ABSTRACT
BACKGROUND
Poorly-executed transitions out of the hospital contribute significant costs to the healthcare system. Several evidence-based interventions can reduce post-discharge utilization.
OBJECTIVE
To evaluate the cost avoidance associated with implementation of the Care Transitions Intervention (CTI).
DESIGN
A quasi-experimental cohort study using consecutive convenience sampling.
PATIENTS
Fee-for-service Medicare beneficiaries hospitalized from 1 January 2009 to 31 May 2011 in six Rhode Island hospitals.
INTERVENTION
The CTI is a patient-centered coaching intervention to empower individuals to better manage their health. It begins in-hospital and continues for 30 days, including one home visit and one to two phone calls.
MAIN MEASURES
We examined post-discharge total utilization and costs for patients who received coaching (intervention group), who declined or were lost to follow-up (internal control group), and who were eligible, but not approached (external control group), using propensity score matching to control for baseline differences.
KEY RESULTS
Compared to matched internal controls (N = 321), the intervention group had significantly lower utilization in the 6 months after discharge and lower mean total health care costs ($14,729 vs. $18,779, P = 0.03). The cost avoided per patient receiving the intervention was $3,752, compared to internal controls. Results for the external control group were similar. Shifting of costs to other utilization types was not observed.
CONCLUSIONS
This analysis demonstrates that the CTI generates meaningful cost avoidance for at least 6 months post-hospitalization, and also provides useful metrics to evaluate the impact and cost avoidance of hospital readmission reduction programs.
Electronic supplementary material
The online version of this article (doi:10.1007/s11606-014-2814-0) contains supplementary material, which is available to authorized users.
KEY WORDS: care transitions, cost avoidance, hospital readmissions, Medicare
INTRODUCTION
Poorly-executed transitions from the hospital contribute to readmissions at an estimated $15–17 billion annual cost to Medicare,1,2 in addition to the physical and emotional toll on patients and families.3,4 A significant number of readmissions may be preventable,2 and the Congressional Budget Office has identified improving care transitions as a key component of advancing healthcare quality and reducing spending.5
Several evidence-based interventions can improve patients’ transitions from the hospital and have decreased readmission rates in randomized, controlled trials.6–10 While effective, these interventions require varying levels of intensity and time commitment from both the patient and from intervention personnel. Previous trials have demonstrated reductions in costs associated with hospital readmissions, but have not explored cost shifting to other types of utilization, such as observation stays, emergency department (ED) visits, skilled nursing facilities (SNFs), and other outpatient services.11,12
In 2008, the Centers for Medicare & Medicaid (CMS) contracted with Rhode Island’s Medicare Quality Improvement Organization, Healthcentric Advisors, to improve care transitions across the state. Healthcentric Advisors implemented the Care Transitions Intervention (CTI) patient coaching model, further demonstrating that the CTI can reduce readmissions in a fee-for-service (FFS) Medicare population.13,14 Using claims data from that prior study, we aimed to demonstrate whether, given the achieved decrease in readmissions, receipt of the intervention was also associated with overall cost avoidance, without significant cost shifting.
METHODS
Design and Setting
We implemented this quality improvement initiative as a quasi-experimental cohort study. From 1 January 2009, to 31 May 2011, we recruited FFS Medicare patients to receive the CTI, using consecutive convenience sampling. Patients were recruited from the general medicine service at six Rhode Island acute care hospitals. The intervention continued through 30 June 2011, and we analyzed data through 31 December 2011.13 Two hospitals’ institutional review boards (IRBs) approved this study; the remaining four hospitals’ IRBs accepted these determinations.
