Abstract
The reaction of 5-(1-adamantyl)-4-phenyl-1,2,4-triazoline-3-thione (compound 5) with formaldehyde and 1-substituted piperazines yielded the corresponding N-Mannich bases 6a–f. The reaction of 5-(1-adamantyl)-4-methyl-1,2,4-triazoline-3-thione 8 with various 2-aminoethyl chloride yielded separable mixtures of the S-(2-aminoethyl) 9a–d and the N-(2-aminoethyl) 10a–d derivatives. The reaction of compound 5 with 1-bromo-2-methoxyethane, various aryl methyl halides, and ethyl bromoacetate solely yielded the S-substituted products 11, 12a–d, and 13. The new compounds were tested for activity against a panel of Gram-positive and Gram-negative bacteria and the pathogenic fungus Candida albicans. Compounds 6b, 6c, 6d, 6e, 6f, 10b, 10c, 10d, 12c, 12d, 12e, 13, and 14 displayed potent antibacterial activity. Meanwhile, compounds 13 and 14 produced good dose-dependent anti-inflammatory activity against carrageenan-induced paw edema in rats.
Keywords: adamantane derivatives; 1,2,4-triazoles; N-Mannich bases; antimicrobial activity; anti-inflammatory activity
Introduction
The incorporation of an adamantyl moiety into several molecules results in compounds with relatively high lipophilicity, which in turn can modify the biological availability of these molecules. In almost all cases, an adamantyl-bearing compound will be more lipophilic than the des-adamantyl analog. Beyond increasing the partition coefficient, the adamantyl group positively modulates the therapeutic index of many experimental compounds through a variety of mechanisms.1,2 After the discovery of amantadine as an antiviral and antiparkinsonian drug in 1960, adamantane derivatives as potential chemotherapeutic agents have attracted the attention of a number of scientists. As a result of this intensive investigation, numerous adamantane derivatives have been synthesized and tested for their biological activity. This has resulted in the discovery of several drugs which are now available on market. Antiviral,3–13 antidiabetic,14 antimicrobial,15–23 anti-inflammatory,21–26 and central nervous system activities27–29 are the most important biological activities of adamantane derivatives. In addition, several 1,2,4-triazoles and their N-Mannich bases were reported to possess potent antimicrobial30–33 and anti-inflammatory34–36 activities. As a continuation of our interest in the chemical and pharmacological properties of adamantane derivatives,13,20–24,37,38 we now report herein on the synthesis and antimicrobial and anti-inflammatory activity of a new series of S-substituted and N-substituted-5-(1-adamantyl)-1,2,4-triazole-3-thiol derivatives.
Materials and methods
Melting points (°C) were measured in open glass capillaries using a Barnstead 9001 electrothermal melting point apparatus (Thermo Fisher Scientific, Waltham, MA, USA) and are uncorrected. NMR spectra were obtained using an AC 500 Ultra Shield NMR spectrometer (Bruker, Fällanden, Switzerland) operating at 500.13 MHz for 1H and 125.76 MHz for 13C. The chemical shifts are expressed in δ (ppm) downfield from tetramethylsilane as the internal standard; coupling constants (J) are expressed in Hz. ESI-MS were recorded on a 6410 Triple Quad tandem mass spectrometer (Agilent Technologies, Santa Clara, CA, USA) at 4.0 kV and 3.5 kV for positive and negative ions, respectively. Elemental analyses (C, H, N, and S) were in agreement with the proposed structures and within ±0.4% of the theoretical values. Monitoring reactions and checking the purity of the final products was done using thin layer chromatography with silica gel-precoated aluminum sheets (60 F254; Merck Schuchardt, Darmstadt, Germany) and visualization with ultraviolet light at 365 nm and 254 nm. The bacterial strains and the C. albicans fungus were obtained from the IFO in Japan. The reference drugs ampicillin trihydrate (CAS 7177-48-2), gentamicin sulfate (CAS 1405-41-0), clotrimazole (CAS 23593-75-1), and indomethacin (CAS 53-86-1) were purchased from Sigma-Aldrich Chemie GmbH (Taufkirchen, Germany). Sprague Dawley rats were purchased from a local animal house (Abu-Rawash, Giza, Egypt). The animal experiments performed to determine the anti-inflammatory activity of the study compounds were carried out in agreement with the pertinent legal and ethical standards of the international guidelines.
5-(1-Adamantyl)-4-phenyl-2-(4-substituted piperazine-1-ylmethyl)-1,2,4-triazoline-3-thiones (6a–f)
A mixture of compound 5 (623 mg, 2.0 mmol), the appropriate N-substituted piperazine (2.0 mmol), and 37% formaldehyde solution (0.5 mL) in ethanol (8 mL) was heated under reflux for 15 minutes until a clear solution was obtained. Stirring was continued for 12 hours at room temperature and the mixture was allowed to stand overnight. Cold water (5 mL) was added and the reaction mixture was stirred for 20 minutes. The precipitated crude products were filtered, washed with water, dried, and crystallized from ethanol or aqueous ethanol.
6a: 1H NMR (CDCl3): δ 1.08 (t, 3H, CH3CH2, J=7.0 Hz), 1.51–1.53 (m, 3H, adamantane-H), 1.63–1.66 (m, 3H, adamantane-H), 1.83–1.86 (m, 6H, adamantane-H), 1.92 (s, 3H, adamantane-H), 2.41 (q, 2H, CH3CH2, J=7.0 Hz), 2.50 (s, 4H, piperazine-H), 2.96 (s, 4H, piperazine-H), 5.21 (s, 2H, CH2), 7.23–7.29 (m, 2H, Ar-H), 7.54–7.56 (m, 3H, Ar-H). 13C NMR: δ 11.91 (CH3CH2), 27.76, 35.81, 36.09, 39.87 (adamantane-C), 50.26, 52.80 (piperazine-C), 52.36 (CH3CH2), 69.25 (CH2), 129.48, 129.65, 130.08, 136.63 (Ar-C), 156.35 (triazole C-5), 171.03 (C=S). ESI-MS, m/z: 438.4 (M+H)+.
6b: 1H NMR (CDCl3): δ 1.17 (t, 3H, CH3, J=7.0 Hz), 1.45–1.47 (m, 3H, adamantane-H), 1.57–1.59 (m, 3H, adamantane-H), 1.77 (s, 6H, adamantane-H), 1.86 (s, 3H, adamantane-H), 2.76 (s, 4H, piperazine-H), 3.43 (s, 4H, piperazine-H), 4.04 (q, 2H, CH2CH3, J=7.0 Hz), 5.11 (s, 2H, CH2), 7.18–7.20 (m, 2H, Ar-H), 7.47–7.49 (m, 3H, Ar-H). 13C NMR: δ 14.67 (CH3), 27.76, 35.89, 36.08, 39.91 (adamantane-C), 43.67, 50.34 (piperazine-C), 61.36 (CH2CH3), 69.51 (CH2), 129.50, 129.66, 130.16, 136.57 (Ar-C), 155.52 (C=O), 156.63 (triazole C-5), 171.13 (C=S). ESI-MS, m/z (relative intensity): 482.3 (M+H)+.
6c: 1H NMR (CDCl3): δ 1.44–1.47 (m, 3H, adamantane-H), 1.56–1.59 (m, 3H, adamantane-H), 1.77 (s, 6H, adamantane-H), 1.86 (s, 3H, adamantane-H), 2.98 (s, 4H, piperazine-H), 3.14 (s, 4H, piperazine-H), 5.17 (s, 2H, CH2), 6.80–6.88 (m, 3H, Ar-H), 7.18–7.19 (m, 4H, Ar-H), 7.46–7.48 (m, 3H, Ar-H). 13C NMR: δ 27.78, 35.89, 36.10, 39.93 (adamantane-C), 49.44, 50.49 (piperazine-C), 69.32 (CH2), 116.35, 120.01, 129.16, 129.53, 129.67, 130.16, 136.62, 151.35 (Ar-C), 156.55 (triazole C-5), 171.14 (C=S). ESI-MS, m/z: 486.4 (M+H)+.
6d: 1H NMR (CDCl3): δ 1.44–1.47 (m, 3H, adamantane-H), 1.56–1.59 (m, 3H, adamantane-H), 1.78 (s, 6H, adamantane-H), 1.86 (s, 3H, adamantane-H), 3.04 (s, 8H, piperazine-H), 3.80 (s, 3H, OCH3), 5.19 (s, 2H, CH2), 6.78–6.96 (m, 4H, Ar-H), 7.19–7.20 (m, 2H, Ar-H), 7.46–7.48 (m, 3H, Ar-H). 13C NMR: δ 27.79, 34.82, 36.13, 39.97 (adamantane-C), 50.57, 50.87 (piperazine-C), 55.24 (OCH3), 69.53 (CH2), 110.79, 118.28, 120.92, 123.14, 129.50, 129.72, 130.11, 135.65, 140.16, 151.11 (Ar-C), 155.41 (triazole C-5), 170.10 (C=S). ESI-MS, m/z: 516.3 (M+H)+.
6e: 1H NMR (CDCl3): δ 1.43–1.46 (m, 3H, adamantane-H), 1.56–1.58 (m, 3H, adamantane-H), 1.76 (s, 6H, adamantane-H), 1.85 (s, 3H, adamantane-H), 2.41–2.43 (m, 4H, piperazine-H), 2.85 (s, 4H, piperazine-H), 3.45 (s, 2H, benzylic CH2), 5.10 (s, 2H, CH2), 7.17–7.20 (m, 3H, Ar-H), 7.22–7.24 (m, 4H, Ar-H), 7.46–7.47 (m, 3H, Ar-H). 13C NMR: δ 27.78, 35.84, 36.11, 39.91 (adamantane-C), 50.37, 53.14 (piperazine-C), 63.30 (benzylic CH2), 69.45 (CH2), 127.13, 128.24, 129.40, 129.48, 129.69, 130.10, 136.66, 137.72 (Ar-C), 156.40 (triazole C-5), 171.08 (C=S). ESI-MS, m/z: 500.3 (M+H)+.
