Abstract
ABCC10, also known as multidrug-resistant protein 7 (MRP7), is the tenth member of the C subfamily of the ATP-binding cassette (ABC) superfamily. ABCC10 mediates multidrug resistance (MDR) in cancer cells by preventing the intracellular accumulation of certain antitumor drugs. The ABCC10 transporter is a 171-kDa protein that is localized on the basolateral cell membrane. ABCC10 is a broad-specificity transporter of xenobiotics, including antitumor drugs, such as taxanes, epothilone B, vinca alkaloids, and cytarabine, as well as modulators of the estrogen pathway, such as tamoxifen. In recent years, ABCC10 inhibitors, including cepharanthine, lapatinib, erlotinib, nilotinib, imatinib, sildenafil, and vardenafil, have been reported to overcome ABCC10-mediated MDR. This review discusses some recent and clinically relevant aspects of the ABCC10 drug efflux transporter from the perspective of current chemotherapy, particularly its inhibition by tyrosine kinase inhibitors and phosphodiesterase type 5 inhibitors.
Keywords: ABCC10, multidrug resistance, antitumor drugs, phosphodiesterase type 5 inhibitors, tyrosine kinase inhibitors
One known cause of multidrug resistance (MDR) to cancer chemotherapy is the overexpression of ATP-binding cassette (ABC) transporters on the membranes of cancer cells[1]. ABC transporters mediate an energy-dependent efflux of structurally and functionally unrelated chemotherapeutic compounds out of cancer cells, significantly decreasing the probability of successful treatment[2]. In mammals, ABC transporters have been divided into subfamilies A-G based on genome sequence similarities[3]. Studies to date have consistently focused on the three major ABC transporters involved in producing MDR in most cancer cells: ABCB1 (MDR1, also known as P-glycoprotein or P-gp), ABCC1 (also known as multidrug-resistant protein 1 or MRP1), and ABCG2 (also known as breast cancer-resistant protein or BCRP)[3]–[7]. However, ABCC10 (MRP7) has only been recently classified as a member of the ABC superfamily that confers the MDR phenotype to cancer cells. This review briefly discusses the emergence of ABCC10 as an important mediator of MDR and the compounds that overcome ABCC10-induced resistance to specific antitumor drugs (Table 1).
Table 1. A timeline of the major discoveries associated with ABCC10.
| Year | Major discovery |
| 2001 | Discovery of ABCC10 as a new member of ABCC subfamily[9] |
| 2002 | cDNA cloning and genomic organization of the murine Abcc10A and Abcc10B[57] |
| 2003 | ABCC10 is a lipophilic anion transporter involved in phase III of detoxification[12] |
| 2004 | First report of ABCC10 as a resistance factor to anticancer drugs like paclitaxel, docetaxel, vincristine, and vinblastine[10] |
| ABCC10 gene expression found to be the highest in the pancreas[14] | |
| 2005 | Peptide derived from ABCC10 reported as an immunoregulator[58] |
| 2007 | ABCC10 is a resistance factor for docetaxel in salivary gland adenocarcinoma[21] |
| 2008 | ABCC10 is established as a biomarker for paclitaxel resistance in non-small cell lung cancer[18] |
| 2009 | ABCC10 is a resistance factor for vinorelbine in non-small cell lung cancer[17] |
| ABCC10 is a resistance factor for epothilone B, and ABCC10 transport does not involve glutathione, unlike ABCB1 and ABCC1[22] | |
| Cepharanthine, a herbal extract, reverses ABCC10-mediated paclitaxel resistance[24] | |
| BCR-Abl tyrosine kinase inhibitors, imatinib and nilotinib, inhibit efflux function of ABCC10 efflux transporter[28] | |
| 2010 | Epidermal growth factor receptor tyrosine kinase inhibitors, erlotinib and lapatinib, inhibit efflux function of ABCC10 efflux transporter[33] |
| 2011 | Docetaxel intermittently increases simultaneous ABCC10 and ABCB1 gene expression[59] |
| ABCC10 transcript was detected in acute myeloid leukemia cell lines[19] | |
| ABCC10 termed as "endogenous resistance factor" for taxanes that confer paclitaxel resistance in vivo in Abcc10-knockout mice[12] | |
| ABCC10 single nucleotide polymorphisms (rs9349256 and rs2125739) and their haplotypes are associated with kidney tubular dysfunction[48] | |
| 2012 | ABCC10 single nucleotide polymorphism rs2125739 is associated with nevirapine-induced hepatotoxicity[54] |
| Phosphodiesterase 5 inhibitors, such as sildenafil and vardenafil, reverse MDR mediated by ABCC10[41] | |
| First murine ABCC10-paclitaxel resistance xenograft model developed[60] | |
| Localization of ABCC10 was found on basolateral cell membrane[11] | |
| 2013 | Tariquidar, a third-generation ABCB1 inhibitor, reverses ABCC10-mediated MDR[61] |
| Tandutinib, an FMS-like tyrosine kinase 3 inhibitor, blocks efflux function of ABCC10 efflux transporter[47] |
ABC, ATP-binding cassette superfamily divided into A-G subfamilies; MDR, multidrug resistance.