Participants
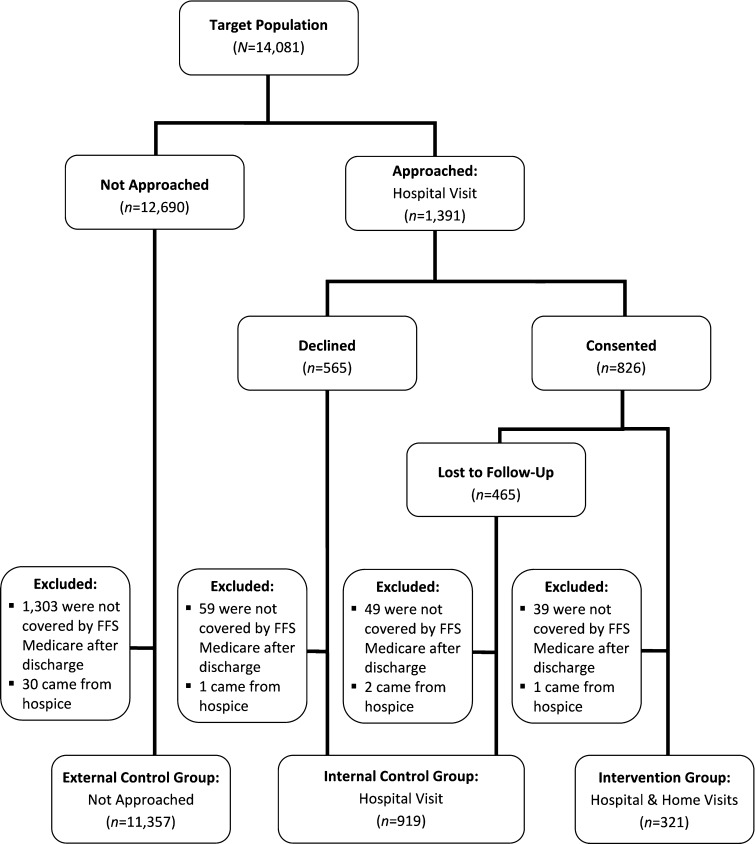
Transitions Coaches used inpatient census lists to identify general medicine FFS Medicare beneficiaries and approached a convenience sample of consecutively identified individuals with planned discharges to the community. Logistical constraints of the CTI team, including the time and day of patient discharge and the coaches’ ability to speak to patients directly without interfering with clinical care or the hospital discharge process, drove convenience. The coaches’ caseload and scheduling also affected sampling; coaches worked 18–24 hours per week, with an average caseload of 12 to 15 patients per coach, comparable to the caseload reported by Coleman et al.6
Transitions Coaches approached eligible hospitalized patients (and family caregivers, if present) to explain the intervention and obtain informed consent. As in Coleman et al.’s randomized, controlled trial of the CTI,6 we excluded patients whose discharge plans included a long-term care or skilled nursing facility; who were referred to hospice; and who had limited English proficiency or documented impaired cognitive function, unless a family caregiver agreed to receive the intervention as a proxy. We have reported additional logistical details elsewhere.13
During the study period, 321 patients received the CTI (intervention group). An additional 919 patients who were eligible declined to participate or were lost to follow-up before completing the home visit (internal control group). Patients who were lost to follow-up fell into two categories: 1) they never scheduled a home visit after consenting to the intervention, or 2) they scheduled a home visit, but were not available when the coaches attempted that visit.
Because of potential selection bias, we also constructed an external control group of 11,357 FFS Medicare patients from the same six hospitals who would have been eligible for participation, but were not approached (Fig. 1). The same criteria applied to the comparison populations, although we could not exclude individuals with limited English proficiency or those with undiagnosed cognitive impairment from the external control group. We excluded patients being discharged to SNF from the external control group using the discharge status code in the claims data. For the internal control group and the intervention group, coaches excluded patients with planned discharge to SNF before approaching them for consent. Prior to data analysis, we checked the discharge status code of every patient who was approached to participate (both in the intervention and in internal control group), to ensure that none of those patients was unexpectedly discharged to SNF after the coach had approached the patient.
Fig. 1.
Study population. FFS = fee-for-service.
Intervention
The CTI is a patient-centered coaching intervention to empower patients to better manage their health and to communicate more effectively with their providers.6,13,15,16 It begins with a hospital visit; the full intervention includes a home visit shortly after discharge and two or three follow-up telephone calls during the 30-day post-discharge period. A trained Transitions Coach focuses on skills transfer for a core set of transition-related skills, such as using a personal health record, making follow-up appointments, and responding to worsening signs and symptoms.6,15
Outcomes and Data Sources
We conducted an analysis based on Medicare claims data, Medicare enrollment data, and a coaching database (developed by the investigators). Medicare Part A and B claims data provided information on costs and healthcare utilization, as well as the following covariates: 1) diagnoses based on ICD-9 codes, 2) discharge date, and 3) self-reported race. Race was included as a covariate because of reported differences in access to care by race for Medicare beneficiaries.17 Medicare enrollment data identified individuals with dual-eligibility status. Information captured in coaching database included, among other things, demographics, presence of a family caregiver, whether patients accepted the intervention, the extent of the intervention received, topics discussed during the coaching interactions, and the number of minutes for each coaching interaction.