6f: 1H NMR (CDCl3): δ 1.40–1.42 (m, 3H, adamantane-H), 1.52–1.55 (m, 3H, adamantane-H), 1.72 (s, 6H, adamantane-H), 1.81 (s, 3H, adamantane-H), 2.89 (s, 4H, piperazine-H), 3.46 (s, 4H, piperazine-H), 5.14 (s, 2H, CH2), 6.50–6.55 (m, 2H, pyridine-H), 7.17–7.16 (m, 2H, Ar-H), 7.36–7.44 (m, 4H, Ar-H and pyridine-H), 8.08–8.09 (m, 1H, pyridine-H). 13C NMR: δ 27.77, 35.88, 36.09, 39.91 (adamantane-C), 45.29, 50.36 (piperazine-C), 69.42 (CH2), 107.15, 113.38, 129.49, 129.67, 130.13, 136.61, 137.52, 147.97, 159.54 (Ar-C), 156.54 (triazole C-5), 171.11 (C=S). ESI-MS, m/z: 487.4 (M+H)+.
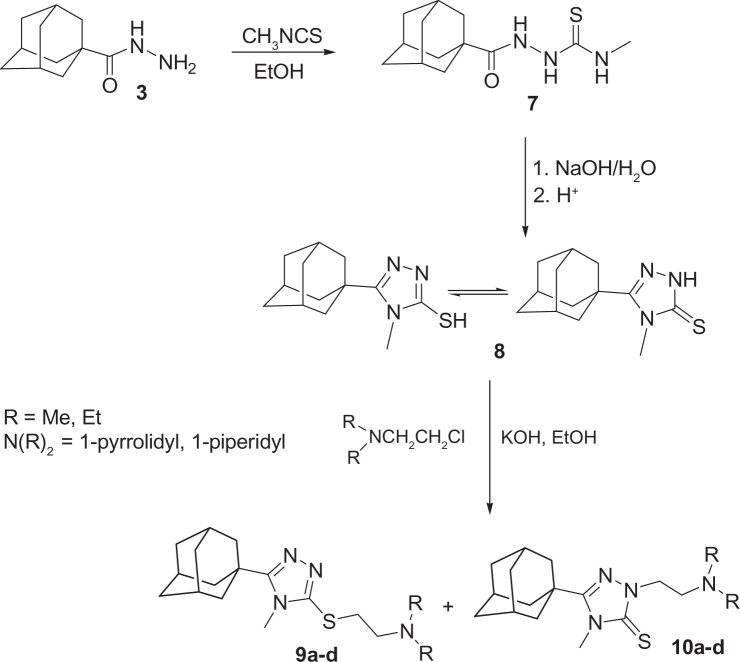
5-(1-Adamantyl)-4-methyl-3-(2-aminoethylthio)-1,2,4-triazoles (9a–d) and 5-(1-adamantyl)-4-methyl-2-(2-aminoethyl)-1,2,4-triazoline-3-thiones (10a–d)
A mixture of compound 8 (2.49 g, 0.01 mol), potassium hydroxide (1.12 g, 0.02 mol) and the appropriate 2-aminoethyl chloride hydrochloride (0.01 mol) in ethanol (15 mL) was heated under reflux with stirring for 3 hours and the solvent was distilled off in vacuo. The residue obtained was washed with water and purified by column chromatography on silica gel column using CHCl3:MeOH (9:1) as an eluent to yield compounds 10a–d followed by 9a–d. The products were then crystallized from aqueous ethanol.
9a: 1H NMR (CDCl3): δ 1.69–1.75 (m, 6H, adamantane-H), 2.04–2.08 (m, 9H, adamantane-H), 2.21 (s, 6H, CH3), 2.62 (t, 2H, SCH2CH2, J=6.5 Hz), 3.28 (t, 2H, SCH2CH2, J=6.5 Hz), 3.59 (s, 3H, CH3). 13C NMR: δ 28.07, 34.98, 36.50, 39.56 (adamantane-C), 31.05 (SCH2CH2), 32.33 (CH3), 45.21 (2 × CH3), 58.24 (SCH2CH2), 152.16 (triazole C-5), 161.21 (triazole C-3). ESI-MS, m/z: 321.2 (M+H)+.
9b: 1H NMR (CDCl3): δ 0.98 (t, 6H, CH2CH3, J=7.0 Hz), 1.70–1.73 (m, 6H, adamantane-H), 1.97–2.08 (m, 9H, adamantane-H), 2.40 (q, 4H, CH2CH3, J=7.0 Hz), 2.82 (t, 2H, SCH2CH2, J=70 Hz), 3.30 (t, 2H, SCH2CH2, J=7.0 Hz), 3.58 (s, 3H, CH3). 13C NMR: δ 12.35 (CH2CH3), 28.0, 35.02, 36.22, 39.68 (adamantane-C), 30.75 (SCH2CH2), 32.16 (CH3), 48.82 (CH2CH3), 57.88 (SCH2CH2), 152.18 (triazole C-5), 161.56 (C-3). ESI-MS, m/z: 349.3 (M+H)+.
9c: 1H NMR (CDCl3): δ 1.59–1.61 (m, 4H, pyrrolidine-H), 1.72–1.76 (m, 6H, adamantane-H), 1.96–2.16 (m, 9H, adamantane-H), 2.36–2.40 (m, 4H, pyrrolidine-H), 2.81 (t, 2H, SCH2CH2, J=7.0 Hz), 3.38 (t, 2H, SCH2CH2, J=7.0 Hz), 3.59 (s, 3H, CH3). 13C NMR: δ 24.44, 55.24 (pyrrolidine-C), 28.20, 35.41, 36.20, 39.58 (adamantane-C), 31.05 (SCH2CH2), 32.0 (CH3), 58.02 (SCH2CH2), 151.98 (triazole C-5), 161.86 (C=S). ESI-MS, m/z (relative intensity): 347.3 (M+H)+.
9d: 1H NMR (CDCl3): δ 1.35–1.37 (m, 2H, piperidine-CH2), 1.48–1.50 (m, 4H, piperidine-CH2), 1.73 (s, 6H, adamantane-H), 2.06 (s, 9H, adamantane-H), 2.36–2.38 (m, 4H, piperidine-CH2), 2.63–2.65 (m, 2H, SCH2CH2), 3.25–3.27 (m, 2H, SCH2CH2), 3.60 (s, 3H, CH3). 13C NMR: δ 24.30, 25.87, 54.28 (piperidine-C), 28.08, 35.02, 35.51, 39.57 (adamantane-C), 30.56 (SCH2CH2), 32.39 (CH3), 58.16 (SCH2CH2), 152.11 (triazole C-5), 161.16 (triazole C-3). ESI-MS, m/z: 361.3 (M+H)+.
10a: 1H NMR (CDCl3): δ 1.70–1.74 (m, 6H, adamantane-H), 1.98–2.03 (m, 9H, adamantane-H), 2.36 (s, 6H, CH3), 2.82 (t, 2H, NCH2CH2N, J=7.0 Hz), 3.66 (s, 3H, CH3), 4.01 (t, 2H, NCH2CH2N, J=7.0 Hz). 13C NMR: δ 28.20, 35.44, 36.32, 39.26 (adamantane-C), 34.52 (CH3), 47.16 (NCH2CH2N), 51.02 (NCH2CH2N), 156.42 (triazole C-5), 167.54 (C=S). ESI-MS, m/z: 321.2 (M+H)+.
10b: 1H NMR (CDCl3): δ 0.97 (t, 6H, CH2CH3, J=7.0 Hz), 1.68–1.75 (m, 6H, adamantane-H), 1.97 (s, 6H, adamantane-H), 2.04 (s, 3H, adamantane-H), 2.53 (q, 4H, CH2CH3, J=7.0 Hz), 2.80 (t, 2H, NCH2CH2N, J=7.0 Hz), 3.70 (s, 3H, CH3), 4.17 (t, 2H, NCH2CH2N, J=7.0 Hz). 13C NMR: δ 12.33 (CH2CH3), 27.95, 35.24, 36.43, 39.14 (adamantane-C), 34.06 (CH3), 47.20 (NCH2CH2N), 47.52 (CH2CH3), 50.86 (NCH2CH2N), 156.44 (triazole C-5), 167.51 (C=S). ESI-MS, m/z: 349.3 (M+H)+.
10c: 1H NMR (CDCl3): δ 1.55–1.77 (m, 4H, pyrrolidine-H), 1.70–174 (m, 6H, adamantane-H), 2.02 (s, 9H, adamantane-H), 2.29–2.40 (m, 4H, pyrrolidine-H), 2.81 (m, 2H, NCH2CH2N, J=7.0 Hz), 3.68 (s, 3H, CH3), 4.15 (t, 2H, NCH2CH2N, J=7.0 Hz). 13C NMR: δ 25.05, 55.95 (pyrrolidine-C), 27.83, 35.15, 35.80, 39.25 (adamantane-C), 34.25 (CH3), 48.0 (NCH2CH2N), 50.82 (NCH2CH2N), 155.99 (triazole C-5), 167.58 (C=S). ESI-MS, m/z: 347.3 (M+H)+.
