Abstract
Purpose
Painful oral mucositis (OM) is a significant toxicity during radiotherapy for head and neck cancers. The aim of this randomized, double-blind, placebo-controlled trial was to test the efficacy of doxepin hydrochloride in the reduction of radiotherapy-induced OM pain.
Patients and Methods
In all, 155 patients were randomly allocated to a doxepin oral rinse or a placebo for the treatment of radiotherapy-related OM pain. Patients received a single dose of doxepin or placebo on day 1 and then crossed over to receive the opposite agent on a subsequent day. Pain questionnaires were administered at baseline and at 5, 15, 30, 60, 120, and 240 minutes. Patients were then given the option to continue doxepin. The primary end point was pain reduction as measured by the area under the curve (AUC) of the pain scale using data from day 1.
Results
Primary end point analysis revealed that the AUC for mouth and throat pain reduction was greater for doxepin (−9.1) than for placebo (−4.7; P < .001). Crossover analysis of patients completing both phases confirmed that patients experienced greater mouth and throat pain reduction with doxepin (intrapatient changes of 4.1 for doxepin-placebo arm and −2.8 for placebo-doxepin arm; P < .001). Doxepin was associated with more stinging or burning, unpleasant taste, and greater drowsiness than the placebo rinse. More patients receiving doxepin expressed a desire to continue treatment than did patients with placebo after completion of each of the randomized phases of the study.
Conclusion
A doxepin rinse diminishes OM pain. Further studies are warranted to determine its role in the management of OM.
INTRODUCTION
Radiation-induced oral mucositis is an inflammatory process of the oral cavity and oropharynx manifested as painful, erythematous ulcerative lesions that develop within 7 to 14 days of the initiation of radiotherapy.1,2 The majority of patients with head and neck cancer treated with radiation, with or without chemotherapy, experience painful oral mucositis as a result of disruption of the normal function and integrity of the oral mucosa.3,4 The pain and associated dysgeusia caused by oral mucositis frequently require treatment with systemic analgesics. This problem can decrease patients' oral intake and nutrition, leading to dehydration, weight loss, and declining performance status that may require intravenous fluid hydration, feeding tube placement, and hospitalization.4,5 When severe, oral mucositis increases the risk of infection and may compromise clinical outcomes by necessitating treatment breaks, dosage reductions, and reduced therapy compliance.1,6 Common clinical management strategies include bland rinses, topical anesthetics and analgesics, mucosal coating agents, and systemic analgesics.1,7 Most patients require opioid analgesia as the severity of oral mucositis increases.8 Topical anesthetics and analgesics, such as lidocaine, benzocaine, and diphenhydramine, typically provide less than 30 minutes of pain relief.9 They can cause burning or stinging pain on first contact with damaged mucosa and then temporarily diminish or abolish taste and the gag reflex.10
Doxepin hydrochloride is a tricyclic antidepressant with anesthetic and analgesic properties when administered topically. These effects may be the result of Na+ channel blockade that limits conduction of noxious stimuli in cutaneous nociceptors.11 Two small pilot trials of an oral doxepin rinse reported a significant short duration of anesthesia followed by more extended analgesia for patients with oral mucositis.12–15 The current randomized placebo-controlled trial was developed to test the efficacy of doxepin oral rinse as an anesthetic or analgesic for oral mucositis pain caused by head and neck cancer therapy.
PATIENTS AND METHODS
Eligibility
Patients were eligible for enrollment if they were age 18 years or older, had histologic proof of a head and neck malignancy, and were currently undergoing radiotherapy (with or without chemotherapy) to a minimum planned dose of 50 Gy including one third of the oral cavity mucosa using 1.6 to 2.2 Gy per fraction. Both three-dimensional conformal and intensity-modulated radiotherapy techniques were allowed. Before enrollment, each patient had an oral examination by an enrolling clinician that confirmed the presence of oral mucositis and the absence of oral infection using the Oral Mucositis Assessment Scale16 and the WHO mucositis grading scale.17 Eligible patients were also required to have mouth pain rated ≥ 4 on a numerical analog questionnaire (0, no oral pain; 10, worst oral pain) and an Eastern Cooperative Oncology Group (ECOG) performance status of 0 to 2. Patients were typically assessed weekly throughout the course of radiotherapy to determine whether mouth pain from mucositis had achieved a score of ≥ 4 of 10 for severity. Key exclusion criteria were known allergies to doxepin or other tricyclic antidepressants, use of a tricyclic antidepressant or monoamine oxidase inhibitor within the 2 weeks before registration, untreated or unresolved oral candidiasis or oral herpes simplex virus infection, untreated narrow angle glaucoma, and untreated urinary retention ≤ 6 weeks before registration. All patients provided written informed consent before enrollment.
