Abstract
Background
Widespread implementation of palliative care treatment plans could reduce suffering in the last days of life by adopting best practices of traditionally home-based hospice care in inpatient settings.
Objective
To evaluate the effectiveness of a multi-modal intervention strategy to improve processes of end-of-life care in inpatient settings.
Design
Implementation trial with an intervention staggered across hospitals using a multiple-baseline, stepped wedge design.
Participants
Six Veterans Affairs Medical Centers (VAMCs).
Intervention
Staff training was targeted to all hospital providers and focused on identifying actively dying patients and implementing best practices from home-based hospice care, supported with an electronic order set and paper-based educational tools.
Main Measures
Several processes of care were identified as quality endpoints for end-of-life care (last 7 days) and abstracted from electronic medical records of veterans who died before or after intervention (n = 6,066). Primary endpoints were proportion with an order for opioid pain medication at time of death, do-not-resuscitate order, location of death, nasogastric tube, intravenous line infusing, and physical restraints. Secondary endpoints were administration of opioids, order/administration of antipsychotics, benzodiazepines, and scopolamine (for death rattle); sublingual administration; advance directives; palliative care consultations; and pastoral care services. Generalized estimating equations were conducted adjusting for longitudinal trends.
Key Results
Significant intervention effects were observed for orders for opioid pain medication (OR: 1.39), antipsychotic medications (OR: 1.98), benzodiazepines (OR: 1.39), death rattle medications (OR: 2.77), sublingual administration (OR: 4.12), nasogastric tubes (OR: 0.71), and advance directives (OR: 1.47). Intervention effects were not significant for location of death, do-not-resuscitate orders, intravenous lines, or restraints.
Conclusions
This broadly targeted intervention strategy led to modest but statistically significant changes in several processes of care, indicating its potential for widespread dissemination to improve end-of-life care for thousands of patients who die each year in inpatient settings.
Electronic supplementary material
The online version of this article (doi:10.1007/s11606-013-2724-6) contains supplementary material, which is available to authorized users.
KEY WORDS: palliative care, end-of-life care, palliative medicine, hospice, inpatient
INTRODUCTION
At life’s end, most people experience physical suffering, as well as significant emotional, spiritual, and social distress.1–7 Often, patients are not recognized as actively or imminently dying,1,8–14 and their suffering may be exacerbated by usual medical care, in which intensive or iatrogenically harmful treatments frequently are continued.15–18 During this time, palliative care treatment plans can be implemented, in place of or in addition to existing care plans, to reduce suffering and improve end-of-life care.12,19,20
Despite the benefits of palliative care18–25 and its growth in recent years,20,25,27 it is not always available for inpatients, partly because the subspecialty palliative care workforce is not currently sufficient to reach all patients dying in hospital settings and partly because practitioners do not recognize shifts in patient trajectory indicating the need for palliative care consultation.26–32 In an environment focusing on “cure,” transition away from disease-modifying treatments to symptom control can seem counterintuitive. Because most Americans are likely to die in a hospital or nursing home,20,33,34 there is a compelling need to address processes of care for actively dying patients in inpatient settings.
This article describes the Best Practices for End-of-Life Care for Our Nation’s Veterans (BEACON) trial (see Online Appendix), a multi-center implementation trial of an intervention strategy to improve the quality of end-of-life care in acute care settings. The multicomponent intervention included training hospital staff to identify actively dying patients, communicate the prognosis to patients/families, and implement best practices of traditionally home-based hospice care in the inpatient setting. The intervention was supported with an electronic order set and other educational tools to prompt and guide implementation. The primary aim of this study was to evaluate the effectiveness of this intervention for improving processes of care provided in the last days of life in Veterans Affairs Medical Centers (VAMCs).
METHODS
Design
The BEACON study was a real-world, multi-site implementation trial. A multiple-baseline, stepped wedge design was used, in which the intervention was initiated sequentially across sites, separated by 6-month intervals.35 Data on processes of end-of-life care before and after intervention were derived from the electronic medical records (EMRs) of deceased veterans.
Sites
Six VAMCs participated in the trial. The sites were identified based on geographic proximity to the coordinating center and availability of an Institutional Review Board. All identified sites agreed to participate and provided letters of agreement/support from the Medical Center Director. All participating VAMCs were affiliated with academic medical centers. The study received Institutional Review Board approval at each site and the coordinating center. The order in which the sites received intervention was determined by order of recruitment and readiness.
Multi-modal intervention strategy
The multi-modal intervention strategy was designed and pilot tested at the coordinating center prior to initiating the trial.36 Intervention was targeted to VAMC inpatient providers and consisted of preparatory site visits, a staff education program supported with printed educational tools, a newly developed Comfort Care order set (CCOS) decision support tool built into the EMR, and follow-up consultation (Table 1). Consistent with the Promoting Action on Research Implementation in Health Services (PARiHS) framework,37 the intervention included elements of facilitation to provide tools, resources, and strategies to fully implement best practices and incorporate them into routine clinical practice. Intervention materials are available on the UAB Center for Palliative and Supportive Care website at the following link, http://www.uab.edu/medicine/palliativecare/training/beacon, and in the e-appendix (see Online Appendix).
Table 1.