ABCC10/MRP7 Transporter
The human ABCC10 gene is located on chromosome 6p12[8],[9]. ABCC10 is a 171-kDa protein that contains three membrane-spanning domains (MSDs) and two nucleotide-binding domains (NBDs) (Figure 1)[10]. ABCC10 belongs to the class of long ABCCs, such as ABCC1, ABCC2, ABCC3, and ABCC6, and is located on the basolateral cell surface[10]–[13]. Using reverse transcription-polymerase chain reaction (RT-PCR), a low level of ABCC10 transcript expression has been found in the skin, testes, spleen, stomach, colon, kidneys, heart, and brain[8],[9]. In addition, the ABCC10 transcript is expressed (in order of highest to lowest) in the pancreas, liver, placenta, lungs, kidneys, brain, ovaries, lymph nodes, spleen, heart, leukocytes, and colon[14]. ABCC10 mRNA is highly expressed in various tissues, including the kidneys, brain, and colon, suggesting that it is involved in the transport of drugs and other endogenous molecules[15]. Kao et al.[16] have discovered a splice variant of ABCC10 that is truncated at its NH2 terminus and has a 15-amino acid deletion between MSD2 and NBD2.
Figure 1. Structure of ABCC10.
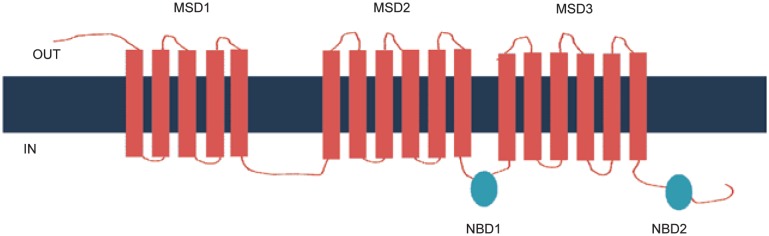
ABCC10 is located on the basolateral cell membrane and consists of 3 membrane-spanning domains (MSDs) and 2 nucleotide-binding domains (NBDs).
Chen et al.[12] have reported that ABCC10 transports leukotriene C4, but not glycocholic acid, taurocholic acid, methotrexate, folic acid, cyclic adenosine monophosphate, or cyclic guanosine monophosphate, which are substrates for other MRP family members. However, the transport of estradiol-17β-D-glucuronide (E217βG), a glucuronide conjugate and the only physical element other than leukotriene C4 to be transported by ABCC10, is competitively inhibited by amphiphiles, such as leukotriene C4, glycolithocholate 3-sulfate, and MK571, as well as lipophilic compounds, such as cyclosporine A[12]. The transfection of HEK293 cells with the ABCC10 gene confers resistance to various chemotherapeutic drugs, including docetaxel, paclitaxel, vincristine, vinblastine, cytarabine, gemcitabine, 2′,3′-dideoxycytidine, 9-(2-phosphonyl methoxyethyl)adenine (PMEA), and epothilone B[10],[13]. Specifically, the presence of ABCC10 is significantly associated with vinorelbine, and paclitaxel resistance in non-small cell lung cancer (NSCLC)[17],[18]. In acute myeloid leukemia (AML) cell lines, ABCC10 protein expression was detected (in highest to lowest order) in ML-2, NB4, MV4, and Kasumi-1 cell lines[19]. The ABCC10 transcript has been found in breast, lung, colon, ovarian, and pancreatic tumor samples, although the interpretation of these studies may be limited due to their small sample size[13],[14]. ABCC10 transcript has been detected in the HepG2 liver cancer cell line and two prostate cancer cell lines, CWR22Rv1 and TSU-PR1[20]. ABCC10 transcript up-regulation has also been shown in salivary gland adenocarcinoma[21].
The ectopic expression of ABCC10 confers resistance to taxanes, which is of particular interest because aside from ABCB1, none of the established cellular efflux pumps produce resistance to clinically used taxanes[22]. Indeed, the role of ABCC10 in taxane resistance is noticeable, as ABCC10 produces high levels of resistance to paclitaxel and docetaxel (116- and 46-fold, respectively) in ABCB1-deficient fibroblasts[22]. In another study, fibroblasts from Abcc10-knockout mice have been shown to be taxane-resistant[13]. In the same study, the mortality of the Abcc10-knockout mice was found to be significantly increased due to neutropenia and marked bone marrow toxicity after paclitaxel treatment[13]. These results suggest that ABCC10 protects cells against paclitaxel toxicity. Recently, it was reported that ABCC10 and ABCB1 gene expression is induced in chemoresistant and chemosensitive tumors by intermittent docetaxel treatment[23], implying that the dosing schedule of chemotherapy affects the development of resistance.
ABCC10 Modulators
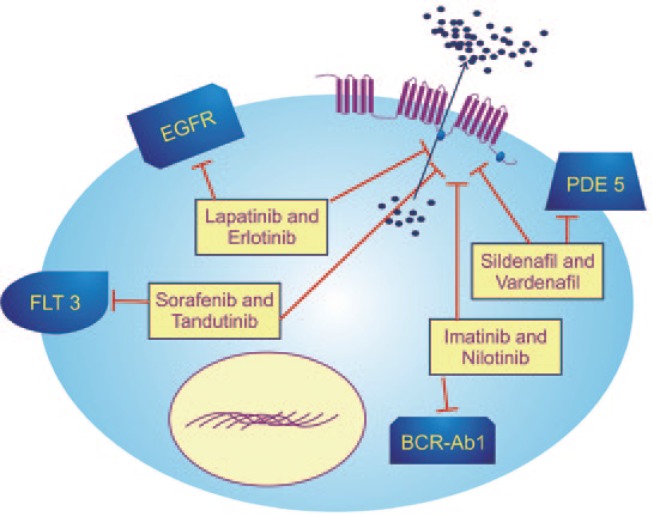
To circumvent ABCC10-induced MDR, various modulators that could significantly reverse the resistance mediated by ABCC10 by increasing the accumulation and decreasing the efflux of antitumor drugs have been tested (Table 2). Various compounds that function as ABCC10 modulators, albeit with different mechanisms of action, will be subsequently discussed (Figure 2).