Randomization, Treatment, and Assessment
Randomization was performed at a central location with stratification according to sex, receipt of concurrent sensitizing chemotherapy, and age (< 60 v ≥ 60 years). Patients were assigned to either doxepin-placebo (arm 1) or placebo-doxepin (arm 2) in a 1:1 ratio using the Pocock and Simon dynamic allocation procedure,18 which balances the marginal distributions of the stratification factors. Group 1 patients received a doxepin rinse prepared in the following manner: doxepin 10 mg/mL × 2.5 mL = 25 mg, diluted to 5 mL with 2.5 mL of sterile or distilled water. Patients in arm 2 received a placebo rinse prepared in a similar manner. Ora-Sweet SF is an alcohol-free flavored sugar-free syrup vehicle that served as the placebo base solution. A designated unblinded pharmacist or nurse at each institution prepared the study dose and then blinded study personnel administered the rinse; the patients swished the solution in their mouth for 1 minute, gargled, and expectorated. Study patients remained at the treating locations for the first hour and completed questionnaires at time zero (before the oral rinse) and 5, 15, 30, and 60 minutes postadministration. Patients were then allowed to leave and were instructed on timing of questionnaire completion at 2 and 4 hours, with reminder phone calls. These questionnaires were based on the Oral Mucositis Daily Questionnaire and the Oral Mucositis Weekly Questionnaire-Head and Neck Cancer,19–21 and they used 11-point numerical analog scales (0 to 10 scores) to measure pain, unpleasant taste, stinging or burning, and drowsiness at the defined intervals following doxepin or placebo (Data Supplement). Patients were maintained on any long-acting analgesia that had been initiated before enrollment; however, no analgesics were allowed for mucositis pain for 60 minutes before and after the study doses. Patients were allowed analgesics after 60 minutes if needed for pain relief and were asked to record this on the questionnaire. No viscous lidocaine, “magic mouthwash,” benzocaine, diphenhydramine, or other medicated oral rinses (except 0.9 normal saline or baking soda rinse) were allowed within 4 hours before or after the study medication. At the end of 4 hours, patients were asked, by questionnaire, whether they would like to continue the mouthwash they received, as needed. Patients returned on a subsequent day when their self-rated mouth pain was again ≥ 4 of 10 on a numerical analog questionnaire and entered the crossover phase, conducted in an identical manner. After completion of the questionnaires from the blinded second dose, patients were unblinded and given the option of continuing treatment with doxepin rinse every 4 hours as needed. Patients who chose to continue with doxepin rinses completed a weekly questionnaire until their oral mucositis pain resolved or they chose to discontinue doxepin. Toxic effects were measured according to the Common Terminology Criteria for Adverse Events (CTCAE) version 4.0.22
Statistical Analysis
This was a randomized, double-blind, placebo-controlled, phase III trial with a crossover phase followed by continued use of the active agent. The modified intent-to-treat principle was adopted to exclude patients from statistical analysis who were ineligible, who cancelled, or who did not have any pain measurement after baseline. The primary end point was the total pain reduction (average of mouth and throat pain) as measured by the numerical analog scale of mouth pain in the questionnaires taken at baseline and at 5, 15, 30, 60, 120, and 240 minutes after doxepin-placebo rinse in the first phase. The area under the curve (AUC) was adjusted for baseline, with the time scale replaced by a numerical scale of 1, 2, 3, 4, 5, and 6 to avoid overweighting the later time points. Proration and imputation were used for terminal and intermittent missing data for AUC, respectively. The AUCs for the two treatment arms (doxepin-placebo and placebo-doxepin) were compared by using the Wilcoxon rank sum test with 95% CIs.
Secondary end points included total stinging or burning from, total unpleasant taste of, and total drowsiness increase from the initial oral rinse, each calculated by the AUC and analyzed in the same way as the primary end point. The incidence of using additional analgesics between 2 and 4 hours after the initial mouthwash was an additional secondary end point and was compared between the arms by using the χ2 test. Additional end points included pain reduction and other adverse event profiles in the blinded crossover phase, whereby the intrapatient change of AUC for the two arms (doxepin-placebo and placebo-doxepin) was compared by using the Wilcoxon rank sum test. The CROS procedure23 with an assumption of no carryover effect after the washout period was used to construct the 95% CIs for the mean differences in the intrapatient change between the two treatments.
By using the empirical rule effect size estimation procedure,24 a clinically meaningful effect size was defined as being roughly equivalent to one half the standard deviation. At the 5% significance level, there would be 80% power to detect a clinically meaningful effect size of 0.5 of a standard deviation with 128 patients (64 patients for each arm) based on the two-sample t test with an equal-variance assumption. This sample size was inflated by 15% to account for patient ineligibility, cancellation, or other major violations. A total of 148 patients (74 patients per arm) was targeted for accrual. Statistical analysis was performed by using SAS version 9.2 (SAS Institute, Cary, NC). P values were based on two-sided comparisons. Analyses were based on the study database frozen on June 26, 2012.
Role of Funding Source
The study was designed and conducted and the data were analyzed by the Alliance for Clinical Trials in Oncology cooperative group, which is funded by the National Cancer Institute. The final protocol, amendments, and informed-consent documents were approved by local institutional review boards or independent ethics committees.
RESULTS
Patients
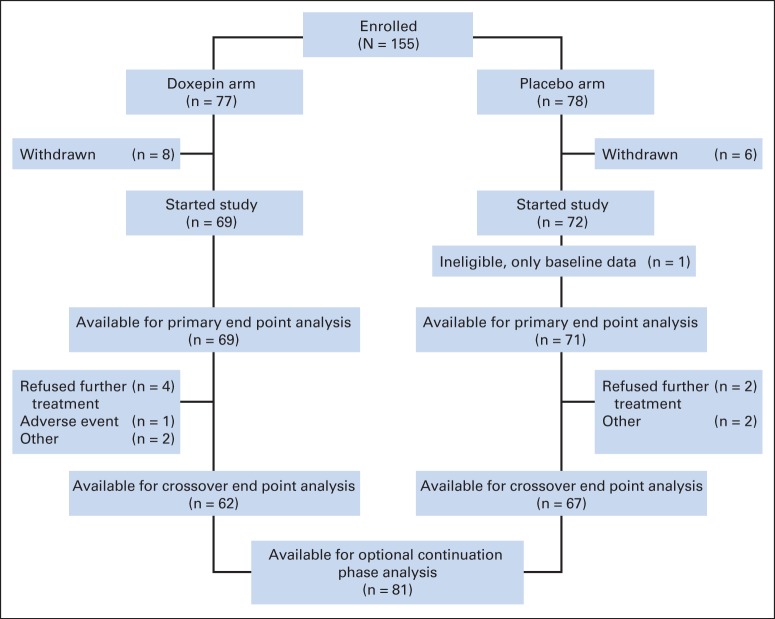
A total of 155 patients at 26 US centers underwent random assignment between December 17, 2010, and May 17, 2012. Figure 1 shows enrollment, random assignment, and follow-up. Table 1 provides the patient characteristics, which were well balanced between the two arms.