Components of the BEACON intervention
| Component | Purpose | Activities | Materials |
|---|---|---|---|
| Preparatory site visits | Orient leadership | Presentations and meetings with administrative leaders | Sample policies and procedures |
| Orient Palliative Care Consult Team (physician, nurse, social worker, chaplain) | Presentations, training |
The Palliative Response (module-based guide) Pocket cards Sample policies and procedures |
|
| Coordinate with Pharmacy to ensure availability of medications | Presentations, training Assistance with policy changes |
Sample policies and procedures | |
| Comfort Care Order Set (CCOS) | Decision support tool designed to facilitate provider behavior change | Order set built into EMR system at each site | Screenshots of order set in EMR system |
| Staff Training program | Teach staff to identify actively dying patients and communicate prognosis to patients/family | Inpatient teaching rounds Morning report Small group sessions Academic detailing |
The Palliative Response (module-based guide) Pocket card: Identifying the Actively Dying Patient |
| Teach staff to implement Comfort Care interventions and navigate the CCOS | Inpatient teaching rounds Morning report Small group sessions Academic detailing |
Screenshots of order set in EMR system CCOS pocket cards |
|
| Train PCCT to teach future residents and physicians | Small group sessions Demonstration by implementation team |
The Palliative Response and pocket cards | |
| Follow-up consultation | Maintain provider behavior change | Trainers available to answer questions for duration of 4-month training period | Additional copies of The Palliative Response and pocket cards as needed |
Preparatory site visits
The interventionists visited each site to orient leadership and understand local context. They met with the Palliative Care Consult Team (PCCT), a VA-mandated team consisting of a physician, nurse, social worker, and chaplain at each medical center. Train-the-trainer sessions were conducted to review implementation strategies and prepare the PCCT to serve as ongoing resources to their institutions.
To develop the infrastructure to support implementation, the interventionists met with key representatives of administration, nursing services, pharmacy, social work, pastoral care, and information resource management. Activities included coordinating with local staff to incorporate the CCOS into the EMR and ensuring the availability of medications needed for comfort care. The interventionists provided assistance with policies, procedures, and skill training needed to implement comfort care interventions. For example, pharmacy policy was added to enable availability of sublingual medications, and nursing policy was altered to include procedures for subcutaneous medication administration.
Staff training program
The interventionists traveled to each site to conduct 2 weeks of staff education targeting all physicians, nurses, chaplains, social workers, dieticians, pharmacists, and other staff throughout the hospital. Members of the Palliative Care Consult Team received intensive training, including recognizing actively dying patients, communicating prognoses to patients/families, implementing Comfort Care interventions, and serving as local trainers and champions in the post-intervention period and beyond.
Physician and resident training was conducted primarily during teaching rounds on the inpatient units. Teaching utilized presentations from The Palliative Response,38 a module-based guide to palliative care interventions (available at http://www.uab.edu/medicine/palliativecare/training/palliative-response). As part of the intervention, the on-site PCCT members were trained to teach the intervention and charged to present the content, so that it could be conveyed to incoming residents and new physicians. To reach other clinical staff, the interventionists circulated through the hospital using academic detailing techniques.39,40 To present all key information to as many staff as possible, sessions were presented on different inpatient units during all shifts.
Initially, training focused on developing skills to identify patients who are entering the dying process9 and should be evaluated for problems with symptom management that may be addressed by the CCOS. A pocket card was developed, “Identifying the Actively Dying Patient,” that included simple criteria for case identification derived from the literature and observation.41,42 Staff received training in several Comfort Care interventions derived from the best practices for hospice care and modified for use in the inpatient care setting.12,43–45 The interventions were based on the American Board of Medical Specialty guidelines and core principles for clinical policy and professional practice in end-of-life care, subsequently endorsed by the National Quality Forum Consensus standards for Palliative Care and End-of-Life Care.46,47 Other pocket cards were used to guide calculation of pain medication doses and reinforce communication techniques.
Comfort Care Order Set (CCOS)
To facilitate implementation of the Comfort Care interventions, the protocols were organized into a CCOS that was integrated into the EMR at each site. Orders could be customized for individual patients, and any or all of the orders could be selected and integrated into existing care plans. To overcome physician reluctance to abandon disease-modifying therapies, the CCOS was designed for use in conjunction with these therapies, with no requirement to discontinue any other treatments.
Measurement
Using a chart abstraction tool designed for the study, a registered nurse derived data on processes of end-of-life care (last 7 days) from the electronic and paper records of veterans who died as inpatients in acute and long-term care units of the participating VAMCs during the study (January 2005 through February 2011). To balance the size of the clusters, we included all deaths before and after the intervention in five of the hospitals and a random sample of deaths that occurred in the largest hospital. Inter-rater reliabilities for the primary outcome measures were established between the chart abstractor and the Director of Palliative Care at the coordinating center.
A priori, several processes of care were identified as primary and secondary endpoints to indicate quality of end-of-life care48 (Table 2) The process of writing an opioid order was chosen as the first endpoint because of the importance of pain management at end of life. We selected presence of an order rather than administration as the primary endpoint, because not every patient will need the medication. However, the process of making it available by maintaining an active order is a quality indicator, because it enables prompt relief of emerging symptoms. Other variables abstracted were diagnoses, locations of care within the medical center, and length of stay. Demographic data were obtained from VA national datasets.
Table 2.
Process of care endpoints
| Primary endpoints | |
| Opioid order present | |
| Do-not-resuscitate order (present) | |
| Location of death (not ICU) | |
| Nasogastric tube (absent) | |
| Intravenous line infusing (absent) | |
| Restraints (absent) | |
| Secondary endpoints | |
| Opioid given | |
| Antipsychotic ordered | |
| Antipsychotic given | |
| Benzodiazepine ordered | |
| Benzodiazepine given | |
| Medication for death rattle | |
| Palliative care consultation | |
| Pastoral care visits | |
| Advance directive | |
| Sublingual administration |
Power
A simulation study was designed to estimate statistical power for a design in which six hospitals were under investigation, the average probability of dying with an opioid order was 0.55 pre-intervention and 0.70 post-intervention, and the intraclass correlation coefficient was 0.01. To meet these assumptions, pre-intervention probabilities among the hospitals were sampled from a beta distribution with a = 44.55, b = 54.55, and post-intervention probabilities were sampled from a beta distribution with a = 9.7, b = 69.3. In the absence of power formulas for generalized estimating equations (GEE), 1,000 simulations were conducted generating 240 observations per hospital (120 pre/120 post) for 6 hospitals. The simulations indicated power to be 92.6 % assuming a type I error rate of 0.05. Simulations were conducted in SAS 9.1 and included recommended degrees of freedom adjustment for small numbers of clusters.