10d: 1H NMR (CDCl3): δ 1.48–1.52 (m, 2H, piperidine-H), 1.52–1.58 (m, 4H, piperidine-H), 1.70–176 (m, 6H, adamantane-H), 2.03 (s, 9H, adamantane-H), 2.32–2.40 (m, 4H, piperidine-H), 2.80 (m, 2H, NCH2CH2N, J=7.0 Hz), 3.67 (s, 3H, CH3), 4.10 (t, 2H, NCH2CH2N, J=7.0 Hz). 13C NMR: δ 24.0, 25.82, 54.22 (piperidine-C), 28.11, 35.35, 35.81, 39.67 (adamantane-C), 34.40 (CH3), 48.02 (NCH2CH2N), 50.74 (NCH2CH2N), 155.65 (triazole C-5), 167.50 (C=S). ESI-MS, m/z: 361.3 (M+H)+.
5-(1-Adamantyl)-3-[(2-methoxyethyl) sulfanyl]-4-phenyl-1,2,4-triazole (11)
A mixture of compound 5 (623 mg, 2 mmol), 1-bromo-2-methoxyethane (278 mg, 2 mmol), and anhydrous potassium carbonate (276 mg, 2 mmol) in N,N-dimethylformamide (5 mL) was stirred at room temperature for 24 hours. Water (15 mL) was added and the mixture was stirred for 30 minutes. The separated crude product was filtered, washed with water, dried, and crystallized from aqueous ethanol to yield 196 mg (53%) of compound 11 as colorless plate-shaped crystals. 1H NMR (CDCl3): δ 1.47–1.49 (m, 3H, adamantane-H), 1.56–1.58 (m, 3H, adamantane-H), 1.84–1.86 (m, 9H, adamantane-H), 3.27–3.35 (m, 5H, SCH2 and OCH3), 3.64 (t, 2H, CH2O, J=6.0 Hz), 7.15–7.20 (m, 2H, Ar-H), 7.44–7.47 (m, 3H, Ar-H). 13C NMR: δ 28.0, 35.82, 36.28, 40.49 (adamantane-C), 31.66 (SCH2), 58.68 (OCH3), 70.87 (CH2O), 128.94, 129.49, 130.22, 135.13 (Ar-C), 152.97 (triazole C-5), 162.05 (triazole C-3). ESI-MS, m/z: 370.4 (M+H)+.
5-(1-Adamantyl)-3-arylmethylsulfanyl-4-phenyl-1,2,4-triazoles (12a–e)
A mixture of compound 5 (623 mg, 2 mmol), benzyl bromide, 4-fluorobenzyl chloride, 4-chlorobenzyl chloride, 3,5-bis(trifluoromethyl) benzyl chloride or 4-nitrobenzyl bromide (2 mmol) and anhydrous potassium carbonate (276 mg, 2 mmol), in dry acetone (10 mL), was heated under reflux with stirring for 2 hours. The solvent was then distilled off in vacuo and the resulted residue was washed with water, dried, and crystallized from ethanol or aqueous ethanol.
12a: 1H NMR (CDCl3): δ 1.46–1.49 (m, 3H, adamantane-H), 1.56–1.58 (m, 3H, adamantane-H), 1.85 (s, 9H, adamantane-H), 4.33 (s, 2H, CH2), 7.03–7.06 (m, 2H, Ar-H), 7.16–7.18 (m, 2H, Ar-H), 7.22–7.40 (m, 6H, Ar-H). 13C NMR: δ 27.02, 36.0, 36.28, 39.88 (adamantane-C), 36.15 (SCH2), 126.82, 127.70, 128.58, 128.80, 129.42, 129.60, 131.42, 138.38 (Ar-C), 151.82 (triazole C-5), 161.10 (triazole C-3). ESI-MS, m/z: 402.4 (M+H)+.
12b: 1H NMR (CDCl3): δ 1.46–1.48 (m, 3H, adamantane-H), 1.56–1.58 (m, 3H, adamantane-H), 1.85 (s, 9H, adamantane-H), 4.30 (s, 2H, CH2), 6.85–6.89 (m, 2H, Ar-H), 7.03–7.04 (m, 2H, Ar-H), 7.21–7.23 (m, 2H, Ar-H), 7.39–7.46 (m, 3H, Ar-H). 13C NMR: δ 27.94, 35.94, 36.32, 40.41 (adamantane-C), 36.19 (SCH2), 115.48, 128.77, 129.50, 130.38, 130.99, 132.57, 134.81, 163.23 (Ar-C), 152.78 (triazole C-5), 161.89 (triazole C-3). ESI-MS, m/z: 420.4 (M+H)+.
12c: 1H NMR (CDCl3): δ 1.47–1.49 (m, 3H, adamantane-H), 1.56–1.59 (m, 3H, adamantane-H), 1.85 (s, 9H, adamantane-H), 4.29 (s, 2H, CH2), 7.04–7.06 (m, 2H, Ar-H), 7.15–7.16 (m, 2H, Ar-H), 7.19–7.21 (m, 2H, Ar-H), 7.39–7.46 (m, 3H, Ar-H). 13C NMR: δ 26.91, 35.16, 35.22, 39.38 (adamantane-C), 36.19 (SCH2), 127.66, 127.73, 128.52, 128.89, 129.42, 129.63, 132.49, 134.33 (Ar-C), 151.70 (triazole C-5), 160.88 (triazole C-3). ESI-MS, m/z (relative intensity): 436.4 (M+H, 100)+, 438.4 (M+H+2, 37)+.
12d: 1H NMR (CDCl3): δ 1.47–1.49 (m, 3H, adamantane-H), 1.57–1.59 (m, 3H, adamantane-H), 1.89 (s, 9H, adamantane-H), 4.50 (s, 2H, CH2), 7.19 (d, 2H, Ar-H, J=8.0 Hz), 7.47–7.54 (m, 5H, Ar-H), 8.05 (d, 2H, Ar-H, J=8.0 Hz). 13C NMR: δ 27.70, 35.73, 36.35, 40.09 (adamantane-C), 35.90 (SCH2), 123.83, 128.54, 129.97, 130.32, 131.28, 133.49, 134.71, 147.47 (Ar-C), 153.25 (triazole C-5), 161.57 (triazole C-3). ESI-MS, m/z: 447.3 (M+H)+.
12e: 1H NMR (CDCl3): δ 1.46–1.49 (m, 3H, adamantane-H), 1.56–1.59 (m, 3H, adamantane-H), 1.85 (s, 9H, adamantane-H), 4.43 (s, 2H, CH2), 7.03 (d, 2H, Ar-H, J=7.5 Hz), 7.41–7.49 (m, 3H, Ar-H), 7.69 (s, 1H, Ar-H), 7.72 (s, 2H, Ar-H). 13C NMR: δ 27.90, 36.02, 36.14, 40.36 (adamantane-C), 36.16 (SCH2), 121.59, 124.18, 128.62, 129.32, 129.66, 130.63, 131.74, 134.54, 139.66 (Ar-C and CF3), 151.79 (triazole C-5), 162.17 (triazole C-3). ESI-MS, m/z: 538.5 (M+H)+.
Ethyl 2-[5-(1-adamantyl)-4-phenyl-1,2, 4-triazol-3-ylsulfanyl]acetate (13)
A mixture of compound 5 (623 mg, 2 mmol), ethyl 2-bromoacetate (334 mg, 2 mmol), and anhydrous potassium carbonate (276 mg, 2 mmol), in dry acetone (10 mL) was heated under reflux with stirring for 2 hours. The solvent was then distilled off in vacuo and the resulting residue was washed with water, dried, and crystallized from aqueous ethanol to yield 645 mg (81%) of compound 13. 1H NMR (CDCl3): δ 1.18 (t, 3H, CH2CH3, J=7.0 Hz), 1.47–1.50 (m, 3H, adamantane-H), 1.57–1.59 (m, 3H, adamantane-H), 1.87 (s, 9H, adamantane-H), 3.95 (s, 2H, SCH2), 4.10 (q, 2H, CH2CH3, J=7.0 Hz), 7.22 (d, 2H, Ar-H, J=7.0 Hz), 7.46–7.50 (m, 3H, Ar-H). 13C NMR: δ 14.10 (CH2CH3), 27.94, 34.54, 35.94, 40.41 (adamantane-C), 36.20 (SCH2), 62.0 (CH2CH3), 128.83, 129.64, 130.53, 134.69 (Ar-C), 152.0 (triazole C-5), 162.04 (triazole C-3), 168.31 (C=O). ESI-MS, m/z: 398.3 (M+H)+.
2-[5-(1-Adamantyl)-4-phenyl-1,2, 4-triazol-3-ylsulfanyl]acetic acid (14)
Compound 13 (795 mg, 2 mmol) was added to a 10% aqueous solution of sodium hydroxide (8 mL) and the mixture was heated under reflux with stirring for one hour. The resulting clear solution was filtered hot, cooled, and acidified with hydrochloric acid to pH 1–2. The precipitated crude product was filtered, washed with water, dried, and crystallized from aqueous ethanol to yield 540 mg (73%) of compound 14. 1H NMR (DMSO-d6): δ 1.48–1.63 (m, 6H, adamantane-H), 1.81–1.86 (s, 9H, adamantane-H), 3.99 (s, 2H, SCH2), 4.28 (s, 1H, OH), 7.53–7.65 (m, 5H, Ar-H). 13C NMR: δ 27.21, 34.18, 35.60, 39.85 (adamantane-C), 35.37 (SCH2), 128.79, 129.73, 130.82, 134.0 (Ar-C), 152.09 (triazole C-5), 160.81 (triazole C-3), 169.07 (C=O). ESI-MS, m/z: 368.3 (M-H)−.