Fig 1.
CONSORT diagram.
Table 1.
Baseline Patient Characteristics
| Characteristic | Doxepin-Placebo(n = 69) |
Placebo-Doxepin(n = 71) |
P | ||
|---|---|---|---|---|---|
| No. | % | No. | % | ||
| Age, years | .24 | ||||
| Mean | 62 | 60 | |||
| Range | 39.0-93.0 | 37.0-86.0 | |||
| Race/ethnicity | .36 | ||||
| White | 62 | 90 | 65 | 92 | |
| Black or African American | 4 | 6 | 3 | 4 | |
| Asian | 3 | 4 | 1 | 1 | |
| American Indian or Alaska Native | 0 | 0 | 2 | 3 | |
| Age, years | .61 | ||||
| < 60 | 33 | 48 | 37 | 52 | |
| ≥ 60 | 36 | 52 | 34 | 48 | |
| Sex | .74 | ||||
| Male | 56 | 81 | 56 | 79 | |
| Female | 13 | 19 | 15 | 21 | |
| Primary tumor site | .53 | ||||
| Oropharyngeal | 41 | 59 | 35 | 49 | |
| Oral cavity | 10 | 15 | 19 | 27 | |
| Laryngeal | 10 | 15 | 10 | 14 | |
| Nasopharyngeal | 1 | 1 | 2 | 3 | |
| Salivary | 1 | 1 | 1 | 1 | |
| Hypopharyngeal | 1 | 1 | 0 | 0 | |
| Not otherwise specified | 0 | 0 | 1 | 1 | |
| Other | 5 | 7 | 3 | 4 | |
| Radiosensitizing chemotherapy | .75 | ||||
| Yes | 55 | 80 | 55 | 78 | |
| No | 14 | 20 | 16 | 23 | |
| Prior surgery | .99 | ||||
| Yes | 29 | 42 | 30 | 42 | |
| No | 39 | 57 | 40 | 56 | |
| Unknown | 1 | 1 | 1 | 1 | |
| Pain score baseline | .39 | ||||
| Mean | 6.0 | 5.7 | |||
| SD | 1.7 | 1.6 | |||
| Range | 4.0-1.0 | 4.0-1.0 | |||
| ECOG performance score | .35 | ||||
| 0 | 22 | 32 | 31 | 44 | |
| 1 | 43 | 62 | 36 | 51 | |
| 2 | 4 | 6 | 4 | 6 | |
| Oral mucositis assessment scale location at baseline (percent with lesion) | |||||
| Upper lip | 1 | 1 | 6 | 8 | .06 |
| Lower lip | 9 | 13 | 8 | 11 | .72 |
| Right cheek | 21 | 30 | 21 | 29 | .87 |
| Left cheek | 18 | 26 | 19 | 27 | .93 |
| Right ventral and lateral tongue | 26 | 38 | 24 | 34 | .63 |
| Left ventral and lateral tongue | 24 | 35 | 22 | 31 | .63 |
| Floor of mouth | 19 | 28 | 11 | 15 | .08 |
| Soft palate/fauces | 38 | 55 | 43 | 60 | .38 |
| Hard palate | 23 | 33 | 22 | 31 | .72 |
| Percent with severe erythema at baseline | |||||
| Upper lip | 1 | 1 | 1 | 1 | .98 |
| Lower lip | 5 | 7 | 1 | 1 | .08 |
| Right cheek | 7 | 10 | 6 | 8 | .54 |
| Left cheek | 7 | 10 | 8 | 11 | .88 |
| Right ventral and lateral tongue | 10 | 14 | 13 | 18 | .54 |
| Left ventral and lateral tongue | 16 | 23 | 12 | 17 | .35 |
| Floor of mouth | 9 | 13 | 5 | 7 | .23 |
| Soft palate/fauces | 22 | 32 | 26 | 37 | .56 |
| Hard palate | 13 | 19 | 15 | 21 | .74 |
| WHO mucositis grade at baseline | .26 | ||||
| 1 (erythema and soreness) | 30 | 44 | 25 | 35 | |
| 2 (ulcers, able to eat solids) | 22 | 32 | 19 | 26 | |
| 3 (ulcers, requires liquid diet) | 13 | 19 | 24 | 33 | |
| 4 (ulcers, alimentation not possible) | 3 | 4 | 4 | 6 | |
Abbreviation: ECOG, Eastern Cooperative Oncology Group.
