenoorganic compound as glutathione peroxidase mimic. Free Radic Biol Med 14: 313–323, 1993 [DOI] [PubMed] [Google Scholar]
- 146.Simons JM T H, art BA, Ip Vai Ching TRAM, van Dijk H, and Labadie RP. Metabolic activation of natural phenols into selective oxidative burst agonists by activated human neutrophils. Free Radic Biol Med 8: 251–258, 1990 [DOI] [PubMed] [Google Scholar]
- 147.Smith SM, Min J, Ganesh T, Diebold B, Kawahara T, Zhu Y, McCoy J, Sun A, Snyder JP, Fu H, Du Y, Lewis I, and Lambeth JD. Ebselen and congeners inhibit NADPH oxidase 2-dependent superoxide generation by interrupting the binding of regulatory subunits. Chem Biol 19: 752–763, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Sorce S. and Krause KH. NOX enzymes in the central nervous system: from signaling to disease. Antioxid Redox Signal 11: 2481–2504, 2009 [DOI] [PubMed] [Google Scholar]
- 149.Sorescu D, Weiss D, LassŠgue B, Clempus RE, Sz”öcs K, Sorescu GP, Valppu L, Quinn MT, Lambeth JD, Vega JD, Taylor WR, and Griendling KK. Superoxide production and expression of nox family proteins in human atherosclerosis. Circulation 105: 1429–1435, 2002 [DOI] [PubMed] [Google Scholar]
- 150.Stielow C, Catar RA, Muller G, Wingler K, Scheurer P, Schmidt HH, and Morawietz H. Novel Nox inhibitor of oxLDL-induced reactive oxygen species formation in human endothelial cells. Biochem Biophys Res Commun 344: 200–205, 2006 [DOI] [PubMed] [Google Scholar]
- 151.Sukumar P, Viswambharan H, Imrie H, Cubbon RM, Yuldasheva N, Gage M, Galloway S, Skromna A, Kandavelu P, Santos CX, Gatenby VK, Smith J, Beech DJ, Wheatcroft SB, Channon KM, Shah AM, and Kearney MT. Nox2 NADPH oxidase has a critical role in insulin resistance-related endothelial cell dysfunction. Diabetes 62: 2130–2134, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Sumimoto H. Structure, regulation and evolution of Nox-family NADPH oxidases that produce reactive oxygen species. FEBS J 275: 3249–3277, 2008 [DOI] [PubMed] [Google Scholar]
- 153.Sun Q-A, Hess DT, Wang B, Miyagi M, and Stamler JS. Off-target thiol alkylation by the NADPH oxidase inhibitor 3-benzyl-7-(2-benzoxazolyl)thio-1,2,3-triazolo[4,5-d]pyrimidine (VAS2870). Free Radic Biol Med 52: 1897–1902, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Surace MJ. and Block ML. Targeting microglia-mediated neurotoxicity: the potential of NOX2 inhibitors. Cell Mol Life Sci 69: 2409–2427, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.t Hart BA, Simons JM, Knaan-Shanzer S, Bakker NP, and Labadie RP. Antiarthritic activity of the newly developed neutrophil oxidative burst antagonist apocynin. Free Radic Biol Med 9: 127–131, 1990 [DOI] [PubMed] [Google Scholar]
- 156.ten Freyhaus H, Huntgeburth M, Wingler K, Schnitker J, Baumer AT, Vantler M, Bekhite MM, Wartenberg M, Sauer H, and Rosenkranz S. Novel Nox inhibitor VAS2870 attenuates PDGF-dependent smooth muscle cell chemotaxis, but not proliferation. Cardiovasc Res 71: 331–341, 2006 [DOI] [PubMed] [Google Scholar]
- 157.Teng L, Fan LM, Meijles D, and Li JM. Divergent effects of p47(phox) phosphorylation at S303–S304 or S379 on tumor necrosis factor-alpha signaling via TRAF4 and MAPK in endothelial cells. Arterioscler Thromb Vasc Biol 32: 1488–1496, 2012 [DOI] [PubMed] [Google Scholar]
- 158.Thakur S, Du J, Hourani S, Ledent C, and Li JM. Inactivation of adenosine A2A receptor attenuates basal and angiotensin II-induced ROS production by Nox2 in endothelial cells. J Biol Chem 285: 40104–40113, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Touyz RM. Reactive oxygen species, vascular oxidative stress, and redox signaling in hypertension: what is the clinical significance? Hypertension 44: 248–252, 2004 [DOI] [PubMed] [Google Scholar]
- 160.Touyz RM. and Briones AM. Reactive oxygen species and vascular biology: implications in human hypertension. Hypertens Res 34: 5–14, 2011 [DOI] [PubMed] [Google Scholar]
- 161.Touyz RM, Chen X, Tabet F, Yao G, He G, Quinn MT, Pagano PJ, and Schiffrin EL. Expression of a functionally active gp91phox-containing neutrophil-type NAD(P)H oxidase in smooth muscle cells from human resistance arteries. Regulation by angiotensin II. Circ Res 90: 1205–1213, 2002 [DOI] [PubMed] [Google Scholar]
- 162.Traber MG. and Stevens JF. Vitamins C and E: beneficial effects from a mechanistic perspective. Free Radic Biol Med 51: 1000–1013, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Ueno N, Takeya R, Miyano K, Kikuchi H, and Sumimoto H. The NADPH oxidase Nox3 constitutively produces superoxide in a p22phox-dependent manner: its regulation by oxidase organizers and activators. J Biol Chem 280: 23328–23339, 2005 [DOI] [PubMed] [Google Scholar]