Statistical analysis plan
Preliminary unadjusted statistical analyses compared the proportions of patients who experienced each of the processes of care in the 12 months before and the 12 months after intervention using GEE.49 Careful adjustment of the degrees of freedom was conducted to account for the known issues of using GEE with small numbers of clusters.50–53
During the trial, the investigative team was cognizant of the possibility of secular trends, changes in palliative care attributable to initiatives other than our intervention, that could bias or confound intervention estimates. Therefore, records were abstracted across the entire 6-year study period for all hospitals. Because of the staggering of intervention, the hospitals had different lengths of baseline and follow-up, but data were collected for all 6 years at all 6 sites. The inclusion of data from the entire study period enabled examination of intervention effects while adjusting for any longitudinal trends that might be found. These models included a time variable, a variable representing whether the hospital had received the intervention yet, and a time by intervention interaction term. Because the interaction term did not achieve significance across any outcome, the final models included variables measuring the effect of time and intervention. The models were not adjusted for covariates such as demographics or terminal conditions. Data were analyzed using SAS 9.3.
RESULTS
Fidelity
Each of the components in Table 1 was delivered to all sites. Sign-in sheets documented that training was attended by at least 1,621 staff, including 131 physicians, residents, and medical students; 66 physician assistants and nurse practitioners, 943 nurses; 135 nursing assistants and students; 53 social workers; 24 chaplains; 100 pharmacy staff; 5 mental health staff; 24 respiratory therapists; 15 dietary staff; 54 other allied health professionals; 44 administrative staff; and 27 other staff.
Characteristics of deceased veterans
The medical records of 6,066 veterans who died in the VAMCs during the study period were abstracted. Table 3 presents the characteristics of the total sample across all 6 years, as well as the subset of 2,213 veterans who died 12 months before and 12 months after intervention. There were no significant differences in patient characteristics between the pre- and post-intervention groups for either sample.
Table 3.
Patient characteristics
| Total sample | 12 months pre- and post-intervention subset | |||||
|---|---|---|---|---|---|---|
| Total (N = 6,066) | Pre-intervention (N = 3,243) | Post-intervention (N = 2,823) | Total (N = 2,213) | 12 months pre-intervention (N = 1,081) | 12 months post-intervention (N = 1,132) | |
| Demographic variables | ||||||
| Site (n, %) | ||||||
| #1 | 1,152 (19.0) | 905 (27.9) | 247 (8.8) | 364 (16.5) | 215 (19.9) | 149 (13.2) |
| #2 | 887 (14.6) | 437 (13.5) | 450 (15.9) | 421 (19.0) | 193 (17.9) | 228 (20.1) |
| #3 | 992 (16.4) | 569 (17.6) | 423 (15.0) | 238 (10.8) | 118 (10.9) | 120 (10.6) |
| #4 | 745 (12.3) | 127 (3.9) | 618 (21.9) | 322 (14.6) | 146 (13.5) | 176 (15.6) |
| #5 | 1,254 (20.7) | 880 (27.1) | 374 (13.3) | 467 (21.1) | 244 (22.6) | 223 (19.7) |
| #6 | 1,036 (17.1) | 325 (10.0) | 711 (25.2) | 401 (18.1) | 165 (15.3) | 236 (20.9) |
| Age, in years (mean, SD) | 71.2 (12.0) | 71.7 (12.1) | 70.7 (11.8) | 71.3 (11.9) | 71.2 (11.9) | 71.4 (11.9) |
| Race (n, %) | ||||||
| Hispanic white | 8 (0.2) | 7 (0.2) | 1 (0.0) | 3 (0.2) | 2 (0.2) | 1 (0.1) |
| Hispanic black | 2 (0.0) | 0 (0.0) | 2 (0.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Native American | 4 (0.1) | 3 (0.1) | 1 (0.0) | 1 (0.1) | 0 (0.0) | 1 (0.1) |
| Black | 1876 (34.6) | 1049 (34.7) | 827 (34.5) | 672 (34.4) | 336 (34.5) | 336 (34.2) |
| Asian | 1 (0.0) | 1 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| White | 3528 (65.1) | 1965 (64.9) | 1563 (65.2) | 1278 (65.3) | 634 (65.2) | 644 (65.5) |
| Native Hawaiian/ Pacific Islander | 4 (0.1) | 1 (0.0) | 3 (0.1) | 2 (0.1) | 1 (0.1) | 1 (0.1) |