Primary Outcome
Analysis of the primary end point revealed that the AUC for the mean mouth and throat pain reduction was greater for doxepin (−9.1) compared with placebo (−4.7; difference, −4.4; 95% CI, −6.7 to −2.1; P < .001). Crossover analysis of the two phases showed intrapatient changes of 4.1 for the doxepin-placebo arm and −2.8 for the placebo-doxepin arm; therefore, the treatment difference of doxepin versus placebo was −3.5 (95% CI, −5.1 to −1.8; P < .001; Fig 2). This translated to an average mouth and throat pain score reduction (on a scale of 0 to 10) of −2.0 (36.3%) from baseline for doxepin compared with −1.0 (18.9%) for placebo at 30 minutes after rinse (P = .0032). This average pain score reduction was statistically significant 1, 2, and 4 hours after study initiation (Fig 3). Although they were treated as stratification factors for randomization, sex and use of concurrent radiosensitizing chemotherapy seemed to interact with the treatment effect in an exploratory analysis; their P values for statistically significant interaction were .021 and .002, respectively. Although female/no concurrent radiosensitizing chemotherapy patients tended to have more pain than male/concurrent radiosensitizing chemotherapy patients in the placebo arm, these relationships were reversed in the doxepin arm (Data Supplement; Figs A1 and A2, online only). Nonetheless, in all situations, the doxepin arm fared better than did the placebo arm.
Fig 2.
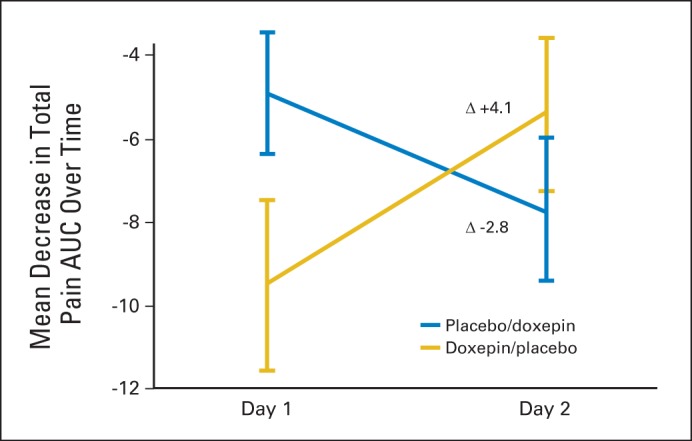
Crossover analysis of area under the curve (AUC) mouth and throat pain score, phases 1 and 2.
Fig 3.
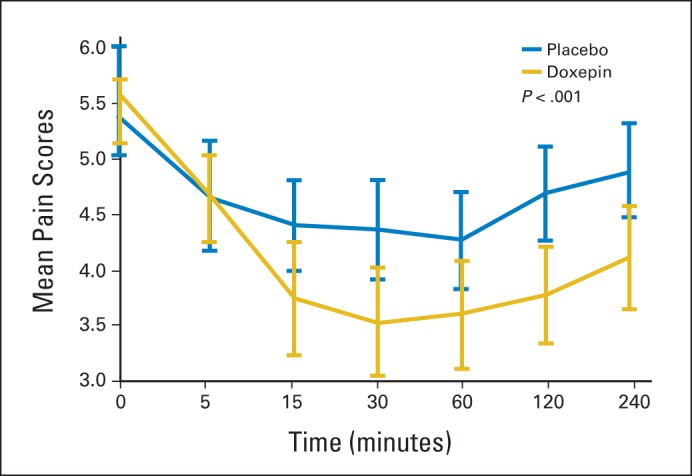
Average mouth and throat pain score over time, doxepin versus placebo, phase 1.
Other Outcomes
Analysis of the crossover data from the second phase revealed findings similar to the first phase with an AUC for the mean mouth and throat pain reduction of −7.9 for doxepin compared with −5.6 for placebo (difference, −2.3; 95% CI, −6.7 to −2.1; P < .001). The mean time between treatment in the first period and the crossover period was 2.1 days. This time was not significantly different between the two arms.
Stinging or burning from the oral rinses was analyzed for the initial dose by using a numerical analog scale of 0 (no stinging or burning) to 10 (worst stinging or burning possible). The AUC for the mean stinging or burning was significantly more for doxepin (9.6) compared with placebo (4.0; difference, 5.6; 95% CI, 2.9 to 8.3; P < .001). The mean scores for stinging or burning were maximal at 5 minutes (3.7 for doxepin v 1.1 for placebo) and decreased but remained statistically different over the 4 hours of assessment, as shown in the Data Supplement and in Figure A3 (online only).
The taste of the oral rinses was also assessed with a numerical analog scale (0 [acceptable] to 10 [terrible]), and AUC analysis showed that patients favored placebo (5.1) rinse over doxepin (7.7; difference, 2.6; 95% CI, 0.1 to 5.1; P = .0018). However, both rinses had taste that was acceptable with a mean score of 2.9 for doxepin compared with 1.6 for placebo at 5 minutes. These scores also declined over time such that by 1 hour, the difference in taste was no longer significant (mean for doxepin 1.1 v 0.9 for placebo).
Drowsiness, a known adverse effect of doxepin, was assessed with a numerical analog scale (0 [no drowsiness] to 10 [extreme drowsiness, leading to sleep]). AUC analysis showed that the placebo rinse (−2.4) made patients less drowsy than the doxepin rinse (−0.7; difference, 1.7; 95% CI, −1.2 to 4.6; P = .0297). The mean drowsiness scores increased from a similar baseline (3.2 for doxepin v 3.1 for placebo) for doxepin and decreased for placebo but this was not significantly different until the 2-hour assessment (3.9 for doxepin v 2.8 for placebo; P = .02).
The use of additional analgesic agents at the 2-hour (8.8% of the patients in the doxepin arm v 2.9% in the placebo arm; P = .1392) and 4-hour (16.9% of the patients in the doxepin arm v 14.5% in the placebo arm; P = .6989) time points was not significantly different between the arms. After each dose was administered, patients were asked if they would like to continue rinses with that particular agent. More patients in the doxepin arm expressed an interest in continuing treatment than patients in the placebo arm (phase 1: 77.3% v 51.5%; P = .0018; phase 2: 69.8% v 43.9%; P = .004). After both doses were administered and results were unblinded, 81 (63%) of the then eligible patients chose to continue with doxepin rinses and completed weekly assessments.