Determination of in vitro antimicrobial activity (agar disc diffusion method)
Sterile filter paper discs (8 mm diameter) were moistened with the compound solution in dimethyl sulfoxide of specific concentration (200 μg/disc), and the antibacterial agents, ie, gentamicin and ampicillin trihydrate (100 μg/disc) and the antifungal drug clotrimazole (100 μg/disc), were carefully placed on agar culture plates that had been previously inoculated separately with the microorganisms. The plates were incubated at 37°C, and the diameters of the growth inhibition zones were measured after 24 hours for bacteria and 48 hours for C. albicans.
Determination of MIC
Compounds 6b, 6c, 6d, 6e, 6f, 10b, 10c, 10d, 12c, 12d, 12e, 13, 14, gentamicin, and ampicillin trihydrate were dissolved in dimethyl sulfoxide at a concentration of 128 μg/mL. The two-fold dilutions of the solution were prepared (128, 64, 32,…, 0.5 μg/mL). Suspensions of the microorganisms at concentrations of 106 colony-forming units per mL were inoculated in the corresponding wells. The plates were then incubated at 36°C for 24 hours. The MIC values were determined as the lowest concentrations that completely inhibited visible growth of the microorganism as detected by the unaided eye.
Determination of in vivo anti-inflammatory activity
Male Sprague Dawley rats weighing 140–190 g were maintained at room temperature (20°C–23°C) and randomly divided into 20 groups containing five animals each. The animals were housed with food and water ad libitum and allowed to become accustomed to their environment for 2 days before testing. Each group was injected with a specific dose of the test compound (20 or 40 mg/kg) or indomethacin 5 mg/kg intraperitoneally as a uniform suspension in 1 mL of 0.5% (w/v) aqueous carboxymethyl cellulose solution one hour before injection of 0.1 mL of carrageenan (1% solution in normal saline) into the plantar tissue of the right hind paw. The left hind paw was injected with 0.1 mL of normal saline solution. Four hours after injection of carrageenan, the volume of paw edema (mL) was determined using a water plethysmometer (Ugo Basile Srl, Varese, Italy). Protection against inflammation was calculated following the formula:
where Vc is the mean percentage increase in paw volume in the absence of the test compound (control) and Vd is the mean percentage increase in paw volume after injection of the test compound. The values are expressed as the mean ± standard error of the mean. Statistical significance between the control and treated groups was assessed using the Student’s t-test.
Determination of oral acute toxicity of compounds 13 and 14
Freshly prepared suspensions of compounds 13 and 14 at concentrations of 1%, 3%, 4%, 6%, 8%, and 12% in 0.5% aqueous carboxymethyl cellulose solution were prepared. Each compound was given to six groups of normal albino mice of either sex (n=6 in each group) by oral intubation at doses of 250, 500, 750, 1,000, 1,250, and 1,500 mg/kg. Percent mortality was recorded 24 hours after administration of the compound and oral lethal doses of LD16, LD50 and LD84 were calculated.
Results and discussion
Chemistry
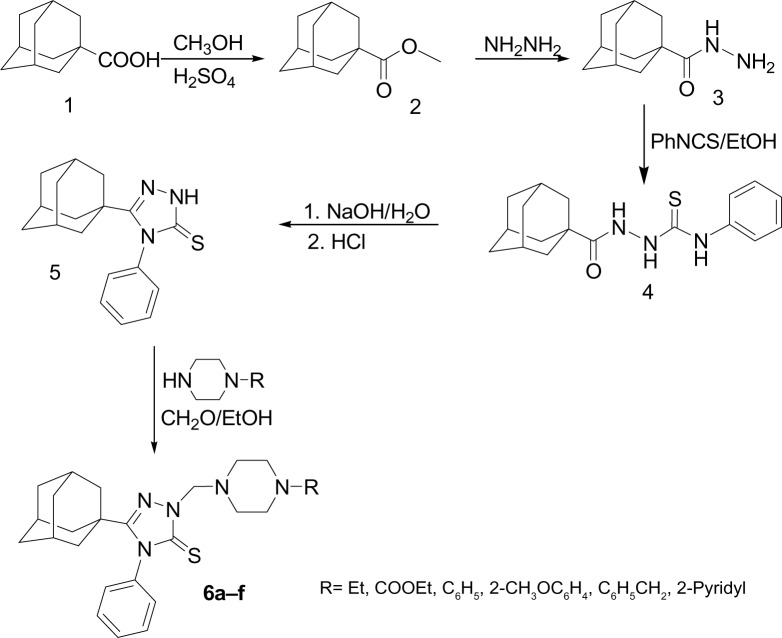
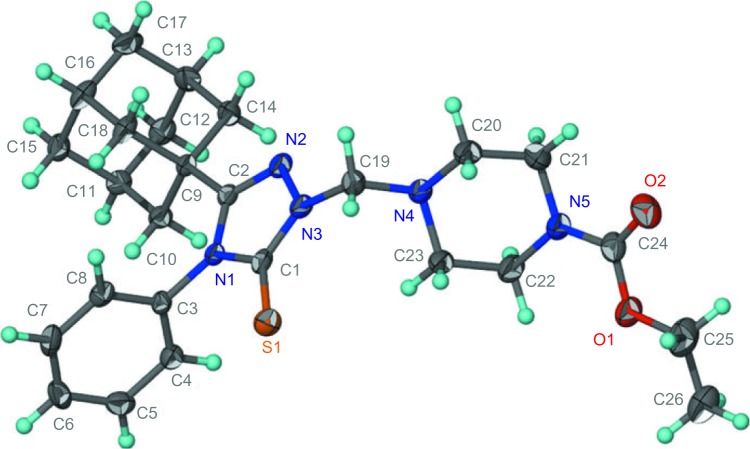
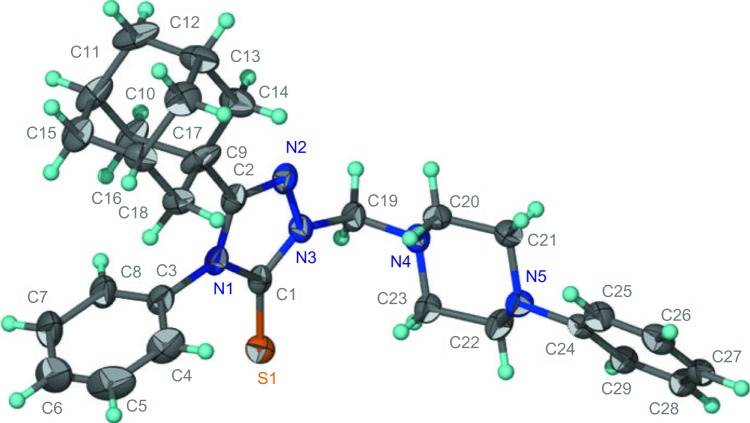
Adamantane-1-carbohydrazide 3 was obtained by prolonged heating of methyl adamantane-1-carboxylate 2 with hydrazine.24 The reaction of compound 3 with phenyl isothiocyanate yielded the intermediate 1-(1-adamantylcarbonyl)-4-phenylthiosemicarbazide 4, which was cyclized to 5-(1-adamantyl)-4-phenyl-1,2,4-triazoline-3-thione 5 via heating in 10% aqueous sodium hydroxide.24 Compound 5 was reacted with the corresponding 1-substituted piperazine and formaldehyde solution in ethanol to yield the corresponding N-Mannich bases 6a–f in good yields. The reaction was carried out by heating the reactants in ethanol for 15 minutes to enhance the solubility of compound 5 (Figure 1 and Table 1). The structures of compounds 6a–f were confirmed by elemental analyses in addition to 1H nuclear magnetic resonance (NMR), 13C NMR, and electrospray ionization mass spectra (ESI-MS) data, which were in full agreement with their structures and the X-ray spectra of compounds 6b (Figure 2),39 6c (Figure 3),40 and 6e (Figure 4).41
Figure 1.
Synthetic approach used for compounds 6a–f.
Table 1.
Melting points, crystallization solvents, yield percentages, molecular formulae, and molecular weights of compounds 6a–f, 9a–d, 10a–d, 11, 12a–e, 13, and 14
| Compound number | R/N(R)2 | Crystalline solvent | Melting point (°C) | Yield (%) | Molecular formula (molecular weight) |
|---|---|---|---|---|---|
| 6a | C2H5 | EtOH/H2O | 231–233 | 51 | C25H35N5S (437.64) |
| 6b | COOC2H5 | EtOH | 192–194 | 80 | C26H35N5O2S (481.65) |
| 6c | C6H5 | EtOH/H2O | 225–227 | 84 | C29H35N5S (485.69) |
| 6d | 2-CH3OC6H4 | EtOH/H2O | 135–137 | 92 | C30H37N5OS (515.71) |
| 6e | C6H5CH2 | EtOH | 197–199 | 80 | C30H37N5S (499.71) |
| 6f | 2-Pyridyl | EtOH/H2O | 145–147 | 76 | C28H34N6S (486.67) |
| 9a | CH3 | EtOH/H2O | 133–135 | 63 | C17H28N4S (320.50) |
| 9b | C2H5 | EtOH/H2O | 111–113 | 69 | C19H32N4S (348.55) |
| 9c | 1-Pyrrolidyl | EtOH/H2O | 132–134 | 59 | C19H30N4S (346.53) |
| 9d | 1-Piperidyl | EtOH/H2O | 155–157 | 66 | C20H32N4S (360.56) |
| 10a | CH3 | EtOH/H2O | 160–162 | 14 | C17H28N4S (320.50) |
| 10b | C2H5 | EtOH/H2O | 148–150 | 11 | C19H32N4S (348.55) |
| 10c | 1-Pyrrolidyl | EtOH/H2O | 144–146 | 14 | C19H30N4S (346.53) |
| 10d | 1-Piperidyl | EtOH/H2O | 167–169 | 19 | C20H32N4S (360.56) |
| 11 | – | EtOH/H2O | 155–157 | 53 | C21H27N3OS (369.52) |
| 12a | H | EtOH/H2O | 139–141 | 65 | C25H27N3S (401.57) |
| 12b | F | EtOH/H2O | 132–134 | 62 | C25H26FN3S (419.56) |
| 12c | cl | EtOH/H2O | 130–132 | 75 | C25H26CIN3S (436.01) |
| 12d | NO2 | EtOH | 188–190 | 85 | C25H26N4O2S (446.56) |
| 12e | 3,5(CF3)2 | EtOH | 156–158 | 92 | C27H25F6N3S (537.56) |
| 13 | – | EtOH/H2O | 156–158 | 81 | C22H27N3O2S (397.53) |
| 14 | – | EtOH/H2O | 288–90 | 73 | C20H23N3O2S (369.48) |
Figure 2.