- 164.Valencia A, Sapp E, Kimm JS, McClory H, Reeves PB, Alexander J, Ansong KA, Masso N, Frosch MP, Kegel KB, Li X, and Difiglia M. Elevated NADPH oxidase activity contributes to oxidative stress and cell death in Huntington's disease. Hum Mol Genet 22: 1112–1131, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 165.Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, and Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol 39: 44–84, 2007 [DOI] [PubMed] [Google Scholar]
- 166.Vaquero EC, Edderkaoui M, Pandol SJ, Gukovsky I, and Gukovskaya AS. Reactive oxygen species produced by NAD(P)H oxidase inhibit apoptosis in pancreatic cancer cells. J Biol Chem 279: 34643–34654, 2004 [DOI] [PubMed] [Google Scholar]
- 167.Vara D, Campanella M, and Pula G. The novel NOX inhibitor 2-acetylphenothiazine impairs collagen-dependent thrombus formation in a GPVI-dependent manner. Br J Pharmacol 168: 212–224, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Vendrov AE, Madamanchi NR, Niu XL, Molnar KC, Runge M, Szyndralewiez C, Page P, and Runge MS. NADPH oxidases regulate CD44 and hyaluronic acid expression in thrombin-treated vascular smooth muscle cells and in atherosclerosis. J Biol Chem 285: 26545–26557, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Verdine GL. and Hilinski GJ. Stapled peptides for intracellular drug targets. Methods Enzymol 503: 3–33, 2012 [DOI] [PubMed] [Google Scholar]
- 170.Walch L, Massade L, Dufilho M, Brunet A, and Rendu F. Pro-atherogenic effect of interleukin-4 in endothelial cells: modulation of oxidative stress, nitric oxide and monocyte chemoattractant protein-1 expression. Atherosclerosis 187: 285–291, 2006 [DOI] [PubMed] [Google Scholar]
- 171.Wang Q, Zhou H, Gao H, Chen SH, Chu CH, Wilson B, and Hong JS. Naloxone inhibits immune cell function by suppressing superoxide production through a direct interaction with gp91phox subunit of NADPH oxidase. J Neuroinflammation 9: 32, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Waris G. and Ahsan H. Reactive oxygen species: role in the development of cancer and various chronic conditions. J Carcinog 5: 14, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Weaver M, Liu J, Pimentel D, Reddy DJ, Harding P, Peterson EL, and Pagano PJ. Adventitial delivery of dominant-negative p67phox attenuates neointimal hyperplasia of the rat carotid artery. Am J Physiol Heart Circ Physiol 290: H1933–H1941, 2006 [DOI] [PubMed] [Google Scholar]
- 174.Wiesel P, Hovsepian L, Mutch PJ, Herve J, Heitz F, and Page P. Safety and pharmacokinetics of single and multiple doses of a first in class dual NADPH oxidase 1 and 4 inhibitor administered orally in healthy subjects. In: Kidney Week 2012, edited by Nephrology ASo. San Diego, CA: Journal of the American Society of Nephrology; 2012. pp. 559A.(Abstract FR-PO831). [Google Scholar]
- 175.Wind S, Beuerlein K, Eucker T, Muller H, Scheurer P, Armitage ME, Ho H, Schmidt HH, and Wingler K. Comparative pharmacology of chemically distinct NADPH oxidase inhibitors. Br J Pharmacol 161: 885–898, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Winterbourn CC. and Hampton MB. Thiol chemistry and specificity in redox signaling. Free Radic Biol Med 45: 549–561, 2008 [DOI] [PubMed] [Google Scholar]
- 177.Xu S, Jiang B, Hou X, Shi C, Bachschmid MM, Zang M, Verbeuren TJ, and Cohen RA. High-fat diet increases and the polyphenol, S17834, decreases acetylation of the sirtuin-1-dependent lysine-382 on p53 and apoptotic signaling in atherosclerotic lesion-prone aortic endothelium of normal mice. J Cardiovasc Pharmacol 58: 263–271, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Zang M, Xu S, Maitland-Toolan KA, Zuccollo A, Hou X, Jiang B, Wierzbicki M, Verbeuren TJ, and Cohen RA. Polyphenols stimulate AMP-activated protein kinase, lower lipids, and inhibit accelerated atherosclerosis in diabetic LDL receptor-deficient mice. Diabetes 55: 2180–2191, 2006 [DOI] [PubMed] [Google Scholar]
- 179.Zhang M, Perino A, Ghigo A, Hirsch E, and Shah AM. NADPH oxidases in heart failure: poachers or gamekeepers? Antioxid Redox Signal 18: 1024–1041, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Zima AV. and Blatter LA. Redox regulation of cardiac calcium channels and transporters. Cardiovasc Res 71: 310–321, 2006 [DOI] [PubMed] [Google Scholar]