Statistical Analysis
This is a secondary analysis of Rhode Island’s efficacy trial of the CTI, in which subjects who received coaching experienced fewer 30-day readmissions (12.8 %; OR: 0.61, 95 % CI: 0.42–0.88).13 In this analysis, we included all post-discharge utilization; we employed an extended time frame (6 months post-discharge vs. 30 days) to examine the durability of the intervention; used propensity score matching to account for differences between groups; and calculated the cost avoidance associated with receiving the CTI.
First, we identified two 6-month periods for each patient: the 6-month baseline period leading to and including the patient’s index hospitalization, and the 6-month period following discharge. Next, we used propensity score matching to control for baseline differences between the intervention and control groups.18 Using one-to-one matching, we identified 321 matches from each of the internal and the external control groups, through multivariate adjustment by subclassification on the propensity score.
Key control variables included year, age, sex, race, dual-eligible status, site of index hospitalization (with smaller hospitals grouped together) and pre-existing conditions and comorbidities based on the CMS Hierarchical Condition Category (HCC) risk adjustment model.19 We controlled for the top 11 HCC groups (Appendix Table 1, available online) and three severity levels based on individual risk scores.
Third, we examined utilization and total costs for 6 months following hospital discharge. Utilization included all hospital admissions during that time period (which were considered readmissions from the initial, index hospitalization), ED visits, and observation stays per 1,000 Medicare beneficiaries. Costs were defined as Medicare payments and included inpatient admissions, ED visits, observation stays, post-acute care (i.e., home health visits, skilled nursing facility stays, and hospice), outpatient physician costs (e.g., office visits), and miscellaneous other outpatient costs. We did not have access to outpatient prescription costs.
Finally, we estimated cost avoidance by calculating the gross difference in total costs between intervention and control subjects and then subtracting intervention costs for the 6-month period following hospital discharge. The total intervention cost was calculated by multiplying the Transitions Coaches’ average hourly rate ($72/hour) by the number of minutes spent with intervention patients, plus estimated average travel time per encounter.
Differences between intervention and control groups were compared using χ2 tests for categorical variables and two-tailed t tests for continuous variables. All analyses were conducted using SAS 9.2.20
This study was supported by a contract from CMS. CMS reviewed the manuscript and provided comments, but did not have any role in the design and conduct of the study; collection, management, analysis, or interpretation of the data; or preparation or approval of the manuscript.
RESULTS
Internal Control Group Characteristics
Compared with internal controls (n = 919), patients in the intervention group (n = 321) were more often female (63.2 % vs. 54.3 %, P = 0.01) and more often classified as having a medium HCC risk severity level (38.0 % vs. 31.5 %, P = 0.03). After propensity score matching, there were no statistically significant differences in any of the patient characteristics between the intervention group and the internal control group (Table 1).
Table 1.
Patient Characteristics
| Unadjusted | Propensity Score Matched* | |||||
|---|---|---|---|---|---|---|
| Characteristics | Internal Control | Intervention | P value | Internal Control | Intervention | P value |
| (n = 919) | (n = 321) | (n = 321) | (n = 321) | |||
| Age, mean (SD), y | 75 (13.4) | 76 (11.9) | 0.33 | 76 (11.9) | 76 (11.9) | 0.96 |
| Female, No. (%) | 499 (54.3) | 203 (63.2) | 0.01 | 196 (61.1) | 203 (63.2) | 0.57 |
| Race, No. (%) | ||||||
| White | 845 (92.0) | 298 (92.8) | 0.61 | 296 (92.2) | 298 (92.8) | 0.76 |
| Black | 41 (4.5) | 15 (4.7) | 0.88 | 16 (5.0) | 15 (4.7) | 0.85 |
| Other race | 31 (3.4) | 7 (2.2) | 0.29 | 8 (2.5) | 7 (2.2) | 0.79 |