Oak Ridge thermal ellipsoid plot of compound 6b showing 70% probability displacement ellipsoids for non-H atoms.
Figure 3.
Oak Ridge thermal ellipsoid plot of compound 6c showing 70% probability displacement ellipsoids for non-H atoms.
Figure 4.
Oak Ridge thermal ellipsoid plot of compound 6e showing 70% probability displacement ellipsoids for non-H atoms.
5-(1-Adamantyl)-4-methyl-1,2,4-triazoline-3-thione 8 was prepared according to a previously reported method24 via the reaction of compound 3 with methyl isothiocyanate in ethanol to yield the intermediate 1-(1-adamantylcarbonyl)-4-methylthiosemicarbazide 7, which was subsequently cyclized to the target compound 8 in good overall yield. The alkylation of 4,5-disubstituted-1,2,4-triazoline-3-thiones (1,2,4-triazol-3-thiols) has been studied by several authors. The reaction product(s) were found to be dependent on the nature of the substituents, the base, the alkyl halide, the reaction solvent, and the reaction temperature. The use of polar protic solvents like ethanol, in a strong alkaline medium like sodium hydroxide, potassium hydroxide, or sodium methoxide favors the formation of both S-alkyl derivatives as major products in addition to N-alkyl derivatives as minor products.42 On the other hand, carrying out the reaction in a polar aprotic solvent like acetone, N,N-dimethylformamide, or acetonitrile in the presence of potassium carbonate at room temperature, favors the formation of S-alkyl derivatives as a sole product.43,44 Thus, the reaction of compound 8 with 2-dimethylamino-, 2-diethylamino-, 2-pyrrolidono- or 2-piperinoethyl chloride hydrochlorides in ethanol in the presence of potassium hydroxide at reflux temperature yielded two separable products identified as the S-(2-aminoethyl) and N-(2-aminoethyl) derivatives (9a–d and 10a–d, respectively) and the S to N-alkyl ratio was approximately 3 to 1 (Figure 5 and Table 1). The structure assignment of compounds 9a–d and 10a–d were based on the 1H NMR and 13C NMR data, which were consistent with the previously reported results and supported by X-ray studies,43–45 in addition to the X-ray spectrum of compound 9a (Figure 6).46
Figure 5.
Synthetic approach used for compounds 9a–d and 10a–d.
Figure 6.
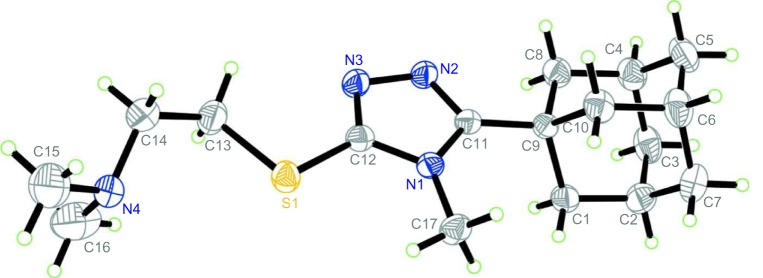
Oak Ridge thermal ellipsoid plot of compound 9a showing 30% probability displacement ellipsoids for non-H atoms.
The reaction of compound 5 with 1-bromo-2-methoxyethane in N,N-dimethylformamide in the presence of anhydrous potassium carbonate at room temperature yielded the corresponding S-(2-methoxyethyl) derivative 11 as confirmed by 1H NMR, 13C NMR, and X-ray spectrum (Figure 7).47 Similarly, reaction of compound 5 with various aryl methyl halides or ethyl bromoacetate in dry acetone in the presence of anhydrous potassium carbonate at reflux temperature yielded the corresponding S-substituted derivatives, 12a–e and 13. Compound 13 was subjected to alkaline hydrolysis via heating in 10% aqueous sodium hydroxide solution for 30 minutes followed by acidification to yield the corresponding carboxylic acid derivative 14 in 73% yield (Figure 8 and Table 1).
Figure 7.
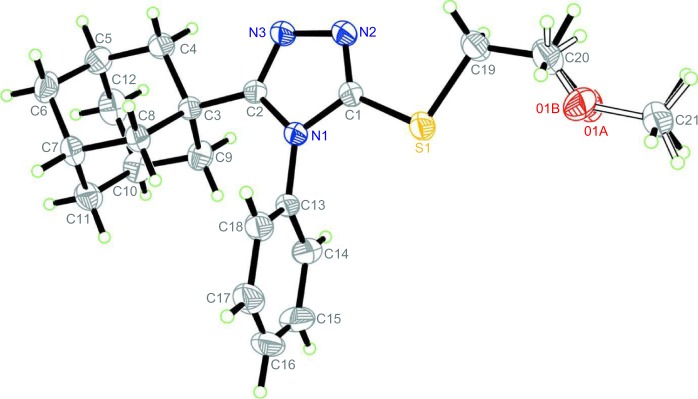
Oak Ridge thermal ellipsoid plot of compound 11 showing 30% probability displacement ellipsoids for non-H atoms.
Figure 8.
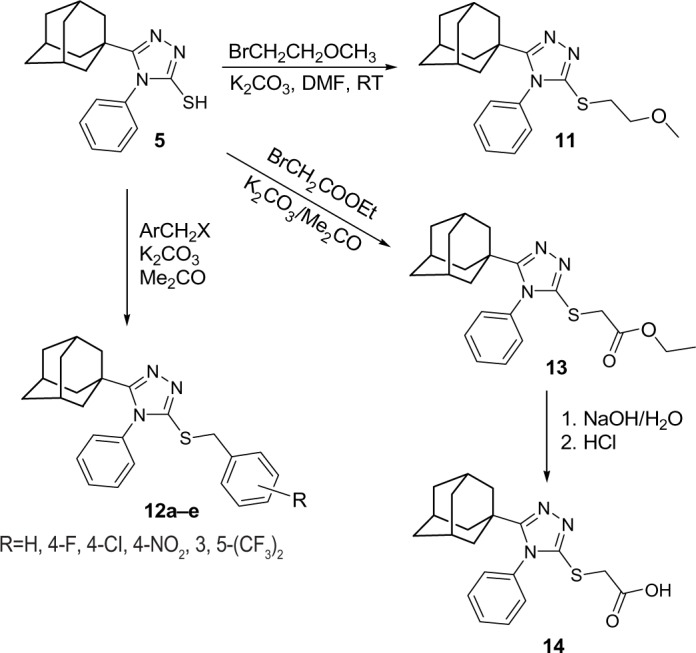
Synthetic approach used for compounds 11, 12a–e, 13, and 14.
In vitro antimicrobial activity
The newly synthesized compounds 6a–f, 9a–d, 10a–d, 11, 12a–e, 13, and 14 were tested for their in vitro growth inhibitory activity against the standard strains of the Institute of Fermentation of Osaka (IFO), ie, Staphylococcus aureus IFO 3060, Bacillus subtilis IFO 3007, Micrococcus luteus IFO 3232 (Gram-positive bacteria), Escherichia coli IFO 3301, Pseudomonas aeruginosa IFO 3448 (Gram-negative bacteria), and the yeast-like pathogenic fungus Candida albicans IFO 0583. The primary screening was carried out using the agar disc diffusion method with Müller–Hinton agar medium.48 The results of the preliminary antimicrobial testing of compounds 6a–f, 9a–d, 10a–d, 11, 12a–e, 13, and 14 (200 μg/disc), the antibacterial agents ampicillin trihydrate and gentamicin (100 μg/disc), the antifungal drug clotrimazole (100 μg/disc), and the calculated log P-values of the tested compounds (calculated using CS ChemOffice® Ultra version 8.0 software, CambridgeSoft, Cambridge, MA, USA) are shown in Table 2.
Table 2.