The crossover data in the second phase also confirmed that doxepin had more stinging and burning and worse taste and also caused more drowsiness (again at the 2-hour time point after administration). Similarly, there was no difference between doxepin and placebo arms in the reported use of additional analgesia at the 2- and 4-hour time points.
During the initial and crossover phases of the trial, there were no other toxic effects that were more common in the active arm than in the placebo arm and none that were higher than grade 3. Of the 81 patients that continued doxepin rinses, 14 patients (17%) discontinued the rinses; adverse effects of burning discomfort and increased drowsiness were the most frequently reported reasons.
DISCUSSION
This randomized controlled trial of doxepin as an anesthetic and analgesic rinse for treatment of oral mucositis pain from head and neck radiotherapy resulted in modest but significant mouth and throat pain score reductions over a 4-hour period after a single doxepin rinse that were confirmed by similar findings with a crossover dose. Pain scores did not return to baseline over the 4-hour assessment period after the single 1-minute oral rinse. The doxepin rinse resulted in more adverse effects than placebo, so it is not clear whether patients chose to continue doxepin rinses because they guessed that they were on doxepin or because pain reduction prevailed over the adverse effect profile. Despite this uncertainty, the majority of patients elected to continue doxepin rinse therapy after the initial test rinses and found repeated doses of doxepin to be beneficial. No significant difference was seen in the minority of patients in the doxepin and placebo arms who required additional analgesia at the 2- and 4-hour time points after the test rinse. Although the doxepin arm did have more oropharyngeal primaries and fewer oral cavity primaries than placebo, these differences were not statistically significant. In addition, the crossover design of the trial showed a reduction in pain for both groups, supporting that there is not a bias caused by an imbalance of one arm versus the other. The results of this trial confirm the beneficial and sustained effect of doxepin rinse in decreasing oral mucositis pain that was seen in the two smaller single-arm trials that preceded it.12–15
The adverse effect profile of doxepin rinse was also similar to that seen in the prior phase I/II trials. Doxepin was well tolerated, although compared with placebo, the doxepin rinse had more stinging and burning and worse taste, and it also caused more drowsiness. These adverse effects were typically mild. Stinging and burning with doxepin rinse was reported in the antecedent single-arm trials.12–15 This mild adverse effect was persistent throughout the 4-hour postrinse assessment. The prolonged duration may be due to a combined agonist-antagonist effect on mucosal nociceptors; however, that did not seem to limit its tolerance. Some patients who continued the rinses noted that the mild sedative effect from doxepin was beneficial as a sleep aid; however, it did limit therapy continuation in a minority of patients. This increased drowsiness seen in this study is likely the result of some systemic doxepin absorption through damaged oral mucosa, even though the rinses were limited to just 1 minute in duration.
Although numerous agents and interventions have been tested over the past three decades to prevent and/or treat painful oral mucositis resulting from cytotoxic therapy—be it radiotherapy, chemotherapy or both—few have been proven to be effective.25–27 Only two agents, the mucosal coating agent benzydamine hydrochloride28 and recombinant human keratinocyte growth factor (palifermin),29,30 have been shown to be more effective than placebo in the prophylaxis of oral mucositis from head and neck radiotherapy. This trial is the largest placebo-controlled trial to date specifically testing the efficacy of a rinse agent in controlling established mucositis pain and the only such trial with positive results. Several smaller phase III trials of other rinse agents, including chlorhexidine,31 “magic mouthwash,”31 phenytoin,32 sucralfate,33 and diclofenac,25 have not demonstrated benefit in controlling pain from established mucositis when compared with placebo.
The limitations of this study include a lack of longitudinal comparison data with placebo beyond one or two rinses and the minimal data collected in the continuation phase of the study with respect to quality of life, weight loss, incidence of supplemental nutrition, need for treatment breaks, and narcotic usage to evaluate possible narcotic dose reduction and any narcotic interactions with doxepin (although none were reported). In addition, this study lacks comparative effectiveness testing with magic mouthwash rinses that are widely prescribed in clinical practice despite the lack of trial data supporting their use.
In conclusion, the results of this randomized phase III trial demonstrate that a doxepin rinse is statistically significantly superior to a placebo in treating oral mucositis pain from head and neck radiotherapy with or without chemotherapy. However, further study is warranted to fully elucidate the use of this doxepin rinse in this setting.
Supplementary Material
Acknowledgment
We thank the patients who participated in the study, the study site personnel, and the members of the data safety monitoring committee.
Presented at the 54th Annual Meeting of the American Society for Therapeutic Radiology and Oncology, Boston, MA, October 28-31, 2012.