Table 2. Tyrosine kinase inhibitors (TKIs) and ABCC10 modulators.
| Compound | Concentrations | Effect on ABCC10 substrate drug | Effect on accumulation of [3H]-paclitaxel | Effect on efflux of [3H]-paclitaxel | Effect on ABCC10 protein expression |
| Cepharanthine[24] | 2 µmol/L | + | ↑ | ↓ | ↔ |
| Imatinib[28] | 1, 2.5, 5 µmol/L | + | ↑ | ↓ | ↔ |
| Nilotinib[28] | 1, 2.5, 5 µmol/L | + | ↑ | ↓ | ↔ |
| Lapatinib[33] | 0.625, 1.25, 2.5 µmol/L | + | ↑ | ↓ | ↔ |
| Erlotinib[33] | 0.625, 1.25, 2.5 µmol/L | + | ↑ | ↓ | ↔ |
| Sorafenib[19] | 5 µmol/L | + | N/A | N/A | N/A |
| Sildenafil[41] | 1.25, 2.5, 5 µmol/L | + | ↑ | ↓ | ↔ |
| Vardenafil[41] | 1.25, 2.5, 5 µmol/L | + | ↑ | ↓ | ↔ |
| Tariquidar[61] | 0.1, 0.3 µmol/L | + | ↑ | ↓ | ↓ (>24 h treatment) |
| Tandutinib[47] | 5, 10 µmol/L | + | ↑ | ↓ | ↔ |
(+): this compound shows reversal activity for the ABCC10 transporter; (-): this compound shows no reversal activity for the ABCC10 transporter; (↑): increase or up-regulate; (↓): decrease or down-regulate; (↔): no significant alterations; (N/A): not applicable.
Figure 2. Modulation of ABCC10.
Overexpression of ABCC10 leads to efflux of antitumor drugs out of cancer cells. Modulation of ABCC10 by different classes of compounds, such as lapatinib, erlotinib, imatinib, nilotinib, sorafenib, tandutinib, sildenafil, and vardenafil, leads to increased accumulation and decreased efflux of antitumor drugs, thus rendering them more efficacious. BCR-Abl, breakpoint cluster region-Abelson; EGFR, endothelial growth factor receptor; FLT3, FMS-like tyrosine kinase 3; PDE5, phosphodiesterase type 5.
Cepharanthine
Cepharanthine [6′12′-dimethoxy-2,2′-dimethyl-6,7-[methylenebis (oxy) oxyacan-than] is isolated from the herbal plant Stephania cepharantha Hayata. Cephanranthine, at 2 µmol/L, has been shown to completely reverse the paclitaxel resistance of ABCC10-transfected HEK293 cells[24]. In contrast, cepharanthine does not significantly alter paclitaxel transport in empty vector-transfected HEK293 cells. Accumulation and efflux studies have indicated that cepharanthine significantly increases the intracellular accumulation of [3H]-paclitaxel and inhibits the efflux of [3H]-paclitaxel from ABCC10-transfected cells, but not in the cells lacking the ABCC10 transporter[24]. The transport of E217βG is competitively inhibited by cepharanthine with a Ki value of 4.86 µmol/L[24].
Imatinib and nilotinib
Imatinib and nilotinib are inhibitors of the tyrosine kinase (TK) breakpoint cluster region-Abelson (BCR-Abl) protein and stem cell factor receptor (c-kit), a class III receptor TK[25]. The abnormal translocation of the BCR-Abl gene is associated with a deregulation of TK function, and its expression subsequently leads to chronic myeloid leukemia (CML)[26]. Previous results from our laboratory suggest that nilotinib significantly inhibits the drug efflux functions of ABCB1 and ABCG2[27]. Subsequently, it has been reported that imatinib and nilotinib reverse ABCC10-mediated MDR[28]. Western blotting analysis has indicated that both imatinib and nilotinib do not significantly affect ABCC10 expression. However, imatinib and nilotinib have been shown to enhance the sensitivity of ABCC10-transfected HEK293 cells to two established ABCC10 substrates, paclitaxel and vincristine, in a dose-dependent fashion[28]. Imatinib and nilotinib significantly increase the accumulation of [3H]-paclitaxel in ABCC10-transfected cells almost to the level of control cells in the absence of imatinib and nilotinib. The efflux of [3H]-paclitaxel from ABCC10-transfected cells is also significantly inhibited by imatinib and nilotinib. In conclusion, imatinib and nilotinib reverse ABCC10-mediated MDR through inhibition of the drug efflux function. If these results can be clinically translated, they suggest that nilotinib and imatinib could be used in combination with paclitaxel to surmount MDR in certain types of cancers[28].
Lapatinib and erlotinib
Lapatinib is a reversible, small-molecule inhibitor of endothelial growth factor receptor (EGFR/HER1) and HER2 receptor approved by the Food and Drug Administration (FDA) in the United States for the treatment of HER2-positive metastatic breast cancers[29]. Erlotinib, another selective small-molecule inhibitor of EGFR, is used clinically for the treatment of chemotherapy resistance in patients with advanced NSCLC as well as advanced pancreatic cancer in combination with gemcitabine[30]. Previously, it has been reported that lapatinib and erlotinib reverse ABCB1- and ABCG2-mediated MDR[31],[32] and that they significantly reduce ABCC10-mediated MDR[33]. For example, lapatinib and erlotinib produce a dose-dependent increase in the sensitivity of ABCC10-transfected HEK293 cells to docetaxel, paclitaxel, vinblastine, and vinorelbine[33]. Furthermore, lapatinib and erlotinib significantly increase the intracellular accumulation of [3H]-paclitaxel by inhibiting the efflux of [3H]-paclitaxel in HEK293/ABCC10 cells[33]. Lapatinib produces a significantly greater inhibitory effect on ABCC10-mediated MDR than erlotinib, which may result from the action of lapatinib on the TKs of EGFR and HER2 receptors, whereas erlotinib only inhibits EGFR[34]. Additionally, Western blotting analysis has indicated that ABCC10 protein expression is not significantly altered by lapatinib or erlotinib. In conclusion, lapatinib and erlotinib reverse ABCC10-mediated MDR through inhibition of the drug efflux function of ABCC10. These findings suggest that these compounds could be used as adjuvant chemotherapeutic drugs in clinical practice[33], although this remains to be verified.