Antimicrobial activity of compounds 6a–f, 9a–d, 10a–d, 11, 12a–e, 13, and 14 (200 μg/8 mm disc), the broad-spectrum antibacterial drugs gentamicin (100 μg/8 mm disc) and ampicillin (100 μg/8 mm disc), and the antifungal drug clotrimazole (100 μg/8 mm disc) against Staphylococcus aureus IFO 3060, Bacillus subtilis IFO 3007, Micrococcus luteus IFO 3232, Escherichia coli IFO 3301, Pseudomonas aeruginosa IFO 3448, and Candida albicans IFO 0583
| Compound number | Clog P | Diameter of growth inhibition zone (mm)a
|
|||||
|---|---|---|---|---|---|---|---|
| SA | BS | ML | EC | PA | CA | ||
| 6a | 5.38 | 15 | 16 | 11 | – | – | – |
| 6b | 6.92 | 17 | 19 (16)b | 14 | – | – | – |
| 6c | 7.08 | 19 (8)b | 18 | 13 | 12 | 10 | – |
| 6d | 6.99 | 22 (2)b | 21 (1)b | 18 | 16 | 12 | – |
| 6e | 8.01 | 19 (8)b | 19 (8)b | 17 | 12 | – | – |
| 6f | 6.13 | 28 (0.5)b | 30 (0.5)b | 20 (8)b | 19 (16)b | 21 (4)b | 13 |
| 9a | 3.59 | 12 | 13 | 10 | – | – | – |
| 9b | 4.65 | 14 | 14 | 10 | – | – | – |
| 9c | 4.28 | 16 | 17 | 11 | – | – | – |
| 9d | 4.84 | 15 | 14 | – | – | – | – |
| 10a | 3.77 | 16 | 17 | 13 | 10 | – | – |
| 10b | 4.83 | 19 (8)b | 20 (16)b | 17 | 12 | – | – |
| 10c | 4.16 | 22 (4)b | 20 (4)b | 18 | 17 | – | – |
| 10d | 4.72 | 23 (1)b | 19 (2)b | 19 (4)b | 14 | – | – |
| 11 | 5.27 | – | – | – | – | – | – |
| 12a | 7.11 | 13 | 14 | 12 | – | – | – |
| 12b | 7.25 | 16 | 16 | 14 | – | – | – |
| 12c | 7.82 | 19 (8)† | 17 | 11 | – | – | – |
| 12d | 6.85 | 21 (4)b | 23 (8)b | 19 (8)b | – | – | 12 |
| 12e | 8.88 | 29 (0.5)b | 32 (0.5)b | 21 (2)b | 20 (2)b | 19 (8)b | 13 |
| 13 | 5.63 | 22 (4)b | 19 (16)b | 14 | – | – | 12 |
| 14 | 4.73 | 23 (8)b | 18 | 18 | – | – | 12 |
| Gentamicin | 26 (2)b | 25 (2)b | 18 (2)b | 20 (0.5)b | 19 (1)b | NT | |
| Ampicillin | 23 (2)b | 21 (0.5)b | 19 (2)b | 17 (2)b | 16 (2)b | NT | |
| Clotrimazole | NT | NT | NT | NT | NT | 21 (2)b | |
Notes: (–), inactive (inhibition zone <10 mm).
Figures shown in parentheses represent the MIC values (μg/mL). Clog P refers to calculated log P-values.
Abbreviations: NT, not tested; MIC, minimum inhibitory concentration; SA, Staphylococcus aureus; BS, Bacillus subtilis; ML, Micrococcus luteus; EC, Escherichia coli; PA, Pseudomonas aeruginosa; CA, Candida albicans.
The tested compounds showed varying degrees of inhibition against the tested microorganisms. Strong antibacterial activity was shown by compounds 6b, 6c, 6d, 6e, 6f, 10b, 10c, 10d, 12c, 12d, 12e, 13, and 14, which produced growth inhibition zones ≥19 mm against one or more of the tested microorganisms. Meanwhile, compounds 6a, 9b, 9c, 9d, 10a, and 12b showed moderate activity (growth inhibition zones 14–18 mm), compounds 9a and 12a showed weak activity (growth inhibition zones 10–13 mm), and compound 11 was practically inactive (growth inhibition zones <10 mm) against the tested bacteria. The Gram-positive bacteria B. subtilis and S. aureus and to a lesser extent M. luteus were considered the most sensitive of the tested microorganisms. Activity against the tested Gram-negative bacteria was generally lower than that of the Gram-positive bacteria, with compound 6f and 12e being strongly active against E. coli and P. aeruginosa. The inhibitory activity of the compounds against C. albicans was rather lower than their antibacterial activity, with only compounds 6f, 12e, 13, and 14 displaying marginal activity compared with clotrimazole. In general, antibacterial activity seemed to be dependent on the nature of the substituents rather than the basic skeleton of the molecules.
The antimicrobial activity of the 5-(1-adamantyl)-4-phenyl-2-(4-substituted-1-piperazinylmethyl)-1,2,4-triazoline-3-thiones 6a–f were dependent on the nature of the 4-piperazino substituents and to a lesser extent on lipophilicity. The aliphatic substituents (C2H5 and COOC2H5) retained good or moderate activity against the tested Gram-positive bacteria. Meanwhile, increasing the lipophilicity by replacing the aliphatic substituents with aromatic, benzyl, or 2-pyridyl substituents enhanced the activity against Gram-positive bacteria and conferred good to marginal activity against Gram-negative bacteria. Despite the lipophilicity, the 2-pyridyl substituent was found to have optimal antibacterial activity because compound 6f displayed good broad-spectrum antibacterial activity in addition to weak activity against C. albicans. Replacement of the 4-phenyl substituent with a methyl group and insertion of S-(2-aminoethyl) substituents greatly decreased the antimicrobial activity, and compounds 9a–d retained only weak to moderate activity against Gram-negative bacteria. On the other hand, the more lipophilic N-(2-aminoethyl) isomeric analogs 10a–d had higher activity against Gram-negative bacteria in addition to weak to moderate activity against E. coli. The S-(2-methoxyethyl) substituent (compound 11) had decreased antimicrobial activity. The antibacterial activity of the 3-arylmethylsulfanyl-4-phenyl-1,2,4-triazoles 12a–e was almost correlated to lipophilicity. Optimal activity was shown by the highly lipophilic 3,5-trifluormethylbenzyl analog 12e which had potent broad-spectrum antibacterial activity in addition to weak activity against C. albicans. Replacement of the aryl methyl substituents with an acetic analog 14 and its ethyl ester derivative 13 retained good activity against Gram-positive bacteria and weak activity against C. albicans. The minimal inhibitory concentration (MIC)49 for the most active compounds, 6b, 6c, 6d, 6e, 6f, 10b, 10c, 10d, 12c, 12d, 12e, 13, and 14, shown in Table 2, was in accordance with the results obtained in the primary screening.
In vivo anti-inflammatory activity
The acute anti-inflammatory activity of nine representative compounds (6a, 6c, 6f, 9b, 10b, 11, 12b, 13, and 14) was determined in vivo using the carrageenan-induced paw edema method in rats.50 The compounds were tested at dose levels of 20 and 40 mg/kg. The results for the anti-inflammatory activity of compounds 6a, 6c, 6f, 9b, 10b, 11, 12b, 13, and 14 (at 20 and 40 mg/kg) and the potent anti-inflammatory drug indomethacin (at 5 mg/kg) are listed in Table 3. The best activity was shown by compounds 13 and 14, which produced strong dose-dependent inhibition of carrageenan-induced paw edema of >50% at the 40 mg/kg dose. Compounds 6c and 6f were moderately active, producing respective reductions in paw edema of 22.50% and 33.52% at the 20 mg/kg dose, with no significant effect seen at the 40 mg/kg dose. Meanwhile, compounds 6a, 10b, and 12b showed weak anti-inflammatory activity, producing a >20% reduction in edema at both dose levels and compounds 9b and 11 did not show any significant anti-inflammatory activity. The structure-activity relationship of the tested adamantyl triazole derivatives revealed that incorporation of an acetic acid or ethyl acetate moiety (compounds 13 and 14) greatly enhanced the anti-inflammatory activity. In addition, the anti-inflammatory activity of the 4-(phenyl and 2-pyridyl) piperazine analogs, 6c and 6f, was higher than that of their ethyl analog, 6a. The anti-inflammatory activity of the N-(2-diethylamino) derivative 10b was superior to that of its S-(2-diethylamino) analog, 9b.
Table 3.
Anti-inflammatory effect of 20 mg/kg and 40 mg/kg intraperitoneal injections of compounds 6a, 6c, 6f, 9b, 10b, 11, 12c, 13, and 14, and indomethacin 5 mg/kg against carrageenan-induced paw edema in rats
| Compound number | Mean percent reduction in paw edema from control valuea
|
|
|---|---|---|
| 20 mg/kg | 40 mg/kg | |
| Controlb | −0.70±0.03d | |
| 6a | 13.16±4.42c | 14.05±5.21c |
| 6c | 22.50±5.25c | 14.05±5.21c |
| 6f | 33.52±0.23d | 38.89±0.14d |
| 9b | −1.42±5.21 c | 2.06±6.01c |
| 10b | 17.32±3.21d | 29.54±2.08d |
| 11 | 2.53±5.21c | 2.46±6.01c |
| 12b | 17.41±1.11d | 26.96±1.91d |
| 13 | 44.62±0.11d | 62.19±0.14d |
| 14 | 28.86±0.09d | 58.62±0.07d |
| Indomethacin 5 mg/kg | 52.79±0.04d | |
Notes:
Results are expressed as the mean percent inhibition ± standard error of the mean (n=5) and compared using the Student’s t-test;
group injected with 1 mL of 0.5% aqueous carboxymethyl cellulose solution;
significant difference at P<0.05;
significant difference at P<0.005.