| Dual eligible, No. (%) | 213 (23.2) | 79 (24.6) | 0.60 | 77 (24.0) | 79 (24.6) | 0.85 |
| Hospital of index admission | ||||||
| Hospitals 1–3, No. (%) | 302 (32.9) | 123 (38.3) | 0.08 | 129 (40.2) | 123 (38.3) | 0.63 |
| Hospital 4, No. (%) | 292 (31.8) | 50 (15.6) | <0.001 | 43 (13.4) | 50 (15.6) | 0.43 |
| Hospital 5, No. (%) | 161 (17.5) | 58 (18.1) | 0.82 | 71 (22.1) | 58 (18.1) | 0.20 |
| Hospital 6, No. (%) | 164 (17.9) | 90 (28.0) | <0.001 | 78 (24.3) | 90 (28.0) | 0.28 |
| HCC risk severity level | ||||||
| Low, No. (%) | 187 (20.4) | 61 (19.0) | 0.60 | 68 (21.2) | 61 (19.0) | 0.49 |
| Medium, No. (%) | 289 (31.5) | 122 (38.0) | 0.03 | 120 (37.4) | 122 (38.0) | 0.87 |
| High, No. (%) | 443 (48.2) | 138 (43.0) | 0.11 | 133 (41.4) | 138 (43.0) | 0.69 |
| Comorbidities | ||||||
| Congestive heart failure, No. (%) | 475 (51.7) | 164 (51.1) | 0.85 | 174 (54.2) | 164 (51.1) | 0.43 |
| COPD, No. (%) | 430 (46.8) | 160 (49.8) | 0.35 | 144 (44.9) | 160 (49.8) | 0.21 |
| Heart arrhythmias, No. (%) | 356 (38.7) | 120 (37.4) | 0.67 | 126 (39.3) | 120 (37.4) | 0.63 |
| Vascular disease, No. (%) | 260 (28.3) | 114 (35.5) | 0.02 | 108 (33.6) | 114 (35.5) | 0.62 |
| Renal failure, No. (%) | 296 (32.2) | 103 (32.1) | 0.97 | 100 (31.2) | 103 (32.1) | 0.80 |
| Diabetes (no complications), No. (%) | 203 (22.1) | 81 (25.2) | 0.25 | 82 (25.6) | 81 (25.2) | 0.93 |
| Cardio-respiratory failure, No. (%) | 213 (23.2) | 66 (20.6) | 0.33 | 58 (18.1) | 66 (20.6) | 0.42 |
| Acute myocardial infarction, No. (%) | 98 (10.7) | 52 (16.2) | 0.01 | 44 (13.7) | 52 (16.2) | 0.38 |
| Diabetes with renal or circulatory manifestation, No. (%) | 133 (14.5) | 50 (15.6) | 0.63 | 46 (14.3) | 50 (15.6) | 0.66 |
| Angina/old MI, No. (%) | 110 (12.0) | 35 (10.9) | 0.61 | 32 (10.0) | 35 (10.9) | 0.70 |
| Breast, prostate, colorectal and other cancers, No. (%) | 98 (10.7) | 34 (10.6) | 0.97 | 35 (10.9) | 34 (10.6) | 0.90 |
HCC Hierarchical Condition Categories; COPD chronic obstructive pulmonary disease; MI myocardial infarction
*Control variables included year, age, sex, race, site of index hospitalization, dual-eligible status, risk severity index, and pre-existing conditions and comorbidities based on the Medicare HCC risk adjustment model
Percentages may not sum to 100 due to rounding
Internal Control Group Utilization and Costs
Mean 6-month readmissions per 1,000 persons were significantly lower for the intervention group than the internal control group (651 per 1,000 vs. 931 per 1,000, P = 0.01), after adjustment. Mean ED visits and mean observation stays were also lower in the intervention group compared to internal controls, but these differences did not achieve statistical significance (Table 2).
Table 2.
Healthcare Utilization in the 6 Months After Discharge, Adjusted*
| Type of Utilization | Internal Control | Intervention | Difference | P value |
|---|---|---|---|---|
| (n = 321) | (n = 321) | |||
| Emergency department visits per 1000 beneficiaries | 495 (1,512) | 439 (1,096) | 56 (1,321) | 0.55 |
| Observation stays per 1000 beneficiaries | 140 (407) | 87 (325) | 53 (368) | 0.07 |
| 6-month readmissions per 1,000 beneficiaries | 931 (1,383) | 651 (1,153) | 280 (1,273) | 0.01 |
*All data are presented as mean (SD). Control variables included year, age, sex, race, site of index hospitalization, dual-eligible status, risk severity index, and pre-existing conditions and comorbidities based on the Medicare HCC risk adjustment model
Compared to matched internal control group subjects, the intervention group had significantly lower mean total health care costs for 6 months post-discharge ($14,729 vs. $18,779, P = 0.03). The intervention group also had lower mean 6-month readmission costs ($8,011 vs. $11,671, P = 0.01). Mean ED visit, observation stay, skilled nursing facility, and outpatient physician visit costs in the 6-month post discharge period were lower in the intervention group compared with the internal controls, but these differences did not achieve statistical significance. The mean gross savings of all costs for 6 months post-discharge is $4,050 for each intervention subject, or $675 per case per month (Table 3).