| Gender | ||||||
| Male | 5,812 (98.2) | 3,180 (98.2) | 2,632 (98.1) | 2,130 (98.1) | 1,059 (98.0) | 1,071 (98.2) |
| Female | 109 (1.8) | 58 (1.8) | 51 (1.9) | 42 (1.9) | 22 (2.0) | 20 (1.8) |
| Most recent income | ||||||
| Below $10,000 | 2,016 (35.3) | 1,150 (37.5) | 866 (32.8) | 770 (36.5) | 414 (39.6) | 356 (33.4) |
| $10,000-$19,999 | 1,647 (28.9) | 880 (28.7) | 767 (29.1) | 587 (27.8) | 267 (25.6) | 320 (30.0) |
| $20,000-$29,999 | 783 (13.7) | 473 (15.4) | 310 (11.8) | 267 (12.7) | 144 (13.8) | 123 (11.5) |
| $30,000 or more | 1,259 (22.1) | 563 (18.4) | 696 (26.4) | 487 (23.1) | 220 (21.1) | 267 (25.1) |
| Clinical characteristics | ||||||
| Terminal condition | ||||||
| Cancer | 1,845 (30.4) | 913 (28.2) | 932 (33.0) | 732 (33.1) | 362 (33.5) | 370 (32.7) |
| Dementia | 618 (10.2) | 369 (11.4) | 249 (8.8) | 208 (9.4) | 108 (10.0) | 100 (8.8) |
| Lung disease | 484 (8.0) | 250 (7.7) | 234 (8.3) | 186 (8.4) | 84 (7.8) | 102 (9.0) |
| Heart disease | 1,165 (19.2) | 645 (19.9) | 520 (18.4) | 430 (19.4) | 202 (18.7) | 228 (20.1) |
| Kidney disease | 306 (5.0) | 158 (4.9) | 148 (5.2) | 106 (4.8) | 51 (4.7) | 55 (4.9) |
| Liver disease | 335 (5.5) | 171 (5.3) | 164 (5.8) | 115 (5.2) | 64 (5.9) | 51 (4.5) |
| Stroke | 451 (7.4) | 255 (7.9) | 196 (6.9) | 146 (6.6) | 61 (5.6) | 85 (7.5) |
| HIV | 93 (1.5) | 52 (1.6) | 41 (1.5) | 36 (1.6) | 19 (1.8) | 17 (1.5) |
| Acute illness | 345 (5.7) | 165 (5.1) | 180 (6.4) | 116 (5.2) | 57 (5.3) | 59 (5.2) |
| None/unexpected | 424 (7.0) | 265 (8.2) | 159 (5.6) | 138 (6.2) | 73 (6.8) | 65 (5.7) |
| Length of stay | ||||||
| <24 h | 376 (6.2) | 227 (7.0) | 149 (5.3) | 136 (6.2) | 62 (5.7) | 74 (6.5) |
| >24 h but <7 days | 2,321 (38.3) | 1,257 (38.8) | 1,064 (37.7) | 873 (39.5) | 424 (39.2) | 449 (39.7) |
| ≥7 days | 3,369 (55.5) | 1,759 (54.2) | 1,610 (57.0) | 1,204 (54.4) | 595 (55.0) | 609 (53.8) |
Changes in processes of care
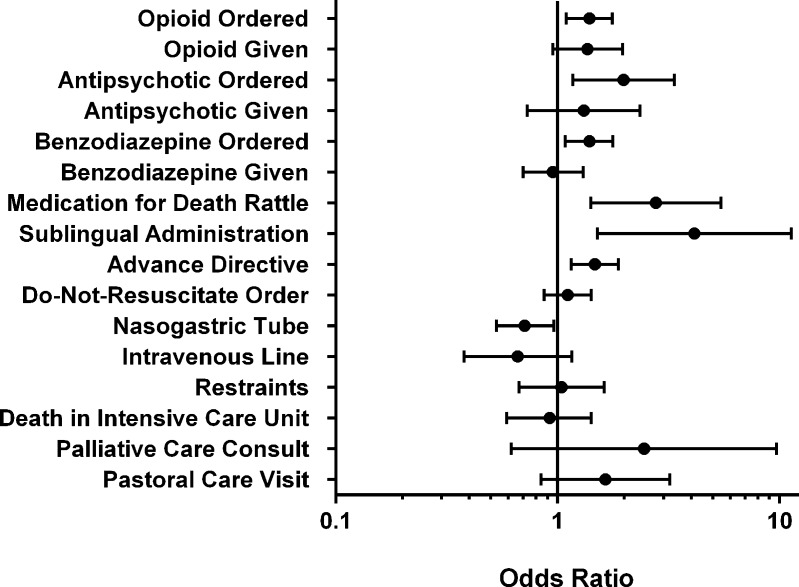
Changes were in the desired direction for all 16 variables. Longitudinal (secular) trends were found to be significant only for the proportions of patients dying with a DNR order (OR: 1.06; 95 % CI: 1.02-1.10) or an order for death rattle medication (OR: 1.16; CI: 1.07-1.24). After adjusting for these longitudinal trends, the intervention effects were significant for the proportion of patients dying with an active opioid order, with an antipsychotic order, and with an order for death rattle medication (Table 4, Fig. 1). In addition, statistically significant intervention effects were found for presence of advance directives, nasogastric tube, benzodiazepine order, and sublingual administration.
Table 4.
Effects of intervention on processes of care adjusting for 6-year longitudinal trends. N = 6,066
| Variable | Pre-intervention (%) n = 3,243 | Post-intervention (%) n = 2,824 | 95 % confidence interval | p-value |
|---|---|---|---|---|
| Opioid ordered | 61.8 | 72.8 | 1.39 (1.09 – 1.76) | 0.009 |
| Opioid given | 48.4 | 58.9 | 1.36 (0.95 – 1.96) | 0.09 |
| Do-not-resuscitate order | 70.4 | 75.3 | 1.11 (0.87 – 1.42) | 0.40 |
| Death in intensive care unit | 40.5 | 37.0 | 0.92 (0.59 – 1.42) | 0.69 |
| Nasogastric tube | 39.9 | 32.7 | 0.71 (0.53 – 0.96) | 0.03 |
| Intravenous line infusing | 69.6 | 58.5 | 0.66 (0.38 – 1.16) | 0.15 |
| Restraints | 16.4 | 12.8 | 1.04 (0.67 – 1.62) | 0.86 |
| Palliative care consultation | 17.2 | 31.3 | 2.45 (0.62 – 9.70) | 0.19 |
| Antipsychotic ordered | 10.4 | 23.4 | 1.98 (1.17 – 3.36) | 0.01 |
| Antipsychotic given | 6.9 | 9.9 | 1.31 (0.73 – 2.35) | 0.36 |
| Benzodiazepine ordered | 34.0 | 42.0 | 1.39 (1.08 – 1.77) | 0.01 |
| Benzodiazepine given | 21.9 | 23.9 | 0.95 (0.70 – 1.30) | 0.76 |
| Medication for death rattle | 3.5 | 22.4 | 2.77 (1.41 – 5.44) | 0.004 |
| Pastoral care visits | 50.3 | 62.5 | 1.64 (0.84 – 3.20) | 0.14 |
| Advance directive | 36.8 | 46.3 | 1.47 (1.15 – 1.88) | 0.003 |
| Sublingual administration | 3.8 | 11.4 | 4.12 (1.51 – 11.28) | 0.007 |
Figure 1.