Oral acute toxicity testing
The Litchfield and Wilcoxon method was used to assess the acute oral toxicity of compounds 13 and 14, which had the best anti-inflammatory activity.51 The acute toxicity results for compounds 13 and 14 and indomethacin are listed in Table 4. The oral LD50 of indomethacin has been reported to be 50 mg/kg in mice.52 Our results show that the ethyl ester derivative, 13, is less toxic relative to its free carboxylic acid derivative, 14. Although the anti-inflammatory potency ratio of compounds 13 and 14 (40 mg/kg) is about 15% of the potency of indomethacin, it could be concluded that compounds 13 and 14 each have a wider therapeutic index than that of indomethacin; the LD50 of compounds 13 and 14 was found to be 5.41% and 9.03% that of indomethacin, respectively.
Table 4.
Acute oral toxicity of compounds 13 and 14 and that of indomethacin in mice
| Compound | LD16 | LD50 | LD84 |
|---|---|---|---|
| 13 | 499 | 924 | 1,435 |
| 14 | 295 | 554 | 1,338 |
| Indomethacin | 50a |
Conclusion
In this study, new N-Mannich bases of 5-(1-adamantyl)-4-phenyl-1,2,4-triazoline-3-thiones (6a–f) and S-substituted and N-substituted 5-(1-adamantyl)-4-methyl or phenyl-1,2,4-triazole-3-thiols (9a–d, 10a–d, 11, 12a–e, 13, and 14) were synthesized and their in vitro antimicrobial activity was determined. Compounds 6b, 6c, 6d, 6e, 6f, 10b, 10c, 10d, 12c, 12d, 12e, 13, and 14 showed potent antibacterial activity. In addition, the in vivo anti-inflammatory activity of compounds 6a, 6c, 6f, 9b, 10b, 11, 12b, 13, and 14 was determined using the carrageenan-induced paw edema method in rats. Compounds 13 and 14 produced good dose-dependent anti-inflammatory activity. Although, the active compounds are considered to be good candidates as newer antibacterial and anti-inflammatory agents, further studies including the mechanism of their biological activity are being undertaken.
Acknowledgments
The authors would like to extend their appreciation to the Deanship of Scientific Research at King Saud University for funding this study (research group project RGP-VPP-274).
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Lamoureux G, Artavia G. Use of the adamantane structure in medicinal chemistry. Curr Med Chem. 2010;17:2967–2978. doi: 10.2174/092986710792065027. [DOI] [PubMed] [Google Scholar]
- 2.Liu J, Obando D, Liao V, Lifa T, Codd R. The many faces of the adamantyl group in drug design. Eur J Med Chem. 2011;46:1949–1963. doi: 10.1016/j.ejmech.2011.01.047. [DOI] [PubMed] [Google Scholar]
- 3.Davies WL, Grunnert RR, Haff RF, et al. Antiviral activity of 1-adamantamine (amantadine) Science. 1964;144:862–863. doi: 10.1126/science.144.3620.862. [DOI] [PubMed] [Google Scholar]
- 4.Wendel HA, Snyder MT, Pell S. Trial of amantadine in epidemic influenza. Clin Pharmacol Ther. 1966;7:38–43. doi: 10.1002/cpt19667138. [DOI] [PubMed] [Google Scholar]
- 5.Togo Y, Hornick RB, Dawkins AT. Studies on induced influenza in man. I. Double blind studies designed to assess prophylactic efficacy of amantadine hydrochloride against A2/Rockville/1/65 strain. JAMA. 1968;203:1089–1094. doi: 10.1001/jama.203.13.1089. [DOI] [PubMed] [Google Scholar]
- 6.Hayden FG, Gwaltney JM, Jr, Van der Castle RL, Adams KF, Giordani B. Comparative toxicity of amantadine hydrochloride and rimantadine hydrochloride in healthy adults. Antimicrob Agents Chemother. 1981;19:226–233. doi: 10.1128/aac.19.2.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ilyushina NA, Bovin NV, Webster RG, Govorkova EA. Combination chemotherapy, a potential strategy for reducing the emergence of drug-resistant influenza A variants. Antiviral Res. 2006;70:121–131. doi: 10.1016/j.antiviral.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 8.Tilley JW, Levitan P, Kramer MJ. Adamantyl thiourea derivatives as antiviral agents. J Med Chem. 1979;22:1009–1010. doi: 10.1021/jm00194a025. [DOI] [PubMed] [Google Scholar]
- 9.Pérez-Pérez MJ, Balzarini J, Hosoya M, De Clercq E, Camarasa MJ. Synthesis of adamantane-spirosultones as potential antiviral agents. Bioorg Med Chem Lett. 1992;2:647–648. [Google Scholar]
- 10.Zoidis G, Fytas C, Papanastasiou I, et al. Heterocyclic rimantadine analogues with antiviral activity. Bioorg Med Chem. 2006;14:3341–3348. doi: 10.1016/j.bmc.2005.12.056. [DOI] [PubMed] [Google Scholar]
- 11.Van Derpoorten K, Balzarini J, De Clercq E, Poupaert JH. Anti-HIV activity of N-1-adamantyl-4-aminophthalimide. Biomed Pharmacother. 1997;51:464–468. doi: 10.1016/s0753-3322(97)82327-x. [DOI] [PubMed] [Google Scholar]
- 12.Balzarini J, Orzeszko B, Mauri JK, Orzeszko A. Synthesis and anti-HIV studies of 2-adamantyl-substituted thiazolidin-4-ones. Eur J Med Chem. 2007;42:993–1003. doi: 10.1016/j.ejmech.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 13.El-Emam AA, Al-Deeb OA, Al-Omar MA, Lehmann J. Synthesis, antimicrobial, and anti-HIV-1 activity of certain 5-(1-adamantyl)-2-substituted thio-1,3,4-oxadiazoles and 5-(1-adamantyl)-3-substituted aminomethyl-1,3,4-oxadiazoline-2-thiones. Bioorg Med Chem. 2004;12:5107–5113. doi: 10.1016/j.bmc.2004.07.033. [DOI] [PubMed] [Google Scholar]
- 14.Villhauer EB, Brinkman JA, Naderi GB, et al. 1-(3-Hydroxy-1-adamantyl)aminoacetyl-2-cyano-(S)-pyrrolidine: a potent, selective, and orally bioavailable dipeptidyl peptidase IV inhibitor with antihyperglycemic properties. J Med Chem. 2003;46:2774–2789. doi: 10.1021/jm030091l. [DOI] [PubMed] [Google Scholar]
- 15.Wang JJ, Wang SS, Lee CF, Chung MA, Chern YT. In vitro antitumor and antimicrobial activity of N-substituents of maliemide by adamantane and diamantane. Chemotherapy. 1997;43:182–189. doi: 10.1159/000239557. [DOI] [PubMed] [Google Scholar]
- 16.Antoniadou-Vyza E, Tsitsa P, Hytiroglou E, Tsantili-Kakoulidou A. New adamantan-2-ol and adamantan-1-methanol derivatives as potent antibacterials, synthesis, antibacterial activity and lipophilicity studies. Eur J Med Chem. 1996;31:105–110. [Google Scholar]
- 17.Omar K, Geronikaki A, Zoumpoulakis P, et al. Novel 4-thiazolidinone derivatives as potential antifungal and antibacterial drugs. Bioorg Med Chem. 2010;18:426–432. doi: 10.1016/j.bmc.2009.10.041. [DOI] [PubMed] [Google Scholar]
- 18.Jia L, Tomaszewski JE, Hanrahan C, et al. Pharmacodynamics and pharmacokinetics of SQ109, a new diamine-based antitubercular drug. Br J Pharmacol. 2005;144:80–87. doi: 10.1038/sj.bjp.0705984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brown JR, North EJ, Hurdle JG, et al. The structure-activity relationship of urea derivatives as anti-tuberculosis agents. Bioorg Med Chem. 2011;19:5585–5595. doi: 10.1016/j.bmc.2011.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.El-Emam AA, Al-Tamimi A-MS, Al-Omar MA, Al-Rashood KA, Habib EE. Synthesis and antimicrobial activity of novel 5-(1-adamantyl)-2-aminomethyl-4-substituted-1,2,4-triazoline-3-thiones. Eur J Med Chem. 2013;68:96–102. doi: 10.1016/j.ejmech.2013.07.024. [DOI] [PubMed] [Google Scholar]
- 21.Al-Deeb OA, Al-Omar MA, El-Brollosy NR, Habib EE, Ibrahim TM, El-Emam AA. Synthesis, antimicrobial, and anti-inflammatory activities of novel 2-[3-(1-adamantyl)-4-substituted-5-thioxo-1,2,4-triazolin-1-yl]acetic acids, 2-[3-(1-adamantyl)-4-substituted-5-thioxo-1,2, 4-triazolin-1-yl]propionic acids and related derivatives. Arzneimittelforschung. 2006;56:40–47. doi: 10.1055/s-0031-1296699. [DOI] [PubMed] [Google Scholar]