Table 3.
Healthcare Costs per Person in the 6 Months After Discharge, Adjusted*
| Type of Cost | Internal Control | Intervention | Gross savings† | Savings per case per month | P value |
|---|---|---|---|---|---|
| (n = 321) | (n = 321) | ||||
| Total costs | $18,779 (25,407) | $14,729 (21,937) | $4,050 (23,736) | $675 (3,956) | 0.03 |
| Emergency department visits | $177 (441) | $142 (329) | $35 (389) | $5 (64) | 0.25 |
| Observation stays | $328 (1,897) | $172 (993) | $155 (1,514) | $25 (252) | 0.19 |
| Inpatient readmissions | $11,671 (20,750) | $8,011 (16,532) | $3,660 (18,760) | $610 (3,126) | 0.01 |
| Post-acute care | |||||
| Home health | $2,092 (3,009) | $2,337 (2,818) | −$246 (2,915) | −$41 (485) | 0.29 |
| Skilled nursing facility | $1,732 (5,867) | $1482 (5,411) | $250 (5,644) | $41 (940) | 0.57 |
| Hospice | $116 (817) | $127 (1,154) | −$12 (1,000) | −$2 (166) | 0.88 |
| Outpatient physician visits | $1,724 (2,984) | $1,447 (2,937) | $276 (2,960) | $46 (493) | 0.24 |
| Outpatient procedures | $937 (2,390) | $1,007 (2,700) | −$71 (2,550) | −$12 (425) | 0.73 |
*All data are presented as mean (SD) in US dollars. Control variables included year, age, sex, race, site of index hospitalization, dual-eligible status, risk severity index, and pre-existing conditions and comorbidities based on the Medicare HCC risk adjustment model
†The difference between internal control group costs and intervention group costs may not exactly equal the gross savings figure due to rounding
We calculated the average intervention cost for each beneficiary to be $298. Thus, on average, the costs avoided per patient receiving the intervention is $3,752 for 6 months post-discharge, compared to the internal control group.
External Control Group Characteristics, Utilization and Costs
We performed the same analyses to compare utilization and costs between the intervention group and the external control group (Appendix Table 2, available online). Compared to matched external controls, 6-month readmissions per 1,000 persons were significantly lower for those in the intervention group (651 per 1,000 vs. 856 per 1,000, P = 0.03) (Appendix Table 3, available online), and the intervention group had significantly lower mean total health care costs ($14,729 vs. $21,248, P < 0.001) (Appendix Table 4, available online). The average costs avoided per patient receiving the intervention are estimated at $6,221, compared to the external control group. External control group utilization and costs were consistent with those of the internal control group and are available online in the Appendix.
DISCUSSION
We found that implementing the CTI in a FFS Medicare population was associated with significant 6-month cost avoidance, with an average of $3,752 avoided for each patient who received the intervention relative to an internal control group. This cost avoidance was driven by significantly lower 6-month rates of hospital admissions, as well as lower ED visits and observation stays, for intervention subjects than for patients in two control populations. The lower rates of ED visits and observation stays in the intervention group suggest that shifting of costs to other types of healthcare utilization did not occur, although these differences did not reach statistical significance. Our study is the first to report on a more comprehensive picture of healthcare utilization in the 6 months following CTI implementation, and to estimate cost avoidance using those data.
Our findings add to previous evidence about the efficacy and effectiveness of the CTI,6,7,13,15 and attest to the durability of the intervention and its financial implications. These findings suggest that the full impact of readmissions reductions programs, such as the CTI, should be captured with measures that broaden the focus from 30-day readmissions to unplanned care over 6 months or more. A recent commentary by four of the present authors and others discusses the potential limitations of focusing solely on hospital readmissions, arguing that doing so not only fails to fully capture post-discharge care and costs, but may also lead to unintended consequences.