Odds ratios and 95 % confidence intervals for process of care endpoints adjusting for longitudinal trends (ICU = intensive care unit)
DISCUSSION
We only die once, and there is only one opportunity to provide excellent care to a patient in the last days of life. The keys to excellent end-of-life care are recognizing the imminently dying patient, communicating the prognosis, identifying goals of care, and anticipating and palliating symptoms. Since it is not possible to predict with certainty which symptoms will arise, it is prudent to have a flexible plan taking into account practical issues, such as loss of the oral or intravenous route for medication. The results show significant changes in several processes of care after intervention, including orders for opioid pain medication and orders for antipsychotic, benzodiazepine, and death rattle medications, sublingual administration, absence of nasogastric tubes, and presence of advance directives, indicating a more comprehensive plan to meet the anticipated distress of patients and families in the last hours of life.
A primary goal of the BEACON intervention was to increase availability and proper use of opioid medications in the inpatient setting. The first step in implementing this process of care is to ensure that a physician’s opioid order is in effect. It would be ideal for all patients to have orders for opioids so that nursing staff could respond promptly to common emergent symptoms rather than having to call for medication orders, which often delays symptom relief. The increase in opioid orders seen in this study is consistent with previous studies showing successful implementation of palliative care order sets in VA and non-VA hospitals.36,54,55
The improvement in orders for antipsychotics is important because it probably indicates increased recognition and treatment of terminal delirium, which affects up to 85 % of patients during the dying process.56 Delirium increases risk of injury, causes difficulty with feeding and delivering medication, and often results in use of restraints. The most appropriate medications for terminal delirium are low-dose antipsychotics. The uptake of the use of medications for rattling respirations is also important because of the distress it causes families and staff. Taken together, these changes represent the development of proactive plans that empower nursing to better control distressing symptoms such as pain, dyspnea, delirium, and secretions with rattling respirations.
Use of intravenous lines and nasogastric tubes is common at the end of life in hospitalized patients and is negatively associated with quality of life.57 We achieved a reduction in the use of nasogastric tubes. We were also able to increase use of the sublingual route. The lack of reduction in intravenous lines could be due to continuation of a routine route of medication administration in hospitalized patients. Physicians routinely order intravenous medications and tend to continue to use this route, even when less burdensome routes are available.
Observed changes were not statistically significant for reducing deaths in ICUs by shifting location of death to other less restrictive locations. This may be due to recent patient-centered practice changes in ICU settings,22,58,59 including permitting longer and more frequent family presence. Transfer to other settings may be advisable in fewer cases, as it may move the patient away from providers who know them well. Further, while the patient may not benefit from the technologically advanced treatments in the ICU, they may benefit from the intensive nursing care, particularly with a palliative care plan in place.
Although changes were observed in some important processes of care, they were modest in magnitude and smaller than what had been achieved in our pilot study.36 This speaks to the difficulty of changing practice patterns of large groups of providers on an institutional level.1,60–62 While the intervention team trained hundreds of people at each medical center, many staff had very little contact with the trainers (≤ an hour), which may have diluted the intervention effects. In addition, staff training lasted only 2 weeks. Efforts were made to use the PCCT members as champions to facilitate and sustain change over time, and follow-up consultation was available. However, this may not have been sufficient for optimal culture change.
Larger changes might be achieved with an expanded model of implementing the BEACON intervention. Future efforts are needed to increase the intensity of the train-the-trainer model to provide local PCCTs with more in-depth training, empowering them to train other providers at their institutions. This approach has potential to provide institutions with a consistent presence of champions working to promote new practice patterns. Nevertheless, the changes seen in this study were based on a large denominator, indicating that hundreds of patients received better care following intervention. This becomes significant when considering the potential impact on over 700,000 patients who die each year in hospitals nationwide.63
Strengths of this study include the large number of patient cases, the completeness of the sampling strategy, multiple process-of-care endpoints, and the longitudinal sampling that allowed analyses adjusted for secular trends. Another strength was the multi-modal intervention designed to optimize change in provider behavior that could be reflected at the institutional level. A drawback to the multi-modal approach is that we cannot discern which components were responsible for the changes observed. However, recent work in implementation science demonstrates that multi-modal approaches are needed to address complex health care systems and that any single method of implementation is inadequate to effectively change practice.
Some may see it as a limitation that the trial was not a randomized controlled trial. However, we chose a stepped wedge design, in which each site served as its own control and intervention effects could be examined in the context of longitudinal trends. This type of design is often considered more appropriate for implementation studies with large clusters.
As an implementation trial, the goal was to change provider practice patterns as reflected in institution-level changes in process. The trial was not designed to evaluate patient outcomes, which would require a different sampling and measurement approach. Thus, while we found evidence of improved patient care, we cannot form conclusions from these data about the impact of this care on patients or whether it enabled a more peaceful death.
In conclusion, this broadly targeted intervention strategy to change practice patterns led to modest but statistically significant changes in several processes of care, indicating its potential for widespread dissemination to improve end-of-life care for the thousands who die each year in inpatient settings. Further research may facilitate dissemination by identifying optimal implementation strategies.