Sorafenib
Sorafenib is an inhibitor of C-rapidly accelerated fibrosarcoma (C-raf), B-raf, c-kit, FMS-like tyrosine kinase 3 (FLT3), platelet-derived growth factor receptor-α and -β, and vascular endothelial growth factor receptor-1, -2, and -3[35]. At a clinically achievable plasma concentration of 10 µmol/L, sorafenib significantly inhibits multiple additional receptor TKs and intracellular kinases[35]. Sorafenib is approved for the treatment of advanced renal cell carcinoma and hepatocellular carcinoma (HCC) and is being evaluated for the treatment of AML[36]. Sorafenib inhibits the cellular efflux function of ABC transporters[37] and re-sensitizes cells to cytarabine, which is a substrate of ABCC10 and ABCC11 transporters[22]. Consequently, we can make the assumption that sorafenib may inhibit the transporter-mediated efflux of cytarabine from AML cells and enhance the accumulation of cytarabine-phosphorylated metabolites, leading to an increase in the sensitivity of AML blast cells to cytarabine. Indeed, sorafenib significantly increases the cellular accumulation of cytarabine and its metabolites, resulting in additive or synergistic anti-leukemic activity[37]. To determine if the expression of ABCC10 and ABCC11 was responsible for the sorafenib-induced cellular retention of cytarabine, gene and protein expression were assessed in 10 human AML cell lines; Western blotting demonstrated that the ABCC10 protein is expressed in 4 of the 10 AML cell lines[19].
Sildenafil and vardenafil
Sildenafil and vardenafil are selective, competitive inhibitors of the phosphodiesterase type 5 (PDE5) enzyme and inhibit the biotransformation of cyclic guanosine monophosphate (cGMP)[38]. These compounds are used to treat erectile dysfunction and certain types of pulmonary hypertension[38]. Sildenafil and vardenafil increase the intracellular accumulation of cGMP, leading to the relaxation of vascular smooth muscle[38]. Currently, several groups have evaluated the anticancer activities of PDE5 inhibitors. Previous results from our laboratory indicate that sildenafil and vardenafil significantly reverse ABCB1-mediated MDR[39],[40]. Furthermore, sildenafil and vardenafil produce a significant dose-dependent reversal of ABCC10-mediated MDR by enhancing the sensitivity of HEK293/ABCC10 cells to paclitaxel, docetaxel, and vinblastine[41]. Sildenafil and vardenafil, at 5 µmol/L, significantly increase the accumulation of [3H]-paclitaxel in HEK/ABCC10 cells. Incubating HEK293/ABCC10 cells with sildenafil or vardenafil (5 µmol/L) also significantly inhibits the efflux of [3H]-paclitaxel. Immunoblotting and immunofluorescence analyses showed that ABCC10 protein expression and localization in HEK293/ABCC10 cells are not significantly altered by 5 µmol/L of sildenafil or vardenafil for up to 72 h. In conclusion, sildenafil and vardenafil reverse ABCC10-mediated paclitaxel resistance, and this is most likely due to the inhibition of the efflux of paclitaxel via ABCC10. These findings suggest that sildenafil or vardenafil, in combination with other antitumor drugs, may be useful in the treatment of certain MDR cancers[41].
Tariquidar
Tariquidar is a third-generation ABCB1 inhibitor with high affinity (Kd = 5 nmol/L), low toxicity, and increased selectivity for ABCB1[42]. Tariquidar is 10- to 30-fold more potent than second-generation ABCB1 inhibitors, such as PSC833[43], and is 100- to 1,000-fold more potent than first-generation ABCB1 inhibitors, such as cyclosporine A and verapamil[44]. Tariquidar is an inhibitor of ABCG2 but does not interact with ABCC1, indicating that it may not be as specific as previously thought[45].
Currently, only a few inhibitors of ABCC10 have been identified, and none of them have progressed to clinical trials. A recent in vitro study reported that tariquidar produces a significant dose-dependent increase in the sensitivity of ABCC10-transfected HEK293 cells to the ABCC10 substrates paclitaxel, docetaxel, vincristine, vinblastine, and vinorelbine. In contrast, tariquidar does not significantly alter the efficacy of the aforementioned substrates in empty vector-transfected HEK293 cells. Tariquidar completely reversed ABCC10-mediated MDR at 0.3 µmol/L, making it a potent ABCC10-reversing agent identified thus far. Furthermore, accumulation assays have demonstrated that tariquidar significantly increases the intracellular accumulation of [3H]-paclitaxel, as well as boron-dipyrromethene (BODIPY)-paclitaxel, in ABCC10-transfected HEK293 cells. The enhanced intracellular accumulation of [3H]-paclitaxel is partly due to the rapid and direct inhibition of ABCC10-mediated drug efflux by tariquidar. Tariquidar also produces a down-regulation of ABCC10 protein expression after 24 h of incubation. Incubating cells with tariquidar for 24 h down-regulates ABCC10 protein expression in a time- and dose-dependent manner. Consequently, the reversal effect of tariquidar on ABCC10-mediated MDR in HEK293/ABCC10 cells is due to the inhibition of ABCC10 efflux function and down-regulation of ABCC10 protein expression. Importantly, the down-regulation of ABCC10 protein expression was not caused by the reduction in ABCC10 mRNA levels or the cellular translocation of ABCC10. In conclusion, tariquidar could be used in combination with specific anti-cancer drugs to treat certain types of cancer, although this remains to be proven.