Several other hospital-initiated interventions to improve care transitions in a general medicine population have also addressed cost.8,9,21–28 Reported intervention costs have ranged from $166 to $1,820 per patient, and reported cost savings have ranged from $430 to $5,314 per patient (converted to 2012 US dollars). We find even more variability in reports of cost savings for hospital-initiated interventions, making it difficult for healthcare administrators and policymakers to compare the cost implications of various transitional care interventions. Various studies included costs for a year post-discharge,24,28 four21 or 6 months,9,22 or 30 days.8 Additionally, some investigators included most healthcare expenditures,8,9,24,26 while others included only hospital costs21,28 or a combination of hospital and ED costs.22 Some used actual costs and others used the average cost of a hospitalization at their facility.22 In addition, many of these interventions were performed in other countries,22–24,26,27 limiting generalizability to the US healthcare system.
A large proportion of patients we approached either declined to participate or were lost to follow-up. Our consent rates are consistent with participation in the CTI efficacy trial and with overall estimates of RCT participation, which range from 35 to 50 %.29,30 To learn how patients who consent and decline differ, we administered a five-question screening tool to a subset of 260 eligible patients approached in this study, prior to consenting them to participate. We found that patients who subsequently declined to participate differed from those who consented in three important ways: they reported higher perceived stress, lower recovery expectation, and higher health literacy.31 These findings further the discussion of how to identify and approach hospitalized patients for participation in complex behavioral interventions, both for research purposes and in routine clinical care. Our high attrition rate may also provide actionable information. This intervention involved study personnel (unaffiliated with their healthcare providers) visiting patients at home, requiring patients to answer a scheduling phone call from a number they did not recognize and to allow a home visit; both the call and visit could be perceived as intrusive or burdensome to patients recovering from an acute illness. Others might consider approaches to mitigate these barriers.
The real-world application of the CTI is one of the strengths of this study. Additionally, we used actual intervention expenses and claims data, rather than estimating post-hospital utilization or costs. Other strengths are the relatively large sample size and control group for this type of intervention and that we were able to capture readmissions, observation stays, and ED visits at any hospital. Finally, using both external and internal control groups mitigates likelihood of the measured benefit being attributable to selection bias.
This study has some limitations. First, because this intervention was implemented as a quality improvement initiative, the design is quasi-experimental, without randomization. Because we used a consecutive convenience sampling method, we were able to approach only 8 % of the eligible population. Those we approached are likely to differ from those we were unable to approach, introducing a sampling bias. Additionally, a large proportion of patients we approached either declined to participate or were lost to follow-up. As a result, there is likely some confounding for which we cannot fully adjust in our analysis, even with our propensity score case matching approach and our inclusion of both internal and external controls. However, others implementing the CTI as a quality improvement intervention are likely to encounter similar logistical constraints and limitations.
Additional limitations include the fact that this work was performed among FFS Medicare beneficiaries in Rhode Island, which may limit generalizability to other patient populations and to regions with different utilization patterns of post-acute services. Finally, we did not evaluate other health outcomes, such as mortality.
In conclusion, this analysis demonstrates significant cost avoidance associated with implementation of the CTI. Our findings are particularly timely for hospitals, given a confluence of policies to improve care transitions, including financial penalties for high readmission rates. Operationalizing true patient-centered care and patient engagement with interventions such as the CTI is essential to lowering healthcare costs.
Electronic supplementary material
(PDF 367 kb)
Acknowledgements
Contributors
The corresponding author affirms that he has listed everyone who contributed significantly to the work. The authors thank the six hospitals where we recruited subjects for the CTI intervention from 2009 to 2011, as well as the other providers and stakeholders who collaborate with Healthcentric Advisors on our ongoing Medicare-funded Safe Transitions Project. We also thank the project team and leadership. Through community collaboration, the Safe Transitions Project aims to transform the Rhode Island healthcare system into one in which discharged patients and their caregivers understand their conditions and medications, know who to contact with questions, and are supported by healthcare professionals who have access to the right information, at the right time. This is our vision statement.
Funders
This study was funded by Contract Number HHSM-500-2008-RI, titled “Utilization and Quality Control Peer Review for the State of Rhode Island,” sponsored by the Centers for Medicare & Medicaid Services (CMS), an agency of the U.S. Department of Health and Human Services. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. government. Li is supported by the National Institute on Aging (Grant Number 2 T32 AG023482-08A1). Coleman is supported by the John A. Hartford Foundation (Grant Number 2012–0047).
Prior Presentations
This work was presented in a poster session at the Academy Health Annual Research Meeting in Baltimore, MD on 24 June 2013 and as an oral abstract at the Gerontological Society of America's Annual Scientific Meeting in New Orleans, LA on 20 November 2013.
Conflict of Interest
The authors declare that they do not have a conflict of interest.