Appendix
The following is a list of principal investigators who participated in the study and their institutions: James Dewitt Bearden III, MD, Spartanburg Regional Medical Center, Spartanburg, SC; Robert J. Behrens, MD, Medical Oncology&Hematology Associates, Des Moines, IA; Jeffrey L. Berenberg, MD, University of Hawaii Cancer Center, Honolulu, HI; Shaker R. Dakhil, MD, Wichita Community Clinical Oncology Program (CCOP), Wichita, KS; Zoneddy Ruiz Dayao, MD, University of New Mexico, Albuquerque, NM; Patrick James Flynn, MD, Metro-Minnesota CCOP, Saint Louis Park, MN; Howard M. Gross, MD, Hematology&Oncology of Dayton, Dayton, OH; Michele Yvette Halyard, MD, Mayo Clinic Arizona, Scottsdale, AZ; Kurt A. Jaeckle, MD, Mayo Clinic Florida, Jacksonville, FL; Anthony John Jaslowski, MD, Green Bay Oncology at St Mary's Hospital Medical Center, Green Bay, WI; Donald James Jurgens, MD, CentraCare Clinic, Saint Cloud, MN; Benjamin T. Marchello, MD, Frontier Cancer Center and Blood Institutes-Billings, Billings, MT; Miroslaw Andrzej Mazurczak, MD, Sanford Cancer Center Oncology Clinic, Sioux Falls, SD; Rex B. Mowat, MD, Toledo Community Hospital Oncology Program CCOP, Toledo, OH; Gilbert D.A. Padula, MD, Saint Mary's Health Care, Grand Rapids, MI; Eduardo R. Pajon Jr, MD, Colorado Cancer Research Program CCOP, Denver, CO: Kendrith M. Rowland Jr, MD, Carle Foundation-Carle Cancer Center, Urbana, IL; Gamini S. Soori, MD, Missouri Valley Cancer Consortium CCOP, Omaha, NE; Preston D. Steen, MD, Sanford Medical Center-Fargo, Fargo, ND; Philip J. Stella, MD, St Joseph Mercy Health System, Ann Arbor, MI; Matthias Weiss, MD, Marshfield Clinic-Minocqua Center, Minocqua, WI; Deborah Weil Wilbur, MD, Mercy Hospital, Cedar Rapids, IA; Robin T. Zon, MD, Michiana Hematology Oncology PC-South Bend, South Bend, IN.
Fig A1.
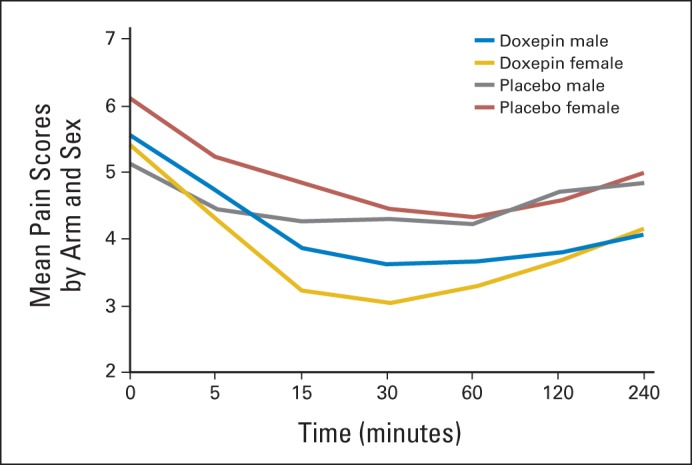
Mean pain scores over time by arm and sex.
Fig A2.
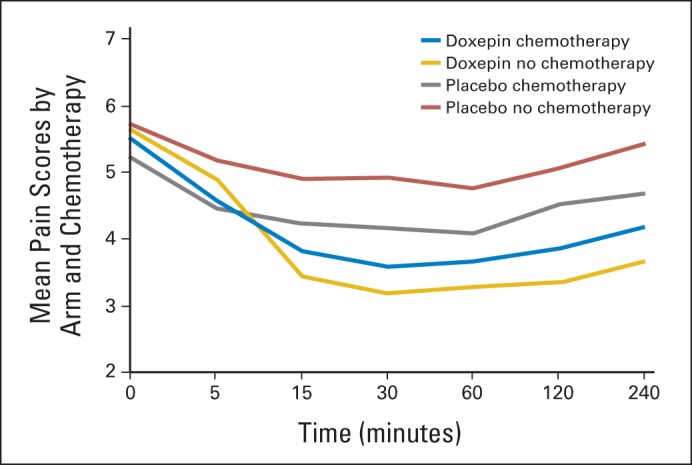
Mean pain scores over time by arm and chemotherapy.
Fig A3.
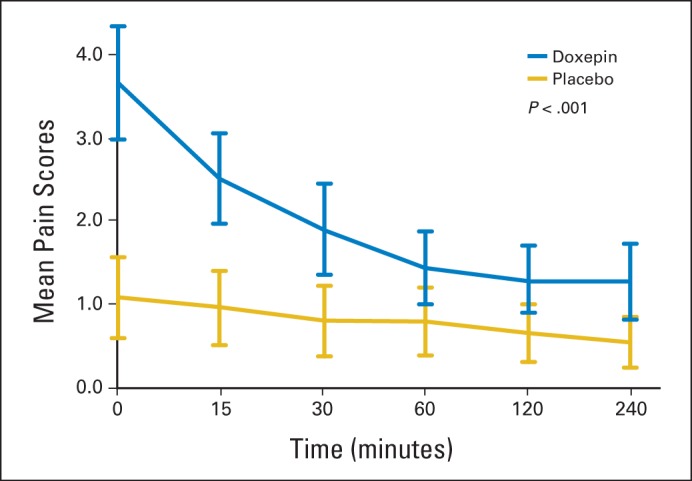
Mean stinging and burning scores over time.
Footnotes
Support information appears at the end of this article.
Written on behalf of the Alliance for Clinical Trials in Oncology.