Tandutinib
Tandutinib is a novel quinazoline-based inhibitor of FLT3 (a transmembrane receptor in the tyrosine kinase family), the platelet-derived growth factor receptor, and c-kit. Tandutinib is approved for the treatment of AML and is currently in phase II clinical trials[46]. A recent study showed that tandutinib reverses ABCC10-mediated MDR[47]. For example, tandutinib significantly sensitizes ABCC10-expressing cells to paclitaxel and vincristine[47]. Moreover, accumulation and efflux experiments have indicated that tandutinib significantly enhances the intracellular accumulation of [3H]-paclitaxel and inhibits the efflux of [3H]-paclitaxel from HEK293/ABCC10 cells[47]. However, Western blotting analysis has indicated that tandutinib does not significantly affect ABCC10 protein expression. These findings suggest that clinical studies should be considered to test the efficacy of tandutinib to reverse ABCC10-mediated MDR in patients[47].
ABCC10 Genetic Variants
Currently, relatively few studies have been published regarding the effects of genomic ABCC10 mutations on the interactions of the ABCC10 protein with various compounds. Pushpakom et al.[48] have found some common variants in ABCC10 in human immunodeficiency virus (HIV)-positive patients receiving tenofovir-containing regimens. This study is the first genetic association analysis reported for ABCC10[48]. Two single nucleotide polymorphisms (SNPs) and their haplotype were significantly associated with renal tubular dysfunction: rs9349256 located in intron 4 and rs2125739, a nonsynonymous SNP, located in exon 12 that results in an amino acid change (Ile920Thr)[48]. Although the functional effects of the polymorphisms identified in ABCC10 are not known, a bioinformatic approach using FastSNP software (http://fastsnp.ibms.sinica.edu.tw/) found rs2125739 in a putative splice site[48]. Previously, splice site polymorphisms have been shown to affect pre-mRNA splicing and may cause the splicing apparatus to use nearby cryptic splice sites or skip exons, leading to altered ABCC10 expression[49]. Based on HapMap data (www.hapmap.org), the minor allele frequency for rs2125739 in various populations was reported as follows: Northern and Western European ancestry (26.7%), Sub-Saharan African (34.2%), Han Chinese (3.4%), and Japanese (8.0%)[48]. ABCC10 rs9349256 is associated with urine phosphorus wasting and β2-microglobulinuria, which are markers for tubular dysfunction[48],[50],[51]. This observation is interesting, as urine phosphorus wasting is among the 3 criteria defining Fanconi syndrome and tenofovir is the drug that is most associated with this syndrome[52],[53], which leads to bone demineralization and osteomalacia[52]. Liptrott et al.[54] have investigated the contribution of ABCC10 SNPs to variability in nevirapine plasma concentration. An exonic SNP in ABCC10, rs2125739, was significantly associated with nevirapine plasma concentration in Caucasians; the variant allele was significantly more prevalent in those with lower nevirapine plasma concentrations[54]. The minor allele frequency for this SNP was similar in both the Caucasians and African-Americans represented in this cohort. Indeed, the same allele was also more prevalent in African-Americans with lower nevirapine plasma concentration, although this association was not statistically significant[54]. This finding is of interest as lower nevirapine plasma concentrations have been reported to be associated with reduced viral suppression[55],[56]. Hence, these SNPs can provide insight for developing modulation strategies of ABCC10 expression to augment the efficacy of therapeutic drugs.
Conclusions
Despite previous reports showing the widespread tissue expression of ABCC10[11],[14], it remains one of the least-characterized ABC family members. It would be of great interest to characterize the mechanism of regulation of ABCC10 expression. Several compounds have recently been shown to interact with ABCC10; thus, ABCC10 can confer resistance to these compounds, both at the tumor cell level and by decreasing their oral bioavailability[28]. Furthermore, the role of ABCC10 needs to be elucidated in both cancer cells and cancer stem cells. Although efflux pump inhibitors have yet to be established as a clinical strategy for sensitizing tumors to chemotherapeutic substances, genetic variations should be considered in the design of new therapeutic strategies.
Acknowledgments
This work was partially supported by funds from the National Institute of Health (No. 1R15CA143701) and St. John's University Research Seed Grant (No. 579-1110-7002) to Z.S. Chen.