REFERENCES
- 1.Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program. N Engl J Med. 2009;360(14):1418–1428. doi: 10.1056/NEJMsa0803563. [DOI] [PubMed] [Google Scholar]
- 2.Report to Congress: Promoting greater efficiency in Medicare [Internet]. Washington, DC: Medicare Payment Advisory Commission, 2007. http://www.medpac.gov/documents/Jun07_EntireReport.pdf. Accessed 6 Feb 2014.
- 3.Krumholz HM. Post-hospital syndrome—an acquired, transient condition of generalized risk. N Engl J Med. 2013;368(2):100–102. doi: 10.1056/NEJMp1212324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Davydow DS, Gifford JM, Desai SV, Bienvenu OJ, Needham DM. Depression in general intensive care unit survivors: a systematic review. Intensive Care Med. 2009;35(5):796–809. doi: 10.1007/s00134-009-1396-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Congressional Budget Office. Issue Brief: Lessons from Medicare’s Demonstration Projects on Disease Management, Care Coordination, and Value-Based Payment. January 2012. http://www.cbo.gov/sites/default/files/cbofiles/attachments/01-18-12-MedicareDemoBrief.pdf. Accessed 6 Feb 2014.
- 6.Coleman EA, Parry C, Chalmers S, Min SJ. The care transitions intervention: results of a randomized controlled trial. Arch Intern Med. 2006;166(17):1822–1828. doi: 10.1001/archinte.166.17.1822. [DOI] [PubMed] [Google Scholar]
- 7.Parry C, Min SJ, Chugh A, Chalmers S, Coleman EA. Further application of the care transitions intervention: results of a randomized controlled trial conducted in a fee-for-service setting. Home Health Care Serv Q. 2009;28:84–99. doi: 10.1080/01621420903155924. [DOI] [PubMed] [Google Scholar]
- 8.Jack BW, Chetty VK, Anthony D, et al. A reengineered hospital discharge program to decrease rehospitalization: a randomized trial. Ann Intern Med. 2009;150(3):178–187. doi: 10.7326/0003-4819-150-3-200902030-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Naylor MD, Brooten D, Campbell R, et al. Comprehensive discharge planning and home follow-up of hospitalized elders: a randomized clinical trial. JAMA. 1999;281(7):613–620. doi: 10.1001/jama.281.7.613. [DOI] [PubMed] [Google Scholar]
- 10.Peikes D, Chen A, Schore J, Brown R. Effects of care coordination on hospitalization, quality of care, and health care expenditures among Medicare beneficiaries: 15 randomized trials. JAMA. 2009;301(6):603–618. doi: 10.1001/jama.2009.126. [DOI] [PubMed] [Google Scholar]
- 11.Feng Z, Wright B, Mor V. Sharp rise in Medicare enrollees being held in hospitals for observation raises concerns about causes and consequences. Health Aff (Millwood). 2012;31(6):1251–1259. doi: 10.1377/hlthaff.2012.0129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rennke S, Nguyen O, Shoeb MH, Magan Y, Wachter R, Ranji SR. Hospital-initiated transitional care interventions as part of a patient safety strategy: a systematic review. Ann Intern Med. 2013;158:433–440. doi: 10.7326/0003-4819-158-5-201303051-00011. [DOI] [PubMed] [Google Scholar]
- 13.Voss R, Gardner R, Baier R, Butterfield K, Lehrman S, Gravenstein S. The care transitions intervention: translating from efficacy to effectiveness. Arch Intern Med. 2011;171(14):1232–1237. doi: 10.1001/archinternmed.2011.278. [DOI] [PubMed] [Google Scholar]
- 14.Brock J, Mitchell J, Irby K, et al. Association between quality improvement for care transitions in communities and rehospitalizations among Medicare beneficiaries. JAMA. 2013;309(4):381–391. doi: 10.1001/jama.2012.216607. [DOI] [PubMed] [Google Scholar]
- 15.Coleman EA, Smith JD, Frank JC, Min SJ, Parry C, Kramer AM. Preparing patients and caregivers to participate in care delivered across settings: the Care Transitions Intervention. J Am Geriatr Soc. 2004;52(11):1817–1825. doi: 10.1111/j.1532-5415.2004.52504.x. [DOI] [PubMed] [Google Scholar]
- 16.Coleman EA, Rosenbek SA, Roman SP. Disseminating evidence-based care into practice. Popul Health Manag. 2013 Mar 28;Epub ahead of print. [DOI] [PubMed]
- 17.Orr N, Elliott MN, Burkhart Q, Haviland A, Weinick RM. Racial/Ethnic Differences in Medicare Experiences and Immunization: The Role of Disease Burden. Med Care. 2013; Epub ahead of print. [DOI] [PubMed]
- 18.Rosenbaum P, Rubin D. The central role of the propensity score in observational studies for causal effects. Biometrika. 1983;70(1):41–55. doi: 10.1093/biomet/70.1.41. [DOI] [Google Scholar]
- 19.Pope GC, Kautter J, Ingber MJ, Freeman S, Sekar R, Newhart C. Evaluation of the CMS-HCC Risk Adjustment Model: Final Report, 2011. http://www.cms.gov/Medicare/Health-Plans/MedicareAdvtgSpecRateStats/downloads/evaluation_risk_adj_model_2011.pdf. Accessed 6 Feb 2014.