The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the National CancerInstitute.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information: NCT01156142.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTSOF INTEREST
Although all authors completed the disclosure declaration, the following author(s) and/or an author's immediate family member(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: None Stock Ownership: None Honoraria: None Research Funding: Robert C. Miller, Pfizer Expert Testimony: None Patents, Royalties, and Licenses: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: James L. Leenstra, Robert C. Miller, Rui Qin, James A. Martenson, Paul J. Novotny, Robert L. Foote
Provision of study materials or patients: James L. Leenstra, Robert C. Miller, James A. Martenson, Kenneth J. Dornfeld, James D. Bearden, Philip J. Stella, Miroslaw A. Mazurczak, Marie D. Klish
Collection and assembly of data: James L. Leenstra, Robert C. Miller, Rui Qin, Paul J. Novotny
Data analysis and interpretation: James L. Leenstra, Robert C. Miller, Rui Qin, James A. Martenson, Kenneth J. Dornfeld, James D. Bearden, Dev R. Puri, Philip J. Stella, Miroslaw A. Mazurczak, Marie D. Klish, Paul J. Novotny, Charles L. Loprinzi
Manuscript writing: All authors
Final approval of manuscript: All authors
Support
Supported, in part, by Grants No. CA31946 from the National Cancer Institute to the Alliance for Clinical Trials in Oncology (North Central Cancer Treatment Group [NCCTG] N09C6) and No. CA33601 from the National Cancer Institute to the Alliance Statistics and Data Center, and by Grant No. CA 124477 from the National Institutes of Health. Funding was provided by the Alliance for Clinical Trials in Oncology and the National Cancer Institute (J.L.L.) and by the Alliance for Clinical Trials in Oncology and the NCCTG (J.A.M.).
REFERENCES
- 1.Lalla RV, Sonis ST, Peterson DE. Management of oral mucositis in patients who have cancer. Dent Clin North Am. 2008;52:61–77. doi: 10.1016/j.cden.2007.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Saadeh CE. Chemotherapy- and radiotherapy-induced oral mucositis: Review of preventive strategies and treatment. Pharmacotherapy. 2005;25:540–554. doi: 10.1592/phco.25.4.540.61035. [DOI] [PubMed] [Google Scholar]
- 3.Wong PC, Dodd MJ, Miaskowski C, et al. Mucositis pain induced by radiation therapy: Prevalence, severity, and use of self-care behaviors. J Pain Symptom Manage. 2006;32:27–37. doi: 10.1016/j.jpainsymman.2005.12.020. [DOI] [PubMed] [Google Scholar]
- 4.Elting LS, Cooksley CD, Chambers MS, et al. Risk, outcomes, and costs of radiation-induced oral mucositis among patients with head-and-neck malignancies. Int J Radiat Oncol Biol Phys. 2007;68:1110–1120. doi: 10.1016/j.ijrobp.2007.01.053. [DOI] [PubMed] [Google Scholar]
- 5.Trotti A, Bellm LA, Epstein JB, et al. Mucositis incidence, severity and associated outcomes in patients with head and neck cancer receiving radiotherapy with or without chemotherapy: A systematic literature review. Radiother Oncol. 2003;66:253–262. doi: 10.1016/s0167-8140(02)00404-8. [DOI] [PubMed] [Google Scholar]
- 6.Russo G, Haddad R, Posner M, et al. Radiation treatment breaks and ulcerative mucositis in head and neck cancer. Oncologist. 2008;13:886–898. doi: 10.1634/theoncologist.2008-0024. [DOI] [PubMed] [Google Scholar]
- 7.Rosenthal DI, Trotti A. Strategies for managing radiation-induced mucositis in head and neck cancer. Semin Radiat Oncol. 2009;19:29–34. doi: 10.1016/j.semradonc.2008.09.006. [DOI] [PubMed] [Google Scholar]
- 8.Bensinger W, Schubert M, Ang KK, et al. NCCN Task Force Report: Prevention and management of mucositis in cancer care. J Natl Compr Canc Netw. 2008;6(suppl 1):S1–S21. [PubMed] [Google Scholar]
- 9.Kramp LF, Eleazer PD, Scheetz JP. Evaluation of prilocaine for the reduction of pain associated with transmucosal anesthetic administration. Anesth Prog. 1999;46:52–55. [PMC free article] [PubMed] [Google Scholar]
- 10.Barasch A, Elad S, Altman A, et al. Antimicrobials, mucosal coating agents, anesthetics, analgesics, and nutritional supplements for alimentary tract mucositis. Support Care Cancer. 2006;14:528–532. doi: 10.1007/s00520-006-0066-1. [DOI] [PubMed] [Google Scholar]
- 11.Sudoh Y, Cahoon EE, Gerner P, et al. Tricyclic antidepressants as long-acting local anesthetics. Pain. 2003;103:49–55. doi: 10.1016/s0304-3959(02)00375-5. [DOI] [PubMed] [Google Scholar]
- 12.Epstein JB, Truelove EL, Oien H, et al. Oral topical doxepin rinse: Analgesic effect in patients with oral mucosal pain due to cancer or cancer therapy. Oral Oncol. 2001;37:632–637. doi: 10.1016/s1368-8375(01)00005-7. [DOI] [PubMed] [Google Scholar]
- 13.Epstein JB, Epstein JD, Epstein MS, et al. Oral doxepin rinse: The analgesic effect and duration of pain reduction in patients with oral mucositis due to cancer therapy. Anesth Analg. 2006;103:465–470. doi: 10.1213/01.ane.0000223661.60471.78. [DOI] [PubMed] [Google Scholar]
- 14.Epstein JB, Epstein JD, Epstein MS, et al. Doxepin rinse for management of mucositis pain in patients with cancer: One week follow-up of topical therapy. Spec Care Dentist. 2008;28:73–77. doi: 10.1111/j.1754-4505.2008.00015.x. [DOI] [PubMed] [Google Scholar]