References
- 1.Szakacs G, Paterson JK, Ludwig JA, et al. Targeting multidrug resistance in cancer. Nat Rev Drug Discov. 2006;5:219–234. doi: 10.1038/nrd1984. [DOI] [PubMed] [Google Scholar]
- 2.Dean M, Allikmets R. Complete characterization of the human abc gene family. J Bioenerg Biomembr. 2001;33:475–479. doi: 10.1023/a:1012823120935. [DOI] [PubMed] [Google Scholar]
- 3.Gottesman MM, Fojo T, Bates SE. Multidrug resistance in cancer: role of ATP-dependent transporters. Nat Rev Cancer. 2002;2:48–58. doi: 10.1038/nrc706. [DOI] [PubMed] [Google Scholar]
- 4.Borst P, Evers R, Kool M, et al. A family of drug transporters: the multidrug resistance-associated proteins. J Natl Cancer Inst. 2000;92:1295–1302. doi: 10.1093/jnci/92.16.1295. [DOI] [PubMed] [Google Scholar]
- 5.Gillet JP, Efferth T, Remacle J. Chemotherapy-induced resistance by ATP-binding cassette transporter genes. Biochem Biophys Acta. 2007;1775:237–262. doi: 10.1016/j.bbcan.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 6.Tiwari AK, Sodani K, Dai CL, et al. Revisiting the ABCs of multidrug resistance in cancer chemotherapy. Curr Pharm Biotechnol. 2011;12:570–594. doi: 10.2174/138920111795164048. [DOI] [PubMed] [Google Scholar]
- 7.Sodani K, Patel A, Kathawala RJ, et al. Multidrug resistance associated proteins in multidrug resistance. Chin J Cancer. 2012;31:58–72. doi: 10.5732/cjc.011.10329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kruh GD, Guo Y, Hopper-Borge E, et al. ABCC10, ABCC11, and ABCC12. Pflugers Arch. 2007;453:675–684. doi: 10.1007/s00424-006-0114-1. [DOI] [PubMed] [Google Scholar]
- 9.Hopper E, Belinsky MG, Zeng H, et al. Analysis of the structure and expression pattern of MRP7 (ABCC10), a new member of the MRP subfamily. Cancer Lett. 2001;162:181–191. doi: 10.1016/s0304-3835(00)00646-7. [DOI] [PubMed] [Google Scholar]
- 10.Hopper-Borge E, Chen ZS, Shchaveleva I, et al. Analysis of the drug resistance profile of multidrug resistance protein 7 (ABCC10): resistance to docetaxel. Cancer Res. 2004;64:4927–4930. doi: 10.1158/0008-5472.CAN-03-3111. [DOI] [PubMed] [Google Scholar]
- 11.Malofeeva EV, Domanitskaya N, Gudima M, et al. Modulation of the ATPase and transport activities of broad-acting multidrug resistance factor ABCC10 (MRP7) Cancer Res. 2012;72:6457–6467. doi: 10.1158/0008-5472.CAN-12-1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen ZS, Hopper-Borge E, Belinsky MG, et al. Characterization of the transport properties of human multidrug resistance protein 7 (MRP7, ABCC10) Mol Pharmacol. 2003;63:351–358. doi: 10.1124/mol.63.2.351. [DOI] [PubMed] [Google Scholar]
- 13.Hopper-Borge EA, Churchill T, Paulose C, et al. Contribution of Abcc10 (Mrp7) to in vivo paclitaxel resistance as assessed in Abcc10(-/-) mice. Cancer Res. 2011;71:3649–3657. doi: 10.1158/0008-5472.CAN-10-3623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Takayanagi S, Kataoka T, Ohara O, et al. Human ATP-binding cassette transporter ABCC10: expression profile and p53-dependent upregulation. J Exp Ther Oncol. 2004;4:239–246. [PubMed] [Google Scholar]
- 15.Bleasby K, Castle JC, Roberts CJ, et al. Expression profiles of 50 xenobiotic transporter genes in humans and pre-clinical species: a resource for investigations into drug disposition. Xenobiotica. 2006;36:963–988. doi: 10.1080/00498250600861751. [DOI] [PubMed] [Google Scholar]
- 16.Kao HH, Chang MS, Cheng JF, et al. Genomic structure, gene expression, and promoter analysis of human multidrug resistance-associated protein 7. J Biomed Sci. 2003;10:98–110. doi: 10.1007/BF02256002. [DOI] [PubMed] [Google Scholar]
- 17.Bessho Y, Oguri T, Ozasa H, et al. ABCC10/MRP7 is associated with vinorelbine resistance in non-small cell lung cancer. Oncol Rep. 2009;21:263–268. [PubMed] [Google Scholar]
- 18.Oguri T, Ozasa H, Uemura T, et al. MRP7/ABCC10 expression is a predictive biomarker for the resistance to paclitaxel in non-small cell lung cancer. Mol Cancer Ther. 2008;7:1150–1155. doi: 10.1158/1535-7163.MCT-07-2088. [DOI] [PubMed] [Google Scholar]
- 19.Hu S, Niu H, Inaba H, et al. Activity of the multikinase inhibitor sorafenib in combination with cytarabine in acute myeloid leukemia. J Natl Cancer Inst. 2011;103:893–905. doi: 10.1093/jnci/djr107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dabrowska M, Sirotnak FM. Regulation of transcription of the human MRP7 gene. Characteristics of the basal promoter and identification of tumor-derived transcripts encoding additional 5′ end heterogeneity. Gene. 2004;341:129–139. doi: 10.1016/j.gene.2004.06.022. [DOI] [PubMed] [Google Scholar]
- 21.Naramoto H, Uematsu T, Uchihashi T, et al. Multidrug resistance-associated protein 7 expression is involved in cross-resistance to docetaxel in salivary gland adenocarcinoma cell lines. Int J Oncol. 2007;30:393–401. [PubMed] [Google Scholar]