Electronic supplementary material
(PDF 10.2 MB)
Acknowledgments
Contributors
The authors thank the participating VAMCs, their Palliative Care Consultation Teams, and chaplains, including: Anna D. Senseney, MD (Site Principal Investigator), Karen Lukacs, RN, APN, and the Palliative Care Team at the Ralph H. Johnson VAMC, Charleston, SC; Adam Herman, MD, Orania Tigaieru, MD, Donna Lewis, CRNP, and the Palliative Care Team at the Atlanta VAMC, Decatur, GA; Debra Layer, CRNP, and the Palliative Care Team at the William Jennings Bryan Dorn VAMC, Columbia, SC; Michael Willoughby, MD, and the Palliative Care Teams at the Charlie Norwood VAMC, Augusta GA, the Malcom Randall VAMC, Gainesville, FL, and the G.V. Sonny Montgomery VAMC, Jackson, MS.
We also thank Rosie Durham, RN, MSN, for assistance with study implementation, Stacey Kovacs, PhD, and Jane E. Castle PhD, RN, for assistance in study design, Janice Taylor, RN, MSN, for chart abstraction, Sandra Broeren, MD, for training assistance, Jean Marie White, BA, for project coordination, and Angelina Wittich, PhD, for assistance with manuscript preparation.
Funders
This research was supported by a Merit Review grant from the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Health Services Research and Development Service (IIR 03-126 “Intervention to Improve Care at Life’s End in VA Medical Centers;” PI: KL Burgio, Co-PI: FA Bailey). The funding source had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript.
ClinicalTrials.gov identifier: NCT00234286
Presentation
This study was presented at the Annual Meeting of the European Association of Palliative Care, Trondheim, Norway, June 2012.
Disclaimer
The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the US government.
Conflict of interest
The authors declare that they do not have a conflict of interest.
References
- 1.Investigators TSP. A controlled trial to improve care for seriously ill hospital patients: The Study to understand prognoses and preferences for outcomes and risks of treatments (SUPPORT) JAMA. 1995;274:1591–1598. doi: 10.1001/jama.1995.03530200027032. [DOI] [PubMed] [Google Scholar]
- 2.Singer PA, Martin DK, Kelner M. Quality end-of-life care patients’ perspectives. JAMA. 1999;281:163–168. doi: 10.1001/jama.281.2.163. [DOI] [PubMed] [Google Scholar]
- 3.Portenoy RK, Thaler HT, Konnblith AB, et al. Symptom prevalence, characteristics and distress in a cancer population. Qual Life Res. 1994;3:183–189. doi: 10.1007/BF00435383. [DOI] [PubMed] [Google Scholar]
- 4.Morris JN, Suissa S, Sherwood S, Wright SM, Greer D. Last days: a study of the quality of life of terminally ill cancer patients. J Chron Dis. 1986;39:47–62. doi: 10.1016/0021-9681(86)90106-2. [DOI] [PubMed] [Google Scholar]
- 5.Christakis N, Lamont EB. Extent and determinants of errors in doctors’ prognoses in terminally ill patients: prospective cohort study. BMJ. 2000;329:469–473. doi: 10.1136/bmj.320.7233.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kehl KA, Kowalkowski JA. A systematic review of the prevalence of signs of impending death and symptoms in the last 2 weeks of life. Am J Hosp Palliat Care. 2013;30:601–616. doi: 10.1177/1049909112468222. [DOI] [PubMed] [Google Scholar]
- 7.Reynolds J, Drew D, Dunwoody C. American Society for pain management nursing position statement: pain management at the end of life. Pain Manag Nurs. 2013;14:172–175. doi: 10.1016/j.pmn.2013.07.002. [DOI] [PubMed] [Google Scholar]
- 8.Billings JA, Gardner M, Putman AT. A one-day, hospital-wide survey of dying inpatients. J Palliat Med. 2002;5:363–374. doi: 10.1089/109662102320135252. [DOI] [PubMed] [Google Scholar]
- 9.Abarshi EA, Echteld MA, Van den Block L, et al. Recognising patients who will die in the near future: a nationwide study via the Dutch Sentinel Network of GPs. Br J Gen Pract. 2011;61:e371. doi: 10.3399/bjgp11X578052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hwang IC, Ahn HY, Park SM, Shim JY, Kim KK. Clinical changes in terminally ill cancer patients and death within 48 h: when should we refer patients to a separate room? Support Care Cancer. 2013;21:835–840. doi: 10.1007/s00520-012-1587-4. [DOI] [PubMed] [Google Scholar]
- 11.Selby D, Chakraborty A, Lilien T, et al. Clinician accuracy when estimating survival duration: the role of the patient’s performance status and time-based prognostic categories. J Pain Symptom Manag. 2011;42:578–588. doi: 10.1016/j.jpainsymman.2011.01.012. [DOI] [PubMed] [Google Scholar]
- 12.Ellershaw J, Ward C. Care of the dying patient: the last hours or days of life. BMJ. 2003;326:30–34. doi: 10.1136/bmj.326.7379.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hui, D. Concepts and definitions for “actively dying,” “end of life,” “terminally ill,” “terminal care,” and “transition of care”: A systematic review. J Pain Symptom Manage. 2013. [DOI] [PMC free article] [PubMed]
- 14.Glare P, Virik K, Jones M, et al. A systematic review of physicians’ survival predictions in terminally ill cancer patients. BMJ. 2003;327:195. doi: 10.1136/bmj.327.7408.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jox RJ, Schaider A, Marckmann G, Borasio GD. Medical futility at the end of life: the perspectives of intensive care and palliative care clinicians. J Med Ethics. 2012;38:540–545. doi: 10.1136/medethics-2011-100479. [DOI] [PubMed] [Google Scholar]
- 16.Jani B, Blane D, Browne S, et al. Identifying treatment burden as an important concept for end of life care in those with advanced heart failure. Curr Opin Support Palliat Care. 2013;7:3–7. doi: 10.1097/SPC.0b013e32835c071f. [DOI] [PubMed] [Google Scholar]
- 17.Piers RD, Van den Eynde M, Steeman E, Vlerick P, Benoit DD, Van Den Noortgate NJ. End-of-life care of the geriatric patient and nurses’ moral distress. J Am Med Dir Assoc. 2012;13:80–87. doi: 10.1016/j.jamda.2010.12.014. [DOI] [PubMed] [Google Scholar]
- 18.Kwok AC, Semel ME, Lipsitz SR, et al. The intensity and variation of surgical care at the end of life: a retrospective cohort study. Lancet. 2011;378:1408–1413. doi: 10.1016/S0140-6736(11)61268-3. [DOI] [PubMed] [Google Scholar]