- 22.Kadi AA, El-Brollosy NR, Al-Deeb OA, Habib EE, Ibrahim TM, El-Emam AA. Synthesis, antimicrobial, and anti-inflammatory activities of novel 2-(1-adamantyl)-5-substituted-1,3,4-oxadiazoles and 2-(1-adamantylamino)-5-substituted-1,3,4-thiadiazoles. Eur J Med Chem. 2007;42:235–242. doi: 10.1016/j.ejmech.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 23.Al-Omar MA, Al-Abdullah ES, Shehata IA, Habib EE, Ibrahim TM, El-Emam AA. Synthesis, antimicrobial, and anti-inflammatory activities of novel 5-(1-adamantyl)-4-arylideneamino-3-mercapto-1,2,4-triazoles and related derivatives. Molecules. 2010;15:2526–2550. doi: 10.3390/molecules15042526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.El-Emam AA, Ibrahim TM. Synthesis, anti-inflammatory and analgesic activity of certain 3-(1-adamantyl)-4-substituted-5-mercapto-1,2, 4-triazole derivatives. Arzneimittelforschung. 1991;41:1260–1264. [PubMed] [Google Scholar]
- 25.Kouatly O, Geronikaki A, Kamoutsis C, Hadjipavlou-Litina D, Eleftheriou P. Adamantane derivatives of thiazolyl-N-substituted amide, as possible non-steroidal anti-inflammatory agents. Eur J Med Chem. 2009;44:1198–1204. doi: 10.1016/j.ejmech.2008.05.029. [DOI] [PubMed] [Google Scholar]
- 26.Baxter A, Bent J, Bowers K, et al. Hit-to-lead studies: the discovery of potent adamantane amide P2X7 receptor antagonists. Bioorg Med Chem Lett. 2003;13:4047–4050. doi: 10.1016/j.bmcl.2003.08.034. [DOI] [PubMed] [Google Scholar]
- 27.Schwab RS, England AC, Jr, Poskanzer DC, Young RR. Amantadine in the treatment of Parkinson’s disease. JAMA. 1969;208:1168–1170. [PubMed] [Google Scholar]
- 28.Abou-Gharbia MA, Childers WE, Fletcher H, et al. Synthesis and SAR of adatanserin: novel adamantly aryl- and heteroarylpiperazines with dual serotonin 5-HT1A and 5-HT2 activity as potential anxiolytic and antidepressant agents. J Med Chem. 1999;42:5077–5094. doi: 10.1021/jm9806704. [DOI] [PubMed] [Google Scholar]
- 29.Bormann J. Memantine is a potent blocker of N-methyl-D-aspartate (NMDA) receptor channels. Eur J Pharmacol. 1989;166:59l–592. doi: 10.1016/0014-2999(89)90385-3. [DOI] [PubMed] [Google Scholar]
- 30.Chai X, Zhang J, Yu S, et al. Design, synthesis, and biological evaluation of novel 1-(1H-1,2,4-triazole-1-yl)-2-(2,4-difluorophenyl)-3-substituted benzylamino-2-propanols. Bioorg Med Chem Lett. 2009;19:1811–1814. doi: 10.1016/j.bmcl.2009.01.048. [DOI] [PubMed] [Google Scholar]
- 31.Xu J, Cao Y, Zhang J, et al. Design, synthesis and antifungal activities of novel 1,2,4-triazole derivatives. Eur J Med Chem. 2011;46:3142–3148. doi: 10.1016/j.ejmech.2011.02.042. [DOI] [PubMed] [Google Scholar]
- 32.Karthikeyan MS, Prasad DJ, Boojary B, Bhat KS, Holla BS, Kumari NS. Synthesis and biological activity of Schiff and Mannich bases bearing 2,4-dichloro-5-fluorphenyl moiety. Bioorg Med Chem. 2006;14:7482–7489. doi: 10.1016/j.bmc.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 33.Almajan GL, Barbuceanu SF, Almajan E, Draghici C, Saramet G. Synthesis, characterization and antibacterial activity of some triazole Mannich bases carrying diphenylsulfone moieties. Eur J Med Chem. 2009;44:3083–3089. doi: 10.1016/j.ejmech.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 34.Wade PC, Vogt BR, Kissick TP, Simpkins LM, Palmers DM. 1-Acyltriazoles as anti-inflammatory agents. J Med Chem. 1982;25:331–333. doi: 10.1021/jm00345a021. [DOI] [PubMed] [Google Scholar]
- 35.Tozkoparan B, Küpeli E, Yeşilada E, Ertan M. Preparation of 5-aryl-3-alkylthio-l,2,4-triazoles and corresponding sulfones with anti-inflammatory-analgesic activity. Bioorg Med Chem. 2007;15:1808–1814. doi: 10.1016/j.bmc.2006.11.029. [DOI] [PubMed] [Google Scholar]
- 36.Navidpour L, Shafaroodi H, Abdi K, et al. Design, synthesis, and biological evaluation of substituted 3-alkylthio-4,5-diaryl-4H-1,2,4-triazoles as selective COX-2 inhibitors. Bioorg Med Chem. 2006;14:2507–2517. doi: 10.1016/j.bmc.2005.11.029. [DOI] [PubMed] [Google Scholar]
- 37.Al-Abdullah ES, Shehata IA, Al-Deeb OA, El-Emam AA. Microwave-assisted dehydrosulphurization: an efficient, solvent-free synthesis of 5-(1-adamantyl)-2-arylamino-1,2,4-triazolo[3,4-b][1,3,4]thiadiazoles. Heterocycles. 2007;71:379–388. [Google Scholar]
- 38.El-Emam AA, Al-Tamimi AMS, Al-Rashood KA, et al. Structural and spectroscopic characterization of a novel potential chemotherapeutic agent 3-(1-Adamantyl)-1-{[4-(2-methoxyphenyl)piperazin-1-yl] methyl}-4-methyl-1H-1,2,4-triazole-5(4H)-thione by first principle calculations. J Mol Struct. 2012;1022:49–60. [Google Scholar]
- 39.Al-Abdullah ES, Asiri HH, El-Emam AA, Ng SW. Ethyl 4-{[3-(adamantan-1-yl)-4-phenyl-5-sulfanylidene-4,5-dihydro-1H-1,2,4-triazol-1-yl]methyl}piperazine-1-carboxylate. Acta Crystallogr Sect E Struct Rep Online. 2012;E68:o531. doi: 10.1107/S1600536811055668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Al-Abdullah ES, Asiri HH, El-Emam AA, Ng SW. 3-(Adamantan-1-yl)-4-phenyl-1-[(4-phenylpiperazin-1-yl)methyl]-4,5-dihydro-1H-triazole-5(4H)-thione. Crystallogr Sect E Struct Rep Online. 2012;E68:o345. doi: 10.1107/S1600536811055711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Al-Abdullah ES, Asiri HH, El-Emam AA, Ng SW. 3-(Adamantan-1-yl)-4-phenyl-1-[(4-benzylpiperazin-1-yl)methyl]-4,5-dihydro-1H-triazole-5(4H)-thione. Crystallogr Sect E Struct Rep Online. 2012;E68:o344. doi: 10.1107/S160053681105570X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sarvá MC, Romeo G, Guerrera F, et al. [1,2,4]Triazole derivatives as 5-HT1A serotonin receptor ligands. Bioorg Med Chem. 2001;10:313–323. doi: 10.1016/s0968-0896(01)00281-4. [DOI] [PubMed] [Google Scholar]
- 43.Alam MM, Nazreen S, Haider S, et al. Synthesis of some new S-alkylated 1,2,4-triazoles, their Mannich bases and their biological activities. Arch Pharm (Weinheim) 2012;345:203–214. doi: 10.1002/ardp.201100128. [DOI] [PubMed] [Google Scholar]
- 44.Fun HK, Yeap CS, Prasad KDJ, Poojary B. N-(2-Chlorophenyl)-2-({5-[4-(methysulfanyl)benzyl]-4-phenyl-4H-1,2,4-triazol-3-yl}sulfanyl) acetamide. Crystallogr Sect E Struct Rep Online. 2011;E67:o2063. doi: 10.1107/S1600536811027565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fun HK, Asik SIJ, Chandrakantha B, Isloor AM, Shetty P. 4-(4-bromophenyl)-1-(2,6-difluorobenzyl)-5-(3,4,5-trimethoxyphenyl)-1H-1,2,4-triazole-5(4H)-thione. Crystallogr Sect E Struct Rep Online. 2011;E67:o3422–o3423. doi: 10.1107/S1600536811048653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.El-Emam AA, Lahsasni S, Asiri HH, Quah CK, Fun HK. 2-{[5-(Adamantan-1-yl)-4-methyl-4H-1,2,4-triazol-3-yl]sulfanyl}-N,N-dimethyl-ethanamine. Crystallogr Sect E Struct Rep Online. 2012;E68:o1356. doi: 10.1107/S160053681201464X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.El-Emam AA, Al-Abdullah ES, Asiri HH, Chantrapromma S, Fun HK. 5-(Adamantan-1-yl)-3-[(2-methoxyethyl)sulfanyl]-4-phenyl-4H-1,2,4-triazole. Crystallogr Sect E Struct Rep Online. 2012;E68:o2326. doi: 10.1107/S1600536812029510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Woods GL, Washington JA. Antibacterial susceptibility tests: dilution and disk diffusion methods. In: Murray PR, Baron EJ, Pfaller MA, Tenover FC, Yolken RH, editors. Manual of Clinical Microbiology. Washington, DC, USA: American Society of Microbiology; 1995. [Google Scholar]
- 49.National Committee for Clinical Laboratory Standards . Approved standard document M-7A. Villanova, PA, USA: National Committee for Clinical Laboratory Standards; 1985. [Google Scholar]
- 50.Winter CA, Risely EA, Nuss GW. Carrageenin-induced paw oedema in hind paw of the rat as an assay for anti-inflammatory drugs. Proc Soc Exp Biol Med. 1962;111:544–457. doi: 10.3181/00379727-111-27849. [DOI] [PubMed] [Google Scholar]
- 51.Litchfield JT, Wilcoxon T. A simplified method of evaluating dose-effect experiments. J Pharmacol Exp Ther. 1949;96:99–104. [PubMed] [Google Scholar]
- 52.Hichens M. In: Anti-Inflammatory Agents. Scherrier RA, Whitehouse MW, editors. II. Academic Press; New York, NY, USA: 1974. [Google Scholar]