- 20.SAS Institute Inc, Cary, North Carolina
- 21.Cowan MJ, Shapiro M, Hays RD, et al. The effect of a multidisciplinary hospitalist/physician and advanced practice nurse collaboration on hospital costs. J Nurs Adm. 2006;36(2):79–85. doi: 10.1097/00005110-200602000-00006. [DOI] [PubMed] [Google Scholar]
- 22.Legrain S, Tubach F, Bonnet-Zamponi D, et al. A new multimodal geriatric discharge-planning intervention to prevent emergency visits and rehospitalizations of older adults: the optimization of medication in AGEd multicenter randomized controlled trial. J Am Geratr Soc. 2011;59(11):2017–2028. doi: 10.1111/j.1532-5415.2011.03628.x. [DOI] [PubMed] [Google Scholar]
- 23.Lim WK, Lambert SF, Gray LC. Effectiveness of case management and post-acute services in older people after hospital discharge. Med J Aust. 2003;178(6):262–266. doi: 10.5694/j.1326-5377.2003.tb05191.x. [DOI] [PubMed] [Google Scholar]
- 24.Nikolaus T, Specht-Leible N, Bach M, Oster P, Schlierf G. A randomized trial of comprehensive geriatric assessment and home intervention in the care of hospitalized patients. Age Ageing. 1999;28(6):543–550. doi: 10.1093/ageing/28.6.543. [DOI] [PubMed] [Google Scholar]
- 25.Palmer HC, Jr, Halperin A, Elnicki M, et al. Effect of a patient care partnership project on cost and quality of care at an academic teaching hospital. South Med J. 2002;95(11):1318–1325. doi: 10.1097/00007611-200211000-00016. [DOI] [PubMed] [Google Scholar]
- 26.Stewart S, Pearson S, Luke CG, Horowitz JD. Effects of home-based intervention on unplanned readmissions and out-of-hospital deaths. J Am Geriatr Soc. 1998;46(2):174–180. doi: 10.1111/j.1532-5415.1998.tb02535.x. [DOI] [PubMed] [Google Scholar]
- 27.Styrborn K. Early discharge planning for elderly patients in acute hospitals–an intervention study. Scand J Soc Med. 1995;23(4):273–285. doi: 10.1177/140349489502300409. [DOI] [PubMed] [Google Scholar]
- 28.Saleh SS, Freire C, Morris-Dickinson G, Shannon T. An effectiveness and cost-benefit analysis of a hospital-based discharge transition program for elderly Medicare recipients. J Am Geriatr Soc. 2012;60:1051–1056. doi: 10.1111/j.1532-5415.2012.03992.x. [DOI] [PubMed] [Google Scholar]
- 29.De Boer SPM, Lenzen MJ, Oemrawsingh RM, et al. Evaluating the ’all-comers’ design: a comparison of participants in two ’all-comers’ PCI trials with non-participants. Eur Heart J. 2011;32(17):2161–2167. doi: 10.1093/eurheartj/ehr126. [DOI] [PubMed] [Google Scholar]
- 30.Stirman SW, DeRubeis RJ, Crits-Christoph P, Rothman A. Can the randomized controlled trial literature generalize to nonrandomized patients? J Consult Clin Psychol. 2005;73(1):127–135. doi: 10.1037/0022-006X.73.1.127. [DOI] [PubMed] [Google Scholar]
- 31.Voss R, Gravenstein S, Baier R, et al. Recruiting hospitalized patients for research: how do participants differ from eligible non-participants? J Hosp Med. 2013;8(4):208–214. doi: 10.1002/jhm.2024. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 367 kb)