- 19.Meier DE. Palliative care in hospitals. J Hosp Med. 2006;1:21–28. doi: 10.1002/jhm.3. [DOI] [PubMed] [Google Scholar]
- 20.Approaching death: improving care at the end of life. Washington: National Academy Press; 1997. [PubMed] [Google Scholar]
- 21.Gade G, Venohr I, Conner D, et al. Impact of an inpatient palliative care team: a randomized controlled trial. J Palliat Med. 2008;11:180–190. doi: 10.1089/jpm.2007.0055. [DOI] [PubMed] [Google Scholar]
- 22.O’Mahony S, McHenry J, Blank AE, et al. Preliminary report of the integration of a palliative care team into an intensive care unit. Palliat Med. 2010;24:154–165. doi: 10.1177/0269216309346540. [DOI] [PubMed] [Google Scholar]
- 23.Higginson IJ, Evans CJ. What is the evidence that palliative care teams improve outcomes for cancer patients and their families? Cancer J. 2010;16:423–435. doi: 10.1097/PPO.0b013e3181f684e5. [DOI] [PubMed] [Google Scholar]
- 24.Lorenz KA, Lynn J, Dy SM, et al. Evidence for improving palliative care at the end of life: a systematic review. Ann Intern Med. 2008;148:147–159. doi: 10.7326/0003-4819-148-2-200801150-00010. [DOI] [PubMed] [Google Scholar]
- 25.Aziz NM, Miller JL, Curtis JR. Palliative and end-of-life care research: embracing new opportunities. Nurs Outlook. 2012;60:384–390. doi: 10.1016/j.outlook.2012.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rabow MW, Pantilat SZ, Kerr K, et al. The intersection of need and opportunity: assessing and capitalizing on opportunities to expand hospital-based palliative care services. J Palliat Med. 2010;13:1205–1210. doi: 10.1089/jpm.2010.0112. [DOI] [PubMed] [Google Scholar]
- 27.Tsuneto S. Past, present, and future of palliative care in Japan. Jpn J Clin Oncol. 2013;43:17–21. doi: 10.1093/jjco/hys188. [DOI] [PubMed] [Google Scholar]
- 28.McCarthy EP, Burns RB, Davis RB, Phillips RS. Barriers to hospice care among older patients dying with lung and colorectal cancer. J Clin Oncol. 2003;21:728–735. doi: 10.1200/JCO.2003.06.142. [DOI] [PubMed] [Google Scholar]
- 29.Goldsmith B, Dietrich J, Du Q, Morrison RS. Variability in access to hospital palliative care in the United States. J Palliat Med. 2008;11:1094–1102. doi: 10.1089/jpm.2008.0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Walling AM, Ettner SL, Barry T, Yamamoto MC, Wenger NS. Missed opportunities: use of an end-of-life symptom management order protocol among inpatients dying expected deaths. J Palliat Med. 2011;14:407–412. doi: 10.1089/jpm.2010.0328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Al-Qurainy R, Collis E, Feuer D. Dying in an acute hospital setting: the challenges and solutions. Int J Clin Pract. 2009;63:508–515. doi: 10.1111/j.1742-1241.2008.01991.x. [DOI] [PubMed] [Google Scholar]
- 32.Pantilat SZ, Isaac M. End-of-life care for the hospitalized patient. Hosp Medicine. 2008;92:349–370. doi: 10.1016/j.mcna.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 33.National Center for Health Statistics (US). Health, United States, 2010: With Special Feature on Death and Dying. Hyattsville (MD) 2011. http://www.ncbi.nlm.nih.gov/books/NBK54372/. Accessed April 1, 2013. [PubMed]
- 34.Gruneir A, Mor V, Weitzen S, Truchil R, Teno J, Roy J. Where people die: A multilevel approach to understanding influences on site of death in America. Med Care Res Rev. 2007;64:351–378. doi: 10.1177/1077558707301810. [DOI] [PubMed] [Google Scholar]
- 35.Brown CA, Lilford RJ. The stepped wedge trial design: a systematic review. BMC Med Res Methodol. 2006;6:54. doi: 10.1186/1471-2288-6-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bailey FA, Burgio KL, Woodby LL, et al. Improving processes of hospital care during the last hours of life. Arch Intern Med. 2005;165:1722–1727. doi: 10.1001/archinte.165.15.1722. [DOI] [PubMed] [Google Scholar]
- 37.Kitson AL, Rycroft-Malone J, Harvey G, McCormack B, Seers K, Titchen A. Evaluating the successful implementation of evidence into practice using the PARiHS framework: theoretical and practical challenges. Implement Sci. 2008;3:1–12. doi: 10.1186/1748-5908-3-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bailey FA. The Palliative Response. Birmingham: Menasha Ridge Press; 2005. [Google Scholar]
- 39.Soumerai SB, Avorn J. Principles of educational outreach (‘academic detailing’) to improve clinical decision making. JAMA. 1990;263:549–556. doi: 10.1001/jama.1990.03440040088034. [DOI] [PubMed] [Google Scholar]
- 40.Davis D. Does CME, work? An analysis of the effect of educational activities on physician performance or health care outcomes. Int J Psychiatry Med. 1998;28:21–39. doi: 10.2190/UA3R-JX9W-MHR5-RC81. [DOI] [PubMed] [Google Scholar]
- 41.Lau F, Maida V, Downing M, et al. Use of the Palliative Performance Scale (PPS) for end-of-life prognostication in a palliative medicine consultation service. J Pain Symptom Manag. 2009;37:965. doi: 10.1016/j.jpainsymman.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 42.Harrold J, Rickerson E, Carroll JT, et al. Is the palliative performance scale a useful predictor of mortality in a heterogeneous hospice population? J Palliat Med. 2005;8:503–509. doi: 10.1089/jpm.2005.8.503. [DOI] [PubMed] [Google Scholar]
- 43.Chan R, Webster J. End-of-life care pathways for improving outcomes in caring for the dying (Review). The Cochrane Library. John Wiley & sons, Ltd. 2010, Issue 3. [DOI] [PubMed]
- 44.Plonk WM, Jr, Arnold RM. Terminal care: the last weeks of life. J Palliat Med. 2005;8:1042. doi: 10.1089/jpm.2005.8.1042. [DOI] [PubMed] [Google Scholar]
- 45.Harlos M, et al. The terminal phase. In: Hanks G, Cherny NI, Christakis NA, et al., editors. Oxford Textbook of Palliative Medicine. 4. Oxford: Oxford University Press; 2010. p. 1551. [Google Scholar]
- 46.Cassel CK, Foley KM. Principles for Care of Patients at the End of Life: An Emerging Consensus Among the Specialties of Medicine. Milbank Memorial Fund, 1999.