- 22.Hopper-Borge E, Xu X, Shen T, et al. Human multidrug resistance protein 7 (ABCC10) is a resistance factor for nucleoside analogues and epothilone B. Cancer Res. 2009;69:178–184. doi: 10.1158/0008-5472.CAN-08-1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.De Souza R, Zahedi P, Badame RM, et al. Chemotherapy dosing schedule influences drug resistance development in ovarian cancer. Mol Cancer Ther. 2011;10:1289–1299. doi: 10.1158/1535-7163.MCT-11-0058. [DOI] [PubMed] [Google Scholar]
- 24.Zhou Y, Hopper-Borge E, Shen T, et al. Cepharanthine is a potent reversal agent for MRP7(ABCC10)-mediated multidrug resistance. Biochem Pharmacol. 2009;77:993–1001. doi: 10.1016/j.bcp.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 25.Shah NP, Nicoll JM, Nagar B, et al. Multiple BCR-ABL kinase domain mutations confer polyclonal resistance to the tyrosine kinase inhibitor imatinib (STI571) in chronic phase and blast crisis chronic myeloid leukemia. Cancer Cell. 2002;2:117–125. doi: 10.1016/s1535-6108(02)00096-x. [DOI] [PubMed] [Google Scholar]
- 26.Gora-Tybor J, Robak T. Targeted drugs in chronic myeloid leukemia. Curr Med Chem. 2008;15:3036–3051. doi: 10.2174/092986708786848578. [DOI] [PubMed] [Google Scholar]
- 27.Tiwari AK, Sodani K, Wang SR, et al. Nilotinib (AMN107, Tasigna) reverses multidrug resistance by inhibiting the activity of the ABCB1/Pgp and ABCG2/BCRP/MXR transporters. Biochem Pharmacol. 2009;78:153–161. doi: 10.1016/j.bcp.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 28.Shen T, Kuang YH, Ashby CR, et al. Imatinib and nilotinib reverse multidrug resistance in cancer cells by inhibiting the efflux activity of the MRP7 (ABCC10) PLoS One. 2009;4:e7520. doi: 10.1371/journal.pone.0007520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cameron D. Lapatinib plus capecitabine in patients with HER2-positive advanced breast cancer. Clin Adv Hematol Oncol. 2007;5:456–458. [PubMed] [Google Scholar]
- 30.Gatzemeier U, Pluzanska A, Szczesna A, et al. Phase III study of erlotinib in combination with cisplatin and gemcitabine in advanced non-small-cell lung cancer: the tarceva lung cancer investigation trial. J Clin Oncol. 2007;25:1545–1552. doi: 10.1200/JCO.2005.05.1474. [DOI] [PubMed] [Google Scholar]
- 31.Dai CL, Tiwari AK, Wu CP, et al. Lapatinib (Tykerb, GW572016) reverses multidrug resistance in cancer cells by inhibiting the activity of ATP-binding cassette subfamily B member 1 and G member 2. Cancer Res. 2008;68:7905–7914. doi: 10.1158/0008-5472.CAN-08-0499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shi Z, Peng XX, Kim IW, et al. Erlotinib (Tarceva, OSI-774) antagonizes ATP-binding cassette subfamily B member 1 and ATP-binding cassette subfamily G member 2-mediated drug resistance. Cancer Res. 2007;67:11012–11020. doi: 10.1158/0008-5472.CAN-07-2686. [DOI] [PubMed] [Google Scholar]
- 33.Kuang YH, Shen T, Chen X, et al. Lapatinib and erlotinib are potent reversal agents for MRP7 (ABCC10)-mediated multidrug resistance. Biochem Pharmacol. 2010;79:154–161. doi: 10.1016/j.bcp.2009.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Burris HA., 3rd Dual kinase inhibition in the treatment of breast cancer: Initial experience with the EGFR/ErbB-2 inhibitor lapatinib. Oncologist. 2004;9(Suppl. 3):10–15. doi: 10.1634/theoncologist.9-suppl_3-10. [DOI] [PubMed] [Google Scholar]
- 35.Fabian MA, Biggs WH, 3rd, Treiber DK, et al. A small molecule-kinase interaction map for clinical kinase inhibitors. Nat Biotechnol. 2005;23:329–336. doi: 10.1038/nbt1068. [DOI] [PubMed] [Google Scholar]
- 36.Mori S, Cortes J, Kantarjian H, et al. Potential role of sorafenib in the treatment of acute myeloid leukemia. Leuk Lymphoma. 2008;49:2246–2255. doi: 10.1080/10428190802510349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hu S, Chen Z, Franke R, et al. Interaction of the multikinase inhibitors sorafenib and sunitinib with solute carriers and ATP-binding cassette transporters. Clin Cancer Res. 2009;15:6062–6069. doi: 10.1158/1078-0432.CCR-09-0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Corbin JD, Francis SH. Molecular biology and pharmacology of PDE-5-inhibitor therapy for erectile dysfunction. J Androl. 2003;24:S38–41. doi: 10.1002/j.1939-4640.2003.tb02744.x. [DOI] [PubMed] [Google Scholar]
- 39.Shi Z, Tiwari AK, Shukla S, et al. Sildenafil reverses ABCB1- and ABCG2-mediated chemotherapeutic drug resistance. Cancer Res. 2011;71:3029–3041. doi: 10.1158/0008-5472.CAN-10-3820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ding PR, Tiwari AK, Ohnuma S, et al. The phosphodiesterase-5 inhibitor vardenafil is a potent inhibitor of ABCB1-P-glycoprotein transporter. PLoS One. 2011;6:e19329. doi: 10.1371/journal.pone.0019329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen JJ, Sun YL, Tiwari AK, et al. PDE5 inhibitors, sildenafil and vardenafil, reverse multidrug resistance by inhibiting the efflux function of multidrug resistance protein 7 (ATP-binding cassette C10) transporter. Cancer Sci. 2012;103:1531–1537. doi: 10.1111/j.1349-7006.2012.02328.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Klinkhammer W, Muller H, Globisch C, et al. Synthesis and biological evaluation of a small molecule library of 3rd generation multidrug resistance modulators. Bioorg Med Chem. 2009;17:2524–2535. doi: 10.1016/j.bmc.2009.01.072. [DOI] [PubMed] [Google Scholar]