- 15.Epstein JB, Epstein JD, Epstein MS, et al. Management of pain in cancer patients with oral mucositis: Follow-up of multiple doses of doxepin oral rinse. J Pain Symptom Manage. 2007;33:111–114. doi: 10.1016/j.jpainsymman.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 16.Sonis ST, Eilers JP, Epstein JB, et al. Validation of a new scoring system for the assessment of clinical trial research of oral mucositis induced by radiation or chemotherapy: Mucositis Study Group. Cancer. 1999;85:2103–2113. doi: 10.1002/(sici)1097-0142(19990515)85:10<2103::aid-cncr2>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 17.World Health Organization. WHO Handbook for Reporting Results of Cancer Treatment. Geneva, Switzerland: 1979. pp. 15–22. [Google Scholar]
- 18.Pocock SJ, Simon R. Sequential treatment assignment with balancing for prognostic factors in the controlled clinical trial. Biometrics. 1975;31:103–115. [PubMed] [Google Scholar]
- 19.Elting LS, Keefe DM, Sonis ST, et al. Patient-reported measurements of oral mucositis in head and neck cancer patients treated with radiotherapy with or without chemotherapy: Demonstration of increased frequency, severity, resistance to palliation, and impact on quality of life. Cancer. 2008;113:2704–2713. doi: 10.1002/cncr.23898. [DOI] [PubMed] [Google Scholar]
- 20.Stiff PJ, Emmanouilides C, Bensinger WI, et al. Palifermin reduces patient-reported mouth and throat soreness and improves patient functioning in the hematopoietic stem-cell transplantation setting. J Clin Oncol. 2006;24:5186–5193. doi: 10.1200/JCO.2005.02.8340. [DOI] [PubMed] [Google Scholar]
- 21.Stiff PJ, Erder H, Bensinger WI, et al. Reliability and validity of a patient self-administered daily questionnaire to assess impact of oral mucositis (OM) on pain and daily functioning in patients undergoing autologous hematopoietic stem cell transplantation (HSCT) Bone Marrow Transplant. 2006;37:393–401. doi: 10.1038/sj.bmt.1705250. [DOI] [PubMed] [Google Scholar]
- 22.U.S. Department of Health and Human Services. CTEP Active Version of the NCI Common Terminology Criteria for Adverse Events. http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_5×7.pdf.
- 23.Freeman PR. The performance of the two-stage analysis of two-treatment, two-period crossover trials. Stat Med. 1989;8:1421–1432. doi: 10.1002/sim.4780081202. [DOI] [PubMed] [Google Scholar]
- 24.Sloan J, Symonds T, Vargas-Chanes D, et al. Practical guidelines for assessing the clinical significance of health-related quality of life changes within clinical trials. Drug Inf J. 2003;37:23–31. [Google Scholar]
- 25.Clarkson JE, Worthington HV, Furness S, et al. Interventions for treating oral mucositis for patients with cancer receiving treatment. Cochrane Database Syst Rev. 2010;8 doi: 10.1002/14651858.CD001973.pub4. CD001973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rodríguez-Caballero A, Torres-Lagares D, Robles-García M, et al. Cancer treatment-induced oral mucositis: A critical review. Int J Oral Maxillofac Surg. 2012;41:225–238. doi: 10.1016/j.ijom.2011.10.011. [DOI] [PubMed] [Google Scholar]
- 27.Worthington HV, Clarkson JE, Bryan G, et al. Interventions for preventing oral mucositis for patients with cancer receiving treatment. Cochrane Database Syst Rev. 2011;4 doi: 10.1002/14651858.CD000978.pub5. CD000978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Epstein JB, Silverman S, Jr, Paggiarino DA, et al. Benzydamine HCl for prophylaxis of radiation-induced oral mucositis: Results from a multicenter, randomized, double-blind, placebo-controlled clinical trial. Cancer. 2001;92:875–885. doi: 10.1002/1097-0142(20010815)92:4<875::aid-cncr1396>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 29.Le QT, Kim HE, Schneider CJ, et al. Palifermin reduces severe mucositis in definitive chemoradiotherapy of locally advanced head and neck cancer: A randomized, placebo-controlled study. J Clin Oncol. 2011;29:2808–2814. doi: 10.1200/JCO.2010.32.4095. [DOI] [PubMed] [Google Scholar]
- 30.Henke M, Alfonsi M, Foa P, et al. Palifermin decreases severe oral mucositis of patients undergoing postoperative radiochemotherapy for head and neck cancer: A randomized, placebo-controlled trial. J Clin Oncol. 2011;29:2815–2820. doi: 10.1200/JCO.2010.32.4103. [DOI] [PubMed] [Google Scholar]
- 31.Dodd MJ, Dibble SL, Miaskowski C, et al. Randomized clinical trial of the effectiveness of 3 commonly used mouthwashes to treat chemotherapy-induced mucositis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000;90:39–47. doi: 10.1067/moe.2000.105713. [DOI] [PubMed] [Google Scholar]
- 32.Baharvand M, Sarrafi M, Alavi K, et al. Efficacy of topical phenytoin on chemotherapy-induced oral mucositis: A pilot study. Daru. 2010;18:46–50. [PMC free article] [PubMed] [Google Scholar]
- 33.Dodd MJ, Miaskowski C, Greenspan D, et al. Radiation-induced mucositis: A randomized clinical trial of micronized sucralfate versus salt&soda mouthwashes. Cancer Invest. 2003;21:21–33. doi: 10.1081/cnv-120016400. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.