- 47.Phillips JL, Halcomb EJ, Davidson PM. End-of-life care pathways in acute and hospice care: an integrative review. J Pain Symptom Manage. 2011;41:940–955. doi: 10.1016/j.jpainsymman.2010.07.020. [DOI] [PubMed] [Google Scholar]
- 48.Lindqvist O, Lundquist G, Dickman A, et al. Four essential drugs needed for quality care of the dying: a Delphi-study based international expert consensus. J Palliat Med. 2013;16:38–43. doi: 10.1089/jpm.2012.0205. [DOI] [PubMed] [Google Scholar]
- 49.Diggle PJ, Liang KY, Zeger SL. Analysis of Longitudinal Data. Oxford, England: Oxford Publishing; 1994. [Google Scholar]
- 50.Fay MP, Graubard BI. Small-sample adjustments for Wald-type tests using sandwich estimators. Biometrics. 2001;57:1198–1206. doi: 10.1111/j.0006-341X.2001.01198.x. [DOI] [PubMed] [Google Scholar]
- 51.Mancl LA, DeRouen TA. A covariance estimator for GEE with improved small-sample properties. Biometrics. 2001;57:126–134. doi: 10.1111/j.0006-341X.2001.00126.x. [DOI] [PubMed] [Google Scholar]
- 52.Pan W, Wall MM. Small-sample adjustments in using the sandwich variance estimator in generalized estimating equations. Stat Med. 2002;21:1429–1441. doi: 10.1002/sim.1142. [DOI] [PubMed] [Google Scholar]
- 53.Kauermann G, Carroll RJ. A note on the efficiency of sandwich covariance estimation. J Am Stat Assoc. 2001;96:1387–1396. doi: 10.1198/016214501753382309. [DOI] [Google Scholar]
- 54.Bookbinder M, Blank AE, Arney E, et al. Improving end-of-life care: development and pilot-test of a clinical pathway. J Pain Symptom Manag. 2005;29:529–543. doi: 10.1016/j.jpainsymman.2004.05.011. [DOI] [PubMed] [Google Scholar]
- 55.Walker KA, Nachreiner D, Patel J, Mayo RL, Kearney CD. Impact of standardized palliative care order set on end-of-life care in a community teaching hospital. J Palliat Med. 2011;14:281–286. doi: 10.1089/jpm.2010.0398. [DOI] [PubMed] [Google Scholar]
- 56.Breitbart W, Alici Y. Agitation and delirium at the end of life: “we couldn’t manage him.". JAMA. 2008;300:2898–2910. doi: 10.1001/jama.2008.885. [DOI] [PubMed] [Google Scholar]
- 57.Zhang B, Nilsson ME, Prigerson HG. Factors important to patients’ quality of life at the end of life. Arch Intern Med. 2012; epub July 9, 2012. [DOI] [PMC free article] [PubMed]
- 58.Nelson JE, Bassett R, Boss RD, et al. Models for structuring a clinical initiative to enhance palliative care in the intensive care unit: a report from the IPAL-ICU Project (Improving Palliative Care in the ICU) Crit Care Med. 2010;38:1765–1772. doi: 10.1097/CCM.0b013e3181e8ad23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.White DB, Ernecoff N, Billings JA, Arnold R. Is dying in an ICU a sign of poor quality end-of-life care? Am J Crit Care. 2013;22:263–266. doi: 10.4037/ajcc2013604. [DOI] [PubMed] [Google Scholar]
- 60.Curtis RJ, Nielsen EL, Treece PD, et al. Effect of a quality-improvement intervention on end-of-life care in the intensive care unit. Am J Respir Crit Care Med. 2011;183:348–355. doi: 10.1164/rccm.201006-1004OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Breuer B, Fleishman SB, Cruciani RA, Portenoy RK. Medical oncologists’ attitudes and practice in cancer pain management: a national survey. J Clin Oncol. 2011;29:4769–4775. doi: 10.1200/JCO.2011.35.0561. [DOI] [PubMed] [Google Scholar]
- 62.Von Roenn JH, von Gunten C. The care people need and the education of physicians. J Clin Oncol. 2011;29:4742–4743. doi: 10.1200/JCO.2011.39.2043. [DOI] [PubMed] [Google Scholar]
- 63.Hall MJ, Levant S, DeFrances CJ. Trends in inpatient hospital deaths: National Hospital Discharge Survey, 2000-2010. NCHS data brief, no. 118. Hyattsville: National Center for Health Statistics; 2013. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 10.2 MB)