- 43.Smith AJ, Mayer U, Schinkel AH, et al. Availability of PSC833, a substrate and inhibitor of P-glycoproteins, in various concentrations of serum. J Natl Cancer Inst. 1998;90:1161–1166. doi: 10.1093/jnci/90.15.1161. [DOI] [PubMed] [Google Scholar]
- 44.Mistry P, Stewart AJ, Dangerfield W, et al. In vitro and in vivo reversal of P-glycoprotein-mediated multidrug resistance by a novel potent modulator, XR9576. Cancer Res. 2001;61:749–758. [PubMed] [Google Scholar]
- 45.Kuhnle M, Egger M, Muller C, et al. Potent and selective inhibitors of breast cancer resistance protein (ABCG2) derived from the p-glycoprotein (ABCB1) modulator tariquidar. J Med Chem. 2009;52:1190–1197. doi: 10.1021/jm8013822. [DOI] [PubMed] [Google Scholar]
- 46.Illmer T, Ehninger G. FLT3 kinase inhibitors in the management of acute myeloid leukemia. Clin Lymphoma Myeloma. 2007;8(Suppl. 1):S24–34. doi: 10.3816/clm.2007.s.030. [DOI] [PubMed] [Google Scholar]
- 47.Deng W, Dai CL, Chen JJ, et al. Tandutinib (MLN518) reverses multidrug resistance by inhibiting the efflux activity of the multidrug resistance protein 7 (ABCC10) Oncol Rep. 2013;29:2479–2485. doi: 10.3892/or.2013.2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pushpakom SP, Liptrott NJ, Rodriguez-Novoa S, et al. Genetic variants of ABCC10, a novel tenofovir transporter, are associated with kidney tubular dysfunction. J Infect Dis. 2011;204:145–153. doi: 10.1093/infdis/jir215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.ElSharawy A, Hundrieser B, Brosch M, et al. Systematic evaluation of the effect of common SNPs on pre-mRNA splicing. Hum Mutat. 2009;30:625–632. doi: 10.1002/humu.20906. [DOI] [PubMed] [Google Scholar]
- 50.Izzedine H, Harris M, Perazella MA. The nephrotoxic effects of HAART. Nat Rev Nephrol. 2009;5:563–573. doi: 10.1038/nrneph.2009.142. [DOI] [PubMed] [Google Scholar]
- 51.Gatanaga H, Tachikawa N, Kikuchi Y, et al. Urinary beta2-microglobulin as a possible sensitive marker for renal injury caused by tenofovir disoproxil fumarate. AIDS Res Hum Retroviruses. 2006;22:744–748. doi: 10.1089/aid.2006.22.744. [DOI] [PubMed] [Google Scholar]
- 52.Izzedine H, Isnard-Bagnis C, Hulot JS, et al. Renal safety of tenofovir in HIV treatment-experienced patients. AIDS. 2004;18:1074–1076. doi: 10.1097/00002030-200404300-00019. [DOI] [PubMed] [Google Scholar]
- 53.Earle KE, Seneviratne T, Shaker J, et al. Fanconi's syndrome in HIV+ adults: report of three cases and literature review. J Bone Miner Res. 2004;19:714–721. doi: 10.1359/jbmr.2004.19.5.714. [DOI] [PubMed] [Google Scholar]
- 54.Liptrott NJ, Pushpakom S, Wyen C, et al. Association of ABCC10 polymorphisms with nevirapine plasma concentrations in the German Competence Network for HIV/AIDs. Pharmacogenet Genomics. 2012;22:10–19. doi: 10.1097/FPC.0b013e32834dd82e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.de Vries-Sluijs TE, Dieleman JP, Arts D, et al. Low nevirapine plasma concentrations predict virological failure in an unselected HIV-1-infected population. Clin Pharmacokinet. 2003;42:599–605. doi: 10.2165/00003088-200342060-00009. [DOI] [PubMed] [Google Scholar]
- 56.Duong M, Buisson M, Peytavin G, et al. Low trough plasma concentrations of nevirapine associated with virologic rebounds in HIV-infected patients who switched from protease inhibitors. Ann Pharmacother. 2005;39:603–609. doi: 10.1345/aph.1E563. [DOI] [PubMed] [Google Scholar]
- 57.Kao HH, Huang JD, Chang MS. cDNA cloning and genomic organization of the murine MRP7, a new ATP-binding cassette transporter. Gene. 2002;286:299–306. doi: 10.1016/s0378-1119(02)00461-4. [DOI] [PubMed] [Google Scholar]
- 58.Wooden SL, Kalb SR, Cotter RJ, et al. Cutting edge: HLA-E binds a peptide derived from the ATP-binding cassette transporter multidrug resistance-associated protein 7 and inhibits NK cell-mediated lysis. J Immunol. 2005;175:1383–1387. doi: 10.4049/jimmunol.175.3.1383. [DOI] [PubMed] [Google Scholar]
- 59.Zahedi P, De Souza R, Huynh L, et al. Combination drug delivery strategy for the treatment of multidrug resistant ovarian cancer. Mol Pharm. 2011;8:260–269. doi: 10.1021/mp100323z. [DOI] [PubMed] [Google Scholar]
- 60.Tiwari AK, Sodani K, Dai CL, et al. Nilotinib potentiates anticancer drug sensitivity in murine ABCB1-, ABCG2-, and ABCC10-multidrug resistance xenograft models. Cancer Lett. 2013;328:307–317. doi: 10.1016/j.canlet.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sun YL, Chen JJ, Kumar P, et al. Reversal of MRP7 (ABCC10)-mediated multidrug resistance by tariquidar. PLoS One. 2013;8:e55576. doi: 10.1371/journal.pone.0055576. [DOI] [PMC free article] [PubMed] [Google Scholar]